Abstract
Several of the most important discoveries in the field of membrane traffic have come from studies of Rab GTPases by Marino Zerial and Peter Novick and their colleagues. Zerial was the first to discover that Rab GTPases represent identity markers for different membrane-bound compartments, and each Rab organizes a collection of specific effectors into function-specifying membrane microdomains to carry out receptor trafficking. Novick discovered that the order (and thus polarity) of Rab GTPases along the secretory and endocytic pathways are established by their specific, cognate guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which partner with one Rab to regulate the subsequent- and prior-acting Rabs. Such so-called Rab cascades have evolved to establish domains that contain unique Rab proteins and their cognate effectors, which drive all steps of membrane trafficking. These findings deserve much broader recognition by the biomedical research community and are highlighted here, along with open questions that require serious attention for full understanding of the molecular basis of Rab GTPase-regulated membrane trafficking in eukaryotic cells.
INTRODUCTION
Dieter Gallwitz was the first to discover a yeast-encoded member of the Rab family in the 1980s, when DNA sequencing was just beginning to be widely adopted and required significant effort. Gallwitz found an essential GTP-binding protein that he named Ypt1. It was related to Ras but could not complement the loss of the yeast RAS1 and RAS2 genes and thus likely played a different role (Schmitt et al., 1986; see also Segev et al., 1988). For his PhD thesis with Randy Schekman, Peter Novick had isolated and described the SEC genes responsible for secretion in yeast (Novick et al., 1980). After a postdoctoral fellowship on the yeast cytoskeleton with David Botstein at MIT, Novick returned to the secretory pathway for his own lab's research and focused on the SEC gene products that are required for the delivery of material from the yeast Golgi to the cell surface. The original screen for yeast mutants uncovered 10 genes that, when mutated, accumulated post-Golgi transport vesicles at a nonpermissive temperature (Novick et al., 1980). Sequencing of the SEC4 gene revealed that it encoded a Ras-related protein (Salminen et al., 1987) that was present on secretory vesicles (Goud et al., 1988) and was later shown to recruit the so-called Exocyst tether (Guo et al., 1999) to help deliver vesicles to the bud tip for fusion (Walch-Solimena et al., 1997). This was the first hint that membrane traffic might require a Ras-like GTPase.
These exciting findings in yeast led several labs to begin the search for mammalian homologues, and Armand Tavitian and Marino Zerial and their colleagues began cloning these relatives. Tavitian named them Ras-like proteins from rat brain (Rabs; Touchot et al., 1987; Chavrier et al., 1990a), in line with the three-letter nomenclature of other Ras-like protein families; the Rabs were numbered in the random order in which their sequences were obtained; Ypt1 was the homologue of Rab1. Eventually, it would become clear that yeast encode 11 Rabs and human cells encode at least 63 (Colicelli, 2004). Most Rabs are stably, C-terminally prenylated on one or (more commonly) two cysteine residues that permit their tight membrane association. Prenylome-wide analysis later revealed that a single cell might contain at least 42 different Rabs (Nguyen et al., 2009); other Rabs are expressed in a more tissue-specific manner.
Segev et al. (1988) showed that Ypt1 was on the Golgi, in contrast to Sec4, which Novick had shown was present on secretory vesicles. Soon thereafter, Zerial and coworkers raised specific antibodies to their newly discovered gene products and found that these proteins each localized to different membrane compartments (Chavrier et al., 1990b). Even more striking was their subsequent discovery, using live-cell microscopy of cells expressing green fluorescent protein (GFP)– and GFP-variant- tagged proteins, that a single compartment might harbor multiple Rabs, each occupying a distinct microdomain of that compartment (Sönnichsen et al., 2000; see also Barbero et al., 2002). The importance of these findings cannot be overstated: this represented the first molecular distinction between membrane-bound compartments within the endocytic pathway and provided a framework for all future molecular analyses of receptor endocytosis and recycling or degradation. Indeed, Zerial and colleagues showed that, like Sec4, Rab5 played a key role in membrane traffic, and its function is rate limiting for endosome fusion and endocytosis (Gorvel et al., 1991). He and his colleagues eventually reconstituted the entire process of early endosome fusion using exclusively Rab5-binding proteins, SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) proteins and their priming factors, liposomes, and a factor (Yip3/PRA1) to deliver prenyl Rab5 onto the liposomes (see later discussion; Ohya et al., 2009).
THE Rab GTPase CYCLE AND MEMBRANE DELIVERY
Yoshimi Takai was studying the biochemistry of a small GTPase, smg p25, which was later identified as Rab3A. He discovered a rat brain cytosol protein that inhibited the ability of smg p35/Rab3A to release GDP, the rate-limiting step in the GTPase cycle (Sasaki et al., 1990). He named this protein GDP-dissociation inhibitor (GDI) and showed that it could extract Rab3A from membranes in vitro but only in the presence of GDP and not GTP (Araki et al., 1990). We now know that there are two GDIs in humans and one in yeast; these proteins have the capacity to bind to all Rab GTPases, with strong preference for their GDP-bound states. At steady state, Rabs distribute themselves about half on membranes and half in cytosol (although this varies considerably between Rab proteins); all cytosolic Rabs exist in complex with GDI. The crystal structure of yeast GDI bound to Ypt1 confirmed that GDI binds to the so-called Rab “switch” regions that report on the status of the bound nucleotide; the so-called C-terminal hypervariable domain of Rab proteins is draped along the surface, and the prenyl groups bind tightly at the bottom of GDI (Pylypenko et al., 2006). Thus GDP-bearing Rabs can be removed from membranes by cytosolic GDI proteins.
My lab was the first to report that GDI binds Rab9 GTPase with ∼20 nM affinity (Shapiro and Pfeffer, 1995); Goody and coworkers confirmed this tight interaction for other Rab proteins (Wu et al., 2007). With this in mind, we and Zerial studied the delivery of Rab proteins onto membranes and showed that purified complexes of Rab proteins bound to GDI contain all of the information needed for accurate membrane delivery (Soldati et al., 1994; Ullrich et al., 1994). Of importance, Rabs were delivered onto membranes in their GDP-bound forms and converted into their GTP-bound forms after a lag of ∼5 min in vitro (Soldati et al., 1994; Ullrich et al., 1994). Because this delivery required protease-sensitive components on the surface of membranes, we postulated that it was catalyzed by a so-called GDI-displacement factor (GDF) that dissociates Rab–GDI complexes. We went on to show that a protein called Yip3/PRA1 can dissociate endosomal Rabs and deliver them to membranes (Sivars et al., 2003); the Zerial lab's complete reconstitution of Rab5-mediated endosome fusion using purified components absolutely requires the presence of Yip3/PRA1 in membranes to accomplish Rab5 membrane association, despite the presence of a Rab5 GEF, Rabex 5, in their reactions (Ohya et al., 2009).
Could a GEF be sufficient for localization? Itzen, Goody, and coworkers found a Legionella protein, DrrA, that can relocalize Rab1 from the early secretory pathway to the inner surface of the plasma membrane (Schoebel et al., 2009). DrrA is a Rab1 GEF that binds Rab1 extremely tightly and was shown to thus bypass any need for a distinct GDF activity. No endogenous Rab GEFs bind their Rab substrates as tightly as DrrA, and thus GDFs may still facilitate some Rab delivery events. Several labs artificially localized GEFs to unusual membranes such as mitochondria and succeeded in moving Rabs to those locations (Gerondopoulos et al., 2012; Blümer et al., 2013; Cabrera and Ungermann, 2013). The most important conclusion from such experiments is that GDI is promiscuous in delivering Rabs to membranes; GDI is also likely to be able to correct mistakes it might make in delivery of Rabs to membranes where the cognate GEF is not present and the Rab is, with GDP bound. Most characterized Rab GEFs are not membrane anchored; they are cytosolic proteins that can associate with other Rab effectors to activate locally specific Rab proteins. Thus one needs Rab domains to maintain Rab domains, and, in cells, Rabs usually show precise localizations (Yoshimura et al., 2007).
These data suggest that Rab proteins are delivered to membranes by GDI and, in some cases, might use the help of GDF factors. Subsequent GEF action is also clearly important to enable Rabs to be stabilized at the site of their activation by loss of susceptibility to GDI extraction, together with active stabilization by subsequent, GTP-dependent effector binding. Several studies showed that effector binding stabilizes Rabs on membrane surfaces (Aivazian et al., 2006).
Rab CASCADES: ESTABLISHING THE POLARITY OF SECRETORY AND ENDOCYTIC PATHWAYS
The role of Rab proteins is to recruit effectors such as motor proteins and tethering factors to facilitate downstream membrane traffic events. If you imagine that membrane flow should go from point A to point B in a pathway, you need a mechanism to template RabB after RabA on different membrane compartments. Zerial was the first to discover that effector proteins can simultaneously bind two adjacently acting Rab GTPases, thereby linking their respective localizations and functions (Vitale et al., 1998; de Renzis et al., 2002). This discovery strongly suggested that Rab effectors organize transitions along the endocytic pathway. Nature has taken this concept one step further, using an incredibly elegant system by which RabA actually recruits to membrane surfaces the GEF that will activate the next acting Rab in the pathway (Figure 1A). Thus, in yeast, Ypt31 binds the Sec 2 GEF for the subsequent-acting Sec4 Rab protein. Active Rabs are then stabilized on membranes by binding to their cognate effector proteins. In this manner, a membrane patch containing one Rab will template the establishment of a nearby domain containing the next Rab. Even better is the fact that the subsequent acting RabB also recruits a GAP for RabA to clear the membrane domain of the prior acting Rab (Figure 1B). Because Rab GTPases each have distinct GEFs and GAPs, evolution has created a system by which the selectivity of each of these proteins coordinates to template an entire membrane-trafficking pathway. Although several labs were obtaining clues to the existence of such interactions, it was Peter Novick who discovered these cascades and revealed their existence in living yeast cells (Ortiz et al., 2002; Rivera-Molina and Novick, 2009; see also, for example, Nottingham et al., 2011; Pusapati et al., 2012; Suda et al., 2013; Rana et al., 2015). This represented a spectacular discovery because it provided the first molecular clues about how cells create and maintain polarized secretory and endocytic pathways.
FIGURE 1:
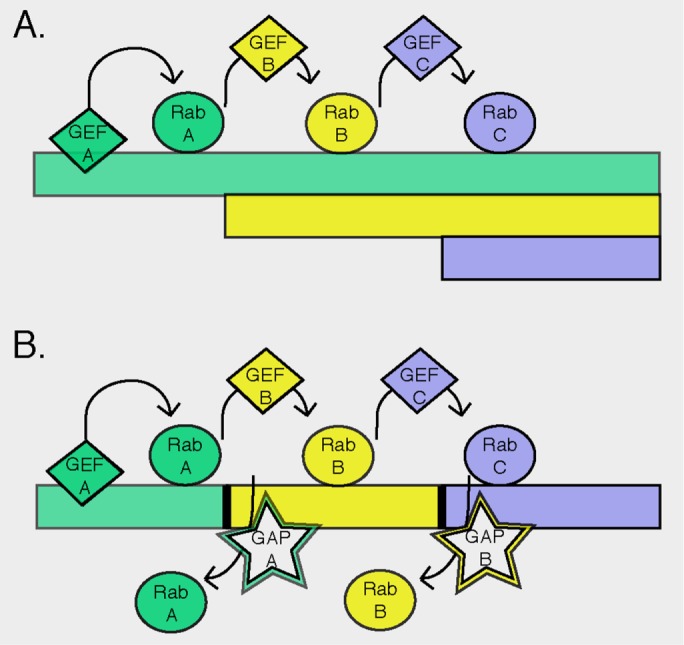
Rab cascade model for the establishment and maintenance of the polarity of the secretory and endocytic pathways. (A) In the first scenario, a Rab GEF specific for Rab A generates active Rab A. That Rab recruits a second GEF, which activates Rab B. Similarly, Rab B recruits a GEF to activate Rab C. In this model, a membrane could have Rabs A–C intermingled or at least on a single compartment. (B) Here GAP proteins are included to remove a previous-acting Rab from a specific membrane domain. The presence of the GAP will sharpen the boundaries between individual Rab domains. Data of Rivera-Molina and Novick (2009) support the model in B. Reprinted from Nottingham and Pfeffer (2009).
SURPRISES AND UNSOLVED MYSTERIES
A Special Topics Subgroup at the 2016 American Society for Cell Biology meeting discussed a number of surprises that await further analysis. We still do not know the localizations or roles of many human Rab proteins, nor do we know all of their cognate GEF or GAP identities or partner effector proteins. Rabs need to be studied in their correct tissue or cell type and at endogenous expression levels. This represents many proteins yet to be discovered because a given Rab may have ∼30 specific effector proteins (Christoforidis et al., 1999). Effector interactions can be weak and missed by affinity chromatography. In addition, GEFs and GAPs can be more promiscuous in vitro than in vivo, and so great care must be taken in all of these analyses.
Are cascades the answer? We need to connect all of the Rab GEFs and GAPs to determine the order of membrane-trafficking events, and we need to test the consequences of altering cascade specificity to verify the predictions of this satisfying model in the broadest physiological sense. Finally, we need to reconstitute a Rab cascade de novo to fully understand how cells establish and maintain secretory and endocytic pathways.
Recently Rab proteins have been shown to be the main phosphorylation substrates for the LRRK2 kinase that is implicated in Parkinson's disease (Steger et al., 2016). What is the physiological consequence of phosphorylation, and how is this modification normally regulated? Transforming growth factor-β–activated kinase 1 (TAK1) also phosphorylates GDP-bearing Rab1 and blocks GDI interaction but not GEF interaction (Levin et al., 2016). This presumably stabilizes active Rab1 on membranes in a totally unanticipated manner that activates membrane trafficking. Pathogens modify Rabs in many unexpected ways, including by AMPylation and phosphocholination (Müller et al., 2010; Mukherjee et al., 2011). Do these posttranslational modifications also occur in uninfected cells, and how (and why) are these events regulated? Finally, Rab GTPases also interact with other small GTPases via shared effector proteins, and their joint coordination is only beginning to be explored. Rab GTPases remain central regulators of membrane-trafficking pathways in healthy cells and disease. Their continued study promises to offer many more important surprises for those who study them.
Abbreviations used:
- GAP
GTPase-activating protein
- GDF
GDI displacement factor
- GDI
GDP-dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- Rab
Ras-like protein from rat brain.
Footnotes
REFERENCES
- Aivazian D, Serrano RL, Pfeffer S. TIP47 is a key effector for Rab9 localization. J Cell Biol. 2006;173:917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y. Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem. 1990;265:13007–13015. [PubMed] [Google Scholar]
- Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-medicated vesicle transport from endosomes to the trans-Golgi in living cells. J Cell Biol. 2002;156:511–518. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümer J, Rey J, Dehmelt L, Mazel T, Wu YW, Bastiaens P, Goody RS, Itzen A. RabGEFS are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200:287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem. 2013;288:28704–28712. doi: 10.1074/jbc.M113.488213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990b;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Vingron M, Sander C, Simons K, Zerial M. Molecular cloning of YPT1/SEC4-Related cDNAs from an epithelial cell line. Mol Cell Biol. 1990a;10:6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renzis S, Sönnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol. 2002;4:124–133. doi: 10.1038/ncb744. [DOI] [PubMed] [Google Scholar]
- Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec 4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RS, Hertz NT, Burlingame AL, Shokat KM, Mukherjee S. Innate immunity kinase TAK1 phosphorylates Rab1 on a hotspot for posttranslational modifications by host and pathogen. Proc Natl Acad Sci USA. 2016;113:E4776–E4783. doi: 10.1073/pnas.1608355113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Liu X, Arasaki K, McDonough J, Galán JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011;477:103–106. doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- Nguyen UT, Guo Z, Delon C, Wu Y, Deraeve C, Fränzel B, Bon RS, Blankenfeldt W, Goody RS, Waldmann H, et al. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol. 2009;5:227–235. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- Nottingham RM, Ganley IG, Barr FA, Lambright DG, Pfeffer SR. RUTBC1 protein, a Rab9A effector that activates GTP hydrolysis by Rab32 and Rab33B proteins. J Biol Chem. 2011;286:33213–33222. doi: 10.1074/jbc.M111.261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham RM, Pfeffer SR. Defining the boundaries: Rab GEFs and GAPs. Proc Natl Acad Sci USA. 2009;106:14185–14186. doi: 10.1073/pnas.0907725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–1097. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec 4p guanine nucleotide exchange factor, Sec 2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusapati GV, Luchetti G, Pfeffer SR. Ric1-Rgp1 complex is a guanine nucleotide exchange factor for the late Golgi Rab6A GTPase and an effector of the medial Golgi Rab33B GTPase. J Biol Chem. 2012;287:42129–42137. doi: 10.1074/jbc.M112.414565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylypenko O, Rak A, Durek T, Kushnir S, Dursina BE, Thomae NH, Constantinescu AT, Brunsveld L, Watzke A, Waldmann H, et al. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI-mediated Rab recycling. EMBO J. 2006;25:13–23. doi: 10.1038/sj.emboj.7600921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana M, Lachmann J, Ungermann C. Identification of a Rab GTPase-activating protein cascade that controls recycling of the Rab5 GTPase Vps21 from the vacuole. Mol Biol Cell. 2015;26:2535–2549. doi: 10.1091/mbc.E15-02-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Novick PJ. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- Schmitt HD, Wagner P, Pfaff E, Gallwitz D. The ras-Related YPT1 gene product in yeast: A GTP-binding protein that might be involved in microtubule organization. Cell. 1986;47:401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A. RabGDI displacement by DrrA from legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell. 2009;36:1060–1072. doi: 10.1016/j.molcel.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Pfeffer SR. Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides. J Biol Chem. 1995;270:11085–11090. doi: 10.1074/jbc.270.19.11085. [DOI] [PubMed] [Google Scholar]
- Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature. 1994;369:76–78. doi: 10.1038/369076a0. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, et al. Phospho-proteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5:e12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Kurokawa K, Hirata R, Nakano A. Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic. Proc Natl Acad Sci USA. 2013;110:18976–18981. doi: 10.1073/pnas.1308627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchot N, Chardin P, Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci USA. 1987;84:8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec 2p mediates nucleotide exchange on Sec 4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Tan KT, Waldmann H, Goody RS, Alexandrov K. Interaction analysis of prenylated Rab GTPase with Rab escort protein and GDP dissociation inhibitor explains the need for both regulators. Proc Natl Acad Sci USA. 2007;104:12294–12299. doi: 10.1073/pnas.0701817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]


