The plant hormone ethylene and its associated signaling pathway are involved in cortical microtubule reassembly, a process that is critical for the salt stress response in Arabidopsis.
Abstract
Regulation of cortical microtubule reorganization is essential for plant cell survival under high salinity conditions. In response to salt stress, microtubules undergo rapid depolymerization followed by reassembly to form a new microtubule network that promotes cell survival; however, the upstream regulatory mechanisms for this recovery response are largely unknown. In this study, we demonstrate that ethylene signaling facilitates salt stress-induced reassembly of cortical microtubules in Arabidopsis (Arabidopsis thaliana). Microtubule depolymerization was not affected under salt stress following the suppression of ethylene signaling with Ag+ or in ethylene-insensitive mutants, whereas microtubule reassembly was significantly inhibited. ETHYLENE-INSENSITIVE3, a key transcription factor in the ethylene signaling pathway, was shown to play a central role in microtubule reassembly under salt stress. In addition, we performed functional characterization of the microtubule-stabilizing protein WAVE-DAMPENED2-LIKE5 (WDL5), which was found to promote ethylene-associated microtubule reassembly and plant salt stress tolerance. These findings indicate that ethylene signaling regulates microtubule reassembly by up-regulating WDL5 expression in response to salt stress, thereby implicating ethylene signaling in salt-stress tolerance in plants.
Abiotic stressors, including high soil salinity, significantly reduce crop growth and yield. High salinity conditions induce rapid hyperosmotic stress and slow ionic stress, thereby reducing plant growth and causing premature senescence and potential plant death. Multiple studies addressing plant cell responses to salt stress focus on the regulation of ion channels, such as by manipulating the expression and activity of the plasma membrane Na+/H+ antiporter SOS1 to remove sodium from the cytosol (Yang et al., 2009; Quintero et al., 2011). Other investigators have found that altered gene expression patterns facilitate plant cell growth under salt stress (Xu et al., 2015; Bahieldin et al., 2016; Zhang et al., 2016). For example, the expression levels of glycosyltransferases UGT79B2 and UGT79B3 are strongly induced by high salinity, which leads to the accumulation of anthocyanins and plant yield that reflects salt-stress tolerance (Li et al., 2017b). A broad investigation into the mechanisms of plant cell responses to salt stress is required to evaluate how plants grow and survive in high salinity conditions.
The phytohormone ethylene plays a crucial role in the regulation of diverse stress responses, including salt stress (Peng et al., 2014; Pan et al., 2016). Ethylene functions via five membrane-associated receptors to activate the downstream signaling pathway mediated by ETHYLENE INSENSITIVE2 (EIN2), EIN3, and EIN3-like 1 (EIL1) (Merchante et al., 2013; Ju and Chang, 2015; Li et al., 2015; Yang et al., 2015). EIN2 is an essential positive regulator of ethylene signaling, and the null mutant ein2-5 is ethylene insensitive (Alonso et al., 1999). Specifically, EIN2 facilitates the functions of the transcription factors EIN3 and EIL1, which in turn activate or repress the expression of ethylene-response target genes by binding to their promoters and thereby modulate ethylene-related responses in plants (Solano et al., 1998; Boutrot et al., 2010; Zhang et al., 2011).
The results of multiple studies have demonstrated that exogenously applied ethylene and its biosynthetic precursor 1-aminocyclopropane-1-carboxylic acid (ACC) can significantly increase salt-stress tolerance in Arabidopsis (Arabidopsis thaliana) seedlings (Cao et al., 2007; Peng et al., 2014). High salinity results in EIN3 accumulation, and ein3eil1 seedlings are hypersensitive to salt stress, thus suggesting that EIN3 and EIL1 are positive regulators of the salt-stress response in plants (Lei et al., 2011; Peng et al., 2014). However, other investigators have demonstrated that EIN3 and EIL1 have diverse roles in stress responses. For example, cold stress induces EIN3 accumulation, and ein3eil1 seedlings have constitutively enhanced freezing tolerance, demonstrating that EIN3 and EIL1 also are negative regulators of the freezing-stress response in plants (Shi et al., 2012). Thus, transcriptional regulation is essential for ethylene-mediated tolerance to various stressors in plants. Although ethylene has been implicated in the salt-stress response in plants, the underlying mechanisms, in particular the cell biological basis, remain largely unknown.
The microtubule cytoskeleton primarily functions to control plant growth and cell morphogenesis (Lloyd, 2011; Bashline et al., 2014). A growing body of evidence has demonstrated that cortical microtubules participate in the adaptation of plants to salt stress (Shoji et al., 2006; Wang et al., 2007; Wang et al., 2011). Decreased levels of α-tubulin and β-tubulin in pfd3 and pfd5 seedlings (i.e. null mutants of prefoldin subunits 3 and 5) confer hypersensitivity to high salinity (Rodríguez-Milla and Salinas, 2009). Cortical microtubule reorganization, including rapid depolymerization and reassembly of new microtubule networks, is considered vital for survival under salt stress (Wang et al., 2007; Zhang et al., 2012). Alteration of biphasic dynamics of the cortical microtubules significantly decreases the survival rate of seedlings under salt stress (Wang et al., 2007; Li et al., 2017a). Several microtubule-associated proteins (MAPs) have been implicated in these processes. The microtubule-stabilizing protein SPIRAL1 (SPR1) is degraded by the 26S proteasome, which favors salinity-induced rapid depolymerization of microtubules and plant salt-stress tolerance (Wang et al., 2011). However, upstream signaling factors that mediate microtubule reorganization in response to salt stress have not been confirmed.
The results of several studies have demonstrated that ethylene regulates the stability and organization of cortical microtubules in cells from roots and etiolated hypocotyls (Le et al., 2004; Verbelen et al., 2008; Sun et al., 2015; Ma et al., 2016). For example, the Arabidopsis microtubule-stabilizing protein WAVE-DAMPENED2-LIKE5 (WDL5) participates in ethylene signaling to inhibit etiolated hypocotyl elongation (Sun et al., 2015). These findings suggest that the regulation of microtubules is crucial for ethylene-mediated responses. In this study, we demonstrate that ethylene signaling promotes microtubule reassembly via the microtubule-stabilizing protein WDL5 in the plant cell response to salt stress.
RESULTS
Ethylene Signaling Is Involved in Microtubule Reassembly in Response to Salt Stress
Given the central role of ethylene signaling in plant salt tolerance and the regulatory role of ethylene and microtubules in plant cell elongation, we hypothesized that ethylene signaling participates in the regulation of cortical microtubule reorganization in response to salt stress in Arabidopsis. To examine this system, we blocked ethylene signaling with the ethylene receptor antagonist Ag+ in the presence of NaCl (Shi et al., 2012). We determined the effect of Ag+ in the ethylene overproduction mutant eto1-1 and in the constitutive ethylene response CTR1 (i.e. constitutive triple response 1) mutant ctr1-1. Seedlings with completely bleached cotyledons were scored as dead. Our findings and those of other researchers have indicated that bleaching is associated with cell death (Supplemental Fig. S1; Jiang et al., 2012; Zhou et al., 2017).
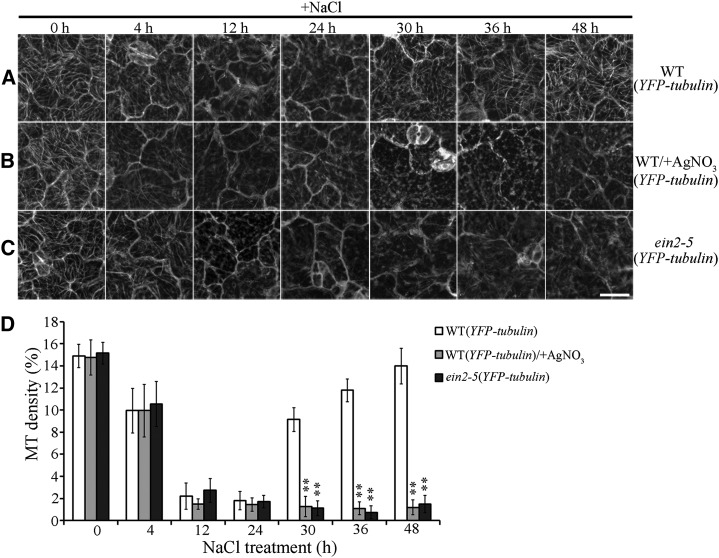
The survival rates of wild-type and eto1-1 seedlings were dramatically decreased in medium containing NaCl with Ag+ compared to those in medium containing NaCl alone. However, ctr1-1 seedlings were resistant to NaCl treatment, even in the presence of Ag+ (Supplemental Figs. S2 and S3). Hence, in the presence of NaCl, Ag+ suppresses ethylene signaling in seedlings without posing significantly toxic ionic effects. We then investigated characteristics of microtubule reorganization in response to salt stress combined with Ag+-induced blockage of ethylene signaling. To quantify the effect of NaCl treatment on cortical microtubules, microtubule density was measured in cotyledon pavement cells as described previously (Higaki et al., 2010). A paired Student’s t test was used to identify significant differences. Results of confocal microscopy showed that cortical microtubules in wild-type (YFP-tubulin) cells are depolymerized after 4 h in medium containing NaCl with or without Ag+ (9.95% ± 2.37% and 9.94% ± 2%). Increasing the duration of NaCl treatment to 12 or 24 h resulted in depletion of most of the cortical microtubules, regardless of Ag+ presence (Fig. 1, A, B, and D). This finding suggested that salt-induced microtubule depolymerization is not dependent on the status of ethylene signaling.
Figure 1.
Blockage of ethylene signaling results in abnormal cortical microtubule reassembly in response to salt stress. A, Cortical microtubules in cotyledon pavement cells from wild-type (YFP-tubulin) seedlings treated with 125 mm NaCl for the indicated times. B, Cortical microtubules in the cotyledon pavement cells from wild-type (YFP-tubulin) seedlings treated with 125 mm NaCl with 20 μm Ag+ for the indicated times. C, Cortical microtubules in the cotyledon pavement cells from mutant ein2-5 (YFP-tubulin) seedlings treated with 125 mm NaCl for the indicated times. Scale bar = 20 μm. D, Density of cortical microtubules in A through C, quantified using ImageJ software. Data represent the mean ± sd of 3 independent experiments with a minimum of 10 cells from 3 seedlings assessed in each experiment. Student’s t test, **P < 0.01.
In wild-type (YFP-tubulin) cells, reassembly of cortical microtubules was observed after 30 h of NaCl exposure (9.14% ± 1.07%). Increasing the duration of NaCl treatment to 36 or 48 h yielded the reappearance of most of the cortical microtubules (36 h, 11.78% ± 1.01%; 48 h, 13.98% ± 1.6%). Although all treated seedlings remained viable 48 h after NaCl treatment, cortical microtubule reassembly was suppressed significantly when NaCl-containing medium was supplemented with Ag+ for 30, 36, or 48 h (30 h, 1.27% ± 0.93%; 36 h, 1.1% ± 0.57%; 48 h, 1.19% ± 0.67%; Fig. 1, B and D). Therefore, blockage of ethylene signaling results in abnormal microtubule reassembly in response to salt stress.
To further validate our findings, the mutant ein2-5 was evaluated in lines that also expressed YFP-tubulin (Ma et al., 2016). The effects of NaCl on cortical microtubule depolymerization were similar in ein2-5 cells and in wild-type cells. In contrast, microtubule reassembly was dramatically inhibited in ein2-5 cells but not in wild-type cells (Fig. 1, C and D). We confirmed this phenomenon by examining cortical microtubules in the same cells of wild-type or ein2-5 seedlings in the presence of NaCl for 0, 12, or 36 h (Supplemental Fig. S4, A and B). An additional method was used for quantification of the total microtubule length per unit surface area (Fujita et al., 2013). The results of this analysis were consistent with those of the density studies (Supplemental Fig. S5). Moreover, abnormal reassembly of cortical microtubules was determined in hypocotyl epidermal cells of ein2-5 seedlings under NaCl treatment (Supplemental Fig. S6, A and B). These findings are consistent with those involving Ag+ treatment and demonstrate that ethylene signaling is critical for microtubule reassembly in response to salt stress.
EIN3 Is Critical for Ethylene Signaling-Mediated Microtubule Reassembly
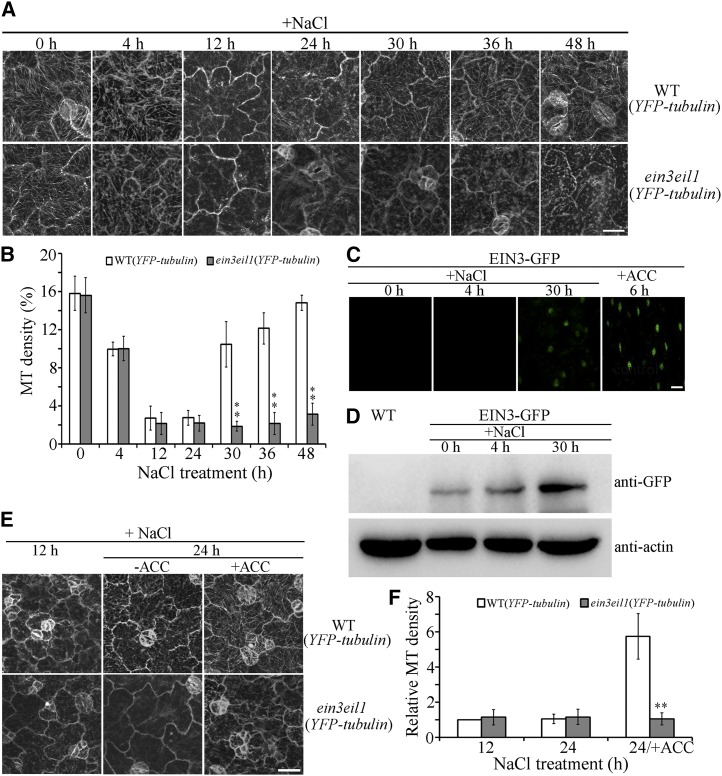
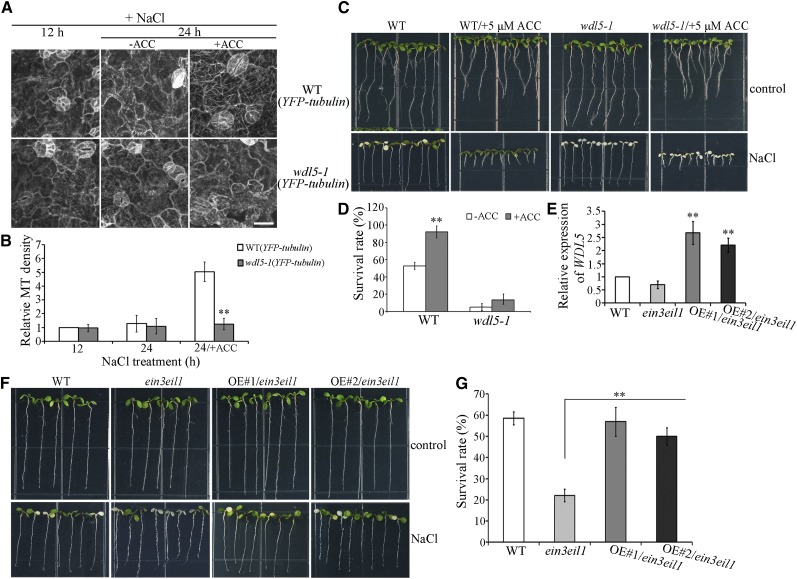
The results of multiple studies have shown that the functionally redundant transcription factors EIN3 and EIL1 are critical for ethylene-mediated responses to salt stress (Asensi-Fabado et al., 2012; Li et al., 2016). The mutant ein3eil1 is hypersensitive to NaCl treatment (Peng et al., 2014). To investigate the role of EIN3/EIL1 in ethylene signaling-mediated microtubule reassembly under salt stress, we crossed ein3eil1 with wild-type expressing YFP-tubulin to create ein3eil1 lines expressing YFP-tubulin. Of the 12 ein3eil1 expressing YFP-tubulin lines obtained, lines 1 and 2 were selected for further analyses. Because these lines exhibited characteristics that were akin to cortical microtubule reorganization in response to salt stress, representative data from line 1 are presented in this study. Results of confocal microscopy indicated no obvious differences in cortical microtubule depolymerization under conditions of NaCl for 4, 12, or 24 h in wild-type or ein3eil1 cells (paired Student’s t test; Fig. 2, A and B). However, cortical microtubule reassembly was observed in wild-type cells, but not in ein3eil1 cells, after NaCl exposure for 30 to 48 h (Fig. 2, A and B). Therefore, EIN3 and EIL1 are crucial for ethylene regulation of microtubule reassembly in response to salt stress.
Figure 2.
EIN3 is essential for microtubule reassembly in response to salt stress. A, Cortical microtubules in the cotyledon pavement cells from wild-type (YFP-tubulin) and ein3eil1 (YFP-tubulin) seedlings treated with 125 mm NaCl for the indicated times. Scale bar = 20 μm. B, Density of cortical microtubules in A quantified using ImageJ software. Data represent the mean ± sd of 3 independent experiments with a minimum of 10 cells from 3 seedlings assessed in each experiment. Student’s t test, **P < 0.01. C, ein3eil1 seedlings expressing 35S:EIN3-GFP were treated with NaCl for 0, 4, or 30 h, and EIN3-GFP fluorescence was examined in hypocotyl epidermal cells. ACC treatment was used as a control. Scale bar = 20 μm. D, Immunoblot analysis of EIN3-GFP accumulation in ein3eil1 seedlings expressing 35S:EIN3-GFP treated with NaCl for 0, 4, or 30 h. Wild-type seedlings served as a comparison. Actin was used as a loading control. E, Cortical microtubules in the cotyledon pavement cells of wild-type (YFP-tubulin) and ein3eil1 (YFP-tubulin) seedlings treated with 125 mm NaCl for 12 h and then transferred onto medium containing 125 mm NaCl with 0 or 10 μm ACC and grown for a further 12 h. Scale bar = 20 μm. F, Density of cortical microtubules in E quantified using ImageJ software. Data represent the mean ± sd of 3 independent experiments with a minimum of 10 cells from 3 seedlings assessed in each experiment. Student’s t test, **P < 0.01.
Researchers demonstrated previously that salt stress activates and stabilizes EIN3 without affecting EIN3 expression (Peng et al., 2014). To investigate the relationship between EIN3 and microtubule reorganization in response to salt stress, ein3eil1 seedlings expressing 35S: EIN3-GFP (i.e. EIN3-green fluorescence protein) were used (Shi et al., 2012). Under confocal microscopy, EIN3-GFP fluorescence was barely detectable in ein3eil1 expressing EIN3-GFP hypocotyl epidermal cells in the absence or presence of NaCl for 4 h. In contrast, an obvious EIN3-GFP signal was observed in cells treated with NaCl for 30 h or with ACC for 6 h (Fig. 2C). Furthermore, immunoblot analysis indicated that only small amounts of EIN3-GFP were detected in ein3eil1 expressing EIN3-GFP seedlings without NaCl or in the presence of NaCl for 4 h. In contrast, EIN3-GFP accumulated significantly in seedlings treated with NaCl for 30 h (Fig. 2D), which is consistent with the salt-induced microtubule reassembly stage.
To further examine the role of EIN3 in ethylene signaling-enhanced microtubule reassembly, ACC was applied to investigate whether the effects of ethylene enhancement were suppressed in ein3eil1 mutant cells. The majority of cortical microtubules disappeared in wild-type and ein3eil1 cells in the presence of NaCl for 12 h or 24 h. We then transferred the seedlings, which had been treated with NaCl for 12 h, to medium containing NaCl with ACC for a further 12 h growth. Whereas the densities of cortical microtubules were similar in wild-type and ein3eil1 cells that had been treated with NaCl for 12 h, growth in NaCl with ACC for a further 12 h resulted in the reappearance of more cortical microtubules in wild-type cells but not in ein3eil1 cells (Fig. 2, E and F). Therefore, microtubule reassembly is insensitive to the effect of ACC in ein3eil1 cells under salt stress. These results demonstrated that transcriptional regulation by EIN3/EIL1 is crucial for ethylene signaling-mediated reassembly of microtubules in response to salt stress.
Salt Stress Induces the Microtubule-Stabilizing Protein WDL5 through Ethylene Signaling
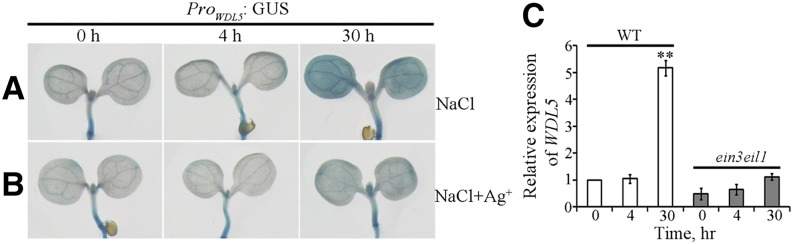
Ethylene is crucial for cortical microtubule reorganization in response to salinity, and transcriptional regulation is important for ethylene signaling-mediated salt tolerance in plants. Hence, we proposed that MAPs likely participate in this physiologic process. Previous studies showed that the microtubule-associated protein WDL5, which is targeted and up-regulated by EIN3, is involved in ethylene signaling-inhibited hypocotyl cell elongation in darkness (Sun et al., 2015; Ma et al., 2016). We assessed levels of the WDL5 transcript to determine whether WDL5 expression was regulated by salt stress. A construct was generated in which the GUS reporter gene was placed under control of the approximately 2.1-kb WDL5 promoter. This construct (ProWDL5:GUS) was introduced into wild-type plants using A. tumefaciens-mediated transformation. The results of GUS staining indicated no obvious WDL5 expression in the cotyledons of seedlings incubated with or without NaCl for 4 h. However, WDL5 expression was substantially increased following seedling incubation with NaCl for 30 h (Fig. 3A), which is consistent with the salt-induced microtubule reassembly stage. To investigate the regulatory relationship between salt-up-regulated WDL5 expression and ethylene signaling, Ag+ was applied to block the ethylene signaling pathway. The results of GUS staining revealed that WDL5 expression was similar following seedling incubation with or without NaCl for 4 h. However, up-regulation of WDL5 expression was significantly inhibited following seedling incubation with NaCl for 30 h (Fig. 3B) when medium was supplemented with Ag+.
Figure 3.
Salt stress activates WDL5 expression via ethylene signaling. A, Histochemical GUS staining of seedlings expressing ProWDL5:GUS treated with NaCl for 0, 4, or 30 h. B, Histochemical GUS staining of seedlings expressing ProWDL5:GUS treated with NaCl with Ag+ for 0, 4, or 30 h. C, WDL5 expression was determined by RT-qPCR using RNA isolated from cotyledons of wild-type or ein3eil11 seedlings that had been treated with NaCl for 0, 4, or 30 h. Error bars represent ± sd (n = 3).
RNA was isolated from cotyledons of wild-type and ein3eil1 seedlings, and mRNA expression was evaluated with reverse transcription quantitative PCR (RT-qPCR). Following seedling incubation with or without NaCl for 4 h, the WDL5 transcript was not obviously altered in wild-type and ein3eil1 cotyledons. However, the WDL5 transcript was abundant in wild-type seedlings, but not in ein3eil1 seedlings, following incubation with NaCl for 30 h (Fig. 3C). Hence, prolonged salt stress induces WDL5 expression via ethylene signaling in the microtubule reassembly stage.
WDL5 Functions as a Positive Regulator in Microtubule Reassembly in Response to Salt Stress
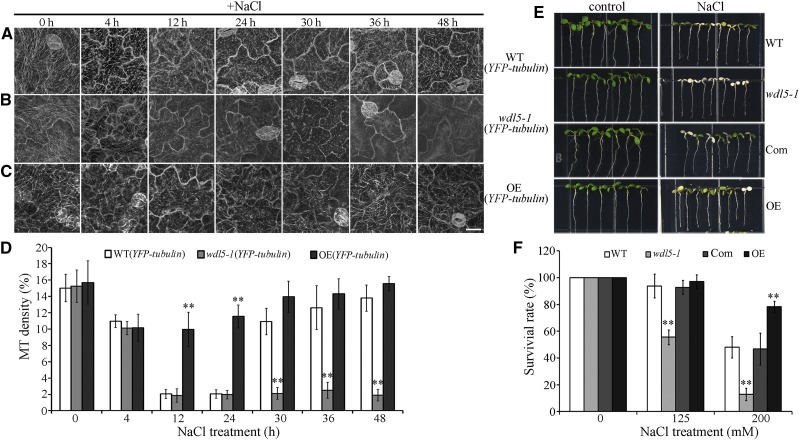
Given that WDL5 expression is related to ethylene signaling and is up-regulated by NaCl treatment, we proposed that WDL5 may be involved in microtubule reassembly during salt stress. To analyze the role of WDL5 regarding microtubules in response to salt stress, a WDL5-null T-DNA insertion mutant was prepared (wdl5-1) in a line with YFP-tubulin expression (Sun et al., 2015). Results of confocal observation showed that cortical microtubules were depolymerized in the presence of NaCl for 4 h in wild-type cells (10.95% ± 0.78%) and in wdl5-1 cells (10.14% ± 0.82%). Increasing the duration of NaCl treatment to 12 or 24 h resulted in the disappearance of most of the cortical microtubules in wild-type cells (12 h, 2.04% ± 0.54%; 24 h, 2.04% ± 0.52%) and in wdl5-1 cells (12 h, 1.86% ± 0.81%; 24 h, 1.97% ± 0.52%). There was no significant difference between wild-type and mutant cells, as determined by paired Student’s t test. Thus, knockout of WDL5 did not substantially affect microtubule depolymerization under salt stress. However, cortical microtubules were found to recover in wild-type cells that were maintained in NaCl for 30 h. Increasing the duration of NaCl treatment to 36 or 48 h resulted in the reappearance of most of the cortical microtubules in wild-type cells (36 h, 12.62% ± 2.7%; 48 h, 13.8% ± 1.6%) but not in wdl5-1 cells (36 h, 2.51% ± 0.99%; 48 h, 1.9% ± 0.71%; Fig. 4, A, B, and D). These findings indicate that WDL5 is a chief component of microtubule reassembly in response to salt stress.
Figure 4.
WDL5 is a positive regulator of microtubule reassembly in response to salt stress. A, Cortical microtubules in the cotyledon pavement cells from wild-type (YFP-tubulin) seedlings treated with 125 mm NaCl for the indicated times. B, Cortical microtubules in the cotyledon pavement cells from mutant wdl5-1 (YFP-tubulin) seedlings treated with 125 mm NaCl for the indicated times. C, Cortical microtubules in cotyledon pavement cells from WDL5-overexpressing (YFP-tubulin, OE) seedlings treated with 125 mm NaCl for the indicated times. Scale bar = 20 μm. D, Density of cortical microtubules in A to C quantified using ImageJ software. Data are the mean ± sd of 3 independent experiments with a minimum of 10 cells from 3 seedlings assessed in each experiment. Student’s t test, **P < 0.01. E, Wild-type (Col-0 ecotype), wdl5-1, wdl5-1 expressing ProWDL5: WDL5 (Com), and WDL5-overexpressing (OE) seedlings were grown for 5 d and then transferred to plates containing 200 mm NaCl and grown for a further 3 d. F, The survival rates of wild-type (Col-0 ecotype), wdl5-1, wdl5-1 expressing ProWDL5: WDL5 (Com), and WDL5-overexpressing (OE) seedlings. Data represent the mean ± sd of 3 independent experiments with a minimum of 80 seedlings assessed in each experiment. Student’s t test, **P < 0.01.
We also examined overexpression of WDL5 in seedlings expressing YFP-tubulin. Of the 10 WDL5-expressing YFP-tubulin lines obtained, lines 1 and 2 were selected for further analysis. Results of RT-qPCR showed that WDL5 transcription levels were considerably increased in seedlings of these lines (Supplemental Fig. S7). Because these lines exhibited similar characteristics of cortical microtubule reorganization in response to salt stress, we included only representative results from line 1 in this report. Results of confocal microscopy and quantification demonstrated that cortical microtubules depolymerize less and recover faster in WDL5-overexpressing cells than in wild-type cells (Fig. 4, A, C, and D). This is concordant with the role of WDL5 as a microtubule stabilizer in salt-induced microtubule reassembly.
The survival rates of wdl5-1 mutant and WDL5-overexpressing seedlings were further explored by transferring 5-d-old seedlings to medium containing 0, 125, or 200 mm NaCl for 3 d. In the presence of 125 mm NaCl, approximately 50% of wdl5-1 seedlings died, whereas almost 100% of wild-type and WDL5-overexpressing seedlings survived. When the concentration of NaCl was increased to 200 mm, approximately 10% of wdl5-1 and 50% of wild-type seedlings survived, which contrasted to the survival of approximately 80% of WDL5-overexpressing seedlings. This wdl5-1 mutant phenotype could be complemented by ProWDL5:WDL5 (Fig. 4, E and F). Therefore, WDL5 functions as a positive regulator in salt stress-induced microtubule reassembly and plant salt tolerance.
WDL5 Knockout Weakens Ethylene-Enhanced Salt Tolerance in Plants
Because salt stress up-regulates WDL5 expression via the ethylene signaling pathway, we hypothesized that the knockout of WDL5 would partially counteract the effects of ethylene-enhanced microtubule reassembly and salt tolerance. The majority of cortical microtubules disappeared in wild-type and wdl5-1 cells following incubation with NaCl for 12 or 24 h. We then transferred seedlings that had been treated with NaCl for 12 h to medium containing NaCl with ACC and allowed the seedlings to grow for a further 12 h. The densities of cortical microtubules were similar in wild-type and wdl5-1 cells following incubation with NaCl for 12 h. In contrast, a further incubation with NaCl and ACC for 12 h yielded the reappearance of more cortical microtubules in wild-type cells but not in wdl5-1 cells (Fig. 5, A and B). This demonstrates that microtubule reassembly is less sensitive to the effect of ACC in wdl5-1 cells under salt stress.
Figure 5.
Knockout of WDL5 partially suppresses ethylene-promoted microtubule reassembly in response to salt stress. A, Cortical microtubules in cotyledon pavement cells from wild-type (YFP-tubulin) and mutant wdl5-1 (YFP-tubulin) seedlings treated with 125 mm NaCl for 12 h and then transferred onto medium containing 125 mm NaCl with 0 or 10 μm ACC for a further 12 h growth. Scale bar = 20 μm. B, Density of cortical microtubules quantified using ImageJ software. Data represent mean ± sd of 3 independent experiments with a minimum of 10 cells from 3 seedlings assessed in each experiment. C, Wild-type (Col-0 ecotype), ACC-pretreated wild-type, wdl5-1, and ACC-pretreated wdl5-1 seedlings were grown for 5 d and then transferred to plates containing 200 mm NaCl and grown for a further 3 d. D, The graph shows the survival rate of seedlings. Data shown are the mean ± sd of 3 independent experiments with a minimum of 80 seedlings assessed in each experiment. E, RT-qPCR analysis of WDL5 transcripts in wild-type, ein3eil1, and 2 ein3eil1 lines expressing pSuper:WDL5-Myc (OE#1/ein3eil1 and OE#2/ein3eil1). F, Wild-type, ein3eil1, OE#1/ein3eil1, and OE#2/ein3eil1 seedlings were grown for 5 d and then transferred to plates containing 200 mm NaCl and grown for a further 3 d. G, The graph shows the survival rate of seedlings. Data represent the mean ± sd of 3 independent experiments with a minimum of 80 seedlings assessed in each experiment. Student’s t test, **P < 0.01.
We then evaluated the sensitivity of wdl5-1 to ACC under salt stress by analyzing the survival rate of seedlings. After ACC pretreatment, the survival rate of wild-type seedlings exposed to NaCl was significantly higher than that of seedlings that were not pretreated. This effect was suppressed in wdl5-1 mutant seedlings (Fig. 5, C and D). In addition, we overexpressed WDL5 in ein3eil1. Of the 11 ein3eil1-expressing WDL5 lines obtained, all exhibited the phenotype of enhanced salt tolerance; lines 1 (OE#1/ein3eil1) and 2 (OE#2/ein3eil1) were selected for further analysis. Results of RT-qPCR showed that WDL5 transcription levels were considerably increased in seedlings of lines 1 and 2 (Fig. 5E). The survival rates of ein3eil1 were substantially increased when WDL5 was overexpressed (Fig. 5, F and G), demonstrating that WDL5 functions as a positive downstream effector in ethylene signaling-mediated salt tolerance.
DISCUSSION
Knowledge of the upstream signals involved in regulation of cortical microtubule reorganization is essential for elucidating the mechanisms of plant cell response to salt stress. In this study, we demonstrate that ethylene positively regulates cortical microtubule reassembly in response to salt stress. The MAP WDL5 participates in ethylene-mediated microtubule reassembly and is a positive regulator of salt tolerance.
Reassembly of Cortical Microtubules Is Crucial for Ethylene-Mediated Salt Tolerance
Investigators have demonstrated previously that ethylene signaling is involved in plant salt tolerance. However, the underlying mechanisms, including the participation of downstream effectors, were not well understood. Results of multiple studies have shown that regulation of cortical microtubules is essential for the plant cell response to salt stress. Our findings support the hypothesis that regulation of microtubule reassembly contributes to salt tolerance and is mediated by ethylene signaling. Cortical microtubule reassembly was significantly suppressed following the inhibition of ethylene signaling and in ein2 and ein3/eil1 loss-of-function mutants. These findings are consistent with ethylene being a positive regulator of salt tolerance. Additionally, WDL5, which encodes a microtubule-stabilizing protein, was up-regulated by NaCl treatment via the ethylene signaling pathway and participated in microtubule reassembly. Knockout of WDL5 partially suppressed ethylene-induced microtubule reassembly, whereas WDL5 up-regulation partially rescued salt sensitivity in ein3eil1 seedlings. Thus, ethylene signaling plays a crucial role in microtubule reassembly and enhances salt tolerance.
Similar to other reports, we found that activation of ethylene signaling greatly alters organization and stability of cortical microtubules in hypocotyl cells (Sun et al., 2015; Ma et al., 2016, 2018). Other phytohormones, such as auxin and brassinosteroid (BR), also affect cortical microtubule organization and stability (Wang et al., 2012; Vineyard et al., 2013). Investigators have demonstrated previously that ethylene interacts with auxin, especially in the regulation of root growth (Růzicka et al., 2007; Swarup et al., 2007; Strader et al., 2010). In addition, seedlings of the BR-deficient mutant det2-1 and the BR-insensitive mutant bin2-1 have increased sensitivity to salt stress (Zeng et al., 2010). Thus, further investigation is needed to elucidate the roles of ethylene/auxin and of BR signaling in the regulation of microtubule reorganization and plant acclimation to salt stress.
Precise regulation of cortical microtubules is necessary in the plant response to salt stress. However, the effect of reorganization of cortical microtubules on plant salt tolerance remains to be elucidated. It is well established that calcium participates in stress signaling in plants (Xiong et al., 2002; Singh et al., 2014; Ohama et al., 2017). Some evidence indicates that microtubule reorganization is associated with increased [Ca2+]cyt levels under salt stress, which benefits plant survival (Thion et al., 1998; Wang et al., 2007). In addition, ethylene has been shown to activate Ca2+-permeable channels of the plasma membrane in tobacco (Nicotiana tabacum) suspension cells (Zhao et al., 2007). Future studies should address whether ethylene signaling enhances microtubule reassembly to facilitate adaptation of plant cells to high salinity via alteration of the [Ca2+]cyt level.
In a previous study, ethylene-mediated salt tolerance was found to occur via an EIN3-dependent manner and an EIN3-independent manner, which was regulated by EIN2 (Peng et al., 2014). We found that microtubule depolymerization in ein2-5 and ein3eil1 was similar to that of wild-type cells, whereas reassembly was abnormal in these mutant backgrounds. Thus, we proposed that regulation of microtubule reorganization might be critical for two pathways involved in salt tolerance. Studies are ongoing to investigate the molecular mechanisms of EIN2-mediated reassembly of cortical microtubules in response to salt stress.
Multiple Layers of Regulation Are Involved in Microtubule Reorganization in Response to Salt Stress
Posttranslational modification of tubulin is involved in the plant response to salt stress. For example, PROPYZAMIDE-HYPERSENSITIVE1 (PHS1), a mitogen-activated protein kinase phosphatase, has been shown to phosphorylate α-tubulin and generate a polymerization-incompetent form, thereby depolymerizing microtubule arrays under salt stress (Ban et al., 2013; Fujita et al., 2013). Several MAPs also participate in microtubule reorganization in response to salt stress. Specifically, certain MAPs alter the organization, dynamics, and stability of cortical microtubules, thereby promoting plant cell adaptation to high salinity. Multiple coordinated pathways modulate the activity and expression levels of these regulators, giving rise to dynamic states of cortical microtubules. Posttranslational regulation is one way in which MAPs can facilitate microtubule reorganization in response to salt stress. Specifically, the microtubule-stabilizing protein SPIRAL1 (SPR1) is cleared by the 26S proteasome degradation pathway to facilitate rapid depolymerization of microtubules in response to excessive salt (Wang et al., 2009, 2011). In high-salinity conditions, phosphatidic acid (PA, a product of phospholipase D) directly binds and promotes the microtubule-stabilizing activity of MAP65-1, which in turn participates in cortical microtubule reassembly (Zhang et al., 2012). Thus, protein degradation and PA-mediated posttranslational regulation appear to contribute to microtubule-mediated salt stress responses. A second mechanism that controls the activity of MAPs to favor plant survival under salt stress is alteration of cellular localization of MAPs. Recently, several investigations determined that the MAP RIC1 plays a negative role in microtubule reassembly under salt stress (Li et al., 2017a). Activation of ROP2 by salt stress resulted in relocalization and dissociation of RIC1 from microtubules, which favored microtubule reassembly and survival of seedlings (Li et al., 2017a). In this study, we demonstrated that transcriptional regulation by upstream signaling factors helps to control the activity of MAPs that function in microtubule reorganization in response to salt stress. Alteration of WDL5 expression through ethylene signaling resulted in abnormal microtubule reassembly and salt tolerance, indicating that transcriptional regulation of WDL5 is involved in ethylene-mediated microtubule reassembly under salt stress. Future studies are planned to identify other MAPs that participate in microtubule depolymerization through transcriptional regulation in response to salt stress.
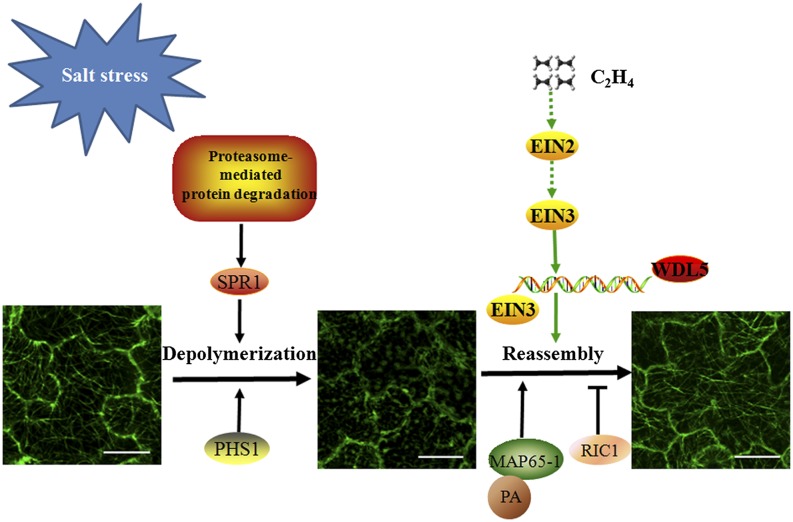
Combined with previous studies involving PHS1, SPR1, MAP65-1, and RIC1, the results of this study provide a clear depiction of the regulation of cortical microtubule reorganization in response to salt stress (Fig. 6). Under salt stress, PHS1-mediated phosphorylation of α-tubulin promotes microtubule depolymerization. The proteasome-mediated protein degradation pathway plays a positive role in rapid depolymerization of microtubules under high salinity via degradation of SPR1. Interaction of MAP65-1 with PA and relocalization of RIC1 facilitate microtubule reassembly, which enhances salt tolerance. We showed that ethylene signaling plays a positive role in regulation of microtubule reassembly and that WDL5, a downstream effector of ethylene signaling, is involved in ethylene-mediated microtubule reassembly under salt stress. We expect that future studies will identify other microtubule regulators that are involved in salt tolerance and are regulated by the proteasome-mediated protein degradation pathway and the ethylene signaling pathway.
Figure 6.
Ethylene signaling participates in microtubule reassembly under salt stress: a working model. Cortical microtubules initially depolymerize and then recover themselves under salt stress. PHS1-mediated phosphorylation of α-tubulin promotes microtubule depolymerization under salt stress. SPR1 is degraded by the proteasome-mediated protein degradation pathway, which facilitates salt-induced rapid depolymerization of cortical microtubules. Ethylene signaling plays a positive role in microtubule reassembly under salt stress. WDL5, as a downstream effector of ethylene signaling, promotes microtubule reassembly and salt tolerance in plants. In addition, PA binding to MAP65-1 and relocalization of RIC1 favor microtubule reassembly under salt stress. Scale bars = 20 μm.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All plant materials used in this study were of the Arabidopsis (Arabidopsis thaliana) Col-0 ecotype background. Seeds were sterilized and placed on 0.5× Murashige and Skoog (MS) medium (Sigma-Aldrich) with 0.8% agar and 1% Suc (w/v). After stratification at 4°C for 2 d, specimens were maintained at 22°C under a 16-h-light/8-h-dark photoperiod until phenotypic analysis.
The following mutants were applied in this study: wdl5-1; WDL5 (overexpression mutant); wdl5-1 expressing ProWDL5:WDL5; wdl5-1 expressing YFP-tubulin (all from Sun et al., 2015); wild-type expressing 35S:YFP-tubulin (from Kirik et al., 2012); ein2-5; ein3eil1; ctr1-1 (all from Peng et al., 2014); eto1-1; ein3eil1 expressing 35S:EIN3-GFP (both from Shi et al., 2012); and ein2-5 expressing YFP-tubulin (from Ma et al., 2016).
Preparation of WDL5 Overexpression Mutant in Arabidopsis
To generate ein3eil1 or 35S:YFP-tubulin seedlings overexpressing WDL5, specific primers (5′-TCTAGAATGGACCCTGAGAGTATCATGG-3′ and 5′-GGTACC ATGCTCAACAGCAACCGCTTCA-3′) were used to amplify the WDL5 coding sequence. The resulting amplicons were fused into a pCAMBIA1300 vector with a pSuper promoter. Constructs then were transformed into ein3eil1 or 35S:YFP-tubulin plants by means of Agrobacterium tumefaciens strain GV3101. Homozygous lines were used for subsequent analyses.
Analysis of Phenotype under Salt Stress
Seedlings were grown for 5 d under normal conditions and were transferred onto medium supplemented with 0, 125, or 200 mm NaCl. The survival rates of the seedlings were scored after 3 d. Complete bleaching of leaves was taken as evidence of salt stress in seedlings.
Pretreatment with ACC under Salt Stress
Seedlings were grown on 0.5× MS medium with or without 10 μm ACC for 5 d and were transferred onto medium supplemented with 0 or 200 mm NaCl. The survival rates of the seedlings were scored after 3 d.
AgNO3 Treatment under Salt Stress
Seedlings were grown on 0.5× MS medium with or without 20 μm AgNO3 for 5 d and were transferred onto medium containing 125 mm or 200 mm NaCl with or without 20 μm AgNO3. The survival rates of the seedlings were determined after 3 d.
GUS Assay of WDL5 Promoter Activity
The promoter of WDL5 was amplified by PCR from Arabidopsis genomic DNA with primers 5′-GTCGACTCTGTAGTCAACGAAATAA-3′ and 5′-GGATCCACCGTATTGTAGAGTATACCT-3′. The resulting fragment, which spanned from base pair 68 to 2,205 upstream of the translation start site of WDL5, was cloned into the pCAMBIA1391 vector to generate ProWDL5:GUS. Plasmids were introduced into Arabidopsis by A. tumefaciens-mediated transformation. Homozygous lines were grown on 0.5× MS medium with or without 20 μm AgNO3 for 5 d and were transferred onto medium containing 125 mm NaCl with or without 20 μm AgNO3 for the indicated time periods. GUS assays were performed as described by Jefferson et al. (1987).
Detection of Cell Death in Leaves
Wild-type seedlings were grown on 0.5× MS medium for 5 d and then were transferred onto medium supplemented with 200 mm NaCl for 3 d. Seedlings that were not treated with NaCl were included as controls. Cotyledons were stained with trypan blue as described by Bowling et al. (1997).
PCR Analysis
RT-qPCR was performed to assess WDL5 transcript levels. Five-day-old seedlings were transferred onto medium supplemented with 125 mm NaCl and were grown for the indicated time periods. Total RNA was isolated from cotyledons with an RNA purification kit (BioTeke), and each time cotyledons were taken and used from at least 20 seedlings. RT-qPCR was carried out with an ABI 7500 PCR system (Applied Biosystems) according to the manufacturer’s instructions. Primers for the detection of WDL5 expression were as follows: 5′-AAATGGTTCTGTTGCTCCTAATGTA-3′ and 5′-TTTGAGACTTTGGTTTCACCTTCT-3′. Actin 2/8 was used as an internal control (primers: 5′-GGTAACATTGTGCTCAGTGGTGG-3′ and 5′-AACGACCTTAATCTTCATGCTGC-3′). Preparation of seedling materials, RNA extraction, and RT-qPCR were repeated three times as different biological replicates, and two or three technical replicates (each biological replicate) were included for each treatment. The average and sd were calculated from the biological replicates.
Immunoblot Assay
ein3eil1 plants expressing 35S:EIN3-GFP were grown on 0.5× MS medium for 5 d and then were transferred onto medium supplemented with 125 mm NaCl for the indicated times. The EIN3-GFP fusion proteins were visualized on immunoblots using an anti-GFP antibody (Roche).
Confocal Imaging
Wild-type, ein2-5, ein3eil1, wdl5-1, and WDL5-overexpressing plants with a 35S:YFP-tubulin background that had been grown on 0.5× MS medium for 5 d were transferred onto medium supplemented with 125 mm NaCl and were grown for the indicated time periods. Cortical microtubules in cotyledon pavement cells and hypocotyl epidermal cells were observed using a confocal laser scanning microscope (LSM 510; Carl Zeiss). YFP was excited at 488 nm, and emissions were collected through a series of filters ranging from 505 to 530 nm.
Quantification of Cortical Microtubules in the Cell
We obtained maximum-intensity projections from serial confocal sections of cortical microtubules from seedlings in a YFP-tubulin background. Algorithms for skeletonization and band-pass filtering (0–1.5 pixels) were run on the projection images by means of ImageJ to perform noise reduction. The resulting images were made binary by thresholding and then were skeletonized with the kbi_bilevelThin ImageJ plug-in (Higaki et al., 2010). To ascertain microtubule density, we determined the density of the YFP-tubulin signal from the skeletonized images and cell regions that had been manually segmented. The density (%) was defined as 100*NMT/NCell, where NMT and NCell represent the pixel numbers constituting the skeletonized cortical microtubules and the cell region, respectively (Fujita et al., 2013). These procedures were carried out using ImageJ software. At least 10 cells from 3 seedlings were measured, and the experiment was repeated three times. Significant differences were ascertained by the paired Student’s t test.
We also quantified microtubules in terms of the total length per unit surface area. We applied the method described previously for this purpose, with some modifications (Fujita et al., 2013). For each image, a fixed region (50 μm × 50 μm) in the cell was selected. The “segmented lines” function in ImageJ then was used to draw outlines of microtubules in this region, and the lengths of the cortical microtubules were measured. Results were expressed as the total length of microtubules per unit surface area. At least 15 cells from 6 seedlings were measured, and the experiment was repeated three times. Significant differences were ascertained by the paired Student’s t test.
Accession Numbers
Sequence data can be found in the Arabidopsis Genome Initiative under accession numbers At3g20770 (EIN3) and At4g32330 (WDL5).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Cell death induced by salt in cotyledons of Arabidopsis seedlings.
Supplemental Figure S2. Suppression of ethylene signaling increases salt sensitivity of seedlings.
Supplemental Figure S3. Full-size images of seedlings.
Supplemental Figure S4. Cortical microtubule reassembly is significantly suppressed in ein2-5 mutant cells under salt stress.
Supplemental Figure S5. Density of cortical microtubules.
Supplemental Figure S6. Cortical microtubule reassembly is greatly suppressed in hypocotyl epidermal cells of ein2-5 seedlings under salt stress.
Supplemental Figure S7. WDL5 expression is increased in seedlings expressing YFP-tubulin.
Acknowledgments
We thank Dr. Shuhua Yang of China Agricultural University who generously provided the ethylene-related Arabidopsis mutant seeds.
Footnotes
Articles can be viewed without a subscription.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Asensi-Fabado MA, Cela J, Müller M, Arrom L, Chang C, Munné-Bosch S (2012) Enhanced oxidative stress in the ethylene-insensitive (ein3-1) mutant of Arabidopsis thaliana exposed to salt stress. J Plant Physiol 169: 360–368 [DOI] [PubMed] [Google Scholar]
- Bahieldin A, Atef A, Edris S, Gadalla NO, Ali HM, Hassan SM, Al-Kordy MA, Ramadan AM, Makki RM, Al-Hajar AS, et al. (2016) Ethylene responsive transcription factor ERF109 retards PCD and improves salt tolerance in plant. BMC Plant Biol 16: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Kobayashi Y, Hara T, Hamada T, Hashimoto T, Takeda S, Hattori T (2013) α-tubulin is rapidly phosphorylated in response to hyperosmotic stress in rice and Arabidopsis. Plant Cell Physiol 54: 848–858 [DOI] [PubMed] [Google Scholar]
- Bashline L, Lei L, Li S, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7: 586–600 [DOI] [PubMed] [Google Scholar]
- Boutrot F, Segonzac C, Chang KN, Qiao H, Ecker JR, Zipfel C, Rathjen JP (2010) Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc Natl Acad Sci USA 107: 14502–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Pytela J, Hotta T, Kato T, Hamada T, Akamatsu R, Ishida Y, Kutsuna N, Hasezawa S, Nomura Y, et al. (2013) An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr Biol 23: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Higaki T, Kutsuna N, Sano T, Kondo N, Hasezawa S (2010) Quantification and cluster analysis of actin cytoskeletal structures in plant cells: role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J 61: 156–165 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JA, Harberd NP (2012) ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J 31: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Chang C (2015) Mechanistic insights in ethylene perception and signal transduction. Plant Physiol 169: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik A, Ehrhardt DW, Kirik V (2012) TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells. Plant Cell 24: 1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbusscheb F, Straetenb DVD, Verbelen JP (2004) Position and cell type-dependent microtubule reorientation characterizes the early response of the Arabidopsis root epidermis to ethylene. Physiol Plant 121: 513–519 [Google Scholar]
- Lei G, Shen M, Li ZG, Zhang B, Duan KX, Wang N, Cao YR, Zhang WK, Ma B, Ling HQ, et al. (2011) EIN2 regulates salt stress response and interacts with a MA3 domain-containing protein ECIP1 in Arabidopsis. Plant Cell Environ 34: 1678–1692 [DOI] [PubMed] [Google Scholar]
- Li C, Lu H, Li W, Yuan M, Fu Y (2017a) A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ 40: 1127–1142 [DOI] [PubMed] [Google Scholar]
- Li P, Li YJ, Zhang FJ, Zhang GZ, Jiang XY, Yu HM, Hou BK (2017b) The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J 89: 85–103 [DOI] [PubMed] [Google Scholar]
- Li W, Ma M, Feng Y, Li H, Wang Y, Ma Y, Li M, An F, Guo H (2015) EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Cell 163: 670–683 [DOI] [PubMed] [Google Scholar]
- Li X, Pan Y, Chang B, Wang Y, Tang Z (2016) NO promotes seed germination and seedling growth under high salt may depend on EIN3 protein in Arabidopsis. Front Plant Sci 6: 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. (2011) Dynamic microtubules and the texture of plant cell walls. Int Rev Cell Mol Biol 287: 287–329 [DOI] [PubMed] [Google Scholar]
- Ma Q, Sun J, Mao T (2016) Microtubule bundling plays a role in ethylene-mediated cortical microtubule reorientation in etiolated Arabidopsis hypocotyls. J Cell Sci 129: 2043–2051 [DOI] [PubMed] [Google Scholar]
- Ma Q, Wang X, Sun J, Mao T (2018) Coordinated regulation of hypocotyl cell elongation by light and ethylene through a microtubule destabilizing protein. Plant Physiol 176: 678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C, Alonso JM, Stepanova AN (2013) Ethylene signaling: simple ligand, complex regulation. Curr Opin Plant Biol 16: 554–560 [DOI] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22: 53–65 [DOI] [PubMed] [Google Scholar]
- Pan YJ, Liu L, Lin YC, Zu YG, Li LP, Tang ZH (2016) Ethylene antagonizes salt-induced growth retardation and cell death process via transcriptional controlling of ethylene-, BAG- and Senescence-associated genes in Arabidopsis. Front Plant Sci 7: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Z, Wen X, Li W, Shi H, Yang L, Zhu H, Guo H (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 10: e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, et al. (2011) Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci USA 108: 2611–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Milla MA, Salinas J (2009) Prefoldins 3 and 5 play an essential role in Arabidopsis tolerance to salt stress. Mol Plant 2: 526–534 [DOI] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24: 2578–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Suzuki K, Abe T, Kaneko Y, Shi H, Zhu JK, Rus A, Hasegawa PM, Hashimoto T (2006) Salt stress affects cortical microtubule organization and helical growth in Arabidopsis. Plant Cell Physiol 47: 1158–1168 [DOI] [PubMed] [Google Scholar]
- Singh A, Kanwar P, Yadav AK, Mishra M, Jha SK, Baranwal V, Pandey A, Kapoor S, Tyagi AK, Pandey GK (2014) Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS J 281: 894–915 [DOI] [PubMed] [Google Scholar]
- Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ma Q, Mao T (2015) Ethylene regulates the Arabidopsis Microtubule-Associated Protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiol 169: 325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion L, Mazars C, Nacry P, Bouchez D, Moreau M, Ranjeva R, Thuleau P (1998) Plasma membrane depolarization-activated calcium channels, stimulated by microtubule-depolymerizing drugs in wild-type Arabidopsis thaliana protoplasts, display constitutively large activities and a longer half-life in ton 2 mutant cells affected in the organization of cortical microtubules. Plant J 13: 603–610 [DOI] [PubMed] [Google Scholar]
- Verbelen JP, Jie L, Vissenberg K, Cnodder TD, Vandenbussche F, Sugimoto K, Straeten DVD (2008) Microtubules and the control of cell elongation in Arabidopsis roots. In YB Blume, WV Baird, AI Yemets, D Breviario, eds, The Plant Cytoskeleton: A Key Tool for Agro-Biotechnology. Springer, Dordrecht, The Netherlands, pp 73–90 [Google Scholar]
- Vineyard L, Elliott A, Dhingra S, Lucas JR, Shaw SL (2013) Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. Plant Cell 25: 662–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li J, Yuan M (2007) Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol 48: 1534–1547 [DOI] [PubMed] [Google Scholar]
- Wang S, Kurepa J, Hashimoto T, Smalle JA (2011) Salt stress-induced disassembly of Arabidopsis cortical microtubule arrays involves 26S proteasome-dependent degradation of SPIRAL1. Plant Cell 23: 3412–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kurepa J, Smalle JA (2009) The Arabidopsis 26S proteasome subunit RPN1a is required for optimal plant growth and stress responses. Plant Cell Physiol 50: 1721–1725 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Yuan M, Ehrhardt DW, Wang Z, Mao T (2012) Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 24: 4012–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl): S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Wang Y, Zheng H, Lu W, Wu C, Huang J, Yan K, Yang G, Zheng C (2015) Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. J Exp Bot 66: 5997–6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Lu X, Ma B, Chen SY, Zhang JS (2015) Ethylene signaling in rice and Arabidopsis: conserved and diverged aspects. Mol Plant 8: 495–505 [DOI] [PubMed] [Google Scholar]
- Yang Q, Chen ZZ, Zhou XF, Yin HB, Li X, Xin XF, Hong XH, Zhu JK, Gong Z (2009) Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol Plant 2: 22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HT, Tang Q, Hua XJ (2010) Arabidopsis brassinosteroid mutants det2-1 and bin2-1 display altered salt tolerance. J Plant Growth Regul 29: 44–52 [Google Scholar]
- Zhang A, Liu D, Hua C, Yan A, Liu B, Wu M, Liu Y, Huang L, Ali I, Gan Y (2016) The Arabidopsis gene zinc finger protein 3 (ZFP3) is involved in salt stress and osmotic stress response. PLoS One 11: e0168367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li Z, Quan R, Li G, Wang R, Huang R (2011) An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol 157: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W (2012) Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24: 4555–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Tian QY, Zhang WH (2007) Ethylene activates a plasma membrane Ca(2+)-permeable channel in tobacco suspension cells. New Phytol 174: 507–515 [DOI] [PubMed] [Google Scholar]
- Zhou S, Chen Q, Sun Y, Li Y (2017) Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ 40: 1512–1530 [DOI] [PubMed] [Google Scholar]