Abstract
The transient receptor potential melastatin 4 (TRPM4) is a Ca2+-activated nonselective monovalent cation channel belonging to the TRP channel superfamily. TRPM4 is widely expressed in various tissues and most abundantly expressed in the heart. TRPM4 plays a critical role in cardiac conduction. Patients carrying a gain-of-function or loss-of-function mutation of TRPM4 display impaired cardiac conduction. Knockout or over-expression of TRPM4 in mice recapitulates conduction defects in patients. Moreover, recent studies have indicated that TRPM4 plays a role in hypertrophy and heart failure. Whereas the role of TRPM4 mediated by cardiac myocytes has been well investigated, little is known about TRPM4 and its role in cardiac fibroblasts. Here we show that in human left ventricular fibroblasts, TRPM4 exhibits typical Ca2+-activation characteristics, linear current–voltage (I–V) relation, and monovalent permeability. TRPM4 currents recorded in fibroblasts from heart failure patients (HF) are more than 2-fold bigger than those from control individuals (CTL). The enhanced functional TRPM4 in HF is not resulted from changed channel properties, as TRPM4 currents from both HF and CTL fibroblasts demonstrate similar sensitivity to intracellular calcium activation and extracellular 9-phenanthrol (9-phen) blockade. Consistent with enhanced TRPM4 activity, the protein level of TRPM4 is about 2-fold higher in HF than that of CTL hearts. Moreover, TRPM4 current in CTL fibroblasts is increased after 24 hours of TGFβ1 treatment, implying that TRPM4 in vivo may be upregulated by fibrogenesis promotor TGFβ1. The upregulated TRPM4 in HF fibroblasts suggests that TRPM4 may play a role in cardiac fibrogenesis under various pathological conditions.
Keywords: TRP channels, TRPM4, Calcium signaling, Human ventricular fibroblasts, Heart failure
Introduction
TRPM4 is a calcium activated nonselective cation channel [43, 44] belonging to the melastatin subfamily of transient receptor potential (TRP) membrane protein superfamily [9, 49, 50]. Among the 28 mammalian TRP channels, TRPM4 and its close homologue, TRPM5, are the only two members which are activated by a rise of intracellular Ca2+ but are calcium-impermeable monovalent cation channels [44, 56, 35]. The channel function of TRPM4 is regulated by PIP2 [75], voltage [51, 35], hypotonic cell swelling [20], decavanadate [52], intracellular nucleotides such as ATP, and polyamines [53]. Recent cryo-EM structure studies have revealed mechanistic insights of monovalent permeability; binding domains for ATP, PIP2, and Ca2+; and how the channel gating can be regulated [1, 18, 70, 30]. Moreover, TRPM4 channel protein is post-translational modified by SUMOylation [42, 46], phosphorylation [11], and glycosylation [67].
TRPM4 is widely expressed in many different types of cells and tissues, and appears to be involved in various physiological functions, including smooth muscle function [19], insulin secretion [8, 62], immune response [68, 61, 2], neuronal degeneration [60], ischemic stroke [7, 71], and cardiovascular functions [41]. TRPM4 is most abundantly expressed in the heart [43, 44]. A gain-of-function mutation at the N-terminal (E7K) of TRPM4 was first found to be associated with progressive familial heart block type I (PFHBI) [42]. A large number of other mutations of TRPM4 were later identified to be associated with PFHBI [12], isolated cardiac conduction block (ICCD) [46, 65], atria-ventricular conduction block (AVB) [65, 66], right bundle branch block (RBBB) [65], bradycardia [45], Brugada syndrome (BrS) [45, 26], and complete heart block (CHB) [4]. Among these mutants, gain- or loss-of-function of TRPM4 seems to be mainly caused by changes of membrane protein level of TRPM4 [4, 41]. Consistent with conduction block in TRPM4 mutation–carrying patients, knockout of TRPM4 or over-expression of TRPM4 in mice results in cardiac arrhythmias [47, 58, 63].
TRPM4 not only plays an essential role in cardiac conduction [27] but also seems to be involved in hypertension, hypertrophy, and heart failure. TRPM4−/− mice are hypertensive [48] and display increased β-adrenergic inotrophy in the ventricular myocardium [47], as TRPM4 deletion causes elevated circulating catecholamine levels due to increased acetylcholine-induced exocytosis in chromaffin cells [48]. TRPM4 deletion mice also exhibit enhanced response to angiotensin II–induced cardiac hypertrophy [40], and left ventricular (LV) eccentric hypertrophy at an older age (32 weeks) [14]. Deletion of TRPM4 in rats aggravates right ventricular hypertrophy induced by right ventricular pressure load [24]. Whereas TRPM4 deficiency appears to exacerbate hypertensive hypertrophy and heart failure, controversial results have been reported about the role of TRPM4 in ischemic heart failure [32, 38, 57]. It was previously shown that TRPM4 knockout improves survival rate and enhances β-adrenergic cardiac reserve after inducing ischemic heart failure [38], and TRPM4 inhibitor 9-phen was also found to reduce ischemia–reperfusion-induced cardiac death in rats [57]. However, a recent study demonstrated that TRPM4 is required for mice to survive in ischemic heart failure [32].
Whereas the function of TRPM4 mediated by cardiac myocytes have been extensively investigated, little is known about TRPM4 in cardiac fibroblasts, the most abundant cells in the heart, which are involved in various pathological processes in heart diseases. A recent study reported functional expression of TRPM4 and its potential role in human atrial fibroblast growth in culture [64]. Our previous study also showed that TRPM4 expression in mouse fibroblasts can be potentiated by fibrosis-promoting factor TGFβ1 [17]. These data imply that TRPM4 may influences fibroblasts’ functions in the heart. In the present study, we demonstrate that TRPM4 is functionally expressed in human ventricular fibroblasts and is markedly enhanced in heart failure patients. Our results suggest that TRPM4 may play a role in cardiac fibrogenesis cascade under pathological conditions.
Materials and methods
Tissue freezing and fibroblast isolation from failing and nonfailing human left ventricular tissues
Human left ventricular tissues were obtained from donor hearts (Table 1) provided by Illinois Gift of Hope (GOH) Organ & Tissue DonorNetwork. These studies were approved by the Human Study Committees of Rush University Medical Center and Illinois GOH. Consent was obtained by GOH from the donors’ families for the use of the donor hearts for research purposes, and studies were performed in accordance with the Declaration of Helsinki. The failing hearts were from the organ donors who had a history of heart failure with an ejection fractioning (EF) of 14.5±2.5 (n=6), reflecting a significantly impaired cardiac function in these failing human hearts (Table 1). The nonfailing control hearts (CTL) were from the donors who had a normal cardiac function with the EF of 62.2±3.6% (n=6) and without a history of any major cardiovascular disease. Those well-procured human hearts were obtained by the Ai lab through the GOH. Following heart explanation, left ventricular tissues were quickly dissected to be flash frozen for western blot (WB) experiments, or to be used immediately for isolation of fibroblasts for functional studies.
Table 1.
Patients’ clinical characteristics
| Non-HF (CTL) | HF | |
|---|---|---|
| N | 6 | 6 |
| Age (years) | 52.8±3.3 | 42.8 ±4.9 |
| Male (%) | 33.3 | 83.3 |
| Cau (%) | 50 | 50 |
| AA (%) | 33.3 | 16.7 |
| Hisp (%) | 16.7 | 0 |
| Unknown race (%) | 0 | 33.3 |
| BMI | 31.9 ± 2.8 | 33.1± 3.4 |
| EF (%) | 62.2 ± 3.6 | 14.5±2.5 |
| HF (%) | 0 | 100 |
| CAD (%) | 0 | 50 |
| MI (%) | 0 | 0 |
| VD (%) | 0 | 0 |
| HTN (%) | 66.7 | 50 |
| HLD (%) | 33.3 | 50 |
| DM (%) | 16.7 | 16.7 |
Fibroblasts were isolated in the Ai lab using the method as we previously described [17]. In brief, left ventricles of CTL or HF heart samples were minced and incubated with collagenase (150~200 U/ml CLS II, Worthington Biochemical, Freehold, NJ, 300 U/mg) in a water bath shaking at 37°C. Enzyme-digested cells were harvested after each 10-min digestion period. After 5 digestion periods, all the digested cells were then centrifuged at 1000 rpm for 10 min. Isolated fibroblasts were re-suspended in the cold (~4 °C) cardioplegic solution modified from a previous recipe [3] containing (in mM) 110 NaCl, 16 KCL, 16 MgCl2, 2 mM Ca2+, 10 HEPES, and 10 mM glucose, and immediately delivered to the Yue lab. Upon arrival to the Yue lab, fibroblasts were re-suspended in DMEM media, and seeded on the coverslips for patch-clamp experiments within 12~18 h, or seeded in the cultured dish and cultured for overnight for other experiments.
Culture of fibroblasts
Some fibroblasts isolated from CTL were seeded in the culture dish, and were cultured in the CO2 cell culture incubator for the experiments of TGFβ1 treatment. Fibroblasts were cultured for overnight, and then serum starved for 12 h, followed by a 24-h incubation in the presence or absence of 10 ng/ml TGFβ1 in 1% serum DMEM medium [17]. After 24 h, fibroblasts were used for patch-clamp recording of TRPM4 currents.
Real-time RT-PCR
Real-time RT-PCR was conducted as we previously reported [17]. In brief, fibroblasts were collected and total RNA was extracted by TRIzol (Invitrogen). Real-time PCR was performed with the SYBR Green method following the protocol suggested by vendor (ABI). Human β-actin was used as an internal control. Primers used for real-time PCR are as follows: TRPM4: forward: TGCGCGCCGAGATGTAT, reverse: AAAGAAGCAGGTCGCTCCAG; β-actin: forward: CACCATTGGCAATGAGCGGTTC, reverse: AGGTCTTTGCGGATGTCCACGT.
Generation of shRNA and knockdown of hTRPM4
TRPM4 shRNA (TRPM4-shRNA) and scramble shRNA (SC-shRNA) were generated as we previously reported [17]. Briefly, hTRPM4-specific shRNA sequence (GCACGACGTTCATAGTTGA: NCBI reference sequence: NM_001321283.2) [36], and scramble shRNA sequence (TGTGCTCCGAACGTGTAGT) [17] were cloned into GFP lenti-virus vector (pLVTHM) using MluI and ClaI sites as we described in the previous study [17]. Lenti-viruses were generated in HEK-293 cells and were used to infect CTL human ventricular fibroblasts in culture. The effects of shRNA were evaluated by a patch clamp of GFP fluorescence fibroblasts 60 to 72 h after lenti-virus infection [17].
Electrophysiology
Whole-cell currents were recorded using an Axopatch 200B amplifier. A voltage ramp ranging from −120 mV to +100 mV at the interval of 1 to 5 s was used to elicit TRPM4 currents. Data were digitized at 5 or 10 kHz, and digitally filtered offline at 1 kHz. Patch electrodes were pulled from borosilicate glass and fire-polished to a resistance of ~3 MΩ when filled with internal solutions. Series resistance (Rs) was compensated up to 90% to reduce series resistance errors to <5 mV. Cells with Rs bigger than 10 MΩ were discarded. A fast perfusion system was used to exchange extracellular solutions, with complete solution exchange achieved in about 1 to 3 s. The internal pipette solution for TRPM4 whole-cell current recordings contained (in mM) 145 Cs-methanesulfonate (CsSO3CH3), 8 NaCl, 1 EGTA, and 10 HEPES, with pH adjusted to 7.2 with CsOH. Ca2+ was adjusted to various concentrations based on calculation using MaxChelator (http://www.stanford.edu/~cpatton/webmaxcS.htm). The standard extracellular Tyrode’s solution for whole-cell recording contained the following (mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 Mg2+, 10 HEPES, and 10 glucose. pH was adjusted to 7.4 with NaOH. The 145-mM NaCl was replaced by NaSO3CH3 in low Cl− Tyrode solution for experiments to determine the potential contribution of the chloride channel to the current recordings, or by N-Methyl-D-glucamine (NMDG) chloride for leak detection. Isotonic Ca2+ solution contained 120 mM Ca2+, 10 mM HEPES, and 10 mM glucose, with pH adjusted to pH 7.4 as we previously reported [39].
Immunoblotting
For western blot analysis, tissue lysates were separated on either 8% or 10% polyacrylamide gels as we reported previously [16]. This was followed by transferring to nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in TBST and then incubated with anti-TRPM4 (Abcam, ab123936) or anti-GAPDH (Sigma, G-9545) antibodies. Blots then were washed with TBST and incubated with HRP-conjugated anti-rabbit or anti-mouse antibodies (Cell Signaling Tochnology, CS7076s and CS7074s). Blots then were washed, enhanced chemiluminescence (ECL; Pierce) was used for detection of signal, and images were captured by using a Fuji LAS-3000 Imaging System.
Data analysis
Pooled data are presented as mean±SEM. Concentration–response curves were fitted by an equation of the following form: E = Emax{1/[1 + (EC50/C)n]}, where E is the effect at concentration C, Emax is the maximal effect, EC50 is the concentration for the half-maximal effect, and n is the Hill coefficient [72]. EC50 is replaced by IC50 if the effect is an inhibitory effect. Statistical comparisons were analyzed using two-way analysis of variance (ANOVA) and a two-tailed t test with Bonferroni correction; p < 0.05 indicated statistical significance.
Results
Functional expression of TRPM4 in human ventricular fibroblasts
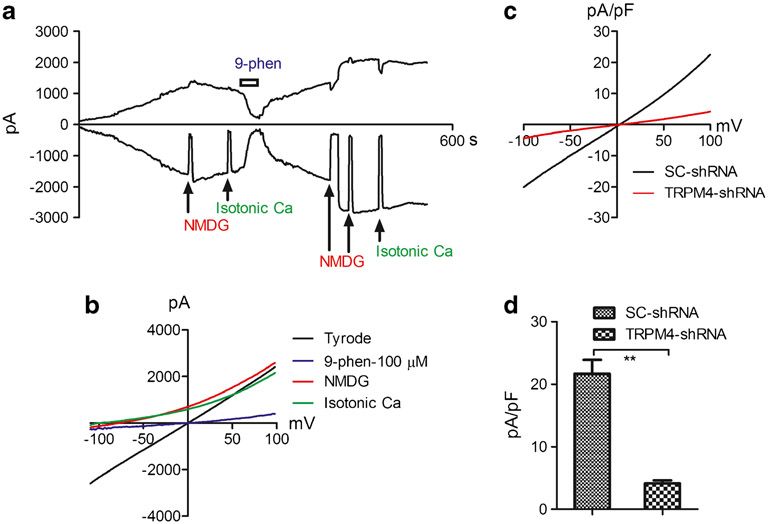
Several TRP channels have been shown to express in cardiac fibroblasts [22, 33]. In order to investigate whether TRPM4 is functionally expressed in human cardiac fibroblasts, we applied whole-cell current recording using pipette solutions containing 100 μM Ca2+ to activate TRPM4, and 3 mM Mg2+ to eliminate potential contamination from the TRPM7 current. As shown in Fig. 1, TRPM4 was activated with time while pipette Ca2+ was dialyzed into the cell (Fig. 1a). NMDG was used to make sure that there was no leak current, and a low-chloride Tyrode solution was used to ensure no Cl− contamination in the current recordings. To determine whether the recorded current was TRPM4 current, we applied extracellular isotonic Ca2+ solution to examine ionic permeation. The isotonic calcium solution did not produce any inward current, indicating that the current recorded in the Tyrode solution was carried by monovalent cations. Among the two calcium nonpermeable TRP channels, TRPM4, but not TRPM5, is highly expressed in the heart [23, 69], suggesting that the currents we recorded were likely carried by TRPM4. We further confirmed that it was TRPM4 current by applying 9-phenanthrol (9-phen) (Fig. 1a, b), which inhibits TRPM4 but not TRPM5 [25]. To further confirm that the recorded currents were TRPM4 currents, we applied TRPM4-shRNA to knock down TRPM4. As shown in Fig. 1c, d, TRPM4-shRNA produced about 80% inhibition, indicating that the recorded current which was blocked by 9-phen was indeed TRPM4 currents.
Fig. 1.
Functional expression of TRPM4 in human ventricular fibroblasts. a Time-dependent activation of TRPM4 recorded in a HF fibroblast, and inhibition by 100 μM 9-phen. Inward and outward currents were measured at −100 mV and +100 mV. TRPM4 currents were elicited by a ramp protocol ranging from −120 to +100 mV with the recording condition of 100 μM Ca2+ in the pipette solution. NMDG was applied to make sure that there was no leak current throughout the recording time. Isotonic Ca2+ solution was used to detect whether there was any Ca2+ permeation. b Representative recordings of TRPM4 in Tyrode solution and after blockade by 9-phen. Note that inward current was eliminated by NMDG and isotonic Ca2+ solution, indicating that there was no leak current and no Ca2+ permeability. c Representative TRPM4 currents recorded in CTL fibroblasts treated with TRPM4-shRNA or SC-shRNA. d Average current amplitude of TRPM4 in fibroblasts treated with TRPM4-shRNA (4.1±0.4 pA/pF, n=11) or SC-shRNA (21.7±2.2, n=12). Note that TRPM4 currents were largely eliminated by TRPM4-shRNA (**p<0.01)
TRPM4 current is upregulated in heart failure patients
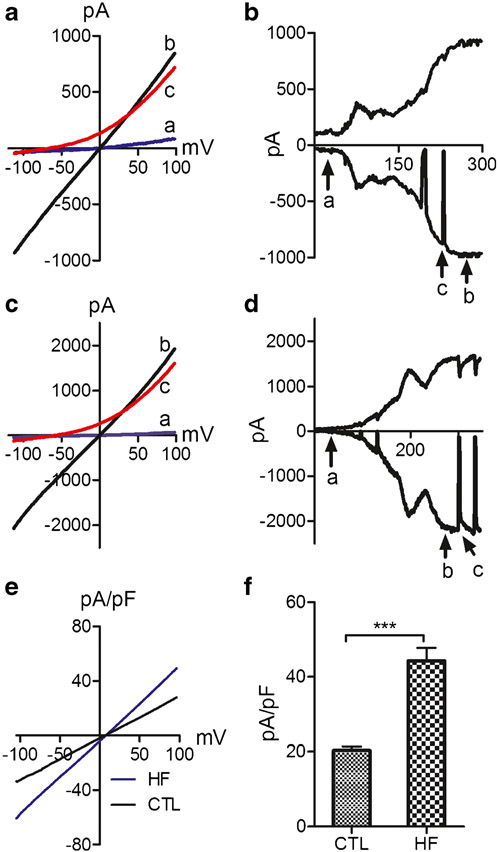
TRPM4 has been shown to play a role in cardiac myocytes under pathological conditions. In order to investigate whether TRPM4 in fibroblasts plays a role under pathological conditions, we determined to investigate if TRPM4 in fibroblasts is regulated by heart failing pathological conditions. Human left ventricular fibroblasts from heart failure patients (HF) and control patients (CTL) were isolated. Using the TRPM4 recording conditions shown in Fig. 1, we did current recording in fibroblasts freshly isolated from left ventricles of CTL and HF patients. The TRPM4 currents recorded from CTL and HF fibroblasts displayed a similar activation process and linear I− V relation after activation (Fig. 2a-d). However, the current amplitude in fibroblasts from HF patients was significantly bigger than that from CTL fibroblasts (Fig. 2e, f).
Fig. 2.
Comparison of TRPM4 currents recorded from fibroblasts of control individuals (CTL) and heart failure patients (HF). a Representative recordings of TRPM4 before and after activation, as well as after NMDG application at the corresponding time points (a, b, c) as shown in b from CTL fibroblasts. b Time-dependent activation of TRPM4 from CTL fibroblasts. Inward and outward current were measured at −100 and +100 mV respectively. c Representative traces of TRPM4 before and after activation, as well as after NMDG application at the corresponding time points (a, b, c) as shown in d from an HF fibroblast. d Time-dependent activation of TRPM4 from HF fibroblasts. Inward and outward currents were measured at −100 and +100 mV, respectively. e, f Comparison of TRPM4 current density from representative cells (c), and average current density of CTL (n=50) and HF (n=38) fibroblasts isolated from 6 CTL and HF hearts. Current density from HF fibroblasts is significantly bigger than that of CTL fibroblasts (***p<0.001)
Calcium sensitivity of TRPM4 in CTL and HF fibroblasts
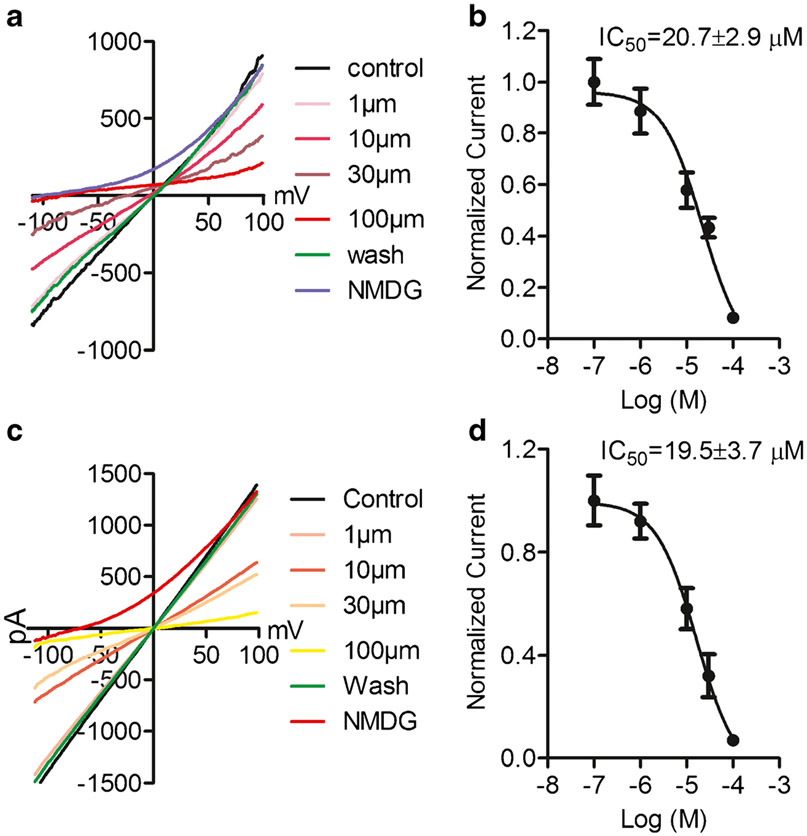
In order to understand the mechanisms underlying the enhanced current amplitude in the HF fibroblasts, we investigated calcium sensitivity for TRPM4 activation. Intracellular pipette solutions with various Ca2+ concentrations were used to activate TRPM4 currents in fibroblasts from both CTL and HF patients (Fig. 3a and c). Concentration-dependent analysis yielded EC50 of 15.5±0.6 μM and 14.4±0.8 μM for activation of TRPM4 in CTL and HF fibroblasts, respectively, indicating that the calcium sensitivity of TRPM4 was not changed by heart failure conditions.
Fig. 3.
Ca2+-sensitivity of TRPM4 from CTL and HF fibroblasts. a, c Representative TRPM4 currents recorded with various concentrations of intracellular Ca2+ in CTL (a) and HF (c) fibroblasts. b, d Concentration-dependent effects of intracellular Ca2+ on TRPM4. Dose–response curves yielded EC50 of 15.5±0.6 μM for CTL fibroblasts (b) and 14.4±0.8 μM for HF fibroblasts (d), respectively. No significant difference in EC50 was observed (n=6 for each Ca2+ concentration in CTL and HF groups)
Effects of 9-phen on TRPM4 from fibroblasts
To further characterize the properties of TRPM4 in fibroblasts from CTL and HF left ventricular fibroblasts, we compared inhibitory effects of 9-phen on TRPM4. TRPM4 currents were recorded with 100 μM Ca2+ in the pipette solution (Fig. 4). We found that 9-phen inhibited TRPM4 in a concentration-dependent manner, with IC50 of 20.7±2.9 μM and 19.5±5.7 μM, respectively, in CTL and HF left ventricular fibroblasts. Thus, TRPM4 channels from CTL and HF left ventricular fibroblasts have similar sensitivity to 9-phen inhibition.
Fig. 4.
Concentration-dependent effects of 9-phen on TRPM4 currents recorded from CTL and HF fibroblasts. a, c Representative recordings of TRPM4 current elicited by voltage ramp ranging from −120 to +100 mV. Effects of 9-phen at 0.1, 1, 10, 30, and 100 μM were tested in CTL (a) and HF (c) fibroblasts. The effect of 9-phen was reversal. b, d Dose–response curves constructed from normalized current amplitude yielded IC50 of 20.7±2.9 μM for CTL fibroblasts (b) and 19.5 ±3.7 μM for HF fibroblasts (d), respectively. No significant difference in IC50 was observed (n=6 for each group)
Upregulation of TRPM4 expression in heart failure patients
Since the increased currents of TRPM4 are independent on intracellular calcium sensitivity, we investigated the expression level of TRPM4 in CTL and HF patients. As the limited number of fresh isolated or cultured fibroblasts does not provide enough proteins, we used left ventricular tissue from CTL and HF patients for western blot experiments. The frozen left ventricular tissues from the same HF patients or CTL as the current recording were used for protein extraction. As shown in Fig. 5, the protein level of TRPM4 in the HF patients was significantly higher than that of CTL (Fig. 5a, b), consistent with the increased functional change of TRPM4 reflected by current amplitude. Moreover, using RNA extracted from the same batches of CTL and HF fibroblasts used for patch-clamp experiments, we did a relative quantification of mRNA expression levels by qPCR. As shown in Fig. 5c, the mRNA level of TRPM4 in HF fibroblasts is more than three-fold higher than that of CTL fibroblasts.
Fig. 5.
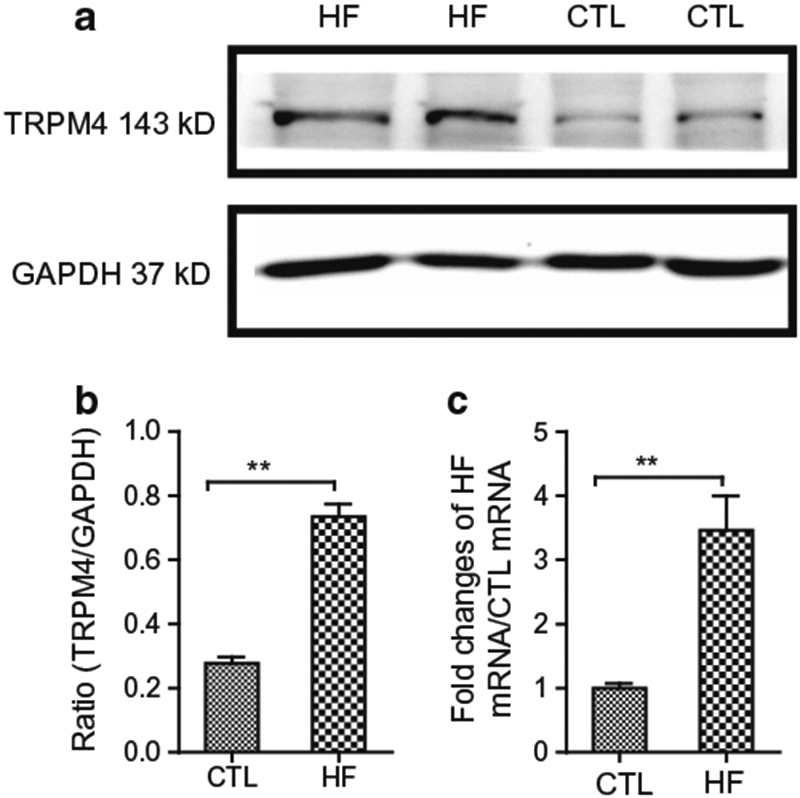
TRPM4 expression detected by western blot (WB) in CTL and HF left ventricle. a WB results of TRPM4 and loading control using GAPDH. b Average ratio of TRPM4 versus GAPDH in CTL and HF hearts. Expression of TRPM4 was significantly bigger in HF hearts than that in CTL hearts (**p<0.01, n=6 for each group). c qPCR quantification of TRPM4 expression levels in fibroblasts of CTL and HF. RNA samples were extracted from the same batches of fibroblasts used for patch-clamp experiments (**p<0.01, n=6 for each group)
Regulation of TRPM4 by TGFβ1
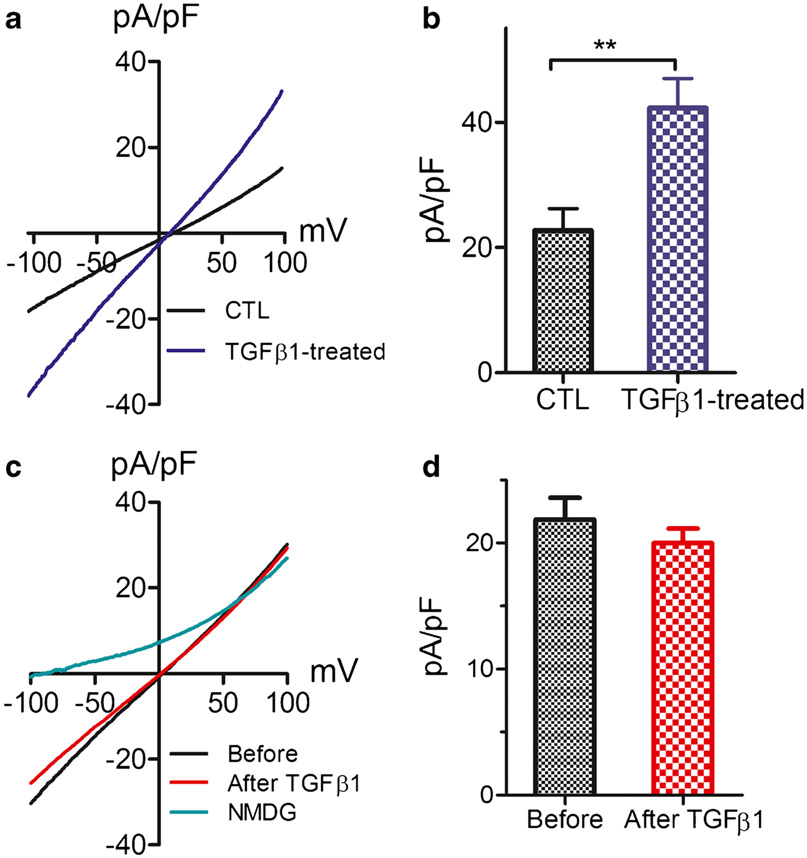
The enhanced TRPM4 currents as well as mRNA and protein expression levels in the HF patients suggest that TRPM4 is upregulated by pathological conditions in vivo in heart failure patients. Among various pathogenesis factors associated with heart failure, cardiac fibrosis plays a detrimental role in heart failure. We therefore investigated whether fibrosis-promoting factor TGFβ1 plays a role in TRPM4 upregulation. Fibroblasts isolated from the CTL left ventricle were cultured and, after serum starving, were treated with 10 ng/ml TGFβ1. Current was recorded 24 h after TGFβ1 treatment. Current density of TRPM4 was increased from 22.7 pA/pF in nontreated fibroblasts to 42.3 pA/pF after TGFβ1 treatment (Fig. 6a, b), indicating that TRPM4 is upregulated by fibrotic conditions. This result is consistent with the upregulated mRNA level of TRPM4 in mouse fibroblasts after treatment with TGFβ1 [17]. Whereas TGFβ1 treatment for 24 h upregulated TRPM4 expression, acute application of TGFβ1 to CTL fibroblasts during patch-clamp experiments did not change the current amplitude (Fig. 6c, d). Thus, it is conceivable that TRPM4 expression is upregulated during the pathogenesis process of heart failure, and the upregulated TRPM4 may have in turn contributed to fibrogenesis in the progression of heart failure.
Fig. 6.
Effect of TGFβ1 on TRPM4 in CTL fibroblasts. a Representative TRPM4 recorded from CTL fibroblasts 24 h after treatment with 10 ng/ml TGFβ1 (blue trace) in comparison with current recorded from nontreated control fibroblasts. b Average current density of TRPM4 in TGFβ1-treated (n=16) and nontreated (n=14) fibroblasts (**p<0.01). c TRPM4 currents recorded from CTL fibroblasts before and after perfusion with 10 ng/ml TGFβ1. NMDG was used to monitor leak current. d Mean current density of TRPM4 before and after TGFβ1 perfusion. No statistical difference was observed (n=8)
Discussion
In this study, we demonstrate an increased current amplitude of TRPM4 in left ventricular fibroblasts from heart failure patients in comparison with CTL individuals. The increased TRPM4 current is not due to the changes of intracellular calcium sensitivity of TRPM4 but is the result of upregulation of the TRPM4 channel protein in HF patients versus CTL individuals. Other properties of TRPM4 channels, such as I–V relation and the sensitivity to extracellular 9-Phen blockade, display no differences between TRPM4 channels recorded in fibroblasts from HF patients and CTL individuals. Moreover, TRPM4 expression in fibroblasts from CTL can be potentiated by TGFβ1, suggesting that TRPM4 may be regulated by fibrogenesis conditions in vivo. Our results of upregulated functional TRPM4 channels in human HF fibroblasts suggest that TRPM4 may play a role in influencing cardiac fibrogenesis cascade, thereby contributing to the pathogenesis of HF.
TRPM4 is a wildly expressed Ca2+-impermeable monovalent cation channel [9, 50, 69]. Long before the cloning of TRPM4 gene, a Ca2+-activated nonselective cation current was recorded in various types of cells including cardiac cells [10, 21, 54]. To date, the TRPM4-like Ca2+-activated nonselective endogenous cation currents have been well characterized in sino-atrial node cells [13], cardiac atrial myocytes [28], Purkinje fiber [34], and ventricular myocytes [29]. The functional TRPM4 expression in the conduction system and cardiac myocytes strongly support the role of TRPM4 in cardiac electrical activity and in the pathogenesis process of hypertrophy and heart failure [27, 41, 73].
In comparison with the functional expression and the known pathophysiological functions of TRPM4 in excitable cells in the heart, little is known about TRPM4 in the cardiac fibroblasts. In cardiac fibroblasts, although mRNA expression of TRPM4 has been detected in human and mouse atrial fibroblasts [17], functional TRPM4 expression in human atrial fibroblasts was not reported until very recently by Simard and colleagues [64]. In the present study, using human ventricular fibroblasts, we demonstrate that TRPM4 is also functionally expressed in human ventricular fibroblasts. We show that the whole cell currents of TRPM4 in CTL and HF exhibit a concentration-dependent activation by intracellular Ca2+ and concentration-dependent block by extracellular application of 9-Phen. More importantly, we found that TRPM4 current density in HF fibroblasts is more than 2-fold bigger than that of CTL fibroblasts.
Our result showing the functional expression of TRPM4 in human ventricular fibroblasts and upregulation by heart failure is the first report to suggest that TRPM4 in cardiac fibroblasts may play a role in the pathogenesis of human heart failure. Consistent with our results of upregulation of TRPM4 in HF fibroblasts, a recent study which thoroughly analyzed the TRP channel in the failing hearts demonstrated that the TRPM4 mRNA level in the left ventricles of a cohort containing 43 patients was 65% higher than that of nonfailing control patients [15]. Given the important role of fibrosis in causing various types of heart diseases, it is plausible that TRPM4 may play a role in fibroblast proliferation and differentiation, thereby contributing to cardiac fibrogenesis. Indeed, in human atrial fibroblasts, Simard and colleagues nicely showed that TRPM4 influences human atrial fibroblast growth [64]. In the present study, we found that TGFβ1, a strong fibrosis-promoting cytokine, increases the TRPM4 current density after a 24-h treatment. In agreement with this result, we previously demonstrated that TGFβ1 treatment enhanced the TRPM4 mRNA level in mouse fibroblasts [17]. The effects of TGFβ1 on increasing TRPM4 expression in the cultured fibroblasts may represent a recapitulation of the in vivo microenvironment where fibroblasts in human ventricles are stimulated by cytokines such as TGFβ1 during the development and progression processes of hypertrophy and heart failure.
TRPM4 is a monovalent cation channel. Unlike the Ca2+-permeable TRP channels [37, 74], such as TRPC3 [31, 55], TRPV4 [59], and TRPM7 [17], which have been shown to play a role in fibrogenesis cascade, it is surprising that the Ca2+-impermeable TRPM4 is highly expressed in cardiac fibroblasts and is drastically upregulated in heart failure patients. How could TRPM4 influence fibroblast biological function and be involved in the fibrogenesis process? TRPM4 conducts inward Na+ current at negative membrane potentials and outward K+ currents at positive membrane potentials under physiological ionic conditions; therefore, TRPM4 can contribute to depolarize fibroblasts as well as hyperpolarize fibroblasts. By depolarizing or hyperpolarizing fibroblasts, TRPM4 can influence Ca2+ signaling mediated by Ca2+-permeable TRP channels, such as TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6, as well as TRPM2 and TRPM7, in human ventricular fibroblasts [15]. Indeed, TRPM4 has been shown to regulate calcium oscillation in different cell types such as T-cells, β-pancreatic cells, and HL-1 cells [5, 43]. Thus, TRPM4 may indirectly influence Ca2+ homeostasis in HF fibroblasts. Alternatively, as the voltage-gated Na+ channel (NaV1.5) only expresses in differentiated myofibroblasts [6], TRPM4 may serve as a depolarization channel in cardiac fibroblasts and therefore contribute to biological function of fibroblasts under normal physiological conditions. Nonetheless, detailed mechanisms by which TRPM4 regulates fibroblast function and fibrosis process need further investigation.
In the present study, we used freshly isolated fibroblasts to evaluate the functional TRPM4 expression level in order to closely reflect the TRPM4 expression level in vivo. However, due to the limited number of isolated fibroblasts, for some experiments, such as western blot, we used left ventricular tissues in order to get enough protein. Moreover, we have only six samples from HF patients and CTL individuals, which did not allow us to investigate whether there is a difference in TRPM4 expression between male and female, as well as whether there is age-dependent difference in TRPM4. Although there are limitations in our study, the discovery in our present study provide novel information about the potential role of TRPM4 in human fibroblasts as well as in human heart failure, and suggest that TRPM4 may serve as a novel target for treating heart failure in patients with cardiac conduction problem.
In summary, our data represent the first report demonstrating the functional expression of TRPM4 in human left ventricular fibroblasts, and the enhanced TRPM4 channel function in the heart failure patients versus control individuals without heart failure. Given the important role of TRPM4 in the cardiac conduction system, our data provide novel insights that targeting on TRPM4 may provide better therapy for heart failure patients complicated with conduction problems.
Funding
This work was partially supported by the National Institute of Health (P01-HL06426, R01-AA024769, and R01-HL146744 to XA; and R01-HL143750 to LY) and American Heart Association (19TPA34890022 to LY).
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- DM
diabetes mellitus
- EF
ejection fraction
- Eth
ethnicity
- Gen
gender
- HF
heart failure
- HLD
hyperlipidemia
- HTN
hypertension
- Med
out-patient medication
- MI
myocardial infarction
- VD
valve diseases
Footnotes
This article is part of the special issue on Calcium Signal Dynamics in Cardiac Myocytes and Fibroblasts: Mechanisms in Pflügers Archiv—European Journal of Physiology
References
- 1.Autzen HE, Myasnikov AG, Campbell MG, Asarnow D, Julius D, Cheng Y (2018) Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359:228–232. 10.1126/science.aar4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbet G, Demion M, Moura IC, Serafini N, Leger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P (2008) The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol 9:1148–1156. 10.1038/ni.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barefield DY, McNamara JW, Lynch TL, Kuster DWD, Govindan S, Haar L, Wang Y, Taylor EN, Lorenz JN, Nieman ML, Zhu G, Luther PK, Varro A, Dobrev D, Ai X, Janssen PML, Kass DA, Jones WK, Gilbert RJ, Sadayappan S (2019) Ablation of the calpain-targeted site in cardiac myosin binding protein-C is cardioprotective during ischemia-reperfusion injury. J Mol Cell Cardiol 129:236–246. 10.1016/j.yjmcc.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi B, Ozhathil LC, Medeiros-Domingo A, Gollob MH, Abriel H (2018) Four TRPM4 cation channel mutations found in cardiac conduction diseases lead to altered protein stability. Front Physiol 9:177. 10.3389/fphys.2018.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt R, Graves BM, Gao M, Li C, Williams DL, Fregoso SP, Hoover DB, Li Y, Wright GL, Wondergem R (2013) 9-Phenanthrol and flufenamic acid inhibit calcium oscillations in HL-1 mouse cardiomyocytes. Cell Calcium 54:193–201. 10.1016/j.ceca.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatelier A, Mercier A, Tremblier B, Theriault O, Moubarak M, Benamer N, Corbi P, Bois P, Chahine M, Faivre JF (2012) A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol 590:4307–4319. 10.1113/jphysiol.2012.233593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Gao Y, Wei S, Low SW, Ng G, Yu D, Tu TM, Soong TW, Nilius B, Liao P (2019) TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Arch Eur J Physiol 471:1455–1466. 10.1007/s00424-019-02326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R (2007) TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 41:51–61. 10.1016/j.ceca.2006.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapham DE (2003) TRP channels as cellular sensors. Nature 426: 517–524 [DOI] [PubMed] [Google Scholar]
- 10.Colquhoun D, Neher E, Reuter H, Stevens CF (1981) Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature 294:752–754 [DOI] [PubMed] [Google Scholar]
- 11.Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S (2010) Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol 299:C682–C694. 10.1152/ajpcell.00101.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daumy X, Amarouch MY, Lindenbaum P, Bonnaud S, Charpentier E, Bianchi B, Nafzger S, Baron E, Fouchard S, Thollet A, Kyndt F, Barc J, Le Scouarnec S, Makita N, Le Marec H, Dina C, Gourraud JB, Probst V, Abriel H, Redon R, Schott JJ (2016) Targeted resequencing identifies TRPM4 as a major gene predisposing to progressive familial heart block type I. Int J Cardiol 207:349–358. 10.1016/j.ijcard.2016.01.052 [DOI] [PubMed] [Google Scholar]
- 13.Demion M, Bois P, Launay P, Guinamard R (2007) TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73:531–538. 10.1016/j.cardiores.2006.11.023 [DOI] [PubMed] [Google Scholar]
- 14.Demion M, Thireau J, Gueffier M, Finan A, Khoueiry Z, Cassan C, Serafini N, Aimond F, Granier M, Pasquie JL, Launay P, Richard S (2014) Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS One 9:e115256. 10.1371/journal.pone.0115256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragun M, Gazova A, Kyselovic J, Hulman M, Matus M (2019) TRP channels expression profile in human end-stage heart failure. Medicina 55. 10.3390/medicina55070380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Xie J, Yue L (2009) Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci 107:7239–7244. 10.1073/pnas.0811725106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L (2010) TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106:992–1003. 10.1161/CIRCRESAHA.109.206771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan J, Li Z, Li J, Santa-Cruz A, Sanchez-Martinez S, Zhang J, Clapham DE (2018) Structure of full-length human TRPM4. Proc Natl Acad Sci U S A 115:2377–2382. 10.1073/pnas.1722038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earley S (2013) TRPM4 channels in smooth muscle function. Arch Eur J Physiol 465:1223–1231. 10.1007/s00424-013-1250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earley S, Waldron BJ, Brayden JE (2004) Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95:922–929. 10.1161/01.RES.0000147311.54833.03 [DOI] [PubMed] [Google Scholar]
- 21.Ehara T, Noma A, Ono K (1988) Calcium-activated non-selective cation channel in ventricular cells isolated from adult guinea-pig hearts. J Physiol 403:117–133. 10.1113/jphysiol.1988.sp017242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng J, Armillei MK, Yu AS, Liang BT, Runnels LW, Yue L (2019) Ca(2+) signaling in cardiac fibroblasts and fibrosis-associated heart diseases. Journal of cardiovascular development and disease 6. 10.3390/jcdd6040034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26: 159–178. 10.1080/10799890600637506 [DOI] [PubMed] [Google Scholar]
- 24.Frede W, Medert R, Poth T, Gorenflo M, Vennekens R, Freichel M, Uhl S (2020) TRPM4 modulates right ventricular remodeling under pressure load accompanied with decreased expression level. J Card Fail 26:599–609. 10.1016/j.cardfail.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R (2008) 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol 153:1697–1705. 10.1038/bjp.2008.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gualandi F, Zaraket F, Malagu M, Parmeggiani G, Trabanelli C, Fini S, Dang X, Wei X, Fang M, Bertini M, Ferrari R, Ferlini A (2017) Mutation load of multiple ion channel gene mutations in Brugada syndrome. Cardiology 137:256–260. 10.1159/000471792 [DOI] [PubMed] [Google Scholar]
- 27.Guinamard R, Bouvagnet P, Hof T, Liu H, Simard C, Salle L (2015) TRPM4 in cardiac electrical activity. Cardiovasc Res 108: 21–30. 10.1093/cvr/cvv213 [DOI] [PubMed] [Google Scholar]
- 28.Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P (2004) Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol 558:75–83. 10.1113/jphysiol.2004.063974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinamard R, Demion M, Magaud C, Potreau D, Bois P (2006) Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 48:587–594. 10.1161/01.HYP.0000237864.65019.a5 [DOI] [PubMed] [Google Scholar]
- 30.Guo J, She J, Zeng W, Chen Q, Bai XC, Jiang Y (2017) Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552:205–209. 10.1038/nature24997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, Ledoux J, Kato T, Naud P, Voigt N, Shi Y, Kamiya K, Murohara T, Kodama I, Tardif JC, Schotten U, Van Wagoner DR, Dobrev D, Nattel S (2012) Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126:2051–2064. 10.1161/CIRCULATIONAHA.112.121830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedon C, Lambert K, Chakouri N, Thireau J, Aimond F, Cassan C, Bideaux P, Richard S, Faucherre A, Le Guennec JY, Demion M (2020) New role of TRPM4 channel in the cardiac excitation-contraction coupling in response to physiological and pathological hypertrophy in mouse. Prog Biophys Mol Biol. 10.1016/j.pbiomolbio.2020.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Hof T, Chaigne S, Recalde A, Salle L, Brette F, Guinamard R (2019) Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol 16:344–360. 10.1038/s41569-018-0145-2 [DOI] [PubMed] [Google Scholar]
- 34.Hof T, Salle L, Coulbault L, Richer R, Alexandre J, Rouet R, Manrique A, Guinamard R (2016) TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres. J Physiol 594:295–306. 10.1113/JP271347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Current biology : CB 13:1153–1158 [DOI] [PubMed] [Google Scholar]
- 36.Holzmann C, Kappel S, Kilch T, Jochum MM, Urban SK, Jung V, Stockle M, Rother K, Greiner M, Peinelt C (2015) Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget 6:41783–41793. doi: 10.18632/oncotarget.6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue R, Kurahara LH, Hiraishi K (2018) TRP channels in cardiac and intestinal fibrosis. Semin Cell Dev Biol. 10.1016/j.semcdb.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 38.Jacobs G, Oosterlinck W, Dresselaers T, Geenens R, Kerselaers S, Himmelreich U, Herijgers P, Vennekens R (2015) Enhanced beta-adrenergic cardiac reserve in Trpm4(−)/(−) mice with ischaemic heart failure. Cardiovasc Res 105:330–339. 10.1093/cvr/cvv009 [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Li M, Yue L (2005) Potentiation of TRPM7 inward currents by protons. J Gen Physiol 126:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kecskes M, Jacobs G, Kerselaers S, Syam N, Menigoz A, Vangheluwe P, Freichel M, Flockerzi V, Voets T, Vennekens R (2015) The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy. Basic Res Cardiol 110:43. 10.1007/s00395-015-0501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruse M, Pongs O (2014) TRPM4 channels in the cardiovascular system. Curr Opin Pharmacol 15:68–73. 10.1016/j.coph.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 42.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O (2009) Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119:2737–2744. 10.1172/JCI38292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet J-P (2004) TRPM4 Regulates calcium oscillations after T cell activation. Science 306:1374–1377. 10.1126/science.1098845 [DOI] [PubMed] [Google Scholar]
- 44.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407 [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Chatel S, Simard C, Syam N, Salle L, Probst V, Morel J, Millat G, Lopez M, Abriel H, Schott JJ, Guinamard R, Bouvagnet P (2013) Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS One 8:e54131. 10.1371/journal.pone.0054131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P (2010) Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3:374–385. 10.1161/CIRCGENETICS.109.930867 [DOI] [PubMed] [Google Scholar]
- 47.Mathar I, Kecskes M, Van der Mieren G, Jacobs G, Camacho Londono JE, Uhl S, Flockerzi V, Voets T, Freichel M, Nilius B, Herijgers P, Vennekens R (2014) Increased beta-adrenergic inotropy in ventricular myocardium from Trpm4−/− mice. Circ Res 114:283–294. 10.1161/CIRCRESAHA.114.302835 [DOI] [PubMed] [Google Scholar]
- 48.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M (2010) Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest 120:3267–3279. 10.1172/JCI41348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montell C (2005) The TRP superfamily of cation channels. Sci STKE 2005:1–24 [DOI] [PubMed] [Google Scholar]
- 50.Nilius B (2007) TRP channels in disease. Biochim Biophys Acta 1772:805–812 [DOI] [PubMed] [Google Scholar]
- 51.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278:30813–30820 [DOI] [PubMed] [Google Scholar]
- 52.Nilius B, Prenen J, Janssens A, Voets T, Droogmans G (2004) Decavanadate modulates gating of TRPM4 cation channels. J Physiol 560:753–765. 10.1113/jphysiol.2004.070839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilius B, Prenen J, Voets T, Droogmans G (2004) Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Arch Eur J Physiol 448:70–75. 10.1007/s00424-003-1221-x [DOI] [PubMed] [Google Scholar]
- 54.Nilius B, Vennekens R (2006) From cardiac cation channels to the molecular dissection of the transient receptor potential channel TRPM4. Arch Eur J Physiol 453:313–321. 10.1007/s00424-006-0088-z [DOI] [PubMed] [Google Scholar]
- 55.Numaga-Tomita T, Oda S, Shimauchi T, Nishimura A, Mangmool S, Nishida M (2017) TRPC3 channels in cardiac fibrosis. Frontiers in cardiovascular medicine 4:56. 10.3389/fcvm.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176 [DOI] [PubMed] [Google Scholar]
- 57.Piao H, Takahashi K, Yamaguchi Y, Wang C, Liu K, Naruse K (2015) Transient receptor potential melastatin-4 is involved in hypoxia-reoxygenation injury in the cardiomyocytes. PLoS One 10:e0121703. 10.1371/journal.pone.0121703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pironet A, Syam N, Vandewiele F, Van den Haute C, Kerselaers S, Pinto S, Vande Velde G, Gijsbers R, Vennekens R (2019) AAV9-mediated overexpression of TRPM4 increases the incidence of stress-induced ventricular arrhythmias in mice. Front Physiol 10: 802. 10.3389/fphys.2019.00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, Moran MM, Schilling WP, Tschumperlin DJ, Olman MA (2014) TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 124:5225–5238. 10.1172/JCI75331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, Flockerzi V, Bruck W, Pongs O, Vennekens R, Kneussel M, Freichel M, Merkler D, Friese MA (2012) TRPM4 cation channel mediates axonal and neuronal degeneration in experimental auto-immune encephalomyelitis and multiple sclerosis. Nat Med 18: 1805–1811. 10.1038/nm.3015 [DOI] [PubMed] [Google Scholar]
- 61.Serafini N, Dahdah A, Barbet G, Demion M, Attout T, Gautier G, Arcos-Fajardo M, Souchet H, Jouvin MH, Vrtovsnik F, Kinet JP, Benhamou M, Monteiro RC, Launay P (2012) The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J Immunol 189:3689–3699. 10.4049/jimmunol.1102969 [DOI] [PubMed] [Google Scholar]
- 62.Shigeto M, Ramracheya R, Tarasov AI, Cha CY, Chibalina MV, Hastoy B, Philippaert K, Reinbothe T, Rorsman N, Salehi A, Sones WR, Vergari E, Weston C, Gorelik J, Katsura M, Nikolaev VO, Vennekens R, Zaccolo M, Galione A, Johnson PR, Kaku K, Ladds G, Rorsman P (2015) GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest 125:4714–4728. 10.1172/JCI81975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simard C, Hof T, Keddache Z, Launay P, Guinamard R (2013) The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J Mol Cell Cardiol 59:11–19. 10.1016/j.yjmcc.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 64.Simard C, Magaud C, Adjlane R, Dupas Q, Salle L, Manrique A, Bois P, Faivre JF, Guinamard R (2020) TRPM4 non-selective cation channel in human atrial fibroblast growth. Arch Eur J Physiol. 10.1007/s00424-020-02476-0 [DOI] [PubMed] [Google Scholar]
- 65.Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hebert JL, Ferrer X, Maugenre S, Schmitz W, Kirchhefer U, Schulze-Bahr E, Guicheney P, Schulze-Bahr E (2012) Mutational spectrum in the Ca(2+)–activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 33:109–117. 10.1002/humu.21599 [DOI] [PubMed] [Google Scholar]
- 66.Syam N, Chatel S, Ozhathil LC, Sottas V, Rougier JS, Baruteau A, Baron E, Amarouch MY, Daumy X, Probst V, Schott JJ, Abriel H (2016) Variants of transient receptor potential melastatin member 4 in childhood atrioventricular block. J Am Heart Assoc 5. 10.1161/JAHA.114.001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syam N, Rougier JS, Abriel H (2014) Glycosylation of TRPM4 and TRPM5 channels: molecular determinants and functional aspects. Front Cell Neurosci 8:52. 10.3389/fncel.2014.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M (2007) Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8:312–320. 10.1038/ni1441 [DOI] [PubMed] [Google Scholar]
- 69.Wang C, Naruse K, Takahashi K (2018) Role of the TRPM4 channel in cardiovascular physiology and pathophysiology. Cells 7. 10.3390/cells7060062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winkler PA, Huang Y, Sun W, Du J, Lu W (2017) Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552: 200–204. 10.1038/nature24674 [DOI] [PubMed] [Google Scholar]
- 71.Yan J, Bengtson CP, Buchthal B, Hagenston AM, Bading H (2020) Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 370. 10.1126/science.aay3302 [DOI] [PubMed] [Google Scholar]
- 72.Yue L, Feng J, Wang Z, Nattel S (2000) Effects of ambasilide, quinidine, flecainide and verapamil on ultra-rapid delayed rectifier potassium currents in canine atrial myocytes. Cardiovasc Res 46: 151–161 [DOI] [PubMed] [Google Scholar]
- 73.Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L (2015) Role of TRP channels in the cardiovascular system. Am J Phys Heart Circ Phys 308:H157–H182. 10.1152/ajpheart.00457.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yue Z, Zhang Y, Xie J, Jiang J, Yue L (2013) Transient receptor potential (TRP) channels and cardiac fibrosis. Curr Top Med Chem 13:270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Okawa H, Wang Y, Liman ER (2005) Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280:39185–39192. 10.1074/jbc.M506965200 [DOI] [PubMed] [Google Scholar]