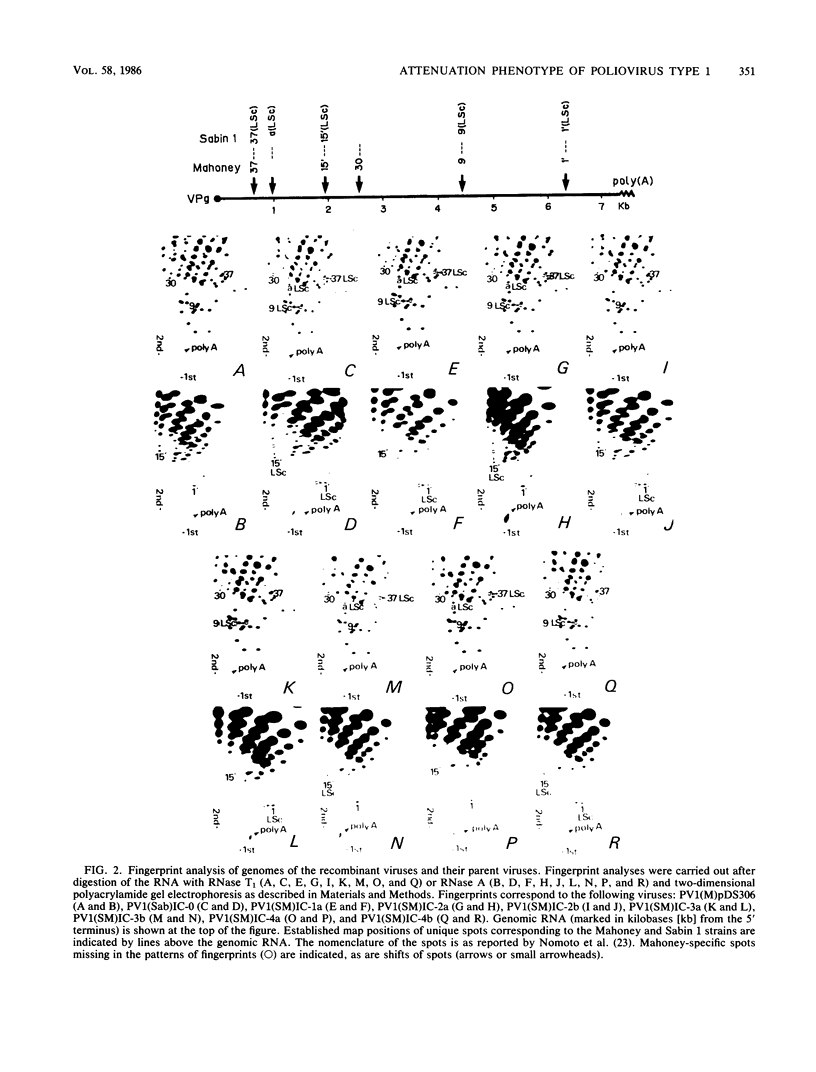
Abstract
Seven different recombinant viruses from the virulent Mahoney and the attenuated Sabin parental strains of type 1 poliovirus were constructed in vitro by using infectious cDNA clones. Monkey neurovirulence tests (lesion score, spread value, and incidence of paralysis) using these recombinant viruses revealed that the loci influencing attenuation were spread over several areas of the viral genome, including the 5' noncoding region. In vitro phenotypic marker tests corresponding to temperature sensitivity of growth (rct marker), plaque size, and dependency of growth on bicarbonate concentration (d marker) were performed to identify the genomic loci of these determinants and to investigate their correlation with attenuation. Determinants of temperature sensitivity mapped to many areas of the viral genome and expressed strong but not perfect correlation with attenuation. Recombinant viruses with Sabin-derived capsid proteins showed a small-plaque phenotype, and their growth was strongly dependent on bicarbonate concentration, suggesting that these determinants map to the genomic region encoding the viral capsid proteins. Plaque size and the d marker, however, were found to be poor indicators of attenuation. Moreover, virion surface characteristics such as immunogenicity and antigenicity had little or no correlation with neurovirulence. Nevertheless, viruses carrying Sabin-derived capsid proteins had an apparent tendency to exhibit less neurovirulence in tests on monkeys compared with recombinants carrying Mahoney-derived capsid proteins. Our results suggest that the extent of viral multiplication in the central nervous system of the test animals might be one of the most important factors determining neurovirulence. Moreover, we conclude that the expression of the attenuated phenotype of the Sabin 1 strain of poliovirus is the result of several different biological characteristics. Finally, none of the in vitro phenotypic markers alone can serve as a good indicator of neurovirulence or attenuation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agol V. I., Drozdov S. G., Frolova M. P., Grachev V. P., Kolesnikova M. S., Kozlov V. G., Ralph N. M., Romanova L. I., Tolskaya E. A., Viktorova E. G. Neurovirulence of the intertypic poliovirus recombinant v3/a1-25: characterization of strains isolated from the spinal cord of diseased monkeys and evaluation of the contribution of the 3' half of the genome. J Gen Virol. 1985 Feb;66(Pt 2):309–316. doi: 10.1099/0022-1317-66-2-309. [DOI] [PubMed] [Google Scholar]
- Agol V. I., Grachev V. P., Drozdov S. G., Kolesnikova M. S., Kozlov V. G., Ralph N. M., Romanova L. I., Tolskaya E. A., Tyufanov A. V., Viktorova E. G. Construction and properties of intertypic poliovirus recombinants: first approximation mapping of the major determinants of neurovirulence. Virology. 1984 Jul 15;136(1):41–55. doi: 10.1016/0042-6822(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Kaesberg P. Determination of the length distribution of poly(A) at the 3' terminus of the virion RNAs of EMC virus, poliovirus, rhinovirus, RAV-61 and CPMV and of mouse globin mRNA. Nucleic Acids Res. 1979 Nov 10;7(5):1195–1204. doi: 10.1093/nar/7.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke T. W., Habermehl K. O., Diefenthal W., Buchholz M. Iodination of poliovirus capsid proteins. J Gen Virol. 1977 Feb;34(2):387–390. doi: 10.1099/0022-1317-34-2-387. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Stanway G., Hughes P. J., Minor P. D., Evans D. M., Schild G. C., Almond J. W. Reversion to neurovirulence of the live-attenuated Sabin type 3 oral poliovirus vaccine. Nucleic Acids Res. 1984 Oct 25;12(20):7787–7792. doi: 10.1093/nar/12.20.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. C., Jameson B. A., Bonin J., Kohara M., Abe S., Itoh H., Komatsu T., Arita M., Kuge S., Nomoto A. Antigenic variation and resistance to neutralization in poliovirus type 1. Science. 1985 Sep 13;229(4718):1090–1093. doi: 10.1126/science.2412292. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Dorner L. F., Larsen G. R., Wimmer E., Anderson C. W. Identification of the initiation site of poliovirus polyprotein synthesis. J Virol. 1982 Jun;42(3):1017–1028. doi: 10.1128/jvi.42.3.1017-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Fiszman M., Reynier M., Bucchini D., Girard M. Thermosensitive block of the Sabin strain of poliovirus type I. J Virol. 1972 Dec;10(6):1143–1151. doi: 10.1128/jvi.10.6.1143-1151.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kohara M., Omata T., Kameda A., Semler B. L., Itoh H., Wimmer E., Nomoto A. In vitro phenotypic markers of a poliovirus recombinant constructed from infectious cDNA clones of the neurovirulent Mahoney strain and the attenuated Sabin 1 strain. J Virol. 1985 Mar;53(3):786–792. doi: 10.1128/jvi.53.3.786-792.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Minor P. D. Characterization of strains of type 3 poliovirus by oligonucleotide mapping. J Gen Virol. 1982 Apr;59(Pt 2):307–317. doi: 10.1099/0022-1317-59-2-307. [DOI] [PubMed] [Google Scholar]
- Nakano J. H., Hatch M. H., Thieme M. L., Nottay B. Parameters for differentiating vaccine-derived and wild poliovirus strains. Prog Med Virol. 1978;24:178–206. [PubMed] [Google Scholar]
- Nomoto A., Jacobson A., Lee Y. F., Dunn J., Wimmer E. Defective interfering particles of poliovirus: mapping of the deletion and evidence that the deletions in the genomes of DI(1), (2) and (3) are located in the same region. J Mol Biol. 1979 Feb 25;128(2):179–196. doi: 10.1016/0022-2836(79)90125-6. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Kitamura N., Lee J. J., Rothberg P. G., Imura N., Wimmer E. Identification of point mutations in the genome of the poliovirus Sabin vaccine LSc 2ab, and catalogue of RNase T1- and RNase A-resistant oligonucleotides of poliovirus type 1 (Mahoney) RNA. Virology. 1981 Jul 15;112(1):217–227. doi: 10.1016/0042-6822(81)90627-9. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Toyoda H., Imura N. Comparative sequence analysis of the 5'terminal noncoding regions of poliovirus vaccine strain Sabin 1, Sabin 2, and Sabin 3 genomes. Virology. 1981 Aug;113(1):54–63. doi: 10.1016/0042-6822(81)90135-5. [DOI] [PubMed] [Google Scholar]
- Omata T., Kohara M., Sakai Y., Kameda A., Imura N., Nomoto A. Cloned infectious complementary DNA of the poliovirus Sabin 1 genome: biochemical and biological properties of the recovered virus. Gene. 1984 Dec;32(1-2):1–10. doi: 10.1016/0378-1119(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALK J. E. Persistence of immunity after administration of formalin-treated poliovirus vaccine. Lancet. 1960 Oct 1;2(7153):715–723. doi: 10.1016/s0140-6736(60)91791-8. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Dorner A. J., Wimmer E. Production of infectious poliovirus from cloned cDNA is dramatically increased by SV40 transcription and replication signals. Nucleic Acids Res. 1984 Jun 25;12(12):5123–5141. doi: 10.1093/nar/12.12.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Reeve P., Minor P. D., Schild G. C., Almond J. W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon 12a1b. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y. V., Maslova S. V., Agol V. I. The genomes of attenuated and virulent poliovirus strains differ in their in vitro translation efficiencies. Virology. 1985 Dec;147(2):243–252. doi: 10.1016/0042-6822(85)90127-8. [DOI] [PubMed] [Google Scholar]
- Totsuka A., Ohtaki K., Tagaya I. Aggregation of enterovirus small plaque variants and polioviruses under low ionic strength conditions. J Gen Virol. 1978 Mar;38(3):519–533. doi: 10.1099/0022-1317-38-3-519. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Mechanism of plaque inhibition of poliovirus possessing the d marker. J Gen Virol. 1968 Dec;3(3):349–357. doi: 10.1099/0022-1317-3-3-349. [DOI] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J Gen Virol. 1979 Aug;44(2):525–534. doi: 10.1099/0022-1317-44-2-525. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Wychowski C., Bruneau P., Blondel B., Crainic R., Horodniceanu F., Girard M. Localization of a poliovirus type 1 neutralization epitope in viral capsid polypeptide VP1. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5080–5084. doi: 10.1073/pnas.80.16.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]



