Significance
Ecologists have embraced modern coexistence theory as a framework for understanding the interspecific differences that drive competitive exclusion versus coexistence. We extend the empirical application of this theory to examine priority effects, in which neither species in a competing pair can invade its competitor due to destabilizing differences that result in positive frequency-dependent growth rates. Using reciprocal invasion experiments between yeast strains across two environmental gradients, we reveal two fundamentally different ways in which environmental conditions alter competitive interactions: high sugar increased the likelihood of priority effects by reducing fitness differences, whereas low acidity increased the neutrality of species interactions by simultaneously reducing both fitness and destabilizing differences. This study demonstrates how modern coexistence theory can systematically explain priority effects.
Keywords: competition, invasion criterion, niche difference, stabilizing difference, fitness difference
Abstract
Modern coexistence theory is increasingly used to explain how differences between competing species lead to coexistence versus competitive exclusion. Although research testing this theory has focused on deterministic cases of competitive exclusion, in which the same species always wins, mounting evidence suggests that competitive exclusion is often historically contingent, such that whichever species happens to arrive first excludes the other. Coexistence theory predicts that historically contingent exclusion, known as priority effects, will occur when large destabilizing differences (positive frequency-dependent growth rates of competitors), combined with small fitness differences (differences in competitors’ intrinsic growth rates and sensitivity to competition), create conditions under which neither species can invade an established population of its competitor. Here we extend the empirical application of modern coexistence theory to determine the conditions that promote priority effects. We conducted pairwise invasion tests with four strains of nectar-colonizing yeasts to determine how the destabilizing and fitness differences that drive priority effects are altered by two abiotic factors characterizing the nectar environment: sugar concentration and pH. We found that higher sugar concentrations increased the likelihood of priority effects by reducing fitness differences between competing species. In contrast, higher pH did not change the likelihood of priority effects, but instead made competition more neutral by bringing both fitness differences and destabilizing differences closer to zero. This study demonstrates how the empirical partitioning of priority effects into fitness and destabilizing components can elucidate the pathways through which environmental conditions shape competitive interactions.
Understanding when competing species coexist or exclude one another is a long-standing goal of ecology. The development and application of modern coexistence theory constitutes a major recent advance toward this goal (1, 2). This theory has been used to determine when species differences promote or preclude diversity (3), which traits underlie competitive interactions (4, 5), and how macroevolutionary processes contribute to coexistence (6, 7).
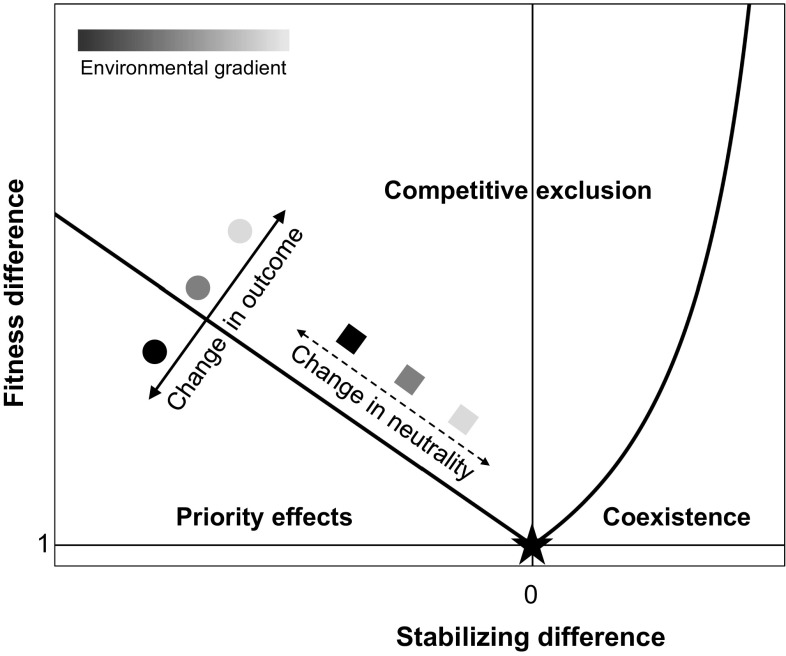
According to modern coexistence theory, competing species stably coexist when each species is able to invade an established population of the other, and this ability to invade is determined by the relative strength of two types of difference between the species (1, 2). First, fitness differences are differences in species’ growth rates and sensitivity to competition that cause one species to outcompete the other (y axis in Fig. 1). Second, stabilizing differences, sometimes called niche differences (1), lower the average effect of interspecific competition relative to intraspecific competition (x axis in Fig. 1). Positive stabilizing differences cause a species to be suppressed more strongly by conspecifics than heterospecifics, which promotes coexistence by allowing both species to invade when rare. In contrast, destabilizing differences (i.e., negative stabilizing differences) cause species to be more negatively impacted by heterospecifics than by conspecifics, which promotes mutual noninvasibility by allowing established species to exclude newly introduced species (2, 8). Destabilizing differences therefore result in historically contingent competitive exclusion, also known as priority effects (8). It is the magnitude of fitness difference relative to stabilizing or destabilizing differences that determines whether species exhibit coexistence, deterministic competitive exclusion (hereafter competitive exclusion), or priority effects (1, 2, 8–10). Strong fitness differences promote competitive exclusion (upper portion of Fig. 1), whereas weak fitness differences lead to either coexistence (lower right side of Fig. 1) or priority effects (lower left side of Fig. 1). In the special case in which stabilizing differences are absent and species have equal fitness (star in Fig. 1), species are functionally equivalent and their competitive interactions are neutral (1, 11).
Fig. 1.
Conceptual framework demonstrating how shifts in fitness differences and stabilizing differences across an environmental gradient alter competitive outcomes and the neutrality of competitive interactions. Circles represent a pair of species competing in three different environments, and squares represent a different pair of species competing in the same three environments. The solid arrowed line shows how positively correlated changes in fitness and stabilizing differences alter the outcome of competition (priority effects vs. competitive exclusion). The dashed arrowed line shows how negatively correlated changes in fitness and stabilizing differences change the neutrality of species interactions (distance to black star). The black star indicates the special case of neutrality in which competing species have equal fitness and no stabilizing differences (fitness difference = 1 and stabilizing difference = 0).
Despite this simple conceptual framework, the link between priority effects and modern coexistence theory remains unexplored in empirical work (8, 9). Empirical tests of modern coexistence theory have reported destabilizing difference for an average of 17% of species interactions (SI Appendix, Table S1), but because these studies focused on coexistence versus competitive exclusion, species pairs exhibiting destabilizing differences were usually excluded from subsequent analyses. Likewise, research on priority effects has demonstrated how environmental conditions determine the degree to which early-arriving species impact late arrivers (12–14), but these studies have not approached this question within the framework of modern coexistence theory (9). Consequently, it remains unknown how environmental conditions alter the fitness and destabilizing differences that drive priority effects.
We suggest that a systematic understanding of the environmental conditions that promote priority effects can be achieved by determining how changes in fitness and stabilizing differences are correlated across environmental gradients. For example, in systems dominated by destabilizing differences (left side of Fig. 1), changes to fitness and stabilizing differences that are positively correlated across environments (solid arrowed line and circles in Fig. 1) will shift species interactions between competitive exclusion and priority effects (light gray vs. black circles in Fig. 1). In contrast, a negative correlation between fitness and stabilizing differences (dashed arrowed line and squares in Fig. 1) should be less likely to affect competitive outcomes, but will instead change the degree to which competition is neutral by moving species pairs closer or further away from neutrality (light gray vs. black squares in Fig. 1).
Here we use nectar-colonizing yeasts as an experimental system to test how environmental conditions drive priority effects by impacting fitness and stabilizing differences. In these yeasts, early-arriving species can suppress the growth of late arrivers by depleting amino acids (a limiting resource) or changing other chemical properties of the shared nectar environment (15, 16). Moreover, the sugar concentration and acidity of nectar vary widely among flowers (17, 18) and influence how yeast populations grow and compete with one another (16, 19). Building on this previous knowledge, we conducted mutual invasibility tests with four yeast strains commonly found in floral nectar: Metschnikowia gruessii, Metschnikowia rancensis, and a “bumpy” and a “smooth” strain of Metschnikowia reukaufii (hereafter referred to as the species Gruessii, Rancensis, Bumpy, and Smooth, respectively). We measured monoculture and invading growth rates for each of six possible species pairs across six nectar environments that characterize the range of acidity and sugar concentration found in floral nectar. Using these growth rates, we determined competitive outcomes and calculated fitness and stabilizing differences (20, 21). We then used these data to determine whether the abiotic environment altered the likelihood of priority effects by changing fitness differences, stabilizing differences, or both.
Results
There was a strong competitive hierarchy among the four species such that Bumpy > Smooth > Gruesssii > Rancensis. In other words, Bumpy could invade all species, Smooth could only invade Gruessii and Rancensis, Gruessii could only invade Rancensis, and Rancensis could not invade any other species (SI Appendix, Table S2). This hierarchy was maintained across all environmental conditions (SI Appendix, Fig. S1) so that an inferior species could never invade a dominant species. Under some conditions, however, an inferior species could prevent a dominant species from invading, resulting in priority effects (SI Appendix, Table S2).
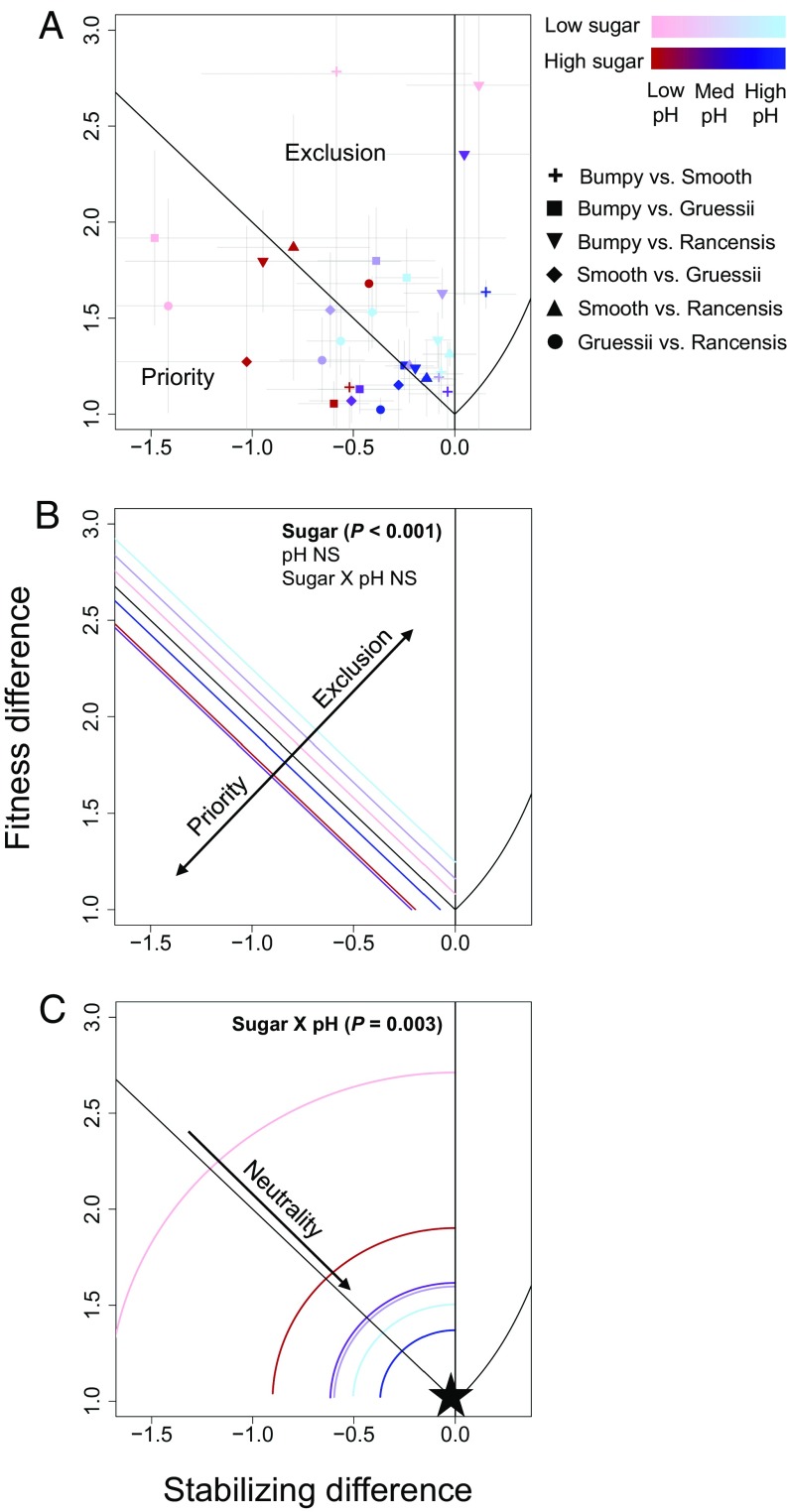
The vast majority of pairwise interactions tested (>90%) exhibited destabilizing differences necessary for priority effects to emerge (Fig. 2A). Of the 32 species pair-by-environment combinations included in our analyses, 13 experienced priority effects (mutual noninvasibility), and 19 experienced competitive exclusion (one species could invade) (Fig. 2A and SI Appendix, Table S2). Just under half of these outcomes were significant (6/13 priority effects and 8/19 competitive exclusion), meaning that the 95% confidence intervals did not cross the boundary line between priority effects and competitive exclusion. Although the effect of sugar and pH on yeast performance (in monoculture) varied across species, growth rates tended to increase with increasing pH, whereas sugar had a more variable effect (SI Appendix, Fig. S1).
Fig. 2.
The effect of pH and sugar on (A) fitness differences and stabilizing differences, (B) the likelihood of priority effects vs. competitive exclusion, and (C) the neutrality of competitive interactions. Points in (A) show fitness differences and stabilizing differences for six species pairs competing in six nectar environments (two levels of sugar × three levels of pH). Four pairs were removed from this analysis because one species in each of these pairs had negative growth in monoculture (Methods). Error bars are 95% confidence intervals, so that error bars falling completely within the priority effects or exclusion zone indicate a significant outcome. Colored lines in B and C show mean values for each environment, calculated from points in A. Colored lines in B show the mean distance that species pairs competing in each nectar environment fall from the line that separates priority effects from competitive exclusion; lines below the black line indicate a greater likelihood of priority effects, and lines above the black line indicate a greater likelihood of competitive exclusion. Arcs in C show the mean distance that species pairs competing in each nectar environment fall from total neutrality (the special case of no fitness or stabilizing differences, indicated by the black star). Arcs close to the star indicate more neutral interactions, and arcs far from the star indicate less neutral interactions.
Changes in both environmental gradients altered competitive dynamics, but only the sugar gradient changed the prevalence of priority effects. Priority effects were more likely at high sugar (dark lines in Fig. 2B), whereas competitive exclusion was more likely at low sugar (light lines in Fig. 2B) (P < 0.001). Although acidity did not affect the likelihood of priority effects (P = 0.21), competition became more neutral with increasing pH (blue lines are closest to the star in Fig. 2C). Interactions were also more neutral at high sugar, but only when pH was low or high (significant sugar × pH interaction, P = 0.003; Fig. 2C).
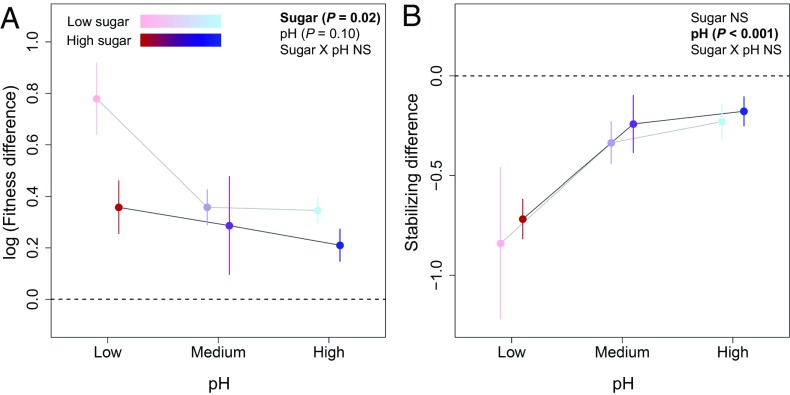
The observed effects of sugar and acidity on competitive outcomes and neutrality were driven by changes in fitness and destabilizing differences across our two environmental gradients. High sugar lowered fitness differences (P = 0.02, points shifted toward zero in Fig. 3A) but had no effect on destabilizing differences (P = 0.18; Fig. 3B), resulting in more priority effects in high-sugar conditions (Fig. 2B). In contrast, increasing pH dampened destabilizing differences (P < 0.001; points shifted toward zero in Fig. 3B) and had a weak dampening effect on fitness differences (P = 0.10; points shifted toward zero in Fig. 3A). This reduction of both types of species differences at high pH meant that under these conditions, species approached equivalency, and interactions became more neutral (Fig. 2C).
Fig. 3.
The effect of pH and sugar on (A) fitness differences and (B) stabilizing differences. Points show mean values in each environment, averaged across six species pairs, and error bars show 1 SE from the mean. Low sugar is shown in light colors (pink, light purple, and light blue) connected by a light gray line, and high sugar treatments are shown in dark colors (red, dark purple, and dark blue) connected by a dark gray line. The dashed lines at zero indicate the absence of fitness differences (A; note the log transformation) or stabilizing differences (B).
Discussion
By quantifying the fitness and destabilizing differences that jointly drive priority effects, our analysis revealed that two environmental gradients had fundamentally different effects on competition. High sugar levels increased the likelihood of priority effects by reducing fitness differences (Figs. 2B and 3A), demonstrating that conditions that equalize species’ competitive abilities can promote historically contingent competitive exclusion, even in the absence of changes to destabilizing differences. This result suggests that natural levels of variation in sugar content among flowers is sufficient to cause competitive exclusion in some communities and priority effects in others (18) (Fig. 2B). In contrast, high pH made competitive interactions more neutral by simultaneously reducing both fitness and destabilizing differences (Figs. 2C and 3 A and B), suggesting that a reduction in nectar pH caused by acidifying bacteria may shift yeast communities from being nearly neutral to being characterized by strong species interactions (Fig. 2C). These results show how the application of modern coexistence theory to priority effects can provide new insight into the ways in which environmental conditions shape competitive dynamics.
Previous research has revealed that productive environments (12, 16), high temperatures (13), and small habitat sizes (22) promote priority effects by allowing early arrivers to more strongly draw down shared resources and suppress late arrivers. However, this research tended to define priority effects broadly, as any negative impact of early arrivers on late arrivers, and to emphasize the role of resource depletion in precipitating the exclusion of the late arriving species (13, 16, 19, 23, 24). Under a mutual noninvasibility definition, priority effects are not caused simply by a depletion of resources that inhibits the invasion of a late arriver, but rather emerge as a consequence of positive frequency-dependent growth (i.e., destabilizing differences) (8–10, 25). In this experiment, our replenishment of amino acid resources (SI Appendix, Fig. S2), the inability of concentrated amino acid additions to reverse most cases of priority effects (SI Appendix, Figs. S3 and S4), and the stable or increasing population sizes of the initial species at 2 d when the invader was added (SI Appendix, Fig. S5) all suggest that resource depletion alone was not the underlying cause of the priority effects we observed. Our use of a mutual noninvasibility definition of priority effects represents an emerging approach to studying historically contingent exclusion that is in line with both coexistence theory (8–10) and research on alternative stable and transient states (26, 27).
In this and other systems, identifying the mechanisms that produce positive frequency-dependent growth and cause environmental conditions to affect fitness and stabilizing differences will require a detailed analysis of how species alter and respond to the environment (28, 29). As with previous modern coexistence research focused on elucidating the consequences of interspecific and intraspecific density dependence (1, 2, 28), our experiment was not designed to uncover these mechanisms. However, classic and contemporary research can inform hypotheses about likely mechanisms. For example, the changes in fitness and stabilizing differences along pH and sugar gradients that we observed are consistent with the theoretical prediction that environmental change that alters species’ requirement for a substitutable resource (e.g., amino acids) will alter fitness and/or stabilizing differences (29). Likewise, positive frequency dependence in other systems has been shown to be the result of a species depleting more of the resource on which a competitor is most limited (30) or altering the local nonresource environment in a way that benefits itself and harms its competitors (e.g., plant–soil feedbacks) (31). In our system, amino acid concentrations, pH, and sugar in nectar are three major determinants of yeast population growth rates (15, 16, 18, 19, 32). Our focal species had only minor effects on pH and sugar levels (SI Appendix), and priority effects were mostly maintained following large additions of amino acids (SI Appendix). These results suggest that rather than feedbacks in pH, sugar, or total resource availability, more subtle shifts in resource composition or chemical inhibitors may have caused the positive frequency dependence that we observed. For example, differences among yeast species in the consumption and requirement of the 22 amino acids provided in our synthetic nectar may underlie the observed destabilizing differences (16). Additionally, some species of yeast produce toxins that inhibit or kill competitors (33), whereas others secrete enzymes that help conspecifics break down sucrose (34). These mechanisms could promote positive frequency dependence by increasing the strength of interspecific competition relative to intraspecific competition. A detailed investigation into the full range of mechanisms that can produce positive frequency dependence in this and other systems will be an important next step for coexistence research.
Priority effects were common in this experiment, with mutual noninvasibility occurring across all environments and all but one species pair and characterizing 41% of competitive outcomes (SI Appendix, Table S2). Because the method that we used to calculate fitness and stabilizing differences and to determine the prevalence of priority effects can be sensitive to the timing of both resource additions and the introduction of the invading species, we took several measures to ensure that our experimental methods matched our model’s assumptions and to verify that any biases were not driving our results (SI Appendix). Specifically, if the invading species were introduced too late or resources were not replenished frequently enough, resources could be depleted to the point of extinction, which would impede invasion and lead to an overestimation of priority effects. The results of a supplementary experiment, in which few cases of priority effects were reversed by the addition of high concentrations of amino acids, indicate that complete resource depletion was not responsible for the low levels of invasibility that we observed (SI Appendix, Fig. S3). In contrast, if the invading species were introduced before the resident species reached equilibrium (which would be most likely under slow-growth conditions), invasion would be facilitated and priority effects underestimated. The observation that our slowest-growth condition (low pH) was associated with low invasion rates, strong destabilizing differences (Fig. 3B), and a high frequency of priority effects (Fig. 2A) indicates that the inability of the first species to reach equilibrium under slow-growth conditions was not driving the trends we observed. The results of supplementary analyses using only the treatments where yeast had reached carrying capacity by 48 h also support this conclusion (SI Appendix, Figs. S7 and S8).
The prevalence of destabilizing differences we report is supported by previous empirical coexistence research that indicates that this outcome occurs regularly in other systems (SI Appendix, Table S1); evidence of destabilizing differences in terrestrial plants (4, 6, 7) and algae (21) highlights the need to incorporate priority effects as a third outcome alongside coexistence and competitive exclusion (8, 9). Mutual noninvasibility may be more common across ecological systems than currently recognized, which could affect patterns of local and regional diversity. For example, no species pairs in this study exhibited mutual invasibility, a prerequisite for local (within-flower) coexistence, despite the fact that regional (across-flower) coexistence of these species is common in the field (35). However, local priority effects can help maintain or even promote regional diversity if different assemblages of species dominate different habitat patches (35, 36). Likewise, metacommunity theory predicts that strong competitive hierarchies, such as those observed here, can promote regional diversity of species dispersing and competing across a landscape of habitat patches whenever strong competitors are also weak dispersers (37). More research is needed to understand how local coexistence outcomes, and priority effects in particular, scale up to influence regional patterns of diversity.
Understanding the conditions under which strong versus weak species’ differences drive competitive interactions can provide insight into the sensitivity of observed competitive outcomes to perturbations (1). For example, neutral species interactions produce competitive outcomes that are sensitive to demographic stochasticity and disturbances that alter species’ abundances, due to the absence of frequency-dependent population regulation (1, 2, 9). Consequently, competitive outcomes characterized by high fitness differences or strong destabilizing differences (e.g., black square in Fig. 1 and pink lines in Fig. 2C) should be less affected by perturbations and drift than interactions with near-zero stabilizing differences and low fitness differences (e.g., light gray square in Fig. 1 and dark blue lines Fig. 2C). Previous research has suggested that neutral interactions are common in hyperdiverse communities (11), among close relatives (38), and when dispersal is limited (39). In our system, both fitness and destabilizing differences were weak at high pH, indicating that benign conditions were associated with reduced species differences and more neutral interactions (Figs. 2C and 3). This finding complements previous work by suggesting a potential association between the harshness of environmental conditions, the neutrality of species interactions, and the sensitivity of competitive outcomes to perturbations. Whether this association emerges in other systems remains to be tested.
Previous efforts to characterize fitness and stabilizing differences have been conducted primarily with annual plants, and usually in a single environment (SI Appendix, Table S1). This is in part because models for determining fitness and stabilizing differences have been developed for annual plants (40), and estimating the parameters required for these metrics is labor-intensive even for a single environment. However, the approach used here [i.e., directly measuring invasibility and using the equations from Carroll et al. (20) to estimate fitness and stabilizing differences] eliminates some of the system specificity and experimental labor of previously used methods. In experimental systems that meet the model assumptions of Carroll et al.’s (20) method, the approach we took here could be used to investigate changes in fitness and stabilizing differences across environments. Any abiotic gradient (e.g., water availability) or biotic gradient (e.g., predator or pathogen abundance) that alters fitness or stabilizing differences could induce shifts in competitive outcomes (6, 41) and neutrality. In systems characterized by positive stabilizing differences (right side of Fig. 1), examining correlated changes in fitness and stabilizing differences could also be informative. In this case, positively correlated shifts in fitness and stabilizing differences would shift the neutrality of competitive interactions, whereas negatively correlated shifts would alter whether pairs experience exclusion or coexistence. Investigating how environmental variation affects species differences and how these differences determine competitive outcomes in a wider range of systems will help link experimental results to patterns of local and regional diversity across heterogeneous landscapes.
Our study builds on a growing body of research demonstrating that partitioning competition into fitness and stabilizing differences facilitates a deeper understanding of species interactions (1, 10, 21, 29, 42). The continued integration of priority effects into modern coexistence theory promises to provide new insights into the full range of coexistence outcomes that manifest from competitive interactions, the role that species differences play in driving these outcomes, and the pathways through which local environmental conditions drive patterns of coexistence and diversity.
Methods
Study System.
We used an experimental system of yeasts that are found in floral nectar. Along with several species of bacteria, these yeasts inhabit the floral nectar of many plant species and are moved between flowers by flower-visiting animals such as hummingbirds (43) and bees (44). These microbes appear to be regularly introduced into floral nectar at low density and rapidly reproduce to reach concentrations of thousands of cells per microliter (16).
Nectar microbe communities are less diverse than most microbial systems, presumably as a result of the harsh nectar environment characterized by high sugar concentrations and low concentrations of the amino acids that constitute these species’ primary resource (15–17, 32). Sugars in nectar reach concentrations of 20–40% (15, 18), and although nectar yeasts consume sugar, high sugar can cause osmotic stress that reduces yeast growth (17, 18). In contrast, nitrogen is limited in nectar (often <0.1 mM concentration) and occurs primarily as amino acids that are consumed by nectar microbes (45). Finally, bacteria that colonize nectar (e.g., species of Acinetobacter and Neokomagataea) produce acidic by-products that cause nectar to range in acidity from pH 8.0 to 2.5 (18, 19). Yeast growth is often reduced when nectar is acidic (19).
We used four taxa of nectar yeasts in our experiments: a strain of M. gruessii; a strain of M. rancensis; and two morphologically and functionally distinct strains of M. reukaufii, which we refer to as smooth and bumpy (46) (SI Appendix). These four strains of yeast can be distinguished based on their colony morphology (SI Appendix, Fig. S9), and we refer to them as species throughout this paper for simplicity. All species were isolated from the floral nectar of Diplacus (formerly Mimulus) aurantiacus plants at Jasper Ridge Biological Preserve in the Santa Cruz Mountains of California (37°24′N, 122°14′W). Each D. aurantiacus flower can contain up to about 10 µL of nectar. All of our four focal yeast species are commonly found in the nectar of D. aurantiacus and other plant species (17, 43).
Experiment.
To mimic floral nectar, we used 200-µL wells in a 96-well PCR plate (USA Scientific), each filled with 9 µL of artificial nectar containing water, amino acids, and sucrose (16). We had six environmental treatments consisting of a fully crossed combination of three pH levels [low pH (3.2), medium pH (5.4), and high pH (6.1)] to simulate the variation in acidity caused by nectar-colonizing bacteria (18, 19) and two sucrose levels [low (20% wt/vol) and high (40% wt/vol)] to simulate natural interplant and intraplant variation in sugar levels (18). We used 0.1 M hydrochloric acid to lower the pH of the low- and medium-pH nectars to the desired levels and measured nectar pH with a pH meter (Accumet excel XL pH meter; Fisher Scientific). To all nectar types, we added amino acids from digested casein, which has a similar amino acid profile as flower nectar (45). We gave all nectars a total amino acid concentration of 0.316 mM, which approximates moderate amino acid availability in floral nectar (16). Nectar was filtered through a 0.2-µm filter before use.
Our experiment included four species, for a total of six possible pairwise combinations of competing species. For each of our six species pairs in each of our six environmental treatments (nectar types), we determined each species’ growth rate in monoculture and growth rate when invading its competitor to calculate fitness and stabilizing differences (ref. 21; see SI Appendix, Fig. S2, for experimental procedure). To do this, we introduced each of our four species at low density into three replicate wells on day 1 of the experiment, allowed these to grow for 48 h at 27 °C, and determined their population sizes at 48 h (see below). At 48 h, we then introduced one of each of the three invader species into each of the three replicate wells, allowed the two competing species to grow together for 48 h at 27 °C, and determined the population sizes of both species at 96 h. The 48-h interval between the introduction of the first and second species was chosen to mimic a realistic frequency of yeast dispersal events (15) (SI Appendix) and to ensure that the assumptions of the model we used to calculate fitness and stabilizing differences were met (SI Appendix). In particular, we ensured that 48 h was generally enough time for nectar yeasts to reach equilibrium by assessing growth curves for a subset of our treatments that we created using instantaneous growth rates from a recent experiment (SI Appendix, Fig. S6) (47). To maintain resource levels throughout the experiment and prevent complete resource depletion, we replaced 2 µL of each microcosm with fresh nectar at 24, 48, and 72 h (SI Appendix, Fig. S2). In one experimental run (well plate), we repeated this sequential invasion protocol in each of our six environments, such that an experimental run contained 4 focal species × 3 invader species × 6 environments, for a total of 72 experimental wells on a well plate. Each well plate therefore contained one replicate per treatment, and we ran two replicates (well plates) simultaneously (over a 96-h period). We repeated this procedure four times for a total of eight replicates per treatment.
We plated samples from the replicates on yeast malt agar (YMA) plates to determine cell densities at 0, 48, and 96 h (SI Appendix, Fig. S2). The number of colony forming units (CFU) on YMA plates has been shown to be correlated to the number of yeast cells in solution (15).
Calculation of Fitness Differences and Stabilizing Differences.
We calculated the growth rate of each species when grown for 48 h in monoculture and when invading a competitor as
| [1] |
where , is 2 d, and and are cell densities (CFU/µL) when the focal species was added (at 0 h for monoculture and at 48 h for invading) and 2 d later (at 48 h when in monoculture and 96 h when invading). We added a constant of 1 to and to allow for growth rate calculations when no cells were detected by plating at either time point due to low cell densities.
We then used the equations for estimating fitness differences and stabilizing differences that were described by Carroll et al. (20) and have been applied empirically in experiments with algae (21) and bacteria (42). This approach uses direct measurements of monoculture and invading growth rates to calculate species’ sensitivities to competition, so it is well suited to rapid systems such as ours where the outcome of competition can be directly observed. The direct measurement of invasion success also eliminates the need for system-specific models to estimate invading growth rates (4, 6, 7). In addition, because competitive effects are evaluated at the extremes (when one species is at equilibrium and the other is at near-zero abundances), this approach is relatively robust to deviations from the assumptions of linear consumer responses and constant competition coefficients underlying Lotka–Volterra dynamics (2). Two related assumptions of these models are that the first species is at equilibrium when the second (invader) species is introduced and that the complete elimination of shared resources by the first species does not prevent the invasion of the second species (20); we conducted several additional experiments and analyses to ensure that these two assumptions were met (Discussion and SI Appendix). We note also that although the models we used assume that competition for resources drives coexistence (20), other mechanisms such as chemical inhibition may also have contributed to competitive interactions in our system (Discussion).
We calculated each species’ sensitivity to competition for each replicate as the extent to which its per capita growth was reduced when invading a population of its competitor , compared with when it was grown in monoculture (20, 21):
| [2] |
When , this indicates that can invade , and when , cannot invade . We used these sensitivities to calculate the fitness difference (FD) and stabilizing difference (SD) for each species pair. Fitness differences cause species’ sensitivities to diverge (1, 2, 20) and are calculated for species indexed such that as the geometric standard deviation of the two species’ sensitivities to competition with one another:
| [3] |
Stabilizing differences are defined as differences that reduce both species’ sensitivities to interspecific competition and are calculated for species pair i and j as 1 minus the geometric mean of both species’ sensitivities:
| [4] |
When both species have low sensitivity to interspecific competition, the term under the square root will be small, and stabilizing differences will be positive. However, when both species are sensitive to interspecific competition such that invading growth rates are negative for both species, the term under the square root will be large, and stabilizing differences will be negative (i.e., destabilizing differences will result). Note that although and are properties of species and , respectively, fitness differences and stabilizing differences are properties of the species pair.
Eqs. 3 and 4 have been used previously to examine cases in which stabilizing differences are positive, and species pairs experience either coexistence or competitive exclusion (20, 21, 42). Here we extend this method to describe cases in which stabilizing differences are negative, to outline the relative values of fitness differences and stabilizing differences that produce priority effects vs. competitive exclusion. For priority effects to occur, both species must be unable to invade in the presence of an established population of the other, meaning that and are both greater than 1. Given that (above), this condition is met when . This inequality can be expressed in terms of fitness and stabilizing differences by dividing both sides by and simplifying, such that . This inequality can then be expressed in terms of fitness and stabilizing differences by using the definitions in Eqs. 3 and 4: . This inequality is represented by the line separating priority effects from competitive exclusion in Figs. 1 and 2. When , neither species can invade when rare, and priority effects occur. When stabilizing differences are negative but , only species (i.e., the species that is less sensitive to competition, as defined in Eq. 3) can invade when rare, and competitive exclusion occurs. We calculated fitness differences and stabilizing differences for each replicate and used these to determine the mean fitness differences and stabilizing differences for each species pair in each environment (SI Appendix).
Data Analysis.
To quantify the likelihood of competitive exclusion vs. priority effects in each nectar environment, we calculated the log ratio of fitness differences to stabilizing differences for each environment across all species pairs as log[FD/(1 − SD)]. When stabilizing differences are negative (all but three pair-by-environment combinations in this experiment), a value of this ratio >1 indicates that in that environment, species pairs fall above the line separating priority effects and competitive exclusion, and competitive exclusion occurs (middle zone of Fig. 1). When this ratio is <1, species pairs fall below the line, and priority effects occur (bottom left zone of Fig. 1). We excluded the three cases in which stabilizing differences were positive from this analysis.
To quantify the neutrality of competitive interactions in each nectar environment, we calculated the Euclidean distance between each point (i.e., the average fitness differences and stabilizing differences for each species pair-by-environment combination) and the point at which SD = 0 and FD = 1. This origin is characterized by fitness equivalence and an absence of stabilizing differences, indicating that species are functionally identical and that interactions are neutral (1).
To determine the effect of our pH and sugar treatments on competitive outcomes, the distance to neutrality, and fitness differences and stabilizing differences, we ran four separate mixed effects models. Each model had pH and sugar as predictors and either the log ratio of fitness differences to stabilizing differences, the distance to neutrality (SD = 0, FD = 1), fitness differences, or stabilizing differences as the response and species pair as a random factor (details in SI Appendix). All analyses were conducted in R (v. 3.2.4). Data supporting this research are available on the Dryad Digital Repository (48).
Supplementary Material
Acknowledgments
We thank M. Dhami and J. Wan for their assistance. We thank P. J. Ke, E. Mordecai, and the T.F., Peay, Mordecai, and B.G. laboratories for feedback. This work was funded by NSF Grants DEB 1149600 and 1737758 (to T.F.) and a Canada Graduate Scholarship and Michael Smith Foreign Study Supplement from the Natural Sciences and Engineering Research Council of Canada (to T.N.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data associated with this study are available on the Dryad Digital Repository (https://doi.org/10.5061/dryad.r5j0s3n).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803122116/-/DCSupplemental.
References
- 1.Adler PB, Hillerislambers J, Levine JM. A niche for neutrality. Ecol Lett. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 2.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 3.Levine JM, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- 4.Kraft NJ, Godoy O, Levine JM. Plant functional traits and the multidimensional nature of species coexistence. Proc Natl Acad Sci USA. 2015;112:797–802. doi: 10.1073/pnas.1413650112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angert AL, Huxman TE, Chesson P, Venable DL. Functional tradeoffs determine species coexistence via the storage effect. Proc Natl Acad Sci USA. 2009;106:11641–11645. doi: 10.1073/pnas.0904512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Germain RM, Weir JT, Gilbert B. Species coexistence: Macroevolutionary relationships and the contingency of historical interactions. Proc Biol Sci. 2016;283:20160047. doi: 10.1098/rspb.2016.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godoy O, Kraft NJ, Levine JM. Phylogenetic relatedness and the determinants of competitive outcomes. Ecol Lett. 2014;17:836–844. doi: 10.1111/ele.12289. [DOI] [PubMed] [Google Scholar]
- 8.Mordecai EA. Pathogen impacts on plant communities: Unifying theory, concepts, and empirical work. Ecol Monogr. 2011;81:429–441. [Google Scholar]
- 9.Fukami T, Mordecai EA, Ostling A. A framework for priority effects. J Veg Sci. 2016;27:655–657. [Google Scholar]
- 10.Ke P-J, Letten AD. Coexistence theory and the frequency-dependence of priority effects. Nat Ecol Evol. 2018;2:1691–1695. doi: 10.1038/s41559-018-0679-z. [DOI] [PubMed] [Google Scholar]
- 11.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Univ Press; Princeton: 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chase JM. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 2010;328:1388–1391. doi: 10.1126/science.1187820. [DOI] [PubMed] [Google Scholar]
- 13.Grainger TN, Rego AI, Gilbert B. Temperature-dependent species interactions shape priority effects and the persistence of unequal competitors. Am Nat. 2018;191:197–209. doi: 10.1086/695688. [DOI] [PubMed] [Google Scholar]
- 14.Kardol P, Souza L, Classen AT. Resource availability mediates the importance of priority effects in plant community assembly and ecosystem function. Oikos. 2013;122:84–94. [Google Scholar]
- 15.Peay KG, Belisle M, Fukami T. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc Biol Sci. 2012;279:249–258. doi: 10.1098/rspb.2011.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannette RL, Fukami T. Historical contingency in species interactions: Towards niche-based predictions. Ecol Lett. 2014;17:115–124. doi: 10.1111/ele.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera CM, Canto A, Pozo MI, Bazaga P. Inhospitable sweetness: Nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc Biol Sci. 2010;277:747–754. doi: 10.1098/rspb.2009.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vannette RL, Gauthier M-PL, Fukami T. Nectar bacteria, but not yeast, weaken a plant–Pollinator mutualism. Proc Biol Sci. 2013;280:20122601. doi: 10.1098/rspb.2012.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker CM, Fukami T. Environmental variability counteracts priority effects to facilitate species coexistence: Evidence from nectar microbes. Proc Biol Sci. 2014;281:20132637. doi: 10.1098/rspb.2013.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll IT, Cardinale BJ, Nisbet RM. Niche and fitness differences relate the maintenance of diversity to ecosystem function. Ecology. 2011;92:1157–1165. doi: 10.1890/10-0302.1. [DOI] [PubMed] [Google Scholar]
- 21.Narwani A, Alexandrou MA, Oakley TH, Carroll IT, Cardinale BJ. Experimental evidence that evolutionary relatedness does not affect the ecological mechanisms of coexistence in freshwater green algae. Ecol Lett. 2013;16:1373–1381. doi: 10.1111/ele.12182. [DOI] [PubMed] [Google Scholar]
- 22.Fukami T. Assembly history interacts with ecosystem size to influence species diversity. Ecology. 2004;85:3234–3242. [Google Scholar]
- 23.Alford RA, Wilbur HM. Priority effects in experimental pond communities: Competition between Bufo and Rana. Ecology. 1985;66:1097–1105. [Google Scholar]
- 24.Rasmussen NL, Van Allen BG, Rudolf VH. Linking phenological shifts to species interactions through size-mediated priority effects. J Anim Ecol. 2014;83:1206–1215. doi: 10.1111/1365-2656.12203. [DOI] [PubMed] [Google Scholar]
- 25.Chesson P. Quantifying and testing species coexistence mechanisms. In: Valladeres F, et al., editors. Unity in Diversity: Reflections on Ecology After the Legacy of Ramon Margalef. Fundacion Banco Bilbao Vizcaya Argentaria; Bilbao, Spain: 2008. pp. 119–164. [Google Scholar]
- 26.Didham RK, Watts CH, Norton DA. Are systems with strong underlying abiotic regimes more likely to exhibit alternative stable states? Oikos. 2005;110:409–416. [Google Scholar]
- 27.Fukami T, Nakajima M. Community assembly: Alternative stable states or alternative transient states? Ecol Lett. 2011;14:973–984. doi: 10.1111/j.1461-0248.2011.01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst. 2012;43:227–248. [Google Scholar]
- 29.Letten AD, Ke PJ, Fukami T. Linking modern coexistence theory and contemporary niche theory. Ecol Monogr. 2017;87:161–177. [Google Scholar]
- 30.Tilman D. Resource Competition and Community Structure. Princeton Univ Press; Princeton: 1982. [PubMed] [Google Scholar]
- 31.Callaway RM, Thelen GC, Rodriguez A, Holben WE. Soil biota and exotic plant invasion. Nature. 2004;427:731–733. doi: 10.1038/nature02322. [DOI] [PubMed] [Google Scholar]
- 32.Dhami MK, Hartwig T, Fukami T. Genetic basis of priority effects: Insights from nectar yeast. Proc Biol Sci. 2016;283:20161455. doi: 10.1098/rspb.2016.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barandica JM, et al. A mathematical model for toxin accumulation by killer yeasts based on the yeast population growth. J Appl Microbiol. 1999;86:805–811. doi: 10.1046/j.1365-2672.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 34.Greig D, Travisano M. The Prisoner’s Dilemma and polymorphism in yeast SUC genes. Proc Biol Sci. 2004;271(Suppl 3):S25–S26. doi: 10.1098/rsbl.2003.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannette RL, Fukami T. Dispersal enhances beta diversity in nectar microbes. Ecol Lett. 2017;20:901–910. doi: 10.1111/ele.12787. [DOI] [PubMed] [Google Scholar]
- 36.Molofsky J, Bever JD. A novel theory to explain species diversity in landscapes: Positive frequency dependence and habitat suitability. Proc Biol Sci. 2002;269:2389–2393. doi: 10.1098/rspb.2002.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leibold MA, et al. The metacommunity concept: A framework for multi‐scale community ecology. Ecol Lett. 2004;7:601–613. [Google Scholar]
- 38.Siepielski AM, Hung K-L, Bein EE, McPeek MA. Experimental evidence for neutral community dynamics governing an insect assemblage. Ecology. 2010;91:847–857. doi: 10.1890/09-0609.1. [DOI] [PubMed] [Google Scholar]
- 39.Hurtt GC, Pacala SW. The consequences of recruitment limitation: Reconciling chance, history and competitive differences between plants. J Theor Biol. 1995;176:1–12. [Google Scholar]
- 40.Godoy O, Levine JM. Phenology effects on invasion success: Insights from coupling field experiments to coexistence theory. Ecology. 2014;95:726–736. doi: 10.1890/13-1157.1. [DOI] [PubMed] [Google Scholar]
- 41.Lanuza JB, Bartomeus I, Godoy O. Opposing effects of floral visitors and soil conditions on the determinants of competitive outcomes maintain species diversity in heterogeneous landscapes. Ecol Lett. 2018;21:865–874. doi: 10.1111/ele.12954. [DOI] [PubMed] [Google Scholar]
- 42.Tan J, Yang X, Jiang L. Species ecological similarity modulates the importance of colonization history for adaptive radiation. Evolution. 2017;71:1719–1727. doi: 10.1111/evo.13249. [DOI] [PubMed] [Google Scholar]
- 43.Belisle M, Peay KG, Fukami T. Flowers as islands: Spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird-pollinated shrub. Microb Ecol. 2012;63:711–718. doi: 10.1007/s00248-011-9975-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pozo MI, Lachance M-A, Herrera CM. Nectar yeasts of two southern Spanish plants: The roles of immigration and physiological traits in community assembly. FEMS Microbiol Ecol. 2012;80:281–293. doi: 10.1111/j.1574-6941.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- 45.Baker H, Baker I. Amino-acids in nectar and their evolutionary significance. Nature. 1973;241:543–545. [Google Scholar]
- 46.Dhami MK, Hartwig T, Letten AD, Banf M, Fukami T. Genomic diversity of a nectar yeast clusters into metabolically, but not geographically, distinct lineages. Mol Ecol. 2018;27:2067–2076. doi: 10.1111/mec.14535. [DOI] [PubMed] [Google Scholar]
- 47.Letten AD, Dhami MK, Ke P-J, Fukami T. Species coexistence through simultaneous fluctuation-dependent mechanisms. Proc Natl Acad Sci USA. 2018;115:6745–6750. doi: 10.1073/pnas.1801846115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grainger TN, Letten A, Gilbert B, Fukami T. 2019 doi: 10.5061/dryad.r5j0s3n. Data from “Applying modern coexistence theory to priority effects.” Dryad Digital Repository. Available at . . Deposited February 14, 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.