Epstein-Barr virus represents an important human pathogen with an etiological role in the development of several cancers. By elucidation of a genome-wide polyadenylation landscape of EBV in JSC-1, Raji, and Akata cells, we have redefined the EBV transcriptome and mapped individual polymerase II (Pol II) transcripts of viral genes to each one of the mapped pA sites at single-nucleotide resolution as well as the depth of expression. By unveiling a new class of viral lytic RNA transcripts antisense to latent EBNAs, we provide a novel mechanism of how EBV might control the expression of viral latent genes and lytic infection. Thus, this report takes another step closer to understanding EBV gene structure and expression and paves a new path for antiviral approaches.
KEYWORDS: antisense RNA, Epstein-Barr virus, genome-wide approach, polyadenylation site mapping, RNA-seq, tumor virus
ABSTRACT
Epstein-Barr virus (EBV) is a ubiquitous human pathogen associated with Burkitt's lymphoma and nasopharyngeal carcinoma. Although the EBV genome harbors more than a hundred genes, a full transcription map with EBV polyadenylation profiles remains unknown. To elucidate the 3′ ends of all EBV transcripts genome-wide, we performed the first comprehensive analysis of viral polyadenylation sites (pA sites) using our previously reported polyadenylation sequencing (PA-seq) technology. We identified that EBV utilizes a total of 62 pA sites in JSC-1, 60 in Raji, and 53 in Akata cells for the expression of EBV genes from both plus and minus DNA strands; 42 of these pA sites are commonly used in all three cell lines. The majority of identified pA sites were mapped to the intergenic regions downstream of previously annotated EBV open reading frames (ORFs) and viral promoters. pA sites lacking an association with any known EBV genes were also identified, mostly for the minus DNA strand within the EBNA locus, a major locus responsible for maintenance of viral latency and cell transformation. The expression of these novel antisense transcripts to EBNA were verified by 3′ rapid amplification of cDNA ends (RACE) and Northern blot analyses in several EBV-positive (EBV+) cell lines. In contrast to EBNA RNA expressed during latency, expression of EBNA-antisense transcripts, which is restricted in latent cells, can be significantly induced by viral lytic infection, suggesting potential regulation of viral gene expression by EBNA-antisense transcription during lytic EBV infection. Our data provide the first evidence that EBV has an unrecognized mechanism that regulates EBV reactivation from latency.
IMPORTANCE Epstein-Barr virus represents an important human pathogen with an etiological role in the development of several cancers. By elucidation of a genome-wide polyadenylation landscape of EBV in JSC-1, Raji, and Akata cells, we have redefined the EBV transcriptome and mapped individual polymerase II (Pol II) transcripts of viral genes to each one of the mapped pA sites at single-nucleotide resolution as well as the depth of expression. By unveiling a new class of viral lytic RNA transcripts antisense to latent EBNAs, we provide a novel mechanism of how EBV might control the expression of viral latent genes and lytic infection. Thus, this report takes another step closer to understanding EBV gene structure and expression and paves a new path for antiviral approaches.
INTRODUCTION
Epstein-Barr virus (EBV) is ubiquitous in the human adult population, with estimates of >90% being infected. Primary infection in early adulthood leads to the establishment of life-long latency in memory B cells (1). Although EBV infection has generally no serious adverse effects on host health, it occasionally leads to cell transformation and carcinogenesis (2). EBV has been associated with a wide range of cancers, including Burkitt’s lymphoma, nasopharyngeal carcinoma, Hodgkin’s lymphoma, large diffuse B-cell lymphoma, gastric carcinoma, and several other rare lymphoid malignancies (3, 4).
Like other herpesviruses, EBV exhibits two distinct forms of infection: latent and lytic. Latent infection, required for virus persistence in an infected host, is a major contributor to EBV-mediated cell transformation (5) and can be grouped into types I, II, and III (6). It is characterized by limited expression of six EBV-encoded nuclear antigens (EBNA-1, -2, -LP [leader protein], -3A, -3B, and -3C), two latent membrane proteins (LMP-1 and -2), two small noncoding RNAs (EBER1 and -2), and a set of viral microRNAs (miRNAs). All EBNA proteins are expressed from a common transcript, spanning a large portion of the EBV genome, via usage of alternative promoters, splicing, and polyadenylation (7, 8). EBNA-1, EBERs, and BamHI A rightward transcripts (miR-BARTs) are expressed in all three types of latency (6). EBNA-1 is essential for maintenance of the episomal viral genome in infected cells during cell division (9–11). EBNA-2 is expressed in type III latency along with all other latent genes. It transactivates LMP-1 expression and functions with EBNA-LP to have a positive effect, and with EBNA-3s to have a negative effect, on EBNA-2 activity (12). LMP-1 and -2 expressed in both type II and III latency are transmembrane proteins promoting the proliferation of latently infected and transformed cells, and B-cell functions, by modulating host signaling pathways (5, 13). The noncoding EBERs and BART-derived viral miRNAs also contribute to latency by modulating host expression at the posttranscriptional level (14–16). The absence of latent gene expression is sometimes regarded as “0” (zero) latency (17). In contrast to latency, lytic infection is characterized by excessive expression of all viral genes and results in active virus replication and production of infectious virions (18).
The EBV genome is ∼170 kb in size and has the capacity to encode more than 100 different gene products, of which 69 EBV-encoded proteins have been recently identified by proteomic analysis (18). The reference EBV genome (GenBank accession no. NC_007605) was constructed using the B95-8 strain (19–21) as the backbone and by the addition of an 11-kb deleted segment from Raji sequences (22, 23). To facilitate all functions required for its complex life cycle and virus-host interactions, EBV encodes numerous gene products from its relatively small genome. Among the common strategies used by all herpesviruses to minimize sequence requirements, a single polyadenylation site (pA site) is often shared by multiple viral genes for polyadenylation of their RNA transcripts (24, 25). As a result, polyadenylation of a viral gene transcript often occurs downstream of several adjacent genes rather than immediately downstream of its gene body as observed in many eukaryotic transcripts. This genome structure resulting in the expression of colinear overlapping transcripts frequently poses a significant challenge for gene annotation, expression profiling, and functional genomic studies.
The majority of EBV transcripts are transcribed by host RNA polymerase II (Pol II) and are polyadenylated at a pA site like their host counterparts. A typical pA site for eukaryotic gene expression is defined by its cis sequence elements, including an upstream polyadenylation signal (PAS), generally represented by the canonical AAUAAA motif, and a downstream distal sequence element (DSE), rich in G or G/U (26, 27). Binding to these cis elements by specific polyadenylation factors facilitates RNA cleavage at a cleavage site (CS) located between the PAS and DSE (28) for RNA polyadenylation. The nontemplated polyadenylation tail is then added to a free 3′ end of the cleavage product to generate a mature polyadenylated mRNA transcript.
The distribution of viral polyadenylation signals was initially predicted in the EBV B95-8 genome (19), and several of the predicted ones were subsequently confirmed to be used for viral gene expression (29–34). The EBV transcriptome has been extensively studied recently by EBV arrays (35) and RNA sequencing (RNA-seq) (36–39). Although RNA-seq provides comprehensive information on the whole transcriptome on a genome-wide scale, it often fails to define the transcription start site (TSS) or RNA pA site due to variations in sequence coverage and overlapping expression in gene cluster regions as well as the lack of a decapping step for adaptor ligation to the RNA 5′ end. To overcome the RNA-seq shortages, a new cap analysis of gene expression (CAGE)-seq technology was recently developed, and 64 TSSs were identified in the EBV genome for viral replication (40). On the other hand, the use of classical techniques to determine a pA site, such as 3′ rapid amplification of cDNA ends (RACE) or RNase protection assays, is impractical as a genome-wide approach. In recent years, various efforts have been made to simultaneously map pA sites of whole transcriptomes (41–44). In this report, we applied a newly developed PA-seq method (44, 45) that was successfully used to map Kaposi’s sarcoma-associated herpesvirus (KSHV) genome-wide pA sites (25, 46) and generated a comprehensive atlas of all pA sites and their usage for EBV genome expression from latency to lytic infection in three EBV-positive (EBV+) cell lines. Analysis of the mapped pA sites in association with currently annotated genes led us to identify a new set of distinct polyadenylated transcripts antisense to various forms of EBNA.
RESULTS
Active EBV expression in JSC-1, Raji, and Akata cells revealed by PA-seq.
To map the genome-wide pA sites and their usage of EBV transcripts, three EBV-positive cell lines, EBV- and KSHV-coinfected JSC-1 (47), EBV nonproducer Raji (48), and EBV producer Akata (49), from latent and lytic infections, were used for the study by PA-seq analysis. The three-EBV-genome alignment in Fig. S1 in the supplemental material shows that the Raji EBV genome has two large deletions, first from nucleotides (nt) 87069 to 90217 (3,148 bp) and then from nt 163986 to 166643 (2,657 bp) (50), but fewer repetitive sequences from nt 170351 to 172550 (22). The Akata EBV genome has fewer repetitive sequences from nt 96351 to 97100 and has two small deletions, first from nt 88144 to 88248 and then from nt 90706 to 90733 (51). The EBV reference genome (GenBank accession no. NC_007605) (19, 22, 23) also has two small deletions, first from nt 88617 to 88772 and then from nt 90706 to 90733, compared with the Raji and Akata genomes (Fig. S1).
EBV lytic reactivation in JSC-1 cells was induced by n-butyrate, that in Raji cells was induced by a combination of both butyrate and 12-O-tetradecanoylphorbol-13-acetate (TPA) (52), and that in Akata cells was induced by surface receptor cross-linking by the addition of goat anti-human serum to the culture medium (49, 53). The most effective method for lytic EBV induction in Akata cells, under multiple tested conditions, was to treat the cells for 24 h with a 1:200 dilution of whole serum, as measured by the expression of the lytic BMRF1/2 gene. The total RNAs from both uninduced (latent) and induced (lytic) cells were harvested for construction of PA-seq libraries (46). The PA-seq libraries from JSC-1 cells were obtained from our previous study and were successfully used to determine KSHV genome-wide pA sites with high levels of accuracy and specificity (25).
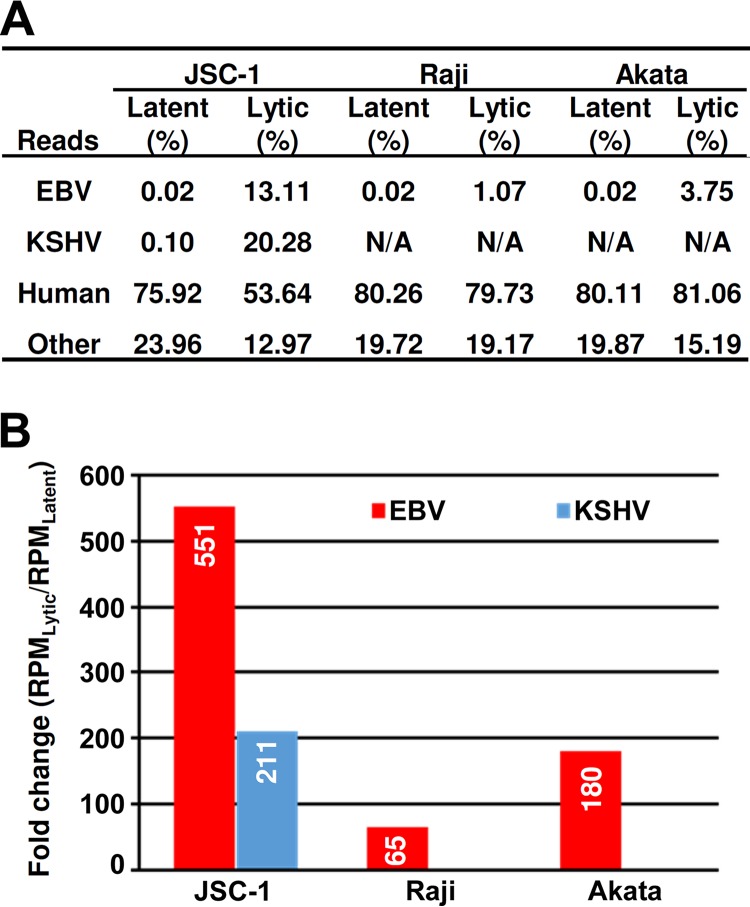
To identify EBV pA sites, we first extracted EBV-specific reads by alignment of the PA-seq reads derived from these libraries to the EBV reference genome (GenBank accession no. NC_007605) (23). We identified 2,001,977 sequence reads in JSC-1 cells, 1,701,835 sequence reads in Raji cells, and 4,925,618 sequence reads in Akata cells uniquely mapped to the EBV genome (Table S1). Among these EBV reads, 3,233 reads in JSC-1, 27,875 reads in Raji, and 29,557 reads in Akata cells were identified from uninduced cells with latent EBV infection, and 1,998,744 reads in JSC-1, 1,673,960 reads in Raji, and 4,896,061 reads in Akata cells were from induced cells with lytic EBV infection (NCBI accession no. SRP157138), representing 0.02% and 1.07% to 13.11% of the total PA-seq reads, respectively (Fig. 1A and Table S1). We noticed that the numbers of EBV reads in JSC-1 cells were smaller than the numbers of KSHV reads obtained from the same libraries. Although the total numbers of EBV-specific reads were lower than those mapped to KSHV, the ratio (>500) of lytic EBV reads from the butyrate-treated cells over the latent EBV reads from the untreated cells was much higher than that (>200) of KSHV lytic reads over the latent reads (Fig. 1B). We also noticed that the level of active expression of EBV lytic genes in JSC-1 cells was much higher than that in Raji and Akata cells upon lytic induction, despite all three cell lines exhibiting similar levels (∼0.02%) of latent gene expression.
FIG 1.
EBV expression in JSC-1, Raji, and Akata cells. (A) Percentage of total reads uniquely mapped to the EBV reference genome (GenBank accession no. NC_007605), the KSHV genome (GenBank accession no. U75698), and human (hg19) reference genomes in PA-seq libraries constructed from JSC-1, Raji, and Akata cells with latent or lytic infection. N/A, not applicable. (B) Simultaneous reactivation of lytic KSHV and EBV infection in JSC-1 cells and EBV lytic infection in Raji and Akata cells followed by lytic reactivation. The level of virus reactivation was determined by the fold change of virus-specific reads between lytic and latent libraries after normalization to the total library size in millions (RPM, reads per million).
Genome-wide distribution of EBV polyadenylation sites.
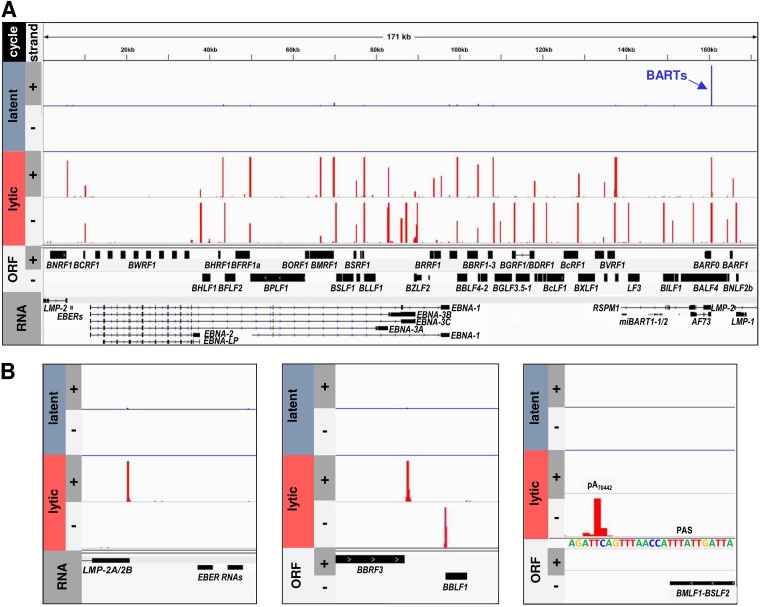
To determine the nucleotide position and usage frequency of individual EBV pA sites, the mapped EBV reads from the cells with latent or lytic infection were first visualized against the EBV reference genome (GenBank accession no. NC_007605) for both viral DNA strands by using IGV software. The EBV reference genome is a hybrid genome sequence composed of the reference EBV B95-8 strain and the Raji strain. As expected, we observed only a few viral reads from each cell line with latent EBV infection but a large number of viral reads from the cell lines with lytic induction, as represented by JSC-1 cells (Fig. 2A). Although the viral reads were scattered along the viral genome, a cluster of the EBV reads can be visualized as a distinct peak on both EBV DNA strands immediately downstream of the majority of annotated open reading frames (ORFs) (Fig. 2). In uninduced JSC-1 cells, a majority of the reads were derived from transcripts of BART, a known EBV latency-associated transcript (54, 55). Analysis of the selected peaks showed that the obtained EBV reads specifically distribute only downstream of polyadenylated Pol II transcripts like LMP-2A/2B. None of the reads were mapped downstream of the nonpolyadenylated Pol III-derived EBER1 and EBER2 RNAs (Fig. 2B, left), two abundant small noncoding EBV RNAs of 167 and 172 nt, respectively (56, 57). Importantly, the clustered reads’ peak could be annotated on the EBV genome in a strand-specific manner and usually downstream of an annotated ORF, such as BBRF3 and BBLF1 (Fig. 2B, middle). In general, the peak heights could be correlated with the expression levels of individual viral genes, as exemplified by BBRF3 by comparing its reads in latent versus lytic infections. Most of the clustered read peaks were mapped to a previously identified pA site (29–34), like the peak downstream of BMLF1-BSLF2 at the position of a previously mapped pA site at nt 70442 (minus strand [−]) (32) (Fig. 2B, right). Thus, this analysis showed that the clustered peaks of PA-seq reads represent the pA sites of EBV RNA transcripts.
FIG 2.
Distribution of PA-seq reads on the linear reference EBV genome. The PA-seq reads from JSC-1 cells without (latent) or with (lytic) butyrate treatment were aligned to the reference EBV genome (GenBank accession no. NC_007605). The nucleotide positions and frequencies of all mapped pA reads in the plus (+) and minus (−) strands of the double-stranded EBV genome (A) or the selected genome regions (B) in latent (blue bars) or lytic (red bars) samples were visualized using IGV software. The scale was set to a maximum of 200 reads.
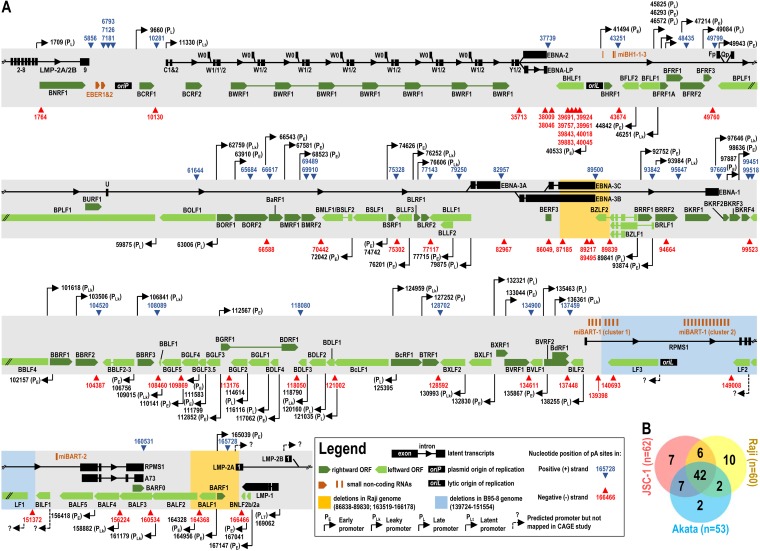
Subsequently, we performed a peak-calling analysis using F-Seq (25, 46). Obtained peaks represent the clusters of PA-seq reads from a specific region where heterogeneity of cleavage sites exists, as normally seen in KSHV and mammalian RNA polyadenylation (25, 58). Each identified peak was then redefined by two additional parameters: peak mode and usage. The peak mode corresponds to the nucleotide position with the highest number of reads within a clustered individual peak. This represents the most prevalent cleavage site used for polyadenylation and, therefore, is further referred to as a “pA site.” The pA site usage is a sum of all reads within each clustered peak and correlates with the relative expression levels of the RNA transcripts. After peak-calling analysis, we eliminated all peaks resulting from internal priming to genomic A-rich regions and those peaks having fewer than 50 reads in all samples. Consequently, the total numbers of PA-seq reads mappable to the identified pA sites were 1,387,309 reads (including 1,725 latent EBV reads) for the JSC-1 EBV genome, 1,621,448 reads (including 18,940 latent EBV reads) for the Raji EBV genome, and 4,797,216 (including 28,590 latent EBV reads) for the Akata EBV genome (see Table S1 in the supplemental material). Using these criteria, we identified a total of 62 pA sites from the JSC-1 EBV genome, 60 pA sites from the Raji EBV genome, and 53 pA sites from the Akata EBV genome (Table S1). We used IGV software to visualize the distribution of individual pA sites across the EBV reference genome (GenBank accession no. NC_007605), mapping 27 pA sites to the plus strand (+) and 35 sites to the minus strand of the JSC-1 EBV genome, 26 pA sites to the plus strand and 34 pA sites to the minus strand of the Raji EBV genome, and 26 pA sites to the plus strand and 27 pA sites to the minus strand of the Akata EBV genome (Fig. 3A). Among them, 42 pA sites were consistently found in all three EBV genomes (Fig. 3B). We also determined genome-specific pA site positions after mapping PA-seq libraries to the complete Raji (GenBank accession no. KF717093) and Akata (GenBank accession no. KC207813) EBV genomes (Table S2) and compared them to the reference EBV genome (GenBank accession no. NC_007605). Although the mapped genome positions of the individual pA sites vary from the reference EBV genome to the Raji and Akata genomes, and to the few previously reported pA sites based on the B95-8 genome (29–34), these data indicate that the mapped pA sites represent a comprehensive atlas of EBV pA sites used for the expression of their corresponding genes in these transformed B cells.
FIG 3.
Genome-wide distributions of the mapped pA sites in the context of the EBV reference genome (GenBank accession no. NC_007605). All EBV pA sites identified by PA-seq in at least one cell line with a frequency of >50 reads are shown in panel A, with a blue or red triangle representing the mapped pA site at a nucleotide position by number from either the plus DNA strand (+) (blue) or the minus DNA strand (−) (red) at the EBV genome. Dark (plus strand) and light green (minus strand) arrows or boxes stand for the lytic gene transcripts, black arrows and boxes indicate the latent gene transcripts, and orange bars or arrows indicate the noncoding small RNA transcripts. The reported promoter positions based on the EBV B95-8 genome (GenBank accession no. V01555) are shown as black arrows according to CAGE-seq analysis of EBV p2089 bacterial artificial chromosome (BAC)mid-infected HEK293 cells (40) after being realigned to the reference EBV genome (GenBank accession no. NC_007605). (B) Cell line-specific use of EBV pA sites. The Venn diagram shows the number of pA sites used in each EBV genome.
Comparing the mapped pA sites in the JSC-1 EBV genome (Fig. 3B), the Raji EBV genome utilizes the 48 pA sites as seen in the JSC-1 EBV genome but misses 6 pA sites from the plus strand at nt 10281, 48435, 79250, 89500, 99451, and 165728 and 8 pA sites from the minus strand at nt 10130, 35713, 66588, 87158, 89217, 89495, 94664, and 164368, whereas the Akata EBV genome shares 49 pA sites as seen in the JSC-1 genome but misses 3 pA sites from the plus strand at nt 37739, 65684, and 99451 and 10 pA sites from the minus strand at nt 35713, 39843, 39961, 66588, 82967, 86049, 87185, 89217, 89495, and 94664 (Table S1). A cluster of the eight mapped pA sites from nt 39691 (−) to nt 40045 (−) in the BHLF1 coding region seen in Raji, but not Akata, cells suggests that they are not essential for lytic EBV induction. A similar implication could also be applied to the pA sites at nt 7126 (+) and nt 7181 (+) seen only in Raji but in neither JSC-1 nor Akata cells. The heterogeneity in the compositions of EBV pA sites among the three cell lines may be due to viral genome polymorphism, variable numbers of internal repeats, and cell line-specific deletions.
Usage of EBV pA sites from viral latent to lytic infection in JSC-1, Raji, and Akata cells.
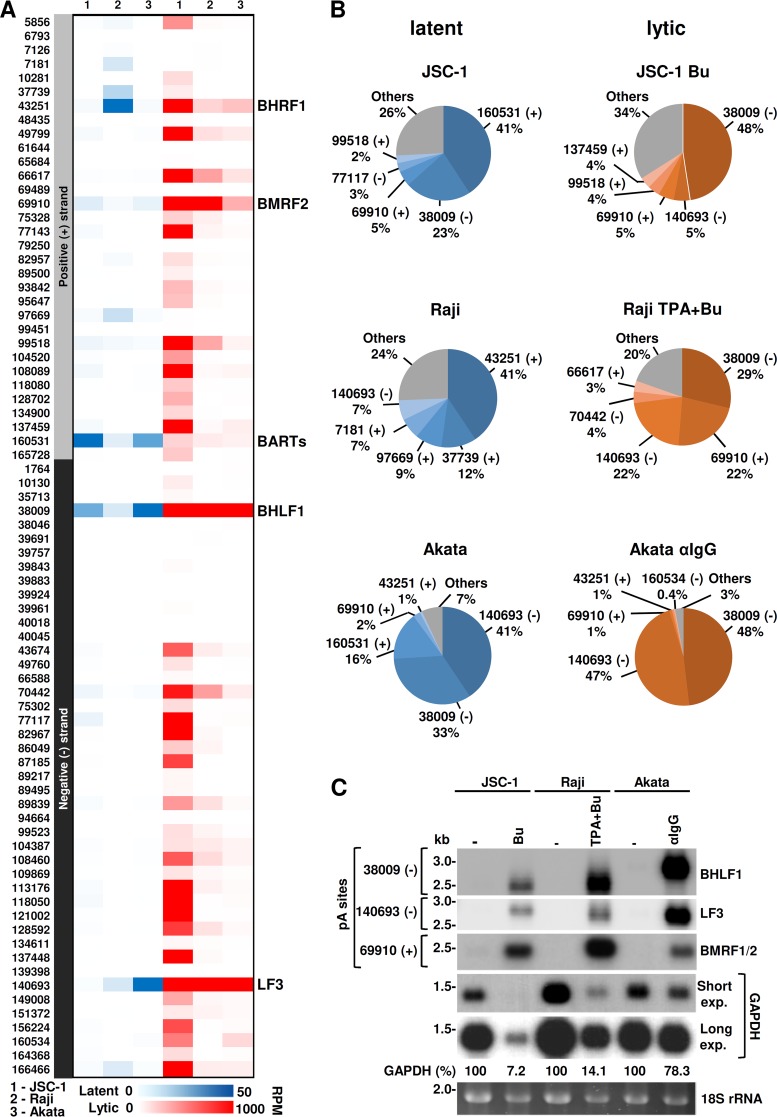
Based on the number of reads mapped to each peak, referred to as pA site usage, we next assessed the relative expression levels of EBV transcripts polyadenylated at each identified pA site. The usage for each pA site was first normalized to library coverage by dividing the number of raw reads by the total number of reads of each library, in millions, to obtain RPM (reads per million). Normalized RPM were then used to compare pA site usages within or between individual samples from the three cell lines. As shown in Fig. 4A and Table S1 in the supplemental material, all three cell lines exhibited a distinct expression pattern of latent and lytic genes, with a limited number of reads in latent infection compared to a large number of reads in lytic induction. Although Raji cells (type III latency) (59) exhibited more expression of the EBV latent genes than JSC-1 (type II latency) (47) or Akata (type I latency) (35, 49) cells, the pA site usage at nt 160531 (+) was found in all three cell lines for the expression of latent BART transcripts (also called RPMS1/A73/BARF0 transcripts). BART transcripts encode a cluster of EBV-derived miRNAs essential for the establishment and maintenance of latency (60). The top five most-used pA sites quantified as a percentage of the total EBV reads from cells with latent infection are shown in Fig. 4B (left): for JSC-1 cells, 41% at nt 160531 (+) for BART expression, 23% at nt 38009 (+) for BHLF1, 5% at nt 69910 (+) for BMRF2, 3% at nt 77117 (−) for BLLF1/2, and 2% at nt 99518 (+) for BKRF4; in Raji cells, 41% at nt 43251 (+) for BHRF1, 11% at nt 37739 (+) for EBNA-2/EBNA-LP, 9% at nt 97669 (+) for EBNA-1, 7% at nt 7181 (+) for an unannotated gene, and 6% at nt 140693 (−) for LF3; and in Akata cells, which displayed the most bias usage of the three pA sites, 41% at nt 140693 (−) for LF3, 33% at nt 38009 (−) for BHLF1, and 16% at nt 160531 (+) for BART.
FIG 4.
Kinetics of EBV gene expressions according to pA site usage. (A and B) Relative expression levels of the annotated viral genes associated with each mapped pA site during EBV latent and lytic infection in individual cell lines. (A) Heat map by color scale showing the gene expression abundance according to the mapped pA site usage in each cell line during latent (blue scale) and lytic (red scale) infection. Numbers on the left are nucleotide positions of the mapped pA sites in the plus (+) or minus (−) strand of the EBV genome. On the right are the representative genes using the mapped pA site. (B) The top five most-used pA sites in latent (blue pies) or lytic (orange pies) EBV infection in each cell line. The pie charts show the percentages of all PA-seq reads mapped to the top five most-used pA sites, with “Others” representing the percentage of combined reads mapped to all remaining pA sites (see details in Table S1 in the supplemental material). (C) Expression of the gene transcripts associated with the three most-used pA sites. Northern blot analysis using a probe derived from the BHLF1 (oVM417), LF3 (oVM418), BMRF1/2 (oVM377), or GAPDH (oZMZ70) gene was performed on total RNA isolated from each cell line with latent (−) or lytic infection induced by butyrate (Bu), 12-O-tetradecanoylphorbol-13-acetate (TPA), or anti-human serum (αIgG). GAPDH RNA and ethidium bromide-stained 18S rRNA served as RNA sample loading controls.
Expression during lytic viral infection was more consistent among all three tested cell lines, although JSC-1 cells displayed usage of the most pA sites for expression of the corresponding lytic genes compared to Raji or Akata cells (Fig. 4A and Table S1). Three of the top five pA sites (Fig. 4B, right) were located at nt 38009 (−) for BHLF1, nt 140693 (−) for LF3 and nt 69910 (+) for BMRF2 transcripts, which accounted for 5% to 48% of the total pA-seq reads. When combined, the read counts from the three pA sites were 58% for JSC-1, 73% for Raji, and 95% for Akata cells (Fig. 4B and Table S1). Surprisingly, in Akata cells, the reads from nt 38009 (−) for BHLF1 and from nt 140693 (−) for LF3 over the total pA site reads accounted for 48% and 47%, respectively. In contrast, variations in the usage of the nt 69910 (+) pA site for BMRF2 expression were noticed from Akata (1%) and JSC-1 (5%) to Raji (22%) cells (Fig. 4B). These pA sites, also expressed during latent infection, displayed a several-100-fold increase in the number of PA-seq reads between latent and lytic infections. Thus, the data suggest that they are truly inducible genes during lytic EBV infection. We also observed that lytic induction of Raji cells, but not JSC-1 or Akata cells, blocked the pA site usage at nt 97669 (+) for the expression of latent EBNA-1 and at nt 37739 (+) for EBNA-2 (Fig. 4A and Table S1).
We next compared the fold changes (FCs) in viral gene expression associated with the switch from latent to lytic infection by calculation of the normalized pA site usage from EBV lytic infection over that from latent infection as a ratio of RPMLytic/RPMLatent. As shown in Table S1 in the supplemental material, expression of the pA site at nt 137448 (−) for BILF2 (viral glycoprotein) had the highest increase of 5,183-fold, followed by the second highest increase of 4,698-fold for the pA site at nt 140693 (−) for LF3 transcripts after EBV reactivation in JSC-1 cells. However, the highest FCs from the latent-to-lytic pA site switch were for the pA sites at nt 89839 (−), with a 2,503-fold increase, for BRLF1 (Rta)/BZLF1 (Zta) and at nt 128592 (−), with a 1,570-fold increase, for BXLF1 (thymidine kinase)/BXLF2 (gH protein) in Raji cells and for the pA sites at nt 38009 (−), with a 262.3-fold increase, for BHLF1 (noncoding RNA) and at nt 140693 (−), with a 210-fold increase, for LF3 (noncoding RNA) in Akata cells. The lowest expression level changes for two pA sites clearly distinguishable from the rest in all three cell lines were the pA sites at nt 97669 (+) for BKRF1/EBNA-1 and at nt 160531 (+) for BART transcripts with FCs of only 2.9- and 3.4-fold in JSC-1, 3.1- and 14.6-fold in Raji, and 1.7- and 1.6-fold in Akata cells after virus reactivation. Therefore, EBNA-1 and BART transcripts are two true latent transcripts in all three cell lines. The pA site usages at nt 166466 (−) and at nt 5856 (+) for the expression of two classical EBV latent genes, LMP-1 and LMP-2A/B (61), were increased by 1,331- and 455-fold in JSC-1 but 11- and 7-fold in Raji and 68- and 33-fold in Akata cells, respectively, during virus lytic induction. These differences in the increased expression of LMP-1 and LMP-2A/B in lytic induction could be due to the induced expression of lytic BNLF2 and BNRF1 for their usage of the same pA site. Similarly, the pA site at nt 43251 (+) for the expression of BHRF1 (bcl-2 homolog)/miBH1-1-3 from latent to lytic infection was increased only 3.7-fold in Raji but 930-fold in JSC-1 and 147-fold in Akata cells. The pA site at nt 82957 (+) for EBNA-3A was relatively inducible in all three cell lines, but the pA sites at nt 89500 (+) for EBNA-3B and -3C expression were also relatively inducible only in JSC-1 and Akata but not in Raji cells with a deletion in this EBV region (62).
To verify if pA site usage truly mirrors EBV lytic expression, we performed Northern blot analysis using total RNA from all three cell lines prepared under the same conditions as those used for PA-seq. The transcripts polyadenylated at the most prevalent pA sites at nt 38009 (−), nt 140693 (−), and nt 69910 (+) were detected by antisense oligonucleotide probes located in BHLF1, LF3, and BMRF1/2 loci. As shown in Fig. 4C, the highly induced expressions of BHLF1, LF3, and BMRF1/2 were detected as single species of RNA transcripts of the expected size from all three cell lines with lytic EBV induction, although more host shutoffs by lytic EBV induction or toxicity of chemical inducers were observed for JSC-1 and Raji cells, as indicated by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA levels. Based on the expected size, the detected BMRF transcripts were viewed as bicistronic BMRF1/2 transcripts because the mapped pA site at nt 69489 (+) for the expression of BMRF1 in Raji cells was not used in JSC-1 cells or little used in Akata cells (Table S1). Moreover, BHLF1 RNA in Akata cells showed a larger band size than that from JSC-1 or Raji cells (Fig. 4C) because of the presence of an additional 125-bp GC-rich repeat in this region in the Akata EBV genome (Fig. S1).
RNA cis elements in regulation of EBV expression and polyadenylation.
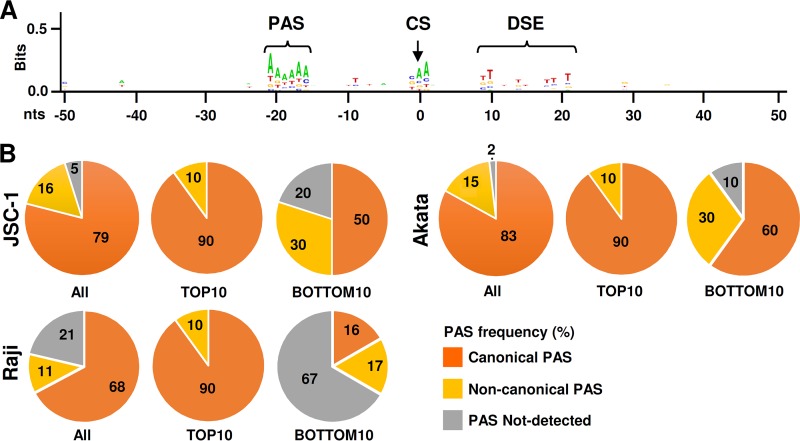
To determine the cis elements in regulation of EBV polyadenylation, we analyzed the sequence flanking each identified pA site within a range of −50 to +50 nt from each pA site. First, we performed an alignment of all sequences using the sequence logo algorithm to identify any consensus motifs surrounding the mapped pA sites. As shown in Fig. 5A, an A/T-rich sequence motif was found to be highly conserved around −20 to −15 nt upstream of the mapped pA site, and a T/G-rich sequence motif was enriched +10 to +20 nt downstream of the mapped pA site. These motifs coincide with the PAS and DSE commonly found in mammalian transcripts (63, 64). Since the PAS plays a critical role in pA site selection, by scanning 50 nt upstream of each mapped pA site for a PAS signal sequence, we identified the presence of a PAS in 59 of the 62 mapped pA sites in the JSC-1 EBV genome, 48 of the 60 pA sites in the Raji EBV genome, and 52 of the 53 pA sites in the Akata EBV genome (see Table S1 in the supplemental material). Among the mapped pA sites, 49 (79%) are the canonical “AAUAAA” PAS and 10 (16%) are noncanonical PAS variants (AAUACA, AUUAAA, GGUAAA, GAUAAA, AGUACA, CAUAAA, CAUAUA, and UAUAAA) in JSC-1 cells, 41 (68%) are canonical and 7 (11%) are noncanonical in Raji cells, and 44 (83%) are canonical and 8 (15%) are noncanonical in Akata cells. We were unable to identify any PAS or PAS variants in 3 pA sites in JSC-1 cells, 13 (including those in the BHLF1 region) in Raji cells, and only 1 in Akata cells (Fig. 5B and Table S1). The overall PAS prevalence in association with the mapped pA sites is close to those observed in KSHV and eukaryotic pA sites (25, 64, 65). Among the top 10 most-used pA sites identified in lytic EBV infection in all three cell lines, 90% bear a canonical PAS upstream. However, in the least-used bottom 10 pA sites, only 50 to 60% in JSC-1 and Akata cells, and 16% in Raji cells, use a canonical PAS (Fig. 5B). We observed a positive correlation between the pA site expression level and PAS sequence conservation in expression of the EBV genome (Table S1). A similar correlation was observed for KSHV expression (25). Together, these data indicate that EBV polyadenylation sites are defined by common regulatory elements found in host transcripts and that the sequence composition surrounding the mapped pA site directly correlates with the level of viral gene expression.
FIG 5.
Regulatory elements of mapped pA sites in the EBV genome. (A) Conservation of the cis regulatory elements surrounding EBV pA sites. The sequence logo was generated from DNA sequences ±50 nt from each pA site using WebLogo (http://weblogo.berkeley.edu/logo.cgi). PAS, polyadenylation signal; CS, cleavage site; DSE, distal sequence element. (B) Roles of polyadenylation signals in EBV expression. Numbers in each pie chart are the frequencies (percent) of PAS variants (canonical, noncanonical, or no detectable PAS) calculated for all pA sites (see Table S1 in the supplemental material).
Verification of PA-seq-identified pA sites.
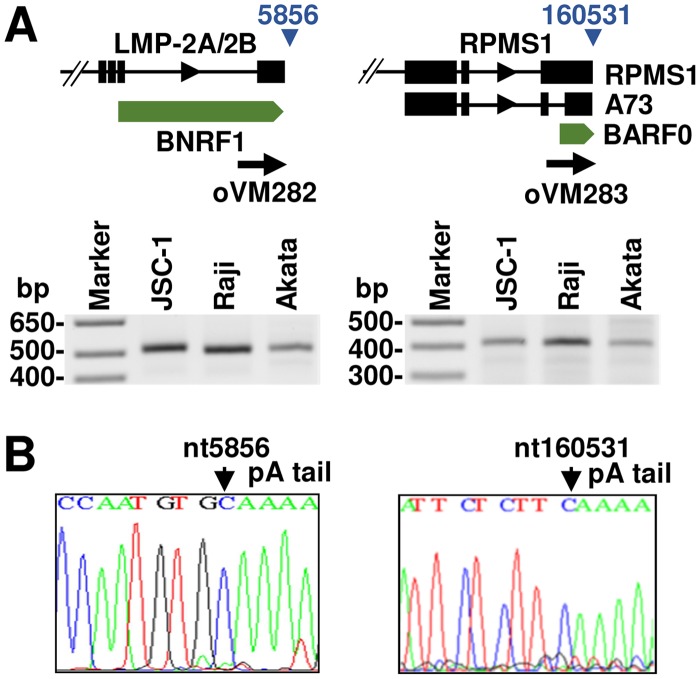
To confirm mapping accuracy, we compared the identified pA sites with several previously reported pA sites, including pA sites for LMP-2A/2B, BZLF1, EB2 (BMFL1-BSLF2), and LF3 transcripts (29–32). In all cases, we observed a high correlation between the PA-seq-identified pA site and the reported pA site. In addition, we experimentally confirmed the pA sites at nt 5856 (+) for LMP-2A/2B expression and at nt 160531 (+) for BART (RPMS1/A73/BARF0) expression by 3′ RACE on total RNA from three EBV-positive cell lines, JSC-1, Raji, and Akata. In all cases, two pA sites determined by 3′ RACE were matched to those mapped by PA-seq and were identical in all three cell lines with lytic EBV infection (Fig. 6), indicating that the EBV pA site usage in JSC-1 cells also coinfected with KSHV is not different from that of Raji or Akata cells infected with only EBV. Altogether, we have confirmed a high accuracy of pA sites identified by PA-seq.
FIG 6.
Experimental validation of selected pA sites. (A) Diagram showing the gene structures of LMP-2A/2B and BART transcript loci and the nucleotide positions of the identified pA sites (blue triangles). 3′ RACE was carried out on total RNA from lytically induced JSC-1, Raji, and Akata cells using gene-specific primers (black thick arrows); the RACE products were separated by agarose gel electrophoresis. (B) The identity of the resulting RACE product (A) was determined by Sanger sequencing after TA cloning.
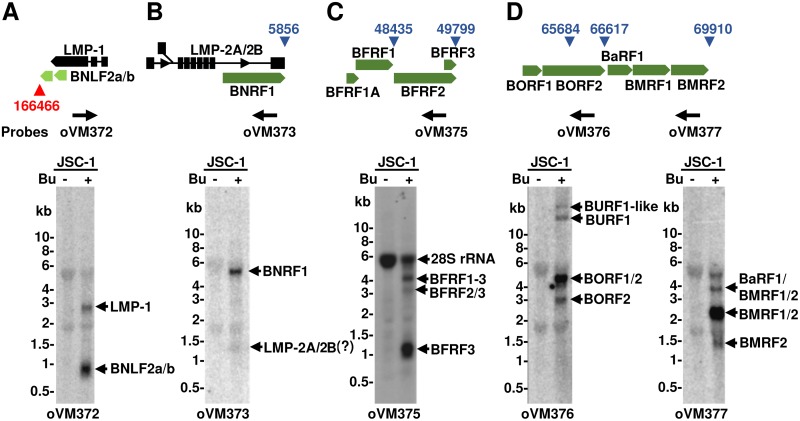
Taking into account the nucleotide position and strand specificity of the pA sites mapped by PA-seq, we next analyzed the position of each identified pA site in the context of annotated EBV genes. As expected, the majority of pA sites were mapped to the regions immediately downstream of the assigned ORFs on both plus (sense) and minus (antisense) DNA strands. Interestingly, many were mapped downstream of a gene clustering locus containing multiple colinear genes. Their unique usage for RNA polyadenylation in this gene cluster leads to the production of multiple polycistronic RNA transcripts commonly observed in all herpesviruses (25) and papillomaviruses (66, 67). To confirm the production of multiple polycistronic RNA transcripts, we chose JSC-1 cells, which exhibit the highest usage of the most pA sites among all three cell lines, and performed Northern blot analysis of five gene clusters on total RNA extracted from JSC-1 cells with or without lytic virus induction using oligonucleotide probes located upstream of the mapped pA sites (Fig. 7). These five pA sites were chosen because of their high expression levels with thousands of mapped reads in the corresponding gene transcripts during lytic EBV infection. First, we investigated the locus at the head-tail junction region of the episomal EBV genome encoding the latent LMP-1 oncoprotein, BNLF2a, and BNLF2b from its minus DNA strand. While BNLF2a functions as an expressible viral immunomodulator (68), the expression and function of BNLF2b remain hypothetical. Northern blotting using a sense oligonucleotide probe upstream of the mapped pA site at nt 166466 (−) revealed the expression of two major transcripts of ∼0.8 and 2.8 kb from this region in JSC-1 cells with only lytic EBV induction and not with latent infection (Fig. 7A). The data are consistent with the expression level determined by PA-seq reads (26,785 lytic reads versus 18 latent reads) in this pA site (see Table S1 in the supplemental material). Based on RNA size, these transcripts represent LMP-1, with a predicted size of ∼2.4 kb (from the ATG codon to the identified pA site), and BNFL2a/b, with a size of ∼0.6 to 0.8 kb. The second locus selected consisted of latent LMP-2A/2B and colinear BNRF1 (major tegument protein) from its plus DNA strand. Using an antisense oligonucleotide probe located in the terminal exon of the LMP-2A/2B locus, we detected one major transcript, BNRF1, with a size of ∼4.5 kb only in JSC-1 cells with lytic EBV induction (Fig. 7B) for expression of the EBV major tegument protein (69, 70). The expression of LMP-2A/2B transcripts was hardly detectable from this gene cluster region (Fig. 7B). These data indicate that the PA-seq reads detected in JSC-1 cells were mainly derived from BNRF1 transcripts expressed from this latent locus.
FIG 7.
Polyadenylation of multiple viral transcripts at a single pA site. Shown are diagrams of the selected gene loci (A, B, C and D) containing multiple colinear genes of which their RNA transcripts are terminated at a common poly(A) site at a specific nucleotide position (triangles in red for antisense transcripts and in blue for sense transcripts). Arrows below the diagram are specific antisense oligonucleotide probes used in Northern blot analyses. The Northern blot gel below each gene cluster region (A, B, C, or D) shows the detected transcripts, as indicated by small black arrows, from this cluster region. A total of ∼5 μg of total RNA from JSC-1 cells with latent (minus butyrate [Bu]) or lytic (plus Bu) EBV infection was used for Northern blot analysis.
Next, we determined the expression of polycistronic transcripts from two lytic loci. One contained BFRF1A (viral DNA packaging protein), BFRF1 (virion egress protein), BFRF2 (uncharacterized protein), and BFRF3 (small capsid protein) and had two pA sites, at nt 48435 (+) and at nt 49799 (+), which were identified by PA-seq. The other locus used encodes BORF1 (minor capsid protein), BORF2 (viral ribonucleoside-diphosphate reductase, large subunit), BaRF1 (viral ribonucleoside-diphosphate reductase, small subunit), BMRF1 (viral DNA polymerase processivity factor), and BMRF2 (glycoprotein) and has three pA sites, at nt 65684 (+), nt 66617 (+), and nt 69910 (+) in JSC-1 cells, which were identified by PA-seq (Table S1). Northern blotting with an oligonucleotide probe upstream of the nt 49799 pA site detected two major transcripts only in the lytic JSC-1 sample, an ∼1-kb BFRF3 transcript (predicted size, ∼0.6 kb) (71) and an ∼3.7-kb BFRF1-3 transcript (72), and one minor ∼3-kb BFRF2/3 transcript (73) (Fig. 7C). Although a mapped pA site with few (only 417) reads at nt 48435 (+) was found downstream of BFRF1, the lack of a canonical poly(A) signal for this pA site usage (Table S1) would make most BFRF1 transcripts skipping the nt 48435 (+) pA site to polyadenylate at the nt 49799 (+) pA site, resulting in the production of a bicistronic BFRF1-3 RNA with a size of ∼3.7 kb, as detected by others with a BFRF1 probe (72) (Fig. 7C). Like the nt 48435 (+) pA site, the mapped nt 65684 (+) pA site also lacks a canonical poly(A) signal and was mapped with few (only 155) reads in the locus (Table S1). Our detection was thus focused only on two highly used pA sites at nt 66617 (+) and nt 69910 (+) in this locus. Northern blotting with a BORF2 probe (oVM376) upstream of the nt 66617 (+) pA site revealed the expression of two major transcripts corresponding to monocistronic BORF2 and bicistronic BORF1/BORF2, with predicted sizes of ∼2.5 and ∼3.7 kb, respectively (Fig. 7D). Two additional bands representing large RNA species (>10 kb in size) were also detected, which were presumably polycistronic BURF1 or BURF1-like RNA transcripts (36) (Fig. 3). The probe oVM377 upstream of the nt 69910 (+) pA site identified 3 major lytic transcripts: the less abundant monocistronic BMRF2 transcript, the most abundant bicistronic BMRF1/BMRF2 transcript, and the less abundant polycistronic BaRF1/BMRF1/BMRF2 transcript, for which the observed sizes were in close agreement with the corresponding predicted sizes of ∼1.1, ∼2.3, and ∼3.3 kb (Fig. 7D).
In conclusion, we have generated a comprehensive atlas of EBV polyadenylation sites and their contribution to the EBV transcriptome in the context of current gene annotation. This study also carefully elucidated that EBV produces multiple bicistronic and polycistronic transcripts from several gene cluster regions by sharing a single pA site for their RNA polyadenylation.
Identification of novel pA sites for polyadenylation of EBV antisense transcripts.
Although most of the mapped pA sites could be assigned downstream of the annotated EBV ORFs, we also identified a subset of pA sites either positioned within the annotated ORFs or not associated with any ORF (Fig. 3; see also Table S1 in the supplemental material). For example, the mapped pA sites in JSC-1 cells assigned to the known ORF regions include the pA site at nt 65684 (+) within the coding region of BORF2 and the pA sites at nt 39843 (−) and nt 39961 (−) in BHLF1, nt 99451 (+) in BKRF4, and nt 140693 (−) in LF3 loci (Fig. 3). In general, these pA sites are characterized by few expression reads, lack a canonical PAS and/or DSE, and are not consistently detected in all three cell lines. Therefore, they represent a group of cryptic pA sites in the coding region that could be activated by noncanonical poly(A) polymerases (74), presumably for regulation of the full-length RNA expression and coding functions. An exception is the pA site at nt 140693 (−), which was mapped to 123 nt upstream of the LF3 stop codon at nt 140570 (−) (Fig. 3). In contrast to other pA sites mentioned above, this pA site was defined by a large number of the mapped reads (Table S1) and has a canonical PAS. In contrast, the pA site at nt 139398 (−) located ∼50 bp downstream of the predicted LF3 protein stop codon (75) was not detectable in either JSC-1 or Akata cells and expressed only 143 reads in lytic Raji cells by using a noncanonical PAS. Therefore, the mapped pA site at nt 140693 (−) is authentic for the production of a noncoding LF3 transcript (30, 38, 51).
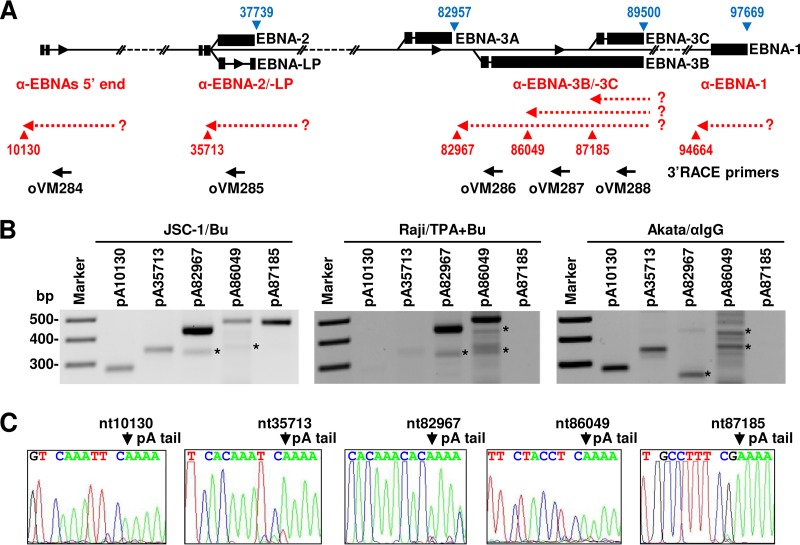
The mapped pA sites not associated with any annotated ORF in the EBV genome are pA sites at nt 6793, 7126, 7181, and 79250 from the plus DNA strand and at nt 1764, 10130, 35713, 66588, 82967, 86049, 87185, and 94664 from the minus DNA strand (Fig. 3 and Table S1). A cluster of pA sites at nt 6793 (+), nt 7126 (+), and nt 7181 (+) in proximity of the EBER locus was detected mainly in Raji cells with low-level usage because they lack a PAS or PAS-like signal upstream. The pA site at nt 79250 (+) without a PAS was detected with 100 reads in JSC-1 and with 44 reads in Akata cells with lytic induction (Table S1) and is located 2.1 kb downstream of the highly expressed BLRF2 pA site at nt 77143 (+). Thus, these pA sites are likely all cryptic pA sites. From the minus EBV DNA strand, the pA site at nt 1764 was mapped only in Akata cells. Although not highly used, it contains a canonical PAS for an antisense transcript to LMP-2A/2B and BNRF1. Interestingly, the remaining other six pA sites from the minus DNA strand are positioned in the antisense direction from individual EBNA transcripts. More precisely, the pA site at nt 10130 (−) is upstream of the EBNA Cp promoter; the pA site at nt 35713 (−) is upstream of the EBNA-2 and EBNA-LP terminal exons; the pA site at nt 66588 (−) is in the intron region of EBNA-3 and EBNA-1 and also upstream of the BMRFs and BaRF1 ORFs; the pA sites at nt 82967 (−), nt 86049 (−), and nt 87185 (−) are in the terminal exons of EBNA-3A, -3B, and -3C; and the pA site at nt 94664 (−) is upstream of the EBNA-1 terminal exon and BKRFs (Fig. 3). Their usage was highly varied among individual pA sites, from a low-use pA site at nt 94664 (−) to a high-use pA site at nt 82967 (−) (Table S1). In addition, all reads mapped to these pA sites came almost exclusively from cells with virus lytic induction. Analysis of the sequences surrounding these pA sites showed that a majority contain a canonical “AAUAAA” PAS, except for the pA sites at nt 86049 (−) with no detectable PAS and the pA site at nt 87185 (−) with a noncanonical “GATAAA” PAS. Their expressions were highly variable among three cell lines. While all pA sites were mapped from JSC-1 cells, only two (pA sites at nt 82967 and 86049) were detected from Raji cells, and one (pA site at nt 10130) was detected from Akata cells (Table S1). These data indicate the presence of several potential polyadenylated transcripts antisense to various forms of EBNA transcripts, as suggested by several laboratories (36, 76, 77).
Given the importance of EBNA proteins in EBV biology and cell transformation, we decided to further verify the mapped pA sites for the expression of EBNA-antisense transcripts by 3′ RACE analysis. The pA site at nt 94664 (−) was excluded from the analysis due to a very low number of associated reads detectable only in JSC-1 cells. Total RNAs from all three cell lines with lytic virus infection were analyzed by 3′ RACE with an EBV-specific primer (oVM284, oVM285, oVM286, oVM287, or oVM288) upstream of the tested pA sites (Fig. 8A). We confirmed the expression of all mapped pA sites in JSC-1 cells but partially in Raji and Akata cells (Fig. 8B). In particular, the pA site at nt 10130 (−) upstream of the EBNA Cp promoter (78) was not detectable or was expressed at a very low level in Raji cells with type III latency (59). The amount of 3′ RACE products was comparable with the calculated pA site usage, with the pA sites at nt 82967 (−) (oVM286 product) being the most prominent in JSC-1 and Raji cells (Fig. 8B). The identity of the final RACE products and pA site positions in JSC-1 cells were further confirmed by Sanger sequencing (Fig. 8C). Raji cells exhibited the main usage, with similar levels, as mapped by PA-seq, of two adjacent pA sites at nt 82967 (−) (oVM286 product) and nt 86049 (−) (oVM287 product) (Fig. 8B). Akata cells showed a usage of two pA sites at nt 10130 (−) (oVM284 product) and nt 35713 (−) (oVM285 product), with a weak 3′ RACE product from the pA site at nt 35713 (−) (oVM285 product). The pA site at nt 35713 (−) was not mapped by PA-seq, probably due to its low expression level. Together, the existence of EBNA-antisense pA sites and their usage during EBV lytic infection in a cell type-dependent manner were verified by using 3′ RACE.
FIG 8.
Identification of the EBNA-antisense transcripts in EBV+ cell lines by 3′ RACE. (A) Diagram showing various parts of the EBNA loci with the mapped pA sites in the plus strand (blue triangles) and the minus strand (red triangles). Hypothetical antisense transcripts (dashed red arrows) transcribed from an unknown (?) promoter are shown in association with each mapped pA site in the minus strand. Black arrows represent DNA oligonucleotides that were used for 3′ RACE. (B) Agarose gel images showing that the detection of 3′ RACE products was carried out with 1 μg of total RNA each from lytic JSC-1, Raji, and Akata cells induced by 48 h of treatment with the corresponding inducers. *, nonspecific products. (C) Sequencing results for each 3′ RACE product from JSC-1 cells. The RACE product was gel purified, and the mapped pA site (cleavage site) for RNA polyadenylation was confirmed by Sanger sequencing.
Detection of polyadenylated transcripts antisense to EBNA in JSC-1 and Raji cells by Northern blot analyses.
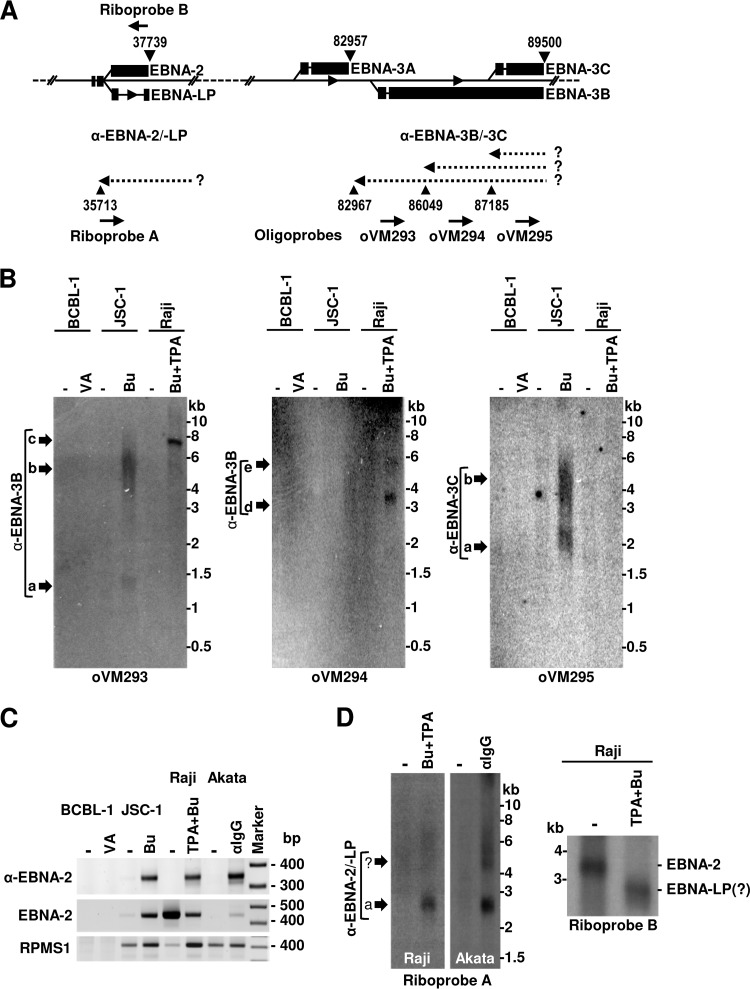
To further understand the nature of EBNA-3-antisense transcripts, we performed Northern blot analysis on poly(A)-selected RNA from three cell lines, with poly(A)+ RNA from the BCBL-1 cell line without EBV infection as a negative control. To determine transcript expression kinetics, we compared their expressions in these cells with latent (no chemical induction) or lytic (with chemical induction) infection. Three specific-sense oligonucleotide probes corresponding to the same locations as those of sense oligonucleotides used for 3′ RACE (Fig. 9A; see also Table S3 in the supplemental material) were used for Northern blot analyses for detection of the antisense transcripts to EBNA-3B and -3C, where the associated pA sites at nt 82967 (−), nt 86049 (−), and nt 87185 (−) expressed a large amount of mapped reads (Table S1). As shown in Fig. 9B, the oVM293 probe located upstream of the pA site at nt 82967 (−) provided a positive signal, as expected, only in JSC-1 and Raji cells. However, the antisense RNA transcripts varied in size and between the two cell lines, with two antisense transcripts with sizes of ∼1.4 and ∼5.5 to 6.0 kb in JSC-1 cells but a single antisense transcript of ∼7.5 kb in Raji cells. The oVM294 probe upstream of the pA site at nt 86049 (−) detected two distinct antisense RNA transcripts only in Raji cells with sizes of ∼3.5 and ∼5.8 kb. In contrast, the oVM295 probe specific for the pA site at nt 87185 (−) detected two antisense RNA transcripts only in JSC-1 cells with estimated lengths of ∼2.0 and ∼3.5 to 4.5 kb but not in Raji cells, as expected, due to the deletion in this region (from nt 86838 to 89830) in the Raji EBV genome (62, 79). The observed variation in the expression of the full-length antisense transcripts to EBNA-3B and -3C between JSC-1 and Raji cells was in agreement with our 3′ RACE results (Fig. 8). According to PA-seq read counts, all three species of antisense RNA transcripts were detected only in cells with lytic infection. Due to their expression characteristics, we named these species of seven antisense RNA transcripts lytic anti-EBNA-3B transcript a (∼1.4 kb), transcript b (∼5 to 6 kb), transcript c (7.5 kb), transcript d (∼3.5 kb), and transcript e (∼5.8 kb) and lytic anti-EBNA-3C transcript a (∼2.0 kb) and transcript b (∼3.5 to 4.5 kb). No such transcripts were detected from EBV-negative BCBL-1 cells, indicating their origin from the EBV genome.
FIG 9.
Northern blot analysis of antisense transcripts to EBNA-3B and -3C and EBNA-2. (A) Schematic diagram of the EBNA loci with the identified pA sites in the plus and minus strands (black triangles with nucleotide positions). The dashed arrows to the left represent the predicted transcripts antisense to various EBNA transcripts, with an unknown (?) promoter. Solid thick arrows to the right, below the diagram, point to riboprobe A or oligonucleotide probes in the sense orientation for detection of the antisense transcripts, or those to the left, above the diagram, point to riboprobe B in the antisense orientation for detection of the EBNA-2 transcript by Northern blotting. (B) Autoradiograms of the antisense EBNA-3 transcripts detected by 32P-labeled sense oligonucleotide probes in Northern blots using the poly(A)-selected RNA from BCBL-1 (KSHV+/EBV-negative), JSC-1, or Raji cells with or without (−) 48 h of treatment with the indicated chemicals. (C) Detection of EBNA-2 and antisense EBNA-2 transcripts by 3′ RACE analyses. Poly(A)-selected RNA from BCBL-1, JSC-1, Raji, and Akata cells with or without (−) lytic EBV induction by the indicated chemicals for 48 h was used for 3′ RACE analyses with an antisense DNA oligonucleotide, oVM285 (Fig. 8A), for detection of antisense EBNA-2 and a sense DNA oligonucleotide, oVM232 (see Table S3 in the supplemental material), for detection of the EBNA-2 transcript. Detection of the EBV RPMS1/BART miRNA transcripts using a sense DNA oligonucleotide, oVM283, served as a control. The products were resolved by agarose gel electrophoresis. (D) Northern blot detection of the transcripts antisense to EBNA-2 and EBNA-2 mRNAs. Poly(A)-selected RNA from Raji and Akata cells with or without (−) lytic EBV infection was used for the analyses with riboprobe A for detection of the antisense transcript to EBNA-2 from Raji and Akata cells (left) or riboprobe B for detection of the EBNA-2 transcripts from Raji cells (right). ?, hypothetical EBNA-LP RNA products.
Considering that the antisense transcripts to EBNA-2 were also detectable from total RNA extracted from all three cell lines during lytic EBV reactivation (Fig. 8), we further showed that these EBNA-2 antisense transcripts could be enriched from poly(A)-selected RNA and became easily detectable by 3′ RACE from all three EBV-positive cell lines only with lytic EBV infection but not from EBV-negative BCBL-1 cells (Fig. 9C). The lytic induction of EBNA-2 antisense transcripts was much stronger than that of the latent RPMS1/BART miRNA transcripts in all three cell lines (Fig. 9C). By Northern blot analysis we also demonstrated that antisense EBNA-2 transcripts were similar in size, at ~2.7-kb, in both Akata and Raji cells with lytic EBV infection (Fig. 9D, left). Although EBNA-2 was inducible in both JSC-1 and Akata cells (Fig. 9C), the highly expressed EBNA-2 in Raji cells during latent infection was largely reduced in lytic EBV infection, as determined by 3′ RACE (Fig. 9C) and Northern blot analyses (Fig. 9D, right). Interestingly, this reduction of EBNA-2 RNA transcripts in Raji cells with lytic EBV infection was accompanied by the appearance of a smaller product, presumably EBNA-LP (Fig. 9D, right). This inverse expression correlation between EBNA-2 and antisense EBNA-2 transcripts suggests that the expression of antisense EBNA-2 transcripts during EBV lytic infection in Raji cells might contribute to the decreased expression of latent EBNA-2 (Fig. 9C and D).
DISCUSSION
We performed a genome-wide analysis of EBV polyadenylation sites using a newly developed PA-seq method (25, 45, 46), an important tool for further refining EBV gene annotation. The unbiased, comprehensive, and quantitative nature of PA-seq allowed us to illustrate an EBV genome landscape of pA sites and their usage during latent and lytic EBV infections in JSC-1, Raji, and Akata cells. As a result, we identified 62 pA sites being used by EBV transcription in JSC-1, 60 in Raji, and 53 in Akata cells, of which 42 pA sites were commonly used in all three cell lines. The viral genome polymorphism, variable numbers of internal repeats, and cell line-specific deletions could be the major factors that contribute to the heterogeneity in the compositions of EBV pA sites and their usage among the three cell lines. Of the 79 canonical PASs available for EBV gene expression in the reference EBV genome, only 51 PASs served for the defined pA sites in this study. Thus, a few pA sites being mapped only in one cell line, often at the repeat EBV regions and lacking consensus polyadenylation sequences, could be cryptic and activated by noncanonical polyadenylation pathways, as observed in KSHV (25). Most of the defined pA sites are highly correlated with the annotated transcriptome, and their usage or expression relates to the depth of PA-seq reads in each pA site, thus reflecting the expression level of the pA site-associated gene(s). By identifying a subset of novel pA sites and polyadenylated antisense RNA transcripts to EBNAs, this study further expands an already larger-than-anticipated repertoire of the EBV transcriptome (39, 76, 77, 80).
The usage of the pA sites identified by PA-seq is highly related to the expression cascade of EBV genes, as reported previously (35, 40). Among the three cell lines examined in this study, JSC-1 cells with EBV and KSHV dual infections and mutual gene regulation of two viruses (14, 47, 81) displayed higher expression levels of more lytic EBV genes than those in Raji and Akata cells with only EBV infection. The lytic EBV expression in butyrate-treated JSC-1 cells was equally as efficient as or better than lytic KSHV expression; our PA-seq data obtained from JSC-1 cells were comparable to those for Raji and Akata cell lines with only EBV infection. Given that the EBV genome in Raji cells is unable to replicate (50, 62), and viral late gene expression depends on viral DNA replication (82–84), it is understandable that our PA-seq study identified only a few true late pA sites for late gene expression in Raji cells (50, 62). Leaky expression of late EBV pA sites was common in all three cell lines, as reported previously for EBV late promoters (40). In addition, some of the mapped pA sites in our study contained at least one early and/or leaky promoter upstream and, therefore, will be expressed and detectable despite the lack of DNA replication.
Although most of the pA sites could be annotated to the known viral genes in the EBV genome, the number of identified pA sites is smaller than the number of annotated EBV genes (19) and suggests the presence of polycistronic viral transcripts, as observed in the closely related KSHV, which employs only 67 pA sites for its genome expression (25). We subsequently verified these bicistronic/polycistronic EBV transcripts derived from several gene clusters, including LMP-1/BNLF2a/BNLF2b, LMP-2A/LMP-2B/BNRF1, BFRF1/BFRF2/BFRF3, BORF1/BORF2, and BaRF1/BMRF1/BMRF2, in the EBV genome. However, because our PA-seq method depends on oligo(T) priming for library construction (45, 46), we were unable to define the 3′ ends of those Pol III transcripts lacking a poly(A) tail. These include both latent EBER1 and EBER2 RNA transcripts, which are highly abundant, small noncoding EBV RNAs transcribed by RNA Pol III (85, 86).
The three most highly expressed genes in lytic EBV infection in all three cell lines were noncoding BHLF1 and LF3 (30, 38, 51) and coding BMRF1/2 (18, 87), as determined by the mapped pA site reads and Northern blot analysis. Interestingly, both BHLF1 and LF3 loci are located in close proximity to the two lytic replication origins (oriL) and were predicted to contain an annotated leftward ORF in the reference EBV genome. However, recent studies indicate that both BHLF1 and LF3 transcripts in Raji and Akata cells contain a stop codon immediately downstream of their initiation codon and are incapable of encoding a full-length protein. Our finding that the mapped pA site for LF3 is positioned upstream of the predicted LF3 stop codon also excludes any possible translation potential of the LF3 ORF. In fact, LF3 in Daudi cells was identified as a noncoding, highly structural nuclear RNA (30). Due to their proximity to oriL, and highly induced expression during lytic replication, LF3 and BHLF1 transcripts had been proposed to be primarily noncoding in other studies (88, 89) and might have a direct role in the regulation of virus replication (89) and pathogenesis. Although BMRF1 and BMRF2 are transcribed from their own promoter (40), we believe that BMRF1 is expressed as a bicistronic BMRF1/2 RNA by using the mapped pA site at nt 69910 downstream of the BMRF2 ORF to encode an EBV DNA polymerase processivity factor, BMRF1, one of the most abundant viral proteins identified by proteomic analysis (18). BMRF1 is essential for EBV lytic replication (87), whereas the less abundant monocistronic BMRF2 encodes a glycoprotein responsible for EBV attachment to susceptible cells for virus entry (90).
Analysis of the unannotated pA sites in our study led to the identification of multiple polyadenylated EBNA-antisense transcripts, some of which the existence was suggested previously by microarray, RNA-seq, and PacBio Iso-seq analyses of the EBV transcriptome (36, 76, 77). Our study provides further evidence that EBNA-antisense transcripts are fully processed RNA transcripts containing a poly(A) tail. These include the antisense RNA transcripts to the EBNA Cp promoter, EBNA-2 and EBNA-LP, EBNA-3B, and EBNA-3C. We verified each antisense transcript by 3′ RACE in all three EBV-positive cell lines and the antisense transcripts to EBNA-3B and -3C by Northern blot analysis in both JSC-1 and Raji cells and antisense transcripts to EBNA-2 in Raji and Akata cells. In general, they are long transcripts, several kilobases in size, and some are spliced (76, 77; our unpublished data). Their size and expression vary among individual cell lines. The reason for this variation remains to be determined. One possible explanation is higher levels of sequence variation among different EBV strains in the EBNA regions than in other parts of the genome (51). For example, the EBV genome in Raji cells lacks a region from nt 86838 to 89830 (79) and does not express two EBNA-3C antisense transcripts from this region in our Northern blot analysis. Although their promoters remain to be determined, all EBNA-antisense transcripts, in contrast to the EBNA RNAs which are expressed mainly during latency, are highly expressed only from cells after virus reactivation and thus are viral lytic transcripts, as proposed previously (76). Their function in the EBV life cycle may negatively regulate EBNA expression during viral lytic infection and promote viral lytic replication. While the proposed functions of these antisense transcripts are under investigation, similar regulation of viral transcripts by antisense transcription was observed in KSHV RTA (91). The increased nuclear retention of EBNA transcripts over to the most lytic transcripts (39) might be one of the functions of antisense EBNA transcripts.
MATERIALS AND METHODS
Cells.
KSHV- and EBV-double-positive JSC-1 (47), EBV-single-positive Raji (92) and Akata (49), and KSHV-single-positive BCBL-1 (93) cell lines were cultivated in HEPES-buffered RPMI 1640 medium (catalog no. 22400-089; Thermo Fisher Scientific) supplemented with 10% HyClone defined fetal bovine serum (catalog no. SH3007.03; GE Healthcare) and 1× penicillin-streptomycin-glutamine (catalog no. 10378-016; Thermo Fisher Scientific). To induce viral lytic replication, all cell lines were adjusted to 5 × 105 cells/ml. To induce lytic replication, JSC-1 cells were treated for 48 h with a final concentration of 3 mM sodium salt of n-butyric acid (sodium butyrate; Bu) (catalog no. B5887; Sigma-Aldrich), Raji cells were treated for 48 h with a combination of 3 mM sodium butyrate and 20 ng/ml of phorbol 12-myristate 13-acetate (TPA) (catalog no. P8139; Sigma-Aldrich), BCBL-1 cells were treated for 48 h with 1 mM sodium salt of valproic acid (VA) for BCBL-1 (catalog no. P4543; Sigma-Aldrich), and Akata cells were treated for 24 h with goat anti-human serum (catalog no. 55087; MP Biologicals) at a 1:200 dilution. After induction, the cells were harvested by centrifugation and lysed in TriPure reagent (catalog no. 11 667 165 001; Roche) for total RNA isolation. The untreated cells were harvested in parallel for analysis of latent gene expression.
PA-seq and data analysis.
The polyadenylation sites were determined by using a PA-seq method described in detail previously (25, 46). The obtained sequence libraries were mapped to human (version hg19; UCSC), KSHV (GenBank accession no. U75698), and EBV (GenBank accession no. NC_007605) reference genomes by the Burrows-Wheeler alignment (BWA) tool (94) and further processed by SAMtools (95). The reads from Raji cells were also mapped to the Raji-specific genome sequence (GenBank accession no. KF717093), and reads from Akata cells were mapped to the Akata-specific genome (GenBank accession no. KC207813). All identified pA cleavage sites were extracted and converted to wiggle (.wig) files for visualization of the nucleotide positions and frequencies using IGV software (http://software.broadinstitute.org/software/igv/). The uniquely mapped reads were then analyzed for viral polyadenylation sites by peak calling using the F-Seq program on combined libraries (25, 46, 96). To eliminate false peaks, we removed all peaks containing a stretch of “A’s” resulting from internal priming and low-abundance peaks with fewer than 50 reads per peak. The peak mode, representing the nucleotide position with the highest number of reads, was then defined as the pA site. To obtain the relative expression levels during latent and lytic infection, the reads were mapped back to the original libraries. The heat map expressing read abundances in individual samples was generated by conditional formatting in Excel software (Microsoft) after normalization to library size and expressed as reads per million (RPM) with maximum scales of 50 RPM for latent and 1,000 RPM for lytic samples. The pA site usage in individual samples was also calculated as a percentage of reads mapped to each pA site from total reads mapped in that particular sample. To identify cis-regulatory sequence elements surrounding viral pA sites, we extracted DNA sequences ±50 nt from each pA site. Sequence conservation was determined by sequence logos (see http://weblogo.berkeley.edu/logo.cgi) (97). The frequencies of PASs were determined by manual inspection of the upstream 50 nt of each pA site.
3′ RACE.
3′ RACE was performed by using the SMARTer RACE 5′/3′ kit (catalog no. 634858; Clontech/TaKaRa), as recommended by the manufacturer (see Table S3 in the supplemental material for primer sequences used). After reverse transcription, 3′ RACE products were amplified for 35 cycles by PCR using a universal primer in combination with a gene-specific primer. The obtained PCR products were analyzed on an agarose gel, gel purified, and directly sequenced by the Sanger sequencing method. Their sequences were mapped back to the EBV genome, and a nucleotide immediately upstream of a pA tail was considered a pA site (cleavage site).
Northern blot analysis.
Northern blotting was carried out as described previously (98), using 5 μg of total RNA or 1 μg of the polyadenylated RNA fraction isolated by using an Illustra QuickPrep mRNA purification kit (catalog no. 27-9254-01; GE Healthcare). After separation on a 1% formaldehyde-containing agarose gel, the RNA was transferred to a nylon membrane and hybridized with an antisense DNA oligonucleotide probe generated by [γ-32P]ATP end labeling (see Table S3 in the supplemental material). The specific signals were collected and processed by using the Typhoon Trio imaging system (GE Healthcare). The riboprobes for detection of EBNA-2 transcripts (probe B) and antisense transcripts to EBNA-2 (probe A) by Northern blotting were synthetized by in vitro transcription using Promega’s T7 riboprobe system (catalog no. P1440) on PCR templates in the presence of [α-32P]GTP and gel purified, and 5 × 106 counts per minute (CMP) were used for hybridization. PCR products were generated by amplification of part of the Raji EBV genome using oVM434 and oVM285 for riboprobe A and oVM432 and oVM433 for riboprobe B.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jeffrey Cohen from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) for providing Raji cells and Akata cells. We thank Deanna Gotte and Cindy Clark for editing and proofreading of the manuscript.
This research was fully supported by the NIH Intramural Research Program, the National Cancer Institute, the Center for Cancer Research, and the National Heart, Lung, and Blood Institute.
Vladimir Majerciak, Jun Zhu, and Zhi-Ming Zheng conceptualized and designed the study. Vladimir Majerciak performed all experiments and worked with Jing Zheng on PA-seq analysis. Wenjing Yang carried out bioinformatics. All authors contributed to data analysis. Vladimir Majerciak and Zhi-Ming Zheng wrote the manuscript, with input from all coauthors.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01593-18.
REFERENCES
- 1.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395–404. doi: 10.1016/S1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JI. 2000. Epstein-Barr virus infection. N Engl J Med 343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 3.Ok CY, Li L, Young KH. 2015. EBV-driven B-cell lymphoproliferative disorders: from biology, classification and differential diagnosis to clinical management. Exp Mol Med 47:e132. doi: 10.1038/emm.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C, Shen H, Weisenberger DJ, Schultz N, Shen R, Weinhold N, Kelsen DP, Bowlby R, Chu A, Kasaian K, Mungall AJ, Gordon Robertson A, Sipahimalani P, Cherniack A, Getz G, Liu Y, Noble MS, Pedamallu C, Sougnez C, Taylor-Weiner A, Akbani R, Lee J-S, Liu W, Mills GB, Yang D, Zhang W, Pantazi A, Parfenov M, Gulley M, Blanca Piazuelo M, Schneider BG, Kim J, Boussioutas A, Sheth M, Demchok JA, Rabkin CS, Willis JE, Ng S, Garman K, Beer DG, Pennathur A, Raphael BJ, et al. 2014. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang MS, Kieff E. 2015. Epstein-Barr virus latent genes. Exp Mol Med 47:e131. doi: 10.1038/emm.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price AM, Luftig MA. 2015. To be or not IIb: a multi-step process for Epstein-Barr virus latency establishment and consequences for B cell tumorigenesis. PLoS Pathog 11:e1004656. doi: 10.1371/journal.ppat.1004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodescot M, Perricaudet M, Farrell PJ. 1987. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol 61:3424–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers RP, Woisetschlaeger M, Speck SH. 1990. Alternative splicing dictates translational start in Epstein-Barr virus transcripts. EMBO J 9:2273–2277. doi: 10.1002/j.1460-2075.1990.tb07398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanda T, Horikoshi N, Murata T, Kawashima D, Sugimoto A, Narita Y, Kurumizaka H, Tsurumi T. 2013. Interaction between basic residues of Epstein-Barr virus EBNA1 protein and cellular chromatin mediates viral plasmid maintenance. J Biol Chem 288:24189–24199. doi: 10.1074/jbc.M113.491167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodin TL, Najrana T, Yates JL. 2013. Efficient replication of Epstein-Barr virus-derived plasmids requires tethering by EBNA1 to host chromosomes. J Virol 87:13020–13028. doi: 10.1128/JVI.01606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deakyne JS, Malecka KA, Messick TE, Lieberman PM. 2017. Structural and functional basis for an EBNA1 hexameric ring in Epstein-Barr virus episome maintenance. J Virol 91:e01046-17. doi: 10.1128/JVI.01046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempkes B, Ling PD. 2015. EBNA2 and its coactivator EBNA-LP. Curr Top Microbiol Immunol 391:35–59. doi: 10.1007/978-3-319-22834-1_2. [DOI] [PubMed] [Google Scholar]
- 13.Vrazo AC, Chauchard M, Raab-Traub N, Longnecker R. 2012. Epstein-Barr virus LMP2A reduces hyperactivation induced by LMP1 to restore normal B cell phenotype in transgenic mice. PLoS Pathog 8:e1002662. doi: 10.1371/journal.ppat.1002662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. 2006. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog 2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee N, Yario TA, Gao JS, Steitz J. 2016. EBV noncoding RNA EBER2 interacts with host RNA-binding proteins to regulate viral gene expression. Proc Natl Acad Sci U S A 113:3221–3226. doi: 10.1073/pnas.1601773113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquitz AR, Mathur A, Edwards RH, Raab-Traub N. 2015. Host gene expression is regulated by two types of noncoding RNAs transcribed from the Epstein-Barr virus BamHI A rightward transcript region. J Virol 89:11256–11268. doi: 10.1128/JVI.01492-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MP, Kurzrock R. 2004. Epstein-Barr virus and cancer. Clin Cancer Res 10:803–821. doi: 10.1158/1078-0432.CCR-0670-3. [DOI] [PubMed] [Google Scholar]
- 18.Ersing I, Nobre L, Wang LW, Soday L, Ma Y, Paulo JA, Narita Y, Ashbaugh CW, Jiang C, Grayson NE, Kieff E, Gygi SP, Weekes MP, Gewurz BE. 2017. A temporal proteomic map of Epstein-Barr virus lytic replication in B cells. Cell Rep 19:1479–1493. doi: 10.1016/j.celrep.2017.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baer R, Bankier AT, Biggin MD, Deininger PL, Farrell PJ, Gibson TJ, Hatfull G, Hudson GS, Satchwell SC, Seguin C. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 20.Arrand JR, Rymo L, Walsh JE, Bjorck E, Lindahl T, Griffin BE. 1981. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res 9:2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raab-Traub N, Dambaugh T, Kieff E. 1980. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell 22:257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- 22.Parker BD, Bankier A, Satchwell S, Barrell B, Farrell PJ. 1990. Sequence and transcription of Raji Epstein-Barr virus DNA spanning the B95-8 deletion region. Virology 179:339–346. doi: 10.1016/0042-6822(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 23.de Jesus O, Smith PR, Spender LC, Elgueta KC, Niller HH, Huang D, Farrell PJ. 2003. Updated Epstein-Barr virus (EBV) DNA sequence and analysis of a promoter for the BART (CST, BARF0) RNAs of EBV. J Gen Virol 84:1443–1450. doi: 10.1099/vir.0.19054-0. [DOI] [PubMed] [Google Scholar]
- 24.Majerciak V, Yamanegi K, Zheng ZM. 2006. Gene structure and expression of Kaposi’s sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J Virol 80:11968–11981. doi: 10.1128/JVI.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majerciak V, Ni T, Yang W, Meng B, Zhu J, Zheng ZM. 2013. A viral genome landscape of RNA polyadenylation from KSHV latent to lytic infection. PLoS Pathog 9:e1003749. doi: 10.1371/journal.ppat.1003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil A, Proudfoot NJ. 1987. Position-dependent sequence elements downstream of AAUAAA are required for efficient rabbit beta-globin mRNA 3' end formation. Cell 49:399–406. doi: 10.1016/0092-8674(87)90292-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Wilusz J. 1998. Auxiliary downstream elements are required for efficient polyadenylation of mammalian pre-mRNAs. Nucleic Acids Res 26:2891–2898. doi: 10.1093/nar/26.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandel CR, Bai Y, Tong L. 2008. Protein factors in pre-mRNA 3'-end processing. Cell Mol Life Sci 65:1099–1122. doi: 10.1007/s00018-007-7474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laux G, Perricaudet M, Farrell PJ. 1988. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J 7:769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Smith PR, Karran L, Lu QL, Griffin BE. 1997. Induction of an exceptionally high-level, nontranslated, Epstein-Barr virus-encoded polyadenylated transcript in the Burkitt’s lymphoma line Daudi. J Virol 71:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rooney CM, Rowe DT, Ragot T, Farrell PJ. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol 63:3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sergeant A, Gruffat H, Manet E. 2008. The Epstein-Barr virus (EBV) protein EB is an mRNA export factor essential for virus production. Front Biosci 13:3798–3813. [DOI] [PubMed] [Google Scholar]
- 33.Furnari FB, Adams MD, Pagano JS. 1993. Unconventional processing of the 3' termini of the Epstein-Barr virus DNA polymerase mRNA. Proc Natl Acad Sci U S A 90:378–382. doi: 10.1073/pnas.90.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadler RH, Raab-Traub N. 1995. Structural analyses of the Epstein-Barr virus BamHI A transcripts. J Virol 69:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan J, Cahir-McFarland E, Zhao B, Kieff E. 2006. Virus and cell RNAs expressed during Epstein-Barr virus replication. J Virol 80:2548–2565. doi: 10.1128/JVI.80.5.2548-2565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dresang LR, Teuton JR, Feng H, Jacobs JM, Camp DG, Purvine SO, Gritsenko MA, Li Z, Smith RD, Sugden B, Moore PS, Chang Y. 2011. Coupled transcriptome and proteome analysis of human lymphotropic tumor viruses: insights on the detection and discovery of viral genes. BMC Genomics 12:625. doi: 10.1186/1471-2164-12-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, Klichinsky M, Leslie C, Lieberman PM. 2012. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 12:233–245. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z, Xu G, Deng N, Taylor C, Zhu D, Flemington EK. 2010. Quantitative and qualitative RNA-Seq-based evaluation of Epstein-Barr virus transcription in type I latency Burkitt’s lymphoma cells. J Virol 84:13053–13058. doi: 10.1128/JVI.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Concha M, Wang X, Cao S, Baddoo M, Fewell C, Lin Z, Hulme W, Hedges D, McBride J, Flemington EK. 2012. Identification of new viral genes and transcript isoforms during Epstein-Barr virus reactivation using RNA-Seq. J Virol 86:1458–1467. doi: 10.1128/JVI.06537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djavadian R, Hayes M, Johannsen E. 2018. CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLoS Pathog 14:e1007114. doi: 10.1371/journal.ppat.1007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jan CH, Friedman RC, Ruby JG, Bartel DP. 2011. Formation, regulation and evolution of Caenorhabditis elegans 3'UTRs. Nature 469:97–101. doi: 10.1038/nature09616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozsolak F, Kapranov P, Foissac S, Kim SW, Fishilevich E, Monaghan AP, John B, Milos PM. 2010. Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell 143:1018–1029. doi: 10.1016/j.cell.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangone M, Manoharan AP, Thierry-Mieg D, Thierry-Mieg J, Han T, Mackowiak SD, Mis E, Zegar C, Gutwein MR, Khivansara V, Attie O, Chen K, Salehi-Ashtiani K, Vidal M, Harkins TT, Bouffard P, Suzuki Y, Sugano S, Kohara Y, Rajewsky N, Piano F, Gunsalus KC, Kim JK. 2010. The landscape of C. elegans 3'UTRs. Science 329:432–435. doi: 10.1126/science.1191244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hafez D, Ni T, Mukherjee S, Zhu J, Ohler U. 2013. Genome-wide identification and predictive modeling of tissue-specific alternative polyadenylation. Bioinformatics 29:i108–i116. doi: 10.1093/bioinformatics/btt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni T, Yang Y, Hafez D, Yang W, Kiesewetter K, Wakabayashi Y, Ohler U, Peng W, Zhu J. 2013. Distinct polyadenylation landscapes of diverse human tissues revealed by a modified PA-seq strategy. BMC Genomics 14:615. doi: 10.1186/1471-2164-14-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni T, Majerciak V, Zheng ZM, Zhu J. 2016. PA-seq for global identification of RNA polyadenylation sites of Kaposi’s sarcoma-associated herpesvirus transcripts. Curr Protoc Microbiol 41:14E.7.1–14E.7.18. doi: 10.1002/cpmc.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, Ambinder RF. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J Virol 74:10187–10193. doi: 10.1128/JVI.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decaussin G, Leclerc V, Ooka T. 1995. The lytic cycle of Epstein-Barr virus in the nonproducer Raji line can be rescued by the expression of a 135-kilodalton protein encoded by the BALF2 open reading frame. J Virol 69:7309–7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147–156. doi: 10.1007/BF00571929. [DOI] [PubMed] [Google Scholar]
- 50.Polack A, Delius H, Zimber U, Bornkamm GW. 1984. Two deletions in the Epstein-Barr virus genome of the Burkitt lymphoma nonproducer line Raji. Virology 133:146–157. doi: 10.1016/0042-6822(84)90433-1. [DOI] [PubMed] [Google Scholar]
- 51.Lin Z, Wang X, Strong MJ, Concha M, Baddoo M, Xu G, Baribault C, Fewell C, Hulme W, Hedges D, Taylor CM, Flemington EK. 2013. Whole-genome sequencing of the Akata and Mutu Epstein-Barr virus strains. J Virol 87:1172–1182. doi: 10.1128/JVI.02517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nutter LM, Grill SP, Li JS, Tan RS, Cheng YC. 1987. Induction of virus enzymes by phorbol esters and n-butyrate in Epstein-Barr virus genome-carrying Raji cells. Cancer Res 47:4407–4412. [PubMed] [Google Scholar]
- 53.Li R, Zhu J, Xie Z, Liao G, Liu J, Chen MR, Hu S, Woodard C, Lin J, Taverna SD, Desai P, Ambinder RF, Hayward GS, Qian J, Zhu H, Hayward SD. 2011. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe 10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD. 2005. Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts. J Virol 79:1724–1733. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skalsky RL, Cullen BR. 2015. EBV noncoding RNAs. Curr Top Microbiol Immunol 391:181–217. doi: 10.1007/978-3-319-22834-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosa MD, Gottlieb E, Lerner MR, Steitz JA. 1981. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol 1:785–796. doi: 10.1128/MCB.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerner MR, Andrews NC, Miller G, Steitz JA. 1981. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A 78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pauws E, van Kampen AH, van de Graaf SA, de Vijlder JJ, Ris-Stalpers C. 2001. Heterogeneity in polyadenylation cleavage sites in mammalian mRNA sequences: implications for SAGE analysis. Nucleic Acids Res 29:1690–1694. doi: 10.1093/nar/29.8.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Day L, Chau CM, Nebozhyn M, Rennekamp AJ, Showe M, Lieberman PM. 2007. Chromatin profiling of Epstein-Barr virus latency control region. J Virol 81:6389–6401. doi: 10.1128/JVI.02172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, Delecluse HJ. 2011. A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus. PLoS Pathog 7:e1001294. doi: 10.1371/journal.ppat.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Contreras-Salazar B, Ehlin-Henriksson B, Klein G, Masucci MG. 1990. Up regulation of the Epstein-Barr virus (EBV)-encoded membrane protein LMP in the Burkitt’s lymphoma line Daudi after exposure to n-butyrate and after EBV superinfection. J Virol 64:5441–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hatfull G, Bankier AT, Barrell BG, Farrell PJ. 1988. Sequence analysis of Raji Epstein-Barr virus DNA. Virology 164:334–340. doi: 10.1016/0042-6822(88)90546-6. [DOI] [PubMed] [Google Scholar]
- 63.Tian B, Graber JH. 2012. Signals for pre-mRNA cleavage and polyadenylation. Wiley Interdiscip Rev RNA 3:385–396. doi: 10.1002/wrna.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beaudoing E, Freier S, Wyatt JR, Claverie JM, Gautheret D. 2000. Patterns of variant polyadenylation signal usage in human genes. Genome Res 10:1001–1010. doi: 10.1101/gr.10.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian B, Hu J, Zhang H, Lutz CS. 2005. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res 33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Meyers C, Wang HK, Chow LT, Zheng ZM. 2011. Construction of a full transcription map of human papillomavirus type 18 during productive viral infection. J Virol 85:8080–8092. doi: 10.1128/JVI.00670-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng ZM, Baker CC. 2006. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci 11:2286–2302. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. 2012. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog 8:e1002704. doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. 2011. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog 7:e1002376. doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feederle R, Neuhierl B, Baldwin G, Bannert H, Hub B, Mautner J, Behrends U, Delecluse HJ. 2006. Epstein-Barr virus BNRF1 protein allows efficient transfer from the endosomal compartment to the nucleus of primary B lymphocytes. J Virol 80:9435–9443. doi: 10.1128/JVI.00473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henson BW, Perkins EM, Cothran JE, Desai P. 2009. Self-assembly of Epstein-Barr virus capsids. J Virol 83:3877–3890. doi: 10.1128/JVI.01733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farina A, Santarelli R, Gonnella R, Bei R, Muraro R, Cardinali G, Uccini S, Ragona G, Frati L, Faggioni A, Angeloni A. 2000. The BFRF1 gene of Epstein-Barr virus encodes a novel protein. J Virol 74:3235–3244. doi: 10.1128/JVI.74.7.3235-3244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aubry V, Mure F, Mariame B, Deschamps T, Wyrwicz LS, Manet E, Gruffat H. 2014. Epstein-Barr virus late gene transcription depends on the assembly of a virus-specific preinitiation complex. J Virol 88:12825–12838. doi: 10.1128/JVI.02139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, Jacquier A. 2005. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 75.Xue SA, Jones MD, Lu QL, Middeldorp JM, Griffin BE. 2003. Genetic diversity: frameshift mechanisms alter coding of a gene (Epstein-Barr virus LF3 gene) that contains multiple 102-base-pair direct sequence repeats. Mol Cell Biol 23:2192–2201. doi: 10.1128/MCB.23.6.2192-2201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Grady T, Cao S, Strong MJ, Concha M, Wang X, Splinter BS, Adams M, Baddoo M, Srivastav SK, Lin Z, Fewell C, Yin Q, Flemington EK. 2014. Global bidirectional transcription of the Epstein-Barr virus genome during reactivation. J Virol 88:1604–1616. doi: 10.1128/JVI.02989-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Grady T, Wang X, Honer Zu Bentrup K, Baddoo M, Concha M, Flemington EK. 2016. Global transcript structure resolution of high gene density genomes through multi-platform data integration. Nucleic Acids Res 44:e145. doi: 10.1093/nar/gkw629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woisetschlaeger M, Strominger JL, Speck SH. 1989. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc Natl Acad Sci U S A 86:6498–6502. doi: 10.1073/pnas.86.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robertson ES, Ooka T, Kieff ED. 1996. Epstein-Barr virus vectors for gene delivery to B lymphocytes. Proc Natl Acad Sci U S A 93:11334–11340. doi: 10.1073/pnas.93.21.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao S, Moss W, O’Grady T, Concha M, Strong MJ, Wang X, Yu Y, Baddoo M, Zhang K, Fewell C, Lin Z, Dong Y, Flemington EK. 2015. New noncoding lytic transcripts derived from the Epstein-Barr virus latency origin of replication, oriP, are hyperedited, bind the paraspeckle protein, NONO/p54nrb, and support viral lytic transcription. J Virol 89:7120–7132. doi: 10.1128/JVI.00608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu D, Coleman T, Zhang J, Fagot A, Kotalik C, Zhao L, Trivedi P, Jones C, Zhang L. 2007. Epstein-Barr virus inhibits Kaposi’s sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J Virol 81:6068–6078. doi: 10.1128/JVI.02743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang S, Yamanegi K, Zheng ZM. 2004. Requirement of a 12-base-pair TATT-containing sequence and viral lytic DNA replication in activation of the Kaposi’s sarcoma-associated herpesvirus K8.1 late promoter. J Virol 78:2609–2614. doi: 10.1128/JVI.78.5.2609-2614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Liu H, Ge H, Ajiro M, Sharma NR, Meyers C, Morozov P, Tuschl T, Klar A, Court D, Zheng ZM. 2017. Viral DNA replication orientation and hnRNPs regulate transcription of the human papillomavirus 18 late promoter. mBio 8:e00713-17. doi: 10.1128/mBio.00713-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D, Fu W, Swaminathan S. 2018. Continuous DNA replication is required for late gene transcription and maintenance of replication compartments in gammaherpesviruses. PLoS Pathog 14:e1007070. doi: 10.1371/journal.ppat.1007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moss WN, Lee N, Pimienta G, Steitz JA. 2014. RNA families in Epstein-Barr virus. RNA Biol 11:10–17. doi: 10.4161/rna.27488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herbert KM, Pimienta G. 2016. Consideration of Epstein-Barr virus-encoded noncoding RNAs EBER1 and EBER2 as a functional backup of viral oncoprotein latent membrane protein 1. mBio 7:e01926-15. doi: 10.1128/mBio.01926-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neuhierl B, Delecluse HJ. 2006. The Epstein-Barr virus BMRF1 gene is essential for lytic virus replication. J Virol 80:5078–5081. doi: 10.1128/JVI.80.10.5078-5081.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xue SA, Griffin BE. 2007. Complexities associated with expression of Epstein-Barr virus (EBV) lytic origins of DNA replication. Nucleic Acids Res 35:3391–3406. doi: 10.1093/nar/gkm170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rennekamp AJ, Lieberman PM. 2011. Initiation of Epstein-Barr virus lytic replication requires transcription and the formation of a stable RNA-DNA hybrid molecule at OriLyt. J Virol 85:2837–2850. doi: 10.1128/JVI.02175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao J, Palefsky JM, Herrera R, Berline J, Tugizov SM. 2008. The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology 370:430–442. doi: 10.1016/j.virol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chandriani S, Xu Y, Ganem D. 2010. The lytic transcriptome of Kaposi’s sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes. J Virol 84:7934–7942. doi: 10.1128/JVI.00645-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pulvertaft JV. 1964. Cytology of Burkitt’s tumour (African lymphoma). Lancet i:238–240. [DOI] [PubMed] [Google Scholar]
- 93.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. 1996. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med 2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 94.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ni T, Corcoran DL, Rach EA, Song S, Spana EP, Gao Y, Ohler U, Zhu J. 2010. A paired-end sequencing strategy to map the complex landscape of transcription initiation. Nat Methods 7:521–527. doi: 10.1038/nmeth.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schneider TD, Stephens RM. 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res 18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majerciak V, Yamanegi K, Nie SH, Zheng ZM. 2006. Structural and functional analyses of Kaposi sarcoma-associated herpesvirus ORF57 nuclear localization signals in living cells. J Biol Chem 281:28365–28378. doi: 10.1074/jbc.M603095200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.