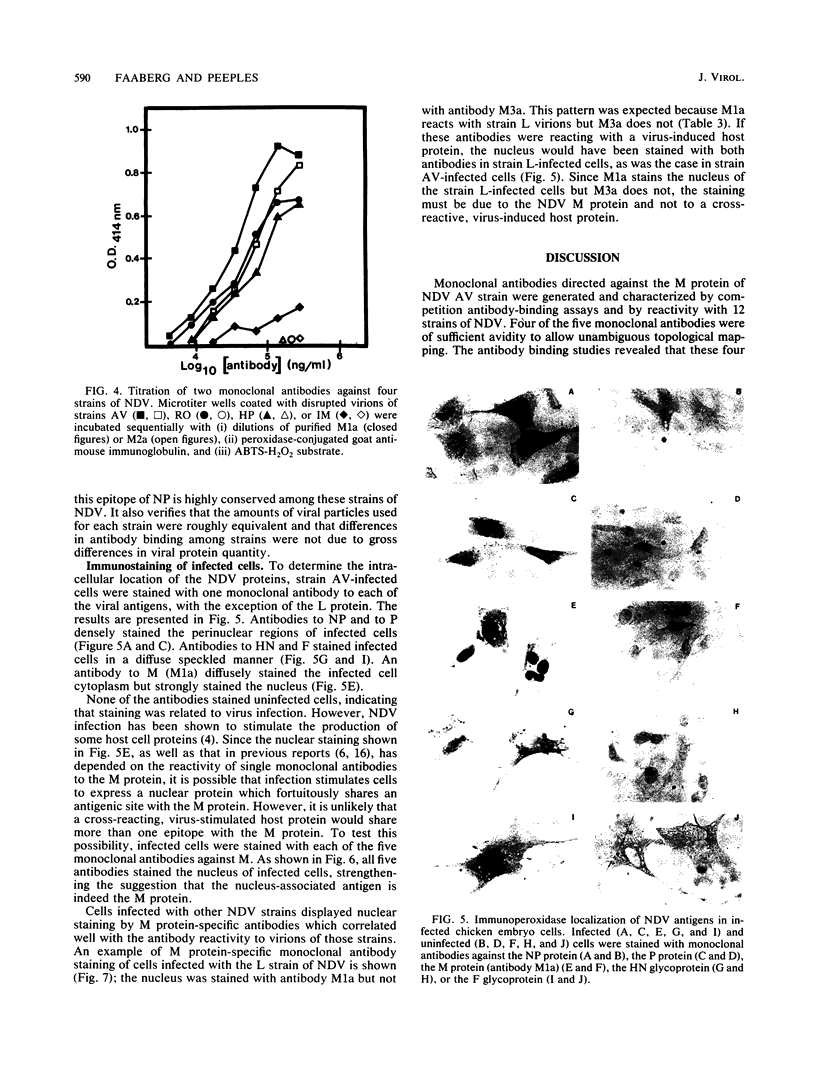
Abstract
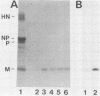
Five monoclonal antibodies to the matrix (M) protein of Newcastle disease virus (NDV) Australia-Victoria (AV) strain were generated and characterized. In competitive antibody-binding assays, the antibodies fell into three discrete groups. The antigenic sites described by these antibody groups were designated M1, M2, and M3. Each antibody reacted with a panel of NDV strains in a manner unique to its group, confirming the grouping by competitive antibody binding. Only site M1 was found on all 12 of the strains tested and may be a "pan-NDV" epitope. A large portion of the M protein of strain AV was detected in the nuclei of infected cells by all five monoclonal antibodies. In addition, the antibodies only stained the nuclei of cells infected with NDV strains expressing M protein containing the corresponding antigenic site. These results confirm that the immunoreactivity in the nucleus is actually caused by the M protein and not by a cross-reacting host protein induced by viral infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Nishikawa K., Toyoda T., Yoshida T., Hanaichi T., Nagai Y. Transcriptive complex of Newcastle disease virus. II. Structural and functional assembly associated with the cytoskeletal framework. Virology. 1985 Dec;147(2):295–308. doi: 10.1016/0042-6822(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Bratt M. A. Monoclonal antibodies to newcastle disease virus: delineation of four epitopes on the HN glycoprotein. J Virol. 1983 Nov;48(2):440–450. doi: 10.1128/jvi.48.2.440-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. M., Lawton K. A., Nicholson P. M., Bratt M. A. Monoclonal antibodies identify a strain-specific epitope on the HN glycoprotein of Newcastle disease virus strain Australia-Victoria. Virus Res. 1984 Oct;1(7):513–525. doi: 10.1016/0168-1702(84)90009-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laurila P., Virtanen I., Wartiovaara J., Stenman S. Fluorescent antibodies and lectins stain intracellular structures in fixed cells treated with nonionic detergent. J Histochem Cytochem. 1978 Apr;26(4):251–257. doi: 10.1177/26.4.207770. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Peeples M. E., McGinnes L. W. Conformational change in a viral glycoprotein during maturation due to disulfide bond disruption. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1020–1024. doi: 10.1073/pnas.84.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M. E., Bratt M. A. Mutation in the matrix protein of Newcastle disease virus can result in decreased fusion glycoprotein incorporation into particles and decreased infectivity. J Virol. 1984 Jul;51(1):81–90. doi: 10.1128/jvi.51.1.81-90.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheshberadaran H., Chen S. N., Norrby E. Monoclonal antibodies against five structural components of measles virus. I. Characterization of antigenic determinants on nine strains of measles virus. Virology. 1983 Jul 30;128(2):341–353. doi: 10.1016/0042-6822(83)90261-1. [DOI] [PubMed] [Google Scholar]
- TRAVER M. I., NORTHROP R. L., WALKER D. L. Site of intracellular antigen production by myxoviruses. Proc Soc Exp Biol Med. 1960 Jun;104:268–273. doi: 10.3181/00379727-104-25803. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Bratt M. A. Polyadenylate sequences on Newcastle disease virus mRNA synthesized in vivo and in vitro. J Virol. 1974 Jun;13(6):1220–1230. doi: 10.1128/jvi.13.6.1220-1230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Nagai Y'Yoshii S., Maeno K., Matsumoto T. Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology. 1976 May;71(1):143–161. doi: 10.1016/0042-6822(76)90101-x. [DOI] [PubMed] [Google Scholar]
- van Wyke K. L., Yewdell J. W., Reck L. J., Murphy B. R. Antigenic characterization of influenza A virus matrix protein with monoclonal antibodies. J Virol. 1984 Jan;49(1):248–252. doi: 10.1128/jvi.49.1.248-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]