Abstract
The incidence of Alzheimer’s disease (AD) has risen exponentially worldwide over the past decade. A growing body of research indicates that AD is linked to diabetes mellitus (DM) and suggests that impaired insulin signaling acts as a crucial risk factor in determining the progression of this devastating disease. Many studies suggest people with diabetes, especially type 2 diabetes, are at higher risk of eventually developing Alzheimer's dementia or other dementias. Despite nationwide efforts to increase awareness, the prevalence of Diabetes Mellitus (DM) has risen significantly in the Middle East and North African (MENA) region which might be due to rapid urbanization, lifestyle changes, lack of physical activity and rise in obesity. Growing body of evidence indicates that DM and AD are linked because both conditions involve impaired glucose homeostasis and altered brain function. Current theories and hypothesis clearly implicate that defective insulin signaling in the brain contributes to synaptic dysfunction and cognitive deficits in AD. In the periphery, low-grade chronic inflammation leads to insulin resistance followed by tissue deterioration. Thus insulin resistance acts as a bridge between DM and AD. There is pressing need to understand on how DM increases the risk of AD as well as the underlying mechanisms, due to the projected increase in age related disorders. Here we aim to review the incidence of AD and DM in the Middle East and the possible link between insulin signaling and ApoE carrier status on Aβ aggregation, tau hyperphosphorylation, inflammation, oxidative stress and mitochondrial dysfunction in AD. We also critically reviewed mutation studies in Arab population which might influence DM induced AD. In addition, recent clinical trials and animal studies conducted to evaluate the efficiency of anti-diabetic drugs have been reviewed.
Keywords: Diabetes mellitus, Alzheimer’s disease, Insulin signaling, Arab population, Anti-diabetic drugs
Abbreviations: AD, Alzheimer’s disease; DM, Diabetes mellitus; Pit-PET, Pittsburgh compound B- positron emission tomography; FDG-PET, Fluorodeoxyglucose- positron emission tomography; MRI, Magnetic resonance imaging; IR, Insulin resistance; CSF, Cerebrospinal fluid; Aβ, Amyloid-beta; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus; MENA, Middle East North African; AGPAT1, 1-acyl-sn-glycerol-3-phosphate acyltransferase alpha; NOTCH4, Neurogenic locus notch homolog protein 4; LPA, Lipophosphatidic acid; MCI, Myocardial infarction; BMI, Body mass index; FTO, Fat Mass and Obesity Associated Gene; TCF7L2, Transcription Factor 7 Like 2; PPAR-γ2, Peroxisome proliferator-activated receptor gamma 2; ADAMTS9, ADAM Metallopeptidase With Thrombospondin Type 1 Motif 9; DUSP9, Dual Specificity Phosphatase 9; GNPDA2, Glucosamine-6-phosphate deaminase 2; MC4R, Melanocortin 4 receptor; TFAP2B, Transcription Factor AP-2 Beta; ApoE, Apolipoprotein E; ABCA1, ATP binding cassette subfamily A member 1; IGF-1, Insulin-like growth factor 1; BACE1, Beta-secretase 1; IDE, Insulin degrading enzyme; BBB, Blood-Brain Barrier; GLP-1, Glucagon like peptide; ECE-1, Endotherin converting enzyme 1; NFT, Neurofibrillary tangles; GSK-3β, Glycogen synthase kinase 3 beta; IR, Insulin receptor; PI3K, Phosphoinositide-3; PP2A, Protein phosphatase 2; AAV, Adeno-associated virus; CIP2A, Cancerous Inhibitor Of Protein Phosphatase 2A; FRMD4A, FERM Domain Containing 4A; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MG-H1, Methylglyoxal-hydroimidazolone isomer trifluoroactic acid salt; NDUFS3, NADH:Ubiquinone Oxidoreductase Core Subunit S3; COX-2, Cyclooxygenase 2; CALR, calreticulin gene; SORT, Sortilin; RAB1A, Ras-related protein 1A; STZ, Streptozotocin
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia worldwide and it affects 3% of the population aged between 65 and 74 years. The most common symptoms of AD include progressive impairment of cognitive abilities such as memory, judgement, communication and ultimately death. Alzheimer’s disease not only affects a person’s living ability but it also causes a substantial economic burden to the society. Globally, it is estimated that the total economic cost for AD raised from US$279.6 billion to US$948 billion in 2016. Pathologically, AD is characterized by (1) specific neuronal loss in temporo-parietal association cortices and in the medial temporal lobe structures (2) presence of extracellular plaques made up of aggregated amyloid-beta protein (Aβ1-42) and (3) presence of intraneuronal tangles composed of truncated and hyperphosphorylated tau (Lashley et al., 2018). In normal physiological state, there is an equilibrium between production and degradation of excess amyloid beta (Aβ) and tau levels but during disease activity, this harmony is disturbed leading to protein aggregation, neuroinflammation and neurodegeneration. Several other neurodegenerative diseases such as Parkinson’s disease dementia (PDD), frontotemporal dementia (FTD) and dementia with lewy bodies (DLB) have symptoms similar to AD. These neurodegenerative diseases often occur with substantial degree of co-morbidity, implying the presence of other pathological proteins co-acting in the same brain causing cognitive loss (Lashley et al., 2008). Though there are numerous studies on AD pathogenesis, the exact neurodegenerative mechanisms are still unclear which makes definitive diagnosis difficult. Until now, treatment of AD using pharmacological interventions (Donepezil, galantamine, memantine, rivastigmine) have been modest and consistent providing symptomatic relief (Yiannopoulou and Papageorgiou, 2013)to the patients but disease-modifying therapies using pharmacological or non-pharmacological approach are considered as a worldwide necessity.
Since 1984, AD has been diagnosed based on National Institute of Neurological and Communicable Disorder and Stroke and the Alzheimer’s Disease and Related Disorders association (NINCDS-ADRDA) criteria which intervenes disease state as “possible”, ”probable” and “definitive” based on clinical as well as neuropathological patterns (Reitz and Mayeux, 2014). Apart from NINCDS-ADRDA scale, clinical diagnosis of AD is performed using three biomarkers tests, (1) cerebrospinal fluid (CSF) Aβ detection and cerebral PiB-PET representing amyloid metabolism (Palmert et al., 1990, Price et al., 2005) (2) FDG-PET representing the status of cerebral glucose metabolism (McKhann et al., 2011) and (3) CSF Tau detection and structural MRI presenting neurodegeneration (Rosenmann, 2012). Though these biomarkers can enhance the possibility of AD diagnosis, due to several shortcomings (expensive, difficult to perform) they are not recommended for conventional diagnosis (Price et al., 2005). In addition, after clinical diagnosis, the mean life expectancy of AD patients is approximately 7 years with only 3% of patients living more than 14 years and by that time it is already too late to treat the disease (Khan and Alkon, 2015). Patients with metabolic disorders such as diabetes mellitus, hypertension, and obesity have higher risk of AD. Hence, specific biomarkers from patients with metabolic disorders that can detect or prevent AD progression at earlier stage are imperative. Various studies have demonstrated that insulin resistance (IR) and impaired insulin signaling acts as a major pathological factor in both DM and AD. The influence of IR on plasma and CSF AD biomarkers were evaluated in 28 IR and 30 non-IR Finnish men recruited from Metabolic Syndrome in Men (METSIM) study. CSF AD biomarkers such as total tau, phosphorylated tau at Thr181 epitope, Aβ were quantified with respect to IR. However, no difference were noted in CSF AD biomarkers between IR and non-IR group but plasma insulin correlated with CSF Aβ/tau in the whole cohort with 487 plasma and 200 CSF proteins differentially expressed in the two groups. This study concluded that in cognitively healthy men, IR is not directly related to level of CSF AD pathology (Westwood et al., 2017). Similarly, levels of Aβ 42, phospoTau, soluble low density lipoprotein receptor related protein 1 (sLRP1) and macrophage-colony stimulating factor (MSCF) were evaluated in CSF and serum of patients with Type 1 Diabetes Mellitus (T1DM) with determinants of cognitive function and white matter integrity. CSF levels of AD biomarkers such as Aβ-42, pTau and sLRP1 were higher in patients with T1DM with no difference in MCSF. However, CSF-sLRP1 were associated with improved attention, information processing and increased white-matter integrity of right inferior fronto-occipital tract. Whereas, elevated tau levels was associated with white-matter integrity of right inferior fronto-occipital tract. However, these observed profile mismatches with the full risk profile as seen in pre-AD patients (Ouwens et al., 2014). Since, DM serves as a major risk factor for AD, various clinical cognitive biomarkers are evaluated in DM patients for early AD diagnosis. Recently, detailed review by Zhao et al summarized most of the available clinical biomarker studies on diabetic patients with cognitive decline that can be further developed and used for diagnostic and therapeutic purposes (Zhao et al., 2017). Thus, to prevent late-stage diagnosis, it is imperative to identify biomarkers at pre-clinical stage (with detectable pathological alteration but without cognitive impairment) to prevent metabolic disease induced AD progression. Various pathological factors such as ApoE mutation, aggregation of amyloid beta and tau, neuroinflammation, oxidative stress and mitochondrial dysfunction plays a major role in increasing the risk of diabetes induced AD. These factors and the pathological mechanisms involved have been discussed in detail in this review.
2. Burden of Alzheimer’s disease in the Middle East
According to a 2014 study, AD affects as many as one in seven of people aged over 60 in the United Arab Emirates (UAE), with an estimated total societal of 403 million dirhams, and the burden and the burden will exponentially increase due to healthcare development (Abyad, 2016). Most individuals with the disease are 65 and older, and after age 65, the risk of AD doubles every five years. After age 85, the risk reaches nearly one-third. In 35–40 years, the adult population in the Middle East will go up in the population pyramid resulting in more number of elderly population in the Middle East, possibly due to increase in longevity and improvements in public health. (Abyad, 2016) World Health organization predicted that between 2000 and 2050, there will be 4–5% increase in the rate of ageing above 65 years with a normal yearly development rate exceeding 5% in Arab nations (Hajjar et al., 2013). Unfortunately, there are only few studies in the Middle East/North Africa (MENA) region evaluating the prevalence of dementia in these regions. A recent multi-language review and feasible analysis on the incidence of dementia in MENA concluded that there is none to little information in dementia incidence (Bhalla et al., 2018). Abyad et al concluded that out of 800 patients, 10% had dementia prevalence in Lebanon. Following this, a similar study was replicated in Dubai and AD prevalence was found to be 3%, 2% rest of dementia and stroke affecting 5% of the study group. However, 40% of the study group had memory problems and 20% had decline in decision making as well (Abyad, 2016). In 2018, Phung et al analyzed dementia prevalence in Lebanon with 502 persons older than 65 years. Illiteracy among the older generations of MENA population is high and hence Arabic version of the one-stage 10/66 Dementia Research group (DRG) diagnostic assessment for dementia was used to minimize the effect of culture and education on cognitive assessment. Crude dementia prevalence was found to be 7%, and 9% had age-standardized dementia prevalence (Phung et al., 2017). Currently, there are no available treatments to stop or reverse the progression of the disease, which worsens as it progresses, and eventually leads to death. Alzheimer disease community organization reported that three thousand cases are diagnosed in the UAE in 2016 alone and they have encouraged elderly population in UAE to engage in cognitive activities as much as possible. Though this value seems to be low, the burden of the disease is projected to increase exponentially. Angiotensin-converting enzyme (ACE) gene polymorphism is closely associated with AD in Israeli Arab community. Angiotensin converting enzyme, an dipeptidyl carboxypeptidase plays a major role in blood pressure regulation. Clinical study have shown that Alu insertion/deletion (I/D) polymorphism of ACE gene (DCP1) increases the risk of DM in Saudi Arabian population (Al-Saikhan et al., 2017). Similarly, fifteen single nucleotide polymorphism in DCP1 was evaluated in 92 patients with AD and 166 non-demented controls. There was a significant association with SNPs rs4343 (P = .00001) and rs4351 (P = .01). Haplotype analysis showed that individual possessing “GA” haplotype derived from these SNPs had 45-fold higher risk of incidence of AD. This study suggests that a variant in close proximity to rs4343 and rs4351 might regulate the risk of AD in Israeli Arab community (Meng et al., 2006). The same group performed autozygosity mapping using Genome wide SNP Data from Israeli-Arab community to identify novel genetic mutations for AD. Though APOE-ε4 frequency is low in Wadi Ara (Arab community in northern Israel), this population have high frequency of AD. Hundred and twenty-four AD cases along with 142 controls were genotyped for a genome wide set of more than 300,000 SNPs to identify the region of autozygosity (identical allele or chromosomal segments of DNA). Seven different chromosomes with eight autozygous regions were frequent in controls and whereas 105 SNPs, mostly in chromosome 6 and 9 were associated with AD. Meta-analysis of four genome wide associated study datasets proved association of SNPs in AGPAT1 AGPAT1 (1-acyl-sn-glycerol-3-phosphate acyltransferase alpha) and NOTCH4 ((Neurogenic locus notch homolog protein 4))., an transmembrane protein of endoplasmic reticulum catalyzes the conversion of lysophosphatidic acid (LPA) to phosphatidic acid (Aguado and Campbell, 1998). Lysophosphatidic acid enhances tau phosphorylation during neurite retraction (Sayas et al., 1999). NOTCH4 is involved in various developmental processes by modulating cell fate decisions. Gamma-secretase (γ-secretase) regulates intramembrane proteolysis of NOTCH4 and γ-secretase is involved in final cleavage of Aβ peptide from amyloid precursor protein, which is responsible for Aβ oligomer toxicity (Lambert et al., 2004). Hence, SNPs in AGPAT1 and NOTCH4 might enhance the risk of AD in Israeli-Arab population. In a similar Wadi Ara population door-to-door survey involving 906 participants, 297 had MCI (33%), 95 had AD (10%) and hypertension was a major risk factor for AD (Inzelberg et al., 2015). However, more clinical studies are needed in the Arab population to understand how metabolic disorders and genetic mutations could contribute to AD. Out of different metabolic disorders, diabetes remains as a major risk factor for AD because insulin-signaling impairment plays a major role in cognitive impairment and AD. Thus, AD is also termed as Type 3 Diabetes since both AD and diabetes share common pathomechanisms. The impact of DM in MENA region is reviewed below to understand the current and forecasted risk of incidence of AD in this region.
3. Diabetes mellitus in the Middle East and its possible links with AD
Worldwide, 382 million people are affected by diabetes in which 90% are Type 2 Diabetes Mellitus (T2DM) (Atlas, 2015). The incidence of T2DM in the UAE is projected to increase from 18.7% to 21.4% by 2030 (Lotfy et al., 2017). Cardiovascular screening program of UAE Emiratis in Abu Dhabi showed more age-standardized prevalence of pre-diabetes (30%) and diabetes (25%) defined as fasting blood glucose (5.6–6.9 mmol/l) or 2 h post oral glucose tolerance test (7.8–11 mmol/l) (Hajat et al., 2012). Similar projection have been reported in most of the MENA regions as well (Alhyas et al., 2012). Saudi Arabia have higher prevalence of DM (23.9%) whereas, Egypt have largest number of DM patients. United Arab Emirates, Kuwait, Qatar and Bahrain were the countries with highest spending ($2000–7000) for DM per person per year (Atlas, 2015). Incidence of DM have increased dramatically due to accelerated economic lifestyle changes that results in reduced physical activity, more intake of refined carbohydrates, obesity and ageing population (Alhyas et al., 2011). Out of 34 million people diagnosed by diabetes in the MENA region, 17 million were undiagnosed that might result in increase in risk of diabetes induced disorders (Majeed et al., 2014). Unusually, prevalence of T2DM is high in Arab children less than 18 years due to high weight and obesity (Al Amiri et al., 2015). Central adiposity is common in Arab population that plays a major role in development of obesity and diabetes. Out of various modifiable risk factors, obesity have major influence on incidence of T2DM in which insulin resistance plays an important pathological role. Though genetic studies in MENA region is limited, it is reported that variation in ADIPOQ gene (encodes for adiponectin) is closely associated with high waist circumference and increased BMI in Arabs (Zadjali et al., 2013). Adiponectin is released by adipocytes and it is observed to be lower in obese subjects. An Emirati population based study involving 259 Emiratis demonstrated that there is association between FTO rs9939609 A/A genotype with insulin resistance and impaired fasting glucose (Saber-Ayad et al., 2019). FTO gene rs9939609 (A > T) is known for its important role in obesity development and insulin resistance (Frayling et al., 2007, Shimaoka et al., 2010). In a similar Emirati population based study, the association between TCF7L2 (Transcription factor 7 like 2) polymorphism and diabetes were analyzed by genotyping two TCF7L2 SNPs rs12255372 and rs7903146 in 368 adult subjects. Diabetes mellitus was slightly significantly associated with frequency of the T variant at rs12255372 but not at rs7303146. Combined DM/Pre-DM data compared to controls resulted in close association of rs12255372 with risk of DM. Hence, TCF7L2 variants are associated with more risk for DM in Emirati subjects but not associated with insulin resistance (Saadi et al., 2008). Conversely, Damcott et al., reported that in Amish subjects, TCF7L2 risk variant may be involved in adipocyte dysfunction resulting in triglycerides deposition and insulin resistance (Damcott et al., 2006). However, some studies report little-to-no association between TCF7L2 risk variant and insulin resistance (Varghese et al., 2016, Alsmadi et al., 2008). Apart from TCF7L2, peroxisome-proliferator-activated receptors-γ2 (PPAR-γ2) have a major effect on T2DM incidence. To understand the influence of PPAR-γ2 on Emirati population, a case control study was performed to predict the association of variants Pro12Ala (rs1801282) of PPAR-γ2 with T2DM risk. This study concluded that Pro12Ala mutation in PPAR-γ2 is not associated with risk of incidence of T2DM in Emirati population (Al-Safar et al., 2015). However, Pro12Ala polymorphism have been reported to be associated with obesity, insulin sensitivity in various population (Sanghera et al., 2008, Herder et al., 2008). Genome-wide genetic analysis of metabolic traits in Arab population is extensively reviewed elsewhere (Hebbar et al., 2019). However, certain T2DM risk loci such as ADAMTS9 (ADAM metallopeptidase with thrombospondin type 1 motif 9), DUSP9 (Dual specificity phosphatase 9) and PPAR-γ that has the ability to modulate insulin action and certain risk loci such as FTO, GNPDA2 (Glucosamine-6-phosphate deaminase 2), MC4R (Melanocortin 4 receptor) and TFAP2B (Transcription Factor AP-2 Beta) associated with obesity, BMI and adiposity have been identified (Hebbar et al., 2019). Apart from genetic risk loci identification, a simple, non-invasive risk score specific for UAE citizens was developed due to differences in lifestyle and ethnicity to predict individuals with higher risk of having undiagnosed T2DM. The study included 872 UAE citizens and their anthropometric measurements, fasting blood glucose and demographic details were analyzed. The risk score was established using stepwise forward repression model. Northern Emirates had 25.1% DM prevalence and the significant risk factors identified were age (≥35 years), hypertension, body mass index ≥30.0, family history of DM and waist-to-hip ratio (≥0.85 for females and ≥0.90 for males). Hence, this scale could be used for earlier detection of DM in high risk population (Sulaiman et al., 2018). Recent observational cross-sectional study in Saudi Arabian population demonstrated that population with prevalence of diabetes and obesity had poorer cognitive performance and these observation was independent of gender or educational background (Alaama et al., 2016). These studies suggest that there is a close association between obesity, insulin resistance and DM. In addition, SNP’s in AD and DM risk genes enhance the risk of incidence neurological disorders such as AD in the Middle East (Table 1). In addition, DM is closely associated with progression of white matter hyperintensities (WMH) volumes in elderly patients and WMH progression is positively correlated with cognitive dysfunction and impairment of activities of daily living (Gouw et al., 2008, Kreisel et al., 2013). Apart from genetic association between insulin resistance and T2DM, various pathological factors such as ApoE, Aβ, Tau, inflammation, mitochondrial dysfunction and oxidative stress enhance the risk of DM patients to neurological disorders. However, more research is needed to understand the pathological mechanisms, role of SNP’s in Middle Eastern population that might play a crucial role in preventing DM induced AD.
Table 1.
Single Nucleotide Polymorphisms in genes that acts as a major risk factor for AD and DM development.
| GENE | PUTATIVE FUNCTION | SNP’s | RISK FACTOR FOR DM/AD | REFERENCE |
|---|---|---|---|---|
| ACE1 | Blood pressure regulation | rs4343, rs4351 | AD | (Meng et al., 2006) |
| FTO | Adipogenesis and energy homoestasis | rs9939609 | DM | (Saber-Ayad et al., 2019) |
| TCF7L2 | Blood pressure homeostasis | rs12255372 | DM | (Saadi et al., 2008) |
| PPAR-γ2 | Regulates fatty acid storage and glucose metabolism | rs1801282 | Insulin resistance & Obesity | (Al-Safar et al., 2015) |
| ApoE | Lipoprotein metabolism | ApoE 2,3,4 | AD | (Moloney et al., 2010) |
| BIN1 | Plasma membrane curvature, membrane shaping and membrane remodeling | rs744373 | AD | (Moloney et al., 2010) |
| CLU | Functions as extracellular chaperone that prevents aggregation of non native proteins | rs11136000 | AD | (Moloney et al., 2010) |
| ABCA7 | Transportation of various molecules across extra-cellular and intra-cellular membranes | rs3764650 | AD | (Moloney et al., 2010) |
| CR1 | Plays major role in capture and clearance of complement-opsonized pathogens | rs3818361 | AD | (Moloney et al., 2010) |
| PICALM | Plays a major role in clathrin-mediated endocytosis | rs3851179 | AD | (Moloney et al., 2010) |
| FRMD4A | Scaffolding protein that regulates epithelial cell polarity | rs7081208, rs2446581, rs17314229 | AD | (Moloney et al., 2010) |
3.1. Role of Apolipoprotein E4 in T2DM and AD
Apolipoproteins which plays a major role in dyslipidemia are having greater attention in T2DM. Apolipoprotein E, a 34 kDa protein is composed of 299 amino acids and their isoforom difference is based on their amino acid residues; ApoE-ε2 (Cys112, Cys158), ApoE-ε3 (Cys112, Arg158), ApoE-ε4 (Arg112, Arg158). Their single amino acid difference modulate their ability to bind to Amyloid-beta (Aβ), lipids and receptors (Frieden and Garai, 2012, Zhong and Weisgraber, 2009). Clinical and pre-clinical studies provide strong evidence that ApoE isoforms affect Aβ aggregation and clearance. Apolipoprotein E4 (ApoE-ε4) carrier status interacts synergistically with T2DM causing cognitive impairment in T2DM patients (Li and Huang, 2016). Although ApoE-ε4 status alone did not have significant effect on dementia but T2DM patients carrying ApoE-ε4 had greater risk of both AD and vascular dementia when compared to T2DM patients without ApoE-ε4 allele (Irie et al., 2008). Pathologically, T2DM and ApoE-ε4 allele synergistically enhanced neuritic plaques and neurofibrillary tangles in hippocampus and Aβ aggregation in brain. Individuals with T2DM and ApoE-ε4 allele had an higher AD risk ratio of 5.5 compared to T2DM alone or ApoE-ε4 allele carriers alone (Peila et al., 2002). Low insulin response was closely associated with higher AD risk and subjects without ApoE-ε4 allele had stronger association (Rönnemaa et al., 2008). Moreover, insulin administration to AD subjects with ApoE-ε4 allele had marked memory facilitation when compared to AD group with ApoE-ε4 allele. ApoE mice fed with high fat diet induced insulin resistance had deficit in peripheral metabolism and cognition, impairment in hippocampus dependent spatial learning and memory. Conversely, these cognitive impairments were not found in Type-1 diabetes model suggesting the role of high fat diet induced obesity and insulin resistance in cognitive impairments. Genome-wide measurements of DNA hydroxymethylation from these samples resulted in alterations in glutamate metabolism, purine metabolism and pentose phosphate pathway. However, these alterations were reversed when E4 mice were fed with low fat diet suggesting functional role was affiliated with same metabolic pathways which is involved in high fat diet induced insulin resistance. Thus, E4 carriers are susceptible to metabolic impairments due to insulin resistance (Johnson et al., 2017). High fat diet induced peripheral insulin resistance and ApoE impair insulin signaling in the brain. However, upon administration of insulin into the periphery, presence of ApoE reduces cerebral and peripheral responsiveness of insulin-induced signaling. This is due to binding of ApoE to insulin receptor and trapping insulin receptor in endosomes and ApoE enhances insulin receptor aggregation, an endocytic abnormality associated with ageing. Binding of ApoE to insulin receptor also impairs insulin internalization. It has been reported that insulin receptor plays an important role in Aβ clearance, hence impairment of insulin receptors leads to cerebral Aβ overload leading to neurodegeneration (Zhao et al., 2009). Hence, AD patients with ApoE-ε4 allele had higher amyloid precursor protein levels upon insulin administration when compared to non-ApoE-ε4 AD patients (Craft et al., 2000) Presence of ApoE-ε4 allele decreases the expression of insulin degrading enzyme (IDE), an endopeptidase enzyme that degrades Aβ (Fernandez-Gamba et al., 2009). Apolipoprotein E is reported to be the non-fibrillar component of systemic amyloid deposits (Hatters et al., 2006) and ApoE-ε4 contributes to less plaque clearance. Oxidative stress is an important initiation factor of toxic pathological events in AD and presence of ApoE-ε4 results in higher oxidative stress (Akter et al., 2011). Furthermore, cholesterol transporter protein ABCA1 (ATP-binding cassette subfamily A member 1) influences lipid binding capacity of ApoE. Lipidated ApoE binds to soluble Aβ and aids in Aβ clearance. Hence, ABCA1 deficiency leads to poor lipidation of ApoE that results in heavier amyloid burden while overexpression of ABCA1 decreased amyloid burden (Hirsch-Reinshagen et al., 2005). In the brain, ApoE is produced by astrocytes to transport cholesterol to neurons via ApoE transporters and hence ApoE is important for synaptogenesis in neurons. Therefore, presence of ApoE isoform may affect synaptic plasticity or neuronal recovery in AD (Mauch et al., 2001, Hayashi et al., 2004). Whereas, in the peripheral tissues ApoE is produced by macrophages and hepatocytes that might be responsible for difference in function of ApoE in periphery and CNS (Zhao et al., 2018). Proteolytic cleavage of ApoE-ε4 by cathepsin D and site-directed antibody cleavage of ApoE-ε4 yielded amino-terminal fragments as seen in AD brain extracts (Rohn et al., 2012, Huang et al., 2001, Zhou et al., 2006). Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial demonstrated that lifestyle modification does not play a significant role in cognitive function between ApoE-ε4 carriers versus non-carriers. However, ApoE-ε4 carriers under treatment had significant improvement in certain cognitive measures compared to ApoE-ε4 carriers without treatment (Solomon et al., 2018). Therefore ApoE-ε4 respond differently to certain interventions when compared to non-carriers and hence specific prevention strategies for ApoE-ε4 carriers is important. Pharmacological AD prevention studies such as the effect of anti-hypertensive drug on ApoE-ε4 carriers had lower risk of AD compared to non-carriers (Guo et al., 2001). It is also important to note that prevalence of hypertension is high in Middle East (Habibzadeh, 2011). Cross-Sectional community based study in Al-Ain city of UAE concluded that 20.8% of 817 subjects had hypertension and 23.3% had DM (Baynouna et al., 2008). Hence, DM and anti-hypertensive drugs are being studied to understand their efficacy in decreasing cognitive deficits in AD. Diabetes drug pioglitazone was recently tested in TOMORROW Phase III AD prevention clinical trial. Regardless of ApoE-ε4 carrier status, pioglitazone does not prevent progression of patients from normal cognition to MCI due to AD. Hence, TOMORROW Phase III AD was recently discontinued. However, Alzheimer’s Prevention Initiative Generation Study will examine the consequence of ApoE-ε4 status on new drugs (CAD106 and CNP520) (Berkowitz et al., 2018). Intranasal delivery of long lasting analog, detemir showed that cognitive improvement is dependent on ApoE status (Claxton et al., 2015). However, more studies are important to understand the pathological mechanisms linking T2DM, ApoE and AD.
3.2. Influence of Amyloid-beta in T2DM and AD
Amyloid beta is produced by sequential cleavage of β-amyloid precursor protein by β and γ-secretase. Pathologically AD is characterized by presence of senile plaques (Aβ) and neurofibrillary tangles (tau). Accumulation of senile plaques (SPs) in the cortices of AD patients are responsible for synaptic degeneration. This results in cognitive impairment due to disturbance in axonal transport of vesicles containing brain derived neurotropic factor and mitochondria (Hardy and Selkoe, 2002, Decker et al., 2010, Tang et al., 2012). Hence, accumulation of cytotoxic Aβ oligomers and fibrillary aggregates is believed to play a major role in various diseases. However, rather than Aβ fibrils, memory loss in AD patients is largely due to Aβ oligomers because long-term potentiation in neurons is inhibited by Aβ oligomers by interacting with glutamate receptors and impair calcium influx in neurons (Walsh and Selkoe, 2004, Walsh et al., 2002, Li et al., 2009). Deposition of Aβ in pancreatic islet cells and brain exhibits pathogenic similarity in T2DM and AD respectively (Beeler et al., 2009). Post-mortem studies in T2DM patients showed presence of islet amyloid polypeptide and hyperphosphorylated tau in pancreatic islet cells (Miklossy et al., 2010). Similarly, amyloid plaques and neurofibrillary tangles were present in hippocampus of T2DM patients (Peila et al., 2002). Hyperphosphorylation of tau leads to intracellular buildup of aggregated tau that leads to neuronal loss and dementia in AD (Mandelkow et al., 1995). The duration of T2DM is directly correlated with the amount of cerebral amyloid load (Janson et al., 2004). Impairment of insulin signaling plays a major role in AD pathology. Post-mortem cases of advanced AD demonstrated deficits in the expression of insulin like growth factor-1 (IGF-1) and IGF-2 peptides, insulin and their receptors followed by abnormalities in downstream insulin signaling mechanisms (Steen et al., 2005). Since, these anomalies were also seen in T1DM or T2DM, de la Monte at al coined AD as “type 3 diabetes”.
Defective insulin secretion in β-cell mass and impaired insulin sensitivity in metabolic organs such as muscle and liver are prime causes of T2DM. In addition to peripheral function of insulin, it is also known to regulate synaptic and neuronal function within the cortex, cerebellum and hippocampus and protect neurons from cell death (Zhao and Alkon, 2001, Chiu et al., 2008). Insulin also acts on β-site amyloid precursor protein cleaving enzyme 1 (BACE1) and γ-secretase to regulate Aβ levels and degrades excess Aβ by modulating insulin-degrading enzyme (IDE) (Eckman and Eckman, 2005, Farris et al., 2003). Therefore, insulin signaling impairment in T2DM and AD have a direct effect on Aβ levels. Mixed mice model of T2DM and AD (APP/PS1xdb/db) showed that aged transgenic mice had impaired metabolic control along with reduced cortical size, reduction in dendritic spine density that leads to learning and memory deficit. These mice also had increased tau phosphorylation, toxic Aβ load, microglia activation and BBB disruption (Infante-Garcia et al., 2016). Similarly, in an mixed AD and DM mice model (APP+ -ob/ob and APP+ -NSY), diabetes exacerbated cognitive impairments, enhanced Aβ levels, decreased brain insulin levels and Akt phosphorylation (important step in insulin signaling) (Takeda et al., 2010). Mechanistically, soluble Aβ oligomers causes loss of insulin receptor (IR) in neuronal processes and inhibits neuronal response to insulin causing deterioration of synaptic function (Zhao et al., 2008). Oligomers binding to synapses (in particular neurons) also causes oxidative stress (De Felice et al., 2007), tau hyperphosphorylation (De Felice et al., 2008)and synapse loss (De Felice et al., 2009). Soluble Aβ oligomers downregulated plasma membrane insulin receptors through calcium calmodulin-dependent kinase II and casein kinase II inhibition. However, insulin administration prevented oxidative stress and synaptic spine deterioration. Submaximal doses of insulin along with rosiglitazone, an insulin-sensitizing drug decreased Aβ oligomers binding to insulin receptors. Surprisingly, inhibition of tyrosine kinase activity prevented the protective effect of insulin (De Felice et al., 2009). However, clinical trials and safety data suggest that rosiglitazone and pioglitazone should not be used for treatment of AD (Miller et al., 2011). Neurons exposed to Aβ oligomers caused serine phosphorylation of insulin receptor substrate-1 (IRS-1), thereby inhibiting downstream insulin signaling and peripheral insulin resistance along with abnormal activation of JNK/TNF-α pathway (Hirosumi et al., 2002). However, exendin-4 (an antidiabetic drug) activates insulin signaling through glucagon-like peptide 1(GLP-1) receptors that resulted in normal insulin signaling, prevented cognitive deficits and inverted insulin pathology (Bomfim et al., 2012). These studies provide evidence that deterioration of insulin signaling might be responsible for T2DM mediated AD progression. Administration of GLP-1 analog to 38 AD patients in randomized, placebo-controlled, double-blind clinical trial prevented cognitive dysfunction, synapse impairment and disease evolution. (Gejl et al., 2016). Recently, patients treated with liraglutide (another GLP-1 agonist) demonstrated a non-significant trend for increased cerebral glucose utilization rate (CMRglc) and the rate of Aβ deposition was not affected by liraglutide (Gejl et al., 2016). However, no firm conclusions on the effect of liraglutide on cognitive scores was drawn from this study. Established animal model showed that intracerebroventricular (icv) injection of streptozotocin (STZ) causes AD like pathology by causing insulin deficiency in pre-clinical studies. For example, icv-injection (3 mg/kg) causes Aβ oligomer accumulation and enhanced production of carboxy-terminal fragments from APP (Lin et al., 2014). STZ injection also decreased the levels of amyloid beta degrading enzyme like endotherin-converting enzyme-1 (ECE-1), insulin degrading enzyme (IDE) in rat cortices (Liu et al., 2011). Accumulation of Aβ in the brain might be due to impairment of endocytosis. Many studies have shown that β-secretase and Aβ are carried intracellulary via endocytosis and Aβ cleavage occurs in endosomes (Grbovic et al., 2003, Koh et al., 2005). Non-human primate T2DM model showed that exacerbated endocytic disturbance causes severe Aβ load in the brain (Okabayashi et al., 2015). Hence, any drug that has the ability to clear or diminish Aβ oligomers may slow the progression of the disease. Solanezumab, a humanized immunoglobulin G1 monoclonal antibody that has the ability to bind to mid-domain of Aβ peptide increases the clearance of soluble Aβ from the brain. In phase three clinical trials (EXPEDITION and EXPEDITION2), solanezumab did not produce significant effect on cognitive function who had mild-to-moderate AD. However, pre-specified pooled analysis showed that solanezumab treated patients had less cognitive decline (34%) and less functional decline (18%) when compared to placebo controls. Third double-blind, placebo-controlled phase 3 trial (EXPEDITION3) study with patients with mild AD was performed. Unfortunately, administration of Solanezumab every 4 weeks with a dose of 400 mg did not significantly affect cognitive decline (Honig et al., 2018). However, efforts to develop drugs that can prevent/disintegrate soluble Aβ oligomers are continuing with a hope for positive clinical trials (Cummings et al., 2018).
3.3. Effect of tau hyperphosphorylation in T2DM and AD
Activation of Glycogen synthase kinase-3β (GSK-3β) leads to tau hyperphosphorylation and resultant neurofibrillary tangles (NFT) toxicity in various neurodegenerative diseases. Early tau alterations may significantly lead to cognitive abnormalities (Hochgräfe et al., 2013). Cleavage of tau at Asp 421 causes pathogenic assembly of tau filaments more rapidly because cleaved tau has greater polymerization kinetics and acts as a nucleation center (Yin and Kuret, 2006, Zilka et al., 2006). There is a strong correlation between tau pathology and degree of dementia in AD patients (Iqbal et al., 2005). Insulin receptor (IR) substrate molecules are phosphorylated by IR tyrosine kinase that leads to activation of phosphoinositide-3 kinase/Akt signaling (Ghasemi et al., 2013). Activation of PI3k/Akt leads to phosphorylation of Ser9 of GSK3β and impairment of its kinase activity resulting in tau phosphorylation. Hence, insulin resistance/deficiency can induce aberrant activation of GSK-3β and hyperphosphorylation of tau in both T1DM and T2DM (Salkovic-Petrisic et al., 2006, Ke et al., 2009). Invitro studies demonstrate that short insulin treatment (<2–3 min) results in quick and short hyperphosphorylation of tau. However, long (upto 60 min) exposure of neurons to insulin had significantly low phosphorylation of tau (Lesort and Johnson, 2000, Lesort et al., 1999). Though the mechanisms involved in diabetic induced tau phosphorylation is not fully understood, protein phosphatase 2A (PP2A) is involved in tau pathology. Surprisingly, PP2A is decreased in AD brains. Streptozotocin injection in a human tau expressing mice model resulted in tau hyperphosphorylation along with inhibition of PP2A, an important tau phosphatase. However, insulin injection significantly restored tau phosphorylation. Recently, Shentu et al demonstrated overexpression of cancerous inhibitor of PP2A (CIP2A) in AD brain. CIP2A-mediated PP2A inhibition not only drives tau/APP hyperphosphorylation but also enhanced Aβ production. PP2A inhibition leads to tau mislocalization to spines and dendrites followed by synaptic loss. This pathomechanism was also reflected in mice injected with AAV-CIP2A with impairments in cognitive deficits and long-term potentiation (Shentu et al., 2018). Similarly, phosphorylation of endogenous specific inhibitor 2 (I2PP2A), also known as SET (PP2A inhibitor) by casein kinase 2 caused tau hyperphosphorylation in neurons and animal models (Zhang et al., 2018). Alzheimer patients have higher deteriorative risk of post-operative cognitive dysfunction after anesthesia. Anesthesia induced rapid and massive hyperphosphorylation of tau, inhibition of PP2A and prolonged hypothermia (Planel et al., 2007). In addition, hypothermia after STZ injection had significant impact on tau hyperphosphorylation when compared to normothermic mice (Gratuze et al., 2017). Hypothermia, an usual experimental outcome in diabetes leads to 80% increase in tau phosphorylation per degree celsius drop under 37 °C in mice (Papon et al., 2013). Under normal condition, loss of tau causes impairment of hippocampal response to insulin and disrupts insulin mediated hypothalamic anorexigenic effect which demonstrated the importance of tau in brain signaling (Marciniak et al., 2017). Phosphatase and tensin homolog (PTEN) and IRS-1 regulation plays a major role in tau mediated hippocampal response to insulin. Tau interacts with PTEN decreasing lipid phosphatase activity and blocks insulin signaling impairment. Thus, loss of tau also results in impaired insulin signaling in AD models (Gratuze et al., 2018). In AD brain, more cytosolic levels of IRS-1 pS312 and pS616 correlate with NFTs but IRS-1 pS312 is confined to the nucleus in controls. Hence, IRS-1 phopho-species might have significant role in building up tau pathology in AD (Moloney et al., 2010). Hence, not only tau hyperphosphorylation leads to NFTs and AD but loss of tau function also causes defective insulin signaling and AD pathology. Endocytic disturbance causes Aβ overload in the brain of T2DM model. Similarly, various AD risk genes such as ApoE, BIN1 (Bridging integrator 1), CLU (Clusterin), ABCA7 (ATP-binding cassette sub-family A member 7), CR1 (Complement C3b/C4b Receptor 1) and PICALM (Phosphatidylinositol Binding Clathrin Assembly Protein) are involved in endocytosis of tau in direct or indirect way. Hence, mutation in any of these genes might increase tau propagation and decrease tau endocytosis in the brain (Avila et al., 2015). Recently, late-onset AD (LOAD) risk gene FRMD4A (FERM domain-containing 4A) was reported to regulate tau secretion via cytohesin-Arfb pathway, a pathway involved in presynaptic vesicle machinery and polarity signaling (Uronen and Huttunen, 2016). However, more genetic studies are needed globally to understand if genetic risk factors could influence tau pathology in AD. Similar to Aβ focused AD therapies, various therapeutic strategies are emerging because tau pathology shows better correlation with cognitive deficits in AD patients than Aβ. These strategies include modulation of tau phosphorylation, targeting tau pos-translational modifications, microtubule stabilizers, tau aggregation inhibitors, anti-tau immunotherapy. These therapeutic strategies have been extensively reviewed recently by Miguel Medina (Medina, 2018). Anti-diabetic drug metformin and its derivative phenformin enhanced PP2A activity and decreases tau hyperphosphorylation in primary neurons and tau transgenic mice model (Kickstein et al., 2010). Oral administration of sodium selenate to tau transgenic mice also prevented tau pathology, activated PP2A and enhanced memory and functional recovery (Corcoran et al., 2010). Phase II clinical study demonstrated the safety and tolerability of sodium selenate in mild-moderate AD patients. Clinical study using leuco-methylthioninium that directly inhibit tau protein assembly yielded positive results (Seripa et al., 2016). However, new avenues targeting tauopathies are in pressing need to prevent and treat AD.
3.4. Inflammatory consequence in T2DM and AD
Inflammation is an early pathological event that leads to neurodegeneration in AD (Blum-Degena et al., 1995)and insulin resistance in T2DM (Kubaszek et al., 2003). Post-mortem AD studies showed that neurofibrillary plaques (NPs) and NFTs are accompanied with inflammation which suggests a possible influence of inflammation in AD pathology. Adipose tissue inflammation is one of the important pathological hallmark of diabetes and obesity (Ouchi et al., 2011, Lumeng et al., 2007). Adipocyte derived proinflammatory factors such as cytokines, chemokines can cross Blood-Brain Barrier (BBB) mediating neuroinflammation (Banks, 2005). Elevated Aβ levels in AD can trigger TNF-α levels by NIk-dependent pathway leading to deficit in synaptic plasticity and cognition (Sims-Robinson et al., 2010, Corona et al., 2012, Carrero et al., 2012). In addition, inflammation suppresses incretin, an effector of reduced insulin resistance, whereas activation of incretin inhibits TNF-α production (Clark and Vissel, 2014). Proinflammatory factors such as IL-1β, IL-6, Macrophage Migration factor, interferon gamma are present at the proximities of Aβ plaques, supporting their role in neuroinflammation (Mehlhorn et al., 2000, D'Andrea et al., 2001). Hyperglycemia induced neuroinflammation plays a major role in insulin resistance by feed-back inhibition of insulin receptor and causes mitochondrial dysfunction through feed-forward mechanisms (Bonnard et al., 2008, Cheng et al., 2010), This mechanism enhances expression of NF-ᴋB inducing kinase (NIk) which again compromises mitochondria function and subsequent insulin resistance (Choudhary et al., 2011, Sheng et al., 2012). Intracerebroventricular injection of STZ in rat hippocampus enhances ROS production and NF-ᴋB activation (Locke and Anderson, 2011)which promotes apoptosis (Jaeschke et al., 2004). In addition, NK-ᴋB regulates insulin sensitivity by modulating the expression of GLUT2 receptor (Patel and Santani, 2009). Clinically, insulin resistance in T2DM is associated with enhanced levels of IL-6, C-reactive protein and α-1-antichymotrypsin (Hak et al., 2001, Van de Ree et al., 2003). Hence, chronic inflammation causes peripheral immune response which enhances cytokine production that crosses BBB causing neuroinflammation (McGeer and McGeer, 2010). Advanced glycation end products (AGE) acts as a significant mediator of inflammation in both AD and insulin resistance in DM (Yamagishi et al., 2012, Unoki et al., 2007). Advanced glycation end products binds to receptor for advanced glycation end products (RAGE) to activate NF-ᴋB, a crucial factor for inflammation. AGE is also elevated in AD patients and they are localized in NPs, NFTs and in neurons and glia. Certain AGE’s such as methyl-glyoxal-imidazolone-H1 (MG-H1) enhance the pro-inflammatory properties of Aβ or tau (Srikanth et al., 2012, Li et al., 2012). Clinically, MG-H1 levels in the circulation or brain are positively correlated with cognitive impairment in older patients (Beeri et al., 2011, Ahmed et al., 2005). Food-derived AGEs contribute largely to chronic diseases due to availability to thermally altered nutrients (Koschinsky et al., 1997, Birlouez-Aragon et al., 2010). Mice fed with MG derivatives increased body weight and systemic insulin resistance, increased brain amyloid deposits and gliosis with low survival factor sirtuin-1 (SIRT-1), which is involved in cellular longevity. Both in mice and humans, dietary MG levels and AGEs correlate with cognitive decline as measured by Mini Mental State Examination (Cai et al., 2014). Hence, AGE acts as a modifiable risk factor for AD and metabolic syndrome by enhancing inflammation. Decrease in sirtuins enhances the risk of AD and insulin resistance which contributes to the interplay between AD and diabetes. Advanced glycation end products decreases SIRT-1 expression which results in insulin resistance, Aβ and tau phosphorylation (Killick et al., 2009). Cross-sectional study involving Mexican adults (18–35 years) showed that AGEs intake subjects were more likely to have impaired fasting glucose and metabolic syndrome regardless of sex, age and family history (Mendoza-Herrera et al., 2018). Hence, blocking or inhibiting AGE expression might be an therapeutic target for AD drug development. Blocking RAGE in rats prevented tau hyperphosphorylation (Lüth et al., 2004). TTP488, an antagonist for RAGE was tested for its phase II clinical efficiency in people with mild to moderate AD. TTP488, at a dose of 5 mg demonstrated significant improvement on Alzheimer-disease-Assessment Scale-cognitive (ADAS-cog11), Clinical dementia rating Sum of Boxes (CDR-sb) and Alzhimer Diseas Cooperative Study-Activities of Daily Living scale (ADCS-ADL) compared to placebo (Burstein et al., 2014). Therapeutically, TTP488 decreased amyloid uptake in brain and lowered glial inflammatory response. Currently, this drug is under Phase III clinical trials PPAR plays a major role in diabetes induced AD and it also modulates insulin-dependent gene expression in response to surface membrane signals (Collino et al., 2008). Furthermore, PPAR has potent anti-inflammatory role owing to (1) reduced expression of PPAR in AD brains (de la Monte and Wands, 2006);( 2) inhibition of PPAR-delta enhances neuroinflammation, Aβ 42 deposition and tau hyperphosphorylation (Barroso et al., 2013). PPAR agonist such as thiazolidinedione class of drugs prevented Aβ induced secretion of pro-inflammatory cytokines such as TNF-α (Combs et al., 2000). Aberrant TNF signaling and activation of JNK pathway plays a major role in peripheral insulin resistance (Hirosumi et al., 2002). Moreover, activation of TNF/JNK pathway leads to endoplasmic reticulum stress, activates stress kinases (IκB kinase) and double-stranded RNA dependent protein kinases which are increased in AD brains (Hoozemans et al., 2009, Chang et al., 2002). Various reports demonstrate that inflammation and endoplasmic reticulum stress are key events in hypothalamic and peripheral insulin resistance in DM (De Souza et al., 2005, Purkayastha et al., 2011). Both IκB kinase and double-stranded RNA dependent protein kinases caused Aβ oligomer induced insulin receptor substrate 1 inhibition (Bomfim et al., 2012). As discussed earlier, transgenic animal model (APP+/ob/ob) had elevated levels of RAGE in the blood and higher expression of TNF-α and IL-6 in the brain. Similarly, 3xTg AD mice raised with high fat diet had enhanced cerebrovascular inflammation and cognitive deficits, although no significant difference in NPs and NFTs were observed (Knight et al., 2014). These studies demonstrate that pathological role of inflammation in diabetes induced AD. Hence, various groups are focusing on identifying pharmacological inhibitors of inflammation to improve insulin action. For example, salicylates inhibits inflammatory kinases and activates insulin signaling within the cell (Kim et al., 2013). Targeting JNK using cell permeable peptide provokes insulin signaling in obese mice (Lee et al., 2003). These studies demonstrate strong correlation between hyperglycemia, insulin resistance and inflammation which eventually leads to neurodegeneration in AD. Recent study by Estella et al demonstrate that not all ApoE-ε4 carriers develop AD but patients with ApoE-ε4 presence along with chronic low-grade neuroinflammation (C-protein measurement) had higher risk of AD (Newcombe et al., 2018). In addition, presence of ApoE influences microglial response to amyloid plaque toxicity. Ulrich et al., 2018 demonstrated that presence of ApoE not only affected amyloid plaque clearance but also induced aggregation of monomeric Aβ and enhanced fibrillary plaque associated microgliosis. Hence, presence of ApoE directly facilitates amyloid plaque formation and activates microgliosis resulting in neuroinflammation. In addition to the inflammatory role, microglia interacts with amyloid plaque, thereby reducing early AD pathology by phagocytic actions. However, presence of ApoE significantly reduced the expression of triggering receptor expressed on myeloid cells 2 (TREM2) which regulates microglial plaque coverage and subsequent phagocytosis (Stephen et al., 2019). Similarly, ApoE activation transforms homeostatic microglia to activated state after phagocytosis of apoptotic neurons. However, targeting ApoE signaling reverses microglia to homeostatic state (Krasemann et al., 2017). Recently, Aburawi et al demonstrated that subclinical inflammation and endothelial dysfunction were common in young diabetic Emirati patients in UAE. Obesity and dyslipidemia were associated with higher levels of TNF-α, IL-6 and lower adiponectin (Aburawi et al., 2016). TNF-α decreases peripheral intake of glucose in response to insulin that causes insulin resistance in DM and obesity (Priyadarshini et al., 2012). Similarly, diabetic UAE citizens had enhanced inflammatory markers with decreased antioxidants with increased body-mass index (BMI) and waist circumference (Gariballa et al., 2014). Hence, chronic low grade inflammation may play pathological role in DM. White blood cells is an important marker of activated immune system. Vozarova et al demonstrated that high WBC count is an independent predictor of insulin impairment and T2DM development in Pima Indian population. Other inflammatory markers such as orosomucoid acid and sialic acid were also associated with later development of T2DM (Vozarova et al., 2002, Schmidt et al., 1999). Metabolic stress such as inflammation and excess nutrients enhance insulin resistance and obesity by increasing expression of mitogen-activated protein kinases (MAPK), c-Jun NH-2-terminal kinase (JNK) and p38 MAPK. Inflammation also induces the expression of dual-specificity phosphatase-1 (DUSP-1) or mitogen-activated protein kinase phosphatase (MKP-1) that inactivates JNK and p38 MAPK. In skeletal muscle, a major tissue involved in glucose metabolism, MPK-1 is upregulated in obese humans and in high-fat diet fed mice. Whereas, knock-down of MKP-1 enhanced the expression of p38 MAPK and JNK along with resistance to diet-induced obesity (Lawan et al., 2018). In cardiovascular diseases (CVD), MPK-1 is shown to be higher in circulating blood cells even after conventional treatments (Kapoor et al., 2014). In a clinical study involving 207 Arab adults extracted from Kuwait Diabetes Epidemiology Program (KDEP), Khadir et al demonstrated that DUSP-1/MKP-1 and high-sensitivity CRP (hsCRP) were higher and they are independently associated with CVD in Arab population in addition to statin treatment (Khadir et al., 2018). Hence, DUSP-1 might be one of the profounding inflammatory factor involved in insulin resistance and obesity in Arab population. Various reports suggest that there is an intricate association between inflammation and mitochondria dysfunction in promoting neurodegeneration (van Horssen et al., 2017, Kolmychkova et al., 2016). Here we provide an overview on how mitochondrial deficits and oxidative stress mediates DM and AD.
3.5. Mitochondrial impairment and oxidative stress in T2DM and AD
Mitochondrial dysfunction, oxidative stress and subsequent antioxidant decline are key pathological players that precede or accompany AD and T2DM (Moreira et al., 2007). Mitochondria has decisive role in ATP production, buffering of cytoplasmic Ca2+, metabolism of ROS and apoptosis (Mattson et al., 2008). Specifically in the neurons, mitochondria are essential to maintain membrane ion gradients, synaptic plasticity and neurotransmission (Fontan-Lozano et al., 2008). Changes in shape of mitochondria perturbs synaptic transmission in presynaptic neurons. For example torus shaped (donut-shaped) mitochondria represents oxidative stress and responsible for cognitive decline in old monkey models (Hara et al., 2014). In diabetic brain, both Aβ and tau can cause mitochondrial alterations leading to neuronal energy deficit, synaptic disturbance and neurodegeneration (Akhter et al., 2017). Dendritic spines of excitatory glutamatergic synapses (NMDA receptor) possess more metabolically active mitochondria that results in high Ca2+ influx which promotes neuronal death (Bezprozvanny and Mattson, 2008). In addition, Aβ oligomers stimulate excitatory NMDA receptors that leads to Ca2+ dependent mitochondrial dysfunction and ROS production (De Felice et al., 2007). Insulin signaling plays a major role in protecting mitochondria. Insulin signaling stimulation protects LAN5 (neuroblastoma) cells against Aβ induced oxidative stress by activating Akt pathway and inhibiting expression of pro-apoptotic transcription factors and maintaining mitochondrial membrane potential (Picone et al., 2011). Hence, there is a close relationship between insulin signaling, mitochondrial oxidative stress and neurodegeneration. In the periphery, physiological stimuli such as insulin facilitates ROS generation, by inhibiting protein phosphatases (Loh et al., 2009). High fat diet causes mitochondrial ROS production in skeletal muscles that causes insulin resistance in T2DM (Anderson et al., 2009). Apart from cellular factors, mutations in mitochondrial DNA are associated with DM. Few mutations in mitochondrial DNA such as A3243G in the mitochondrial DNA-encoded transfer RNA (tRNA) gene are closely associated with diabetes. Carriers of A3243G showed marked decrease in insulin secretion when compared to non-carriers which might be due to decrease in cytosol ADP/ATP levels resulting in resetting glucose sensor such as maturity-onset diabetes of the young (MODY)-2 with glucokinase mutation in pancreatic β cell (Maassen et al., 2004). Oxidative stress and antioxidant activity was evaluated in serum samples collected from Japanese patients carrying A3243G mutation. Mean diacron-reactive oxygen metabolites (d-ROMs), a marker for oxidative stress was significantly higher in carriers than controls concluding that carriers of A3243G mutation is always exposed to constant oxidative stress (Ikawa et al., 2012). Mitochondrial proteome study using mitochondria isolated from skeletal muscle of T2DM patients resulted in 335 differentially expressed proteins (DEPs) between diabetic and non-diabetic groups. The study concluded that two DEPs (NDUFS3 and COX2) for downregulated oxidative phosphorylation and three DEPs (CALR, SORT and RAB1A) for upregulated calcium and protein transport representing mitochondria-associated ER membrane functions could be used as a potential protein profile for dysregulation of mitochondrial function in T2DM (Chae et al., 2018). Interestingly, point mutation in mitochondrial DNA nt3243 in Japanese women resulted in AD brain pathology. During the last ten years, the women was diagnosed with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke like episodes (MELAS). Post-mortem studies showed presence of senile plaques through the brain, mostly in frontal and temporal lobes and NFTs in parahippocampal gyrus (Kaido et al., 1996). However, mutations in codons 693, 713 and 717 of β-amyloid precursor protein were not found. This study proved a possible link between mitochondria DNA abnormality and AD like pathology. Nonetheless, the presence of A3243 mutation as a risk factor for diabetes or AD in Arab population is not yet reported. Amount of ROS produced by mitochondria directly contributes to apoptosis and since pancreatic-β cells have poor regeneration capacity after cell loss, insulin deficiency occurs. Similarly, inhibition of insulin receptor by Aβ oligomers causes NMDA receptor dysregulation, oxidative stress and impaired insulin signaling in hippocampal neurons. Oxidative changes in mitochondrial proteins, lipids and nucleic acids enhances ROS production, Aβ accumulation and tau phosphorylation (Swerdlow and Khan, 2004). Intracerebroventricular injection of STZ causes mitochondrial dysfunction (Xu et al., 2003), impairs insulin/IGF signaling through PI3 kinase-Akt leading to enhanced GSK-3β activity (de la Monte and Wands, 2008). As mentioned earlier, high GSK-3β activity leads to tau hyperphosphorylation and neuronal death. In addition of hyperglycemia, hypoglycemia also leads to mitochondria deficits (Cardoso et al., 2013)and cognitive decline (Strachan et al., 2000). Insulin induced acute hypoglycemia impairs antioxidant defense, oxidative damage and release of excitatory amino acids by cortical neurons (Cardoso et al., 2010, Cardoso et al., 2011). Long-term hyperglycemia and recurrent hypoglycemia impairs antioxidant defense in brain hippocampal and cortical mitochondria resulting in oxidative stress (Cardoso et al., 2013). A retrospective population study using Western Australian hospital inpatient, mental health outpatient, and death records showed that patients with cognitive impairment or dementia were likely to be admitted for hypoglycemia (Zilkens et al., 2013). Hence, repeated hypoglycemia leads to cognitive decline and acts as a major contributor of dementia. Mitochondrial uncoupling proteins (UPs) regulate ROS production and it acts as a key defense mechanisms against brain damage (Cardoso et al., 2015). Pharmacological inhibition of UP2 aggravates glucose mediated neuronal damage, mitochondrial dysfunction and enhances oxidative stress (Cardoso et al., 2018). Zucker diabetic fatty rat model demonstrated mitochondrial dysfunction and oxidative stress (Raza et al., 2015). Similarly, Goto-Kakizaki non-obese T2DM rat model also showed age-dependent redox perturbation which enhances neurodegenerative events (Carvalho et al., 2014). These studies suggest that metabolic disturbances associated with DM causes oxidative stress and mitochondrial deficits as seen in AD. Huang et al demonstrated that aberrant mitochondrial morphology, impaired mitochondrial complex I activity, decline in ATP production are seen in obese T2DM mice model. These alterations were due to impaired mitochondrial fusion and fission through GSK-3β/Drp-1 (dynamin-related protein 1) mechanism. Further, supporting the link between metabolic and cognitive diseases, Akhtar et al demonstrated that amyloid beta oligomers and high glucose concentration causes S-nitrosylation of Drp-1 and IDE as well as hyperactivating mitochondria fission resulting in dysfunctional synaptic plasticity (Akhtar et al., 2016). Intracerebroventricular (icv) injection of sub-diabetogenic STZ dose caused mitochondria abnormalities by decreasing mitochondria membrane potential, repolarization levels, respiratory state 3, ATP content and increase in lag phase of repolarization. Also, icv injection decreased pyruvate and α-ketoglutarate dehydrogenase, cytochrome c oxidase activities and impaired antioxidant defense mechanisms (Correia et al., 2013). Though these reports demonstrate the pathological relationship between insulin resistance and mitochondrial dysfunction in diabetes induced AD, the exact molecular mechanisms through which mitochondrial dysfunction causes diabetes induced AD is still not clear. Thus, anti-diabetic drug might have profound effect on mitochondria deficit. Anti-diabetic drugs such as metformin (Pintana et al., 2012)and dipeptidyl-peptidase-4 inhibitors (Pintana et al., 2013)prevented mitochondrial deficits and cognitive decline in high fat insulin resistance rats. Similarly, GLP-1 analogues prevented tau phosphorylation and apoptosis, increased brain insulin and insulin like growth factor 1 levels in T2DM rats (Xu et al., 2015, Yang et al., 2016). Few mitochondria enhancing drugs such as MP-101, rasagiline and oxaloacetate are currently under clinical trials for AD treatment (Cummings et al., 2018).
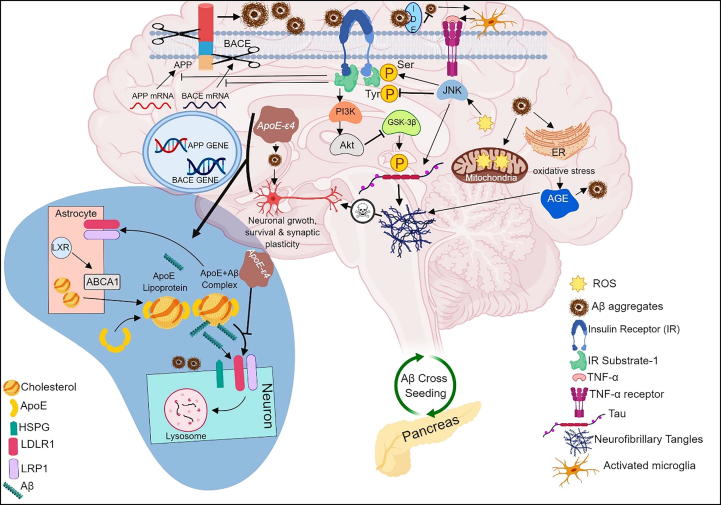
Overall, impairment of downstream insulin signaling by either Aβ oligomers or ApoE protein causes mitochondrial dysfunction, oxidative stress, enhanced expression of advanced glycation end products (AGEs) and inflammation in AD. Accumulation of Aβ oligomers in both pancreas and brain represent the ability of Aβ oligomers to cross seed between pancreas and brain promoting cellular/neuronal dysfunction in both T2DM and AD. Both Aβ oligomers and ApoE proteins blocks insulin receptors resulting in deficits in downstream signaling pathways such as PI3K and Akt. These deficits reduces glucose metabolism, enhances oxidative stress and alters JNK activity. Under normal physiological conditions, insulin signaling pathway reduces excess Aβ through insulin degrading enzyme (IDE) and by inhibiting translation of BACE and APP gene that results in less cleavage of BACE1 and APP. Moreover, insulin signaling pathway reduces GSK-3β phosphorylation which in turn reduces neurofibrillary tangle formation and subsequent neuronal death. Intracellular accumulation of Aβ oligomers is prevented by trafficking Aβ oligomers from golgi and trans-golgi network to extracellular milieu. Aβ oligomers also causes activation of microglia, production of pro-inflammatory factors (primarily TNF-α) and initiating downstream JNF pathway that again blocks brain’s insulin receptors. In addition, oxidative stress causes activation of AGEs which inturn causes NF-ᴋB activation, tau hyperphosphorylation and Aβ oligomerization. Apart from Aβ oligomers, ApoE also binds to insulin receptor blocking downstream signaling. In addition of proteolytic cleavage of Aβ oligomers by IDE, Aβ oligomers are also degraded by receptor mediated uptake by glia and neurons. Primarily ApoE is synthesized in the glia, lapidated by ABCA1 transporter forming lipoprotein particles. Soluble Aβ binds to lapidated ApoE and facilitates neuronal uptake of Aβ through LRP1, LDLR and HSPG cell surface receptors where Aβ is degraded by lysosomal pathway. However, ApoE modification (ApoE-ε4) or ABCA1 deficiency impairs this process causing intracellular accumulation of Aβ oligomers and neuronal death (Fig. 1).
Fig. 1.
Overview of different pathomechanisms linking DM and AD. Amyloid beta oligomers and ApoE mutation causes dysregulation of downstream insulin signaling pathway resulting in oxidative stress, mitochondrial dysfunction, AGE expression and inflammation. Cross-seeding of Aβ oligomers between the pancreas and the brain promotes cellular dysfunction in both T2DM and AD. Aβ oligomers and ApoE protein blocks insulin receptors resulting in impairment of PI3K and Akt pathways and activates microglia mediated JNK downstream pathway. Further, dysregulation of insulin signaling pathway enhances Aβ production due to translation of BACE and APP, increases GSK-3β phosphorylation resulting in NFT formation and neuronal death. In addition, Aβ oligomers are degraded by lysosomal pathway. However, ApoE mutation impairs lysosomal degradation resulting in intracellular accumulation of Aβ and cell death.
4. Conclusion
Traditionally, AD and DM were thought to be independent disorders. However, various clinical and pre-clinical studies have demonstrated that AD and DM share common pathological mechanisms. However, DM cannot be assumed to be sufficient to cause AD but could act as a cofactor in AD progression due to selective impairments in insulin signaling accompanied by significant upregulation of Aβ aggregation, tau hyperphosphorylation, inflammation, oxidative stress and mitochondrial dysfunction. Due to shared pathology, it has been suggested that anti-diabetic drugs may have therapeutic potential in treating AD and currently tested in clinical trials. Some anti-diabetic drugs were already reported to be beneficial against few hallmark AD pathology and they also promoted neurogenesis. Although, these drugs showed promising results, precise knowledge on common pathomechanisms between DM and AD, molecular action of drugs both centrally and peripherally, influence of demographic changes and genetic mutations on AD progression are urgently needed for diagnostic and therapeutic purposes. Particularly, in the MENA region, the influence of genetic mutations in AD risk genes such as ACE, DCP1, FTO, TCF7L2, PPAR-γ2, ApoE, BIN1, CLU, ABCA7, CR1, PICALM, FRMD4A that plays a major role in diabetes, insulin resistance, obesity and AD progression needs to be screened to prevent AD at the pre-clinical stage. In addition, increase in AD awareness, diagnosis and treatment strategies and population based studies are much needed to avert DM induced dementia in high-risk population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Not applicable.
Funding
This work was funded by United Arab Emirates University Start-up Grant (31M411).
Availability of data and materials
Not applicable.
Author’s contributions
This manuscript was written by RJ. Modifications were suggested by RB. AS and RB critically edited the final manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Richard L. Jayaraj, Email: richardlj@uaeu.ac.ae.
Sheikh Azimullah, Email: azim.sheikh@uaeu.ac.ae.
Rami Beiram, Email: rbeiram@uaeu.ac.ae.
References
- Aburawi E.H., AlKaabi J., Zoubeidi T., Shehab A., Lessan N., Al Essa A. Subclinical inflammation and endothelial dysfunction in young patients with diabetes: a study from United Arab Emirates. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0159808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abyad A. Alzheimer’s the road ahead in the middle east. J Alzheimers Dis Parkinsonism. 2016;6(241) pp. 2161–0460.1000241. [Google Scholar]
- Aguado B., Campbell R.D. Characterization of a human lysophosphatidic acid acyltransferase that is encoded by a gene located in the class III region of the human major histocompatibility complex. J. Biol. Chem. 1998;273(7):4096–4105. doi: 10.1074/jbc.273.7.4096. [DOI] [PubMed] [Google Scholar]
- Ahmed N., Ahmed U., Thornalley P.J., Hager K., Fleischer G., Münch G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment. J. Neurochem. 2005;92(2):255–263. doi: 10.1111/j.1471-4159.2004.02864.x. [DOI] [PubMed] [Google Scholar]
- Akhtar M.W., Sanz-Blasco S., Dolatabadi N., Parker J., Chon K., Lee M.S. Elevated glucose and oligomeric beta-amyloid disrupt synapses via a common pathway of aberrant protein S-nitrosylation. Nat. Commun. 2016;7:10242. doi: 10.1038/ncomms10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter F., Chen D., Yan S.F., Yan S.S. Mitochondrial perturbation in Alzheimer's disease and diabetes. Prog. Mol. Biol. Transl. Sci. 2017;146:341–361. doi: 10.1016/bs.pmbts.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter K., Lanza E.A., Martin S.A., Myronyuk N., Rua M., Raffa R.B. Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br. J. Clin. Pharmacol. 2011;71(3):365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Amiri E., Abdullatif M., Abdulle A., Al Bitar N., Afandi E.Z., Parish M. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health. 2015;15(1):1298. doi: 10.1186/s12889-015-2649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaama, T., Basheikh, M., Khiyami, A., Mutwalli, M., Batawi, S., Watfa, G., 2016. Diabetes status is associated with poor cognitive performace in Saudi population at high metabolic risk.
- Alhyas L., McKay A., Balasanthiran A., Majeed A. Prevalences of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: systematic review. JRSM Short Rep. 2011;2(7):1–16. doi: 10.1258/shorts.2011.011019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhyas L., McKay A., Majeed A. Prevalence of type 2 diabetes in the States of the co-operation council for the Arab States of the Gulf: a systematic review. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Safar H., Hassoun A., Almazrouei S., Kamal W., Afandi B., Rais N. Association of the genetic polymorphisms in transcription factor 7-like 2 and peroxisome proliferator-activated receptors-γ2 with type 2 diabetes mellitus and its interaction with obesity status in Emirati Population. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/129695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saikhan F.I., Abd-Elaziz M.A., Ashour R.H. Association between risk of type 2 diabetes mellitus and angiotensin-converting enzyme insertion/deletion gene polymorphisms in a Saudi Arabian population. Biomed. Rep. 2017;7(1):56–60. doi: 10.3892/br.2017.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsmadi O., Al-Rubeaan K., Mohamed G., Alkayal F., Al-Saud H., Al-Saud N.A. Weak or no association of TCF7L2 variants with Type 2 diabetes risk in an Arab population. BMC Med. Genet. 2008;9(1):72. doi: 10.1186/1471-2350-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas D. seventh ed. International Diabetes Federation; Brussels, Belgium: 2015. International Diabetes Federation. IDF Diabetes Atlas. [Google Scholar]
- Avila J., Gómez-Ramos A., Bolós M. AD genetic risk factors and tau spreading. Front. Aging Neurosci. 2015;7:99. doi: 10.3389/fnagi.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 2005;11(8):973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- Barroso E., del Valle J., Porquet D., Santos A.M.V., Salvadó L., Rodríguez-Rodríguez R. Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARβ/δ-null mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013;1832(8):1241–1248. doi: 10.1016/j.bbadis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Baynouna L.M., Revel A.D., Nagelkerke N.J., Jaber T.M., Omar A.O., Ahmed N.M. High prevalence of the cardiovascular risk factors in Al-Ain, United Arab Emirates. An emerging health care priority. Saudi Med. J. 2008;29(8):1173–1178. [PubMed] [Google Scholar]
- Beeler N., Riederer B.M., Waeber G., Abderrahmani A. Role of the JNK-interacting protein 1/islet brain 1 in cell degeneration in Alzheimer disease and diabetes. Brain Res. Bull. 2009;80(4–5):274–281. doi: 10.1016/j.brainresbull.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Beeri M.S., Moshier E., Schmeidler J., Godbold J., Uribarri J., Reddy S. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech. Ageing Dev. 2011;132(11–12):583–587. doi: 10.1016/j.mad.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz C.L., Mosconi L., Rahman A., Scheyer O., Hristov H., Isaacson R.S. Clinical application of APOE in Alzheimer's prevention: a precision medicine approach. J. Prevent. Alzheimer's Dis. 2018;5(4):245–252. doi: 10.14283/jpad.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla D., Lotfalinezhad E., Amini F., Salmannejad M., Reza Borhani Nezhad V., Rezai Kooshalshah S.F. Incidence and risk profile of dementia in the regions of middle east and North Africa. Neuroepidemiology. 2018;50(3–4):144–152. doi: 10.1159/000487761. [DOI] [PubMed] [Google Scholar]
- Birlouez-Aragon I., Saavedra G., Tessier F.J., Galinier A., Ait-Ameur L., Lacoste F. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutrit. 2010;91(5):1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- Blum-Degena D., Müller T., Kuhn W., Gerlach M., Przuntek H., Riederer P. Interleukin-1β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci. Lett. 1995;202(1–2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bomfim T.R., Forny-Germano L., Sathler L.B., Brito-Moreira J., Houzel J.-C., Decker H. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomers. J. Clin. Investig. 2012;122(4):1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard C., Durand A., Peyrol S., Chanseaume E., Chauvin M.-A., Morio B. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008;118(2):789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein A.H., Grimes I., Galasko D.R., Aisen P.S., Sabbagh M. Mjalli AMM. Effect of TTP488 in patients with mild to moderate Alzheimer's disease. BMC Neurol. 2014;14:12-. doi: 10.1186/1471-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Uribarri J., Zhu L., Chen X., Swamy S., Zhao Z. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA. 2014;111(13):4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S., Santos M.S., Seica R., Moreira P.I. Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. BBA. 2010;1802(11):942–951. doi: 10.1016/j.bbadis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Cardoso S., Carvalho C., Santos R., Correia S., Santos M.S., Seica R. Impact of STZ-induced hyperglycemia and insulin-induced hypoglycemia in plasma amino acids and cortical synaptosomal neurotransmitters. Synapse (New York, NY) 2011;65(6):457–466. doi: 10.1002/syn.20863. [DOI] [PubMed] [Google Scholar]
- Cardoso S., Santos R.X., Correia S.C., Carvalho C., Santos M.S., Baldeiras I. Insulin-induced recurrent hypoglycemia exacerbates diabetic brain mitochondrial dysfunction and oxidative imbalance. Neurobiol. Dis. 2013;49:1–12. doi: 10.1016/j.nbd.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Cardoso S., Correia S.C., Santos R.X., Carvalho C., Candeias E., Duarte A.I. Hyperglycemia, hypoglycemia and dementia: role of mitochondria and uncoupling proteins. Curr. Mol. Med. 2013;13(4):586–601. doi: 10.2174/1566524011313040010. [DOI] [PubMed] [Google Scholar]
- Cardoso S., Correia S., Carvalho C., Candeias E., Placido A.I., Duarte A.I. Perspectives on mitochondrial uncoupling proteins-mediated neuroprotection. J. Bioenerg. Biomembr. 2015;47(1–2):119–131. doi: 10.1007/s10863-014-9580-x. [DOI] [PubMed] [Google Scholar]
- Cardoso S., Seica R.M., Moreira P.I. Uncoupling protein 2 inhibition exacerbates glucose fluctuation-mediated neuronal effects. Neurotox. Res. 2018;33(2):388–401. doi: 10.1007/s12640-017-9805-y. [DOI] [PubMed] [Google Scholar]
- Carrero I., Gonzalo M., Martin B., Sanz-Anquela J., Arevalo-Serrano J., Gonzalo-Ruiz A. Oligomers of beta-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1beta, tumour necrosis factor-alpha, and a nuclear factor kappa-B mechanism in the rat brain. Exp. Neurol. 2012;236(2):215–227. doi: 10.1016/j.expneurol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Carvalho C., Correia S.C., Santos M.S., Baldeiras I., Oliveira C.R., Seica R. Vascular, oxidative, and synaptosomal abnormalities during aging and the progression of type 2 diabetes. Curr. Neurovasc. Res. 2014;11(4):330–339. doi: 10.2174/1567202611666140903122801. [DOI] [PubMed] [Google Scholar]
- Chae S., Kim S.-J., Do Koo Y., Lee J.H., Kim H., Ahn B.Y. A mitochondrial proteome profile indicative of type 2 diabetes mellitus in skeletal muscles. Exp. Mol. Med. 2018;50(9):129. doi: 10.1038/s12276-018-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R.C.C., Suen K.C., Ma C.H., Elyaman W., Ng H.K., Hugon J. Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2α in neuronal degeneration. J. Neurochem. 2002;83(5):1215–1225. doi: 10.1046/j.1471-4159.2002.01237.x. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Tseng Y., White M.F. Insulin signaling meets mitochondria in metabolism. Trends Endocrinol. Metab. 2010;21(10):589–598. doi: 10.1016/j.tem.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.-L., Chen C.-M., Cline H.T. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58(5):708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S., Sinha S., Zhao Y., Banerjee S., Sathyanarayana P., Shahani S. NF-κB-inducing kinase (NIK) mediates skeletal muscle insulin resistance: blockade by adiponectin. Endocrinology. 2011;152(10):3622–3627. doi: 10.1210/en.2011-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I.A., Vissel B. Inflammation-sleep interface in brain disease: TNF, insulin, orexin. J. Neuroinflam. 2014;11(1):51. doi: 10.1186/1742-2094-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton A., Baker L.D., Hanson A., Trittschuh E.H., Cholerton B., Morgan A. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J. Alzheimers Dis. 2015;44(3):897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- Collino M., Patel N.S., Thiemermann C. PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Therap. Adv. Cardiovasc. Dis. 2008;2(3):179–197. doi: 10.1177/1753944708090924. [DOI] [PubMed] [Google Scholar]
- Combs C.K., Johnson D.E., Karlo J.C., Cannady S.B., Landreth G.E. Inflammatory mechanisms in Alzheimer's disease: inhibition of β-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. J. Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran N.M., Martin D., Hutter-Paier B., Windisch M., Nguyen T., Nheu L. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci. 2010;17(8):1025–1033. doi: 10.1016/j.jocn.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Corona A.W., Fenn A.M., Godbout J.P. Cognitive and behavioral consequences of impaired immunoregulation in aging. J. Neuroimmune Pharmacol. 2012;7(1):7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Correia S.C., Santos R.X., Santos M.S., Casadesus G., Lamanna J.C., Perry G. Mitochondrial abnormalities in a streptozotocin-induced rat model of sporadic Alzheimer's disease. Curr. Alzheimer Res. 2013;10(4):406–419. doi: 10.2174/1567205011310040006. [DOI] [PubMed] [Google Scholar]
- Craft S., Asthana S., Schellenberg G., Baker L., Cherrier M., Boyt A.A. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann. N. Y. Acad. Sci. 2000;903(1):222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- Cummings J., Lee G., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2018. Alzheimer's Dementia: Transl. Res. Clin. Intervent. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damcott C.M., Pollin T.I., Reinhart L.J., Ott S.H., Shen H., Silver K.D. Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes. 2006;55(9):2654–2659. doi: 10.2337/db06-0338. [DOI] [PubMed] [Google Scholar]
- D'Andrea M., Reiser P., Gumula N., Hertzog B., Andrade-Gordon P. Application of triple immunohistochemistry to characterize amyloid plaque-associated inflammation in brains with Alzheimer's disease. Biotech. Histochem. 2001;76(2):97–106. [PubMed] [Google Scholar]
- De Felice F.G., Velasco P.T., Lambert M.P., Viola K., Fernandez S.J., Ferreira S.T. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- De Felice Fernanda G., Vieira Marcelo N.N., Bomfim Theresa R., Decker Helena, Velasco Pauline T., Lambert Mary P., Viola Kirsten L., Zhao Wei-Qin, Ferreira Sergio T., Klein William L. Protection of synapses against Alzheimer's-linked toxins: Insulin signaling prevents the pathogenic binding of Aβ oligomers. PNAS. 2009;106(6):1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F.G., Wu D., Lambert M.P., Fernandez S.J., Velasco P.T., Lacor P.N. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging. 2008;29(9):1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S.M., Wands J.R. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J. Alzheimers Dis. 2006;9(2):167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- de la Monte S.M., Wands J.R. Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008;2(6):1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Decker H., Lo K.Y., Unger S.M., Ferreira S.T., Silverman M.A. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30(27):9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman E., Eckman C. Aβ-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. 2005;33(5):1101–1105. doi: 10.1042/BST20051101. [DOI] [PubMed] [Google Scholar]
- Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. 2003;100(7):4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gamba A., Leal M., Morelli L., Castano E. Insulin-degrading enzyme: structure-function relationship and its possible roles in health and disease. Curr. Pharm. Des. 2009;15(31):3644–3655. doi: 10.2174/138161209789271799. [DOI] [PubMed] [Google Scholar]
- Fontan-Lozano A., Lopez-Lluch G., Delgado-Garcia J.M., Navas P., Carrion A.M. Molecular bases of caloric restriction regulation of neuronal synaptic plasticity. Mol. Neurobiol. 2008;38(2):167–177. doi: 10.1007/s12035-008-8040-1. [DOI] [PubMed] [Google Scholar]
- Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (New York, NY) 2007 doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C., Garai K. Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer’s disease. Proc. Natl. Acad. Sci. 2012;109(23):8913–8918. doi: 10.1073/pnas.1207022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariballa S., Kosanovic M., Yasin J., Essa A.E. Oxidative damage and inflammation in obese diabetic emirati subjects. Nutrients. 2014;6(11):4872. doi: 10.3390/nu6114872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl M., Gjedde A., Egefjord L., Møller A., Hansen S.B., Vang K. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front. Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi R., Haeri A., Dargahi L., Mohamed Z., Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol. Neurobiol. 2013;47(1):145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- Gouw A.A., van der Flier W.M., Fazekas F., van Straaten E.C., Pantoni L., Poggesi A. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39(5):1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Gratuze M., Julien J., Petry F.R., Morin F., Planel E. Insulin deprivation induces PP2A inhibition and tau hyperphosphorylation in hTau mice, a model of Alzheimer’s disease-like tau pathology. Sci. Rep. 2017;7:46359. doi: 10.1038/srep46359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratuze M., Joly-Amado A., Vieau D., Buée L., Blum D. Mutual relationship between tau and central insulin signalling: consequences for AD and Tauopathies? Neuroendocrinology. 2018;107(2):181–195. doi: 10.1159/000487641. [DOI] [PubMed] [Google Scholar]
- Grbovic O.M., Mathews P.M., Jiang Y., Schmidt S.D., Dinakar R., Summers-Terio N.B. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular levels of betaCTFs and Abeta production. J. Biol. Chem. 2003 doi: 10.1074/jbc.M304122200. [DOI] [PubMed] [Google Scholar]
- Guo Z., Fratiglioni L., Viitanen M., Lannfelt L., Basun H., Fastbom J. Apolipoprotein E genotypes and the incidence of Alzheimer's disease among persons aged 75 years and older: variation by use of antihypertensive medication? Am. J. Epidemiol. 2001;153(3):225–231. doi: 10.1093/aje/153.3.225. [DOI] [PubMed] [Google Scholar]
- Habibzadeh F. Hypertension in the Middle East. Acta Biomed. 2011;82:223–229. [Google Scholar]
- Hajat C., Harrison O., Shather Z. A profile and approach to chronic disease in Abu Dhabi. Globalization Health. 2012;8(1):18. doi: 10.1186/1744-8603-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R.R., Atli T., Al-Mandhari Z., Oudrhiri M., Balducci L., Silbermann M. Prevalence of aging population in the Middle East and its implications on cancer incidence and care. Ann. Oncol. 2013;24(suppl 7):vii11–vii24. doi: 10.1093/annonc/mdt268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak A.E., Pols H.A., Stehouwer C.D., Meijer J., Kiliaan A.J., Hofman A. Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J. Clin. Endocrinol. Metabol. 2001;86(9):4398–4405. doi: 10.1210/jcem.86.9.7873. [DOI] [PubMed] [Google Scholar]
- Hara Y., Yuk F., Puri R., Janssen W.G., Rapp P.R., Morrison J.H. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. PNAS. 2014;111(1):486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science (New York, NY) 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hatters D.M., Zhong N., Rutenber E., Weisgraber K.H. Amino-terminal domain stability mediates apolipoprotein E aggregation into neurotoxic fibrils. J. Mol. Biol. 2006;361(5):932–944. doi: 10.1016/j.jmb.2006.06.080. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Campenot R.B., Vance D.E., Vance J.E. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J. Biol. Chem. 2004;279(14):14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Hebbar P., Abubaker J.A., Abu-Farha M., Tuomilehto J., Al-Mulla F., Thanaraj T.A. A perception on genome-wide genetic analysis of metabolic traits in Arab populations. Front. Endocrinol. 2019;10:8. doi: 10.3389/fendo.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C., Rathmann W., Strassburger K., Finner H., Grallert H., Huth C. Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Horm. Metab. Res. 2008;40(10):722–726. doi: 10.1055/s-2008-1078730. [DOI] [PubMed] [Google Scholar]
- Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V., Maia L.F., Burgess B.L., Blain J.-F., Naus K.E., McIsaac S.A. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J. Biol. Chem. 2005;280(52):43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Hochgräfe K., Sydow A., Mandelkow E.M. Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology. FEBS J. 2013;280(18):4371–4381. doi: 10.1111/febs.12250. [DOI] [PubMed] [Google Scholar]
- Honig L.S., Vellas B., Woodward M., Boada M., Bullock R., Borrie M. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med. 2018;378(4):321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- Hoozemans J.J., van Haastert E.S., Nijholt D.A., Rozemuller A.J., Eikelenboom P., Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 2009;174(4):1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Liu X.Q., Wyss-Coray T., Brecht W.J., Sanan D.A., Mahley R.W. Apolipoprotein E fragments present in Alzheimer's disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc. Natl. Acad. Sci. 2001;98(15):8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M., Arakawa K., Hamano T., Nagata M., Nakamoto Y., Kuriyama M. Evaluation of systemic redox states in patients carrying the MELAS A3243G mutation in mitochondrial DNA. Eur. Neurol. 2012;67(4):232–237. doi: 10.1159/000336568. [DOI] [PubMed] [Google Scholar]
- Infante-Garcia C., Ramos-Rodriguez J.J., Galindo-Gonzalez L., Garcia-Alloza M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology. 2016;65:15–25. doi: 10.1016/j.psyneuen.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Inzelberg R., Massarwa M., Schechtman E., Strugatsky R., Farrer L.A., Friedland R.P. Estimating the risk for conversion from mild cognitive impairment to Alzheimer's disease in an elderly Arab community. J. Alzheimer's Dis.: JAD. 2015;45(3):865–871. doi: 10.3233/JAD-142871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Alonso A.d.C., Chen S., Chohan M.O., El-Akkad E., Gong C.-X. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005;1739(2–3):198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Irie F., Fitzpatrick A.L., Lopez O.L., Kuller L.H., Peila R., Newman A.B. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4: the Cardiovascular Health Study Cognition Study. Arch. Neurol. 2008;65(1):89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke A., Czech M.P., Davis R.J. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18(16):1976–1980. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson J., Laedtke T., Parisi J.E., O’Brien P., Petersen R.C., Butler P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- Johnson L.A., Torres E.R., Impey S., Stevens J.F., Raber J. Apolipoprotein E4 and insulin resistance interact to impair cognition and alter the epigenome and metabolome. Sci. Rep. 2017;7:43701. doi: 10.1038/srep43701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaido M., Fujimura H., Soga F., Toyooka K., Yoshikawa H., Nishimura T. Alzheimer-type pathology in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) Acta Neuropathol. 1996;92(3):312–318. doi: 10.1007/s004010050524. [DOI] [PubMed] [Google Scholar]
- Kapoor D., Trikha D., Vijayvergiya R., Kaul D., Dhawan V. Conventional therapies fail to target inflammation and immune imbalance in subjects with stable coronary artery disease: a system-based approach. Atherosclerosis. 2014;237(2):623–631. doi: 10.1016/j.atherosclerosis.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Ke Y.D., Delerue F., Gladbach A., Götz J., Ittner L.M. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer's disease. PLoS ONE. 2009;4(11) doi: 10.1371/journal.pone.0007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadir A., Kavalakatt S., Dehbi M., Alarouj M., Bennakhi A., Tiss A. DUSP1 is a potential marker of chronic inflammation in arabs with cardiovascular diseases. Dis. Markers. 2018;2018 doi: 10.1155/2018/9529621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T.K., Alkon D.L. Peripheral biomarkers of Alzheimer's disease. J. Alzheimer's Dis.: JAD. 2015;44(3):729–744. doi: 10.3233/JAD-142262. [DOI] [PubMed] [Google Scholar]
- Kickstein E., Krauss S., Thornhill P., Rutschow D., Zeller R., Sharkey J. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. 2010;107(50):21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick R., Scales G., Leroy K., Causevic M., Hooper C., Irvine E.E. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem. Biophys. Res. Commun. 2009;386(1):257–262. doi: 10.1016/j.bbrc.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Yamamoto Y., Kim K., Kamei N., Shimada T., Liu L. Regulation of diet-induced adipose tissue and systemic inflammation by salicylates and pioglitazone. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0082847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E.M., Martins I.V., Gumusgoz S., Allan S.M., Lawrence C.B. High-fat diet-induced memory impairment in triple-transgenic Alzheimer's disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol. Aging. 2014;35(8):1821–1832. doi: 10.1016/j.neurobiolaging.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh Y.H., von Arnim C.A., Hyman B.T., Tanzi R.E., Tesco G. BACE is degraded via the lysosomal pathway. J. Biol. Chem. 2005;280(37):32499–32504. doi: 10.1074/jbc.M506199200. [DOI] [PubMed] [Google Scholar]
- Kolmychkova K.I., Zhelankin A.V., Karagodin V.P., Orekhov A.N. Mitochondria and inflammation. Patologicheskaia fiziologiia i eksperimental'naia terapiia. 2016;60(4):114–121. [PubMed] [Google Scholar]
- Koschinsky T., He C.-J., Mitsuhashi T., Bucala R., Liu C., Buenting C. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. 1997;94(12):6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3) doi: 10.1016/j.immuni.2017.08.008. 566 81.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel S.H., Blahak C., Bäzner H., Inzitari D., Pantoni L., Poggesi A. Deterioration of gait and balance over time: the effects of age-related white matter change-the LADIS study. Cerebrovasc. Dis. 2013;35(6):544–553. doi: 10.1159/000350725. [DOI] [PubMed] [Google Scholar]
- Kubaszek A., Pihlajamäki J., Komarovski V., Lindi V., Lindström J., Eriksson J. Promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2003;52(7):1872–1876. doi: 10.2337/diabetes.52.7.1872. [DOI] [PubMed] [Google Scholar]
- Lambert J.C., Mann D., Harris J., Araria-Goumidi L., Chartier-Harlin M.C., Cottel D. Association study of Notch 4 polymorphisms with Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2004;75(3):377–381. doi: 10.1136/jnnp.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T., Holton J.L., Gray E., Kirkham K., O'Sullivan S.S., Hilbig A. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol. 2008;115(4):417–425. doi: 10.1007/s00401-007-0336-0. [DOI] [PubMed] [Google Scholar]
- Lashley T., Schott J.M., Weston P., Murray C.E., Wellington H., Keshavan A. Molecular biomarkers of Alzheimer's disease: progress and prospects. Dis. Models Mech. 2018;11(5) doi: 10.1242/dmm.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawan A., Min K., Zhang L., Canfran-Duque A., Jurczak M.J., Camporez J.P.G. Skeletal muscle-specific deletion of MKP-1 Reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes. 2018;67(4):624–635. doi: 10.2337/db17-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Giraud J., Davis R.J., White M.F. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 2003;278(5):2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- Lesort M., Johnson G. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99(2):305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Lesort M., Jope R.S., Johnson G.V. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3β and Fyn tyrosine kinase. J. Neurochem. 1999;72(2):576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J. Alzheimers Dis. 2016;53(2):393–402. doi: 10.3233/JAD-160114. [DOI] [PubMed] [Google Scholar]
- Li X.-H., Xie J.-Z., Jiang X., Lv B.-L., Cheng X.-S., Du L.-L. Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. NeuroMol. Med. 2012;14(4):338–348. doi: 10.1007/s12017-012-8191-0. [DOI] [PubMed] [Google Scholar]
- Lin F., Jia J., Qin W. Enhancement of beta-amyloid oligomer accumulation after intracerebroventricular injection of streptozotocin, which involves central insulin signaling in a transgenic mouse model. NeuroReport. 2014;25(16):1289–1295. doi: 10.1097/WNR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu L., Lu S., Wang D., Liu X.-d., Xie L. Impaired amyloid β-degrading enzymes in brain of streptozotocin-induced diabetic rats. J. Endocrinol. Invest. 2011;34(1):26–31. doi: 10.1007/BF03346691. [DOI] [PubMed] [Google Scholar]
- Locke M., Anderson J. NF-κB activation in organs from STZ-treated rats. Appl. Physiol. Nutr. Metab. 2011;36(1):121–127. doi: 10.1139/H10-094. [DOI] [PubMed] [Google Scholar]
- Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfy M., Adeghate J., Kalasz H., Singh J., Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr. Diabetes Rev. 2017;13(1):3–10. doi: 10.2174/1573399812666151016101622. [DOI] [PubMed] [Google Scholar]
- Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüth H.-J., Ogunlade V., Kuhla B., Kientsch-Engel R., Stahl P., Webster J. Age-and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer's disease brains. Cereb. Cortex. 2004;15(2):211–220. doi: 10.1093/cercor/bhh123. [DOI] [PubMed] [Google Scholar]
- Maassen J.A., ’T Hart L.M., Van Essen E., Heine R.J., Nijpels G., Jahangir Tafrechi R.S. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- Majeed A., El-Sayed A.A., Khoja T., Alshamsan R., Millett C., Rawaf S. Diabetes in the Middle-East and North Africa: an update. Diabetes Res. Clin. Pract. 2014;103(2):218–222. doi: 10.1016/j.diabres.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Mandelkow E.M., Biernat J., Drewes G., Gustke N., Trinczek B., Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging. 1995;16(3):355–362. doi: 10.1016/0197-4580(95)00025-a. [DOI] [PubMed] [Google Scholar]
- Marciniak E., Leboucher A., Caron E., Ahmed T., Tailleux A., Dumont J. Tau deletion promotes brain insulin resistance. J. Exp. Med. 2017;214(8):2257–2269. doi: 10.1084/jem.20161731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P., Gleichmann M., Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch D.H., Nägler K., Schumacher S., Göritz C., Müller E.-C., Otto A. CNS synaptogenesis promoted by glia-derived cholesterol. Science (New York, NY) 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- McGeer E.G., McGeer P.L. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. J. Alzheimers Dis. 2010;19(1):355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia: J. Alzheimer's Assoc. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M. An overview on the clinical development of tau-based therapeutics. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn G., Hollborn M., Schliebs R. Induction of cytokines in glial cells surrounding cortical β-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int. J. Dev. Neurosci. 2000;18(4–5):423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Mendoza-Herrera K., Aradillas-García C., Mejía-Diaz M., Alegría-Torres J., Garay-Sevilla M., Luevano-Contreras C. Association of dietary advanced glycation end products with metabolic syndrome in young mexican adults. Medicines. 2018;5(4):128. doi: 10.3390/medicines5040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Baldwin C.T., Bowirrat A., Waraska K., Inzelberg R., Friedland R.P. Association of polymorphisms in the Angiotensin-converting enzyme gene with Alzheimer disease in an Israeli Arab community. Am. J. Hum. Genet. 2006;78(5):871–877. doi: 10.1086/503687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J., Qing H., Radenovic A., Kis A., Vileno B., Làszló F. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol. Aging. 2010;31(9):1503–1515. doi: 10.1016/j.neurobiolaging.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.W., Willett K.C., Desilets A.R. Rosiglitazone and pioglitazone for the treatment of Alzheimer's disease. Ann. Pharmacother. 2011;45(11):1416–1424. doi: 10.1345/aph.1Q238. [DOI] [PubMed] [Google Scholar]
- Moloney A.M., Griffin R.J., Timmons S., O’Connor R., Ravid R., O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging. 2010;31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Moreira P.I., Santos M.S., Seica R., Oliveira C.R. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J. Neurol. Sci. 2007;257(1–2):206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Newcombe E.A., Camats-Perna J., Silva M.L., Valmas N., Huat T.J., Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer's disease. J. Neuroinflam. 2018;15(1):276. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi S., Shimozawa N., Yasutomi Y., Yanagisawa K., Kimura N. Diabetes mellitus accelerates Aβ pathology in brain accompanied by enhanced GAβ generation in nonhuman primates. PLoS ONE. 2015;10(2) doi: 10.1371/journal.pone.0117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11(2):85. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens D.M., van Duinkerken E., Schoonenboom S.N.M., de Wiza D.H., Klein M., van Golen L. Cerebrospinal fluid levels of Alzheimer’s disease biomarkers in middle-aged patients with type 1 diabetes. Diabetologia. 2014;57(10):2208–2214. doi: 10.1007/s00125-014-3333-6. [DOI] [PubMed] [Google Scholar]
- Palmert M.R., Usiak M., Mayeux R., Raskind M., Tourtellotte W.W., Younkin S.G. Soluble derivatives of the beta amyloid protein precursor in cerebrospinal fluid: alterations in normal aging and in Alzheimer's disease. Neurology. 1990;40(7):1028–1034. doi: 10.1212/wnl.40.7.1028. [DOI] [PubMed] [Google Scholar]
- Papon M.-A., El Khoury N.B., Marcouiller F., Julien C., Morin F., Bretteville A. Deregulation of protein phosphatase 2A and hyperphosphorylation of τ protein following onset of diabetes in NOD mice. Diabetes. 2013;62(2):609–617. doi: 10.2337/db12-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep.: PR. 2009;61(4):595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- Peila R., Rodriguez B.L., Launer L.J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Phung K.T.T., Chaaya M., Prince M., Atweh S., El Asmar K., Karam G. Dementia prevalence, care arrangement, and access to care in Lebanon: A pilot study. Alzheimer's Dementia: J. Alzheimer's Assoc. 2017;13(12):1317–1326. doi: 10.1016/j.jalz.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone P., Giacomazza D., Vetri V., Carrotta R., Militello V., San Biagio P.L. Insulin-activated Akt rescues Abeta oxidative stress-induced cell death by orchestrating molecular trafficking. Aging Cell. 2011;10(5):832–843. doi: 10.1111/j.1474-9726.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- Pintana H., Apaijai N., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11–12):409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Pintana H., Apaijai N., Chattipakorn N., Chattipakorn S.C. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J. Endocrinol. 2013;218(1):1–11. doi: 10.1530/JOE-12-0521. [DOI] [PubMed] [Google Scholar]
- Planel E., Richter K.E., Nolan C.E., Finley J.E., Liu L., Wen Y. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci.: Off. J. Soc. Neurosci. 2007;27(12):3090–3097. doi: 10.1523/JNEUROSCI.4854-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.C., Klunk W.E., Lopresti B.J., Lu X., Hoge J.A., Ziolko S.K. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metabolism: Off. J. Int. Soc. Cerebral Blood Flow Metabol. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Priyadarshini M., Kamal M.A., Greig N.H., Reale M., Abuzenadah A.M., Chaudhary A.G. Alzheimer's disease and type 2 diabetes: exploring the association to obesity and tyrosine hydroxylase. CNS Neurol. Disord.: Drug Targets. 2012;11(4):482–489. doi: 10.2174/187152712800792767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S., Zhang H., Zhang G., Ahmed Z., Wang Y., Cai D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc. Natl. Acad. Sci. 2011;108(7):2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H., John A., Howarth F.C. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol., Biochem., Pharmacol. 2015;35(3):1241–1251. doi: 10.1159/000373947. [DOI] [PubMed] [Google Scholar]
- Reitz C., Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T.T., Catlin L.W., Coonse K.G., Habig J.W. Identification of an amino-terminal fragment of apolipoprotein E4 that localizes to neurofibrillary tangles of the Alzheimer's disease brain. Brain Res. 2012;1475:106–115. doi: 10.1016/j.brainres.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Rönnemaa E., Zethelius B., Sundelöf J., Sundström J., Degerman-Gunnarsson M., Berne C. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71(14):1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- Rosenmann H. CSF biomarkers for amyloid and tau pathology in Alzheimer's disease. J. Mol. Neurosci.: MN. 2012;47(1):1–14. doi: 10.1007/s12031-011-9665-5. [DOI] [PubMed] [Google Scholar]
- Saadi H., Nagelkerke N., Carruthers S.G., Benedict S., Abdulkhalek S., Reed R. Association of TCF7L2 polymorphism with diabetes mellitus, metabolic syndrome, and markers of beta cell function and insulin resistance in a population-based sample of Emirati subjects. Diabetes Res. Clin. Pract. 2008;80(3):392–398. doi: 10.1016/j.diabres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Saber-Ayad M., Manzoor S., El Serafi A., Mahmoud I., Hammoudeh S., Rani A. The FTO rs9939609 “A” allele is associated with impaired fasting glucose and insulin resistance in Emirati population. Gene. 2019;681:93–98. doi: 10.1016/j.gene.2018.09.053. [DOI] [PubMed] [Google Scholar]
- Salkovic-Petrisic M., Tribl F., Schmidt M., Hoyer S., Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J. Neurochem. 2006;96(4):1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- Sanghera D.K., Ortega L., Han S., Singh J., Ralhan S.K., Wander G.S. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med. Genet. 2008;9(1):59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas C.L., Moreno-Flores M.T., Avila J., Wandosell F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer's disease-like Tau phosphorylation. J. Biol. Chem. 1999;274(52):37046–37052. doi: 10.1074/jbc.274.52.37046. [DOI] [PubMed] [Google Scholar]
- Schmidt M.I., Duncan B.B., Sharrett A.R., Lindberg G., Savage P.J., Offenbacher S. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet (London, England). 1999;353(9165):1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Seripa D., Solfrizzi V., Imbimbo B.P., Daniele A., Santamato A., Lozupone M. Tau-directed approaches for the treatment of Alzheimer's disease: focus on leuco-methylthioninium. Expert Rev. Neurother. 2016;16(3):259–277. doi: 10.1586/14737175.2016.1140039. [DOI] [PubMed] [Google Scholar]
- Sheng L., Zhou Y., Chen Z., Ren D., Cho K.W., Jiang L. NF-κB–inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat. Med. 2012;18(6):943. doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu Y.P., Huo Y., Feng X.L., Gilbert J., Zhang Q., Liuyang Z.Y. CIP2A causes tau/APP phosphorylation, synaptopathy, and memory deficits in Alzheimer's disease. Cell Rep. 2018;24(3):713–723. doi: 10.1016/j.celrep.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka I., Kamide K., Ohishi M., Katsuya T., Akasaka H., Saitoh S. Association of gene polymorphism of the fat-mass and obesity-associated gene with insulin resistance in Japanese. Hypertens. Res. 2010;33(3):214. doi: 10.1038/hr.2009.215. [DOI] [PubMed] [Google Scholar]
- Sims-Robinson C., Kim B., Rosko A., Feldman E.L. How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 2010;6(10):551. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A., Turunen H., Ngandu T., Peltonen M., Levälahti E., Helisalmi S. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75(4):462–470. doi: 10.1001/jamaneurol.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V., Westcott B., Forbes J., Phan T.G., Beare R., Venn A. Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2012;68(1):68–73. doi: 10.1093/gerona/gls100. [DOI] [PubMed] [Google Scholar]
- Steen E., Terry B.M., Rivera J.E., Cannon J.L., Neely T.R., Tavares R. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease–is this type 3 diabetes? J. Alzheimer's Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stephen T., Cacciottolo M., Balu D., Morgan T., LaDu M., Finch C. APOE genotype and sex affect microglial interactions with plaques in Alzheimer’s disease mice. Acta Neuropath. Commun. 2019;7(1):82. doi: 10.1186/s40478-019-0729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan M.W., Deary I.J., Ewing F.M., Frier B.M. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23(3):305–312. doi: 10.2337/diacare.23.3.305. [DOI] [PubMed] [Google Scholar]
- Sulaiman N., Mahmoud I., Hussein A., Elbadawi S., Abusnana S., Zimmet P. Diabetes risk score in the United Arab Emirates: a screening tool for the early detection of type 2 diabetes mellitus. BMJ Open Diabetes Res. Care. 2018;6(1) doi: 10.1136/bmjdrc-2017-000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R.H., Khan S.M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Takeda S., Sato N., Uchio-Yamada K., Sawada K., Kunieda T., Takeuchi D. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Aβ deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. 2010;107(15):7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Scott D.A., Das U., Edland S.D., Radomski K., Koo E.H. Early and selective impairments in axonal transport kinetics of synaptic cargoes induced by soluble amyloid beta-protein oligomers. Traffic (Copenhagen, Denmark) 2012;13(5):681–693. doi: 10.1111/j.1600-0854.2012.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki H., Bujo H., Yamagishi S.-I., Takeuchi M., Imaizumi T., Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res. Clin. Pract. 2007;76(2):236–244. doi: 10.1016/j.diabres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Uronen R.-L.E., Huttunen H.J. Genetic risk factors of Alzheimer’s disease and cell-to-cell transmission of Tau. J. Neurol. Neuromed. 2016 [Google Scholar]
- Van de Ree M., Huisman M., Princen H., Meinders A., Kluft C., Group D.-S. Strong decrease of high sensitivity C-reactive protein with high-dose atorvastatin in patients with type 2 diabetes mellitus. Atherosclerosis. 2003;166(1):129–135. doi: 10.1016/s0021-9150(02)00316-7. [DOI] [PubMed] [Google Scholar]
- van Horssen J., van Schaik P., Witte M. Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.06.050. [DOI] [PubMed] [Google Scholar]
- Varghese R.T., Viegas I., Barosa C., Marques C., Shah M., Rizza R.A. Diabetes-associated variation in TCF7L2 Is not associated with hepatic or extrahepatic insulin resistance. Diabetes. 2016;65(4):887–892. doi: 10.2337/db15-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozarova B., Weyer C., Lindsay R.S., Pratley R.E., Bogardus C., Tataranni P.A. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(2):455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Selkoe D.J. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44(1):181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Westwood S., Liu B., Baird A.L., Anand S., Nevado-Holgado A.J., Newby D. The influence of insulin resistance on cerebrospinal fluid and plasma biomarkers of Alzheimer’s pathology. Alzheimer's Res. Therapy. 2017;9(1):31. doi: 10.1186/s13195-017-0258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Yang Y., Yuan G., Zhu W., Ma D., Hu S. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J. Invest. Med.: Off. Publ. Am. Feder. Clin. Res. 2015;63(2):267–272. doi: 10.1097/JIM.0000000000000129. [DOI] [PubMed] [Google Scholar]
- Yamagishi S-i, Maeda S., Matsui T., Ueda S., Fukami K., Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta (BBA)-Gen. Sub. 2012;1820(5):663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Yang Y., Ma D., Xu W., Chen F., Du T., Yue W. Exendin-4 reduces tau hyperphosphorylation in type 2 diabetic rats via increasing brain insulin level. Mol. Cell. Neurosci. 2016;70:68–75. doi: 10.1016/j.mcn.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Yiannopoulou K.G., Papageorgiou S.G. Current and future treatments for Alzheimer's disease. Therap. Adv. Neurol. Disord. 2013;6(1):19–33. doi: 10.1177/1756285612461679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Kuret J. C-terminal truncation modulates both nucleation and extension phases of τ fibrillization. FEBS Lett. 2006;580(1):211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- Zadjali F., Al-Yahyaee S., Hassan M., Albarwani S., Bayoumi R. Association of adiponectin promoter variants with traits and clusters of metabolic syndrome in Arabs: family-based study. Gene. 2013;527(2):663–669. doi: 10.1016/j.gene.2013.06.057. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xia Y., Wang Y., Shentu Y., Zeng K., Mahaman Y.A.R. CK2 phosphorylating I(2)(PP2A)/SET mediates tau pathology and cognitive impairment. Front. Mol. Neurosci. 2018;11:146. doi: 10.3389/fnmol.2018.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-Q., Alkon D.L. Role of insulin and insulin receptor in learning and memory. Mol. Cell. Endocrinol. 2001;177(1–2):125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Zhao W.-Q., De Felice F.G., Fernandez S., Chen H., Lambert M.P., Quon M.J. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- Zhao X., Han Q., Lv Y., Sun L., Gang X., Wang G. Biomarkers for cognitive decline in patients with diabetes mellitus: evidence from clinical studies. Oncotarget. 2017;9(7):7710–7726. doi: 10.18632/oncotarget.23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.-Q., Lacor P.N., Chen H., Lambert M.P., Quon M.J., Krafft G.A. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric abeta. J. Biol. Chem. 2009;jbc doi: 10.1074/jbc.M109.011015. M109. 011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Liu C.-C., Qiao W., Bu G. Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol. Psychiatry. 2018;83(4):347–357. doi: 10.1016/j.biopsych.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Weisgraber K.H. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J. Biol. Chem. 2009;284(10):6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Scott S., Shelton S., Crutcher K. Cathepsin D-mediated proteolysis of apolipoprotein E: possible role in Alzheimer’s disease. Neuroscience. 2006;143(3):689–701. doi: 10.1016/j.neuroscience.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Zilka N., Filipcik P., Koson P., Fialova L., Skrabana R., Zilkova M. Truncated tau from sporadic Alzheimer's disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006;580(15):3582–3588. doi: 10.1016/j.febslet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Zilkens R.R., Davis W.A., Spilsbury K., Semmens J.B., Bruce D.G. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am. J. Epidemiol. 2013;177(11):1246–1254. doi: 10.1093/aje/kws387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.