Abstract
Aims
To estimate the impact of existing high‐coverage needle and syringe provision (HCNSP, defined as obtaining more than one sterile needle and syringe per injection reported) and opioid substitution therapy (OST) on hepatitis C virus (HCV) transmission among people who inject drugs (PWID) in three UK settings and to determine required scale‐up of interventions, including HCV treatment, needed to reach the World Health Organization (WHO) target of reducing HCV incidence by 90% by 2030.
Design
HCV transmission modelling using UK empirical estimates for effect of OST and/or HCNSP on individual risk of HCV acquisition.
Setting and participants
Three UK cities with varying chronic HCV prevalence (Bristol 45%, Dundee 26%, Walsall 19%), OST (72–81%) and HCNSP coverage (28–56%).
Measurements
Relative change in new HCV infections throughout 2016–30 if current interventions were stopped. Scale‐up of HCNSP, OST and HCV treatment required to achieve the WHO elimination target.
Findings
Removing HCNSP or OST would increase the number of new HCV infections throughout 2016 to 2030 by 23–64 and 92–483%, respectively. Conversely, scaling‐up these interventions to 80% coverage could achieve a 29 or 49% reduction in Bristol and Walsall, respectively, whereas Dundee may achieve a 90% decrease in incidence with current levels of intervention because of existing high levels of HCV treatment (47–58 treatments per 1000 PWID). If OST and HCNSP are scaled‐up, Walsall and Bristol can achieve the same impact by treating 14 or 40 per 1000 PWID annually, respectively (currently two and nine treatments per 1000 PWID), while 18 and 43 treatments per 1000 PWID would be required if OST and HCNSP are not scaled‐up.
Conclusions
Current opioid substitution therapy and high‐coverage needle and syringe provision coverage is averting substantial hepatitis C transmission in the United Kingdom. Maintaining this coverage while getting current drug injectors onto treatment can reduce incidence by 90% by 2030.
Keywords: HCV treatment scale‐up, hepatitis C virus, mathematical model, needle and syringe provision, opioid substitution therapy, people who inject drugs
Introduction
Hepatitis C virus (HCV) is a major cause of morbidity world‐wide 1. Approximately 85% of HCV infections in the United Kingdom are acquired through injection drug use 2, 3, therefore prevention of HCV transmission among people who inject drugs (PWID) is crucial for reducing the HCV disease burden.
Primary prevention interventions for HCV are opioid substitution therapy (OST) and needle and syringe programmes (NSP) among PWID 4. OST and high‐coverage needle and syringe provision (HCNSP, defined as obtaining more than one sterile needle and syringe per injection) can reduce the risk of HCV acquisition by 40–80% 5, 6, 7, 8, 9, 10. Although low in many settings 11, 12, UK coverage levels for OST (70%) and HCNSP (48%) are high 2. Further, the emergence of highly efficacious anti‐viral HCV drugs 13 has raised the possibility of scaling‐up HCV treatment as a prevention strategy among PWID 14, 15. Consequently, the World Health Organization (WHO) recently produced a global strategy for eliminating HCV 1.
Modelling has suggested that OST and HCNSP has reduced HCV prevalence in the United Kingdom, but further scale‐up will have limited impact 16, with HCV treatment being needed to reduce HCV prevalence markedly 15, 17, 18. However, no analyses have considered how the impact of OST or HCNSP may vary between regional settings, or what combined scale‐ups of HCV treatment with OST and HCNSP are required to achieve the WHO HCV elimination targets.
In this paper, a HCV transmission model incorporating improved empirical evidence for the effectiveness of harm reduction interventions is used to evaluate the impact of current levels of OST and HCNSP on cumulative numbers of incident HCV infections in three UK settings (Bristol, Dundee and Walsall), and the required scale‐up of these interventions with HCV treatment to reach WHO's targets of reducing HCV incidence by 90% by 2030 1.
Methods
Model description and assumptions
We developed a dynamic deterministic model of HCV transmission and disease progression among PWID, similar to other HCV transmission models 16, 17 using principles outlined by recent guidelines for HIV or economic infectious disease models 19, 20. The model simulates the movement of current PWID through different stages of injecting duration, intervention coverage, risk and HCV infection states, as shown in Fig. 1. Further model details are in the Supporting information.
Figure 1.
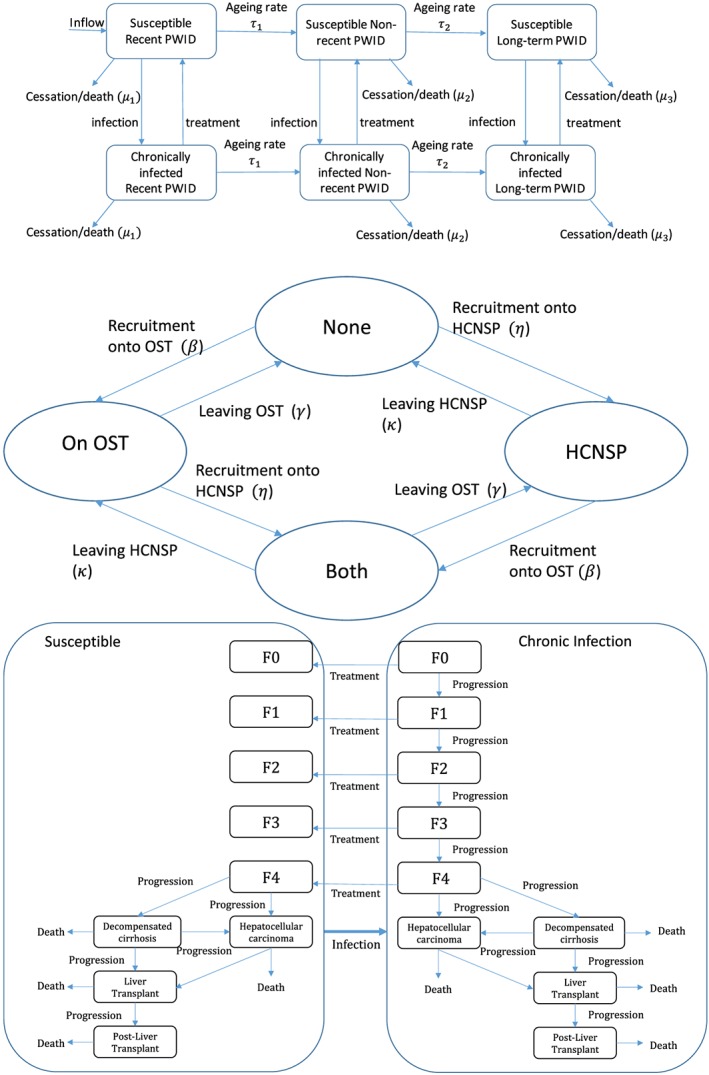
Schematics of different model components. (a) Schematic of injecting duration and infection components of model. Susceptible individuals are free from disease and upon infection move to the chronically infected category. Successfully treated individuals move back into the susceptible category. Injecting duration is modelled as three categories; recently initiated people who inject drugs (PWID) (denoted recent PWID, < 3 years), non‐recent PWID (≥ 3 and < 10 years) and long‐term PWID (≥ 10 years), with PWID transitioning through these categories at rates τi, where i = 1, 2 for recent and non‐recent injectors, respectively. Injectors cease injecting (cessation or death) at rate μi where i = 1, 2, 3 for recent (< 3 years of injecting), non‐recent (≥ 3 years and < 10 years) and long‐term injectors (> = 10 years) respectively. (b) Schematic of intervention component of model. It is assumed the recruitment rates β and η are independent of the current intervention state. OST = opioid substitution therapy; HCNSP = high coverage needle and syringe provision (defined as at least one clean needle for every injection). (c) Schematic of disease progression component of the model. Each of the disease states is stratified by injecting duration n, risk category m, OST category i and needle and syringe provision (NSP) category j. Progression through the disease states occurs at a rate determined by the current disease state, as are the disease related death rates. Metavir states F0, F1 (mild HCV disease), F2, F3 (moderate HCV disease), compensated cirrhosis (also denoted as metavir state F4), decompensated cirrhosis, hepatocellular carcinoma (HCC), liver transplant and post‐liver transplant. All states have a cessation rate from injecting and a non‐disease related background death rate. Infection can occur between all disease states, but not shown for clarity. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Stratifications by injecting duration are included to incorporate increased injecting cessation and HCV acquisition risk among people recently initiated into injecting 5, 21, 22, with the chosen category in line with reporting from the unlinked anonymous monitoring (UAM) survey of PWID 23. PWID are also stratified into different intervention states that influence HCV transmission risk: no intervention, OST only, HCNSP only, or both. PWID enter the model as recent initiates with no intervention coverage. They transition through successive injecting duration categories with rates of injecting cessation and non‐HCV‐related death. Due to a lack of data, we assumed that recruitment and leaving rates onto and off OST and HCNSP were independent of the current intervention state; previous modelling suggests that this should not affect our model projections 16. The model is stratified further by high and low HCV transmission risk, with a proportion starting injecting in the high‐risk category 24 and PWID transitioning between these categories. PWID were defined as high‐risk if they had been homeless in the last year and/or injected crack in the last 4 weeks (low‐risk otherwise), which was associated with increased HCV transmission risk 25.
New initiates into injecting are initially susceptible to HCV and become infected at a per‐capita rate depending on their intervention state, injecting duration category, risk category and prevalence of HCV infection in the population. Previous analyses suggest that incorporating like‐with‐like mixing (individuals with the same risk behaviour or characteristics being more likely to form injecting contacts with each other than with other individuals) will have little effect on our model projections 16, with data suggesting that it only occurs weakly in Bristol 26, and so random mixing was assumed between all subgroups.
Once infected, some PWID clear infection spontaneously 27, with the remainder becoming chronically infected, which is life‐long unless treated. Chronically infected PWID progress through disease states (Fig. 1c), with HCV disease‐related death occurring from the decompensated cirrhosis, hepatocellular carcinoma, liver transplant and post‐liver transplant stages.
HCV treatment is only allowed in the F0–F3 and compensated cirrhosis states, as it was contraindicated for more severe liver disease for interferon (IFN)‐based therapy 28 and evidence is only now emerging of its benefits with new direct‐acting anti‐viral (DAA) therapies 29. An annual number of PWID are treated, with a proportion achieving a sustained virological response (SVR‐effective cure) and the remainder returning to their prior infection category. Following successful treatment, no further disease progression occurs in the F0–F3 states 30, 31, but continued slower progression occurs among those with compensated cirrhosis 31, 32. We allow re‐infection of those who have attained SVR and retreatment of those who fail treatment or become re‐infected in line with current recommendations 13.
Model parameterization
The model was parameterized for three UK settings: Bristol, Dundee and Walsall. These sites were chosen because of the availability of survey data and to provide a range of epidemic settings. The model parameters and uncertainty ranges are given in Table 1 and Supporting information, Table S1. Effect estimates for how HCV transmission risk are modified by OST and/or HCNSP, or injecting duration, were taken from a pooled analysis of UK and Australian data 25 and a Cochrane systematic review 10. Estimated leaving rates from the high‐risk category (1.16 per year), OST (1.65 per year) or HCNSP (0.52 per year) came from two UK studies 24, 38.
Table 1.
Model parameters.
| Parameters | Symbol | Value/range | Reference |
|---|---|---|---|
| Epidemiological and demographic parameters | |||
| Number of new injectors per year | θ | Fitted to obtain population sizes | Bristol 33, 34, Walsall 34 and unpublished estimates, Dundee 35. See Table S2 and supporting information |
| Combined mortality and injecting cessation rates per year | μi | Fitted to obtain injecting duration profiles for each setting | Lower bounds of 0.004 and 0.008 chosen to ensure leaving rate greater than the death rate 21, 36. See Table S2 and supporting information |
| Infection rate per year | π | Fitted to obtain HCV prevalence for each setting | See Table S2 and supporting information |
| Proportion of new infections which spontaneously clear | δ | Sampled from uniform distribution (0.22–0.29) | 27 |
| Annual leaving rate from high‐ to low‐risk behaviour | ζ | Sampled range (0.6761–1.617) | Data from cohort study 24 found 78/145 injectors no longer homeless after 8 months Transition probability sampled from beta distribution (α=78, β = 67) and converted to yearly rate |
| Annual recruitment rate from low‐ to high‐risk behaviour | σ | Fitted to obtain required high‐risk proportions in each setting | See Table S2 and supporting information |
| Intervention‐related parameters | |||
| Annual OST leaving rate | γ | 1–3 | Duration on OST was 8 months (4–12 months) in cohort of PWID in UK 37 |
| Annual HCNSP leaving rate | κ | 0.37–0.77 | Welsh cohort study 61% PWID still HCNSP after 1 year, so estimated duration on HCNSP as 1.3–2.7 years 38 |
| Annual recruitment rate into OST | β | Fitted to obtain required OST coverage proportions in each setting | See Table S2 and supporting information |
| Annual recruitment rate onto HCNSP | η | Fitted to obtain required high NSP coverage proportions in each setting | See Table S2 and supporting information |
| Proportion of treatments achieving SVR prior to 2015 | α | Sampled from uniform distribution (0.40–0.67) | Weighted mean of pooled intention to treat SVR for genotypes 1 and 2/3 taken from UK treatment data for PWID 39 |
| Proportion of treatments achieving SVR post‐2015 | α | Sampled from uniform distribution (0.86–0.92) | 40 Weighted mean of SVR for genotype 1 (90%) and genotypes 2/3 (82–93%) from 41 |
| Number of PWID treated per year | Φ |
Bristol: 18 (2009 onwards) Dundee: 34 (2009 to 2015), and then 40 (2015 onwards) Walsall: (2009 onwards) |
Number of HCV treatments in 2011 Assumed treatment of PWID commenced in 2009 39. More recent estimate for Dundee (personal communication, John Dillon). Walsall assumed same rate as Bristol |
| Relative transmission risk parameters | |||
| Risk associated with being on OST only | Γ | 0.41 (0.22–0.75) sampled from log‐normal distribution | Odds ratio and 95% CI from pooled analysis 25 |
| Risk associated with being on HCNSP only | Π | 0.59 (0.36–0.96) sampled from log‐normal distribution | Odds ratio and 95% CI from pooled analysis 25 |
| Risk associated with being on both OST and HCNSP | Γ × Π | 0.26 (0.09–0.64) | Calculated as product of risk associated with being solely on OST or NSP. Compares well to estimate from systematic review 0.29 (0.13–0.65) 25 |
| Risk associated with being a recent injector compared to a long‐term injector | Χ1 | 1.53 (0.93–2.52) sampled from Lognormal distribution | Odds ratio from pooled analysis 25 |
| Risk associated with being in the high‐risk category | Ξ |
Scotland: 2.13 (1.40–3.24) Bristol and Walsall: 2.75 (1.97–4.22). Both sampled from log‐normal distribution |
Odds ratio from pooled analysis 25. For Scotland, the OR is just for homelessness, because there is little crack injection, whereas it is for crack injection or homelessness for Bristol and Walsall |
OST = opioid substitution therapy; HCNSP = high coverage needle syringe provision; SVR = sustained virological response, high‐risk defined as crack injecting in last 4 weeks or homeless in the last year; HCV = hepatitis C virus; PWID = people who inject drugs; CI = confidence interval.
HCV treatment was initiated in 2009 at rates determined by local data, except for Walsall, where Bristol treatment rates were used. Before 2015, UK‐specific PWID SVR rates 39 for pegylated IFN (pegIFN) and ribavirin (RBV) were assumed, whereas post‐2015 a weighted average of SVRs for genotypes 1 and 2/3 40, 41 for direct‐acting anti‐viral drugs was assumed.
HCV disease progression rates were calculated from two meta‐analyses 28, 42 (Supporting information, Table S1). Non‐HCV related death rates were derived from two UK studies 21, 36.
Model calibration and uncertainty
The model was calibrated to temporal data on HCV prevalence, recent estimates of coverage of OST and HCNSP, proportion of PWID with high‐risk attributes, population‐size estimates of PWID and their distribution by injecting duration (Table 2 and Supporting information, Table S2) 23, 26, 33, 34, 35, 43, 46, 47, 48, 49. Data on HCV incidence for Dundee and Bristol and HCV prevalence after 2006 for Bristol and Walsall were used for model validation, with both extracted from routine surveys of PWID [needle exchange surveillance initiative in Scotland (NESI) 43, unlinked anonymous monitoring survey (UAM) in England and Wales 23 and two additional community surveys using respondent‐driven sampling from Bristol 26; see Supporting information for details of the surveys].
Table 2.
Summary of baseline characteristics of people who inject drugs for each setting (minimum‐maximum values).
| Baseline characteristics (2014 unless stated) | Setting | ||
|---|---|---|---|
| Bristol | Dundee | Walsall | |
| Chronic HCV prevalence | 26% (19–32%)b | 19% (11–26%)a | |
| HCV incidence | 10.0 per 100 py, 95% CI 9.7–14.0 26 in 2009 | 14.3 per 100 py, 95% CI 4.9–25.9 43 | Not available |
| Population size (2011) | 2025–2564 44 | 675–825 35 | 1296–1623 unpublished estimates |
| Proportion high risk | 80–95%a | 26–42%b | 50–65%a |
| Proportion on OST | 81% (77–86%)26 | 72% (65–79%)b | 72% (61–82%)a |
| Proportion with HCNSP | 56% (38–82%)a 33 | 48% (34–79%)b | 28% (21–42%)a |
| Treatments per year | 18 39 | 40 (from 2015) (personal communication, John Dillon) | 2 (assumed similar rate per infected PWID as Bristol) |
Data extracted from unlinked anonymous monitoring survey 45;
data extracted from Needle Exchange Surveillance Initiative 43. OST = opioid substitution therapy, HCNSP = high coverage needle syringe provision, high‐risk defined as crack injecting in last 4 weeks or homeless in the last year; CI = confidence interval; py = person‐years.
A sequential Bayesian method was used to calibrate the model, each to have 1000 model fits. First, a demographic submodel was fitted to data on the population size of PWID for each setting and their distribution by injecting duration, which was assumed to be stable in Dundee 43, but decreasing 33, 34, 46, 50 and ageing 26, 47, 48, 49, 51 in Bristol and Walsall. Secondly, an intervention submodel was fitted to changing trends in the coverage of OST and HCNSP from each setting 26, 43, 47, 51, with OST coverage increasing in each setting during recent years from 40 to 70–81%, and the proportion with HCNSP remaining stable in Bristol (ranging between 38 and 82%) and Walsall (ranging between 21 and 42%), but increasing in Dundee (up to 34–79%, see Supporting information, Table S2). Thirdly, a high‐/low‐risk submodel was fitted to setting‐specific data on the trends in crack injecting and/or homelessness 43, 51, which has remained stable in Dundee (33% high‐risk) and Walsall (52% high‐risk), but increased in Bristol during recent years (88% high‐risk in 2014).
Lastly, the full model was calibrated to HCV prevalence data from each setting. For each of the 1000 combined parameter sets from the previous calibration steps, the model's infection rate was calibrated to an initial sampled prevalence estimate for each city (Bristol 2004, Walsall 2006 and Dundee 2008; Supporting information, Table S2 and Fig. S1), assuming a stable epidemic at that time. For Walsall and Bristol, this infection rate captured the subsequent epidemic dynamics accurately (Supporting information, Fig. S1), whereas for Dundee a second infection rate [median = 2.0‐fold (95% credibility interval (95% CrI) = 1.8–2.7‐fold greater than initial infection rate] was used to capture the HCV prevalence in 2014. Table S2 summarizes the model parameters obtained through model‐fitting, and the Supporting information includes further details.
Model analyses
The model estimated the impact of current coverage levels of OST, HCNSP and HCV treatment from 2016 to 2030 by comparing the baseline model with a counterfactual where the effect of these interventions was removed from 2016. Impact was assessed in terms of the relative change in the cumulative number of incident infections from 2016 to 2030. Results were obtained for each parameter set and given in terms of 95% CrI (see Supporting information for details).
We then estimated the impact to 2030 of scaling‐up both OST and HCNSP to 80% coverage. This maximum coverage was based partially on the fact that 85% of PWID inject opioids 51, and so would derive a benefit from OST, and that higher intervention coverage levels are probably unsustainable 16. Additionally, the HCNSP target of 80% coverage is a current target of needle and syringe providers (personal communication, Rachel Ayres). Following this, we estimated what additional HCV treatment scale‐up is needed to reduce HCV incidence by 90% by 2030. We also considered how these projections were modified if the heightened transmission risks associated with our high‐risk categories were halved.
Uncertainty analysis
A linear regression analysis of covariance (ANCOVA) 52 was undertaken to determine which parameter uncertainties (exposure variables in the linear regression analysis) contributed most to variability in the percentage reduction in incident HCV infections over 15 years due to current levels of HCNSP or OST (outcome variable). The proportion of each model outcome's sum‐of‐squares contributed by each parameter was calculated to estimate the importance of individual parameters to the overall uncertainty.
Results
Baseline epidemic projections
The chronic HCV prevalence pre‐2016 was projected to be stable in all three settings (Fig. 2). From 2016 to 2030, HCV prevalence will decrease slightly in Bristol (by 5%) and Walsall (by 0.4%), but reduce markedly in Dundee (by 99%). These decreases are due to the current scale‐up of new treatments from 2015, with heightened impact in Dundee due to treatment already being scaled‐up (47–58 per 1000 PWID). The projected HCV incidence in 2014 varied between settings. The highest incidence was in Dundee [7.7 per 100 person‐year (py), 95% CrI = 4.0–12.6] and Bristol (6.9 per 100 py, 95% CrI = 3.9–11.5), both lower but comparable to recent empirical estimates from these settings [Dundee: 14.3 per 100 py, 95% confidence interval (CI) = 4.9–25.9 43 and Bristol: 10.0 per 100 py, 95% CI = 9.7–14.0 26]. Incidence was 3.4 per 100 py in Walsall (95% CrI = 1.7–6.6), corresponding to the low prevalence in that setting. Incidence is expected to decrease slightly in Walsall (by 1%) and Bristol (by 11%) during 2016–2030, but will decrease by more than 90% in Dundee (99.97%, 95% CrI = 99.0–99.99).
Figure 2.
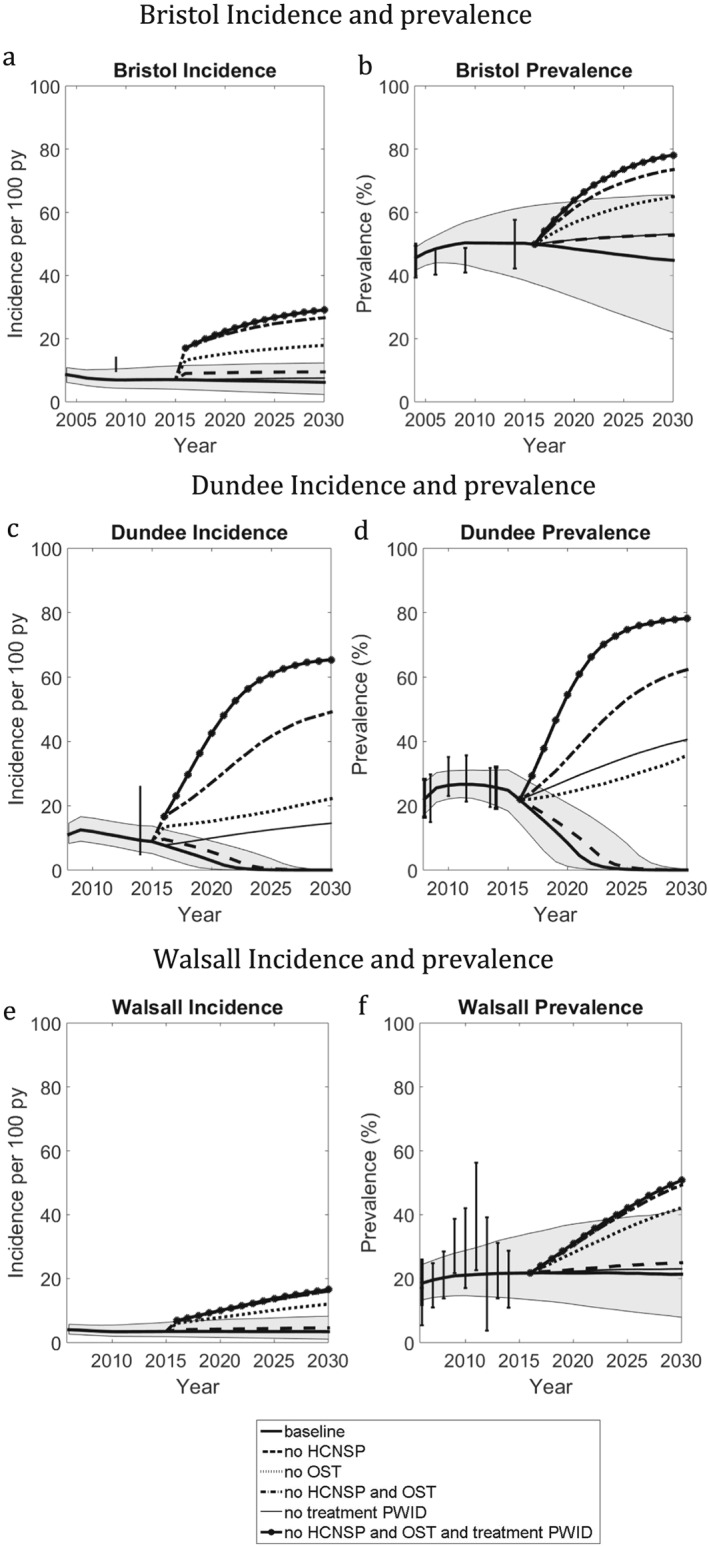
Impact of each intervention scenario on hepatitis C virus (HCV) incidence and prevalence in Bristol (a,b), Walsall (c,d) and Dundee (e,f). Thick solid line is median baseline scenario, with shaded region the 95% credible intervals. The black points with thin whiskers are the data points [with 95% credible interval (CrI)] that were not fitted to, whereas the black points with thick whiskers are the data points used for model calibration. (a,b) Bristol incidence and prevalence. (c,d) Dundee incidence and prevalence. (e,f) Walsall incidence and prevalence
Impact of existing interventions
Figures 2 and 3 (and Supporting information, Table S3) show that, regardless of setting, removing HCNSP and/or OST would lead to a large increase in the number of incident infections and increased HCV prevalence and incidence by 2030. Removing OST has greater impact than removing HCNSP (92–483% increase in infections compared with 23–64% respectively), with less impact being achieved from removing HCV treatment. This differential impact is due both to the differing coverage of these interventions and their relative effectiveness. For example, in Walsall the number of incident infections from 2016 to 2030 would increase by 23, 129 or 176% if HCNSP, OST or both interventions were removed, respectively, compared with 3% if HCV treatment were removed. In Dundee, a greater increase (380%) would result from removing treatment because of the higher treatment rate in that setting.
Figure 3.
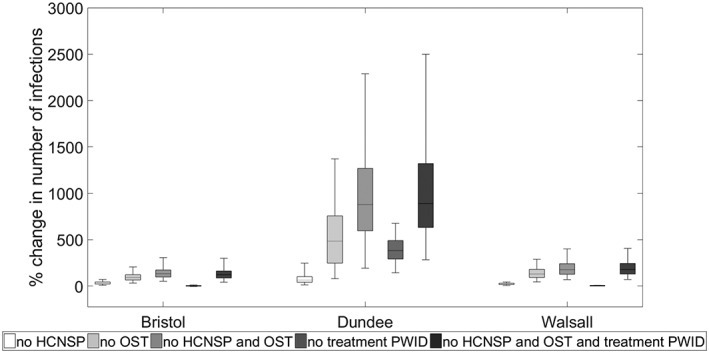
Relative increase in new hepatitis C virus (HCV) infections (2016–30) resulting from removing existing coverage levels of needle and syringe provision (NSP), opioid substitution therapy (OST), both NSP and OST, HCV treatment of people who inject drugs (PWID) or all interventions in each city. The box‐plots signify the uncertainty (middle line is the median, the limits of the box are 25and 75% percentiles and the whiskers 2.5 and 97.5% percentiles)
Impact of combined OST/NSP interventions and treatment scale‐up on HCV incidence
Through scaling‐up HCNSP and OST to 80% coverage from the current levels, it is possible to reduce HCV incidence by 29% (95% CrI = 4.7–58%) in Bristol, 100% (95% CrI = 99–100%) in Dundee and 49% (95% CrI = 1.2–77%) in Walsall by 2030 compared to 2015 levels (Supporting information, Fig. S2). Greater impact is achieved in Dundee due to recent HCV treatment scale‐up. In all settings, most impact (> 80%) is achieved from scaling‐up HCNSP due to its lower baseline coverage (28–56%, depending on setting).
With current levels of HCNSP and OST, the annual number of HCV treatments needed to reduce incidence by 90% by 2030 (WHO elimination target) is 43 (95% CrI = 26–61), 29 (95% CrI = 14–45) and 18 (95% CrI = 8–36) per 1000 PWID for Bristol, Dundee and Walsall, respectively (Fig. 4), which translates to 7.5–13.2% of infected PWID in the first year (see supporting information Figure S3). This would require considerable scale‐up of treatment in Bristol (fivefold from nine annual treatments per 1000 PWID) and Walsall (ninefold from two annual treatments per 1000 PWID), while treatment numbers could be reduced by 45% (95% CrI = 11–63%) in Dundee and still achieve this target.
Figure 4.
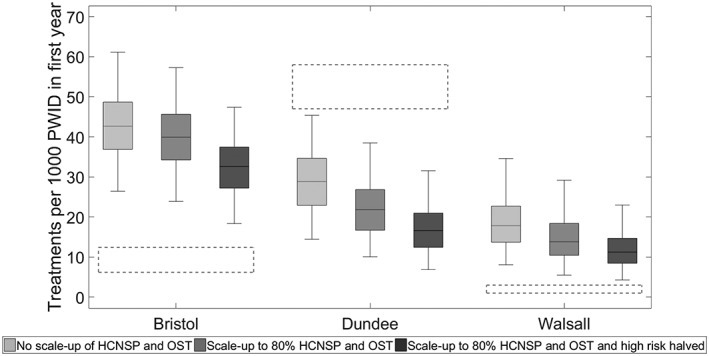
Required annual number of hepatitis C virus (HCV) treatments per 1000 PWID needed to reduce incidence by 90%, with or without high coverage needle syringe provision (HCNSP) and opioid substitution therapy (OST) scaling up to 80% coverage. The box‐plots signify the uncertainty in the model projections (middle line is the median, the limits of the box are 25 and 75% percentiles and the whiskers 2.5 and 97.5% percentiles). The dashed boxes show the uncertainty range in the current treatment rate per 1000 PWID in each setting
Concurrent scale‐up of HCNSP and OST to 80% coverage decreases the yearly treatments required to reach the WHO target (Fig. 4); from 43 to 40 per 1000 PWID for Bristol, 29 to 22 for Dundee and 18 to 14 for Walsall. If, additionally, the transmission risk associated with high‐risk injecting (homelessness and crack injecting) was also halved, then the required number of treatments would reduce further by one‐fifth (19–22%; see Fig. 4) in each setting. In all scenarios, fewer treatments are always needed in Dundee than the current number of treatments (47–58 per 1000 PWID).
Uncertainty analysis
Analyses of covariance (Supporting information, Fig. S4) suggest that most of the variability in the effect of removing HCNSP on increasing the number of infections between 2016 and 2030 is due to uncertainty in the efficacy of HCNSP (accounting for 41, 4 and 49% of variability in Bristol, Dundee and Walsall, respectively), the coverage of HCNSP in 2014 (32% of variation in Bristol, 39% in Walsall and < 1% in Dundee) and HCV prevalence in 2014 (77% in Dundee, 0.1% in Bristol, 3% in Walsall). Increasing effectiveness and coverage of HCNSP increases the impact of removing the intervention (Bristol and Walsall) and in Dundee the lower the 2014 HCV prevalence the greater the increase in infections (Supporting information, Fig. S5). All other parameters and inputs had little effect. For the impact of removing OST (Supporting information, Fig. S6), most variability is due to uncertainty in the efficacy of OST (45% in Bristol, 44% in Dundee and 38% in Walsall).
Discussion
Main findings
Current levels of HCNSP and OST in the United Kingdom are averting considerable HCV transmission, with their removal likely to double the number of new HCV infections occurring during the next 15 years. If HCNSP and OST levels are maintained, only moderate rates of HCV treatment (18–43 per 1000 PWID annually) are needed to reduce HCV incidence by 90% by 2030, therefore achieving the WHO elimination target. This scale‐up has already been achieved in Dundee. Although scaling‐up HCNSP and OST further (to 80% coverage) will reduce these treatment targets (by a fifth), their existing moderate coverage in the United Kingdom limits the additional impact they can have.
Strengths and limitations
Our detailed modelling of three contrasting settings provides insights into how the impact of existing interventions varies throughout the United Kingdom, with our projections being strengthened by using detailed local data and utilizing new synthesized estimates for the efficacy of OST and HCNSP. Additionally, calibrating our model to temporal trends in injecting duration and PWID population size allowed us to incorporate possible changes in injecting over recent years, increasing the potential realism of our model projections.
However, limitations exist, relating primarily to the data used to inform our model. First, despite synthesizing the best available international evidence 10, there remains substantial uncertainty in the intervention effectiveness of HCNSP, which our uncertainty analyses show was the most important contributor to uncertainty in our model projections. Further data collection of individual injecting frequency, real‐life syringe provision and blood‐borne virus status could improve these estimates.
Secondly, self‐reported data on NSP coverage derived from surveys recruiting from needle and syringe providers are likely to overestimate coverage in the population as a whole 43, 45, so instead we estimated the HCNSP coverage in each city as the ratio of the number of syringes distributed to the estimated total number of injections undertaken by PWID in that city. Unfortunately, this measure utilized three uncertain pieces of information (PWID population size, yearly injecting frequency and the number of new needles distributed), with the resulting uncertainty in HCNSP coverage contributing considerably to the variability in our projections. Less biased estimates of syringe coverage could only come from better monitoring of service users.
Thirdly, our use of homelessness or crack use as a marker of high transmission risk is incomplete, because it does not explicitly incorporate differences in injecting risk. This was conducted because of the inherent difficulty in using data on specific injecting behaviours to estimate reliably the variability in transmission risk between different PWID, and so we used proxy markers that have been found to be related to increased transmission risk. Importantly, previous analyses 15, 16 suggest that risk heterogeneity rarely plays an important role in determining the impact of HCV interventions, so this simplification should not have affected our projections.
Fourthly, we assumed that all PWID were eligible for OST, despite a growing proportion (3.9% in 2004 and 12% in 2014) injecting non‐opioids 2, for which OST is not an appropriate treatment. Although this was accounted for partially by assuming that the coverage of OST could not be greater than 80%, future analyses could improve upon this by modelling non‐opioid injecting PWID explicitly as a separate group, where HCNSP would be the only harm reduction strategy available. This could mean that existing interventions would have less prevention benefit.
Fifthly, we assumed that HCV treatment was not allowed for individuals with severe liver disease (decompensated cirrhosis and beyond), despite recent treatment guidelines allowing it 13. This assumption is unlikely to affect our impact projections, because fewer than 7% of all chronically infected PWID have this level of liver disease.
Lastly, we assumed that Walsall had the same low treatment rate among PWID as Bristol, due to lack of data. This will have had little impact on our results. which show that treatment needs to be scaled‐up substantially to have a large impact on HCV incidence. In contrast, current treatment numbers in Dundee are very high (25% of infected per year from 2015), with our modelling suggesting that they will achieve HCV elimination by 2030 if maintained at this high level. To determine if this may have affected our impact projections for OST and HCNSP, we undertook a scenario analysis (not shown) which halved treatment rates from 2016 and showed similar impacts for the same levels of HCNSP and OST coverage.
Comparison with other studies
Few model analyses have estimated the impact of HCNSP or OST on HCV transmission, with previous analyses either not using empirical intervention effect estimates 53 and/or not using detailed context‐specific data to evaluate how impact may vary across settings 16, 17. This analysis is consistent with previous findings that HCNSP and OST can have substantial impact 16, with our analysis also highlighting how impact can vary depending on local levels of intervention coverage and epidemic trends. Additionally, our analysis is the first to estimate the required scale‐up of OST, HCNSP and HCV treatment needed to achieve WHO's HCV‐elimination targets among PWID in a European setting, with previous analyses either considering their required scale‐up in a rural U. setting 54, or just the treatment scale‐up requirements in Australia 55. Without scale‐up of OST and HCNSP, our analyses suggest that similar treatment rates (43/1000 PWID annually) are needed in Bristol as for Australia (59 of 1000 PWID annually), which has a similar chronic prevalence of HCV, but much lower than in the rural US setting (89 of 1000 PWID annually) due to the increasing HCV epidemic occurring there. However, further scale‐up of OST and HCNSP results in lower treatment rates being needed in the United Kingdom (14–44 of 1000 PWID annually) than in Australia, although the reduction is smaller (20% decrease in treatment requirements) than for the rural US (halves treatment requirements) due to the negligible current coverage of these interventions in the US setting.
Conclusions and implications
Our projections highlight the considerable impact that existing harm reduction interventions are having in high coverage settings, such as Europe and Australia, emphasizing the need to maintain current coverage levels of these interventions, avoid reductions in prevention funding or changes in drug treatment policy away from harm reduction towards abstinence. There is also an urgent imperative to continue funding for NSPs, as changes in drug use favouring non‐opioid use may reduce the impact of OST.
Our projections suggest that benefits could be achieved from scaling‐up OST and HCNSP further, especially in lower coverage settings such as Walsall. This highlights the need to initiate strategies for increasing the coverage of OST and HCNSP in these and similar settings, which should be conducted in close consultation with service users. Evidence shows the benefits of extending opening hours 56 and promotion of secondary distribution via peers 57, 58 or vending machines for increasing coverage 59. Additionally, better monitoring at the local district level is needed to identify lower coverage settings.
Although our analyses emphasized the importance of OST and HCNSP for reducing HCV transmission, a combined approach utilizing HCV treatment is needed to reduce HCV incidence to low levels, as advocated by WHO. This scale‐up will require policy‐driven expansion of case‐finding interventions in settings such as drug treatment centres and NSPs, as already undertaken in Dundee, emphasizing their crucial role for any scaled‐up HCV prevention response. Strategies also need to be developed to reduce the harms associated with structural factors, such as homelessness and incarceration, which modelling suggests could be heightening HCV transmission among PWID 25, 60, 61. Such a multi‐pronged approach must be prioritized to achieve the HCV‐elimination targets set by WHO 1.
Declaration of interest
None
Supporting information
Table S1 Disease progression parameters.
Table S2 Summary of data collated for each setting for model calibration.
Table S3 Impact of interventions on prevalence, incidence and the number of new infections. Median projections with 95% credibility intervals in brackets.
Figure S1 Graphs showing fitting of the baseline scenarios in each setting. Error bars in black are data points from surveys, error bars in red are the ranges used for model calibration.
Figure S2 Reduction in incidence by 2030 compared with 2016 through scaling‐up high‐coverage needle and syringe provision (HCNSP) and opioid substitution therapy (OST) to 80% coverage. The box‐plots signify the uncertainty (middle line is the median, the limits of the box are 25 and 75% percentiles and the whiskers 2.5 and 97.5% percentiles.
Figure S3 Percentage of Infected PWID which need to be treated in the first year of treatment scale up to reduce incidence by 90% by 2030, with or without high coverage needle syringe provision (HCNSP) and opioid substitution therapy (OST) scaling up to 80% coverage. The box‐plots signify the uncertainty in the model projections (middle line is the median, the limits of the box are 25 and 75% percentiles and the whiskers 2.5 and 97.5% percentiles). The dashed boxes show the uncertainty range in the current treatment numbers as a percentage of infected PWID in each setting.
Figure S4 Analysis of covariance (ANCOVA) results of the contribution of each model parameter or input to the overall variation in the relative change in number of infections when HCNSP is removed (only those aspects with greater than 3% contribution for any setting are shown).
Figure S5 Analysis of covariance (ANCOVA) results of the contribution of each model parameter or input to the overall variation in the relative change in number of infections when opioid substitution therapy (OST) is removed (only those aspects with greater than 3% contribution for any setting are shown).
Figure S6 Impact of important parameters on the percentage increase in infections when needle and syringe provision (NSP) is removed in each setting.
Acknowledgements
This study was supported by National Institute of Health Research Public Health Research Programme (grant number PHP Project: 12/3070/13) and the National Institute for Health Research Health Protection Research Unit (HPRU‐2012‐10026) in Evaluation of Interventions at the University of Bristol in partnership with Public Health England. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The opinions expressed in this paper are solely those of the authors and do not necessarily represent the opinions of the University of Bristol. N.K.M., P.V. and M.H. were additionally supported by the National Institute for Drug Abuse (grant number R01 DA037773‐01A1), and N.M. was partially funded by the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) ‐ funded program (grant number P30 AI036214). P.V. and M.H. acknowledge support from the National Institute of Health Research Health Protection Research Unit in Evaluation of Interventions. L.M. is supported by an Australian National Health and Medical Research Council (NHMRC) Fellowship. N.K.M. and P.V. have received unrestricted research grants from Gilead unrelated to this work, and N.K.M. has received honoraria from Merck, AbbVie and Janssen. M.H. has received honoraria unrelated to this work from Merck, Abbvie and Gilead and declares no conflicts of interest.
Ward, Z. , Platt, L. , Sweeney, S. , Hope, V. D. , Maher, L. , Hutchinson, S. , Palmateer, N. , Smith, J. , Craine, N. , Taylor, A. , Martin, N. , Ayres, R. , Dillon, J. , Hickman, M. , and Vickerman, P. (2018) Impact of current and scaled‐up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings—what is required to achieve the WHO's HCV elimination targets?. Addiction, 113: 1727–1738. 10.1111/add.14217.
References
- 1. World Health Organization (WHO) . Combating Hepatitis B and C to Reach Elimination by 2030. Geneva: WHO; 2016.
- 2. Public Health England . Hepatitis C in the UK 2015 report. London, UK: Public Health England; 2015.
- 3. Harris R. J., Ramsay M., Hope V. D., Brant L., Hickman M., Foster G. R. et al Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012; 22: 187–192. [DOI] [PubMed] [Google Scholar]
- 4. Palmateer N., Kimber J., Hickman M., Hutchinson S., Rhodes T., Goldberg D. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010; 105: 844–859. [DOI] [PubMed] [Google Scholar]
- 5. Turner K. M., Hutchinson S., Vickerman P., Hope V., Craine N., Palmateer N. et al The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011; 106: 1978–1988. [DOI] [PubMed] [Google Scholar]
- 6. Palmateer N. E., Taylor A., Goldberg D. J., Munro A., Aitken C., Shepherd S. J. et al Rapid decline in HCV incidence among people who inject drugs associated with national scale‐up in coverage of a combination of harm reduction interventions. PLOS ONE 2014; 9: e104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nolan S., Dias Lima V., Fairbairn N., Kerr T., Montaner J., Grebely J. et al The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109: 2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White B., Dore G. J., Lloyd A. R., Rawlinson W. D., Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS‐c study. Med J Aust 2014; 201: 326–329. [DOI] [PubMed] [Google Scholar]
- 9. Tsui J. I., Evans J. L., Lum P. J., Hahn J. A., Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174: 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Platt L., Reed J., Minozzi S., Vickerman P., Hagan H., French C. et al Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016; CD12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathers B. M., Degenhardt L., Ali H., Wiessing L., Hickman M., Mattick R. P. et al HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 2010; 375: 1014–1028. [DOI] [PubMed] [Google Scholar]
- 12. Harm Reduction International . The Global State of Harm Reduction 2016. London, UK: Harm Reduction International; 2016. [Google Scholar]
- 13. European Association for the Study of the Liver (EASL) EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66: 153–194. [DOI] [PubMed] [Google Scholar]
- 14. Grebely J., Dore G. J. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res 2014; 104: 62–72. [DOI] [PubMed] [Google Scholar]
- 15. Martin N. K., Vickerman P., Grebely J., Hellard M., Hutchinson S. J., Lima V. D. et al Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale‐up in the age of direct‐acting antivirals. Hepatology 2013; 58: 1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vickerman P., Martin N., Turner K., Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction 2012; 107: 1984–1995. [DOI] [PubMed] [Google Scholar]
- 17. Martin N. K., Hickman M., Hutchinson S. J., Goldberg D. J., Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis 2013; 57: S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin N. K., Vickerman P., Miners A., Foster G. R., Hutchinson S. J., Goldberg D. J. et al Cost‐effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 2012; 55: 49–57. [DOI] [PubMed] [Google Scholar]
- 19. Delva W., Wilson D. P., Abu‐Raddad L., Gorgens M., Wilson D., Hallett T. B. et al HIV treatment as prevention: principles of good HIV epidemiology modelling for public health decision‐making in all modes of prevention and evaluation. PLOS Med 2012; 9: e1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitman R., Fisman D., Zaric G. S., Postma M., Kretzschmar M., Edmunds J. et al Dynamic transmission modeling: a report of the ISPOR–SMDM Modeling Good Research Practices Task Force Working Group‐5. Med Decis Making 2012; 32: 712–721. [DOI] [PubMed] [Google Scholar]
- 21. Kimber J., Copeland L., Hickman M., Macleod J., McKenzie J., De Angelis D. et al Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 2010; 341: 3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutton A. J., Gay N. J., Edmunds W. J., Hope V. D., Gill O. N., Hickman M. Modelling the force of infection for hepatitis B and hepatitis C in injecting drug users in England and Wales. BMC Infect Dis 2006; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Public Health England . People Who Inject Drugs: HIV and Viral Hepatitis Unlinked Anonymous Monitoring Survey Tables (Pyschoactive): 2016 Update. London, UK: Public Health England; 2016.
- 24. Kemp P. A., Neale J., Robertson M. Homelessness among problem drug users: prevalence, risk factors and trigger events. Health Soc Care Community 2006; 14: 319–328. [DOI] [PubMed] [Google Scholar]
- 25. Platt L., Sweeney S., Ward Z., Guinness L., Hickman M., Hope V. et al Assessing the impact and cost‐effectiveness of needle/syringe provision on hepatitis C transmission among people who inject drugs in the United Kingdom: analysis of pooled datasets and economic modelling. Public Health Research 2017; 5(5). [PubMed] [Google Scholar]
- 26. Mills H. L., Colijn C., Vickerman P., Leslie D., Hope V., Hickman M. Respondent driven sampling and community structure in a population of injecting drug users, Bristol, UK. Drug Alcohol Depend 2012; 126: 324–332. [DOI] [PubMed] [Google Scholar]
- 27. Micallef J. M., Kaldor J. M., Dore G. J. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepatol 2006; 13: 34–41. [DOI] [PubMed] [Google Scholar]
- 28. Shepherd J., Jones J., Hartwell D., Davidson P., Price A., Waugh N. Interferon alfa (pegylated and non‐pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007; 11: 1–205. [DOI] [PubMed] [Google Scholar]
- 29. Guarino M., Morisco F., Valvano M. R., Ippolito A. M., Librandi M., Andriulli N. et al Systematic review: interferon‐free regimens for patients with HCV‐related Child C cirrhosis. Aliment Pharmacol Ther 2017; 45: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 30. Bruno S., Zuin M., Crosignani A., Rossi S., Zadra F., Roffi L. et al Predicting mortality risk in patients with compensated HCV‐induced cirrhosis: a long‐term prospective study. Am J Gastroenterol 2009; 104: 1147–1158. [DOI] [PubMed] [Google Scholar]
- 31. Morgan R. L., Baack B., Smith B. D., Yartel A., Pitasi M., Falck‐Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med 2013; 158: 329–337. [DOI] [PubMed] [Google Scholar]
- 32. van der Meer A. J., Veldt B. J., Feld J. J., Wedemeyer H., Dufour J. F., Lammert F. et al Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis c and advanced hepatic fibrosis. JAMA 2012; 308: 2584–2593. [DOI] [PubMed] [Google Scholar]
- 33. Jones H. E., Welton N. J., Ades A., Pierce M., Davies W., Coleman B. et al Problem drug use prevalence estimation revisited: heterogeneity in capture–recapture and the role of external evidence. Addiction 2015; 111: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hay G., Rael dos Santos A., Millar T. Estimates of the Prevalence of Opiate Use and/or Crack Cocaine Use, 2010/11: Sweep 7 Report. Liverpool; John Moores University; 2013.
- 35. King R., Bird S. M., Overstall A., Hay G., Hutchinson S. J. Injecting drug users in Scotland, 2006: listing, number, demography, and opiate‐related death‐rates. Addict Res Theory 2013; 21: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierce M., Bird S. M., Hickman M., Millar T. National record linkage study of mortality for a large cohort of opioid users ascertained by drug treatment or criminal justice sources in England, 2005–2009. Drug Alcohol Depend 2015; 146: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornish R., Macleod J., Strang J., Vickerman P., Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 2010; 341: c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Craine N., Hickman M., Parry J. V., Smith J., Walker A. M., Russell D. et al Incidence of hepatitis C in drug injectors: the role of homelessness, opiate substitution treatment, equipment sharing, and community size. Epidemiol Infect 2009; 137: 1255–1265. [DOI] [PubMed] [Google Scholar]
- 39. Martin N. K., Foster G. R., Vilar J., Ryder S., Cramp M. E., Gordon F. et al HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepatol 2015; 22: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris R. J., Thomas B., Griffiths J., Costella A., Chapman R., Ramsay M. et al Increased uptake and new therapies are needed to avert rising hepatitis C‐related end stage liver disease in England: modelling the predicted impact of treatment under different scenarios. J Hepatol 2014; 61: 530–537. [DOI] [PubMed] [Google Scholar]
- 41. Kohli A., Shaffer A., Sherman A., Kottilil S. Treatment of hepatitis C: a systematic review. JAMA 2014; 312: 631–640. [DOI] [PubMed] [Google Scholar]
- 42. Smith D. J., Combellick J., Jordan A. E., Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta‐analysis. Int J Drug Policy 2015; 26: 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Information Services Division Scotland . Injecting Equipment Provision in Scotland Survey 2013/14. Edinburgh, UK: Information Services Division Scotland; 2015.
- 44. Jones H. E., Welton N. J., Ades A., Pierce M., Davies W., Coleman B. et al Problem drug use prevalence estimation revisited: heterogeneity in capture–recapture and the role of external evidence. Addiction 2016; 111: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency Northern Ireland . Accompanying Data Tables Shooting up: Infections Among People Who Inject Drugs in the UK 2015. An update: November 2016. London: Public Health England; 2016.
- 46. Hay G., Gannon M., MacDougall J., Millar T., Eastwood C., McKeganey N. National and Regional Estimates of the Prevalence of Opiate Use and/or Crack Cocaine Use 2006/07: a Summary of Key Findings. Home Office Research Report 9. London: Home Office; 2008.
- 47. Hickman M., Hope V., Brady T., Madden P., Jones S., Honor S. et al Hepatitis C virus (HCV) prevalence, and injecting risk behaviour in multiple sites in England in 2004. J Viral Hepatol 2007; 14: 645–652. [DOI] [PubMed] [Google Scholar]
- 48. Hope V. D., Hickman M., Ngui S. L., Jones S., Telfer M., Bizzarri M. et al Measuring the incidence, prevalence and genetic relatedness of hepatitis C infections among a community recruited sample of injecting drug users, using dried blood spots. J Viral Hepatol 2011; 18: 262–270. [DOI] [PubMed] [Google Scholar]
- 49. Mills H. L., Johnson S., Hickman M., Jones N. S., Colijn C. Errors in reported degrees and respondent driven sampling: implications for bias. Drug Alcohol Depend 2014; 142: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hickman M., Hope V., Coleman B., Parry J., Telfer M., Twigger J. et al Assessing IDU prevalence and health consequences (HCV, overdose and drug‐related mortality) in a primary care trust: implications for public health action. J Public Health (Oxf) 2009; 31: 374–382. [DOI] [PubMed] [Google Scholar]
- 51. Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency Northern Ireland . Shooting Up: Infections Among People Who Inject Drugs in the UK, 2015. London, UK: Public Health England; 2016.
- 52. Briggs A., Sculpher M., Claxton K. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 53. Kwon J. A., Anderson J., Kerr C. C., Thein H. H., Zhang L., Iversen J. et al Estimating the cost‐effectiveness of needle‐syringe programs in Australia. AIDS 2012; 26: 2201–2210. [DOI] [PubMed] [Google Scholar]
- 54. Fraser H., Zibbell J., Hoerger T., Hariri S., Vellozzi C., Martin N. et al Scaling‐up HCV prevention and treatment interventions in rural USA—model projections for tackling an increasing epidemic. Addiction 2018; 113: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scott N., McBryde E. S., Thompson A., Doyle J. S., Hellard M. E. Treatment scale‐up to achieve global HCV incidence and mortality elimination targets: a cost‐effectiveness model. Gut 2017; 66: 1507–1515. [DOI] [PubMed] [Google Scholar]
- 56. Treloar C., Hopwood M., Bryant J. ‘Does anyone know where to get fits from around here?’ Policy implications for the provision of sterile injecting equipment through pharmacies in Sydney, Australia. Drugs Educ Prev Policy 2010; 17: 72–83. [Google Scholar]
- 57. Newland J., Newman C., Treloar C. ‘We get by with a little help from our friends’: small‐scale informal and large‐scale formal peer distribution networks of sterile injecting equipment in Australia. Int J Drug Policy 2016; 34: 65–71. [DOI] [PubMed] [Google Scholar]
- 58. Irwin K., Karchevsky E., Heimer R., Badrieva L. Secondary syringe exchange as a model for HIV prevention programs in the Russian Federation. Subst Use Misuse 2006; 41: 979–999. [DOI] [PubMed] [Google Scholar]
- 59. Islam M., Wodak A., Conigrave K. M. The effectiveness and safety of syringe vending machines as a component of needle syringe programmes in community settings. Int J Drug Policy 2008; 19: 436–441. [DOI] [PubMed] [Google Scholar]
- 60. Csete J., Kamarulzaman A., Kazatchkine M., Altice F., Balicki M., Buxton J. et al Public health and international drug policy. Lancet; 387: 1427–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stone J., Martin N. K., Hickman M., Hutchinson S., Aspinall E. J., Taylor A. et al The potential prevention impact of scaling up Hepatitis C Virus treatment for people who inject drugs in prison: a modeling analysis for Scotland. Hepatology 2015; 62; 1082A‐A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Disease progression parameters.
Table S2 Summary of data collated for each setting for model calibration.
Table S3 Impact of interventions on prevalence, incidence and the number of new infections. Median projections with 95% credibility intervals in brackets.
Figure S1 Graphs showing fitting of the baseline scenarios in each setting. Error bars in black are data points from surveys, error bars in red are the ranges used for model calibration.
Figure S2 Reduction in incidence by 2030 compared with 2016 through scaling‐up high‐coverage needle and syringe provision (HCNSP) and opioid substitution therapy (OST) to 80% coverage. The box‐plots signify the uncertainty (middle line is the median, the limits of the box are 25 and 75% percentiles and the whiskers 2.5 and 97.5% percentiles.
Figure S3 Percentage of Infected PWID which need to be treated in the first year of treatment scale up to reduce incidence by 90% by 2030, with or without high coverage needle syringe provision (HCNSP) and opioid substitution therapy (OST) scaling up to 80% coverage. The box‐plots signify the uncertainty in the model projections (middle line is the median, the limits of the box are 25 and 75% percentiles and the whiskers 2.5 and 97.5% percentiles). The dashed boxes show the uncertainty range in the current treatment numbers as a percentage of infected PWID in each setting.
Figure S4 Analysis of covariance (ANCOVA) results of the contribution of each model parameter or input to the overall variation in the relative change in number of infections when HCNSP is removed (only those aspects with greater than 3% contribution for any setting are shown).
Figure S5 Analysis of covariance (ANCOVA) results of the contribution of each model parameter or input to the overall variation in the relative change in number of infections when opioid substitution therapy (OST) is removed (only those aspects with greater than 3% contribution for any setting are shown).
Figure S6 Impact of important parameters on the percentage increase in infections when needle and syringe provision (NSP) is removed in each setting.


