Abstract
Regulatory T (Treg) cells are found at elevated densities in many human cancers, and are thought to be a major barrier to the generation of robust anti-tumor T cell responses. Here, we review recent advances in the understanding of tumor-associated Treg cell diversity and function. Emerging evidence indicates that the transcriptional program of Treg cells infiltrating human cancers may represent a composite program blending a tissue-associated expression signature with an additional tumor-specific signature common to Treg cells from multiple cancer types. Studies in mouse models have defined unique molecular pathways required for Treg cell function in the tumor context that can be manipulated to selectively dampen intratumoral Treg cell activity. Finally, an expanding body of work has revealed diverse functions for Treg cells in non-lymphoid tissues that are unrelated to immune suppression, suggesting a need to explore functions of intratumoral Treg cells beyond the regulation of anti-tumor immunity.
INTRODUCTION
The development and progression of cancer can be profoundly impacted by tumor cell-extrinsic factors such as cells of the immune system, which are thought to either promote or restrict tumor progression in different contexts (1). Many human tumors contain immune cells localized diffusely or clustered within distinct regions, indicative of ongoing inflammatory reactions or anti-tumor immune responses. Regulatory T (Treg) cells expressing the transcription factor Foxp3 are common protagonists in these reactions, and are often found at elevated densities in tumor lesions relative to lymphoid and non-lymphoid sites. Treg cells throughout the body are essential for the prevention of autoimmunity and the maintenance of immune homeostasis, and function by suppressing the activation and differentiation of CD4+ helper T cells and CD8+ cytotoxic T cells reactive to autologous, environmental, or tumor-expressed antigens. Numerous correlative studies have revealed that for some cancers, the density of tumor-infiltrating Treg cells has prognostic significance (2, 3), suggesting that Treg cells may have a functional impact on tumor development and progression. Interestingly, in some cancers such as hepatocellular carcinoma, a high Treg cell density is predictive of poor clinical outcome, consistent with the paradigm that Treg cells promote tumor progression by suppressing tumor-specific T cell responses. In contrast, a high Treg cell density is predictive of improved clinical outcome in other cancers such as colorectal carcinoma. While the precise mechanisms driving this association are undefined, it has been proposed that the favorable effect of Treg cells in colorectal carcinoma may reflect a role for Treg cells in suppressing tumor-promoting inflammation in response to gut microbes (4). These disparate findings suggest that the role of Treg cells in shaping tumorigenesis may be highly context-dependent, varying considerably at different organ sites.
Given the pivotal role of Treg cells in immune suppression and the prevalence of these cells in many human cancers, it is thought that Treg cells constitute a major barrier to therapeutic efforts to mobilize the immune system to induce tumor regression. This idea has spurred concerted efforts to develop modalities to enhance cancer immunotherapies by inducing the selective depletion or modulation of intratumoral Treg cells, while simultaneously leaving Treg cells elsewhere in the body unaffected. In this Brief Review, we highlight recent studies that advance our understanding of tumor-associated Treg cell biology and reveal potential paths for the selective manipulation of these cells. First, we discuss evidence suggesting that therapeutic antibodies specific for T cell-expressed receptors such as CTLA-4 may function in part by inducing the specific depletion of intratumoral Treg cells. We then review recent surveys of Treg cells isolated from human tumors, which suggest that intratumoral Treg cells are broadly imprinted by the tissue microenvironment, but also express a conserved tumor-specific signature that may be common to intratumoral Treg cells from multiple cancer types. Next, we discuss work indicating that intratumoral Treg cells require unique molecular programs to function and thrive within tumor lesions, and that these programs can be selectively perturbed to modulate intratumoral Treg cell activity in preclinical animal models. Finally, we discuss mounting evidence that Treg cells resident in non-lymphoid organs can function to regulate diverse processes such as tissue homeostasis, repair, and metabolism, and speculate about the potential implications of these findings on our understanding of tumor-associated Treg cells. We conclude by highlighting critical gaps in knowledge in the field and outlining future inquiries needed to gain a more complete understanding of intratumoral Treg cells at different organ sites.
Do “checkpoint blockade” antibodies function by depleting intratumoral Treg cells?
In the past decade, antibodies specific for the T cell co-inhibitory receptors CTLA-4 and PD-1 have shown striking success in inducing durable clinical benefit in a fraction of cancer patients spanning a variety of cancer types (5). Early in their development, these antibodies were dubbed “checkpoint blockade” antibodies based on the idea that they were thought to function by blocking the binding of CTLA-4 or PD-1 to their ligands, thereby releasing tumor-specific T cells from checkpoints limiting their activation and effector function (6). However, recent work has challenged this idea, suggesting that some of these antibodies may function instead by inducing the depletion of CTLA-4-expressing cells in the tumor environment by binding to target cells and inducing antibody-dependent cellular cytotoxicity (ADCC) (7, 8). Given that Treg cells generally express high amounts of cell-surface CTLA-4, PD-1, and other immunomodulatory receptors such as OX40, 4-1BB, and ICOS (discussed below), this suggests that therapeutic antibodies targeting these proteins may function in part by driving the selective depletion of intratumoral Treg cells.
In support of this idea, Simpson and colleagues demonstrated in mice that administration of distinct anti-CTLA-4 antibody clones induced the depletion of intratumoral Treg cells without impacting intratumoral conventional T cells or Treg cells outside of the tumor, resulting in slowed outgrowth of transplantable melanomas (9). Interestingly, the efficacy of these antibodies was dependent on the presence of tumor-infiltrating myeloid cells expressing activating Fc receptors, implying a role for Fc receptor-dependent, ADCC-mediated Treg cell depletion. In addition, the authors suggested that the selectivity of anti-CTLA-4 antibody in driving the preferential depletion of Treg cells was largely due to the elevated cell-surface expression of CTLA-4 by intratumoral Treg cells relative to conventional T cells within the tumor, a concept that may be broadly relevant for therapeutic antibodies targeting other T cell-expressed receptors. Working in parallel, Selby and colleagues performed similar studies in mice using an anti-CTLA-4 antibody clone of fixed antigen-binding specificity in which the constant region of the antibody was changed to different isotypes known to engage activating Fc receptors with varying affinities (10). It was found that the therapeutic efficacy of anti-CTLA-4 antibody treatment correlated with the ability of the Fc region to bind activating Fc receptors, again suggesting a role for ADCC in determining in vivo antibody activity. These two studies highlight the importance of both the Fab and Fc regions of an antibody in determining the therapeutic efficacy of an antibody of interest. Consistent with these findings, additional studies in mice have demonstrated similar requirements for therapeutic antibodies targeting other T cell-expressed co-receptors such as GITR (11, 12) and OX40 (13), for which specific antibodies are currently under clinical development for the treatment of human cancer (7).
Despite clear mechanistic evidence in mice that antibodies can promote tumor rejection by inducing intratumoral Treg cell depletion, the relevance of these concepts to the efficacy of antibody-based immunotherapies in human cancer patients remains undefined. The clearest available evidence in support of this idea comes from the studies of Romano et al., which compared samples from human melanoma patients who did or did not respond to therapy using the anti-CTLA-4 antibody ipilimumab (14). In ex vivo assays, it was found that non-classical monocytes expressing the activating Fc receptor CD16 can engage ipilimumab and induce ADCC-mediated lysis of Treg cells in vitro. Importantly, clinical responses to ipilimumab were associated with Treg cell depletion in tumor lesions, as well as elevated densities of non-classical monocytes in the peripheral blood at baseline compared to non-responders (14), suggestive of ADCC-dependent intratumoral Treg cell ablation. Taken together, the studies above support the idea that antibodies specific for T cell-expressed receptors may function in part by inducing the selective elimination of tumor-infiltrating Treg cells in circumstances in which the antibody isotype can avidly engage activating Fc receptors, and Fc receptor-expressing myeloid cells are present at sufficient densities.
The idea that intratumoral Treg cell depletion may underlie the efficacy of therapeutic antibodies in some settings highlights the functional importance of Treg cells in the tumor context. Moreover, the notion that antibodies that had previously been thought of as “blocking” reagents may function in some contexts by inducing ADCC has revitalized enthusiasm for older strategies for therapeutic Treg cell ablation. For example, an attractive marker for Treg cell targeting is the IL-2 receptor alpha chain CD25; relative to conventional T cells, Foxp3+ Treg cells express high amounts of CD25 (15), and CD25 expression is required for optimal Treg cell survival and fitness (16, 17). However, previous studies in mice and humans have failed to demonstrate consistent activity of anti-CD25 antibodies in the tumor context (15). To re-examine this phenomenon, Arce Vargas et al. demonstrated in mice that administration of a common anti-CD25 antibody clone reduced Treg cell frequencies in the blood and secondary lymphoid organs (SLOs) but failed to induce intratumoral Treg cell depletion (15). In contrast, use of a variant of this clone engineered to preferentially engage activating Fc receptors (at the expense of binding to inhibitory Fc receptors) induced robust intratumoral Treg cell depletion and enhanced anti-tumor immunity (15). These findings provide additional evidence that the design of antibody Fc regions is a key determinant of biological activity and in vivo efficacy of antibody therapeutics, and suggests a need to re-evaluate antibodies that had previously been deemed to be ineffective in the tumor setting. Moreover, the collective findings discussed above suggest that antibodies targeting different T cell-expressed cell-surface receptors may function by a common mechanism - binding avidly to Treg cells and marking these cells for elimination by ADCC (7).
Analysis of tumor-infiltrating Treg cells in human cancers
Beyond CTLA-4, CD25, and other well-defined immunomodulatory receptors, an improved understanding of the unique features of tumor-infiltrating Treg cells may provide key insight into the function of these cells in the tumor context, and reveal novel targets for the selective modulation of Treg cells for therapeutic benefit. In this light, three recent papers report high-resolution surveys of Treg cells infiltrating human cancers. In one study, Plitas et al. performed transcriptional and phenotypic analysis of Treg cells isolated from breast cancer lesions, and compared these to Treg cells isolated from normal breast parenchyma or peripheral blood (18). The authors found that breast tumors contained elevated percentages of Treg cells relative to normal breast tissue, and that intratumoral Treg cells were highly proliferative and expressed high amounts of CD25, CTLA-4, and PD-1 proteins. RNA sequencing of purified cell populations revealed that the transcriptional profiles of intratumoral Treg cells and Treg cells from normal breast tissue were very similar, suggesting that much of the transcriptional program of breast cancer-infiltrating Treg cells may be associated with residency in mammary tissue. The analysis also revealed that CCR8, a receptor for chemokines such as CCL1 and CCL18, was selectively expressed by both intratumoral Treg cells and Treg cells in normal breast parenchyma, suggesting that CCR8-dependent signals may be important for Treg cell recruitment, positioning, or function at these sites. Notably, the authors demonstrated that CCR8 protein was also expressed by intratumoral Treg cells from human colorectal carcinoma (CRC), melanoma, and lung adenocarcinoma samples, suggesting that CCR8 may be relevant in multiple cancer types.
In parallel studies, De Simone and colleagues analyzed bulk Treg cells and CD4+ T helper cell subsets isolated from CRCs and non-small-cell lung cancers (NSCLC), enabling a direct comparison of the transcriptomes of Treg cells from cancers originating at different organ sites (19). Interestingly, this revealed a set of transcripts that were preferentially upregulated by Treg cells in both CRC and NSCLC compared to Treg cells and conventional T cells from normal non-lymphoid tissues and blood. Single-cell analysis of intratumoral Treg cells using quantitative PCR confirmed that many of these signature genes were also upregulated in Treg cells isolated from other cancer types, including breast cancer, gastric cancer, and metastases of NSCLC and CRC. More recently, Zheng and colleagues performed single-cell RNA sequencing of T cells isolated from the blood, tumor, and normal tissue of patients with hepatocellular carcinoma (20). Transcriptional analysis of single FOXP3-expressing Treg cells identified ~400 genes that were preferentially upregulated by intratumoral Treg cells relative to Treg cells isolated from normal adjacent tissue. Importantly, Zheng et al. performed a meta-analysis and noted that 31 genes in the intratumoral Treg cell signature were identified in the three human studies highlighted here, as well as a fourth study of cells isolated from human melanomas (21), indicating that intratumoral Treg cells isolated from different cancer types may express a conserved “tumor specific” Treg cell signature. This signature includes many genes encoding well characterized immunomodulatory cell-surface receptors, including CTLA-4, GITR, 4-1BB, TIGIT, OX40, ICOS, and CD27. Given that these markers are also expressed by a fraction of Treg cells throughout the body, these proteins may represent the “usual suspects” that appear in the conserved tumor-specific Treg cell signature based on upregulation in the tumor environment. Importantly, the conserved 31-gene signature also includes genes encoding proteins not previously implicated in tumor-infiltrating Treg cell biology, such as MAGEH1 (encoding a type II MAGE family protein of unknown function), IL1R2 (encoding a decoy receptor for IL-1), TFRC (encoding a transferrin receptor), FCRL3 (encoding an Fc receptor-like protein), and CCR8. It remains unknown whether these proteins are important for Treg cell function in the tumor environment, or reflect adaptation required to survive and thrive within neoplastic lesions.
Beyond the characterization of transcriptional profiles, the single-cell RNA sequencing studies of Zheng et al. provided a unique glimpse into Treg cell clonality within human tumors. The investigators were able to determine αβTCR sequences for the majority of single Treg cells, which revealed that a substantial fraction of intratumoral Treg cells expressed αβTCRs that were found recurrently in a given tumor. In contrast, Treg cells isolated from peripheral blood and intratumoral conventional T cells exhibited diverse αβTCR sequences with few recurrent clones. In accordance with this, Plitas et al. utilized βTCR sequencing of bulk T cell populations to demonstrate that breast cancer-infiltrating Treg cells exhibited reduced clonal diversity relative to naive-phenotype peripheral Treg cells (18). These findings provide direct evidence of expansion or enrichment of distinct Treg cell clones in human tumors, a finding that is consistent with previous work in mice suggesting that developing tumors drive the enrichment of oligoclonal Treg cell populations (22).
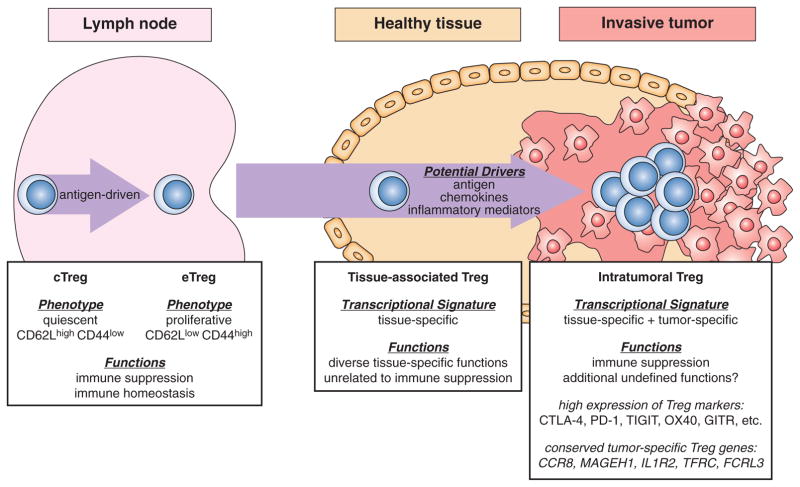
Taken together, these studies demonstrate that in the human cancers analyzed thus far, the transcriptional program of tumor-infiltrating Treg cells may represent a composite signature blending a tissue-specific signature associated with the organ of cancer origin with an additional tumor-specific signature that may be common to Treg cells from many cancer types (Figure 1). Theses findings pave the way for future mechanistic studies probing the functional role of these pathways in coordinating intratumoral Treg cell activity, and determining the feasibility of targeting these pathways using antibodies or small-molecule agents.
Figure 1. Hallmarks of tumor-infiltrating Treg cells.
Conceptual model highlighting the phenotypic and functional diversity of Treg cells in lymphoid organs, non-lymphoid tissue sites, and tumor lesions. Central Treg cells (cTregs) and effector Treg cells (eTregs) in the lymph nodes and spleen are thought to mediate the suppression of autoimmune reactions and the maintenance of immune homeostasis. cTreg cells are characterized by a quiescent “naive” phenotype, and can differentiate into activated-phenotype eTreg cells in an antigen-dependent process. Tissue-associated Treg cells present in different non-lymphoid sites exhibit tissue-specific transcriptional programs unique to the tissue of residence, and can mediate diverse context-dependent functions that are independent of immune suppression. The mechanisms driving Treg cell enrichment and expansion within tumors are unknown, but may involve responsiveness to antigen, chemokines, and inflammatory mediators. Intratumoral Treg cells are characterized by the following hallmarks: 1) evidence of oligoclonal expansion; 2) a composite transcriptional signature that blends a tissue-specific Treg cell signature associated with the tissue of cancer origin with a conserved tumor-specific signature that is common to Treg cells from different cancer types; 3) high expression of common Treg markers known to function as immunomodulatory receptors, including CTLA-4, PD-1, TIGIT, OX40, and GITR; 4) expression of transcripts encoding proteins with undefined roles in intratumoral Treg cell biology, including CCR8, MAGEH1, IL1R2, TFRC, and FCRL3. Beyond suppression of anti-tumor T cell responses, additional functions of intratumoral Treg cells have yet to be clearly defined. The arrow suggests a precursor-product relationship, which has been defined for the cTreg-to-eTreg transition, but remains hypothetical for tissue-associated and intratumoral Treg cell populations.
Intratumoral Treg cells exhibit unique requirements that may enable selective targeting or modulation
In recent years, there has been a growing appreciation that Treg cells exhibit substantial phenotypic and functional diversity (Reviewed extensively by others (23–25)). In the SLOs, Treg cells can be broadly categorized as quiescent, naïve-phenotype “central” Tregs (cTregs), or highly proliferative “effector” Tregs (eTregs) exhibiting an activated phenotype (26, 27) (Figure 1). Conditional deletion of the TCR on Foxp3-expressing cells blocks the differentiation of cTregs into eTreg cells and leads to the development of autoimmunity, indicating that eTreg differentiation is required for Treg-mediated immune suppression (28, 29). In addition, eTreg cells in the SLOs exhibit specialized transcriptional programs associated with common T helper cell differentiation pathways such as Th1, Th2, Th17, and Tfh programs, and in some cases, adoption of these programs by Treg cells is essential for the regulation of inflammatory responses. Beyond Treg cell diversity in the SLOs, as discussed below, it is now evident that additional differentiation steps are required for the trafficking, function, and fitness of Treg cells infiltrating non-lymphoid organs and inflammatory sites. Building from these concepts, the demonstration that Treg cells infiltrating human tumors express unique transcriptional signatures suggests that Treg cells may have distinct requirements to function and thrive in the unique inflammatory milieus associated with cancer, including tissue remodeling, angiogenesis, hypoxia, and altered metabolic states (1). This raises the possibility that unique requirements of intratumoral Treg cells, if identified, could be targeted to specifically modulate tumor-infiltrating Treg cells without impacting Treg cells elsewhere in the body. As discussed below, recent advances have identified such pathways and provided functional evidence in support of this idea.
Some of the first work demonstrating this concept came from studies examining the role of neuropilin-1 (Nrp1) expression by Treg cells. Nrp1 is a type I transmembrane protein that is highly expressed by Treg cells and has been suggested to play a key role in Treg cell function (30, 31). Nrp1 functions as a receptor for both vascular endothelial growth factor (VEGF), a critical regulator of blood vessel formation, and semaphorin-4a (Sema4a), a cell-surface protein that has well-defined roles in axonal guidance but is also expressed by dendritic cells, B cells, and T cells. Work from Hansen and colleagues demonstrated that Nrp1-expressing Treg cells migrate in response to tumor-derived VEGF in vitro, and showed that Treg-specific deletion of Nrp1 slowed the outgrowth of transplantable and autochthonous tumors without inducing systemic autoimmunity, suggesting a role for Nrp1 expression on tumor-associated Treg cells (32). Although a direct connection between VEGF production and Treg-specific Nrp1 function has yet to be demonstrated in vivo, these findings raise the intriguing possibility that Nrp1 may play a key role in coordinating Treg cell responsiveness to VEGF-dependent angiogenic signals in the tumor environment. In parallel studies, work from Vignali and colleagues demonstrated that Nrp1 functions as a receptor for Sema4a on Treg cells, and revealed a key role for Nrp1 ligand engagement in maintaining Treg cell functionality within tumors (33, 34). Using mutant mice in which Nrp1 is conditionally deleted on Treg cells, they showed that Nrp1 expression was dispensable for Treg-mediated maintenance of systemic tolerance and immune homeostasis (33). However, challenge of Nrp1 mutant mice with transplantable B16 melanomas revealed slower tumor outgrowth and enhanced anti-tumor T cell responses, consistent with the findings of Hansen et al. Blockade of the Sema4a-Nrp1 axis using monoclonal antibodies significantly slowed tumor growth, indicating that transient Nrp1 blockade can have measurable effects. Mechanistic analysis showed that tumor-infiltrating Treg cells in Nrp1 mutant mice maintained normal levels of Foxp3 expression, but exhibited a dysregulated transcriptional program and aberrant production of cytokines such as IFN-γ (34). Interestingly, this effect was dominant, in that conditional deletion of Nrp1 on 50% of Treg cells induced T cell-intrinsic defects but also led to the dysregulation of the remaining Nrp1-expressing intratumoral Treg cells (34). Together, these studies in mice demonstrate that disruption of Nrp1 expression by Treg cells specifically affects Treg cell activity in the tumor environment without impacting Treg cell function elsewhere in the body, revealing the existence of a distinct pathway that can be specifically targeted to modulate intratumoral Treg cell activity. Further work will be needed to determine whether Nrp1 signaling plays a key role in Treg cell function in human cancers, and to assess the relative contributions of VEGF and Sema4a-signals in coordinating the activity of Nrp1-expressing Treg cells in the tumor environment.
In other areas of inquiry, Luo et al. demonstrated that the transcription factor Foxo1 antagonizes the eTreg transcriptional program, and must be downregulated for proper eTreg differentiation and tumor infiltration (35). Using mutant mice in which Foxo1 expression in Treg cells is resistant to downregulation, the authors observed that the homozygous mutant mice exhibited defects in eTreg differentiation associated with decreased Treg cell trafficking to non-lymphoid organs, resulting in autoimmune reactions mediated by CD8+ T cells. Moreover, the outgrowth of transplantable and genetically driven tumors was slowed in homozygous mutant mice, indicative of impaired Treg cell activity and enhanced anti-tumor immunity. Interestingly, the investigators also identified a “sweet spot” in which heterozygosity of the constitutively-activate Foxo1 mutant allele led to enhanced anti-tumor immunity without inducing widespread autoimmunity. Thus, this study identified Foxo1 activity as a key determinant of the differentiation of eTregs, and demonstrated that this axis can be modulated genetically to restrict the trafficking and fitness of intratumoral Treg cells without altering Treg-mediated immune regulation in the SLOs. Given that Foxo1 is an intracellular transcription factor that is widely expressed by many cell types and functions downstream of the PI3K-Akt pathway, it is unclear whether the “tuning” of Foxo1 activity in genetic mouse models can be recapitulated using small-molecule drugs targeting this pathway. Regardless, these data reveal that subtle perturbations in Treg cell signaling can induce major functional changes that dissociate Treg cell function in the tumor environment from Treg cell activity elsewhere in the body.
Collectively, these studies demonstrate that in mice, intratumoral Treg cells exhibit unique requirements for proper function and fitness in the tumor context, and show that these requirements are divergent from the functional requirements of Treg cells tasked with preventing autoimmunity and maintaining immune homeostasis in the SLOs. Moving forward, it is possible that the intratumoral Treg cell signatures identified in the studies discussed above may help illuminate molecular pathways required for Treg cell activity in human cancers, providing new opportunities to selectively target intratumoral Treg cells while maintaining systemic immune regulation.
Beyond immune suppression: newly defined functions of Treg cells in non-lymphoid tissues
In recent years, a growing number of studies have revealed unique functions of Foxp3+ Treg cells in non-lymphoid sites that appear to be independent of their well-defined roles in suppressing adaptive immunity and maintaining immune homeostasis. While the relevance of these functions to tumor-associated Treg cells is highly speculative at this time, these findings expand our view of Treg cell functional diversity, suggest that the functions of intratumoral Treg cells may be manifold and context-dependent, and force a re-visitation of the paradigm that the primary function of tumor-infiltrating Treg cells is to suppress anti-tumor immunity.
In seminal work, Mathis and Benoist demonstrated that Treg cells play a key role in regulating obesity-related inflammation of the visceral adipose tissue (VAT) (36–38). The investigators showed that the densities of Treg cells in the VAT are greatly diminished in insulin-resistant mouse models of obesity, and that sustained expansion of Treg cells induced normalization of blood glucose levels in mice fed a high fat diet, indicating that Treg cells can affect adipose-associated inflammation and metabolic function. The investigators used transcriptional profiling to reveal that VAT Treg cells preferentially express an array of transcripts not typically expressed by Treg cells at other sites, and showed that at least two of these factors are critical for the optimal accumulation of Treg cells in VAT. First, it was shown that PPAR-γ, a receptor that is important for adipocyte differentiation, is highly expressed by VAT Treg cells and is required for Treg cell enrichment in adipose tissue. Second, it was shown that the majority of VAT Treg cells express the IL-33 receptor ST2, and that Treg cell density in the VAT was diminished in ST2-deficient mice. Notably, IL-33 has been shown to function as an “alarmin” that triggers inflammatory responses when released from stressed or dying cells (39), suggesting a role for the IL-33/ST2 axis in driving the homing and/or expansion of Treg cells at sites of tissue damage. Together, these studies laid the groundwork for a paradigm suggesting that Treg cells at non-lymphoid sites mediate functions that are independent of immune suppression, express unique gene expression profiles that are required for optimal function and fitness in the local environment, and are responsive to antigen-independent inflammatory mediators (25).
In other areas, additional work has defined a unique role for Treg cells in promoting tissue repair in non-lymphoid organs (40, 41), and identified Amphiregulin (Areg) as a key factor produced by Treg cells at sites of tissue damage. In one study, Burzyn and colleagues demonstrated that clonally expanded Treg populations accumulate in acutely injured muscle tissue and in the damaged muscle of Dmd mutant mice, a mouse model of muscular dystrophy. Transient depletion of Treg cells impaired muscle repair, and was associated with increased cellular infiltrates, increased fibrosis, and a failure of myeloid cells to switch to a pro-regenerative phenotype. As with VAT-associated Treg cells, muscle-infiltrating Treg cells exhibited a unique transcriptional program that was distinct from that of splenic Treg cells. One of the genes preferentially expressed by muscle-infiltrating Treg cells was the gene encoding Areg, an epidermal growth factor (EGF) family ligand that signals through the EGF receptor system. Treg cells were found in close proximity to regenerating muscle myofibers in situ, and promoted the differentiation of muscle satellite cells in vitro, suggesting that Treg cells may function to promote muscle regeneration in part by direct communication with muscle progenitor cells. In a separate study, Arpaia and colleagues generated mice with Treg-specific deletion of Areg, and examined the impact of this deficiency on immune regulation and tissue repair in the lung in the context of an ongoing viral infection (41). Notably, the authors found that Areg expression by Treg cells was dispensable for the maintenance of immune homeostasis and the regulation of virus-specific T cell responses. In contrast, Areg mutant mice exhibited increased tissue damage and a rapid decline in lung function following influenza virus infection, demonstrating that Treg cells restrict tissue damage via Areg production in this setting. Additional mechanistic data revealed that Areg production by Treg cells in vitro was triggered by exposure to IL-18 and IL-33, but not by TCR stimulation, suggesting that TCR-independent cues in the microenvironment may be the primary drivers of Areg production by Treg cells. Taken together, these findings raise the intriguing possibility that a key function of intratumoral Treg cells in lung cancers and other solid cancers may lie in regulating tissue remodeling and repair through the production of Areg and other factors. Notably, the studies of Plitas et al. revealed that Areg is significantly upregulated by a subset of intratumoral Treg cells in breast cancer lesions (18), lending credence to this idea.
Finally, a recent study by Ali et al. further expanded the newly defined roles of tissue-associated Treg cells, demonstrating a pivotal role for skin-resident Treg cells in promoting the differentiation of hair follicle stem cells (HFSCs) (42). The authors demonstrated that Treg cells are localized in close proximity to the HFSC niche within hair follicles, and that inducible ablation of Treg cells suppressed hair growth. Interestingly, it was also shown that Treg cell expression of Jagged1 (Jag1), a Notch ligand that is highly expressed by Treg cells at this site, was required for optimal HFSC proliferation and induction of the active growth stage of hair follicle formation. Thus, these findings suggest a model in which skin-associated Treg cells function at steady state to promote hair follicle development via engagement of Notch receptors on HFSCs. Interestingly, Notch pathway activation or inhibition has been implicated as a driver of tumorigenesis in some human malignancies, including melanoma (43–45). These concepts raise the possibility that Treg cells may shape cancer development by direct cell-cell crosstalk with cancer cells or cancer stem cells via Jag1-Notch interactions.
Collectively, the studies discussed above highlight our expanding view of the functional diversity and specialization of Treg cells in distinct tissue environments throughout the body, and reveal broad principles regarding Treg cell biology in non-lymphoid tissues (Figure 1). Specifically, tissue-associated Treg cells can respond to antigen-independent inflammatory mediators, and express unique gene expression profiles that are required for optimal function and survival in the local environment. From a functional standpoint, tissue-associated Treg cells exhibit diverse functions that are independent of immune suppression; these functions may include conserved mechanisms such as the augmentation of tissue repair via Areg production, or specialized tissue-specific functions such as metabolic regulation or stimulation of stem cell differentiation. The finding that the transcriptional programs of intratumoral Treg cells from various human cancer types are similar to the programs of their Treg cell counterparts in normal adjacent tissue suggests that the principles defined for tissue-associated Treg cells may be directly relevant for Treg cell activity in the tumor context. This suggests that the functions of intratumoral Treg cells may be diverse and highly context-dependent, and highlights a key need to examine additional functions of tumor-infiltrating Treg cells beyond their defined roles in the suppression of anti-tumor immunity.
CONCLUSIONS
Evidence that intratumoral Treg cell depletion may underlie the clinical efficacy of therapeutic antibodies such as anti-CTLA-4 serves as a testament to the functional importance of Treg cells in the tumor context, and provides the impetus for continued efforts to understand the fundamental biology of Treg cells and the functional diversity of these cells in different cancers. Given that many current and emerging therapeutic antibodies target cell-surface receptors that are expressed at high amounts by tumor-infiltrating Treg cells, it will be critical to determine the extent to which these antibodies function by direct action on Treg cells, either through modulation of Treg cell activity or through ADCC-mediated Treg cell depletion. In this regard, it will be interesting to compare the clinical efficacy of antibodies bearing isotypes that vary with respect to ADCC triggering (7). Such mechanistic understanding may be crucial for identifying patients who are likely to benefit from treatment, versus those who are not. In addition, for cancers such as CRC in which Treg cells may play a key role in restricting cancer progression, if a given antibody is shown to directly dampen intratumoral Treg cell activity, it may be unwise to utilize such antibodies in these clinical contexts.
Moving forward, mechanistic experiments in tractable mouse models of autochthonous cancer and human cell culture systems are needed to elucidate the role of key molecular pathways implicated in intratumoral Treg cell biology. For example, the human studies discussed above demonstrated that CCR8 is upregulated by tumor-infiltrating Treg cells in multiple cancer types, suggesting that CCR8-dependent signals may be important for Treg cell homing, positioning, or function within tumor lesions. However, given that there are additional chemokine receptors expressed by tumor-associated Treg cells (18), and considerable redundancy in many chemokine-receptor axes, further investigation will be needed to determine the requirement for CCR8 in intratumoral Treg cell activity. Furthermore, CCR8 expression is not unique to tumor-infiltrating Treg cells, but is also expressed by tumor-infiltrating NKT cells and a fraction of Treg cells resident in some healthy tissues (18). Future studies in preclinical cancer models can be used to gauge the feasibility of targeting CCR8 to either dampen intratumoral Treg cell function or induce the selective depletion of these cells.
Finally, an undefined facet of intratumoral Treg cell biology lies in understanding the cellular partners that Treg cells interface with in the tumor environment. Do Treg cells directly interact with tumor cells or cancer progenitors cells, or is their activity limited to interactions with antigen-presenting cell populations such as dendritic cells or macrophages? Several imaging-based studies have demonstrated direct Treg cell interactions with antigen-bearing dendritic cells within transplantable and genetically engineered murine tumors (46–48). However, given emerging data indicating that Treg cells may be responsive to antigen-independent environmental cues within non-lymphoid tissues, it will be important to determine whether additional antigen-independent liaisons take place in distinct regions of a tumor. Multi-color immunofluorescence analysis of cellular positioning in human tumor samples and mechanistic loss-of-function experiments in mice may provide insight into these questions.
Acknowledgments
We would like to thank Daniel Leventhal for critical reading of the manuscript.
This work was supported R01-AI126756 (to P.A.S.) and R01-AI110507 (to P.A.S.). J.L.C. was supported by T32 AI007090.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Deleeuw RJ, Kost SE, Kakal JA, Nelson BH. The Prognostic Value of FoxP3+ Tumor-Infiltrating Lymphocytes in Cancer: A Critical Review of the Literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 3.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune Modulation in Cancer with Antibodies. Annual review of medicine. 2013 doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 6.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends in immunology. 2014;35:290–298. doi: 10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.DiLillo DJ, Ravetch JV. Fc-Receptor Interactions Regulate Both Cytotoxic and Immunomodulatory Therapeutic Antibody Effector Functions. Cancer Immunol Res. 2015;3:704–713. doi: 10.1158/2326-6066.CIR-15-0120. [DOI] [PubMed] [Google Scholar]
- 9.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 11.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 14.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P, Speiser DE. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A, Ben Aissa A, Franz D, Werner Sunderland M, Wong YNS, Henry JY, O’Brien T, Nicol D, Challacombe B, Beers SA, Melanoma TC, Renal TC, Lung TC, Turajlic S, Gore M, Larkin J, Swanton C, Chester KA, Pule M, Ravetch JV, Marafioti T, Peggs KS, Quezada SA. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 17.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 18.Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJ, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell. 2017;169:1342–1356. e1316. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, Savage PA. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 31.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, Helfrich I. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-gamma Drives Treg Fragility to Promote Anti-tumor Immunity. Cell. 2017;169:1130–1141. e1111. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529:532–536. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. 2017;198:1395–1402. doi: 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- 40.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, Rosenblum MD. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell. 2017;169:1119–1129. e1111. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 44.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Fukunaga-Kalabis M, Li L, Herlyn M. Developmental pathways activated in melanocytes and melanoma. Arch Biochem Biophys. 2014;563:13–21. doi: 10.1016/j.abb.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425–2440. doi: 10.1172/JCI66375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, Robbins R, Crowley DM, Bronson RT, Jacks T. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep. 2017;20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]