Abstract
Problem
Premature birth complicates 10%–12% of deliveries. Infection and inflammation are the most common etiologies and are associated with increased offspring morbidity and mortality. We hypothesize that lipopolysaccharide (LPS)-induced maternal inflammation causes direct placenta injury and subsequent injury to the fetal intestine.
Method of study
Pregnant C57Bl6 mice were injected intraperitoneally on day 15.5 with 100 μg/kg LPS or saline. Maternal serum, amniotic fluid, placental samples, and ileal samples of offspring were obtained assessed for inflammation and/or injury. Maternal placental ultrasounds were performed. Placental DNA was isolated for microbiome analysis.
Results
Maternal injection with LPS caused elevated IL- 1β, IL-10, IL-6, KC-GRO, and TNF. Placental tissue showed increased IL-1β, IL-6, and KC-GRO and decreased IL-10, but no changes were observed in amniotic fluid. Placental histology demonstrated LPS-induced increases in mineralization and necrosis, but no difference in placental blood flow. Most placentas had no detectable microbiome. Exposure to maternal LPS induced significant injury to the ilea of the offspring.
Conclusion
Lipopolysaccharide causes a maternal inflammatory response that is mirrored in the placenta. Placental histology demonstrates structural changes; however, placental blood flow is preserved. LPS also induces an indirect intestinal injury in the offspring that lasts beyond the neonatal period.
Keywords: cytokines, lipopolysaccharide, microbiome, mouse, placenta
1 | INTRODUCTION
Intrauterine infection and inflammation, which most commonly occurs following ascending microbial invasion into the amniotic cavity, are major causes of preterm birth,1 and perinatal morbidity and mortality.2 Preterm infants exposed to intrauterine infection have higher rates of neonatal sepsis, respiratory distress syndrome, necrotizing enterocolitis, patent ductus arteriosus, intraventricular hemorrhage, bronchopulmonary dysplasia, hearing loss, and cerebral palsy.3–5 However, the mechanisms linking maternal inflammation to these adverse infant outcomes remain unclear. The effect of maternal inflammation and infection on the placenta is largely unknown. Clinical studies indicate that cellular changes on the maternal and fetal sides of the placenta are stereotypical and can be graded for severity,6 but how this relates to placental function and clinical outcome of the newborn fetus has yet to be elucidated.
Emerging evidence suggests that when an intrauterine infection is present, it is detected by the maternal innate immune system.7,8 Activation of the innate immune system results in downstream synthesis of pro-inflammatory cytokines and chemokines,9 with the most significant elevations seen in IL-6, IL-8, IL-1β, TNF, and macrophage inflammatory proteins.9,10 In severe cases, maternal infection can elicit fetal inflammatory response syndrome (FIRS), which is defined as a fetal plasma concentration of IL-6 >11 pg/mL,11 or result in development of preterm labor.12 Neonates who are diagnosed with FIRS have an increased risk for severe neonatal morbidity.7,11,13 However, it is unclear if FIRS is generated in response to direct microorganism exposure, by trans-placental passage of maternal cytokines and chemokines, or by a direct alteration of the placental structure or function resulting in a secondary effect on the newborn.
To begin to understand the association between maternal inflammation and subsequent adverse neonatal outcomes, we aimed to investigate the effects of maternal inflammation on inflammatory cytokine levels, placental structure, placental blood flow, the placental microbiome, and the intestinal health in a murine model. We hypothesized that in an established model,14,15 lipopolysaccharide (LPS)-induced maternal inflammation will induce direct injury to the placenta, resulting in altered blood flow and subsequent fetal injury.
2 | MATERIALS AND METHODS
2.1 | Animals and sample collection
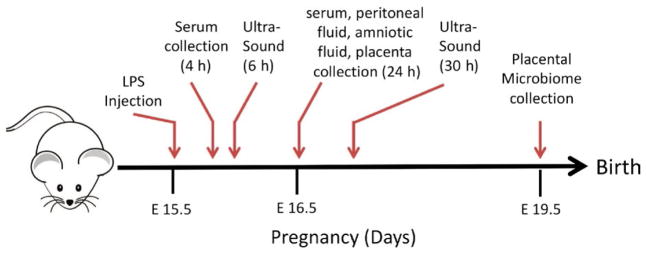
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Usage Committees at the University of Iowa (Iowa City, IA) and in accordance with ARRIVE guidelines. Experiments were performed using C57BL/6j mice whose founders were purchased from Jackson Laboratories. The mice were housed in the University of Iowa AAALAC-approved vivarium and bred to achieve timed pregnancy. On day 15.5 gestation, pregnant mice were given a single 100 μg/kg intraperitoneal injection of LPS derived from Escherichia coli O55:B5 (Sigma Aldrich) or equivalent volume of saline (Sham) unless otherwise noted based on established models.14,15 Unless prenatal samples were collected, pregnant mice were monitored for preterm delivery and gestation was allowed to continue uninterrupted until term gestation (E20/P0). Maternal serum samples were obtained 4 hours following LPS injection via venous puncture of the submandibular vein with a 4 mm Goldenrod animal-bleeding lancet. Samples were centrifuged at 8000 g for 6 minutes, and serum was collected for analysis. A schematic of the timing of injections and sample harvesting is presented as Figure 1.
FIGURE 1.
Schematic representation of experimental time points
On E19.5, pregnant mice underwent carbon dioxide euthanasia and the uterus was exteriorized via a midline laparotomy to identify individual gestational sacs. Amniotic fluid was aspirated under sterile conditions using a 28-gauge insulin syringe. Gestational sacs that yielded <1 μL of amniotic fluid were pooled for analysis. The gestational sacs were then opened, and individual placentas were collected. Placental samples underwent tissue homogenization with a TissueLyser LT Bead Beater (Qiagen) or were fixed in 10% buffered formalin for 48 hours prior to embedding in paraffin for histologic analysis. Samples were normalized for the amount of protein obtained and maternal serum, placental homogenates, and amniotic fluid samples were quantified for cytokine levels (IL-1β, IL-6, IL-10, KC-GRO TNF, IL-2, IL-4, IL-5, IL-12, and INFγ) using a Meso Scale Discovery technology as previously described.16 Non-significant cytokines differences are not shown.
2.2 | Placental histology
Placental samples were H&E-stained and scored by a single-blinded board-certified veterinary pathologist using the following system, based on prior rodent placental histologic assessments.17 Placental tissue was scored for mineralization (0 = no mineralization in field of view; 1 = rare, multifocal mineralization; 2 = multifocally, scattered mineralization; 3 = large zones of diffuse mineralization) and necrosis (0 = no necrosis in field of view; 1 = multifocal placental necrosis exclusively within the marginal placenta; 2 = multifocal necrosis throughout the placenta; 3 = multifocal to coalescing, large zones of necrosis throughout the placenta; 4 = diffuse placental necrosis).
2.3 | Intestinal histology
Neonatal offspring were raised under standard conditions in the University of Iowa AAALAC-approved vivarium and had ad lib access to food and water. The distal 1/3 of the intestine between the stomach and the cecum was collected following euthanasia at ages listed. Tissue samples were fixed in neutral-buffered 10% formalin, embedded in paraffin, and sectioned at 5 μm thickness. The samples were stained with hemotoxin and eosin (H&E) stain and evaluated microscopically by a single, blinded investigator. Injury scores were determined via a 3-point intestinal injury scoring scale (0 = normal, 1 = mild, 2 = severe) based on degree of villi vacuolization, mucosal ulceration, lamina propria damage, and the presence of hemorrhage within villi as previously described.18
2.4 | Quantification of LPS
All samples analyzed for LPS quantification were obtained 24 hours following maternal intraperitoneal injection with LPS. Maternal serum samples were obtained as above from submandibular venipuncture immediately prior to euthanasia. Immediately following euthanasia, a midline laparotomy was performed and 200 μL saline was pipetted into the peritoneal cavity. The peritoneal fluid was re-aspirated and collected for analysis as peritoneal wash. The uterus was then exteriorized, and individual gestational sacs were identified. Amniotic fluid was aspirated using a 28-gauge insulin syringe. Lastly, the gestational sacs were opened and individual placentas were collected and homogenized as above with a new bead for each sample to prevent cross-contamination. The serum, peritoneal, amniotic fluid, and placental samples were then analyzed for the presence of LPS using a commercially available limulus amebocyte lysate assay that detects >0.03 EU/mL of LPS (E-toxate; Sigma Aldrich).
2.5 | Placental blood flow analysis
Abdominal ultrasounds were performed at 6 and 30 hours following maternal LPS injection by a single-blinded investigator. Immediately prior to ultrasound, mice were sedated with midazolam (0.1 mg subcutaneous injection). The anterior thorax and abdomen were shaved. The animal was grasped by the nape of the neck and cradled in the recumbent position in the imager’s hand. Warmed gel was applied to the ultrasound probe to optimize the acoustic interface. Maternal cardiac, uterine, and fetal sonography was performed using a 40 MHz linear array probe coupled to a Vevo 2100R imaging system (VisualSonics, Toronto, ON, Canada), generating ~180–200 2- dimensional (2-D) frames per second. Maternal heart rates were measured using M- mode echocardiography, which reports the time between heartbeats in milliseconds. Fetal cardiac activity was visualized using 2-D images. Discrimination between uterine (maternal) and fetal arterial flow was accomplished by noting differences in heart rate (~600 vs ~200, respectively).19 Maternal uterine and umbilical artery flow velocity were identified and interrogated using 2-D images as previously described.20 S:D ratio was calculated using the peak systolic pressure/end diastolic pressure. Resistive index was calculated as 1 – (1/pulsatility index), with pulsatility index being defined as peak systolic/end diastolic flow velocity by Doppler.20
2.6 | Analysis of the placental microbiome
On E19, pregnant mice underwent carbon dioxide euthanasia. Immediately following euthanasia, midline laparotomy was performed under sterile conditions and the uterus was exteriorized and individual gestational sacs were identified. Gestational sacs were opened, and individual placentas were collected. DNA was isolated from placental samples using Fecal DNA MiniPrep™ (Zymo Research). The extracted DNA was stored at −20°C and analyzed as previously described.21 Bacterial 16s rRNA amplification of the V4 domain was performed using the following primers: F515 (5′-NNNNNNNNGTGTGCCAFCMGCCGCCGCGGTAA-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′), with the forward primer modified to contain a unique 8 nucleotide linker sequence (italicized poly-N section of the primer above) and a 2 nucleotide linker sequence (bold, underlined portion) at the 5′ end. PCR reactions used 5–100 ng DNA template, 1× GoTaq Green Master Mix (Promega, Madison, WI, USA), 1 mmol/L MgCl2, and 2 pmol of each primer. PCR was performed at 94°C for the initial 3 minutes followed by 35 cycles of 94°C for 45 seconds, 50°C for 60 seconds, and 72°C for 90 seconds, with a final extension of 72°C for 10 minutes. PCR amplicons were grouped at approximately equal amplification intensity ratios and were purified using the Qiaquick PCR purification kit (Qiagen). The PCR amplicons were submitted to the UC Davis Genome Center DNA Technologies Core for Illumina paired-end library preparation, cluster generation, and 250 bp paired-end Illumina MiSeq sequencing. Data from the sequencing run were analyzed using the QIIME software package (version 1.7.0; University of Colorado, Boulder, CO, USA).22 Sequences were quality filtered and demultiplexed, and the UCLUST (drive5.com; Tiburon, CA, USA) was used to assign operational taxonomic units (OTUs), based on a 97% pairwise identity.23,24 Secondary filtration of 0.005% was used to remove low-abundance OTUs.23 The filtered OTUs were taxonomically classified based on the Ribosomal Database Project classifier (Michigan State University, East Lansing, MI, USA)25 against a representative subset of the Greengenes 16s rRNA database (gg_13_5 release; Second Genome, South San Francisco, CA, USA).26 The data were analyzed using linear discriminate analysis (LDA) effect size to determine the significantly differentiated taxa.
2.7 | Statistical analysis
All experiments were performed in at least triplicate, and specific sample sizes are denoted in the Results section. Non-parametric ANOVA with Kruskal-Wallis multiple comparisons or Mann-Whitney non-parametric t testing was performed as appropriate to determine statistical significance using GraphPad Prism v7. Significance of LPS presence was determined using Chi-square with Fisher’s exact testing using GraphPad Prism v7. Significance was set as P < .05 for all experiments. Microbiome statistics were performed as described above.
3 | RESULTS
3.1 | LPS-induced maternal inflammation has a dose- dependent effect on maternal and neonatal survival
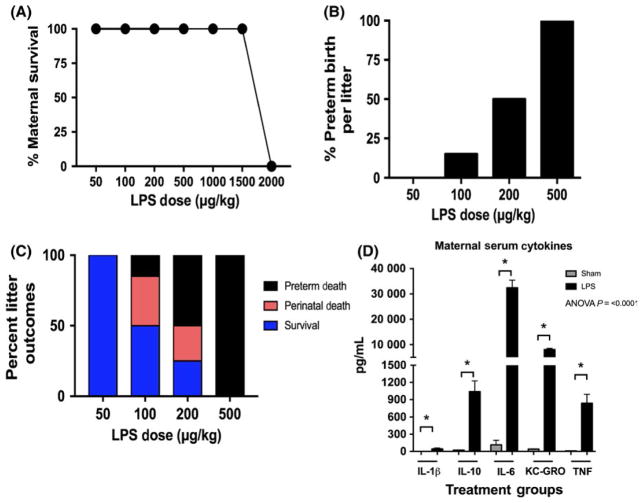
To determine the optimal dose for our experiments, a dose-response curve was generated to evaluate maternal survival, rates of preterm birth, newborn survival, and maternal serum cytokines (Figure 2, n = 60 pregnant mice). Maternal survival was 100% at doses ranging from 50 to 1500 μg/kg. When LPS was administered at 2000 μg/kg, maternal survival precipitously dropped to 0% (Figure 2A). Preterm birth rates increased with increasing doses of LPS—the rates were 0%, 15%, 50%, and 100% at doses of 50, 100, 200, and 500 μg/kg, respectively (Figure 2B, n = 55 litters). The proportion of litters that survived to birth and then through 7 days of life also varied by maternal LPS dose. Litters that were exposed to 50 μg/kg survived to 7 days in 100% of cases, while those exposed to 100, 200, and 500 μg/kg only survived 50%, 25%, and 0% of all cases (Figure 2C). Based on the results of these experiments, we chose to use 100 μg/kg for the remainder of our experiments to maximize inflammatory phenotypes while minimizing the risk of preterm delivery and maternal mortality. To ensure that pregnant mice were exhibiting a response to the intraperitoneal LPS dosed at 100ug/kg, maternal serum was collected 4 hours following LPS injection. Pregnant mice exposed to intraperitoneal LPS had a significant rise in the levels of IL-1β, IL-10, IL-6, KC-GRO, and TNF (P < .0009 for all serum cytokines; Sham n = 5, LPS n = 10 pregnant mice) (Figure 2D).
FIGURE 2.
Increased doses of intraperitoneal lipopolysaccharide (LPS) result in higher rates of preterm birth without significant effect on maternal survival. A, Pregnant mice were given increasing doses of LPS to assess effect on maternal and neonatal survival. There was no effect on maternal survival below a dose of 2000 μg/kg (n = 60 pregnant mice). B, Rates of preterm birth were 0%, 15%, 50%, and 100% for doses of 50, 100, 200, and 500 μg/kg, respectively (n = 55 litters). C, The proportion of litters that survived to term and then 7 d of life were 100%, 50%, 25%, and 0% at doses of 50, 100, 200, and 500 μg/kg, respectively. D, To assess maternal response to injection, serum cytokines were obtained 4 h following injection. There were significant elevations in the levels of IL-1β, IL-10, IL-6, KC-GRO, and TNF (P < .0009 for all serum cytokines; Sham n = 5, LPS n = 10 pregnant mice, P < .0001)
3.2 | Maternal inflammation induces cytokine upregulation in the placenta, but not the amniotic fluid
To assess whether the maternal serum response was mirrored in the placenta and amniotic fluid, we quantified cytokines levels in both the placenta and amniotic fluid compartments. Pregnant mice exposed to LPS had increased levels of IL-1β, IL-6, and KC-GRO and decreased levels of IL-10 within the placenta when compared to controls (P = .0001; n = 8 pregnant dams per group, only 1 placenta was used from each animal) (Figure 3A). However, when the amniotic fluid was analyzed, there were no differences between levels of IL-1β, IL-10, IL-6, KC-GRO, and TNF in the 2 treatment groups (n = 8 pregnant dams per group with roughly 3 amniotic fluid compartments sampled and pooled per mother) (Figure 3B). To understand the localization of cytokines, placental samples were stained for IL-6 (Figure 3C). Animals exposed to LPS had increased staining compared to sham controls. While IL-6 staining is present in both intra- and extracellular spaces, there was a trend for increased placental tissue staining.
FIGURE 3.
Maternal injection with lipopolysaccharide (LPS) increases inflammatory cytokines in the placenta, but not in the amniotic fluid. A, IL-1B, IL-6, and KC-GRO were significantly elevated, and IL-10 was decreased in placental tissue (P = .0001; n = 8 pregnant dams per group, only 1 placenta was used from each animal). B, There were no significant differences in cytokine levels in the amniotic fluid compared to sham controls (P < .0001; n = 8 pregnant dams per group with roughly 3 amniotic fluid compartments sampled and pooled per mother). C, Placental tissue samples were stained for IL-6 and examined at 6× (total placenta) and 20× (intervillous space). LPS-exposed animals had increased staining compared to sham with a trend toward intracellular staining (n = 6)
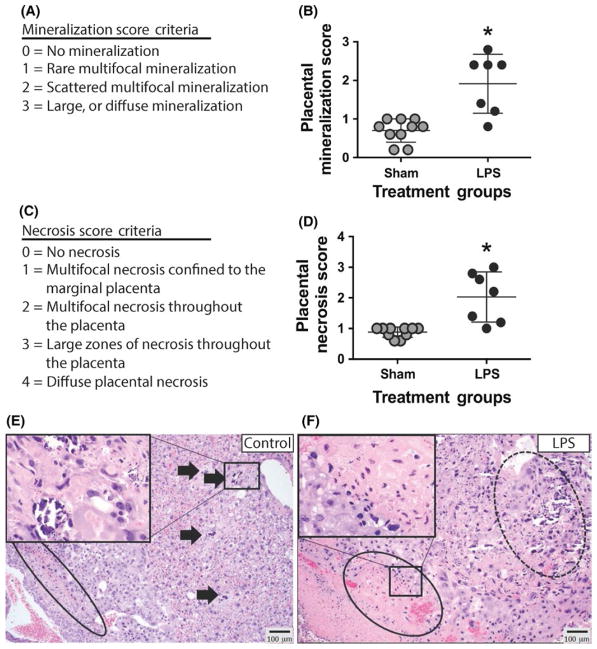
3.3 | Maternal LPS exposure induces placental injury
To examine whether the placental structure was altered as a result of maternal inflammation, we performed histologic analysis following exposure to LPS and compared this to controls. A histologic scoring system was developed based on prior studies9 to quantify the degree of placental mineralization and necrosis (Figure 4A, C). Placental tissue exposed to LPS had significantly higher scores for mineralization, most significantly within the spongiotrophoblastic layer (P = .001; LPS n = 8, Sham n = 10) (Figure 4B, D). There also was significant multifocal necrosis within the giant cell layer, the spongiotrophoblastic layer, and the maternal vascular layer of the placental tissue exposed to LPS (P = .0004; n = 8 pregnant dams in the sham group and 10 pregnant dams in the LPS group, only 1 placenta was used from each animal) (Figure 4E, F), as compared to controls.
FIGURE 4.
Placental histopathology demonstrates increased levels of mineralization and necrosis in placental tissue exposed to maternal lipopolysaccharide (LPS). A, Mineralization scoring criteria. B, Analysis of placental mineralization demonstrates increased mineralization in tissue exposed to LPS (P = .001; n = 8 pregnant dams in the sham group and 10 pregnant dams in the LPS group, only 1 placenta was used from each animal). C, Necrosis scoring criteria. D, Analysis of placental necrosis demonstrates increased necrosis in tissue exposed to LPS (P = .0004; n = 8 pregnant dams in the sham group and 10 pregnant dams in the LPS group, only 1 placenta was used from each animal). E, Sham example: rare zones of coagulation necrosis (circle). Variable-sized zones of mineral accumulation in vascular spaces (arrows). Inset (40× magnification) highlights an area of mineralization characterized by accumulation of a basophilic granular material. F, LPS-exposed example: extensive, multifocal necrosis (circle) with severe, extensive mineralization (dashed circle). Inset (40× magnification) highlights multifocal, variably sized zones of placental necrosis
3.4 | LPS is not detected in the fetal compartment 24 hours after maternal injection
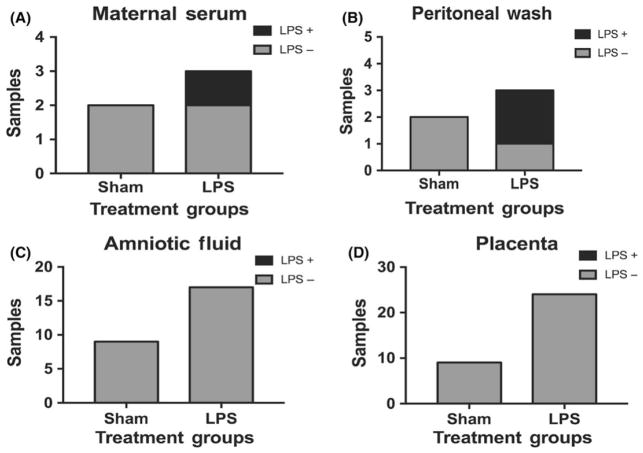
After demonstrating that maternal administration of LPS caused differences in maternal serum cytokines, placental cytokines, and placental structure, we next asked whether LPS in our model crossed the placenta and thus could be exerting direct effects on the fetus. To answer this, we obtained maternal serum, a saline washing of the peritoneal cavity, placenta, and amniotic fluid samples 24 hours following intraperitoneal injection of the pregnant mother. Maternal serum from LPS mothers continues to have detectible LPS in 33% of cases (Figure 5A). Similarly, LPS was detected in 66% of peritoneal wash samples of mothers injected with LPS compared to controls (Figure 5B). However, we were unable to detect any evidence of LPS within the placenta (n = 26) or amniotic fluid samples (n = 33) in either treatment group (Figure 5C, D), suggesting that LPS does not cross the placenta All samples were obtained from 2 separate sham-treated and 3 separate LPS-treated pregnant dams.
FIGURE 5.
Intraperitoneal injections of lipopolysaccharide (LPS) do not cross the placenta. A, LPS was detected in 1 of 3 maternal serum and in (B) 2 of 3 maternal peritoneal fluid in the LPS-treated dams but not in the 2 sham-treated dams. C, LPS was not detected in either the amniotic fluid (n = 45) or (D) placenta (n = 45). All samples were obtained from 2 separate sham-treated and 3 separate LPS-treated pregnant dams
3.5 | LPS-induced maternal inflammation does not alter placental blood flow patterns
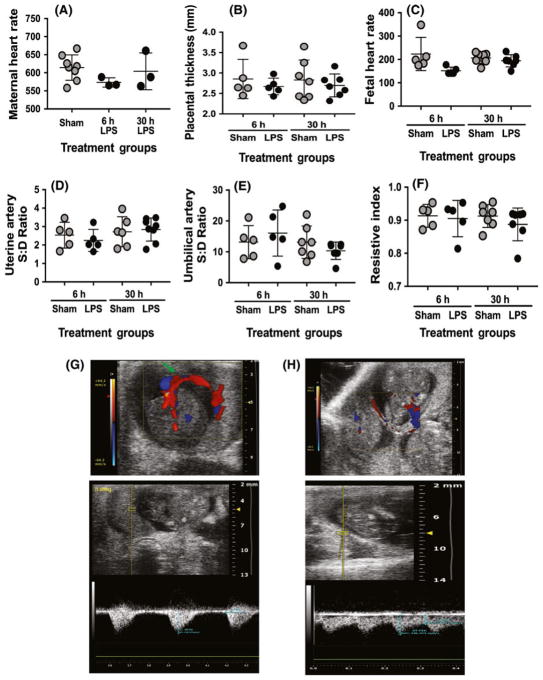
To examine whether LPS-induced maternal inflammation injures the placenta through alteration of blood flow, we performed Doppler ultrasounds of the umbilical artery and small branches of the uterine artery 6 and 30 hours following exposure to LPS (Figure 6) (6 hours: n = 5 Sham pregnant dams, 5 LPS pregnant dams; and 30 hours: n = 6 Sham pregnant dams, 8 LPS pregnant dams). To determine the overall health of the mother, maternal heart rate and placental thickness were measured (Figure 6A, B). There were no significant differences between LPS-treated and Sham mothers. We next examined the fetal heart rates (Figure 6C). One Sham fetus developed tachycardia 6 hours post-procedure. Excluding that 1 outlier, there were no significant differences in fetal heart rate in LPS-exposed vs Sham, at either time point. We lastly examined the blood flow velocity of the placenta (Figure 6D–F). There was no difference in the systolic:diastolic flow velocity ratio or resistive index in the umbilical arteries at either 6 (P = .42) or 30 hours (P = .32) following LPS compared to Sham controls. There also were no differences in the systolic:diastolic flow velocity ratio or resistive index in the branches of the uterine arteries at either 6 (P = .42) or 30 hours (P > .99), as compared to controls. Sample pulse wave Doppler tracings are shown in Figure 6G, H.
FIGURE 6.
Maternal exposure to lipopolysaccharide (LPS) does not alter umbilical or uterine artery blood flow. At 6 (n = 5 Sham pregnant dams, 5 LPS pregnant dams) and 30 h (n = 6 Sham pregnant dams, 8 LPS pregnant dams) following injection, Doppler ultrasounds of the umbilical and uterine artery were performed. A, Maternal heart rate (beats/min) was not significantly different in the LPS-treated group compared to Sham controls. B, Placental thickness (mm) was not significantly different between LPS-treated mothers and Sham controls. C, Fetal heart rate was significantly lower in the LPS-treated group compared to the sham group at 6 h (P = .026), but if the single outlier was removed, this significance is lost. There were no significant differences at 30 h (P = .88). No changes were seen in (D) umbilical artery blood flow (P = .42 [6 h], P = .32 [30 h]), in (E) uterine artery blood flow (P = .42 [6 h], P > .99 [30 h]) following maternal LPS exposure, or in (F) calculated systolic:diastolic ratio. Sample pulse wave Doppler tracings are shown for umbilical (G) and uterine (H) arteries
3.6 | LPS-induced maternal inflammation has no effect on the limited bacterial flora of the placenta
Recently, there has been discrepancy in the literature regarding the presence of a placental microbiome.27–29 To determine if there was a detectible microbiome in our model and further if LPS-induced maternal inflammation had an effect on it, placental samples were collected for microbiome analysis under careful sterile conditions. Of the 17 samples obtained (9 sham and 8 LPS, 1 placenta sampled per pregnant mother), bacterial DNA was only isolated in 3 of the sham samples and 1 of the LPS samples (Figure 7A). Among these 4 specimens, the majority of bacteria in both groups were bacteroides and firmicutes with small proportions of tenericutes, actinobacteria, and proteobacteria. Statistical analysis could not be performed based on the inadequate sample size; however, large alterations in the microbiome do not appear to result from LPS exposure (Figure 7B).
FIGURE 7.
Rare bacterial DNA was isolated from placental tissues, but lipopolysaccharide (LPS) did not significantly alter detected bacterial ratios. Placental DNA was isolated from both treatment groups under sterile conditions (n = 9 Sham placentas and 8 LPS placentas, 1 placenta sampled per pregnant dam). A, DNA was only isolated in 3 of 9 of sham samples and 1 of 8 LPS samples. B, The majority of bacteria in both groups were bacteroides and firmicutes with small proportions of tenericutes, actinobacteria, and proteobacteria. Statistical analysis could not be performed based on inadequate sample size
3.7 | Fetal exposure to LPS-induced maternal inflammation induces neonatal intestinal injury
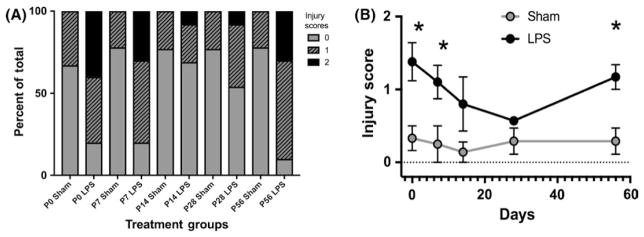
As there is a known association between maternal chorioamnionitis and later development of neonatal necrotizing enterocolitis in her infants, we lastly wanted to assess whether fetal exposure to maternal LPS-induced inflammation would injure the developing intestinal tract. To assess this, we harvested small intestinal tissues from infants at weekly intervals and assessed them for injury using an intestinal injury score as previously described17 (Figure 8). Significantly increased injury scores were seen in the exposed pups at P0, P7, and P56 (P = .0259, 0.0134 and 0.0031) compared to sham controls (n = 8 sham-treated and 8 LPS-treated pups from at least 3 separate pregnant dams at each time point). Although they did not reach significance, injury scores at P14 and P28 also remained elevated from baseline.
FIGURE 8.
Exposure to lipopolysaccharide (LPS)-induced maternal inflammation generates intestinal injury in her offspring. Intestinal ileal samples showed significantly higher injury scores in mice who had been exposed to maternal LPS-induced inflammation compared with controls (n = 8 Sham and 8 LPS offspring from at least 3 separate pregnant dams at each time point). Significant differences in injury score were seen at PO (P = .0259), P7 (P = .0134), and P56 (P = .0031). Differences at P14 and P21 were not statistically different. Scores as shown as a percentage of the total (A) and as an average for each age (B)
4 | DISCUSSION
Preterm birth is commonly associated with intrauterine infection and inflammation. In patients who deliver between 25 and 28 weeks, the incidence of chorioamnionitis is 40%, as compared to 4% in those who deliver at term.10 Clinical studies have demonstrated that infants who are born preterm with intrauterine infection are at increased risk of neonatal complications.3–5 Several prior animal studies have used maternal injection with LPS to induce maternal inflammation and induce preterm birth,30–32 or neonatal injury.14,15 While it is known that administering LPS can generate preterm birth, we aimed to investigate the consequences of creating an environment of maternal inflammation, short of causing preterm birth. While this model does not replicate the ascending bacterial translocation component thought to induce human chorioamnionitis, it does allow us to understand the effects caused by isolated maternal inflammation that is induced during chorioamnionitis and is important and relevant to understanding the human condition. Our hypothesis was that LPS-induced maternal inflammation will cause direct injury to the placenta, resulting in altered blood flow, and subsequent damage to the offspring. Our novel findings presented in this manuscript show that injecting intraperitoneal LPS into a pregnant mouse causes an expected inflammatory response within the maternal serum but spares the amniotic fluid compartment suggesting that neonatal consequences of maternal LPS-induced inflammation are secondary and not directly due to LPS exposure. Furthermore, our data show that LPS-induced maternal inflammation induces direct damage to the placenta, but does not alter resistance in uterine arteries. Importantly, we show that in our study, most placentas do not have a detectible microbiome, and what is present, is not altered by LPS-induced maternal inflammation. Lastly, we show that exposure to maternal LPS induces intestinal injury through an indirect method in the offspring that lasts beyond the perinatal period. Based on these data, we speculate that neonatal morbidities following chorioamnionitis are more likely due to transmission of maternal cytokines and/or alterations in placental function (unrelated to placental blood flow) than a direct effect of the inflammatory agent on the fetus or of alterations to placental blood flow.
The inflammatory response seen in maternal serum following LPS exposure is similar to what is clinically seen in pregnant patients who are subsequently diagnosed with histologic chorioamnionitis.33,34 Furthermore, the increase in maternal serum cytokines is mirrored in the cytokine levels within the placental tissue.34 However, no elevation was seen within the amniotic fluid (Figure 2B). The human placenta has the capacity to both produce and express virtually all known cytokines.35 Prior research has demonstrated that in vitro administration of LPS to first-trimester cytotrophoblast cells results in increased cytotrophoblastic production of TNF-alpha, IL-1B, IL-6, and IL-8.36 LPS injections in pregnant sheep have also been shown to induce systemic inflammation; however, similar to our results, in sheep, LPS was not found to cross the placenta into the fetal compartment.37,38 The elevated levels of placental cytokines seen in our study (IL-1B, IL-6, and KC-GRO) match historical data and could be the result of inflammatory cell transfer from uteroplacental circulation or by innate placental generation.
Our histologic analysis of the placentas determined that mice exposed to maternal inflammation have significantly increased mineralization and necrosis within the placental tissue. Cytokines are known to alter environmental conditions and can alter the function of placental cells.39,40 While it is likely that these parenchymal changes can be attributed to pro-inflammatory cytokines, a relevant question is whether or not the placental changes could also be attributed to direct damage by LPS. Our data show no evidence of LPS within either the placenta or amniotic fluid 24 hours following maternal injection. Given this result, it is more likely that the placenta is directly damaged by transmission of cytokines. This correlates with clinical literature demonstrating that placental cellularity and vascular structure are compromised in the setting of infection and inflammation.41 However, it remained possible the LPS was altering blood flow in the placenta which then caused a secondary effect on the fetus due to in utero hypoxemia. To examine this possibility, we quantified the blood flow of both umbilical and uterine arteries. We could find no significant differences in systolic:diastolic ratios or resistive index in either arterial systems, making it unlikely that adverse neonatal outcomes are related to alterations in blood flow. Recent data have shown that intraperitoneal vs intrauterine administration of infectious or inflammatory agents can affect the severity of sequelae to the fetus.42 Importantly, our data demonstrate that exposure to intraperitoneally injected LPS into pregnant dams induced a sustained injury pattern in the intestinal tract of the fetuses. This is despite no evidence that LPS was able to cross the placenta.
Lastly, we wanted to evaluate any effects that LPS-induced maternal inflammation would have on the placental microbiome. Historically, the placenta and the developing fetus were thought to be sterile43; however, over the last several years, several studies have introduced the idea that the placenta may harbor a unique low-abundance microbiome.29,44 This concept has recently been called into question based on studies that have revealed no differences in the microbiome of the placenta as compared to sterile swabs, air swabs, and blank DNA purification kits, raising the concern for contamination.27,28 Our data support this recent evidence given that such a low proportion of our samples revealed any bacteria for analysis. In the context of our study, these results suggest that the placental microbiome likely does not have a significant role in the increased risk that accompanies pregnancies complicated by intrauterine infection and inflammation.
5 | CONCLUSION
This study demonstrates use of a highly relevant mouse model that is developmentally similar to the early third trimester in human gestation, when the majority of cases of preterm birth occur. We have shown that this method reliably introduces maternal and placental inflammation. Although structural changes were noted in the placenta, this is seemingly unrelated to placental perfusion. Based on our results, the leading hypothesis for adverse neonatal intestinal outcomes in pregnancies complicated by antenatal infection and inflammation is fetal exposure to inflammatory cytokines. Future studies investigating the role of individual cytokines and further defining the effects of exposure on the neonatal intestine are warranted to determine the molecular mechanisms by which maternal inflammation impacts the fetus. Understanding of these mechanisms has the potential to reveal potential preventive therapies.
Acknowledgments
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: HD081121; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK097335; NIH Office of the Director, Grant/Award Number: OD019941
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interests to report.
References
- 1.Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 7. Philadelphia, PA: Elsevier/Saunders; 2017. [Google Scholar]
- 2.Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore T, Greene MF. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice: Expert Consult Premium Edition - Enhanced Online Features. Houston, TX: Elsevier Health Sciences; 2013. [Google Scholar]
- 3.Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. 2009;22:780–784. doi: 10.3109/14767050902922581. [DOI] [PubMed] [Google Scholar]
- 4.Lau J, Magee F, Qiu Z, Hoube J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. 2005;193:708–713. doi: 10.1016/j.ajog.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- 6.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213:S21–S28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Blank V, Hirsch E, Challis JR, Romero R, Lye SJ. Cytokine signaling, inflammation, innate immunity and preterm labour–a workshop report. Placenta. 2008;29(Suppl A):S102–S104. doi: 10.1016/j.placenta.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Prearo MN, Camargo BR, Fernandes ML, et al. Toll-like receptor-2 and -4 expression by maternal neutrophils in preterm labor. Gynecol Obstet Invest. 2018;83:1–8. doi: 10.1159/000468930. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836.e1–836.e18. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 12.Filipovich Y, Klein J, Zhou Y, Hirsch E. Maternal and fetal roles in bacterially induced preterm labor in the mouse. Am J Obstet Gynecol. 2016;214:386.e1–386.e9. doi: 10.1016/j.ajog.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofer N, Kothari R, Morris N, Muller W, Resch B. The fetal inflammatory response syndrome is a risk factor for morbidity in preterm neonates. Am J Obstet Gynecol. 2013;209:542.e1–542.e11. doi: 10.1016/j.ajog.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–294. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- 16.White JR, Gong H, Pope B, Schlievert P, McElroy SJ. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis Model Mech. 2017;10:727–736. doi: 10.1242/dmm.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline JM, Dixon D, Ernerudh J, et al. The placenta in toxicology. Part III: pathologic assessment of the placenta. Toxicol Pathol. 2014;42:339–344. doi: 10.1177/0192623313482207. [DOI] [PubMed] [Google Scholar]
- 18.Wynn JL, Wilson CS, Hawiger J, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A. 2016;113:E2627–E2635. doi: 10.1073/pnas.1515793113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rada CC, Pierce SL, Nuno DW, et al. Overexpression of the SK3 channel alters vascular remodeling during pregnancy, leading to fetal demise. Am J Physiol Endocrinol Metab. 2012;303:E825–E831. doi: 10.1152/ajpendo.00165.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dokras A, Hoffmann DS, Eastvold JS, et al. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod. 2006;75:899–907. doi: 10.1095/biolreprod.106.053603. [DOI] [PubMed] [Google Scholar]
- 21.Underwood MA, Arriola J, Gerber CW, et al. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res. 2014;76:326–333. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salminen A, Paananen R, Vuolteenaho R, et al. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res. 2008;63:280–286. doi: 10.1203/PDR.0b013e318163a8b2. [DOI] [PubMed] [Google Scholar]
- 31.Mijovic JE, Zakar T, Zaragoza DB, Olson DM. Tyrphostins inhibit lipopolysaccharide induced preterm labor in mice. J Perinat Med. 2002;30:297–300. doi: 10.1515/JPM.2002.043. [DOI] [PubMed] [Google Scholar]
- 32.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy. 2013;2013:412831. doi: 10.1155/2013/412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Ray I, Mace G, Sediki M, et al. Changes in maternal blood inflammatory markers as a predictor of chorioamnionitis: a prospective multicenter study. Am J Reprod Immunol. 2015;73:79–90. doi: 10.1111/aji.12323. [DOI] [PubMed] [Google Scholar]
- 34.Oros D, Strunk M, Breton P, et al. Altered gene expression in human placenta after suspected preterm labour. Placenta. 2017;55:21–28. doi: 10.1016/j.placenta.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Szukiewicz D. Cytokines in placental physiology and disease. Mediators Inflamm. 2012;2012:640823. doi: 10.1155/2012/640823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Tu J, Jiang Y, Zhou J, Yabe S, Schust DJ. Effects of lipopolysaccharide on human first trimester villous cytotrophoblast cell function in vitro. Biol Reprod. 2016;94:33. doi: 10.1095/biolreprod.115.134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfs TG, Derikx JP, Hodin CM, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16:68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
- 38.Wolfs TG, Kramer BW, Thuijls G, et al. Chorioamnionitis-induced fetal gut injury is mediated by direct gut exposure of inflammatory mediators or by lung inflammation. Am J Physiol Gastrointest Liver Physiol. 2014;306:G382–G393. doi: 10.1152/ajpgi.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003;1:119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gayle DA, Beloosesky R, Desai M, Amidi F, Nunez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1024–R1029. doi: 10.1152/ajpregu.00664.2003. [DOI] [PubMed] [Google Scholar]
- 41.Redline RW. Placental inflammation. In: Keeling JW, Khong TY, editors. Fetal and Neonatal Pathology. London, UK: Springer London; 2007. pp. 90–101. [Google Scholar]
- 42.Vermillion MS, Lei J, Shabi Y, et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun. 2017;8:14575. doi: 10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elgin TG, Kern SL, McElroy SJ. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin Ther. 2016;38:706–715. doi: 10.1016/j.clinthera.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers. J Reprod Immunol. 2014;104–105:12–19. doi: 10.1016/j.jri.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]