Abstract
Organic anion transporter 3 (OAT3) plays an important role in the disposition of various anionic drugs which impacts the pharmacokinetics and pharmacodynamics of the therapeutics, thus influencing the pharmacological effects and toxicity of the drugs. In this study, we investigated the effect of insulin on the regulation of OAT3 function, expression, and SUMOylation. We demonstrated that insulin induced an increase in OAT3 transport activity through a dose- and time-dependent manner in COS-7 cells. The insulin-induced elevation in OAT3 function was blocked by PKA inhibitor H89, which correlated well with OAT3 protein expression. Moreover, both PKA activator Bt2-cAMP-induced increase and insulin-induced increase in OAT3 function were blocked by PKB inhibitor AKTi1/2. To further investigate the involvement of SUMOylation, we treated OAT3-expressing cells with insulin in presence or absence of H89 or AKTi1/2 followed by examining OAT3 SUMOylation. We showed that insulin enhanced OAT3 SUMOylation, and such enhancement was abrogated by H89 and AKTi1/2. Lastly, insulin increased OAT3 function and SUMOylation in rat kidney slice. In conclusion, our investigations demonstrated that insulin regulated OAT3 function, expression, and SUMOylation through PKA/PKB signaling pathway.
Keywords: Organic Anion Transporter 3, Drug Transport, Regulation, Insulin, SUMOylation
Introduction
Drug transporters are membrane proteins that are expressed on the physiological barriers of different tissues (e.g., intestine, brain, liver, and kidney), which are key players for translocating various endogenous and exogenous substances across the cell membrane including nutrients, toxins, and therapeutic drugs (1–5). Organic anion transporter 3 (OAT3), a member of solute carrier (SLC) transporter family, interacts with and transports a variety of therapeutic drugs including anti-tumor drugs, anti-inflammatory drugs, antibiotics, antiviral therapy, and antihypertensive therapy, therefore impacting the pharmacokinetics and pharmacodynamics of those therapeutics. As a result, the understanding of OAT3 regulation is of great significance (6–8).
SUMOylation, a reversible and dynamic post-translational modification (PTM), refers to the addition of small ubiquitin-like modifier (SUMO) to the target protein on lysine residue(s), catalyzed by specific enzymes. SUMOylation is a crucial regulatory mechanism for both cell surface proteins and nuclear proteins. SUMO1–3, the members of the ubiquitin-like protein family, have been identified in mammals so far. SUMO 2 and SUMO 3 share 97% identity in sequences and are able to form polySUMO chains through internal SUMO consensus motifs. However, SUMO1 only shares 50% homology with SUMO2/3 and is not capable of forming the polySUMO chains. OAT3 has been identified as the substrate of SUMO2/3, but not of SUMO1 (9–13).
Human insulin, produced by beta cells of the pancreas, is an anabolic hormone consisting of 51 amino acids. Insulin promotes the absorption of glucose (small molecules) from the blood into tissue cells and converts the absorbed glucose into glycogen or fats (large molecules), therefore decreasing the blood glucose. Furthermore, insulin is also involved in protein and lipid synthesis in various tissues. On the contrary, low blood insulin levels promote widespread catabolism of the whole body. Increased nutrients in the blood triggers insulin secretion, followed by elevated uptake and conversion of amino acid, glucose, and fatty acids into large molecules (protein, glycogen, and lipids) for storage. Diabetic patients fail to uptake and store nutrients from blood into tissues (14–16).
Diabetes can be classified into two types including type-1 diabetes and type-2 diabetes. Type-1 diabetes, characterized by the body’s inability to produce insulin, are also known as insulin-dependent diabetes and juvenile diabetes. The possible cause of type-1 diabetes is that the insulin-producing beta cells are mistakenly destroyed by the body’s own immune system, but the exact cause needs further exploration. The treatment for type-1 diabetes focuses on managing blood sugar levels with insulin therapy and diet to prevent complications and progression. Type-2 diabetes, also known as adult-onset diabetes, is characterized by insulin resistance or lack of enough insulin. The exact cause of type-2 diabetes is unknown but being overweight and inactive seem to be contributing factors. There are three main types of insulin therapy including short–acting insulin (such as regular insulin), intermediate–acting insulin (such as NPH insulin), and long-acting insulin (such as insulin glargine) (17–20). Diabetic kidney disease is a renal complication of both type-1 and type-2 diabetes, which is found to be related with oxidative stress in renal tubular cells, due to excessive reactive oxygen species (ROs). Diabetic kidney disease, the major cause of end-stage kidney failure, is one of the most severe complications which leads to aggravation and mortality of diabetic patients (21). Diabetic kidney disease results in renal transport dysfunction and alters the disposition of therapeutic drugs in which several renal drug transporters were reported to be involved, such as organic cation transporters and organic anion transporters. It was previously reported that the accumulation of OAT3 substrate, fluorescein, was observed in diabetic mice (22).
Insulin receptor is composed of two transmembrane β subunits and two extracellular α subunits. Insulin binds to the α subunits of the receptor and induces a conformational change and β subunit autophosphorylation which activates the downstream signaling transduction. Several protein kinases are involved in the insulin signaling pathways, such as protein kinase A (PKA), Protein kinase B (PKB, also known as AKT), Phosphoinositide 3-kinase (PI3K), etc (23–26). It is recently demonstrated that PKA activation stimulated OAT3 transport activity and protein expression by altering the rates of transporter recycling and degradation possibly through SUMOylation (13).
In the present study, we examined the regulatory effect and mechanism of insulin on OAT3. We demonstrated that insulin stimulated OAT3 function, expression, and SUMOylation through PKA/PKB signaling.
Results
Insulin increases OAT3 transport activity –
To explore the effect of insulin on OAT3-mediated uptake of estrone sulfate (ES), a prototypical substrate, OAT3-expressing cells were treated with insulin for various concentrations or various time points, followed by the measurement of 4-min OAT3-mediated [3H] ES uptake. As shown in Fig. 1a, insulin stimulated OAT3 transport activity in a dose-dependent manner. Compared with control cell, insulin-treated cells demonstrated an over two-fold stimulation at insulin concentration of 1 μM. For time-dependent study, Fig. 1b demonstrated that insulin increased OAT3 function in a time-dependent manner. Treatment with insulin at 100 nM for 0, 1, 2, 4, and 6 h stimulated ES uptake with ~10%, ~15%, ~35%, and ~60% increase.
Fig. 1.
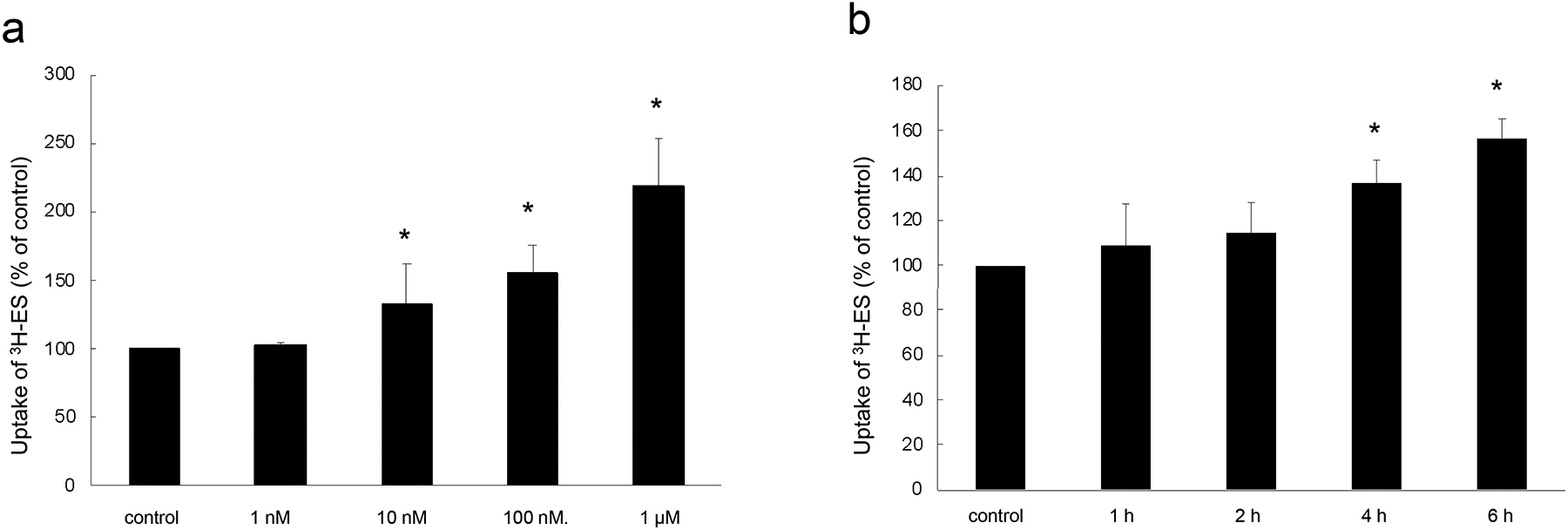
Insulin increases OAT3 transport activity. (a). Dose dependence. OAT3-expressing cells were treated for 6 h with insulin at various doses (0–1 μM). 4-min uptake of [3H]-ES (300 nM) was then measured. Uptake activity was expressed as folds of the uptake measured in control cells. The data represent uptake into OAT3-expressing cells minus uptake into mock cells (parental cells). Values are mean ± S.E. (n = 3). *P < 0.05. (b). Time dependence. OAT3-expressing cells were treated with insulin (100 nM) for 0–6 h. 4-min uptake of [3H]-ES (300 nM) was then measured. Uptake activity was expressed as folds of the uptake measured in control cells. The data represent uptake into OAT3-expressing cells minus uptake into mock cells (parental cells). Values are mean ± S.E. (n = 3). *P < 0.05. Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Insulin alters the kinetics of OAT3-mediated transport of estrone sulfate (ES) –
To explore how insulin alters the kinetics of OAT3-mediated ES uptake, OAT3-expressing cells were treated with insulin, followed by the measurement of [3H] ES uptake at the concentration of 0.1–10 μM. As shown in Fig. 2, insulin treatment resulted in an increase in maximal transport velocity Vmax of OAT3 (73.0 ± 13.4 pmol·mg–1·4min–1 for control cells and 116.9 ± 18.7 pmol·mg–1·4min–1 for insulin-treated cells), without altering the substrate-binding affinity Km of the transporter.
Fig. 2.
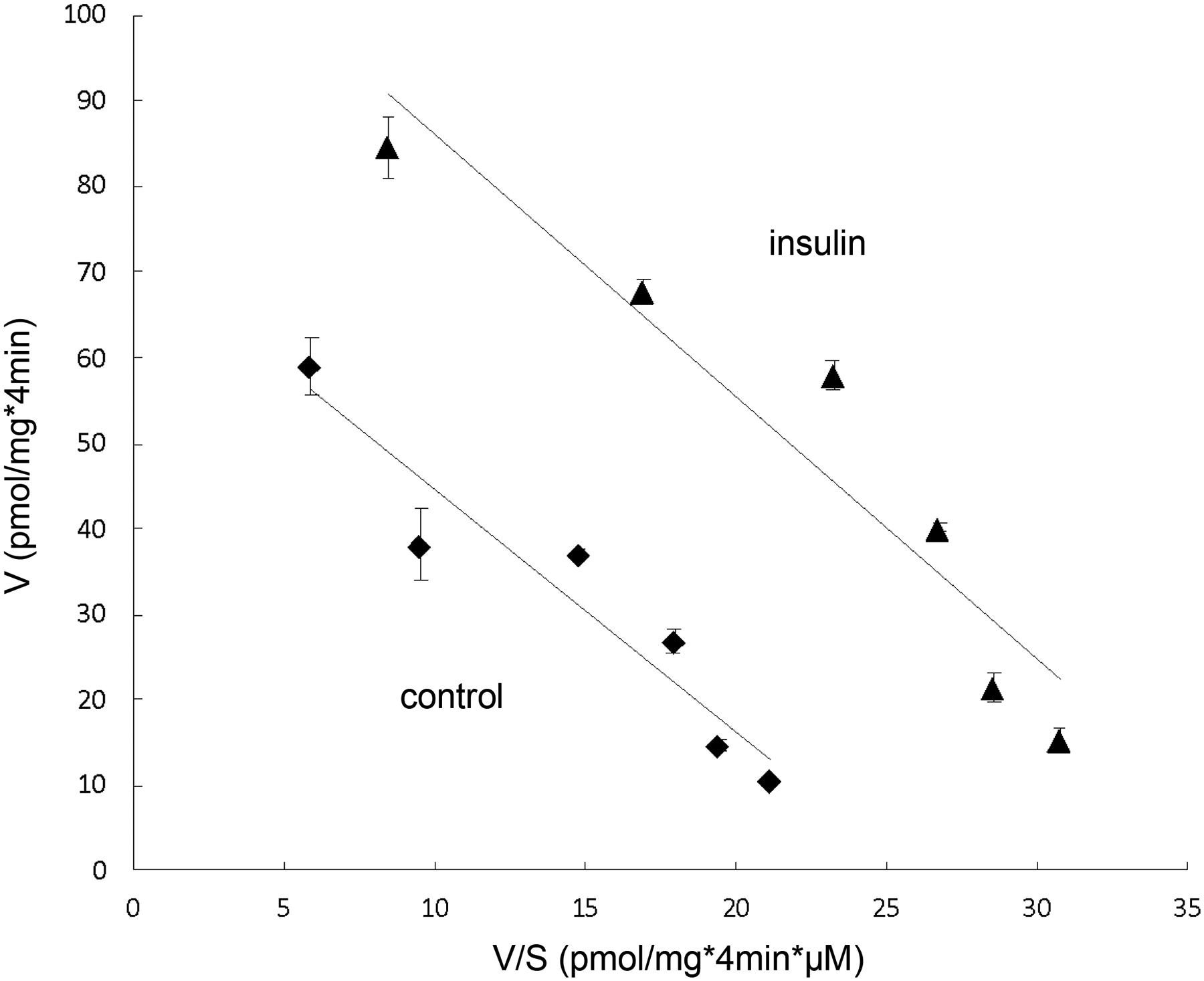
Insulin alters the kinetics of OAT3-mediated estrone sulfate transport. OAT3-expressing cells were treated with the insulin (100 nM, 6h), and initial uptake (4 min) of [3H]-estrone sulfate was measured at the concentration of 0.1–10 μM. The data represent uptake into OAT3-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are means ± SD (n = 3). V, velocity; S, substrate concentration. Transport kinetic values were calculated using the Eadie–Hofstee transformation.
The effects of protein kinase A (PKA) inhibitor H89 on insulin stimulation of OAT3-mediated transport –
To explore the underlying mechanism of insulin-induced increase in OAT3 function, we treat OAT3-expressing cells with insulin in presence or absence of PKA inhibitor H89. As shown in Fig. 3, PKA inhibitor H89 significantly blocked the stimulatory effect of insulin on OAT3 function. Cells treated with H89 alone did not show any toxicity. The results indicated that PKA is an important player in the regulation of insulin-stimulated OAT3 function.
Fig. 3.
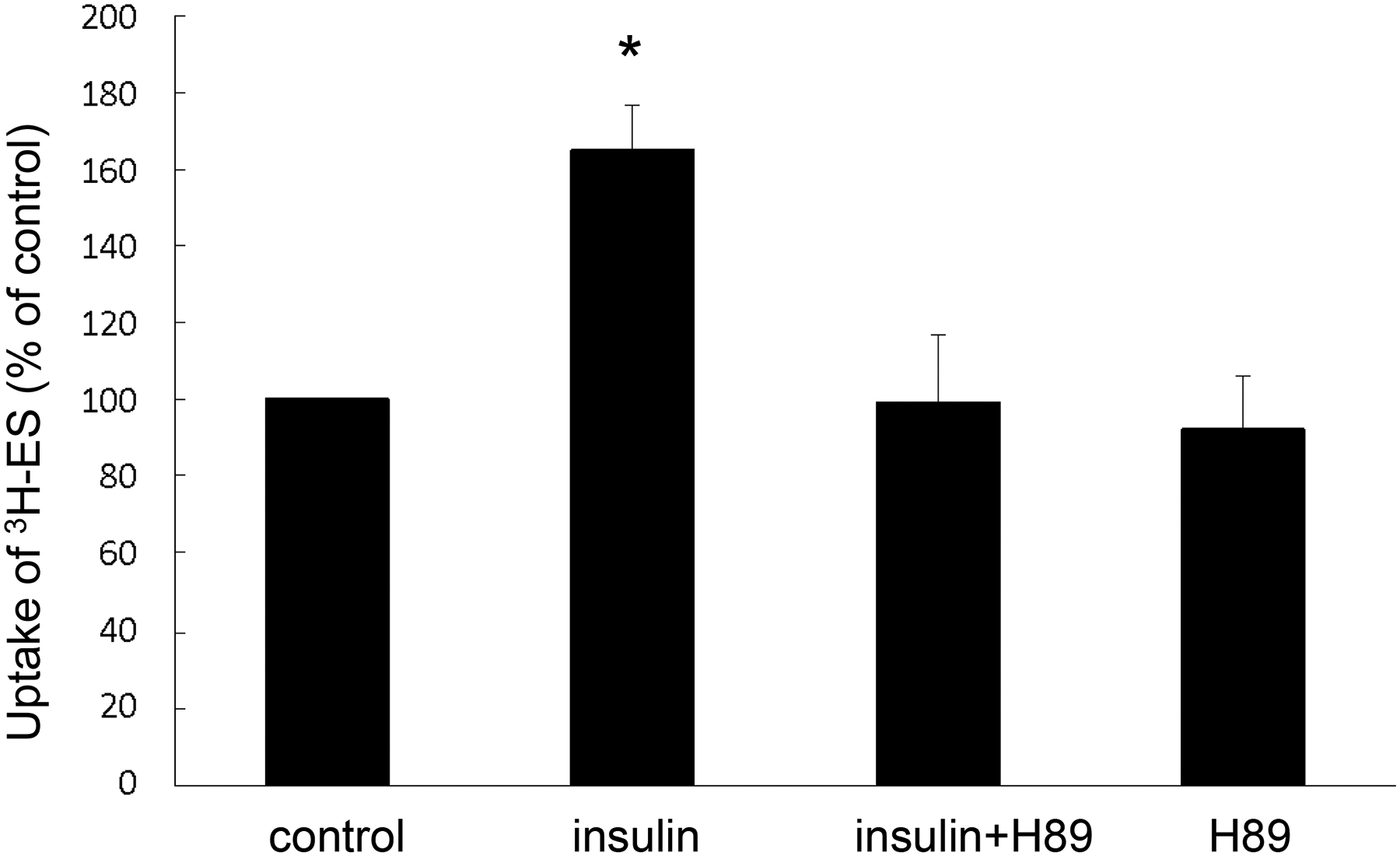
The effects of PKA inhibitor H89 on insulin stimulation of OAT3-mediated transport. OAT3-expressing cells were treated with insulin (100 nM, 6h) with or without H89 (10 μM) followed by measuring the uptake of [3H]-ES (4 min, 300 nM). Uptake activity was expressed as a percentage of the uptake determined in control cells. The data represent uptake into OAT3-expressing cells minus uptake into mock cells. Values are mean ± SD (n = 3). *P<0.05. Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Insulin regulates OAT3 expression through PKA pathway –
An augment in OAT3 cell surface expression or turnover rate of substrate transport could contribute to the elevated maximal transport velocity Vmax shown in Fig. 2. We therefore explored the effect of insulin on OAT3 expression. Insulin treatment elevated the OAT3 expression at cell surface (Fig. 4a, top panel) and in its total lysate (Fig. 5a, top panel), and such elevations were significantly blocked by PKA inhibitor H89. The expressions of cell surface protein marker E-cadherin (Fig. 4a, bottom panel) and total cell protein marker GAPDH (Fig. 5a, bottom panel) were not altered under these treatments, thus the changes induced by insulin and H89 were not because of the overall cellular disturbance.
Fig. 4.
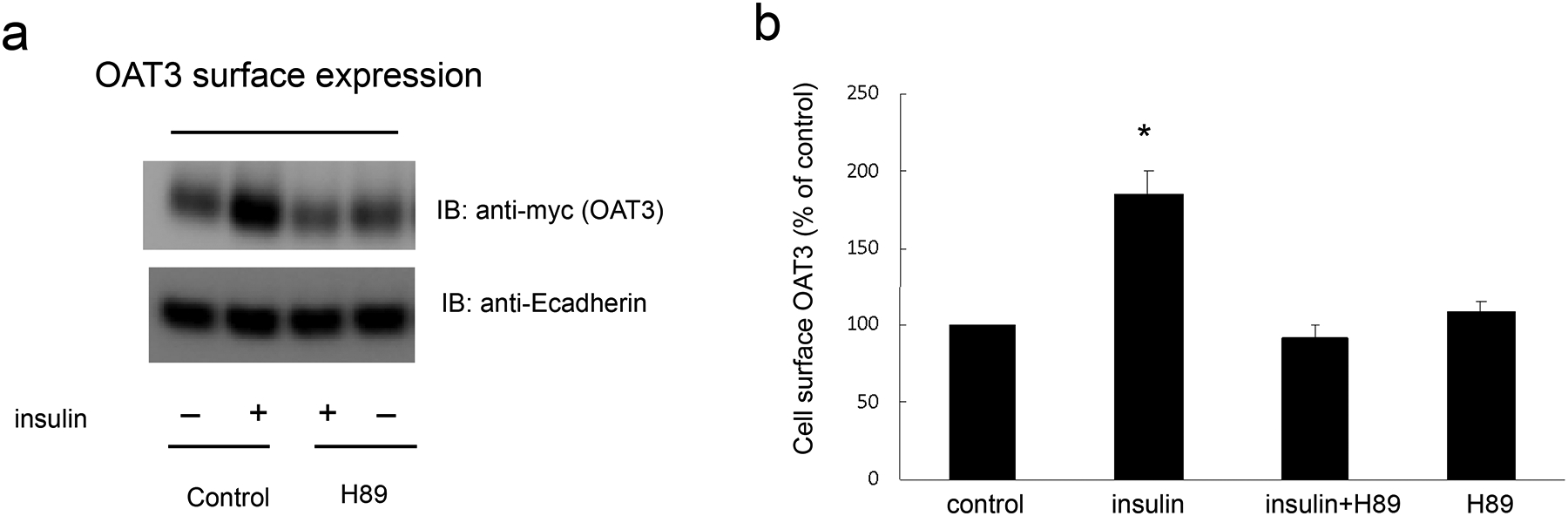
Insulin regulates OAT3 cell surface expression through PKA pathway. (a) Top panel: OAT3-expressing cells were treated with insulin (100 nM) with or without PKA inhibitor H89 (10 μM) for 6 h. Cells were labeled with membrane impermeable biotin. Biotinylated cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with anti-myc antibody (OAT3 was tagged with epitope myc for immunodetection). Bottom panel: The identical blot of the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a marker for cell membrane proteins. (b) Densitometry analysis of blot results from Fig. 4a top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Fig. 5.
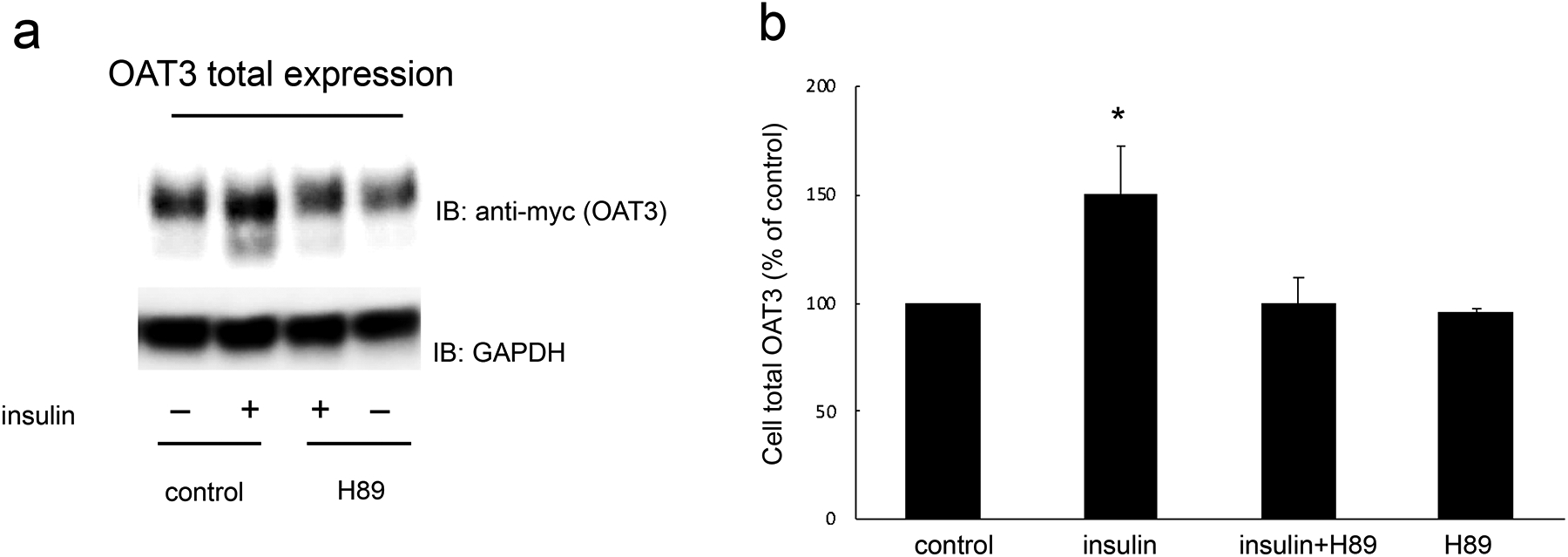
Insulin regulates OAT3 total expression through PKA pathway. (a). Top panel: OAT3-expressing cells were treated with insulin (100 nM) with or without H89 (10 μM) for 6 h. The cells were collected and lysed, followed by immunoblotting (IB) with anti-myc antibody (OAT3 was tagged with epitope myc for immunodetection). Bottom panel: The identical blot of the top panel was re-probed with anti-GAPDH antibody. GAPDH is a marker for total cell proteins. (b). Densitometry analyses of blot results from Fig. 5a top panel as well as from other experiments. The values are mean ± SD (n = 3); *P < 0.05. Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Insulin regulates OAT3 transport activity through PKA/PKB pathway –
It is reported that several protein kinases, such as PKA and PKB, are key regulators in insulin signaling pathway. In addition, PKA has been reported to activate PKB (27). To explore the role of PKA and PKB in the regulation of insulin on OAT3 transport activity, we treated OAT3-expressing cells with insulin or PKA activator Bt2-cAMP in the presence or absence of PKB inhibitor AKTi1/2. As shown in Fig. 6a, PKB inhibitor AKTi1/2 blocked the stimulatory effect of insulin without showing toxicity. Furthermore, PKA activation by Bt2-cAMP elevated OAT3 transport activity, and such elevation was reversed by AKTi1/2 (Fig. 6b).
Fig. 6.
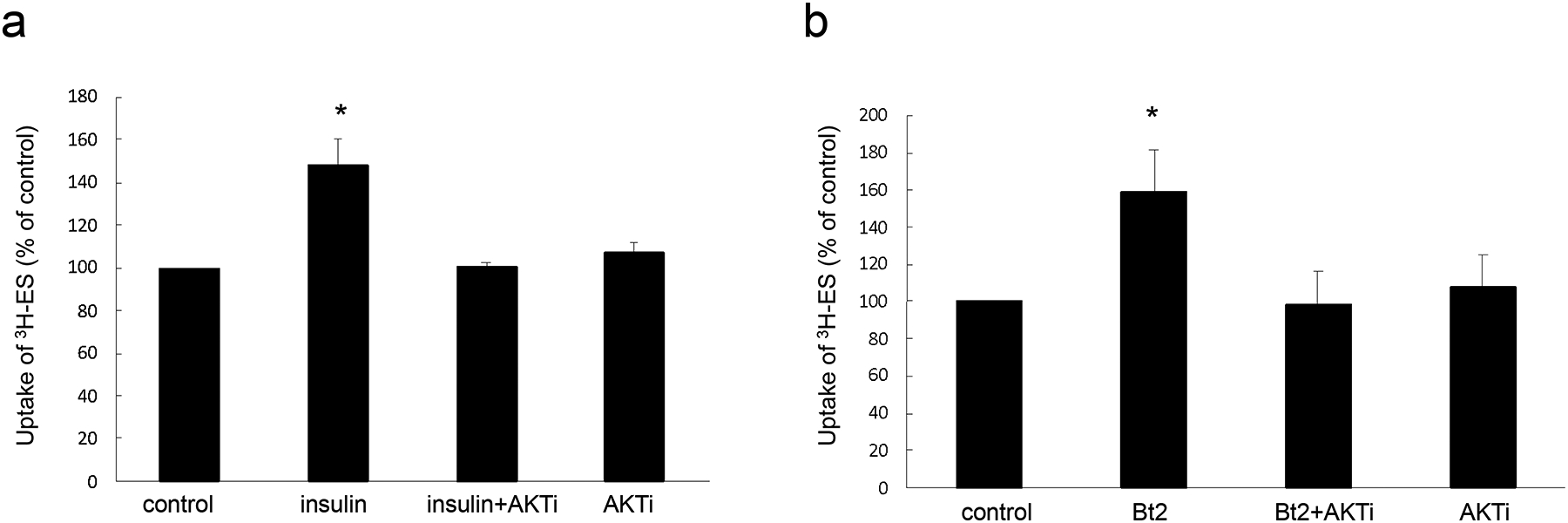
Insulin regulates OAT3 transport activity through PKA/PKB pathway. (a). OAT3-expressing cells were treated with insulin (100 nM, 6h) with or without AKTi1/2 (5 μM, PKB inhibitor) followed by measuring the uptake of [3H]-ES (4 min, 300 nM). Uptake activity was expressed as a percentage of the uptake determined in control cells. The data represent uptake into OAT3-expressing cells minus uptake into mock cells. Values are mean ± SD (n = 3). *P<0.05. (b). OAT3-expressing cells were treated with Bt2-cAMP (20 μM, 2h) with or without AKTi1/2 (5 μM, PKB inhibitor) followed by measuring the uptake of [3H]-ES (4 min, 300 nM). Uptake activity was expressed as a percentage of the uptake determined in control cells. The data represent uptake into OAT3-expressing cells minus uptake into mock cells. Values are mean ± SD (n = 3). *P<0.05. Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Insulin stimulates OAT3 SUMOylation through PKA/PKB pathway –
It has been reported that OAT3 is the substrate of SUMO2 and SUMO3 which share 97% identity and are referred as SUMO2/3. To examine whether insulin changes OAT3 SUMOylation and the underlying signaling pathway, we transfected OAT3-expressing cells with epitope HA-tagged SUMO-2. Transfected cells were treated with insulin in the presence or absence of PKA inhibitor H89 or PKB inhibitor AKTi1/2. OAT3 was immunoprecipitated followed by immunoblotting (IB) with anti-HA antibody to detect SUMOylated OAT3. As shown in Fig. 7a (top panel) and Fig. 7c (top panel), insulin increased OAT3 SUMOylation, and such increase were blocked by both PKA inhibitor H89 and PKB inhibitor AKTi1/2. The changes in SUMOylation induced by insulin and inhibitors were not because of the variances in amount of OAT3, as the amount of OAT3 pulled down in all the samples were similar (Fig. 7a, bottom panel and Fig. 7c, bottom panel).
Fig. 7.
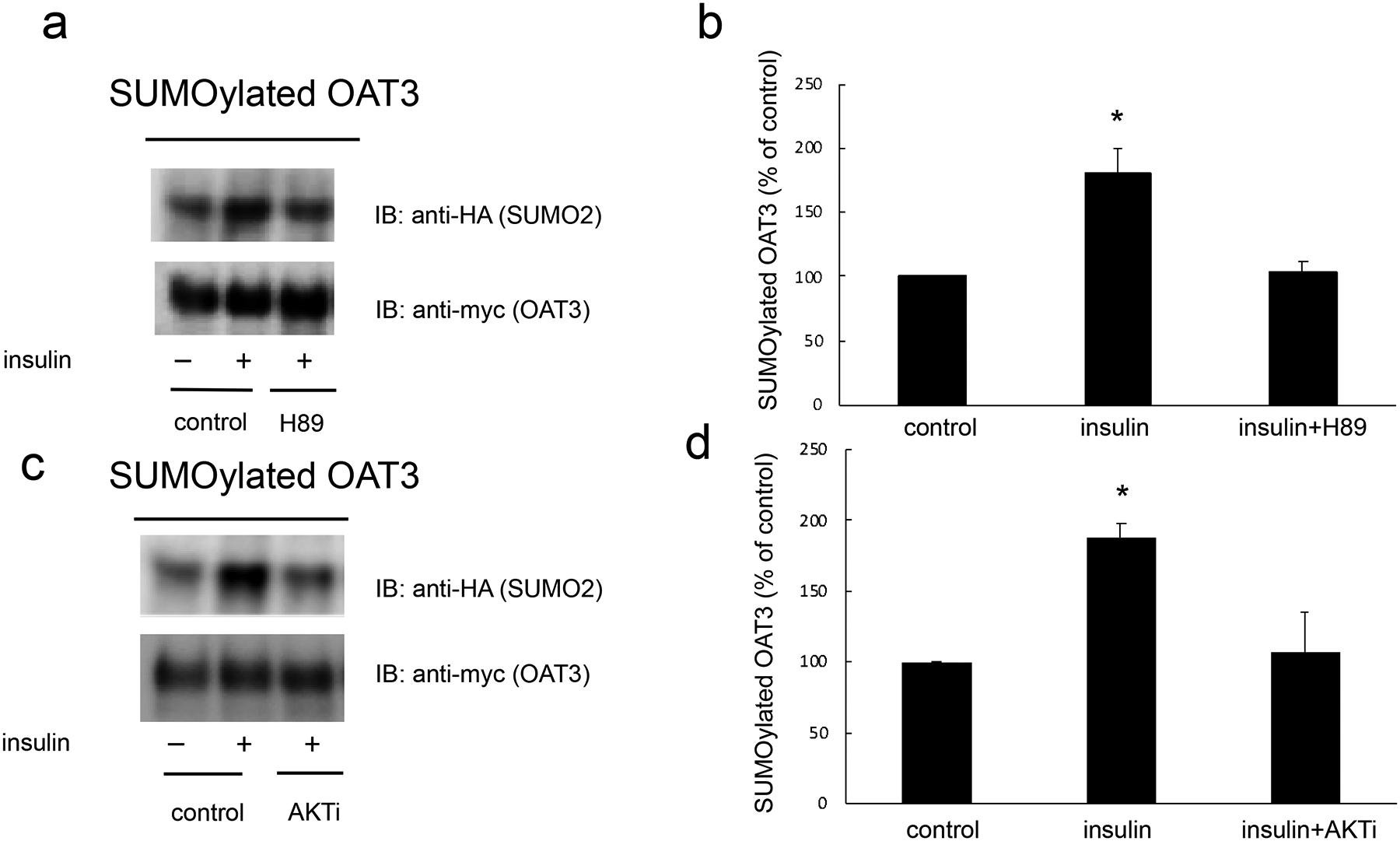
Insulin stimulated OAT3 SUMOylation through PKA/PKB pathway. (a). OAT3-expressing cells were transfected with HA-SUMO2 for 48h, then treated with the insulin (100 nM, 6 h) in the presence and absence of PKA inhibitor H89 (10 μM). The treated cells were lysed. OAT3 was immunoprecipitated by anti-myc antibody, followed by immunoblotting (IB) with anti-HA antibody. (b) Densitometry plot of results from Fig. 7a, as well as from other experiments. Values are mean ± SD (n = 3). (c). OAT3-expressing cells were transfected with HA-SUMO2 for 48h, then treated with the insulin (100 nM, 6 h) in the presence and absence of PKA inhibitor AKTi1/2 (5 μM). The treated cells were lysed. OAT3 was immunoprecipitated by anti-myc antibody, followed by immunoblotting (IB) with anti-HA antibody. (d) Densitometry plot of results from Fig. 7c, as well as from other experiments. Values are mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA (GraphPad Software Inc., San Diego, CA, USA).
Insulin stimulates OAT3 transport activity and SUMOylation in rat kidney slice –
To examine the effects of insulin on OAT3 function in native epithelium, we collected fresh kidneys from rats under anesthesia, and then we treated the fresh kidneys with insulin (8 μM, 1h) followed by measuring the ES uptake. As shown in Fig. 8a, insulin stimulated OAT3 transport activity in kidney slice. Furthermore, to explore whether insulin could stimulate OAT3 SUMOylation in rat kidney slice, kidney slices were treated with insulin (8 μM, 1h), and then SUMO2/3 was immunoprecipitated (IP) followed by immunoblotting (IB) with anti-OAT3 antibody to detect SUMOylated OAT3. As shown in Fig. 8b, insulin significantly stimulated OAT3 SUMOylation. Protein concentrations for immunoprecipitation were measured in all samples and adjusted to equal amount in order to make sure the changes induced by insulin were not due to protein loading variances.
Fig. 8.
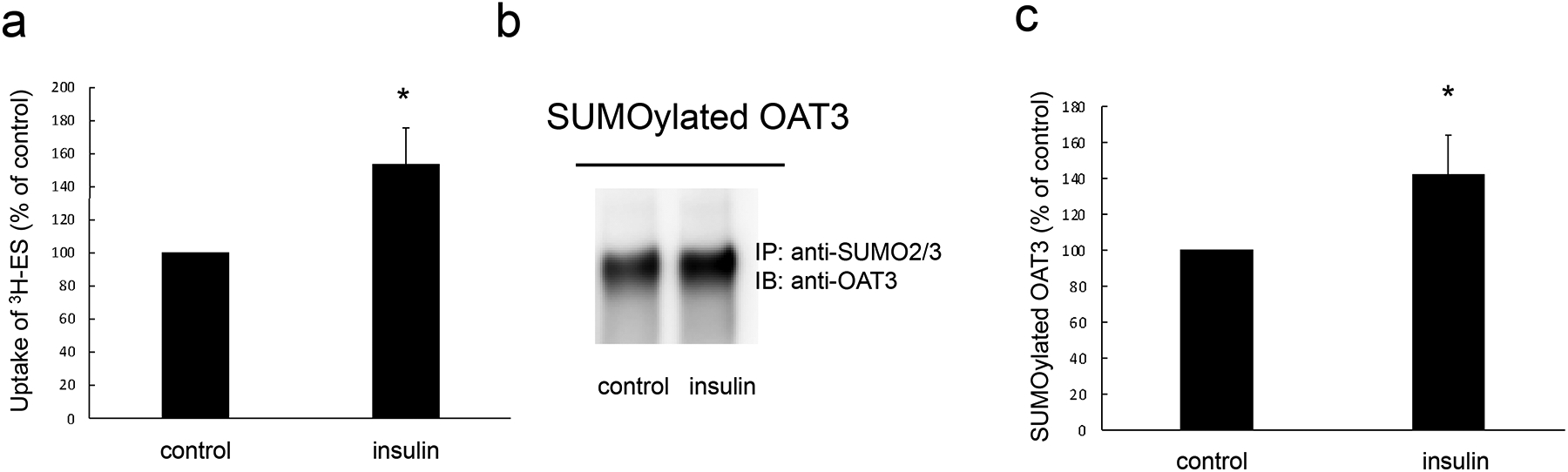
Insulin stimulated OAT3 transport activity and SUMOylation in rat kidney slice. (a). Kidney slices were treated with the insulin (8 μM, 1h) followed by measuring the uptake of [3H]-ES (25 min, 300 nM). The data represent uptake into kidney slice minus background value. Values are means ± SD (n = 3). (b). Kidney slices were treated with the insulin (8 μM, 1h), and then the slices were lysed. SUMO2/3 was immunoprecipitated by anti-SUMO2/3 antibody, followed by immunoblotting (IB) with anti-OAT3 antibody. (c) Densitometry plot of results from Fig. 8b, as well as from other experiments. Values are mean ± SD (n = 3). Statistical analysis was performed using Student’s paired t-tests.
Discussion
Organic anion transporter 3 (OAT3) plays a vital role in the toxicity and therapeutic efficacy of many anionic drugs. Thus, the investigations of mechanisms underlying the OAT3 regulation at molecular and cellular level is of great value (28). The current study showed that insulin, a peptide hormone produced by beta cells of the pancreas, is a critical player in the regulation of OAT3 function, expression, and SUMOylation both in vitro and in native epithelium.
COS-7 cell line, a heterologous cell system, was chosen as the in vitro system for our current study. COS-7 cells, originated from African green monkey kidney tissue, have been proven to be an excellent in vitro system for exploring the mechanisms at molecular and cellular level of numerous renal transporters (29–32). Its high transfection efficiency and many intact signaling pathways make this cell line fit to be a great exploratory in vitro model. In addition, this cell line does not express endogenous OATs which allows us to explore OAT3 regulation without being interfered by other members of OAT family. Lastly, it has been reported that COS-7 cells demonstrated similar characteristics and properties of OATs as compared to those in vivo. Thus, the studies in these cells could pave the way for the further investigations of the roles of insulin in OAT3 regulation in vivo.
In the recent years, a hypothesis called remote sensing and signaling has attracted a lot of attentions. The hypothesis indicates that the roles of transporters form a network which allows the communications between cells, as well as between organs. Such communications at the intercellular and interorgan levels regulate the local and whole-body homeostasis (5, 33, 34). There are several factors contribute to the formation of the remote sensing and signaling network. The regulations of transporters function and expression mediated by signaling molecules secreted from remote organs into the body fluid is one of the essential factors. Insulin, a peptide hormone secreted from pancreas, is released into the blood stream and arrives at kidney to regulate the function of various renal transporters, such as epithelium sodium channel, Na+/H+ exchanger 3, and urate transporter 1 (35–37). The regulation by insulin in cellular processes could be through multiple signaling pathways, and several protein kinases are involved in the signaling pathways (23–26). Protein kinase A (PKA) and protein kinase B (PKB) have been reported to play important roles in the signaling pathways mediated by insulin. Furthermore, it has been indicated that PKA is an upstream activator of PKB (27).
In the current study, we demonstrated that insulin stimulated OAT3 transport activity at a dose-dependent and time-dependent manner (Fig. 1a and 1b). We further showed that insulin induced an increase in maximal transport velocity Vmax without altering the substrate-binding affinity Km of the transporter (Fig. 2). The increased Vmax could be attributed to the increased OAT3 cell surface expression or increased turnover rate of substrate transport. Therefore, we explored the effect of insulin on OAT3 protein expression. It is shown insulin increased OAT3 protein expression (Fig. 4a and 5a). Such increase was reversed by PKA inhibitor H89, which paralleled well with OAT3 transport activity (Fig. 3). To further explore the mechanisms underlying the regulation of insulin on OAT3, we treated OAT3-expressing cells with insulin or PKA activator Bt2-cAMP in the presence or absence of PKB inhibitor AKTi1/2. The results demonstrated that the augmented OAT3 function induced by insulin or Bt2-cAMP was abrogated by PKB inhibitor AKTi1/2 (Fig. 6a and 6b). Our results suggest that insulin regulates OAT3 by activating PKA followed by the activation of PKB. In our previous publication, OAT3 was identified as a SUMO2/3 substrate, and the conjugation of SUMO2/3 to OAT3 was PKA-dependent. PKA activation by Bt2-cAMP increased OAT3 SUMOylation, and such increase were abrogated by H89. To explore the effect of insulin on OAT3 SUMOylation, we treated OAT3-expressing cells with insulin in the presence or absence of H89 or AKTi1/2, followed by the examination of OAT3 SUMOylation. We showed that the effect of insulin on OAT3 SUMOylation correlated well with the effects of insulin on OAT3 function and expression. In addition, insulin-induced OAT3 SUMOylation was abrogated by both H89 and AKTi1/2 (Fig. 7a and 7c), suggesting that both PKA and PKB play important roles in insulin regulation of OAT3 SUMOylation. Lastly, insulin increased OAT3-mediated transport of ES and SUMOylation in rat kidney slice (Fig. 8a and 8b), validating our observations from in vitro system in the native epithelium. Of course, we cannot exclude the potential role of other transporters in mediating ES transport under the regulation of insulin. To summary, Insulin increased OAT3 SUMOylation, protein expression, and function. Thus, diabetic patients, lack of insulin, could have impaired OAT3-mediated transport activity, and therefore altered therapeutic drug disposition and potential drug toxicity should be taken into consideration in diabetic patients.
Most protein substrates for SUMOylation bear the consensus motif, Ψ-K-x-D/E (where ψ is a hydrophobic residue, K is the lysine conjugated to SUMO, x is any amino acid, E is a glutamic acid, and D is an aspartic acid) (38, 39). Using program TMpred, we predicted the membrane spanning regions of OAT3 and their orientation, and we identified ten intracellular lysine residues on OAT3. K285 and K518 lie within the consensus motif Ψ-K-x-D/E. In addition, using program SUMOplot™, we predicted that K69, K285, K286, K515, and K518 could potentially be the SUMOylation sites. However, SUMOylation can also occur outside the conventional motif, and the presence of conventional motif does not guarantee the SUMOylation. The mapping of SUMO-conjugation sites on OAT3 under PKA activation is our future step.
Conclusion
In conclusion, this current study uncovered the vital role of insulin in the regulation of OAT3 function, expression, and SUMOylation. Insulin activated PKA followed by the activation of PKB, which resulted in the enhanced OAT3 transport activity, protein expression, and SUMOylation (Fig. 9).
Fig. 9.
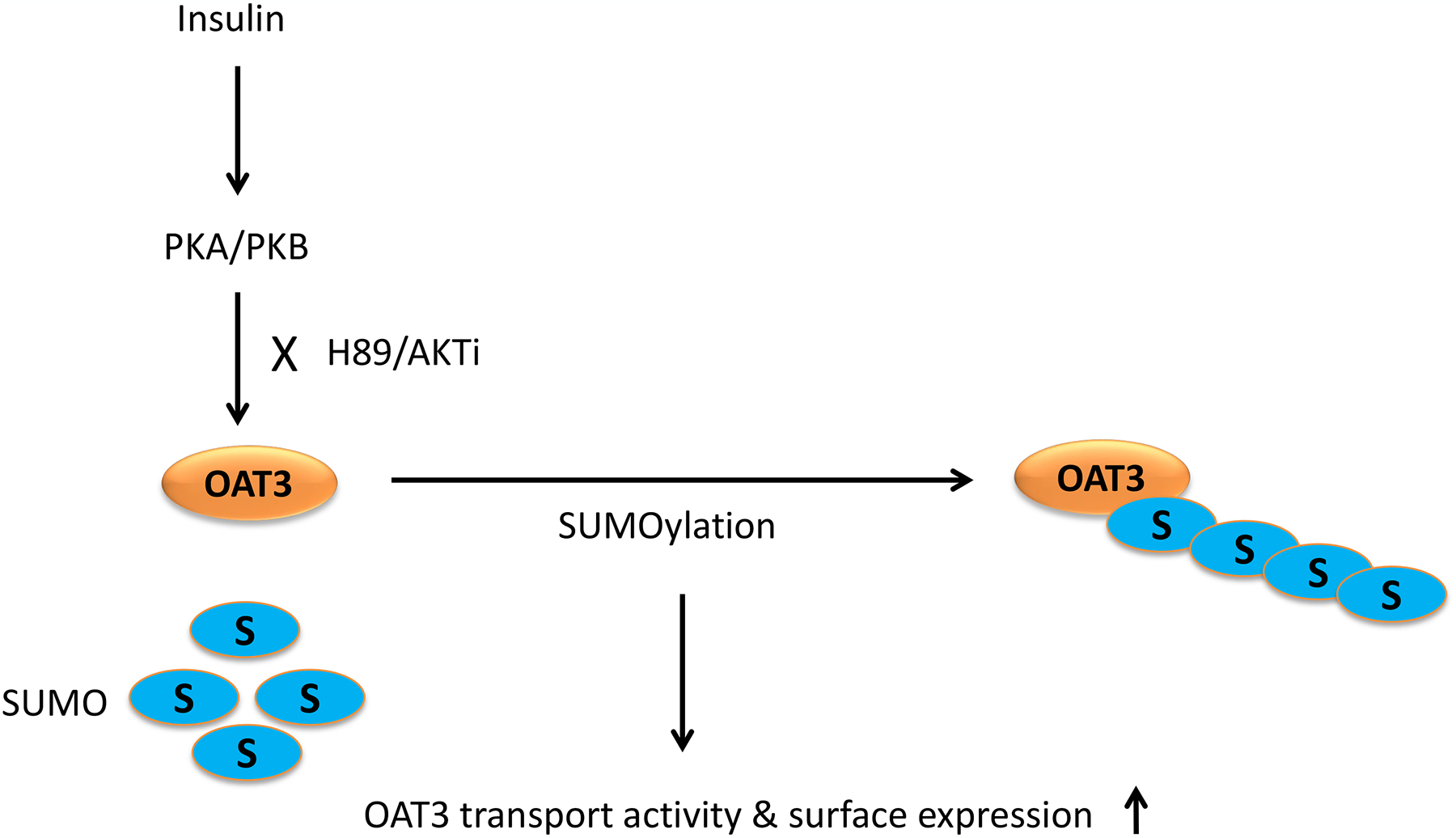
Insulin regulated OAT3 function, expression, and SUMOylation through PKA/PKB signaling pathway.
Acknowledgement
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
We would like to thank Dr. Kexin Liu and his group from Provincial Key Laboratory for Pharmacokinetics and Transport, Liaoning Dalian Medical University for their technical help on kidney slice preparation.
Footnotes
Conflict of interest
The authors have declared that there is no conflict of interest.
Reference
- 1.You G. Structure, function, and regulation of renal organic anion transporters. Med Res Rev. 2002;22(6):602–16. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 2.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta. 2003;1618(2):185–93. doi: 10.1016/j.bbamem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38(7–8):889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31(1):1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 5.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol. 2009;76(3):481–90. doi: 10.1124/mol.109.056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Sweet DH. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013;15(1):53–69. doi: 10.1208/s12248-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wang H, Fan Y, Yu Z, You G. Regulation of organic anion transporters: Role in physiology, pathophysiology, and drug elimination. Pharmacol Ther. 2020:107647. doi: 10.1016/j.pharmthera.2020.107647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T, Inui K. Gene expression and regulation of drug transporters in the intestine and kidney. Biochem Pharmacol. 2007;73(3):440–9. doi: 10.1016/j.bcp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11(12):861–71. doi: nrm3011 [pii] 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill G SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18(17):2046–59. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich HD. The fast-growing business of SUMO chains. Mol Cell. 2008;32(3):301–5. doi: 10.1016/j.molcel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, He Y, Wang X, Liang Z, He G, Zhang P, et al. Protein SUMOylation modification and its associations with disease. Open Biol. 2017;7(10). doi: 10.1098/rsob.170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Zhang J, You G. Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3. AAPS J. 2019;21(2):30. doi: 10.1208/s12248-019-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnieli E, Zarnowski MJ, Hissin PJ, Simpson IA, Salans LB, Cushman SW. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981;256(10):4772–7. [PubMed] [Google Scholar]
- 15.Kono T, Suzuki K, Dansey LE, Robinson FW, Blevins TL. Energy-dependent and protein synthesis-independent recycling of the insulin-sensitive glucose transport mechanism in fat cells. J Biol Chem. 1981;256(12):6400–7. [PubMed] [Google Scholar]
- 16.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34(Pt 2):213–6. doi: 10.1042/BST20060213. [DOI] [PubMed] [Google Scholar]
- 17.Guo S Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220(2):T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234(6):8152–61. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 19.Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17(2):109–15. doi: 10.1080/07315724.1998.10718735. [DOI] [PubMed] [Google Scholar]
- 20.Baumgard LH, Hausman GJ, Sanz Fernandez MV. Insulin: pancreatic secretion and adipocyte regulation. Domest Anim Endocrinol. 2016;54:76–84. doi: 10.1016/j.domaniend.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–40. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lungkaphina A, Arjinajarna P, Srimaroenga C, Chatsudthipong V. Function and expression of renal organic anion transporters in experimental diabetes in mice. ScienceAsia. 2012;38:18–23. doi: 10.2306/scienceasia1513-1874.2012.38.018. [DOI] [Google Scholar]
- 23.Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol. 2018;217(7):2273–89. doi: 10.1083/jcb.201802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelis RM, Wright SH. Renal transport of organic anions and cations. Compr Physiol. 2011;1(4):1795–835. doi: 10.1002/cphy.c100084. [DOI] [PubMed] [Google Scholar]
- 25.Barros SA, Srimaroeng C, Perry JL, Walden R, Dembla-Rajpal N, Sweet DH, et al. Activation of protein kinase Czeta increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J Biol Chem. 2009;284(5):2672–9. doi: 10.1074/jbc.M808078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lungkaphin A, Arjinajarn P, Pongchaidecha A, Srimaroeng C, Chatsudthipong L, Chatsudthipong V. Impaired insulin signaling affects renal organic anion transporter 3 (Oat3) function in streptozotocin-induced diabetic rats. PLoS One. 2014;9(5):e96236. doi: 10.1371/journal.pone.0096236. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Filippa N, Sable CL, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19(7):4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100(5):411–26. doi: 10.1254/jphs.crj06006x. [DOI] [PubMed] [Google Scholar]
- 29.Duan P, Li S, You G. Angiotensin II inhibits activity of human organic anion transporter 3 through activation of protein kinase Calpha: accelerating endocytosis of the transporter. Eur J Pharmacol. 2010;627(1–3):49–55. doi: 10.1016/j.ejphar.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, et al. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277(22):19665–72. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 31.Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N, Saad M, Knipp GT. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur J Pharm Sci. 2006;27(5):533–42. doi: 10.1016/j.ejps.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284(3):F467–73. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 33.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95(1):83–123. doi: 10.1152/physrev.00025.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nigam SK. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu Rev Pharmacol Toxicol. 2018;58:663–87. doi: 10.1146/annurev-pharmtox-010617-052713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem. 2007;282(41):29866–73. doi: 10.1074/jbc.M701923200. [DOI] [PubMed] [Google Scholar]
- 36.Klisic J, Hu MC, Nief V, Reyes L, Fuster D, Moe OW, et al. Insulin activates Na(+)/H(+) exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol. 2002;283(3):F532–9. doi: 10.1152/ajprenal.00365.2001. [DOI] [PubMed] [Google Scholar]
- 37.Toyoki D, Shibata S, Kuribayashi-Okuma E, Xu N, Ishizawa K, Hosoyamada M, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol. 2017;313(3):F826–F34. doi: 10.1152/ajprenal.00012.2017. [DOI] [PubMed] [Google Scholar]
- 38.Chang CC, Tung CH, Chen CW, Tu CH, Chu YW. SUMOgo: Prediction of sumoylation sites on lysines by motif screening models and the effects of various post-translational modifications. Sci Rep. 2018;8(1):15512. doi: 10.1038/s41598-018-33951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matic I, Schimmel J, Hendriks IA, van Santen MA, van de Rijke F, van Dam H, et al. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol Cell. 2010;39(4):641–52. doi: 10.1016/j.molcel.2010.07.026. [DOI] [PubMed] [Google Scholar]


