Abstract
Polyphenols, due to their high biocompatibility and wide occurrence in nature, have attracted increasing attention in the engineering of functional materials ranging from films, particles, to bulk hydrogels. Colloidal particles, such as nanogels, hollow capsules, mesoporous particles and core-shell structures, have been fabricated from polyphenols or their derivatives with a series of polymeric or biomolecular compounds through various covalent and non-covalent interactions. These particles can be designed with specific properties or functionalities, including multi-responsiveness, radical scavenging capabilities, and targeting abilities. Moreover, a range of cargos (e.g., imaging agents, anticancer drugs, therapeutic peptides or proteins, and nucleic acid fragments) can be incorporated into these particles. These cargo-loaded carriers have shown their advantages in the diagnosis and treatment of diseases, especially of cancer. In this review, we summarize the assembly of polyphenol-based particles, including polydopamine (PDA) particles, metal-phenolic network (MPN)-based particles, and polymer-phenol particles, and their potential biomedical applications in various diagnostic and therapeutic applications.
Keywords: polydopamine particles, metal-phenolic networks, self-assembly, diagnosis, drug delivery
Introduction
Polyphenols are a big family of organic compounds with multiple phenol units and wide occurrence in nature (e.g., most of plants and many families of animals) 1-3. They play important roles in both plants and animals, including regulation of hormones as signaling molecules, UV screening, antioxidant effects, and prevention of microbial infections 4-6. Polyphenols have also been widely used in diverse chemical industries for several decades. For example, they have been used as dyes or inks in the printing and dyeing industries, for tanning leather in the leather industry, as ingredients to make adhesives, and as additives in food industries 1. However, polyphenols were not popular as building blocks for material engineering until the mussel-inspired coating strategies independent of substrates came into sight over a decade ago 7. Ever since then, a series of compounds containing dihydroxyphenyl (catechol) and/or or trihydroxyphenyl (galloyl) groups have been intensively investigated for interface engineering and particle assembly.
Due to the existence of catechol and/or galloyl groups in polyphenols, various covalent and non-covalent interactions can be involved in the assembly of polyphenol-based materials. For instance, catechol or galloyl groups can partially be oxidized to quinone groups in the presence of oxygen under neutral or weak alkaline conditions (e.g., in the physiological or marine environment), which could result in self-polymerization of the precursors to form oligomeric/polymeric polyphenols and self-assembly of polyphenols into films or particles 4, 7. The quinone groups are also reactive to other functional groups (e.g., amine and thiol groups) through Michael addition or Schiff base reactions, enabling the addition of extra functions to the polyphenol-based materials 7. Catechol or galloyl groups can be engaged in multivalent coordination interactions with metal ions to form metal-phenolic network (MPN) complexes for the assembly of films, particles, and hydrogels 8-11. It has been approved that there are big libraries of both polyphenols and metal ions for the construction of multifunctional MPN-based particles 12-15. The catechol group as a special cis-diol can also form a reversible boronate ester bond with boronic acid, which is both pH and cis-diol responsive 16, 17. In addition, catechol or galloyl groups have been reported being engaged in other non-covalent interactions, including hydrogen bonding, hydrophobic interactions, electrostatic interactions, π-π stacking and cation-π interactions 18-21. Therefore, polyphenols are easy to interact with a wide range of polymers, including the synthetic poly(ethylene glycol) (PEG), poly(ethyleneimine) (PEI), poly(N-vinylpyrrolidone) (PVPON), and polypeptides (e.g., polyglutamic acid, PGA), as well as the natural polysaccharides, proteins and nucleic acids, forming polymer-phenol-based complexes, capsules, particles and hydrogels 18, 22-25. In addition to natural polyphenols, a series of catechol- or galloyl-functionalized polymers have been synthesized for the assembly of polymeric materials based on versatile polyphenol chemistry 26-30.
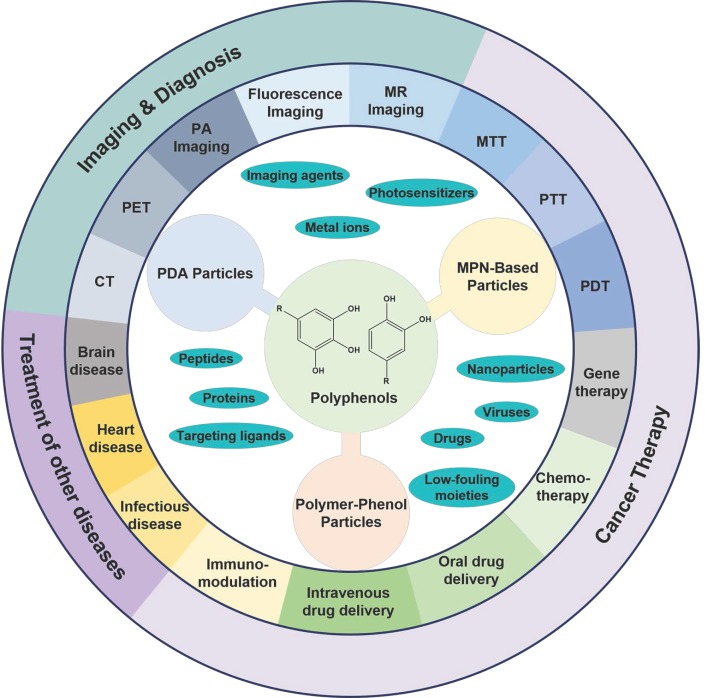
Over the past decade, a wide range of polyphenol-based materials, especially polyphenol-based colloidal particles have been designed 31-33. Via controlling the physiological properties (e.g., size, shape, and surface chemistry) of polyphenol-based particles they have exhibited their potential in a large variety of biological and biomedical applications, including drug delivery, antibacterial applications, as well as the diagnosis and treatment of various diseases, especially cancer (Figure 1) 33-35. For instance, mussel-inspired melanin-like polydopamine (PDA) particles have been engineered for photoacoustic (PA) imaging and photothermal therapy (PTT) of cancer 36-38. PDA particles showed high biocompatibility, physiological stability, and photostability in both in vitro and in vivo tests 34, 37, 38. In addition, PDA particles could be incorporated with a variety of cargos, including anti-cancer drugs for chemotherapy, metal ions as imaging agents, photosensitizers for photodynamic therapy (PDT), siRNA for gene therapy, as well as different functional nanoparticles as imaging or therapeutic agents 39-43. Thereby, the PDA-based particle systems have been demonstrated for a series of imaging-guided combination therapy of cancer 32, 41, 44, 45. MPN-based particles are another family of particles which have shown great promising in imaging and therapeutic applications 12, 46, 47. Similar to PDA particles, MPN-based particles could encapsulate a wide range of cargoes, such as therapeutic agents and imaging agents 47-50. Moreover, MPN-based particles allow for the addition of extra functions, such as targeting abilities, via either pre-functionalization of the building blocks or post-functionalization of the fabricated particles 14, 51, 52. MPN-based particles have shown their potential in the development of universal blood, the efficient inhibition of tumor growth in a tumor-bearing model, and the successful prevention of tumor metastasis or recurrence 48, 53, 54. Polymer-phenol particles assembled based on the interactions between polyphenols and a large variety of natural or synthetic polymers have also shown advantages in the delivery of therapeutic and/or imaging agents for the treatment of diseases 26, 30, 33, 55. In particular, the construction of protein-polyphenol capsules, conjugates, and self-assembled nanoparticles have resulted in a series of drug delivery carriers for both oral and intravenous delivery of therapeutic agents for the treatment of different diseases, including colon cancer, infectious diseases, heart diseases and Alzheimer's disease 55-59. Besides, due to the biocompatibility and biodegradability of the building blocks, the protein-polyphenol particles displayed responsiveness to biological enzymes with negligible acute or long-term toxicity 55.
Figure 1.
The design of polyphenol-based particles for diagnosis and therapy of cancer and many other diseases. (Abbreviations: PDA, polydopamine; MPN, metal-phenolic networks; PDT, photodynamic therapy; PTT, photothermal therapy; MTT, microwave thermal therapy; MR, magnetic resonance; PA, photoacoustic imaging; PET, positron emission tomography; CT, X-ray computed tomography.)
The fascinating properties and prosperous functions of polyphenols have attracted extensive interests to design polyphenol-based materials, especially nanostructured particles, for diverse biomedical applications with enhanced efficacy but reduced side effects. In previous reviews, specific types of polyphenol-based particles, such as melanin-like PDA nanoparticles, or a broader range of polyphenol-based materials, including particles, films and hydrogels, were discussed with the focuses on their structures, properties, and assembly mechanisms 26, 31-33, 35. Herein, we focus on the composition, design, and therapeutic and/or diagnostic applications of polyphenol-based particles (Figure 1). On the basis of particle composition, the description of polyphenol-based particles is divided into three sections, which are PDA particles, MPN-based particles, and polymer-phenol particles. The pros and cons of each type of particles will be discussed. In addition, prospects for further improvement and advanced applications of each particle systems are also covered in this review.
Mussel-Inspired PDA Particles
Melanin-like PDA nanoparticles
Dopamine with a catechol and an amine groups has been widely used for the engineering of surface coatings with diverse functionalities due to the easy occurrence of oxidation and self-polymerization of dopamine into PDA, which enabled quick surface coating and further surface functionalization 7, 60-63. In addition, PDA has been extensively investigated in the synthesis of colloidal nanoparticles for biomedical applications. Artificial melanin nanoparticles are an important type among the PDA particles. Melanin, one of the most ubiquitous pigments, has a variety of important functions in the biosystem, including photoprotection, free radical scavenging, thermoregulation, antibiotic abilities, and as neurotransmitter involved in the neural regulation 32, 36, 37, 64, 65. The mostly used methods to synthesize melanin-like PDA particles are the oxidation of dopamine or 3,4-dihydroxyphenylalanine (DOPA) under basic conditions, which allow the occurrence of self-polymerization and the growth of melanin-like particles (Figure 2) 32, 36, 37, 66, 67. Lee and co-workers reported a simple method to synthesize melanin-like PDA particles, through spontaneous air oxidation of dopamine 36. The existence of oxygen in the solution and the basic condition were essential for the synthesis process via oxidation of dopamine followed by the intra/intermolecular cross-linking reactions. The size of melanin-like nanoparticles were well controlled from tens to hundreds of nanometers by tuning the concentration of dopamine, the amount of NaOH added into the reaction solution, and the reaction temperature. Subsequent surface functionalization of the melanin-like particles with thiol-terminated methoxy-PEG (mPEG-SH) further improved their dispersity in aqueous solutions as well as in biological media. Moreover, the surface functionalization did not influence their radical scavenging ability of the melanin-like nanoparticles. This study provides a simple and feasible method for the synthesis of melanin-like PDA particles and promotes the research on properties and potential biomedical applications of these artificial melanin-like particles.
Figure 2.
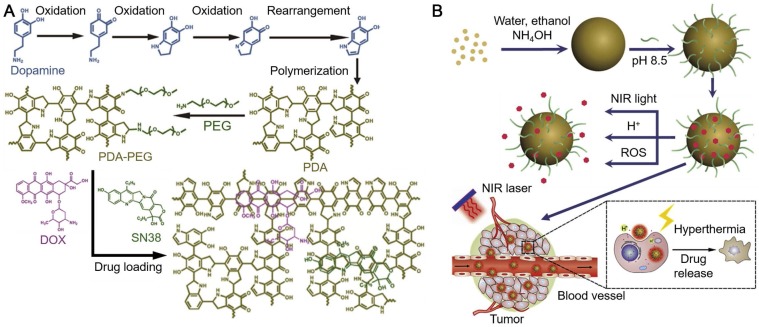
(A) Schematics showing the formation of melanin-like PDA particles loaded with anti-cancer drugs (doxorubicin, DOX and 7-ethyl-10-hydroxycamptothecin, SN38). (B) Triggered-drug release by near-infrared light, pH, or ROS from PDA particles for cancer therapy. Adapted with permission 38. Copyright 2015 Elsevier.
Due to the broad light adsorption of PDA from UV to near-infrared (NIR) wavelengths, PDA particles have been used as photosensitizing agents for PTT of cancer 32, 37, 38. The NIR laser irradiation at 808 nm, under which PDA exhibited high absorption and photothermal conversion efficacy, is usually used to assist PDA for PTT 37, 38, 44, 45. While for certain cases in which other photosensitizers were incorporated into the PDA-based particles, lasers with different wavelengths were also used to achieve the highest absorption of the loaded photosensitizers 40, 68. Based on the oxidation and self-polymerization of dopamine mentioned above, melanin-like PDA nanoparticles were synthesized in a mixture of water, ethanol and ammonia 37. The size of the synthesized PDA nanoparticles could be controlled via tuning either the molar ratio of ammonia to dopamine or the reaction time. The PDA nanoparticles exhibited high photothermal conversion efficiency and high photostability superior to gold nanorods (AuNRs) which easily aggregated under laser irradiation and lost NIR absorbance. Instead, PDA nanoparticles could continuously adsorb light and convert light into heat to raise the local temperature during laser irradiation. Although the measured NIR photoadsorption capability of the synthesized PDA nanoparticles was not as high as porphysomes, it was much higher than most of the reported photothermal therapeutic agents (e.g., copper selenide, carbon nanotubes and Malachite Green) 37, 69-71. As PDA nanoparticles were highly biocompatible, they could only destroy cancer cells with the assistance of NIR irradiation. After intratumoral injection into tumor bearing mice, PDA particles facilitated by laser irradiation could cause the necrosis of tumor tissues and the subsequent ablation of tumor. In addition, magnetic resonance (MR) imaging agents (e.g., Gadolinium, GdIII) could be embeded into PDA nanoparticles (Gd-PDA nanoparticles) for both MR imaging and PTT of tumor in vivo. The surface of PDA nanoparticles could be further modified by using amine-terminated mPEG (mPEG-NH2) for enhanced dispersity 38. Moreover, a series of anticancer drugs, including DOX and SN38, could be loaded in the PDA nanoparticles via hydrogen bonding and/or π-π stacking (Figure 2). The drug-loaded PDA particles showed good drug-retaining ability in physiological conditions and multi-responsiveness to multi-stimuli including pH, NIR irradiation and reactive oxygen species (ROS) for triggered drug release (Figure 2). The drug-loaded PDA nanoparticles were applied for photothermal-chemotherapy assisted by NIR irradiation, which exhibited synergetic effect for cancer treatment with minimal toxicity to normal tissues. These studies indicate that the synthetic melanin-like PDA particles with functional modification can act as good candidates for efficient cancer therapy.
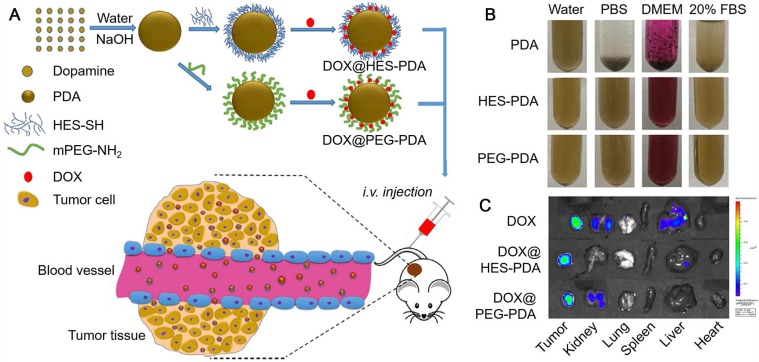
Except for PEG, hydroxyethyl starch (HES) was also used for the surface modification of PDA nanoparticles to enhance their dispersity under physiological conditions (Figure 3) 72. The HES-functionalized PDA (HES-PDA) nanoparticles showed similar stability, drug loading efficiency and biocompatibility compared to the conventional PEGylated PDA (PEG-PDA) nanoparticles. Moreover, similar to PEG-PDA nanoparticles, DOX-loaded HES-PDA nanoparticles exhibited high tumor accumulation and tumor inhibition efficacy with minimal side effect (Figure 3). Therefore, the polysaccharide HES could act as an efficient substitute of PEG for the surface modification of PDA nanoparticles. Apart from the good dispersity, PDA nanoparticles could obtain targeting specificity via surface functionalization with targeting moieties, such as hyaluronic acid (HA) and arginine-glycine-aspartic-cysteine acid peptide (RGDC) 40, 45. Na and co-workers reported a surface functionalization process of PDA nanoparticles via the iron-mediated coordination chemistry by using the photosensitizer-conjugated HA (HA-photosensitizer), resulting in the HA-photosensitizer shielded PDA (PHPD) nanoparticles 40. When HA-photosensitizer was deposited onto the surface of PDA particles, both the fluorescence emissions and the ROS generation of the photosensitizer were quenched by the PDA core. Upon the internalization of PHPD nanoparticles by tumor cells via HA receptor-mediated endocytosis, the HA-photosensitizer shells were degraded by hyaluronidase, a cancer-selective enzyme, releasing photosensitizers from PHPD nanoparticles. The released photosensitizers restored their fluorescence and ROS generation ability for PDT of cancer. On the other hand, the PDA cores, assisted by laser irradiation, showed efficient photothermal conversion ability for PTT of cancer. This system could act as an efficient combined therapy of PDT and PTT for cancer treatment. RGDC peptides could also be functionalized onto the surface of PDA particles via covalent bonding by using a bi-functionalized PEG linker, providing the PDA particles with targeting specificity to integrin αVβ3-positive cancer cells 45. DOX as the model drug for chemotherapy of cancer was then loaded into PDA nanoparticles and could be subsequently released with the trigger of pH and NIR light. Furthermore, the PDA nanoparticles with high NIR absorbance showed high PA imaging efficiency and PTT of cancer. Therefore, this PDA nanoparticle system with targeting ability could achieve PA imaging-guided cancer therapy through the synergistic effect of PTT and chemotherapy.
Figure 3.
(A) Schematic illustration of the preparation of DOX-loaded HES-PDA and PEG-PDA nanoparticles for cancer chemotherapy. (B) Photographs of PDA, HES-PDA and PEG-PDA nanoparticles suspended in H2O, PBS, DMEM, and 20% FBS for 24 h. (C) Ex vivo fluorescence images of tumor, kidney, lung, spleen, liver, and heart showing the distribution of DOX (24 h post-injection). Adapted with permission 72. Copyright 2018 Elsevier.
Besides chemotherapeutic agents (e.g., DOX), other cargos, such as photosensitizers for PDT and PTT, contrast agents for MR imaging, and siRNA for gene therapy, can be loaded into PDA nanoparticles 42-44, 68, 73. For example, Chlorin e6 (Ce6) was loaded into the polymer matrix of PDA nanoparticles during the synthesis of particles via π-π stacking 68. The loading process did not change the surface charge or hydrophilicity of the resulted Ce6-loaded PDA (PDA-Ce6) nanoparticles. Besides, the PDA-Ce6 nanoparticles showed prolonged release of Ce6 through passive release and enhanced cell uptake by endocytosis. In vitro assay results demonstrated the therapeutic efficacy of the PDA-Ce6 nanoparticles under laser irradiation via the combined PDT and PTT. Indocyanine green (ICG), a photothermal agent, could be loaded into PDA nanoparticles via both electrostatic and hydrophobic interactions, which increased the photostability of ICG 44. DOX as the model drug for chemotherapy was adsorbed onto the surface of PDA nanoparticles through π-π stacking and hydrophobic interactions following the nanoparticle surface modification with PEG-grafted poly(maleic anhydride-alt-1-octadecene) (C18PMH-PEG). The release of DOX could be triggered by both pH and the NIR laser irradiation. Manganese ion (MnII) was then loaded into the nanoparticles via coordination interactions and was used as the contrast agent for MR imaging. The MnII-doped PDA nanoparticles exhibited both brightening effect under T1-weighted MR imaging and darkening effect under T2-weighted MR imaging in a concentration-dependent manner. Moreover, the temperature changes caused by ICG and the PDA core during laser irradiation could be monitored by the infrared (IR) camera, allowing the IR thermal imaging of tumor sites. Overall, this multi-cargo-doped PDA nanoparticle achieved imaging-guided combination cancer therapy, with high synergistic efficacy of PTT and chemotherapy in a mouse tumor model. Similar to MnII, other metal ions, including iron (FeIII) and copper (CuII), could also be loaded into PDA nanoparticles for the application as MR imaging agents. The encapsulation amount of FeIII could be tuned by using different doping strategies, either post-doping strategy or pre-polymerization doping strategy 73. Therefore, the FeIII-doped PDA nanoparticles could achieve optimized MR contrast for efficient MR imaging via controlling the amount of the doped FeIII. The loading of cytotoxic CuII in PDA nanoparticles could be achieved during the oxidation and self-polymerization of dopamine 42. The loaded CuII, which release was mainly stimulated in acidic environment, could be used not only as the contrast agent for MR imaging, but also as the chemotherapeutic agent for cancer treatment. Thus, the CuII-doped PDA nanoparticles with long blood circulation and stability would be potential for MR imaging-guided PTT and chemotherapy of cancer. Gene therapy has been reported by loading siRNA onto the surface of tertiary amine-modified mesoporous PDA nanoparticles via electrostatic interactions 43. An out layer coating of calcium phosphate (CaP) was then formed via PDA-induced biomineralization and acted as the protecting layer to prevent the premature release of the loaded siRNA. Upon cell internalization, the CaP layer on PDA nanoparticles was degraded due to the low pH in lysosomes, which resulted in the release of the siRNA and the expose of tertiary amine and catechol groups on PDA nanoparticles. The amine and catechol groups worked synergistically, achieving wet adhesion and resulting in the destabilization of lysosome membranes. Hence, the released siRNA could efficiently escape from lysosomes and consequently exhibited high gene silencing efficacy. In addition, PDA nanoparticles could be utilized as photothermal conversion agents for PTT. Therefore, the reported particles could serve as a platform for the combination of gene therapy and PTT for efficient tumor ablation.
PDA capsules and core-shell particles
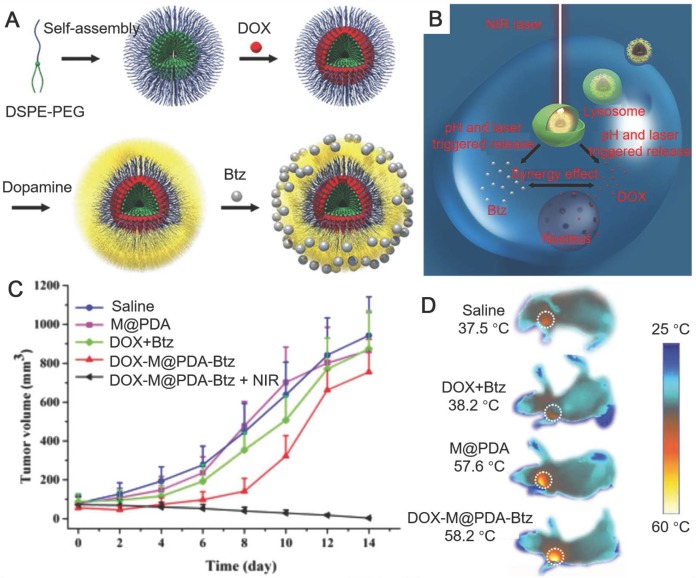
In addition to PDA nanoparticles, PDA capsules with a hollow structure could also be fabricated via templated processes for cancer therapy 41, 74-77. PDA capsules could be prepared by coating PDA on emulsion droplets followed by the removal of the templates 74, 78. The hydrazone bond-linked polymer-drug conjugates (DOX conjugated to thiolated poly(methacrylic acid), PMASH-DOX) were then deposited onto the surface of PDA capsules via thiol-catechol reactions. The DOX-doped PDA capsules showed triggered release of DOX at lysosomal/endosomal pH and higher cancer cell toxicity compared to free DOX, indicating their ability for anticancer drug delivery. PDA shells could also form on DOX-loaded polymer micelles 75. The PDA shells not only acted as photothermal conversion agents for PTT, but also provided immobilization sites for the administration of another anti-tumor drug bortezomib (Btz) (Figure 4). The loaded DOX and Btz could be released within tumor cells triggered by pH and NIR. Such a core-shell nanoparticle platform was anticipated to combine PTT and chemotherapy for efficient cancer treatment. PDA capsules could also be prepared by using sacrificial silica nanoparticles as templates 76. Ionic liquids (ILs), as microwave susceptible agents, were then loaded into the cavity of PDA capsules through ultrasonication under vacuum environment. The IL-doped PDA capsules exhibited efficient anti-tumor effect through microwave thermal therapy. DOX was then loaded into the IL-doped PDA capsules to achieve the combination of chemotherapy and microwave thermal therapy with minimal acute tissue toxicity 41.
Figure 4.
(A) The design of Dox- and Btz-loaded PDA-coated nanoparticles. (B) Schematic illustration of the PDA-coated nanoparticles for dual drug delivery and photothermal therapy. (C) Antitumor activities of drug formulations in a xenograft model of human breast cancer in BALB/c athymic nude mice. (D) IR thermographic images of tumors after laser irradiation for 8 min. Adapted with permission 75. Copyright The Royal Society of Chemistry 2015.
PDA shells could also form on functional particles, resulting in the composite core-shell particles. Dopamine polymerization on the surface of branched gold-silver (Au-Ag) nanoparticles resulted in the PDA-coated Au-Ag nanoparticles (Au-Ag@PDA NPs) 79. PDA shells significantly improved the properties of Au-Ag NPs, including their biocompatibility, structural stability, and photothermal transduction efficiency. Au-Ag@PDA NPs with much higher photothermal transduction efficiency (up to 70%) compared to traditional Au nanostructures would be competitive as a PTT agent for tumor ablation. PDA-capped Fe3O4 superparticles (Fe3O4@PDA SPs) were fabricated via dopamine polymerization on a cluster of pre-assembled Fe3O4 nanoparticles 39. Compared to the pre-assembled Fe3O4 nanoparticles, Fe3O4@PDA SPs exhibited enhanced photothermal and MR imaging performances due to the agglomeration of Fe3O4 nanoparticles in the superparticles. Besides, the PDA shell provided the Fe3O4@PDA SPs with highly improved biocompatibility and stability, as well as extra photothermal efficacy. In a tumor-bearing mouse model, the Fe3O4@PDA SPs demonstrated their great potential as the in vivo MR imaging probe and photothermal agent for both the diagnosis and ablation of tumor. Moreover, similar to PDA nanoparticles, the PDA shells could also be incorporated with imaging or therapeutic agents (e.g., CuII), introducing extra functions to the composite core-shell particles. Zhang and co-workers designed the CuII-doped PDA shell-coated AuNRs (AuNR@Cu-PDA) for dual-model imaging and combined cancer therapy 80. In addition to CT imaging using AuNRs, the CuII doped in the PDA shell provided the AuNR@Cu-PDA with the ability for MR imaging. Furthermore, upon accumulation at the acidic tumor microenvironment or internalization by tumor cells, the cytotoxic CuII could be released from PDA shells and acted as the chemotherapeutic agent to kill cancer cells. Assisted by NIR laser irradiation, the AuNR@Cu-PDA could induce efficient PTT due to the high photothermal efficiency of AuNR enhanced by the PDA shell. After intravenous injection of AuNR@Cu-PDA into tumor bearing mice, tumors could be completely ablated without recurrence through CT and MR imaging-guided PTT and chemotherapy.
PDA particles with unique nanostructures
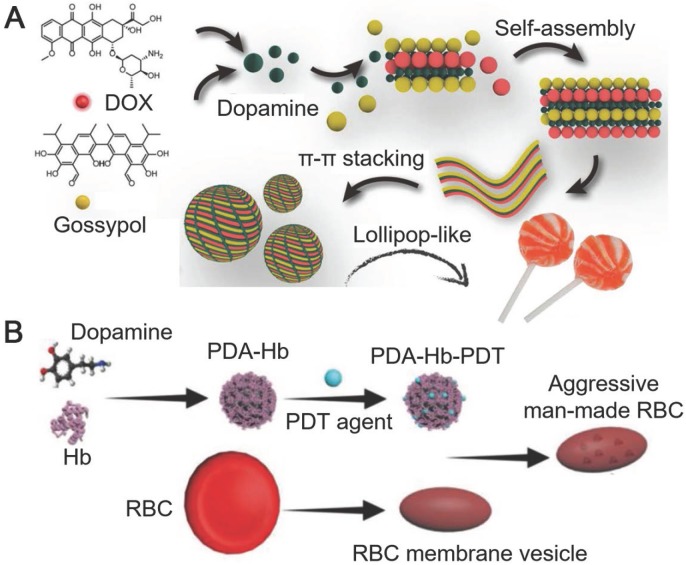
PDA particles with different nanostructures have also been engineered. Wu and co-workers reported lollipop-like PDA nanoparticles (50-70 nm) loaded with two anti-cancer drugs, which showed pH-responsiveness and ultra-long circulation in vivo 81. In the reported system, PDA acted as a cross-linker between the hydrophilic DOX (hydrochloride) and the hydrophobic gossypol via π-π stacking and hydrogen bonds. Dense lollipop-like PDA nanoparticles loaded with dual drugs showed high stability in physiological environments (Figure 5A). At acidic pH, the amine groups on PDA and the DOX were protonated, which partially broke the π-π stacking between PDA and the drugs, resulting in the pH-triggered drug release 81. Therefore, the low pH in the acidic intracellular compartments (e.g., endosome and lysosome) could trigger the release of both drugs from lollipop-like PDA nanoparticles, which enabled the inhibition of tumor by these two drugs synergistically. In addition, the lollipop-like PDA nanoparticles showed ultra-long blood circulation time (>8 days) resulting in 12% tumor accumulation on the 8th day post injection via enhanced permeability and retention (EPR) effect. These advantages allowed the lollipop-like PDA nanoparticles to be an excellent candidate for tumor synergistic therapy with low administration dosages. To overcome the biological barriers and achieve improved therapeutic efficiency, PDA nanoparticles could be functionalized by endogenously derived substances, such as the components of red blood cells (RBCs). For example, PDA could interact with hemoglobin (Hb), the oxygen-carrying protein in RBCs, to form Hb-PDA complexes 82. PDA in the complexes not only protected Hb from oxidation during circulation in vivo, taking the advantage of their antioxidant activity, but also serve as mounting sites for the loading of aromatic compounds (e.g., photodynamic agents) via π-π stacking. Further coating of RBC membranes on the photodynamic agent-loaded Hb-PDA complexes resulted in the artificial RBCs with capabilities for PDT (Figure 5B). The artificial RBCs could retain the properties and functions of natural RBCs, including the biocompatibility, low immune responses, as well as the ability and efficiency of oxygen carrying and releasing. Upon the light irradiation, photodynamic agents loaded in the artificial RBCs could generate ROS to damage tumor cells at tumor site for PDT. Meanwhile, Hb in the artificial RBCs supplied oxygen effectively to boost the generation of ROS for enhanced PDT. The in situ supply of Hb-carried oxygen was essential for PDT efficacy, where hypoxia exists at tumor sites. The reported system could efficiently eliminate 4T1 tumor, and was expected as an oxygen-self-supplied platform for other hypoxia-limited therapeutic applications.
Figure 5.
(A) Schematic illustration of the assembly of DOX-PDA-gossypol nanoparticles. Adapted with permission 81. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Schematic illustration of the preparation of aggressive man-made RBCs for cancer therapy. Adapted with permission 82. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
PDA-based particles derived from naturally occurring monomers maintain high biosafety and biocompatibility. The application of PDA-based particles did not cause significant immune responses or obvious damage to normal tissues or major organs in vivo 32, 39, 44, 75, 81. Therefore, a series of PDA particles have been reported for diverse diagnostic or therapeutic applications. However, the disadvantages of PDA particles should not be ignored. For example, PDA films cross-linked by degradable intermolecular linkers can be disassembled under certain stimuli conditions, however, the PDA itself cannot be fully degraded into small molecules at physiological condition due to the covalent formation of non-degradable carbon-carbon bonds during the self-polymerization of dopamine 67, 83, 84. Therefore, the long-term or high-dosage administration of PDA therapeutic agents may increase the burden of liver and kidney to eliminate fragments of PDA particles 67, 85-87. Hence, introducing degradable intramolecular linkers into the system is desirable for improving the properties of the PDA particles 34, 84. The balance between stability and biodegradability of the PDA particles should be well tuned to obtain the optimized system for subsequent biomedical applications. Due to the existence of aromatic rings and catechol groups in the PDA particles, PDA particles can easily interact with biomolecules in the biological environment via a series of covalent and non-covalent interactions 34, 83, 84. These unexpected interactions could increase nonspecific interactions with normal tissues, causing serious side effects and changing the in vivo fate of PDA particles. Therefore, it is essential to take these interactions into consideration at the early stage of material designing. Effective surface modification can provide the PDA particles with capability to reduce the non-specific interactions 34, 38, 44, 72, 77, 88. Although in vitro and ex vivo analysis of the interactions between PDA particles and biomolecules or cells play important roles in bio-nano interactions, it may not completely predict the in vivo results for effective therapeutic delivery.
MPN-Based Particles
MPN hollow capsules
As the development of material science and nanotechnology, inorganic-organic hybrid structures generated based on metal-organic coordination became a big family of materials under extensive investigations for biomedical applications. MPN-based materials on the basis of metal-phenolic coordination are an important type of materials with promising potential for a wide range of applications. By using the simple, cheap and scalable assembly process of MPN reported by the Caruso group, MPN-based core-shell particles and hollow capsules have been engineered as vehicles for diverse biomedical applications 8, 47, 52, 89. Generally, MPN hollow capsules are assembled by depositing MPN films on a sacrificial template, followed by the removal of the templates 8, 12. The most advantages of the MPN system lies in its biodegradability at low pH. Ubiquitous natural polyphenol, tannic acid (TA), can coordinate with various metal ions, including aluminium (AlIII), CuII, MnII, FeIII, vanadium (VIII), europium (EuIII) and GdIII, to generate robust MPN films and to form a library of functional capsules (Figure 6A and 6B) 12. The properties and functions of MPN capsules could be customized by selecting different metal ions for theranostic applications, including drug delivery, MR imaging, luminescence imaging, and positron emission tomography (PET) 12. Furthermore, mixed metal ions can be incorporated into a single MPN capsule, which provides the capsules with tunable degradation profiles via varying the ratio of the metal ion mixtures 12. In addition to TA, many other natural plant polyphenols or flavonoids, including epigallocatechin (EGC), epigallocatechin gallate (EGCG), myricetin, quercetin, fisetin, and luteolin have also been reported as building blocks for the MPN formation (Figure 6C) 8, 15. Simple phenolic ligands, the monomeric building blocks of natural polyphenols, such as gallic acid (GA), pyrogallol (PG) and pyrocatechol (PC), can also be used for the fabrication of MPN hollow capsules (Figure 6D) 13. Moreover, the library of phenolic compounds for MPN assembly could be further expanded to polyphenol-functionalized polymers, such as catechol-functionalized PEG (PEG-catechol), galloyl-functionalized PEG (PEG-galloyl), catechol-functionalized HA (HA-catechol), and catechol-functionalized chitosan (Chitosan-catechol) 14, 51. These synthetic polymers provide the assembled hollow capsules with extra functions, such as active targeting to CD44-expressing breast cancer cells 14, 51.
Figure 6.
(A) Schematic illustration of the assembly of MPN capsules; (B) Differential interference contrast microscopy (DIC) images of MPN capsules prepared from TA and different metal ions. Insets are photographs of MPN capsule suspensions. Scale bars are 5 μm. (A,B) are adapted with permission 12. Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) Molecular structures of some flavonoids: myricetin from green tea, quercetin from onion, fisetin from strawberry, and luteolin from celery. Adapted with permission 15. Copyright the Royal Society of Chemistry 2017. (D) Structures of GA, PG, PC and DIC images of MPN capsules prepared from related phenols and FeIII. Adapted with permission 13. Copyright 2015 American Chemical Society.
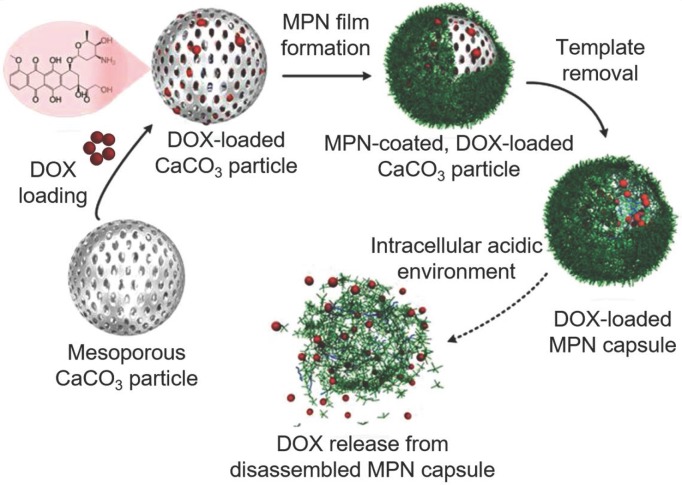
One of the mostly used templates for MPN capsule assembly is calcium carbonate (CaCO3) particles due to its easy synthesis process and the mild condition for template removal 14, 46, 51. For example, FeIII-TA capsules were prepared using CaCO3 particles as templates and could convert hydrogen peroxide (H2O2) into oxygen (O2) molecules in physiological conditions 46. The resulted gas nucleation of O2 formed microbubbles, which changed the acoustic impedance with nonlinear oscillations created. These changes in acoustic impedance could be detected by ultrasound imaging in real time both ex vivo and in vivo. The cumulative production of O2 linearly correlated to the concentration of H2O2 over a wide range. Therefore, FeIII-TA capsules could be used for the rapid detection of endogenous H2O2 levels, which demonstrated the potential of MPN capsules for diagnostic applications via advanced ultrasound imaging. The pH-responsive MPN hollow capsules have also been reported as a carrier for anticancer drug delivery. Due to large surface area of the mesoporous CaCO3 particles, large amount of anticancer drugs (e.g., DOX) could be loaded into the porous interior of the particles based on electrostatic interactions via simply mixing the drug with the templates in the aqueous solution 47. Drug-loaded MPN capsules could be obtained after AlIII-TA coating on the CaCO3 particles and subsequent dissolution of the templates by using ethylenediamine tetraacetic acid (EDTA) solution (Figure 7) 47. The DOX release kinetics were associated with the disassembly kinetics of the MPN capsules, which was pH dependent. Although the permeability of the AlIII-TA capsule shells would allow the partial diffusion of free DOX, more than 70% of the loaded DOX were remained within the stable capsules at extracellular pH. Under acidic microenvironments (e.g., endosomes or lysosomes), the AlIII-TA capsules disassembled and released the encapsulated drugs. This would favor the intracellular delivery of anticancer drugs and enhance the drug delivery efficacy. Except for anticancer drugs, photosensitizer (e.g., hematoporphyrin monomethyl ether, HMME) could also be incorporated into the CaCO3 template via co-precipitation during the synthesis of templates for the assembly of folic acid-functionalized MPN capsules composed of FeIII and PEG-catechol 52. The resulted HMME-encapsulated MPN capsules could targeted to cancer cells with high folate receptor expression while maintained resistance to normal tissue cells. Upon internalization into cancer cells, the capsules disassembled and released the encapsulated HMME. Assisted by laser irradiation at specific sites, HMME would generate ROS both in lysosomes and cytoplasm of cells to induce cell apoptosis. This targeted delivery strategy increased the specific interaction with cancer cells, and enhanced the PDT efficacy of HMME while reducing side effects caused by the nonspecificity of HMME.
Figure 7.
Schematic representation of the fabrication of DOX-loaded MPN capsules and release mechanism of DOX from MPN capsules. Adapted with permission 47. Copyright 2015 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
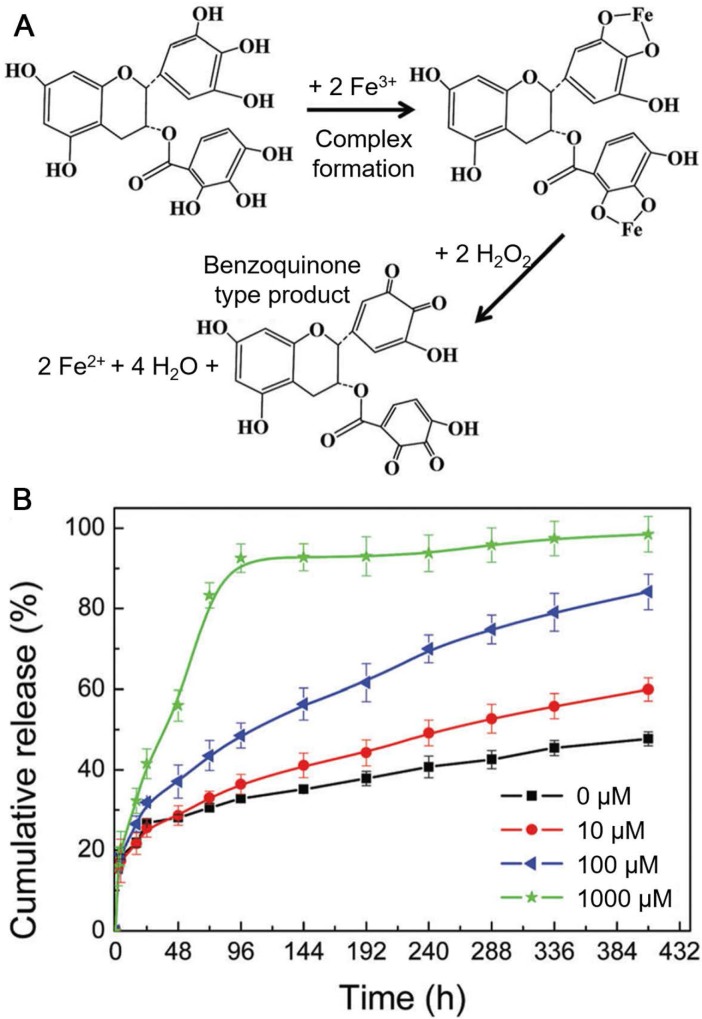
It has been widely accepted that the sizes of nanocarriers below 200 nm are suitable for accumulation at tumor sites via EPR effect 90. Although MPN capsules have been assembled using CaCO3 particles as templates for drug delivery, their sizes are typically in micrometer range, which is not ideal for the delivery in vivo. Therefore, to obtain MPN capsules smaller than 200 nm for in vivo drug delivery, the development of alternative sacrificial templates is required. Recently, metal-organic frameworks (MOFs) have been widely used for cargo delivery due to their large pore volumes and multiple architectures 91. Both the size and shape of MOF particles could be finely controlled via tuning the type and amount of metal ions and ligands. Besides, a wide range of compounds, including proteins, peptides, nucleic acids, carbohydrates and synthetic polymers, could be loaded into MOF particles through co-precipitation during the preparation of MOF particles 91. In addition, MOF particles could be degraded under mild conditions, such as acidic or EDTA solutions. Therefore, MOF particles are excellent candidates for the assembly of MPN capsules with the sizes smaller than 200 nm. Sun and co-workers reported the fabrication of DOX-loaded FeIII-EGCG capsules by using zeolitic imidazolate framework-8 (ZIF-8) particles as templates 92. DOX was first encapsulated into ZIF-8 particles through co-precipitation and FeIII-EGCG films were then deposited onto the templates. DOX-loaded FeIII-EGCG capsules were obtained after the removal of ZIF-8 templates by using EDTA solution. The obtained FeIII-EGCG capsules showed high potential in ROS scavenging and could be disassembled due to the oxidation of EGCG after ROS elimination, leading to the release of the encapsulated DOX (Figure 8). The high level of endogenous ROS in tumor cells accelerated the degradation of ROS-responsive FeIII-EGCG capsules and enhanced the intracellular release of DOX in tumor cells 92. The DOX-loaded FeIII-EGCG capsules showed improved tumor inhibition, compared to free DOX, while acute toxicity to normal tissues or organs was negligible. MPN hollow capsules could also be synthesized by etching MOF particles with natural polyphenol (e.g., TA or ellagic acid, EA) solutions under weak basic condition 93. By using different types of MOF particles, the size, morphology, roughness and composition of the MPN capsules could be tuned. The high stability and slow release of encapsulated cargos (~11%) under the physiological condition (pH 7.4), as well as the disassembled feature of the hollow capsules under weak acidic conditions (e.g., at intracellular endosomal pH) enabled their potential for anticancer drug delivery. Besides, by using FeIII/II, MnII, or cobalt ion (CoII)/MnII-based MOF particles for the capsule assembly, these metal ions could be coordinated into the MPN capsules and exploited as contrast agents for MR imaging. Therefore, MPN capsules assembled through the etching of MOF particles have the potential for theranostic applications.
Figure 8.
(A) Illustration of the coordination of FeIII by EGCG followed by ROS (e.g., H2O2) scavenging, FeIII reduction and the formation of benzoquinone species. (B) In vitro cumulative release of DOX from DOX-loaded FeIII-EGCG capsules at pH 7.4 with varying H2O2 concentrations (0-1 mM). Adapted with permission 92. Copyright the Royal Society of Chemistry 2018.
MPN core-shell particles
In addition to using synthetic particles as templates, cells were coated with MPN films, resulting in the cell-in-shell structures. The Choi group reported the coating of TA-Fe films on individual RBCs 54. The formation of MPN shells on RBCs would not destroy the oxygen-carrying capability of RBCs or cause any aggregation/hemolysis. However, a reasonably thick MPN shell formed on the RBC could shield the epitopes on cell membranes. Since the epitopes on RBC membranes are one of the key factors provoking serious immune responses during transfusion, the formation of MPN shells on RBCs could reduce the immune responses caused by membrane epitopes. Therefore, this strategy provided a simple and fast method for the development of universal blood which could overcome the agglutination and hemolysis caused by cross-type transfusion. Moreover, this cell surface modification method could be extended to other cell-in-shell structures for a range of biomedical applications, such as cell therapy or gene therapy for cancer treatment.
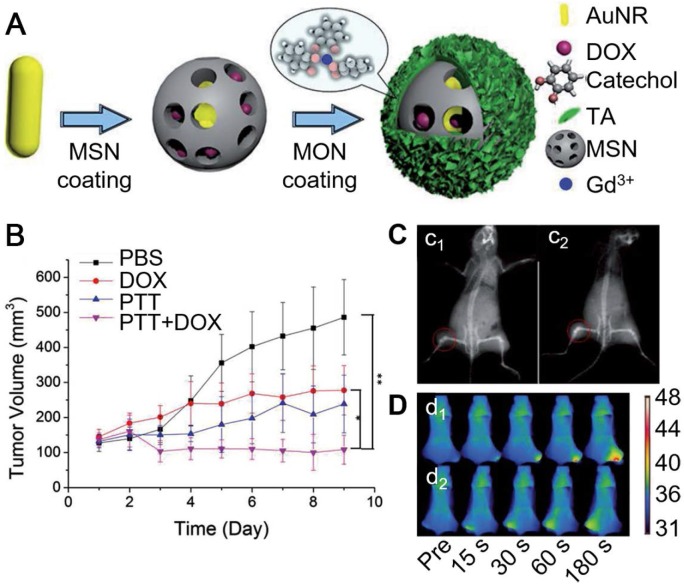
Besides hollow capsules, MPN-based core-shell particles also showed high potential for imaging-guided cancer treatment. Zein-quaternized chitosan nanoparticles have been used as a promising drug delivery system due to their high biocompatibility, biodegradability and responsiveness to diverse physiological variations 94, 95. The formation of a MPN film layer onto the surface of these nanoparticles could further enhance the drug encapsulation efficiency of the nanoparticles and provide a protective layer for the drug-doped nanoparticles 95. By adjusting the type and amount of metals in the MPN layer on the nanoparticles, the pH-responsive degradability of the MPN films were tunable, resulting in the controllable releasing profile of anti-cancer drugs (e.g., DOX). Therefore, the MPN-coated nanoparticles showed potential in intracellular delivery and controlled release of anti-cancer drugs. Moreover, the formation of EuIII-TA film provided the nanoparticles with fluorescence properties for biomedical imaging applications. Similarly, EuIII-TA could be deposited on BaGdF5 nanoparticles to enhance the biocompatibility and reduce the cytotoxicity of bare BaGdF5 nanoparticles used as contrast agents for X-ray computed tomography (CT), while providing the particles with luminescence properties 89. Except for EuIII, GdIII ions have also been used for the formation of MPN films on the surfaces of nanoparticles for MR imaging. Zhang and co-workers reported the MPN coated-mesoporous silica nanoparticle system loaded with AuNRs and DOX for diagnosis and treatment of metastatic tumors (Figure 9) 48. Due to the degradability of GdIII-TA films at acidic pH, the release of the loaded DOX in the mesoporous silica particles could be triggered by the acidic tumor microenvironment 48. The released DOX together with AuNRs, under NIR irradiation, synergistically enhanced cytotoxicity to tumor cells (Figure 9B). On the other hand, the presence of TA down-regulated the overexpression levels of matrix metalloproteinases (MMPs, such as MMP-2 and MMP-9), which played important roles in tumor invasion and vascularization. Therefore, the MPN coated-nanoparticles could effectively inhibit the progression, migration and angiogenesis of metastatic tumors. Since the disassembly of MPN films released free GdIII ions, whose longitudinal relaxivity was increased compared to the coordinated form, it resulted in the enhanced MR imaging ability at tumor sites (Figure 9C). The reported particles could also be used as a contrast agent for CT and enabled the photothermal imaging to evaluate the photothermal efficiency (Figure 9D). Therefore, this system provided a promising theranostic platform for both the primary and metastasis tumor-guided therapy.
Figure 9.
(A) Schematic illustration of the assembly of GdIII-TA coated mesoporous silica nanoparticles loaded with DOX and gold nanoparticles. (B) Relative tumor volume of different groups after treatment with different materials. (C) In vivo CT imaging before (c1) and after (c2) the injection of the GdIII-TA coated nanoparticles. (D) In vivo photothermal imaging after subcutaneous injection of the nanoparticles at 0, 15, 30, 60, and 180 s intervals at the tumor site (d1) or at the normal tissue (d2). Adapted with permission 48. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Self-assembled MPN nanoparticles
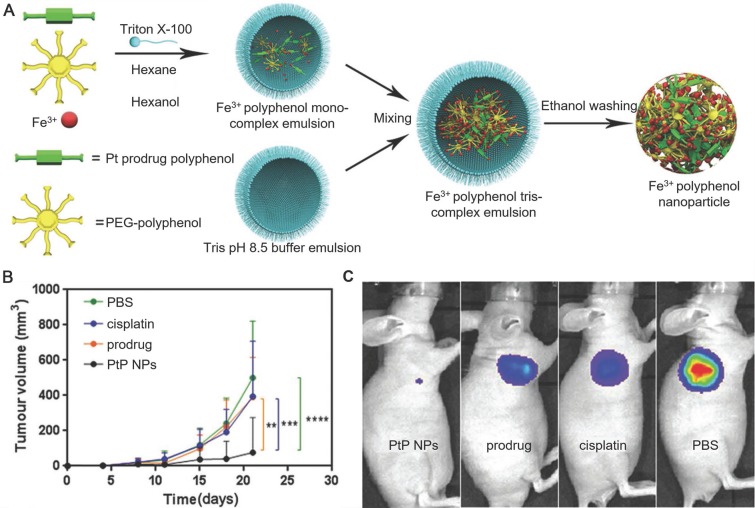
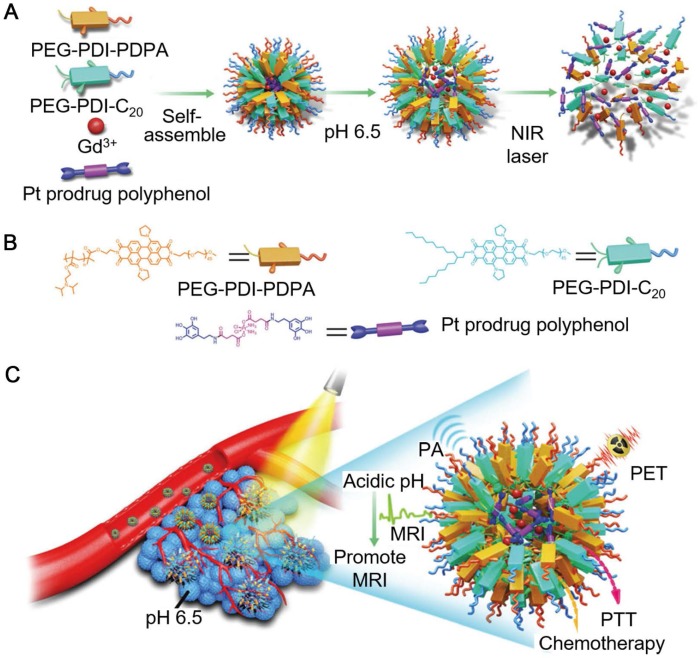
Polyphenols and their derivatives have also been fabricated into self-assembled nanoparticles in the presence of metal ions via metal-phenolic coordination. For instance, PEG-polyphenol and platinum (Pt)-polyphenol were assembled into nanoparticles (PtP NPs) by using FeIII ions as stabilizers via emulsification assisted metal-phenolic coordination (Figure 10) 53. Fluorescence imaging agents (e.g., Ce6) were loaded into the PtP NPs simultaneously for imaging. The incorporation of PEG engaged the nanoparticles with low-fouling properties and therefore enabled the long circulation time of the nanoparticles. These PtP NPs, upon the accumulation at tumor sites and internalized into the tumor cells, showed promising tumor cell inhibition with no significant side effects (Figure 10). Except for cancer therapy, Caruso and co-workers anticipated other diagnostic/imaging applications of the tailored PtP NP system by replacing FeIII in the above system with other metal ions, such as MnII and GdIII for MR imaging, ytterbium (YbIII) and gold (AuIII) for CT. Based on this strategy, many other self-assembled MPN nanoparticles have also been reported. DOX could be loaded into the PtP NP system during the self-assembly process, resulting in the DOX-loaded Pt prodrug FeIII nanoparticles (DPPF NPs) 50. Different components in the DPPF NPs, including DOX, Pt, polyphenols and FeIII, synergistically induced the generation of ROS, which could activate a cascade of bio-reactions, resulting in the damage of cell membrane and DNA. Upon labeling with zirconium-89 (89Zr), the DPPF NPs could be detected by PET in vivo, and showed long blood circulation with high tumor accumulation. The 89Zr-labelled DPPF NPs exhibited high performances in imaging-guided tumor inhibition with reduced side effects caused by anticancer drugs. In addition to DOX, a phagocytic enzyme myeloperoxidase (MPO) was also loaded in the PtP NPs 49. The resulted MPO-loaded MPN nanoparticles (MPP NPs) protected the MPO from degradation during circulation in blood and released the MPO and cisplatin in the intracellular microenvironment upon degradation. The released MPO, which could catalyze the conversion of H2O2 to HOCl, assisted cisplatin to activate the ROS cascade bioreactions and enhanced the chemotherapy efficiency. By using PET imaging, the long circulation and high tumor accumulation of 89Zr-labelled MPP NPs were traced. This strategy showed high potential in controlling the highly toxic HOCl for efficient cancer therapy. Similarly, ratiometric PA imaging probes could be incorporated into the self-assembled Pt prodrug nanoparticles to control the production of ROS and monitor in vivo ROS levels 96. The rod-like nanoparticles were generated by the self-assembly of semiconducting perylene diimide (PDI)-based cisplatin prodrug functionalized with a galloyl group, chelated FeIII, and a ROS activatable IR dye (IR790s). Both the cisplatin and FeIII synergistically achieved the combination cancer therapy by catalyzing the production of ROS. While the ROS generation in vivo was accurately visualized through ratiometric PA imaging measured at two IR excitation wavelengths: 680 nm for PDI and 790 nm for IR790s. These nanoparticles showed high potential for real-time imaging-guided cancer chemotherapy. In addition, other imaging agents, including GdIII for MR imaging, PDI for PA imaging, and 64Cu for PET imaging, could be incorporated into the self-assembled Pt prodrug nanoparticles (GPDPA NPs) for multimodal imaging-guided cancer therapy (Figure 11) 97. The design of pH-sensitive polymers as building blocks provided the GPDPA NPs with responsiveness to acidic tumor microenvironment while reducing drug leakage in the physiological environment.
Figure 10.
(A) Schematic representation of the PtP NP self-assembly process. (B) Tumor volume of luciferase-expressing PC3 cells treated with different groups at the same Pt dose. (C) In vivo luminescence images of mice bearing luciferase PC3 cell xenograft tumors post 10 d treatment with different groups. Adapted with permission 53. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figure 11.
Schematic illustration of the formulation and cancer theranostics mechanism of the nanoparticles. (A) The self-assembly process of GPDPA NPs with mild acidic pH/NIR light-sensitive properties. (B) The molecular structures of building blocks used for GPDPA NP assembly. (C) The multimodal imaging-guided chemo-photothermal combination therapy of GPDPA NPs with NIR irradiation. Adapted with permission 97. Copyright The Royal Society of Chemistry 2019.
Besides, due to strong NIR absorption of PDI, the GPDPA NPs also exhibited high photothermal conversion efficiency, which favored the combined chemo-photothermal cancer therapy. The administration of GPDPA NPs into mouse models effectively achieved tumor inhibition through synergistic effect of chemotherapy and PTT under NIR laser irradiation, which could be monitored via PA/MR/PET multimodal imaging.
Self-assembled MPN nanoparticles could be easily prepared by mixing the FeIII and TA solutions 98. FeIII-TA nanoparticles with the size of a few nanometers in diameter induced autophagic cell death by initiating the formation of autophagosomes. Due to the higher uptake level of FeIII-TA nanoparticles by hepatocellular carcinoma cells compared to normal hepatocytes, FeIII-TA nanoparticles showed the capability in inducing carcinoma cell death by triggering the autophagy process and enhancing the MR imaging contrast of carcinoma cells, which indicated their potential application for diagnosis and treatment of hepatocellular carcinoma. MPN nanoparticles generated from natural polyphenols and FeIII could be incorporated with boronic acid-containing therapeutic agents, such as the anti-tumor drug Btz, through the formation of boronate ester bonds 99. The Btz-loaded MPN nanoparticles demonstrated efficient delivery and controlled release of Btz at tumor sites, while FeIII in the nanoparticles enabled the detection of nanoparticles via MR imaging for MR imaging-guided chemotherapy. Synthetic amphiphilic block copolymers with catechol groups could also be utilized for the self-assembly of MPN nanoparticles in the presence of FeIII 100. The spherical and cylindrical shapes of the resulted MPN nanoparticles could be tuned by changing the volume fraction of the hydrophobic domain in the block copolymers. MPN nanoparticles with both shapes could be used as T1-weighted MR imaging contrast agents. However, the biological behaviors of MPN nanoparticles were proved to be shape-dependent when interacting with HeLa cells. Compared to the spherical MPN nanoparticles, cylindrical ones showed higher cell uptake and better resolution as the MR imaging contrast.
Taking advantages of the intrinsic properties of natural polyphenols, including the antibacterial and antioxidant properties, MPN-based particles have shown high potential for a range of biomedical applications, including drug delivery, antibacterial application, as well as diagnosis and therapy of cancer. The minimal toxicity to normal organs of animal models enabled them to be engaged in further studies for practical applications 48-50, 92. Although a wide range of MPN-based particles have been engineered, more efforts are still essential in the development of MPN carriers for efficient theranostic applications. One important point is to design MPN-based particles by using functional polyphenols with anticancer activities, such as EGCG, resveratrol and curcumin 101-107. Many natural plant polyphenols with therapeutic capabilities, such as curcumin, have poor solubility in aqueous solutions and will undergo rapid hydrolytic degradation at physiological conditions, resulting in low bio-activity when used in physiological solutions 108-110. Assembly of these plant polyphenols into polyphenol-based particles would allow efficient delivery and controlled release of these plant polyphenols in the body, therefore, resulting in the improvement of their bioavailability and the enhancement of their therapeutic activities 110. In addition, it has been reported that the coordination between polyphenols and transition metal ions would induce the oxidation of polyphenols and provide the polyphenols with pro-oxidant effect, resulting in mitochondrial dysfunction, DNA damage, and cell apoptosis 102, 103, 106, 107. The transition metal ions, such as FeIII and CuII, could also induce cell apoptosis by raising the level of toxic ROS via Fenton reaction and causing DNA damage 42, 50, 80, 106. Therefore, the two major components of polyphenol and metal ions in the MPN-based particle systems could act as chemotherapeutic agents and work synergistically for tumor ablation.
Polymer-phenol particles
Boronate bond-stabilized particles
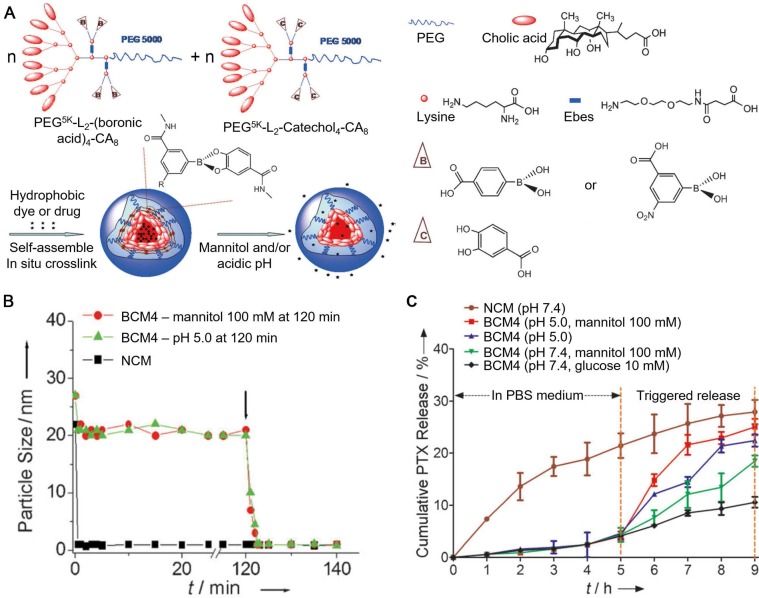
As a family of compounds with high affinity to a wide range of materials, polyphenols and their derivatives are easy to be exploited for the assembly of particles upon the interaction with a series of synthetic or biological polymers. These polymer-phenol particles constructed through covalent and non-covalent interactions have been studied for different biomedical applications. Polyphenols can form boronate bonds between boronic acids and catechol groups to fabricate particles 17, 111, 112. Since the boronate bonds are responsive to both pH and cis-diols, particles assembled based on the boronate cross-linking strategy are dual responsive and can disassemble with either acidic pH or exogenous competing diols 17, 111. For example, boronic acids and catechol groups were conjugated on PEG-dendritic cholic acid (CA) block copolymers (telodendrimers) by using a hydrophilic linker, resulting in the boronic acid-/catechol-functionalized telodendrimers, respectively 111. Interactions between the two types of telodendrimers at neutral pH (pH 7.2) resulted in the formation of micelles cross-linked by boronate bonds (BCMs), with dual-responsiveness (Figure 12). Hydrophobic drugs (e.g., paclitaxel, PTX) were encapsulated into the core of the BCMs during the self-assembly process with high loading efficiency (> 98%). The release of PTX from PTX-loaded BCMs, as a result of the disassembly of BCMs, was dependent on the pH and the concentration of competing diols (e.g., mannitol) in the release medium (Figure 12). Therefore, the reported BCMs could be used for the two-stage release strategy, where premature drug could be minimized during blood circulation at physiological pH while rapid drug release could be triggered by the acidic tumor microenvironment and further triggered by the intravenous administration of mannitol. The Caruso group reported another type of boronate bond-stabilized particles, the boronate-phenolic network (BPN) capsules, which were fabricated using CaCO3 particles as templates, with TA and benzene-1,4-diboronic acid (BDBA) as the building blocks 17. The model anticancer drug DOX could be pre-loaded into the templates, resulting in the DOX-loaded BPN capsules upon template removal. BPN capsules showed good stability in physiological conditions even in the presence of competing carbohydrates (e.g., glucose) in the blood. However, BPN capsules could be disassembled in the acidic microenvironment or with the addition of mannitol, which could be administrated intravenously on demand to trigger the burst release of the encapsulated anti-cancer drugs. Due to their responsiveness to cis-diols, these BPN capsules could also be used for closed-loop insulin delivery and biological targeting to furanoside carbohydrates, or be used as biomimics responsive to different environmental changes. In addition, EGCG has been used to interact with phyenylboronic acid (PBA) moieties conjugated on the poly(ethylene glycol)-block-poly(L-lysine) (PEG-b-PLys), leading to the formation of polyion complex (PIC) micelles cross-linked with boronate bonds 113. Meanwhile, DOX could be encapsulated into the core of PIC micelles via π-π stacking interactions with EGCG. The DOX-loaded PIC micelles showed high stability in physiological environment and released DOX and EGCG after cell internalization. The released EGCG interacted with the P-glycoprotein (P-gp) and inhibit its activity as a transporter to pump DOX out of the cells. Therefore, DOX could accumulate in drug resistant cancer cells in the presence of EGCG. The conversion of DOX to its derivative would induce the generation of ROS, which could cause cardiomyocytes damage in the heart. Due to the ROS scavenging ability of EGCG, the DOX-induced cardiotoxicity of PIC micelles was efficiently inhibited.
Figure 12.
(A) Schematic representation of the assembly of BCMs in response to mannitol and/or acidic pH. (B) Continuous dynamic light scattering measurements of the non-cross-linked micelles (NCMs) and BCMs in SDS with the addition of mannitol or adjusting the pH to 5.0 at 120 min (shown by the arrow). (C) PTX release profiles of BCMs and NCMs in response to diols (mannitol and glucose) and/or pH 5.0. Adapted with permission 111. Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Via introducing targeting molecules, the boronate bond-stabilized systems can be further optimized for targeted drug delivery. HA, a natural carbohydrate which can be recognized specifically by CD44 on cell membranes, has been incorporated into polyphenol-based particles or capsules, providing these structures with active targeting ability 14, 51. Tirelli and co-workers designed the nanoparticles fabricated with TA and boronic acid-modified HA via the formation of boronate bonds for antibacterial applications 112. Boronic acid-modified HA, upon the interaction between boronic acid and catechol or galloyl groups at neutral/basic pH, formed HA-TA nanoparticles. The resulted HA-TA nanoparticles were stable at physiological pH and could be disassembled in acidic microenvironment (e.g., endosomes), resulting in the release of TA to exhibit antibacterial activities. The reported particles not only worked as the specific carrier for targeted TA delivery, but also protected the catechols or galloyls of TA from being oxidized before reaching the desired sites. Hence, the boronate bond-stabilized HA-TA nanoparticles were anticipated to be applied for the treatment of diseases caused by intracellular pathogens.
Polymer-phenol multilayer capsules
Due to the existence of a high portion of benzene rings and hydroxyl groups, polyphenols are easy to be engaged into a series of non-covalent interactions, including hydrogen bonding, electrostatic interactions, and hydrophobic interactions. Therefore, polyphenols are good candidates of building blocks for the fabrication of hollow capsules via layer-by-layer (LbL) assembly technique 22, 57, 114, 115. For instance, EGCG and the proline-rich protein of type A gelatin have been used for the assembly of LbL capsules using MnCO3 particles as templates 22. The EGCG maintained its antioxidant property upon the formation of gelatin-EGCG shells, which were stabilized mainly by hydrophobic interactions between EGCG and gelatin. Drug-encapsulated capsules could be prepared by using drug-loaded particles or drug crystals as templates. Moreover, the permeability of gelatin-EGCG capsules were dependent on molecular weight, which enabled the controlled release of encapsulated cargoes. Lvov and co-workers reported the gelatin-EGCG capsules could be a representative of new formulations containing polyphenols for drug delivery and/or antioxidant applications. Bovine serum albumin (BSA) and TA could also be used to form drug-loaded LbL capsules by using drug-loaded CaCO3 particles or drug-doped emulsion droplets as templates 57. BSA-TA capsules were degraded selectively by the enzyme α-chymotrypsin while resistant to trypsin, which would be suitable for intravenous drug delivery and achieve enzyme-triggered release of the delivered cargoes. In addition, BSA-TA capsules also showed great potential for the oral delivery of bioactive compounds, such as lactoferrin 58. By protecting the encapsulated cargoes against gastric digesting, BSA-TA capsules could significantly enhance the absorption of bioactive cargoes in small intestine. The BSA-TA formulation was then further optimized by incorporation of gastro-adhesive proteins or other specific targeting moieties onto the BSA-TA shells for the site-specific accumulation and cargo release 59. Furthermore, the building blocks of LbL capsules could be extended to a wide range of natural polyphenols and proteins, such as blood serum proteins from individual patient, resulting in the customized drug carriers for the patient and avoiding the harmful side effects (e.g., acute immune responses and thrombosis) 57.
Except for natural proteins, synthetic polymers, including PVPON, poly(dimethyldiallylamide) (PDDA), poly(allylamine) (PAH), and poly(N-vinylcaprolactam) (PVCL), together with polyphenols have been used for the fabrication of LbL capsules 114-117. Polycation-TA LbL capsules showed pH-dependent permeability and could be further tuned by using different types of polycations, which has the potential for the post-loading and controlled release of cargoes 115. Ionic strength could also influence the permeability of PVPON-TA capsules 114. A higher salt concentration induces TA ionization and mutual repulsion between the neighbor layers of TA, which reduces the thickness of LbL shells and hence results in higher permeability of the PVPON-TA shells. By using DOX-loaded mesoporous silica particles as templates, DOX were encapsulated in the PVPON-TA capsules after template removal. The capsules showed negligible release of DOX at physiological pH and could be degraded by enzymes in cytoplasm resulting in the release of DOX. In addition, TA maintained its antioxidant properties in polyelectrolyte-TA capsules (e.g., PVPON-TA and PVCL-TA LbL capsules), which could scavenge free radicals for immunomodulation 117. The TA-based capsules were potentially used as mediators of T-cell immunity for the protection of physical transplant. Living pancreatic islets could also be encapsulated in the PVPON-TA and PVCL-TA capsules to decrease the risk of xenograft and allograft rejection 117, 118. Furthermore, TA-incorporated capsules could be formed by capping a TA layer on the polymersomes derived from PVCL-PVPON diblock copolymers for imaging-guided chemotherapy 116. DOX and fluorescently labelled dextran were encapsulated into the TA-capped polymersomes by mixing the cargoes with the diblock copolymers in solution. The TA coatings acted as the stabilizer of the polymersome and prevented cargo leakage. The encapsulated DOX was used as anticancer therapeutic agent and the fluorescently labelled dextran was for fluorescence imaging to track the transportation and accumulation of the TA-capped polymersomes. The cargos were released via the degradation of the capping layer of TA in the intracellular microenvironment, which demonstrated their potential as drug carriers for tumor chemotherapy.
Self-assembled nanoparticles
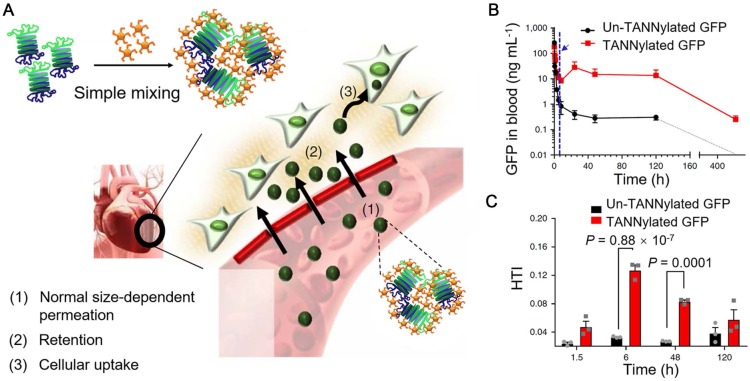
As mentioned above, polyphenols can interact with biomolecules, such as proteins, carbohydrates, and nucleic acids, based on non-covalent interactions (e.g., hydrogen bonding, hydrophobic and electrostatic interactions) 18, 25, 55, 119, 120, which also provides the possibility for the self-assembly of biomacromolecule-phenol nanoparticles. Protein-phenol nanoparticles were assembled by mixing the solutions of TA and green fluorescent protein (GFP) and the nanoparticle size was tunable via adjusting the relative stoichiometric ratios of TA and GFP (Figure 13) 55. Based on fluorescence imaging, GFP-TA nanoparticles could exhibit long blood circulation time and permeation capability from blood vessels to tissues (Figure 13B). Due to the high affinity of TA to proline-rich proteins (e.g., elastin and type I collagen abundant in the cardiac extracellular matrix), GFP-TA nanoparticles showed robust retention in the cardiac extracellular matrix and could be internalized efficiently by myocardial cells, resulting in the specific heart targeting (Figure 13A and 13C). Other therapeutic proteins or peptides (e.g., substance P, SP) or viruses with gene delivery functionalities (e.g., adeno-associated virus 9, AAV9) could also interact with TA, forming the peptide-TA or virus-TA nanoparticles, respectively, by using the reported simple mixing approach. These protein-TA, peptide-TA and virus-TA particles with high cardiosafety were anticipated to be applied for the treatment of heart disease or heart damage via chemotherapy or gene therapy. Due to the high affinity of polyphenols to metal-based materials, imaging agents (e.g., AuNRs for CT, 64Cu for PET, and FeIII or Fe3O4 nanoparticles for MR imaging) can be incorporated into the polyphenol-based particles for theranostic applications of heart diseases.
Figure 13.
(A) Schematic illustration demonstrating the formation of protein-TA nanoparticles and the potential heart-targeting mechanisms. (B) Pharmacokinetics of pure GFP and GFP-TA nanoparticles in the blood stream after intravenous injection. The blue arrow and dashed vertical line indicate the onset time for heart accumulation 6 h after injection. (C) Heart accumulation of TA-GFP nanoparticles as a function of time (1.5, 6, 48 and 120 h). Adapted with permission 55. Copyright 2018 Macmillan Publishers Limited, part of Springer Nature.
Natural polyphenols, such as GA, EGCG and curcumin, can be used as therapeutic agents for the treatment of disease (e.g., colon cancer and Alzheimer's disease) 56, 121. The incorporation of these polyphenols into biomacromolecule-phenol particles can achieve the efficient delivery of these therapeutic polyphenols. GA-grafted chitosan (CS-GA) and caseinophosphopetides (CPP) were mixed for the self-assembly of CS-GA-CPP nanoparticles 121. The presence of GA endowed the nanoparticles with both antioxidant and anticancer activities. Moreover, EGCG was encapsulated into the CS-GA-CPP nanoparticles during the self-assembly process with high loading efficiency (> 80%). The nanoparticles could protect EGCG, which was labile under neutral and alkaline conditions, from being oxidized during the delivery process. The controlled release of EGCG could be achieved under simulated gastrointestinal environment and the released EGCG showed anticancer activity against colon cancer cells. Therefore, the reported nanoparticles could be used for oral delivery of bioactive polyphenols for the treatment of digestive tract diseases and colon cancer. Curcumin with the ability to recognize and bind to β-amyloid (Aβ) peptides were conjugated to a constrained cyclopeptide scaffold together with another Aβ-binding motif (the KLVFFA peptide), resulting in the peptide-phenol conjugates 56. The reported conjugates efficiently bond to Aβ peptides and prevented them from abnormal aggregating into the toxic amyloid fibrils. The peptide-phenol conjugates were anticipated to play important roles in the therapy of Alzheimer's disease and other amyloid related disorders.
Polymer-phenol particles, constructed from natural polyphenols or their derivatives (e.g., TA, GA, EGCG and dopamine functionalized chitosan) and a range of biomacromolecules (e.g., proteins, polypeptides, carbohydrates and nucleic acids) or synthetic polymers (e.g., PEG, PVPON and PVCL) exhibited not only minimal acute immune responses or toxicity to normal tissues but also negligible long-term toxicity to major organs 17, 55, 111, 121. However, the potential applications of polymer-phenol particles have not been fully investigated, as they are mostly used as cargo delivery systems or antibacterial agents in previous studies. Multifunctional polymer-phenol particles for diverse biomedical applications, including the diagnosis and treatment of different diseases, are still highly desirable. Due to the complexity of interactions between polyphenols and biomacromolecules, the formation mechanisms of biomacromolecule-phenol particles have not been fully understood 18, 55, 56. This brings the difficulties in fully controlling the physicochemical properties, functionalities, and ultimate in vivo fate of the constructed biomacromolecule-phenol particles. Therefore, more efforts are also required for basic understandings of polymer-phenol interactions and bio-nano interactions to engineer new generations of polyphenol-based particles for efficient theranostics.
Conclusion and Perspective
Polyphenol-based particles with high biocompatibility, tunable physicochemical properties, and diverse functionalities are an important family of carriers for biomedical applications, especially for the diagnosis and treatment of cancer (Table 1). Mussel-inspired PDA particles are good photothermal conversion agents for PTT. A range of cargoes, including anticancer drugs for chemotherapy, contrast agents for MR imaging, siRNA for gene therapy, and photosensitizers for PDT have been successfully loaded in PDA particles for imaging-guided cancer therapy. MPN-based vehicles, including hollow capsules, core-shell nanoparticles, and self-assembled nanoparticles, have shown the high potential for theranostics. The modification of targeting moieties on the surfaces of MPN-based particles can improve the specific interactions and diagnostic efficacy. In addition, polymer-phenol particles have been fabricated upon the interaction between polyphenols and different synthetic or natural polymers, which combine the functional properties of both polyphenols and polymers. The protection and in vivo transportation of natural polyphenols, which are good ROS scavengers, could be achieved by using the polymer-phenol formulations. Except for cancer therapy, the application of polymer-phenol particles has been expanded to the treatment of different diseases, such as heart damage, infectious diseases, Alzheimer's disease or other related disorders.
Table 1.
The summary of polyphenol-based particles for diagnosis and therapeutic agent delivery.
| Structure | Building block | Cargo | Application | Ref. | |
|---|---|---|---|---|---|
| Mussel-inspired PDA particles | Melanin-like NPs | PDA | / | PTT | 37 |
| PDA | Gd, DOX, SN38 | MR imaging, PTT, chemotherapy | 38 | ||
| PDA | DOX | Chemotherapy | 72 | ||
| PDA | Photosensitizer | PDT, PTT | 40 | ||
| PDA | DOX | Chemotherapy, PTT, PA imaging | 45 | ||
| PDA | ICG, DOX, MnII | MR imaging, PTT, chemotherapy | 44 | ||
| PDA | Ce6 | PDT, PTT | 68 | ||
| PDA | FeIII | MR imaging | 73 | ||
| PDA | CuII | MR imaging, PTT, chemotherapy | 42 | ||
| PDA | siRNA | PTT, gene therapy | 43 | ||
| Hollow capsules | PDA | DOX | Drug delivery, chemotherapy | 74 | |
| PDA | Ionic liquids, DOX | Microwave thermal therapy, chemotherapy | 41, 76 | ||
| Core-shell particles | Polymer micelle@PDA | DOX, Btz | PTT, chemotherapy | 75 | |
| Au-Ag@PDA | / | PTT | 79 | ||
| Fe3O4@PDA | / | MR imaging, PTT | 39 | ||
| AuNR@PDA | CuII | CT, MR imaging, PTT, chemotherapy | 80 | ||
| Lollipop-like NPs | PDA | DOX, gossypol | Chemotherapy | 81 | |
| Artificial RBC particles | Hb-PDA | Photodynamic agent | PDT | 82 | |
| MPN-based particles | Hollow capsules | TA, metal ions | DOX | Ultrasound imaging, MR imaging, drug delivery, chemotherapy | 12, 46, 47, 93 |
| PEG-catechol, FeIII | Photosensitizer | Targeted drug delivery, PDT | 52 | ||
| EGCG, FeIII | DOX | Chemotherapy | 92 | ||
| Core-shell particles | RBC@TA-Fe | / | Universal blood for cross-type transfusion | 54 | |
| Zein-chitosan@TA-metal ion | DOX | Fluorescence imaging, chemotherapy | 95 | ||
| BaGdF5@TA-Eu | / | CT, fluorescence imaging | 89 | ||
| Silica@TA-Gd | AuNR, DOX | CT, MR imaging, photothermal imaging, chemotherapy, PTT | 48 | ||
| Self-assembled NPs | PEG-polyphenol, (PDI)-Pt-polyphenol, metal ions | Ce6, DOX, MPO, IR790s | Chemotherapy, CT, PET, MR imaging, PA imaging, fluorescence imaging | 49, 50, 53, 96 | |
| PEG-PDI, Pt-polyphenol, metal ions | / | PET, PA imaging, MR imaging, chemotherapy, PTT | 97 | ||
| TA, FeIII | / | MR imaging, hepatocellular carcinoma treatment | 98 | ||
| Natural polyphenols, FeIII | Btz | MR imaging, chemotherapy | 99 | ||
| Catechol-modified block copolymers, FeIII | / | MR imaging | 100 | ||
| Polymer-phenol particles | Micelles | Boronic acid-functionalized telodendrimers, catechol-functionalized telodendrimers | PTX | Chemotherapy | 111 |
| EGCG, boronic acid functionalized block copolymers | DOX | Chemotherapy, reducing cardiotoxicity, overcoming drug resistance | 113 | ||
| Hollow capsules | TA, BDBA | DOX | Chemotherapy, drug delivery | 17 | |
| EGCG, gelatin | Dextran | Cargo delivery | 22 | ||
| TA, BSA | Lactoferrin, TRITC, THCP | Oral delivery, intravenous delivery | 57-59 | ||
| TA, PVPON, PVCL, PDDA, PAH | DOX, dextran | Drug delivery, immunomodulation | 114-117 | ||
| Self-assembled NPs | TA, boronic acid-functionalized HA | / | Antibacterial applications | 112 | |
| TA, therapeutic proteins/viruses | / | Treatment of heart disease via chemotherapy or gene therapy | 55 | ||
| GA-grafted chitosan, peptides | EGCG | Oral delivery, treatment of colon cancer | 121 | ||
| Polymer conjugates | Curcumin, peptides | / | Treatment of AD | 56 | |
Abbreviations: NPs: nanoparticles; Ref.: reference; TRITC: tetramethylrhodamine-isothiocyanate; THCP: hydrophobic 3,4,9,10-tetra-(hectoxycarbonyl)-perylene.
Although fascinating outcomes have been obtained in the engineering of polyphenol-based particles, there is still a huge space in the exploration of these particle systems for improved theranostics. One of the most important points is to introduce both low-fouling and targeting components into the polyphenol-based particle systems, which aims to provide the particles with reduced non-specific accumulation and improved targeting ability for enhanced tumor accumulation, respectively 122, 123. However, the optimized balance between low-fouling properties and specific targeting capabilities requires repeated trials within the design and modification - in vitro and in vivo test - re-design and re-modification cycle. With a wide range of polyphenol-based particles designed, some of these particles were constructed in a black box with unclear mechanisms. Therefore, the detailed polyphenol-engaged interactions should be considered to fully understand the assembly mechanisms for controllable engineering of the polyphenol-based particles. Polyphenol-based particles for in vitro and in vivo studies have been widely investigated in previous studies. However, much more efforts are required to utilize these materials for practical applications in daily medical treatment, which include but not limited to establishing an optimized formulation, producing the materials in large-scale, selecting the best methods and amount for administration, as well as dealing with the potential side effects. In addition, the design of customized or personalized therapeutic formulations is possible by using polyphenol-based particles incorporating proteins or other biomolecules from individual patient. Although there is a long way from the concept to practical commercialized product, polyphenols have open up a new path for the design of functional materials for biomedical applications.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (J.C., 21603120, 21872085; Q.D., 21802088). This work was also supported by China Postdoctoral Science Foundation (Q.D., 2018M632662) and Natural Science Foundation of Shandong Province (Q.D., ZR2018BB036; J.H. ZR2018ZA0547).
References
- 1.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 2011;50:586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 2.Haslam E, Cai Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat Prod Rep. 1994;11:41–66. doi: 10.1039/np9941100041. [DOI] [PubMed] [Google Scholar]
- 3.Wigglesworth VB. The source of lipids and polyphenols for the insect cuticle: The role of fat-body, enocytes and enocytoids. Tissue Cell. 1988;20:919–32. doi: 10.1016/0040-8166(88)90033-x. [DOI] [PubMed] [Google Scholar]
- 4.Sileika TS, Barrett DG, Zhang R, Lau KHA, Messersmith PB. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew Chem Int Ed Engl. 2013;52:10766–70. doi: 10.1002/anie.201304922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan G. Phenolic tanning and pigmentation of the cuticle in Carcinus maenas. Q J Microsc Sci. 1951;92:333–42. [Google Scholar]
- 6.Huber B, Eberl L, Feucht W, Polster J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z Naturforsch C. 2003;58:879–84. doi: 10.1515/znc-2003-11-1224. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK. et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013;341:154–7. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Tardy BL, Christofferson AJ, Dai Y, Richardson JJ, Zhu W. et al. Modular assembly of superstructures from polyphenol-functionalized building blocks. Nat Nanotechnol. 2016;11:1105–11. doi: 10.1038/nnano.2016.172. [DOI] [PubMed] [Google Scholar]
- 10.Rahim MA, Björnmalm M, Suma T, Faria M, Ju Y, Kempe K. et al. Metal-phenolic supramolecular gelation. Angew Chem Int Ed Engl. 2016;55:13803–7. doi: 10.1002/anie.201608413. [DOI] [PubMed] [Google Scholar]
- 11.Yun G, Besford QA, Johnston ST, Richardson JJ, Pan S, Biviano M. et al. Self-assembly of nano- to macroscopic metal-phenolic materials. Chem Mater. 2018;30:5750–8. [Google Scholar]
- 12.Guo J, Ping Y, Ejima H, Alt K, Meissner M, Richardson JJ. et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew Chem Int Ed Engl. 2014;53:5546–51. doi: 10.1002/anie.201311136. [DOI] [PubMed] [Google Scholar]
- 13.Rahim MA, Kempe K, Müllner M, Ejima H, Ju Y, van Koeverden MP. et al. Surface-confined amorphous films from metal-coordinated simple phenolic ligands. Chem Mater. 2015;27:5825–32. [Google Scholar]
- 14.Ju Y, Cui J, Sun H, Müllner M, Dai Y, Guo J. et al. Engineered metal-phenolic capsules show tunable targeted delivery to cancer cells. Biomacromolecules. 2016;17:2268–76. doi: 10.1021/acs.biomac.6b00537. [DOI] [PubMed] [Google Scholar]
- 15.Bertleff-Zieschang N, Rahim MA, Ju Y, Braunger JA, Suma T, Dai Y. et al. Biofunctional metal-phenolic films from dietary flavonoids. Chem Commun. 2017;53:1068–71. doi: 10.1039/c6cc08607a. [DOI] [PubMed] [Google Scholar]
- 16.Springsteen G, Wang B. A detailed examination of boronic acid-diol complexation. Tetrahedron. 2002;58:5291–300. [Google Scholar]
- 17.Guo J, Sun H, Alt K, Tardy BL, Richardson JJ, Suma T. et al. Boronate-phenolic network capsules with dual response to acidic pH and cis-diols. Adv Healthc Mater. 2015;4:1796–801. doi: 10.1002/adhm.201500332. [DOI] [PubMed] [Google Scholar]
- 18.Le Bourvellec C, Renard CMGC. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit Rev Food Sci Nutr. 2012;52:213–48. doi: 10.1080/10408398.2010.499808. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Yu D, Sun J, Liu X, Jiang L, Guo H. et al. Interaction of plant phenols with food macronutrients: characterisation and nutritional-physiological consequences. Nutr Res Rev. 2014;27:1–15. doi: 10.1017/S095442241300019X. [DOI] [PubMed] [Google Scholar]
- 20.Lim C, Huang J, Kim S, Lee H, Zeng H, Hwang DS. Nanomechanics of poly(catecholamine) coatings in aqueous solutions. Angew Chem Int Ed Engl. 2016;55:3342–6. doi: 10.1002/anie.201510319. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Wang Y, Park SY, Lee H. Progressive fuzzy cation-π assembly of biological catecholamines. Sci Adv. 2018;4:eaat7457. doi: 10.1126/sciadv.aat7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shutava TG, Balkundi SS, Lvov YM. (-)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J Colloid Interface Sci. 2009;330:276–83. doi: 10.1016/j.jcis.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 23.Kozlovskaya V, Harbaugh S, Drachuk I, Shchepelina O, Kelley-Loughnane N, Stone M. et al. Hydrogen-bonded LbL shells for living cell surface engineering. Soft Matter. 2011;7:2364–72. [Google Scholar]
- 24.Kim K, Shin M, Koh MY, Ryu JH, Lee MS, Hong S. et al. TAPE: A medical adhesive inspired by a ubiquitous compound in plants. Adv Funct Mater. 2015;25:2402–10. [Google Scholar]
- 25.Shin M, Ryu JH, Park JP, Kim K, Yang JW, Lee H. DNA/Tannic acid hybrid gel exhibiting biodegradability, extensibility, tissue adhesiveness, and hemostatic ability. Adv Funct Mater. 2015;25:1270–8. [Google Scholar]
- 26.Hu Q, Luo Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr Polym. 2016;151:624–39. doi: 10.1016/j.carbpol.2016.05.109. [DOI] [PubMed] [Google Scholar]
- 27.Cirillo G, Curcio M, Vittorio O, Iemma F, Restuccia D, Spizzirri UG. et al. Polyphenol conjugates and human health: A perspective review. Crit Rev Food Sci Nutr. 2016;56:326–37. doi: 10.1080/10408398.2012.752342. [DOI] [PubMed] [Google Scholar]
- 28.Vittorio O, Curcio M, Cojoc M, Goya GF, Hampel S, Iemma F. et al. Polyphenols delivery by polymeric materials: challenges in cancer treatment. Drug Deliv. 2017;24:162–80. doi: 10.1080/10717544.2016.1236846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu FG, Ma CC, McClements DJ, Gao YX. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of beta-carotene emulsions. Food Hydrocoll. 2016;61:578–88. [Google Scholar]
- 30.Pawlaczyk-Graja I, Balicki S, Ziewiecki R, Matulová M, Capek P, Gancarz R. Polyphenolic-polysaccharide conjugates of Sanguisorba officinalis L. with anticoagulant activity mediated mainly by heparin cofactor II. Int J Biol Macromol. 2016;93:1019–29. doi: 10.1016/j.ijbiomac.2016.09.082. [DOI] [PubMed] [Google Scholar]
- 31.Rahim MA, Kristufek SL, Pan S, Richardson JJ, Caruso F. Phenolic building blocks for the assembly of functional materials. Angew Chem Int Ed Engl. 2018;57:2–26. doi: 10.1002/anie.201807804. [DOI] [PubMed] [Google Scholar]
- 32.Qi C, Fu LH, Xu H, Wang TF, Lin J, Huang P. Melanin/polydopamine-based nanomaterials for biomedical applications. Sci China Chem. 2019;62:162–88. [Google Scholar]
- 33.Foegeding EA, Plundrich N, Schneider M, Campbell C, Lila MA. Protein-polyphenol particles for delivering structural and health functionality. Food Hydrocoll. 2017;72:163–73. [Google Scholar]
- 34.Mrówzyński R. Polydopamine-based multifunctional (nano)materials for cancer therapy. ACS Appl Mater Interfaces. 2018;10:7541–61. doi: 10.1021/acsami.7b08392. [DOI] [PubMed] [Google Scholar]
- 35.Conte R, Calarco A, Napoletano A, Valentino A, Margarucci S, Di Cristo F. et al. Polyphenols nanoencapsulation for therapeutic applications. J Biomol Res Ther. 2016;5:1000139. [Google Scholar]
- 36.Ju KY, Lee Y, Lee S, Park SB, Lee JK. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules. 2011;12:625–32. doi: 10.1021/bm101281b. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Ai K, Liu J, Deng M, He Y, Lu L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater. 2013;25:1353–9. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Zhang J, Wang Y, Wang C, Xiao J, Zhang Q. et al. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials. 2016;81:114–24. doi: 10.1016/j.biomaterials.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Ge R, Li X, Lin M, Wang D, Li S, Liu S. et al. Fe3O4@polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl Mater Interfaces. 2016;8:22942–52. doi: 10.1021/acsami.6b07997. [DOI] [PubMed] [Google Scholar]
- 40.Han J, Park W, Park SJ, Na K. Photosensitizer-conjugated hyaluronic acid-shielded polydopamine nanoparticles for targeted photomediated tumor therapy. ACS Appl Mater Interfaces. 2016;8:7739–47. doi: 10.1021/acsami.6b01664. [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Liu B, Wang S, Liu T, Fu C, Ren X. et al. Doxorubicin-loaded ionic liquid-polydopamine nanoparticles for combined chemotherapy and microwave thermal therapy of cancer. RSC Adv. 2016;6:32434–40. [Google Scholar]
- 42.Ge R, Lin M, Li X, Liu S, Wang W, Li S. et al. Cu2+-loaded polydopamine nanoparticles for magnetic resonance imaging-guided pH- and near-infrared-light-stimulated thermochemotherapy. ACS Appl Mater Interfaces. 2017;9:19706–16. doi: 10.1021/acsami.7b05583. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Wang L, Prabhakar N, Xing Y, Rosenholm JM, Zhang J. et al. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019;86:416–28. doi: 10.1016/j.actbio.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Dong Z, Gong H, Gao M, Zhu W, Sun X, Feng L. et al. Polydopamine nanoparticles as a versatile molecular loading platform to enable imaging-guided cancer combination therapy. Theranostics. 2016;6:1031–42. doi: 10.7150/thno.14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Jiang C, Zhang D, Wang Y, Ren X, Ai K. et al. Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor. Acta Biomater. 2017;47:124–34. doi: 10.1016/j.actbio.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Guo J, Wang X, Henstridge DC, Richardson JJ, Cui J, Sharma A. et al. Nanoporous metal-phenolic particles as ultrasound imaging probes for hydrogen peroxide. Adv Healthc Mater. 2015;4:2170–5. doi: 10.1002/adhm.201500528. [DOI] [PubMed] [Google Scholar]
- 47.Ping Y, Guo J, Ejima H, Chen X, Richardson JJ, Sun H. et al. pH-Responsive capsules engineered from metal-phenolic networks for anticancer drug delivery. Small. 2015;11:2032–6. doi: 10.1002/smll.201403343. [DOI] [PubMed] [Google Scholar]
- 48.Fan JX, Zheng DW, Mei WW, Chen S, Chen SY, Cheng SX. et al. A metal-polyphenol network coated nanotheranostic system for metastatic tumor treatments. Small. 2017;13:1702714. doi: 10.1002/smll.201702714. [DOI] [PubMed] [Google Scholar]
- 49.Dai Y, Cheng S, Wang Z, Zhang R, Yang Z, Wang J. et al. Hypochlorous acid promoted platinum drug chemotherapy by myeloperoxidase-encapsulated therapeutic metal phenolic nanoparticles. ACS Nano. 2018;12:455–63. doi: 10.1021/acsnano.7b06852. [DOI] [PubMed] [Google Scholar]
- 50.Dai Y, Yang Z, Cheng S, Wang Z, Zhang R, Zhu G. et al. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv Mater. 2018;30:1704877. doi: 10.1002/adma.201704877. [DOI] [PubMed] [Google Scholar]
- 51.Ju Y, Dai Q, Cui J, Dai Y, Suma T, Richardson JJ. et al. Improving targeting of metal-phenolic capsules by the presence of protein coronas. ACS Appl Mater Interfaces. 2016;8:22914–22. doi: 10.1021/acsami.6b07613. [DOI] [PubMed] [Google Scholar]
- 52.Wei Y, Wei Z, Luo P, Wei W, Liu S. pH-sensitive metal-phenolic network capsules for targeted photodynamic therapy against cancer cells. Artif Cells Nanomed Biotechnol. 2018;46:1552–61. doi: 10.1080/21691401.2017.1377724. [DOI] [PubMed] [Google Scholar]
- 53.Dai Y, Guo J, Wang TY, Ju Y, Mitchell AJ, Bonnard T. et al. Self-assembled nanoparticles from phenolic derivatives for cancer therapy. Adv Healthc Mater. 2017;6:1700467. doi: 10.1002/adhm.201700467. [DOI] [PubMed] [Google Scholar]
- 54.Park T, Kim JY, Cho H, Moon HC, Kim BJ, Park JH. et al. Artificial spores: Immunoprotective nanocoating of red blood cells with supramolecular ferric ion-tannic acid complex. Polymers. 2017;9:140. doi: 10.3390/polym9040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin M, Lee HA, Lee M, Shin Y, Song JJ, Kang SW. et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat Biomed Eng. 2018;2:304–17. doi: 10.1038/s41551-018-0227-9. [DOI] [PubMed] [Google Scholar]
- 56.Ouberai M, Dumy P, Chierici S, Garcia J. Synthesis and biological evaluation of clicked curcumin and clicked KLVFFA conjugates as inhibitors β-amyloid fibril formation. Bioconjug Chem. 2009;20:2123–32. doi: 10.1021/bc900281b. [DOI] [PubMed] [Google Scholar]
- 57.Lomova MV, Brichkina AI, Kiryukhin MV, Vasina EN, Pavlov AM, Gorin DA. et al. Multilayer capsules of bovine serum albumin and tannic acid for controlled release by enzymatic degradation. ACS Appl Mater Interfaces. 2015;7:11732–40. doi: 10.1021/acsami.5b03263. [DOI] [PubMed] [Google Scholar]
- 58.Kilic E, Novoselova MV, Lim SH, Pyataev NA, Pinyaev SI, Kulikov OA. et al. Formulation for oral delivery of lactoferrin based on bovine serum albumin and tannic acid multilayer microcapsules. Sci Rep. 2017;7:44159. doi: 10.1038/srep44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lau HH, Murney R, Yakovlev NL, Novoselova MV, Lim SH, Roy N. et al. Protein-tannic acid multilayer films: A multifunctional material for microencapsulation of food-derived bioactives. J Colloid Interface Sci. 2017;505:332–40. doi: 10.1016/j.jcis.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science. 2010;328:216–20. doi: 10.1126/science.1181044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng H, Hwang DS, Israelachvili JN, Waite JH. Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water. Proc Natl Acad Sci U S A. 2010;107:12850–3. doi: 10.1073/pnas.1007416107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Lee Y, Statz AR, Rho J, Park TG, Messersmith PB. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv Mater. 2008;20:1619–23. doi: 10.1002/adma.200702378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You I, Seo YC, Lee H. Material-independent fabrication of superhydrophobic surfaces by mussel-inspired polydopamine. RSC Adv. 2014;4:10330–3. [Google Scholar]
- 64.Simon JD. Spectroscopic and dynamic studies of the epidermal chromophores trans-urocanic acid and eumelanin. Acc Chem Res. 2000;33:307–13. doi: 10.1021/ar970250t. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Wang D, Dai T, Xu P, Wu P, Zou Y. et al. Skin pigmentation-inspired polydopamine sunscreens. Adv Funct Mater. 2018;28:1802127. [Google Scholar]
- 66.Herlinger E, Jameson RF, Linert W. Spontaneous autoxidation of dopamine. J Chem Soc Perkin 2. 1995;2:259–63. [Google Scholar]
- 67.Wang X, Sheng J, Yang M. Melanin-based nanoparticles in biomedical applications: From molecular imaging to treatment of diseases. Chin Chem Lett. 2019;30:533–40. [Google Scholar]
- 68.Poinard B, Neo SZY, Yeo ELL, Heng HPS, Neoh KG, Kah JCY. Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl Mater Interfaces. 2018;10:21125–36. doi: 10.1021/acsami.8b04799. [DOI] [PubMed] [Google Scholar]
- 69.Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW. et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011;11:2560–6. doi: 10.1021/nl201400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kam NWS, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–5. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du H, Fuh RCA, Li J, Corkan LA, Lindsey JS. PhotochemCAD: A computer-aided design and research tool in photochemistry. Photochem Photobiol. 1998;68:141–2. [Google Scholar]
- 72.Wu H, Hu H, Wan J, Li Y, Wu Y, Tang Y. et al. Hydroxyethyl starch stabilized polydopamine nanoparticles for cancer chemotherapy. Chem Eng J. 2018;349:129–45. [Google Scholar]
- 73.Li Y, Xie Y, Wang Z, Zang N, Carniato F, Huang Y. et al. Structure and function of iron-loaded synthetic melanin. ACS Nano. 2016;10:10186–94. doi: 10.1021/acsnano.6b05502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui J, Yan Y, Such GK, Liang K, Ochs CJ, Postma A. et al. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules. Biomacromolecules. 2012;13:2225–8. doi: 10.1021/bm300835r. [DOI] [PubMed] [Google Scholar]
- 75.Zhang R, Su S, Hu K, Shao L, Deng X, Sheng W. et al. Smart micelle@polydopamine core-shell nanoparticles for highly effective chemo-photothermal combination therapy. Nanoscale. 2015;7:19722–31. doi: 10.1039/c5nr04828a. [DOI] [PubMed] [Google Scholar]
- 76.Tan L, Tang W, Liu T, Ren X, Fu C, Liu B. et al. Biocompatible hollow polydopamine nanoparticles loaded ionic liquid enhanced tumor microwave thermal ablation in vivo. ACS Appl Mater Interfaces. 2016;8:11237–45. doi: 10.1021/acsami.5b12329. [DOI] [PubMed] [Google Scholar]
- 77.Zhao H, Chao Y, Liu J, Huang J, Pan J, Guo W. et al. Polydopamine coated single-walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics. 2016;6:1833–43. doi: 10.7150/thno.16047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui J, Wang Y, Postma A, Hao J, Hosta-Rigau L, Caruso F. Monodisperse polymer capsules: Tailoring size, shell thickness, and hydrophobic cargo loading via emulsion templating. Adv Funct Mater. 2010;20:1625–31. [Google Scholar]
- 79.Li J, Wang W, Zhao L, Rong L, Lan S, Sun H. et al. Hydroquinone-assisted synthesis of branched Au-Ag nanoparticles with polydopamine coating as highly efficient photothermal agents. ACS Appl Mater Interfaces. 2015;7:11613–23. doi: 10.1021/acsami.5b02666. [DOI] [PubMed] [Google Scholar]
- 80.Liu S, Wan L, Lin M, Wang D, Song Z, Li S. et al. Cu(II)-doped polydopamine-coated gold nanorods for tumor theranostics. ACS Appl Mater Interfaces. 2017;9:44293–306. doi: 10.1021/acsami.7b13643. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Wu Y, Li K, Shen S, Liu Z, Wu D. Ultralong circulating lollipop-like nanoparticles assembled with gossypol, doxorubicin, and polydopamine via π-π stacking for synergistic tumor therapy. Adv Funct Mater. 2019;29:1805582. [Google Scholar]
- 82.Liu WL, Liu T, Zou MZ, Yu WY, Li CX, He ZY. et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater. 2018;30:1802006. doi: 10.1002/adma.201802006. [DOI] [PubMed] [Google Scholar]
- 83.Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW. Perspectives on poly(dopamine) Chem Sci. 2013;4:3796–802. [Google Scholar]
- 84.Lynge ME, van der Westen R, Postma A, Städler B. Polydopamine-a nature-inspired polymer coating for biomedical science. Nanoscale. 2011;3:4916–28. doi: 10.1039/c1nr10969c. [DOI] [PubMed] [Google Scholar]
- 85.Eisenhofer G, Åneman A, Hooper D, Holmes C, Goldstein DS, Friberg P. Production and metabolism of dopamine and norepinephrine in mesenteric organs and liver of swine. Am J Physiol Gastrointest Liver Physiol. 1995;268:G641–9. doi: 10.1152/ajpgi.1995.268.4.G641. [DOI] [PubMed] [Google Scholar]
- 86.Hagenbuch B. Drug uptake systems in liver and kidney: A historic perspective. Clin Pharmacol Ther. 2010;87:39–47. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hiraoka H, Yamamoto K, Miyoshi S, Morita T, Nakamura K, Kadoi Y. et al. Kidneys contribute to the extrahepatic clearance of propofol in humans, but not lungs and brain. Br J Clin Pharmacol. 2005;60:176–82. doi: 10.1111/j.1365-2125.2005.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jo SD, Ku SH, Won YY, Kim SH, Kwon IC. Targeted nanotheranostics for future personalized medicine: Recent progress in cancer therapy. Theranostics. 2016;6:1362–77. doi: 10.7150/thno.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu W, Liang S, Wang J, Yang Z, Zhang L, Yuan T. et al. Europium-phenolic network coated BaGdF5 nanocomposites for tri-modal computed tomography/magnetic resonance/luminescence imaging. J Mater Sci Mater Med. 2017;28:74. doi: 10.1007/s10856-017-5888-5. [DOI] [PubMed] [Google Scholar]
- 90.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 91.Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N. et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun. 2015;6:7240. doi: 10.1038/ncomms8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Li X, Liang X, Liang J, Zhang C, Yang J. et al. ROS-responsive capsules engineered from green tea polyphenol-metal networks for anticancer drug delivery. J Mater Chem B. 2018;6:1000–10. doi: 10.1039/c7tb02688a. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Zhu W, Ping Y, Wang C, Gao N, Yin X. et al. Controlled fabrication of functional capsules based on the synergistic interaction between polyphenols and MOFs under weak basic condition. ACS Appl Mater Interfaces. 2017;9:14258–64. doi: 10.1021/acsami.7b01788. [DOI] [PubMed] [Google Scholar]
- 94.Liang H, Zhou B, He L, An Y, Lin L, Li Y. et al. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015;5:13891–900. [Google Scholar]
- 95.Liang H, Li J, He Y, Xu W, Liu S, Li Y. et al. Engineering multifunctional films based on metal-phenolic networks for rational pH-responsive delivery and cell imaging. ACS Biomater Sci Eng. 2016;2:317–25. doi: 10.1021/acsbiomaterials.5b00363. [DOI] [PubMed] [Google Scholar]
- 96.Yang Z, Dai Y, Yin C, Fan Q, Zhang W, Song J. et al. Activatable semiconducting theranostics: Simultaneous generation and ratiometric photoacoustic imaging of reactive oxygen species in vivo. Adv Mater. 2018;30:1707509. doi: 10.1002/adma.201707509. [DOI] [PubMed] [Google Scholar]
- 97.Yang Z, Dai Y, Shan L, Shen Z, Wang Z, Yung BC. et al. Tumour microenvironment-responsive semiconducting polymer-based self-assembling nanotheranostics. Nanoscale Horiz. 2019;4:426–33. doi: 10.1039/C8NH00307F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saowalak K, Titipun T, Somchai T, Chalermchai P. Iron(III)-tannic molecular nanoparticles enhance autophagy effect and T1 MRI contrast in liver cell lines. Sci Rep. 2018;8:6647. doi: 10.1038/s41598-018-25108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C, Sang H, Wang Y, Zhu F, Hu X, Wang X. et al. Foe to friend: Supramolecular nanomedicines consisting of natural polyphenols and bortezomib. Nano Lett. 2018;18:7045–51. doi: 10.1021/acs.nanolett.8b03015. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Huang Y, Wang Z, Carniato F, Xie Y, Patterson JP. et al. Polycatechol nanoparticle MRI contrast agents. Small. 2016;12:668–77. doi: 10.1002/smll.201502754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W, Tang Q, Yu T, Li X, Gao Y, Li J. et al. Surfactant-free preparation of Au@resveratrol hollow nanoparticles with photothermal performance and antioxidant activity. ACS Appl Mater Interfaces. 2017;9:3376–87. doi: 10.1021/acsami.6b13911. [DOI] [PubMed] [Google Scholar]
- 102.Sandoval-Acuña C, Ferreira J, Speisky H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 103.Dayem AA, Choi HY, Yang GM, Kim K, Saha SK, Cho SG. The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients. 2016;8:581. doi: 10.3390/nu8090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiang S, Yang P, Guo H, Zhang S, Zhang X, Zhu F. et al. Green tea makes polyphenol nanoparticles with radical-scavenging activities. Macromol Rapid Commun. 2017;38:1700446. doi: 10.1002/marc.201700446. [DOI] [PubMed] [Google Scholar]
- 105.Lu QY, Zhang L, Yee JK, Go VLW, Lee WN. Metabolic consequences of LDHA inhibition by epigallocatechin gallate and oxamate in MIA PaCa-2 pancreatic cancer cells. Metabolomics. 2015;11:71–80. doi: 10.1007/s11306-014-0672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–61. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Perron NR, García CR, Pinzón JR, Chaur MN, Brumaghim JL. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J Inorg Biochem. 2011;105:745–53. doi: 10.1016/j.jinorgbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Mancarella S, Greco V, Baldassarre F, Vergara D, Maffia M, Leporatti S. Polymer-coated magnetic nanoparticles for curcumin delivery to cancer cells. Macromol Biosci. 2015;15:1365–74. doi: 10.1002/mabi.201500142. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Ma W, Tu P. Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci. 2015;15:1252–61. doi: 10.1002/mabi.201500043. [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Zou Q, Yuan C, Li S, Xing R, Yan X. Amino acid coordination driven self-assembly for enhancing both the biological stability and tumor accumulation of curcumin. Angew Chem Int Ed Engl. 2018;57:17084–8. doi: 10.1002/anie.201810087. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Xiao W, Xiao K, Berti L, Luo J, Tseng HP. et al. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew Chem Int Ed Engl. 2012;51:2864–9. doi: 10.1002/anie.201107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montanari E, Gennari A, Pelliccia M, Gourmel C, Lallana E, Matricardi P. et al. Hyaluronan/tannic acid nanoparticles via catechol/boronate complexation as a smart antibacterial system. Macromol Biosci. 2016;16:1815–23. doi: 10.1002/mabi.201600311. [DOI] [PubMed] [Google Scholar]
- 113.Cheng T, Liu J, Ren J, Huang F, Ou H, Ding Y. et al. Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics. 2016;6:1277–92. doi: 10.7150/thno.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu F, Kozlovskaya V, Zavgorodnya O, Martinez-Lopez C, Catledge S, Kharlampieva E. Encapsulation of anticancer drug by hydrogen-bonded multilayers of tannic acid. Soft Matter. 2014;10:9237–47. doi: 10.1039/c4sm01813c. [DOI] [PubMed] [Google Scholar]
- 115.Shutava T, Prouty M, Kommireddy D, Lvov Y. pH responsive decomposable layer-by-layer nanofilms and capsules on the basis of tannic acid. Macromolecules. 2005;38:2850–8. [Google Scholar]
- 116.Kozlovskaya V, Liu F, Xue B, Ahmad F, Alford A, Saeed M. et al. Polyphenolic polymersomes of temperature-sensitive poly(N-vinylcaprolactam)-block-poly(N-vinylpyrrolidone) for anticancer therapy. Biomacromolecules. 2017;18:2552–63. doi: 10.1021/acs.biomac.7b00687. [DOI] [PubMed] [Google Scholar]
- 117.Kozlovskaya V, Xue B, Lei W, Padgett LE, Tse HM, Kharlampieva E. Hydrogen-bonded multilayers of tannic acid as mediators of T-cell immunity. Adv Healthc Mater. 2015;4:686–94. doi: 10.1002/adhm.201400657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pham-Hua D, Padgett LE, Xue B, Anderson B, Zeiger M, Barra JM. et al. Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking. Biomaterials. 2017;128:19–32. doi: 10.1016/j.biomaterials.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Song IT, Lee M, Lee H, Han J, Jang JH, Lee MS. et al. PEGylation and HAylation via catechol: alpha-amine-specific reaction at N-terminus of peptides and proteins. Acta Biomater. 2016;43:50–60. doi: 10.1016/j.actbio.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 120.Shin M, Lee H. Gallol-rich hyaluronic acid hydrogels: Shear-thinning, protein accumulation against concentration gradients, and degradation-resistant properties. Chem Mater. 2017;29:8211–20. [Google Scholar]
- 121.Hu B, Wang Y, Xie M, Hu G, Ma F, Zeng X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J Funct Foods. 2015;15:593–603. [Google Scholar]
- 122.Cui J, Richardson JJ, Björnmalm M, Faria M, Caruso F. Nanoengineered templated polymer particles: Navigating the biological realm. Acc Chem Res. 2016;49:1139–48. doi: 10.1021/acs.accounts.6b00088. [DOI] [PubMed] [Google Scholar]
- 123.Cui J, Björnmalm M, Ju Y, Caruso F. Nanoengineering of poly(ethylene glycol) particles for stealth and targeting. Langmuir. 2018;34:10817–27. doi: 10.1021/acs.langmuir.8b02117. [DOI] [PubMed] [Google Scholar]