Abstract
The study aims to develop a new mathematical model in order to explain the dynamics of viral infections in vivo such as HIV infection. The model includes three classes of cells, takes into account the cure of infected cells in latent period and also incorporates three modes of transmission. The mention modes are modeled by three general incidence functions covering several special cases available in the literature. The basic properties of the model as well as its stability analysis have been carried out rigorously. Further, an application is given and also numerical simulation results have been incorporated supporting the analytical results.
Keywords: Viral infection, Latently infected cells, Mathematical modeling, Asymptotic stability
1. Introduction
Dangerous viruses have been appearing from time to time attacking the human body and causing several health problems, deaths and socio-economic damage worldwide. For instance, a novel coronavirus (nCoV) was identified on January 7, 2020 responsible for the initial cases of pneumonia occurred in Wuhan, China. This virus has recently been reported as extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses [7]. Because its genetic sequence is very similar to that of severe acute respiratory syndrome (SARS), another coronavirus that appeared for first time in 2002 and caused a pandemic with more than 8000 infected people and 800 deaths. Another coronavirus that causes severe disease in humans is middle east respiratory syndrome (MERS), identified for the first time in 2012 in the Middle East having connection with camels. As of 24 March 2020, more than 372,757 cases of SARS-CoV-2 have been confirmed in 196 countries and territories, including 195,511 cases in European region, and the number of deaths from the virus has reached 16,231 globally, according to the World Health Organization (WHO) [27]. Also, already existing viruses like hepatitis B virus (HBV) and human immunodeficiency virus (HIV) are still causing large numbers of deaths worldwide. As per an estimation in 2015, 257 million people were living with chronic hepatitis B infection (defined as hepatitis B surface antigen positive), and death of 887,000 people, mostly due to cirrhosis and hepatocellular carcinoma (i.e. primary liver cancer) [28]. Also, 770,000 people died from HIV-related causes and 1.7 million people were newly infected in 2018 [29].
The rapid spread of viruses has been attracting the attention of researchers to carry out new researches to understand various associated aspects. It has been shown biologically that viruses can spread by both modes, viz., virus-to-cell mode via the extracellular environment and through cell-to-cell transmission mode by direct cell-cell contact [13], [14], [23]. During cell-to-cell transmission, it is possible for several viral particles to be transferred from infected to uninfected cells via structures called infectious or virological synapses [13]. In the case of HIV infection, cell-to-cell transmission may be 10–1000 times more effective than the passive dissemination of virons via the extracellular environment [2], [4], [5]. In [30], HIV is used as a model virus in understanding the processes that consequence in viral spread via virus-to-cell or cell contact dependent mode. In the same work, it is demonstrated that HIV can spread by virus-to-cell mode if all steps of the viral replication cycle are efficient. If any of these steps is impaired, then co-cultures of HIV donor cells with specific target cells allow effective viral spreading.
Latently infected cells are those cells which include the viruses but do not producing them due to time lag between both the moment of infection and the moment when the infected cell becomes active to create new infectious viruses. For some viruses like herpesvirus, latency is an essential mechanism for immune evasion and viral persistence [16], [17]. One of the major hurdles in eliminating HIV infection completely is the presence of a small reservoir of latently infected cells that can survive through long-term suppression of viral replication by extremely active anti-retroviral therapy (HAART) [6], [21], [22]. A recent study found that cell-to-cell transmission contributes to the production of latently infected cells [1]. This mode of viral transmission is found to contribute to both the spread and pathogenesis of the virus in vivo and to the production and maintenance of latent reservoirs that represent a serious barrier to the curing of the infection [19].
The above biological studies inspire to propose the following model:
| (1) |
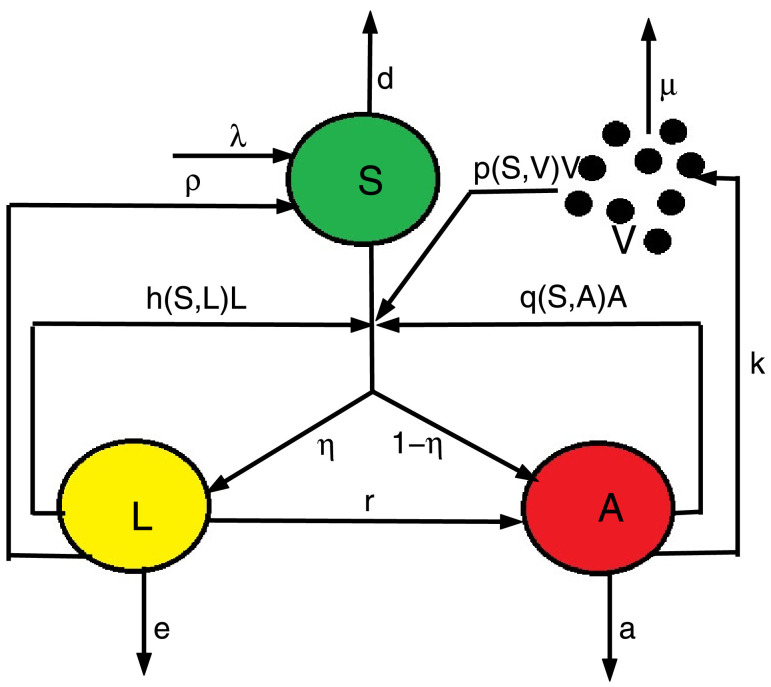
where S(t), L(t), A(t) and V(t) respectively indicate the concentration of susceptible cells, latently infected cells, actively infected cells and free virus particles at time t. It is also considered that susceptible cells are generated at a constant rate of λ, die at a rate of dS and become infected either through free virus at a rate of p(S, V)V, or through direct contact with actively infected cells at a rate of q(S, A)A, or by direct contact with latently infected cells at a rate of h(S, L)L. The term then describes the total infection rate of susceptible cells. It is assumed a fraction η of infected cells to be latently infected cells and the remaining becomes actively infected cells, where 0 < η ≤ 1. We take e and a as the parameters for the death rates of latently and actively infected cells. Also, r, k and μ are the parameters respectively for the production rate of virus from actively infected cells, the rate at which the latently infected cells are transmitted to actively infected cells, and the clearance rate of virus. Fig. 1 is an illustration of the schematic diagram of model (1).
Fig. 1.
Schematic diagram of model (1).
As in Hattaf and Yousfi [10], 11], the general incidences p(S, V), q(S, A) and h(S, L) are assumed to be continuously differentiable in the interior of and also satisfy the following hypotheses:
-
(H1)
∀ S, V ≥ 0.
-
(H2)
∀ S, A ≥ 0.
-
(H3)
∀ S, L ≥ 0.
It should be noted that model (1) extends as well as generalizes many special cases available in the literature. As example, one can get the latent infection model of Pankavich [18] with and where β 1 is the virus-to-cell infection rate. Also, the model of Callaway and Perelson [3] with and where ϵ is an overall drug efficacy. Furthermore, the model (1) includes the class of HIV infection models for different rates of infection and latently infected cells as investigated in Wang et al. [26].
The model (1) reduces to
| (2) |
when all of infected cells become latently infected cells (i.e. ).
This system includes the following viral models:
-
•
Model of Rong et al. [20] with and .
-
•
Model of Hu et al. [9] with and .
-
•
Model of Sun et al. [24] with and .
-
•
Model of Wang et al. [25] with and .
-
•
Model of Maziane et al. [15] with and where α 1, α 2 and α 3 are nonnegative constants measuring the saturation or inhibitory effect.
The paper has been organized as follows: In Section 2, the existence of equilibria and the well-posedness of the model including nonnegativity and boundedness of the solutions have been established. The stability analysis of the equilibria has been presented in Section 3. An application and illustrative numerical simulations have been given in Section 4. The Section 5 is for conclusion in terms of both mathematical and biological perspectives of the findings.
2. Well-posedness and equilibria
This section first establishes the well-posedness of our model (1) by proving the nonnegativity and boundedness of solutions. Then we derive the threshold parameter for the existence of equilibria.
Theorem 2.1
All solutions of model (1) with nonnegative initial conditions remain nonnegative and bounded ∀ t > 0.
Proof
We have
Then is positively invariant with regard to (1). Next, we prove the boundedness of solutions. Denote
Then
where . Therefore,
which implies that S(t), L(t), A(t) and V(t) are bounded. □
It is obvious that model (1) has always one infection-free equilibrium Ep(S 0, 0, 0, 0), where . Then we define the basic reproduction number of (1) as follows:
| (3) |
where . This number can be rewritten as follows
where
The other equilibrium of model (1) satisfies the following system
| (4) |
| (5) |
| (6) |
| (7) |
Since we have . This implies that there is no biological equilibrium when . Let G be a function defined on the closed interval as follows
We have and
Then the equation admits a unique solution if . Therefore, model (1) has a unique chronic infection equilibrium E*(S*, L*, A*, V*) when .
The pervious discussions are summarized in the following theorem.
Theorem 2.2
3. Stability analysis
This section deals with the stability analysis of two equilibria Ep and E*. For more details, the local stability is obtained by analyzing the characteristic equation. However, the global stability is established by using two techniques, the first is based on the definition which consists to prove that the solution of the model converges to the equilibrium point independently of the initial conditions. The second technique is based on the direct Lyapunov method which aims to construct an appropriate Lyapunov functional.
Theorem 3.1
The infection-free equilibrium Ep is locally asymptotically stable if and unstable if .
Proof
The characteristic equation of model (1) at Ep is given by
which lead to
(8) where
It is obvious that is a negative root of Eq. (8). The other roots are determined by the following equation:
(9) If then a 1 > 0, a 2 > 0, a 3 > 0 and
By the Routh-Hurwitz Theorem [8], we know that all roots of (9) have negative real parts. Thus, the infection-free equilibrium Ep is locally asymptotically stable if .
If then a 3 < 0. So, Eq. (9) has at least one positive root. This implies that the infection-free equilibrium Ep becomes unstable when . □
The following theorem characterizes the global stability of the infection-free equilibrium Ep when .
Theorem 3.2
The infection-free equilibrium Ep is globally asymptotically stable when .
Proof
We have the following inequality from the first equation of (1)
which implies that
Then for arbitrary ϵ > 0, there exist a t 1 > 0 such that
From the last three equations of (1), for t ≥ t 1, we have
Consider the following comparison system
This system can be rewritten as follows
where and
The eigenvalues of the matrix M satisfy the following equation:
with
and
Clearly, . Since we can fix an ϵ > 0 small enough such that R 0(ϵ) < 1. Thus, b 1 > 0, b 2 > 0, b 3 > 0 and
It follows from the Routh-Hurwitz Theorem that all eigenvalues of M have negative real parts. Therefore,
Hence, we conclude that (L(t), A(t), V(t)) → (0, 0, 0) as for .
From the first equation of (1), for t ≥ t 1, we can get
Consequently,
This completes the proof of Theorem 3.2. □
Next, we investigate the global asymptotic stability of the chronic infection equilibrium E*(S*, L*, A*, V*) by assuming that and the incidence functions p, q and h satisfy, ∀ S, L, A, V > 0, the following further hypothesis:
Theorem 3.3
Suppose that and (H 4 ) holds. Then the chronic infection equilibrium E* is globally asymptotically stable for and .
Proof
Consider the following Lyapunov functional
where for z > 0. It is clear that Φ(z) ≥ 0 for all z > 0, and if and only if . Thus, W(S, L, A, V) > 0 for all S, L, A, V > 0 and . By a simple computation, we get
Since the incidence function p(S, V) is strictly monotonically increasing with respect to S, we deduce that
By (H 4), we obtain
and
Since Φ(x) ≥ 0, we have with equality if and only if and . From LaSalle’s invariance principle [12], we conclude that the chronic infection equilibrium E* is globally asymptotically stable when . □
4. Application and numerical simulations
In this section, we first apply our main results to the following HIV infection model:
| (10) |
which is a special case of model (1) by choosing and with β 2 and β 3 are the cell-to-cell transmission rates through actively and latently infected cells, respectively.
Obviously, the hypotheses are satisfied. Further, we have
and
Then the assumption (H 4) is also satisfied. In this special case, the basic reproduction number has the following form:
| (11) |
According to Theorems 3.2 and 3.3, we deduce the following result.
Corollary 4.1
Let defined by (11) .
Now, we carry out numerical simulations to confirm the above analytical results concerning the dynamics of model with the parameter values given in Table 1 .
Table 1.
Parameter values of model (10).
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| λ | 10 | a | 0.27 |
| d | 0.0139 | k | 50 |
| β1 | Varied | μ | 3 |
| β2 | e | 0.06 | |
| β3 | ρ | Varied | |
| α1 | 0.1 | r | 0.01 |
| α2 | 0.01 | η | Varied |
| α3 | 0.01 |
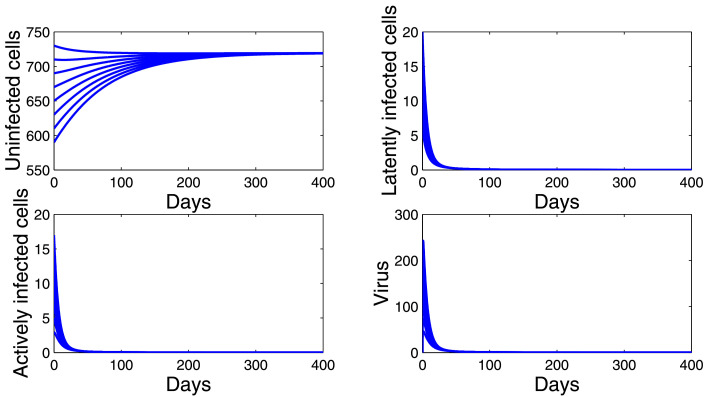
Firstly, we choose and . By a simple computation, we have . Hence, model (10) has an infection-free equilibrium Ep(719.4245, 0, 0, 0). Fig. 2 shows that the trajectories of (10) starting from different initial values tend to Ep. This supports the global stability result given by Corollary 4.1(i).
Fig. 2.
Dynamics of the model (10) when .
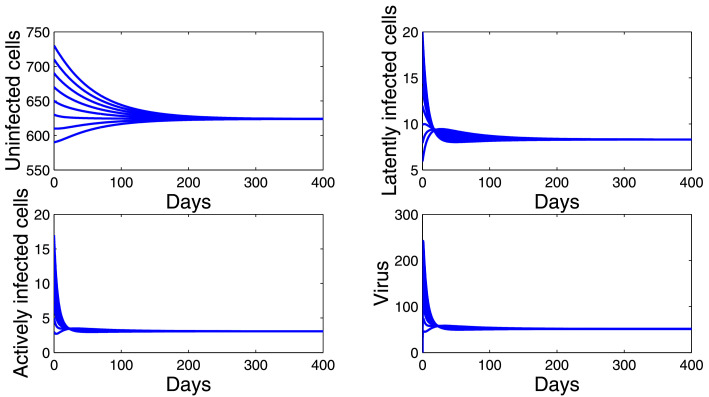
Secondly, we choose and . In this case, we have . Fig. 3 shows that the trajectories of model (10) starting from different initial values converge to E*(628.2521, 8.3904, 3.1097, 51.8294). This confirms the global stability result given by Corollary 4.1(ii).
Fig. 3.
Dynamics of the model (10) when .
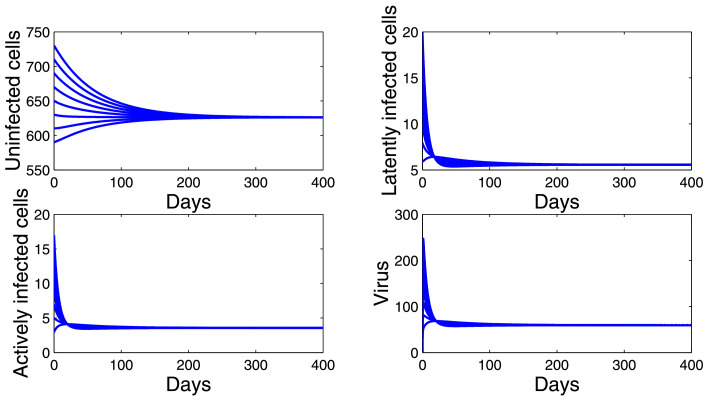
Thirdly, we choose and . Then . Fig. 4 shows that E*(618.5765, 5.4708, 3.5012, 58.3445) is globally asymptotically stable despite ρ ≠ 0 and η ≠ 1. Thus, the conditions and are not necessary for the global asymptotic stability of the chronic infection equilibrium E*. We will prove analytically this open problem in future work.
Fig. 4.
Dynamics of the model (10) when .
5. Conclusions
The work developed a model by using tools and techniques of mathematics for infection by viruses with latently infected cells and cell-to-cell transmission. The well-posedness of the model, including nonnegativity and boundedness of solutions have been established. It also identified two equilibria, viz. an infection-free equilibrium Ep and chronic infection equilibrium E*. It is proved that Ep is globally asymptotically stable if the basic reproduction number is less than one, which implies that the virus is eradicable and the infection is curable. And, when the basic reproduction number is greater than one, Ep becomes unstable and E* is globally asymptotically stable for and . In this case, the virus persists in the host. On the other hand, the models and results presented in Callaway and Perelson [3], Hu et al. [9], Maziane et al. [15], Pankavich [18], Rong et al. [20], Sun et al. [24], Wang et al. [25], 26] are improved and generalized.
It is observed from the explicit formula of the basic reproduction number given in (3) that is as sum of the basic reproduction numbers for the classical virus-to-cell infection mode cell-to-cell transmission mode via actively infected cells and cell-to-cell transmission mode via latently infected cells . Hence, ignoring the cell-to-cell transmission in viral infections dynamics will underestimate . Further, the study of this paper can be of help for the biologists to develop effective treatments against viral infections in order to reduce the patients’ basic reproduction number to less than one and eliminate the latent reservoirs which represent the main obstacle to the eradication of virus from the human body.
Credit statement
Author 1 discussed the research problem with Author 2 first. Author 2 gave his opinion on the formulation of the model and possible solutions. All the authors contributed in developing the main results and their proofs. All authors contributed in each section of the paper and approved the paper for submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Karmina K Ali, University of Zakho for reading the manuscript and help in formatting the paper. The authors are also grateful to the editor and anonymous reviewers for their careful reading and helpful suggestions.
Contributor Information
Khalid Hattaf, Email: k.hattaf@yahoo.fr.
Hemen Dutta, Email: hemen_dutta08@rediffmail.com.
References
- 1.Agosto L.M., Herring M.B., Mothes W., Henderson A.J. HIV-1-infected CD4T cells facilitate latent infection of resting CD4T cells through cell-cell contact. Cell Rep. 2018;24(8):2088–2100. doi: 10.1016/j.celrep.2018.07.079. [DOI] [PubMed] [Google Scholar]
- 2.Carr J.M., Hocking H., Li P., Burrell C.J. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–329. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 3.Callaway D.S., Perelson A.S. HIV-1 infection and low steady state viral loads. Bull Math Biol. 2002;64:29–64. doi: 10.1006/bulm.2001.0266. [DOI] [PubMed] [Google Scholar]
- 4.Chen P., Hubner W., Spinelli M.A., Chen B.K. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitrov D.S., Willey R.L., Sato H., Chang L.J., Blumenthal R., Martin M.A. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993;67(4):2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 7.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species severe acute respiratory syndromerelated coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020:1–9. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gradshteyn I.S., Ryzhik I.M. Academic Press; 2000. Routh-Hurwitz theorem, in tables of integrals, series, and products. [Google Scholar]
- 9.Hu Z., Pang W., Liao F., Ma W. Analysis of a CD4T cell viral infection model with a class of saturated infection rate. Discrete Contin Dyn Syst Ser B. 2014;19(3):735–745. [Google Scholar]
- 10.Hattaf K., Yousfi N. A generalized virus dynamics model with cell-to-cell transmission and cure rate. Adv Differ Equ. 2016;2016 [Google Scholar]; Article 174
- 11.Hattaf K., Yousfi N. Qualitative analysis of a generalized virus dynamics model with both modes of transmission and distributed delays. Int J Differ Equ. 2018;2018 [Google Scholar]; Article ID 9818372.
- 12.LaSalle J.P. Regional conference series in applied mathematics. SIAM Philadelphia; 1976. The stability of dynamical systems. [Google Scholar]
- 13.Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mothes W., Sherer N.M., Jin J., Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–8368. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maziane M., Lotfi E.M., Hattaf K., Yousfi N. Dynamics of a class of HIV infection models with cure of infected cells in eclipse stage. Acta Biotheor. 2015;63(4):363–380. doi: 10.1007/s10441-015-9263-y. [DOI] [PubMed] [Google Scholar]
- 16.Speck S.H., Ganem D. Viral latency and its regulation: lessons from the g-herpesviruses. Cell Host Microbe. 2010;8:100–115. doi: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perng G.C., Jones C. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdiscip Perspect Infect Dis. 2010;2010:1–18. doi: 10.1155/2010/262415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankavich S. The effects of latent infection on the dynamics of HIV. Differ Equ Dyn Syst. 2016;24(3):281–303. [Google Scholar]
- 19.Pedro K.D., Henderson A.J., Agosto L.M. Mechanisms of HIV-1 cell-to-cell transmission and the establishment of the latent reservoir. Virus Res. 2019;265:115–121. doi: 10.1016/j.virusres.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong L., Gilchrist M.A., Feng Z., Perelson A.S. Modeling within-host HIV-1 dynamics and the evolution of drug resistance: trade-offs between viral enzyme function and drug susceptibility. J Theor Biol. 2007;247:804–818. doi: 10.1016/j.jtbi.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruelas D.S., Greene W.C. An integrated overview of HIV-1 latency. Cell. 2013;155:519–529. doi: 10.1016/j.cell.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strain M.C., Günthard H.F., Havlir D.V., Ignacio C.C., Smith D.M., Leigh-Brown A.J., Macaranas T.R., Lam R.Y., Daly O.A., Fischer M., Opravil M., Levine H., Bacheler L., Spina C.A., Richman D.D., Wong J.K. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q., Min L., Kuang Y. Global stability of infection-free state and endemic infection state of a modified human immunodeficiency virus infection model. IET Syst Biol. 2015;9(3):95–103. doi: 10.1049/iet-syb.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J., Lang J., Liu X. Global dynamics for viral infection model with Beddington-DeAngelis functional response and an eclipse stage of infected cells. Discrete Contin Dyn Syst B. 2015;20(9):3215. [Google Scholar]
- 26.Wang H., Xu R., Wang Z., Chen H. Global dynamics of a class of HIV-1 infection models with latently infected cells. Nonlinear Anal Model Control. 2015;20(1):21–37. [Google Scholar]
- 27.WHO, coronavirus disease. 2019a. (COVID-19) Situation Report-64. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200324-sitrep-64-covid-19.
- 28.WHO, hepatitis b, july. 2019b. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 29.WHO, HIV/AIDS, november. 2019c. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
- 30.Zhong P., Agosto L.M., Ilinskaya A., Dorjbal B., Truong R., Derse D., Uchil P.D., Heidecker G., Mothes W. Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV. PLoS One. 2013;8(1):E53138. doi: 10.1371/journal.pone.0053138. [DOI] [PMC free article] [PubMed] [Google Scholar]