Summary
Objective
We previously discovered a role for the extracellular domain of the transmembrane protein Sema4D as a fast-acting, selective, and positive regulator of functional GABAergic synapse formation in hippocampal neuronal culture. We also demonstrated that Sema4D treatment increases inhibitory tone and suppresses hyperexcitability in an organotypic hippocampal slice culture model of epilepsy. Here, we investigate the ability of Sema4D to promote GABAergic synapse formation and suppress seizure activity in vivo in adult mice.
Methods
We performed a three-hour, intra-hippocampal infusion of Sema4D or control protein into the CA1 region of adult mice. To quantify GABAergic presynaptic bouton density, we performed immunohistochemistry on hippocampal tissue sections isolated from these animals using an antibody that specifically recognizes the glutamic acid decarboxylase isoform 65 protein (GAD65), which is localized to presynaptic GABAergic boutons. To assess seizure activity, we employed two in vivo mouse models of epilepsy, intravenous (i.v.) pentylenetetrazol (PTZ) and hippocampal electrical kindling, in the presence or absence of Sema4D treatment. We monitored seizure activity by behavioral observation or EEG. To assay the persistence of the Sema4D effect, we monitored seizure activity and measured the density of GAD65-positive presynaptic boutons three or 48 hours after Sema4D infusion.
Results
Sema4D treated mice displayed an elevated density of GABAergic presynaptic boutons juxtaposed to hippocampal pyramidal neuron cell bodies, consistent with the hypothesis that Sema4D promotes the formation of new inhibitory synapses in vivo. Further, Sema4D acutely suppressed seizures in both the PTZ and electrical kindling models. When we introduced a 48-hour gap between Sema4D treatment and the seizure stimulus, seizure activity was indistinguishable from controls. Moreover, immunohistochemistry on brain sections or hippocampal slices isolated three hours, but not 48 hours, after Sema4D treatment displayed an increase in GABAergic bouton density, demonstrating temporal correlation between the effects of Sema4D on seizures and GABAergic synaptic components.
Significance
Our findings suggest a novel approach to treating acute seizures: harnessing synaptogenic molecules to enhance connectivity in the inhibitory network.
Keywords: Epilepsy, Sema4D, Synaptogenesis, GABAergic, Hippocampus
Introduction
Despite a substantial increase in the number of new antiepileptic drugs (AEDs) over the past twenty years, the prevalence of drug resistant epilepsy remains at 20–30% of epileptic individuals1; thus, there is an urgent need for the development of novel AEDs. Historically, many epilepsy treatments have relied on enhancing inhibition. A number of first generation antiepileptic drugs such as diazepam and clonazepam increase the chloride conductance of GABAA receptors, boosting the hyperpolarizing effect of GABAergic synaptic transmission. Recent studies demonstrated seizure suppression in rodent models through local and/or temporally restricted control of neuronal inhibition using optogenetics to silence excitatory neurons or activate interneurons, DREADDs to silence excitatory neurons, and transplantation of interneuron progenitor cells2–4. Thus, finding new ways to augment inhibitory tone in the seizing brain appears to be a viable option for new AED development.
Our lab identified a novel regulator of the hippocampal inhibitory network: the transmembrane protein Semaphorin 4D (Sema4D)5. Sema4D is required for proper formation of GABAergic synapses onto hippocampal pyramidal neurons6,7. Further, bath application of a soluble human Sema4D ectodomain/mouse Fc fusion protein (Sema4D-Fc) induced a dramatic increase in GABAergic, but not glutamatergic, synapse density and synaptic activity within two hours of application to cultured hippocampal neurons and organotypic slice5. The Sema4D-Fc induced increase in synapse density requires the transmembrane protein Plexin B1, a high affinity Sema4D receptor5.
Given these findings, we sought to determine if Sema4D application could have similar synaptogenic effects on the intact brain in adult animals and, further, if Sema4D treatment could ameliorate network hyperexcitability in rodent epilepsy models. To investigate these questions, we treated adult mice by intra-hippocampal infusion of Sema4D-Fc. We observed an increase in presynaptic GABAergic bouton density at the infusion site, as well as significant suppression of seizures in two well-established rodent models of epilepsy. We further found a correlation between the time course of Sema4D-mediated seizure suppression and the increase in GABAergic bouton density. Together with our previous work on the pro-synaptogenic effects of Sema4D in vitro5, our results are consistent with a model in which administration of Sema4D promotes the rapid assembly of GABAergic synapses in the hippocampus, suggesting that inhibitory synapse formation can occur quickly in the adult brain. Further, Sema4D application in a discrete hippocampal region ameliorated seizure activity, supporting the hypothesis that spatially restricted modulation of the inhibitory network can limit recurrent excitation in the temporal lobe2,8,9. Our findings suggest that a novel anti-epilepsy therapy could enhance network inhibition by increasing the number of GABAergic synapses through treatment with a synaptogenic molecule such as Sema4D.
Results
Sema4D increases GABAergic bouton density in vivo
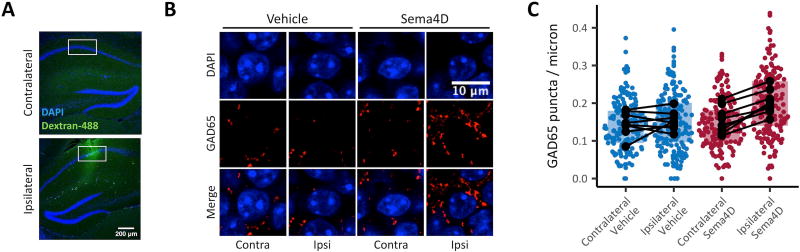
Mice received unilateral hippocampal infusions of 100nM Sema4D-Fc in artificial Cerebral Spinal Fluid (aCSF) with 5% dextran-488. Three hours after the start of infusion, the mice were sacrificed and their brains processed for immunohistochemistry (Fig. 1A). This treatment time frame is consistent with the amount of time required for Sema4D-mediated formation of active synapses in cultured hippocampal neurons and hippocampal slice5. To quantify GABAergic synapse density, we performed immunostaining on coronal brain sections using an antibody that specifically recognizes the GABA synthesizing enzyme GAD65, which is concentrated at the presynaptic terminal and is regularly used in immunohistochemistry of rodent brain tissue to visualize presynaptic boutons of GABAergic synapses10–15. We also treated the sections with DAPI to label cell nuclei. We imaged sections containing hippocampus proximal to the infusion site on a confocal microscope; infusion sites were identified by dextran-488 florescence (Fig. 1A). Single plane images were taken of the ipsilateral infusion site and a matching contralateral position in the section. The nuclei of layer CA1 cells were identified by DAPI fluorescence. Presynaptic GABAergic boutons in the principal cell layer are found around neuronal somas and therefore appear as rings of punctate GAD65 immunoreactivity in close proximity to DAPI-stained nuclei (Fig. 1B). GAD65 puncta density was assessed as the number of puncta in a ring divided by the perimeter of an ellipse fit to the ring. To ask if Sema4D treatment led to an increase in GABAergic bouton density, we calculated the effect of treatment within a mouse by taking the difference between the mean bouton density on the treated, ipsilateral side of the brain and the mean density on the untreated, contralateral side. We found that this difference was significantly increased in mice treated with Sema4D-Fc compared to mice treated with the vehicle solution, indicating that Sema4D treatment increases GABAergic bouton density (Fig 1B, C). We found no effect of Sema4D treatment on the intensity of the GAD65 immunostaining (Fig. S1A) or the diameter of the GAD65 puncta (Fig. S1B). These data are consistent with a recent time-lapse imaging study demonstrating that Sema4D application promotes the stabilization of GABAergic presynaptic boutons in hippocampal slice16. Taken together, these data suggest that Sema4D promotes the formation of new GABAergic synapses in the mouse hippocampus in vivo, most likely by stabilizing nascent presynaptic boutons.
Fig. 1. Sema4D treatment increases GABAergic bouton density in vivo.
Mice (C57Bl/6, male, P42–P59) were anesthetized and treated with Sema4D or vehicle solution by unilateral intra-hippocampal infusion over 10 minutes. The mice were sacrificed 3 hours following treatment. (A) Coronal sections through mouse hippocampus contralateral (top) and ipsilateral (bottom) to the infusion site. The infusion is marked by dextran-488 (green), and nuclei are labelled by DAPI (blue). White boxes denote regions of interest from which cells analyzed as in C are selected. Scale bar represents 200 µm. (B) CA1 principal layer cells in coronal sections through Sema4D or vehicle treated brain hemispheres. Sections are stained with an antibody that specifically recognizes GAD65 (red) and with DAPI (blue). Scale bar represents 10 µm. (C) Density of GAD65 puncta on the perimeter of CA1 principal cell layer somas. In all cases, ipsilateral is the site of infusion and contralateral was not treated. Colored points represent individual cells and black points represent means for individual animals; black lines connecting dots indicate the same animal. Boxplots summarize individual cell data. In this and all figures: in boxplots, hinges represent the first and third quartile. Statistics were performed on delta scores calculated within animal by subtracting the mean bouton density on the contralateral side from the mean bouton density on the ipsilateral side. Delta scores were significantly greater in Sema4D-treated mice than in vehicle-treated mice (p<0.01, unpaired, two-tailed t test). n=8 mice and ≥174 cells per condition.
Sema4D treatment protects against PTZ-induced seizures
We next evaluated the hypothesis that a Sema4D-mediated increase in GABAergic synapse density would counteract the abnormal excitatory activity present during seizures. C57Bl/6 male mice were implanted with cannulas targeting CA1. After recovering from surgery, the mice were treated for three hours by infusion of 100nM of Sema4D-Fc with 5% dextran-488 in aCSF or with vehicle 100nM Fc control protein with 5% dextran-488 in aCSF. After treatment, the mice were subjected to intravenous infusion of 1% PTZ in PBS for a maximum of five minutes at a rate of 0.1ml/min (Fig. 2A). PTZ is a GABAA receptor antagonist and proconvulsant agent that induces a stereotyped, dose-dependent progression of seizure classes (Materials and Methods Table 1)17–19. We chose the PTZ model in part because of the reproducible and quantitative nature of the timed i.v. PTZ infusion seizure paradigm as well as the paradigm’s frequent use as a model for assessing anti-convulsant drug effects17.
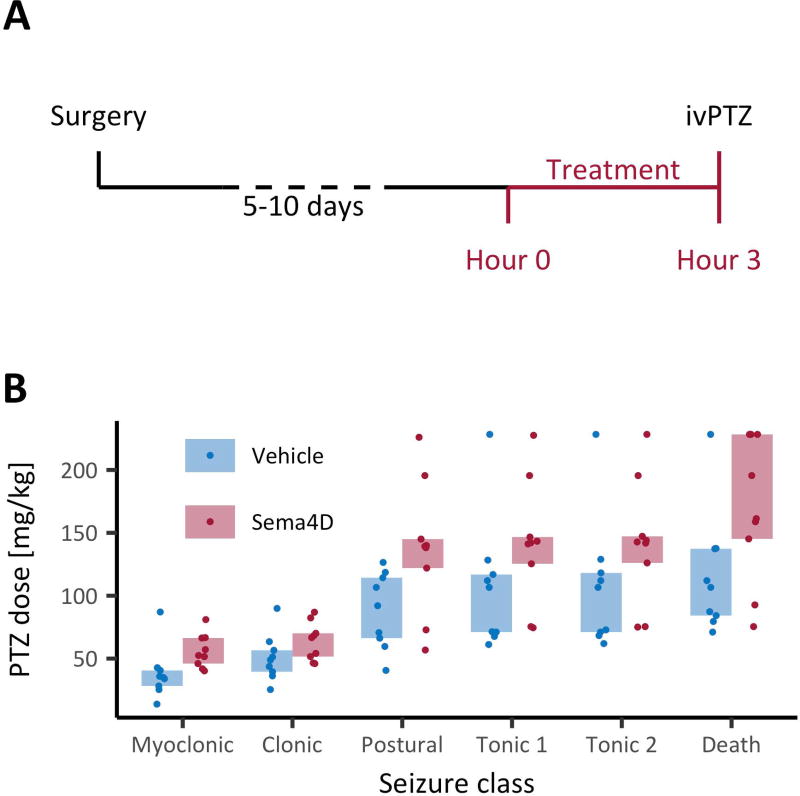
Fig. 2. Sema4D treatment increases i.v. PTZ-induced seizure thresholds.
(A) Timeline of the experiment. Mice (C57Bl/6, male, P50–P80) were implanted unilaterally with cannulas targeting CA1, then given 5–10 days to recover. After recovery, a 3-hour intra-hippocampal Sema4D or vehicle treatment was administered. Immediately following treatment, PTZ was infused through the tail vein for up to 5 min. (B) PTZ dose (d=tcr/w, where d is the PTZ dose received, t is the time since the start of infusion, c is the concentration of PTZ, r is the rate of infusion, and w is the weight of the animal) versus seizure class (see methods for description of PTZ seizure classes). There was a significant main effect of treatment on dose, p<0.01, mixed-design ANOVA on ranks. There was no significant interaction between treatment and seizure class. n=9 mice per condition.
Table 1.
Behavioral seizure classes used for analysis of PTZ-induced seizures.
| Seizure behavior by severity index | ||
|---|---|---|
|
| ||
| Name | Behavior | Severity index |
| Myoclonic | Myoclonic jerk | 1 |
| Clonic | Clonic limb movement | 2 |
| Postural | Loss of postural control | 3 |
| Tonic 1 | Tonic forelimb extension | 4 |
| Tonic 2 | Tonic hindlimb extension | 5 |
| Death | Death | 6 |
We found that PTZ seizure thresholds were significantly higher in mice treated with Sema4D-Fc, compared to mice treated with vehicle Fc control protein (Fig. 2B), suggesting that Sema4D application to a discrete region of the hippocampus can suppress behavioral seizures in this model. The mean effect of Sema4D treatment on PTZ dose at myoclonic seizures (47%) is comparable to the effects of other routinely employed anticonvulsants including Valproate (51%) and Levetiracetam (51%) (tested in CD-1 mice)20.
Sema4D treatment confers a temporally restricted increase in GABAergic bouton density and suppression of seizures
Next, we turned our attention to assessing the long-term effect of a three-hour Sema4D treatment on seizure activity. Once again, we implanted mice with cannulas targeting CA1 and infused 100nM Sema4D-Fc or 100nM vehicle Fc in aCSF for three hours. Forty-eight hours after treatment, the mice were subjected to i.v. infusion of 1% pentylenetetrazol (PTZ) in PBS for five minutes at a rate of 0.1mL/min (Fig. 3A). We observed no difference in PTZ seizure thresholds between Sema4D-Fc treated and Fc vehicle treated mice forty-eight hours after Sema4D treatment (Fig. 3B).
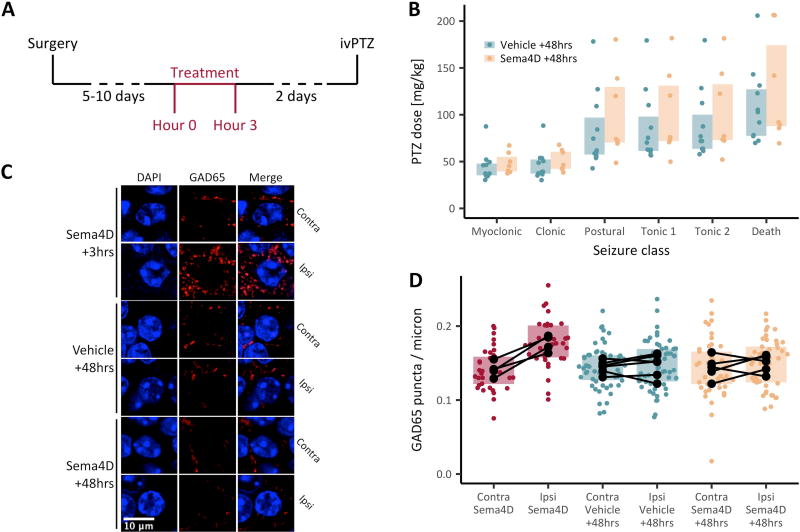
Fig. 3. The anti-seizure and increased GABAergic density-promoting effects of Sema4D are temporally correlated.
(A) Timeline of the experiment shown in B. Mice (C57Bl/6, male, P50–P80) were implanted unilaterally with cannulas targeting CA1, then given 5–10 days to recover. After recovery, an intra-hippocampal Sema4D or vehicle treatment was administered. Forty-eight hours after treatment, PTZ was infused through the tail vein for up to 5 min. (B) PTZ dose versus seizure class. There was no significant main effect of treatment on dose, and there was no significant interaction between treatment and seizure class. Mixed-design ANOVA on ranks; n=11 vehicle-treated and n=7 Sema4D-treated mice. (C, D) Mice (C57Bl/6, male, P49–P59) were anesthetized and treated with Sema4D or vehicle solution by unilateral, intra-hippocampal infusion over 10 minutes. The mice were sacrificed 3 hours (n=4 mice, all Sema4D-treated) or 48 hours (n=6 mice, vehicle-treated and n=5 mice, Sema4D-treated) following treatment. (C) CA1 principal layer cells in coronal sections through Sema4D or vehicle treated brains. Sections are stained with an antibody that specifically recognizes GAD65 (red) and with DAPI (blue). Scale bar represents 10 µm. (D) Density of GAD65 puncta on the perimeter of CA1 principal cell layer somas. Colored points represent individual cells and black points represent means for individual animals; black lines connecting dots indicate the same animal. Boxplots summarize individual-cell data. In all cases, ipsilateral is the site of infusion and contralateral was not treated. Statistics were performed by ANOVA with post-hoc, Bonferroni-corrected, unpaired, two-tailed, t tests on delta scores calculated within animal by subtracting the mean bouton density on the contralateral side from the mean bouton density on the ipsilateral side. Delta scores were significantly greater in Sema4D-treated mice sacrificed 3 hours after treatment than mice of either treatment group sacrificed 48 hours after treatment (p<0.05). Delta scores did not differ between Sema4D and vehicle-treated mice sacrificed 48 hours after treatment (p>0.05).
To evaluate the persistence of the Sema4D-mediated change in GABAergic bouton density, we treated mice with Sema4D-Fc or vehicle solution as described above (Fig. 1). Forty-eight hours after the start of infusion, the mice were sacrificed and their brains processed for immunohistochemistry; PTZ was not administered. As a positive control, a cohort of mice received the Sema4D treatment and were sacrificed three hours post-treatment, as in Figure 1. GAD65 immunostaining and quantification of presynaptic GABAergic bouton density were performed as above. To ask if Sema4D increased GABAergic bouton density, the effect of treatment was calculated for each mouse as the mean puncta density on the treated side of the brain minus the mean puncta density on the untreated side. We found no significant difference when comparing Sema4D-treated and vehicle-treated mice sacrificed forty-eight hours after treatment (Fig 3C, D). As expected, the difference in puncta density between the ipsilateral and contralateral side was significantly increased in mice infused with Sema4D and sacrificed after three hours compared to mice in either 48-hour treatment group (Fig 3C, D).
To corroborate our in vivo findings, we sought to determine the persistence of the Sema4D-mediated change in GABAergic bouton density in cultured organotypic hippocampal slices. To this end, we cultured hippocampal slices from P6–7 rat pups and treated with 2nM Sema4D-Fc or vehicle control Fc for two hours. After treatment, the slices were either fixed immediately or washed once with culture media to the remove Sema4D-Fc and incubated for an additional 48 hours. Immunostaining for GAD65 revealed a Sema4D-dependent increase in the density of GABAergic boutons juxtaposed to the cell bodies of CA1 pyramidal cells in cultures fixed immediately after treatment, compared to organotypic cultures treated with vehicle Fc protein (Fig. S2A, B). In contrast, we found no difference in GABAergic bouton density between Sema4D-Fc and vehicle treated cultures after a 48-hour incubation following Sema4D-Fc or vehicle withdrawal (“+48hrs” in Figure S2) (Fig. S2A, B). This result agrees with our previous electrophysiological study showing that two hours of Sema4D treatment suppresses neuronal hyperexcitability and increases inhibitory tone in organotypic hippocampal slice5. Further, our data demonstrate a temporal correlation between the increased GABAergic bouton density as detected by immunostaining both in vitro and in vivo, and the seizure suppressing effect of Sema4D treatment.
Sema4D treatment protects against electrically kindled seizures
To evaluate the generalizability of Sema4D-mediated seizure suppression, we turned to a second in vivo seizure model: rapid hippocampal electrical kindling. C57Bl/6 male mice were implanted with bilateral cannulas targeting the dentate gyrus (DG) of the hippocampus, as well as bilateral, bipolar electrodes for stimulating and recording at the infusion sites. After a five to ten day recovery period, the mice were subjected to three days of kindling by serial, 0.4 mA stimulation at 20 Hz, followed by a 48-hour stimulus-free rest period (Fig. 4A)21.
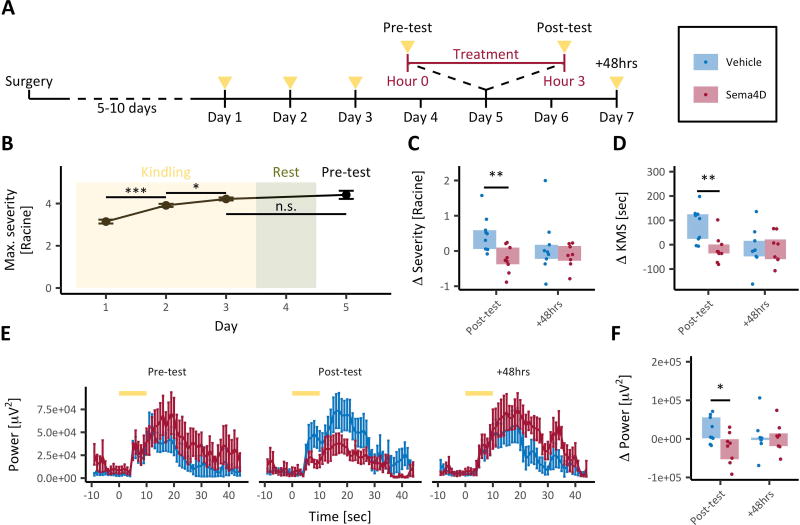
Fig. 4. Acute Sema4D treatment protects against electrically kindled seizures.
(A) Timeline of the experiment. Mice (C57Bl/6, male, P56–P80) were implanted bilaterally with cannulas and electrodes targeting DG, then given 5–10 days to recover. The rapid kindling protocol (see methods) was conducted over a three-day period (yellow triangles denote periods of stimulus delivery). Kindling was followed by a 48-hour rest period, then a baseline evoked seizure test (pre-test), 3 hours of Sema4D or vehicle treatment, and a post-treatment evoked seizure test (post-test). Another post-test was administered 48-hours post-treatment (+48hrs). (B) Maximal behavioral seizure score per kindling stimulus versus experimental day (see methods for description of Racine seizure scores). *p<0.05, ***p<0.001, unpaired two-tailed t tests with Bonferroni corrections. Error bars represent SEM. (C) Delta (post-test minus pre-test or +48hrs minus pre-test) mean seizure scores for post-test and +48hrs. **p<0.01, mixed-design ANOVA on ranks with post-hoc unpaired two-tailed t tests with Bonferroni corrections. (D) Delta (calculated as described above) time spent in kindled motor seizures (KMS) for post-test and +48hrs. **p<0.01, mixed-design ANOVA on ranks with post-hoc unpaired two-tailed t tests with Bonferroni corrections. (E) Total EEG power (0.5–30Hz) versus time during the evoked seizure tests. Recordings were contralateral to the site of stimulation. Yellow bars denote the time of stimulus presentation. Error bars indicate SEM. (F) Delta (calculated as described above) after-discharge EEG power for post-test and +48hrs. *p<0.05, mixed-design ANOVA on ranks with post-hoc unpaired two-tailed t tests with Bonferroni corrections. n=9 mice per condition (all panels).
After the stimulus-free period, the mice were given a single stimulus to determine baseline evoked seizure response (“pre-test”), as measured by changes in EEG power and behavioral seizure score. We recorded EEG and video from each mouse for a total of ten minutes centered on the time of stimulus delivery. Evoked responses were assessed during the five minutes following stimulus onset. Kindling was demonstrated by an observed increase in the magnitude of evoked behavioral seizures that was not diminished after the stimulus-free period (Fig. 4B, compare the magnitude of response to day three kindling stimulus to day five pre-test stimulus).
Next, the mice were treated for three hours by bilateral infusion of 100nM Sema4D-Fc in aCSF or 100nM vehicle Fc protein in aCSF. Treatment group assignments were random, and a retrospective analysis revealed that the time course and degree of kindling did not differ between treatment groups during the three-day kindling period (see Materials and methods for statistical analysis). Absolute values for seizure severity for each animal can be found in Fig. S3.
While a post-hoc analysis shows that pre-test severity scores were higher among Sema4D-treated mice than vehicle-treated mice (p=0.02906, uncorrected, unpaired, two-tailed t test) (Fig. S3), mice were randomly assigned to treatment groups without this knowledge, therefore we interpret this difference as the product of random sampling. Importantly, the effect of kindling day did not differ between treatment groups during the three-day kindling period.
After treatment, the mice were given a second stimulus (“post-test”) and evoked seizure response was recorded as previously described. Sema4D-Fc treated mice exhibited a reduction in post-test behavioral seizure magnitude compared to vehicle treated mice (Fig. 4C, “post-test”). Further, compared to vehicle treated mice, mice treated with Sema4D-Fc spent less time in kindled motor seizures (KMS), advanced seizures classified as three or higher on the modified Racine scale (Fig. 4D, “post-test”)21. Further, the change in score from pre-test to post-test cannot be explained by regression to the mean because mice tended to return to individual baselines (rather than a global mean) at the +48hrs time point (Fig. S3).
Stimulating the DG with the seizure-evoking stimulus triggers a high amplitude EEG event called an after-discharge (Fig. S4A)22. Compared to vehicle treatment, Sema4D-Fc treatment did not significantly alter the duration of the after-discharge (Fig. S4B). However, Sema4D-Fc treated mice experienced a reduction in total EEG power at the infusion site during the after-discharge event compared to control mice (Fig. 4E, F “post-test”). Taken together with the effect of Sema4D on PTZ-induced seizures, these results demonstrate that the anti-epileptic effect of Sema4D treatment generalizes to a chronic and non-pharmacological epilepsy model.
We also assayed the persistence of the effect of Sema4D treatment on seizures in the kindled mice forty-eight hours after Sema4D or vehicle Fc treatment. We allowed the mice a 48-hour stimulus free period after the post-treatment test stimulus on experimental day five (Fig. 4A). The mice were then given a second stimulus, and evoked seizure response was measured as previously described. We observed no difference in behavioral seizure severity in Sema4D-Fc compared to vehicle treated mice 48 hours after treatment (Fig. 4C, D “+48hrs”). In addition, the change from baseline in after-discharge EEG power did not differ between treatment groups at the 48-hour time point (Fig. 4E, F “+48hrs”). Together with our PTZ-induced seizure data (Fig. 3B), these data indicate that the anti-seizure effects of three hours of Sema4D treatment are restricted to a window of less than forty-eight hours. The correlation between the time course of the Sema4D-mediated increase in GABAergic bouton density and anti-seizure activity is consistent with the hypothesis that these two effects are related.
Discussion
Here we demonstrate that application of Sema4D to discrete hippocampal regions both increases the density of presynaptic GABAergic boutons and suppresses seizure activity in vivo. We also show that the effects of Sema4D on bouton density and seizures are temporally correlated. Based on these results, we propose that increased GABAergic synapse formation in response to Sema4D underlies the seizure suppressing effect of the in vivo Sema4D treatment. This model is consistent with our previous findings in cultured neurons and hippocampal slice which demonstrated that a two-hour Sema4D treatment is sufficient to promote functional GABAergic synapse formation5.
Defining the precise cell biological and molecular mechanisms that underlie GABAergic synapse formation remains an active area of investigation. Time-lapse imaging studies of GABAergic synapse formation in hippocampal slice revealed that a nascent GABAergic synapse is marked by the appearance of a new, stable presynaptic GABAergic bouton that is positive for GAD65 immunoreactivity23. Next, postsynaptic components of GABAergic synapses, such as the scaffolding protein gephyrin, become localized in opposition to the newly formed presynaptic bouton within two-to-three hours after detection of the GABAergic bouton24,25.
One interesting unanswered question in our study is: what are the molecular mechanisms by which Sema4D treatment increases the density of GABAergic synaptic boutons? Our earlier studies indicate that Sema4D is localized to the synaptic membrane, signals through its conserved, extracellular Sema domain7, and requires the presence of the Plexin B1 receptor to promote GABAergic synapse formation5. Also, Sema4D promotes GABAergic synapse formation onto both dendrites and somas of hippocampal neurons, suggesting that multiple classes of interneurons may signal through Sema4D/Plexin B1 to regulate synapse formation5.
Time-lapse imaging studies of GAD65-GFP labelled presynaptic boutons in organotypic hippocampal slice demonstrate that boutons are dynamic structures that appear, disappear, and re-appear within a timeframe of a few hours16,24. Interestingly, Sema4D treatment promotes the stabilization of a subpopulation of these dynamic boutons, suggesting a mechanism by which Sema4D increases the density of GABAergic boutons16. We posit that a population of pre-synaptic boutons undergo similar dynamics in the adult hippocampus in vivo and that Sema4D treatment promotes the stabilization and subsequent maturation of these boutons. Further, we postulate that Sema4D-dependent stabilization of presynaptic GABAergic boutons ultimately leads to increased density of functional, GABAergic synapses as GABA receptors and other components of the GABAergic postsynaptic specialization are recruited to newly stabilized boutons. A formal test of this hypothesis awaits further study.
Another conclusion of our study is that administration of Sema4D unilaterally, in a discrete region of hippocampus (CA1), is effective at suppressing seizures caused by systemic administration of PTZ. This result is in line with other studies which demonstrate that increased activation of inhibitory neurons in discrete hippocampal sub-regions is sufficient to suppress behavioral seizures in mice2,8,9,26. For example, using the unilateral, intra-hippocampal kainate mouse model of TLE, the Soltesz group demonstrated that optogenetic activation of less than 5% of parvalbumin-positive interneurons significantly reduced seizure duration even when the activation occurred in the hippocampal region contralateral to the site of kainate injection2. In addition, the same group showed that cerebellar purkinje cell activation, in either hemisphere, can also inhibit hippocampal seizures in the same TLE mouse model27. The success of these studies and others28 support the long-held belief that the hippocampus can function as a gate or “choke-point” for seizure activity in the brain29,30. Our data also suggest that an anti-seizure effect can be achieved by increasing GABAergic synapse density in a constrained area of the hippocampus.
In sum, our data provide strong evidence for the feasibility of a novel class of anti-epileptic drugs that increase inhibitory synapse density in pro-epileptic brain structures. In the future, it may be fruitful to explore the potential for synergistic interactions between synaptogenic therapeutics such as Sema4D and traditional therapies. In particular, one might expect that interactions with drugs and treatment strategies that enhance GABAAR chloride currents or GABAergic neuronal activity would have multiplicative effects on total inhibitory current. For example, co-administration of Sema4D and a benzodiazepine could increase the efficacy of this first line treatment31. This may be particularly relevant to epilepsies such as Status Epilepticus, given the discrete time window in which Sema4D appears to suppress seizure activity.
Materials and methods
Animals
C57Bl/6, male mice and pregnant Nile rats were purchased from the Charles River Laboratories and housed in the animal facility at Brandeis University. Animals were maintained with a 12-hr light-dark cycle. Food and water were available ad libitum. Six to 11-week-old male mice were used for in vivo studies and P6–7 male and female rat pups were used for organotypic cultures. Animal procedures were performed with approval from the Institutional Care and Use Committee at Brandeis University.
Surgeries for cannula and electrode implantations
Mice were anesthetized by intraperitoneal injection of a ketamine/xylazine cocktail (100mg/kg ketamine, 10mg/kg xylazine) or isoflurane gas inhalation (4% induction and 1.5–2% maintenance). An incision was made along the scalp midline and the periosteum over the skull was removed. Stereotaxic coordinates for the hippocampus were identified according to the protocol described by Huang et al. (2012)32. A craniotomy was opened using a dental drill. A 22-gauge guide cannula (a dulled 26-gauge needle) was slowly lowered to −1.2mm (CA1) or −1.5mm (DG). For kindling experiments, cannulas were placed bilaterally, and twisted bipolar electrodes (MS303/1, Plastics One, Roanoke, VA) were lowered alongside cannulas. Cannulas and electrodes were affixed to the skull with cyanoacrylate glue. A ground electrode screw (8209, Pinnacle) was placed −2mm lateral and 2mm anterior to bregma. A small hole was opened in the skull by twisting a 21-gauge needle and the electrode was screwed into place. Incisions were closed with dental cement and cannulas were plugged with blunted 30-gauge needles.
In vivo Sema4D treatments
For seizure experiments: after recovering from surgery for 5–10 days, mice were gently restrained and connected to infusion tubing tipped with a 26-gauge infusion cannula (a dulled 26-gauge needle) by insertion of the infusion cannula into the guide cannula. The infusion cannula was bent at a 45-degree angle so as to extend 0.5 mm below the end of the guide cannula when inserted. The infusion cannula was fixed to the mouse’s head with a drop of cyanoacrylate glue. 100 nM Sema4D-Fc (5235-S4-050, R&D Systems, Minneapolis, MN) in aCSF (125mM NaCl, 23mM NaHCO3, 2.3mM KCl, 1.26mM KH2PO4, 2mM CaCl2, 2mM MgSO4, 10mM glucose, 10mM HEPES, adjusted to pH 7.4 +/− 0.05 and 285 +/− 0.05 mOsm) with 5% dextran-488 (D22910, Thermo Fisher Scientific, Waltham, MA) or 100 nM vehicle Fc (4460MG number, R&D Systems) control protein in aCSF with 5% dextran-488 was infused through the intra-hippocampal cannula for 3 hours at a rate of 0.2 µl per hour. After three hours, the cannula was removed by twisting to break the glue fix or by cutting through the infusion tubing. In the case of electrically kindled mice, infusions were performed bilaterally.
For immunohistochemistry experiments: mice were placed in an induction chamber with isoflurane administered at 3–4%, then transferred to a nose cone with isoflurane administered at 3–4%. A small hole was drilled in the skull and a cannula was lowered to the right hippocampus. When the cannula was positioned, the isoflurane rate was adjusted to 1–4%. We then infused the mice with 100 nM Sema4D-Fc in aCSF with 5% dextran-488 or 100 nM vehicle Fc control protein in aCSF with 5% dextran-488 at a rate of 100nL per minute for 10 minutes. After infusion, the incision was sealed with dental cement, and the animal was allowed to wake from anesthesia. Three hours after the start of Sema4D infusion, mice were perfused with cold aCSF as described below.
Intravenous PTZ model of epilepsy
After recovering from surgery for 5–10 days, mice were treated with Sema4D-Fc or vehicle for 3 hours as described above. After treatment, the mice were warmed for 15 minutes on a heating pad to induce blood vessel dilation. Tail vein catheters (Monoject IV Catheter, polyurethane, 26g, Patterson, Devens, MA) were inserted and fixed in place with veterinary tape (Patterson). The mice were placed individually in a Plexiglas enclosure. PTZ (18682, Cayman Chemical, Ann Arbor, MI) (1% in PBS, pH 7.4) was infused at a rate of 0.1mL per minute for 5 minutes using a syringe pump. At the end of the five-minute period, animals that had not already expired were humanely sacrificed. All animals were weighed after PTZ infusion. Measured weights were adjusted by subtracting the weight of the infused PTZ solution. Video was recorded throughout the infusion.
Analysis of PTZ-induced seizures
Two observers were blinded to treatment condition and scored videos separately. The seizure classes used for PTZ analysis are defined in Table 1. Prior to analysis, time thresholds from the two observers were combined by the following method: disagreements between observers of less than 2 seconds were resolved by random selection, and disagreements of greater than 2 seconds were resolved by discussion between the observers. Seizure threshold doses were calculated for each seizure class as d=tcr/w, where d is the PTZ dose received, t is the time since the start of infusion, c is the concentration of PTZ, r is the rate of infusion, and w is the weight of the animal. Some mice failed to display one or more specific seizure classes. In these cases, threshold dose was assigned by taking that mouse’s dose at the next most severe seizure class, such that doses were reported at all classes for all mice. Some animals did not die within the 5-minute infusion period. For these animals, dose at death was imputed as the maximum dose experienced by any animal.
Rapid hippocampal kindling model of temporal lobe epilepsy
Kindling was initiated 5–10 days after bilateral cannula and electrode implant surgeries. At the start of each stimulus session, the mice were placed in individual cages. The kindling stimulus, a 10-second duration, 20Hz train of 1ms, 0.4mA square pulses, was delivered 12 times per session at intervals of 30–60 minutes21. The mice were observed for 5 minutes from the time of delivery of each stimulus. The maximum seizure class achieved on the modified Racine scale by each mouse was recorded for each stimulus22. The stimulus session was repeated on three consecutive days. The mice were returned to their home cages between sessions. Mice were considered kindled after experiencing four consecutive stimulus-evoked seizures of class 3 or greater21. All mice achieved the kindling criterion. All mice underwent the full kindling protocol, 12 stimuli per day for 3 days, regardless of when the kindling criterion was achieved.
After 3 days of kindling, the mice were returned to their home cages for a 2-day stimulus-free period. After the stimulus-free period the mice were given a single stimulus (10-second duration, 20Hz train of 1ms, 0.4mA square pulses) to determine baseline evoked seizure response, as measured by changes in EEG power and behavioral seizure score. The mice were recorded for a total of 10 minutes centered on the time of stimulus delivery. Evoked responses were assessed during the 5 minutes following stimulus onset. After the baseline stimulus, mice were randomly chosen for treatment with either Sema4D-Fc or vehicle for 3 hours as described above. After treatment, the mice were given a second stimulus to determine evoked seizure response. After the second stimulus, mice were returned to their home cage for 2 days. After these 2 days, the evoked seizure response was again recorded. This time point is called “+48hrs”.
We performed retrospective statistical analysis to determine whether the time course and degree of kindling differed between treatment groups during the three-day kindling period. Repeated measures ANOVA with Greenhouse-Geisser corrections for departure from sphericity using future treatment as a between-subjects factor, protocol day as a within-subject factor, and maximum Racine class exhibited on each day as the outcome variable revealed a significant effect of protocol day (p<0.001) but not of treatment group (p>0.05) or the interaction between protocol day and treatment group (p>0.05).
EEG recording
EEG was recorded using the PAL 8200 three-channel monitoring system (Pinnacle Technologies, Lawrence, KS) at a 400 Hz sampling frequency and with a 25 Hz lowpass filter. The pre-amplifier was connected to the bipolar electrodes and ground screw through a custom connector. EEG was recorded for 5 minutes prior to and 5 minutes post-electrical stimulation.
Analysis of EEG data
Power was calculated using Welch’s method during the after-discharge event (10 to 30 seconds from stimulus delivery)33. Power was computed in the following frequency bands: delta [0.5–3Hz), theta [3–8Hz), alpha [8–12Hz), and beta [12–30Hz]. The after-discharge duration was defined as the time from the end of the stimulus presentation to the first time that the EEG signal dropped to baseline amplitude for at least two seconds. The effects of treatment, frequency band, and the interaction of treatment and frequency band on pre-test to post-test gain in power were assessed by mixed-design ANOVA and post-hoc t tests with Bonferroni corrections. One mouse was removed from the dataset prior to analysis because the recording equipment failed during the +48hrs recording session.
Behavioral analysis of electrically kindled seizures
The scorer was blinded to treatment condition and test time (pre-test, post-test, or +48hrs). Seizure behavior was scored with one-second resolution according to a modified Racine scale (Table 2)22. Mean seizure scores for each animal were calculated over the first 5 minutes post-stimulus thus the reported scores are the average of 300 higher-resolution scores.
Table 2.
Modified Racine scale for behavioral analysis of evoked seizures in kindled mice.
| Seizure behavior by modified Racine scores | |
|---|---|
|
| |
| Behavior | Score |
| Myoclonic jerking | 1 |
| Absence seizure | 1 |
| Head bobbing | 2 |
| Agitated walking and head bobbing | 2 |
| Forelimb clonus | 3 |
| Agitated walking with forelimb clonus | 3 |
| Rapid hindlimb automata | 3 |
| Rearing | 4 |
| Rearing and falling | 5 |
| Tonic-clonic convulsions | 6 |
Whole brain immunohistochemistry
Mice were anesthetized by intraperitoneal injection of ketamine/xylazine cocktail and transcardially perfused with ice-cold aCSF. Brains were dissected and fixed overnight in 4% formalin, then transferred to 30% sucrose for >24hrs. This fixation procedure is adapted from a protocol developed by Notter et al. (2014)34. The brains were sectioned coronally at 40 microns on a microtome. Sections were washed 3 times for 10 minutes in 0.1% Triton/PBS. Tissue was incubated in blocking solution (0.1% Triton/PBS, 20% bovine serum albumin) on a shaker for 1 hour at room temperature. Primary antibody solution was added to the blocking solution (final concentration [1:500] mouse anti-GAD65 (Millipore, MAB351, Billerica, MA)) and sections were incubated overnight at 4°C. The following day, the slices were washed 3 times for 10 minutes in 0.1% Triton/PBS, then incubated in secondary antibody solution (anti-rabbit Cy3 [1:500]) on a shaker for 2 hours at room temperature. The slices were washed 3 times with PBS, with DAPI [1:500] added to the second wash. The slices were mounted on glass slides with #1 coverslips and Aqua-Mount (Lerner Laboratories, Pittsburgh, PA).
Organotypic hippocampal culture and Sema4D treatment
P6–7 rat pup brains were dissected into the cutting solution (4 mM KCl, 1 mM CaCl2, 8 mM NaHCO3, 200 mM sucrose, 30 mM d-glucose and 25 mM HEPES free acid, at 320 mOsm). Hippocampi were isolated and sliced at thickness of 380µm using a tissue chopper (Stoelting, Wood Dale, IL). Individual slices were placed on cell culture inserts (0.4 µm pore size, Millipore). Organotypic culture media (25% HBSS (Hyclone, Logan, UT), 50% OptiMEM (Thermo Fisher Scientific), 25% horse serum, 25 units/mL penicillin, and 25 units/mL streptomycin at pH 7.35 and 315–320 mOsm) was added outside of the inserts. Slices were maintained for 7 DIV at 35°C and 5% CO2 with media replacements every 2–3 days.
Slices were treated with either 2nM (final concentration in the well) Sema4D-Fc (R&D Systems, 5235-S4-050) or 2nM Fc (R&D Systems, 4460MG) control protein for 2 hours. After treatment, half of the slices were fixed with 1mL cold 4% paraformaldehyde solution with 4% sucrose in PBS, followed by 1mL cold 20% Methanol/PBS. For the remaining slices, the treatment media was replaced with slice culture media. Slices were maintained for an additional 48 hours before fixation.
Organotypic culture immunocytochemistry
Fixed slices were permeabilized overnight in 0.5% Triton X-100 in PBS placed above and beneath the cell culture inserts. After permeabilization, the slices were incubated in blocking solution (20% BSA in PBS) at room temperature for 4 hours or overnight at 4°C. The fixed slices were cut out of the culture inserts, placed on a lid of a 12-well plate and covered with 5% BSA/PBS. Cutouts were blocked with [1:50] donkey anti-mouse Fc antibody (715-005-150, Jackson ImmunoResearch, West Grove, PA) in 5% BSA/PBS for 3 hours. The slices were transferred to a 12-well plate and washed 3 times with 5% BSA/PBS solution.
Slices were incubated with primary antibody solution (5% BSA/PBS, [1:200] mouse anti-GAD65 (Millipore, MAB351)) for 4 hours at room temperature or overnight in a hydration chamber at 4° C. The slices were washed 3 times with 5% BSA/PBS, then incubated in secondary antibody solution (5%BSA/PBS, [1:500] anti-mouse Cy3) for 3 hours at room temperature or overnight in a hydration chamber at 4° C. Afterwards, the next primary antibody (5% BSA/PBS, [1:200] mouse anti-NeuN (Millipore, MAB377)) and secondary antibody (5% BSA/PBS, [1:500] anti-mouse 488) incubations were performed as described. All slices were washed 3 times with PBS for 15 minutes, with DAPI (1:500 in PBS) in the second wash. Cutouts were mounted onto glass slides with #1 coverslips and Aqua-Mount (Lerner Laboratories).
Confocal imaging and image Analysis
Image acquisition and quantification was performed by an experimenter blinded to condition. Analyzed images were acquired on a Nikon Eclipse Ti using a 60× objective. Representative images in Figure 2A were acquired using a 10× objective. Identical acquisition settings were maintained within experiments.
For organotypic culture experiments z-stacks were acquired with a step size of 0.5 microns beginning at the bottom of each slice where the tissue contacted the cell culture membrane inserts. Each slice was imaged 3 times in different locations in CA1. The brightest image from each stack was used for analysis. At least 3 cells from each image were analyzed using FIJI. Neuronal somas in the CA1 principal layer were identified by NeuN staining. The perimeter of the fit ellipse to each cell was approximated as, where p is the perimeter, a is the semi-major axis, and b is the semi-minor axis. GAD65 puncta were counted manually along the periphery of each soma. The per-cell puncta density was calculated by dividing the number of puncta by the soma perimeter.
For whole brain sections produced after in vivo Sema4D infusion, single plane images were acquired of the infusion site and a contralateral matched position in the section. Three sections were imaged per mouse, with 3 images taken per sections in non-overlapping regions of CA1 within the infusion site. At least 3 cells from each image were analyzed. Somas in the CA1 principal layer were identified by DAPI staining. The puncta ring around each soma was identified as the concave hull of GAD65 staining around the nucleus. The per-cell puncta density was calculated as described above. For individual puncta measurements, diameters and mean intensities were measured using the straight selection and measure tools in FIJI.
Statistical analysis
All statistical analysis was performed using the R programming language. Specific statistical tests are described in the text and figure legends. Prior to applications of ANOVA, normality was assessed by visual inspection of boxplots and by Shapiro’s tests, homoscedasticity was assessed by visual inspection of boxplots and by Levene’s tests, and sphericity was assessed by Mauchly tests. Rank transforms were applied when normality or homoscedasticity assumptions were violated. Where appropriate, we applied Greenhouse-Geisser corrections for departure from sphericity.
Supplementary Material
Fig. S1. Characterization of GAD65 puncta in Sema4D treated mice. (A) Mean intensities of GAD65 immunoreactivity for GABAergic boutons. (B) Diameters of GAD65 puncta. In all panels, colored points represent individual puncta and black points represent means for individual animals; black lines connecting dots indicate the same animal. n=8 mice and 200 puncta per condition (all panels). Statistics were performed by unpaired, two-tailed, t tests on delta scores calculated within animal by subtracting the mean puncta intensity or diameter on the contralateral side from the mean value on the ipsilateral side. Delta scores were not significantly different when comparing puncta intensity (p=0.0828) or diameter (p=0.5690) in Sema4D-treated and vehicle-treated mice. Analyzed images were the same used to count puncta density in Figure 1C.
Fig S2. Time course of Sema4D-dependent increase in GABAergic bouton density in vitro. Organotypic hippocampal cultures from rats (P6–P7) were treated for 2 hours with Sema4D or vehicle solution. After two hours of treatment, half of the slices in each treatment condition were fixed and processed for immunostaining. The remaining slices were cultured for an additional 2 days before being fixed and processed. (A) CA1 principal layer neurons in organotypic culture fixed immediately after Sema4D or vehicle treatment or 48 hours after Sema4D or vehicle withdrawal (+48hrs). Cells are stained with DAPI (blue) and antibodies that specifically recognize NeuN (green) and GAD65 (red). Scale bar represents 10 µm. (B) Density of GAD65 puncta on the perimeter of CA1 principal cell layer somas in organotypic culture fixed either immediately after Sema4D or vehicle treatment or after 48 hours withdrawal. ***p<0.001, Kruskal-Wallis test with post-hoc Mann-Whitney U tests with Bonferroni corrections. n≥6 pups and ≥59 neurons per condition.
Figure S3. Absolute seizure severity scores of electrically kindled mice. Absolute (non-delta) mean Racine seizure scores of electrically kindled mice are plotted by treatment condition and experimental stage. Dots represent scores for individual animals and lines show means scores by condition. Error bars represent the mean +/− S.E.M for each treatment condition and experimental stage. Post-hoc analysis showed that pre-test severity scores were higher among Sema4D-treated mice than vehicle-treated mice (p=0.02906, uncorrected, unpaired, two-tailed t test). These mice are the same as those used in the experiment shown in Fig. 4.
Fig. S4. Representative EEG and duration of the after-discharge event in electrically kindled mice. (A) (Top) Representative EEG surrounding an after-discharge event, recorded from the hippocampus contralateral to stimulation. (Bottom) Expanded view of the grey region in top panel (EEG within the after-discharge event). The gold bar denotes the time of stimulus presentation. (B) Delta (post-test minus pre-test or +48hrs minus pre-test) after-discharge duration for post-test and +48hrs. p>0.05 for all effects, mixed-design ANOVA.
Key points.
Ectopic Semaphorin 4D (Sema4D) promotes increased GABAergic bouton density in vivo in the adult hippocampus.
Intra-hippocampal Sema4D treatment suppresses seizures in two mouse models: intravenous pentylenetetrazol and electrical kindling.
The effects of Sema4D on seizure activity and GABAergic bouton density are correlated in time with onset within 3 and no effect after 48 hours.
Acknowledgments
We are grateful to members of the Paradis lab, in particular Dr. Marissa Kuzirian and Dr. Aram Raissi, for helpful comments and suggestions throughout the project. We also thank Dr. Jamie Maguire of Tufts University Medical School for experimental advice and critical reading of the manuscript. This work was supported by NIH grant R01NS065856 (S.P.) and a NARSAD Independent Investigator grant number 23405 (S.P.).
Footnotes
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Competing interests
Author Suzanne Paradis has submitted Provisional US Patent Application No. 61/756,809 entitled "Methods of Modulating GABAergic Inhibitory Synapse Formation and Function Using Sema4D." Co-inventors: Kuzirian, Marissa; Moore, Anna; Paradis, Suzanne. The remaining authors have no conflicts of interest.
References
- 1.Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ. 2014;348:g254. doi: 10.1136/bmj.g254. [DOI] [PubMed] [Google Scholar]
- 2.Krook-Magnuson E, Armstrong C, Oijala M, et al. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraban SC, Southwell DG, Estrada RC, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci. 2009;106:15472–7. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzel D, Nicholson E, Schorge S, et al. Chemical–genetic attenuation of focal neocortical seizures. Nat Commun. 2014;5:3847. doi: 10.1038/ncomms4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuzirian MS, Moore AR, Staudenmaier EK, et al. The class 4 Semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus. J Neurosci. 2013;33:8961–73. doi: 10.1523/JNEUROSCI.0989-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paradis S, Harrar DB, Lin Y, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–32. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raissi AJ, Staudenmaier EK, David S, et al. Sema4D localizes to synapses and regulates GABAergic synapse development as a membrane-bound molecule in the mammalian hippocampus. Mol Cell Neurosci. 2013;57:23–32. doi: 10.1016/j.mcn.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang C-C, Ladas TP, Gonzalez-Reyes LE, et al. Seizure Suppression by High Frequency Optogenetic Stimulation Using In Vitro and In Vivo Animal Models of Epilepsy. Brain Stimulat. 2014;7:890–9. doi: 10.1016/j.brs.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Zhong C, Wang L, et al. Optogenetic dissection of ictal propagation in the hippocampal–entorhinal cortex structures. Nat Commun. 2016;7:10962. doi: 10.1038/ncomms10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuy ST, Houser CR. Prominent Expression of Two Forms of Glutamate Decarboxylase in the Embryonic and Early Postnatal Rat Hippocampal Formation. J Neurosci. 1996;16:6919–32. doi: 10.1523/JNEUROSCI.16-21-06919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang ZJ, Kirkwood A, Pizzorusso T, et al. BDNF Regulates the Maturation of Inhibition and the Critical Period of Plasticity in Mouse Visual Cortex. Cell. 1999;98:739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Wu H, Osterhaus G, et al. Demonstration of functional coupling between γ-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–8. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyaya B, Di Cristo G, Wu CZ, et al. GAD67-mediated GABA Synthesis and Signaling Regulate Inhibitory Synaptic Innervation in the Visual Cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazzari P, Paternain AV, Valiente M, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–80. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 15.Hartman KN, Pal SK, Burrone J, et al. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–9. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- 16.Frias CP, Bresser T, Scheefhals L, et al. Molecular pathway underlying bouton stabilization by Semaphorin4D during inhibitory synapse formation. bioRxiv. 2017:100271. [Google Scholar]
- 17.Löscher W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur J Pharmacol. 2009;610:1–11. doi: 10.1016/j.ejphar.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Tollner K, Twele F, Löscher W. Evaluation of the pentylenetetrazole seizure threshold test in epileptic mice as surrogate model for drug testing against pharmacoresistant seizures. Epilepsy Behav. 2016;57:95–104. doi: 10.1016/j.yebeh.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Lüttjohann A, Fabene PF, van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579–86. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: A comparative analysis with s.c. PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44. doi: 10.1016/j.seizure.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Lothman EW, Williamson JM. Rapid kindling with recurrent hippocampal seizures. Epilepsy Res. 1993;14:209–20. doi: 10.1016/0920-1211(93)90045-9. [DOI] [PubMed] [Google Scholar]
- 22.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 23.Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci. 2008;11:1044–52. doi: 10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- 24.Wierenga CJ. Live imaging of inhibitory axons: Synapse formation as a dynamic trial-and-error process. Brain Res Bull. 2017;129:43–9. doi: 10.1016/j.brainresbull.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Dobie FA, Craig AM. Inhibitory Synapse Dynamics: Coordinated Presynaptic and Postsynaptic Mobility and the Major Contribution of Recycled Vesicles to New Synapse Formation. J Neurosci. 2011;31:10481–93. doi: 10.1523/JNEUROSCI.6023-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a new tool to interrupt seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krook-Magnuson E, Szabo GG, Armstrong C, et al. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro. 2014;1 doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy M, Duffy BA, Lee JH. Optogenetic study of networks in epilepsy. J Neurosci Res. 2017;95:2325–35. doi: 10.1002/jnr.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krook-Magnuson E, Armstrong C, Bui A, et al. In vivo evaluation of the dentate gate theory in epilepsy. J Physiol. 2015;593:2379–88. doi: 10.1113/JP270056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paz JT, Huguenard JR. Microcircuits and their interactions in epilepsy: Is the focus out of focus? Nat Neurosci. 2015;18:351–9. doi: 10.1038/nn.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinka E, Höfler J, Leitinger M, et al. Pharmacotherapy for Status Epilepticus. Drugs. 2015;75:1499–521. doi: 10.1007/s40265-015-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Yang S, Hu ZY, et al. A new approach to location of the dentate gyrus and perforant path in rats/mice by landmarks on the skull. Acta Neurobiol Exp (Warsz) 2012;72:468–72. doi: 10.55782/ane-2012-1917. [DOI] [PubMed] [Google Scholar]
- 33.Welch P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoustics. 1967;15:70–3. [Google Scholar]
- 34.Notter T, Panzanelli P, Pfister S, et al. A protocol for concurrent high-quality immunohistochemical and biochemical analyses in adult mouse central nervous system. Eur J Neurosci. 2014;39:165–75. doi: 10.1111/ejn.12447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Characterization of GAD65 puncta in Sema4D treated mice. (A) Mean intensities of GAD65 immunoreactivity for GABAergic boutons. (B) Diameters of GAD65 puncta. In all panels, colored points represent individual puncta and black points represent means for individual animals; black lines connecting dots indicate the same animal. n=8 mice and 200 puncta per condition (all panels). Statistics were performed by unpaired, two-tailed, t tests on delta scores calculated within animal by subtracting the mean puncta intensity or diameter on the contralateral side from the mean value on the ipsilateral side. Delta scores were not significantly different when comparing puncta intensity (p=0.0828) or diameter (p=0.5690) in Sema4D-treated and vehicle-treated mice. Analyzed images were the same used to count puncta density in Figure 1C.
Fig S2. Time course of Sema4D-dependent increase in GABAergic bouton density in vitro. Organotypic hippocampal cultures from rats (P6–P7) were treated for 2 hours with Sema4D or vehicle solution. After two hours of treatment, half of the slices in each treatment condition were fixed and processed for immunostaining. The remaining slices were cultured for an additional 2 days before being fixed and processed. (A) CA1 principal layer neurons in organotypic culture fixed immediately after Sema4D or vehicle treatment or 48 hours after Sema4D or vehicle withdrawal (+48hrs). Cells are stained with DAPI (blue) and antibodies that specifically recognize NeuN (green) and GAD65 (red). Scale bar represents 10 µm. (B) Density of GAD65 puncta on the perimeter of CA1 principal cell layer somas in organotypic culture fixed either immediately after Sema4D or vehicle treatment or after 48 hours withdrawal. ***p<0.001, Kruskal-Wallis test with post-hoc Mann-Whitney U tests with Bonferroni corrections. n≥6 pups and ≥59 neurons per condition.
Figure S3. Absolute seizure severity scores of electrically kindled mice. Absolute (non-delta) mean Racine seizure scores of electrically kindled mice are plotted by treatment condition and experimental stage. Dots represent scores for individual animals and lines show means scores by condition. Error bars represent the mean +/− S.E.M for each treatment condition and experimental stage. Post-hoc analysis showed that pre-test severity scores were higher among Sema4D-treated mice than vehicle-treated mice (p=0.02906, uncorrected, unpaired, two-tailed t test). These mice are the same as those used in the experiment shown in Fig. 4.
Fig. S4. Representative EEG and duration of the after-discharge event in electrically kindled mice. (A) (Top) Representative EEG surrounding an after-discharge event, recorded from the hippocampus contralateral to stimulation. (Bottom) Expanded view of the grey region in top panel (EEG within the after-discharge event). The gold bar denotes the time of stimulus presentation. (B) Delta (post-test minus pre-test or +48hrs minus pre-test) after-discharge duration for post-test and +48hrs. p>0.05 for all effects, mixed-design ANOVA.