Abstract
Discovery and characterization of serologic biomarkers has revolutionized the diagnostic framework of systemic and paraneoplastic autoimmune neuro-ophthalmic diseases. Expanding recognition of the multiple ocular and visual manifestations of these conditions highlights the important role of the referring provider in identifying potential cases. Increasing ease of access to serologic testing also enables these practitioners to initiate the diagnostic work-up in suspected cases. We aimed to provide an update on the current knowledge surrounding and use of relevant autoimmune biomarkers by correlating specific clinical neuro-ophthalmic manifestations with autoantibody biomarkers. The utility of select biomarkers for myasthenia gravis, neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein-IgG-associated disorder, opsoclonus–myoclonus syndrome, anti-collapsin-response mediator protein-5 optic neuropathy, and glial fibrillary acidic protein-IgG-associated disease are discussed with particular focus on the clinical contexts in which to consider testing.
Keywords: Autoantibodies, autoimmune neuro-ophthalmic disorders, neuro-ophthalmology, paraneoplastic syndromes, serum biomarkers
Introduction
Autoimmune disorders have been gaining attention within neuro-ophthalmology over the last two decades. The discovery of new serologic biomarkers has aided the characterization and re-categorization of systemic and paraneoplastic autoimmune neuro-ophthalmic diseases. These diseases can manifest in the afferent, efferent, or both arms of the visual system, so patients are likely to initially present to a range of ophthalmic subspecialists. Given the profound systemic health implications of these entities, it is imperative that ophthalmologists recognize these conditions to facilitate expedient diagnosis and management.
In the last decade alone, the diagnostic work-up for optic neuritis has significantly changed with the discovery of the association between atypical forms of optic neuritis clinically categorized into neuromyelitis optic spectrum disorder (NMOSD) with anti-aquaporin-4 (AQP4) antibodies in 2004.1 Notwithstanding prior investigation as a marker of multiple sclerosis (MS) in 2003,2 the association between anti-myelin oligodendrocyte glycoprotein antibodies in other clinically atypical optic neuritis was subsequently discovered in 2014.2–4 These discoveries reshaped the neuro-ophthalmic approach to patients with acute optic neuritis, from diagnosis to management. Autoimmune conditions with neuro-ophthalmic manifestations, including NMOSD, can also arise in the setting of malignancy.5,6 Ectopic protein expression by the underlying tumor can trigger autoimmunity, giving rise to specific paraneoplastic syndromes that share considerable clinical overlap with other autoimmune disorders. Distinguishing these etiologies requires clinical judgment aided by identification of specific serologic biomarkers.
This review provides an overview correlating autoimmune systemic neurologic and paraneoplastic diseases with clinical neuro-ophthalmic manifestations and their autoantibody biomarkers. Acknowledging overlap, we separately discuss systemic autoimmune diseases, specifically myasthenia gravis (MG), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein (MOG)-IgG-related disorder, followed by paraneoplastic syndromes including those associated with antineuronal antibody types 1 and 2, anti-collapsin-response mediator protein-5 (CRMP5), and anti-glial fibrillary acidic protein (GFAP) antibodies. A primary objective is to provide a perspective on the clinical scenarios in which specific autoimmune/paraneoplastic syndromes and their corresponding serologic markers should be considered.
SYSTEMIC AUTOIMMUNE DISORDERS
Myasthenia Gravis – LRP4/Agrin
Myasthenia gravis causes fatigable weakness due to an autoimmune disruption of neuromuscular synaptic transmission. Approximately 40% of patients diagnosed with myasthenia gravis present with ocular symptoms including diplopia and ophthalmic signs including fatigable ptosis, Cogan’s lid twitch, and pupil-sparing ophthalmoplegia.7 Of these, approximately 38% of cases remain ocular. The mechanism of antibodies targeting the muscle-specific nicotinic acetylcholine receptor (the post-synaptic receptor required for synaptic transmission at the neuromuscular junction) was uncovered by Patrick and Lindstrom in 1973.8 Despite a clear pathogenic link, acetylcholine receptor (AChR) antibodies are only detectable in about 50% of patients with ocular myasthenia gravis. Muscle-specific kinase (MuSK), a tyrosine kinase required for formation and maintenance of the neuromuscular junction, was identified as another marker of MG, and according to a retrospective study of 82 patients seropositive for MuSK, only 3 (3.7%) of the patients presented with isolated ocular myasthenia gravis.9 Among patients with generalized myasthenia gravis, AChR antibodies are positive in about 80% of patients, and MuSK antibodies are positive in 10%.9–12
To address the need for additional biomarkers, antibodies targeting proteins that associate with the AChR and its downstream targets have recently been investigated in patients negative for both AChR and MuSK antibodies, termed double-seronegative myasthenia gravis (DNMG). Agrin is a proteoglycan that activates MuSK and aggregates AChRs at the neuromuscular junction.13–15 0–50% of DNMG patients test positive for agrin antibodies.16–18 Notably, mutations in the AGRN gene, which encodes agrin, have been implicated in congenital myasthenic syndrome.19,20 Agrin also binds low-density lipoprotein receptor–related protein 4 (LRP4), which serves to activate MuSK and facilitates clustering and stabilization of the AChR at the neuromuscular junction.21 The reported prevalence for LRP4 antibodies among DNMG patients also ranges widely between 0.14% and 50%.16,17 Rivner et al. tested 181 DNMG patients for LRP4 and agrin antibodies and found 154 (85.1%) were negative for both, 27 (14.9%) were positive for at least one, and 23 (12.7%) patients were positive for both LRP4 and agrin antibodies.16 LRP4 antibody positivity has a female predominance of 59% and approximately 20% maintain isolated ocular manifestations years after initial presentation.7,16 LRP4-IgG is less commonly associated with thymus abnormalities (1 of 15 patients who underwent chest imaging in the Rivner et al. study had thymic hyperplasia and 5 underwent thymectomy prior to study entry) and typically portends a good response to pyridostigmine and immunosuppressant therapy.16 The clinical implications of these markers are still being elucidated, and ongoing work in this area will reveal the diagnostic and management importance of identifying these markers.
When should LRP4 and agrin antibody tests be sent? The LRP4 antibody test should be sent out for patients presenting with isolated ocular myasthenia gravis for whom the AChR antibodies (binding, blocking, and modulating) have already returned negative. That being said, single fiber EMG is the most sensitive test for isolated ocular myasthenia gravis.22–24 Agrin antibodies are not yet commercially available, and their clinical utility may be limited in DNMG patients.
NMOSD – AQP4
Neuromyelitis optica spectrum disorder (NMOSD) is characterized by optic neuritis (bilateral in 20% of cases) with longitudinally extensive transverse myelitis, area postrema syndrome, and syndrome of inappropriate secretion of anti-diuretic hormone.1,25–27 Lennon et al. identified antibodies targeting the astrocytic transmembrane water channel aquaporin-4 (AQP4) as the pathologic marker for neuromyelitis optica, thereby definitively distinguishing NMOSD from related disorders including MS.1 Approximately 80% of patients who meet clinical criteria for NMOSD are seropositive for antibodies targeting AQP4. AQP4 antibodies are pathogenic based on animal studies showing that injection of AQP4-IgG into rats with incipient experimental autoimmune encephalomyelitis results in immunoglobulin deposition, complement activation, and granulocyte influx.1,28,29
MRI of the brain is more likely to show enhancement of the intracranial optic nerve, optic tract, and optic chiasm in NMOSD as compared to MS.30,31 Imaging may also reveal lesions in the posterior fossa, periaqueductal gray, and hypothalamus. NMOSD is typically seen in older adults with a female predominance (up to 83%), and it is less common in Caucasians as compared to MS.32 Unlike MS, cerebrospinal fluid (CSF) is unlikely to show oligoclonal bands,1 but may show eosinophils or polymorphonuclear cells. Vision loss in NMOSD is often more severe than is typical for optic neuritis associated with MS (mean central visual acuity under 20/400). Altitudinal visual field defects may be more common, and OCT often reveals more significant loss of the retinal nerve fiber layer after NMOSD-related optic neuritis.33–36 Treatment is guided by distinguishing NMOSD from MS, as visual loss in NMOSD is much more responsive to plasma exchange than IV steroids in the acute setting. Patients with NMOSD are treated more aggressively using immunosuppressive therapy given the high relapse rate in patients with seropositive NMOSD.26,27,37 Moreover, certain standard disease-modifying treatments for MS, including interferons, fingolimod, and natalizumab, may not only be ineffective but may even increase the flare rate in NMOSD.26,38,39 Therefore, early identification and distinction of NMOSD from MS carry significant clinical implications, underscoring the importance of serologic markers. AQP4 antibodies have also been associated with breast carcinomas, intestinal carcinoid, and thymomas.5,29
When should AQP4 antibody testing be sent? Though routine testing for AQP4-IgG in all patients with clinical optic neuritis is a debated topic, it is clear that AQP4 antibody testing should be sent in patients with severe optic neuritis with the aforementioned clinical and paraclinical features that make MS less likely. AQP4-IgG is generated outside of the central nervous system, so serum testing is sufficient.
MOG-IgG
Myelin oligodendrocyte glycoprotein (MOG)-IgG-associated disorder is characterized by optic neuritis (bilateral in 38% of seropositive patients), often in association with optic disc edema, myelitis, and acute disseminated encephalomyelitis (ADEM).40 There is a slight female predominance (57%) and wide age range of 2–79 years. Younger adults are more likely to present with unilateral and recurrent disease and older adults (>45 years) with bilateral optic neuritis.40 Children can have recurrent optic neuritis and ADEM; in the Chen et al. study, 12/16 MOG-IgG-positive patients with ADEM were under 18 years old.40,41 MRI often shows enhancement of the intraorbital optic nerve and nerve sheath and is more likely to exhibit extensive, longitudinal (>50% nerve length) retrobulbar nerve lesions than MS or even NMOSD (Figure 1).30,31 Enhancement of the perineural orbital tissue (“perineuritis”) is common. Lesions of the deep gray nuclei may also be present.30,31 Compared to NMOSD, spinal cord imaging is more likely to reveal thoracolumbar and conus lesions than cervical lesions. In a study by Sato et al., all six MOG seropositive patients with spinal cord lesions involved the thoracic and/or lumbar region, and only one had a cervical lesion.42 A systematic review performed by Vosoughi et al. on 62 patients with MOG-associated disease reported 29 patients with afferent visual manifestations beyond optic neuritis that included uveitis, peripheral ulcerative keratitis, acute macular neuroretinopathy, neuroretinitis, venous stasis retinopathy, large pre-retinal macular hemorrhage, orbital inflammatory syndrome, and orbital apex syndrome.43
Figure 1.
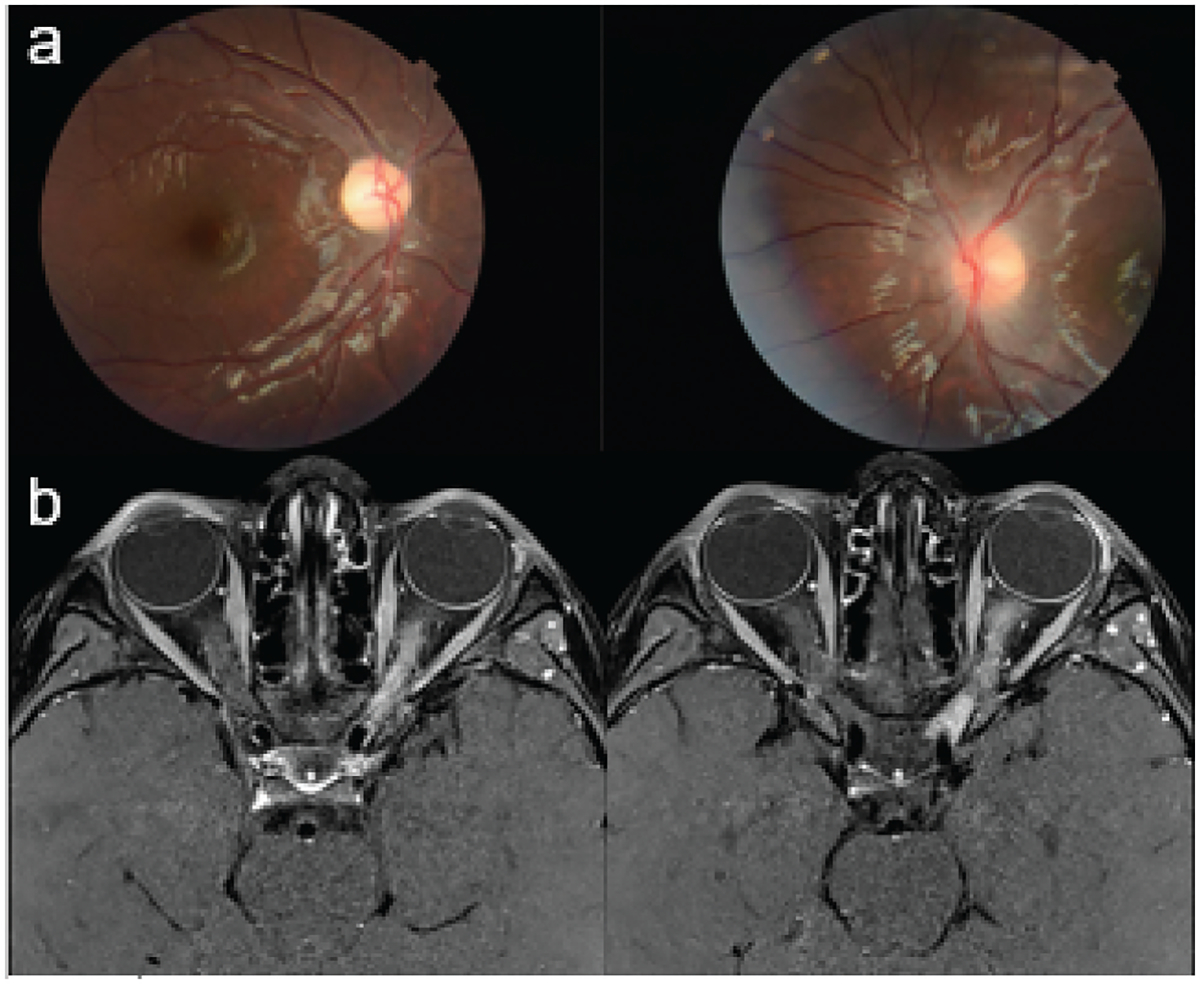
Seven-year-old girl with MOG-IgG-associated disorder. Fundus photos: right: optic disc has sharp margins with temporal pallor; Left: optic disc is edematous, especially superior and inferior, with hazy borders and gliosis. Post-contrast MRI brain, axial T1, fat suppressed: diffuse left optic nerve T2 signal abnormality, edema, and enhancement.
Recurrent attacks of optic neuritis occur in 80% of MOG-IgG-positive patients within 3 years of their first attack and are frequently associated with severe vision loss (often to the count-fingers level). Optical coherence tomography may demonstrate peripapillary retinal nerve fiber layer thinning and ganglion cell layer/complex thinning that worsens with each recurrence.40 Significant CSF pleocytosis (>100 cells/mm3) and the absence of oligoclonal bands can be suggestive of MOG-IgG-associated disease. Patients are both corticosteroid-responsive and frequently suffer recurrent flares with attempted tapering. Corticosteroid-sparing agents including azathioprine, rituximab, and mycophenolate mofetil are recommended.41,44
When should MOG antibody testing be sent? MOG antibody testing should be sent in patients with atypical optic neuritis who have optic disc edema, corticosteroid-responsive vision loss, with CSF pleocytosis, and the aforementioned neuroimaging findings. Given its more recent discovery, the utility of routine testing for MOG-IgG in all patients with clinical optic neuritis is unclear.
Paraneoplastic Autoimmune Syndromes
Clinical manifestations of paraneoplastic syndromes result from autoimmune attack on normal tissue that is triggered by ectopic protein expression by an underlying neoplasm. Neuro-ophthalmic manifestations of paraneoplastic conditions often precede a cancer diagnosis, highlighting the importance of early recognition and timely work-up.
Opsoclonus–Myoclonus Syndrome
Opsoclonus (high-frequency, conjugate, and chaotic multidirectional saccadic movements) and myoclonus (involuntary sudden jerking movements of the limbs) are hallmark features of opsoclonus–myoclonus syndrome (OMS), which is strongly associated with malignancy.45–48 In the pediatric population, OMS manifests in the context of neuroblastoma in 50% of cases (with the remainder believed to be post-infectious).45,49,50 In adults, OMS is most commonly associated with: small cell lung cancer, ovarian cancer, and breast cancer.51,52 Opsoclonus is suspected to result from antibody-mediated dysfunction of omnipause neurons that gate saccadic eye movement).51,53
Paraneoplastic antibodies associated with OMS were discovered in 1985 and 1988, respectively: antineuronal nuclear antibody type 1 (ANNA-1) (seen with SCLC and neuroblastoma) and antineuronal nuclear antibody type 2 (ANNA-2) (seen with small cell lung cancer and breast carcinoma).51,54 The pathologic mechanism by which ANNA-1 and 2 mediate their effects remains unclear, but postmortem studies have uncovered an associated loss of cerebellar Purkinje cells and pontine neurons in the parapontine reticular formation.55 ANNA-1-positive OMS also manifests with limbic encephalitis, encephalomyelitis, polyneuropathy and sensory neuronopathies, and dysautonomia. In the Lucchinetti et al. study, 2/162 (1.25%) ANNA-1-positive patients had OMS. ANNA-2-positive OMS is associated with jaw dystonia, larygospasm, and brainstem encephalitis. In the work-up of OMS, urine catecholamine levels should be sent for homovanillic and vanillylmandelic acids that may suggest neuroblastoma. Even if these antibodies are negative, a comprehensive evaluation for cancer should be performed, including imaging of the chest, abdomen and pelvis, mammography, and (if unrevealing) whole-body fludeoxyglucose positron emission tomography (FDG-PET).
Treatment is targeted to the underlying malignancy, in addition to IV corticosteroids, intravenous immune globulin, or plasma exchange for the acute manifestations, with subsequent corticosteroid-sparing immunotherapy long term.55
When should ANNA-1 and ANNA-2 be sent? When patients present with clinical manifestations that are suspected to represent OMS. Negative results do not exclude the possibility of malignancy.
Paraneoplastic Optic Neuropathy
Paraneoplastic optic neuropathies are typically seen in middle-aged and older patients presenting with painless, subacute visual loss that can be bilaterally simultaneous or rapidly sequential. The characteristic features vary according to the underlying autoantibody, many of which are actively being studied.
CRMP5 – Optic Neuropathy with Retinitis and Vitritis
Cross et al. and Sawyer et al. reported the phenotypic overlap between lung cancer patients with carcinoma-associated retinopathy and CRMP5-IgG-associated optic neuropathy.56,57 CRMP5 is a neuronal cytoplasmic protein that regulatesneurite outgrowth during development and regeneration. CRMP5-IgG-associated disease presents with retinitis and often bilateral optic neuropathy without optic nerve enhancement on MRI.56
Cohen et al. reviewed 29 CRMP5-IgG seropositive patients with neuro-ophthalmic manifestations, the majority of which preceded a cancer diagnosis.58 Of the 59% with anterior visual pathway or intraocular findings, initial median visual acuity was 20/50, 82% were diagnosed with an optic neuropathy, and all who underwent fundoscopic examination at onset had optic disc edema, often associated with uveitis, retinitis or both. None of the patients showed retrobulbar optic nerve enhancement on MRI,58 consistent with prior studies,56,59–61 suggesting that CRMP5-IgG does not cause optic neuritis as was initially asserted. Ocular motility dysfunction secondary to central nystagmus was present in 41% of patients.58 Visual function improved in 50% of the cohort with immunosuppressive therapy. Associated neurologic manifestations (in order of decreasing frequency) included neuropathic disorders (predominantly peripheral neuropathy and polyradiculoneuropathy), cerebellar ataxia, limbic encephalitis, cognitive decline, autonomic dysfunction, choreoathetosis, myelopathy, and Lambert–Eaton syndrome.
Systemic work-up for malignancy is positive in 62–87% of the patients positive for CRMP5-IgG.56,58 Small-cell carcinoma is the most common underlying malignancy (83%), though it can also be seen in mixed small-cell lung cancer and non-small-cell lung cancer, breast ductal carcinoma in situ, and thymoma.56,58
When should CRMP5-IgG testing be sent? Serologic testing for CRMP5-IgG should be sent in patients with optic disc edema without optic nerve enhancement on MRI, particularly in conjunction with retinitis and vitritis.
GFAP – Optic Papillitis
IgG antibodies targeting glial fibrillary acidic protein (GFAP), an astrocytic cytoplasmic intermediate filament protein, have been associated with inflammatory optic disc edema in 40% of patients with corticosteroid-responsive GFAP-IgG-positive meningoencephalitis, an entity originally described by Fang et - al.62–64 Chen et al. reviewed the ophthalmic findings of 10 patients with GFAP IgG-associated meningoencephalitis with bilateral disc edema.64 Among those, three (30%) had mild vitritis and preserved central visual acuity, but two (20%) developed arcuate visual field defects with corresponding ganglion cell layer thinning and clinical optic atrophy upon resolution of the disc edema.64 Fluorescein angiography revealed venular leakage in one patient.64 Two patients (20%) had mildly elevated opening pressures on lumbar puncture (265 and 298 mm H2O).
Neurologic manifestations of GFAP-IgG-associated disease include headache, encephalopathy, postural tremor, and cerebellar ataxia.64 MRI brain classically shows perivascular radial enhancement extending from the ventricles or cerebellum without associated retrobulbar optic nerve signal change.62–65 Associated malignancies are reported in 14–38% of patients with GFAP-IgG-associated disease, including teratomas, prostate and GI adenocarcinomas, melanomas, and carcinoid tumors.63,64,66 IV methylprednisolone should be initiated on presentation followed by high-dose oral corticosteroids with a duration based upon clinical judgment. Though we have no intention of conflating the two paraneoplastic syndromes, there have been case reports of optic neuritis with N-methyl-D-aspartate receptor (NMDAR) antibody-associated encephalitis, which was the first cell-surface antibody-mediated autoimmune encephalitis reported.67–69 There are also reports of efferent manifestations in NMDAR antibody-mediated encephalitis, including ocular bobbing and OMS, but this antibody is better known for its association with neuropsychiatric symptoms, seizures, and movement disorders.70,71
When should GFAP-IgG testing be sent? Serum and CSF GFAP-IgG should be sent in patients with bilateral optic papillitis without associated retrobulbar optic nerve enhancement on MRI. Radial perivascular enhancement extending from the ventricles or cerebellum on brain MRI, certainly in the setting of meningoencephalitis, should raise suspicion for GFAP-IgG-related disease.
Conclusion
The discovery of and availability to test for disease-specific antibody biomarkers has reshaped neuro-ophthalmic practice, both with respect to the work-up and management of our patients (Table 1). A common theme is the importance of recognizing the clinically atypical variants of more traditional syndromes with which patients more commonly present – from that of atypical optic neuritis in patients with NMOSD or MOG-IgG-associated disorder to optic disc edema with retinitis and vitritis associated with CRMP5-IgG. Antibody biomarkers cannot replace clinical judgment or gold-standard diagnostic testing in certain conditions. As neuroimmunology labs continue to gather and test samples from patients with atypical neuro-ophthalmic presentations, the number of distinct clinical entities will continue to grow and become more refined over time. Advancements in autoantibody biomarker research are therefore integral to the future of neuro-ophthalmology.
Table 1.
Summary of select antibodies included in this review.
| Antibody/Antibody target | Associated diseasea | Function of antibody target | When to test |
|---|---|---|---|
|
| |||
| LRP4 | Myasthenia gravis (MG) | Activate MuSK kinase and facilitates clustering and stabilization of the Ach receptor at the neuromuscular junction | Isolated ocular MG with negative AChR antibodies (binding, blocking, and modulating) |
| AQP4 | NMOSD | Facilitates water transport through the cell membrane at the blood-brain barrier | Severe optic neuritis and transverse myelitis with features atypical for MS, including greater responsiveness to PLEX than steroids |
| MOG | MOG-IgG-associated disorder | Protein located on the surface of myelin sheaths | Atypical optic neuritis with optic disc edema, corticosteroid- responsive vision loss, and CSF pleocytosis |
| ANNA-1 and 2 | Opsoclonus myoclonus syndrome (OMS) | Exact target and pathologic mechanism by which ANNA-1 and 2 mediate their effects remain unclear | Patients with OMS, though negative results do not exclude the possibility of malignancy |
| CRMP-5 | Paraneoplastic optic neuropathy | Neuronal cytoplasmic protein involved in regulation of neurite outgrowth in development and regeneration | Optic disc edema without optic nerve enhancement on MRI, particularly in conjunction with retinitis and vitritis |
| GFAP | Optic papillitis | Astrocytic cytoplasmic intermediate filament protein | Bilateral optic papillitis without associated retrobulbar optic nerve enhancement on MRI, particularly if instead there is radial perivascular enhancement extending from the ventricles or cerebellum |
As it pertains to this review article.
.AChR: acetylcholine receptor; ANNA: antineuronal nuclear antibody; CRMP5: collapsin-response mediator protein-5; CSF: cerebrospinal fluid; LRP4: lipoprotein-4; MOG: Myelin oligodendrocyte glycoprotein; MuSK: muscle-specific kinase; NMOSD: neuromyelitis spectrum disorder; PLEX: plasma exchange.
Footnotes
Disclosure Statement
EDG: Luminopia, Inc. (equity; patent; advisor); Stoke Therapeutics, Inc. (consultant).
REFERENCES
- 1.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 2.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349(2):139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan S, Reddel SW, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2014;1(4):e40. doi: 10.1212/NXI.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S-M, Woodhall MR, Kim J-S, et al. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e163. doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sepúlveda M, Sola-Valls N, Escudero D, et al. Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin-4 antibodies. Mult Scler. 2018;24 (13):1753–1759. doi: 10.1177/1352458517731914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2014;175(3):408–518. doi: 10.1111/cei.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14 (10):1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 8.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- 9.Evoli A, Alboini PE, Iorio R, et al. Pattern of ocular involvement in myasthenia gravis with MuSK antibodies. Journal of Neurology, Neurosurgery & Psychiatry. 2017;88(9):761. doi: 10.1136/jnnp-2017-315782. [DOI] [PubMed] [Google Scholar]
- 10.Gilhus NE, Skeie GO, Romi F, et al. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–268. doi: 10.1038/nrneurol.2016.44. [DOI] [PubMed] [Google Scholar]
- 11.Kerty E, Elsais A, Argov Z, et al. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol. 2014;21(5):687–693. doi: 10.1111/ene.12359. [DOI] [PubMed] [Google Scholar]
- 12.Sanders DB, El-Salem K, Massey JM, et al. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60 (12):1978–1980. doi: 10.1212/01.WNL.0000065882.63904.53. [DOI] [PubMed] [Google Scholar]
- 13.Pevzner A, Schoser B, Peters K, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259(3):427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- 14.Zong Y, Zhang B, Gu S, et al. Structural basis of agrin-LRP4-MuSK signaling. Genes Dev. 2012;26(3):247–258. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higuchi O, Hamuro J, Motomura M, et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69(2):418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 16.Rivner MH, Quarles BM, Pan JX, et al. Clinical features of LRP4/agrin-antibody-positive myasthenia gravis: a multicenter study. Muscle Nerve. 2020;62(3):333–343. doi: 10.1002/mus.26985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Tzartos JS, Belimezi M, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69(4):445–451. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Shen C, Bealmear B, et al. Autoantibodies to agrin in myasthenia gravis patients. PLoS One. 2014;9(3):e91816. doi: 10.1371/journal.pone.0091816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huzé C, Bauché S, Richard P, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. The American Journal of Human Genetics. 2009;85 (4):536. doi: 10.1016/j.ajhg.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasperi C, Melms A, Schoser B, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology. 2014;82(22):1976–1983. doi: 10.1212/WNL.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 21.Barik A, Lu Y, Sathyamurthy A, et al. LRP4 is critical for neuromuscular junction maintenance. J Neurosci. 2014;34 (42):13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padua L, Stalberg E, LoMonaco M, et al. SFEMG in ocular myasthenia gravis diagnosis. Clin Neurophysiol. 2000;111 (7):1203–1207. doi: 10.1016/S1388-2457(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 23.Cui LY, Guan YZ, Wang H, et al. Single fiber electromyography in the diagnosis of ocular myasthenia gravis: report of 90 cases. Chin Med J (Engl). 2004; 117(6): 848–851. [PubMed] [Google Scholar]
- 24.Gonzalez-Hidalgo M, Carcedo CF. Single fiber electromyography by axonal stimulation in orbicularis oculi muscle: technique and results in the diagnosis of ocular myasthenia gravis. Clin Neurophysiol. 2009;120(4):e139–e140. doi: 10.1016/j.clinph.2008.09.056. [DOI] [Google Scholar]
- 25.Pittock SJ, Weinshenker BG, Lucchinetti CF, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006;63(7):964–968. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 26.Kleiter I, Hellwig K, Berthele A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69 (2):239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 27.Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206–216. doi: 10.1002/ana.24554. [DOI] [PubMed] [Google Scholar]
- 28.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zekeridou A, Lennon VA. Aquaporin-4 autoimmunity. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramanathan S, Prelog K, Barnes EH, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016;22(4):470–482. doi: 10.1177/1352458515593406. [DOI] [PubMed] [Google Scholar]
- 31.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study: table 1. J Neurol Neurosurg Psychiatry. 2016;87(4):446–448. doi: 10.1136/jnnp-2014-310206. [DOI] [PubMed] [Google Scholar]
- 32.Brody J, Hellmann MA, Marignier R, et al. Neuromyelitis optica spectrum disorder: disease course and long-term visual outcome. J Neuroophthalmol. 2016;36(4):356–362. doi: 10.1097/WNO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 33.Merle H, Olindo S, Jeannin S, et al. Treatment of optic neuritis by plasma exchange (add-on) in neuromyelitis optica. Arch Ophthalmol. 2004;364(9451):858–862. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima H, Hosokawa T, Sugino M, et al. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010;10(1):45. doi: 10.1186/1471-2377-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72(12):1077–1082. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73(4):302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89(4):346–351. doi: 10.1136/jnnp-2017-316286. [DOI] [PubMed] [Google Scholar]
- 38.Min J-H, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler. 2012;18(1):113–115. doi: 10.1177/1352458511431973. [DOI] [PubMed] [Google Scholar]
- 39.Kim S-H, Kim W, Li XF, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler. 2012;18(10):1480–1483. doi: 10.1177/1352458512439439. [DOI] [PubMed] [Google Scholar]
- 40.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin oligodendrocyte glycoprotein antibody–positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. 2012;69 (4):8–15. doi: 10.1016/j.ajo.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody–associated disease. JAMA Neurol. 2018;75 (4):478–487. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vosoughi AR, Ling J, Tam KT, et al. Ophthalmic manifestations of myelin oligodendrocyte glycoprotein-IgG-associated disorder other than optic neuritis: a systematic review. Br J Ophthalmol. 2020. doi: 10.1136/bjophthalmol-2020-317267. [DOI] [PubMed] [Google Scholar]
- 44.Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89(2):127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hero B, Schleiermacher G. Update on pediatric opsoclonus myoclonus syndrome. Neuropediatrics. 2013;44(6):324–329. doi: 10.1055/s-0033-1358604. [DOI] [PubMed] [Google Scholar]
- 46.Laroumagne S, Elharrar X, Coiffard B, et al. “Dancing eye syndrome” secondary to opsoclonus-myoclonus syndrome in small-cell lung cancer. Case Rep Med. 2014;2014:545490. doi: 10.1155/2014/545490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaas JP, Ahlskog JE, Pittock SJ, et al. Adult-onset opsoclonus-myoclonus syndrome. Arch Neurol. 2012;69 (12):1598–1607. doi: 10.1001/archneurol.2012.1173. [DOI] [PubMed] [Google Scholar]
- 48.Sahu JK, Prasad K. The opsoclonus-myoclonus syndrome. Pract Neurol. 2011;11(3):160–166. doi: 10.1136/practneurol-2011-000017. [DOI] [PubMed] [Google Scholar]
- 49.Singhi P, Sahu JK, Sarkar J, et al. Clinical profile and outcome of children with opsoclonus-myoclonus syndrome. J Child Neurol. 2014;29(1):58–61. doi: 10.1177/0883073812471433. [DOI] [PubMed] [Google Scholar]
- 50.Wells EM, Dalmau J. Paraneoplastic neurologic disorders in children. Curr Neurol Neurosci Rep. 2011;11(2):187–194. doi: 10.1007/s11910-010-0169-4. [DOI] [PubMed] [Google Scholar]
- 51.Panzer JA, Anand R, Dalmau J, et al. Antibodies to dendritic neuronal surface antigens in opsoclonus myoclonus ataxia syndrome. J Neuroimmunol. 2015;286:86–92. doi: 10.1016/j.jneuroim.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popławska-Domaszewicz K, Florczak-Wyspiańska J, Kozubski W, et al. Paraneoplastic movement disorders. Reviews in the Neurosciences. 2018;29(7):745–755. doi: 10.1515/revneuro-2017-0081. [DOI] [PubMed] [Google Scholar]
- 53.Kim JS, Choi K-D, Oh S-Y, et al. Double saccadic pulses and macrosaccadic oscillations from a focal brainstem lesion. J Neurol Sci. 2007;263(1–2):118–123. doi: 10.1016/j.jns.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Sutton IJ, Barnett MH, Watson JDG, et al. Paraneoplastic brainstem encephalitis and anti-Ri antibodies. J Neurol. 2002;249 (11):1597–1598. doi: 10.1007/s00415-002-0863-5. [DOI] [PubMed] [Google Scholar]
- 55.Hormigo A, Dalmau J, Rosenblum MK, et al. Immunological and pathological study of anti-Ri-associated encephalopathy. Ann Neurol. 1994;36(6):896–902. doi: 10.1002/ana.410360615. [DOI] [PubMed] [Google Scholar]
- 56.Cross S, Salomao DR, Parisi JE, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Am J Ophthalmol. 2003;136(6):1200. doi: 10.1016/j.ajo.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81(5):606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 58.Cohen DA, Bhatti MT, Pulido JS, et al. Collapsin response-mediator protein 5–associated retinitis, vitritis, and optic disc edema. Ophthalmology. 2020;127(2):221–229. doi: 10.1016/j.ophtha.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 59.De La Sayette V, Bertran F, Honnorat J, et al. Paraneoplastic cerebellar syndrome and optic neuritis with anti-CV2 antibodies: clinical response to excision of the primary tumor. Arch Neurol. 1998;55(3):405–408. doi: 10.1001/archneur.55.3.405. [DOI] [PubMed] [Google Scholar]
- 60.Pulido J, Cross SA, Lennon VA, et al. Bilateral autoimmune optic neuritis and vitreitis related to CRMP-5-IgG: intravitreal triamcinolone acetonide therapy of four eyes. Eye (Lond). 2008. Sep;22(9):1191–3. doi: 10.1038/sj.eye.6702959. [DOI] [PubMed] [Google Scholar]
- 61.Rogemond V, Honnorat J. Anti-CV2 autoantibodies and paraneoplastic neurological syndromes. Clin Rev Allergy Immunol. 2000;19 (1):51–59. doi: 10.1385/CRIAI:19:1:51. [DOI] [PubMed] [Google Scholar]
- 62.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 63.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol. 2017;81(2):298–309. doi: 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 64.Chen JJ, Aksamit AJ, McKeon A, et al. Optic disc edema in glial fibrillary acidic protein autoantibody–positive meningoencephalitis. J Neuroophthalmol. 2018;38(3):276–281. doi: 10.1097/WNO.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 65.Iorio R, Damato V, Evoli A, et al. Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J Neurol Neurosurg Psychiatry. 2018;89 (2):138–146. doi: 10.1136/jnnp-2017-316583. [DOI] [PubMed] [Google Scholar]
- 66.Bohm PE, Chen JJ, Bhatti TM, et al. Neuro-ophthalmic features of autoimmune encephalitides. J Neuroophthalmol. 2020;40 (3):385–397. doi: 10.1097/WNO.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 67.Dalmau J, Tüzün E, Wu H-Y, et al. Paraneoplastic anti- N -methyl -D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruer MC, Koch TK, Bourdette DN, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology. 2010;74 (18):1473–1475. doi: 10.1212/WNL.0b013e3181dc1a7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mugavin M, Mueller BH, Desai M, et al. Optic neuropathy as the initial presenting sign of N-methyl-d-aspartate (NMDA) Encephalitis. Neuro-ophthalmology. Amsterdam: : Aeolus Press. 1980. 2017;41(2):90–93. doi: 10.1080/01658107.2016.1262431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimazaki H, Morita M, Nakano I, et al. Inverse ocular bobbing in a patient with encephalitis associated with antibodies to the N-methyl-D-aspartate Receptor. Arch Neurol. 2008;65(9):1251. doi: 10.1001/archneur.65.9.1251. [DOI] [PubMed] [Google Scholar]
- 71.Kurian M, Lalive PH, Dalmau JO, et al. Opsoclonus-myoclonus syndrome in anti-N-methyl-D-aspartate receptor encephalitis. Arch Neurol. 2010;67 (1):118–121. doi: 10.1001/archneurol.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]


