Abstract
Mesenchymal stem/stromal cells (MSCs) are a promising resource for cell-based therapy because of their high immunomodulation ability, tropism towards inflamed and injured tissues, and their easy access and isolation. Currently, there are more than 1200 registered MSC clinical trials globally. However, a lack of standardized methods to characterize cell safety, efficacy, and biodistribution dramatically hinders the progress of MSC utility in clinical practice. In this review, we summarize the current state of MSC-based cell therapy, focusing on the systemic safety and biodistribution of MSCs. MSC-associated risks of tumor initiation and promotion and the underlying mechanisms of these risks are discussed. In addition, MSC biodistribution methodology and the pharmacokinetics and pharmacodynamics of cell therapies are addressed. Better understanding of the systemic safety and biodistribution of MSCs will facilitate future clinical applications of precision medicine using stem cells.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12929-021-00725-7.
Keywords: Mesenchymal stem/stromal cell, Cell therapy, Systemic safety, biodistribution, Single cell imaging
Introduction
Cell therapy has become one of the most important emerging medical treatments in the world. Treatments utilizing stem cells, induced pluripotent stem cells (iPSCs), somatic cells, and immune cells are well documented [1]. Many cell therapy products have already received global market approval. Among them, the mesenchymal/stromal stem cells (MSCs) present a promising tool for the treatment of various diseases.
MSCs were first isolated and described by Friedenstein and his colleagues as adherent and highly replicative cells that can differentiate into mesodermal lineages including osteoblasts, chondrocytes, adipocytes, and hematopoietic stroma [2]. Since then, these cells have gained attention in the field of cell therapy for their tropism towards injured/inflamed tissues, their immunomodulatory capabilities [3], and their relative ease of isolation and expansion [4]. MSCs can be isolated from many sources, including bone marrow [5], umbilical cord [6], adipose tissue [7], cord blood [6], placenta [8], dental pulp [9], endometrium [10], amniotic fluid [11], skeletal muscle tissue [12], lung tissue [13], liver tissue [7, 12] and dermal tissue [12], and many of these cells have been used in clinical studies (Fig. 1a). The characteristics of MSCs make them attractive as cellular therapeutic agents for regenerative medicine and immune-related diseases.
Fig. 1.
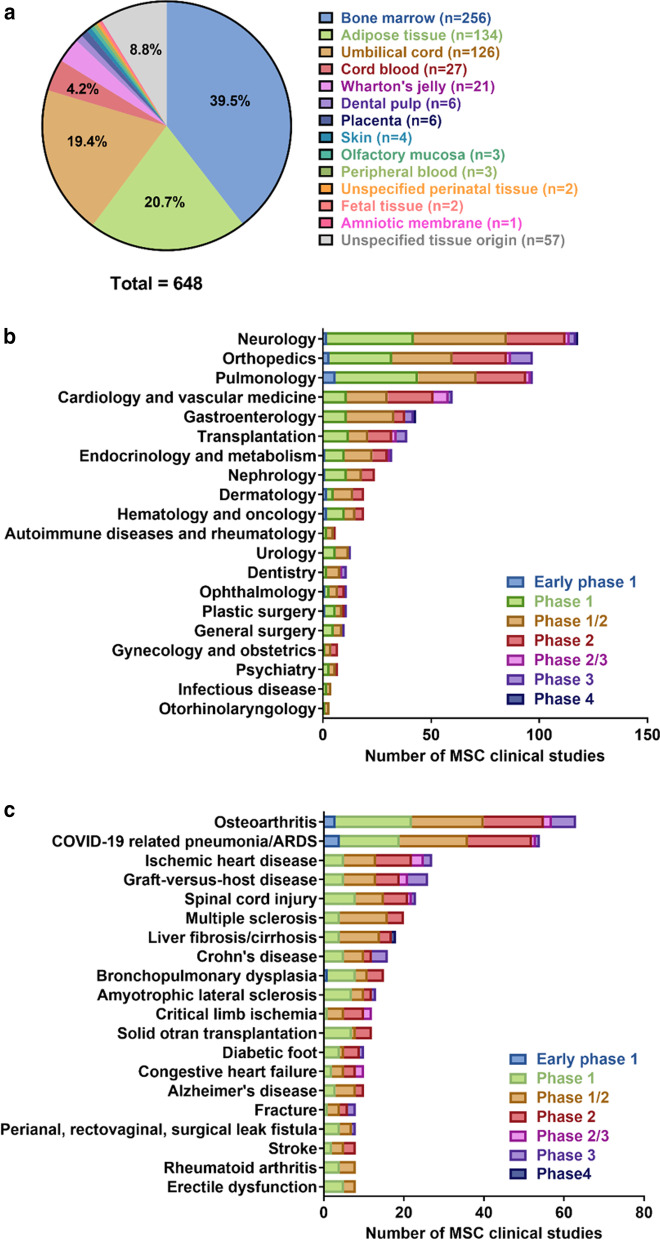
MSC sources and clinical indications in clinical studies. As of October 11, 2020, 1,242 registered studies were identified on clinicaltrials.gov by searching keywords “mesenchymal stem cell” or “mesenchymal stromal cell” (Additional file 1). After excluding studies with no longer available/ suspended/ temporarily not available/ terminated/ unknown/ withdrawn status, unknown phase information, and studies that did not use MSCs in their intervention arm, 639 studies remained. Nine of these 639 studies investigated MSCs from two tissue origins, generating a total of 648 studies for analysis. a Tissue origins of MSCs in clinical studies, b number of MSC-related clinical studies by medical specialty, and c the top 20 disease indications of MSC-related clinical studies
The first clinical trial of MSCs was reported in 1995 in patients with hematologic malignancies. Lazarus et al. demonstrated that ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (BMMSCs) in patients caused no severe adverse effects [14]. Subsequently, treatment with BMMSCs was shown to provide clinical improvement in the rare skeletal disease osteogenesis imperfecta [15]. Furthermore, many clinical trials have examined the feasibility and efficacy of MSCs for the treatment of various conditions, including acute organ failure [16–18], graft-versus-host disease (GVHD) [19–21], ischemic heart disease [22, 23], cardiovascular disease [24, 25], liver cirrhosis [26], diabetes [27, 28], spinal cord injury [29–31], and bone/cartilage injury [32–37] (Table 1). According to the National Institutes of Health (http://www.clinicaltrial.gov/), the number of registered MSC-based clinical trials was over 1,200 as of October 11, 2020, of which approximately 600 had defined phase and status (Fig. 1b, c, Additional file 1 and Additional file 2). Most of the studies to date are phase 1 and phase 2 trials which evaluate safety and feasibility, and evidence of therapeutic efficacy is still lacking (Fig. 1). The most common indications of MSC-based cellular therapy include osteoarthritis, ischemic heart disease, graft-versus-host disease, spinal cord injury, and multiple sclerosis (Fig. 1c). In addition, since the elevation of coronavirus disease-19 (COVID-19) outbreak to pandemic status on March 11, 2020 [38], numerous MSC-based studies have been registered, and COVID-19 related pneumonia and acute respiratory distress syndrome (ARDS) has risen as the second most common indication as of October 11, 2020 (Fig. 1c). The rapid global response and increase of COVID-19 related MSC trials highlighted the promise of MSCs in treatment of inflammatory and immune diseases.
Table 1.
Summary of MSC-based clinical/preclinical trials
| Indication | Cell source | Model | Quantification modality | In vivo distribution |
|---|---|---|---|---|
| Acute organ failure | Bone marrow, Bone | Rat [17, 18] | Histology/RT-PCR | More exogenous human MSCs localized to injured tissues |
| Graft-versus-host disease (GvHD) | Bone marrow | Patients [19] | PCR | MSC DNA detected in lymph nodes |
| Ischemic disease | Bone marrow | Swine [22, 23] | Histology/qPCR | DAPI staining confirmed rapid cell loss after transplantation |
| Lung cancer | Umbilical cord | Mouse [252] | PET-CT | MSCs remained in the lungs up to 1 week after injection |
| Liver cirrhosis | Bone marrow | Patients [26] | Planar whole-body acquisitions/SPECT | MSCs accumulated in the lung first, MSCs in the liver increased from 0.0%–2.8% to 13.0%–17.4% in 10 days |
| Diabetes | Bone marrow | Rat [28] | Histology/qPCR | MSCs detected in the diabetic kidneys at 24 and 48 h after cell infusion. Cell engraftment also observed in spleen and thymus at 24 h |
| Spinal cord injury | Bone marrow | Rat [299] | CT/MRI | After transplantation of BMMSCs, the hypersignal emerged in spinal cord in T1WI starting at day 7 that was focused at the injection site, which then increased and extended until day 14 |
| Cartilage/bone injury | Adipose | Rabbit [37] | MRI | Representative tibial joint, regenerated meniscus and joint surface of tibia at 6 and 12 weeks after surgery |
BM bone marrow, MSCs mesenchymal stem/stromal cells, PCR polymerase chain reaction, PET positron emission tomography, SPECT single-photon emission computed tomography, MRI magnetic resonance imaging, CT computed tomography
Although studies on MSCs are well-documented, MSC-based cellular products still have not been approved by the US Food and Drug Administration. The lack of consistent and standardized methods for characterizing the safety and efficacy of MSC products is a major concern, which dramatically slows the progress of MSC therapy towards clinical use. The safety of cellular products is always the first priority. Although some MSCs have been shown to be safe for clinical use in a previous meta-analysis, whether this conclusion can be extended to MSCs from other tissue origins or different culture conditions is still uncertain (Fig. 1a) [39]. The risk associated with MSC products centers around their capability to initiate and promote tumors. These risks, as well as the biodistribution of systemically administered cells must be better clarified before the widespread use of MSCs in clinical practice. In this review article, we focus on the effects of MSCs on tumor promotion and suppression, and discuss methods to study their biodistribution.
MSC-based mechanisms of action
Several possible mechanisms by which MSCs exert their beneficial effects have been proposed. Early studies reported that MSCs could migrate to sites of injury and then differentiate into functional cells [40], or that they could fuse with compromised cells to regenerate damaged tissues [41, 42]. More recent studies have demonstrated that paracrine factors [43, 44], mitochondrial transfer [45], and extracellular vesicle secretion [46] have important roles in mediating the effects of MSCs.
Paracrine effects
MSCs secrete paracrine factors, including cytokines, chemokines, growth factors, and miRNAs. MSC transplantation or administration of isolated secreted factors enables MSC paracrine factors to get to injured tissues, to help restore a healthy microenvironment to promote tissue repair [47] (Table 2). MSC paracrine factors play important roles in immunomodulation [48, 49], tissue regeneration and healing [50, 51], anti-fibrosis [52, 53], anti-apoptosis [54], and angiogenesis [55]. As such, many studies have focused on altering culture conditions in order to steer the secretome of MSCs towards therapeutic agents. Alterations have included using MSCs from different types of tissue [56, 57], oxygen concentration [58], growth factor incubation or cytokine pretreatment [59], passage number [60–62], three-dimensional spheroid culturing [63], and mechanical strain [64].
Table 2.
MSC secreted factors involved in tumor promotion
| Factors involved in tumor promotion | ||
|---|---|---|
| Cytokines | IL6, TGF-β1, IL-8 | [125, 132, 133, 139, 147, 150, 159, 162, 165] |
| Chemokines | SDF-1, CXCL1, CCL2, CCL5 | [123, 124, 136, 142–144, 150, 160, 162] |
| Angiogenic factors | VEGF, Ang-1, PDGF, IGF | [148, 162] |
| Growth factor | NRG1 | [135] |
| Other factors | periostin, PAI-1, Sema-7A | [134, 162] |
| microRNAs | miR-21-5p, miR-410, MiR-142-3p, miR-23b | [126, 136, 145, 158] |
The capability of MSCs for immunomodulation has made them a useful treatment approach for inflammatory disorders such as multiple sclerosis [65], Crohn’s disease [66], GVHD [67], systemic lupus erythematosus [67], and type I diabetes [68]. Immunomodulation is dependent on crosstalk between MSCs and the immune microenvironment of the target tissue. In an inflammatory microenvironment, proinflammatory cytokines, including IL-1β, IL-6, IL-23, IFN-γ, and TNF-α, can stimulate MSCs to secrete anti-inflammatory factors such as TNFα stimulated gene (TSG)-6 [69], nitric oxide (NO) [70], IL-10 [71], galectins [72], prostaglandin E2 (PGE2) [73], and transforming growth factor (TGF)-β [3, 71]. Upon exposure to these MSC-secreted anti-inflammatory signals, nuclear factor (NF)-κB activity and consequent inflammatory cytokine expression in macrophages, dendritic cells, and T cells are inhibited, and immune cells will express higher levels of anti-inflammatory cytokine IL-10 as a result [3, 74]. The MSC paracrine factors also interact with other immune cells and have been reported to skew macrophage polarization towards the M2 phenotype, which downregulates both innate and adaptive immune responses [75]. Regulatory T cells (Treg) were also reported to stimulate MSCs to secrete indoleamine 2,3-dioxygenase (IDO), thereby augmenting the Treg response and attenuating acute liver injury [3, 76].
In addition to their immunomodulation ability, MSCs are able to secrete factors that can promote cell proliferation, increase angiogenesis, and reduce cell apoptosis. For example, MSCs can secrete growth and angiogenesis-promoting factors such as basic fibroblast growth factor (bFGF) [77], insulin-like growth factor (IGF) [78], TGF-β [3, 55], stromal cell-derived factor (SDF)-1α [79], secreted frizzled-related protein-1/2 (SFRP1/2) [80, 81], angiopoietins, and vascular endothelial growth factor (VEGF) [82, 83].
It has been demonstrated that MSCs can inhibit fibrosis via paracrine factors [84]. Chronic inflammation is a major factor that drives the fibrosis process, which can alter the normal architectural structure of tissues and lead to deteriorated functioning. Because MSCs can be used to reduce inflammation, they have become an attractive therapeutic strategy for suppressing fibrosis. MSC-derived conditioned medium (CM) was shown to attenuate liver fibrosis by reducing Th17 cells in a IDO-dependent manner [85]. MSC-secreted interleukin 1 receptor antagonist (IL-1Ra) was also shown to inhibit stellate cell activation and decrease type I collagen expression, a key component of liver fibrosis [86]. Administration of MSC-CM also reduced fibrotic score and collagen deposition in both bleomycin- and silica-induced lung injury models [87, 88]. In MSC-treated cells, levels of HGF, KGF, and BMP-7 increased while levels of TGF-β1 and TNF-α decreased. These results suggest that the anti-fibrotic effect of MSCs may be mediated via paracrine mechanisms [88]. In support of this, a bleomycin-induced lung injury model showed that the stanniocalcin-1 (STC-1) secreted by MSCs in response to TGF-β1 exerted antifibrotic effects by reducing oxidative stress, endoplasmic reticulum (ER) stress, and TGF-β1 production in alveolar epithelial cells [89]. Likewise, MSCs were able to decrease the expression of fibrosis-associated tissue inhibitor of matrix metalloproteinase 1 (TIMP)-1, to improve cardiac function in a myocardial infarction model [90].
Mitochondrial transfer
Mitochondrial dysfunction is a hallmark of the aging process, and has been implicated in the pathogenesis of numerous diseases [91]. MSC-based mitochondrial transfer has therefore been a promising therapeutic strategy, by either replenishing or replacing the damaged mitochondria in targeted diseased cells [92]. Studies have observed increased tunneling nanotube (TNT) and gap junction formation with mitochondrial transfer between MSCs and injured epithelial/endothelial cells under inflammatory or hypoxic conditions, and MSC-derived mitochondria transfers could prevent apoptosis of recipient cells [93–95]. In addition, it was found that iPSC-derived MSCs could attenuate alveolar damage and fibrosis via mitochondrial transfer by TNT [96]. The tissue origin of MSCs may affect mitochondrial transfer ability. For example, iPSC-derived MSCs were shown to be more effective at mitochondria transfer compared with MSCs derived from bone marrow [96]. Mechanistically, mitochondrial transfer was found to alleviate epithelial injury through mitochondrial Rho-GTPase Miro1 regulation in an asthma model [97].
Despite these beneficial findings of MSC-mediated mitochondrial transfer, there are also potential risks, as mitochondrial transfer can increase the risk of tumor promotion. In acute myeloid leukemia (AML), NOX2 stimulated mitochondrial transfer from BMMSCs to cancer cells, and this promoted the survival of the cancer cells [98]. Mitochondrial transfer also increased the resistance of leukemic cells to chemotherapeutic agents, and transfer occurred bidirectionally [99, 100]. In an in vitro co-culture of BMMSCs and T cell acute lymphoblastic leukemia (T-ALL) cells, upon induction of oxidative stress by the addition of chemotherapeutic agents, T-ALL cells transferred their mitochondria to BMMSCs, but received few mitochondria from the BMMSCs, raising the chemoresistance of the T-ALL cells [99]. Neutralizing the cell adhesion molecule ICAM-1 and disrupting intercellular mitochondrial transfer restored the sensitivity of the T-ALL cells to the chemotherapeutic agent [99].
Extracellular vesicle (EV) transfer
MSC-derived extracellular vesicles (EVs) have raised increasing interest as a non-cellular alternative to MSC-based therapy, as this approach eliminates concerns of unintended lineage differentiation [101]. EVs refer to exosomes, microvesicles, and apoptotic bodies, and are membrane-enclosed entities secreted by a cell in response to stimulation or apoptosis. The size and contents of these vesicles are highly variable and heterogeneous, involving proteins, mRNAs, and miRNAs [101]. Their role in MSC-mediated cellular therapy remains elusive due to their heterogeneous nature, but it is currently believed that they play an important role in many biological processes and intercellular communication [101].
Exosomes from MSCs have shown beneficial effects in disease models of autoimmune uveitis [102], retinal detachment [103], myocardial infarction [104], type 1 diabetes [105], wound healing [106], bone repair [107], burn injury [46], traumatic brain injury [108], spinal cord injury [109], and several other conditions [110]. The most commonly suggested mechanism responsible for the effects of exosomes is via their capability to regulate immune cells and immune microenvironments. MSC-derived exosomes can suppress the expression of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, IL-17, IFN-γ, and MIP-1α in immune cells [103, 105, 109, 111]. Additionally, MSC-derived exosomes significantly increased the levels of anti-inflammatory cytokines IL-4, IL-10, and TGF-β in a type 1 diabetes animal model [105]. In a drug-induced liver injury model, MSC-derived exosomes enhanced the local expression of cytokines TGF-β and HGF, both of which are key factors in liver regeneration [112]. The underlying mechanism involved changes in the immune cell population, including increased M2 polarization [106, 108, 109], increased Th2 and regulatory T cell differentiation [105, 112], decreased Th17 differentiation [111], and decreased local immune cell infiltration [102].
In addition to promoting immunomodulation, MSC-derived exosomes participate in other biological processes. MSC-derived exosomes were found to promote neoangiogenesis in diabetic and burn wounds via increased VEGF-A expression, the Wnt4/β-catenin pathway, and increased tube formation and proliferation of endothelial cells [106, 113]. MSC-derived exosomes also activate Akt, ERK, and STAT3 pathways and induce expression of HGF, IGF1, NGF, SDF1, and TGF-β, which critically regulate wound healing and tissue repair [114]. In addition, MSC-derived exosomes can aid in tissue repair by enhancing autophagy and inhibiting apoptosis [103].
In contrast to microvesicles and exosomes from MSCs, apoptotic bodies are entities specifically generated by cells during apoptosis. Apoptotic bodies containing ubiquitin ligase RNF146 and miR328-3p were shown to help maintain MSC multipotency via the Wnt/β-catenin pathway [115]. In support, it was recently shown that apoptotic bodies released from donor MSCs improved myocardial infarction via autophagy regulation in recipient cells [116].
The lack of consistent or standardized methods to isolate and identify EVs presents a challenge for current therapeutics. A recent study has shown that compared to EVs, MSC-CM resulted in more effective immunomodulation [117]. Further studies are necessary to decipher the optimal MSC culture conditions and the specific subpopulations of secreted components that contribute to the most effective therapeutic benefit.
Clinical applications of MSC-derived EVs have gained increasing interest, as many of the safety concerns of MSC-based therapy might be avoided, including undesired differentiation of implanted cells in tumor formation/promotion risks, and the cell-derived secondary ischemic damage by vessel clotting. As MSC-derived EVs are still in their clinical infancy, there is currently little information on clinical safety. To monitor biodistribution, most of the in vivo studies utilize lipophilic dyes to label the EVs [118, 119]. While the injected MSC-derived EVs migrated and accumulated at the injured tissue, they also aggregated in the lung, liver, and spleen [118, 119].
MSC safety consideration: Tumor initiation, promotion, and suppression
MSC-related cell therapy is a promising therapeutic strategy because of the high immune modulation ability and the absence of tumor initiation risk of MSCs. However, there is still concern that MSCs can pose a risk for promoting tumor cell growth [120, 121]. MSCs share some characteristics with fibroblast cells, which are able to transform into cancer-associated fibroblasts (CAFs) in tumor niches. The tumor niche involves local fibroblasts, endothelial cells, immune cells, and cancer associated MSCs. Increasing evidence shows that the tumor niche is not only trophic to cancer cells, but also highly associated with tumor initiation and growth, and is able to increase cancer stemness-related properties, including the capacity for cell migration, invasion, and chemotherapy resistance. Therefore, cancer treatment strategies have expanded from solely targeting the tumor cells, to altering the tumor milieu.
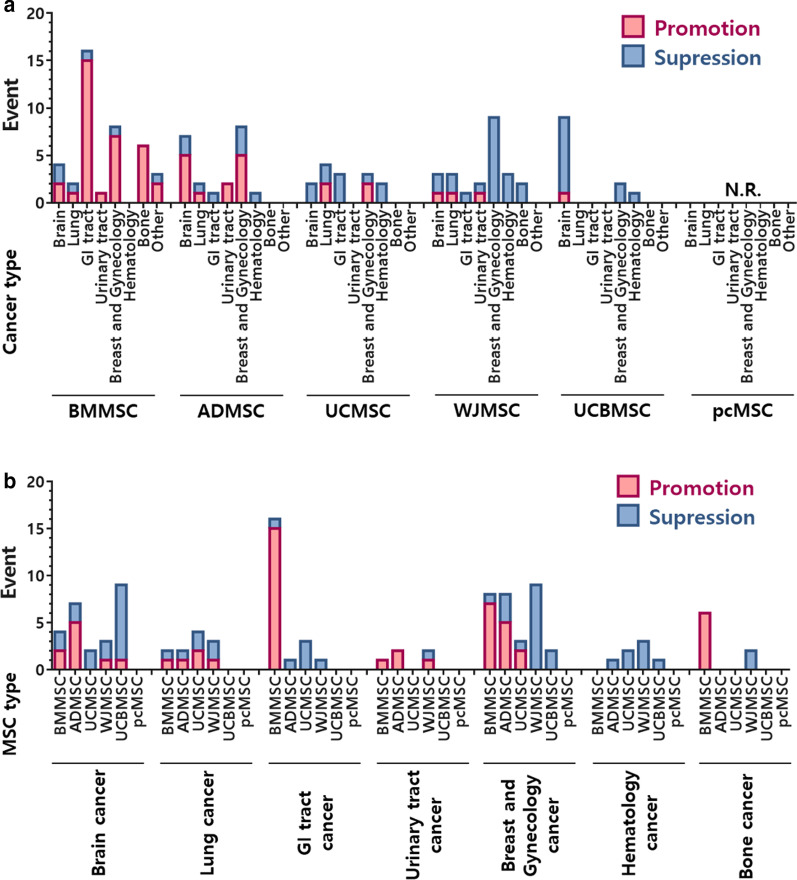
Since MSCs have an excellent ability for homing to tumor sites, the possibility for therapeutic MSCs to transform into cancer-associated MSCs exists. Several studies have examined the effect of MSCs on different types of tumor cells. Not surprisingly, conclusions among these studies are unclear (Fig. 2). Studies using MSCs from different tissue origins, different cultivation processes, and different cancers can lead to diverse results and interpretations.
Fig. 2.
Promotion and suppression effects of MSCs on different cancer types. Data analysis from published studies listed in Tables 4 and 5, but excluding engineered MSCs. N.R. not reported
On the other hand, taking advantage of the ability of MSCs to home to tumor sites enables MSCs to serve as therapeutic carriers that deliver anticancer agents to appropriate sites [122]. As highly progressive and late stage malignancies constitute a major health burden, for which current treatments are unsatisfactory and curative therapies are unavailable, MSC-related drug carriers may provide new hope for cancer treatments, particularly for late stage cancers.
MSC Promotion effects on tumor cell growth and metastasis
The underlying mechanisms responsible for MSC tumor promotion are complicated and diverse (Table 3). They are classified below according to MSC type and signaling pathway, and are listed systematically in Table 4 and summarized in Fig. 3.
Table 3.
MSC tumor promoting signaling pathways
| Tumor promoting signaling pathways | ||
|---|---|---|
|
TGF-β1 IL6 IL-8 |
Smad2/3, Akt/GSK-3β/β-catenin, PI3K/Akt, NF-κB, p38 MAPK JAK2/STAT3 FAK |
[159] [132] [140] |
|
SDF-1 CXCL1 CXCR4 CCL5 |
CXCR4, CXCR7 CXCR1/2 PI3K/Akt, Ras/Erk CCR1/β-catenin/Slug, CCR5 → CSF1 secretion → recruitment of TAM and MDSC |
[124] [150] [127] |
| NRG1 | HER2(HER3)/PI3K/AKT | [41] |
|
miR-21-5p miR-410 miR-142-3p miR-23b |
Downregulation of PTEN, PDCD4 and RECK; M2 polarization Downregulation of PTEN Activating Notch signalling by downregulation of Numb Downregulation of MARCKS |
[126] [158] [136] [145] |
| Direct contact | NOTCH | [136, 144, 149] |
Table 4.
MSC promotion effects in cancers
| Cancer type/MSC source | Surface marker | Effect | Factors/mechanisms | Ref. |
|---|---|---|---|---|
| Brain cancer | ||||
| Human bone marrow from ScienCell Research Laboratories | CD73+ CD90+ CD105+ | tumor-like phenotype transformation |
Glioma cell-derived exosomes, upregulating the levels of Glut-1, HK-2, and PKM-2, activating glycolysis in MSCs |
[125] |
| Human bone marrow of healthy donor | SH2+ SH3+ CD29+ CD44+ CD71+ CD90+ CD106+ CD120a+ CD124+ CD14− CD34− CD45− | Increased tumor metastasis | SDF-1/CXCR4 and SDF-1/CXCR7 signaling | [124] |
| Human adipose tissue from trochanteric fat of healthy donor | CD73+ CD90+ CD105+ CD45− | Increased tumor cell proliferation | MSC-EV | [281] |
| Human adipose tissue | CD31− CD45− | Increased tumor growth | Higher mRNA expression levels of angiogenic factors (VEGF, Ang-1, PDGF, and IGF) and SDF-1(CXCL12) in MSCs | [148] |
| Mouse adipose tissue | Not stated | Increased tumor migratory capacity | MSC-secreted conditioned medium increases vimentin, MMP2, and NRAS expression | [282] |
| Human adipose tissue from individuals receiving abdominoplasty | CD44+ CD90+ CD34− | Increased tumor growth; decreased apoptosis and H2O2-induced cancer cell death |
Co-injection in vivo Co-culture or MSCs-CM |
[154] |
| Human adipose tissue of individuals receiving abdominoplasty or mammoplasty | CD44+ CD105+ CD14− CD34− CD45− | Increased cancer cell migration; no significant effect on cancer cell proliferation, TMZ response, CSC traits | MSCs-CM | [282] |
| Human umbilical cord Wharton's jelly (human umbilical cord perivascular cells) | Sh2+ SH3+ Thy-1+ CD44+ CD34− CD45− | Increased tumor growth and migration; no significant effect on TMZ response | Cytokines from MSCs-CM (ex. CCL2, PDGF-C, Sema-7A, periostin, IL6) | [162] |
| Human umbilical cord blood of healthy donors | CD29 CD44 CD73 CD90 CD105 CD166 | Increased tumor growth |
Exosomes CD133+ GBM secreted MCP-1/CCL2 and SDF-1/CXCL12 induce migration of MSCs |
[160] |
| Lung cancer | ||||
| Human bone marrow | Not stated | Increased tumor growth, cancer cell proliferation, intra-tumoral angiogenesis and M2 polarization of macrophages |
Downregulated PTEN, PDCD4 and RECK gene expression in cancer cells largely through miR-21-5p, which derived from EVs of MSCs pre-challenged with hypoxia |
[126] |
| Human adipose tissue stem cell line | Not stated | Increased migration capacity | ADMSC-differentiated CAFs promoted cancer EMT by NOTCH pathway | [149] |
| Human umbilical cord of healthy donor | CD133+ CD271+ CD105+ CD3− CD14− CD19− CD38− CD66b− | Increased tumor EMT, invasion, and migration; decreased tumor proliferation and increased tumor apoptosis | Exosomes derived from MSCs activated Smad2/3, Akt/GSK-3β/β-catenin, NF-kB, ERK, JNK, and p38 MAPK signaling pathway by TGF-β1 | [159] |
| Human umbilical cord of healthy donor | CD105 CD73 CD90 CD45 CD34 CD14 CD19 HLA-DR | Increased tumor cell proliferation and decreased tumor cell apoptosis | Reduced PTEN expression mediated by the MSC-EV-transmitted miR-410 | [158] |
| Human umbilical cord Wharton's jelly | CD105+ CD90+ CD166+ CD73+ CD45− CD31− CD34− | Increased AC-LCSC tumor cell proliferation and expression of CSC markers (ALDH+ and CD133+ cell population) | MSC-CM and in vivo co-transplantation | [161] |
| Liver cancer | ||||
| Human bone marrow MSC cell line | CD44+ CD90+ | Increased tumor progression, but decreased pulmonary metastasis | Decreased TGFβ1 and MMP2 expression in cancer cells | [165] |
| Human bone marrow from patients with orthopedic surgery | CD44+ CD73+ CD90+ CD105+ CD146+ CD34− CD45− | Increased tumor growth, migration and invasion | MSC-dependent activation of the CXCR4, PI3K/Akt, Ras/Erk pathways | [127] |
| Human bone marrow from ATCC | Not stated | Increased tumor growth and metastasis | Activated pERK signaling pathway and over-expressed integrin α5 in HCC; decreased NK cell marker CD56 and increased IL-6 and TNF-α in tumor niche | [128] |
| Colorectal cancer | ||||
| Human bone marrow of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ MHC-DR+ CD14− CD34− Flk-1− | Increased tumor growth | MSC-differentiated CAFs expressed PDGFR to mediate tumor growth and metastasis | [130] |
| Human bone marrow of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ MHC-DR+ CD14− CD34− Flk-1− | Increased tumor growth, angiogenesis, and metastasis | MSC-differentiated into CAFs; paracrine effects of MSCs; | [129] |
| Human bone marrow of healthy donor | CD73+ CD90+ CD105+ CD34− CD45− | Increased migration capacity | MSC-secreted PAI-1 promoted cancer cell migration | [134] |
| Human bone marrow of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ | Increased tumor-initiating ability and tumor growth | MSC-secreted IL6 increased cancer cell CD133 expression by activation of JAK2/STAT3 | [132] |
| Human bone marrow from sternum of healthy donor | CD49c+ CD73+ CD90+ CD105+ CD34− CD45− CD184− CD106− | Increased tumor growth, invasion; decreased survival | MSC-secreted NRG1 activated HER2/HER3-dependent PI3K/AKT pathway | [135] |
| Human bone marrow from iliac crest of healthy donor | Not stated | Increased tumor growth and angiogenesis | MSC-secreted IL6 induced ET1/AKT or ERK pathway in endothelial cells to promote angiogenesis | [133] |
| Human bone marrow from the iliac crest of the patient following bone defect reconstruction | Not stated | Increased tumor growth, cell proliferation, invasion and cancer stemness-related properties | Suppressed RNA-binding protein PTBP1 by indirect co-culture with MSCs | [136] |
| Human bone marrow from the femoral head during hip-replacement surgery | Not stated | Increased cancer cells stem cell-like traits | MiR-142-3p contained in exosomes derived from MSCs promoted the Notch signalling pathway by downregulating Numb in cancer cells | [136] |
| Human bone marrow from ATCC | Not stated | Increased tumor cell proliferation, EMT, migration, and invasion | TNF-α-primed-MSCs secreted high level of CCL5 which further involved cancer cells’ CCl5/CCR1/β-catenin/Slug signaling pathway | [137] |
| Renal cancer | ||||
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Increased tumor growth | Induction of HGF synthesis via RNA transferred by MSC-MVs activated AKT and ERK1/2 signaling | [163] |
| Human adipose tissue from liposuction | CD44+ CD90+ CD34− CD45− | Increased Ciprofloxacin resistance | Not explored | [151] |
| Human amniotic fluid from healthy pregnant women | CD44+ CD90+ CD34− CD45− | Increased Ciprofloxacin resistance | Not explored | [151] |
| Ovarian cancer | ||||
| Healthy human donor | CD90 CD105 CD106 CD117 CD146 CD56 CD166 CD29 CD44 CD14 CD31 CD34 HLA-DR | Increased tumor growth and angiogenesis | LL-37 recruited MSCs, which release trophic factors that initiate angiogenesis and/or differentiate into blood vessel-supporting cells | [153] |
| Human adipose tissue from omentum of normal donors | CD105+ CD73+ CD90+ CD34− | Increased tumor growth and metastasis | Elevated the expression of MMP2 and MMP9 | [152] |
| Endometrial cancer | ||||
| Human adipose tissue from omentum of patient with recurrent adenocarcinoma of endometrium and ovary | CD44+ CD29+ CD90+ CD105+ CD34− CD45− CD11b− | Increased tumor growth and cell proliferation | Not explored | [283] |
| Breast cancer | ||||
| Human bone marrow of healthy donor | Not stated | Increased tumor metastasis | MSCs involve in driving recruitment of TAMs and MDSCs | [143] |
| Human bone marrow of healthy donor | Not stated | Increased skin invasion and metastasis | Activation of EGFR signaling pathway | [284] |
| Human bone marrow of healthy donor | Not stated | Increased tumor cell migration and invasion | Tumor and stroma physical interactions activated Notch1 by TNFα or IL-1β, which further lead to CXCL8 production | [144] |
| Human bone marrow from Lonza |
CD29+ CD44+ CD105+ CD166+ CD90+ CD73+ CD14− CD34− CD45−HLA-DR− CD19− |
Increased tumor lung metastases | Elevated CXCL8 (IL-8), CCL2 (MCP-1), and CCL5 (RANTES) by TNFα/IL-1β primed TNBC:MSC co-cultures | [144] |
| Human bone marrow of healthy donor | CD105+ CD45− |
Increased tumor motility, invasion and metastasis |
CCL5 secreted by MSCs activated CCL5-CCR5 signaling in cancer cells | [142] |
| Human bone marrow of healthy donor | Not stated | Increased tumor growth and bone metastasis | Not explored | [285] |
| Human bone marrow of healthy donor | Not stated | Acquisition of dormant phenotypes including decreased tumor cells proliferation, the abundance of stem cell–like surface markers, invasion capacity, and sensitivity to docetaxel |
miR-23b contained in exosomes derived from MSCs suppressed MARCKS expression |
[145] |
| Human adipose tissue from women (BMI > 30) undergoing liposuction from the subcutaneous abdominal adipose tissue |
CD29 CD166 CD73 CD105 CD90 CD11b CD31 CD34 CD45 HLA-DR |
Increased tumor growth and angiogenesis | CXCL1/8 secreted by MSCs activated CXCL1/8-CXCR1/2 signaling in cancer cells | [150] |
| Human adipose tissue from patients undergoing tumescent liposuction |
CD44+ CD105+ HLA-ABC+ CD29+ Fik1+ CD45− CD31− CD34− CD106− CD184− |
Increased tumor cell migration | Exosomes derived from MSCs activated Wnt signaling pathway | [155] |
| Human adipose tissue from ScienCell Research Laboratories (Carlsbad, CA) | CD73+ CD90+ CD105+ | Increased Doxorubicin resistance | MSC-secreted conditioned medium promoted BCRP protein expression in cancer cells | [156] |
| Human adipose tissue from liposuction | CD73+ CD90+ CD105+ CD34− | Increased tumor metastatic spread | MSC dose dependent | [286] |
| Human umbilical cord of healthy donor | Not stated | Increased tumor cell invasion and metastasis |
IL-8 and IL-6 secreted by MSCs activated the autocrine IL-8 and IL-6 signaling in cancer cells and induced CD44 + /CD24 − cells |
[286] |
| Human umbilical cord of healthy donors | CD29+ CD44+ CD90+ CD34− HLA-DR− | Increased tumor cells proliferation and migration | MSC-CM inhibited E-cadherin expression, increased the expression of N-cadherin, ZEB1 and PCNA through activation of the ERK pathway | [157] |
| Prostate cancer | ||||
| Mouse bone marrow from femurs | CD90+ CD34−/CD45− | Increased tumor cell migration and invasion | SDF1a secreted by MSCs | [123] |
| Melanoma | ||||
| Resident MSCs of bone and liver | Not stated | Increased tumor metastasis to bone and liver | MCC interact with resident MSCs at the perivascular space through co-expressed CD146 and CXCL12-CXCR4 signaling | [287] |
| Bone cancer | ||||
| Human bone marrow from the proximal femur during orthopedic surgery | Not stated | Increased tumor growth and metastasis to lung | MSC-secreted IL6 activated tumor STAT3 signaling pathway | [139] |
| Human bone marrow from patients with orthopedic surgery | CD44+ CD73+ CD90+ CD105+ CD146+ CD34− CD45− | Increased tumor growth, migration and invasion | MSCs-dependent activation of the CXCR4, PI3K/Akt, Ras/Erk pathways | [127] |
| Human bone marrow |
CD29+ CD44+ CD105+ CD45− HLA-DR− |
Increased tumor cell growth | Activation of Hedgehog signaling pathway by MSC-derived exosomes | [138] |
| Human bone marrow from TaKaRa Biotechnology | Not stated | Increased tumor cell growth and metastasis | Paracrine effect of IL-8 through activation of FAK pathway | [140] |
| Human bone marrow of healthy donor |
CD73+ CD90+ CD105+ CD34− CD45− CD19− CD14− |
Increased tumor cell invasion and transendothelial migration | MSCs trans-differentiate into CAFs, increasing GRO-a, MCP-1, IL-6 and IL-8 levels in the tumor microenvironment | [141] |
| Human bone marrow |
CD73+ CD90+ CD105+ CD34− CD45− CD3− |
Increased tumor cell proliferation | Not explored | [288] |
| Gastric cancer | ||||
| Human bone marrow |
CD29+ CD44+ CD105+ CD45− HLA-DR− |
Increased tumor cell growth | Activation of Hedgehog signaling pathway by MSC-derived exosomes | [138] |
| Human bone marrow from ATCC | Not stated | Increased tumor cells viability and invasion | MSCs recruitment and presence of CAF-like myofibroblastic phenotypes by tumor-derived HDGF | [131] |
| Head and neck cancer | ||||
| Human bone marrow from patients during hip-replacement surgery | CD73 + CD90 + CD105 + CD19- CD34- | Increased tumor cell invasion | Induction of ALP and MMP9 activity | [146] |
| Esophageal cancer | ||||
| Human bone marrow | CD29+ CD44+ CD73+ CD90+ CD45− CD31− | Increased tumor cell proliferation, viability and invasion | Gremlin1 derived from MSC-CM is related to TGF-β/BMP signaling pathway | [147] |
| Bladder cancer | ||||
| Human adipose tissue from liposuction | CD44+ CD90+ CD34− CD45− | Increased Ciprofloxacin resistance | Not explored | [151] |
| Human amniotic fluid from healthy pregnant women | CD44+ CD90+ CD34− CD45− | Increased Ciprofloxacin resistance | Not explored | [151] |
Fig. 3.
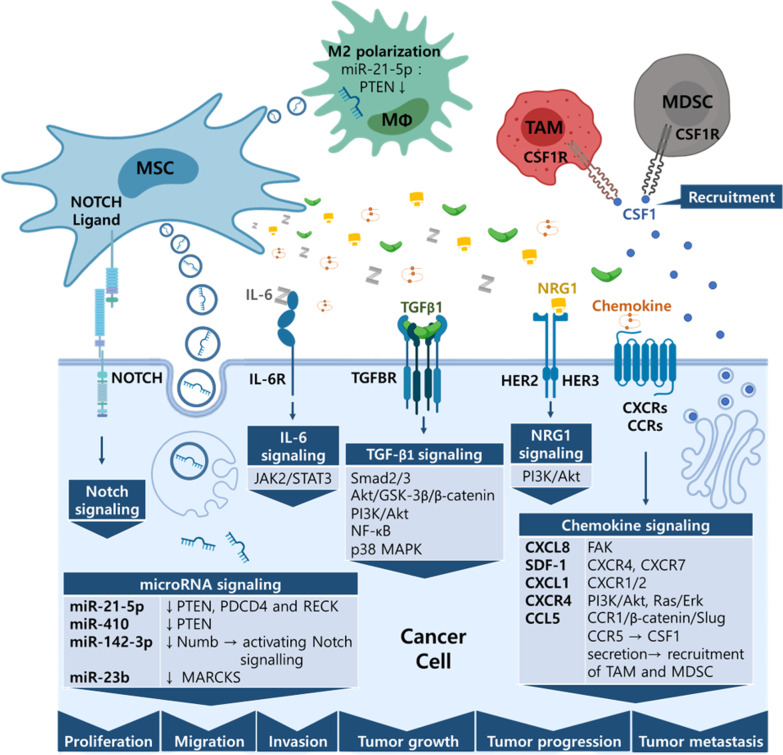
Schematic diagram of tumor promoting mechanisms of MSCs. MSCs influence cancer cells and immune cells to promote tumor cell proliferation, invasion, migration and metastasis. Secreted microRNA-containing exosomes, soluble factors, and contact-dependent signaling pathways are summarized
Cell type
BMMSCs
Several studies have examined the effects of MSCs on tumor cell growth (Fig. 2). MSCs derived from human bone marrow (hBMMSCs) have been shown to enhance the motility of prostate cancer cells via SDF-1 regulation in vitro [123]. Additionally, hBMMSCs were reported to promote glioblastoma bone metastasis in vivo through the activation of SDF-1/CXCR4 and SDF-1/CXCR7 signaling [124]. It has also been shown that exosomes derived from glioma cells induce hBMMSC transformation to a tumor-like phenotype by activating glycolysis [125]. hBMMSCs that were pre-challenged with hypoxia increased tumor growth, cell proliferation, intra-tumoral angiogenesis and M2 polarization of macrophages in lung adenocarcinomas. The underlying mechanism involved downregulation of PTEN, PDCD4 and RECK gene expression by miR-21-5p derived from hBMMSCs exosomes [126]. Furthermore, hBMMSCs were shown to mediate osteosarcoma and hepatocellular carcinoma (HCC) cell migration and invasion through the regulation of CXCR4 [127]. Human MSCs (hMSCs) promote HCC tumor growth via the MAPK pathway and promote metastasis by epithelial-mesenchymal transition (EMT) and integrin α5. Furthermore, hMSC treatment promoted HCC progression, increased IL-6 and TNF-α expression, and decreased the number of natural killer (NK) cells in tumor niches [128].
In addition to their paracrine effect, hBMMSCs also promote colorectal carcinoma (CRC) and gastric cancer progression by directly differentiating to CAFs and exerting their trophic effects [129–131]. In colorectal adenocarcinomas, IL6 secreted from hBMMSCs not only increased cancer cell CD133 expression via activation of the JAK2/STAT3 pathway [132], but also activated Akt and ERK in endothelial cells by inducing the secretion of endothelin-1 (ET-1) [133]. Furthermore, hBMMSC-secreted PAI-1 and NRG1 were shown to promote CRC progression; the latter activates the PI3K/AKT pathway in a HER2/HER3-dependent manner [134, 135]. Indirect co-culture of CRCs with hBMMSCs enhanced the invasiveness of CRCs via suppression of RNA-binding protein PTBP1 [136]. The up-regulation of cancer stemness-related properties in CRCs is correlated with activation of the Notch signalling pathway by miR-142-3p, which downregulates Numb expression and is transmitted via hBMMSC exosomes[136].
One approach to mimic the inflammatory niche is to generate TNF-α-primed-hBMMSCs that secrete high levels of CCL5, which is involved in the CRC-related CCl5/CCR1/β-catenin/Slug signaling pathway that promotes tumor cell proliferation, EMT, migration, and invasion [137]. Activation of the Hedgehog signaling pathway by hBMMSC-derived exosomes leads to increased tumor cell growth in both gastric cancer and in osteosarcoma [138]. hBMMSC-secreted IL6 and IL-8 have been shown to increase tumor growth and metastasis in osteosarcomas by activation of the STAT3 and FAK signaling pathways, respectively [139, 140]. Meanwhile, elevated levels of GRO-a, MCP-1, IL-6 and IL-8 in the tumor microenvironment promoted osteosarcoma invasion and transendothelial migration via cross-talk between tumor cells and CAFs from hBMMSCs [141]. CCL5 secreted by hBMMSCs increased the motility of breast cancer cells (BCCs) by activation of CCL5-CCR5 signaling [142]. This signalling also promotes BCCs to secret CSF1, which will bind to the CSF1 receptor on MSCs, tumor-associated macrophages and myeloid-derived suppressor cells, and drive recruitment of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophage (TAMs) [143]. Elevated CCL5 (RANTES), CCL2 (MCP-1), and CXCL8 (IL-8) in TNFα/IL-1β primed triple-negative subtype of breast cancer cells (TNBCs): hBMMSCs co-cultures increase BCC lung metastases [144]. Moreover, physical interactions between TNBCs and hBMMSCs primed with TNFα or IL-1β, activates Notch1, which leads to CXCL8 production and increased tumor cell migration and invasion [144]. Exosomes derived from hBMMSCs promote the acquisition of dormant phenotypes by suppressing MARCKS expression in a bone marrow-metastatic human breast cancer cell line through miR-23b [145]. In head and neck squamous cell carcinoma (HNSCC) and esophageal squamous cell carcinoma (ESCC), increased tumor cell invasion was correlated with induction of ALP and MMP9 activity by direct contact between tumor cells and hBMMSCs, and by activation of the Gremlin1-dependent TGF-β/BMP signaling pathway by hBMMSC-CM, respectively [146, 147]
ADMSCs
The effect of MSCs on promoting tumor cell growth may be mediated via angiogenic factors VEGF, Ang-1, PDGF, and IGF and SDF-1 [148]. In addition, adipose tissue-derived mesenchymal stem cells (ADMSCs)-differentiated CAFs promote the EMT of lung cancers by activating the NOTCH pathway [149]. hADMSC-secreted CXCL1/8 enhances the growth and angiogenesis of BCCs by activating CXCL1/8-CXCR1/2 signaling [150]. hADMSCs and human amniotic fluid‐derived stem cells (hAFMSCs) increase ciprofloxacin resistance in renal cell carcinomas (RCCs) and bladder cancer cells [151]. Additionally, elevating the expression of MMP2 and MMP9 in ovarian cancer cells causes increased tumor growth and metastasis in both direct and indirect co-cultures with hADMSCs [152]. LL-37, which is usually overexpressed in ovarian cancer, can recruit and stimulate MSCs to release trophic factors, which increase tumor growth and angiogenesis [153]. In addition to MSCs, the CM and the EVs derived from human ADMSCs showed the ability to increase tumor growth and migration and to decrease H2O2–induced tumor cell apoptosis [154]. Meanwhile, the hADMSC-CM and exosomes were shown to increase doxorubicin resistance and tumor cell migration either by increasing breast cancer resistance protein (BCRP) levels or by activating the Wnt signaling pathway in BCCs, respectively [155, 156].
UCMSCs and WJMSCs
hUCMSCs promote proliferation and migration of BCCs by activating ERK signaling, including down-regulating E-cadherin expression, and up-regulating N-cadherin, ZEB1 and PCNA expression [157].
The EVs derived from hUCMSCs also have the ability to increase tumor cell proliferation and to decrease tumor cell apoptosis in lung adenocarcinomas via transmission of miR-410, which reduces PTEN expression [158]. Additionally, exosomes derived from hUCMSCs increased tumor EMT, invasion, and migration through TGF-β1-mediated signaling pathways [159]. Furthermore, CD133+ glioblastoma stem cells exhibited the ability to recruit hUCBMSCs, which can further promote tumor growth in vivo, via exosomes containing MCP-1/CCL2 and SDF-1/CXCL12 [160].
An increase in the cancer stemness-related ALDH+ and CD133+ cell populations was observed in lung adenocarcinomas treated with Wharton's Jelly mesenchymal stem cell CM (WJMSC-CM) [161]. WJMSC-CM also showed effects of increasing tumor growth and migration of glioblastoma cells by secreted cytokines (eg. CCL2, PDGF-C, Sema-7A, periostin, IL6) [162]. Besides the cytokines and chemokines secreted by MSCs, WJMSC microvesicles (MVs) transfer RNA to RCCs, which induces HGF synthesis and further activates AKT and ERK1/2 signaling [163].
Signaling pathways
Chemokine signaling
Chemokine signaling plays an important role in MSC-dependent tumor promotion (Fig. 3). CD133+ glioblastoma stem cells induce hUCMSC migration to tumor regions by secreting CCL2 and CXCL12. Once in the tumor region, MSCs then promote tumor proliferation and glial invasiveness [160]. In addition, SDF-1 secreted from hBMMSCs promotes neuroblastoma migration and invasion via CXCR4 and CXCR7 [124]. hBMMSCs also enhance osteosarcoma and HCC cell migration and invasion by activating the AKT and ERK pathways of tumor cells via CXCR4 [127]. These observations suggest that chemokine signaling may be involved in bone metastasis. Furthermore, Chaturvedi et al. demonstrated that there is a delicate crosstalk among BCCs, hBMMSCs and TAMs/MDSCs involving chemokine signaling, and that there are two signaling loops among these cell types. In the second loop, CCL5 secreted from MSCs activates BCCs via CCR5, which promotes the BCCs to secret CSF1 and further recruits TAMs and MDSCs to the tumor region [143]. In addition, hBMMSCs weakly enhance the invasiveness and metastasis of metastatic human BCCs through CCL5-CCR5 signaling regulation [142]. CCL5 secreted from TNF-α-primed hBMMSCs also showed the ability to promote CRC progression and EMT via the CCL5/CCR1/β-catenin/Slug signaling pathway [137]. In addition to tumor and immune cells, chemokine signaling affects other cells in tumor niches. For example, CXCL1/8 derived from hADMSCs can enhance the migration and tube formation of human umbilical vein endothelial cells (HUVECs) in vitro by CXCR1 and CXCR2, which promote angiogenesis in a breast tumor xenograft mouse model [150]. CXCL8 derived from hBMMSCs was also shown to activate FAK signaling in osteosarcomas and to promote tumor metastasis [140].
TGF-β signaling
TGF-β is well known as an EMT promotor, but it can also induce cell cycle arrest and apoptosis [164]. In lung cancer cells, hUCMSCs have been shown to promote tumor cell EMT, invasion, and migration, but also to decrease tumor proliferation and promote tumor apoptosis by TGF-β1 from exosomes secreted by MSCs. The TGF-β1 activates Smad2/3, Akt/GSK-3β/β-catenin, NF-kB, ERK, JNK, and the p38 MAPK signaling pathway in cancer cells. Silencing TGF-β1 or inhibiting exosome secretion can eliminate the MSC-dependent effects on cancer cells described above [159]. hBMMSCs also increased tumor progression, but decreased pulmonary metastasis with decreased TGFβ1 levels in HCC [165]. Furthermore, Hong et al. demonstrated that hBMMSC-CM can enhance the proliferation, viability and invasiveness of esophageal cancer cells via Gremlin1, which activates the TGF-β/Smad2/3 signaling pathway by inhibiting the BMP4/Smad1/5/8 signaling pathway in cancer cells [147].
MicroRNA signaling
Accumulating evidence shows that EV-derived miRNA contributes to tumor initiation, angiogenesis, drug resistance, metastasis and immune suppression in cancer [166]. EVs derived from hBMMSCs pre-challenged with hypoxia can promote tumor growth, cancer cell proliferation, invasion, intra-tumoral angiogenesis and M2 polarization of macrophages in non-small cell lung cancer cells. This occurs via miR-21-5p, which decreases PTEN, PDCD4 and RECK protein levels in cancer cells while enriching for CD163+CD206+, M2 macrophage-related cell surface marker macrophages, and decreasing the CD40+CD86+, M1 macrophage-related cell surface marker macrophage population. Transfecting miR-21-5p inhibitor or re-overexpressing PTEN abrogated the tumor promoting and M2 polarization effects that the hypoxia pre-challenged EVs induced [126]. Dong et al. also reported that miR-410 derived from hUCMSC-secreting EVs repressed PTEN protein levels in lung adenocarcinoma cells, further increased tumor cell proliferation, and decreased tumor cell apoptosis [158].
miRNA is also reported to be involved in the dynamics of the cancer stem cell population. Increased cancer stem cell-like traits, including sphere formation, Lgr5+CD133+ population, colony formation, drug resistance, and tumourigenesis, were reported in CRCs upon treatment with hBMMSC-derived exosomes that transmitted miR-142-3p. Mechanistically, it was found that miR-142-3p inhibits the expression of the Numb gene, which results in increased mRNA and protein levels of Notch target genes Hes1, P21, and cyclin D3 mRNA [136]. On the other hand, Ono et al. demonstrated that miR-23b delivered via hBMMSC-derived exosomes caused bone marrow–metastatic human breast cancer cells to acquire dormant phenotypes, characterized by decreases in tumor cell proliferation, tumourigenic capacity, CD44+ population, invasion capacity, and sensitivity to docetaxel. The miR-23b may exert its effects by targeting MARCKS [145].
MSC suppression effects on tumor growth
While MSCs utilize diverse mechanisms for tumor promotion, they suppress tumor growth mainly by inducing apoptosis of tumor cells. MSCs have been shown to suppress the growth of breast [167–169], brain [148, 170–174], lung [170, 175], liver [175, 176], ovarian [167, 177, 178], bone [167, 179], esophageal [168], bladder [180], colorectal [170] and hematological malignancies [181–183]. The underlying mechanisms responsible for MSC tumor suppression are classified below as well in Table 5, and are summarized in Fig. 4.
Table 5.
MSC suppression effects in cancers
| Cancer type/MSC source | Surface marker | Effect | Factors/mechanisms | Ref. |
|---|---|---|---|---|
| Brain cancer | ||||
| Human bone marrow from femoral head of individuals undergoing hip-replacement surgery | CD44+ CD90+ CD105+ | Decreased tumor growth and angiogenesis | Coculture with hBMMSCs decreased PDGF-BB and IL1β secretion | [184] |
| Human bone marrow from Lonza |
CD29+ CD44+ CD105+ CD166+ CD90+ CD73+ CD14− CD34− CD45−HLA-DR− CD19− |
Decreased tumor cell proliferation | MSC-EV | [281] |
| Human bone marrow of healthy donor | Not stated | Inducing apoptosis in CD133-positive primary glioma cells | Engineered TRAIL-expressing MSCs induced apoptosis of glioma cells | [198] |
| Human bone marrow of healthy donor | CD44+ CD105+ CD34− CD38− CD45− | Decreased cancer cell proliferation and increased animal survival | Engineered IFNβ-expressing MSCs | [201] |
| Human subcutaneous adipose tissue of 18–30 years old mothers receiving cesarean sections | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45− | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Human adipose tissue from liposuction | CD29+ CD44+ CD90+ CD105+ | Decreased tumor growth and formation | Paracrine effects by MSC-secreted cytokines | [289] |
| Human adipose tissue from patients receiving liposuctions (commercial) | CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ CD14− CD31− CD45− Lin1− | Decreased tumor growth, migration; induced differentiation; no significant effect in survival | Engineered BMP4-secreting MSCs had additional effect of improved survival | [204] |
| Human umbilical cords of 18–30 years old mothers receiving cesarean section | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45− | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Human umbilical cord of healthy donor | CD73 CD105 CD90 CD45 CD34 CD14 CD11b HLA-DR | Decreased cancer stem cell proliferation | Direct cell interaction | [290] |
| Human umbilical cord from the cell library of the Chinese Academy of Science | CD29+ CD105+ CD34− CD45− | Decreased tumor growth | Engineered IL-24-secreting MSCs induced tumor cell apoptosis | [200] |
| Human umbilical cord Wharton's jelly of healthy donor | CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA-DR− | Decreased cancer stem-like cells proliferation | MSC-CM mediated cell cycle arrest and senescence of cancer cells by downregulating cyclin D1 and upregulating expression of p21 and p16 | [171] |
| Human umbilical cord Wharton's jelly from CryoSave | Not stated | Decreased tumor cell proliferation | MSC-EV | [281] |
| Human umbilical cord blood of healthy donor | CD29+ CD81+ | Decreased tumor growth and migration capacity | Co-culture with MSCs increased PTEN expression and downregulated PI3K/AKT pathway in cancer cells | [187] |
| Human umbilical cord blood of healthy donor | CD29+ CD81+ | Decreased tumor growth and angiogenesis | Co-culture with MSCs downregulated FAK, VEGF, AKT | [185] |
| Human umbilical cord blood of healthy donor | CD29+ CD81+ | Decreased invasion capacity | Increased cancer cell Mad1 expression represses c-Myc activity and further decreases activity of ERK | [188] |
| Human umbilical cord blood of healthy donor | CD29+ CD81+ | Decreased migration and invasion capacity | Decreased EGFR and c-Met expression, activity, and physical association | [188] |
| Human umbilical cord blood of healthy donors | CD29+ CD81+ | Decreased tumor growth | G0/G1 cell cycle arrest; decreased cyclin D1/Cdk4 and cyclin D1/Cdk6 expression | [172] |
| Human umbilical cord blood of healthy donors | CD29+ CD81+ | Decreased tumor growth and increased apoptosis | Decreased XIAP expression resulted in activation of caspase-3 and caspase-9, downregulated AKT pathway and activated Smac/DIABLO | [173] |
| Human umbilical cord blood of healthy donors | CD31− CD45− | Decreased tumor growth and increased tumor cell apoptosis | TRAIL signaling | [148] |
| Human umbilical cord blood of healthy donors | CD29+ CD44+ HLA-ABC+ CD34− CD45− HLA-DR− | Decreased tumor growth and angiogenesis | Reduced the number of cyclin D1-positive cancer cells | [174] |
| Human umbilical cord blood of healthy donors | Not stated | Decreased tumor growth and angiogenesis; increased the survival of glioma-bearing mice and tumor-specific long-term T-cell immunity | Engineered IL-12 M-secreting MSCs | [199] |
| Human umbilical cord blood of healthy donors | Not stated | Enhanced tropism of MSCs towards tumor site | Engineered CXCR4- overexpressing MSCs | [196] |
| Human umbilical cord blood from Medipost Co | Not stated | Increased tumor cell apoptosis | Engineered TRAIL-expressing MSCs induced apoptosis of glioma cells | [197] |
| Human umbilical cord blood of healthy donors | Not stated | Enhanced tropism of MSCs towards tumor site | Engineered CXCR1- overexpressing MSCs | [196] |
| Lung cancer | ||||
| Human bone marrow of healthy donor | CD29+ CD73+ CD90+ CD105+ CD166+ CD14− CD31− CD34− CD45− | Decreased tumor tumorigenicity and EMT | MSCs-secreted OSM activates OSM/STAT1 signaling pathway | [191] |
| Human subcutaneous adipose tissue of 18–30 years old mothers receiving cesarean sections | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45− | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Human umbilical cord of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA-DR− | Decreased cancer cell proliferation and migration capacity | Cell cycle arrest; induced apoptosis; downregulated Bcl-2, pro-caspase-7, β-catenin and c-Myc | [175] |
| Human umbilical cords of 18–30 years old mothers receiving cesarean section | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45− | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Human umbilical cord Wharton's jelly | CD105+ CD90+ CD166+ CD73+ CD45− CD31− CD34− | Decreased SCC-LCSC tumor cell proliferation and expression of CSC markers | MSC-CM and in vivo co-transplantation | [161] |
| Human umbilical cord Wharton's jelly | CD105+ CD90+ CD73+ CD45− CD34− | No effect on AC-A549 | Secretome has no effect on AC-A549 | [291] |
| Human umbilical cord Wharton's jelly | Not stated | Decreased tumor cell growth and increased apoptosis | Engineered IFNβ-expressing MSCs | [202] |
| Human endometrium of women with uterine fibroids | CD13+ CD29+ CD44+ CD49+ CD49b+ CD73+ CD90+ HLA-ABC+ CD9− CD14− CD31− CD34− CD40− CD45− CD54− CD117− CD133− HLA-DR− | Decreased migration capacity | Not explored | [191] |
| Liver cancer | ||||
| Human fetal bone marrow at 4 months of gestation from abortion (BMMS-03) | CD105+ CD166+ CD34− | Decreased cancer cell proliferation | Decreased NF-κB expression and activity | [192] |
| Human bone marrow of healthy donor | CD44 + CD90 + CD34- CD45- | Decreased tumor growth | Engineered IFNβ-expressing MSCs induced cell cycle arrest and increasing expression of p21, p27 and FOXO3a as well as decreasing protein levels of cyclin D1, pRb and AKT | [203] |
| Human fetal dermal tissues at 4 months gestation from abortion (Z3) | CD105+ CD166+ CD34− | Decreased cancer cell proliferation | Decreased NF-κB expression and activity | [192] |
| Human fetal dermal tissues at 4 months gestation from abortion (Z3) | CD29+ CD44+ CD105+ CD166+ CD31− CD34− CD45− HLA-DR− vWF− | Decreased tumor growth | Induced apoptosis and cell cycle arrest; Bcl-2, c-Myc, PCNA and survivin were downregulated | [176] |
| Human umbilical cord of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA-DR− | Decreased cancer cell proliferation and migration capacity | Cell cycle arrest; induced apoptosis; downregulated Bcl-2, pro-caspase-7, β-catenin and c-Myc | [175] |
| Human umbilical cord Wharton's jelly | Not stated | Decreased tumor growth | Engineered AFP promoter driving-sTRAIL- expressing MSCs | [208] |
| Bile duct cancer | ||||
| Human umbilical cord of healthy donors | CD29+ CD44+ CD105+ CD34− CD45− | Decreased tumor growth and cell proliferation | MSC-CM inhibits Wnt/β-catenin signaling pathway by GSK-3β | [190] |
| Pancreatic cancer | ||||
| Human umbilical cord blood of healthy donors | CD44+ CD29+ HLA-I+ CD34− CD38− HLA-DR− | Decreased tumor growth and prolonged survival of tumor-bearing mice | Engineered IL-15- expressing MSCs mediate NK and CD8-positive T cells accumulation in tumor site | [211] |
| Colorectal cancer | ||||
| Human subcutaneous adipose tissue of 18–30 years old mothers receiving cesarean sections | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45- | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Human adipose tissue from liposuction | CD29 + CD44 + CD90 + CD105 + | Decreased tumor growth | Engineered CD-expressing MSCs sensitized colon cancer cells to 5-FC | [212] |
| Human umbilical cords of 18–30 years old mothers receiving cesarean section | CD13+ CD44+ CD90+ CD105+ CD14− CD34− CD45− | Decreased cancer cell proliferation |
Increased apoptosis; G0/G1 cell cycle arrest Upregulation of caspase-3 and caspase-9 and downregulation of survivin and XIAP |
[170] |
| Ovarian cancer | ||||
| Human bone marrow | CD105+ CD90+ CD44+ CD73+ CD45− CD34− CD24− HLA-DR− CD14− | Increased tumor cell apoptosis | MSCs-CM induced reduction in the level of CA-125, LDH and beta-hCG and expression of MMP-2, MMP-9, and CA-125 as well as increased TIMP 1, 2, and 3 mRNA expression | [177] |
| Human adipose tissue from liposuction | CD105+ CD90+ CD44+ CD73+ CD45− CD34− CD24− HLA-DR− CD14− | Increased tumor cell apoptosis | MSCs-CM induced reduction in the level of CA-125, LDH and beta-hCG and expression of MMP-2, MMP-9, and CA-125 as well as increased TIMP 1, 2, and 3 mRNA expression | [177] |
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased tumor cell growth and migration |
hWJSC-CL and hWJSC-CM induced apoptosis Upregulation of pro-apoptotic BAX and downregulation of anti-apoptotic BCL2 and SURVIVIN genes |
[167] |
| Human umbilical cord Wharton's jelly |
CD73+ CD90+ CD105+ CD34− CD45− CD14− CD20− |
Decreased tumor cells proliferation | MSC-CM and MSC-CL decreased the expression of oncogenic cytokines, chemokines and growth factors in cancer cells | [292] |
| Human umbilical cord Wharton's jelly | CD44+ CD90+ CD105+ CD73+ CD29+ CD34− CD45− | Decreased tumor cells expression of CSC markers; increased cell cycle arrest and apoptosis | MSC-CM and MSC-CL decreased expression of cell cycle regulatory genes (cyclin A2, Cyclin E1), prostaglandin receptor signaling genes (EP2, EP4) and the pro-inflammatory genes (IL-6, TNF-α) in cancer cells | [178] |
| Human umbilical cord Wharton's jelly | Not stated | Decreased tumor cells proliferation | Not explored | [293] |
| Human umbilical cord Wharton's jelly | CD105+ CD90+ CD44+ CD73+ CD45− CD34− CD24− HLA-DR− CD14− | Increased tumor cell apoptosis |
MSCs-CM induced reduction in the level of CA-125, LDH and beta-hCG and MMP-2, MMP-9, and CA-125 mRNA expression as well as increased TIMP 1, 2, and 3 mRNA expression |
[177] |
| Human umbilical cord blood of healthy donors | Decreased tumor growth and prolonged survival of tumor-bearing mice | Engineered IL-21-secreting MSCs | [210] | |
| Human endometrium of healthy women | CD73+ CD90+ CD34− | Decreased tumor growth and pro-angiogenetic ability | Inhibition of AKT phosphorylation and decreasing the expressions of VEGFA and HIF-1α in cancer cells, probably by activation of FoxO3a in cancer cells | [186] |
| Breast cancer | ||||
| Resident MSCs of vascular wall | Not stated | Decreased the risk of lung metastasis after radiation-induced injury | Downregulation of radiation-induced expression of endothelial MMP2 and of the SASP factors CCL2 and Plau/uPA | [193] |
| Human adipose tissue from normal (non-diabetic) adult lipoaspiration | CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ CD14− CD31−CD45− | Decreased tumor cell viability and migration | MSC-secreted conditioned medium inhibits canonical Wnt signalling | [189] |
| Human adipose tissue of individuals receiving mammoplasty | Not stated | Decreased tumor cell growth and lung metastasis | Not explored | [294] |
| Human umbilical cord of healthy donors | CD73+ CD90+ CD105+ CD45− CD14− | Increased tumor cells apoptosis | MSCs secretome | [295] |
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased tumor cell growth and migration |
hWJSC-CL and hWJSC-CM induced apoptosis Upregulation of pro-apoptotic BAX and downregulation of anti-apoptotic BCL2 and SURVIVIN genes |
[167] |
| Human umbilical cord Wharton's jelly of healthy donor | CD73+ CD90+ CD105+ CD44+ CD45− CD14− CD34− | Increased tumor cells apoptosis |
Upregulating p21 Downregulating PCNA, cyclin D1, Bcl-2, Bcl-xL, and MMPs and upregulating p53 and p21 |
[168] |
| Human umbilical cord Wharton's jelly of healthy donor | CD44+ CD90+ CD105+ CD34− | Decreased tumor cell growth | Intra-tumoral injections of MSC-CM and MSC | [179] |
| Mouse umbilical cord Wharton's jelly | Not stated | Decreased tumor cell growth | Increased activated caspase-3 | [169] |
| Human umbilical cord blood of healthy donors | Not stated | Decreased tumor cell proliferation | MSC-ECM | [296] |
| Human umbilical cord blood of healthy donors | Not stated | Decreased tumor cell growth and lung metastasis | Not explored | [294] |
| Melanoma | ||||
| Human bone marrow of healthy donor | Not stated | Decreased the risk of lung metastasis after radiation-induced injury | Downregulation of radiation-induced expression of endothelial MMP2 and of the SASP factors CCL2 and Plau/uPA | [193] |
| Resident MSCs of vascular wall | Not stated | Decreased the risk of lung metastasis after radiation-induced injury | Downregulation of radiation-induced expression of endothelial MMP2 and of the SASP factors CCL2 and Plau/uPA | [193] |
| Bone cancer | ||||
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased tumor cell growth and migration | MSC-CL and MSC-CM induced apoptosis and autophagy; upregulation of pro-apoptotic BAX and autophagy genes (ATG5, ATG7, and BECLIN1); downregulation of anti-apoptotic BCL2, SURVIVIN genes | [167] |
| Human umbilical cord Wharton's jelly of healthy donor | CD44+ CD90+ CD105+ CD34− | Decreased tumor cell growth | MSC-CL and MSC-CM induced apoptosis and autophagy | [179] |
| Gastric cancer | ||||
| Human umbilical cord blood |
CD44+ CD105+ CD29+ CD90+ CD38− CD117− CD45− CD34− |
Increased tumor cell apoptosis | Engineered TNFSF14-secreting MSCs promote tumor cell apoptosis with elevated caspase-3 expression | [209] |
| Esophageal cancer | ||||
| Human umbilical cord Wharton's jelly of healthy donor | CD73+ CD90+ CD105+ CD44+ CD45− CD14− CD34− | Increased tumor cells apoptosis | Upregulate p21 downregulate PCNA, cyclin D1, Bcl-2, Bcl-xL, and MMPs and upregulate p53 and p21 | [168] |
| Bladder cancer | ||||
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased tumor growth | MSC-MVs induced down-regulated phosphorylation of Akt protein kinase and up-regulated cleaved Caspase 3 | [180] |
| Hematological cancer | ||||
| Human adipose tissue from 3liposuction | CD73+ CD90+ CD105+ CD45− |
Decreased tumor cell clonogenicity and growth |
Not explored | [297] |
| Human umbilical cord of healthy donor |
CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD166+ HLA-ABC+ CD14− CD31− CD34− CD38− CD45− HLA-DR− |
Inducing granulocytic differentiation of APL cells |
MSCs secreted IL-6 activates MEK/ERK pathways | [194] |
| Human umbilical cord of healthy donor | CD73+ CD90+ CD105+ CD45− |
Decreased tumor cell clonogenicity and growth |
Not explored | [297] |
| Human umbilical cord of healthy donor | CD73 CD90 CD105 CD14 CD19 CD34 CD45 and HLA-DR | Decreased tumor growth | Engineered Tandab -expressing MSCs combined with IDO pathway inhibitor inhibit expression of CD98 and Jumonji | [205] |
| Human umbilical cord of healthy donor | Not stated | Decreased tumor cell proliferation | Engineered IDO -secreting MSCs abolish the anti-apoptotic effect of MSCs | [206] |
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased tumor growth and increased tumor cell apoptosis | Engineered scFvCD20-sTRAIL fusion protein -secreting MSCs | [207] |
| Human umbilical cord Wharton's jelly of healthy donor | CD105+ CD73+ CD90+ CD45− CD34− CD14− CD11b− CD79α−/ CD19− | Decreased tumor cells viability; increased tumor cells apoptosis | MSC-CM induced CD47 and PD-L1 expression decreased in the tumor cells | [181] |
| Human umbilical cord Wharton's jelly of healthy donor | Not stated | Decreased cell viability and mitochondrial membrane potential; increased apoptosis | MSC-CM-3 kDa MWCO regulates cellular H2O2 levels | [182] |
| Human umbilical cord Wharton's jelly of healthy donor | CD105+ CD73+ CD90+ CD34− CD45− | Decreased tumor cell proliferation | IFNγ stimulated MSC secretome | [298] |
| Human umbilical cord blood of healthy donor | Not stated | Decreased tumor cell proliferation | Direct cell-to-cell contact caused arrest of the growth of cancer cells in the G0/G1 phase | [183] |
Fig. 4.
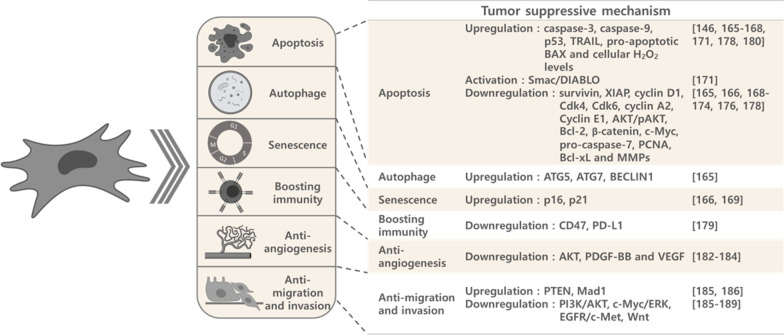
Schematic diagram of tumor suppressing mechanisms of MSCs. MSCs suppress tumor progression predominantly by promoting tumor cell apoptosis, autophagy, and senescence; and by boosting immunity, anti-angiogenesis, and anti-tumor cell migration and invasion
Apoptosis, autophagy and senescence
The majority of MSC tumor suppressing mechanisms involve increasing tumor cell apoptosis and impeding cell cycle progression. Upregulation of caspase-3, caspase-9, p16, p21, p53, TRAIL, pro-apoptotic BAX, ATG5, ATG7, BECLIN1 and cellular H2O2 levels [148, 167–170, 173, 180, 182]; activation of Smac/DIABLO [173]; and downregulation of survivin, XIAP, cyclin D1, Cdk4, Cdk6, cyclin A2, cyclin E1, AKT/pAKT, Bcl-2, β-catenin, c-Myc, pro-caspase-7, PCNA, Bcl-xL and MMPs have been demonstrated to be involved in the MSC-dependent tumor cell apoptosis seen with MSC-CM, MSC cell lysate (CL) and with direct cell–cell interaction [167, 168, 170–176, 178, 180].
Boosting immunity
The immunomodulation ability of MSCs is also correlated with tumor suppression. Lin et al. demonstrated that 3 kDa MWCO-WJMSC-CM concentrate can induce immunogenic cell death in lymphoma cells, which showed decreased viability and increased apoptosis, as well as increased levels of the ER stress markers eLF2a and XBP-1. Increased levels of surface damage-associated molecular pattern markers ecto-CRT, ecto-Hsp70 and ecto-Hsp90, as well as extracellular ATP and high mobility group box 1 were also observed. When cocultured with WJMSC-CM-treated lymphoma cells, dendritic cells had enhanced CD80 and CD86 expression. Yet lymphoma cells treated with WJMSC-CM concentrate had decreased CD47 and PD-L1 expression [181].
Anti-angiogenesis
In addition to directly inhibiting tumor cell growth, coculturing with hBMMSCs or hUCBMSCs decreased angiogenesis in glioblastoma. The underlying mechanism may involve the down-regulation of PDGF-BB and IL1β secretion or decreases in FAK, VEGF or Akt [184, 185]. Another attractive source of MSCs, human endometrial mesenchymal stem cells (EnSCs), also show an ability to decrease tumor growth and to increase angiogenesis in ovarian cancer by inhibiting AKT phosphorylation and decreasing expression of VEGFA and HIF-1α, possibly via nuclear translocation of FoxO3a [186].
Anti-migration and invasion
hUCBMSCs are also reported to decrease glioblastoma cell invasion and migration by increasing PTEN or Mad1 expression and downregulating PI3K/AKT, c-Myc/ERK or EGFR/c-Met activities [187, 188]. Inhibition of Wnt signaling has been shown to decrease tumor growth and migration after treatment with hUCMSC- or hADMSC- CM in bile duct cancer and breast cancer, respectively [189, 190].
Other mechanisms
Human BMMSC-secreted oncostatin M (OSM) has been reported to inhibit tumorigenicity and EMT by activating the OSM/STAT1 signaling pathway in lung adenocarcinoma cells [191]. Decreased cancer cell proliferation was also correlated with suppressed NF-κB expression and activity in HCCs and BCCs by MSCs derived from fetal bone marrow or fetal dermal tissue [192]. Vascular wall-resident MSCs as well as hBMMSCs displayed a capacity for decreasing the risk of lung metastasis after radiation-induced injury in breast cancer and melanoma by downregulating endothelial MMP2 and SASP factors CCL2 and Plau/uPA, which were induced by radiation injury [193]. In addition to suppressing tumor progression, hUCMSCs promote granulocytic differentiation of immature myeloid cancer cells in acute promyelocytic leukemia (APL), which drives the disease into remission by activating MEK/ERK pathways [194].
Engineered MSCs
Another promising strategy to treat progressive malignancy is the use of engineered MSCs, which show a remarkable ability to suppress tumor progression [195]. UCBMSCs with exogenous overexpression of CXCR1 and CXCR4 displayed enhanced tropism towards gliomas [196]. In addition, irradiation of glioma cells enhanced IL-8 expression, which promoted the tropism of hUCBMSCs equipped with TRAIL migration to tumors, and further induced tumor cell apoptosis [197]. hBMMSCs overexpressing TRAIL can also induce apoptosis in CD133-positive primary glioma cells in vitro [198]. Modified interleukin-12 (IL-12p40N220Q; IL-12 M), which enhances expression of the IL-12p70 heterodimer that is necessary for induction of Th1 and CTL immunity, was overexpressed in hUCBMSCs and found to significantly decrease tumor growth and angiogenesis, as well as to increase the survival of glioma-bearing mice and to confer tumor-specific long-term T-cell immunity [199].
In human glioma studies, IL-24-hUCMSCs promoted tumor cell apoptosis, and IFN-beta-hBMMSCs were shown to prolong animal survival [200, 201]. Meanwhile, IFN-beta-WJMSCs and IFN-beta-hBMMSCs exhibited the ability to suppress tumor growth in bronchioloalveolar carcinomas [202] and HCCs, respectively, the latter exerting its effect by increasing expression of p21, p27 and FOXO3a, as well as decreasing protein levels of cyclin D1, pRb and AKT [203]. In addition, engineered BMP4-secreting hADMSCs could suppress tumor cell migratory ability and increase survival in glioblastoma [204]. As for hematological cancers, treatment with hUCMSCs equipped with Tandab (a tetravalent bispecific tandem diabody with two binding sites for CD3 and two for CD19) combined with IDO pathway inhibitor showed significantly decreased B cell lymphoma growth by way of decreasing CD98 and Jumonji, and by restoring the proliferation of T cells [205]. Another study demonstrated that UC-MSCs overexpressing IDO can inhibit proliferation of leukemia cells [206]. hWJMSCs engineered with scFvCD20-sTRAIL fusion protein, which targets CD20-positive cells and induces apoptosis through sTRAIL, inhibited proliferation in B cell lymphoma [207]. Another study showed that hWJMSCs transfected with vector coding sTRAIL driven by AFP promoter had significant antitumor activity in HCC [208]. Decreased tumor growth was also observed in gastric cancer and in epithelial ovarian cancer using hUCBMSCs delivering TNFSF14 or IL-21, respectively [209, 210]. In a syngeneic pancreatic tumor mouse model, IL15-hUCBMSCs inhibited tumor growth and increased survival of tumor-bearing mice. The IL15-hUCBMSCs induced NK- and T-cell accumulation at the tumor site and established tumor-specific T-cell memory immunity [211]. Cytosine deaminase-expressing hADMSCs serving as a prodrug converting vehicle, showed significant decreases in colorectal cancer growth in the presence of prodrug 5-fluorocytosine [212].
Summary of promotion and suppression effects of MSCs in cancer
MSCs can contribute to tumor promotion as well as to tumor suppression. Although it may appear that these effects occur randomly, closer examination provides a more promising picture. Summarizing a total of 110 reports, (excluding engineered MSCs) reveals that in 58.6% of the studies, BMMSCs promoted tumor growth, while 9.8% of studies found that BMMSCs suppressed growth. Although the tendency of ADMSCs is not as obvious as that of BMMSCs, they also exhibit a preference for tumor promotion (Fig. 2). In general, MSCs derived from reproduction-related sources, including placenta, umbilical cord, Wharton’s jelly, and umbilical cord blood, show a higher likelihood for tumor suppression (Fig. 2). In regards to tumor type, we found that BMMSCs show an overwhelming promoting effect on cancers of the bone (100%, 6/6), breast (100%, 7/7) and GI tract, (liver, bile duct, colorectal, gastric and esophageal; 93.75%, 15/16) (Fig. 2a).
MSCs demonstrate an impressive suppressive ability in hematological cancers. In all 7 studies, MSCs from different tissue types showed tumor suppression. Similarly, in a total of 8 studies of MSCs and ovarian cancer, only one study reported that MSCs promoted tumor growth (Fig. 2b). To date, there is no report showing a tumor promoting effect for MSCs from placental tissue.
MSCs can exert their effects directly by contacting tumor cells, or indirectly by secreting soluble factors and microRNAs that the affect the tumor cells. The mechanisms by which different types of MSCs promote or suppress the growth of different tumor types are complicated (Tables 2, 3, 5, Fig. 4). Factors that may affect the properties of MSCs and cause different outcomes, include (1) the origin of the MSCs; (2) different processes of isolation, purification, and expansion of MSCs; and (3) different culture conditions and passages of the MSCs. Most of the results described herein were derived from direct or indirect in vitro co-culture systems or from in vivo co-injection experiments, but the underlying mechanisms were not always examined. It will be necessary to elucidate these underlying mechanisms, as well as to find potential biomarkers of MSC-tumor interactions for future clinical applications of MSCs.
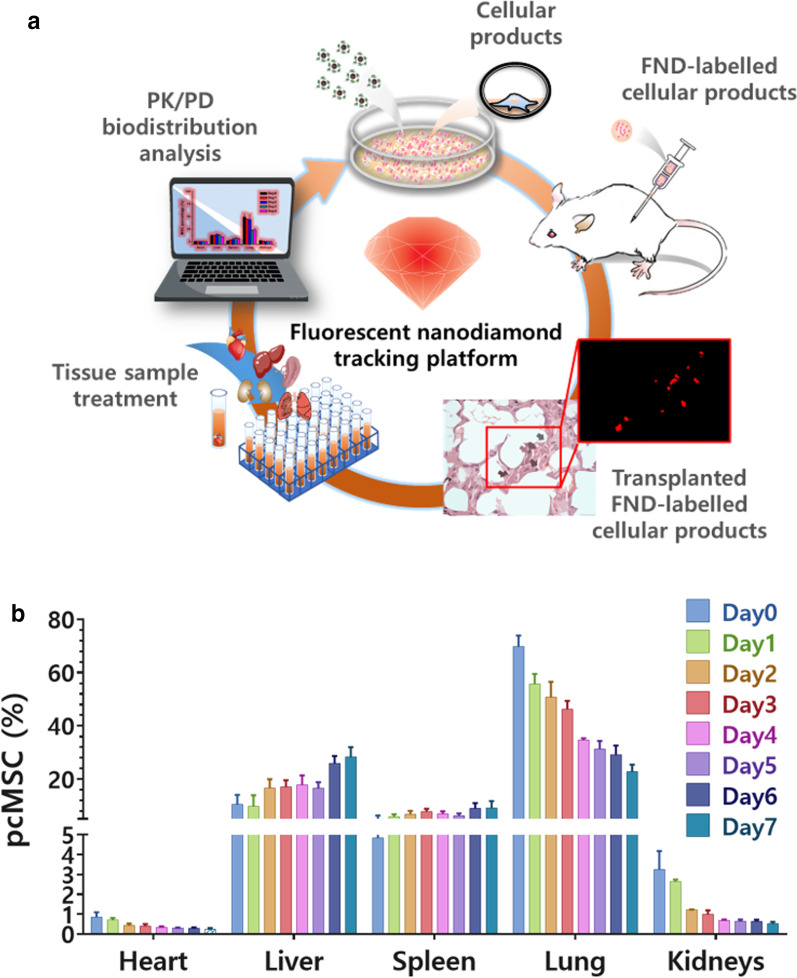
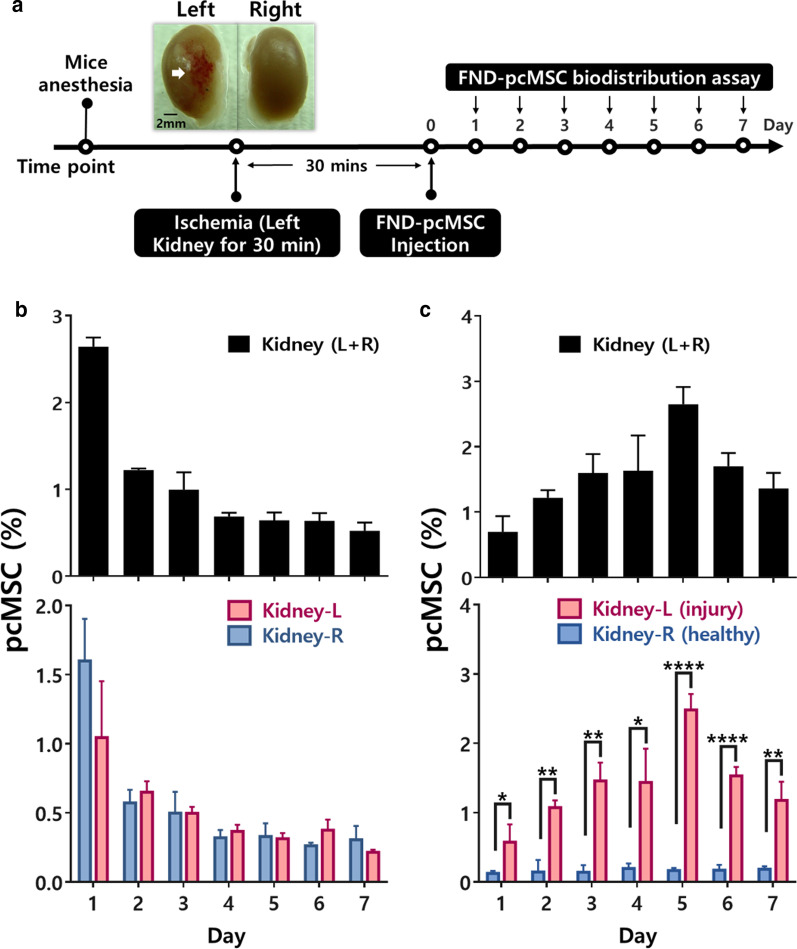
Biodistribution of therapeutic cells in a preclinical evaluation
In light of the tremendous potential of MSCs for treating various diseases, it is necessary to define the systemic distribution and to quantify the administered cells in order to facilitate our understanding of the safety and efficacy of MSC-based cell therapy. This information is critical in clinical trials since it is vitally important to know whether the transplanted cell products home to the target diseased sites to deliver their intended effects. Indeed, several factors can affect the pharmacokinetics (PK) of the administered MSCs, including cell size, cell source, immunological features and labeling, detection methods, route of administration, and size of the animal model.
Factors that affect the biodistribution of MSCs
The typical diameter of a MSC is between 15–30 μm; in comparison, lymphocytes have a diameter of only 4–12 μm [213]. Furthermore, MSCs become larger after serial ex vivo cell passaging [214]. The relatively large size of MSCs explains their initial mechanical entrapment at lung capillary systems after intravenous administration, a phenomenon referred to as the pulmonary first-pass effect [26, 215]. Redistribution to liver, spleen, and other inflamed tissues subsequently takes place in the following hours to days, with gradual clearance from the lungs [26]. In some studies, MSCs were still detected in the lungs up to 150 days after transplantation in vivo [216]. MSCs retained at the lungs potentially decrease the number of cells available for therapeutic effects [217]. To decrease the mechanical entrapment of MSCs at the lungs, several strategies may be implemented, including pretreatment with the vasodilator sodium nitroprusside in order to increase the effective diameter of the pulmonary capillary system; delivery via an extravascular route; or delivery via multiple smaller doses [215, 217, 218]. Although administering MSCs intra-arterially may decrease the extent of mechanical entrapment at the lungs [219], the effect of cell size still has important implications, as larger MSCs may be associated with vascular occlusions that could cause subsequent ischemia and infarcts of unintended tissues and organs [220, 221]. Engineering of MSCs might potentially alter this adverse effect. For example, by overexpressing integrin α4 (ITGA4), which mediates leukocyte trafficking of MSCs, Cui et al. observed that cell aggregation of MSCs were significantly decreased, and MSC-associated cerebral embolism was ameliorated in rat model of stroke [222]. Furthermore, the risk of embolism has been found to be positively associated with cell dose of infusion and low infusion velocity [223].
In addition, aging of either donor or recipient could affect the biodistribution of inoculated MSCs, with decreased transplantation efficiency observed with aged donor MSCs and recipients [224]. Furthermore, when MSCs were extracted from older donors, they exhibited lower proliferative and differentiation capabilities [225, 226]. The culture condition also plays a role in the kinetics of administered MSCs. For example, hypoxic preconditioning increased MSC migration to injured tissue via enhanced HGF/cMET signaling and MSC recruitment, thus affecting biodistribution of the administered cells [227].
Immunogenic reactions also affect clearance and biodistribution of injected cells, as the allogeneic MSCs are not completely immune-privileged [228]. When MSCs are transplanted in an allogeneic host, the transplanted MSCs have decreased survival compared with their survival in a syngeneic host [229]. Formation of antibodies against injected MSCs could explain the reduced effectiveness and increased adverse effects that were observed with repeated inoculations in some studies [230].
Furthermore, the injected cells can also trigger an instant blood-mediated inflammatory reaction (IBMIR), which causes reduced graft survival and thromboembolism [231]. A portion of injected MSCs do not reach their intended destination due to the host’s immune reaction, embolization, and micro-ischemia [232]. Previous literature has demonstrated that the extent of IBMIR is related to the level of tissue factor (TF) expressed by MSCs; expression levels vary among different tissue origins of MSCs, and with culture conditions [233]. Compared with ADMSCs and UCMSCs, BMMSCs express lower levels of TF [233]. Thus, selecting TF-deficient BMMSCs may reduce the risk of IBMIR and improve the chances for clinical success. Otherwise, co-treatment with an anticoagulant may be an important consideration for clinical applications [234].
Methods of tracking MSCs in vivo
A critical step in generating pharmacokinetic models of cell products is tracking the fate of cells following transplantation. An ideal quantification technique should have the following features: high sensitivity and specificity; long-term detection and monitoring; and spatiotemporal resolution. The advantages and disadvantages of currently available methods for quantitative MSC detection are summarized in Table 6. Polymerase chain reaction (PCR) has been used to track human MSCs in murine xenogeneic models by detecting human DNA [19, 235–237]. The low limit of detection of quantitative PCR enables detection of 100 MSCs per gram of organ tissue, making it feasible to detect MSCs in patient biopsies. Both flow cytometry and optical imaging require labeling MSCs with fluorescent dyes or proteins. Flow cytometry enables estimation of the number of live MSCs per weight unit of tissue, and optical imaging uses a variety of dyes, such as 4′,6-diamidino-2-phenlindole (DAPI), that can bind reversibly or irreversibly to the MSCs [238–241]. The use of red fluorescent protein (RFP) or green fluorescent protein (GFP) expressing MSCs has the advantage of providing viability information of transplanted cells [242]. However, the transfection efficiency is not consistent, and the transfected cells could have altered potency and expression and cannot be accurately tracked over time [243]. Therefore, the biodistribution and quantitative data produced by fluorescent protein labeling methods may be incomplete. Bioluminescence imaging (BLI) which utilizes luciferase reactions also has the advantage of providing viability information of transplanted cells, but this method suffers from poor tissue penetration and low spatial resolution. MSCs can also be labeled with gold nanoparticle and tracked by computed tomography (CT) image in vivo [244, 245]. These gold nanoparticles have advantage of exerting negligible influence on viability, proliferation, and differentiation ability of labeled MSCs, and offer good spatial resolution and long-term tracking when used in conjunction with CT modality [244]. However, sensitivity is relatively poor, and there is still difficulty deriving quantitative information from CT images [246].
Table 6.
Comparison of methods used for quantitative mesenchymal stem/stromal cells (MSC) detection (Adapted from ref. [300])
| Technique | Detection | Advantages | Disadvantages |
|---|---|---|---|
| PCR/histology | Transplanted-cell specific DNA sequences or antigens |
High sensitivity No need to label the cells |
Need animal sacrifice, biopsy, Postmortem samples from patients |
| Optical imaging | Fluorescent dyes/proteins |
High throughput Good for longitudinal studies |
Small animals only, Low resolution, Non-stable |
| Flow cytometry | Fluorescent dyes/proteins |
High specificity, Quantification of live cells |
Preclinical use only |
| MRI | Contrast agents |
Clinically useful High spatial resolution Whole-body scanning |
Quantification is difficult Cytotoxicity of certain labeling agents |
| Radionuclear | Radioisotope labels |
Quantification feasible using SPECT Whole-body scanning High sensitivity |
Limited spatial resolution Ionizing radiation |
| FND | Fluorescence |
Large animal models (pigs) PK/PD of transplanted cells Biodistribution of transplanted cells Background-free imaging Single-cell detection sensitivity High throughput quantification No interference with cell potency |
Need animal sacrifice |
PCR polymerase chain reaction, PET positron emission tomography, SPECT single-photon emission computed tomography, FND fluorescent nanodiamond, PK pharmacokinetic, PD pharmacodynamics
Magnetic resonance imaging (MRI) can be used to track MSCs in vivo by labeling MSCs with superparamagnetic iron oxide nanoparticles (SPIONs) or fluorine-19 (19F). Direct labeling of MSCs with SPIONs is possible as these agents are readily taken up by MSCs and show up as hypointense signals on MRI [247]. However, some studies have shown that proliferative and differentiation capabilities of MSCs could be affected when labeled at higher concentrations [247]. The downside of SPION labeling is that the specificity of SPION-labeled cells could be low and the signals could be hard to differentiate from acutely injured tissues containing hemorrhages. In contrast, 19F-labeling offers better specificity as endogenous fluorine level is low, minimizing background interference and is a better labeling agent when the targeting lesion involves hemorrhage [248]. In general, MRI offers good spatial resolution but suffers from poor temporal resolution. Positron emission tomography (PET), single-photon emission computed tomography (SPECT) [26, 249–251] and radioisotope labeling [26, 252, 253] have been used to image and track the migration dynamics, and inter-patient variability of MSCs in clinical patients, but quantifying cell numbers with these methods is difficult and only semi-quantitative information on the biodistribution of the transplanted cells can be obtained. Photoacoustic imaging, which combines ultrasonography with optical imaging, is another attractive approach, as ultrasonography has the unique advantage of providing real-time information while still maintaining good spatial resolution. By using gold nanorods coated with reactive oxygen species (ROS) sensitive dye as probe, Dhada et al. were able to also detect viability of implanted cells [254]. However, photoacoustic imaging suffers from operator dependent variability [255]. More recently, multimodal imaging probes that combine the advantage of different imaging modalities have been developed, including PET/MRI imaging agent [256], SPECT/MRI/fluorescent imaging agent [257], and SPECT/MRI/BLI imaging agent [258].
An ideal cell tracking method should be biocompatible and nontoxic, require no genetic modification, have single-cell detection sensitivity, and permit quantification of cell numbers at any anatomic location. Optical imaging utilizing nanoparticles as exogenous contrast agents is suitable for this purpose, although the technique is mainly used for animal models in preclinical experimentation due to the limited penetration depth of visible photons into tissue. Among various exogenous contrast agents, fluorescent nanodiamond (FND) has emerged as an attractive option because it is chemically inert and inherently biocompatible [259, 260]. A viable application of FNDs for background-free imaging and quantitative tracking of MSCs in animal models beyond rodents has been demonstrated using magnetic modulation [261–263]. The magnetic modulation fluorescence (MMF) method uses magnets to modulate the fluorescence intensity of FNDs. This technique, which allows background-free imaging, together with the inertness of FNDs and the large quantity of the nanoparticles taken up by the cells, has permitted studies of the biodistribution and pharmacokinetics of FND-labeled MSCs in preclinical settings. This strategy can also be applied to the characterization of cell-based products in order to accelerate their progression towards commercialization to meet the needs of patients. The technique has excellent compatibility with time-gated fluorescence imaging, which has been shown to be a powerful means of acquiring high-contrast fluorescence imaging of FND-labeled cells in tissues. The ability to find single cells is particularly valuable for ex vivo histological detection of MSCs in clinical trials. This combined approach represents an appealing alternative to hazardous radioisotope labeling techniques in cell tracking applications. The technique can be used with immune cells, stem cells, and other cell types used for cell therapy. Here, we put these technologies together, and describe how they could be used to contribute to the development of pharmacokinetic modeling of MSC-based cell products.
An FND-based platform to track therapeutic cells in vivo
The ability to monitor the behavior of transplanted cells in vivo is required for cell therapy. When cellular products are submitted for investigational new drug (IND) status, pharmaceutical studies must provide evidence of not only the safety of the cell product, but also information regarding cell location, cell migration, PK and pharmacodynamics (PD), and cell biodistribution after transplantation in animal models. There are three critical issues that must be addressed for cell therapy: (1) whether therapeutic cells maintain their potency after transplantation, (2) the appropriate dosage for curing diseases and (3) a route of administration and a formulation that permits successful drug delivery. Over the past decade, the traditional concepts, confined to low molecular weight organic compounds and large biomolecules, have been challenged with the advent of new drugs based upon cells, which we refer to here as cell therapy. As for all drugs, understanding the pharmacology of cell-therapy products is critical for their effective application in the clinical setting. For example, tissue section and PCR does not provide sufficient information of cell behavior in vivo, because these procedures select a sample from a population, making it difficult to provide PK and PD information for the whole animal. In contrast, the FND-labelled tracking technique provides a new method to achieve high throughput whole organ treatment and analysis, providing accurate pharmacology information, such as PK, PD and biodistribution of the cellular therapy (Fig. 5a). This method not only provides immediate and highly specific cell localization data after gathering histological sections from the animal, but also provides a one-step, one-tube analysis for any kind of animal tissue. Compared to the qPCR sampling method, this protocol can provide more accurate data for whole organ/tissue analysis and takes less time for validation and analysis.
Fig. 5.
Workflow of fluorescent nanodiamond (FND)-labelled tracking platform and biodistribution analysis of FND-labelled pcMSCs. a The FND-labelled tracking platform for cell biodistribution analysis. This platform can provide analysis for transplanted cell localization, pharmacokinetics (PK), and pharmacodynamics (PD). FND-labelled cells are delivered through intravenous injection. The transplanted cells can be pinpointed to specific locations with background-free imaging by Leica SP8 microscopy using a time-gating technique. PK and PD analyses can be performed with a magnetic modulation fluorescence (MMF) machine after tissue/organ digestion. b Distribution of FND-labelled pcMSCs among different organs in a healthy mouse model. Experiments were repeated in triplicate and error bars represent the standard deviation
We use a healthy mouse model to demonstrate that the FND-labelled platform can provide evidence of cell biodistribution. Figure 5b shows the biodistribution analysis of FND-labelled placenta choriodecidual membrane-derived MSCs (pcMSCs) for one week in a mouse model using the FND-based labelling platform. Our results show that the majority (up to 70%) of FND-labelled pcMSCs localized to the lungs after intravenous administration, which is consistent with the pulmonary first-pass effect [217, 264]. The trapping of MSCs in the lungs is due to space restriction [265], as pcMSCs are more than ~ 20 μm in diameter and much larger than the width of the micro-capillaries of the lung. After intravenous infusion, FND-labelled pcMSCs disappeared from the lungs as time passed, and migrated to other tissues/organs such as the liver and spleen, or to injured sites. Nevertheless, the number of FND-labelled pcMSCs decreased in the heart and kidneys (Fig. 5b).
As it has been reported that MSCs will migrate to injured sites [266], we induced an ischemia–reperfusion injury to the left kidney in our animal model (Fig. 6a) and examined whether FND-labelled pcMSCs injected into the portal vein would appear in the injured kidney, to test the concept that MSCs will migrate to sites of injury. In our mouse model with healthy kidneys, the number of pcMSCs in the kidneys decreased over time (Fig. 6b, upper panel) and the decrease was evident in both the left and right kidneys. (Fig. 6b, lower panel). In contrast, in the mouse model with the injury the number of FND-labelled pcMSCs in the injured kidney was highest on day 5 (3%; Fig. 6c). As seen in the lower panel of Fig. 6c, the injured kidney (L kidney) had significantly more FND-labelled pcMSCs than the healthy kidney (R kidney). The percent of FND-labelled pcMSCs remained consistent over time (~ 0.25%) in the healthy right kidney (R kidney) (*P < 0.5, **P < 0.01, ***P < 0.001, ****P < 0.0001.) (Fig. 6c, lower panel). Given these data, it appears that the percentage of MSCs that migrate to kidneys is limited to about 4%, and it appears that the kidneys have the ability to redistribute MSCs in vivo. In addition to providing fast and accurate results, this technique is completely safe to the cell tissue. The FND-labelling technique does not alter any properties of the cell, including cell viability, proliferation, differentiation and immunomodulation, making this method very biocompatible.
Fig. 6.
Fluorescent nanodimond (FND)-labelled pcMSC biodistribution analysis in mouse model with a kidney ischemia–reperfusion injury. a Timeline of the ischemia–reperfusion kidney injury mouse model. The ischemia–reperfusion injury was created on the left-hand side kidney (L) in a mouse, then FND-labelled pcMSCs were injected through the portal vein. b Bodistribution of FND-labelled pcMSCs in healthy kidney mouse model. Experiments were repeated in triplicate and error bars represent the standard deviation of the measurements. c Biodistribution of FND-labelled pcMSCs in ischemia–reperfusion kidney injury mouse model. Experiments were repeated in triplicate and error bars represent the standard deviation of uncertainty. Data are presented as mean ± standard deviation. Data were analyzed using Student’s t-test. *P value of < 0.05. **P value of < 0.01. ***P value of < 0.001. ****P value of < 0.0001
Clinical applications of MSCs in cell therapy: safety and potency
The potential and promise of MSC therapy is highly anticipated in recent and coming decades. As with all emerging new medical technologies, patient safety is always the first priority. As we have discussed, although the ability to modulate immune environment and promote tissue regeneration have been well reported in preclinical studies, the aspect regarding tumor induction or promotion is still one of the many concerns. The MSCs derived from different tissue origins or expanded under different culture conditions present different immune profiles which may result in tumor promotion [126]. Additionally, as the double sided blades of the MSCs’ strong immune modulation ability [262], evaluation of both the specific MSC properties as well as the patient’s immune conditions is strongly needed. The patient’s immune condition both before, during, and after treatment should be closely monitored.
Some reports showed that artificial engineering process may decrease the tumor induction and increase tumor-suppressing function of MSCs [263]. However, genetically engineered MSCs also raise other safety concerns. Although several clinical trials claimed the safety of MSC-treated patients, however, most of the trials only showed short-term safety and are without the examination of tumor-associated biomarkers [267, 268].
A recent systematic review and meta-analysis reappraised 55 randomized controlled trials and over 2000 patients to investigate the safety of systemically inoculated MSCs [39]. The risk of fever was significantly greater in the group of patients receiving MSCs. There was no significant increase in the risk of infection, thrombo-embolic events, malignancy or ectopic tissue formation, while the risk of death was significantly lower in the MSC-treated patients. Among the included studies, severe adverse events, including treatment related fever, in-stent thrombosis with death, acute coronary artery occlusions after intra-coronary delivery, grade 1 anaphlyactoid reaction, gastric ulcer perforation, hypersensitivity reaction, and anal cancer, have been reported to be possibly related to MSC treatment. Although the conclusion of the meta-analysis ends on a promising note, it was also emphasized that an a priori plan to monitor safety should be outlined in every clinical study design, including immediate allergic reactions, local complications (hematoma formation, local infection), vascular obstructions (dyspnea, oliguria, myocardial infarction, venous thromboembolic events), systemic complications (systemic infection, abnormal liver or renal function), malignancy or ectopic manifestation of implanted MSCs, and other disease-specific safety considerations [39].
Additionally, patients with medical history of ischemic diseases, cardiovascular diseases, lung fibrosis, concurrent neoplasm, and family history of hereditary cancer should be carefully reviewed during MSC treatment. The cell dose, infusion route and rate should be documented. The product profiles of the MSCs from different tissues and different generation processes, such as transcriptome, epigenome, proteomic data, cell populations, potential potency biomarkers, preclinical data from cell and animal studies, should be provided.
The therapeutic efficacy of MSCs in different disease indication is still under evaluation, as most of the studies to date have been limited to phase 1 and phase 2 studies (Fig. 1b and 1c, Additional file 2). As we have discussed in this review, the differences in MSC tissue origins and the variety of cell culture conditions would be some of the important factors determining MSC potency in vivo [269]. Thus, the development of surrogate potency assays using preclinical animal model is needed [270]. Recently the International Society for Cellular Therapy (ISCT) have announced some strategies to identify the potential effective factors of MSC action mechanism, including the combined the matrix assay and multiple techniques, such as quantitative RNA analysis for the specific genes, flow cytometry analysis for cell surface markers, and the protein-based assay of secretome [271]. Potency assessments in evaluating cell pharmacology, cell delivery route, as well as the cell-drug interaction are still under development to improve the MSC precision therapy [272–275]. Although the matrix assays were reported to serve as a platform to identify the biomarkers for MSC potency in vitro [276, 277], whether this in vitro assays are able to identify the MSC potency are still under discussion. For example, the use of allogeneic human peripheral blood mononuclear cells for mixed lymphocyte reaction (MLR) assays is a popular assay to demonstrate the MSC immunomodulation capacity. However, the lack of robustness, accuracy, and reproducibility is of concern [278–280]. Additionally, the correlation between the in vitro assays and in vivo pre-clinical/clinical data requires further evaluation.
Cryopreservation could be another factor affecting MSC potency. It has been documented that the MSC cryostorge, the so-called “cryo stun effect”, may decrease MSC therapeutic efficacy, leading to failures in MSC clinical trials [278]. Recently, a systematic review regarding the impact of cryopreservation on BMMSCs showed that the cryopreservation appears to affect the cell viability, apoptosis, cellular attachment, immunomodulation, and metabolism of BMMSCs [279]. Furthermore, these impaired viability or functions of the MSCs can be restored, partially or totally, by following an acclimation period [279–281], or by IFNγ licensing before cryopreservation [282].
In summary, the use of standardized potency assays should be incorporated into future MSC product release criteria. Thus, development of surrogate potency assays for different disease indications should be highlighted. The optimal process of cryopreservation and thawing may be another important factor requiring further attention.
Conclusions
MSCs are a major cornerstone to the advancement of cell therapy, yet much remains to be learned about their pharmacokinetics and pharmacodynamics after systemic application in vivo. The different tissue origins of MSCs not only confer different biological activities that affect their therapeutic usefulness, but also raise the concern of different safety profiles. Many methods, including herein discussed fluorescent nanodiamond, are available for tracking inoculated MSCs in vivo, each with different advantages and disadvantages. These imaging platforms will facilitate future studies to discern and optimize the use of different MSCs for future clinical therapies.
Supplementary Information
Additional file 1. Flowchart of MSC clinical study inclusion. As of October 11, 2020, 1,242 registered studies were identified on clinicaltrials.gov by searching keywords “mesenchymal stem cell” or “mesenchymal stromal cell”. After excluding studies with no longer available/ suspended/ temporarily not available/ terminated/ unknown/ withdrawn status, unknown phase information, and studies that did not use MSCs in their intervention arm, 639 studies remained. Nine of these 639 studies investigated MSCs from two tissue origins, generating a total of 648 studies for analysis.
Additional file 2. Breakdown of MSC-related clinical studies by disease indication. The included studies for analysis are as illustrated in Additional file 1.
Acknowledgements
We thank Dr. Houng-Chi Liou of Department of Pharmacology, College of Medicine of National Taiwan University for his excellent technical support with kidney ischemia/reperfusion animal model.
Abbreviations
- AC-LCSC
Adenocarcinomas-lung cancer stem cells
- AFP
Alpha-fetoprotein
- ALDH
Aldehyde dehydrogenase
- ALP
Alkaline phosphatase
- Ang-1
Angiopoietin-1
- APL
Acute promyelocytic leukemia
- ATG5
Autophagy related 5
- ATG7
Autophagy related 7
- BAX
Bcl-2-associated X
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- BCRP
Breast cancer resistance protein
- BMP
Bone morphogenetic protein
- CA-125
Cancer antigen 125
- CAFs
Cancer-associated fibroblasts
- CCL2
C–C motif chemokine 2 (MCP-1)
- CCL5
C–C motif chemokine 5
- CCR1
C–C chemokine receptor type 1
- CD
Cytosine deaminase
- CL
Cell lysate
- CM
Conditioned medium
- CSC
Cancer stem cells
- CTL
Cytotoxic T-cell
- CXCL1
C-X-C Motif Chemokine Ligand 1 (GRO-a)
- CXCL8
C-X-C Motif Chemokine Ligand 8 (IL8)
- CXCL12
C-X-C Motif Chemokine Ligand 12 (SDF-1)
- CXCR1
C-X-C chemokine receptor type 1
- CXCR2
C-X-C chemokine receptor type 2
- CXCR4
C-X-C chemokine receptor type 4
- CXCR7
C-X-C chemokine receptor type 7
- CXCR12
C-X-C chemokine receptor type 12
- DIABLO
Direct IAP-Binding protein with Low PI
- ECM
Extracellular matrix
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial–mesenchymal transition
- EP
Prostaglandin E2 receptor
- ERK
Extracellular signal–regulated kinase
- ET1
Endothelin-1
- EVs
Extracellular vesicles
- Exos
Exosomes
- FAK
Focal adhesion kinase
- FND
Fluorescent nanodiamond
- FoxO3a
Forkhead box class O 3a
- GBM
Glioblastoma Multiforme
- Glut-1
Glucose transporter type 1
- GRO-a
Growth-regulated oncogene-alpha (CXCL1)
- GSK3β
Glycogen synthase kinase 3β
- hCG
Human chorionic gonadotropin
- HDGF
Hepatoma-derived growth factor
- HGF
Hepatocyte growth factor
- HIF-1α
Hypoxia-inducible factor 1alpha
- HK-2
Hexokinase-2
- IBMIR
Instant blood-mediated inflammatory reaction
- IDO
Indoleamine-2,3-dioxygenase
- IGF
Insulin-like growth factor
- IL1β
Interleukin 1 beta
- IL6
Interleukin 6
- IL8
Interleukin 8
- IFN
Interferon
- JAK
Janus kinase
- JNK
C-Jun N-terminal kinase
- LDH
Lactic dehydrogenase
- Mad1
Mitotic arrest deficient 1
- MAPK
Mitogen-activated protein kinase
- MARCKS
Myristoylated Alanine Rich Protein Kinase C Substrate
- MCP-1
Monocyte chemoattractant protein-1 (CCL2)
- MDSC
Myeloid-derived suppressor cells
- MMF
Magnetic modulation fluorescence
- MMP
Matrix metalloproteinase
- MSC
Mesenchymal stem/stromal cell
- MWCO
Molecular weight cut off
- NRG1
Neuregulin 1
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural killer
- OSM
Oncostatin M
- PAI-1
Plasminogen activator inhibitor-1
- PCNA
Proliferating cell nuclear antigen
- PDCD4
Programmed cell death 4
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- PD-L1
Programmed death-ligand 1
- PI3K
Phosphoinositide 3-kinase
- PD
Pharmacodynamics
- PK
Pharmacokinetics
- PKM-2
Pyruvate Kinase M2
- Plau/uPA
Urokinase-type plasminogen activator
- pRb
Phosphorylated retinoblastoma protein
- PTBP1
Polypyrimidine tract-binding protein 1
- PTEN
Phosphatase and tensin homolog
- RECK
Reversion-inducing-cysteine-rich protein with kazal motifs
- SCC
Squamous cell carcinomas
- SASP
Senescence-associated secretory phenotype
- SDF-1
Stromal cell-derived factor-1(CXCL12)
- Sema-7A
Semaphorin-7A
- Smac
Second mitochondria-derived activator of caspases
- STAT
Signal transducer and activator of transcription
- TAM
Tumor-associated macrophage
- TF
Tissue factor
- TGF-β
Transforming growth factor beta
- Th1
T-helper 1
- TIMP
Tissue inhibitors of metalloproteinase
- TMZ
Temozolomide
- TNF-α
Tumor Necrosis Factor-α
- TNFSF14
Tumor necrosis factor superfamily member 14
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- VEGF
Vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis protein
- ZEB1
Zinc finger E-box binding homeobox 1
Authors' contributions
Conception and design: WZZ, YHL, TYL and YHH; Development of methodology: HCC, LJS, TYL; Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): HCC, LJS, TYL, YHH; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): WZZ, YHL, LJS, TYL and YHH; Writing, review, and/or revision of the manuscript: WZZ, YHL, HYJ, TYL and YHH; All authors read and approved the final manuscript.
Funding
This study is supported by research grants from Ministry of Science and Technology, Taiwan (Grant numbers: MOST 107-2321-B-038-002, MOST 107-2314-B-038-061, MOST108-2314-B-038-006, MOST108-2321-B-038-003, MOST109-2314-B-038-135, MOST109-2321-B-038-003 to YHH; and MOST105-2325-B-002-040, MOST106-3114-B-038-001, MOST107-2321-B-038 -002, MOST108-2321-B-038-003 to TYL).
Availability of data and materials
All relevant data are included in this published article.
Declarations
Ethics approval and consent to participate
The kidney ischemia/reperfusion animal model and its study was approved by the Institutional Animal Care and Use Committee of National Taiwan University (with IACUC Approval No. 20180123) and conducted with compliance of the standards established in the Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei-Zhan Zhuang and Yi-Heng Lin contributed equally to this work
Contributor Information
Yen-Hua Huang, Email: rita1204@tmu.edu.tw.
Thai-Yen Ling, Email: tyling@ntu.edu.tw.
References
- 1.Aijaz A, Li M, Smith D, Khong D, LeBlon C, Fenton OS, et al. Biomanufacturing for clinically advanced cell therapies. Nat Biomed Eng. 2018;2(6):362–376. doi: 10.1038/s41551-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- 3.Diehl R, Ferrara F, Müller C, Dreyer AY, McLeod DD, Fricke S, et al. Immunosuppression for in vivo research: state-of-the-art protocols and experimental approaches. Cell Mol Immunol. 2017;14(2):146–179. doi: 10.1038/cmi.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin I, Galipeau J, Kessler C, Le Blanc K, Dazzi F. Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aat2189. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 7.Heidari B, Shirazi A, Akhondi MM, Hassanpour H, Behzadi B, Naderi MM, et al. Comparison of proliferative and multilineage differentiation potential of sheep mesenchymal stem cells derived from bone marrow, liver, and adipose tissue. Avicenna J Med Biotechnol. 2013;5(2):104–117. [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 9.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 11.in `t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FHJ, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–9. [DOI] [PubMed]
- 12.Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264(1):51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Sun Z, Cui D, Xu X, Zhu H, Wang L, et al. Isolation and characterization of lung resident mesenchymal stem cells capable of differentiating into alveolar epithelial type II cells. Cell Biol Int. 2014;38(4):405–411. doi: 10.1002/cbin.10240. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–564. [PubMed] [Google Scholar]
- 15.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 16.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011;27(3):719–733. doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan SF, Jiang T, Sun LH, Zheng RJ, Cao GQ, Ahat NZ, et al. Use of bone mesenchymal stem cells to treat rats with acute liver failure. Genet Mol Res. 2014;13(3):6962–6980. doi: 10.4238/2014.April.30.10. [DOI] [PubMed] [Google Scholar]
- 18.Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22(3):325–332. [PubMed] [Google Scholar]
- 19.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 22.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30(22):2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai M, Shen R, Song L, Lu M, Wang J, Zhao S, et al. Bone Marrow Mesenchymal Stem Cells (BM-MSCs) Improve Heart Function in Swine Myocardial Infarction Model through Paracrine Effects. Sci Rep. 2016;6:28250. doi: 10.1038/srep28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(19 Suppl):Ii247–Ii256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 25.Pei Z, Zeng J, Song Y, Gao Y, Wu R, Chen Y, et al. In vivo imaging to monitor differentiation and therapeutic effects of transplanted mesenchymal stem cells in myocardial infarction. Sci Rep. 2017;7(1):6296. doi: 10.1038/s41598-017-06571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961–967. doi: 10.1016/j.nucmedbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26(1):244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41(5):2629–2639. doi: 10.3892/ijmm.2018.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 30.Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. doi: 10.3390/ijms20112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cyranoski D. Japan's approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature. 2019;565(7741):544–545. doi: 10.1038/d41586-019-00178-x. [DOI] [PubMed] [Google Scholar]
- 32.Delling U, Brehm W, Metzger M, Ludewig E, Winter K, Julke H. In vivo tracking and fate of intra-articularly injected superparamagnetic iron oxide particle-labeled multipotent stromal cells in an ovine model of osteoarthritis. Cell Transplant. 2015;24(11):2379–2390. doi: 10.3727/096368914X685654. [DOI] [PubMed] [Google Scholar]
- 33.Pak J, Lee JH, Pak N, Pak Y, Park KS, Jeon JH, et al. Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status. Int J Mol Sci. 2018;19(7):2146. doi: 10.3390/ijms19072146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam Y, Rim YA, Lee J, Ju JH. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int. 2018;2018:8490489. doi: 10.1155/2018/8490489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang CZ, Eswaramoorthy R, Lin TH, Chen CH, Fu YC, Wang CK, et al. Enhancement of chondrogenesis of adipose-derived stem cells in HA-PNIPAAm-CL hydrogel for cartilage regeneration in rabbits. Sci Rep. 2018;8(1):10526. doi: 10.1038/s41598-018-28893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satue M, Schuler C, Ginner N, Erben RG. Intra-articularly injected mesenchymal stem cells promote cartilage regeneration, but do not permanently engraft in distant organs. Sci Rep. 2019;9(1):10153. doi: 10.1038/s41598-019-46554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y, Yang Z, Ding Q, Zhao T, Huang Z, Feng G. Targeted transplantation of iron oxide-labeled, adipose-derived mesenchymal stem cells in promoting meniscus regeneration following a rabbit massive meniscal defect. Exp Ther Med. 2016;11(2):458–466. doi: 10.3892/etm.2015.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinMed. 2020 doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. doi: 10.1016/S1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 41.Mandel K, Yang Y, Schambach A, Glage S, Otte A, Hass R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 2013;22(23):3114–3127. doi: 10.1089/scd.2013.0249. [DOI] [PubMed] [Google Scholar]
- 42.Kouris NA, Schaefer JA, Hatta M, Freeman BT, Kamp TJ, Kawaoka Y, et al. Directed fusion of mesenchymal stem cells with cardiomyocytes via VSV-G facilitates stem cell programming. Stem Cells Int. 2012;2012:414038. doi: 10.1155/2012/414038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354(3):700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY, et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem Cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23(3):687. doi: 10.3390/molecules23030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol. 2016;1416:123–146. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- 48.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 49.Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98(6):888–895. doi: 10.3324/haematol.2012.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91(3):402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, et al. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regen Med. 2012;6(7):559–569. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 52.Hu CH, Tseng YW, Chiou CY, Lan KC, Chou CH, Tai CS, et al. Bone marrow concentrate-induced mesenchymal stem cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther. 2019;10(1):275. doi: 10.1186/s13287-019-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW, et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton) 2018;23(8):728–736. doi: 10.1111/nep.13099. [DOI] [PubMed] [Google Scholar]
- 54.Faezi M, Nasseri Maleki S, Aboutaleb N, Nikougoftar M. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J Chem Neuroanat. 2018;94:21–31. doi: 10.1016/j.jchemneu.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Kwon HM, Hur SM, Park KY, Kim CK, Kim YM, Kim HS, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63(1):19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Terunuma A, Ashiba K, Takane T, Sakaguchi Y, Terunuma H. Comparative transcriptomic analysis of human mesenchymal stem cells derived from dental pulp and adipose tissues. J Stem Cells Regen Med. 2019;15(1):8–11. doi: 10.46582/jsrm.1501003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7(1):163. doi: 10.1186/s13287-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overath JM, Gauer S, Obermüller N, Schubert R, Schäfer R, Geiger H, et al. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res. 2016;342(2):175–183. doi: 10.1016/j.yexcr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93(4):814–825. doi: 10.1016/j.kint.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 60.von Bahr L, Sundberg B, Lönnies L, Sander B, Karbach H, Hägglund H, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18(4):557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11(1):166–174. doi: 10.3892/mmr.2014.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi MR, Kim HY, Park JY, Lee TY, Baik CS, Chai YG, et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2010;472(2):94–98. doi: 10.1016/j.neulet.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Shi T, Xu A, Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20(7):1203–1213. doi: 10.1111/jcmm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumanasinghe RD, Pfeiler TW, Monteiro-Riviere NA, Loboa EG. Expression of proinflammatory cytokines by human mesenchymal stem cells in response to cyclic tensile strain. J Cell Physiol. 2009;219(1):77–83. doi: 10.1002/jcp.21653. [DOI] [PubMed] [Google Scholar]
- 65.Karussis D, Kassis I. The potential use of stem cells in multiple sclerosis: An overview of the preclinical experience. Clin Neurol Neurosurg. 2008;110(9):889–896. doi: 10.1016/j.clineuro.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial. Gut Liver. 2018;12(1):73–78. doi: 10.5009/gnl17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018;32(12):2672–2684. doi: 10.1038/s41375-018-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donzelli E, Scuteri A. Mesenchymal stem cells: a trump card for the treatment of diabetes? Biomedicines. 2020;8(5):112. doi: 10.3390/biomedicines8050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163–76.e20. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Simovic Markovic B, Gazdic M, Arsenijevic A, Jovicic N, Jeremic J, Djonov V, et al. Mesenchymal stem cells attenuate cisplatin-induced nephrotoxicity in iNOS-dependent manner. Stem Cells Int. 2017;2017:1315378. doi: 10.1155/2017/1315378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, et al. The role of TNF-alpha induced MSCs on suppressive inflammation by increasing TGF-beta and IL-10. Open Access Maced J Med Sci. 2018;6(10):1779–1783. doi: 10.3889/oamjms.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Muller I. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol. 2013;43(10):2741–2749. doi: 10.1002/eji.201343335. [DOI] [PubMed] [Google Scholar]
- 73.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118(2):330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, et al. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells. 2016;34(7):1909–1921. doi: 10.1002/stem.2369. [DOI] [PubMed] [Google Scholar]
- 76.Gazdic M, Markovic BS, Arsenijevic A, Jovicic N, Acovic A, Harrell CR, et al. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl. 2018;24(5):687–702. doi: 10.1002/lt.25049. [DOI] [PubMed] [Google Scholar]
- 77.Wu L, Leijten J, van Blitterswijk CA, Karperien M. Fibroblast growth factor-1 is a mesenchymal stromal cell-secreted factor stimulating proliferation of osteoarthritic chondrocytes in co-culture. Stem Cells Dev. 2013;22(17):2356–2367. doi: 10.1089/scd.2013.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18(11):2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 79.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229–236. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 80.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, et al. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26(11):2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]
- 81.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 83.Ong HT, Dilley RJ. Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev. 2018;44:69–79. doi: 10.1016/j.cytogfr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 84.Li X, An G, Wang Y, Liang D, Zhu Z, Tian L. Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats. Stem Cell Res Ther. 2018;9(1):335. doi: 10.1186/s13287-018-1083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells - an experimental study. Transpl Int. 2018;31(1):102–115. doi: 10.1111/tri.13023. [DOI] [PubMed] [Google Scholar]
- 86.Meier RP, Mahou R, Morel P, Meyer J, Montanari E, Muller YD, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62(3):634–641. doi: 10.1016/j.jhep.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 87.Shen Q, Chen B, Xiao Z, Zhao L, Xu X, Wan X, et al. Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol Med Rep. 2015;11(4):2831–2837. doi: 10.3892/mmr.2014.3092. [DOI] [PubMed] [Google Scholar]
- 88.Li X, Wang Y, An G, Liang D, Zhu Z, Lian X, et al. Bone marrow mesenchymal stem cells attenuate silica-induced pulmonary fibrosis via paracrine mechanisms. Toxicol Lett. 2017;270:96–107. doi: 10.1016/j.toxlet.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 89.Ono M, Ohkouchi S, Kanehira M, Tode N, Kobayashi M, Ebina M, et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol Ther. 2015;23(3):549–560. doi: 10.1038/mt.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu X, Xu Z, Xu Y, Cui G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16(4):245–255. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Javadov S, Kozlov AV, Camara AKS. Mitochondria in health and diseases. Cells. 2020;9(5):1177. doi: 10.3390/cells9051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin HY, Liou CW, Chen SD, Hsu TY, Chuang JH, Wang PW, et al. Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44. doi: 10.1016/j.mito.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180(9):5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51(3):455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 97.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marlein CR, Zaitseva L, Piddock RE, Robinson SD, Edwards DR, Shafat MS, et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood. 2017;130(14):1649–1660. doi: 10.1182/blood-2017-03-772939. [DOI] [PubMed] [Google Scholar]
- 99.Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11(1):11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128(2):253–264. doi: 10.1182/blood-2015-07-655860. [DOI] [PubMed] [Google Scholar]
- 101.Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20(9):509–510. doi: 10.1038/s41580-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 102.Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7(1):4323. doi: 10.1038/s41598-017-04559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma M, Li B, Zhang M, Zhou L, Yang F, Ma F, et al. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Exp Eye Res. 2020;191:107899. doi: 10.1016/j.exer.2019.107899. [DOI] [PubMed] [Google Scholar]
- 104.Zou L, Ma X, Lin S, Wu B, Chen Y, Peng C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp Ther Med. 2019;18(4):2574–2582. doi: 10.3892/etm.2019.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119(11):9433–9443. doi: 10.1002/jcb.27260. [DOI] [PubMed] [Google Scholar]
- 106.Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 107.Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 2016;5(12):1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Yang YY, Ren JL, Xu F, Chen FM, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8(1):198. doi: 10.1186/s13287-017-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 110.Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep. 2015;11(1):150–160. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 111.Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 112.Tamura R, Uemoto S, Tabata Y. Immunosuppressive effect of mesenchymal stem cell-derived exosomes on a concanavalin A-induced liver injury model. Inflamm Regen. 2016;36:26. doi: 10.1186/s41232-016-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5):513–522. doi: 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018;28(9):918–933. doi: 10.1038/s41422-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. 2020 doi: 10.1080/15548627.2020.1717128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tokhanbigli S, Baghaei K, Asadirad A, Hashemi SM, Asadzadeh-Aghdaei H, Zali MR. Immunoregulatory impact of human mesenchymal-conditioned media and mesenchymal derived exosomes on monocytes. Mol Biol Res Commun. 2019;8(2):79–89. doi: 10.22099/mbrc.2019.33346.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wen S, Dooner M, Papa E, Del Tatto M, Pereira M, Borgovan T, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a radiation injury bone marrow murine model. Int J Mol Sci. 2019;20(21):5468. doi: 10.3390/ijms20215468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, et al. Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and MicroRNA study. Transl Stroke Res. 2019;10(5):509–521. doi: 10.1007/s12975-018-0668-1. [DOI] [PubMed] [Google Scholar]
- 120.Li JH, Fan WS, Wang MM, Wang YH, Ren ZG. Effects of mesenchymal stem cells on solid tumor metastasis in experimental cancer models: a systematic review and meta-analysis. J Transl Med. 2018;16(1):113. doi: 10.1186/s12967-018-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin YH, Chen YH, Chang HY, Au HK, Tzeng CR, Huang YH. Chronic niche inflammation in endometriosis-associated infertility: current understanding and future therapeutic strategies. Int J Mol Sci. 2018;19(8):2385. doi: 10.3390/ijms19082385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol. 2018;9:259. doi: 10.3389/fphar.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mognetti B, La Montagna G, Perrelli MG, Pagliaro P, Penna C. Bone marrow mesenchymal stem cells increase motility of prostate cancer cells via production of stromal cell-derived factor-1alpha. J Cell Mol Med. 2013;17(2):287–292. doi: 10.1111/jcmm.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma M, Ye JY, Deng R, Dee CM, Chan GC. Mesenchymal stromal cells may enhance metastasis of neuroblastoma via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Lett. 2011;312(1):1–10. doi: 10.1016/j.canlet.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 125.Ma Z, Cui X, Lu L, Chen G, Yang Y, Hu Y, et al. Exosomes from glioma cells induce a tumor-like phenotype in mesenchymal stem cells by activating glycolysis. Stem Cell Res Ther. 2019;10(1):60. doi: 10.1186/s13287-019-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res. 2019;38(1):62. doi: 10.1186/s13046-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fontanella R, Pelagalli A, Nardelli A, D'Alterio C, Ierano C, Cerchia L, et al. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016;370(1):100–107. doi: 10.1016/j.canlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 128.Chen J, Ji T, Wu D, Jiang S, Zhao J, Lin H, et al. Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin α5 in hepatocellular carcinoma. Cell Death Dis. 2019;10(6):425. doi: 10.1038/s41419-019-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127(10):2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- 130.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Onoyama M, Ohnishi M, et al. Stroma-directed imatinib therapy impairs the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic transplantation model of colon cancer. Int J Cancer. 2013;132(4):813–823. doi: 10.1002/ijc.27735. [DOI] [PubMed] [Google Scholar]
- 131.Liu CJ, Wang YK, Kuo FC, Hsu WH, Yu FJ, Hsieh S, et al. Helicobacter pylori infection-induced hepatoma-derived growth factor regulates the differentiation of human mesenchymal stem cells to myofibroblast-like cells. Cancers (Basel). 2018;10(12):479. doi: 10.3390/cancers10120479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141(3):1046–1056. doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 133.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013;32(37):4343–4354. doi: 10.1038/onc.2012.458. [DOI] [PubMed] [Google Scholar]
- 134.Hogan NM, Joyce MR, Murphy JM, Barry FP, O'Brien T, Kerin MJ, et al. Impact of mesenchymal stem cell secreted PAI-1 on colon cancer cell migration and proliferation. Biochem Biophys Res Commun. 2013;435(4):574–579. doi: 10.1016/j.bbrc.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 135.De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut. 2013;62(4):550–560. doi: 10.1136/gutjnl-2011-301393. [DOI] [PubMed] [Google Scholar]
- 136.Fu X, Xie F, Gong F, Yang Z, Lv X, Li X, et al. Suppression of PTBP1 signaling is responsible for mesenchymal stem cell induced invasion of low malignancy cancer cells. Biochim Biophys Acta Mol Cell Res. 2018;1865:1552–1565. doi: 10.1016/j.bbamcr.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Chen K, Liu Q, Tsang LL, Ye Q, Chan HC, Sun Y, et al. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/beta-catenin/Slug pathway. Cell Death Dis. 2017;8(5):e2819. doi: 10.1038/cddis.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem. 2017;42(6):2242–2254. doi: 10.1159/000479998. [DOI] [PubMed] [Google Scholar]
- 139.Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325(1):80–88. doi: 10.1016/j.canlet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 140.Kawano M, Tanaka K, Itonaga I, Iwasaki T, Tsumura H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun Signal. 2018;16(1):13. doi: 10.1186/s12964-018-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pietrovito L, Leo A, Gori V, Lulli M, Parri M, Becherucci V, et al. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol Oncol. 2018;12(5):659–676. doi: 10.1002/1878-0261.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 143.Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci U S A. 2014;111(20):E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liubomirski Y, Lerrer S, Meshel T, Rubinstein-Achiasaf L, Morein D, Wiemann S, et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Front Immunol. 2019;10:757. doi: 10.3389/fimmu.2019.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 146.Wessely A, Waltera A, Reichert TE, Stockl S, Grassel S, Bauer RJ. Induction of ALP and MMP9 activity facilitates invasive behavior in heterogeneous human BMSC and HNSCC 3D spheroids. FASEB J. 2019 doi: 10.1096/fj.201900925R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hong D, Liu T, Huang W, Liao Y, Wang L, Zhang Z, et al. Gremlin1 delivered by mesenchymal stromal cells promoted epithelial-mesenchymal transition in human esophageal squamous cell carcinoma. Cell Physiol Biochem. 2018;47(5):1785–1799. doi: 10.1159/000491060. [DOI] [PubMed] [Google Scholar]
- 148.Akimoto K, Kimura K, Nagano M, Takano S, To'a Salazar G, Yamashita T, et al. Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 2013;22(9):1370–1386. doi: 10.1089/scd.2012.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park YM, Yoo SH, Kim SH. Adipose-derived stem cells induced EMT-like changes in H358 lung cancer cells. Anticancer Res. 2013;33(10):4421–4430. [PubMed] [Google Scholar]
- 150.Wang Y, Liu J, Jiang Q, Deng J, Xu F, Chen X, et al. Human adipose-derived mesenchymal stem cell-secreted CXCL1 and CXCL8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells. 2017;35(9):2060–2070. doi: 10.1002/stem.2643. [DOI] [PubMed] [Google Scholar]
- 151.Maj M, Bajek A, Nalejska E, Porowinska D, Kloskowski T, Gackowska L, et al. Influence of mesenchymal stem cells conditioned media on proliferation of urinary tract cancer cell Lines and their sensitivity to ciprofloxacin. J Cell Biochem. 2017;118(6):1361–1368. doi: 10.1002/jcb.25794. [DOI] [PubMed] [Google Scholar]
- 152.Chu Y, Tang H, Guo Y, Guo J, Huang B, Fang F, et al. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp Cell Res. 2015;337(1):16–27. doi: 10.1016/j.yexcr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 153.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009;106(10):3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17(3):463–473. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 155.Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 156.Chen DR, Lu DY, Lin HY, Yeh WL. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. Biomed Res Int. 2014;2014:532161. doi: 10.1155/2014/532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li T, Zhang C, Ding Y, Zhai W, Liu K, Bu F, et al. Umbilical cord-derived mesenchymal stem cells promote proliferation and migration in MCF-7 and MDA-MB-231 breast cancer cells through activation of the ERK pathway. Oncol Rep. 2015;34(3):1469–1477. doi: 10.3892/or.2015.4109. [DOI] [PubMed] [Google Scholar]
- 158.Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9(2):218. doi: 10.1038/s41419-018-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao X, Wu X, Qian M, Song Y, Wu D, Zhang W. Knockdown of TGF-beta1 expression in human umbilical cord mesenchymal stem cells reverts their exosome-mediated EMT promoting effect on lung cancer cells. Cancer Lett. 2018;428:34–44. doi: 10.1016/j.canlet.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 160.Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SMF, Cabral FR, et al. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. 2018;9(1):310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vulcano F, Milazzo L, Ciccarelli C, Eramo A, Sette G, Mauro A, et al. Wharton's jelly mesenchymal stromal cells have contrasting effects on proliferation and phenotype of cancer stem cells from different subtypes of lung cancer. Exp Cell Res. 2016;345(2):190–198. doi: 10.1016/j.yexcr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 162.de Vieira Castro J, Gomes ED, Granja S, Anjo SI, Baltazar F, Manadas B, et al. Impact of mesenchymal stem cells' secretome on glioblastoma pathophysiology. J Transl Med. 2017;15(1):200. doi: 10.1186/s12967-017-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Du T, Ju G, Wu S, Cheng Z, Cheng J, Zou X, et al. Microvesicles derived from human Wharton's jelly mesenchymal stem cells promote human renal cancer cell growth and aggressiveness through induction of hepatocyte growth factor. PLoS ONE. 2014;9(5):e96836. doi: 10.1371/journal.pone.0096836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20(11):2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren N, et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci. 2010;101(12):2546–2553. doi: 10.1111/j.1349-7006.2010.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma P, Pan Y, Li W, Sun C, Liu J, Xu T, et al. Extracellular vesicles-mediated noncoding RNAs transfer in cancer. J Hematol Oncol. 2017;10(1):57. doi: 10.1186/s13045-017-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gauthaman K, Yee FC, Cheyyatraivendran S, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton's jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J Cell Biochem. 2012;113(6):2027–2039. doi: 10.1002/jcb.24073. [DOI] [PubMed] [Google Scholar]
- 168.Dzobo K, Vogelsang M, Thomford NE, Dandara C, Kallmeyer K, Pepper MS, et al. Wharton's jelly-derived mesenchymal stromal cells and fibroblast-derived extracellular matrix synergistically activate apoptosis in a p21-dependent mechanism in WHCO1 and MDA MB 231 cancer cells in vitro. Stem Cells Int. 2016;2016:4842134. doi: 10.1155/2016/4842134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, et al. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009;69(5):1815–1820. doi: 10.1158/0008-5472.CAN-08-2750. [DOI] [PubMed] [Google Scholar]
- 170.Yang C, Lei D, Ouyang W, Ren J, Li H, Hu J, et al. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int. 2014;2014:109389. doi: 10.1155/2014/109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kolosa K, Motaln H, Herold-Mende C, Korsic M, Lah TT. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015;24(4):631–644. doi: 10.3727/096368915X687787. [DOI] [PubMed] [Google Scholar]
- 172.Velpula KK, Dasari VR, Tsung AJ, Gondi CS, Klopfenstein JD, Mohanam S, et al. Regulation of glioblastoma progression by cord blood stem cells is mediated by downregulation of cyclin D1. PLoS ONE. 2011;6(3):e18017. doi: 10.1371/journal.pone.0018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dasari VR, Velpula KK, Kaur K, Fassett D, Klopfenstein JD, Dinh DH, et al. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP) PLoS ONE. 2010;5(7):e11813. doi: 10.1371/journal.pone.0011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jiao H, Guan F, Yang B, Li J, Shan H, Song L, et al. Human umbilical cord blood-derived mesenchymal stem cells inhibit C6 glioma via downregulation of cyclin D1. Neurol India. 2011;59(2):241–247. doi: 10.4103/0028-3886.79134. [DOI] [PubMed] [Google Scholar]
- 175.Yuan Y, Zhou C, Chen X, Tao C, Cheng H, Lu X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: a possible role for apoptosis and Wnt signaling. Oncol Lett. 2018;15(6):8536–8544. doi: 10.3892/ol.2018.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18(4):500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 177.Khalil C, Moussa M, Azar A, Tawk J, Habbouche J, Salameh R, et al. Anti-proliferative effects of mesenchymal stem cells (MSCs) derived from multiple sources on ovarian cancer cell lines: an in-vitro experimental study. J Ovarian Res. 2019;12(1):70. doi: 10.1186/s13048-019-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kalamegam G, Sait KHW, Ahmed F, Kadam R, Pushparaj PN, Anfinan N, et al. Human Wharton's jelly stem cell (hWJSC) extracts inhibit ovarian cancer cell lines OVCAR3 and SKOV3 in vitro by inducing cell cycle arrest and apoptosis. Front Oncol. 2018;8:592. doi: 10.3389/fonc.2018.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gauthaman K, Fong CY, Arularasu S, Subramanian A, Biswas A, Choolani M, et al. Human Wharton's jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice. J Cell Biochem. 2013;114(2):366–377. doi: 10.1002/jcb.24367. [DOI] [PubMed] [Google Scholar]
- 180.Wu S, Ju GQ, Du T, Zhu YJ, Liu GH. Microvesicles derived from human umbilical cord Wharton's jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS ONE. 2013;8(4):e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin DH, Biswas A, Choolani M, Fong CY, Bongso A. Induction of immunogenic cell death in lymphoma cells by Wharton's jelly mesenchymal stem cell conditioned medium. Stem Cell Rev Rep. 2017;13(6):801–816. doi: 10.1007/s12015-017-9767-8. [DOI] [PubMed] [Google Scholar]
- 182.Lin HD, Fong CY, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton's jelly stem cell conditioned medium induces tumoricidal effects on lymphoma cells through hydrogen peroxide mediation. J Cell Biochem. 2016;117(9):2045–2055. doi: 10.1002/jcb.25501. [DOI] [PubMed] [Google Scholar]
- 183.Fonseka M, Ramasamy R, Tan BC, Seow HF. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line) Cell Biol Int. 2012;36(9):793–801. doi: 10.1042/CBI20110595. [DOI] [PubMed] [Google Scholar]
- 184.Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31(1):146–155. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- 185.Dasari VR, Kaur K, Velpula KK, Dinh DH, Tsung AJ, Mohanam S, et al. Downregulation of Focal Adhesion Kinase (FAK) by cord blood stem cells inhibits angiogenesis in glioblastoma. Aging (Albany NY) 2010;2(11):791–803. doi: 10.18632/aging.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bu S, Wang Q, Zhang Q, Sun J, He B, Xiang C, et al. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci Rep. 2016;6:37019. doi: 10.1038/srep37019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, et al. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS ONE. 2010;5(4):e10350. doi: 10.1371/journal.pone.0010350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 188.Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS. Transcriptional repression of Mad-Max complex by human umbilical cord blood stem cells downregulates extracellular signal-regulated kinase in glioblastoma. Stem Cells Dev. 2012;21(10):1779–1793. doi: 10.1089/scd.2011.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Visweswaran M, Arfuso F, Dilley RJ, Newsholme P, Dharmarajan A. The inhibitory influence of adipose tissue-derived mesenchymal stem cell environment and Wnt antagonism on breast tumour cell lines. Int J Biochem Cell Biol. 2018;95:63–72. doi: 10.1016/j.biocel.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 190.Liu J, Han G, Liu H, Qin C. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS ONE. 2013;8(4):e62844. doi: 10.1371/journal.pone.0062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang ML, Pan CM, Chiou SH, Chen WH, Chang HY, Lee OK, et al. Oncostatin m modulates the mesenchymal-epithelial transition of lung adenocarcinoma cells by a mesenchymal stem cell-mediated paracrine effect. Cancer Res. 2012;72(22):6051–6064. doi: 10.1158/0008-5472.CAN-12-1568. [DOI] [PubMed] [Google Scholar]
- 192.Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD. NF-kappaB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin. 2008;29(3):333–340. doi: 10.1111/j.1745-7254.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 193.Klein D, Schmetter A, Imsak R, Wirsdorfer F, Unger K, Jastrow H, et al. Therapy with multipotent mesenchymal stromal cells protects lungs from radiation-induced injury and reduces the risk of lung metastasis. Antioxid Redox Signal. 2016;24(2):53–69. doi: 10.1089/ars.2014.6183. [DOI] [PubMed] [Google Scholar]
- 194.Chen F, Zhou K, Zhang L, Ma F, Chen D, Cui J, et al. Mesenchymal stem cells induce granulocytic differentiation of acute promyelocytic leukemic cells via IL-6 and MEK/ERK pathways. Stem Cells Dev. 2013;22(13):1955–1967. doi: 10.1089/scd.2012.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sage EK, Thakrar RM, Janes SM. Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy. 2016;18(11):1435–1445. doi: 10.1016/j.jcyt.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park SA, Ryu CH, Kim SM, Lim JY, Park SI, Jeong CH, et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int J Oncol. 2011;38(1):97–103. [PubMed] [Google Scholar]
- 197.Kim SM, Oh JH, Park SA, Ryu CH, Lim JY, Kim DS, et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28(12):2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 198.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, Water JA van de, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106(12):4822–7. [DOI] [PMC free article] [PubMed]
- 199.Ryu CH, Park SH, Park SA, Kim SM, Lim JY, Jeong CH, et al. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther. 2011;22(6):733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 200.Fan S, Gao H, Ji W, Zhu F, Sun L, Liu Y, et al. Umbilical cord-derived mesenchymal stromal/stem cells expressing IL-24 induce apoptosis in gliomas. J Cell Physiol. 2019 doi: 10.1002/jcp.29095. [DOI] [PubMed] [Google Scholar]
- 201.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 202.Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70(1):28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xie C, Xie DY, Lin BL, Zhang GL, Wang PP, Peng L, et al. Interferon-beta gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. Br J Cancer. 2013;109(5):1198–1205. doi: 10.1038/bjc.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li Q, Wijesekera O, Salas SJ, Wang JY, Zhu M, Aprhys C, et al. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin Cancer Res. 2014;20(9):2375–2387. doi: 10.1158/1078-0432.CCR-13-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang X, Yang Y, Zhang L, Lu Y, Zhang Q, Fan D, et al. Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor D-1-methyl-tryptophan. J Hematol Oncol. 2017;10(1):56. doi: 10.1186/s13045-017-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yuan Y, Lu X, Tao CL, Chen X, Shao HW, Huang SL. Forced expression of indoleamine-2,3-dioxygenase in human umbilical cord-derived mesenchymal stem cells abolishes their anti-apoptotic effect on leukemia cell lines in vitro. Vitro Cell Dev Biol Anim. 2013;49(10):752–758. doi: 10.1007/s11626-013-9667-4. [DOI] [PubMed] [Google Scholar]
- 207.Yan C, Li S, Li Z, Peng H, Yuan X, Jiang L, et al. Human umbilical cord mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion protein delivery: a double-target therapy against non-Hodgkin's lymphoma. Mol Pharm. 2013;10(1):142–151. doi: 10.1021/mp300261e. [DOI] [PubMed] [Google Scholar]
- 208.Yan C, Yang M, Li Z, Li S, Hu X, Fan D, et al. Suppression of orthotopically implanted hepatocarcinoma in mice by umbilical cord-derived mesenchymal stem cells with sTRAIL gene expression driven by AFP promoter. Biomaterials. 2014;35(9):3035–3043. doi: 10.1016/j.biomaterials.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 209.Zhu X, Su D, Xuan S, Ma G, Dai Z, Liu T, et al. Gene therapy of gastric cancer using LIGHT-secreting human umbilical cord blood-derived mesenchymal stem cells. Gastric Cancer. 2013;16(2):155–166. doi: 10.1007/s10120-012-0166-1. [DOI] [PubMed] [Google Scholar]
- 210.Hu W, Wang J, He X, Zhang H, Yu F, Jiang L, et al. Human umbilical blood mononuclear cell-derived mesenchymal stem cells serve as interleukin-21 gene delivery vehicles for epithelial ovarian cancer therapy in nude mice. Biotechnol Appl Biochem. 2011;58(6):397–404. doi: 10.1002/bab.63. [DOI] [PubMed] [Google Scholar]
- 211.Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y, et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther. 2014;13(8):2127–2137. doi: 10.1158/1535-7163.MCT-14-0175. [DOI] [PubMed] [Google Scholar]
- 212.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue–derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67(13):6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 213.Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: The good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651–663. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Oja S, Komulainen P, Penttila A, Nystedt J, Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther. 2018;9(1):6. doi: 10.1186/s13287-017-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 216.Niyibizi C, Wang S, Mi Z, Robbins PD. The fate of mesenchymal stem cells transplanted into immunocompetent neonatal mice: implications for skeletal gene therapy via stem cells. Mol Ther. 2004;9(6):955–963. doi: 10.1016/j.ymthe.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 217.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 219.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev Rep. 2014;10(2):295–303. doi: 10.1007/s12015-013-9492-x. [DOI] [PubMed] [Google Scholar]
- 221.Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P, et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep. 2017;7:40758. doi: 10.1038/srep40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cui LL, Nitzsche F, Pryazhnikov E, Tibeykina M, Tolppanen L, Rytkönen J, et al. Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017;48(10):2895–2900. doi: 10.1161/STROKEAHA.117.017809. [DOI] [PubMed] [Google Scholar]
- 223.Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fabian C, Naaldijk Y, Leovsky C, Johnson AA, Rudolph L, Jaeger C, et al. Distribution pattern following systemic mesenchymal stem cell injection depends on the age of the recipient and neuronal health. Stem Cell Res Ther. 2017;8(1):85. doi: 10.1186/s13287-017-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 230.Joswig AJ, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, 3rd, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. 2017;8(1):42. doi: 10.1186/s13287-017-0503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 232.Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104(3):398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Oeller M, Laner-Plamberger S, Hochmann S, Ketterl N, Feichtner M, Brachtl G, et al. Selection of tissue factor-deficient cell transplants as a novel strategy for improving hemocompatibility of human bone marrow stromal cells. Theranostics. 2018;8(5):1421–1434. doi: 10.7150/thno.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wu Z, Zhang S, Zhou L, Cai J, Tan J, Gao X, et al. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. Transplant Proc. 2017;49(7):1656–1658. doi: 10.1016/j.transproceed.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 235.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 236.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25(1):220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Toupet K, Maumus M, Peyrafitte JA, Bourin P, van Lent PL, Ferreira R, et al. Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 2013;65(7):1786–1794. doi: 10.1002/art.37960. [DOI] [PubMed] [Google Scholar]
- 238.Wang H, Liang X, Xu ZP, Crawford DHG, Liu X, Roberts MS. A physiologically based kinetic model for elucidating the in vivo distribution of administered mesenchymal stem cells. Sci Rep. 2016;6:22293. doi: 10.1038/srep22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8(11):677–688. doi: 10.1038/nrclinonc.2011.141. [DOI] [PubMed] [Google Scholar]
- 240.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33(5):1055–1063. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7(1):160. doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kumar M, Yasotha T, Singh RK, Singh R, Kumar K, Ranjan R, et al. Generation of transgenic mesenchymal stem cells expressing green fluorescent protein as reporter gene using no viral vector in caprine. Indian J Exp Biol. 2013;51(7):502–509. [PubMed] [Google Scholar]
- 243.Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, et al. Cellular GFP toxicity and immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev Rep. 2016;12(5):553–559. doi: 10.1007/s12015-016-9670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ning X, Bao H, Liu X, Fu H, Wang W, Huang J, et al. Long-term in vivo CT tracking of mesenchymal stem cells labeled with Au@BSA@PLL nanotracers. Nanoscale. 2019;11(43):20932–20941. doi: 10.1039/C9NR05637H. [DOI] [PubMed] [Google Scholar]
- 245.Huang J, Huang J, Ning X, Luo W, Chen M, Wang Z, et al. CT/NIRF dual-modal imaging tracking and therapeutic efficacy of transplanted mesenchymal stem cells labeled with Au nanoparticles in silica-induced pulmonary fibrosis. J Mater Chem B. 2020;8(8):1713–1727. doi: 10.1039/C9TB02652E. [DOI] [PubMed] [Google Scholar]
- 246.Bose RJC, Mattrey RF. Accomplishments and challenges in stem cell imaging in vivo. Drug Discov Today. 2019;24(2):492–504. doi: 10.1016/j.drudis.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 247.Ohki A, Saito S, Fukuchi K. Magnetic resonance imaging of umbilical cord stem cells labeled with superparamagnetic iron oxide nanoparticles: effects of labelling and transplantation parameters. Sci Rep. 2020;10(1):13684. doi: 10.1038/s41598-020-70291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muhammad G, Xu J, Bulte JWM, Jablonska A, Walczak P, Janowski M. Transplanted adipose-derived stem cells can be short-lived yet accelerate healing of acid-burn skin wounds: a multimodal imaging study. Sci Rep. 2017;7(1):4644. doi: 10.1038/s41598-017-04484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14(4):431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126(4):430–439. doi: 10.1161/CIRCULATIONAHA.111.087684. [DOI] [PubMed] [Google Scholar]
- 251.Chin BB, Nakamoto Y, Bulte JW, Pittenger MF, Wahl R, Kraitchman DL. 111In oxine labelled mesenchymal stem cell SPECT after intravenous administration in myocardial infarction. Nucl Med Commun. 2003;24(11):1149–1154. doi: 10.1097/00006231-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 252.Patrick PS, Kolluri KK, Zaw Thin M, Edwards A, Sage EK, Sanderson T, et al. Lung delivery of MSCs expressing anti-cancer protein TRAIL visualised with (89)Zr-oxine PET-CT. Stem Cell Res Ther. 2020;11(1):256. doi: 10.1186/s13287-020-01770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bulte JWM, Daldrup-Link HE. Clinical tracking of cell transfer and cell transplantation: trials and tribulations. Radiology. 2018;289(3):604–615. doi: 10.1148/radiol.2018180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dhada KS, Hernandez DS, Suggs LJ. In vivo photoacoustic tracking of mesenchymal stem cell viability. ACS Nano. 2019;13(7):7791–7799. doi: 10.1021/acsnano.9b01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu H, Liu S, Liang Y, Deng Z, Wang Y, Yan F. Real-time imaging tracking of mesenchymal stem cells labeled with lipid-poly(lactic-co-glycolic acid) nanobubbles. J Biomed Nanotechnol. 2019;15(11):2271–2280. doi: 10.1166/jbn.2019.2852. [DOI] [PubMed] [Google Scholar]
- 256.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49(8):1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 257.Tang Y, Zhang C, Wang J, Lin X, Zhang L, Yang Y, et al. MRI/SPECT/fluorescent tri-modal probe for evaluating the homing and therapeutic efficacy of transplanted mesenchymal stem cells in a rat ischemic stroke model. Adv Funct Mater. 2015;25(7):1024–1034. doi: 10.1002/adfm.201402930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zaw Thin M, Allan H, Bofinger R, Kostelec TD, Guillaume S, Connell JJ, et al. Multi-modal imaging probe for assessing the efficiency of stem cell delivery to orthotopic breast tumours. Nanoscale. 2020;12(31):16570–16585. doi: 10.1039/D0NR03237A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Su LJ, Lin HH, Wu MS, Pan L, Yadav K, Hsu HH, et al. Intracellular delivery of luciferase with fluorescent nanodiamonds for dual-modality imaging of human stem cells. Bioconjug Chem. 2019;30(8):2228–2237. doi: 10.1021/acs.bioconjchem.9b00458. [DOI] [PubMed] [Google Scholar]
- 260.Vaijayanthimala V, Tzeng YK, Chang HC, Li CL. The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology. 2009;20(42):425103. doi: 10.1088/0957-4484/20/42/425103. [DOI] [PubMed] [Google Scholar]
- 261.Wu T-J, Tzeng Y-K, Chang W-W, Cheng C-A, Kuo Y, Chien C-H, et al. Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat Nanotechnol. 2013;8:682. doi: 10.1038/nnano.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Su L-J, Wu M-S, Hui YY, Chang B-M, Pan L, Hsu P-C, et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci Rep. 2017;7:45607. doi: 10.1038/srep45607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wu YC, Wang YC, Wang WT, Wang HD, Lin HH, Su LJ, et al. Fluorescent nanodiamonds enable long-term detection of human adipose-derived stem/stromal cells in an in vivo chondrogenesis model using decellularized extracellular matrices and fibrin glue polymer. Polymers (Basel). 2019;11:9. doi: 10.3390/polym11091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Harting MT, Jimenez F, Cox CS., Jr The pulmonary first-pass effect, xenotransplantation and translation to clinical trials–a commentary. Brain. 2008;131(Pt 8):e100. doi: 10.1093/brain/awn142. [DOI] [PubMed] [Google Scholar]
- 265.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19(12):1843–1853. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- 266.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 267.Blanco JF, Villarón EM, Pescador D, da Casa C, Gómez V, Redondo AM, et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res Ther. 2019;10(1):63. doi: 10.1186/s13287-019-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9(1):17–27. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Introna M, Golay J. Tolerance to bone marrow transplantation: do mesenchymal stromal cells still have a future for acute or chronic GvHD? Front Immunol. 2020;11:609063. doi: 10.3389/fimmu.2020.609063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI, et al. Stem cells as an emerging paradigm in stroke 4: advancing and accelerating preclinical research. Stroke. 2019;50(11):3299–3306. doi: 10.1161/STROKEAHA.119.025436. [DOI] [PubMed] [Google Scholar]
- 271.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jackson ML, Ruppert KA, Kota DJ, Prabhakara KS, Hetz RA, Aertker BM, et al. Clinical parameters affecting multipotent adult progenitor cells in vitro. Heliyon. 2019;5(10):e02532. doi: 10.1016/j.heliyon.2019.e02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Giri J, Galipeau J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020;4(9):1987–1997. doi: 10.1182/bloodadvances.2020001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tan Y, Salkhordeh M, Wang JP, McRae A, Souza-Moreira L, McIntyre L, et al. Thawed mesenchymal stem cell product shows comparable immunomodulatory potency to cultured cells in vitro and in polymicrobial septic animals. Sci Rep. 2019;9(1):18078. doi: 10.1038/s41598-019-54462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chinnadurai R, Rajan D, Qayed M, Arafat D, Garcia M, Liu Y, et al. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018;22(9):2504–2517. doi: 10.1016/j.celrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lehman N, Cutrone R, Raber A, Perry R, Van't Hof W, Deans R, et al. Development of a surrogate angiogenic potency assay for clinical-grade stem cell production. Cytotherapy. 2012;14(8):994–1004. doi: 10.3109/14653249.2012.688945. [DOI] [PubMed] [Google Scholar]
- 278.Hematti P. Characterization of mesenchymal stromal cells: potency assay development. Transfusion. 2016;56(4):32S–S35. doi: 10.1111/trf.13569. [DOI] [PubMed] [Google Scholar]
- 279.Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17(2):125–127. doi: 10.1016/j.jcyt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 280.Bloom DD, Centanni JM, Bhatia N, Emler CA, Drier D, Leverson GE, et al. A reproducible immunopotency assay to measure mesenchymal stromal cell-mediated T-cell suppression. Cytotherapy. 2015;17(2):140–151. doi: 10.1016/j.jcyt.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, et al. Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 2015;15(4):495–504. doi: 10.1517/14712598.2015.997706. [DOI] [PubMed] [Google Scholar]
- 282.Iser IC, Ceschini SM, Onzi GR, Bertoni AP, Lenz G, Wink MR. Conditioned medium from adipose-derived stem cells (ADSCs) promotes epithelial-to-mesenchymal-like transition (EMT-Like) in glioma cells in vitro. Mol Neurobiol. 2016;53(10):7184–7199. doi: 10.1007/s12035-015-9585-4. [DOI] [PubMed] [Google Scholar]
- 283.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18(3):771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lacerda L, Debeb BG, Smith D, Larson R, Solley T, Xu W, et al. Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model. Breast Cancer Res. 2015;17:42. doi: 10.1186/s13058-015-0549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010;70(24):10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kamat P, Schweizer R, Kaenel P, Salemi S, Calcagni M, Giovanoli P, et al. Human adipose-derived mesenchymal stromal cells may promote breast cancer progression and metastatic spread. Plast Reconstr Surg. 2015;136(1):76–84. doi: 10.1097/PRS.0000000000001321. [DOI] [PubMed] [Google Scholar]
- 287.Correa D, Somoza RA, Lin P, Schiemann WP, Caplan AI. Mesenchymal stem cells regulate melanoma cancer cells extravasation to bone and liver at their perivascular niche. Int J Cancer. 2016;138(2):417–427. doi: 10.1002/ijc.29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Avril P, Le Nail LR, Brennan MA, Rosset P, De Pinieux G, Layrolle P, et al. Mesenchymal stem cells increase proliferation but do not change quiescent state of osteosarcoma cells: potential implications according to the tumor resection status. J Bone Oncol. 2016;5(1):5–14. doi: 10.1016/j.jbo.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bajetto A, Pattarozzi A, Corsaro A, Barbieri F, Daga A, Bosio A, et al. Different effects of human umbilical cord mesenchymal stem cells on glioblastoma stem cells by direct cell interaction or via released soluble factors. Front Cell Neurosci. 2017;11:312. doi: 10.3389/fncel.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hendijani F, Javanmard ShH, Rafiee L, Sadeghi-Aliabadi H. Effect of human Wharton's jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res Pharm Sci. 2015;10(2):134–142. [PMC free article] [PubMed] [Google Scholar]
- 292.Kalamegam G, Sait KHW, Anfinan N, Kadam R, Ahmed F, Rasool M, et al. Cytokines secreted by human Wharton's jelly stem cells inhibit the proliferation of ovarian cancer (OVCAR3) cells in vitro. Oncol Lett. 2019;17(5):4521–4531. doi: 10.3892/ol.2019.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kalamegam G, Pushparaj PN, Khan F, Sait KH, Anfinan N, Al-Qahtani M. Primary ovarian cancer cell inhibition by human Wharton's Jelly stem cells (hWJSCs): mapping probable mechanisms and targets using systems oncology. Bioinformation. 2015;11(12):529–534. doi: 10.6026/97320630011529.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11(3):289–98. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 295.Mirabdollahi M, Haghjooyjavanmard S, Sadeghi-Aliabadi H. An anticancer effect of umbilical cord-derived mesenchymal stem cell secretome on the breast cancer cell line. Cell Tissue Bank. 2019;20(3):423–434. doi: 10.1007/s10561-019-09781-8. [DOI] [PubMed] [Google Scholar]
- 296.Sun B, Yu KR, Bhandari DR, Jung JW, Kang SK, Kang KS. Human umbilical cord blood mesenchymal stem cell-derived extracellular matrix prohibits metastatic cancer cell MDA-MB-231 proliferation. Cancer Lett. 2010;296(2):178–185. doi: 10.1016/j.canlet.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 297.Ciavarella S, Caselli A, Tamma AV, Savonarola A, Loverro G, Paganelli R, et al. A peculiar molecular profile of umbilical cord-mesenchymal stromal cells drives their inhibitory effects on multiple myeloma cell growth and tumor progression. Stem Cells Dev. 2015;24(12):1457–1470. doi: 10.1089/scd.2014.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hendijani F, Javanmard SH, Sadeghi-aliabadi H. Human Wharton's jelly mesenchymal stem cell secretome display antiproliferative effect on leukemia cell line and produce additive cytotoxic effect in combination with doxorubicin. Tissue Cell. 2015;47(3):229–234. doi: 10.1016/j.tice.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 299.Zhang H, Wang L, Wen S, Xiang Q, Xiang X, Xu C, et al. Magnetic resonance imaging tracking and assessing repair function of the bone marrow mesenchymal stem cells transplantation in a rat model of spinal cord injury. Oncotarget. 2017;8(35):58985–58999. doi: 10.18632/oncotarget.19775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Brooks A, Futrega K, Liang X, Hu X, Liu X, Crawford DHG, et al. Concise review: quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl Med. 2018;7(1):78–86. doi: 10.1002/sctm.17-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Flowchart of MSC clinical study inclusion. As of October 11, 2020, 1,242 registered studies were identified on clinicaltrials.gov by searching keywords “mesenchymal stem cell” or “mesenchymal stromal cell”. After excluding studies with no longer available/ suspended/ temporarily not available/ terminated/ unknown/ withdrawn status, unknown phase information, and studies that did not use MSCs in their intervention arm, 639 studies remained. Nine of these 639 studies investigated MSCs from two tissue origins, generating a total of 648 studies for analysis.
Additional file 2. Breakdown of MSC-related clinical studies by disease indication. The included studies for analysis are as illustrated in Additional file 1.
Data Availability Statement
All relevant data are included in this published article.