Abstract
Purpose
In recent years, considerable progress has been made in the use of gallium-68 labeled receptor-specific peptides for imaging oncologic diseases. The objective was to examine the stability and pharmacokinetics of [68Ga]NODAGA and DOTA-peptide conjugate targeting VPAC [combined for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP)] receptors on tumor cells.
Procedures
A VPAC receptor-specific peptide was chosen as a model peptide and conjugated to NODAGA and DOTA via solid-phase synthesis. The conjugates were characterized by HPLC and MALDI-TOF. Following Ga-68 chelation, the radiochemical purity of Ga-68 labeled peptide conjugate was determined by radio-HPLC. The stability was tested against transmetallation using 100 nM Fe3+/Zn2+/Ca2+ ionic solution and against transchelation using 200 μM DTPA solution. The ex vivo and in vivo stability of the Ga-68 labeled peptide conjugate was tested in mouse plasma and urine. Receptor specificity was determined ex vivo by cell binding assays using human breast cancer BT474 cells. Positron emission tomography (PET)/X-ray computed tomography (CT) imaging, tissue distribution, and blocking studies were performed in mice bearing BT474 xenografts.
Results
The chemical and radiochemical purity was greater than 95 % and both conjugates were stable against transchelation and transmetallation. Ex vivo stability at 60 min showed that the NODAGA-peptide-bound Ga-68 reduced to 42.1 ± 3.7 % (in plasma) and 37.4 ± 2.9 % (in urine), whereas the DOTA-peptide-bound Ga-68 was reduced to 1.2 ± 0.3 % (in plasma) and 4.2 ± 0.4 % (in urine) at 60 min. Similarly, the in vivo stability for [68Ga]NODAGA-peptide was decreased to 2.1 ± 0.2 % (in plasma) and 2.2 ± 0.4 % (in urine). For [68Ga]DOTA-peptide, it was decreased to 1.4 ± 0.3 % (in plasma) and 1.2 ± 0.4 % (in urine) at 60 min. The specific BT474 cell binding was 53.9 ± 0.8 % for [68Ga]NODAGA-peptide, 25.8 ± 1.4 % for [68Ga]-DOTA-peptide, and 18.8 ± 2.5 % for [68Ga]GaCl3 at 60 min. Inveon microPET/CT imaging at 1 h post-injection showed significantly (p < 0.05) higher tumor to muscle (T/M) ratio for [68Ga]NODAGA-peptide (3.4 ± 0.3) as compared to [68Ga]DOTA-peptide (1.8 ± 0.6). For [68Ga]GaCl3 and blocked mice, their ratios were 1.5 ± 0.6 and 1.5 ± 0.3 respectively. The tissue distributions data were similar to the PET imaging data.
Conclusion
NODAGA is superior to DOTA in terms of radiolabeling kinetics. The method of radiolabeling was reproducible and yielded higher specific activity. Although both agents have relatively low in vivo stability, PET/CT imaging studies delineated BC tumors with [68Ga]NODAGA-peptide, but not with [68Ga]DOTA-peptide.
Keywords: Chelating agents, Gallium-68, Molecular imaging, Radiochemistry, Tumor imaging
Introduction
In recent years, considerable progress has been made in developing Ga-68 labeled small molecules such as peptides, for imaging oncologic diseases [1–5]. To facilitate Ga-68 labeling effectively, a variety of bifunctional chelating agents (BFCAs) have been developed and used [6–10]. Many studies have shown NODAGA (1,4,7-triazacyclononane-1-glutamic acid-4,7-diacetic acid) superiority over other BFCAs such as DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid), or HBED-CC (N,N′-bis-[2-hydroxy-5-(carboxyethyl) benzyl] ethylenediamine-N,N’-diacetic acid) [11, 12]. Our previous study has reported that NODAGA has faster labeling kinetics and permits Ga-68 labeling at room temperature under acidic conditions [13]. In vivo stability governs the success of these conjugates in animal and patient imaging. However, only a few investigators reported on the stability of various Ga-68 labeled agents. In this investigation, we have examined the stability of [68Ga]NODAGA and DOTA-peptide conjugates and studied their pharmacokinetics in tumor xenografts.
Our laboratory has successfully designed and characterized a peptide-chelator conjugate (TP-3805) which has a high affinity for VPAC [combined for vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP)] receptors. Radiolabeled with copper-64, TP-3805 has been used for PET imaging of breast cancer (BC) and prostate cancer (PC) in humans [14, 15]. This peptide is an analog of pituitary adenylate cyclase-activating peptide (PACAP)—a 27 amino acid peptide which has a high affinity for VPAC receptors expressed on (100 %) malignant BC and PC cells [16–21]. VPAC receptors which encode a G protein involved in cell proliferation and cell differentiation are also expressed in high density on the cell surface of cancers such as those of urinary bladder (100 %), colon (96 %), pancreas (65 %), lung (58 %), stomach (54 %), and liver (49 %) [22–24]. Only a few VPAC receptors are expressed on stroma, normal cells, and benign masses.
With the virtue of Germanium-68 (68Ge)/[68Ga] generator availability and high positron emission (β+, 88 %), Ga-68 is a suitable radionuclide for diagnostic PET imaging. The short physical half-life also induces a low radiation burden to the patients, which strengthens its choice for imaging [25–27]. For the stability and pharmacokinetic studies, we chose TP-3805 as a model peptide, conjugated with NODAGA and DOTA, and chelated with Ga-68. This study provides the details of ex vivo and in vivo stability, cell binding assay, positron emission tomography/computed tomography (PET/CT) imaging, and tissue distribution in mice bearing BT474 xenografts.
Materials and Methods
Reagents
Fmoc amino acids, solvents, and reagents for peptide-chelator synthesis were purchased from Fluka chemicals (St. Louis, MO). The bifunctional chelator DOTA was purchased from Macrocyclics (Dallas, TX) and NODAGA from Chematech (Dijon, France). Diethylenetriamine pentaacetic acid (DTPA), ferric chloride (FeCl3), calcium chloride (CaCl2), zinc chloride (ZnCl2), hydrochloric acid solutions, and acetone were purchased from Thermo Fisher Scientific (Waltham, MA). Transferrin was obtained from Sigma-Aldrich (St. Louis, MO) and cation exchange columns from Agilent Technologies (Santa Clara, CA). Sodium chloride solution (0.9 %) was prepared in our laboratory using deionized water. All reagents were of analytical grade and were used without further purification.
Instruments
The PACAP analog with C-terminal NODAGA and DOTA chelators was synthesized on a Wang resin using a PS3 peptide synthesizer (Protein Technologies, Tucson, AZ, USA) and purified using a preparative reversed phase C18 column (Grace Davison, Deerfield, IL, USA) on a high-pressure liquid chromatography (HPLC) system (Shimadzu Corporation, Kyoto, Japan) equipped with gradient pumps and ultraviolet/visible (UV/Vis) detector. Radiochemical purity was monitored by radio-HPLC (Shimadzu Corporation, Kyoto, Japan) equipped with gradient pumps and UV/ Vis detector, a sodium iodide-thallium [NaI(Tl)] radioactivity monitor, and a rate meter. Mass spectrometry (MS) was performed on a 4800 matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) instrument (SciEx, Framingham, MA). Instant thin layer chromatography (ITLC) was performed on pre-coated aluminum sheets with silica gel which were purchased from Merck (Kenilworth, NJ) and radioactivity was measured on a Wizard2 2480 automatic gamma counter (Perkin Elmer, Waltham, MA). The pH of the solutions was measured using either a pH meter (Sartorius, Gottingen, Germany) or pH strips (Fisher Scientific Inc.). In vivo PET imaging was performed on an Inveon microPET/CT (Siemens, Knoxville, TN). A 370-MBq [68Ge/68Ga] generator was purchased from Eckert and Zeigler (Berlin, Germany) for eluting [68Ga]GaCl3.
Synthesis of Peptide-Chelator Conjugate
Using methods previously described [13], Fmoc-Lys(ivDde) was first introduced as the first residue on the resin, forming the C terminus of the peptide, followed by 4-aminobutyric acid (γ-Aba). The 27-amino-acid long PACAP sequence was then extended C→N by standard Fmoc coupling with the final histidyl residue being a t-Boc-protected His(Trt) derivative. The t-Boc capping function was necessary to ensure that the N-terminal amino group remained protected during subsequent deprotection and coupling cycles performed at the γ-amino group of the C-terminal lysine. The ivDde group at the C-terminal lysine was then selectively removed with 2 % hydrazine, followed by coupling with NODAGA/DOTA. The peptide-chelator was then cleaved from the resin, deprotected using CF3CO2H/water/phenol/thioanisole/ethanedithiol (82.5/5/5/5/2.5), and precipitated with diethyl ether. The crude peptide was purified to homogeneity by HPLC using a preparative C18 reversed phase column. The purified peptide-chelators were characterized by MALDI-MS.
Radiochemistry
[68Ga]GaCl3 was eluted from the generator using 5 ml of 0.1 M HCl and passed through a cation exchange column (CEX). The column was washed with 80 % acetone (in 0.1 M HCl) which removed cationic impurities like Fe3+, or Ge3+, whereas [68Ga]GaCl3 was retained on the column. [68Ga]GaCl3 then was eluted with 1 ml of 98 % acetone (in 0.05 M HCl) into a test tube. For labeling, the excess acetone was evaporated by heating at 70 °C for 5 min facilitated with gentle flow of nitrogen. Twenty micrograms of peptide conjugate in 200 μl of deionized water and 4.5 ± 0.5 mCi (20 μl of [68Ga]GaCl3 were incubated at 90 °C for 30 min and pH was adjusted to 7.2 ± 0.2 with 0.1 M NaOH (post-incubation). Radiochemical purity was monitored by radio-HPLC on a reversed phase C18 micro-bound column (4.6 mm × 250 mm, 5 μm, ZORBAX 300SB-C-18) eluted with a gradient from 10 % CH3CN in aqueous 0.1 % CF3CO2H to 100 % CH3CN in 0.1 % CF3CO2H at a flow rate of 1 ml/min over 28 min. Specific activity was measured after the end of preparation (approximately 30 min after the start of synthesis) and decay corrected for the time elapsed (30 min) after the start of synthesis.
Transchelation and Transmetallation Studies
Aliquots of [68Ga]NODAGA-peptide and DOTA-peptide solutions (0.3 ml, ~ 1.5 MBq) were mixed with (1) 0.3 ml of 0.9 % NaCl, (2) 0.3 ml of 100 nM Fe3+/Zn2+/Ca2+ ionic salt solution (to measure transmetallation), and (3) 0.3 ml of a 200 μM DTPA solution, or with 0.3 ml of a 50-μM transferrin solution (to measure transchelation). The mixtures were incubated at 37 °C and analyzed at 5, 15, 30, 60, and 120 min of incubation, 10 μl (in 100 μl of normal saline) aliquots of preparations (1), and (2) were used for radio-HPLC, while preparation (3) was analyzed by ITLC using aqueous 0.1 M citric acid as a solvent.
Stability Studies (ex vivo and in vivo)
For testing ex vivo stability, fresh cardiac blood was collected (n = 3) in heparinized vials after sacrificing athymic nude mice. The blood was centrifuged at 3000×g for 10 min at room temperature to separate the plasma. Urine was collected from the bladders. Twenty microliters of [68Ga]NODAGA-peptide and DOTA-peptide was mixed with 180 μl of plasma or urine and incubated for 5, 15, 30, and 60 min at 37 °C in a water bath. An equal volume of separated plasma or urine and chloroform/methanol (4:1, v/v) were mixed and centrifuged at 6000×g for 5 min to precipitate the proteins. The supernatant was used for HPLC analysis.
For testing in vivo stability, 100 ± 20 μCi of [68Ga]NODAGA and DOTA-peptide conjugate was injected intravenously (i.v.) into athymic nude mice (n = 3). Blood samples (into heparinized vials) and urine samples (into polymer vials) were collected at predetermined time interval (5, 15, 30, and 60 min) and were processed using the same procedure as described above.
Cell Binding Assay and Specific Binding
Human BT474 breast tumor cells were purchased from American Type Culture Collection (ATCC). Cells were grown in the RPMI-1640 medium with 2 mM l-glutamine containing 10 mM HEPES, 1.0 mM sodium pyruvate, 1.5 g/l sodium bicarbonate, and 4.5 g/l glucose, supplemented with 0.2 U/ml of 90 % bovine insulin and 10 % fetal bovine serum in a T75 flask, which was placed in a humidified incubator at 37 °C under 5 % CO2/95 % air. The cells, when confluent, were detached using 0.25 % trypsin–EDTA, washed, and re-suspended in RPMI 1640 medium. The cells were centrifuged at 3000×g for 10 min and pellet was resuspended in phosphate buffered saline (pH—7.0), at a concentration of ~5 × 106cells/ml. Each test tube contained 1 ml of cell suspension into which 10 μl (≈ 0.74 MBq) of [68Ga]NODAGA, [68Ga]DOTA-peptide, and [68Ga]GaCl3 (as a control) was added and incubated for 15, 30, 60, and 120 min at 37 °C in a water bath.
Non-specific binding was calculated by incubating 1 ml of BT474 cell suspension (~ 5 × 106 cells/ml) in triplicate (n = 3) with a 400-fold excess of non-radioactive NODAGA-peptide or DOTA-peptide for 15 min at 37 °C in a water bath. After incubation, 10 μl of [68Ga]NODAGA-peptide and DOTA-peptide (≈ 0.74 MBq) was added to each vial. The mixtures with labeled peptides were further incubated for 15, 30, 60, and 120 min at 37 °C. The incubation was terminated by adding 0.5 ml of cold normal saline. The mixtures were centrifuged at 3000×g for 10 min. The supernatant from each wash was collected into marked test tubes. The pellet was washed thrice with normal saline and supernatants were collected separately in marked tubes. The radioactivity associated with cells and supernatant was counted in a gamma counter (Wizard2 2480, Perkin Elmer) and the percentage of radioactivity bound to the cells was calculated. The specific binding was determined by subtracting the non-specific binding from total binding.
Animal Studies
PET/CT Imaging and Tissue Distribution
Tumor xenografts were generated by subcutaneously injecting approximately 1.5 × 106 BT474 cells, suspended in 100 μl serum-free medium supplemented with Matrigel, into the right thigh of 6–8 week old, female, athymic nude mice (n = 20). When tumors had grown to a diameter of 6–8 mm (usually 4–6 weeks after inoculation), 100 ± 20 μCi of [68Ga]NODAGA-peptide, [68Ga]DOTA-peptide, or [68Ga]GaCl3 as a control were injected i.v. into tail vein of each mouse (n = 5 per group) and 60 min later subjected to PET/CT imaging. In another group of mice bearing tumor BT474 xenografts (n = 5), 100 μg of cold peptide was administered i.v. 30 min prior the injection of [68Ga]peptide conjugate in order to block the VPAC receptors. Briefly, 100 ± 20 μCi of [68Ga]NODAGA-peptide was then injected i.v. in the tail vein and PET/CT imaging was performed at 1 h post-injection. A low-dose CT scan without contrast agent was acquired for anatomic localization and attenuation correction. The CT scan was acquired in step-and-shoot acquisition mode with full rotation at 360 projections. The exposure X-ray settings were set to a voltage of 80 kV and a current of 500 μA. The whole body PET scan was acquired from head to tail at 2–3 min per bed position in 3-dimensional mode. Inveon Research Workplace, version (Siemens, Knoxville, TN), was used for image processing and analysis. An automatic rigid registration algorithm with weighted mutual information as a measure of similarity was used for registering PET and CT datasets. An ordered-subset expectation maximization (OSEM) 3-dimensional (3D) algorithm with five iterations and eight subsets was used for reconstruction. The standardized uptake value (SUV) was calculated for each organ.
Mice were then euthanized by carbon dioxide inhalation, and tissues were dissected, washed free of blood, dried, and weighed. Concomitant radioactivity was counted together with a Ga-68 standard; and injected dose per gram (% ID/g) was then calculated.
Results
Purity of Peptide-Chelator Conjugates
The chemical purity of NODAGA-peptide and DOTA-peptide conjugates was greater than 95.0 %. The molecular mass for the NODAGA-peptide conjugate was 3716 Da vs. the calculated molecular mass of 3718 Da. For the DOTA-peptide conjugate, observed mass was 3787 vs. the calculated molecular mass of 3785 Da. The MALDI mass spectra for the peptide conjugates are shown in Fig. 1.
Fig. 1.
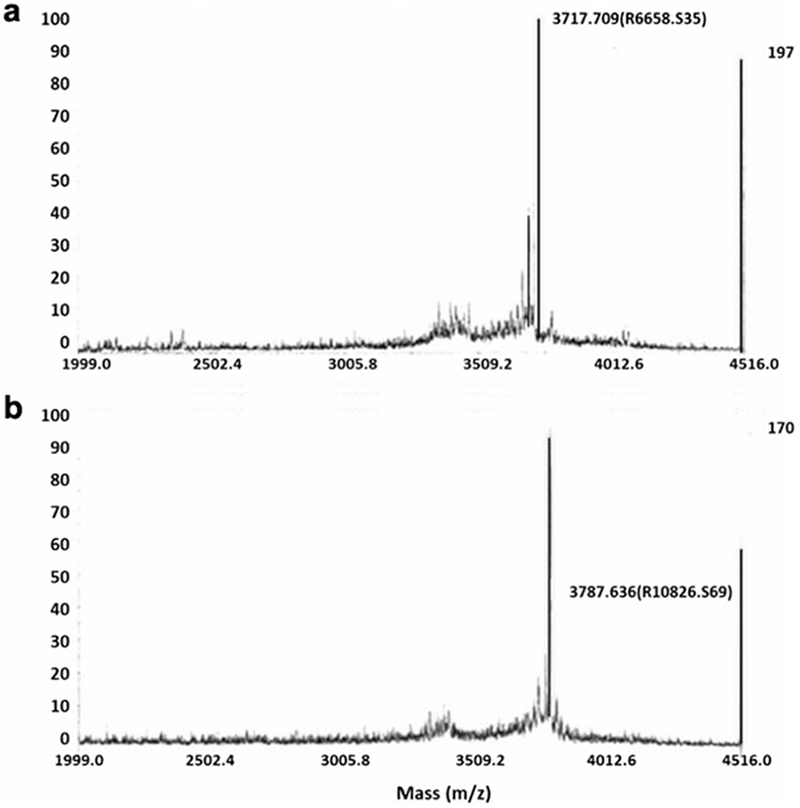
MALDI-TOF MS analysis of a NODAGA-peptide and b DOTA-peptide conjugates showing respective molecular weights (Da). The major peaks at 3787 Da for the NODAGA-peptide and 3717 Da for the DOTA-peptide are in concurrence with the calculated masses.
Radiochemical Purity
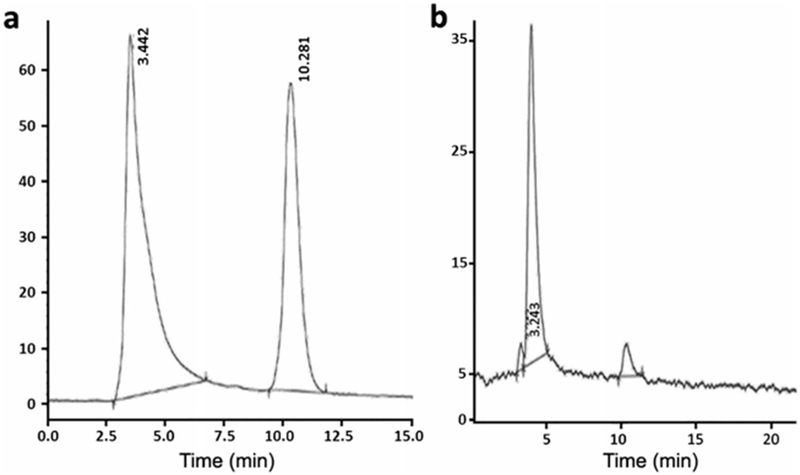
The radiochemical purity as analyzed by radio-high performance liquid chromatography (HPLC) was 96.3 ± 0.5 % for [68Ga]-NODAGA-peptide and 92.7 ± 5.8 % for [68Ga]DOTA-peptide. The retention time for the [68Ga]NODAGA-peptide and DOTA-peptide was ± 0.3 min, whereas for [68Ga]GaCl3 was 3.4 ± 0.4 min (Fig. 2). The specific activity of [68Ga]NODAGA/DOTA-peptide each, based on the availability of [68Ga]GaCl3, varied from 37.2 ± 2.1 to 49.2 ± 2.3 GBq/μmol.
Fig. 2.

Radio-HPLC showing peaks for Ga-68Cl3 at 3.1 min and [68Ga]NODAGA and DOTA-peptide conjugate at 10.2 ± 0.3 min.
Transchelation and Transmetallation Study
Both the [68Ga]NODAGA-peptide and DOTA-peptide were stable in 0.9 % normal saline and showed (< 2 %) transmetallation with FeCl3, CaCl2, and ZnCl2 at 4 h incubation. The transchelation study with DTPA and transferrin solution showed that both radio complexes remained intact over four incubation time. ITLC showed a ratio factor value of 0.0–0.1 for the [68Ga]NODAGA-peptide and DOTA-peptide and 0.9–1.0 for [68Ga]GaCl3.
Stability (ex vivo and in vivo)
The ex vivo stability examined in plasma and urine at specified time intervals at 37 °C for [68Ga]NODAGA-peptide showed a reduction to 45.2 ± 1.8 % at 5 min and then remained the same (42.1 ± 3.7 %) at 60 min. For the [68Ga]DOTA-peptide, the radioactivity de-chelated rapidly down to 4.9 ± 0.9 % at 5 min and further degraded to 1.4 ± 0.3 % at 60 min. In urine, [68Ga]NODAGA-peptide retained ± 2.3 % radioactivity at 5 min and slightly decreased further to 37.4 ± 2.9 % at 60 min. [68Ga]DOTA-peptide radioactivity was only 6.1 ± 1.1 % at 5 min and further decreased to 4.2 ± 0.4 % at 60 min. (Table 1). HPLC analysis of the reaction mixture performed post-incubation suggests that the radioactivity was released as ionic [68Ga](III) (Fig. 3a, b).
Table 1.
Stability of [68Ga]NODAGA and DOTA-peptide conjugate in plasma and urine on incubation at 37 °C
| Time (min) | [68Ga]NODAGA-peptide |
[68Ga]DOTA-peptide |
||||||
|---|---|---|---|---|---|---|---|---|
| Plasma |
Urine |
Plasma |
Urine |
|||||
| % bound | % free | % bound | % free | % bound | % free | % bound | % free | |
| 5 | 45.2 ± 1.8 | 54.8 ± 1.8 | 41.2 ± 2.3 | 58.8 ± 2.3 | 4.9 ± 0.9 | 95.1 ± 0.9 | 6.1 ± 1.1 | 93.9 ± 1.1 |
| 15 | 42.1 ± 3.2 | 42.1 ± 3.2 | 39.9 ± 1.6 | 60.1 ± 1.6 | 5.6 ± 0.1 | 94.4 ± 01. | 6.8 ± 0.9 | 93.2 ± 0.9 |
| 30 | 47.9 ± 5.6 | 52.1 ± 5.6 | 38.9 ± 2.1 | 61.1 ± 2.1 | 3.3 ± 0.4 | 96.7 ± 0.4 | 5.4 ± 0.7 | 94.6 ± 0.7 |
| 60 | 42.1 ± 3.7 | 57.9 ± 3.7 | 37.4 ± 2.9 | 62.6 ± 2.9 | 1.2 ± 0.3 | 98.8 ± 0.3 | 4.2 ± 0.4 | 95.8 ± 0.4 |
Fig. 3.
HPLC analysis of plasma showed decrease in the radiolabeling of a [68Ga]NODAGA-peptide and b [68Ga]DOTA-peptide conjugate upon incubation at 37 °C.
The in vivo stability in plasma at 37 °C showed a different pattern than ex vivo stability; [68Ga]NODAGA-peptide degraded to 6.9 ± 0.9 % at 5 min and further decreased to 2.1 ± 0.2 % at 60 min. [68Ga]DOTA-peptide degraded to 4.9 ± 0.9 % at 5 min and further reduced to 2.2 ± 0.4 %. In urine, [68Ga]NODAGA-peptide was 6.1 ± 1.1 % at 5 min and further reduced to 1.4 ± 0.3 % at 60 min. [68Ga]DOTA-peptide was 2.6 ± 0.8 % at 5 min and degraded further to 1.2 ± 0.4 % at 60 min. (Table 2). The HPLC showed decrease in area under curve at 10.2 ± 0.3 min.
Table 2.
Stability of [68Ga]NODAGA-peptide and DOTA-peptide conjugate in plasma and urine collected at 1 h after i.v. injection of the Ga-68 peptide conjugates
| Time (min) | [68Ga]NODAGA-peptide |
[68Ga]DOTA-peptide |
||||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Urine | Plasma | Urine | |||||
| % bound | % free | % bound | % free | % bound | % free | % bound | % free | |
| 5 | 6.9 ± 0.9 | 93.1 ± 0.9 | 6.1 ± 1.1 | 93.9 ± 1.1 | 4.9 ± 0.9 | 95.1 ± 0.9 | 2.6 ± 0.8 | 97.4 ± 0.8 |
| 15 | 5.9 ± 0.7 | 94.1 ± 0.7 | 5.8 ± 0.8 | 94.2 ± 0.8 | 2.1 ± 0.1 | 97.9 ± 0.1 | 1.9 ± 0.9 | 98.1 ± 0.9 |
| 30 | 6.1 ± 1.2 | 93.9 ± 1.2 | 5.4 ± 0.6 | 94.6 ± 0.6 | 3.2 ± 0.4 | 96.8 ± 0.4 | 2.1 ± 0.7 | 97.9 ± 0.7 |
| 60 | 2.1 ± 0.2 | 97.9 ± 0.2 | 2.2 ± 0.4 | 97.8 ± 1.4 | 1.4 ± 0.3 | 98.6 ± 0.3 | 1.2 ± 0.4 | 98.8 ± 0.4 |
Cell Binding Assay
The specific cell binding was calculated by subtracting the non-specific binding from total binding of [68Ga]NODAGA-peptide and DOTA-peptide. The non-specific binding, evaluated after incubation with excess of cold peptide, was 18.0 ± 3.2 % at 1 h and 18.1 ± 2.2 % at 2 h. The specific binding for [68Ga]-NODAGA-peptide was 52.2 ± 1.1 % at 1 h and 53.9 ± 0.9 % at 2 h. For [68Ga]DOTA-peptide, it was 18.2 ± 1.1 % at 1 h and 25.8 ± 1.4 % at 2 h. For [68Ga]Cl3, it was 18.8 ± 2.5 % at 2 h (Fig. 4). [68Ga]NODAGA-peptide has significantly (p < 0.05) higher affinity for BT474 cells than [68Ga]DOTA-peptide or [68Ga]GaCl3 at 2 h.
Fig. 4.
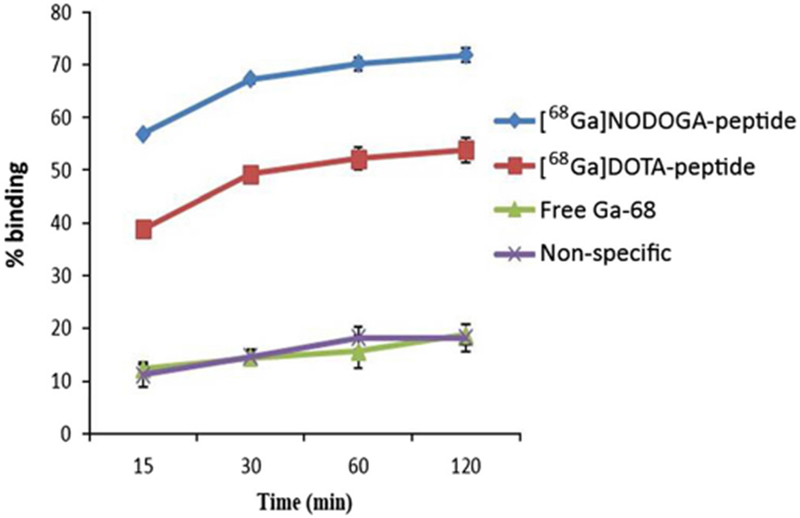
Cell uptake of [68Ga]NODAGA-peptide, [68Ga]DOTA-peptide, and free Ga-68(III) at various time points.
PET/CT Imaging and Tissue Distribution
PET/CT images were obtained on mice bearing BT474 xenografts at 1 h post-i.v. injection (Fig. 5). Both [68Ga]NODAGA-peptide and DOTA-peptide showed major uptake in kidneys followed by the liver. On comparing the standard uptake value (SUV), the tumor to muscle (T/M) ratio was 3.4 ± 0.3 for [68Ga]NODAGA-peptide as compared to 1.8 ± 0.6 for [68Ga]DOTA-peptide and 1.5 ± 0.6 for [68Ga]GaCl3. For [68Ga]NODAGA-peptide, T/M ratio in receptor-blocked mice decreased to 1.6 ± 0.3 as compared to 3.4 ± 0.3 for non-blocked mice (p < 0.05) (Fig. 6). In tumors, [68Ga]NODAGA-peptide showed significantly (p < 0.05) higher uptake as compared to those of [68Ga]DOTA-peptide and [68Ga]GaCl3.
Fig. 5.
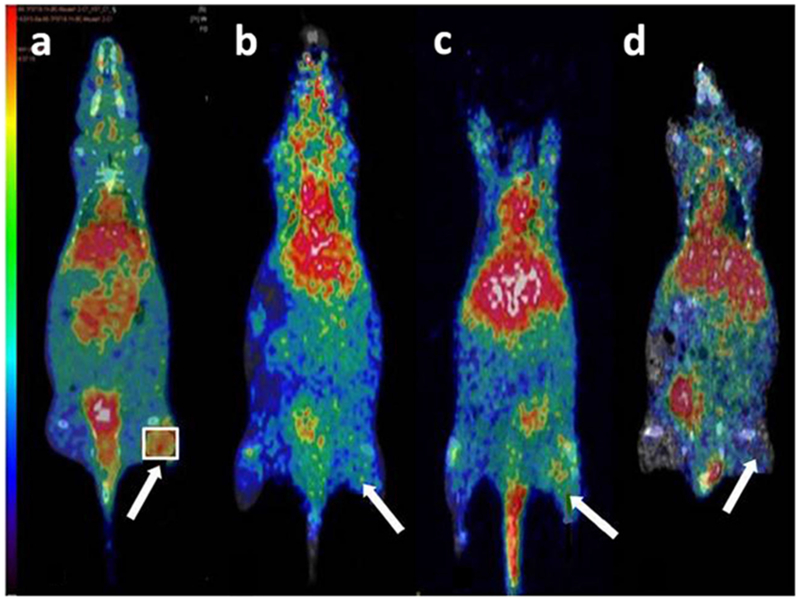
PET/CT images show biodistribution of a [68Ga]NODAGA-peptide, b [68Ga]DOTA-peptide, c blocked mouse, and d [68Ga]Cl3. The tumor to muscle (T/M) ratio for [68Ga]NODAGA-peptide was 3.4 ± 0.3, 1.8 ± 0.6 for [68Ga]DOTA-peptide, 1.6 ± 0.3 in receptor-blocked mice, and 1.5 ± 0.6 for [68Ga]Cl3. T/M ratios were significantly (p < 0.05) greater for NODAGA-peptide than for the DOTA-peptide. Arrow indicates the tumor.
Fig. 6.
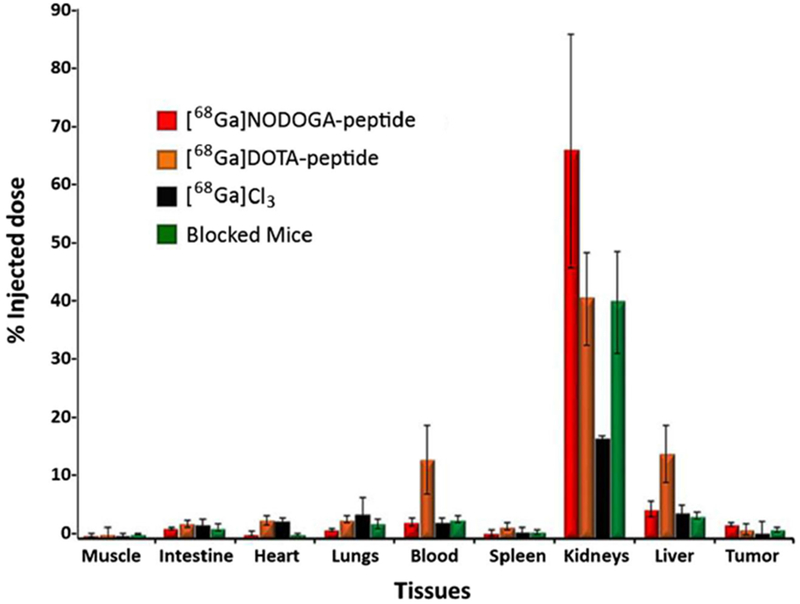
Tissue distribution %ID/g of [68Ga]NODAGA-peptide, [68Ga]DOTA-peptide, [68Ga]GaCl3, and blocked mouse.
The mice were euthanized after PET/CT imaging and injected dose/gram (%ID/g) was calculated for each organ. For [68Ga]NODAGA-peptide, the tissue distribution data (Fig. 6) showed a similar pattern of distribution as observed on PET imaging. The maximum uptake was seen in kidneys (65.3 ± 19.7 %), followed by liver (4.9 ± 1.3 %). The tumor uptake was 2.4 ± 0.3 % as compared to 0.7 ± 0.2 % in the contralateral muscle; the T/M ratio was 3.4 ± 0.3. For [68Ga]DOTA-peptide (Fig. 6), the major uptake was seen in kidneys (40.3 ± 5.8 %) followed by liver (14.3 ± 4.8 %) and blood (13.2 ± 5.8 %). The uptake in tumor was 2.9 ± 0.9 % vs. 1.8 ± 1.1 % in contralateral muscle, and thus the T/M ratio was 1.9 ± 0.9, which was significantly (p < 0.05) lower as compared to [68Ga]-NODAGA-peptide. The tissue distribution of [68Ga]GaCl3 (Fig. 6) showed higher uptake in the lungs (4.2 ± 2.6 %) and liver (4.3 ± 1.3 %) at 1 h. The tumor uptake was 2.2 ± 1.7 % as compared to 1.2 ± 0.4 % in contralateral muscle; T/M ratio was 1.7 ± 0.8. The tissue distribution of [68Ga]NODAGA-peptide in blocked mice (Fig. 6) showed same tissue distribution pattern as of non-blocked mice. However, the uptake decreased significantly (p < 0.05), decreasing the T/M ratio to 1.7 ± 0.3 as compared to 3.4 ± 0.3 in non-blocked mice.
Discussion
Advancements in molecular imaging have contributed extensively to the early detection of BC. The development of novel radiopharmaceuticals to detect BC has showed promising results. However, an unmet need exists for development of more specific radiopharmaceuticals for early detection of oncologic diseases. Our laboratory has successfully designed and characterized a peptide that has high affinity for VPAC receptors expressed in high density on BC cells. The peptide sequence has already been given in our earlier publication [15]. The peptide was conjugated with bis(thiosemicarbazone)-[N2S2], named as TP-3805 and radiolabeled with Cu-64. Cu-64-TP-3805 has showed success in PET imaging of BC, PC, and urothelial bladder cancer (UBC) in humans [28, 29].
We conjugated the same peptide with two different bifunctional chelating agents (NODAGA and DOTA) to radiolabel it with Ga-68. Ga-68 was the choice of radionuclide due to its many advantages over Cu-64. Previously, we have studied the difference in the radiolabeling kinetics of [68Ga]NODAGA-peptide and DOTA-peptide conjugate and found that NODAGA-peptide has more flexibility in radiolabeling with Ga-68 over wide a range of pH and temperature [13]. The possible reason for more favorable Ga-68 (III) kinetics with NODAGA is its coordination number (VI). Ga(III) uses all its coordination sites to form a complex with NODAGA, whereas with DOTA, two sites remain uncoordinated [30].
The chemical and radiochemical purities of the Ga-68 labeled NODAGA-peptide and DOTA-peptide were greater than 95 %. No post-labeling purification was required. The current method of labeling gave three times higher specific activity (37.2 ± 2.1 GBq/μmol) as compared to our previously reported method (12.6 ± 0.4 GBq/μmol) [13]. However, the actual specific activity would be very high if calculated by the HPLC method, which determines the exact amount of labeled peptide. Higher specific activity shows high uptake in small tumor tissue compartments [31]. Both radio complexes were stable against transchelation and transmetallation. Considering ex vivo stability in plasma and urine, [68Ga]NODAGA-peptide is more stable (42.1 ± 3.7 %) as compared to [68Ga]DOTA-peptide (1.2 ± 0.3 %) after 2 h incubation at 37 °C. The in vivo stability results indicated the low in vivo stability of both radioligands.
A number of studies have reported the in vivo stability of Ga-68 labeled DOTA and NODAGA in human or rat serum but the results of this work speak otherwise. There are various reported methods for testing stability. Asti et al. reported stability (> 95 %) of [68Ga]NODAGA, DOTA, and NOTA on incubation with mouse serum by UPLC method [32]. Persson et al. have tested in vivo stability of [68Ga]DOTA and DTPA-PA and found [68Ga]DOTA-PA was 85 % stable and [68Ga]DTPA-PA complex was 27 % stable at 4 h by HPLC method [33]. Kang el al. used the PD-10 column to test the stability of [68Ga]NODAGA-VEGF in mouse serum [34]. Mirzaei et al. have used ITLC method to test stability of [68Ga]ECC using fresh human serum [35]. Many studies have used either HPLC or TLC method to test the in vivo and ex vivo stability [36–41]. We have precipitated plasma proteins using chloroform/ethanol (4/1) and analyzed the supernatant by HPLC. The HPLC (Fig. 3) from plasma and serum showed a peak at 3.4 ± 0.4 min which indicated free Ga-68 (Fig. 2) dechelated (> 90 %) from the complex. At retention time 10.2 ± 0.3, the UV/Vis detector showed elution of the peptide conjugate which confirmed Ga-68 dechelating from the complex. A further detailed investigation is needed to find out the exact reason for such low in vivo instability.
The cell binding was studied in human BT474 breast cancer cells, showing higher 53.9 ± 0.8 % affinity for [68Ga]NODAGA-peptide than for [68Ga]DOTA-peptide conjugate (25.8 ± 1.4 %) at 2 h (p < 0.05). The PET/CT imaging SUVs for [68Ga]NODAGA-peptide showed significantly (p < 0.05) higher T/M ratio (3.4 ± 0.3) as compared to [68Ga]DOTA-peptide (1.8 ± 0.6) and [68Ga]Cl3 (1.5 ± 0.6). The images (Fig. 5) showed renal clearance of [68Ga]NODAGA-peptide and DOTA-peptide with elimination of Ga-68 through the bladder. The excretion of the radioactivity through renal system helps in rapid elimination from background tissues, which facilitates delineation of BC and reduces the radiation burden. The kidneys however are the major dose limiting organ. The tissue distribution (Fig. 6) showed a similar distribution pattern as observed in PET images. [68Ga]NODAGA showed higher T/M ratio (3.4 ± 0.3) as compared to 1.9 ± 0.9 for [68Ga]DOTA-peptide and 1.7 ± 0.8 for [68Ga]GaCl3. The receptor blocking studies showed a similar pattern of tissue distribution but lower T/M ratio (1.7 ± 0.3) as compared to those in non-blocked mice (3.4 ± 0.3), which suggested the specificity of [68Ga]NODAGA-peptide towards VPAC expressing tumors.
However, the uptake of [68Ga]NODAGA-peptide conjugate in tumor mice was not significantly higher as expected after observing the in vitro cell binding data; this may be due to low in vivo stability. In the same peptide conjugated to a different chelating agent, a different distribution was observed. This may be due to change in the overall biological properties of the molecule after conjugation and radiolabeling. The overall chemical structure of the compound may be responsible for in vivo distribution of the [68Ga]NODAGA-peptide and DOTA-peptide [42]. Although both agents have relatively low in vivo stability, the studies suggested that [68Ga]NODAGA-peptide has relatively better stability and greater affinity for VPAC-expressing BT474 breast cancer cells than [68Ga]DOTA-peptide.
Conclusion
The post-processing of [68Ga]GaCl3 ensured removal of cationic impurities and yielded a higher radiochemical purity. The labeling method was reproducible and yielded higher specific activity for [68Ga]NODAGA-peptide and [68Ga]DOTA-peptide. The in vitro, ex vivo studies, and in vivo imaging showed that [68Ga]NODAGA-peptide has higher affinity for breast cancer cells expressing VPAC than [68Ga]DOTA-peptide and [68Ga]GaCl3. PET imaging studies delineated the BC tumors with the [68Ga]NODAGA-peptide but not with the [68Ga]DOTA-peptide. Future studies are needed to elucidate the possible reasons for instability of [68Ga]NODAGA and [68Ga]DOTA in mice.
Acknowledgements.
We thank Kim Lee for providing technical assistance on this project.
Funding Information The research, in part, was supported by NIH/NCI RO1CA157372 (MLT), NIH/NCI 1S10OD012406 (MLT), and NIH/NCI S10RR23709 (MLT).
Footnotes
Conflict of Interest
Mathew L. Thakur is consultant to NuView and Zevacor, Inc. No other potential conflicts of interest relevant to this article are reported.
References
- 1.Al-Nahhas A, Win Z, Szyszko T et al. (2007) Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res 27:4087–4094 [PubMed] [Google Scholar]
- 2.Banerjee SR, Pullambhatla M, Byun Y et al. (2010) [68Ga]-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Med Chem 53:5333–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosini V, Campana D, Tomassetti P et al. (2011) PET/CT with [68Ga]gallium-DOTA-peptides in NET: an overview. Eur J Radiol 80:116. [DOI] [PubMed] [Google Scholar]
- 4.Baum RP, Kulkarni HR, Carreras C (2012) Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med 42:190–207 [DOI] [PubMed] [Google Scholar]
- 5.Weiner RE, Thakur ML (2005) Radiolabeled peptides in oncology: role in diagnosis and treatment. BioDrugs 19:145–163 [DOI] [PubMed] [Google Scholar]
- 6.Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES (2014) The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 4:426–34 [PMC free article] [PubMed] [Google Scholar]
- 7.Notni J, Pohle K, Wester HJ (2012) Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfob CH, Ziegler S, Graner FP, Kohner M, Schachoff S, Blechert B, Wester HJ, Scheidhauer K, Schwaiger M, Maurer T, Eiber M (2016) Biodistribution and radiation dosimetry of 68Ga-PSMA HBED CC-a PSMA specific probe for PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging 43:1962–1970 [DOI] [PubMed] [Google Scholar]
- 9.Prasad V, Ambrosini V, Hommann M, Hoersch D, Fanti S, Baum RP (2010) Detection of unknown primary neuroendocrine tumours (CUP-NET) using 68Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging 37:67–77 [DOI] [PubMed] [Google Scholar]
- 10.Prata MI (2012) Gallium-68: a new trend in PET radiopharmacy. Curr Radiopharm 5:142–149 [DOI] [PubMed] [Google Scholar]
- 11.Altai M, Strand J, Rosik D, Selvaraju RK, Eriksson Karlstrom A, Orlova A, Tolmachev V (2013) Influence of nuclides and chelators on imaging using affibody molecules: comparative evaluation of recombinant affibody molecules site-specifically labeled with 68Ga and 111In via maleimido derivatives of DOTA and NODAGA. Bioconjug Chem 24:1102–1109 [DOI] [PubMed] [Google Scholar]
- 12.Trencsenyi G, Denes N, Nagy G et al. (2017) Comparative preclinical evaluation of [68Ga]-NODAGA and [68Ga]-HBED-CC conjugated procainamide in melanoma imaging. J Pharm Biomed Anal 139:54–64 [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Tripathi SK, Chen CP, Mehta N, Paudyal B, Wickstrom E, Thakur ML (2016) Evaluation of a PACAP peptide analogue labeled with 68Ga using two different chelating agents. Cancer Biother Radiopharm 31:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tripathi S, Trabulsi EJ, Gomella L, Kim S, McCue P, Intenzo C, Birbe R, Gandhe A, Kumar P, Thakur M (2016) VPAC targeted (64)Cu-TP3805 positron emission tomography imaging of prostate cancer: preliminary evaluation in man. Urology 88:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakur ML, Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C, Frangos AJ, Wickstrom E, Intenzo CM (2013) VPAC receptors for imaging breast cancer: a feasibility study. J Nucl Med 54:1019–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reubi JC, Laderach U, Waser B et al. (2000) Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60:3105–3112 [PubMed] [Google Scholar]
- 17.Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC, Fridkin M, Gozes I, Moody TW (1996) Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res 56:3486–3489 [PubMed] [Google Scholar]
- 18.Valdehita A, Carmena MJ, Bajo AM, Prieto JC (2012) RNA interference-directed silencing of VPAC receptor inhibits VIP effects on both EGFR and HER2 transactivation and VEGF secretion in human breast cancer cells. Mol Cell Endocrinol 348:241–246 [DOI] [PubMed] [Google Scholar]
- 19.Valdehita A, Bajo AM, Fernandez-Martinez AB et al. (2010) Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides 31:2035–2045 [DOI] [PubMed] [Google Scholar]
- 20.Moody TW, Gozes I (2007) Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Curr Pharm Des 13:1099–1104 [DOI] [PubMed] [Google Scholar]
- 21.Leyton J, Gozes Y, Pisegna J et al. (1999) PACAP(6-38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat 56:177–186 [DOI] [PubMed] [Google Scholar]
- 22.Schulz S, Rocken C, Mawrin C et al. (2004) Immunocytochemical identification of VPAC, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res 10:8235–8242 [DOI] [PubMed] [Google Scholar]
- 23.Reubi JC (1997) Regulatory peptide receptors as molecular targets for cancer diagnosis and therapy. Q J Nucl Med 41:63–70 [PubMed] [Google Scholar]
- 24.Reubi JC (1995) Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 36:1825–1835 [PubMed] [Google Scholar]
- 25.Asti M, De Pietri G, Fraternali A et al. (2008) Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl Med Biol 35:721–724 [DOI] [PubMed] [Google Scholar]
- 26.Decristoforo C, Knopp R, von Guggenberg E, Rupprich M, Dreger T, Hess A, Virgolini I, Haubner R (2007) A fully automated synthesis for the preparation of [68Ga]-labelled peptides. Nucl Med Commun 28:870–875 [DOI] [PubMed] [Google Scholar]
- 27.Fani M, Andre JP, Maecke HR (2008) [68Ga]-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol Imaging 3:67–77 [DOI] [PubMed] [Google Scholar]
- 28.Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C, Kim C, McCue P, Wickstrom E, Thakur ML (2008) PET imaging of VPAC expression in experimental and spontaneous prostate cancer. J Nucl Med 49:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakur ML, Devadhas D, Zhang K, Pestell RG, Wang C, McCue P, Wickstrom E (2010) Imaging spontaneous MMTVneu transgenic murine mammary tumors: targeting metabolic activity versus genetic products. J Nucl Med 51:106–111 [DOI] [PubMed] [Google Scholar]
- 30.Viola-Villegas N, Doyle R (2009) The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N, N’, N”, N‴-tetraacetic acid (H4DOTA): structural overview and analyses on structure-stability relationships. Coord Chem Rev 253:1906–1925 [Google Scholar]
- 31.Notni J, Steiger K, Hoffmann F, Reich D, Schwaiger M, Kessler H, Wester HJ (2016) Variation of specific activities of [68Ga]-Aquibeprin and [68Ga]-Avebetrin enables selective PET imaging of different expression levels of integrins alpha5beta1 and alphavbeta3. J Nucl Med 57:1618–1624 [DOI] [PubMed] [Google Scholar]
- 32.Asti M, Iori M, Capponi PC, Atti G, Rubagotti S, Martin R, Brennauer A, Muller M, Bergmann R, Erba PA, Versari A (2014) Influence of different chelators on the radiochemical properties of a 68-gallium labelled bombesin analogue. Nucl Med Biol 41:24–35 [DOI] [PubMed] [Google Scholar]
- 33.Gourni E, Demmer O, Schottelius M et al. (2011) PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J Nucl Med 52:1803–1810 [DOI] [PubMed] [Google Scholar]
- 34.Kang CM, Koo HJ, Choe YS, Choi JY, Lee KH, Kim BT (2014) 68Ga-NODAGA-VEGF121 for in vivo imaging of VEGF receptor expression. Nucl Med Biol 41:51–57 [DOI] [PubMed] [Google Scholar]
- 35.Mirzaei A, Jalilian AR, Aghanejad A, Mazidi M, Yousefnia H, Shabani G, Ardaneh K, Geramifar P, Beiki D (2015) Preparation and evaluation of 68Ga-ECC as a PET renal imaging agent. Nucl Med Mol Imaging 49:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson M, Madsen J, Ostergaard S et al. (2012) [68Ga]-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl Med Biol 39:560–569 [DOI] [PubMed] [Google Scholar]
- 37.Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ (2012) [68Ga]-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol 39:777–784 [DOI] [PubMed] [Google Scholar]
- 38.Prinsen K, Cona MM, Cleynhens BJ, Li J, Vanbilloen H, Dyubankova N, Lescrinier E, Ni Y, Bormans GM, Verbruggen AM (2013) Synthesis and biological evaluation of [68Ga]-bis-DOTA-PA as a potential agent for positron emission tomography imaging of necrosis. Nucl Med Biol 40:816–822 [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Ma X, Zhang Z, Sun Z, Loft M, Ding B, Liu C, Xu L, Yang M, Jiang Y, Liu J, Xiao Y, Cheng Z, Hong X (2016) Preclinical study on GRPR-targeted 68Ga-probes for PET imaging of prostate cancer. Bioconjug Chem 27:1857–1864 [DOI] [PubMed] [Google Scholar]
- 40.Ujula T, Salomaki S, Virsu P et al. (2009) Synthesis, [68Ga] labeling and preliminary evaluation of DOTA peptide binding vascular adhesion protein-1: a potential PET imaging agent for diagnosing osteomyelitis. Nucl Med Biol 36:631–641 [DOI] [PubMed] [Google Scholar]
- 41.Vilche M, Reyes AL, Vasilskis E, Oliver P, Balter H, Engler H (2016) 68Ga-NOTA-UBI-29–41 as a PET tracer for detection of bacterial infection. J Nucl Med 57:622–627 [DOI] [PubMed] [Google Scholar]
- 42.Domnanich KA, Muller C, Farkas R et al. (2016) 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: preclinical in vitro and in vivo investigations. EJNMMI Radiopharmacy Chem 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]


