To the best of our knowledge, there is no published study on the use of interferon β-1a (IFN β-1a) in the treatment of severe COVID-19. In this randomized clinical trial, the efficacy and safety of IFN β-1a were evaluated in patients with severe COVID-19. Forty-two patients in the interferon group received IFN β-1a in addition to the national protocol medications (hydroxychloroquine plus lopinavir-ritonavir or atazanavir-ritonavir). Each 44-μg/ml (12 million IU/ml) dose of interferon β-1a was subcutaneously injected three times weekly for two consecutive weeks.
KEYWORDS: COVID-19, clinical response, interferon, mortality
ABSTRACT
To the best of our knowledge, there is no published study on the use of interferon β-1a (IFN β-1a) in the treatment of severe COVID-19. In this randomized clinical trial, the efficacy and safety of IFN β-1a were evaluated in patients with severe COVID-19. Forty-two patients in the interferon group received IFN β-1a in addition to the national protocol medications (hydroxychloroquine plus lopinavir-ritonavir or atazanavir-ritonavir). Each 44-μg/ml (12 million IU/ml) dose of interferon β-1a was subcutaneously injected three times weekly for two consecutive weeks. The control group consisted of 39 patients who received only the national protocol medications. The primary outcome of the study was time to reach clinical response. Secondary outcomes were duration of hospital stay, length of intensive care unit stay, 28-day mortality, effect of early or late administration of IFN on mortality, adverse effects, and complications during the hospitalization. Between 29 February and 3 April 2020, 92 patients were recruited, and a total of 42 patients in the IFN group and 39 patients in the control group completed the study. As the primary outcome, time to the clinical response was not significantly different between the IFN and the control groups (9.7 ± 5.8 versus 8.3 ± 4.9 days, respectively, P = 0.95). On day 14, 66.7% versus 43.6% of patients in the IFN group and the control group, respectively, were discharged (odds ratio [OR], 2.5; 95% confidence interval [CI], 1.05 to 6.37). The 28-day overall mortality was significantly lower in the IFN than the control group (19% versus 43.6%, respectively, P = 0.015). Early administration significantly reduced mortality (OR, 13.5; 95% CI, 1.5 to 118). Although IFN did not change the time to reach the clinical response, adding it to the national protocol significantly increased discharge rate on day 14 and decreased 28-day mortality. (This study is in the Iranian Registry of Clinical Trials under identifier IRCT20100228003449N28.)
INTRODUCTION
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is a novel virus that has been introduced as the first pandemic of the century since its first detection in late December 2019 in China (1). The disease is named coronavirus disease 2019 (COVID-19) and manifests with dyspnea, fever, cough, myalgia, and other flu-like symptoms (2). However, it can progress to more severe disease and cause acute respiratory distress syndrome (ARDS), organ failure, and death (3). In the absence of definite treatment, the disease has caused more than 500,000 deaths worldwide in nearly 6 months (4).
Some antivirals are being examined in the treatment of COVID-19. However, the results are not sound. Data in terms of efficacy of lopinavir-ritonavir were promising at first, but a recent randomized trial failed to show benefits, especially in later stages of the disease (5). Hydroxychloroquine is another available choice that has been included in many national protocols (6). However, practitioners have been advised to restrict the administration of this drug for clinical trials due to its questionable efficacy and risk of adverse effects (7). The race is still on to find an effective treatment for COVID-19. Most of our experience came from other coronavirus epidemics, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (8).
Interferon (IFN) subtypes were previously examined in the treatment of SARS and MERS. Primary in vitro experience showed antiviral effects of IFNs, especially IFN-β and IFN-γ, on SARS-CoV (9). The same results were reported for IFN-β against MERS-CoV (10, 11). Later, in an animal model, higher antiviral activity of IFN-β compared to that of lopinavir-ritonavir was shown against MERS-CoV (12). The efficacy of IFN-β on MERS is still being investigated (13).
The antiviral effects of IFNs are expressed through activating interferon-stimulated genes (ISGs) (14). Additionally, by decreasing the vascular leakage, IFN β-1a improved ARDS complications, regardless of its antiviral properties (15). The higher expression of a protein named CD-73 also could lead to a better prognosis in ARDS. However, these data were not replicated in a later trial (16).
To the best of our knowledge, there is no published study regarding the use of IFN β-1a in the treatment of severe COVID-19. In this randomized clinical trial, the efficacy and safety of IFN β-1a were evaluated in patients with severe COVID-19.
RESULTS
Patients and baseline features.
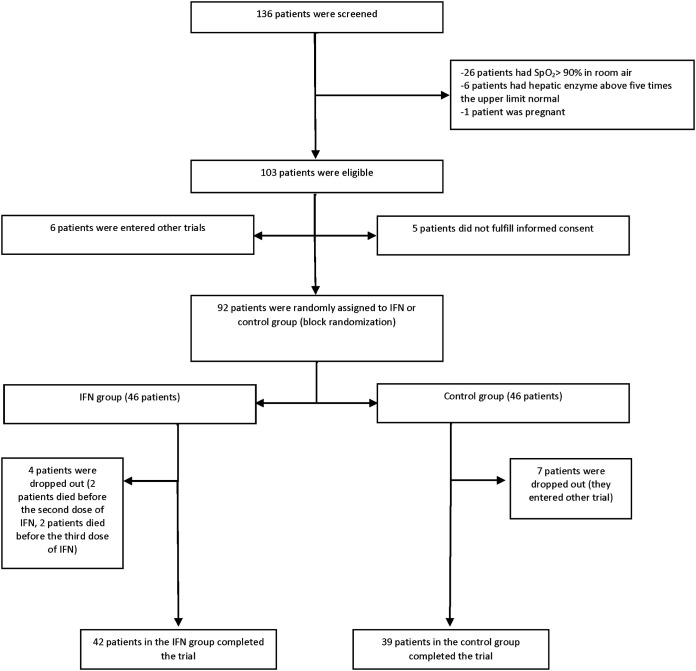
During the study period, 139 patients were screened, of whom 103 subjects were eligible. Considering dropouts, 81 patients (42 in the IFN and 39 in the control group) completed the treatment for further analysis (Fig. 1). Males were 54.3% of patients. The mean age ± standard deviations (SD) in the IFN and control groups was 56.0 ± 16 and 59.5 ± 14 years, respectively. Fifty-two (64.19%) patients had positive nasopharyngeal real-time PCR (RT-PCR) for SARS-CoV-2, and 29 (35.81%) patients were diagnosed according to the clinical signs/symptoms along with the imaging findings. Hypertension (38.3%), cardiovascular diseases (28.4%), diabetes mellitus (27.2%), endocrine disorders (14.8%), and malignancy (11.1%) were common baseline diseases. Endocrine disorders were dyslipidemia and hypothyroidism. There was no significant difference in terms of demographic data and baseline diseases between the groups. The most frequent chief complaints of patients were cough, fever, and dyspnea (Table 1). APACHE II score at the time of intensive care unit (ICU) admission was not significantly different between the two groups (15.3 ± 4 in the IFN group versus 14.5 ± 3 in the control group, P = 0.79).
FIG 1.
Consort flowchart of the study.
TABLE 1.
Baseline characteristics of patients
| Variablea | Value(s) for |
|
|---|---|---|
| IFN group (n = 42) | Control group (n = 39) | |
| Age (median with IQR) (yr) | 56.50 (47.25–67.25) | 61.00 (50.00–70.00) |
| Male, n (%) | 22 (52.38) | 22 (56.4) |
| Female, n (%) | 20 (47.61) | 17 (43.58) |
| BMI (median with IQR) kg/m2 | 25.00 (23.00–29.00) | 25.00 (22.00–29.00) |
| Baseline diseases, n (%) | ||
| Any comorbidity | 32 (76.19) | 31 (79.48) |
| Hypertension | 15 (35.71) | 16 (41.02) |
| Diabetes mellitus | 13 (30.95) | 9 (23.07) |
| Ischemic heart disease | 11 (26.19) | 12 (30.76) |
| Endocrine disorder | 6 (14.28) | 6 (15.38) |
| Malignancy | 4 (9.52) | 5 (12.82) |
| Neuropsychiatric disorders | 3 (7.14) | 2 (5.12) |
| Hematologic disorder | 2 (4.76) | 0 |
| Rheumatoid disorder | 1 (2.38) | 2 (5.12) |
| Renal disease | 1 (2.38) | 2 (5.12) |
| Liver disease | 1 (2.38) | 2 (5.12) |
| Rheumatoid arthritis | 1 (2.38) | 1 (2.56) |
| Asthma | 1 (2.38) | 0 |
| Transplantation | 1 (2.38) | 0 |
| COPD | 0 | 1 (2.56) |
| Symptoms at admission, n (%) | ||
| Cough | 37 (88.09) | 25 (64.10) |
| Fever | 30 (71.42) | 20 (51.28) |
| Dyspnea | 29 (69.04) | 27 (69.23) |
| Myalgia | 16 (38.09) | 20 (51.28) |
| Chills | 16 (38.09) | 5 (12.82) |
| Anorexia | 12 (28.57) | 6 (15.38) |
| Diarrhea | 8 (19.04) | 2 (5.12) |
| Malaise | 6 (14.28) | 8 (20.51) |
| Nausea/vomiting | 4 (9.52) | 10 (25.64) |
| Headache | 3 (7.14) | 4 (10.25) |
| Chest discomfort | 1 (2.38) | 4 (10.25) |
| Duration, in days, of symptoms before admission (mean ± SD) | 8.33 ± 4.5 | 6.57 ± 3.6 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; SD, standard deviations.
Vital signs and laboratory data.
Median time from the onset of symptoms (according to patients’ reports) to the administration of IFN was 10 days (interquartile range [IQR], 8 to 13). The vital signs at the time of hospital admission were not statistically different, except respiratory rate was significantly higher in the IFN group (22 versus 20, respectively, P = 0.009). Comparing the baseline laboratory data at the time of hospital admission revealed that the median blood urea nitrogen level was higher in the IFN than in the control group. The median INR value also was significantly higher in the control group than in the IFN group. Other laboratory findings were comparable (Table 2).
TABLE 2.
Initial vital signs and laboratory data
| Variable | Value(s) [median (IQR)] for: |
|
|---|---|---|
| IFN group | Control group | |
| Temp (°C) | 37.30 (36.70–38.72) | 37.25 (36.97–38.00) |
| Heart rate (beats/min) | 95 (83–105) | 92 (80–101) |
| Respiratory rate (breaths per min) | 22 (19–29) | 20 (18–24) |
| Systolic blood pressure (mm Hg) | 120 (117–130) | 120 (110–130) |
| SPO2 (%) | 89 (83–90) | 87 (84–90) |
| White blood cell (cells/μl) | 7,200.00 (5,175.00–9,250.00) | 6,650.00 (4,900.00–9,550.00) |
| Acute lymphocyte count (cells/μl) | 1,017.00 (689.00–1,321.25) | 850.00 (650.00–1,150.00) |
| Hemoglobin (g/dl) | 13.10 (11.45–14.50) | 13.40 (11.97–14.72) |
| Platelet count (cells ×103/μl) | 187.50 (160.50–281.50) | 196.00 (136.25–245.00) |
| Blood urea nitrogen (mg/dl) | 28.50 (22.00–48.50) | 15.00 (11.00–21.00) |
| Creatinine (mg/dl) | 1.10 (0.97–1.30) | 1.10 (0.90–1.30) |
| Sodium (mEq/liter) | 139.00 (136.00–141.25) | 138.00 (134.00–140.00) |
| Potassium (mEq/liter) | 4.10 (3.90–4.62) | 4.10 (3.80–4.40) |
| Calcium (mg/dl) | 8.00 (7.70–8.50) | 8.10 (7.80–8.50) |
| Phosphorus (mg/dl) | 3.15 (2.70–3.95) | 2.80 (2.50–3.10) |
| Magnesium (mg/dl) | 2.05 (1.80–2.27) | 2.00 (1.77–2.12) |
| Aspartate aminotransferase (U/liter) | 40. (28–65) | 43 (30–58) |
| Alanine aminotransferase (U/liter) | 36 (24–52) | 33 (26–54) |
| Alkaline phosphatase (U/liter) | 158 (115–203) | 152 (118–202) |
| Total bilirubin (mg/dl) | 0.60 (0.40–1.20) | 0.8 (0.50–1.02) |
| International normalized ratio (INR) | 1.06 (1.02–1.12) | 1.10 (1.05–1.20) |
| C-reactive protein (mg/dl) | 149 (91–20) | 122 (45–187) |
| Erythrocyte sedimentation rate (mm/h) | 78 (58–87) | 65 (45–81) |
| Lactate dehydrogenasea (U/liter) | 642 (454–899) | 758 (582–870) |
| Creatine phosphokinaseb (U/liter) | 146 (69–334) | 139 (100–233) |
| Troponin-Ic (ng/liter) | 5.80 (1.70–13.90) | 4.50 (1.50–20.40) |
Lactate dehydrogenase was measured for 66% and 58% of patients in the IFN and control groups, respectively.
Creatinine phosphokinase was measured in 42% and 58% of patients in the IFN and control groups, respectively.
Troponin was measured in 35% and 69% of patients in the IFN and control groups, respectively.
Treatment strategies.
Hydroxychloroquine is the main medication in Iran’s national protocol for the treatment of COVID-19. Lopinavir-ritonavir or atazanavir-ritonavir may be added to hydroxychloroquine in severe cases. The antiviral regimens were not significantly different between the two groups. All antivirals were continued for 10 days. Deep-vein thrombosis prophylaxis and stress ulcer prophylaxis were considered for patients where indicated. Based on patients’ clinical conditions, azithromycin, intravenous ascorbic acid, antibiotics, intravenous immunoglobulin (IVIG), or a corticosteroid was added to the antiviral regimens. A corticosteroid (methyl prednisolone, hydrocortisone, or dexamethasone) was administered for 61.9% and 43.6% of patients in the IFN and the control groups, respectively. The corticosteroid dose was equivalent to 250 mg methylprednisolone daily for 3 days. In addition, 35.7% and 25.6% of patients in the IFN and the control group, respectively, received IVIG. The dose of IVIG was 5 g daily for 3 days. Supportive care modalities and administered medications are summarized in Table 3.
TABLE 3.
Supportive care interventions and medications
| Variablea | Value(s) for: |
|
|---|---|---|
| IFN group (n = 42) | Control group (n = 39) | |
| Time from starting symptoms to start of interventions (means ± SD) | 11.70 ± 5.71 | 9.31 ± 4.45 |
| ICU admission, n (%) | 19 (45.23) | 23 (58.97) |
| Respiratory support, n (%) | ||
| Nasal cannula | 2 (4.76) | 1 (2.56) |
| Face mask | 24 (57.14) | 21 (53.84) |
| NIPPV | 1 (2.38) | 0 |
| IMV | 15 (35.71) | 17 (43.58) |
| Medications, n (%) | ||
| Hydroxychloroquine | 40 (95.23) | 39 (100.0) |
| Antiviral regimen | 42 (100) | 39 (100) |
| Atazanavir-ritonavir | 17 (40.47) | 19 (48.71) |
| Lopinavir-ritonavir | 25 (59.52) | 20 (51.28) |
| Azithromycin | 8 (19.04) | 5 (12.82) |
| Vitamin C | 13 (30.95) | 12 (30.76) |
| Broad-spectrum antibiotics | 33 (78.57) | 27 (69.23) |
| Diphenhydramine | 17 (40.47) | 26 (66.66) |
| Antiemetic | 11 (26.19) | 6 (15.38) |
| Opioid | 14 (33.33) | 17 (43.58) |
| Stress ulcer prophylaxis | 42 (100) | 39 (100) |
| Deep-vein thrombosis prophylaxis | 41 (97.61) | 37 (94.87) |
| Statins | 9 (21.42) | 6 (15.38) |
| ARBs | 10 (23.80) | 4 (10.25) |
| Beta-blockers | 8 (19.04) | 2 (5.12) |
| Calcium channel blockers | 7 (16.66) | 5 (12.82) |
| ACEIs | 1 (2.38) | 2 (5.12) |
| Corticosteroid | 26 (61.90) | 17 (43.58) |
| Immunoglobulin | 15 (35.71) | 10 (25.64) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; IMV, invasive mechanical ventilation; NIPPV, noninvasive positive pressure ventilation; SD, standard deviations.
Outcomes and complications.
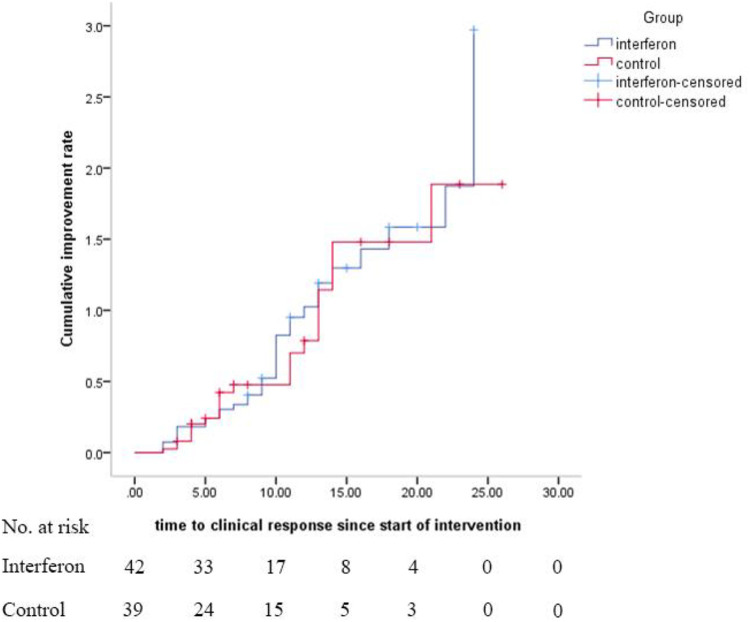
As a primary outcome, the time to clinical response was not significantly different between the IFN and control groups (9.7 ± 5.8 versus 8.3 ± 4.9 days, respectively, P = 0.95), which is shown in the Kaplan-Meier plot (Fig. 2). The log rank test also revealed that there was no statistically significant difference between the groups, considering time to clinical response (hazard ratio [HR], 1.10; 95% CI, 0.64 to 1.87; P = 0.72).
FIG 2.
Kaplan-Meier plot for time to clinical response. Hazard ratio was calculated as 1.10 with 95% CI of 0.64 to 1.87.
The six-category ordinal scale was assessed at days 0, 7, 14, and 28 (Table 4). On day 0, there was no significant difference between the groups in terms of the components of this scale. On day 7 of therapy, 19% of patients in the IFN group were discharged with no deaths. At this time, 28% of patients in the control group were discharged and 25% died. However, the difference was not statistically significant (odds ratio [OR], 0.60; 95% CI, 0.21 to 1.69). On day 14, the results were statistically significant, and 66.7% versus 43.6% of patients in the IFN group and the control group, respectively, were discharged (OR, 2.5; 95% CI, 1.05 to 6.37). When the administration of IVIG and corticosteroid was considered a probable confounding factor, the adjusted odds ratio was 4.05 (95% CI, 1.42 to 11.55).
TABLE 4.
Findings based on the six-category scale at days 0, 7, 14, and 28
| Parameter | Value(s) [no. (%)] for: |
OR (if calculated) | |
|---|---|---|---|
| IFN group (n = 42) | Control group (n = 39) | ||
| Day 0 | |||
| 1-Discharge | |||
| 2-Hospital admission not requiring supplemental oxygen | 1 (2.38) | 0 | |
| 3-Hospital admission, requiring supplemental oxygen | 29 (69.04) | 27 (69.23) | |
| 4-Hospital admission, requiring high-flow nasal cannula or noninvasive mechanical ventilation | 3 (7.14) | 1 (2.56) | |
| 5-Hospital admission, requiring invasive mechanical ventilation | 9 (21.42) | 11 (28.20) | |
| 6-Death | |||
| Day 7 | |||
| 1-Discharge | 8 (19.04) | 11 (28.20) | 0.60 (0.21–1.69) |
| 2-Hospital admission not requiring supplemental oxygen | 2 (4.76) | 0 | |
| 3-Hospital admission, requiring supplemental oxygen | 21 (50.00) | 12 (30.76) | |
| 4-Hospital admission, requiring high-flow nasal cannula or noninvasive mechanical ventilation | 1 (2.38) | 0 | |
| 5-Hospital admission, requiring invasive mechanical ventilation | 10 (23.80) | 6 (15.38) | |
| 6-Death | 0 | 10 (25.64) | |
| Day 14 | |||
| 1-Discharge | 28 (66.66) | 17 (43.58) | 2.5 (1.05–6.37) |
| 2-Hospital admission not requiring supplemental oxygen | 1 (2.38) | 0 | |
| 3-Hospital admission, requiring supplemental oxygen | 5 (11.90) | 6 (15.38) | |
| 4-Hospital admission, requiring high-flow nasal cannula or noninvasive mechanical ventilation | 1 (2.38) | 0 | |
| 5-Hospital admission, requiring invasive mechanical ventilation | 3 (7.14) | 2 (5.12) | |
| 6-Death | 4 (9.52) | 14 (35.89) | |
| Day 28 | |||
| 1-Discharge | 31 (73.80) | 23 (58.97) | 1.96 (0.76–5.01) |
| 2-Hospital admission not requiring supplemental oxygen | 2 (4.76) | 0 | |
| 3-Hospital admission, requiring supplemental oxygen | 1 (2.38) | 0 | |
| 4-Hospital admission, requiring high-flow nasal cannula or noninvasive mechanical ventilation | 0 | 0 | |
| 5-Hospital admission, requiring invasive mechanical ventilation | 0 | 1 (2.56) | |
| 6-Death | 8 (19.04) | 15 (38.46) | |
Regarding the time of IFN initiation, the analysis showed that early administration significantly reduced mortality (OR, 13.5; 95% CI, 1.5 to 118). However, late administration of IFN did not show significant effects (OR, 2.1; 95% CI, 0.48 to 9.6).
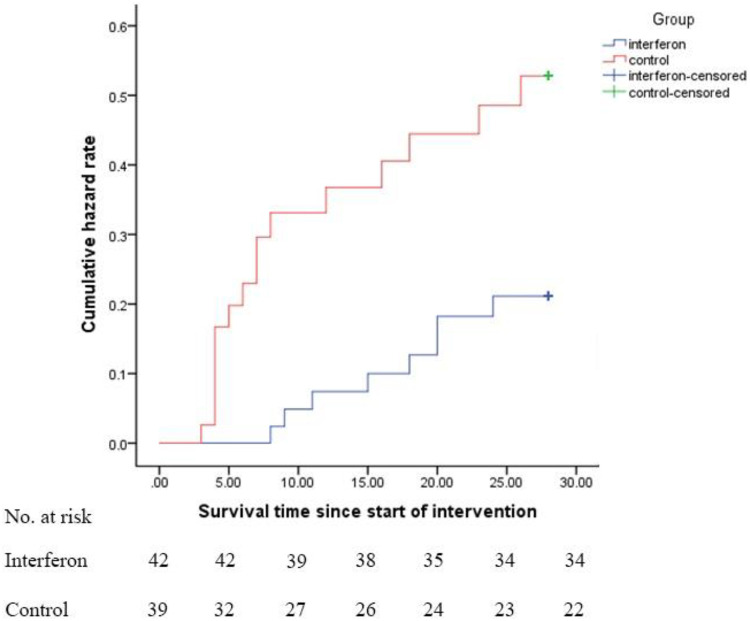
Other secondary outcomes, such as duration of hospital stay, length of ICU stay, and duration of mechanical ventilation, were not statistically different. However, more patients were extubated in the IFN group (P = 0.019). Additionally, the 28-day overall mortality was significantly lower in the IFN than the control group (19% versus 43.6%, respectively, P = 0.015). In multivariate analysis, the effect of IFN on the reduction of mortality was shown (OR, 2.95; 95% CI, 1.08 to 8.03). When the model was adjusted for administration of IVIG and corticosteroid as confounding factors, the effect not only remained but also became stronger (OR, 6.65; 95% CI, 1.67 to 26.45). Kaplan-Meier plot for assessment of survival was also determined (HR, 0.375; 95% CI, 0.16 to 0.87; P = 0.024) (Fig. 3).
FIG 3.
Kaplan-Meier plot for survival. Hazard ratio was calculated as 0.375 with 95% CI of 0.16 to 0.87.
Complications during the hospitalization course, incidence of organ failure, and adverse effects were not different between the groups. Injection-related side effects (fever, chills, myalgia, and headache a few hours after injection of IFN) happened in 8 (19%) patients (Table 5).
TABLE 5.
Outcomes of the study
| Outcome | Value(s) [means ± SD or n (%)] |
P value | |
|---|---|---|---|
| IFN group (n = 42) | Control group (n = 39) | ||
| Time from starting the interventions to the clinical response (days) | 9.74 ± 5.8 | 8.39 ± 4.9 | 0.95 |
| Required invasive mechanical ventilation | 15 (35.71) | 17 (43.58) | 0.30 |
| Extubation ratec (%) | 8 (53.33) | 2 (11.76) | 0.019 |
| Duration of mechanical ventilation (days) | 10.86 ± 5.38 | 7.82 ± 7.84 | 0.47 |
| Duration of ICU stay (days) | 7.71 ± 8.75 | 8.52 ± 7.48 | 0.42 |
| Duration of hospital stay (days) | 14.80 ± 8.45 | 12.25 ± 7.48 | 0.69 |
| Death in hospital | 8 (19.04) | 16 (41.02) | 0.027 |
| Death in general wardsa | 0 | 2 (12.50) | 0.17 |
| Death in ICUb | 8 (42.10) | 14 (60.86) | 0.14 |
| 28-Day mortality | 8 (19) | 17 (43.6) | 0.015 |
| Complications | |||
| Acute kidney injury | 12 (28.57) | 11 (28.20) | 0.58 |
| Nosocomial infections | 11 (26.19) | 5 (12.82) | 0.09 |
| Septic shock | 10 (23.80) | 7 (17.94) | 0.35 |
| Hepatic failure | 5 (11.90) | 9 (23.07) | 0.15 |
| Deep vein thrombosis/pulmonary thromboembolism | 1 (2.38) | 0 | 0.51 |
| Adverse events | |||
| Hypersensitivity reactions | 1 (2.38) | 0 | 0.51 |
| IFN-related injection reactions | 8 (19.04) | 0 | |
| Neuropsychiatric problems | 4 (9.52) | 0 | 0.06 |
| Indirect hyperbilirubinemia | 1 (2.38) | 1 (2.56) | 0.73 |
Twenty-two patients in the IFN group and 16 patients in the control group were in the general ward.
Nineteen patients in the IFN group and 23 patients in the control group were in the intensive critical unit.
The percentage of extubated patients was calculated according to the number of intubated patients.
A hypersensitivity reaction occurred in one patient who received IFN. The reaction presented with maculopapular rash on the trunk and both upper and lower limbs. However, the patient was taking herbal medicine for cough consisting of thyme and honey and other medications, like hydroxychloroquine and lopinavir-ritonavir, concomitantly. Interferon was discontinued after the fourth dose, and rashes began to disappear within 3 days. According to the Naranjo score, the reaction possibly was due to IFN.
Neuropsychiatric problems were detected in 4 patients in the IFN group. Two cases experienced severe agitation and two cases complained of mood swings (mostly depression). One of the patients with mood swings had a history of mild depressive disorder in past years. Out of 4 cases, two patients were in the hospital for nearly 1 month. Neuropsychiatric side effects of IFN are unlikely to happen in short-term use. All patients received a psychiatric consult. According to the Naranjo score, IFN possibly and probably caused neuropsychiatric problems in three and one patients, respectively.
DISCUSSION
The present study was the first randomized, open-label, controlled trial that assessed the efficacy and safety of IFN β-1a in the treatment of patients diagnosed with severe COVID-19. Time to reach the clinical response did not change following adding IFN to the national protocol medications. However, IFN significantly improved the discharge rate by day 14. The 28-day mortality also was significantly lower in the IFN group. Patients who received IFN in the early phase of the disease experienced significantly more benefits from the treatment. Some injection-related adverse effects of IFN occurred, and all were tolerable.
As of now, no effective therapy has been introduced for COVID-19. Some anti-inflammatory agents and cytokine release inhibitors, like corticosteroids and tocilizumab (acting against IL-6), have been proposed (17, 18). However, increasing risk of secondary infections, activation of latent tuberculosis, and other adverse effects are serious concerns (19). Lopinavir-ritonavir did not improve time to the clinical improvement and mortality (5). The efficacy of other therapeutic modalities, like convalescent plasma, is not clear (20).
Among the coronavirus family, the efficacy of IFNs at first was reported in SARS (21). After the subsidence of the SARS epidemic, IFN was again proposed for treatment of another coronavirus, MERS. However, different subtypes of IFN (alpha and beta) in combination with ribavirin did not show significant efficacy in critically ill patients with MERS (22). Due to promising primary effects of IFN β-1 in MERS, a trial for evaluating its efficacy is still running (13). Thus, IFNs, especially type I, are still interesting options for recent epidemics. One study evaluated the effects of IFN β-1b in combination with lopinavir-ritonavir and ribavirin on mild to moderate COVID-19 (23). In addition, nebulized IFN α-2b in combination with oral arbidol was examined for the treatment of the disease (24).
IFNs may have multifunctional roles in the pathogenesis of SARS-CoV-2. IFNs are natural cytokines that are produced in response to viral infections. They can activate interferon-stimulated genes (ISGs) and increase the expression of angiotensin-converting enzyme inhibitor 2 (ACE2). Although the upregulation of ACE2 might increase the risk of SARS-CoV-2 infection, by inactivating angiotensin II, it might protect lung host cells from further injury (25). Viral infections, including SARS-CoV-2, can suppress the production of IFN types 1 and 3 but induce the production of IL-6 and TNF. IFN type 1 might remarkably reduce viral replication (26). Interestingly, SARS-CoV showed the ability to act against the effects of IFNs (27, 28). SARS-CoV encoded a family of proteins, the open reading frame (ORF) family, that inhibits STAT1 transporter from entering the nucleus and blocks interferon signaling (28). However, recently it has been shown that the function of some proteins in this family (ORF6 and ORF3b) had changed in SARS-CoV-2. This may have changed the pathogenesis of SARS-CoV-2 and its interaction with IFN (29).
Beside the antiviral effects of IFNs, the potential role of IFN β-1a in improving ARDS complications was proposed. Expression of CD-73 proteins in lung cells and decrease in vascular leakage in ARDS and subsequent mortality were reported following treatment with intravenous IFN β-1a (15). However, the results were not repeated in the next, larger trial (16). This may be related to the extensive use of glucocorticoids in the latter trial that can interfere with the effect of IFN. The antagonist effects of corticosteroids are considerable (30).
The mean age of patients in the present study was 57 ± 15 years. The mean or median ages were different in published studies, from 46 to 65 years (31, 32). The male gender was dominant in our study, resembling other studies of COVID-19 (5, 32). Although gender difference is evident in many studies of COVID-19, it does not affect outcomes. However, in one study, critically ill males had higher mortality (33). This difference was first explained by the increase in expression of ACE2 (the receptor for entrance of SARS-CoV2 to the cell) in Asian men (34). Later, the issue was attributed to the higher rate of cigarette smoking in Asian men than Asian women. However, both hypotheses should be confirmed in future studies. At present, neither gender nor smoking is certainly correlated with severity of COVID-19 (35).
Baseline vital signs and laboratory data were almost comparable between the groups, with some exceptions. The respiratory rate was significantly higher in the IFN group. The mean blood urea nitrogen level was higher in this group. This may be due to the higher rate of diarrhea as an initial symptom of COVID-19 in this group compared to the control one (19% versus 5.1%). Diarrhea may cause dehydration and lead to higher blood urea nitrogen.
Two patients in the control group were under treatment with warfarin, which may explain the higher INR (international normalized ratio) mean value in this group. Before interpretation of the laboratory results, it should be noted that serum levels of lactate dehydrogenase, creatine phosphokinase, and troponin were not measured for all included patients.
As a primary outcome of the study, time to reach the clinical response was not significantly different between the groups. Considering the dysregulated inflammatory response in the pathogenesis of the late phase of COVID-19, it is not surprising that antivirals do not have immediate effects in relieving the main symptoms at this stage. In the study of Hung et al., as a secondary outcome clinical improvement occurred significantly faster in the IFN combination therapy group (lopinavir-ritonavir plus interferon β-1b plus ribavirin) than in the control group, i.e., 7 versus 12 days (23). However, it should be noted that most of the patients in this study had mild disease. Remdesivir also shortened time to recovery in mild cases, i.e., 11 days compared to 15 days in the placebo group. The definition of time to recovery was somewhat different from that of our study (36). In severe cases, remdesivir showed better results in a 5-day than 10-day course. However, this arm of the study did not have a control group (37).
The length of ICU and hospital stays and duration of mechanical ventilation were not statistically different between the groups, like the other interferon trial (23). However, according to the six-category ordinal scale, more patients were discharged following IFN therapy at day 14. This scale was also used in the study of remdesivir, but the same results were not detected (32). One of the remarkable findings in our study was a decrease in 28-day mortality in the IFN group that was not achieved in other studies on COVID-19 (23, 32). Neither in early use (within 10 days of the onset of the symptoms) nor in the late phase (10 days after the onset of the symptoms) did remdesivir significantly change mortality in patients with severe COVID-19 (32). Although the effect of IFN on decreasing the mortality of patients with severe COVID-19 is surprising, the results should be interpreted with caution and considering the limitations of the study. Four patients in the IFN group died before receiving the fourth dose of IFN. According to the protocol of the study, these patients were excluded from the final analysis for assessing mortality. Further studies are needed to confirm the results.
As in previous reports, the mortality rate in our patients in the control group was high. In patients with severe COVID-19, mortality rate has been reported between 62% and 81% (38, 39). About half of the included patients had severe conditions and were transferred to the ICU. Most were intubated and were under mechanical ventilation.
The clinical course of COVID-19 is divided into early viral replication phase and late cytokine release phase (40). It was suggested that early administration of antiviral medications (within 7 to 10 days of the onset of the symptoms) would improve outcomes of patients with COVID-19 (32). Additionally, early administration of IFNs was recommended in the treatment of MERS (41). The early administration of antiviral agents in viral infections can accelerate viral clearance and postpone neutrophil infiltration. Early administration of IFN β-1a, even in severely ill, mechanically ventilated patients, led to a higher survival rate. Late administration did not show more benefits.
Regarding the safety of IFN therapy in patients with COVID-19, injection-related reactions, including fever, chills, headache, and fatigue (early after injection), were detected in 19% of the patients. All of these symptoms responded to the supportive therapy (acetaminophen) and change in the time of injection to late night. No erythema or injection site reaction, or any reaction that caused treatment interruption, was reported. Considering the duration of the intervention, the incidence rate of IFN adverse reactions was lower than that reported in patients with multiple sclerosis (42). However, it should be accounted that some patients in our study were under mechanical ventilation, and the exact evaluation of these reactions was not feasible. As a component of the supportive care in COVID-19, most patients received analgesic and antipyretic concomitant with antiviral agents and IFN. These medications might mask the adverse reactions of IFN, too.
Nausea, vomiting, and abdominal pain were the most common gastrointestinal complications in our patients, and the incidence rates were not different between the groups. Although two cases experienced slight elevation in serum amylase and lipase levels, in further evaluations, no pancreatitis was confirmed. COVID-19 can cause several gastrointestinal symptoms. However, gastrointestinal symptoms that started after the hospital admission may be related to the medications. The incidence rates of AKI and hepatic impairment were not significantly different between the IFN and the control groups. Both renal and liver injuries can be COVID-19-associated organ dysfunction (43). The nephrotoxicity of medications like antibiotics and furosemide (which were frequently prescribed in our patients) and hepatotoxicity of antiviral agents also should be taken into account. No case of hepatotoxicity that led to the discontinuation of interferon was detected. Indirect hyperbilirubinemia is one of the adverse effects of atazanavir-ritonavir (44).
This study had some limitations. In this open-label, randomized clinical trial, patients in general and intermediate wards and intensive care units were recruited. Most of the general wards actually were intermediate wards, but the accurate classification was not possible due to special and emergent conditions. Due to restrictions in each pandemic event and low experience, the diagnosis of COVID-19 was according to either positive RT-PCR or signs/symptoms plus imaging findings highly suggestive for the disease. In addition, in assessing viral load, follow-up PCR and imaging was not possible due to limitations and emergent conditions.
Conclusions.
Although IFN did not change the time to reach a clinical response, added to the national protocol, it significantly increased the discharge rate on day 14 and decreased 28-day mortality. Improved survival rate was significant when patients received IFN β-1a in the early phase of the disease. Adverse effects of IFN β-1a were injection-related, neuropsychiatric problems, and hypersensitivity reactions that all were tolerable and resolved during the follow-up period.
MATERIALS AND METHODS
Study design.
This open-label, randomized clinical trial was conducted to assess the efficacy and safety of IFN-β-1a in the treatment of adult (aged ≥18 years) patients diagnosed with COVID-19. Patients were admitted from 29 February to 3 April 2020 in Imam Khomeini Hospital Complex, the main central hospital in Tehran, capital of Iran.
Eligibility criteria.
The diagnosis of COVID-19 was according to either a positive real-time PCR (RT-PCR) of the deep nasopharyngeal secretions or clinical signs/symptoms plus imaging findings highly suspicious for COVID-19. RNA extraction was performed using the viral nucleic acid extraction kit (no. YVN50/YVN100) provided by RBC Bioscience, Taipei, Taiwan. After processing nasopharyngeal samples, rapid RT-PCR was performed by a novel coronavirus (2019-nCOV) nucleic acid diagnostic kit (PCR-fluorescence probing) from Sansure Biotech (S3102E) (Changsha, China) according to the instructions of the manufacturer.
Cough, fever, myalgia, dyspnea, headache, chills, anorexia, gastrointestinal problems, and chest discomfort were considered symptoms of the disease. More than 50% involvement of the field of examination with typical findings of COVID-19, including peripheral, bilateral ground glass opacities (GGO) or multifocal GGO of rounded morphology with or without consolidations or crazy paving, was defined as a positive computed tomography scan.
Patients with severe COVID-19 were included (45). These patients had at least one of the following conditions: (i) hypoxemia (need for noninvasive or invasive respiratory support to provide capillary oxygen saturation above 90%), (ii) hypotension (systolic blood pressure less than 90 mm Hg or vasopressor requirement), (iii) renal failure secondary to COVID-19 (according to KDIGO definition) (46), (iv) neurologic disorder secondary to COVID-19 (decrease of 2 or more scores on the Glasgow Coma Scale), (v) thrombocytopenia secondary to COVID-19 (platelet count less than 150,000/mm3), and (vi) severe gastrointestinal symptoms secondary to COVID-19 (vomiting/diarrhea that caused at least mild dehydration).
Allergy to IFNs, receiving IFNs for any other reason, severe depression, previous suicide attempts, alanine amino transferase (ALT) >5× the upper limit of the normal range, and pregnant women were defined as the exclusion criteria of the study.
The Ethics Committee of Tehran University of Medical Sciences approved the study (approved ID IR.TUMS.VCR.REC.1398.1052), and the protocol of the trial was registered (registered ID IRCT20100228003449N28). The protocol of the study was explained to the patients, and written informed consents were obtained from them or from their next of kin.
Sample size and randomization.
Due to the lack of a similar study at the start of this trial, for producing significant difference, the estimated time to clinical response was assumed. By estimating 15 days to reach clinical response and to detect a difference of 5 days, the sample size was estimated. A number of 46 patients was estimated for each group.
Randomization was performed for all patients. An allocation ratio of 1:1 was accounted between IFN and the control group in randomization, but the patients had to receive at least four injections of IFN to be included in the final analysis. The randomization was performed by permuted block randomization and block sizes of 2, 4, and 6, used randomly. The statistician prepared a list of numbers for randomization. Sequentially numbered opaque envelopes were prepared according to the list, and envelopes were delivered to the clinical investigators. The statistician that performed randomization and analysis was unaware of the treatment and follow-up of patients.
Treatment protocols.
Patients in the IFN group received IFN β-1a in addition to the national protocol medications. Each 44-μg/ml (12 million IU/ml) dose of interferon β-1a (ReciGen, CinnaGen Co., Iran) was subcutaneously injected three times weekly for two consecutive weeks. The control group received only the standard of care. The standard of care (the hospital protocol) consisted of hydroxychloroquine (400 mg twice a day [BID] on the first day and then 200 mg BD) plus lopinavir-ritonavir (400 and 100 mg, respectively, BD) or atazanavir-ritonavir (300 and 100 mg, respectively, daily) for 7–10 days. Primary care, respiratory support, fluid, electrolyte, analgesic, antipyretic, corticosteroid, and antibiotic treatments were recommended in the hospital protocol if indicated. The duration of the study was 2 weeks, and the patients were monitored for 4 weeks.
Demographic data, baseline characteristics, and laboratory data were recorded for each patient. APACHE II score at the time of ICU admission was calculated for critically ill patients. All patients were daily monitored in terms of vital signs, medical interventions, and clinical conditions during the study course.
The need for respiratory support (invasive, noninvasive, or not required) was assessed by a physician regularly. Patients were assessed to fit in one of the six categories of the ordinal scale at days 0, 7, 14, and 28 of inclusion (47). If discharged, the patient was followed up by phone. Readmission was surveyed until 3 May.
Outcomes assessment.
The primary outcome of the study was time to reach clinical response. Clinical response was defined according to the six-category ordinal scale (47). This scale classifies patients into six categories according to the severity of the viral pneumonia: (1) discharge; (2) hospital admission, not requiring oxygen; (3) hospital admission, requiring oxygen; (4) hospital admission, requiring noninvasive positive pressure ventilation; (5) hospital admission, requiring invasive mechanical ventilation; (6) death. Time to clinical response was considered the number of days required to at least two scores of improvement on the scale or patient’s discharge, whichever occurred sooner. Secondary outcomes were duration of mechanical ventilation, duration of hospital stay, length of ICU stay, 28-day mortality, effect of early or late (before or after 10 days of the onset of symptoms) administration of IFN on mortality, adverse effects, and complications during the hospitalization. The following adverse effects of the antiviral regimen/IFN β-1a and complications during the hospitalization course were assessed: gastrointestinal (nausea, vomiting, diarrhea, abdominal pain, and pancreatitis), anaphylaxis and allergic reactions (rash, urticaria, angioedema, bronchospasm, and dyspnea related to medication administration), IFN injection-related reaction (skin erythema and necrosis, chills, fever, and flu-like symptoms after injection), neuropsychiatric (sleep disorder, psychosis, agitation, depression, and mania), renal impairment (according to KDIGO definition) (46), hepatic impairment (hepatic aminotransferase serum levels raised more than three times the upper limit of normal or serum total bilirubin above 2 mg/dl) (48), indirect hyperbilirubinemia (direct bilirubin level less than 15% of the total bilirubin) (49), incidence of thromboembolism (deep-vein thrombosis or pulmonary thromboembolism), incidence of nosocomial infections, and diagnosis of septic shock (according to the surviving sepsis campaign guidelines) (50). The Naranjo scale was used for evaluation of adverse effects of IFN. In this standard scale, several items, including previous reports, relationship with starting the agent, improvement after discontinuation, challenge result, alternative causes, data of drug assay, dose dependency, and patient’s history of the same reaction were considered. Total scores of >8, 5 to 8, and 1 to 4 were considered definite, probable, and possible correlations between the use of IFN and the adverse drug reaction, respectively (51).
Statistical analysis.
The quantitative variables were reported as means ± standard deviations (SD) if they had normal distributions or as median with IQR if they did not pass the normality test. The qualitative ones were reported as number (percent). For comparing the quantitative variables, t test or Mann-Whitney test was used. The qualitative variables were compared by chi-square test.
The analysis was performed on a per-protocol basis, and patients who did not receive at least four doses of IFN were not included. The Kaplan-Meier plot and log rank test were used to compare the number of days to reach the clinical response and survival time between the groups. The HR and 95% CI for clinical improvement and death were estimated by Cox proportional hazards model. The odds ratio was also calculated for patients who received IFN early versus late. The multiple logistic regression model was performed for the variables that were significantly different between the groups according to the univariate analysis. In the multiple logistic regression model, corticosteroid and immunoglobulin were adjusted as confounding factors, and the adjusted odds ratio was reported. SPSS software (version 24) was used for statistical analyses.
ACKNOWLEDGMENTS
We thank the nurses and other staff members of Imam Khomeini Hospital Complex for their kind support and Ava Khalili for proofreading the manuscript.
We did not receive any funding for this work. ReciGen was a generous gift from CinnaGen Co.
We have no conflict of interest to declare.
REFERENCES
- 1.Wang C, Horby PW, Hayden FG, Gao GF. 2020. A novel coronavirus outbreak of global health concern. Lancet 395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2020. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland: https://covid19.who.int/?gclid=Cj0KCQjwl4v4BRDaARIsAFjATPmRcsB7VPlJAxJMhXU5bnYKH2jMgjcLtozUR_fRAeH7rKbf_PV7IbIaAuU6EALw_wcB. Accessed 6 July 2020. [Google Scholar]
- 5.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. 2020. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Tian Z, Yang X. 2020. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 7.Meyerowitz E, Vannier AGL, Friesen MGN, Schoenfeld S, Gelfand JA, Callahan MV, Kim AY, Reeves PM, Poznansky MC. 2020. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J 34:6027–6037. doi: 10.1096/fj.202000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham AC, Goh HP, Koh D. 2020. Treatment of COVID-19: old tricks for new challenges. Crit Care 24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scagnolari C, Vicenzi E, Bellomi F, Stillitano MG, Pinna D, Poli G, Clementi M, Dianzani F, Antonelli G. 2004. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther 9:1003–1011. [PubMed] [Google Scholar]
- 10.Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, Hayden FG, Yuen KY. 2013. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect 67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart BJ, Dyall J, Postnikova E, Zhou H, Kindrachuk J, Johnson RF, Olinger GG, Frieman MB, Holbrook MR, Jahrling PB, Hensley L. 2014. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J Gen Virol 95:571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. 2020. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Assiri AM, Al-Hameed F, AlSaedi A, Mandourah Y, Almekhlafi GA, Sherbeeni NM, Elzein FE, Memon J, Taha Y, Almotairi A, Maghrabi KA, Qushmaq I, Al Bshabshe A, Kharaba A, Shalhoub S, Jose J, Fowler RA, Hayden FG, Hussein MA, the MIRACLE trial group. 2018. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials 19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. 2020. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellingan G, Maksimow M, Howell DC, Stotz M, Beale R, Beatty M, Walsh T, Binning A, Davidson A, Kuper M, Shah S, Cooper J, Waris M, Yegutkin GG, Jalkanen J, Salmi M, Piippo I, Jalkanen M, Montgomery H, Jalkanen S. 2014. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. Lancet Respir Med 2:98–107. doi: 10.1016/S2213-2600(13)70259-5. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri VM, Pettilä V, Karvonen MK, Jalkanen J, Nightingale P, Brealey D, Mancebo J, Ferrer R, Mercat A, Patroniti N, Quintel M, Vincent JL, Okkonen M, Meziani F, Bellani G, MacCallum N, Creteur J, Kluge S, Artigas-Raventos A, Maksimow M, Piippo I, Elima K, Jalkanen S, Jalkanen M, Bellingan G, INTEREST Study Group. 2020. Effect of intravenous interferon β-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. JAMA 323:725. doi: 10.1001/jama.2019.22525. [DOI] [PubMed] [Google Scholar]
- 17.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. 2020. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol 92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. 2020. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Li L, Shen A, Chen Y, Qi Z. 2020. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig 40:511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roback JD, Guarner J. 2020. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 323:1561. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 21.Cinat J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. 2003. Treatment of SARS with human interferons. Lancet 362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, Jose J, Alraddadi B, Almotairi A, Al Khatib K, Abdulmomen A, Qushmaq I, Sindi AA, Mady A, Solaiman O, Al-Raddadi R, Maghrabi K, Ragab A, Al Mekhlafi GA, Balkhy HH, Al Harthy A, Kharaba A, Gramish JA, Al-Aithan AM, Al-Dawood A, Merson L, Hayden F, Fowler R. 2020. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: a multicenter observational study. Clin Infect Dis 70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung IF, Lung KC, Tso EY, Liu R, Chung TWH, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AKL, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CCY, Zhang RR, Fung AYF, Yan EYW, Leung KH, Ip JD, Chu AWH, Chan WM, Ng ACK, Lee R, Fung K, Yeung A, Wu TC, Chan JWM, Yan WW, Chan WM, Chan JFW, Lie AKW, Tsang OTY, Cheng VCC, Que TL, Lau CS, Chan KH, To KKW, Yuen KY. 2020. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Chen V, Shannon CP, Wei XS, Xiang X, Wang X, Wang ZH, Tebbutt SJ, Kollmann TR, Fish EN. 2020. Interferon-α2b treatment for COVID-19. Front Immunol 11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su S, Jiang S. 2020. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal Transduct Target Ther 5:71. doi: 10.1038/s41392-020-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. 2020. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopecky-Bromberg SA, Martínez-Sobrido L, Frieman M, Baric RA, Palese P. 2007. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol 81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS. 2007. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol 81:9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokugamage KG, Schindewolf C, Menacher VD. 2020. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv doi: 10.1101/2020.03.07.982264. [DOI]
- 30.Flammer JR, Dobrovolna J, Kennedy MA, Chinenov Y, Glass CK, Ivashkiv LB, Rogatsky I. 2010. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol 30:4564–4574. doi: 10.1128/MCB.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Zheng F, Qing Y, Ding S, Yang D, Lei C, Yin Z, Zhou X, Jiang D, Zuo Q, He J, Lv J, Chen P, Chen Y, Peng H, Li H, Xie Y, Liu J, Zhou Z, Luo H. 2020. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv doi: 10.1101/2020.03.03.20030353. [DOI] [Google Scholar]
- 32.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. 2020. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicenter trial. Lancet 395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shan Y. 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. 2020. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv doi: 10.1101/2020.01.26.919985. [DOI] [PMC free article] [PubMed]
- 35.Cai H. 2020. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, de Castilla DL, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members. 22 May 2020. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A, GS-US-540–5773 Investigators. 16 May 2020. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss P, Murdoch DR. 2020. Clinical course and mortality risk of severe COVID-19. Lancet 395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. 2020. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao W, Li T. 2020. COVID-19: towards understanding of pathogenesis. Cell Res 30:367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, Sompallae R, McCray PB, Meyerholz DK, Perlman S. 2019. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Investig 129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, Zhu Y, Crossman M, Nestorov I, Gronke RS, Baker DP, Rogge M, Subramanyam M, Davar G. 2012. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol 52:798–808. doi: 10.1177/0091270011407068. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. 2020. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institute of Diabetes and Digestive and Kidney Diseases. 2017. LiverTox: clinical and research information on drug-induced liver injury. Atanazir. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: https://www.ncbi.nlm.nih.gov/books/NBK547918/. [PubMed] [Google Scholar]
- 45.Wu Z, McGoogan JM. 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 46.Khwaja A. 2012. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 47.Peterson RL, Vock DM, Powers JH, Emery S, Cruz EF, Hunsberger S, Jain MK, Pett S, Neaton JD, INSIGHT FLU-IVIG Study Group. 2017. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials 14:264–276. doi: 10.1177/1740774517697919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lescot T, Karvellas C, Beaussier M, Magder S. 2012. Acquired liver injury in the intensive care unit. Anesthesiology 117:898–904. doi: 10.1097/ALN.0b013e318266c6df. [DOI] [PubMed] [Google Scholar]
- 49.Singh A, Jialal I. 2020. Unconjugated hyperbilirubinemia In StatPearls. StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- 50.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 51.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. 1981. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]