Abstract
Previous studies have found GABA in vestibular end organs. However, existence of GABA receptors or possible GABAergic effects on vestibular nerve afferents has not been investigated. The current study was conducted to determine whether activation of GABAB receptors affects calyx afferent terminals in the central region of the cristae of semicircular canals. We used patch-clamp recording in postnatal day 13–18 (P13–P18) Sprague-Dawley rats of either sex. Application of GABAB receptor agonist baclofen inhibited voltage-sensitive potassium currents. This effect was blocked by selective GABAB receptor antagonist CGP 35348. Application of antagonists of small (SK)- and large-conductance potassium (BK) channels almost completely blocked the effects of baclofen. The remaining baclofen effect was blocked by cadmium chloride, suggesting that it could be due to inhibition of voltage-gated calcium channels. Furthermore, baclofen had no effect in the absence of calcium in the extracellular fluid. Inhibition of potassium currents by GABAB activation resulted in an excitatory effect on calyx terminal action potential firing. While in the control condition calyces could only fire a single action potential during step depolarizations, in the presence of baclofen they fired continuously during steps and a few even showed repetitive discharge. We also found a decrease in threshold for action potential generation and a decrease in first-spike latency during step depolarization. These results provide the first evidence for the presence of GABAB receptors on calyx terminals, showing that their activation results in an excitatory effect and that GABA inputs could be used to modulate calyx response properties.
NEW & NOTEWORTHY Using in vitro whole cell patch-clamp recordings from calyx terminals in the vestibular end organs, we show that activation of GABAB receptors result in an excitatory effect, with decreased spike-frequency adaptation and shortened first-spike latencies. Our results suggest that these effects are mediated through inhibition of calcium-sensitive potassium channels.
Keywords: calcium-sensitive potassium channel, efferent, GABAB, supporting cell
INTRODUCTION
Vestibular end organs in the inner ear provide inputs to the vestibular nuclei in the brain stem through afferent vestibular nerve fibers. This information is used to provide vestibular reflexes that stabilize the gaze (the vestibulo-ocular reflex, VOR) and posture (the vestibulospinal reflexes) during daily activities. Particularly, afferents with more irregular resting discharge are shown to have high-pass properties and carry information about fast head movements. The most irregular afferents receive inputs from type I hair cells through a specialized nerve terminal that covers the basolateral walls of the hair cell and are located in the central region of the cristae of the semicircular canals or maculae of the otolithic organs (reviewed in Goldberg 2000). In contrast, afferents with highly regular resting discharges receive inputs from type II hair cells through bouton terminals and are located in the most peripheral regions of the neuroepithelia. The majority of the afferents receive inputs from both type I and type II hair cells through calyx and bouton terminals, respectively. These “dimorphic” afferents are located throughout the neuroepithelium. However, dimorphic afferents that innervate the peripheral zones of the end organs tend to have a more regular resting discharge, and those innervating the central zones are more irregular (Goldberg et al. 1990; Lysakowski et al. 1995).
Vestibular afferents carry signals to the vestibular nuclei in the brain stem, and in turn, an efferent pathway carries signals from the brain stem back to the end organs. In mammals, efferent neurons receive inputs from the reticular formation (Metts et al. 2006) and mainly from the vestibular nuclei (Plotnik et al. 2002; Sadeghi et al. 2009). Efferent fibers project bilaterally to innervate afferent fibers and processes (including calyx terminals) and type II hair cells (Lysakowski and Goldberg 2008). Interestingly, the effect of efferents on afferents is directly related to their resting discharge regularity, with the largest effects observed on the most irregular fibers (Goldberg and Fernández 1980; Marlinski et al. 2004; Raghu et al. 2019; Sadeghi et al. 2009). In vivo studies have shown that efferent stimulation results in an increase in resting discharge and a decrease in sensitivity of afferents (Goldberg and Fernández 1980; Highstein and Baker 1985). This has been suggested to play a role in avoiding saturation of responses during fast self-generated head movements in toadfish (Highstein and Baker 1985), but not in primates (Cullen and Minor 2002; Sadeghi et al. 2007). Efferents have been shown to play a role in normal development of the vestibulo-ocular reflex (Hübner et al. 2015; Luebke et al. 2014). It has also been suggested that following unilateral labyrinthectomy, efferents play a role in shifting the distribution of afferents toward those with more irregular resting discharges and high-pass properties as a compensatory mechanism on the contralesional side (Sadeghi et al. 2007) and play a role in normal compensation (Hübner et al. 2017).
The above-mentioned studies have challenged the traditional idea that the vestibular periphery is a sensor with fixed properties, and recent studies have shown that the properties of hair cells and calyx terminals can be modified. Nicotinic (nAChR) and muscarinic acetylcholine receptors (mAChR) are present on type II hair cells and calyx afferent terminals, and their activation results in changes in response properties of these cells (Holt et al. 2015, 2017; Kong et al. 2005, 2006; Lee et al. 2017; Li et al. 2007; Parks et al. 2017; Poppi et al. 2018, 2020; Yu et al. 2020). In addition to changing the gain of the response (e.g., type II hair cell inhibition; Parks et al. 2017; Poppi et al. 2018; Yu et al. 2020), cholinergic inputs can inhibit potassium channels in afferents and affect their membrane properties (Holt et al. 2017; Pérez et al. 2009; Ramakrishna et al. 2020) and regularity of resting discharge (Kalluri et al. 2010).
The results of studies on γ-amino butyric acid (GABA) in the vestibular periphery are less conclusive. Some of the older studies suggested GABA as a neurotransmitter in the afferent pathway (Flock and Lam 1974), while others contradicted these results (Annoni et al. 1984). In mammals, GABA has been shown in efferent fibers (Kong et al. 1998a, 1998b; Matsubara et al. 1995; Schrott-Fischer et al. 2007) and a recent study has found glutamic acid decarboxylase 67 (GAD67), which is required for generation of GABA, and GABA itself in supporting cells in mice vestibular end organs (Tavazzani et al. 2014). Another study in mice has found GAD2 in supporting cells and mainly hair cells in mice (Holman et al. 2019). While the presence of GAD2 does not necessarily mean that GABA is produced, it indicates the possibility of having GABA in these cells. In most brain areas and sensory organs, GABA has an inhibitory effect. Typically, activation of ionotropic GABAA receptors result in activation of an inward chloride current and hyperpolarization of cells. In the cochlea, it has been shown that the presence of GABAA receptors is required for long-term maintenance of hair cells and neurons (Maison et al. 2006). Metabotropic GABAB receptors are also known to provide inhibitory effects in most brain areas through pre- and postsynaptic effects (reviewed in Kantamneni 2016). A common presynaptic mechanism for the inhibitory effect of GABAB receptor activation is inhibition of voltage-gated calcium channels and a resultant decrease in vesicular release. In the cochlea, GABAB is expressed by efferent terminals and plays a role in presynaptic inhibition of acetylcholine release from efferents (Wedemeyer et al. 2013). Postsynaptic GABAB receptors typically induce inhibitory effects through G protein-mediated pathways that activate GIRK and TREK-2 potassium channels, resulting in outward potassium currents and hyperpolarization of cells. Two exceptions have been reported where postsynaptic GABAB activation results in an excitatory effect in the retina and substantia nigra. In the retina, this is mediated through a decrease in the activity of calcium channels, resulting in a decrease in large-conductance potassium (BK) channel activity (Garaycochea and Slaughter 2016). In substantia nigra, GABAB activation directly decreases the activity of small-conductance potassium (SK) channels, through a cAMP/PKA-mediated pathway (Estep et al. 2016). Interestingly, a previous study has also found excitatory GABAergic effects on vestibular afferents (Vega et al. 1987).
Previous studies have also shown the existence of different potassium channels in the vestibular periphery, including inward rectifier K+ (Kir) channels (Udagawa et al. 2012), SK channels (Meredith et al. 2011), and BK channels (Limón et al. 2005; Schweizer et al. 2009), which could be affected by GABAB activation. A previous study has shown that activation of GABAB receptors in type I hair cells results in a decrease in calcium conductances and calcium-dependent potassium conductances (Lapeyre et al. 1993). Here, we used whole cell patch-clamp recording to investigate the presence of GABAB receptors on vestibular afferent calyx terminals and whether they affected the activity of any of the potassium channels. We found that almost all calyces showed a GABAB-mediated response that consisted of a decrease in outward voltage-sensitive potassium currents. GABAB agonist (baclofen) application affected membrane properties to decrease spike-frequency adaptation: calyx terminals that showed a single spike firing at the beginning of a step depolarization would fire many action potentials at a lower threshold in the presence of baclofen. Furthermore, the first-spike latency decreased at the beginning of step depolarizations. We provide evidence that this effect is mediated through inhibition of BK and SK channels. These results provide the first evidence for the presence of GABAB receptors on calyx terminals with an unusual excitatory effect and show that the response properties of calyx terminals can be modified by GABA.
MATERIALS AND METHODS
Animals.
Seventy two Sprague-Dawley rats (Charles River Laboratories), 13–18 days old and of either sex, were used for the experiments. All animals were handled in accordance with animal protocols approved by the Institutional Animal Care and Use Committee at the University at Buffalo and carried out in accordance with NIH guidelines.
Tissue preparation.
Dissection of the end organ and tissue preparation were performed as previously described (Ramakrishna et al. 2020; Sadeghi et al. 2014; Songer and Eatock 2013). Briefly, rats were decapitated after being deeply anesthetized by isoflurane inhalation. The skull was opened in the midline, and the bony labyrinth was removed and placed in extracellular solution. Under the microscope, the ampullae of the horizontal and superior canals were opened. Attachments to bone were carefully dissected, and the membranous labyrinth containing the cristae of the horizontal canal, anterior canal, and the macula of the utricle were taken out of the bone. The membranous labyrinth was opened over the two cristae and the utricle. The preparation was secured on a coverslip under a pin, transferred to the recording chamber, and perfused with extracellular solution at 1.5–3 mL/min.
Electrophysiology recording.
The extracellular solution contained (in mM) 5.8 KCl, 144 NaCl, 0.9 MgCl2, 1.3 CaCl2, 0.7 NaH2PO4, 5.6 glucose, and 10 HEPES, 300 mosmol/kgH2O, pH 7.4 (NaOH). The intracellular solution contained (in mM) 20 KCl, 110 K-methanesulfonate, 0.1 CaCl2, 5 EGTA, 5 HEPES, 5 Na2-phosphocreatine, 4 MgATP, and 0.3 Tris-GTP, 290 mosmol/kgH2O, pH 7.2 (KOH). To perform patch-clamp recording, tissue was visualized with a ×40 water-immersion objective and differential interference contrast (DIC) optics (Examiner D1 Zeiss microscope), and viewed on a monitor via a video camera (optiMOS, Qimaging). To make patch-clamp recording pipettes, borosilicate glass (1-mm inner diameter, 1B100F-4; WPI) was pulled with a multistep horizontal puller (P-1000; Sutter), coated with Sylgard (Dow Corning), and fire polished. Pipette resistances were 5–10 MΩ. All recordings were performed at room temperature (~23°C). Application of drug solutions was performed (VC-6 channel valve controller; Warner Instruments, Hamden, CT) using a gravity-driven flow pipette (~100-μm tip diameter) placed at ~45° angle with the top of the crista near the area where a calyx was recorded from. The above solutions result in a liquid junction potential of −9 mV, which was corrected offline. Drugs were dissolved daily in the extracellular solution to their final concentrations from frozen stocks. R-baclofen (100 µM), CGP 35348 (300 µM), iberiotoxin (IBTX; 150 nM), apamin (Apa; 300 nM), cadmium chloride (CdCl2; 0.5–1 mM), and XE-991 (10 µM) were purchased from Tocris Bioscience. Barium chloride (BaCl2; 100 µM) was purchased from Sigma.
Experiment protocol.
Whole cell patch-clamp recordings were performed from calyx terminals in the central region of the cristae of anterior or horizontal canals. All measurements were acquired using Multiclamp 700B amplifier (Molecular Devices), digitized at 50 kHz with Digidata 1440A, and filtered at 10 kHz by pCLAMP10.7 software. Calyces innervated type I hair cells with typical morphology. Membrane capacitance and series resistance were electronically compensated during recordings (correction and prediction circuits set to 75–80% with a bandwidth of 10–15 kHz). Cells were held at a holding potential of −70 mV, resulting in a holding current of <200 pA. Leak subtraction was not used. To minimize time-dependent changes (Hurley et al. 2006), all data were collected starting ~10 min after the membrane was ruptured. Since the effect of drugs increased with duration of application for the first ~10 min, to avoid time-dependent changes in drug effects, all data were collected 9–12 min after start of application (Ramakrishna et al. 2020). All drug effects were reversible.
During voltage-clamp recordings, a voltage step protocol was used to study calyx properties (Fig. 1A), which consisted of an initial holding potential of −79 mV (100 ms), a hyperpolarizing step to −129 mV (100 ms), 20-mV steps between −129 and +11 mV (300 ms), and return to holding potential of −79 mV. Depolarizing steps resulted in initial large Na+ inward currents and slower voltage-sensitive outward (potassium) currents as previously described (Horwitz et al. 2014; Meredith and Rennie 2018; Ramakrishna et al. 2020; Sadeghi et al. 2014; Songer and Eatock 2013). To study the effect of various drugs on responses to the voltage step protocol, current amplitudes were averaged for the final 100 ms of steps. Peak amplitude of tail currents was measured after the return to −79 mV at the end of steps and was plotted against holding potential at the preceding step (denoted as “activation step” on x-axis in figures).
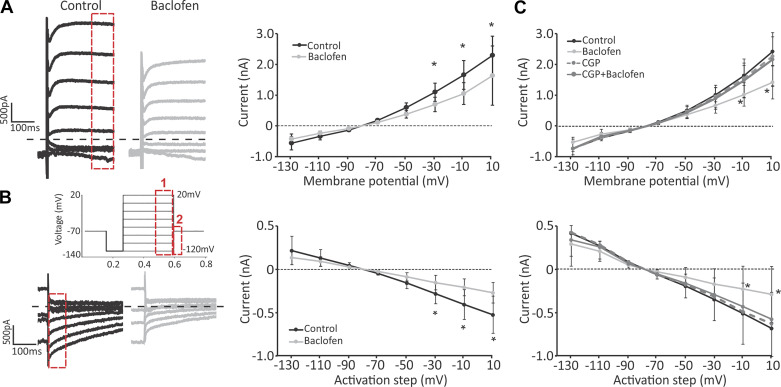
Fig. 1.
Activation of GABAB receptors attenuates voltage‐activated currents. A: response of a calyx terminal to the voltage step protocol (20-mV steps between −129 mV and +11 mV) before (black) and after application of baclofen (gray). Calyx recording exhibited delayed rectifier type outward currents during depolarizing voltage steps (left; red box 1). Average responses (right) show a significant decrease in voltage-sensitive currents at the 3 most depolarized steps (marked by asterisks). Note, in all panels, changes in average currents are presented relative to the current at the initial holding potential of −79 mV. B: the amplitude of tail currents after the steps decreased during baclofen application in an example recording (left; red box 2). Average tail currents (right) significantly decreased after the 3 largest depolarization steps (marked by asterisks). C: application of CGP 35348 (CGP; antagonist of GABAB receptors) blocked the effect of baclofen, confirming that the effect is mediated through GABAB receptors. All traces are from the same calyces recorded in the presence of different drug combinations.
During current-clamp recordings, we first measured the resting membrane potential (i.e., 0-pA current injection). A current was then injected to hold the cells at −79 mV (for 5–10 s), and then current steps of 100 pA, 200 pA, 300 pA, 400 pA, and 500 pA (2-s duration) were injected with 3–5 s between different steps. None of the recorded calyces in the present study had spontaneous firing. The smallest of the five steps that generated an action potential (AP) was considered as the AP generation threshold. The latency of the first spike was measured as the time from the beginning of the step to the peak of first AP for the threshold step. Finally, the number of APs during the steps was also counted. Results were compared before and after application of drugs.
Data analysis.
Clampfit 10 (Molecular Devices), Prism (GraphPad), and MATLAB (The MathWorks) software were used for analysis of data. Results are reported as means ± SD. Paired t test was used for comparison between two parameters, and repeated measures two-way ANOVA with Bonferroni or Tukey post hoc test was used for comparisons of more than two conditions. Level of statistical significance was set at α = 0.05.
RESULTS
Recordings were performed from the central zone of the crista of the horizontal or superior canals at postnatal days 13–18 so that type I hair cells and their calyx afferent terminals had acquired their characteristic morphological and electrophysiological properties (Favre and Sans 1979; Hurley et al. 2006; Lysakowski and Goldberg 2008; Rüsch et al. 1998). Patch-clamp recordings were performed from calyx terminals (n = 73) as previously described (Ramakrishna et al. 2020; Sadeghi et al. 2014). The average resting membrane potential of calyces during whole cell recordings was −63.2 ± 13.6 mV (n = 73), similar to that reported previously in mammals (Lim et al. 2014; Ramakrishna et al. 2020; Sadeghi et al. 2014; Songer and Eatock 2013). Voltage step protocols were used to investigate voltage-dependent changes in membrane currents (Fig. 1A). The effect of drugs were measured on currents at the last 100 ms of step depolarizations (Fig. 1A, red box 1) and on tail currents (Fig. 1B, red box 2). During depolarizing steps, calyces displayed outward currents mainly due to activation of delayed rectifier type currents by KCNQ and ether-a-go-go-related gene (erg) channels (Hurley et al. 2006; Lysakowski et al. 2011), calcium-sensitive SK channels (Meredith et al. 2011), and probably voltage-sensitive BK channels (Limón et al. 2005). After the depolarizing step, a tail current was observed (Contini et al. 2017; Lim et al. 2011).
GABAB activation suppressed voltage-gated currents.
To investigate whether GABAB receptors are present on the calyx terminal and their possible effect on membrane properties of calyces, we applied R-baclofen, an agonist of GABAB receptors, during calyx recordings. Baclofen application (100 µM) did not affect the resting membrane potential (control, −67.2 ± 1.4 mV; baclofen, −63.0 ± 8.1 mV; paired t test, P = 0.58), but resulted in a decrease in voltage-dependent currents during depolarizing steps in every recorded calyx (n = 15), suggesting the presence of GABAB receptors on these afferent terminals (Fig. 1A) (response to the largest depolarizing step of +11 mV: control response, 2,466.3 ± 183.0 pA; baclofen, 1,633.0 ± 305.0 pA after baclofen application). For the population of recorded calyx terminals, application of baclofen resulted in an inhibition of currents for the more depolarized voltage steps greater than −29 mV (repeated measures ANOVA, P < 0.0001; Bonferroni post hoc test, P < 0.001 for −29 mV, −9 mV, and +11 mV). As expected, tail currents were also suppressed during baclofen application following depolarizing steps larger than −29 mV (repeated measures ANOVA, P = 0.0001; Bonferroni post hoc test, P < 0.01 for −29 mV and P < 0.001 for −9 mV and +11 mV).
The above-described changes were completely blocked in the presence of CGP 35348 (300 µM), a GABAB antagonist. Figure 1C shows the average response for four calyx recordings in which voltage-sensitive currents were inhibited with baclofen application (repeated measures ANOVA, P < 0.001 for −9 mV and +11 mV steps). After baclofen was washed, CGP 35348 was applied, which by itself had no effect on the currents in the same calyces (repeated measures ANOVA, P > 0.05 for all steps). This was then followed by application of a combination of CGP 35348 with baclofen, which showed no effect (repeated measures ANOVA, P > 0.05 for all steps). These results strongly suggest that calyx terminals (at least in the central region of the cristae) have functional GABAB receptors.
Kir channels are not affected by GABAB activation in calyx terminals.
Previous studies have shown that GABAB activation in different neurons and glial cells typically increases inward rectifying potassium (Kir) currents (O’Callaghan et al. 1996, Isomoto et al. 1997), including those mediated by Kir4.1 channels (Takeda et al. 2015). Kir4.1 channels are also expressed in calyx terminals (Udagawa et al. 2012), and it has also been proposed that, similarly to glial cells, they play a role in clearance of K+ from the closed synaptic space between type I hair cells and calyx terminals. Despite baclofen having a clear inhibitory effect on potassium currents at depolarized membrane potentials in the above-described experiments, we still tested whether GABAB has any possible effect on Kir channel activity in the calyx. Voltage steps similar to those shown in Fig. 1 were used before and after baclofen application in the absence and presence of barium chloride (BaCl2; 100 µM), a voltage-independent blocker of Kir channels (O’Callaghan et al. 1996). Addition of baclofen decreased the currents by ~38% in response to the more depolarized steps with or without BaCl2 (e.g., for +11 mV, control currents were 1,977 ± 377 pA, decreased to 1,217 ± 247 pA after baclofen application, and stayed at 1,249 ± 344 pA after addition of BaCl2) (n = 6, repeated measures ANOVA, Bonferroni multiple comparison test; control vs. baclofen: P < 0.01 for −29 mV and P < 0.001for −9 mV and +11 mV; control vs. baclofen + BaCl2: P < 0.01 for −29 mV and P < 0.001for −9 mV and +11 mV; baclofen vs. baclofen + BaCl2: P > 0.05 for all steps), suggesting that Kir channels were not affected by GABAB activation (data not shown).
GABAB inhibits Ca2+-sensitive K+ channels.
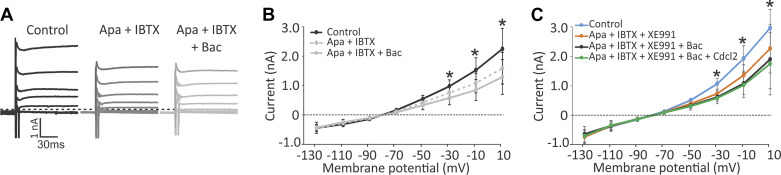
Two recent studies have shown GABAB-mediated inhibition of BK (large current) potassium channels in the retinal ganglion cells (Garaycochea and Slaughter 2016) and SK (small current) channels in the substantia nigra (Estep et al. 2016). Since calyx terminals also express SK (Meredith et al. 2011) and probably BK (Limón et al. 2005) channels, we studied whether a similar GABAB-mediated inhibition was also present in the calyx terminal. We first used a combination of antagonists for SK channel (apamin, 300 nM) and BK channel (iberiotoxin, 150 nM). As expected, the outward voltage-dependent currents (Fig. 2A) and tail currents (not shown) were both inhibited in the presence of apamin and iberiotoxin. For the population of recorded calyces (n = 6), the outward current was decreased by 16–26% (for steps larger than −69 mV) with application of apamin and iberiotoxin. The decrease in voltage-dependent currents was significant for the largest depolarizing steps (Fig. 2B). Addition of baclofen resulted in a nonsignificant decrease in currents by 10–20% (repeated measures ANOVA, P = 0.33). Similar effects were observed for tail currents at the end of voltage steps (not shown). Application of apamin (n = 5) or iberiotoxin (n = 4) alone also resulted in a decrease in voltage-dependent currents in response to the three largest steps (repeated measures ANOVA, P < 0.001). Addition of baclofen resulted in an extra 10–20% reduction [for 11-mV step: apamin + baclofen, 11% (re apamin alone); iberiotoxin + baclofen, 20% reduction (re iberiotoxin alone)]. The latter change was statistically nonsignificant compared with apamin or iberiotoxin alone (repeated measures ANOVA, P > 0.05 for all comparisons) but was significant compared with control conditions for the three largest steps (repeated measures ANOVA, P < 0.001). These results show that both SK and BK channels are expressed by the calyx and that both are involved in mediating the effect of baclofen.
Fig. 2.
Effect of baclofen could be inhibited by blocking small (SK)- and large-conductance potassium (BK) channels. A: example calyx recording showing a decrease in outward currents during the step protocol in the presence of apamin (Apa; SK blocker) and iberiotoxin (IBTX; BK blocker), which blocked the effect of baclofen (Bac). B: average of the recorded currents during the step protocol shows that apamin and iberiotoxin application blocked some of the voltage-sensitive currents and that application of baclofen resulted in a small nonsignificant further decrease in the currents for the most depolarized steps. *Significant differences by repeated measures ANOVA, post hoc Bonferroni test: control vs. Apa + IBTX, P < 0.001 at +11 mV and P < 0.05at −9 mV; control vs. Apa + IBTX + Bac, P < 0.001 at +11 mV and −9 mV, and P < 0.05 at −29 mV. C: application of a cocktail of apamin, iberiotoxin, and XE-991 (KCNQ channel blocker) resulted in a decrease in voltage-sensitive currents. Addition of baclofen resulted in a further small nonsignificant decrease in the currents at depolarized steps, which was blocked by cadmium chloride (Cdcl2; a general blocker of calcium channels), suggesting that baclofen inhibits voltage-sensitive calcium channels. *Significant differences between control and other conditions with P values similar to those in B.
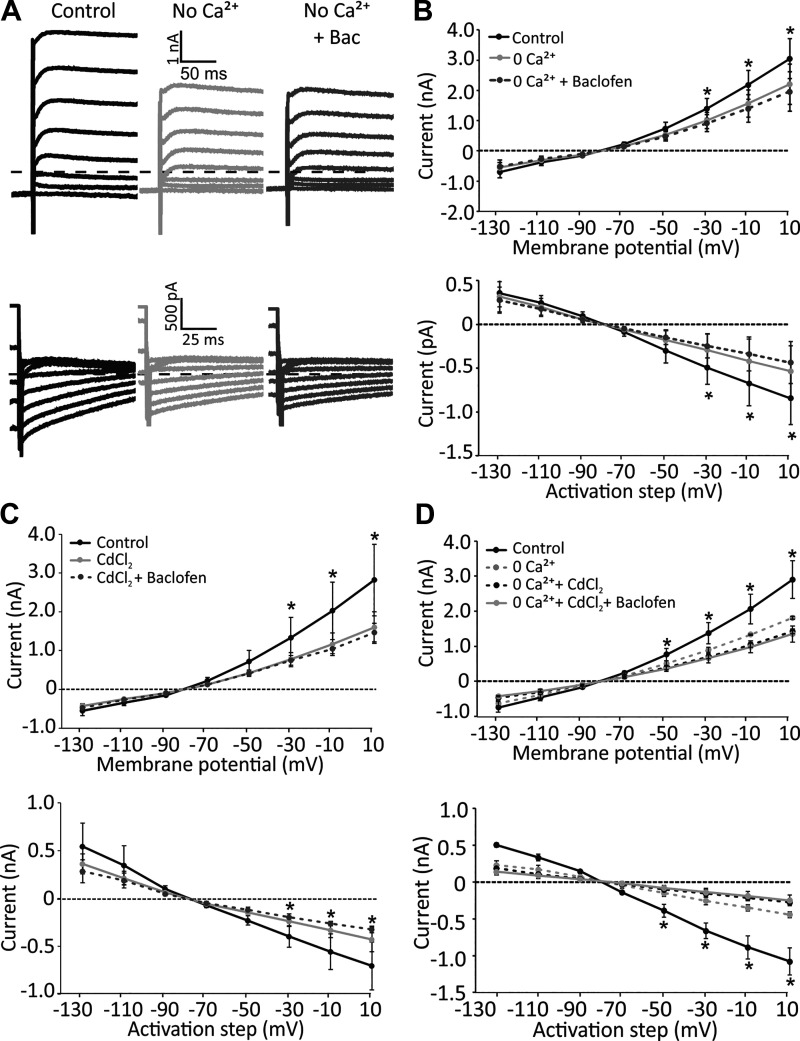
A common pathway that could affect both of these potassium channels is inhibition of voltage-sensitive calcium channels. As a first step, to investigate whether the baclofen effect was due to inhibition of a voltage-sensitive calcium current, we added XE-991 (KCNQ channel blocker) to the cocktail to inhibit all major voltage-activated K+ currents in the calyx (response to +11-mV step: control, 2,977 ± 285 pA; apamin and iberiotoxin, 2,276 ± 338 pA; apamin and iberiotoxin and XE-991, 1,914 ± 410 pA). Again, baclofen resulted in a small decrease in the average current (n = 5; largest decrease of 163 ± 115 pA for +11-mV step; Fig. 2C). A similar decrease in currents was observed with addition of the nonspecific Ca2+ channel blocker cadmium chloride (CdCl2; Fig. 2C), suggesting that baclofen inhibits voltage-sensitive Ca2+ channels. Furthermore, in the absence of calcium in the external solution (0 Ca2+), baclofen did not have any effect on the depolarizing steps and tail currents (Fig. 3A). Note that currents were decreased with 0 Ca2+ due to the inhibition of voltage-sensitive Ca2+ currents and Ca2+-activated potassium currents, but there was no further decrease with baclofen application (for +11 mV step: control, 2,901 ± 310; no calcium, 1,811 ± 25 pA; no calcium and CdCl2, 1,424 ± 40 pA, addition of baclofen, 1,349 ± 135 pA). For the population of recordings in control external solution, 0-Ca2+ external solution, and subsequent baclofen application (n = 7), voltage-sensitive currents and tail currents decreased in 0 Ca2+ compared with control, but there was no further change with baclofen application in 0 Ca2+ (Fig. 3B). In a second set of experiments in the control external solution, we applied CdCl2 to block calcium channels (n = 4), which decreased the amplitude of currents during depolarizing steps as well as the tail currents and occluded the effect of baclofen (Fig. 3C). In three recordings, we used CdCl2 with 0-Ca2+ external solution to completely block all sources of external calcium to the cell. This resulted in inhibition of voltage-activated and tail currents and complete block of the effect of baclofen (Fig. 3D). These results further emphasize the role of Ca2+-sensitive SK and BK channels in baclofen-mediated effects.
Fig. 3.
The effect of baclofen was blocked in the absence of calcium. A: example calyx recording showing a decrease in voltage-sensitive currents during the step protocol and in tail currents in 0-Ca2+ external solution, most likely due to inhibition of Ca2+-dependent K+ currents. The effect of baclofen was blocked under these conditions. B: average currents recorded from calyces during and after (tail currents) the step protocol. Application of 0-Ca2+ external solution decreased the currents and almost completely blocked the effect of baclofen. C: application of calcium channel blocker cadmium chloride resulted in a decrease in voltage-sensitive currents and tail currents and almost completely blocked the effect of baclofen. D: application of the combination of 0-Ca2+ external solution and cadmium chloride resulted in a complete block of baclofen effect on voltage-sensitive currents and tail currents. *Significant differences from control condition (repeated measures ANOVA, post hoc Bonferroni test, P < 0.03).
GABAB activation has an excitatory effect on calyx terminals.
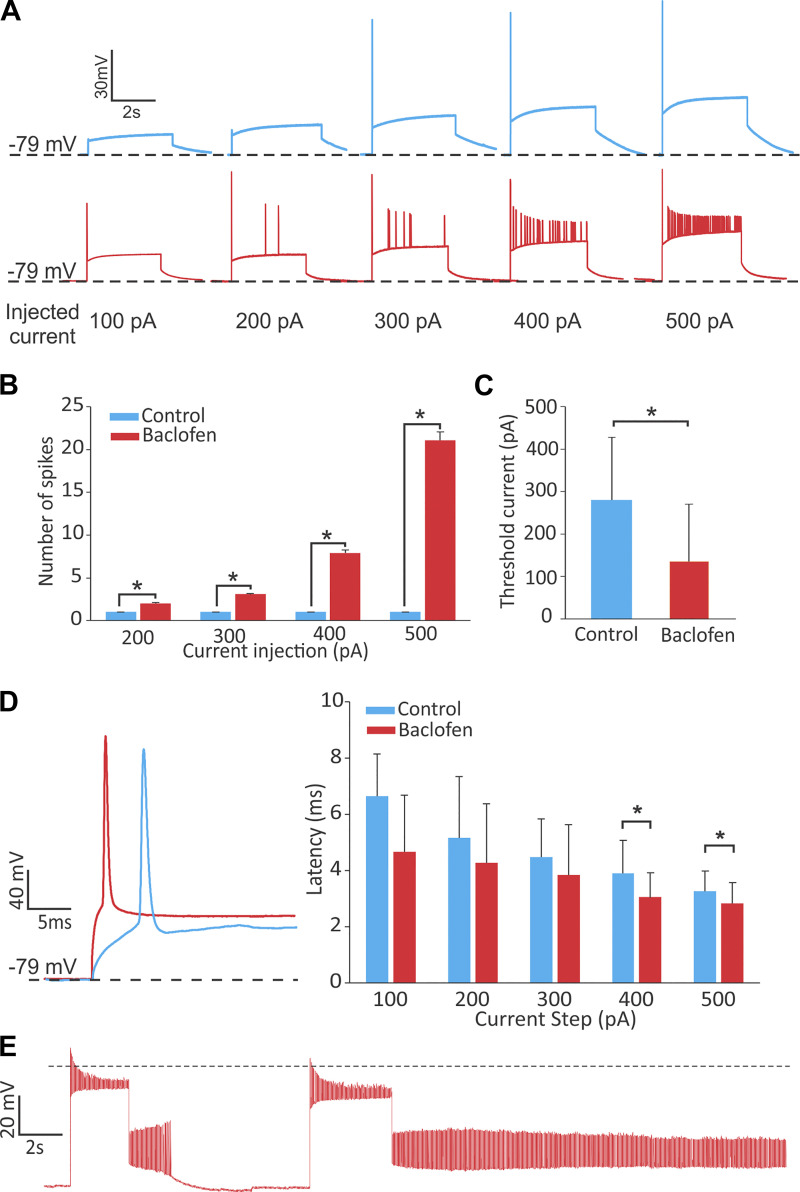
We next used current clamp to investigate the effect of GABAB activation on firing of action potentials (AP). For all recordings, we injected currents (if necessary) to have an initial calyx membrane potential at −79 mV. We then injected current steps of 100–500 pA in 100-pA steps. In control condition (i.e., before application of drugs), none of the recorded calyces (n = 15) showed spontaneous firing of APs, and the threshold for generating an AP was reached by injecting 320 ± 147 pA. At threshold and more depolarized membrane potentials, all recorded calyces fired only a single AP at best, as previously reported (Ramakrishna et al. 2020; Songer and Eatock 2013). The latency of this single spike was 6.8 ± 2.1 ms for the threshold current. After baclofen application, calyces fired more than one spike during depolarization (Fig. 4A). On average, calyces generated more than one spike after baclofen application compared with control condition for 200-pA, 300-pA, 400-pA, and 500-pA steps (Fig. 4B). The amplitude of the current step required for spike generation also significantly decreased (control, 320 ± 147 pA; baclofen, 219 ± 122 pA; paired t test, P = 0.009; Fig. 4C), and action potential generation threshold decreased from −50 ± 1.37 mV in control condition to −58.3 ± 1.65 mV after baclofen application (paired t test, P < 0.0009). Furthermore, the responses became faster with GABAB activation as quantified by the first-spike latency at the beginning of step depolarizations (Fig. 4D). For the threshold current, the latency showed a nonsignificant decrease (~2 ms) from 6.8 ± 2.1 ms to 5.0 ± 0.9 ms (paired t test, P = 0.12). However, the latency of the first spike decreased with increasing amplitude of step currents (for the control condition as well as in the presence of baclofen), and the difference between the two conditions was significant for the 400-pA and 500-pA steps (paired t test, P > 0.05 for 100- to 300-pA steps, P = 0.028 for 400-pA step, and P = 0.003 for 500-pA step).
Fig. 4.
Calyces become more sensitive and respond faster following activation of GABAB receptors. A: examples of current-clamp recordings from calyces that at best fire a single action potential (AP) in the control condition in response to injection of 100- to 500-pA current steps, but in the presence of baclofen fired many APs during the step. B: the average number of spikes during steps was higher after baclofen application for all current steps. The control condition had 0 or 1 spikes for all steps, while after baclofen, calyces fired at least 1 spike, even for the smallest current step. C: the amplitude of the current required for AP generation (i.e., current threshold) decreased significantly during baclofen application. D: the latency of the first spike decreased during application of baclofen compared with control condition. The decreases were significant for the two largest steps. E: some calyces showed repetitive AP firing during baclofen application (dashed line represents 0 mV). In A–E, control results are shown in blue and baclofen condition in red. *Significant changes from control condition (P < 0.05).
Previous studies have shown that both SK and BK channels affect neuronal spike-frequency adaptation and action potential firing patterns in different brain areas and animal models (e.g., Deemyad et al. 2011, 2012; Gittis et al. 2010; Smith et al. 2002). Although we expected to see changes in firing patterns, it was surprising to see excitatory effects with GABAB activation. Furthermore, while none of the recordings showed spontaneous AP firing in control condition, 8 of 15 recorded calyces in current clamp exhibited repeated firing of AP in the presence of baclofen (Fig. 4E). Note that the other two reports of excitatory GABAB effects in retinal ganglion cells and substantia nigra neurons did not show such repetitive firing in the presence of baclofen (Estep et al. 2016; Garaycochea and Slaughter 2016).
DISCUSSION
In this study, we provide evidence for an unusual excitatory effect of GABAB receptors on membrane properties of a group of sensory nerve terminals in the vestibular periphery. Application of GABAB agonist baclofen resulted in inhibition of K+ currents at depolarized membrane potentials in calyx afferent nerve terminals. This effect was occluded by exhausting available Ca2+ sources through application of 0-Ca2+ external solution and using the calcium channel blocker CdCl2. Baclofen effect was also decreased in the presence of SK and BK channel antagonists. Together, these results suggests that GABAB acts through inhibition of Ca2+-sensitive SK and BK potassium channels. These changes also resulted in faster responses (i.e., decreased first-spike latency in response to step currents), increased sensitivity (i.e., decreased threshold), and increased gain (i.e., more spikes in response to depolarization). Notably, some of the calyx terminals showed repeated (spontaneous) firing in the presence of baclofen, a property that is not typically seen in the in vitro preparation in the central regions of the cristae (Ramakrishna et al. 2020; Sadeghi et al. 2014) and maculae (Songer and Eatock 2013).
Excitatory GABAergic activity in the vestibular periphery.
Typically, activation of the metabotropic GABAB receptors results in an inhibitory effect. On the presynaptic side, this is mediated by inhibition of voltage-gated Ca2+ channels and a resultant decrease in synaptic vesicular release (reviewed in Kantamneni 2016). On the postsynaptic side, inhibition is achieved through activation of K+ channels. Only two previous studies have reported an excitatory GABAB-mediated effect: 1) in retinal ganglion sensory neurons through inhibition of postsynaptic voltage-sensitive Ca2+ channels, resulting in inhibition of BK channels (Garaycochea and Slaughter 2016), and 2) in substantia nigra through inhibition of SK channels through a direct pathway involving adenylyl cyclase and protein kinase A (Estep et al. 2016). Notably, baclofen was most effective at membrane potentials between −29 mV and +11 mV (i.e., highest voltages tested), consistent with the peak activity range of voltage-sensitive calcium channels in the vestibular (Vincent et al. 2014) and cochlear (Goutman and Glowatzki 2007) periphery. Because SK and BK channels are both present in the calyx, it is inevitable that both get affected by lack of Ca2+ or inhibition of voltage-sensitive Ca2+ channels. Although in the present study we did not investigate the underlying pathway(s) for the GABAB-mediated effect, either or both of the above-mentioned pathways in the retina and substantia nigra could be present in the calyx terminals. It should be noted that we only studied the effect of GABAB on the two most likely candidates, SK and BK channels. However, it is possible that other Ca2+-dependent K+ channels are also expressed by the calyx terminals. For example, intermediate-conductance K+ channels (KCa3.1) or KCa4.1 channels could theoretically be present in the calyx. The above-mentioned possibilities could explain the further (albeit nonsignificant) decrease in K+ currents with addition of CdCl2 after block of SK/BK channels. However, this further decrease could also be due to incomplete block of SK/BK channels by antagonists.
Finally, we did not investigate whether GABAB activation had any effects on sodium channels. Such effects are particularly likely since calyces might have persistent sodium currents (Liu et al. 2016), and a recent study (Li et al. 2017) has shown that baclofen could inhibit persistent sodium currents and subsequently inhibit a Na+-dependent K+ current in rat olfactory bulb mitral cells. Such effects could result in an excitatory effect, depending on the balance between inhibition of the sodium and potassium currents.
Source of GABA in the vestibular periphery.
Although previous studies have found GABAA subunits on afferents in rats, mice, and hamsters (Foster et al. 1995; Kitahara et al. 1994) and our results provide ample evidence for the presence of GABAB receptors on calyx terminals, a source of GABA in the vestibular periphery has not been unequivocally identified. There is evidence for existence of GABAergic efferent fibers and terminals in the peripheral vestibular neuroepithelium in mouse (Kong et al. 2002a), rats (Kong et al. 1998b; Matsubara et al. 1995), squirrel monkey (Usami et al. 1987), and humans (Kong et al. 1998a, 2002b; Schrott-Fischer et al. 2007). However, the main vestibular efferent cell group in the brain stem (near the genu of the facial nerve) was not labeled with anti-GABA antibodies (Perachio and Kevetter 1989). GABA has also been shown to be present in some of the hair cells in toadfish (Holstein et al. 2004) and guinea pigs (López et al. 1990).
Recent studies have suggested supporting cells as a source of GABA in the vestibular periphery. One study used GAD67-GFP or GAD65-GFP mice that express green fluorescent protein (GFP) along with the two isoforms of the enzyme glutamate decarboxylase (GAD), which transform glutamate to GABA (Tavazzani et al. 2014). The results of this study showed that only GAD67 was expressed in vestibular end organs and only in supporting cells in the peripheral zones of the cristae. Since GAD67 is responsible for generating cytoplasmic GABA (compared with GAD65 for vesicular GABA) (Martin and Rimvall 1993; Soghomonian and Martin 1998), supporting cells could have nonvesicular release of GABA similarly to glial cells (Attwell et al. 1993; Héja et al. 2009; Wu et al. 2007). Interestingly, with the use of anti-GABA antibodies, this study found that GABA was present in the supporting cells in both central and peripheral regions of the cristae. Another recent study used GAD2-tdTomato mice, which expressed red fluorescence with GAD2 and found fluorescence in both hair cells and supporting cells in the peripheral regions of the cristae and maculae (Holman et al. 2019). However, presence of GAD2 does not necessarily indicate generation of GABA in hair cells, and it is unlikely that GABA is an afferent neurotransmitter as AMPA receptor blockers suppress all synaptic events in calyx terminals (Sadeghi et al. 2014). Furthermore, GABA antibodies in the mouse failed to label hair cells (Tavazzani et al. 2014).
From all the above-described previous studies, the most likely sources for GABA in the vestibular periphery seem to be supporting cells, efferent fibers, and, possibly, some of the hair cells.
Function of GABAB activation in the vestibular periphery.
The firing properties of afferents are decided by presynaptic as well as postsynaptic factors. Typically, vesicular release properties of the hair cell such as number of released vesicles, quantal size, and probability of release are the main deciding presynaptic elements. A previous study has shown an excitatory GABAB activation effect on type I hair cells in guinea pigs (Lapeyre et al. 1993). Here, we did not set up for proper measurement of such effects and cannot rule out similar excitatory effects in rat hair cells. Furthermore, depolarization of type I hair cells could also affect the nonquantal transmission to the calyx terminals in this unique synapse and result in further depolarization of the calyces (Contini et al. 2017; Lim et al. 2011). In the present study, we did not observe any effect on resting membrane potential with baclofen application, thus ruling out the latter effect. On the postsynaptic side, the number and type of glutamate receptors as well as the calyx membrane properties are the main factors affecting the firing properties of the calyx. Here, we propose that modulation of calcium-dependent potassium channels through activation of GABAB receptors results in a change in firing properties of calyces as observed in their responses to step depolarizations, including a change in action potential threshold, which will affect calyx responses to presynaptic inputs and could partly explain the repetitive firing observed during depolarizing steps as well as with resting membrane potential.
Presence of different types of potassium (e.g., SK, BK, KCNQ, Kir, etc.) channels in calyx terminals decreases their membrane resistances, making it difficult to depolarize the calyx. We recently showed that muscarinic acetylcholine receptors (mAChR) inhibit KCNQ channels in calyx terminals, resulting in similar excitatory effects (Ramakrishna et al. 2020), supporting the findings of previous studies in turtle (Holt et al. 2017). Calcium currents as well as potassium currents (here, SK and BK channels) are involved in the hyperpolarization phase of action potentials (including the after hyperpolarization) and have been shown to affect the spike-frequency adaptation, firing patterns, and filtering properties of the membrane in other brain areas and animal models (e.g., Beraneck and Straka 2011; Deemyad et al. 2011, 2012; Gittis et al. 2010; Smith et al. 2002). Interestingly, calyces in the central regions of the in vitro preparation of the vestibular neuroepithelium can only fire a single AP in response to a step depolarization. This could be due to the fact that all (e.g., KCNQ, SK, BK) potassium channels expressed by the calyx are fully active in the absence of spontaneous efferent inputs (i.e., absence of or decreased acetylcholine and GABA), which is the case in the in vitro preparation since efferent fibers are cut and separated from their cell bodies in the brain stem. This notion is further supported by our recent in vivo results that showed suppression of efferents results in a decrease or complete shutdown of mainly irregular afferents in mice (Raghu et al. 2019). Note that the low release rates from hair cells onto the calyx most likely also contribute to the lack of spontaneous firing; however, under the same conditions, changing the membrane properties of the calyx resulted in repetitive (spontaneous) firing of action potentials in some of our recordings.
A second function for GABAB receptors can be a gain control mechanism. Inhibition of SK/BK channels and the resulting increase in the number of APs during step depolarizations is akin to an increase in the response gain. Similar increases in the spontaneous firing and response gain have been observed in the vestibular nuclei following inhibition of BK channels (Nelson et al. 2005, 2017; Smith et al. 2002). In addition to direct gain control, changes in the activity of potassium channels affect membrane filtering properties and its resonance at subthreshold potentials (Beraneck and Straka 2011).
Interaction between cholinergic efferents and GABAergic cells.
Whether and how cholinergic and GABAergic inputs interact in the vestibular periphery remain to be explored. Recently, it was shown that supporting cells that contain GAD2 are activated by acetylcholine in the vestibular periphery (Holman et al. 2019). A recent study has shown that GABAB activation inhibits cholinergic efferent terminals in contact with inner and outer hair cells (Wedemeyer et al. 2013). It is possible that a similar effect is present in efferents that synapse onto type II hair cells, which are similar to cochlear efferents in that they hyperpolarize type II hair cells through a9-containing receptors (Parks et al. 2017; Poppi et al. 2018; Yu et al. 2020). However, an interaction between the two inputs seems unlikely for the efferent-calyx synapse, since cholinergic efferents have an excitatory effect on calyces (Holt et al. 2015, 2017; Lee et al. 2017; Ramakrishna et al. 2020), and therefore, GABAergic inhibition of these efferents would not have resulted in an excitatory effect on calyces.
Conclusion.
Overall, our results provide evidence for an unusual excitatory effect of GABAB receptors on calyx terminals, which modulates their resting discharge, sensitivity, and membrane properties. This could potentially affect the response properties of irregular afferents, which play a major role in carrying information about fast head movements. The expression of other GABA receptors by the calyx as well as the presence of any GABA-mediated responses in type II hair cells and bouton afferent terminals remains to be explored. Our findings question the traditional notion that only central vestibular pathways are modulated during adaptation and compensation and suggest that GABAergic inputs along with cholinergic efferent inputs can provide the means for continuous fast adjustments of response properties of the vestibular periphery.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grant R03 DC015091 and a Research Grant from the American Otological Society (to S.G.S).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.R. and S.G.S. conceived and designed research; Y.R. performed experiments; Y.R. analyzed data; Y.R. and S.G.S. interpreted results of experiments; Y.R. and S.G.S. prepared figures; Y.R. and S.G.S. drafted manuscript; Y.R. and S.G.S. edited and revised manuscript; Y.R. and S.G.S. approved final version of manuscript.
REFERENCES
- Annoni JM, Cochran SL, Precht W. Pharmacology of the vestibular hair cell-afferent fiber synapse in the frog. J Neurosci 4: 2106–2116, 1984. doi: 10.1523/JNEUROSCI.04-08-02106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron 11: 401–407, 1993. doi: 10.1016/0896-6273(93)90145-H. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Straka H. Vestibular signal processing by separate sets of neuronal filters. J Vestib Res 21: 5–19, 2011. doi: 10.3233/VES-2011-0396. [DOI] [PubMed] [Google Scholar]
- Contini D, Price SD, Art JJ. Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. J Physiol 595: 777–803, 2017. doi: 10.1113/JP273060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22: RC226, 2002. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Kroeger J, Chacron MJ. Sub- and suprathreshold adaptation currents have opposite effects on frequency tuning. J Physiol 590: 4839–4858, 2012. doi: 10.1113/jphysiol.2012.234401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Maler L, Chacron MJ. Inhibition of SK and M channel-mediated currents by 5-HT enables parallel processing by bursts and isolated spikes. J Neurophysiol 105: 1276–1294, 2011. doi: 10.1152/jn.00792.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep CM, Galtieri DJ, Zampese E, Goldberg JA, Brichta L, Greengard P, Surmeier DJ. Transient activation of GABAB receptors suppresses SK channel currents in substantia nigra pars compacta dopaminergic neurons. PLoS One 11: e0169044, 2016. doi: 10.1371/journal.pone.0169044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Sans A. Morphological changes in afferent vestibular hair cell synapses during the postnatal development of the cat. J Neurocytol 8: 765–775, 1979. doi: 10.1007/BF01206675. [DOI] [PubMed] [Google Scholar]
- Flock A, Lam DM. Neurotransmitter synthesis in inner ear and lateral line sense organs. Nature 249: 142–144, 1974. doi: 10.1038/249142a0. [DOI] [PubMed] [Google Scholar]
- Foster JD, Drescher MJ, Drescher DG. Immunohistochemical localization of GABAA receptors in the mammalian crista ampullaris. Hear Res 83: 203–208, 1995. doi: 10.1016/0378-5955(95)00006-P. [DOI] [PubMed] [Google Scholar]
- Garaycochea J, Slaughter MM. GABAB receptors enhance excitatory responses in isolated rat retinal ganglion cells. J Physiol 594: 5543–5554, 2016. doi: 10.1113/JP272374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Moghadam SH, du Lac S. Mechanisms of sustained high firing rates in two classes of vestibular nucleus neurons: differential contributions of resurgent Na, Kv3, and BK currents. J Neurophysiol 104: 1625–1634, 2010. doi: 10.1152/jn.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 791–804, 1990. doi: 10.1152/jn.1990.63.4.791. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43: 986–1025, 1980. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci USA 104: 16341–16346, 2007. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héja L, Barabás P, Nyitrai G, Kékesi KA, Lasztóczi B, Toke O, Tárkányi G, Madsen K, Schousboe A, Dobolyi A, Palkovits M, Kardos J. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One 4: e7153, 2009. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highstein SM, Baker R. Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol 54: 370–384, 1985. doi: 10.1152/jn.1985.54.2.370. [DOI] [PubMed] [Google Scholar]
- Holman HA, Poppi LA, Frerck M, Rabbitt RD. Spontaneous and acetylcholine evoked calcium transients in the developing mouse utricle. Front Cell Neurosci 13: 186, 2019. doi: 10.3389/fncel.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein GR, Martinelli GP, Henderson SC, Friedrich VL Jr, Rabbitt RD, Highstein SM. γ-Aminobutyric acid is present in a spatially discrete subpopulation of hair cells in the crista ampullaris of the toadfish Opsanus tau. J Comp Neurol 471: 1–10, 2004. doi: 10.1002/cne.11025. [DOI] [PubMed] [Google Scholar]
- Holt JC, Jordan PM, Lysakowski A, Shah A, Barsz K, Contini D. Muscarinic acetylcholine receptors and M-currents underlie efferent-mediated slow excitation in calyx-bearing vestibular afferents. J Neurosci 37: 1873–1887, 2017. doi: 10.1523/JNEUROSCI.2322-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Kewin K, Jordan PM, Cameron P, Klapczynski M, McIntosh JM, Crooks PA, Dwoskin LP, Lysakowski A. Pharmacologically distinct nicotinic acetylcholine receptors drive efferent-mediated excitation in calyx-bearing vestibular afferents. J Neurosci 35: 3625–3643, 2015. doi: 10.1523/JNEUROSCI.3388-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Holt JR. Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons. J Gen Physiol 143: 481–497, 2014. doi: 10.1085/jgp.201311126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in the high-frequency response and short-term adaptation of the vestibuloocular reflex. J Neurophysiol 114: 3154–3165, 2015. doi: 10.1152/jn.00307.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in vestibulo-ocular reflex compensation after unilateral labyrinthectomy. J Neurophysiol 117: 1553–1568, 2017. doi: 10.1152/jn.01049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26: 10253–10269, 2006. doi: 10.1523/JNEUROSCI.2596-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol 47: 11–39, 1997. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Xue J, Eatock RA. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol 104: 2034–2051, 2010. doi: 10.1152/jn.00396.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantamneni S. Modulation of neurotransmission by the GABAB receptor. In: GABAB Receptors, edited by Colombo G. New York: Humana, 2016, p. 109–128. doi: 10.1007/978-3-319-46044-4. [DOI] [Google Scholar]
- Kitahara T, Takeda N, Ohno K, Araki T, Kubo T, Kiyama H. Expression of GABAA receptor γ1 and γ2 subunits in the peripheral vestibular system of the rat. Brain Res 650: 157–160, 1994. doi: 10.1016/0006-8993(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Cheng HM, van Cauwenberge P. Expression of nicotinic acetylcholine receptor subunit α9 in type II vestibular hair cells of rats. Acta Pharmacol Sin 27: 1509–1514, 2006. doi: 10.1111/j.1745-7254.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Guo CK, Zhang S, Hao J, Wang YJ, Li ZW. The properties of ACh-induced BK currents in guinea pig type II vestibular hair cells. Hear Res 209: 1–9, 2005. doi: 10.1016/j.heares.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the human utricular macula. Hear Res 119: 104–112, 1998a. doi: 10.1016/S0378-5955(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Hussl B, Thumfart WF, Schrott-Fischer A. Ultrastructural localization of GABA-like immunoreactivity in the vestibular periphery of the rat. Acta Otolaryngol 118: 90–95, 1998b. doi: 10.1080/00016489850155198. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Scholtz AW, Hussl B, Kammen-Jolly K, Schrott-Fischer A. Localization of efferent neurotransmitters in the inner ear of the homozygous Bronx waltzer mutant mouse. Hear Res 167: 136–155, 2002a. doi: 10.1016/S0378-5955(02)00382-9. [DOI] [PubMed] [Google Scholar]
- Kong WJ, Scholtz AW, Kammen-Jolly K, Glückert R, Hussl B, von Cauvenberg PB, Schrott-Fischer A. Ultrastructural evaluation of calcitonin gene-related peptide immunoreactivity in the human cochlea and vestibular endorgans. Eur J Neurosci 15: 487–497, 2002b. doi: 10.1046/j.0953-816x.2001.01880.x. [DOI] [PubMed] [Google Scholar]
- Lapeyre PN, Kolston PJ, Ashmore JF. GABAB-mediated modulation of ionic conductances in type I hair cells isolated from guinea-pig semicircular canals. Brain Res 609: 269–276, 1993. doi: 10.1016/0006-8993(93)90882-N. [DOI] [PubMed] [Google Scholar]
- Lee C, Holt JC, Jones TA. Effect of M-current modulation on mammalian vestibular responses to transient head motion. J Neurophysiol 118: 2991–3006, 2017. doi: 10.1152/jn.00384.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Kevetter GA, Leonard RB, Prusak DJ, Wood TG, Correia MJ. Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neuroscience 146: 384–402, 2007. doi: 10.1016/j.neuroscience.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Stewart R, Butler A, Gonzalez-Cota AL, Harmon S, Salkoff L. GABA-B controls persistent Na+ current and coupled Na+-activated K+ current. eNeuro 4: ENEURO.0114-17.2017, 2017. doi: 10.1523/ENEURO.0114-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Drury HR, Camp AJ, Tadros MA, Callister RJ, Brichta AM. Preliminary characterization of voltage-activated whole-cell currents in developing human vestibular hair cells and calyx afferent terminals. J Assoc Res Otolaryngol 15: 755–766, 2014. doi: 10.1007/s10162-014-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Kindig AE, Donne SW, Callister RJ, Brichta AM. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp Brain Res 210: 607–621, 2011. doi: 10.1007/s00221-011-2592-4. [DOI] [PubMed] [Google Scholar]
- Limón A, Pérez C, Vega R, Soto E. Ca2+-activated K+-current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol 94: 3751–3761, 2005. doi: 10.1152/jn.00177.2005. [DOI] [PubMed] [Google Scholar]
- Liu XP, Wooltorton JR, Gaboyard-Niay S, Yang FC, Lysakowski A, Eatock RA. Sodium channel diversity in the vestibular ganglion: NaV1.5, NaV1.8, and tetrodotoxin-sensitive currents. J Neurophysiol 115: 2536–2555, 2016. doi: 10.1152/jn.00902.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López I, Juiz JM, Altschuler RA, Meza G. Distribution of GABA-like immunoreactivity in guinea pig vestibular cristae ampullaris. Brain Res 530: 170–175, 1990. doi: 10.1016/0006-8993(90)90677-4. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Holt JC, Jordan PM, Wong YS, Caldwell JS, Cullen KE. Loss of α-calcitonin gene-related peptide (αCGRP) reduces the efficacy of the vestibulo-ocular reflex (VOR). J Neurosci 34: 10453–10458, 2014. doi: 10.1523/JNEUROSCI.3336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Gaboyard-Niay S, Calin-Jageman I, Chatlani S, Price SD, Eatock RA. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J Neurosci 31: 10101–10114, 2011. doi: 10.1523/JNEUROSCI.0521-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511: 47–64, 2008. doi: 10.1002/cne.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernández C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- Maison SF, Rosahl TW, Homanics GE, Liberman MC. Functional role of GABAergic innervation of the cochlea: phenotypic analysis of mice lacking GABAA receptor subunits α1, α2, α5, α6, β2, β3, or δ. J Neurosci 26: 10315–10326, 2006. doi: 10.1523/JNEUROSCI.2395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, Plotnik M, Goldberg JM. Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol 5: 126–143, 2004. doi: 10.1007/s10162-003-4029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem 60: 395–407, 1993. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Usami S, Fujita S, Shinkawa H. Expression of substance P, CGRP, and GABA in the vestibular periphery, with special reference to species differences. Acta Otolaryngol Suppl 519: 248–252, 1995. doi: 10.3109/00016489509121916. [DOI] [PubMed] [Google Scholar]
- Meredith FL, Li GQ, Rennie KJ. Postnatal expression of an apamin-sensitive K(Ca) current in vestibular calyx terminals. J Membr Biol 244: 81–91, 2011. doi: 10.1007/s00232-011-9400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Rennie KJ. Regional and developmental differences in Na+ currents in vestibular primary afferent neurons. Front Cell Neurosci 12: 423, 2018. doi: 10.3389/fncel.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metts BA, Kaufman GD, Perachio AA. Polysynaptic inputs to vestibular efferent neurons as revealed by viral transneuronal tracing. Exp Brain Res 172: 261–274, 2006. doi: 10.1007/s00221-005-0328-z. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Faulstich M, Moghadam S, Onori K, Meredith A, du Lac S. BK channels are required for multisensory plasticity in the oculomotor system. Neuron 93: 211–220, 2017. doi: 10.1016/j.neuron.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 46: 623–631, 2005. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JF, Jarolimek W, Lewen A, Misgeld U. (−)-Baclofen-induced and constitutively active inwardly rectifying potassium conductances in cultured rat midbrain neurons. Pflugers Arch 433: 49–57, 1996. doi: 10.1007/s004240050247. [DOI] [PubMed] [Google Scholar]
- Parks XX, Contini D, Jordan PM, Holt JC. Confirming a role for α9nAChRs and SK potassium channels in type II hair cells of the turtle posterior crista. Front Cell Neurosci 11: 356, 2017. doi: 10.3389/fncel.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perachio AA, Kevetter GA. Identification of vestibular efferent neurons in the gerbil: histochemical and retrograde labelling. Exp Brain Res 78: 315–326, 1989. doi: 10.1007/BF00228903. [DOI] [PubMed] [Google Scholar]
- Pérez C, Limón A, Vega R, Soto E. The muscarinic inhibition of the potassium M-current modulates the action-potential discharge in the vestibular primary-afferent neurons of the rat. Neuroscience 158: 1662–1674, 2009. doi: 10.1016/j.neuroscience.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Marlinski V, Goldberg JM. Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol 88: 1234–1244, 2002. doi: 10.1152/jn.2002.88.3.1234. [DOI] [PubMed] [Google Scholar]
- Poppi LA, Holt JC, Lim R, Brichta AM. A review of efferent cholinergic synaptic transmission in the vestibular periphery and its functional implications. J Neurophysiol 123: 608–629, 2020. doi: 10.1152/jn.00053.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppi LA, Tabatabaee H, Drury HR, Jobling P, Callister RJ, Migliaccio AA, Jordan PM, Holt JC, Rabbitt RD, Lim R, Brichta AM. ACh-induced hyperpolarization and decreased resistance in mammalian type II vestibular hair cells. J Neurophysiol 119: 312–325, 2018. doi: 10.1152/jn.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu V, Salvi R, Sadeghi SG. Efferent inputs are required for normal function of vestibular nerve afferents. J Neurosci 39: 6922–6935, 2019. doi: 10.1523/JNEUROSCI.0237-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna Y, Manca M, Glowatzki E, Sadeghi SG. Cholinergic modulation of membrane properties of calyx terminals in the vestibular periphery. bioRxiv 2020.04.15.041491, 2020. doi: 10.1101/2020.04.15.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Goldberg JM, Minor LB, Cullen KE. Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J Neurophysiol 101: 988–1001, 2009. doi: 10.1152/jn.91112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Pyott SJ, Yu Z, Glowatzki E. Glutamatergic signaling at the vestibular hair cell calyx synapse. J Neurosci 34: 14536–14550, 2014. doi: 10.1523/JNEUROSCI.0369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott-Fischer A, Kammen-Jolly K, Scholtz A, Rask-Andersen H, Glueckert R, Eybalin M. Efferent neurotransmitters in the human cochlea and vestibule. Acta Otolaryngol 127: 13–19, 2007. doi: 10.1080/00016480600652123. [DOI] [PubMed] [Google Scholar]
- Schweizer FE, Savin D, Luu C, Sultemeier DR, Hoffman LF. Distribution of high-conductance calcium-activated potassium channels in rat vestibular epithelia. J Comp Neurol 517: 134–145, 2009. doi: 10.1002/cne.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol 87: 2031–2042, 2002. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 19: 500–505, 1998. doi: 10.1016/S0165-6147(98)01270-X. [DOI] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. doi: 10.1523/JNEUROSCI.4067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Nasu M, Kanazawa T, Shimazu Y. Activation of GABAB receptors potentiates inward rectifying potassium currents in satellite glial cells from rat trigeminal ganglia: in vivo patch-clamp analysis. Neuroscience 288: 51–58, 2015. doi: 10.1016/j.neuroscience.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Tavazzani E, Tritto S, Spaiardi P, Botta L, Manca M, Prigioni I, Masetto S, Russo G. Glutamic acid decarboxylase 67 expression by a distinct population of mouse vestibular supporting cells. Front Cell Neurosci 8: 428, 2014. doi: 10.3389/fncel.2014.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T, Tatsumi N, Tachibana T, Negishi Y, Saijo H, Kobayashi T, Yaguchi Y, Kojima H, Moriyama H, Okabe M. Inwardly rectifying potassium channel Kir4.1 is localized at the calyx endings of vestibular afferents. Neuroscience 215: 209–216, 2012. doi: 10.1016/j.neuroscience.2012.04.037. [DOI] [PubMed] [Google Scholar]
- Usami S, Igarashi M, Thompson GC. GABA-like immunoreactivity in the squirrel monkey vestibular endorgans. Brain Res 417: 367–370, 1987. doi: 10.1016/0006-8993(87)90466-5. [DOI] [PubMed] [Google Scholar]
- Vega R, Soto E, Budelli R, González-Estrada MT. Is GABA an afferent transmitter in the vestibular system? Hear Res 29: 163–167, 1987. doi: 10.1016/0378-5955(87)90164-X. [DOI] [PubMed] [Google Scholar]
- Vincent PF, Bouleau Y, Safieddine S, Petit C, Dulon D. Exocytotic machineries of vestibular type I and cochlear ribbon synapses display similar intrinsic otoferlin-dependent Ca2+ sensitivity but a different coupling to Ca2+ channels. J Neurosci 34: 10853–10869, 2014. doi: 10.1523/JNEUROSCI.0947-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer C, Zorrilla de San Martín J, Ballestero J, Gómez-Casati ME, Torbidoni AV, Fuchs PA, Bettler B, Elgoyhen AB, Katz E. Activation of presynaptic GABAB(1a,2) receptors inhibits synaptic transmission at mammalian inhibitory cholinergic olivocochlear-hair cell synapses. J Neurosci 33: 15477–15487, 2013. doi: 10.1523/JNEUROSCI.2554-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56: 851–865, 2007. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, McIntosh JM, Sadeghi SG, Glowatzki E. Efferent synaptic transmission at the vestibular type II hair cell synapse. J Neurophysiol 124: 360–374, 2020. doi: 10.1152/jn.00143.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]