Abstract
Toll-like receptor 4 (TLR4) activation contributes to vascular dysfunction in pathological conditions such as hypertension and diabetes, but the role of chronic TLR4 activation on renal autoregulatory behavior is unknown. We hypothesized that subclinical TLR4 stimulation with low-dose lipopolysaccharide (LPS) infusion increases TLR4 activation and blunts renal autoregulatory behavior. We assessed afferent arteriolar autoregulatory behavior in male Sprague-Dawley rats after prolonged LPS (0.1 mg·kg−1·day−1 sq) infusion via osmotic minipump for 8 or 14 days. Some rats also received daily cotreatment with either anti-TLR4 antibody (1 μg ip), competitive antagonist peptide (CAP; 3 mg/kg ip) or tempol (2 mmol/l, drinking water) throughout the 8-day LPS treatment period. Autoregulatory behavior was assessed using the in vitro blood-perfused juxtamedullary nephron preparation. Selected physiological measures, systolic blood pressure and baseline diameters were normal and similar across groups. Pressure-dependent vasoconstriction averaged 72 ± 2% of baseline in sham rats, indicating intact autoregulatory behavior. Eight-day LPS-treated rats exhibited significantly impaired pressure-mediated vasoconstriction (96 ± 1% of baseline), whereas it was preserved in rats that received anti-TLR4 antibody (75 ± 3%), CAP (84 ± 2%), or tempol (82 ± 2%). Using a 14-day LPS (0.1 mg·kg−1·day−1 sq) intervention protocol, CAP treatment started on day 7, where autoregulatory behavior is already impaired. Systolic blood pressures were normal across all treatment groups. Fourteen-day LPS treatment retained the autoregulatory impairment (95 ± 2% of baseline). CAP intervention starting on day 7 rescued pressure-mediated vasoconstriction with diameters decreasing to 85 ± 1% of baseline. These data demonstrate that chronic subclinical TLR4 activation impairs afferent arteriolar autoregulatory behavior through mechanisms involving reactive oxygen species and major histocompatibility complex class II activation.
Keywords: inflammation, major histocompatibility complex class II, oxidative stress, renal microcirculation, Toll-like receptor 4
INTRODUCTION
Renal autoregulation is critical for maintaining a stable renal blood flow, glomerular capillary pressure, and glomerular filtration rate over a wide range of arterial pressures (8, 39). Autoregulatory control uses precise pressure/flow-dependent changes in preglomerular vascular resistance, particularly afferent arterioles (AAs) (8, 39). Loss of autoregulatory capability allows the transmission of inappropriately high pressure downstream to the glomerular capillaries, which can lead to glomerular sclerosis, nephron resorption, and chronic kidney disease (CKD) (8).
Toll-like receptors (TLRs) are expressed by most tissues, including immune cells, the vasculature, and the kidney (55, 56). Generally, renal TLR expression predominates in glomeruli and the tubular epithelium (3, 54). TLR4, in particular, is expressed in the renal cortex and medulla (1, 51, 55, 56) and localized to the tubular epithelium, glomeruli, endothelium, and vascular smooth muscle cells (10, 14, 18, 55). Renal TLR4 is constitutively expressed at low levels and increases in response to pathogens [lipopolysaccharide (LPS)] and damage-associated molecular patterns (DAMPs) or in pathological conditions such as hypertension (6, 10, 17). Renal TLR4 stimulation releases proinflammatory cytokines including TNF-α, IL-6, tranforming growth factor-β, and IL-1β, suggesting a mechanistic connection with renal inflammation (12, 15, 25, 32). Recently, TLR4 activation has been linked to cardiovascular and renal pathologies (21, 33, 48). Souza et al. (49) reported that TLR4 mutant mice were protected against glomerulosclerosis and renal fibrosis in a progressive CKD model. Hypertension causes tissue injury and cell death that allows the release of intracellular contents and debris that can act as endogenous TLR4 ligands (DAMPs) (33, 48). TLR4 was the first TLR implicated in vascular dysfunction and hypertension (6). TLR4 mRNA expression was increased in vascular smooth muscle cells from spontaneously hypertensive rats compared with normotensive controls. Anti-TLR4 antibody treatment in spontaneously hypertensive rats decreased blood pressure and preserved mesenteric vascular function (6). Furthermore, Bomfim et al. (6) demonstrated that anti-TLR4 antibody treatment prevented the increase in proinflammatory cytokine expression in the mesenteric vasculature. Finally, our laboratory (53) demonstrated that acute LPS treatment impairs AA autoregulatory behavior through TLR4-mediated reactive oxygen species (ROS) production. Clinically, TLR4 activation has been implicated in CKD, with TLR4 expression correlating directly with inflammatory markers and disease severity (3, 30, 32, 33, 35, 48). Although evidence suggests that TLR4 activation participates in the development of vascular dysfunction (16, 43, 49, 53), no studies have investigated the impact of persistent TLR4 activation on the function of AAs.
The major histocompatibility complex (MHC), primarily expressed on innate immune cells, binds peptide fragments from exogenous or endogenous sources and displays them for antigenic presentation to the adaptive immune system (5). A vital component of the transition between innate and adaptive immunity is the processing and presentation of antigenic peptide fragments associated with MHCs (5). In the MHC-II compartment, the invariant chain is digested, leaving a residual MHC class II-associated peptide (CLIP) in the binding groove of MHC-II molecules (5, 40). CLIP fills the MHC-II peptide-binding groove, rendering MHC-II inactive until HLA-DM, an MHC class II-like catalyst, facilitates the exchange of CLIP with antigenic fragments for presentation to CD4+ T cells (5). Several studies have provided evidence for mechanistic and functional links between TLRs and MHC-II molecules (20, 29, 41, 46). TLR2 and/or TLR4 reportedly colocalize with MHC-II molecules in human embryonic kidney cells (20). Furthermore, macrophages from MHC-II−/− mice exhibit impaired responsiveness to both bacterial lipoprotein, a TLR2 agonist, and LPS, a known TLR4 ligand (20). Kissner et al. (29) reported that LPS or the MHC-II agonist Staphylococcus enterotoxin B upregulated myeloid differentiation primary response protein MyD88, a key signaling component in most TLR signaling, in cultured B cells. In contrast, MHC-II deficient B cells did not upregulate MyD88 (29). These studies suggested a direct mechanistic linkage between MHC-II and TLRs, specifically TLR4, and implicated TLR4 and MHC-II as possible therapeutic targets for transitioning from innate to adaptive immune responses in acute and chronic inflammatory diseases.
To extend our previous acute study (53), we postulated that sustained subclinical TLR4 stimulation with low-dose LPS can increase TLR4 activation and blunt AA autoregulatory responsiveness. Experiments were performed to determine whether chronic low-dose LPS treatment leads to TLR4-dependent impairment of AA autoregulatory behavior.
METHODS
Animal Model and Monitoring
Male Sprague-Dawley rats (n = 120, Charles River Breeding Laboratories, Raleigh, NC) were maintained in institutional animal facilities in a 12:12-h light-dark cycle-controlled room. Rats received standard chow ad libitum (Purina Mills LabDiet 5L3Z, PMI Nutrition, Brentwood, MO) and free access to water. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committees of the University of Alabama at Birmingham.
LPS (0.1 mg·kg−1·day−1, Sigma-Aldrich, St. Louis, MO) was administered using osmotic minipumps implanted subcutaneously (model 2002, Alzet, Duret, Cupertino, CA). Conscious systolic blood pressure (SBP) was measured in both kidney and blood donor rats (n = 12 rats/group) using tail-cuff plethysmography (IITC Life Science, Woodland Hills, CA). SBP was measured on days 0, 3, and 7 covering the treatment period before kidneys were harvested for study in the 8-day LPS infusion protocol and extended to cover days 10 and 13 of the 14-day LPS protocol before kidney harvest.
Metabolic Cage Experiments
Rats were individually housed in metabolic cages (Laboratory Products, Seaford, DE) over an 8-day protocol. The first 24 h were for acclimation. Urine was collected on days 0, 3, and 7. Food and water consumption as well as body weights were measured daily. Collected urine was centrifuged at (1,000 g, 4°C, 10 min) to remove food particles. The supernatant was collected, aliquoted, and stored at −80°C for later analysis.
In Vitro Blood-Perfused Juxtamedullary Nephron Preparations
Experiments were performed using the juxtamedullary nephron technique (11, 24, 28). For each experiment, two identically treated rats (blood donor and kidney donor) were anesthetized by pentobarbital sodium (50 mg/kg ip, Diamondback Drugs, Scottsdale, AZ). The right kidney of the kidney donor was cannulated via the superior mesenteric artery and continuously perfused with Tyrode buffer containing 5.2% BSA (Calbiochem, La Jolla, CA).
Blood from kidney and blood donor rats was collected into heparinized syringes (500 IU, West Ward Pharmaceuticals, Eatontown, NJ) via a carotid artery catheter and prepared for centrifugation. The plasma and washed erythrocytes were mixed and passed through a 5-μm nylon mesh filter to form a reconstituted blood perfusate (hematocrit ≈ 33%).
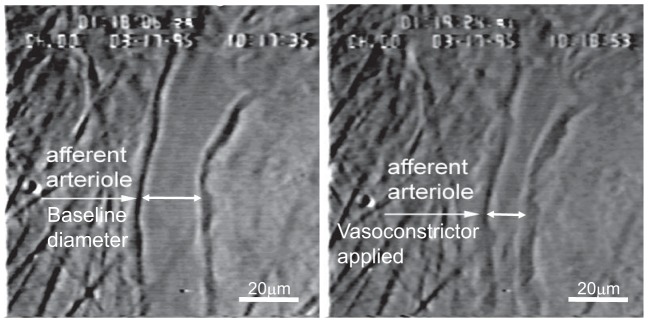
The perfused kidney was harvested from the kidney donor and prepared for videomicroscopy (27, 28). After the completion of microdissection, the perfused kidney was visualized by light microscopy (Nikon Optiphot2-UD, Nikon, Tokyo, Japan) with a Zeiss water-immersion objective (×40, Oberkohen, Germany). The inner cortical kidney surface was superfused with Tyrode buffer containing 1% BSA at 37°C. The perfusate was switched from Tyrode buffer containing 5.2% BSA to the reconstituted blood delivered from a reservoir pressurized with 95% O2-5% CO2. Perfusion pressure was monitored using a pressure cannula positioned near the tip of perfusion cannula and connected to a pressure transducer (model TRN005, Kent Scientific, Torrington, CT). The focused image of the inner cortical surface and AA was displayed on a video monitor and recorded on digital video disk for later analysis (Fig. 1). AAs were monitored at a single site, and diameters were measured every 12 s using an image shearing monitor (model 908, Vista Electronics, Valencia, CA). The mean AA diameter was calculated using the diameter measurements obtained in the final 2 min of each treatment or pressure period.
Fig. 1.
Example images of an afferent arteriole in the in vitro blood-perfused juxtamedullary nephron preparation before (left) and during (right) exposure to a vasoconstrictor stimulus.
Experimental Protocols
Protocol 1: effect of 8-day LPS infusion on AA autoregulatory behavior.
Experiments were performed to determine the impact of prolonged low-dose LPS on AA autoregulatory behavior. All LPS-treated groups were implanted with osmotic minipumps containing LPS (0.1 mg·kg−1·day−1) on day 0 and administered for 8 days. Some rats received anti-TLR4 antibody (TLR4 MTS510, sc13591, 1 μg/day ip, Santa Cruz Biotechnologies, Dallas, TX), competitive antagonist peptide (CAP; 3 mg/kg ip, VG Life Sciences, Santa Barbara, CA), or tempol (2 mmol/l, Sigma-Aldrich) treatment in drinking water. The antibody was selected using the specificity data from the supplier, which included flow cytometry results using peripheral blood leukocytes compared with normal rat IgG. We also validated it in our system by comparing the effectiveness of the anti-TLR4 antibody against the actions of IgG. IgG had no effect on LPS-induced autoregulatory impairment. In addition, we compared the effectiveness of the anti-TLR4 antibody against a TLR4 receptor antagonist. The antagonist and antibody yielded the same results (53). Eight groups were studied (n = 5–6 rats/group): saline (0.9% NaCl sq), LPS (0.1 mg·kg−1·day−1 sq), saline + anti-TLR4 antibody (1 μg ip), LPS + anti-TLR4 antibody (1 μg ip), LPS + CAP (3 mg/kg ip) (41, 50), saline + CAP, saline + tempol (2 mmol/l, drinking water), and LPS + tempol. Twenty-four-hour metabolic parameters and conscious SBP were measured on days 0, 3, and 7 in all groups before juxtamedullary nephron experimentation.
AA autoregulatory behavior was assessed by measuring changes in luminal diameter in response to stepwise changes in renal perfusion pressure. Autoregulatory behavior was assessed by reducing perfusion pressure from 100 to 65 mmHg and then increasing perfusion pressure from 65 to 170 mmHg in 15-mmHg increments at 5-min intervals.
Protocol 2: effect of 14-day LPS infusion on AA autoregulatory behavior.
Experiments were performed to determine if chronic 14-day LPS treatment impairs AA autoregulatory behavior. LPS-treated groups were implanted with osmotic minipumps filled with LPS (0.1 mg·kg−1·day−1) on day 0 and administered for 14 days. The LPS + CAP group received injections of CAP (3 mg·kg−1·day−1 ip) on days 7–14. SBP was measured on days 0, 3, 7, 10, and 13 in all groups. Three groups were studied (n = 6 rats/group): saline (0.9% NaCl sq), LPS (0.1 mg·kg−1·day−1 sq), and LPS + CAP (3 mg/kg ip). Kidneys were harvested on day 14 for juxtamedullary nephron experimentation. AA autoregulatory behavior was assessed by measuring changes in arteriole diameter in response to stepwise changes in perfusion pressure as described above (protocol 1).
Urinary Electrolytes
All urine samples and reagents were equilibrated to room temperature before assessment. Urine samples were diluted 1:10 [70 µl of sample to 630 µl of Medica Urine Diluent (Medica, Bedford, MA)], and urine electrolyte concentrations (Na+, K+, and Cl−) were measured using the EasyLyte system (Medica).
Competitive Antagonist Peptide
CAP is a propriety MHC-II-targeted peptide synthesized as previously described by Newell-Rogers et al. (41, 50). Briefly, Newell-Rogers et al. computationally derived a 9mer peptide (CAP) that was designed by MHCPred and NetMHC server-based quantitative prediction database software for peptide-MHC binding to have a higher binding constant than the invariant peptide CLIP for the binding groove of MHC-II molecules. CAP competitively antagonizes the antigen-binding site of MHC-II molecules (41, 50). CAP was generously provided by M. K. Newell-Rogers and VG Lifesciences (South Pasadena, CA).
Statistical Analysis
Data are expressed as means ± SE. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparison test. Significant differences between groups were determined using one-way ANOVA and Dunnett’s multiple-comparison test or Student’s t-test. For analysis of SBP, selected physiological parameters, and urinary electrolyte excretion, significant differences were determined using two-way ANOVA and Bonferroni’s multiple-comparison test. P values of <0.05 were considered to indicate significant difference.
RESULTS
Effects of 8-Day LPS Treatment on SBP
SBP was measured in all kidney and blood donor groups (n = 12 rats/group). Baseline SBPs (day 0) were similar between saline-, LPS-, LPS + anti-TLR4-, LPS + CAP-, LPS + tempol-, saline + anti-TLR4-, saline + CAP-, and saline + tempol-treated rats (Table 1). SBPs remained unchanged throughout the 8-day treatment period in all groups. Thus, sustained TLR4 activation with low-dose LPS does not significantly alter SBP in these treatment groups.
Table 1.
SBP is normal during chronic 8-day LPS treatment
| Baseline SBP, mmHg | Day 3 SBP, mmHg | Day 7 SBP, mmHg | |
|---|---|---|---|
| Saline | 123 ± 4 | 131 ± 3 | 130 ± 6 |
| LPS | 133 ± 3 | 123 ± 2 | 124 ± 2 |
| LPS + anti-TLR4 antibody | 128 ± 5 | 131 ± 2 | 133 ± 4 |
| LPS + CAP | 127 ± 5 | 128 ± 3 | 138 ± 2 |
| LPS + tempol | 131 ± 8 | 131 ± 4 | 131 ± 7 |
| Saline + anti-TLR4 antibody | 134 ± 2 | 133 ± 3 | 132 ± 4 |
| Saline + CAP | 130 ± 3 | 127 ± 1 | 125 ± 1 |
| Saline + tempol | 119 ± 6 | 130 ± 4 | 130 ± 6 |
Values are means ± SE. SBP, systolic blood pressure; LPS, lipopolysaccharide; CAP, competitive antagonist peptide; TLR4, Toll-like receptor 4. Two-way ANOVA with Bonferroni’s multiple-comparison test was used to analyze significance.
Effects of 8-Day LPS Treatment on Physiological Parameters
Experiments were performed to assess the physiological status of rats during LPS treatment. Rats gained body weight normally in all groups throughout the 8-day treatment period. Food and water consumption and urine production were similar between all groups throughout the treatment period (Table 2). In the LPS + anti-TLR4-treated group on day 0, water intake was significantly lower compared with all other treatment groups. This difference returned to normal on day 3 and remained similar to all other treatment groups on day 7.
Table 2.
Physiological parameters during 8-day low-dose LPS treatment
|
Day 0 |
Day 3 |
Day 7 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight, g | Food intake, g/day | Water intake, ml/day | Urine output, ml/day | Body weight, g | Food intake, g/day | Water intake, ml/day | Urine output, ml/day | Body weight, g | Food intake, g/day | Water intake, ml/day | Urine output, ml/day | |
| Saline | 302 ± 6 | 27 ± 3 | 26 ± 2 | 12 ± 1 | 305 ± 1 | 22 ± 2 | 22 ± 1 | 9 ± 1 | 316 ± 5 | 26 ± 2 | 20 ± 2 | 10 ± 1 |
| LPS | 295 ± 7 | 26 ± 1 | 30 ± 2 | 11 ± 2 | 300 ± 8 | 22 ± 1 | 26 ± 2 | 8 ± 1 | 323 ± 10 | 23 ± 2 | 23 ± 2 | 9 ± 1 |
| LPS + anti-TLR4 antibody | 293 ± 4 | 25 ± 3 | 17 ± 1a,b,c,d,e | 12 ± 1 | 303 ± 4 | 18 ± 3 | 22 ± 3 | 9 ± 1 | 322 ± 4 | 17 ± 3 | 21 ± 1 | 11 ± 1 |
| LPS + CAP | 275 ± 5 | 18 ± 4 | 26 ± 2 | 9 ± 1 | 285 ± 6 | 19 ± 2 | 19 ± 2 | 15 ± 1 | 308 ± 10 | 23 ± 2 | 28 ± 2 | 15 ± 3 |
| LPS + Tempol | 290 ± 7 | 26 ± 1 | 29 ± 2 | 11 ± 1 | 301 ± 5 | 24 ± 2 | 28 ± 1 | 9 ± 1 | 322 ± 7 | 26 ± 1 | 29 ± 4 | 10 ± 2 |
| Saline + Tempol | 287 ± 7 | 22 ± 2 | 28 ± 1 | 8 ± 1 | 298 ± 8 | 23 ± 2 | 27 ± 1 | 8 ± 0 | 317 ± 9 | 25 ± 3 | 23 ± 2 | 9 ± 1 |
Values are means ± SE. LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; CAP, competitive antagonist peptide.
P < 0.05 vs. the saline-treated group on the same day;
P < 0.05 vs. the LPS-treated group on the same day;
P < 0.05 vs. the LPS + CAP-treated group on the same day;
P < 0.05 vs. the LPS + tempol-treated group on the same day;
P < 0.05 vs. the saline + tempol-treated group on the same day. Two-way ANOVA with Bonferroni’s multiple-comparison test was used to analyze significance. The rats used for the microvascular experiments for the saline + anti-TLR4 antibody- and saline + CAP-treated groups were not put in metabolic cages because there were no significant effects noted in the LPS + anti-TLR4 antibody- or LPS + CAP-treated groups.
We also performed experiments to assess the renal handling of Na+, K+, and Cl− during 8-day TLR4 stimulation with LPS. Baseline urinary electrolyte excretion rates were similar across all groups. Urinary electrolyte excretion rates were essentially unchanged during the 8-day treatment period (Table 3). Collectively, these data indicate that common physiological variables and renal handling of electrolytes were normal in rats that received 8-day LPS treatment (0.1 mg·kg−1·day−1). These data also indicate that rats in all treatment groups were healthy and behaved normally.
Table 3.
Urinary electrolyte excretion rates during low-dose 8-day LPS treatment
|
Day 0 |
Day 3 |
Day 7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ excretion, meq/24 h | K+ excretion, meq/24 h | Cl− excretion, meq/24 h | Na+ excretion, meq/24 h | K+ excretion, meq/24 h | Cl− excretion, meq/24 h | Na+ excretion, meq/24 h | K+ excretion, meq/24 h | Cl− excretion, meq/24 h | |
| Saline | 1.5 ± 0.2 | 2.4 ± 0.3 | 1.5 ± 0.2 | 1.7 ± 0.2 | 2.4 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.1 | 2.6 ± 0.2 | 1.8 ± 0.1 |
| LPS | 1.9 ± 0.1 | 2.5 ± 0.1 | 1.9 ± 0.1 | 1.5 ± 0.1 | 2.3 ± 0.2 | 1.5 ± 0.1 | 1.7 ± 0.1 | 2.5 ± 0.1 | 1.7 ± 0.1 |
| LPS + anti-TLR4 antibody | 1.5 ± 0.2 | 2.3 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | 2.1 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.1 | 2.4 ± 0.2 | 1.7 ± 0.1 |
| LPS + CAP | 1.3 ± 0.1 | 2.0 ± 0.1 | 1.3 ± 0.1 | 2.0 ± 0.2 | 2.4 ± 0.1 | 2.0 ± 0.2 | 1.8 ± 0.1 | 2.3 ± 0.1 | 1.8 ± 0.1 |
| LPS + tempol | 1.7 ± 0.1 | 2.3 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 2.4 ± 0.2 | 1.5 ± 0.1 | 1.7 ± 0.2 | 2.5 ± 0.2 | 1.7 ± 0.1 |
| Saline + tempol | 1.4 ± 0.2 | 1.9 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.1 | 2.3 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.1 | 1.5 ± 0.1 |
Values are means ± SE. LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; CAP, competitive antagonist peptide. Two-way ANOVA with Bonferroni’s multiple-comparisons test was used to analyze significance. The rats used for the microvascular experiments for the saline + anti-TLR4- and saline + CAP-treated groups were not put in metabolic cages because there were no significant effects noted in the LPS+ anti-TLR4 antibody- or LPS + CAP-treated groups.
Eight-Day Low-Dose LPS Infusion Impairs AA Autoregulatory Behavior
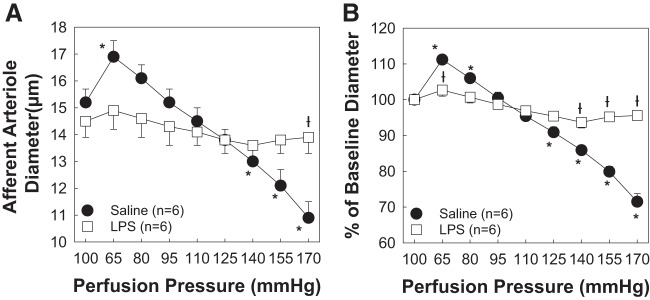
Experiments were performed to determine the impact of persistent, low-dose, LPS-dependent, TLR4 activation on AA autoregulatory behavior (Fig. 2). Baseline diameters at 100 mmHg averaged 15.2 ± 0.5 and 14.5 ± 0.6 µm for arterioles from kidneys of saline- and LPS-treated rats, respectively (Fig. 2A). When renal perfusion pressure was reduced from 100 to 65 mmHg, AA diameter increased to 111 ± 1% (16.9 ± 0.6 μm) in the saline-treated group but remained essentially unchanged in the LPS-treated group, averaging 103 ± 2% (14.9 ± 0.7 μm) of baseline diameter in the LPS-treated group. Increasing renal perfusion pressure from 65 to 170 mmHg in 15-mmHg increments resulted in AAs exhibiting stepwise reductions in diameter, reaching 72 ± 2% (10.9 ± 0.6 μm, P < 0.05) of baseline diameter. In contrast, arteriole diameter in kidneys from LPS-treated rats decreased to just 96 ± 1% (13.9 ± 0.6 μm) of baseline diameter, demonstrating impaired pressure-mediated vasoconstriction (Fig. 2B).
Fig. 2.
Eight-day lipopolysaccharide (LPS) treatment impairs afferent arteriolar autoregulatory behavior. A: effect of renal perfusion pressure changes on afferent arteriolar diameter in kidneys from saline- and LPS (0.1 mg·kg−1·day−1 sq)-treated male rats. B: data are expressed as percentages of baseline diameter at 100 mmHg. Each data point represents the mean ± SE of n = 6 rats/group. *P < 0.05 vs. baseline diameter in the same group; †P < 0.05 vs. the-treated saline group at the same perfusion pressure. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparisons test. Differences between groups were determined using a Student’s t-test.
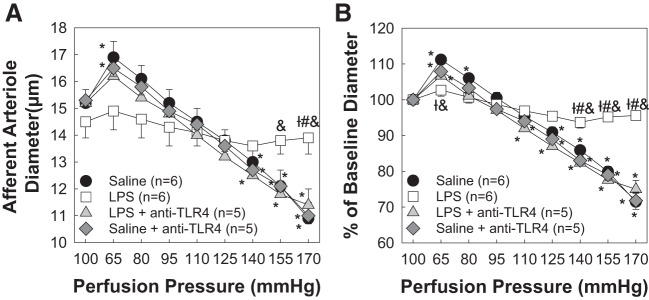
Cotreatment With Anti-TLR4 Antibody Preserves Autoregulatory Behavior During 8-Day LPS Treatment
Experiments were performed to determine the impact of TLR4 inhibition on AA autoregulatory behavior during 8-day LPS treatment. Baseline AA diameters averaged 15.2 ± 0.5 μm in the LPS + anti-TLR4 antibody-treated group (Fig. 3A). When perfusion pressure was reduced from 100 to 65 mmHg, arteriole diameter increased to 107 ± 2% (16.2 ± 0.7 μm) of baseline diameter (Fig. 3B). Increasing renal perfusion pressure to 170 mmHg decreased arteriole diameter to 75 ± 3% of baseline diameter (11.4 ± 0.6 μm, P < 0.05; LPS vs. LPS + anti-TLR4), similar to responses obtained from the saline-treated control group. The saline + anti-TLR4-treated group exhibited an autoregulatory curve indistinguishable from the saline control group (Fig. 3B). These data indicate that inhibition of TLR4 activation during chronic low-dose LPS treatment preserved AA autoregulatory behavior.
Fig. 3.
Cotreatment with anti-Toll-like receptor 4 (TLR4) antibody preserves afferent arteriolar autoregulatory behavior during lipopolysaccharide (LPS) treatment. A: effect of perfusion pressure changes on afferent arteriolar diameter in kidneys from saline-, LPS (0.1 mg·kg−1·day−1 sq)-, LPS + anti-TLR4 (1 μg ip)-, and saline + anti-TLR4-treated male rats. B: data are expressed as percentages of baseline diameter at 100 mmHg during the equilibration period. Saline and LPS data are included from Fig. 1 for reference. Each data point represents the mean ± SE of n = 5–6 rats/group. *P < 0.05 vs. baseline diameter in the same group; †P < 0.05 vs. the saline-treated group at the same perfusion pressure; #P < 0.05 vs. the LPS + anti-TLR4 antibody-treated group at the same perfusion pressure; &P < 0.05 vs. the saline + anti-TLR4 antibody-treated group at the same perfusion pressure. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparisons test. Differences between groups were determined using one-way ANOVA with post hoc analysis using Dunnett’s multiple-comparisons test.
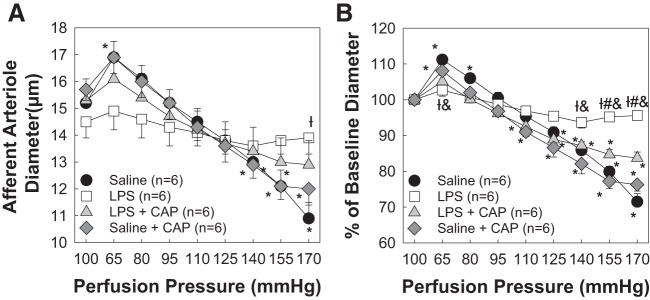
MHC-II-Associated Invariant Peptide Antagonism by CAP Preserves AA Autoregulatory Behavior During 8-Day LPS Treatment
Renal inflammation plays a critical role in kidney injury and impairment of AA autoregulatory behavior (22, 23, 47). Antigen processing and presentation via MHC-II is a critical step in initiating an adaptive, and antigen-specific, response (5). Using CAP, a CLIP antagonist (41, 50), we hypothesized that inhibition of MHC-II presentation of CLIP preserves AA autoregulatory behavior. Baseline arteriole diameters for the LPS + CAP-treated group at 100 mmHg averaged 15.3 ± 0.8 µm (Fig. 4A). Decreasing renal perfusion pressure to 65 mmHg increased AA diameter to 105 ± 2% (16.1 ± 0.9 μm) of baseline diameter. Increasing renal perfusion pressure to 170 mmHg reduced arteriole diameter to 84 ± 2% (12.9 ± 0.9 μm, P < 0.05; Fig. 4B) of baseline diameter, indicating preservation of AA autoregulatory behavior. Importantly, the saline + CAP-treated group exhibited autoregulatory responses indistinguishable from the saline control group (76 ± 2% of baseline diameter; Fig. 4B). These data demonstrate that antagonization of cell surface CLIP in the peptide binding groove of MHC-II regulated AA autoregulatory behavior; thus, the transition between the innate and adaptive immune system could provide a novel therapeutic target for the treatment of renal dysregulation.
Fig. 4.
Major histocompatibility complex class II-associated invariant peptide antagonism by competitive antagonist peptide (CAP) preserves afferent arteriolar autoregulatory behavior during 8-day lipopolysaccharide (LPS) treatment. A: effect of renal perfusion pressure changes on afferent arteriolar diameter in kidneys from saline (0.9% NaCl sq)-, LPS (0.1 mg·kg−1·day−1 sq)-, LPS + CAP (3 mg/kg ip daily)-, and saline + CAP-treated male rats. B: data are expressed as percentages of baseline diameter at 100 mmHg. Saline and LPS data are included from Fig. 1 for reference. Each data point represents the mean ± SE of n = 6 rats/group. *P < 0.05 vs. baseline diameter in the same group; †P < 0.05 vs. the saline-treated group at the same perfusion pressure; #P < 0.05 vs. the LPS + CAP-treated group at the same perfusion pressure; &P < 0.05 vs. the saline + CAP-treated group at the same perfusion pressure. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparisons test. Differences between groups were determined using one-way ANOVA with post hoc analysis using Dunnett’s multiple-comparisons test.
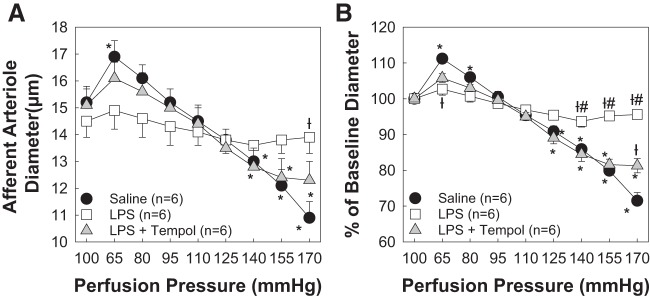
Tempol Cotreatment Preserves AA Autoregulatory Behavior During 8-Day LPS Treatment
ROS generation and/or accumulation is linked to impaired renal function and AA autoregulatory behavior (19, 47). Reportedly, activation of TLR4 can increase ROS generation (10, 16, 37, 45). Baseline AA diameter averaged 15.1 ± 0.7 μm in the LPS + tempol-treated group (Fig. 5A). When perfusion pressure was reduced to 65 mmHg, AA diameter increased to 106 ± 1% of baseline diameter in the LPS + tempol-treated group (P < 0.05, LPS vs. LPS + tempol). Increasing renal perfusion pressure to 170 mmHg decreased arteriole diameter to 82 ± 2% of baseline diameter (P < 0.05, LPS vs. LPS + tempol; Fig. 5B). Thus, scavenging ROS preserves AA autoregulatory behavior during LPS treatment.
Fig. 5.
Cotreatment with tempol preserves afferent arteriolar autoregulatory behavior. A: effect of renal perfusion pressure changes on afferent arteriolar diameter in saline (0.9% NaCl sq)-, lipopolysaccharide (LPS; 0.1 mg·kg−1·day−1 sq)-, and LPS + tempol (2 mmol/l, drinking water)-treated male rats. Saline and LPS-treated groups are the same as those shown in Fig. 1. B: data are expressed as percentages of baseline diameter at 100 mmHg during the equilibration period. Each data point represents the mean ± SE of n = 6 rats/group. *P < 0.05 vs. baseline diameter in the same group; †P < 0.05 vs. the saline-treated group at the same perfusion pressure; #P < 0.05 vs. the LPS + tempol-treated group at the same perfusion pressure. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparisons test. Differences between groups were determined using one-way ANOVA with post hoc analysis using Dunnett’s multiple-comparisons test.
Effects of 14-Day LPS Treatment on SBP
Because chronic 8-day LPS treatment impaired AA autoregulatory behavior, we developed an intervention protocol using 14-day LPS treatment. Experiments were performed to assess the effect of 14-day low-dose LPS treatment on SBP. Baseline SBPs (day 0) were similar across saline (128 ± 4 mmHg)-, LPS (131 ± 3 mmHg)-, and LPS + CAP (131 ± 4 mmHg)-treated rats (Table 4). SBPs remained unchanged throughout the 14-day treatment period. These data indicate that 14-day low-dose LPS did not significantly alter SBP in any of the treatment groups. Accordingly, any impairment of renal microvascular function must involve a mechanism other than a negative impact of blood pressure.
Table 4.
Fourteen-day LPS treatment does not significantly alter SBP
| Baseline SBP, mmHg | Day 3 SBP, mmHg | Day 7 SBP, mmHg | Day 10 SBP, mmHg | Day 13 SBP, mmHg | |
|---|---|---|---|---|---|
| Saline | 128 ± 4 | 129 ± 1 | 129 ± 1 | 129 ± 3 | 129 ± 1 |
| LPS | 131 ± 3 | 126 ± 2 | 126 ± 2 | 128 ± 2 | 128 ± 2 |
| LPS + CAP | 131 ± 4 | 132 ± 2 | 132 ± 2 | 128 ± 2 | 128 ± 1 |
Values are means ± SE. SBP, systolic blood pressure; LPS, lipopolysaccharide; CAP, competitive antagonist peptide. Two-way ANOVA with Bonferroni’s multiple-comparisons test were used to analyze significance.
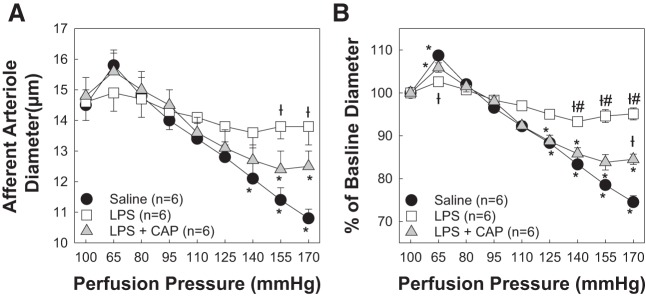
Renal Autoregulatory Behavior Is Rescued With 7 Days of CAP Treatment During 14-Day LPS Treatment
Cotreatment with LPS and CAP preserved AA autoregulatory behavior (Fig. 4). We used a 14-day LPS treatment (0.1 mg·kg−1·day−1) protocol to determine if CAP intervention could rescue AA autoregulatory behavior. Starting on day 7, where we know that autoregulatory behavior is impaired (Fig. 2), we started CAP (3 mg/kg ip) treatment to determine if we could correct dysfunctional AAs to normal autoregulatory capability. Baseline arteriole diameters at 100 mmHg averaged 14.5 ± 0.5, 14.6 ± 0.6, and 14.8 ± 0.6 μm for saline-, LPS-, and LPS + CAP-treated groups, respectively (Fig. 6A). When perfusion pressure was reduced to 65 mmHg in saline-treated kidneys, arteriole diameter increased to 109 ± 1% (15.7 ± 0.6 μm), but diameters remained essentially unchanged in the LPS-treated group (103 ± 1% of baseline diameter, 14.9 ± 0.6 μm). Interestingly, arteriole diameter increased to 106 ± 1% (15.6 ± 0.6 μm) in the LPS + CAP-treated group, similar to the saline-treated group. Increasing perfusion pressure to 170 mmHg in the saline-treated group revealed pressure-induced vasoconstriction reaching 71 ± 2% (10.3 ± 0.5 μm) of baseline diameter, signifying normal autoregulatory reactivity. In contrast, arteriole diameter in the LPS-treated group remained unchanged (95 ± 2%, 13.8 ± 0.6 μm, P < 0.05 compared with saline controls; Fig. 6B), demonstrating impaired pressure-mediated vasoconstriction. Intervention with CAP led to a reduction in arteriole diameter to 85 ± 1% (12.5 ± 0.5 μm, P < 0.05 vs. LPS) of baseline diameter at 170 mmHg. These data indicate that intervention with CAP, a MHC class II invariant peptide competitive antagonist, reversed the functional impairment known to occur with LPS for 8–14 days and returned these arterioles to essentially normal autoregulatory capability compared with saline controls. Together, these data suggest that CAP intervention blocks the proinflammatory effect of MHC-II processing and presentation of CLIP and rescues AA autoregulatory behavior in an already impaired kidney.
Fig. 6.
Competitive antagonist peptide (CAP) intervention rescues afferent arteriolar autoregulatory behavior during chronic 14-day lipopolysaccharide (LPS) treatment. Intervention with CAP, a major histocompatibility complex class II-associated invariant chain peptide antagonist, rescued renal autoregulatory behavior during 14-day LPS administration. A: effect of perfusion pressure changes on afferent arteriolar diameter in kidneys from saline (0.9% NaCl sq)-, LPS (0.1 mg·kg−1·day−1 sq)-, and LPS + CAP (3 mg/kg ip daily)-treated male rats. B: data are expressed as percentages of baseline diameter at 100 mmHg. Each data point represents the mean ± SE of n = 6 rats/group. *P < 0.05 vs. baseline diameter in the same group; †P < 0.05 vs. the saline-treated group at the same perfusion pressure; #P < 0.05 vs. the LPS + CAP-treated group at the same perfusion pressure. Within-group analysis was conducted using one-way ANOVA for repeated measures with post hoc analysis using Dunnett’s multiple-comparisons test. Differences between groups were determined using one-way ANOVA with post hoc analysis using Dunnett’s multiple-comparisons test.
DISCUSSION
Previous studies have suggested that pathological conditions such as hypertension and CKD can lead to TLR activation (1, 6, 10, 16, 26, 35, 38, 49), but a direct link between persistent TLR4 activation and renal microvascular dysfunction remains to be elucidated. We previously reported that acute LPS treatment impairs AA autoregulatory behavior through TLR4-mediated ROS production (53). The present study was conducted to extend our previous acute experiments (53) to a more realistic chronic setting to determine the impact of chronic, low-dose TLR4 stimulation on AA function. We hypothesized that persistent subclinical TLR4 activation would impair AA autoregulatory behavior. Accordingly, we developed a subclinical TLR4 activation model using low-dose LPS (0.1 mg·kg−1·day−1 sq) to reflect low grade inflammation. The present study has several main findings: 1) low-dose LPS treatment did not significantly alter the basic physiological parameters that we measured (SBP and electrolyte excretion rates), 2) 8- and 14-day low-dose LPS treatment impaired AA autoregulatory behavior through a TLR4-dependent mechanism, and 3) competitive antagonism of CLIP from the groove of MHC-II via CAP treatment preserved and rescued AA autoregulatory behavior during the 8- and 14-day LPS protocols, respectively. These novel findings suggest that subclinical activation of TLR4, with low-dose LPS, impairs AA autoregulatory behavior through mechanisms that also involve ROS.
Autoregulation is an intrinsic property of AAs that gives the renal microvasculature an exquisite ability to maintain a stable renal blood flow and glomerular capillary pressure over a wide range of arterial pressures (7, 8, 52). Renal autoregulation maintains renal blood flow in a normal kidney over a range of mean arterial pressures, due to precise adjustments in preglomerular resistance, most of which reflects AA resistance (7, 19). For instance, as arterial pressure increases, there is a proportional decrease in renal arteriole diameter causing renal vascular resistance to increase and maintain renal blood flow and glomerular capillary pressure constant. With complete loss of autoregulatory efficiency, the pressure-renal blood flow relationship is passive such that diameter increases as pressure increases and decreases as pressure decreases. This results in large pressure-dependent fluctuations in renal blood flow and glomerular capillary pressure. In the present study, we observed impaired autoregulatory efficiency such that as arterial pressure increased, AA diameter remained relatively unchanged compared with control kidneys. Moreover, loss of renal autoregulatory behavior leads to the transmission of inappropriately high pressures downstream to delicate glomeruli, ultimately leading to glomerular injury, nephron resorption, and CKD. Impairment of renal autoregulation occurs in pathological conditions such as ANG II- and DOCA-salt-induced hypertension, high dietary salt, and diabetes (9, 19, 22–24, 31, 44). Fellner et al. (19) demonstrated that renal function, AA autoregulatory behavior, and whole kidney renal blood flow autoregulation are significantly impaired with high dietary salt intake. Furthermore, autoregulatory efficiency is compromised in individuals with hypertension and in animal models of hypertension (8, 23, 24). In the present study, chronic, low-dose LPS treatment impaired AA autoregulatory behavior compared with saline controls, and this impairment was sustained in rats that received LPS for 14 days. Impaired AA autoregulatory behavior was observed despite blood pressure being normal between the groups tested. The flat pressure-diameter relationship observed from AAs of LPS-treated rats is consistent with previous studies demonstrating impaired AA autoregulatory behavior in models of high dietary salt and two models of hypertension (19, 22–24). Our results demonstrate that chronic subclinical LPS treatment can lead to renal vascular dysfunction as indicated by impaired renal autoregulation. Furthermore, impaired renal autoregulation is possibly a contributing factor associated with the development of renal injury in various pathological states.
Important roles for the innate immune system and TLR4 activation on renal functional impairment have been reported in humans and experimental models of vascular dysfunction and CKD (6, 16, 26, 32, 34, 35, 49). McIntyre et al. (34) showed that serum endotoxin concentration increases significantly in patients with CKD (stages 3−5) and strongly correlated with reduced survival in these patients with CKD. Souza et al. (49) investigated the role of TLR4 using TLR4 mutant mice (CH3/HeJ) expressing a missense point mutation in the TLR4 gene. TLR4 mutation prevented glomerulosclerosis, increase serum creatinine, and renal fibrosis after 5/6 nephrectomy compared with wild-type control mice. Furthermore, Bomfim et al. (6) reported that mesenteric arteries from SHRs had vascular dysfunction that was TLR4 dependent. Collectively, these studies provided evidence in humans and animal models that TLR4 activation by pathogen-associated molecular patterns (endotoxin) and/or DAMPs (high-mobility group box 1 protein, heat shock proteins, etc.) can cause vascular dysfunction and end-organ damage under pathological conditions. Accordingly, we sought to determine the impact of TLR4 activation on AA autoregulatory behavior using a low-dose LPS treatment model. We found that cotreatment with anti-TLR4 antibody (1μg/day ip) preserved AA autoregulatory behavior in rats that received LPS infusion, such that it mimicked control rats. The demonstration that prevention of TLR4 activation preserved microvascular function in our LPS-treated rats agrees with our previous work in an acute endotoxemia setting and agrees with other reports for different vascular beds (6, 16, 26, 53). Consequently, even low doses of LPS can impose subclinical activation of TLR4 that changes renal microvascular function, and this change could contribute to the progression of renal injury and CKD.
Previous studies have established a link between TLR4 activation and ROS in pathological experimental models (6, 10, 16, 26, 36, 38). Carrillo-Sepulveda et al. (10) reported that mesenteric vascular smooth muscle cells exposed to high glucose significantly increase TLR4 and MyD88 expression, interaction, and ROS production. Treatment with a TLR4 antagonist, CLI-095, prevented TLR4-MyD88 interaction and also attenuated increased ROS production evoked by high glucose. In another study, De Batista et al. (16) reported that aortas of SHRs exhibited significant vascular dysfunction that was linked to TLR4 activation, increased NADPH oxidase 4 expression, and increased superoxide production, all of which could be prevented with the TLR4 antagonist CLI-0951. More recently, it has been reported that aortic extracellular SOD protein expression increased significantly in TLR4-deficient (TLR4−/−) mice with ANG II-induced hypertension, indicating that TLR4 inhibition prevents vascular oxidative stress in a murine model of hypertension (38). Together, these studies suggest that a TLR4-dependent ROS mechanism contributes to vascular dysfunction in models of chronic inflammation. In the present study, we found that cotreatment with anti-TLR4 antibody preserved AA autoregulatory behavior during LPS treatment for 8 days. Furthermore, cotreatment with a SOD mimetic, tempol (LPS + tempol), resulted in autoregulatory profiles that mimicked vehicle controls. Therefore, TLR4-dependent ROS generation may play an important role in the renal microvascular autoregulatory impairment caused by chronic low-dose LPS treatment.
Activation of the family of TLRs is a vital step in innate immune system activation. This step precedes, and can potentiate, antigen processing and presentation through MHC-II molecules (4, 5, 40, 42). MHC-II processing and presentation is critical for bridging acute responses involving the innate immune system, with the long-term adaptive immune response. Over the years, research has formed the basis for mechanistic and functional links between TLRs and MHC-II molecules (13, 20, 29, 41, 46). Piani et al. (46) reported that human and murine macrophages that lack, or express few, MHC-II molecules have diminished secretion of proinflammatory cytokines when exposed to LPS. Coexpression of MHC-II in cultured human embryonic kidney cells (human embryonic kidney-293 cells) enhanced TLR-dependent activation by LPS (20). More recently, Chatterjee et al. (13) showed that CAP treatment prevented, and reversed, TLR-induced preeclampsia-like features in wild-type mice. These studies suggest a mechanistic link between MHC-II, CLIP, and TLRs; however, it is unclear whether TLR4-dependent CLIP presentation is critical for impaired AA autoregulatory behavior. We hypothesized that CLIP antagonism with CAP in the MHC-II-binding groove would preserve autoregulatory behavior in LPS-treated rats. In this study, we found that cotreatment with CAP during 8-day LPS treatment preserved AA autoregulatory behavior compared with saline-treated controls. Since CAP cotreatment preserved renal autoregulatory behavior, we used an intervention protocol to mimic clinical scenarios, starting CAP treatment on day 7, where we have established renal autoregulatory impairment. Notably, we found that 14-day treatment alone impaired pressure-mediated reductions in AA diameter. The flat pressure-diameter relationship observed after 14 days of continuous LPS infusion was essentially identical to the impaired autoregulatory profile observed with the 8-day LPS treatment protocol. Interestingly, we found that CAP intervention, beginning on day 8, rescued AA autoregulatory behavior and returned it to a pressure-diameter relationship essentially identical to kidneys from normal saline-treated controls. Therefore, these data indicate that intervention with CAP, an MHC-II antagonist, can rescue an already impaired kidney during chronic subclinical 14-day LPS treatment. Furthermore, these data may reveal a novel therapeutic target for inhibiting the transition between the innate and adaptive immune systems. Further studies are needed with CAP to better understand how this system contributes to the autoregulatory impairment that occurs in conditions of hypertension, high dietary salt, and diabetes (9, 19, 22–24). Furthermore, more work is needed with this peptide to assess its therapeutic potential on other renal pathologies including hypertensive kidney injury, diabetic kidney injury, acute kidney injury, and CKD.
GRANTS
This work was supported by American Heart Association Greater Southeast Affiliate Predoctoral Fellowship 14PRE20460061 (to J. P. Van Beusecum), National Institutes of Health Grants DK-44628 and HL-098135 (to E. W. Inscho), the Scott and White Foundation (to M. K. Newell-Rogers), and the Texas A&M Health Science Center (to M. K. Newell-Rogers).
DISCLOSURES
M. K. Newell-Rogers and R. P. Tobin have a financial interest in the work. M. K. Newell-Rogers and R. P. Tobin are inventors on patents related to CAP and the use of the peptide. The peptide has been licensed to BCell Solutions Incorporated and VG Life Sciences Incorporated.
AUTHOR CONTRIBUTIONS
J.P.V.B., R.P.T., M.K.N.-R., and E.W.I. conceived and designed research; J.P.V.B., S.Z., E.B., and A.K.C. performed experiments; J.P.V.B., S.Z., A.K.C., R.P.T., M.K.N.-R., and E.W.I. analyzed data; J.P.V.B., S.Z., R.P.T., M.K.N.-R., and E.W.I. interpreted results of experiments; J.P.V.B. prepared figures; J.P.V.B. drafted manuscript; J.P.V.B., R.P.T., M.K.N.-R., and E.W.I. edited and revised manuscript; J.P.V.B., S.Z., E.B., A.K.C., R.P.T., M.K.N.-R., and E.W.I. approved final version of manuscript.
ACKNOWLEDGMENTS
CAP was provided by M. K. Newell-Rogers.
Present address of M. K. Newell-Rogers: BCell Solutions Incorporated, 4755 Forge Rd., Colorado Springs, CO 80907.
REFERENCES
- 1.Anderberg SB, Luther T, Frithiof R. Physiological aspects of Toll-like receptor 4 activation in sepsis-induced acute kidney injury. Acta Physiol (Oxf) 219: 573–588, 2017. doi: 10.1111/apha.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders HJ, Banas B, Schlöndorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004. doi: 10.1097/01.ASN.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MS, Miller J. Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc Natl Acad Sci USA 89: 2282–2286, 1992. doi: 10.1073/pnas.89.6.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol 31: 443–473, 2013. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 122: 535–543, 2012. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 12: 845–858, 2014. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlström M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens 19: 85–90, 2010. doi: 10.1097/MNH.0b013e32833240fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo-Sepulveda MA, Spitler K, Pandey D, Berkowitz DE, Matsumoto T. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J Mol Med (Berl) 93: 1341–1354, 2015. doi: 10.1007/s00109-015-1318-7. [DOI] [PubMed] [Google Scholar]
- 11.Casellas D, Carmines PK, Navar LG. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int 28: 752–759, 1985. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- 12.Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi EJ, Correa-Silva R, Elias RM, Salvador F, Moraes-Vieira PM, Cenedeze MA, Reis MA, Hiyane MI, Pacheco-Silva Á, Gonçalves GM, Saraiva Câmara NO. TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS One 7: e37584, 2012. doi: 10.1371/journal.pone.0037584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee P, Chiasson VL, Seerangan G, De Guzman E, Milad M, Bounds KR, Gasheva O, Tobin RP, Hatahet M, Kopriva S, Jones KA, Newell-Rogers MK, Mitchell BM. Depletion of MHC class II invariant chain peptide or γ-δ T-cells ameliorates experimental preeclampsia. Clin Sci (Lond) 131: 2047–2058, 2017. doi: 10.1042/CS20171008. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Hartono JR, John R, Bennett M, Zhou XJ, Wang Y, Wu Q, Winterberg PD, Nagami GT, Lu CY. Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int 80: 504–515, 2011. doi: 10.1038/ki.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol 172: 2629–2635, 2004. doi: 10.4049/jimmunol.172.4.2629. [DOI] [PubMed] [Google Scholar]
- 16.De Batista PR, Palacios R, Martín A, Hernanz R, Médici CT, Silva MA, Rossi EM, Aguado A, Vassallo DV, Salaices M, Alonso MJ. Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One 9: e104020, 2014. doi: 10.1371/journal.pone.0104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 18.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007. doi: 10.1152/ajprenal.00217.2007. [DOI] [PubMed] [Google Scholar]
- 19.Fellner RC, Cook AK, O’Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol 307: F33–F40, 2014. doi: 10.1152/ajprenal.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frei R, Steinle J, Birchler T, Loeliger S, Roduit C, Steinhoff D, Seibl R, Büchner K, Seger R, Reith W, Lauener RP. MHC class II molecules enhance Toll-like receptor mediated innate immune responses. PLoS One 5: e8808, 2010. doi: 10.1371/journal.pone.0008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulopoulou S, McCarthy CG, Webb RC. Toll-like receptors in the vascular system: sensing the dangers within. Pharmacol Rev 68: 142–167, 2016. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276–F1284, 2010. doi: 10.1152/ajprenal.00743.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X(1) receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol 304: F801–F807, 2013. doi: 10.1152/ajprenal.00286.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan Z, Singletary ST, Cha H, Van Beusecum JP, Cook AK, Pollock JS, Pollock DM, Inscho EW. Pentosan polysulfate preserves renal microvascular P2X1 receptor reactivity and autoregulatory behavior in DOCA-salt hypertensive rats. Am J Physiol Renal Physiol 310: F456–F465, 2016. doi: 10.1152/ajprenal.00110.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M, Li Y, Liu M, Li Y, Cong B. Renal neutrophil gelatinase associated lipocalin expression in lipopolysaccharide-induced acute kidney injury in the rat. BMC Nephrol 13: 25, 2012. doi: 10.1186/1471-2369-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernanz R, Martínez-Revelles S, Palacios R, Martín A, Cachofeiro V, Aguado A, García-Redondo L, Barrús MT, de Batista PR, Briones AM, Salaices M, Alonso MJ. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol 172: 3159–3176, 2015. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 57: 780–787, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kissner TL, Ruthel G, Alam S, Ulrich RG, Fernandez S, Saikh KU. Activation of MyD88 signaling upon staphylococcal enterotoxin binding to MHC class II molecules. PLoS One 6: e15985, 2011. doi: 10.1371/journal.pone.0015985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koc M, Toprak A, Arikan H, Odabasi Z, Elbir Y, Tulunay A, Asicioglu E, Eksioglu-Demiralp E, Glorieux G, Vanholder R, Akoglu E. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant 26: 955–963, 2011. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- 31.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension 55: 983–989, 2010. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepenies J, Eardley KS, Kienitz T, Hewison M, Ihl T, Stewart PM, Cockwell P, Quinkler M. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-β1 in patients with chronic kidney disease. Nephron Clin Pract 119: c97–c104, 2011. doi: 10.1159/000324765. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 306: H184–H196, 2014. doi: 10.1152/ajpheart.00328.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair AR, Ebenezer PJ, Saini Y, Francis J. Angiotensin II-induced hypertensive renal inflammation is mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial cells. Exp Cell Res 335: 238–247, 2015. doi: 10.1016/j.yexcr.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Nair AR, Elks CM, Vila J, Del Piero F, Paulsen DB, Francis J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: potential mechanism of TLR4-MAPK signaling pathway. PLoS One 9: e111976, 2014. doi: 10.1371/journal.pone.0111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair AR, Masson GS, Ebenezer PJ, Del Piero F, Francis J. Role of TLR4 in lipopolysaccharide-induced acute kidney injury: protection by blueberry. Free Radic Biol Med 71: 16–25, 2014. doi: 10.1016/j.freeradbiomed.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima T, Umemoto S, Yoshimura K, Matsuda S, Itoh S, Murata T, Fukai T, Matsuzaki M. TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res 38: 649–655, 2015. doi: 10.1038/hr.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol 234: F357–F370, 1978. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 40.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 11: 823–836, 2011. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 41.Newell MK, Tobin RP, Cabrera JH, Sorensen MB, Huckstep A, Villalobos-Menuey EM, Burnett M, McCrea E, Harvey CP, Buddiga A, Bar-Or A, Freedman MS, Nalbantoglu J, Arbour N, Zamvil SS, Antel JP. TLR-mediated B cell activation results in ectopic CLIP expression that promotes B cell-dependent inflammation. J Leukoc Biol 88: 779–789, 2010. doi: 10.1189/jlb.0410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordeng TW, Bakke O. The bio-logical role of invariant chain (Ii) in MHC class II antigen presentation. Immunol Lett 43: 47–55, 1994. doi: 10.1016/0165-2478(94)00159-6. [DOI] [PubMed] [Google Scholar]
- 43.O’Neill S, Humphries D, Tse G, Marson LP, Dhaliwal K, Hughes J, Ross JA, Wigmore SJ, Harrison EM. Heat shock protein 90 inhibition abrogates TLR4-mediated NF-κB activity and reduces renal ischemia-reperfusion injury. Sci Rep 5: 12958, 2015. doi: 10.1038/srep12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohishi K, Okwueze MI, Vari RC, Carmines PK. Juxtamedullary microvascular dysfunction during the hyperfiltration stage of diabetes mellitus. Am J Physiol 267: F99–F105, 1994. doi: 10.1152/ajprenal.1994.267.1.F99. [DOI] [PubMed] [Google Scholar]
- 45.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 46.Piani A, Hossle JP, Birchler T, Siegrist CA, Heumann D, Davies G, Loeliger S, Seger R, Lauener RP. Expression of MHC class II molecules contributes to lipopolysaccharide responsiveness. Eur J Immunol 30: 3140–3146, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 47.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005. doi: 10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- 48.Singh MV, Abboud FM. Toll-like receptors and hypertension. Am J Physiol Regul Integr Comp Physiol 307: R501–R504, 2014. doi: 10.1152/ajpregu.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souza AC, Tsuji T, Baranova IN, Bocharov AV, Wilkins KJ, Street JM, Alvarez-Prats A, Hu X, Eggerman T, Yuen PS, Star RA. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol Rep 3: e12558, 2015. doi: 10.14814/phy2.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobin RP, Mukherjee S, Kain JM, Rogers SK, Henderson SK, Motal HL, Newell Rogers MK, Shapiro LA. Traumatic brain injury causes selective, CD74-dependent peripheral lymphocyte activation that exacerbates neurodegeneration. Acta Neuropathol Commun 2: 143, 2014. doi: 10.1186/s40478-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 52.Van Beusecum J, Inscho EW. Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr Opin Pharmacol 21: 82–88, 2015. doi: 10.1016/j.coph.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Beusecum JP, Zhang S, Cook AK, Inscho EW. Acute toll-like receptor 4 activation impairs rat renal microvascular autoregulatory behaviour. Acta Physiol (Oxf) 221: 204–220, 2017. doi: 10.1111/apha.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Gou SJ, Zhao MH, Chen M. The expression of Toll-like receptors 2, 4 and 9 in kidneys of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin Exp Immunol 177: 603–610, 2014. doi: 10.1111/cei.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van ’t Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 56.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol 168: 554–561, 2002. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]