Abstract
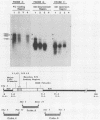
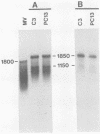
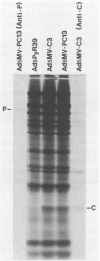
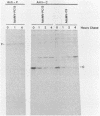
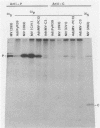
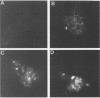
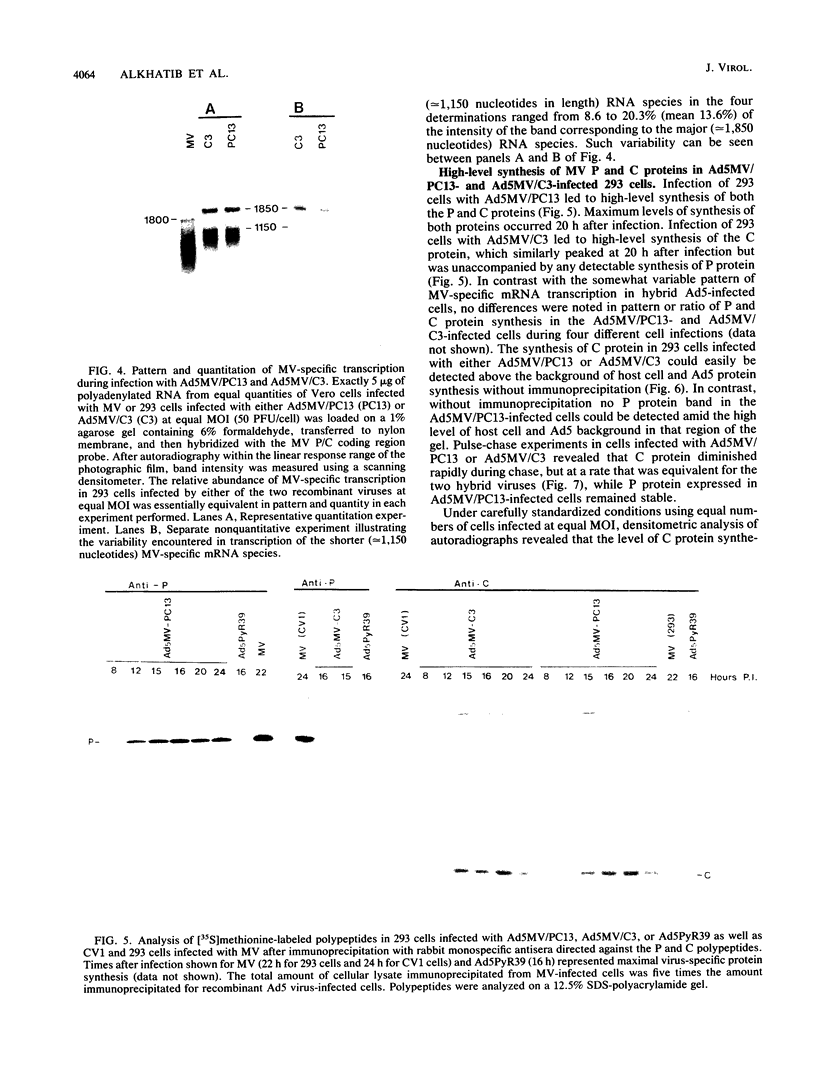
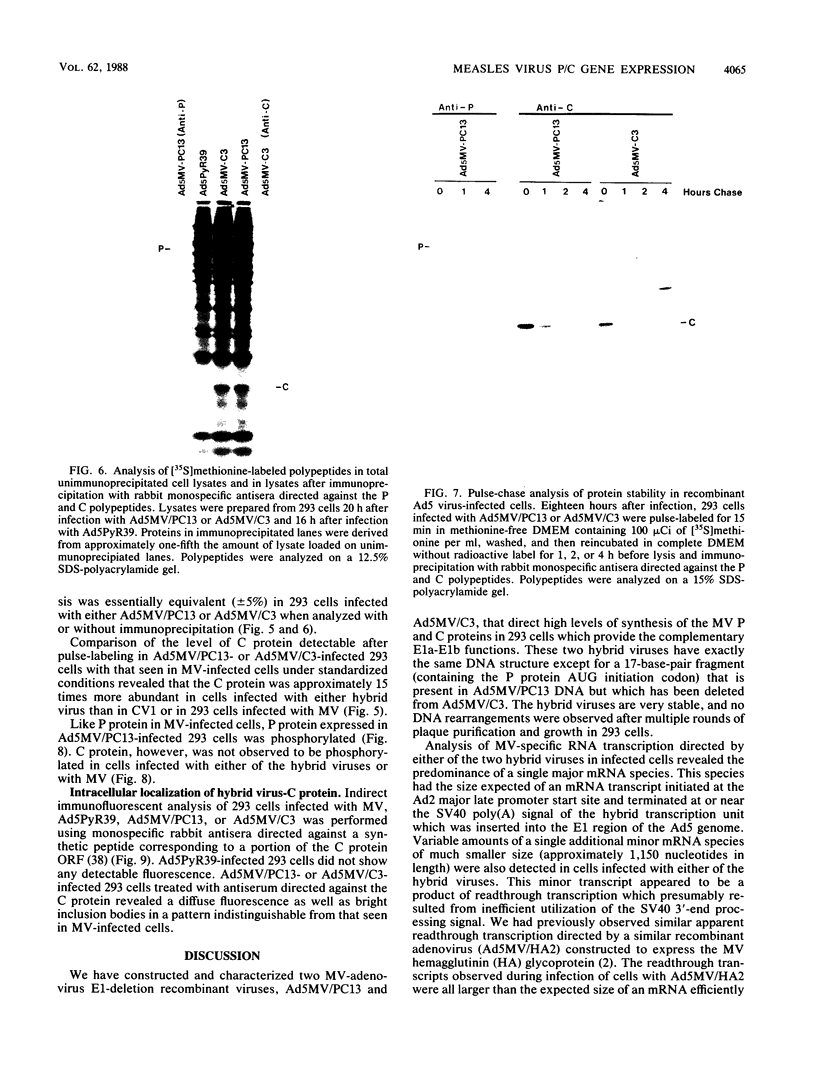
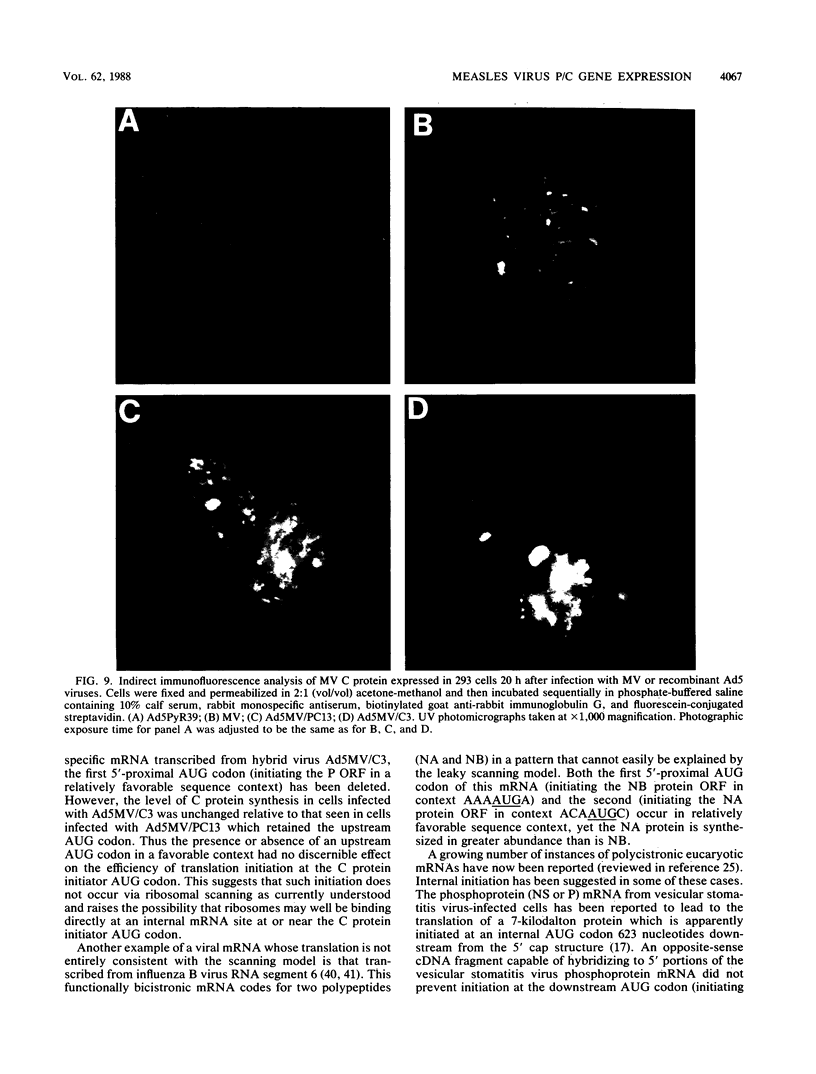
The measles virus (MV) P/C mRNA is functionally bicistronic. Translation is presumed to initiate at both the first and second 5'-proximal AUG codons, leading, respectively, to synthesis of the P and C polypeptides from different overlapping reading frames. To study the function and differential expression of these polypeptides, we have constructed hybrid human adenoviruses capable of expressing high levels of P and C together or of C alone. Cloned cDNA corresponding to the MV P/C gene was coupled to the adenovirus type 2 (Ad2) major late promoter, most of the Ad2 tripartite leader sequence, and the simian virus 40 3'-end processing signal and then used to replace most of the E1a-E1b region of the Ad5 genome in two hybrid adenoviruses: one (Ad5MV/PC13) which contained both 5'-proximal AUG codons of the P/C mRNA and another (Ad5MV/C3) which retained only the second. The sequence context for the P protein initiator AUG codon in Ad5MV/PC13 was made more favorable (GAGAUGG) than the relatively unfavorable context (CCGAUGG) seen in the native MV P/C mRNA. After infection of 293 cells (which provide complementary E1a-E1b functions), both viruses directed equal amounts of P/C-specific mRNA transcription. Ad5MV/PC13 directed the synthesis of both P and C proteins, while Ad5MV/C3 directed the synthesis of C protein alone. Ad5-expressed P protein was phosphorylated, while C was not. C protein had a similar diffuse cytoplasmic localization in both MV and Ad5-infected cells. Ad5MV/C3 and Ad5MV/PC13 directed equal amounts of C protein expression in 293 cells at a level approximately 15 times greater than that seen in MV-infected cells. Thus the level of C protein expression was unaffected by the presence or absence of an out-of-frame upstream AUG codon in a favorable sequence context. This observation cannot be explained by the scanning model for ribosomal initiation and suggests that ribosomes may be binding directly at an internal mRNA site at or near the initiator AUG codon for the C protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. High-level eucaryotic in vivo expression of biologically active measles virus hemagglutinin by using an adenovirus type 5 helper-free vector system. J Virol. 1988 Aug;62(8):2718–2727. doi: 10.1128/jvi.62.8.2718-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
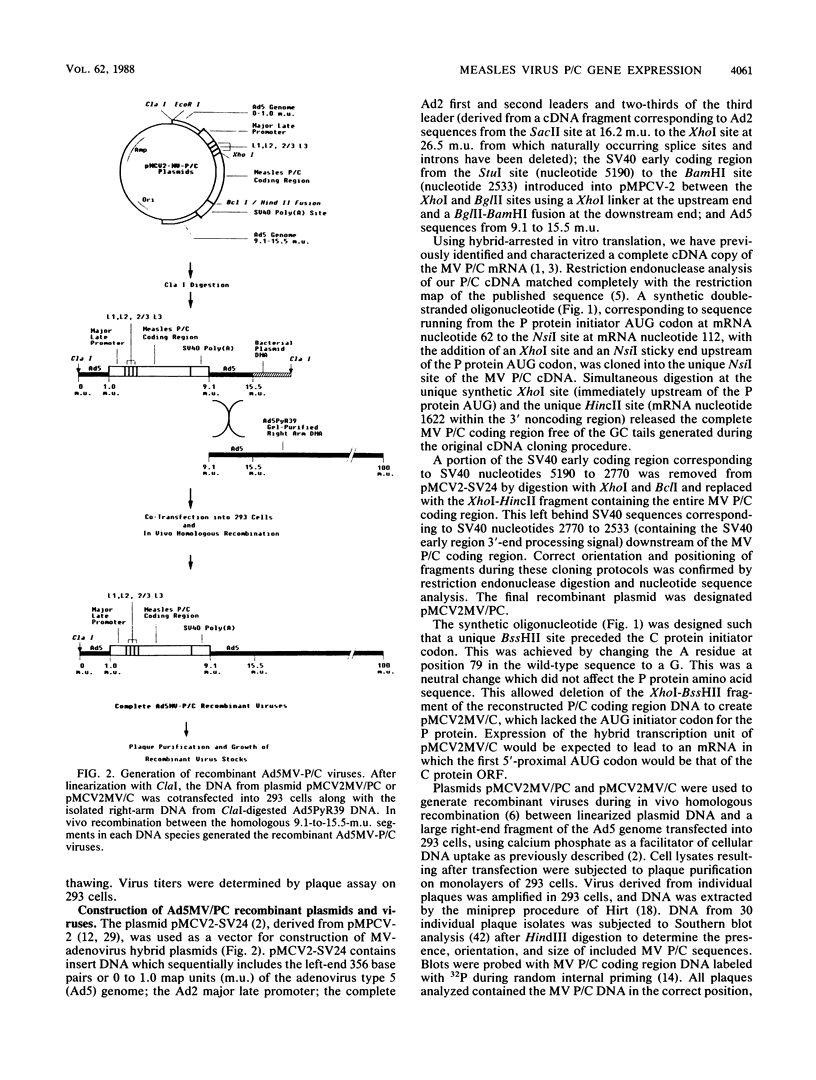
- Berkner K. L., Sharp P. A. Expression of dihydrofolate reductase, and of the adjacent EIb region, in an Ad5-dihydrofolate reductase recombinant virus. Nucleic Acids Res. 1984 Feb 24;12(4):1925–1941. doi: 10.1093/nar/12.4.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briedis D. J., Lamb R. A., Choppin P. W. Influenza B virus RNA segment 8 codes for two nonstructural proteins. Virology. 1981 Jul 30;112(2):417–425. doi: 10.1016/0042-6822(81)90289-0. [DOI] [PubMed] [Google Scholar]
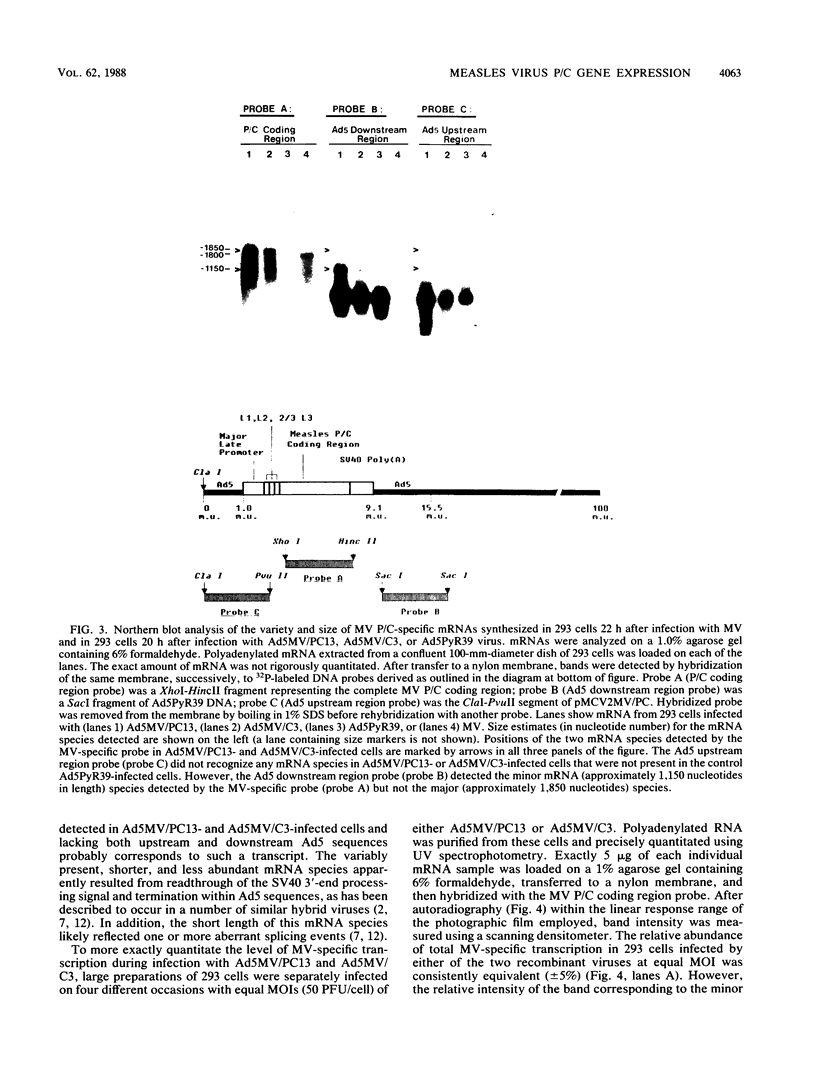
- Briedis D. J., Lamb R. A. Influenza B virus genome: sequences and structural organization of RNA segment 8 and the mRNAs coding for the NS1 and NS2 proteins. J Virol. 1982 Apr;42(1):186–193. doi: 10.1128/jvi.42.1.186-193.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Baczko K., ter Meulen V., Billeter M. A. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987 Oct;160(2):523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Davidson D., Hassell J. A. Overproduction of polyomavirus middle T antigen in mammalian cells through the use of an adenovirus vector. J Virol. 1987 Apr;61(4):1226–1239. doi: 10.1128/jvi.61.4.1226-1239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Hassin D., Korn R., Horwitz M. S. A major internal initiation site for the in vitro translation of the adenovirus DNA polymerase. Virology. 1986 Nov;155(1):214–224. doi: 10.1016/0042-6822(86)90181-9. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Internal initiation of translation on the vesicular stomatitis virus phosphoprotein mRNA yields a second protein. J Virol. 1986 Jun;58(3):797–804. doi: 10.1128/jvi.58.3.797-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the "scanning model" for initiation of protein synthesis in eucaryotes. Cell. 1980 Nov;22(1 Pt 1):7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J., Choppin P. W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk D., Sánchez A., Banerjee A. K. Messenger RNA encoding the phosphoprotein (P) gene of human parainfluenza virus 3 is bicistronic. Virology. 1986 Sep;153(2):318–325. doi: 10.1016/0042-6822(86)90036-x. [DOI] [PubMed] [Google Scholar]
- Massie B., Gluzman Y., Hassell J. A. Construction of a helper-free recombinant adenovirus that expresses polyomavirus large T antigen. Mol Cell Biol. 1986 Aug;6(8):2872–2883. doi: 10.1128/mcb.6.8.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Sakuma T., Kuwajima S., Yamamoto T. K., Iida H. Characterization of measles viruses in establishment of persistent infections in human lymphoid cell line. J Gen Virol. 1976 Dec;33(3):361–379. doi: 10.1099/0022-1317-33-3-361. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Choppin P. W. A comparison of the polypeptides of four measles virus strains. Virology. 1977 May 15;78(2):463–474. doi: 10.1016/0042-6822(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Gupta K. C., Seyer J. M., Beachey E. H., Kingsbury D. W. Localization and characterization of Sendai virus nonstructural C and C' proteins by antibodies against synthetic peptides. Virus Res. 1986 Nov;6(2):109–121. doi: 10.1016/0168-1702(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Berkovich A., Rozenblatt S., Bellini W. J. Use of antibodies directed against synthetic peptides for identifying cDNA clones, establishing reading frames, and deducing the gene order of measles virus. J Virol. 1985 Apr;54(1):186–193. doi: 10.1128/jvi.54.1.186-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A., Erickson B. W., Briedis D. J., Choppin P. W. Complete nucleotide sequence of the neuraminidase gene of influenza B virus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Sequence analysis of the P and C protein genes of human parainfluenza virus type 3: patterns of amino acid sequence homology among paramyxovirus proteins. J Gen Virol. 1986 Dec;67(Pt 12):2705–2719. doi: 10.1099/0022-1317-67-12-2705. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N. Segments of simian virus 40 DNA spanning most of the leader sequence of the major late viral messenger RNA are dispensable. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2556–2560. doi: 10.1073/pnas.76.6.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Cook K. A. Isolation and characterization of measles virus intracellular nucleocapsid RNA. J Virol. 1984 Jan;49(1):57–65. doi: 10.1128/jvi.49.1.57-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. C., Hirano A. Functional cDNA library for efficient expression of measles virus-specific gene products in primate cells. J Virol. 1986 Jan;57(1):343–348. doi: 10.1128/jvi.57.1.343-348.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]