Abstract
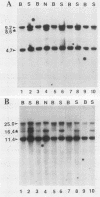
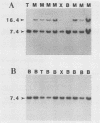
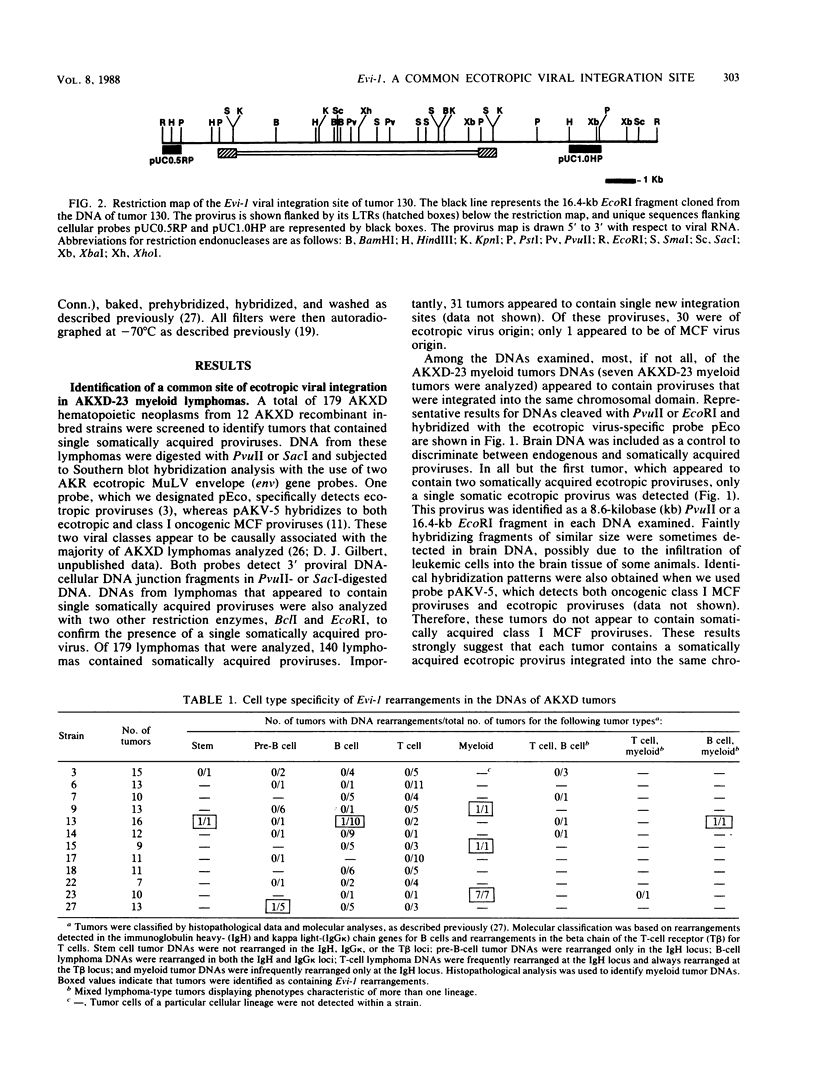
AKXD-23 recombinant inbred mice develop myeloid tumors at a high frequency, unlike other AKXD recombinant inbred strains which develop B-cell lymphomas, T-cell lymphomas, or both. AKXD-23 myeloid tumors are monoclonal, and their DNA contains somatically acquired proviruses, suggesting that they are retrovirally induced. We identified a common site of ecotropic proviral integration that is present in the DNA of all AKXD-23 myeloid tumors that were analyzed and in the DNA of all myeloid tumors that occur in AKXD strains other than AKXD-23. We designated this locus Evi-1 (ecotropic viral integration site 1). Rearrangements in the Evi-1 locus were also detected in the DNA of a number of myeloid tumors and myeloid cell lines isolated from strains other than AKXD. In contrast, few Evi-1 rearrangements were detected in the DNA of T- or B-cell tumors. Evi-1 may thus identify a new proto-oncogene locus that is involved in myeloid disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel J. M., Bedigian H. G. Expression of murine leukemia viruses in B-cell lymphomas of CWD/Agl mice. J Virol. 1984 Nov;52(2):691–694. doi: 10.1128/jvi.52.2.691-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedigian H. G., Johnson D. A., Jenkins N. A., Copeland N. G., Evans R. Spontaneous and induced leukemias of myeloid origin in recombinant inbred BXH mice. J Virol. 1984 Sep;51(3):586–594. doi: 10.1128/jvi.51.3.586-594.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. The tandem direct repeats within the long terminal repeat of murine leukemia viruses are the primary determinant of their leukemogenic potential. J Virol. 1984 Dec;52(3):945–952. doi: 10.1128/jvi.52.3.945-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson T. N., Morse H. C., 3rd, Rowe W. P. Spontaneous tumors of NFS mice congenic for ecotropic murine leukemia virus induction loci. J Natl Cancer Inst. 1984 Aug;73(2):521–524. doi: 10.1093/jnci/73.2.521. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., O'Neill R. R., Rutledge R. A., Theodore T. S., Martin M. A., Ruscetti S. K., Austin J. B., Hartley J. W. Biologic and molecular characterization of two newly isolated ras-containing murine leukemia viruses. J Virol. 1987 Jul;61(7):2109–2119. doi: 10.1128/jvi.61.7.2109-2119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M., Adams J. M., Cory S. Murine T lymphomas with retroviral inserts in the chromosomal 15 locus for plasmacytoma variant translocations. 1985 Apr 25-May 1Nature. 314(6013):740–743. doi: 10.1038/314740a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. L., Langdon W. Y., Fredrickson T. N., Coffman R. L., Hoffman P. M., Hartley J. W., Morse H. C., 3rd Analysis of neoplasms induced by Cas-Br-M MuLV tumor extracts. J Immunol. 1986 Jul 15;137(2):679–688. [PubMed] [Google Scholar]
- Holmes K. L., Palaszynski E., Fredrickson T. N., Morse H. C., 3rd, Ihle J. N. Correlation of cell-surface phenotype with the establishment of interleukin 3-dependent cell lines from wild-mouse murine leukemia virus-induced neoplasms. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6687–6691. doi: 10.1073/pnas.82.19.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak I., Lee J. C., Enjuanes L., Ihle J. N. Characterization of a unique defective type C virus associated with a Moloney leukemia virus-induced splenic T-cell lymphoma cell line. J Virol. 1980 Nov;36(2):299–308. doi: 10.1128/jvi.36.2.299-308.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison K. W., Copeland N. G., Jenkins N. A. Dilute-coat-color locus of mice: nucleotide sequence analysis of the d+2J and d+Ha revertant alleles. Mol Cell Biol. 1984 Dec;4(12):2899–2904. doi: 10.1128/mcb.4.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Greenberger J. S., Henderson L., Yetter R. A., Morse H. C., 3rd Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982 Oct;129(4):1377–1383. [PubMed] [Google Scholar]
- Ishimoto A., Adachi A., Sakai K., Matsuyama M. Long terminal repeat of Friend-MCF virus contains the sequence responsible for erythroid leukemia. Virology. 1985 Feb;141(1):30–42. doi: 10.1016/0042-6822(85)90180-1. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. R., Weinstein Y., Hursey M., Ihle J. N. Interleukins 2 and 3 regulate the in vitro proliferation of two distinguishable populations of 20-alpha-hydroxysteroid dehydrogenase-positive cells. J Immunol. 1985 Sep;135(3):1864–1871. [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Holland C. A., Hartley J. W., Hopkins N. Viral integration near c-myc in 10-20% of mcf 247-induced AKR lymphomas. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6808–6811. doi: 10.1073/pnas.81.21.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Copeland N. G., Jenkins N. A. Characterization of somatically acquired ecotropic and mink cell focus-forming viruses in lymphomas of AKXD recombinant inbred mice. J Virol. 1987 Sep;61(9):2929–2933. doi: 10.1128/jvi.61.9.2929-2933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Jenkins N. A., Copeland N. G. AKXD recombinant inbred strains: models for studying the molecular genetic basis of murine lymphomas. Mol Cell Biol. 1986 Dec;6(12):4236–4243. doi: 10.1128/mcb.6.12.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Cleveland J. L., Fredrickson T. N., Holmes K. L., Morse H. C., 3rd, Jansen H. W., Patschinsky T., Bister K. Rapid induction of hemopoietic neoplasms in newborn mice by a raf(mil)/myc recombinant murine retrovirus. J Virol. 1985 Jul;55(1):23–33. doi: 10.1128/jvi.55.1.23-33.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten G., Cuypers H. T., Zijlstra M., Melief C., Berns A. Involvement of c-myc in MuLV-induced T cell lymphomas in mice: frequency and mechanisms of activation. EMBO J. 1984 Dec 20;3(13):3215–3222. doi: 10.1002/j.1460-2075.1984.tb02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Takano T. Long terminal repeat (LTR)-derived recombination of retroviral DNA: sequence analyses of an aberrant clone of baboon endogenous virus DNA which carries an inversion from the LTR to the gag region. Nucleic Acids Res. 1982 Sep 11;10(17):5333–5343. doi: 10.1093/nar/10.17.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Quint W., Cuypers T., Radaszkiewicz T., Schoenmakers H., de Goede R., Melief C. Ecotropic and mink cell focus-forming murine leukemia viruses integrate in mouse T, B, and non-T/non-B cell lymphoma DNA. J Virol. 1986 Mar;57(3):1037–1047. doi: 10.1128/jvi.57.3.1037-1047.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., de Goede R. E., Schoenmakers H., Radaszkiewicz T., Melief C. J. Ecotropic and dualtropic mink cell focus-inducing murine leukemia viruses can induce a wide spectrum of H-2 controlled lymphoma types. Virology. 1984 Oct 30;138(2):198–211. doi: 10.1016/0042-6822(84)90345-3. [DOI] [PubMed] [Google Scholar]