Abstract
The objective of this study was to assess adherence and costs-benefits of colorectal cancer (CRC) screenings from an accountable care organization/population health perspective. We performed a retrospective review of 94 patients (50–75 years of age) in an integrated safety net system for whom fecal CRC screening was abnormal for the period of June 1, 2014, to June 1, 2016. A cost-benefit model was constructed using Medicare payment rates and a sensitivity analysis. Most patients included in the study (64/94, 68%) received or were offered a colonoscopy. Of those receiving a colonoscopy, 24 of 45 (53%) had an abnormal finding. Total direct medical costs avoided by screening the patient panel was $32,926 but could have exceeded $63,237 had more patients received follow-up colonoscopies. A sensitivity analysis with 1000 patients demonstrated total monetary benefits between $2.2 million and $8.16 million when follow-up and colonoscopy rates were allowed to vary. Although the resulting rates of follow-up were within the range reported in the literature, there is room for improvement, especially considering the monetary benefit that could be used on other diseases. Health systems and payers should work cooperatively to structure payment models to better incentivize CRC screenings.
Keywords: Colorectal cancer screening, cost-benefit model, preventive care
An estimated 95,520 cases of colorectal cancer (CRC) occur annually in the USA. CRC is the second leading cause of cancer deaths, with an estimated 50,260 per year, and is one of the most costly types of cancer, with nearly $14 billion spent on direct medical care in 2010.1–4 Costs of CRC the first year after diagnosis are estimated to range from $12,757 to $58,704 depending on comorbidities, stage, location, and other factors.4–9 Evidence suggests that screening—invasive exams, like colonoscopy, and noninvasive stool tests, like fecal immunochemical test (FIT)—reduces CRC incidence and mortality and is cost effective.2,10–15 Patients are more likely to complete screening when FIT is recommended or when given a choice between fecal methods and colonoscopy, but the rate of diagnostic colonoscopy after an abnormal fecal test result ranges from 42% to 83%.16–36 Improving follow-up after abnormal FIT represents a significant population health opportunity in reducing incidence, mortality, and costs. The goal of this study was to create a theoretical understanding of how accountability through cost avoidance could be included in the development of a new payment and delivery model. Using data from an integrated delivery system in Central Texas and a cost-benefit model (CBM), this study assessed how adherence to CRC screenings could influence the allocation of resources from CRC treatment to other health initiatives.
METHODS
In this retrospective electronic medical record review, the initial search screened for patients aged 50 to 75 years who completed FIT between June 1, 2014, and June 1, 2016 (Figure 1). Exclusion criteria were (1) not having an assigned primary care physician, (2) having a positive FIT test or colonoscopy within the year prior to completion of the FIT test results used for this study, and (3) dying within the follow-up period. Two authors independently screened patient charts and abstracted the following data: order date for FIT in outpatient setting, electronic documentation of communicating abnormal results to the patient, the ordering of and completion of diagnostic colonoscopy, the ordering of and completion of gastroenterology clinic visit, and the time to and results of diagnostic colonoscopy. Additionally, the following demographic and protected health information data were extracted: age, gender, ethnicity, insurance status, ZIP code, and previously positive FIT. This was an exploratory investigation, so a smaller sample was used as a proof of concept. Simple random sampling was conducted and used to explore the relation between abnormal FIT tests and rate of follow-up within 1 year. Measures of central tendencies were used to describe results.
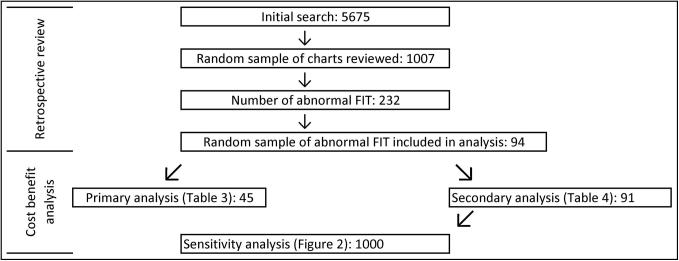
Figure 1.
Populations used for retrospective review and cost-benefit analysis.
A CBM explored the relation between monetary benefit of screening adherence via the (1) follow-up rate after abnormal FITs and (2) rate of colonoscopies after abnormal FITs. The primary outcome of interest was total monetary benefit in terms of avoided direct medical costs within 1 year of diagnosis, less the cost of screening, complications, and missed incidences. A population health perspective was chosen because health systems are increasingly taking accountability for a population, and assessing the monetary impact of various innovations on a population is an important tool for investment purposes. Additionally, the analysis focused on initial treatment costs instead of continuing, last year of life, or lifetime costs for two primary reasons: (1) annual FIT screening aligns with first-year costs and (2) payment model performance is typically calculated annually and therefore aligns with annual screening.
Financial calculations were carried out in a separate Excel file. Medicare’s 2017 physician, clinical diagnostic library, and anesthesia fee schedules were used with national payment instead of geographic-specific amounts (Table 1). The most frequent complications and rates associated with colonoscopy were estimated with diagnosis-related group codes using the Texas PricePoint tool (Table 1).37,38 Authors identified six CRC treatment cost studies, inflated the average reported in the studies to 2017 dollars using the Consumer Price Index, and calculated the mean cost.38,39 The cost of distant (stages 3 and 4) CRC ($42,039.70) treatment was adjusted downward, as demonstrated by Zhehui et al, to obtain the cost of local (stages 1 and 2) CRC treatment by 0.9204.40 In constructing the CBM, multiple assumptions were made and are listed under corresponding tables. Further, dysplastic polyps were categorized as local CRC, adenomatous polyps were estimated to have a 25% annualized risk of progression to CRC, and hyperplastic polyps were estimated to have a negligible risk of progression to CRC.41–47 Findings from the quality improvement/statistical analysis were used to inform the cost-benefit analysis.
Table 1.
Breakdown of costs and benefits of screening for and treating colorectal cancer
| Variable | Amount |
|---|---|
| Costsa | |
| Colonoscopy with foreign body removal | $416.31 |
| Colonoscopy with biopsy | $412.72 |
| Colonoscopy with lesion removal | $457.94 |
| Diagnostic colonoscopy | $322.64 |
| Colonoscopy with polypectomy | $357.45 |
| Tissue exam by pathologist (per jar/specimen) | $69.62 |
| Fecal blood screen immunoassay | $21.82 |
| Anesthesia low intestine scope | $198.41 |
| Complication: perforation (DRG 921, incidence 0.19/1000) | $26,159.00 |
| Complication: gastrointestinal bleeding with and without transfusion (DRG 921, incidence 0.79 and 1.59/1000, respectively) | $26,159.00 |
| Complication: diverticulitis (DRG 329, incidence 0.23/1000) | $19,029.00 |
| Complication: angina or myocardial infarction (DRG 281, incidence 0.56/1000) | $31,352.00 |
| Complication: stroke or transient ischemic attack (DRG 062, incidence 0.33/1000) | $53,452.00 |
| Other complication requiring observation (DRG 99219, incidence 0.09/1000) | $138.60 |
| Benefits | |
| Direct medical CRC costs avoidedb | $42,039.70 |
CRC indicates colorectal cancer; DRG, diagnosis-related group.
Values calculated from 2017 Medicare physician, clinical diagnostic lab, and anesthesia fee schedules without geographic adjustments. DRG values calculated from Texas Hospital PricePoint tool.
Median cost calculated from literature review and inflated and standardized dollars.
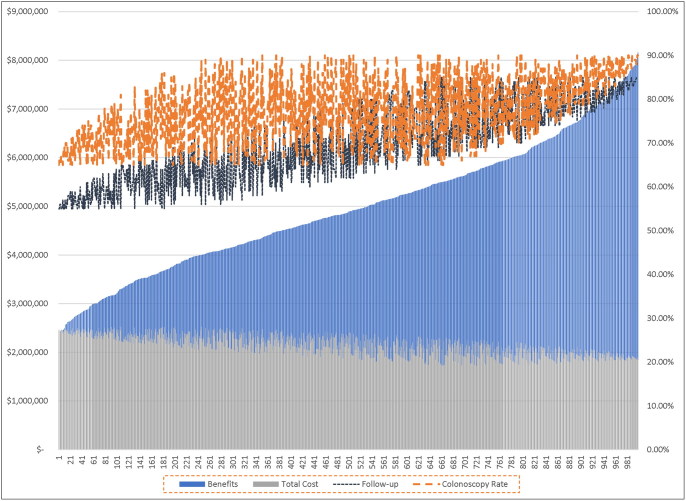
A sensitivity analysis was conducted to investigate the robustness of benefit generated (Figure 2). In the first simulation, we allowed the rate of follow-ups to vary between 55% and 85%, holding all other variables constant. In the second simulation, we allowed both the rate of follow-up and the rate of follow-up colonoscopies to vary between 55% and 85% and 65% and 90%, respectively. In addition, the second simulation allowed the rate of complications to vary between the bounds of the confidence intervals of their incidence rates based on the literature.38 In both simulations, the panel of patients was grown from 94 to 1000 patients to better fit rates of complications.
Figure 2.
Sensitivity analysis with varied rates of follow-up, colonoscopies, and complications. Total costs represent the cost of screening, treatment, and complications, and total benefits represent avoided cancer costs achieved through adherence. As the adherence to screening and appropriate follow-up improves across a population, total cancer costs avoided (i.e., benefit) increase.
RESULTS
The initial search revealed 5675 FITs with results that were randomized and divided among the abstractors (Table 2, Figure 1). Of these, 1007 charts were screened for abnormal results, revealing 232 abnormal FIT results. The first 94 charts with an abnormal FIT test that were ordered for colonoscopy screening were included in this analysis.
Table 2.
Breakdown of included patients
| Variable | N |
|---|---|
| Total FITs ordered June 2014–2016 | 5675 |
| Charts screened for abnormal results | 1007 |
| Total abnormal results found | 232 (23.0%) |
| Charts reviewed | 94 |
| Median age (years) | 64.5 |
| Female | 51 (54.3%) |
| White | 65 (69.2%) |
| Black | 17 (18.1%) |
| Other/unknown | 12 (12.8%) |
| Received follow-up care | 64 (68.1%) |
| Received follow-up colonoscopy | 45 (47.9%) |
| Declined colonoscopy | 16 (17.0%) |
| Health precluded colonoscopy | 3 (3.2%) |
| Did not receive appropriate follow-up care | 30 (31.9%) |
| No/inadequate communication of FIT results to patient | 19 (20.2%) |
| Repeat FIT ordered | 7 (7.5%) |
| Colonoscopy supposedly obtained elsewhere but no records in our EMR | 2 (2.1%) |
| Lacked transportation to colonoscopy | 2 (2.1%) |
EMR indicates electronic medical record; FIT, fecal immunochemical test.
In total, 64 of the 94 patients received appropriate follow-up care; however, less than half (45/94, 47.87%) of all patients received a follow-up diagnostic colonoscopy. Sixteen of the 94 patients (17.02%) received communication regarding abnormal results and were offered a colonoscopy but declined. Three of the 94 patients (3.19%) received communication regarding abnormal results, but health precluded them from being offered a colonoscopy. Other results are indicated in Table 2.
Approximately one-half of colonoscopies (24/45, 53.33%) had an abnormal colonoscopy finding, with 3/24 (12.5%) dysplastic polyps, 14/24 (58.33%) adenomatous polyps, and 7/24 (29.17%) hyperplastic polyps. Among the three patients with dysplastic polyps, one was eventually diagnosed as a stage 3 malignancy after a wait of 90 days from abnormal FIT to diagnostic colonoscopy. Another was diagnosed as a stage 4 malignancy after a 719-day wait from positive FIT to diagnostic colonoscopy. The third case of dysplastic polyp was attributed to hemorrhoids; when the patient presented with a complaint of anemia with unintentional weight loss approximately 2 years following the abnormal FIT, the diagnostic colonoscopy was performed.
The primary CBM demonstrated a population health benefit of $32,926 in the annual screening by avoiding possible CRC (Table 3). The largest cost components of CRC screenings were the average cost of a colonoscopy with any intervention ($9441) and anesthesia ($8928). The two patients diagnosed with distant CRC contributed an estimated $84,079.40 to costs. Total direct medical costs avoided by screening the 94-patient panel was $189,178, which was generated by 1 dysplastic plus 3.5 adenomatous (14 patients multiplied by 25% estimated rate of progression to malignancy as described in Methods section) for 4.5 total patient equivalents who received a colonoscopy after an abnormal FIT and had adenomatous polyps removed.
Table 3.
Cost-benefit analysis of patient panel with actual follow-up ratea
| Variable | Unit cost | Units | Total |
|---|---|---|---|
| Costs of CRC screenings | |||
| FIT screening | $21.82 | 94.00 | ($2051.08) |
| Diagnostic colonoscopy | $322.64 | 21.00 | ($6775.44) |
| Colonoscopy with intervention | $357.41 | 24.00 | ($8577.81) |
| Anesthesia for low intestine | $198.41 | 45.00 | ($8928.45) |
| Pathology | $69.62 | 33.60 | ($2339.23) |
| Complication (perforation) | $26,159.00 | 0.01 | ($223.66) |
| Complication (bleeding, hospitalization) | $26,159.00 | 0.07 | ($1871.68) |
| Complication (bleeding, transfusion) | $26,159.00 | 0.04 | ($929.95) |
| Complication (diverticulitis, hospitalization) | $19,029.00 | 0.01 | ($196.95) |
| Other GI complication (observation) | $138.60 | 0.00 | ($0.56) |
| Angina or myocardial infarction | $31,352.00 | 0.03 | ($790.07) |
| Stroke or transient ischemic attack | $53,452.00 | 0.01 | ($793.76) |
| Total | ($33,478.65) | ||
| Costs of CRC | |||
| Direct initial CRC costs (distant-stage CRC) | $42,039.70 | 2.00 | ($84,079.40) |
| Direct initial CRC costs (local-stage CRC) | $38,694.28 | 1.00 | ($38,694.28) |
| Total | ($122,773.68) | ||
| Total costs | ($156,252.32) | ||
| Benefits of CRC screening | |||
| Direct initial CRC costs avoided | $42,039.70 | 4.5 | $189,178.65 |
| Total benefits for CRC screening | $189,178.65 | ||
| Total cost-benefit for CRC screening | $32,926.33 | ||
CRC indicates colorectal cancer; FIT, fecal immunochemical test; GI, gastrointestinal.
Assumptions: All patients received a full colonoscopy instead of a sigmoidoscopy. Pathology costs represent the product of the average number of jars per patient (1.4) and total patients (24). All unit costs were based on 2017 Medicare payment rates. Anesthesia unit costs were calculated assuming a 45-minute/three units of time interval per unit. Complication costs were estimated using diagnosis-related groups and the Texas PricePoint tool. Direct initial CRC costs avoided are a median of six previous studies of initial direct medical costs for treating CRC. Three patients were excluded from the 100% follow-up due to assumed precluded health. All colonoscopy costs with any intervention were averaged. Units represent the number of patients applicable based on the results from the data analysis and applied rates from the literature review.
A secondary CBM was performed to ascertain the possible maximum benefits and costs for the studied panel of patients (Table 4). In this evaluation, each of the 91 patients received a colonoscopy (excluding an equal proportion of patients who would not be healthy enough for the procedure), and equal proportions from the improvement analysis were assumed to have abnormal colonoscopies. The costs associated with the expected number of patients with pre-existing local or distant CRC were added to the costs side of the model. Specifically, 53.33% of the 91 patients receiving a colonoscopy would have abnormal findings, meaning that 42 patients would have a colonoscopy without an intervention and 49 patients would receive a colonoscopy with an intervention. Further, whereas each of the 49 patients who received a colonoscopy with intervention would have a pathology, only 9.17 patients (2.09 dysplastic and 7.08 adenomatous patient equivalents) would receive the full cost avoidance benefit of screening. In this situation, total benefits through costs avoided increased from $189,178 to $385,363. Total annual benefits less costs were calculated to be over $63,237 for the year in which the patients were screened.
Table 4.
Cost-benefit analysis of patient panel with full follow-up ratea
| Variable | Unit cost | Units | Total |
|---|---|---|---|
| Costs of CRC screenings | |||
| FIT screening | $21.82 | 94.00 | ($2051.08) |
| Diagnostic colonoscopy | $322.64 | 42.00 | ($13,550.88) |
| Colonoscopy with intervention | $357.41 | 49.00 | ($17,513.04) |
| Anesthesia for low intestine | $198.41 | 91.00 | ($18,055.31) |
| Pathology | $69.62 | 68.60 | ($4775.93) |
| Complication (perforation) | $26,159.00 | 0.02 | ($452.29) |
| Complication (bleeding, hospitalization) | $26,159.00 | 0.14 | ($3784.95) |
| Complication (bleeding, transfusion) | $26,159.00 | 0.07 | ($1880.57) |
| Complication (diverticulitis, hospitalization) | $19,029.00 | 0.02 | ($398.28) |
| Other GI complication (observation) | $138.60 | 0.01 | ($1.14) |
| Angina or myocardial infarction | $31,352.00 | 0.05 | ($1597.70) |
| Stroke or transient ischemic attack | $53,452.00 | 0.03 | ($1605.16) |
| Total cost for CRC screenings | ($65,666.32) | ||
| Costs of CRC | |||
| Direct initial CRC costs (distant-stage CRC) | $42,039.70 | 4.18 | ($175,632.52) |
| Direct initial CRC costs (local-stage CRC) | $38,694.28 | 2.09 | ($80,828.04) |
| Total costs for CRC | ($256,460.57) | ||
| Total costs | ($322,126.88) | ||
| Benefits of CRC screenings | |||
| Direct initial CRC costs avoided | $42,039.70 | 9.17 | $385,363.92 |
| Total benefits for CRC screenings | $385,363.92 | ||
| Total cost-benefit for CRC screenings | $63,237.03 | ||
CRC indicates colorectal cancer; FIT, fecal immunochemical test; GI, gastrointestinal.
aAssumptions: All patients received a full colonoscopy instead of a sigmoidoscopy. Pathology costs represent the product of the average number of jars per patient (1.4) and total patients (24). All unit costs were based on 2017 Medicare payment rates. Anesthesia unit costs were calculating assuming a 45-minute/three units of time interval per unit. Complication costs were estimated using diagnosis-related groups and the Texas PricePoint tool. Direct initial CRC costs avoided are a median of six previous studies of initial direct medical costs for treating CRC. Three patients were excluded from the 100% follow-up due to assumed precluded health. All colonoscopy costs with any intervention were averaged. Units represent the number of patients applicable based on the results from the data analysis and applied rates from the literature review.
In a follow-up sensitivity analysis, the rate of follow-up colonoscopies appears to be the main driver in costs avoided/savings instead of overall rate of follow-ups (Figure 2). Total complication rates were not a major driver of cost; however, at the model’s minimum follow-up and colonoscopy rates, the overall costs appear greater than the benefits.
DISCUSSION
This two-part evaluation of CRC screening in an integrated health system demonstrates that improving screening guideline adherence can increase CRC cost avoided and, therefore, total monetary benefit for population. We found that though 68.09% of patients received appropriate follow-up care, in terms of being communicated results and offered a colonoscopy, only 47.87% of all patients had a follow-up colonoscopy as recommended by current treatment guidelines. For the assessed population, this equated to a benefit of over $189,178, less $33,478 in screening and $122,773 in two patients with distant and local CRC. Had CRC screening guidelines been followed appropriately, the net benefit would have exceeded $63,237. Additionally, a population simulation of 1000 patients that examined the sensitivity of monetary benefits across follow-up rates resulted in total monetary benefit, demonstrating that as follow-up and colonoscopy rates improved, overall monetary benefit significantly improved, because costs appear largely fixed or stagnant. Said differently, benefits of improving screening far outweigh the costs and demonstrate the need to incentivize higher rates of follow-up and colonoscopies to avoid costs.
Though follow-up rates fall within the range cited in the literature and above the national average for 2016 performance results for accountable care organizations (ACOs; ACO #19, 61.52%) and merit-based incentive payment system benchmark averages (ACO #113/PPRNET18, 57.48%), these results are underwhelming, especially given the increasing incidence of CRC in younger populations, the increasing number of baby boomers retiring into Medicare, and more pressure from payers and health systems to improve follow-up. A recent analysis of a large integrated urban safety net system in North Texas had similar results, where 42.30% of its study cohort failed to undergo a colonoscopy 1 year after positive screening compared to our 52.13%.23 In that study, however, 57% of cases of failing to undergo a colonoscopy were attributable to patient failures, primarily patient refusal, compared to our 17% of patients who refused and 3% who were unable to undergo a colonoscopy because of poor health. The remaining 30% of cases that failed to receive appropriate follow-up colonoscopies were due to provider or system-level failures. This suggests an opportunity to improve adherence through improved outreach and patient and physician education. Addressing patient preferences, such as accuracy of the test versus frequency of testing, through shared patient-provider decision making may increase compliance.50
To our knowledge, this is the first evaluation that utilizes a CBM from a population health perspective to explore the financial impact of CRC screening rates. Though there is a rich literature on patient-level benefits and cost-effectiveness studies of CRC screenings, no analysis has attempted to measure the potential monetary benefits to health system or production efficiency potential of appropriate screenings.13–15,38,51,52 Our results indicate that despite only 47% of patients receiving follow-up colonoscopies after abnormal FITs, the total benefit net of costs exceeded costs from complications or increased resources devoted to screening.
Several studies have already explored the relationship between new delivery models (ACOs, patient-centered medical homes, and integrated delivery systems) and cancer screenings.54–57 However, more policies, pilots, and evaluations of the aforementioned are necessary to refine new care and payment models where cost-avoided dollars could be accounted for vis-à-vis shared savings. Though population health screening rate quality measures are already in use by payers and ACOs, policymakers should consider more closely linking CRC screening rates with alternative payment models.48,49 One example would be using shared savings of avoided CRC costs as an incentive to improve compliance. This would involve shifting screening from a process measure to more of an outcome measure, because measures could be linked with incidence rates to ensure that incentive payments are appropriately rewarded. Through these alternative payment models, benefits could therefore be reallocated by ACOs and other community health providers, who take accountability in other areas like diabetes prevention, community interventions for asthma, and reductions in obesity rates.
A limitation to this study relates to external validity (generalizability), in that only charts within the system (limited by geography, hospital not-for-profit status) were selected. Results may be biased because random sampling was used instead of cluster sampling based on ordering physician; the authors did not investigate whether there was a relationship between follow-up rates and ordering physician. Moreover, the sample size was too small to perform formal statistical analysis. Patients who died were excluded due to the time frame of analysis. Additionally, complication rates were not collected, and estimates for the incidence rates and costs associated with each complication had to be estimated from previous literature using populations that may not exactly match the population in this study. We could not find data on what percentage of precancerous lesions, if not removed, would eventually be discovered as local CRC versus distant CRC. Thus, we calculated that all of these would be discovered as distant CRC, although this is probably not the case. Although we did our best to stay true to appropriate estimations for hospital costs, many of the primary source estimations vary widely. Our analysis did not consider the cost-benefit scenario of uninsured or underinsured populations. This population is less likely to receive CRC screening, and any malignancies found tend to be in a later stage (distant CRC) compared to malignancies found in those with health insurance. However, many hospitals, physicians, and pharmaceutical companies provide a degree of charity care annually, and it would not be unreasonable to think that providing charity care for screening would be more cost-effective from a population health and integrated health system perspective. Finally, this investigation assumed that all lesions were removed through endoscopy, but larger lesions may need to be removed through colectomy. However, this is relatively uncommon compared to endoscopic removal and, given the small size used in this investigation, would not be likely to impact the financial outcome.
Colorectal cancer is a devastating, prevalent, and expensive disease, but appropriate screening can significantly reduce its incidence and mortality. Adherence to screening guidelines, however, remains suboptimal. Though the assessed rate of follow-up after abnormal FIT (68.09%) is consistent with that found at other institutions, there is room for improvement, especially given the likely increase in new incidence and mortality rate. Health systems, ACOs, and payers should work together to structure financial incentives in a way that rewards providers for improved outreach and adherence guidelines. Optimized CRC screening could translate to the allocation of health care dollars toward other diseases.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Yabroff KR, Mariotto AB, Feuer E, Brown ML. Projections of the costs associated with colorectal cancer care in the United States, 2000–2020. Health Econ. 2008;17:947–959. doi: 10.1002/hec.1307. [DOI] [PubMed] [Google Scholar]
- 4.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelsattar ZM, Birkmeyer JD, Wong SL. Variation in Medicare payments for colorectal cancer surgery. J Oncol Pract. 2015;11:391–395. doi:doi: 10.1200/JOP.2015.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Z, Bradley CJ, Dahman BA, Gardiner JC. Colon cancer treatment costs for Medicare and dually eligible beneficiaries. Health Care Financ Rev. 2010;31:35–50. [PMC free article] [PubMed] [Google Scholar]
- 7.Lang K, Lines LM, Lee DW, Korn JR, Earle CC, Menzin J. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clin Gastroenterol Hepatol. 2009;7:198–204. doi: 10.1016/j.cgh.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Yabroff KR, Borowski L, Lipscomb J. Economic studies in colorectal cancer: challenges in measuring and comparing costs. J Natl Cancer Inst Monogr. 2013;2013:62–78. doi: 10.1093/jncimonographs/lgt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gani F, Cerullo M, Canner JK, et al. Defining payments associated with the treatment of colorectal cancer. J Surg Res. 2017;220:284–292. doi: 10.1016/j.jss.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 11.Chiu HM, Chen SL, Yen AM, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the one million Taiwanese screening program. Cancer. 2015;121:3221–3229. doi: 10.1002/cncr.29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zauber AG. Cost-effectiveness of colonoscopy. Gastrointest Endosc Clin N Am. 2010;20:751–770. doi: 10.1016/j.giec.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinh T, Ladabaum U, Alperin P, Caldwell C, Smith R, Levin TR. Health benefits and cost-effectiveness of a hybrid screening strategy for colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:1158–1166. doi: 10.1016/j.cgh.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595–2609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel SS, Kilgore ML. Cost-effectiveness of colorectal cancer screening strategies. Cancer Control. 2015;22:248–258. doi: 10.1177/107327481502200219. [DOI] [PubMed] [Google Scholar]
- 16.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; and the US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: a patient demographic study. South Med J. 2004;97:224–230. doi: 10.1097/01.SMJ.0000078619.39604.3D. [DOI] [PubMed] [Google Scholar]
- 18.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Cancer Society Colorectal Cancer Facts & Figures 2017–2019. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 20.US Preventive Services Task Force Final recommendation statement: colorectal cancer: screening. 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2.
- 21.Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160:171–181. doi: 10.7326/M13-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BT, Bay C, Xu Y, et al. Test characteristics of faecal immunochemical tests (FIT) compared with optical colonoscopy. J Med Screen. 2014;21:133–143. doi: 10.1177/0969141314541109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130:93.e1–93.e7. doi: 10.1016/j.amjmed.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol. 2017;112:375–382. doi: 10.1038/ajg.2016.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening. Ann Intern Med. 2016;164:456–463. doi: 10.7326/M15-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers RE, Turner B, Weinberg D, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38:375–381. doi: 10.1016/j.ypmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Oluloro A, Petrik AF, Turner A, et al. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Community Health. 2016;41:864–870. doi: 10.1007/s10900-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med. 2011;171:249–256. doi: 10.1001/archinternmed.2010.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol Biomarkers Prev. 2016;25:344–350. doi: 10.1158/1055-9965.EPI-15-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 31.Jensen CD, Quinn VP, Doubeni CA, et al. Colonoscopy delay after a positive fecal test and risk of colorectal cancer–related outcomes. Gastroenterology. 2016;150:S45. doi: 10.1016/S0016-5085(16)30276-1. [DOI] [Google Scholar]
- 32.Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317:1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cyhaniuk A, Coombes ME. Longitudinal adherence to colorectal cancer screening guidelines. Am J Manag Care. 2016;22:105–111. [PubMed] [Google Scholar]
- 34.Etzioni DA, Ponce NA, Babey SH, et al. A population-based study of colorectal cancer test use: results from the 2001 California Health Interview Survey. Cancer. 2004;101:2523–2532. doi: 10.1002/cncr.20692. [DOI] [PubMed] [Google Scholar]
- 35.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46:228–236. doi: 10.1016/j.amepre.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Qiu F, Gregg A, et al. Barriers and facilitators of colorectal cancer screening for patients of rural accountable care organization clinics: a multilevel analysis. J Rural Health. 2018;34:202–212. doi: 10.1111/jrh.12248. [DOI] [PubMed] [Google Scholar]
- 37.Texas Hospital Association. PricePoint—comprehensive query. http://www.txpricepoint.org/Report.aspx. Accessed July 15, 2019.
- 38.Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2010;8:166–173. doi: 10.1016/j.cgh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhehui L, Bradley CJ, Dahman BA, Gardiner JC. Colon cancer treatment costs for Medicare and dually eligible beneficiaries. Health Care Financ Rev. 2009;31:35–50. [PMC free article] [PubMed] [Google Scholar]
- 41.Brenner H, Hoffmeister M, Stegmaier C, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan C, Pickhardt PJ, Kim DH, et al. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Ther. 2010;31:210–217. [DOI] [PubMed] [Google Scholar]
- 43.Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 44.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 45.Amersi F, Agustin M, Ko CY. Colorectal cancer: epidemiology, risk factors, and health services. Clin Colon Rectal Surg. 2005;18(03):133–140. doi: 10.1055/s-2005-916274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coleman HG, Loughrey MB, Murray LJ, et al. Colorectal cancer risk following adenoma removal: a large prospective population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2015;24:1373–1380. doi: 10.1158/1055-9965.EPI-15-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Medicare and Medicaid Services 2016 Shared Savings Program ACO interactive dataset. https://data.cms.gov/Special-Programs-Initiatives-Medicare-Shared-Savin/2016-Shared-Savings-Program-SSP-Accountable-Care-O/3jk5-q6dr. August 30, 2018.
- 49.Centers for Medicare and Medicaid Services 2018 Quality Benchmarks. Baltimore, MD: Centers for Medicare and Medicaid Services; 2017. [Google Scholar]
- 50.Schroy PC III, Lal S, Glick JT, Robinson PA, Zamor P, Heeren TC. Patient preferences for colorectal cancer screening: how does stool DNA testing fare? Am J Manag Care. 2007;13:393–400. [PubMed] [Google Scholar]
- 51.Lee KS, Park EC. Cost effectiveness of colorectal cancer screening interventions with their effects on health disparity being considered. Cancer Res Treat. 2016;48:1010–1019. doi: 10.4143/crt.2015.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong MC, Ching JY, Chan VC, Sung JJ. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs colonoscopy. Sci Rep. 2015;5:13568. doi: 10.1038/srep13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mostashari F, Sanghavi D, McClellan M. Health reform and physician-led accountable care: the paradox of primary care physician leadership. JAMA. 2014;311:1855–1856. doi: 10.1001/jama.2014.4086. [DOI] [PubMed] [Google Scholar]
- 54.Kim J, Young L, Bekmuratova S, et al. Promoting colorectal cancer screening through a new model of delivering rural primary care in the USA: a qualitative study. Rural Remote Health. 2017;17:4187. doi: 10.22605/RRH4187. [DOI] [PubMed] [Google Scholar]
- 55.Verma M, Sarfaty M, Brooks D, Wender RC. Population-based programs for increasing colorectal cancer screening in the United States. CA Cancer J Clin. 2015;65:497–510. [DOI] [PubMed] [Google Scholar]
- 56.Mehta SJ, Jensen CD, Quinn VP, et al. Race/ethnicity and adoption of a population health management approach to colorectal cancer screening in a community-based healthcare system. J Gen Intern Med. 2016;31:1323–1330. doi: 10.1007/s11606-016-3792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howard DH. Capsule commentary on Mehta et al. Race/ethnicity and adoption of a population health management approach to colorectal cancer screening in a community-based healthcare system. J Gen Intern Med. 2016;31:1356. doi: 10.1007/s11606-016-3803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]