Summary
GATA2 is essential for the endothelial-to-hematopoietic transition (EHT) and generation of hematopoietic stem cells (HSCs). It is poorly understood how GATA2 controls the development of human pluripotent stem cell (hPSC)-derived HS-like cells. Here, using human embryonic stem cells (hESCs) in which GATA2 overexpression was induced by doxycycline (Dox), we elucidated the dual functions of GATA2 in definitive hematopoiesis before and after the emergence of CD34+CD45+CD90+CD38– HS-like cells. Specifically, GATA2 promoted expansion of hemogenic precursors via the EHT and then helped to maintain HS-like cells in a quiescent state by regulating cell cycle. RNA sequencing showed that hPSC-derived HS-like cells were very similar to human fetal liver-derived HSCs. Our findings will help to elucidate the mechanism that controls the early stages of human definitive hematopoiesis and may help to develop a strategy to generate hPSC-derived HSCs.
Keywords: GATA2, hESCs, hematopoietic stem cells, definitive hematopoiesis, cell cycle
Graphical Abstract

Highlights
-
•
hESC-derived HS-like cells were highly similar to human fetal liver (FL) HSCs
-
•
Overexpression of GATA2 promoted expansion of hemogenic precursors through EHT
-
•
Overexpression of GATA2 enhanced the maintenance of HS-like cells
-
•
GATA2 overexpression maintained HS-like cells by inducing cell-cycle arrest
In this article, Professor Ma and his colleagues at IBT of CAMS & PUMC show that overexpression of GATA2 promoted expansion of hemogenic precursors through EHT, and thereafter enhanced the maintenance of HS-like cells in dormancy by cell-cycle arrest. hESC-derived HS-like cells were highly similar to human fetal liver (FL) HSCs.
Introduction
Primitive hematopoiesis initiates in the yolk sac with a transient wave of primitive erythroid progenitors; however, definitive hematopoiesis occurs via a temporally and spatially dynamic pattern (Palis et al., 1999). The definitive hematopoiesis firstly generates erythroid with adult-type features but does not produce hematopoietic stem cells (HSCs) in the yolk sac (Böiers et al., 2013). The second wave of definitive hematopoiesis is marked by the emergence of HSCs originated from hemogenic endothelium (HE) in the aorto-gonad-mesonephros (AGM) region of the embryo through the endothelial-to-hematopoietic transition (EHT) (Boisset et al., 2010, Ivanovs et al., 2011, Medvinsky and Dzierzak, 1996, Swiers et al., 2013, Taoudi et al., 2008), while more pre-HSCs generated from AGM region move to the fetal liver (FL) and then acquire a mature HSC property (Rybtsov et al., 2016). Developmental hematopoiesis has been mainly studied in mouse models, but early human hematopoiesis is poorly understood. The establishment of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) led to the development of models that are ideal to explore early hematopoiesis in humans (Costa et al., 2012, Takahashi et al., 2007, Thomson et al., 1998, Yoder, 2014). Several recent studies reported the development of hPSC-derived early mesodermal precursors into mature hematopoietic stem/progenitor cells (HSPCs) in vitro (Ng et al., 2016, Slukvin, 2016). The latter cells can be traced as CD34+CD43+CD45+CD90+CD38– cells; these surface markers are typical of human HSCs emerging in AGM and FL in vivo (Rowe et al., 2016). Although phenotypically similar to their adult-type counterparts in AGM, hPSC-derived HSPCs generated in vitro cannot reconstitute hematopoiesis in NOG-SCID mice (Ng et al., 2016). Gene manipulation has been performed to enhance the HSC property of hPSC-derived HS-like cells. Overexpression of medial HOXA genes prolongs the maintenance of hESC-derived HSPCs but does not confer self-renewal in hESC-derived HSPCs (Dou et al., 2016). A more recent study showed that hESC-derived CD34+KDR+CD43−GPA– HE cells with a definitive hematopoietic potential reconstitute hematopoiesis in NOG-SCID mice when transfected with five transcription factors (HOXA9, ERG, RORA, SOX4, and MYB) (Sugimura et al., 2017). However, this previous study did not purify HSCs in vitro. It remains challenging to generate hPSC-derived HSCs in vitro.
The transcription factor GATA2 plays a unique role in the development, maintenance, and quiescence of HSCs, particularly in the AGM. In mouse embryos, Gata2 is expressed in lateral plate mesoderm (LPM)-, AGM-, and FL-derived HSCs (Minegishi et al., 1999, Minegishi et al., 2003). Knockout (KO−/–) of Gata2 blocks the development of murine HSCs in the AGM region. Deletion of a cis element located 9.5 kb downstream of the Gata2 promoter (+9.5) in mice decreases Gata2 expression in HE in AGM, and abrogates the EHT (Gao et al., 2013). RNA sequencing (RNA-seq) demonstrated that GATA2 and other HSC-specific genes are upregulated in human CD34+CD45+CD38– (34+45+38–) HSPCs derived from HE-5TF cells (Sugimura et al., 2017). GATA2-knockout hESCs exhibit marked defects in the EHT and conversion into HSPCs, while specification of HE is unaffected (Kang et al., 2018). By contrast, GATA2/eGFP+ hESCs give rise to an increased number of HSPCs (Huang et al., 2016). During the development of hPSCs into HSPCs in vitro, expression of GATA2 is upregulated along with the KDR+APLNR+CD34−CD43– early mesodermal precursors (Choi et al., 2012). On the other hand, overexpression of GATA2 (GATA2-OE) hampers, rather than enhances, HSC activity in human cord blood (CB)-derived CD34+ HSCs by inducing cell-cycle arrest at the G0/G1 phase (Tipping et al., 2009). Thus, GATA2 has diverse effects on the early stages of mesodermal hematopoietic lineage specification, the EHT, and maintenance of HSCs. It is unknown how GATA2 elicits these effects and at what level GATA2 influences the cell fate decision.
We previously reported an efficient coculture system with AGM-S3 cells that facilitates definitive hematopoiesis of hPSCs in vitro (Mao et al., 2016, Wang et al., 2018). In the current study, we established hESCs in which GATA2-OE was induced by treatment with doxycycline (Dox) using a PiggyBac (PB)-based Tet-on system, and the functional role of GATA2 in the early stages of hematopoiesis was analyzed. Our data revealed that GATA2 functions at different time points to initiate, enhance, and maintain definitive hematopoiesis of hESCs by regulating the cell cycle. Specifically, GATA2 exerts dual functions by promoting the generation of definitive hematopoietic progenitors before and after the EHT and arresting CD34+CD45+CD90+CD38– (34+45+90+38–) HS-like cells in a quiescent state.
Results
Generation of an hESC Line with Inducible GATA2 Overexpression
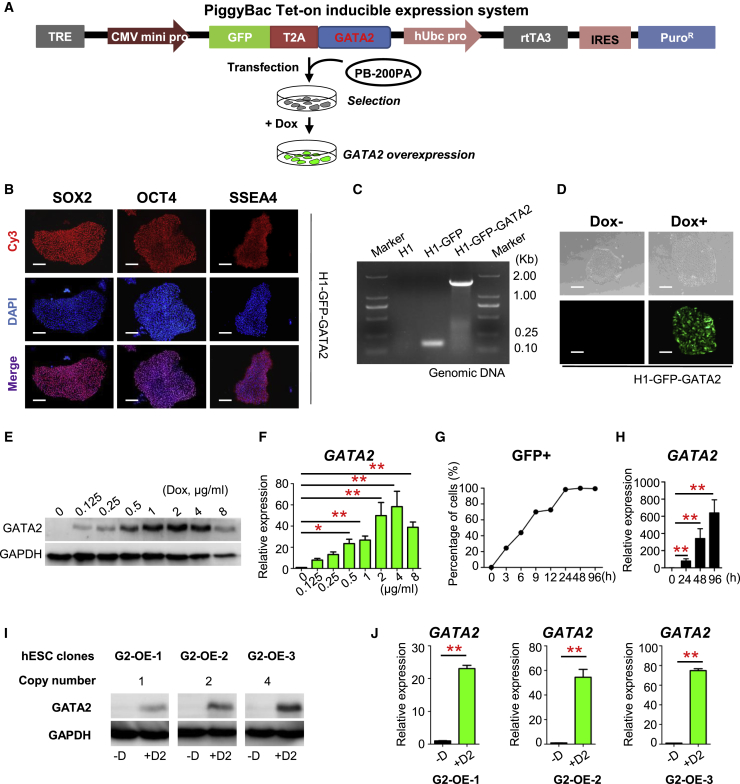
To explore the function of GATA2 in hematopoietic fate determination, we generated H1-GFP-GATA2 hESCs (G2/hESCs) in which exogenous expression of GATA2 was induced on treatment with Dox using a previously described PB-based Tet-on system (Figure 1A) (Chen et al., 2017). In the absence of Dox, G2/hESCs expressed key stemness-specific markers, such as SOX2, OCT4, and SSEA4 (Figure 1B). PCR analysis verified that the coding sequence (CDS) of GATA2 was integrated into the genome of G2/hESCs (Figure 1C). G2/hESCs could be repeatedly passaged and retained a hESC phenotype without Dox (Figure 1D). Western blotting and qRT-PCR showed increased expression of GATA2 in G2/hESCs and AGM-S3 cocultures (G2/hESC cocultures) with increased concentration of Dox from 0 to 4 μg/mL (Figures 1E and 1F). Treatment with Dox (1μg/mL) increased the percentage of GFP+ cells and the mRNA expression of GATA2 in G2/hESCs from 0 to 96 h (Figures 1D, 1G, and 1H). There were three hESC clones used for the experiments with 1, 2, and 4 copies of transgene detected by qPCR, respectively (Figure 1I) (Christodoulou et al., 2016); The three hESC clones showed increased expression of GATA2 mRNA and protein with increased copies of transgene detected by qRT-PCR and western blotting (Figures 1I and 1J). These results demonstrated that G2/hESCs were pluripotent and had a normal phenotype, that the CDS of GATA2 was integrated into the genome of these cells, and that treatment with Dox-induced GATA2-OE from 0 to 4 μg/mL.
Figure 1.
Generation and Analysis of G2/hESCs
(A) Schematic illustration of the PB Tet-on inducible expression system and the strategy used to establish G2/hESCs.
(B) Immunofluorescence staining of pluripotency markers in G2/hESCs. Scale bars, 50 μm.
(C) Confirmation of homologous recombination in G2/hESCs by PCR. Genomic DNA was isolated from wild-type hESCs, GFP-expressing hESCs, and G2/hESCs.
(D) Fluorescence and phase-contrast images of G2/hESCs cultured in mTeSR1 medium containing and lacking Dox. Scale bars, 50 μm.
(E) Western blotting and qRT-PCR analysis of GATA2 expression in various concentrations of Dox (0–8 μg/mL)-treated G2/hESCs and AGM-S3 cocultures (G2/hESC cocultures) on day 7.
(F) qRT-PCR analysis of GATA2 expression in various concentrations of Dox (0–8 μg/mL)-treated G2/hESCs and AGM-S3 cocultures (G2/hESC cocultures) on day 7.
(G) Flow cytometric analysis of the percentage of GFP+ cells among Dox-treated G2/hESCs.
(H) qRT-PCR analysis of GATA2 expression in G2/hESCs treated with Dox for 0–96 h.
(I and J) Western blotting (I) and qRT-PCR (J) analysis of GATA2 expression in G2/hESC cocultures on day7 derived from three different G2/hESC clones with various transgene copy number.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
GATA2 Overexpression in hESCs Promotes Definitive Hematopoiesis
We firstly elucidated the hematopoietic cells in the H1 hESCs and AGM-S3 cocultures (Figure 2A). KDR+ cells, which are mesodermal precursors of hematopoietic and endothelial lineages, gradually express CD34 during human embryonic development (Cortés et al., 1999). In H1 hESCs and AGM-S3 coculture, the KDR+CD34−CD43– (K+34–43–) cells mainly expressed mesodermal proteins and genes compared with the KDR+CD34+CD43– (K+34+43–) cells (Figures S1A and S1B). CD43 was previously used as a marker of hematopoietic progenitors in cocultures of hPSCs and OP9 cells (Vodyanik et al., 2006). CD34+CD43+ (34+43+) cells expressed markers of hematopoietic progenitors and upregulated hematopoietic genes compared with CD34+CD43– (34+43–) cells (Figures S1C and S1D). In cocultures of H1 hESCs and AGM-S3 cells, the former cells differentiate into (APLNR+KDR−/+CD34–, A+K−/+34–) mesodermal progenitors (CD34+CD43–, 34+43–) endothelial cells, and (CD34+CD43+CD45−/+, 34+43+45−/+) hematopoietic progenitors in a stepwise manner (Figures S1E and S1F), as occurs in other hematopoietic systems (Slukvin, 2013). GATA2 expression was upregulated during hematopoiesis (Figure S1G). GATA2 expression in 34+43+ and 34+45+ hematopoietic cells derived from H1 hESCs was comparable with that in human CB-derived CD34+ cells (Figure S1H). These data demonstrate that the AGM-S3 stromal cell line efficiently induced hematopoietic differentiation of hESCs and simulated the early stages of hematopoiesis (Figure S1I) (Mao et al., 2016, Wang et al., 2018). Expression of GATA2 increased during the development of 34+43+45−/+ hematopoietic cells from hESCs.
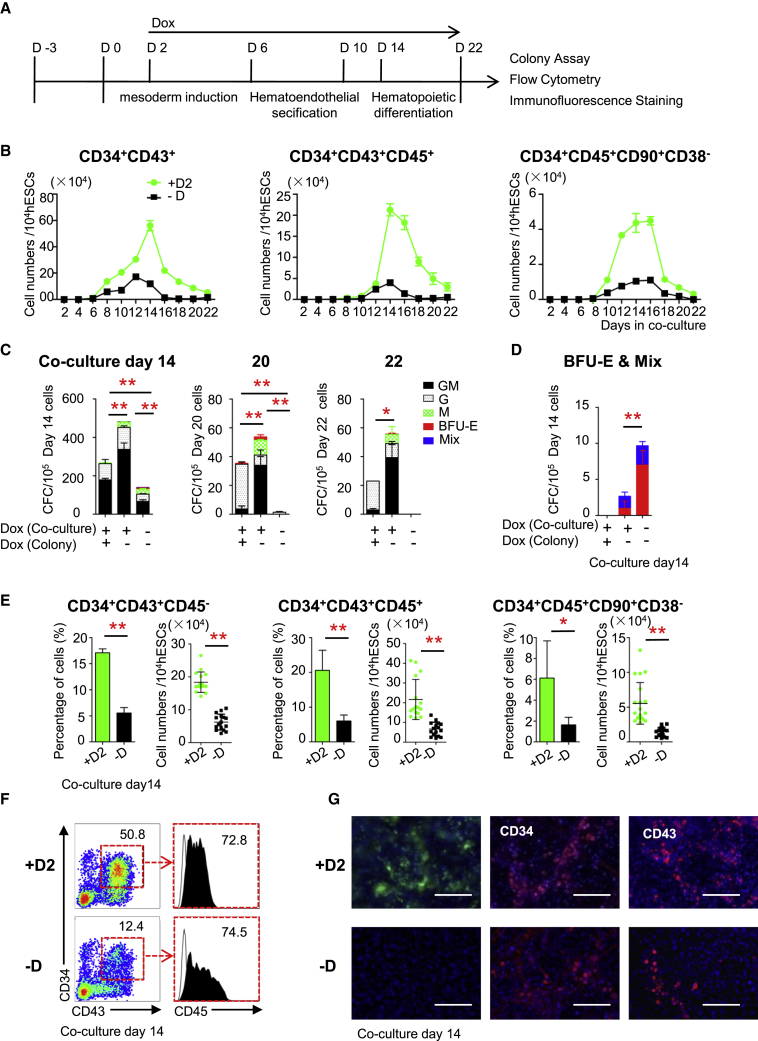
Figure 2.
GATA2-OE in hESCs Promotes Definitive Hematopoiesis
(A) Experimental scheme to study the stage-wise effect of GATA2 on hematopoietic development.
(B) Absolute numbers of 34+43+, 34+43+45+, and 34+45+90+38− cells in untreated and Dox-treated G2/hESC cocultures on days 2–22.
(C) Colony formation by total cells collected from untreated and Dox-treated G2/hESC cocultures on days 14, 20, and 22.
(D) Formation of BFU-E and Mix colonies by total cells collected from untreated and Dox-treated G2/hESC cocultures on day 14.
(E) Percentages and absolute numbers of 34+43+45–, 34+43+45+, and 34+45+90+38– cells in untreated and Dox-treated G2/hESC cocultures on day 14.
(F) Flow cytometric analysis of 34+43+45+ cells in untreated and Dox-treated G2/hESC cocultures on day 14.
(G) Immunofluorescence staining of CD34 and CD43 in untreated and Dox-treated G2/hESC cocultures on day 14. Scale bars, 100 μm.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
We further evaluated the role of GATA2 in hematopoiesis by three different culture systems. The hematopoietic efficiency in the promotion of 34+45+90+38– cells was improved in AGM-S3 coculture than in OP9 and stromal cell-free culture (Figure S1J). Transition of 34+K+43– cells into 34+43+ cells was predominantly enhanced in AGM-S3 cocultures (Figure S1K). A comparable increase of GATA2 expression was paralleled to the increase of Dox dose from 0 to 4 μg/mL, accompanying by an enhanced promotion of 34+43+ cells in G2/hESC cocultures (Figure S2A). We found 1 μg/mL Dox was enough to trigger GATA2 overexpression, and this dose was used in further experiments. We found that all three hESC lines with different transgene copy numbers demonstrated similar efficacy in promoting hematopoiesis; however, there was a slight difference in enhancement (Figures S2B and S2C). On the other hand, the vector control hESCs showed no difference in differentiation with and without Dox (Figure S2C). To identify barriers to the generation of hematopoietic cells from GATA2-overexpressing hESCs, we analyzed the proportion of hematopoietic cells among G2/hESC cocultures at days 6, 10, and 14, when Dox treatment was initiated at different time points (Figure S2D). Addition of Dox from day 2 until the day of collection promoted the development of K+34–43–, 34+K+43–, 34+43+, and 34+43+45+ cells (Figure S2D).
We next evaluated the effect GATA2-OE on hematopoiesis from day 2 to 22. The increased level of GATA2 expression mainly relied on exogenous GATA2 expression both at the various times of differentiation and in the 34+43+-sorted cells with Dox (Figures S2E and S2F). The total cell number was not markedly affected (data not shown). GATA2-OE prolonged the growth of 34+43+45−/+ and 34+45+90+38– cells in cocultures over 22 days (Figures 2B and S2G). Moreover, GATA2-OE increased the generation of 34+45+90+38– HS-like cells by more than 4.8-fold in cocultures. Total cells collected from Dox-treated cocultures on days 14, 20, and 22 formed an increased number of colonies, which coincided with the increase in hematopoietic cells; however, more colonies were observed when Dox was removed during the colony-formation assay (Figure 2C). BFU-E and Mix colonies recovered when Dox was removed in cloned cultures (Figure 2D).
Fluorescence-activated cell sorting (FACS) analysis demonstrated that the levels of 34+43+45– cells (pre-HSPCs), 34+43+45+ cells (HSPCs), and 34+45+90+38– cells (HS-like cells) were increased in Dox-treated cocultures at day 14 (Figures 2E and 2F). Immunofluorescence staining of CD34 and CD43 showed that dense networks of blood vessels and many hematopoietic cells formed in Dox-treated cocultures on day 14 (Figure 2G).These data demonstrate that GATA2-OE promotes the generation of hematopoietic cells and prolongs the colony-forming unit (CFU) potential of cocultured cells.
GATA2 Overexpression Promotes Definitive Hematopoiesis via Differentiation of hESC-Derived Mesodermal KDR+CD34– (K+34–) Cells and the EHT
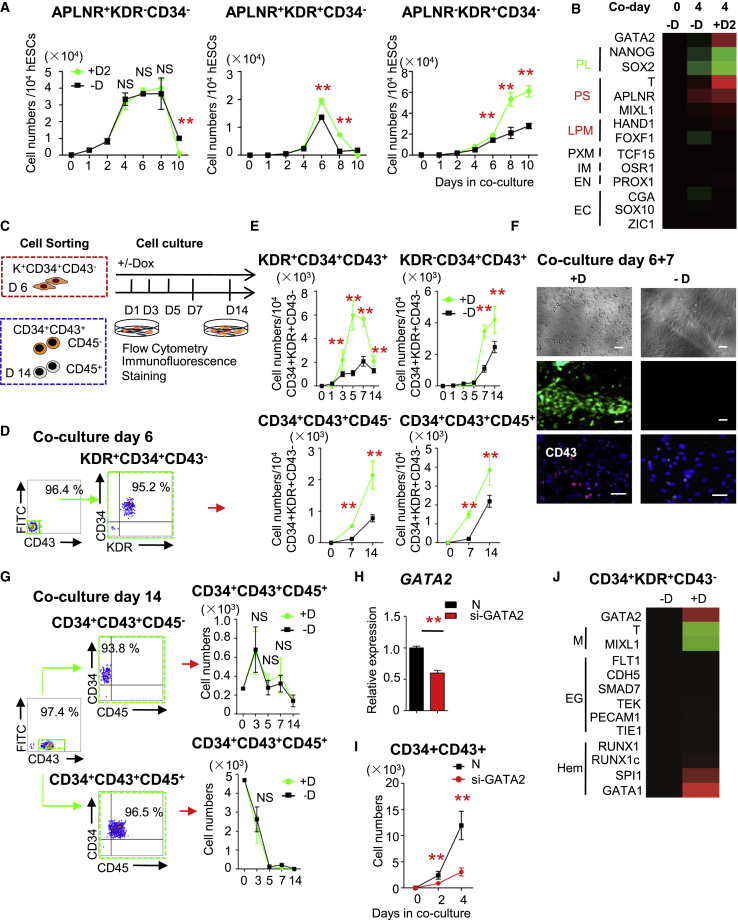
The percentage and number of early mesodermal cells with an A+K–34– phenotype did not markedly differ. However, the number of K+34– cells, including definitive mesodermal cells with an A+K+34– phenotype, was higher in Dox-treated cocultures than in untreated cocultures (Figures 3A and S3A). Expression of primitive streak (PS) and LPM genes increased on Dox treatment (Figure 3B). Thus, GATA2-OE promoted the generation of hESC-derived mesodermal K+34– cells, including A+K+34– and A−K+34– cells.
Figure 3.
GATA2-OE Promotes Development of 34+43+45+ HSPCs via Mesodermal Differentiation and the EHT
(A) Absolute numbers of A+K–34–, A+K+34–, and A−K+34– cells in untreated and Dox-treated G2/hESC cocultures on days 0–10.
(B) Heatmap of expression of genes associated with the development of germ layers in untreated and Dox-treated G2/hESC cocultures on day 4. PL, pluripotency genes; PS, primitive streak genes; LPM, lateral plate mesoderm genes; PXM, paraxial mesoderm genes; IM, intermediate mesoderm genes; EN, endodermal genes; EC, ectodermal genes.
(C) Experimental scheme. 34+K+43− cells and 34+43+45–/+cells were isolated from coculture at days 6 and 14, respectively, then recultured for an additional 14 days onto AGM-S3-coated plates with and without Dox.
(D) Flow cytometric analysis of K+34+43– cells sorted from G2/hESC cocultures on day 6.
(E) The K+34+43– cells sorted from G2/hESC cocultures on day 6 were recultured on AGM-S3 cells with and without Dox. The numbers of K+/–34+43+ and 34+43+45−/+ cells were determined by flow cytometry.
(F) Microscopic analysis and immunofluorescence staining of CD43 in K+34+43– cells cocultured with AGM-S3 cells with and without Dox for 7 days. Scale bars, 50 μm.
(G) 34+43+45– and 34+43+45+ cells were sorted from G2/hESC cocultures on day 14 and recultured on AGM-S3 cells for 14 days with and without Dox. The numbers of 34+43+45+ cells were determined by flow cytometry.
(H) qRT-PCR analysis of relative GATA2 expression in siRNA-transfected cells from day 4 G2/hESC cocultures.
(I) The absolute number of 34+43+ cells in cocultures treated with si-GATA2 (20 nM) or control siRNA for 2 and 4 days.
(J) Heatmap of gene expression in 34+K+43– cells derived from untreated and Dox-treated G2/hESC cocultures on day 6. M, mesodermal genes; EG, endothelial genes; Hem, hematopoietic genes.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
The increase in 34+43+ cells in cocultures on GATA2-OE may be because of the promotion of EHT and after EHT. To determine whether GATA2-OE promotes EHT, 34+K+43– cells were sorted from cocultures on day 6 and recultured on AGM-S3 cells in the presence or absence of Dox (Figures 3C and 3D). 34+K+43– cells gave rise to more 34+43+ cells, including 34+43+K+/– and 34+43+45−/+ cells, in the presence of Dox (Figures 3E and S3B). Immunofluorescence staining revealed that 34+K+43– cells gave rise to more CD43+ cells on GATA2-OE for 7 days (Figure 3F). To investigate the effect of GATA2-OE on expansion of 34+43+45– and 34+43+45+ cells after the EHT, both cell populations were sorted from cocultures on day 14 and recultured on AGM-S3 cells (Figure 3C). GATA2-OE did not markedly affect the expansion of 34+43+45+ cells (Figure 3G).
To further elucidate the effect of GATA2-OE on EHT, cocultures on day 4, which lacked emerging CD43+ cells, were transfected with GATA2-targeting (si-GATA2: GUCCAAGAAGAGCAAGAAATT) or control siRNA (si-N:UUCUCCGAACGUGUCACGUTT) (Figure S3C). Transfection of 20 nM si-GATA2 significantly decreased endogenous GATA2 expression by more than 42.3% (Figure 3H). Downregulation of GATA2 decreased the emergence of 34+43+ cells (Figures 3I and S3C).
Relative gene expression profiles differed between 34+K+43– cells sorted from untreated and Dox-treated cocultures on day 6. Hematopoietic genes were upregulated in 34+K+43– cells on GATA2-OE (Figure 3J).
These data suggest that GATA2-OE promotes the generation of 34+43+ cells through the EHT in cocultures of hESCs and AGM-S3 cells, and that GATA2 is essential for the EHT during hematopoiesis.
GATA2 Overexpression Inhibits the Differentiation of 34+45+ HSPCs
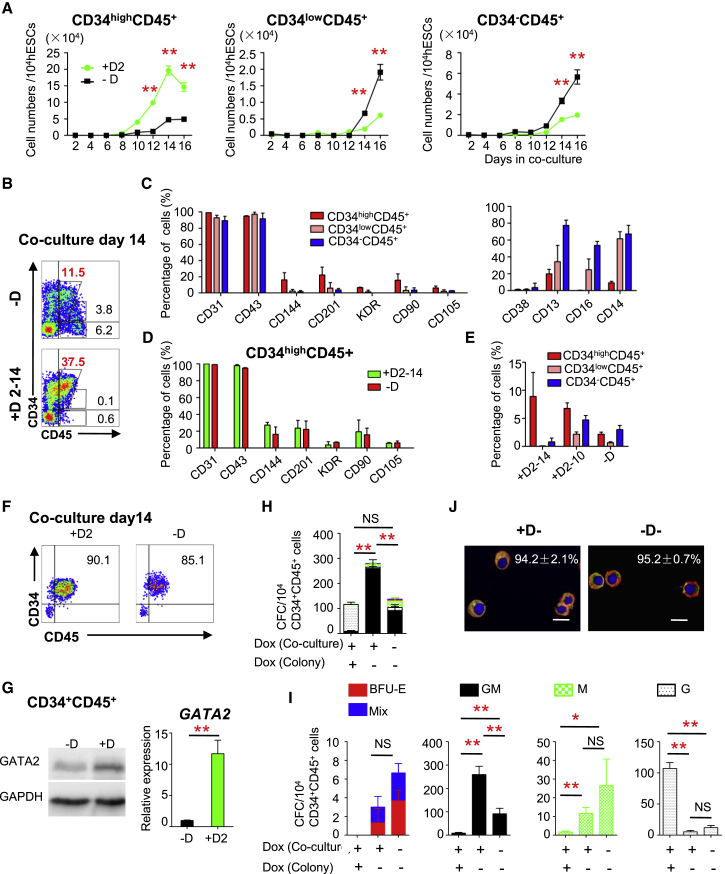
GATA2-OE increased production of CD34highCD45+ (34high45+) cells and inhibited production of 34low45+ and 34–45+ cells until day 16 of coculture (Figures 4A and S4A). Flow cytometric analysis demonstrated that the percentages of cells positive for CD144, CD201, KDR, CD90, and CD105 were higher among 34high45+ cells than among 34low45+ and 34–45+ cells, whereas the percentages of cells positive for lineage-specific markers such as CD38, CD13, CD16, and CD14 were lower (Figures 4B and 4C). GATA2-OE increased production of 34high45+ cells and inhibited their differentiation into 34low45+ and 34–45+ cells (Figures 4A, 4B, and S4A). GATA2-OE did not obviously change the immunophenotype of 34high45+ cells (Figure 4D). When Dox was removed from day 10, differentiation of 34low45+ and 34–45+ cells was restored on day 14 (Figure 4E). Thus, GATA2-OE promoted the generation of 34high45+ cells and inhibited their differentiation.
Figure 4.
GATA2-OE Inhibits the Differentiation of CD34+CD45+ HSPCs
(A) Absolute numbers of 34high45+, 34low45+, and 34–45+ cells in untreated and Dox-treated G2/hESC cocultures on days 2–16.
(B) Flow cytometric analysis of 34+45+ cells in untreated and Dox-treated G2/hESCs co-cultures on day 14.
(C) Flow cytometric analysis of the subphenotype of 34high45+, 34low45+, and 34−45+ cells in Dox-untreated G2/hESC cocultures on day 14.
(D) Flow cytometric analysis of the subphenotype of 34high45+ cells in untreated and Dox-treated G2/hESC cocultures on day 14.
(E) The percentage of 34high45+, 34low45+, and 34–45+ cells in untreated and Dox-treated G2/hESC cocultures on day 14.
(F–J) Properties of 34+45+ cells sorted from G2/hESC cocultures on day 14. (F) Flow cytometric analysis of 34+45+ cells sorted from untreated and Dox-treated cocultures on day 14. (G) Western blotting and RT-qPCR analysis of GATA2 in 34+45+ cells sorted from untreated and Dox-treated cocultures on day 14. (H–I) Colony formation by 34+45+ cells sorted from untreated and Dox-treated cocultures on day 14. (J) β-Globin expression in BFU-E colonies derived from 34+45+ cells with and without Dox. Scale bars, 20 μm.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
To further elucidate the effect of GATA2-OE on differentiation of 34+45+ cells, these cells were sorted from cocultures on day 14 (Figure 4F). Western blotting and qRT-PCR analysis demonstrated that GATA2 expression was upregulated in 34+45+ cells sorted from Dox-treated cocultures (Figure 4G). Removal of Dox increased the numbers of BFU-E, Mix, GM, and M colonies, while GATA2-OE increased the number of G colonies (Figures 4H and 4I). More CD71+GPA+ erythroid cells were detected by FACS analysis when Dox was removed during the colony-formation assay (Figure S4B). BFU-E formed from GATA2-overexpressing cocultured cells generated erythroid cells that predominantly expressed adult β-globin (Figure 4J). These data demonstrate that GATA2-OE inhibits the differentiation of hematopoietic cells.
To further investigate the effect of GATA2-OE on the differentiation of 34+45+ cells, we recultured 34+45+ cells on day 14 in IMDM containing 10% fetal bovine serum (FBS) and seven cytokines. More cells with a 34–45+ phenotype were produced in the presence of Dox (Figures S4C and S4D). Upon suspension of cultures to induce differentiation, Dox treatment decreased the percentage of CD163+CD14+ cells (macrophages), but increased the percentages of c-KIT+CD45+ (mast cells) and Siglec-8+CD88+ cells (eosinophils) (Figures S4C–S4E).
Thus, GATA2-OE increases production of 34+43+45+ cells, but prevents their differentiation in cocultures and the colony-formation assay. By contrast, GATA2-OE increases the granulocytic and mast cell potential of 34+45+ cells in suspension culture and the colony-formation assay.
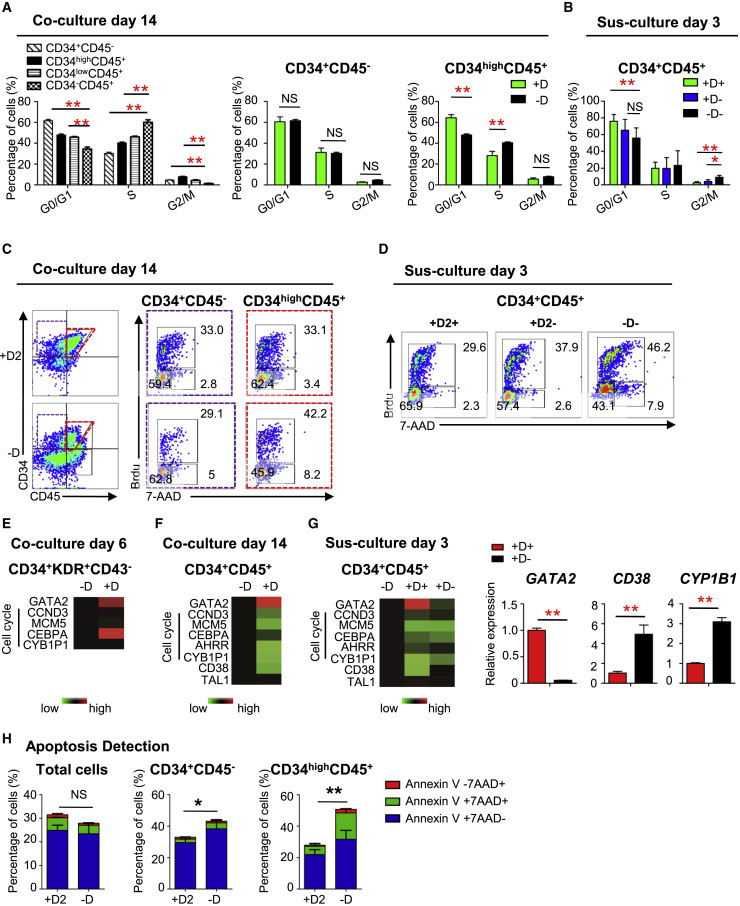
GATA2 Overexpression Maintains HSPCs by Inducing Cell-Cycle Arrest
5′-Bromo-2′-deoxyuridine (BrdU) incorporation analysis suggestd that 34+45– cells differentiated into 34high45+ cells, which was accompanied by a decreased percentage of cells in G0/G1 phase and an increased percentage of cells in S stage (Figure 5A). The cell cycle of 34+45– cells did not obviously differ between untreated and Dox-treated cocultures at day 14 (Figure 5A). However, the cell cycle of 34+45+ cells differed between untreated and Dox-treated samples at day 14 of coculture and day 3 of suspension culture (Figures 5A–5D). The decreased generation of hematopoietic cells from 34+45+ cells on Dox treatment was due to induction of quiescence, while removal of Dox promoted progression of 34+45+ cells into S and G2/M phase (Figures 5B and 5D).
Figure 5.
GATA2-OE Promotes the Maintenance and Inhibits the Differentiation of HSPCs by Inducing Cell-Cycle Arrest
(A–D) Flow cytometric analysis and quantification of BrdU incorporation and 7-AAD staining to determine the cell-cycle distribution of (A and C) 34+45+ HSPCs on day 14 of coculture and (B and D) day 3 of suspension culture.
(E–G) Gene expression profile of 34+K+43– cells (E) on day 6 of coculture, and (F) 34+45+ cells on 14 of coculture and (G) day 3 of suspension culture.
(H) Apoptosis detection of hematopoietic cells from G2/hESC cocultures on day14 with and without Dox through flow cytometric analysis.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
Despite confirming GATA2-OE in 34+K+43– and 34+45+ cells treated with Dox, qRT-PCR analysis demonstrated that the expression of cell-cycle-related genes was markedly altered in the latter cells (Figures 5E–5G). Expression of genes encoding cell-cycle activators, such as CCND3, MCM5, and CEBPA, was decreased in GATA2-OE 34+45+ cells. In addition, AHRR and CYB1P1 expression were reduced in GATA2-OE 34+45+ cells (Figures 5F and 5G). On removal of Dox from suspension cultures, expression of GATA2 decreased in 34+45+ cells, while that of CD38 and CYB1P1 increased (Figure 5G). Enforced GATA-2 expression showed no increase in apoptosis of coculture cells. GATA2-OE inhibited apoptosis in CD34highCD45+ cells on coculture day14 (Figure 5H). These data suggest that GATA2-OE prevents the expansion and differentiation of 34+45+ cells isolated from cocultures on day 14 by inducing quiescence and inhibiting apoptosis.
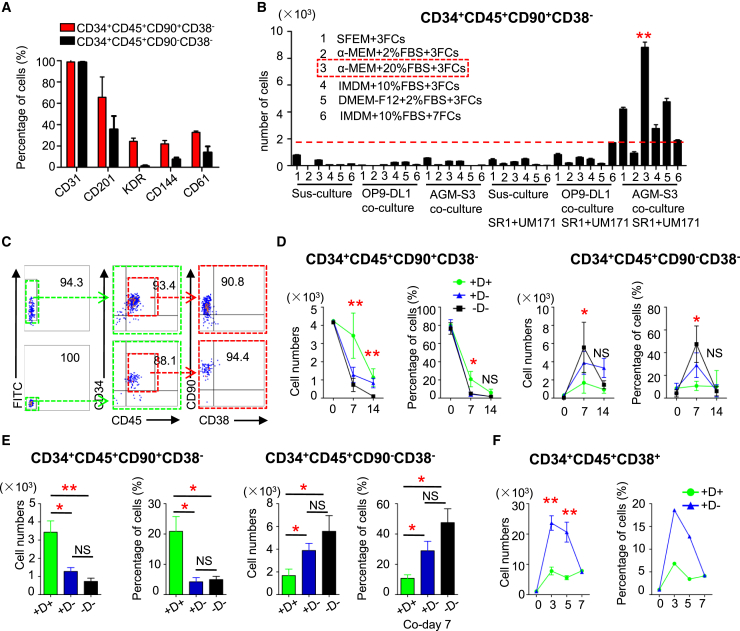
GATA2 Overexpression Selectively Promotes the Maintenance of 34+45+90+38– HS-like Cells
CD90+ cells were enriched among 34high45+ cells (Figure 4C). The percentages of cells positive for endothelial markers, such as CD201, KDR, CD144, and CD61, were higher among 34+45+90+38– HS-like cells than among 34+45+90–38– cells (Figure 6A). A two-step differentiation protocol was applied to develop HS-like cells from H1 hESC cocultures on day14 (Figure 6B). Medium was replaced by α-minimum essential medium containing 20% FBS, thrombopoietin, stem cell factor, and FMS-like tyrosine kinase 3 ligand (FL), which supports the maintenance of multipotent human HS-like cells (Figure 6B). To investigate the functional properties of GATA2-OE HS-like cells, 34+45+90+38– HS-like cells were sorted and replated onto AGM-S3 cells (Figure 6C). Although 34+45+90+38– cells were detected in the presence of Dox, the percentage of these cells rapidly decreased when Dox was removed (Figures 6D, 6E, and S5A). To further elucidate the effect of GATA2-OE on differentiation of hematopoietic cells, cocultured cells on day 14 were cultured in differentiation medium and their ability to differentiate into CD34+CD45+CD38+ (34+45+38+) progenitors was investigated (Figures 6F and S5B). The percentage of 34+45+38+ cells by FACS and relative expression of CD38 in 34+45+ cells by qRT-PCR increased when Dox was removed (Figures 5G, 6F, and S5B).
Figure 6.
GATA2-OE Selectively Promotes the Maintenance of 34+45+90+38– HS-like Cells
(A) Flow cytometric analysis of the subphenotype of 34+45+90+38– and 34+45+90–38– cells from Dox untreated G2/hESC cocultures on day 14.
(B) Analysis of the maintenance of HS-like 34+45+90+38– cells derived from H1 hESC cocultures on day 14. The absolute number of 34+45+90+38– cells on day 14 + 7 is shown.
(C) Flow cytometric analysis of 34+45+90+38– HS-like cells sorted from untreated and Dox-treated G2/hESC cocultures on day 14.
(D and E) Percentages and absolute numbers of (D) 34+45+90+38– and (E) 34+45+90–38– cells derived from sorted 34+45+90+38– cells treated with and without Dox for 14 days.
(F) Expansion of 34+45+38+ cells derived from untreated and Dox-treated G2/hESC cocultures on day 14 in medium containing FBS and cytokines.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
GATA2-OE promoted the development and maintenance of 34+45+90+38– HS-like cells and inhibited their differentiation into 34+45+38+ hematopoietic cells. These data show that GATA2 selectively promotes the development and maintenance of HS-like cells when expressed in the correct cellular context.
GATA2 Overexpression Maintains 34+45+90+38– HS-like Cells by Regulating Expression of Cell-Cycle-Related Genes
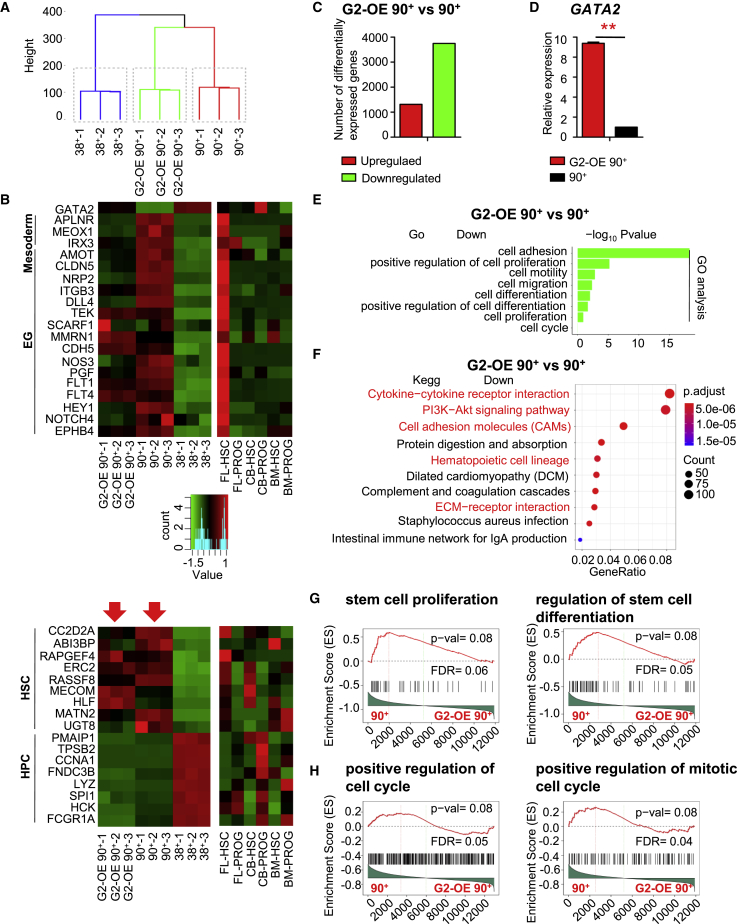
To dissect the transcriptional features of 34+45+90+38– HS-like cells, we isolated 34+45+90+38– and 34+45+38+ cells from Dox-treated and -untreated G2/hESC cocultures and performed RNA-seq. Thereafter, we compared the transcriptional profiles of these cells with those of HSCs (CD34+CD38−CD90+CD45RA−) and progenitors (CD34+CD38+) derived from FL, CB, and bone marrow (BM) (Cesana et al., 2018).
Unsupervised hierarchical clustering of GATA2-OE 34 + 45+90 + 38− cells (G2-OE 90+), 34 + 45+90 + 38− cells (90+) and CD34+CD45+CD38+ cells (38+) showed that G2-OE 90+ and 90+ were positioned next to each other (Figure 7A).
Figure 7.
GATA2-OE Maintains 34+45+90+38– HS-like Cells by Regulating Expression of Cell-Cycle-Related Genes
(A) Unsupervised hierarchical clustering of RNA-seq data of 34+45+38+ (38+), 34+45+90+38– (90+) and G2-OE 34 + 45+90 + 38− (G2-OE 90+) cells. RNA-seq of 38+, 90+, and G2-OE 90+ cells was performed on day 14 of G2/hESC cocultures. Data are from three representative experiments (GEO: GSE122207).
(B) Heatmaps showing mesodermal, endothelial, HSC, and hematopoietic progenitor cell (HPC) genes that were differentially expressed in G2-OE 90+, 90+, and 38+ cells. The scale is log2 RPKM (reads per kilobase of transcript per million). The data are from three FACS experiments. The heatmaps showed significant differences in gene expression in 90+ and 38+ cells, p < 0.05. Expression of sample genes previously reported to be regulators of mesodermal cells, endothelial cells, HSCs, and HPCs is shown. Expression profiles were investigated in HSCs and progenitor cells derived from FL, CB, and BM (Cesana et al., 2018).
(C) Numbers of differentially expressed genes in G2-OE 90+ cells (fold change >2, p < 0.05).
(D) qRT-PCR analysis of GATA2 expression in 90+ cells and G2-OE 90+ cells.
(E and F) (E) Gene ontology categories of biological processes and (F) KEGG signaling pathways that were significantly downregulated in G2-OE 90+ cells.
(G and H) Gene set enrichment analysis of (G) the stem cell proliferation and differentiation and (H) positive regulation of mitotic cell-cycle genes were repressed in G2-OE 90+ cells.
Error bars represent mean ± SD of samples from at least three independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01.
Gene expression patterns of hematopoietic markers, cell surface markers and HSC regulators were very similar in 34 + 45+90 + 38− cells, 34 + 45+38+ cells and that cells derived from FL, CB, and BM (Figures 7B and S6A). Expression of mesodermal and endothelial genes was similar in 90+ cells and FL-derived HSCs (Figure 7B). Taken together, the profiling data suggest that 90+ cells generated in vitro are transcriptionally very similar to HSCs and are most similar to human FL-derived HSCs.
HSC-specific gene expression was similar in untreated and Dox-treated hESC-derived HS-like cells, suggesting that GATA2-OE did not affect the HSC properties of these cells (Figure 7B). We next assessed how GATA2-OE affects the proliferation and differentiation of 90+ cells. In total, 5,058 genes (3,744 downregulated and 1,314 upregulated) were significantly differentially expressed between G2-OE 90+ and 90+ cells (2.85-fold; Figures 7C and 7D). Repressed genes in G2-OE 90+ cells were mostly enriched in gene ontology categories related to cell adhesion, motility, migration, proliferation, and differentiation (Figure 7E). Kyoto Encyclopedia of Genes and Genome (KEGG) analysis suggestd that several pathways important for regulation of HSCs were downregulated in G2-OE 90+ cells (Figure 7F). Gene set enrichment analysis (GSEA) showed that the stem cell proliferation and differentiation genes were repressed in G2-OE 90+ cells (Figures 7G and S6B). GSEA showed that the positive regulation of mitotic cell-cycle and apoptosis genes were repressed in G2-OE 90+ cells (Figures 7H and S6C). Expression of genes related to the AHR signaling pathway, which was previously reported to be inhibited by SR1 (Rentas et al., 2016), was also inhibited in G2-OE 90+ cells (Figure S6D).
Discussion
The mechanism that controls the development and maturation of hPSC-derived HSCs during definitive hematopoiesis remains to be elucidated, and its characterization is hindered by the lack of a serial observation method. GATA2 is expressed in a spatial- and temporal-dependent manner during definitive hematopoiesis and is thus a good marker to monitor early hematopoiesis of hPSCs. Using a PB-based Tet-on system to monitor GATA2-OE at different stages of hematopoiesis, we found that GATA2 plays dual roles before and after the emergence of 34+43+45+ HSPCs during definitive hematopoiesis.
Lugus et al. (2007) reported that GATA2-OE promotes the generation of mesodermal cells from mouse embryonic stem cells. Forced expression of GATA2 stimulates hematopoietic specification at the mesodermal stage in a cell-autonomous manner in Xenopus (Maeno et al., 1996). However, knockout of GATA2 in hESCs does not markedly affect specification of mesodermal and endothelial lineages before hematopoiesis (Kang et al., 2018).
In our coculture system, GATA2-OE enhanced the generation of K+34− cells, which further developed into K+34+ hemogenic cells, suggesting GATA2 may promote early mesoderm differentiation toward endothelium and hematopoiesis. The discrepancy in the effects of GATA2 knockout and our overexpression system in mesodermal development might be because of the differentiation conditions used, the transient nature of K+34− mesodermal cells, and the level of GATA2. A culture system favoring mesoderm differentiation will be more suitable for clarifying it.
Analysis of definitive hematopoiesis in the AGM region in conditional GATA2-knockout mice demonstrated that GATA2 is essential for the EHT (de Pater et al., 2013, Gao et al., 2013). The EHT is markedly inhibited in GATA2−/− hESCs, and this effect is rescued by GATA2 overexpression (Kang et al., 2018). Consistently, in our coculture system, GATA2-OE-induced expansion of K+34+43+ cells followed by K–34+43+ cells. Definitive hematopoiesis-related genes, including GATA1, RUNX1c, and SPI1, were also upregulated in GATA2-OE 34+K+43– cells. RUNX1c marked a subset of cells expressing CD34, a cell surface marker present on human AGM-derived HSCs (Ivanovs et al., 2014). RUNX1c tracked EHT in the hematopoiesis of hESCs, while transcription factor GATA2, GATA1, and SPI1 abundantly expressed in RUNX1c+ cells compared with RUNX1c− cells (Ng et al., 2016). Thus, our data suggest that GATA2-OE enhances definitive hematopoiesis through the promotion of EHT with a conserved cooperation of these critical genes for definitive hematopoiesis.
The CD34medCD45+ fraction showed higherhematopoietic potential compared with CD34lowCD45+ and CD34−CD45+ cells from hESCs (Ditadi et al., 2015). In our coculture system, the 34high45+ cell subset expressed a low percentage of differentiation makers and resided more in G0/G1 stage than CD34lowCD45+ and CD34−CD45+ cell subsets, reflecting the hematopoietic potential was enhanced in 34high45+ cell subset. Since maintenance of dormancy and recovery on stimulation are the most important characteristics for sustaining HSCs throughout life (Nakamura-Ishizu et al., 2014), the quiescence of 34high45+ GATA2-OE HSPCs and recovery in full hematopoietic activity when released from Dox showed their HS-like properties. The results are consistent with the previous finding that GATA2 alters hematopoietic lineage specification and cell differentiation (Hirasawa et al., 2002, Huang et al., 2016, Tsai et al., 1994). Besides, GATA2-OE promotes quiescence in human CB-HSCs, sacrificing their proliferation and performance in long-term culture-initiating cell assays (Tipping et al., 2009). Enforced expression of GATA2 also blocks proliferation and differentiation of murine BM cells (Persons et al., 1999). GATA2 seems to elicit a critical dose-dependent effect on maintenance of HSCs during early and late hematopoiesis. A recent study demonstrated that pulsatile changes in GATA2 expression subtly regulated the generation of HSCs (Eich et al., 2017). The mechanisms by which GATA2 regulates the generation and maintenance of HSCs at different developmental stages must be further elucidated.
Lin−CD34+CD43+CD45+/−CD90+CD38– cells in cocultures of hESCs and OP9 cells were previously defined as multipotent definitive hematopoietic progenitors (Slukvin, 2013). Recent studies showed that hESC-derived hematopoietic cells are very similar to human AGM- and FL-derived HSCs in terms of their gene profiles and immunophenotypes, and thus these cells are defined as HS-like cells (Dou et al., 2016, Ng et al., 2016). In the current study, GATA2-OE selectively maintained 34+45+90+38– HS-like cells in cocultures for 2 weeks and inhibited their differentiation into 34+45+38+ progenitor cells. RNA-seq demonstrated that the maintenance of hESC-derived HS-like cells on GATA2-OE was due to downregulation of stem cell proliferation, differentiation, cell-cycle, and apoptosis pathways. Phosphatidylinositol 3-kinase-Akt signaling, which maintains the properties of HSCs, were downregulated on GATA2-OE (Juntilla et al., 2010). AHR antagonists such as SR1 promoted the expansion of human HSCs (Boitano et al., 2010). The downregulation of genes related to the AHR pathway in G2-OE 90+ cells suggests that GATA2 maintains HS-like cells by inhibiting AHR signaling.
AGM-derived stromal cells were reported to predominantly support definitive hematopoiesis of hESCs (Ledran et al., 2008). AGM-S3 cells induce maturation of immature embryonic yolk sac cells and para-aortic splanchnopleure cells into reconstituting HSCs (Matsuoka et al., 2001). In the present study, AGM-S3 cells provided an ideal microenvironment for maintenance of 34+45+90+38– HS-like cells. However, we failed to detect long-term engraftment of these cells in immunodeficient mice (data not shown), suggesting that intrinsic maturity and exogenous factors are further required for gaining transplantable ability in hESC-derived HS-like cells.
We provide evidence that complex and subtle regulation of GATA2 is important for the development, expansion, and maintenance of hESC-derived HS-like cells. GATA2 plays two functionally distinct roles in the generation and expansion of HSCs in the AGM region and the proliferation of HSCs in adult BM in mice (Ling et al., 2004). In our system, the dual effects GATA2-OE on promoting early hemogenic endothelial precursors (typically 34+43+45– cells) through the EHT and later arresting 34+45+ cells in a quiescent stage by cell-cycle regulation provided new insight into its function in the maturation of hPSC definitive hematopoiesis. Although we lack evidence of the reconstitution potential of HS-like cells derived from cocultures, the similar gene expression profiles for GATA2-OE HS-like cells and human FL-derived HSCs suggest the possibility to achieve functional HSCs by manipulating GATA2 expression. Our findings should help to elucidate the mechanism controlling definitive hematopoiesis in humans by GATA2 regulation and may assist in future clinical applications.
Experimental Procedures
Maintenance of hESCs
H1 hESCs were provided by Professor Tao Cheng and maintained on Matrigel-coated plates in mTeSR1 medium (STEMCELL Technologies). The cells were dissociated into clumps using ReLeSR (STEMCELL Technologies) every 4–5 days.
Establishment of G2/hESCs
H1 hESCs were seeded into a 24-well plate with 70%–90% confluent at the time of transfection. Plasmid DNA-lipid complexes (containing 0.5 μg of the PB-Tet-on-GFP-T2A-hGATA2 vector, and 0.5 μg of PB200PA vector containing catalytic component) were added to the cells per well and incubated for 2–4 days according to the manufacturer’s description of lipofectamine 3000 (Invitrogen). Transfected cells were selected by culture in medium containing 1 μg/mL puromycin (Sigma). For single-cell culture, cells were dissociated into single cells with Accutase (Gibco) and maintained in mTeSR1 medium supplemented with Y27632 (Cayman). Puromycin-resistant clones were picked, and successful targeting was confirmed by western blotting, qRT-PCR (F1:5′-CAGCAAGGCTCGTTCCTGTTCA-3′, R1:5′-ATGAGTGGTCGGTTCTGCCCAT-3′) and PCR (F2: 5′GAGGAAGTCTTCTAACATGCGGT-3′, R2: 5′CCCTTCGTCTGACGTGGCAG-3′). To minimize variation owing to long-term selection, multiple stable cell lines were used and all experiments were repeated at least three times. The study was approved by the Ethics Committee of the Institute of Blood Transfusion, CAMS & PUMC.
Coculture of hESCs and AGM-S3 Cells
Approximately 50–60 clumps of hESCs, each of which contained 3–4 × 102 cells, were seeded onto 2–3 ×105 irradiated AGM-S3 cells in each well of a 6-well plate and cultured in hPSC maintenance medium. hESCs were differentiated into HSPCs as described previously (Mao et al., 2016). The medium was changed every day.
Colony Assay
Hematopoietic potential of cells was assessed by culture on methylcellulose (H4320, STEMCELL Technologies) supplemented with 1% penicillin/streptomycin (Invitrogen) and cytokines, which were described previously (Ma et al., 2008). BFU-E, CFU-Mix, CFU-GM, CFU-G, and CFU-M colonies were assessed after 10–16 days.
Flow Cytometry and Cell Sorting
Cocultured cells were dissociated with 0.25% trypsin-EDTA solution (Invitrogen) and filtered through a 70-μm nylon mesh to obtain a single-cell suspension. Flow cytometry was performed using a FACSCanto II system (BD Biosciences), and data were analyzed using FlowJo software (v.10.0.8.). Cell sorting was performed using a MoFlo Astrios High Speed Cell Sorter (Beckman Coulter). The antibodies used are presented in Table S1.
Cell-Cycle Analysis
Cells were treated with BrdU for 6 h, stained for surface antigens, and processed using an APC-BrdU Flow Kit (BD) according to the manufacturer's instructions.
Apoptosis Assays
Cells were detected by flow cytometry of cells stained according to the manufacturer's instructions with annexin V–APC and 7-AAD (BioLegend).
Statistical Analysis
All data are presented as the mean ± SD. Statistical analyses were performed using the Student's t test. p < 0.05 was considered significant.
Author Contributions
Conception and Design, F.M., Y.Z., J.X.Z., and M.W.; Performance of Research, Y.Z., X.P., B.M., M.W.L., B.C., Y.D., Y.M.Z., Y.J.C., Q.X.Z., Y.G.Z., and G.H.B.; Collection and Assembly of Data, Y.Z. and Y.G.Z.; Data Analysis and Interpretation, Y.Z., F.M., J.X.Z., and Y.G.Z.; Manuscript Writing, Y.Z., M.W., and F.M.; Final Approval of Manuscript, all authors.
Acknowledgments
We thank Professor Tao Cheng at the State Key Laboratory of Experimental Hematology, CAMS & PUMC for generously providing the H1 hESC line, Professor Emery Bresnick at University of Wisconsin-Madison for his critical comments and reading our manuscript. This work was supported by the National Basic Research Program (973 Program: 2015CB964902), the National Natural Science Foundation of China (H81170466 and H81370597), and the CAMS Initiatives for Innovative Medicine (2016-I2M-1-018) awarded to F.M., and by the Union Youth Fund of the Chinese Academy of Medical Sciences (2014-10023-1001-1011) awarded to Y.Z.
Published: June 6, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.05.007.
Contributor Information
Yonggang Zhang, Email: biozyg@163.com.
Feng Ma, Email: mafeng@hotmail.co.jp.
Accession Numbers
The accession number for the RNA-seq data reported in this paper is GEO: GSE122207.
Supplemental Information
References
- Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C., Azzoni E., Woll P.S., Mead A.J., Hultquist A., Swiers G. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Boisset J.C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Boitano A.E., Wang J., Romeo R., Bouchez L.C., Parker A.E., Sutton S.E., Walker J.R., Flaveny C.A., Perdew G.H., Denison M.S. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesana M., Guo M.H., Cacchiarelli D., Wahlster L., Barragan J., Doulatov S., Vo L.T., Salvatori B., Trapnell C., Clement K. A CLK3-HMGA2 alternative splicing axis impacts human hematopoietic stem cell molecular identity throughout development. Cell Stem Cell. 2018;22:575–588. doi: 10.1016/j.stem.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Teng J., Liu H., Pan X., Zhou Y., Huang S., Lai M., Bian G., Mao B., Sun W. Inducible overexpression of RUNX1b/c in human embryonic stem cells blocks early hematopoiesis from mesoderm. J. Mol. Cell Biol. 2017;9:262–273. doi: 10.1093/jmcb/mjx032. [DOI] [PubMed] [Google Scholar]
- Choi K.D., Vodyanik M.A., Togarrati P.P., Suknuntha K., Kumar A., Samarjeet F., Probasco M.D., Tian S., Stewart R., Thomson J.A. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou I., Patsali P., Stephanou C., Antoniou M., Kleanthous M., Lederer C.W. Measurenment of lentiviral vector titre and copy number by cross-species duplex quantitative PCR. Gene Ther. 2016;23:113–118. doi: 10.1038/gt.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés F., Debacker C., Péault B., Labastie M.C. Differential expression of KDR/VEGFR-2 and CD34 during mesoderm development of the early human embryo. Mech. Dev. 1999;83:161–164. doi: 10.1016/s0925-4773(99)00030-1. [DOI] [PubMed] [Google Scholar]
- Costa G., Kouskoff V., Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Ditadi A., Sturgeon C.M., Tober J., Awong G., Kennedy M., Yzaguirre A.D., Azzola L., Ng E.S., Stanley E.G., French D.L. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat. Cell Biol. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D.R., Calvanese V., Sierra M.I., Nguyen A.T., Minasian A., Saarikoski P., Sasidharan R., Ramirez C.M., Zack J.A., Crooks G.M. Medial HOXA genes demarcate haematopoietic stem cell fate during human development. Nat. Cell Biol. 2016;18:595–606. doi: 10.1038/ncb3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich C., Arlt J., Vink C.S., Solaimani Kartalaei P., Kaimakis P., Mariani S.A., van der Linden R., van Cappellen W.A., Dzierzak E. In vivo single cell analysis reveals Gata2 dynamics in cells transitioning to hematopoietic fate. J. Exp. Med. 2017;215:233–248. doi: 10.1084/jem.20170807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Johnson K.D., Chang Y., Boyer M.E., Dewey C.N., Zhang J., Bresnick E.H. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 2013;210:2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa R., Shimizu R., Takahashi S., Osawa M., Takayanagi S., Kato Y., Onodera M., Minegishi N., Yamamoto M., Fukao K. Essential and instructive roles of GATA factors in eosinophil development. J. Exp. Med. 2002;195:1379–1386. doi: 10.1084/jem.20020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Gao J., Du J., Ma N., Zhu Y., Wu P., Zhang T., Wang W., Li Y., Chen Q. Generation and analysis of GATA2w/eGFP human ESCs reveal ITGB3/CD61 as a reliable marker for defining hemogenic endothelial cells during hematopoiesis. Stem Cell Reports. 2016;7:854–868. doi: 10.1016/j.stemcr.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Welch L., Anderson R.A., Turner M.L., Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J. Exp. Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovs A., Rybtsov S., Anderson R.A., Turner M.L., Medvinsky A. Identification of the niche and phenotype of the first human hematopoietic stem cells. Stem Cell Reports. 2014;2:449–456. doi: 10.1016/j.stemcr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla M.M., Patil V.D., Calamito M., Joshi R.P., Birnbaum M.J., Koretzky G.A. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactiveoxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Mesquitta W.T., Jung H.S., Moskvin O.V., Thomson J.A., Slukvin I.I. GATA2 is dispensable for specification of hemogenic endothelium but promotes endothelial-to-hematopoietic transition. Stem Cell Reports. 2018;11:197–211. doi: 10.1016/j.stemcr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledran M.H., Krassowska A., Armstrong L., Dimmick I., RenstroÖm J., Lang R., Yung S., Santibanez-Coref M., Dzierzak E., Stojkovic M. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Ling K.W., Ottersbach K., van Hamburg J.P., Oziemlak A., Tsai F.Y., Orkin S.H., Ploemacher R., Hendricks R.W., Dzierzak E. GATA2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 2004;200:871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugus J.J., Chung Y.S., Mills J.C., Kim S.I., Grass J., Kyba M., Doherty J.M., Bresnick E.H., Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Ma F., Ebihara Y., Umeda K., Sakai H., Hanada S., Zhang H., Zaike Y., Tsuchida E., Nakahata T., Nakauchi H. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc. Natl. Acad. Sci. U S A. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno M., Mead P.E., Kelley C., Xu R.H., Kung H.F., Suzuki A., Ueno N., Zon L.I. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1965–1972. [PubMed] [Google Scholar]
- Mao B., Huang S., Lu X., Sun W., Zhou Y., Pan X., Yu J., Lai M., Chen B., Zhou Q. Early development of definitive erythroblasts from human pluripotent stem cells defined by expression of glycophorin A/CD235a, CD34, and CD36. Stem Cell Reports. 2016;7:869–883. doi: 10.1016/j.stemcr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Tsuji K., Hisakawa H., Xu Mj., Ebihara Y., Ishii T., Sugiyama D., Manabe A., Tanaka R., Ikeda Y. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98:6–12. doi: 10.1182/blood.v98.1.6. [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Minegishi N., Ohta J., Yamagiwa H., Suzuki N., Kawauchi S., Zhou Y., Takahashi S., Hayashi N., Enge J.D., Yamamoto M. The mouse GATA-2 gene is expressed in the para-aortic splanchnopleura and aorta-gonads and mesonephros region. Blood. 1999;93:4196–4207. [PubMed] [Google Scholar]
- Minegishi N., Suzuki N., Yokomizo T., Pan X., Fujimoto T., Takahashi S., Hara T., Miyajima A., Nishikawa S., Yamamoto M. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 2003;102:896–905. doi: 10.1182/blood-2002-12-3809. [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Takizawa H., Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141:4656–4666. doi: 10.1242/dev.106575. [DOI] [PubMed] [Google Scholar]
- Ng E.S., Azzola L., Bruveris F.F., Calvanese V., Phipson B., Vlahos K., Hirst C., Jokubaitis V.J., Yu Q.C., Maksimovic J. Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat. Biotechnol. 2016;34:1168–1179. doi: 10.1038/nbt.3702. [DOI] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C., Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- de Pater E., Kaimakis P., Vink C.S., Yokomizo T., Yamada-Inagawa T., van der Linden R., Kartalaei P.S., Camper S.A., Speck N., Dzierzak E. Gata2 is required for HSC generation and survival. J. Exp. Med. 2013;210:2843–2850. doi: 10.1084/jem.20130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons D.A., Allay J.A., Allay E.R., Ashmun R.A., Orlic D., Jane S.M., Cunningham J.M., Nienhuis A.W. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–499. [PubMed] [Google Scholar]
- Rentas S., Holzapfel N., Belew M.S., Pratt G., Voisin V., Wilhelm B.T., Bader G.D., Yeo G.W., Hope K.J. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016;532:508–511. doi: 10.1038/nature17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.G., Mandelbaum J., Zon L.I., Daley G.Q. Engineering hematopoietic stem cells: lessons from development. Cell Stem Cell. 2016;18:707–720. doi: 10.1016/j.stem.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Ivanovs A., Zhao S., Medvinsky A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development. 2016;143:1284–1289. doi: 10.1242/dev.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I.I. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013;12:720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I.I. Generating human hematopoietic stem cells in vitro -exploring endothelial to hematopoietic transition as a portal for stemness acquisition. FEBS Lett. 2016;590:4126–4143. doi: 10.1002/1873-3468.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R., Jha D.K., Han A., Soria-Valles C., da Rocha E.L., Lu Y.F., Goettel J.A., Serrao E., Rowe R.G., Malleshaiah M. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545:432–438. doi: 10.1038/nature22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiers G., Baumann C., O'Rourke J., Giannoulatou E., Taylor S., Joshi A., Moignard V., Pina C., Bee T., Kokkaliaris K.D. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat. Commun. 2013;4:2924. doi: 10.1038/ncomms3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taoudi S., Gonneau C., Moore K., Sheridan J.M., Blackburn C.C., Taylor E., Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tipping A.J., Pina C., Castor A., Hong D., Rodrigues N.P., Lazzari L., May G.E., Jacobsen S.E., Enver T. High GATA2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood. 2009;113:2661–2672. doi: 10.1182/blood-2008-06-161117. [DOI] [PubMed] [Google Scholar]
- Tsai F.Y., Keller G., Kuo F.C., Weiss M., Chen J., Rosenblatt M., Alt F.W., Orkin S.H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Vodyanik M.A., Thomson J.A., Slukvin I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu C., Liu X., Wang M., Wu D., Gao J., Su P., Nakahata T., Zhou W., Xu Y. MEIS1 regulates hemogenic endothelial generation, megakaryopoiesis, and thrombopoiesis in human pluripotent stem cells by targeting TAL1 and FLI1. Stem Cell Reports. 2018;10:447–460. doi: 10.1016/j.stemcr.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder M.C. Inducing definitive hematopoiesis in a dish. Nat. Biotechnol. 2014;32:539–541. doi: 10.1038/nbt.2929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.