Abstract
A paradigm shift in drug delivery systems have been noted recently. The focus nowadays is to obtain maximum benefit with lower side effects. It is a monetary burden to launch newer molecules hence the industry is concentrating on improving the efficacy of existing molecules. Thus controlled release, target controlled infusion and closed loop infusion have entered the scene. Applying pharmacokinetic principles, instead of mathematically calculating drug dose could improve safety and maintain steady drug levels in the body. When computers are applied to an efficient operating system, it will only magnify the efficiency. Most of these technologies which were earlier limited to research only have entered clinical practice. This has made it mandatory for the practicing clinician to familiarize themselves with these technologies. Our focus in this review has been to discuss newer drug delivery systems available for anesthesiology practice.
Keywords: Controlled released, newer drug delivery system, target control release
Introduction
Drug delivery systems (DDSs) are developed to deliver the required amount of drugs effectively to appropriate target sites and to maintain the desired drug levels. Research in newer DDS is being carried out in liposomes, nanoparticles, niosomes, transdermal drug delivery, implants, microencapsulation, and polymers.
Advantages of drug delivery system
Increases bioavailability
Can be used for long-term treatments of chronic illness
Sustained maintenance of plasma drug levels
Decrease in the total amount of drugs required thus reducing side effects
Improved patient compliance due to reduction in number and frequency of doses required
There is less damage sustained by normal tissue due to targeted drug delivery
Reduction in cost by developing newer delivery systems for existing molecules.[3,4]
Alternate Route of Drug Delivery
Intranasal drug delivery
In addition to being convenient and painless, there is no reduction in bioavailability of drugs administered nasally.[5] Direct deliveries to the cerebrospinal fluid due to nose-brain pathway reduce the onset time. Highly lipophilic drugs of low molecular weight easily cross the nasal mucosa.[6,7,8,9] It does not require coupling with carrier nor any modification of therapeutic agent.[5] To avoid runoff, 0.25–0.3 ml of concentrated drug per nostril is used. One of the limitations is that in patients with bloody nose or increased mucus production there may be decreased absorption. Intranasal drug takes about 3–5 min to be absorbed, and drug levels achieved rarely cause respiratory depression. However, sufentanil is an exception where toxic levels can reach very rapidly.[10]
Pulmonary drug delivery system
Metered dose inhalers, nebulizers, and dry powder inhalers are used for pulmonary drug delivery.[11] They offer several advantages including a larger surface area and closer proximity to blood flow.[11,12] Lower doses can be used thus avoiding systemic toxicity. However, disadvantages include shorter duration and only 10–40% of the drugs delivered become available for systemic absorption, and to overcome this limitation, nanoparticles have been developed. There has been a constant endeavor to deliver opioids by inhalation route.[13] Bioavailability of fentanyl when administered by this route has been found to be 20%.[14,15] Iloprost, a newer prostacyclin analog used to treat pulmonary hypertension, has a very short half-life requiring frequent dosing regimens. Thus, to improve patient compliance an aerosolized controlled release formulation is an available alternative.[10,16,17] The use of pulmonary DDS using colloidal carrier systems has more physiological components.[18] The drugs used to treat asthma and certain lung infections as well as inhaled insulin are also being developed with newer technology using carriers.[19,20]
Buccal mucosal drug delivery system
In addition to ease of administration, it avoids the first pass effect and presystemic elimination.[21,22] Toxicity or undesired side effects are significantly reduced. Drugs for chronic pain management and breakthrough pains[23] including fentanyl (lozenges, tablets, and films)[24] and buprenorphine hydrochloride (tablets) are available for delivery through this route. The buccal mucosa is less permeable with a large immobile surface which results in slower onset and is more suitable for sustained release preparations, whereas sublingual drug delivery has a more dramatic onset.[25,26,27]
Intra-articular drug delivery
The size of the drug molecule has to be 3–5 μ. The residence time of drugs in intra-articular tissues may be prolonged by microspheres that are designed to improve their uptake by the synovium.[28]
Controlled Release Drug Therapy
Drug impregnated lozenges, nasal and buccal aerosol sprays, and transdermal and transmucosal DDS are the various controlled release formulations available.
Transdermal drug delivery system
Fentanyl, clonidine, glyceryl trinitrate, lisuride, and buprenorphine are available as transdermal preparations. Advantage of this route is, it avoids first pass metabolism and large variations in plasma drug concentrations.[29] Decreased gastrointestinal side effect improves compliance.[3] Constant drug levels are maintained which avoids ups and downs in plasma concentration levels seen with oral and parenteral route.[30] The stratum corneum is the greatest barrier to transport of drugs; hence, drugs need to be lipid soluble and have a low molecular weight.[3,31,32] Factors such as drug permeability, molecular weight, total body clearance, and therapeutic plasma concentrations have to be taken into consideration when calculating transdermal doses.[33] Reservoir patch and matrix patch are the two types of patches [Figure 1].[33,34,35]
Figure 1.
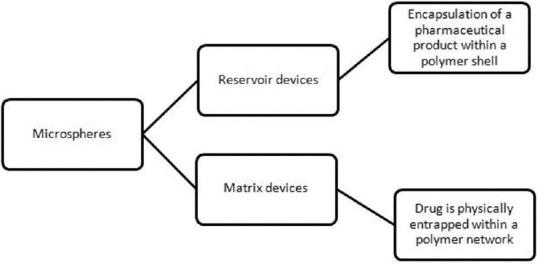
Types of transdermal delivery devices
Fentanyl patches are of the matrix design from which a constant amount of drug is released per unit time. The diffusion occurs at a constant rate in the direction of the lower concentration. After application of patch, the skin under the system absorbs fentanyl, and a depot of fentanyl concentrates in the upper skin layers.[34,36] Plasma fentanyl concentrations gradually increase following initial application. A steady-state serum concentration is reached between 12 and 24 h and remains relatively constant, with some fluctuation, for the remainder of the 72 h application period.[37] By the end of the second 72 h application, serum concentration remains nearly steady and this is maintained during subsequent applications of a patch of the same size. The fentanyl patches are available to deliver the drug at a constant rate of 25, 50, 75, and 100 μg/h spread over a period of 72 h.[38] The elimination half-life after patch removal is 13–22 h due to slow release of the fentanyl from skin depots. Thus, in cases of adverse effects, the patient needs to be monitored for a further period of 24 h after removal of the patch. The most frequent side effects observed are nausea, vomiting, and constipation. Less frequent ones include hypoventilation and rash at application site.[39]
A newer patient-controlled fentanyl transdermal system, the size of a credit card has been designed, which has to be worn on the upper arm or the chest. Iontophoresis is utilized to deliver fixed drug boluses.[40] There is no background infusion and also passive absorption of the drug is negligible.[35,40,41] Ahmad et al. compared intravenous patient-controlled analgesia morphine with fentanyl iontophoretic transdermal system and found the latter to be associated with lesser analgesic gaps.[42,43]
Although the transdermal delivery of drug is the most patient compliant mode of delivery only lipophilic, low molecular weight drugs can be delivered by this route.[44,45] To overcome this shortcoming, second and third generation DDS has been developed. In the second generation, enhancers are incorporated into the DDS, to reversibly disrupt the stratum corneum and thus promote the transfer of lesser lipid soluble drugs. In case of hydrophilic drugs, active energy-dependent methods have been used to drive drug across. This can be achieved using eutectic mixtures of local anesthetics, controlled heat, magnetophoresis, iontophoresis,[46,47] electroporation, or sonophoresis.[48] Using iontophoresis dermal anesthesia with lignocaine can be achieved.[49,50] The effect can be enhanced by adding adrenaline. Controlled release preparations of tramadol and buprenorphine are available. Beta blocker delivered in this way synchronized with continuous Holter monitoring is a novel application.[51]
Targeted Drug Delivery
Conventional DDS may cause reduction in potencies of drug before they reach the target tissues of the body due to partial degradation. Goal of all DDS is to deploy the active pharmaceutical compound to the particular targeted areas of the body. Past decade has witnessed the development of polymeric micelles, microspheres, etc., which effectively lower the systemic drug toxicity, enhance the ability of the drugs to target the specific sites, improve the absorption rates, and retard the biochemical degradation of the drug before reaching the target site.
Liposomes
Liposomes are nanovesicles encapsulated in phospholipid bilayer. They are biodegradable, nontoxic, and nonimmunogenic thus making them favorable carriers for drug delivery. Although major advances of liposomal drugs are in anticancer treatment, efficiency of anesthetic drugs can also be enhanced. Multimodal approach to postoperative pain management is the key to early recovery. One of the major limitations to the array of local anesthetics available is their relative short duration of action and the risk of systemic toxicity. Liposomal bupivacaine is an important advance in the delivery of local anesthetics formulations.[52] Up to 96 h of therapeutically active concentration can be achieved after single administration with delay in peak plasma concentrations.[53] However, phase 3 trials need to be conducted before they can be used in clinical practice for field blocks.[54] Liposomal formulation of epidural morphine DepoDur can provide up to 2 days of analgesia.[55,56] This was also confirmed in a study by Viscusi et al. using extended release morphine.[56] Liposomal encapsulated inhaled fentanyl having onset of action similar to intravenous preparation is also being developed[57] Liposomal drugs though in clinical research since last 50 years have not made much strides in clinical practice due to the various hurdles faced in quality assurance and costs. Furthermore, clinical trials related to them are more complex than conventional molecules.[58]
Transient targeted thromboprophylaxis
Thromboprophylaxis in the immediate postoperative period has always remained a gray area. Bleeding in the postoperative period is a real danger and at the same time thrombosis risk is also highest during this period when the patient is immobile. The novel DDS for thromboprophylaxis includes flow sensitive nanoparticles which are coated with tissue plasminogen activator (tPA) which release it at the site of clot.[59,60,61,62,63]
Postoperative Pain Relief
Local anesthetic drugs can be delivered to the body by various routes.[64] It can be delivered directly into the surgical incision or in the perineural[65] tissues with benefits of improved analgesia, reduced consumption of opioids, and reduced hospital stay [Figure 2]. The concerns regarding wound infections are unwarranted. The drugs can be delivered either using patient-controlled syringe pumps or elastomeric pumps. Patient-controlled syringe pumps are electronic pumps which can deliver the prescribed amount of drug at specific intervals on demand. Elastomeric pumps are nonelectronic medication pumps.[51] It consists of a balloon which deflates at a specified rate and this pushes the drug through the intravenous tubing and then into the catheter. It is available as variable or fixed rate module and can deliver the drug for 12 h to 7 days durations. The driving pressures in these pumps are from 260 to 520 mmHg, and the infusion rates can vary from 0.5 to 500 ml/h.[66] The delivery rates are limited to the set values due to the presence of flow restrictors. The variable rate infusor can be adjusted to deliver 5, 7, or 12 ml/h drug, whereas the fixed infusors are designed to deliver 1.5, 2, 5, 7, and 12 ml/h of the drug volume. The infusor rate is most accurate when the temperature of the infusion can be maintained at 92°F or 33.3°C. Increase in temperature increases the infusor rate.[67,68] To achieve this, infusor should be strapped to the torso. Furthermore, diluents used influence the rate of delivery which is most accurate when 5% dextrose is used. The drug should be filled to the prescribed nominal volume to maintain accuracy. The balloon reservoir and the Luer Lock connector should be adjusted to the same height to ensure delivery of the drug at the desired volume. Their advantages include small size, light weight, not dependent on electrical source. However, disadvantages include costs, inaccurate flow rates,[69,70] and lack of flexibility with flow rates.[71]
Figure 2.
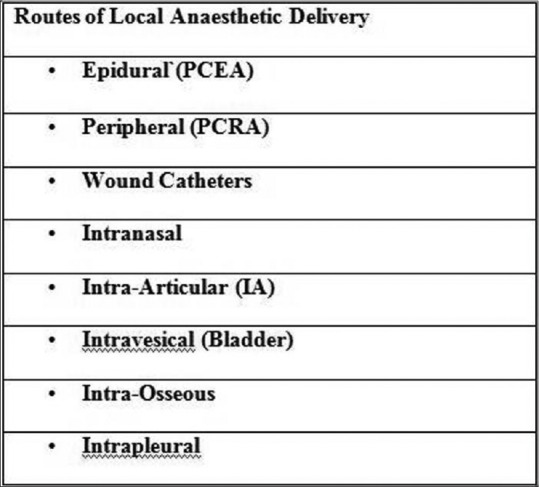
Various routes of local anaesthetic delivery
Computer-Controlled Local Anesthetic Delivery Devices
Computer-controlled local anesthetic delivery devices (C-CLAD) forms important add-ons in dental anesthesia. Pulpal nerve block through the palatal approach can be performed using C-CLAD. The slow local anesthetic infusion rate using this device significantly reduces the discomfort and thus may find a niche in cosmetic and pediatric dentistry.[72]
Computerized Drug Delivery System
Total intravenous anesthesia is becoming the norm due to rising environmental concerns and availability of newer shorter-acting anesthetic drugs. This is further aided with the advent of computerized DDS.[73]
Target-controlled infusion pumps (open-loop)
In the routine practice in drug dose calculations, age, sex, or creatinine clearance is unaccounted for due to complex calculations.[74] The distribution of the drug to the peripheral tissues must be taken into account and only after there is equilibrium with the peripheral tissue can clearance be taken for calculation.
Pharmacokinetic simulation of certain opioids has shown that initial boluses usually result in drug concentrations far in excess than that is in the therapeutic range, and subsequent boluses result in the drug concentration periodically falling in the subtherapeutic range. Ideally, the drug concentration should always be in the therapeutic range which can be achieved by continuous infusions. However, the currently available sophisticated infusion pumps fall short of the convenience and accuracy of volatile anesthetic agents the delivery of which is guided by the minimum alveolar concentration.[75,76] Development of infusion devices for intravenous drugs which are the prototype of the vaporizers would be desirable.[77,78,79]
A step toward this goal is the development of target-controlled infusion (TCI) devices. In open-loop system, the plasma or the drug concentrations at the site of effect are calculated using a computer and the knowledge of the pharmacokinetic and the pharmacodynamic models of the drug.[76,80] Conventional infusion pumps result in continuous drug uptake, whereas TCI pumps gradually decrease the rate of drug delivery based on the pharmacokinetics of the drug. In TCI, the anesthesiologist sets desired target concentration, and the computer calculates the infusion rate that is required to deliver the necessary target concentration [Figure 3].
Figure 3.

Delivery of a drug via a computer controlled infusion pump
Measurements obtained from clinical studies in various subgroups are used to first formulate the pharmacokinetic profile of the drug which is then fed into the computer system. The drug delivery in TCI pumps is based on bolus elimination and transfer principle.[76] The initial bolus is given to reach the target concentration followed by infusion rate that replaces the amount of drug that is eliminated and the drug that is transferred to the peripheral tissue using an exponentially decreasing infusion rate. At present, only models for propofol, fentanyl, sufentanil, alfentanil, and remifentanil are available.[74] Using pharmacokinetic data models for dexmedetomidine are also in the process of being developed.[81]
Paedfusor TCI for pediatric use has shown promising results in cardiac surgery.[82] Pediatric TCI models currently available can be used in healthy children over 3 years of age. However, further research is needed to develop a system for optimal drug delivery in children.
Closed-loop systems
The closed-loop systems have real-time monitoring of the patients’ variables such as muscle relaxation, hypnosis,[83] analgesia, and a computer-controlled feedback mechanism which precisely delivers the drugs based on these parameters.[76] Muscle relaxation is monitored using peripheral nerve stimulator and recording the responses using accelerograph.[84] Hypnosis is monitored using the bispectral (BIS) monitor and a BIS index between 40 and 60 is generally advised as adequate hypnosis for general anaesthesia.[85,86,87,88,89,90,91,92] The most challenging parameter to monitor is the adequacy of analgesia as there are no objective parameters to verify. Since a surgical stimulus results in strong sympathetic activation, physical parameters such as heart rate and blood pressure can be used to monitor the level of analgesia.[93,94] Newer parameter, heart rate variability, has been used to quantify the degree of analgesia.[95] Hence, these patient parameters are fed to the computer which in turn using the inbuilt drug pharmacodynamic data adjusts the dose of intravenous drugs. Postoperative delivery of sedative doses of propofol after cardiac surgeries can also be delivered more precisely using closed-loop system.[88,96] With robotic surgery being the norm of the day, closed-loop anesthesia can be compared to pharmacological robot.[97] Teleanesthesia is also not far away where not only the patient's fitness will be done by distant preoperative assessment but also anesthesia will be provided at distant locations.[98]
Pitfalls of drug delivery system
Final drug product could turn out to be costly when high-end technology is being used
Toxicity of the various carriers
Lack of knowledge about the degradation products
Trained staff to administer personalized treatment and to use various DDS
Patient compliance may be affected due to complex devices.
Conclusion
DDS is a highly evolving field with the multidisciplinary involvement. The anesthesiologist in future will thus have a safer, simpler, and faster anesthetic practice with novel DDSs and monitoring equipment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Buvanendran A, Kroin JS. Useful adjuvants for postoperative pain management. Best Pract Res Clin Anaesthesiol. 2007;21:31–49. doi: 10.1016/j.bpa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Rosen H, Abribat T. The rise and rise of drug delivery. Nat Rev Drug Discov. 2005;4:381–5. doi: 10.1038/nrd1721. [DOI] [PubMed] [Google Scholar]
- 3.Kshirsagar NA. Drug delivery systems. Indian J Pharmacol. 2000;32:S54–61. [Google Scholar]
- 4.Dhote V, Bhatnagar P, Mishra PK, Mahajan SC, Mishra DK. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci Pharm. 2012;80:1–28. doi: 10.3797/scipharm.1108-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talegaonkar S, Mishra PR. Intranasal delivery: An approach to bypass the blood brain barrier. Indian J Pharmacol. 2004;36:140–7. [Google Scholar]
- 6.Sakane T, Akizuki M, Yoshida M, Yamashita S, Nadai T, Hashida M, et al. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol. 1991;43:449–51. doi: 10.1111/j.2042-7158.1991.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 7.Westin UE, Boström E, Gråsjö J, Hammarlund-Udenaes M, Björk E. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res. 2006;23:565–72. doi: 10.1007/s11095-006-9534-z. [DOI] [PubMed] [Google Scholar]
- 8.Illum L. Nasal drug delivery: New developments and strategies. Drug Discov Today. 2002;7:1184–9. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 9.Lu CT, Zhao YZ, Wong HL, Cai J, Peng L, Tian XQ. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int J Nanomedicine. 2014;9:2241–57. doi: 10.2147/IJN.S61288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav N, Lohani A. A dry powder inhaler: A review. Indo Glob J Pharm Sci. 2013;3:142–55. [Google Scholar]
- 12.Shaikh S, Nazim S, Khan T, Shaikh A, Zameeruddin M, Quazi A. Recent advances in pulmonary drug delivery system: A review. Int J Appl Pharm. 2010;2:27–31. [Google Scholar]
- 13.Mather LE, Woodhouse A, Ward ME, Farr SJ, Rubsamen RA, Eltherington LG. Pulmonary administration of aerosolised fentanyl: Pharmacokinetic analysis of systemic delivery. Br J Clin Pharmacol. 1998;46:37–43. doi: 10.1046/j.1365-2125.1998.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worsley MH, MacLeod AD, Brodie MJ, Asbury AJ, Clark C. Inhaled fentanyl as a method of analgesia. Anaesthesia. 1990;45:449–51. doi: 10.1111/j.1365-2044.1990.tb14331.x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins MJ, Asbury AJ, Brodie MJ. Inhaled nebulised fentanyl for postoperative analgesia. Anaesthesia. 1991;46:973–6. doi: 10.1111/j.1365-2044.1991.tb09862.x. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2:2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51(2):161–9. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siobal MS. Pulmonary vasodilators. Respir Care. 2007;52:885–99. [PubMed] [Google Scholar]
- 19.Fala L. Afrezza (Insulin Human) inhalation powder approved for the treatment of patients with type 1 or type 2 diabetes. Am Health Drug Benefits. 2015;8:40–3. [PMC free article] [PubMed] [Google Scholar]
- 20.Patton JS, Byron PR. Inhaling medicines: Delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 21.Bruschi ML, de Freitas O. Oral bioadhesive drug delivery systems. Drug Dev Ind Pharm. 2005;31:293–310. doi: 10.1081/ddc-52073. [DOI] [PubMed] [Google Scholar]
- 22.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: A review. J Pharm Pharm Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- 23.Stanley TH. Fentanyl. J Pain Symptom Manage. 2005;29(5 Suppl):S67–71. doi: 10.1016/j.jpainsymman.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Gilhotra RM, Ikram M, Srivastava S, Gilhotra N. A clinical perspective on mucoadhesive buccal drug delivery systems. J Biomed Res. 2014;28:81–97. doi: 10.7555/JBR.27.20120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathbone MJ, Drummond BK, Tucker G. The oral cavity as a site for systemic drug delivery. Adv Drug Deliv Rev. 1994;13:1–22. [Google Scholar]
- 26.Sangeetha S, Venkatesh ND, Krishan PN, Saraswathi R. Mucosa as a route for systemic drug delivery. Res J Pharm Biol Chem Sci. 2010;1:178–87. [Google Scholar]
- 27.Smart JD. Buccal drug delivery. Expert Opin Drug Deliv. 2005;2:507–17. doi: 10.1517/17425247.2.3.507. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Huang G. Intra-articular lornoxicam loaded PLGA microspheres: Enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Deliv. 2012;19:255–63. doi: 10.3109/10717544.2012.700962. [DOI] [PubMed] [Google Scholar]
- 29.Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1:109–31. doi: 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: Overcoming the skin's barrier function. Pharm Sci Technolo Today. 2000;3:318–326. doi: 10.1016/s1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 31.Panchagnula R. Transdermal delivery of drugs. Indian J Pharmacol. 1997;29:140–56. [Google Scholar]
- 32.Bajaj S, Whiteman A, Brandner B. Transdermal drug delivery in pain management. Contin Educ Anaesth Crit Care Pain. 2011;11:39–43. [Google Scholar]
- 33.Sinatra R. The fentanyl HCl patient-controlled transdermal system (PCTS): An alternative to intravenous patient-controlled analgesia in the postoperative setting. Clin Pharmacokinet. 2005;44(Suppl 1):1–6. doi: 10.2165/00003088-200544001-00002. [DOI] [PubMed] [Google Scholar]
- 34.Viscusi ER, Reynolds L, Tait S, Melson T, Atkinson LE. An iontophoretic fentanyl patient-activated analgesic delivery system for postoperative pain: A double-blind, placebo-controlled trial. Anesth Analg. 2006;102:188–94. doi: 10.1213/01.ane.0000183649.58483.77. [DOI] [PubMed] [Google Scholar]
- 35.Lehmann KA, Zech D. Transdermal fentanyl: Clinical pharmacology. J Pain Symptom Manage. 1992;7(3 Suppl):S8–16. doi: 10.1016/0885-3924(92)90048-m. [DOI] [PubMed] [Google Scholar]
- 36.Richard BM, Rickert DE, Doolittle D, Mize A, Liu J, Lawson CF. Pharmacokinetic compatibility study of lidocaine with EXPAREL in yucatan miniature pigs. ISRN Pharm 2011. 2011:582351. doi: 10.5402/2011/582351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margetts L, Sawyer R. Transdermal drug delivery: Principles and opioid therapy. Contin Educ Anaesth Crit Care Pain. 2007;7:171–6. [Google Scholar]
- 38.Muijsers RB, Wagstaff AJ. Transdermal fentanyl: An updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs. 2001;61:2289–307. doi: 10.2165/00003495-200161150-00014. [DOI] [PubMed] [Google Scholar]
- 39.Milligan K, Lanteri-Minet M, Borchert K, Helmers H, Donald R, Kress HG, et al. Evaluation of long-term efficacy and safety of transdermal fentanyl in the treatment of chronic noncancer pain. J Pain. 2001;2:197–204. doi: 10.1054/jpai.2001.25352. [DOI] [PubMed] [Google Scholar]
- 40.Viscusi ER, Reynolds L, Chung F, Atkinson LE, Khanna S. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: A randomized controlled trial. JAMA. 2004;291:1333–41. doi: 10.1001/jama.291.11.1333. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad S, Hewitt DJ, Damaraju CV. Fentanyl HCl iontophoretic transdermal system versus intravenous morphine pump after gynecologic surgery. Arch Gynecol Obstet. 2007;276:251–8. doi: 10.1007/s00404-007-0339-z. [DOI] [PubMed] [Google Scholar]
- 42.Panchal SJ, Damaraju CV, Nelson WW, Hewitt DJ, Schein JR. System-related events and analgesic gaps during postoperative pain management with the fentanyl iontophoretic transdermal system and morphine intravenous patient-controlled analgesia. Anesth Analg. 2007;105:1437–41. doi: 10.1213/01.ane.0000281442.36582.81. [DOI] [PubMed] [Google Scholar]
- 43.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandrashekar NS, Shobha Rani RH. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J Pharm Sci. 2008;70:94–6. doi: 10.4103/0250-474X.40340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Thakur R, Fan Q, Michniak B. Transdermal iontophoresis: Combination strategies to improve transdermal iontophoretic drug delivery. Eur J Pharm Biopharm. 2005;60:179–91. doi: 10.1016/j.ejpb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: Application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin Drug Deliv. 2010;7:1415–32. doi: 10.1517/17425247.2010.538679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eberhart L. The safety and tolerability of the fentanyl HCl iontophoretic transdermal system: An alternative to currently available analgesic modalities. J Opioid Manag. 2007;3:249–56. doi: 10.5055/jom.2007.0012. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita M, Yamamoto R, Kominami K. In-vitro and in-vivo transdermal iontophoretic delivery of tramadol, a centrally acting analgesic. J Pharm Pharmacol. 2011;63:1437–45. doi: 10.1111/j.2042-7158.2011.01355.x. [DOI] [PubMed] [Google Scholar]
- 49.Bose S, Ravis WR, Lin YJ, Zhang L, Hofmann GA, Banga AK. Electrically-assisted transdermal delivery of buprenorphine. J Control Release. 2001;73:197–203. doi: 10.1016/s0168-3659(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 50.Okave K, Yamaguchi H, Kawai Y. New iontophoretic transdermal administration of beta-blocker metoprolol. J Control Release. 1986;4:79–85. [Google Scholar]
- 51.Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83:11–25. [PMC free article] [PubMed] [Google Scholar]
- 52.Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107–16. doi: 10.2147/JPR.S30861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard BM, Rickert DE, Newton PE, Ott LR, Haan D, Brubaker AN, et al. Safety evaluation of EXPAREL (DepoFoam Bupivacaine) administered by repeated subcutaneous injection in rabbits and dogs: Species comparison. J Drug Deliv 2011. 2011:467429. doi: 10.1155/2011/467429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilfeld BM, Malhotra N, Furnish TJ, Donohue MC, Madison SJ. Liposomal bupivacaine as a single-injection peripheral nerve block: A dose-response study. Anesth Analg. 2013;117:1248–56. doi: 10.1213/ANE.0b013e31829cc6ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madni A, Sarfraz M, Rehman M, Ahmad M, Akhtar N, Ahmad S, et al. Liposomal drug delivery: A versatile platform for challenging clinical applications. J Pharm Pharm Sci. 2014;17:401–26. doi: 10.18433/j3cp55. [DOI] [PubMed] [Google Scholar]
- 56.Viscusi ER, Martin G, Hartrick CT, Singla N, Manvelian G EREM Study Group. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology. 2005;102:1014–22. doi: 10.1097/00000542-200505000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Hung OR, Whynot SC, Varvel JR, Shafer SL, Mezei M. Pharmacokinetics of inhaled liposome-encapsulated fentanyl. Anesthesiology. 1995;83:277–84. doi: 10.1097/00000542-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greineder CF, Howard MD, Carnemolla R, Cines DB, Muzykantov VR. Advanced drug delivery systems for antithrombotic agents. Blood. 2013;122:1565–75. doi: 10.1182/blood-2013-03-453498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Runge MS, Bode C, Matsueda GR, Haber E. Antibody-enhanced thrombolysis: Targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci U S A. 1987;84:7659–62. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peter K, Graeber J, Kipriyanov S, Zewe-Welschof M, Runge MS, Kübler W, et al. Construction and functional evaluation of a single-chain antibody fusion protein with fibrin targeting and thrombin inhibition after activation by factor Xa. Circulation. 2000;101:1158–64. doi: 10.1161/01.cir.101.10.1158. [DOI] [PubMed] [Google Scholar]
- 62.Modery CL, Ravikumar M, Wong TL, Dzuricky MJ, Durongkaveroj N, Sen Gupta A. Heteromultivalent liposomal nanoconstructs for enhanced targeting and shear-stable binding to active platelets for site-selective vascular drug delivery. Biomaterials. 2011;32:9504–14. doi: 10.1016/j.biomaterials.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 63.Bode C, Meinhardt G, Runge MS, Freitag M, Nordt T, Arens M, et al. Platelet-targeted fibrinolysis enhances clot lysis and inhibits platelet aggregation. Circulation. 1991;84:805–13. doi: 10.1161/01.cir.84.2.805. [DOI] [PubMed] [Google Scholar]
- 64.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Chelly JE, Delaunay L, Williams B, Borghi B. Outpatient lower extremity infusions. Best Pract Res Clin Anaesthesiol. 2002;16:311–20. doi: 10.1053/bean.2002.0240. [DOI] [PubMed] [Google Scholar]
- 66.Mohseni M, Ebneshahidi A. The flow rate accuracy of elastomeric infusion pumps after repeated filling. Anesth Pain Med. 2014;4:e14989. doi: 10.5812/aapm.14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ilfeld BM, Morey TE, Enneking FK. The delivery rate accuracy of portable infusion pumps used for continuous regional analgesia. Anesth Analg. 2002;95:1331–6. doi: 10.1097/00000539-200211000-00043. [DOI] [PubMed] [Google Scholar]
- 68.Ilfeld BM, Morey TE, Enneking FK. Delivery rate accuracy of portable, bolus-capable infusion pumps used for patient-controlled continuous regional analgesia. Reg Anesth Pain Med. 2003;28:17–23. doi: 10.1053/rapm.2003.50008. [DOI] [PubMed] [Google Scholar]
- 69.Chung IS, Cho HS, Kim JA, Lee KH. The flow rate of the elastomeric balloon infusor is influenced by the internal pressure of the infusor. J Korean Med Sci. 2001;16:702–6. doi: 10.3346/jkms.2001.16.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizuuchi M, Yamakage M, Iwasaki S, Kimura A, Namiki A. The infusion rate of most disposable, non-electric infusion pumps decreases under hypobaric conditions. Can J Anaesth. 2003;50:657–62. doi: 10.1007/BF03018707. [DOI] [PubMed] [Google Scholar]
- 71.Skryabina EA, Dunn TS. Disposable infusion pumps. Am J Health Syst Pharm. 2006;63:1260–8. doi: 10.2146/ajhp050408. [DOI] [PubMed] [Google Scholar]
- 72.Clark TM, Yagiela JA. Advanced techniques and armamentarium for dental local anesthesia. Dent Clin North Am. 2010;54:757–68. doi: 10.1016/j.cden.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Michel M, Struys RF, Aslom AR, Shafer SL. Intravenous drug delivery systems. In: Miller RD, editor. Miller's Anesthesia. 8th ed. Ch. 13. Elsevier: Saunders published; 2015. pp. 945–56. [Google Scholar]
- 74.Struys MM, De Smet T, Glen JI, Vereecke HE, Absalom AR, Schnider TW. The history of target-controlled infusion. Anesth Analg. 2016;122:56–69. doi: 10.1213/ANE.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 75.Egan TD. Target-controlled drug delivery: Progress toward an intravenous “vaporizer” and automated anesthetic administration. Anesthesiology. 2003;99:1214–9. doi: 10.1097/00000542-200311000-00031. [DOI] [PubMed] [Google Scholar]
- 76.Glen JB. The development of ‘Diprifusor’: A TCI system for propofol. Anaesthesia. 1998;53(Suppl 1):13–21. doi: 10.1111/j.1365-2044.1998.53s115.x. [DOI] [PubMed] [Google Scholar]
- 77.Weiskopf RB. Target-controlled drug delivery progress toward an intravenous “vaporizer” and automated anesthetic administration. Anesthesiology. 2003;99:1214–9. doi: 10.1097/00000542-200311000-00031. [DOI] [PubMed] [Google Scholar]
- 78.Guarracino F, Lapolla F, Cariello C, Danella A, Doroni L, Baldassarri R, et al. Target controlled infusion: TCI. Minerva Anestesiol. 2005;71:335–7. [PubMed] [Google Scholar]
- 79.Egan TD. Intravenous drug delivery systems: Toward an intravenous “vaporizer”. J Clin Anesth. 1996;8(3 Suppl):8S–14S. doi: 10.1016/s0952-8180(96)90005-7. [DOI] [PubMed] [Google Scholar]
- 80.Schnider TW, Minto CF, Struys MM, Absalom AR. The safety of target-controlled infusions. Anesth Analg. 2016;122:79–85. doi: 10.1213/ANE.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 81.Hannivoort LN, Eleveld DJ, Proost JH, Ryntines KM, Absalom AR, Vereecke HE, et al. Development of an optimized pharmacokinetic model of dexmedetomidine using target controlled infusions in healthy volunteers. Anesthesiology. 2015;123:357–67. doi: 10.1097/ALN.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 82.Wilson G. Target controlled infusion anaesthesia in children. South Afr J Anaesth Analg. 2010;16:124–6. [Google Scholar]
- 83.Schmidt GN, Bischoff P, Standl T, Lankenau G, Hellstern A, Hipp C, et al. SNAP index and Bispectral index during different states of propofol/remifentanil anaesthesia. Anaesthesia. 2005;60:228–34. doi: 10.1111/j.1365-2044.2004.04120.x. [DOI] [PubMed] [Google Scholar]
- 84.Fan X, Ma M, Li Z, Gong S, Zhang W, Wen Y. The relationship between the target effective site concentration of rocuronium and the degree of recovery from neuromuscular blockade in elderly patients. Int J Clin Exp Med. 2015;8:16369–73. [PMC free article] [PubMed] [Google Scholar]
- 85.Struys MM, De Smet T, Versichelen LF, Van De Velde S, Van den Broecke R, Mortier EP. Comparison of closed-loop controlled administration of propofol using Bispectral Index as the controlled variable versus “standard practice” controlled administration. Anesthesiology. 2001;95:6–17. doi: 10.1097/00000542-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Agarwal J, Puri GD, Mathew PJ. Comparison of closed loop vs. manual administration of propofol using the Bispectral index in cardiac surgery. Acta Anaesthesiol Scand. 2009;53:390–7. doi: 10.1111/j.1399-6576.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 87.Rigouzzo A, Girault L, Louvet N, Servin F, De-Smet T, Piat V, et al. The relationship between bispectral index and propofol during target-controlled infusion anesthesia: A comparative study between children and young adults. Anesth Analg. 2008;106:1109–16. doi: 10.1213/ane.0b013e318164f388. [DOI] [PubMed] [Google Scholar]
- 88.Short TG, Hannam JA, Laurent S, Campbell D, Misur M, Merry AF, et al. Refining target-controlled infusion: An assessment of pharmacodynamic target-controlled infusion of propofol and remifentanil using a response surface model of their combined effects on Bispectral index. Anesth Analg. 2016;122:90–7. doi: 10.1213/ANE.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 89.Sakai T, Matsuki A, White PF, Giesecke AH. Use of an EEG-bispectral closed-loop delivery system for administering propofol. Acta Anaesthesiol Scand. 2000;44:1007–10. doi: 10.1034/j.1399-6576.2000.440819.x. [DOI] [PubMed] [Google Scholar]
- 90.De Smet T, Struys MM, Greenwald S, Mortier EP, Shafer SL. Estimation of optimal modeling weights for a Bayesian-based closed-loop system for propofol administration using the bispectral index as a controlled variable: A simulation study. Anesth Analg. 2007;105:1629–38. doi: 10.1213/01.ane.0000287269.06170.0f. [DOI] [PubMed] [Google Scholar]
- 91.De Smet T, Struys MM, Neckebroek MM, Van den Hauwe K, Bonte S, Mortier EP. The accuracy and clinical feasibility of a new bayesian-based closed-loop control system for propofol administration using the bispectral index as a controlled variable. Anesth Analg. 2008;107:1200–10. doi: 10.1213/ane.0b013e31817bd1a6. [DOI] [PubMed] [Google Scholar]
- 92.Liu N, Chazot T, Genty A, Landais A, Restoux A, McGee K, et al. Titration of propofol for anesthetic induction and maintenance guided by the bispectral index: Closed-loop versus manual control: A prospective, randomized, multicenter study. Anesthesiology. 2006;104:686–95. doi: 10.1097/00000542-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 93.Gentilini A, Schaniel C, Morari M, Bieniok C, Wymann R, Schnider T. A new paradigm for the closed-loop intraoperative administration of analgesics in humans. IEEE Trans Biomed Eng. 2002;49:289–99. doi: 10.1109/10.991156. [DOI] [PubMed] [Google Scholar]
- 94.Hemmerling TM, Arbeid E, Wehbe M, Cyr S, Taddei R, Zaouter C. Evaluation of a novel closed-loop total intravenous anaesthesia drug delivery system: A randomized controlled trial. Br J Anaesth. 2013;110:1031–9. doi: 10.1093/bja/aet001. [DOI] [PubMed] [Google Scholar]
- 95.Simanski O, Schubert A, Kaehler R, Janda M, Bajorat J, Hofmockel R, et al. Automatic Drug Delivery in Anesthesia – The Design of an Anesthesia Assistant System. Proceedings of the 17th World Congress the International Federation of Automatic Control Seoul, Korea. 2008:9601–6. [Google Scholar]
- 96.Solanki A, Puri GD, Mathew PJ. Bispectral index controlled post operative sedation in cardiac surgery patients: A comparative trial between closed loop and manual administration of propofol. Eur J Anaesthesiol. 2010;27:708–13. doi: 10.1097/EJA.0b013e328335b2d4. [DOI] [PubMed] [Google Scholar]
- 97.Hemmerling TM, Taddei R, Wehbe M, Morse J, Cyr S, Zaouter C. Robotic anesthesia – A vision for the future of anesthesia. Transl Med UniSa. 2011;1:1–20. [PMC free article] [PubMed] [Google Scholar]
- 98.Hemmerling TM. Automated anesthesia. Curr Opin Anaesthesiol. 2009;22:757–63. doi: 10.1097/ACO.0b013e328332c9b4. [DOI] [PubMed] [Google Scholar]


