Significance
Schizophrenia is a debilitating psychiatric disorder characterized by positive, negative, and cognitive symptoms. Current antipsychotic drugs, including D2 receptor (D2R) partial agonist aripiprazole, antagonize excess striatal dopamine (DA) neurotransmission and reverse positive symptoms but are not efficacious at reversing cortical-related cognitive symptoms. Here, we show using pharmacological, behavioral, and electrophysiological approaches that a β-arrestin2 (βarr2)-biased D2R ligand has opposite antagonist and agonist actions in the striatum and cortex, respectively. This phenomenon is regulated by differential expression levels of signal transducer proteins G protein-coupled receptor kinase 2 and βarr2. Thus, D2R-βarr2–biased ligands have the potential to simultaneously target excess striatal and deficient cortical DA neurotransmission and provide more broadly effective therapies for schizophrenia.
Keywords: arrestin, antipsychotics, biased signaling, dopamine D2R, fast-spiking interneurons
Abstract
The current dopamine (DA) hypothesis of schizophrenia postulates striatal hyperdopaminergia and cortical hypodopaminergia. Although partial agonists at DA D2 receptors (D2Rs), like aripiprazole, were developed to simultaneously target both phenomena, they do not effectively improve cortical dysfunction. In this study, we investigate the potential for newly developed β-arrestin2 (βarr2)-biased D2R partial agonists to simultaneously target hyper- and hypodopaminergia. Using neuron-specific βarr2-KO mice, we show that the antipsychotic-like effects of a βarr2-biased D2R ligand are driven through both striatal antagonism and cortical agonism of D2R-βarr2 signaling. Furthermore, βarr2-biased D2R agonism enhances firing of cortical fast-spiking interneurons. This enhanced cortical agonism of the biased ligand can be attributed to a lack of G-protein signaling and elevated expression of βarr2 and G protein-coupled receptor (GPCR) kinase 2 in the cortex versus the striatum. Therefore, we propose that βarr2-biased D2R ligands that exert region-selective actions could provide a path to develop more effective antipsychotic therapies.
G protein-coupled receptors (GPCRs) represent the largest family of receptors in the human genome and are one of the most common targets of pharmaceutical drugs (1, 2). Upon ligand binding, GPCRs activate downstream G protein-dependent signaling pathways followed by phosphorylation of the receptor by G protein-coupled receptor kinases (GRKs) (3). Phosphorylation enhances association of the GPCR with β-arrestins (βarrs), and this combined process mediates desensitization of G-protein signaling (4) and internalization of GPCRs (5–7). Two isoforms of βarrs, βarr1 and βarr2, are widely coexpressed in most tissues in mammals and are 80% identical, but they can have either overlapping or distinct functions (8, 9). It is now firmly established that GPCRs activate downstream signaling pathways through not only canonical G-protein pathways but also, the ability of βarrs to scaffold distinct intracellular signaling complexes (10–12). Elucidation of these distinct G-protein and βarr signaling pathways has provided support for the concept of functional selectivity or biased signaling, wherein each signaling pathway has the ability to mediate distinct physiological responses (13). There are now several physiologically relevant examples of selective engagement of signaling pathways or selective GPCR ligands that target these different signaling pathways (13–15). Therefore, leveraging the concept of GPCR functional selectivity holds promise for the development of more selective therapeutic approaches.
Dopamine (DA) is a catecholamine neurotransmitter that has been implicated in movement, reward, and cognition (16–19) as well as CNS disorders, such as schizophrenia, attention deficit hyperactivity disorder, Parkinson’s disease, and obsessive–compulsive disorder (20–23). DA mediates its effects via GPCRs belonging to two major subclasses of receptors: the D1 class [D1 receptor (D1R) and D5 receptor] and the D2 class [D2 receptor (D2R), D3 receptor, and D4 receptor] (24), a classification based on their ability to activate the stimulatory G protein Gαs/olf or inhibitory G protein Gαi/o signaling pathway, respectively. In the brain, D2Rs activate canonical Gαi/o-mediated signaling to inhibit adenylyl cyclase, cyclic adenosine monophosphate (cAMP) production, and the protein kinase A/dopamine and cAMP regulated phosphoprotein Mr 32 KDa (PKA/DARPP32) pathway to mediate many of the behavioral effects of DA (23, 25, 26). However, based on the initial observation that the DA-dependent locomotor response to amphetamine (AMPH) was markedly attenuated in mice globally lacking βarr2 but not βarr1 (9), we provided biochemical and genetic evidence for a βarr2-dependent signaling pathway downstream of D2Rs that is separate from Gαi/o signaling (27). We have shown that this D2R-βarr2 signaling pathway inhibits protein kinase B (PKB or AKT) activity, activates glycogen synthase kinase 3 beta (GSK3β), and can mediate specific DA-dependent behaviors (28–30).
D2Rs are the major target for most antipsychotic drugs (APDs), which are the first-line treatment for schizophrenia (31, 32). APDs do not treat all symptoms of schizophrenia effectively and have several side effects that are thought to be associated with Gαi/o signaling (26, 30). APDs that selectively target the D2R-βarr2 pathway could be therapeutically beneficial without producing extrapyramidal side effects. To further investigate the potential role of functional selectivity of the D2R-βarr2 pathway in APD action, we have recently generated βarr2-biased D2R ligands based on the scaffold of the partial agonist APD aripiprazole (ARI) (33, 34). These biased ligands show antipsychotic-like profile in pharmacological [phencyclidine (PCP) and AMPH] and genetic (NMDA receptor NR1 subunit knockdown) mouse models of schizophrenia-like phenotypes that depend on their D2R-βarr2 selectivity, because their activity is lost in global βarr2-KO mice (33, 35). Although classical APDs, like haloperidol, and newer APDs, like ARI, reverse the postulated striatal hyperdopaminergic tone associated with schizophrenia, none of these drugs effectively correct cortical dysfunction (36–38). It is currently not known whether targeting D2R-βarr2 signaling might represent an alternative strategy to identify more broadly effective APDs.
In this study, we used βarr2-biased D2R ligands and behavioral and electrophysiological approaches in mice lacking βarr2 in various D2R-expressing neuronal populations to investigate whether region-specific D2R-βarr2 signaling contributes to unique antipsychotic-like effects in vivo.
Results
Determinants of in Vitro D2R-βarr2 Functional Selectivity.
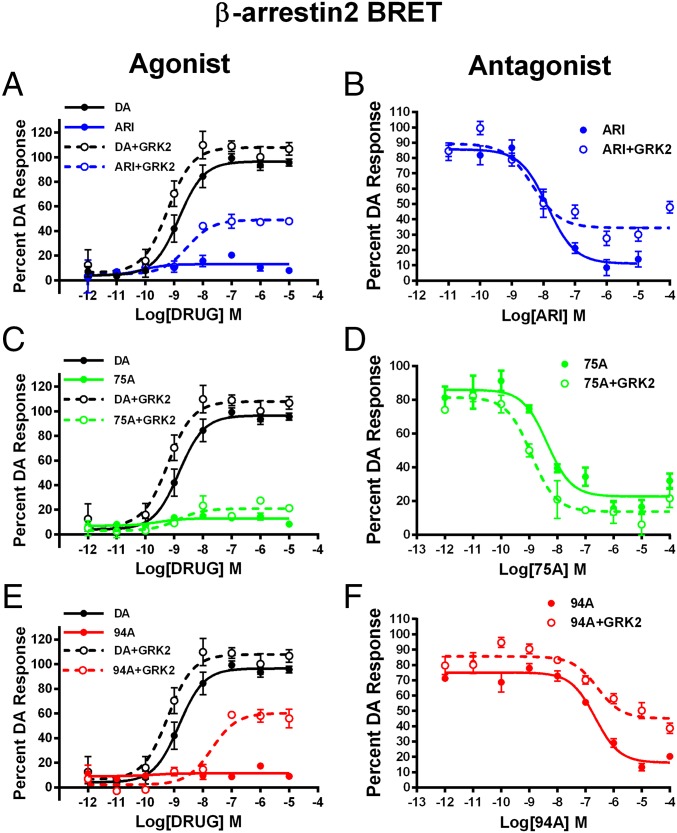
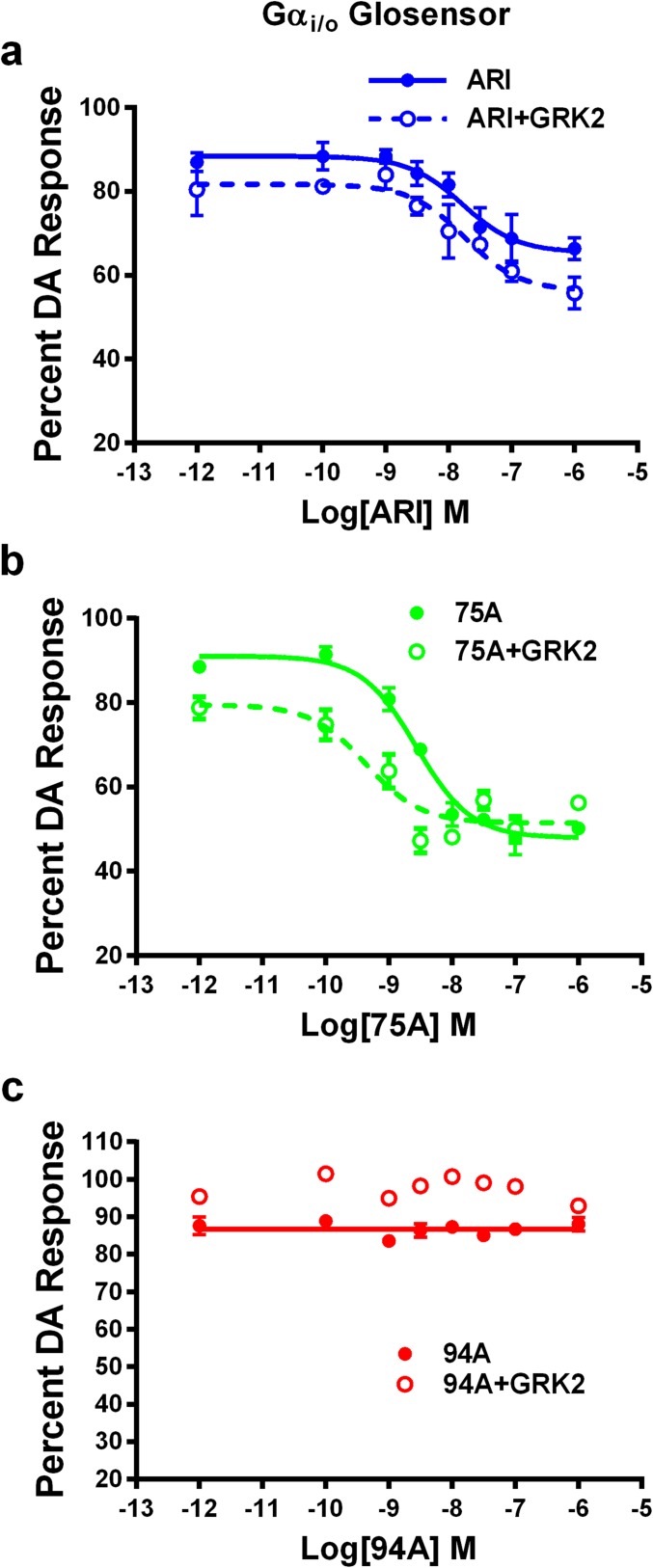
Clinically effective APDs are either antagonists or partial agonists at both D2R-G protein and D2R-βarr2 signaling pathways (39, 40). We have previously shown that the βarr2-biased D2R ligands UNC9994A (94A) and UNC9975A (75A), which are based on the scaffold of ARI, are weak selective partial agonists at D2R-βarr2 interactions and have little or no agonist activity at D2R-G protein signaling (33). However, we show in Fig. 1 that the agonist versus antagonist profiles of these ligands at D2R-βarr2 interactions in HEK293 cells can be modulated by the complement of GRK2 and βarr2. In a bioluminescence resonance energy transfer (BRET)-based assay (Fig. 1 A, C, and E), compared with DA ARI, 94A and 75A are very weak partial agonists at mediating D2R-βarr2 interactions, but increasing GRK2 levels in these cells markedly enhanced the partial agonist activity of only ARI and 94A. Consistent with pharmacological principles, when tested as antagonists (Fig. 1 B, D, and F), all three ligands fully antagonized DA-mediated D2R-βarr2 interactions, and high GRK2 levels reduced the antagonist efficacy of only ARI and 94A. The profile of 75A was not significantly changed by GRK2, suggesting that it may have slightly different properties than ARI or 94A. However, in a D2R–G protein (GloSensor) assay, ARI and 75A but not 94A behaved as antagonists (Fig. S1 and Table S1), suggesting that only 94A is a completely selective D2R-βarr2 ligand. These results are consistent with the established concept that βarr-dependent GPCR functions are not only dependent on agonist activation but also, enhanced by phosphorylation of the receptor by GRKs (11, 41) and that the expression levels of βarr2 and GRK2 can regulate GPCR signaling and the pharmacological profile of ligands (42–44).
Fig. 1.
Effects of GRK2 overexpression on the agonist or antagonist profile of D2R ligands in HEK293 cells. (A) ARI, (C) UNC9975A (75A), and (E) UNC9994A (94A) were tested in agonist mode in a D2R-βarr2 interaction BRET assay with endogenous GRK2 (solid lines) or GRK2 overexpression (dashed lines) compared with DA (black lines) in HEK293 cells. D2R-βarr2 BRET antagonist assays for (B) ARI, (D) UNC99975A (75A), and (F) UNC9994A (94A) with endogenous GRK2 (solid lines) or overexpressed GRK2 (dashed lines) levels in HEK293 cells. Data are presented as inhibition of the total DA response (mean ± SEM).
Fig. S1.
D2R-Gαi/o GloSensor antagonist assay. In vitro D2R-Gαi/o GloSensor antagonist assays for (A) ARI, (B) UNC99975A (75A), and (C) UNC9994A (94A) with endogenous GRK2 (solid lines) or overexpressed GRK2 (dashed lines) levels in HEK293 cells. Data are presented as inhibition of the total DA response (mean ± SEM).
Table S1.
IC50 and Emax of antipsychotic compounds on antagonist assays
| Compound | eGRK2 | oeGRK2 | ||||
| IC50 (average) | pIC50 | Inhibition (%) | IC50 (average) | pIC50 | Inhibition (%) | |
| Gαi/o GloSensor | ||||||
| ARI | 11.5 | 8.10 ± 0.28 | 40 ± 8 | 3.5 | 8.48 ± 0.12 | 32 ± 2 |
| UNC9975 | 4.5 | 8.37 ± 0.11 | 48 ± 6 | 3.7 | 8.54 ± 0.24 | 47 ± 5 |
| UNC9994A | N.I. | N.I. | ||||
| βarr2 BRET | ||||||
| ARI | 12.8 | 8.10 ± 0.28 | 71 ± 9 | 3.2 | 8.59 ± 0.13 | 63 ± 9 |
| UNC9975 | 2.8 | 8.58 ± 0.10 | 59 ± 8 | 4.1 | 8.60 ± 0.25 | 79 ± 5 |
| UNC9994A | 200 | 6.72 ± 0.12 | 89 ± 5 | 186 | 6.77 ± 0.08 | 64 ± 7 |
eGRK2, endogenous G protein-coupled receptor kinase 2; N.I., no inhibition; oeGRK2, overexpressed G protein-coupled receptor kinase 2; pIC50, negative log of the IC50 value in molar.
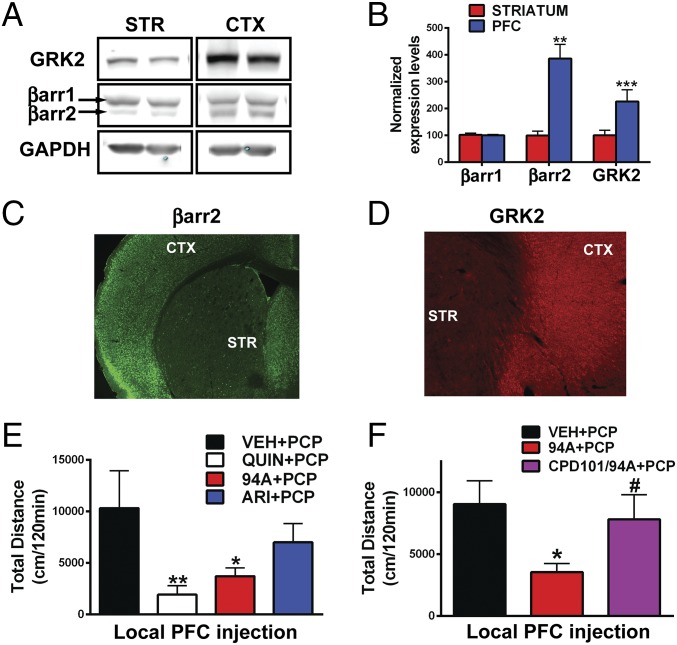
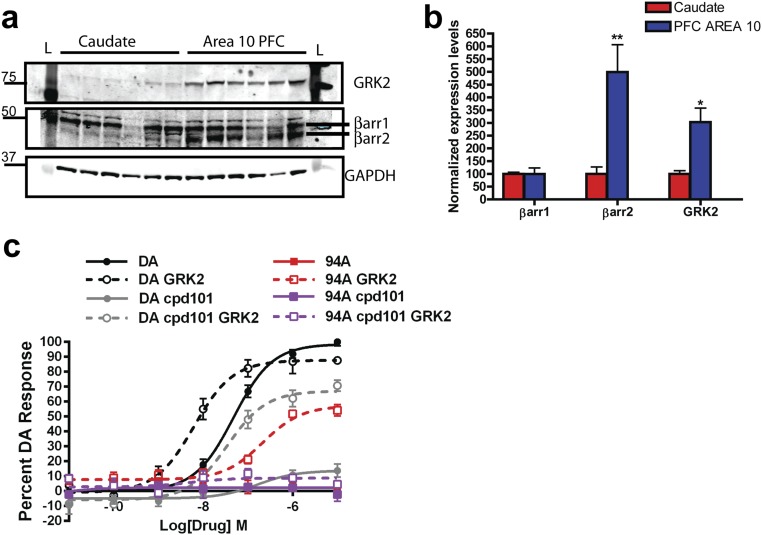
Previous studies have suggested that the levels of GRKs and βarrs can vary significantly between tissues and brain areas (45, 46). Protein levels of βarr2 and GRK2 but not βarr1 are higher in the prefrontal cortex (PFC) compared with the striatum (STR) in mice (Fig. 2 A–D) and humans (Fig. S2 A and B) (Ps < 0.05). Because ARI and 94A gain D2R-βarr2 partial agonism on GRK2 overexpression, we hypothesized that these compounds might behave as agonists in the PFC (high βarr2/GRK2). Previous studies have shown that local injection of the D2R agonist quinpirole can exert antipsychotic-like effects, because it inhibits locomotion induced by the NMDA receptor antagonist PCP (47). PCP-induced locomotion is a pharmacologically induced behavior commonly inhibited by APDs, and the behavioral effects of PCP are thought to be mediated by cortical disinhibition (48). To assess whether PFC D2R-βarr2 agonism can elicit antipsychotic-like effects, we measured the effects of injecting quinpirole, ARI, and 94A locally into the PFC of WT mice on PCP-induced locomotion. Consistent with previous findings, local bilateral PFC injection of the unbiased full-agonist quinpirole significantly inhibited PCP-induced locomotion (Fig. 2E, QUIN) (P < 0.01); 94A mimicked this D2R agonist action (Fig. 2E, 94A) (P < 0.05) and inhibited PCP-induced locomotion, whereas the effect of ARI was weaker and did not reach significance. Coadministration of a pharmacological GRK2 inhibitor [compound 101 (cpd101)] prevented the 94A-mediated inhibition of PCP-induced locomotion (Fig. 2F, cpd101/94A). These results are consistent with our in vitro data, showing that, in a D2R-βarr2 interaction BRET assay, the ability of GRK2 to enhance the agonist effects of both DA and UNC9994A is lost in presence of the GRK2 inhibitor (cpd101) (Fig. S2C). These observations show that D2R-βarr2 agonism in the PFC is sufficient to inhibit PCP-induced locomotion, and this effect is dependent on βarr and GRK2.
Fig. 2.
Cortical and striatal expression patterns of βarr2 and GRK2 as well as D2R PFC agonism. (A) Western blot analysis from WT mice probed with antibodies to GRK2, βarr2, and βarr1 and GAPDH in cortex (CTX) compared with STR and (B) quantification of Western blot band intensities normalized to GAPDH (loading control; Ps < 0.05). IHC images of mouse brain sections (cortical-striatal) stained with antibodies to (C) βarr2 and (D) GRK2. Locomotor responses to (E) bilateral local PFC injection 1 μg per side quinpirole (QUIN), UNC9994A (94A), and aripiprazole (ARI) in WT mice followed by systemic PCP injection (6 mg/kg i.p.; n = 8–11) or (F) bilateral local PFC injection of 1 μg per side UNC9994A (94A) with or without cpd101 (0.5 μg per side) in WT mice followed by systemic PCP injection (6 mg/kg i.p.; n = 8–10). *P < 0.05, compared with VEH + PCP; **P < 0.01, compared with VEH + PCP; #P < 0.05, compared with 94A + PCP.
Fig. S2.
GRK2 and βarr2 expression in PFC compared with STR in human postmortem tissue and effect of GRK2 inhibition on D2R-βarr2 BRET agonist responses. (A) Human tissue samples from caudate and PFC (area 10) were lysed and analyzed by Western blot. Antibodies to GRK2, βarr1/2 (a2ct), and GAPDH (loading control) were used (n = 6). (B) Quantification of βarr1, βarr2, and GRK2 levels in PFC (blue bars) and caudate (red bars) normalized to GAPDH. Caudate levels were set to 100%. *P < 0.05, compare caudate with PFC using a two-way ANOVA (Bonferroni) test; **P < 0.01, compare caudate with PFC using a two-way ANOVA (Bonferroni) test. (C) DA and 94A were tested in agonist mode in a D2R-βarr2 BRET assay with endogenous GRK2 (solid lines) or GRK2 overexpression (dashed lines) in HEK293 cells with or without pretreatment of GRK2/3 inhibitor 10 μM cpd101 or VEH for 1 h. Cpd101 inhibits the D2-βarr2 interaction with endogenous GRK2 levels or inhibits the increased potency of D2-βarr2 interaction with GRK2 overexpression.
Distinct Striatal Vs. Cortical Role of D2R on PCP-Mediated Behavioral Response.
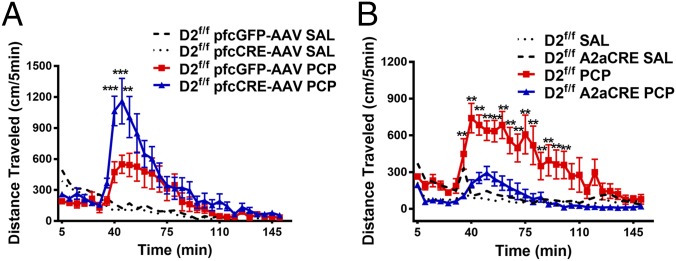
Although D2R PFC agonism inhibits PCP-induced locomotion, striatal D2R antagonism is thought to be the primary mechanism of action of most APDs (49, 50). To broadly explore this paradoxical role of D2R function behaviorally, we selectively deleted D2Rs in either the PFC by injecting an mCherry-Cre adeno-associated virus (AAV) in the previously described D2R floxed (D2f/f) mice (51) or the STR by crossing D2f/f mice with adenosine 2A receptor Cre (A2aCre) mice (Fig. S3), and we tested the effect on PCP-induced locomotion. We observed that, compared with controls, deletion of D2Rs in the PFC resulted in an enhanced response to acute PCP injection (Fig. 3A) (Ps < 0.01), consistent with D2R agonism playing an inhibitory role on the PCP response (47). However, deletion of D2Rs in the STR attenuated the PCP response (Fig. 3B) compared with controls (Ps < 0.01), consistent with striatal D2R antagonism inhibiting PCP-mediated responses (49, 50). These pharmacological and behavioral data suggest distinct roles for D2R signaling in the PFC versus the STR in mediating antipsychotic-like effects.
Fig. S3.
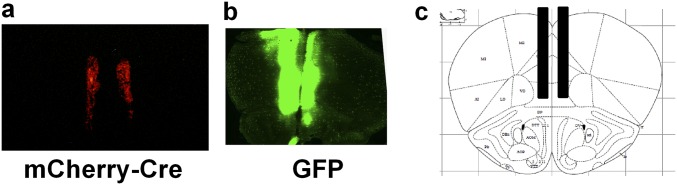
AAV injection in PFC of D2f/f mice. Representative image of coronal sections of (A) mCherry-Cre and (B) GFP AAV injection in the PFC of D2f/f mice. (C) Stereotaxic atlas image (Paxinos and Franklin Atlas) of coordinates for PFC injection of AAVs in D2f/f mice.
Fig. 3.
Distinct contribution of striatal and cortical D2Rs to the behavioral effects of PCP. (A) D2f/f mice were injected with a control GFP virus (GFP-AAV) or Cre-AAV in the PFC and 3 wk later, injected systemically with either saline (SAL) or PCP (4 mg/kg i.p.); their locomotor response was recorded (n = 8–9). **P < 0.01; ***P < 0.001 compare D2f/f pfcGFP-AAV with D2f/f pfcCre-AAV (PCP) using a three-way RMANOVA [genotype × treatment × time interaction, F(29, 720) = 1.947, P < 0.01] with post hoc Bonferroni tests. (B) D2f/f mice were crossed with A2aCre mice (D2f/f A2aCre) to delete D2Rs in the STR and injected systemically with either SAL or PCP (6 mg/kg i.p.); their locomotor response was recorded (n = 8–13). **P < 0.01; ***P < 0.001 compare D2f/f with D2f/f A2aCre (PCP) using a three-way RMANOVA [genotype × treatment × time interaction, F(29, 840) = 3.722, P < 0.001] with post hoc Bonferroni tests.
Generation and Characterization of βarr2 Floxed Mice.
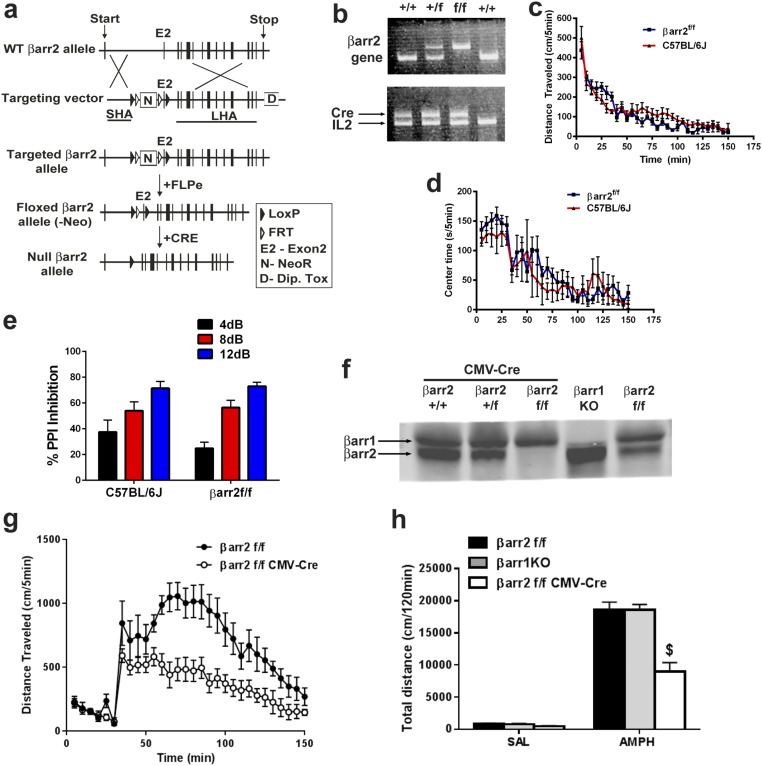
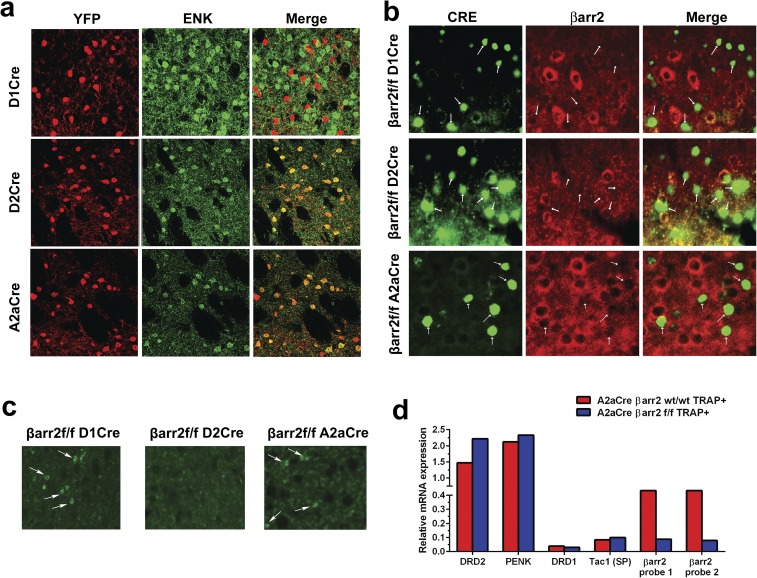
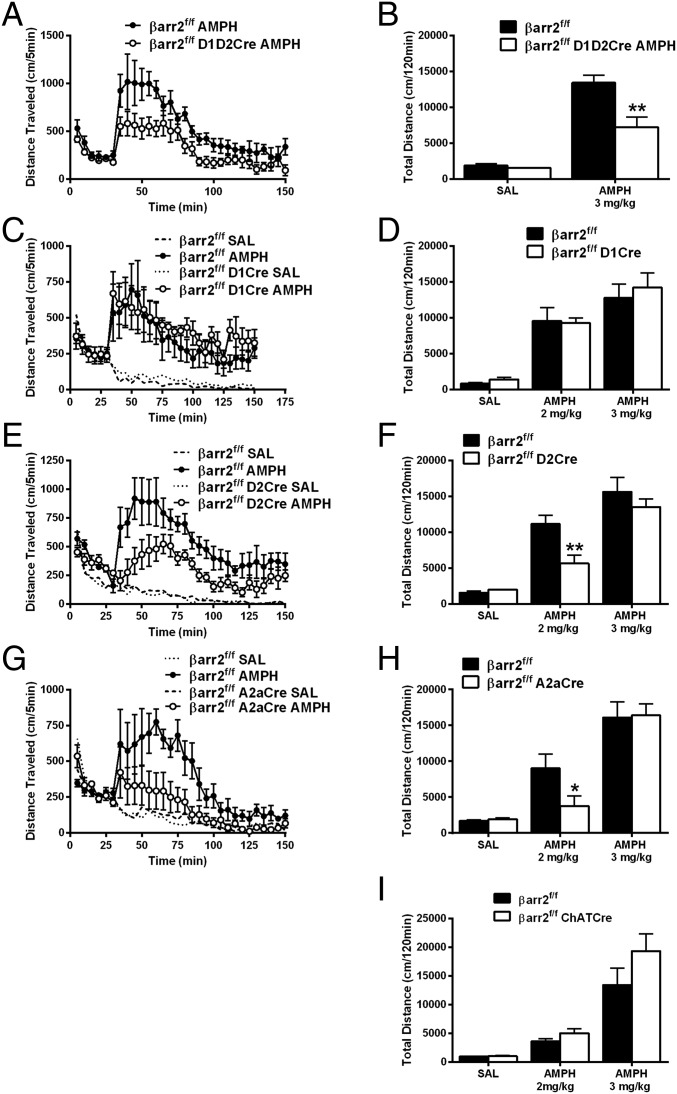
To further evaluate the potential opposite role of D2R-βarr2 signaling in the PFC and STR in antipsychotic-like effects, we generated a βarr2 floxed (βarr2f/f) mouse line for region- and cell-specific Cre-dependent deletion of βarr2 (details are in Materials and Methods and SI Materials and Methods; Fig. S4 A and B); βarr2f/f mice were indistinguishable from C57BL/6J mice in standard behavioral tests (Fig. S4 C–E). Deleting βarr2 in all neurons (βarr2f/f CMV-Cre) recapitulated the decrease in the AMPH locomotor response originally observed in whole-body βarr2KO mice (27) (Fig. S4 F–H). We have previously shown that the AMPH locomotor response is mediated at least in part by D2Rs in a βarr2-dependent manner (27, 29), but D2Rs are expressed in multiple regions of the brain, such as midbrain, STR, and PFC (24, 52–57). To confirm which D2R+ population of neurons is responsible for the AMPH response, we deleted βarr2 in several distinct neuronal populations; βarr2f/f mice were crossed with either D2Cre (all D2R+ neurons) or A2aCre (postsynaptic D2R+ striatal neurons) mice as well as the D1Cre (D1R+ neurons) or ChaTCre (cholinergic interneurons) mice as controls. Using immunohistochemistry (IHC) and real-time quantitative PCR techniques, we confirmed selective deletion of βarr2 in appropriate neuronal populations of all Cre lines crossed with the βarr2f/f mice (Fig. S5). As shown in Fig. 4, βarr2 in striatal D2R+ neurons seems to play the most prominent role in the AMPH response, because the locomotor response is reduced only in D2R+ or A2aR+ neurons lacking βarr2 (Fig. 4 E–H) but not affected by deletion of βarr2 in either D1R+ neurons alone or cholinergic interneurons (Fig. 4 C, D, and I). These data suggest that deleting βarr2 in D2R striatal neurons is sufficient to mimic antipsychotic-like activity. Importantly, the mice in which βarr2 is inactivated in select D2R+ neuronal populations provide unprecedented models to critically examine the in vivo antipsychotic-like function of our D2R-βarr2–biased compounds.
Fig. S4.
Generation and characterization of floxed βarr2 mice. (A) Schematic of targeting strategy to generate the βarr2f/f mice (SI Materials and Methods). (B) PCR confirmation of floxed alleles from βarr2+/+ (WT), βarr2+/f (floxed heterozygote), and βarr2f/f (floxed homozygote) mice crossed with CMV-Cre mice. IL-2 is used as an internal control. (C) Basal locomotor activity in βarr2f/f mice compared with C57BL/6J mice. (D) Time spent in the center of the open field (center time) as a measure of anxiety-like behavior in βarr2f/f mice compared with C57BL/6J mice. (E) Basal PPI in βarr2f/f mice (SI Materials and Methods); n = 7–9 mice. Mice were tested with 4-, 8-, or 12-dB noise above a 64-dB white noise background. (F) Western blot analysis of cortical tissue from βarr2f/f CMV-Cre but not βarr2+/+ CMV-Cre or βarr2+/f CMV-Cre mice shows deletion of βarr2. (G) Locomotor response of controls (βarr2f/f) and βarr2f/f CMV-Cre mice injected with 3 mg/kg AMPH after 30 min of habituation. Data were analyzed by two-way RMANOVA [genotype × time interaction, F(29, 232) = 3.393, P < 0.0001] with post hoc Bonferroni comparison. (H) Postinjection total distance traveled after 3 mg/kg AMPH injection in controls (n = 4) and βarr2f/f CMV-Cre mice (n = 7) or βarr1KO (n = 4) mice. $P < 0.0001, compared with βarr2f/f using a two-way ANOVA (Bonferroni) test.
Fig. S5.
IHC and TRAP analysis to confirm deletion of βarr2 in neuron-specific KO mice. (A) Representative images of IHC analyses for YFP used as a reporter for Cre recombinase activity (YFP; red) and D2 neuron marker enkephalin (ENK) (green) show no colocalization in D1Cre (Top) but show colocalization in D2Cre (Middle) and A2aCre mice (Bottom), confirming appropriate neuron-specific expression of Cre recombinase in all lines. (B) Representative image of IHC staining for βarr2 in the STR shows labeling of morphologically identified cholinergic interneurons (arrows) in D1 βarr2f/f and A2a βarr2f/f but not D2 βarr2f/f mice, consistent with presence of Cre recombinase expression in cholinergic interneurons in only D2Cre mice. (C) Representative image of IHC staining for βarr2 (green) and Cre recombinase (red) shows deletion of βarr2 (arrows) in all lines. (D) A2aCre+ βarr2f/f mice and A2Acre+ βarr2 wt/wt mice were crossed with a Cre-sensitive TRAP mouse line to isolate mRNA from D2R+ MSNs. Real-time quantitative PCR for D2 MSN markers (Drd2 and Penk) and D1 MSN markers (Drd1 and Tac1) confirmed respective enrichment and depletion of these markers from TRAP isolates. D2 MSNs from βarr2 wt/wt mice (red bars) and βarr2f/f mice (blue bars) have similar expression of D2R MSN genes. In contrast, D2R+ MSNs from βarr2f/f mice have a clear decrease in expression for βarr2 as measured via Taqman probes for exon 2 of the Arrb2 gene. These observations indicate that, as expected, recombination has occurred specifically in the D2R+ MSN population and resulted in loss of exon 2 of the Arrb2 transcript.
Fig. 4.
Deletion of βarr2 in D2R-expressing neurons inhibits the AMPH response. (A) βarr2f/f mice were crossed with both D1Cre and D2Cre mice to delete βarr2 in all striatal neurons simultaneously to generate βarr2f/f D1D2Cre and injected with 3 mg/kg AMPH; distance traveled was calculated for 120 min after 30 min of habituation compared with control βarr2f/f mice. Data were analyzed using a two-way RMANOVA test [genotype × time interaction, F(29, 145) = 1.956, P < 0.01] followed by Bonferroni comparisons. (B) Total cumulative distance after injection of saline (SAL) and 3 mg/kg AMPH was calculated for βarr2f/f D1D2Cre and βarr2f/f controls. **P < 0.01, using a two-way ANOVA (Bonferroni) test (n = 7 mice for each genotype). (C) βarr2f/f D1Cre, (E) βarr2f/f D2Cre, or (G) βarr2f/f A2aCre and their respective Cre-negative βarr2f/f controls were injected with SAL or 2 mg/kg AMPH after 30 min of habituation, and locomotor activity was measured for 120 min. Data were analyzed using a three-way RMANOVA for D1βarr2 [genotype × time interaction, F(29, 116) = 0.4045, P = 0.9967; genotype × treatment interaction, F(1, 480) = 3.463, P = 0.0634], A2aβarr2 [genotype × time interaction, F(87, 435) = 4.760; genotype × treatment interaction, F(1, 600) = 47.15, P < 0.001], and D2βarr2 [genotype × time interaction, F(87, 261) = 3.324, P < 0.001; genotype × treatment interaction, F(1, 360) = 66.37, P < 0.001] with post hoc Bonferroni tests. (D, F, and H) Total cumulative (120 min) postinjection distance after SAL or 2 or 3 mg/kg AMPH; n = 8 mice for each group. *P < 0.05, compared with βarr2f/f using a two-way ANOVA (Bonferroni) test; **P < 0.01, compared with βarr2f/f using a two-way ANOVA (Bonferroni) test. (I) ChAT-Cre mice were crossed with βarr2f/f mice to generate βarr2f/f ChATCre or βarr2f/f controls, and total cumulative distance after postinjection of SAL or 2 or 3 mg/kg AMPH was calculated; n = 8 mice for each group.
Region-Specific Responses of Antipsychotics and βarr2-Biased D2R Ligands.
AMPH- and PCP-induced hyperlocomotion are the two commonly used pharmacological models to test APD efficacy. Most APDs are D2R partial agonists or antagonists with varying potencies and efficacies (31, 32, 40, 58) and inhibit either the AMPH- or PCP-induced locomotor response in mice (59). The AMPH-induced locomotor response is dependent on striatal DA release, whereas the behavioral effects of PCP are thought to be mediated by cortical disinhibition and activation of the corticostriatal pathway (48, 50). We tested the ability of representative first, second, and third generation APDs, such as haloperidol, clozapine, and ARI, respectively, along with the βarr2-biased D2R ligands 94A and 75A in both of these pharmacological models. Optimal doses for APDs and the D2R-βarr2–biased ligands were based on previous studies (30, 33, 35).
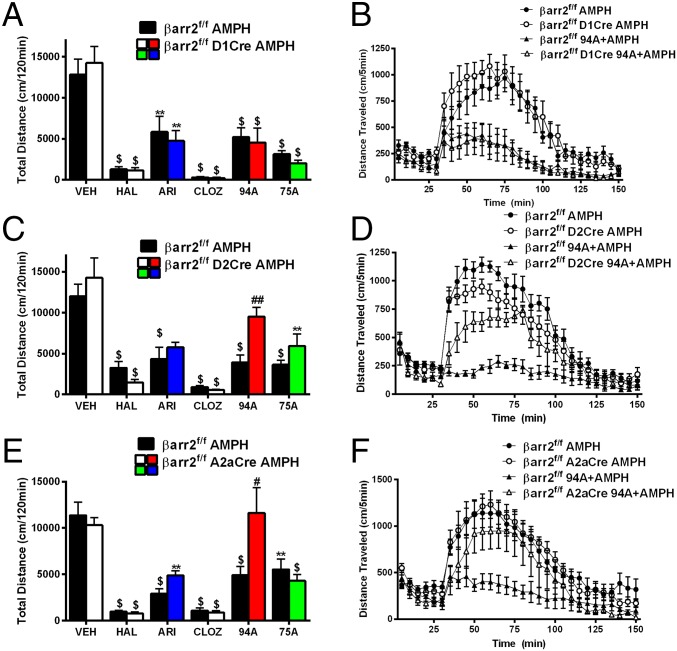
For the AMPH-induced locomotor response, AMPH was injected at a dose (3 mg/kg) at which there were no significant genotype differences between mice (Fig. 4 C–H). This higher dose allowed us to achieve better separation of effects when treating with APDs and biased compounds. Consistent with their antagonist activity, all tested APDs and biased D2R ligands significantly inhibited AMPH-induced locomotion in all control βarr2f/f mice (Fig. 5, black bars) (Ps < 0.01). No genotype differences were observed in mice lacking βarr2 in D1R+ neurons for all compounds (Fig. 5 A and B) (Ps < 0.01). All three APDs and 75A also inhibited the AMPH response (Ps < 0.01) in mice lacking βarr2 in either all D2R+ (Fig. 5C) or striatal A2aR+ neurons (Fig. 5E). However, in these mice, 94A showed markedly diminished antipsychotic-like activity compared with genotype controls (Ps < 0.05), consistent with this compound being a selective βarr2-biased D2R antagonist. Compared with other APDs, the observations with 94A indicate that D2R-βarr2 antagonism is sufficient but not necessary for efficacious antipsychotic-like activity in the AMPH pharmacological model, which is consistent with previous observations (60).
Fig. 5.
UNC9994A loses its antipsychotic-like activity in response to AMPH in D2R+ and A2aR+ neuron-specific βarr2KO mice. Control mice (βarr2f/f) and (A) βarr2f/f D1Cre, (C) βarr2f/f D2Cre, or (E) βarr2f/f A2aCre mice were injected with vehicle (VEH); the antipsychotics haloperidol (HAL; 0.5 mg/kg), ARI (0.5 mg/kg), or clozapine (CLOZ; 2 mg/kg); or βarr2-biased drugs UNC9994A (94A; 2 mg/kg) or UNC9975A (75A; 0.5 mg/kg) followed by 3 mg/kg AMPH injection. Total cumulative distance postinjection of AMPH for 120 min was calculated and shows that all APDs and drugs, except UNC9994A, are able to inhibit the AMPH response in the βarr2f/f D2Cre and βarr2f/f A2aCre mice. **P < 0.01, compared with respective VEH control; $P < 0.001, compared with respective VEH control; #P < 0.05 compare 94A between genotypes using a two-way ANOVA (Bonferroni) test; ##P < 0.01 compare 94A between genotypes using a two-way ANOVA (Bonferroni) test. Representative graphs of AMPH inhibition by 94A for (B) βarr2f/f D1Cre, (D) βarr2f/f D2Cre, or (F) βarr2f/f A2aCre mice compared with controls (βarr2f/f); n = 8 mice for each group. Data were analyzed by two-way RMANOVA [genotype × treatment interaction, F(1, 420) = 2.053, P = 0.1526, for βarr2f/f D1Cre; genotype × treatment interaction, F(29, 420) = 3.858, P < 0.05, for βarr2f/f A2aCre; and genotype × treatment interaction, F(29, 420) = 2.285, P < 0.01, for βarr2f/f D2Cre] with post hoc Bonferroni tests.
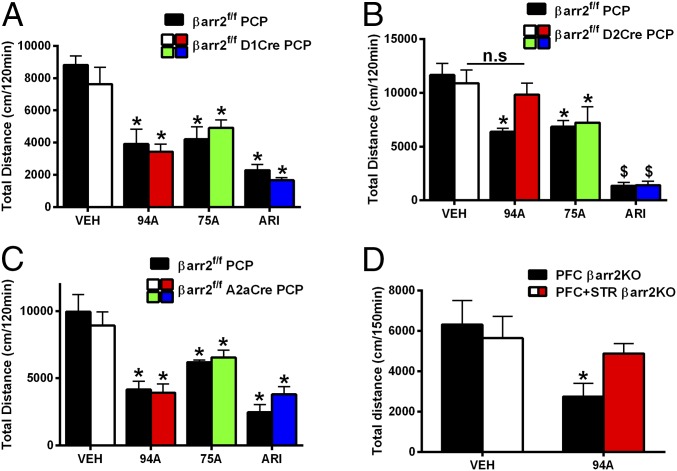
For PCP-induced responses, in mice lacking βarr2 in D1R+ neurons, all drugs significantly inhibited PCP-induced locomotion compared with vehicle (VEH)-treated controls (Fig. 6A) (Ps < 0.05), and there were no genotype differences. ARI and 75A also inhibited PCP-induced locomotion in mice lacking βarr2 in D2R+ striatal neurons (Fig. 6B) (Ps < 0.05) or all D2R+ neurons (Fig. 6C) (Ps < 0.05) compared with genotype and VEH controls. In contrast to the AMPH response (Fig. 4), however, we observed a loss of 94A activity only in mice lacking βarr2 in all D2R+ neurons (Fig. 6B) (P = 0.2526) but not in striatal D2R+ neurons (Fig. 6C) (P < 0.05), suggesting a role for βarr2 outside the STR, because A2aCre is essentially STR-selective. Although the D2Cre is expressed in STR, PFC, and midbrain, a cortical role for βarr2 in 94A-mediated inhibition of PCP-induced locomotion is most likely, because evidence suggests against a role for midbrain DA neuron βarr2 (61) and the primary site of action for PCP is in the PFC. However, to further rule out the contribution of midbrain presynaptic βarr2 and confirm a role for cortical βarr2, we deleted βarr2 in PFC and STR or PFC alone by injecting mCherry-Cre AAV in the PFC of βarr2f/f A2aCre (PFC + STR βarr2KO) or βarr2f/f mice (PFC βarr2KO) (Fig. S6); 94A inhibited PCP-induced locomotion in PFC βarr2KO mice (Fig. 6D) (P < 0.05) but lost its antipsychotic-like activity when βarr2 was deleted in both PFC and STR (Fig. 6D), thus confirming a dual dependence on cortical and striatal βarr2. These data suggest that cortical and striatal βarr2 are necessary for the antipsychotic-like effect of 94A. Thus, our behavioral data further support our initial supposition that distinct mechanisms might regulate the antipsychotic-like effect of D2R-βarr2 signaling in the PFC (agonism) versus the STR (antagonism). We next wanted to confirm the neuronal mechanism of these distinct phenomena electrophysiogically in the PFC and STR.
Fig. 6.
UNC9994A loses antipsychotic-like activity to PCP in D2R+ but not A2aR+ neuron-specific βarr2KO mice. Control mice (βarr2f/f) and (A) βarr2f/f D1Cre, (B) βarr2f/f D2Cre, or (C) βarr2f/f A2aCre mice were injected with VEH, ARI (0.5 mg/kg), or βarr-biased drugs UNC9994A (94A; 2 mg/kg) and UNC9975A (75A; 0.5 mg/kg) followed by 6 mg/kg PCP injection 10 min later. Total cumulative distance postinjection of PCP for 120 min was calculated; n = 8–10 mice for each group. One mouse each from the βarr2f/f A2aCre 94A- and 75A-treated groups was discarded based on criterion set in Statistical Analyses. *P < 0.05, compared with respective VEH controls using a two-way ANOVA (Bonferroni) test; $P < 0.001, compared with respective VEH controls using a two-way ANOVA (Bonferroni) test. (D) mCherry-Cre AAV8 was injected into the PFC of βarr2f/f A2aCre (PFC + STR βarr2KO) or βarr2f/f (PFC βarr2KO) mice followed by injection 3 wk later with 94A (2 mg/kg i.p.) and PCP (6 mg/kg i.p.). Total cumulative distance postinjection of PCP for 120 min was calculated; n = 8 mice for each group. *P < 0.05, compared with respective VEH controls using a two-way ANOVA (Bonferroni) test.
Fig. S6.
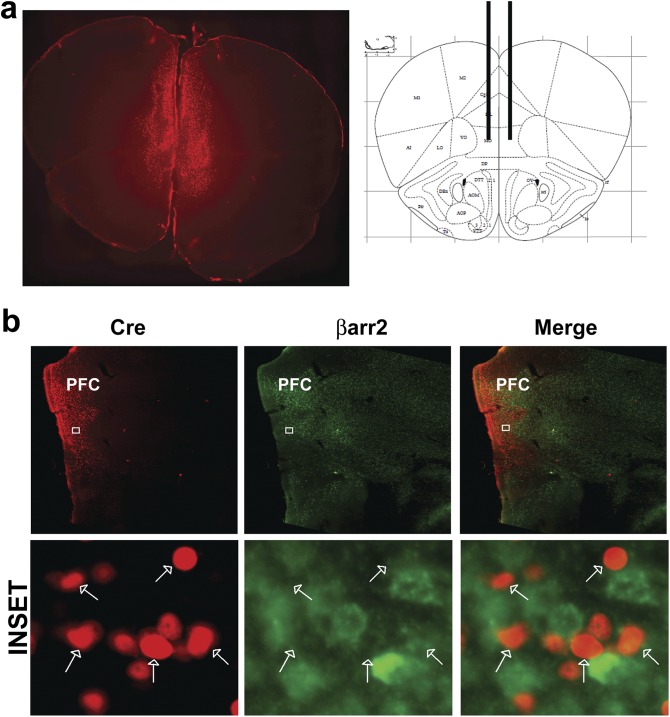
Representative coronal images of βarr2f/f A2aCre mice injected with mCherry-CRE AAV in PFC. (A) Coronal vibratome sections were taken from fixed brains of A2aβarr2 mice injected with virus, and images were taken using a Zeiss Axiozoom microscope (Left). Stereotaxic location (Paxinos and Franklin Atlas) of virus injection site in the PFC (Right). (B) IHC analysis showing deletion of βarr2 in PFC of A2aβarr2−/− mice injected with mCherry-Cre AAV. Upper shows lack of βarr2 in areas labeled for Cre in PFC, and Lower shows Cre (arrows) and βarr2 labeling at the cellular level.
Effect of 94A on Excitability of Cortical D2R+ Fast-Spiking Interneurons.
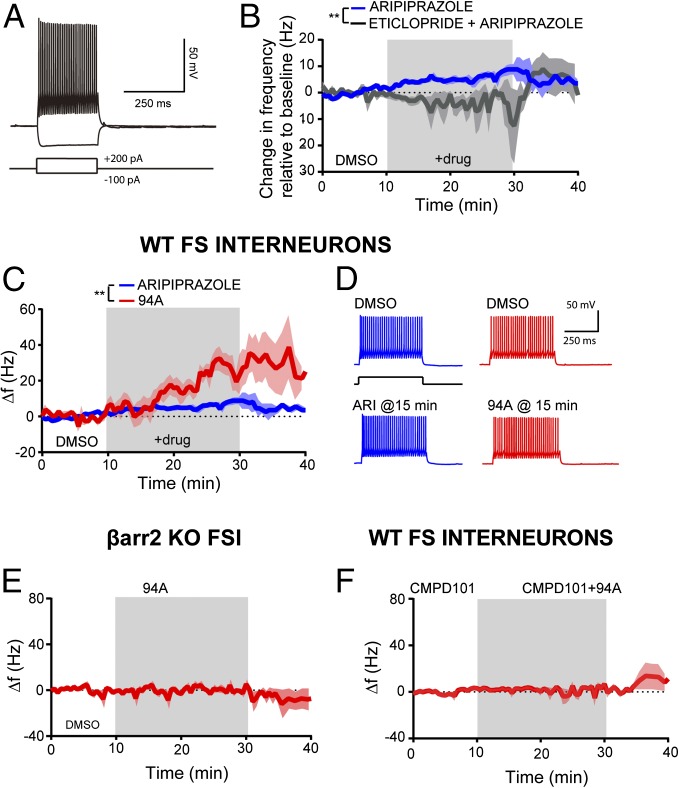
The PFC comprises multiple neuronal cell types, and many of these neurons, in particular GABA interneurons and pyramidal glutamatergic neurons, express D2Rs (56, 62–64). GABAergic interneurons are thought to play a critical role in schizophrenia pathophysiology in humans and animal models of schizophrenia. Postmortem brain analyses of patients (65–71) and behavioral studies in rodents have implicated GABAergic parvalbumin+ (PV+) fast-spiking interneurons (FSIs) in altered excitation–inhibition imbalance and cognitive impairment in schizophrenia (72, 73). The D2R agonist quinpirole increases FSI excitability in adult rodents in a similar fashion as D1R agonists (64), suggesting that this excitatory effect is not mediated by the canonical inhibitory Gαi/o activation but presumably, is through a G protein-independent pathway. Our data suggest that the higher levels of βarr2 and GRK2 in the cortex might support this G protein-independent agonist signaling of D2R ligands (Figs. 1 and 2). Additionally, the elevated βarr2 and GRK2 expression is present in PFC PV+ FSI (Fig. S7) and presumably, pyramidal neurons (non-PV+ cells) as well. Given the pharmacological, genetic, and behavioral evidence for the importance of FSIs in schizophrenia pathophysiology, we chose to determine the functional impact of higher cortical levels of βarr2 and GRK2 in FSIs. We performed whole-cell, current clamp slice recordings in prefrontal GAD67+ FSI from control (βarr2+/+) and global βarr2KO mice and assessed the effects of UNC9994A. FSIs were visually identified in acute slices from Gad1-eGFP adult mice and further identified by their responses to hyperpolarizing and depolarizing current injections (Fig. 7A). As expected from previous studies with D2R agonists, like quinpirole (64), ARI and 94A increased prefrontal FSI excitability as measured by changes in FSI action potential frequency in response to minimal amounts of depolarizing current injection. The D2R partial agonist ARI (10 µM) elicited a modest increase in action potential firing (+6.2 ± 1.4 Hz after 20 min of exposure relative to a 10 min predrug baseline; n = 6) (Fig. 7B). This effect was prevented by the D2R antagonist eticlopride (10 µM) and even led to a slight decrease in FSI excitability (−2.1 ± 2.2 Hz after 20 min of exposure of 10 µM eticlopride and 10 µM ARI; n = 4) (Fig. 7B). These data suggest that ARI has modest D2R agonist-like activity on FSIs in the PFC and are consistent with the partial agonist activity observed with ARI when GRK2 and βarr2 are overexpressed in HEK293 cells (Fig. 1 B, D, and F). Interestingly, 94A (10 µM) elicited a significantly more robust increase in FSI excitability compared with ARI (+25.8 ± 3.8 Hz after 20 min of 94A; +6.1 ± 1.4 Hz after 20 min of 10 µM ARI; n = 4 and n = 6, respectively; P < 0.01) (Fig. 7 C and D). Bath application of eticlopride (10 µM) also prevented the increase in FSI excitability by 94A and again, led to a slight decrease in FSI excitability [−5.0 ± 12.6 Hz after 20 min of exposure to eticlopride and 94A (10 µM); n = 3]. Additionally, the effects of 94A were dependent on βarr2-signaling, because UNC9994A failed to enhance FSI excitability in βarr2KO mice [+1.3 ± 1.6 Hz after 20 min of 94A (10 µM)] (Fig. 7E). Finally, the presence of the membrane-permeable GRK2 inhibitor cpd101 (30 μM) prevented the increase in FSI excitability elicited by 94A (Fig. 7F) (−2.3 ± 3.2 Hz after 20 min of exposure of cpd101 and 94A; n = 5). These data further highlight that 94A requires GRK2 in addition to βarr2 to produce its D2R-dependent, agonist-like effects in cortical FSI.
Fig. S7.
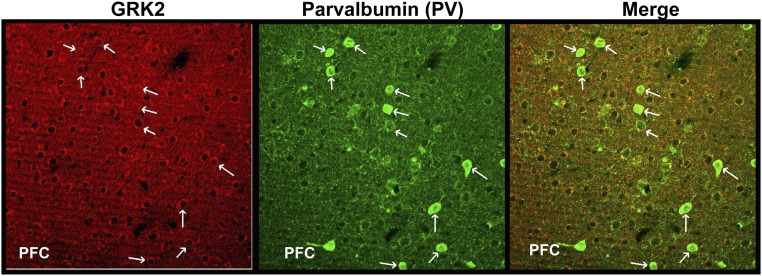
Elevated expression levels of GRK2 in PV+ neurons in the PFC. IHC images of mouse brain sections (PFC) stained with antibodies to GRK2 (red) and PV (green) and colocalization (merge).
Fig. 7.
UNC9994A has βarr2- and GRK2-dependent, agonist-like effects in prefrontal GABAergic FSIs. (A) Sample recording from a prefrontal FSI showing responses to hyperpolarizing or depolarizing current injection. (B) ARI (10 μM) increases action potential firing in prefrontal FSIs [+6.2 ± 1.4 Hz (relative to a 10-min predrug baseline) after 20 min of exposure; n = 6]. Bath application of eticlopride (10 μM) prevented the increase in excitability elicited by ARI (−2.08 ± 2.2 Hz after 15 min of exposure of eticlopride + ARI; n = 4). **P < 0.01. (C) UNC9994A (94A; 10 μM) elicited a greater increase in FSI excitability (+25.86 ± 3.8 Hz after 20 min of exposure; n = 4) compared with ARI. Data were analyzed using a standard two-way ANOVA test. **P < 0.01. (D) Sample responses of FSIs to depolarizing current pulses in various pharmacologic conditions showing an increase in FSI excitability after bath application of 94A or ARI. (E) The 94A-mediated increase in FSI excitability is absent in βarr2KO mice (+1.32 ±1.68 Hz after 20 min of exposure; n = 4). (F) Bath application of the GRK2 inhibitor cpd101 (30 μM) prevented the increase in excitability elicited by 94A (−2.3 ± 3.2 Hz after 20 min of exposure of cpd101 + 94A; n = 5). FS, fast spiking.
Effect of 94A on Excitability of Striatal D2R+ Medium Spiny Neurons.
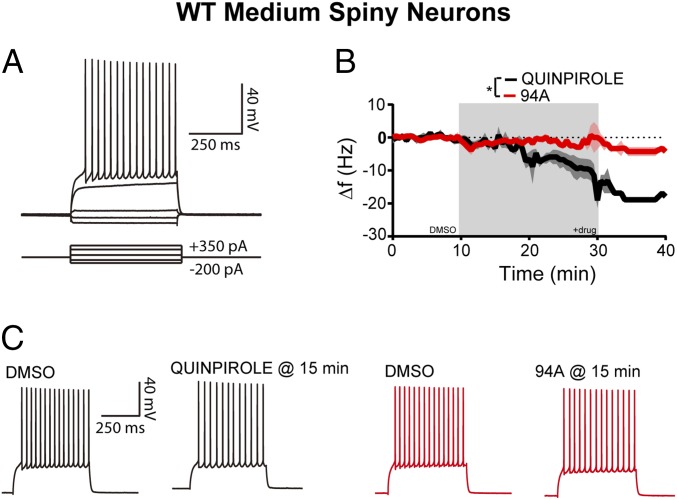
Based on the above behavioral and biochemical observations, because βarr2 and GRK2 levels are significantly lower in the STR than in the PFC, one might expect that the agonist activity of 94A would be reduced in striatal neurons. The D2R agonist quinpirole decreased excitability of striatal medium spiny neurons [MSNs; −6.8 ± 2.2 Hz after 20 min of quinpirole (10 µM); n = 8] (Fig. 8B, black line). These findings are consistent with previous reports showing that D2R agonists reduce the excitability of striatal MSNs (74). Unlike quinpirole, 94A produced a negligible reduction in striatal MSN excitability [−2.6 ± 1.0 Hz after 20 min of 94A (10 µM); n = 8; P = 0.0137 compared with quinpirole] (Fig. 8 B and C). Thus, 94A lacks agonist-like activity in striatal MSNs, showing that D2R-βarr2–biased ligands can have distinct electrophysiological actions in the PFC and STR.
Fig. 8.
UNC9994A lacks agonist-like effects in the STR. (A) Sample recording from striatal MSNs showing responses to hyperpolarizing or depolarizing current injection. (B) The D2R agonist quinpirole (10 μM) decreases action potential firing in striatal MSNs (−6.8 ± 2.2 Hz relative to a 10-min predrug baseline after 20 min of exposure; n = 8). UNC9994A (94A; 10 μM) elicits a significantly smaller decrease in MSN excitability compared with quinpirole (−2.6 ± 1.0 Hz after 20 min of exposure; n = 8). Data were analyzed using a standard two-way ANOVA test. *P = 0.0137. (C) Representative traces of striatal MSNs to depolarizing current pulses in control conditions and after quinpirole or 94A application.
SI Materials and Methods
Animals and Drugs.
Mice were age- and sex-matched, and for all experiments, at least eight mice were used for each genotype/treatment group (unless specified in the figures). All experiments were performed in an unblinded fashion during the light cycle and in drug-naïve animals. D1Cre, D2Cre, and A2aCre were all obtained from GENSAT and backcrossed with the C57BL/6J strain for at least 10 generations (30). The Cre lines were crossed with the βarr2f/f mice (C57BL/6J:129SvJ mixed strain), such that littermates were βarr2f/f (controls) and βarr2f/f Cre (knockouts). The D2f/f mice were previously described (51), and the ChAT-IRES-Cre mice were obtained for Jackson Laboratories (stock 018957) (89). AMPH, PCP, and haloperidol were purchased from Sigma-Aldrich and freshly prepared in saline (SAL); ARI was purchased from Sequoia Biosciences, dissolved in minimal glacial acetic acid, brought up to volume with distilled water, and dilutions were made in SAL. Clozapine was dissolved in a stock solution of Na-tartrate, and additional dilutions were made in SAL. University of North Carolina biased compound stock solutions were prepared in a minimal amount of DMSO, sonicated, and dissolved in 20% (vol/vol) hydroxylpropyl cyclodextrin (Sigma-Aldrich), and dilutions were subsequently made in SAL. All drugs were injected i.p. at a volume of 5 μL/g body weight, and the doses chosen were based on previous studies (30, 33).
Generation of βarr2 Targeting Vector and Floxed βarr2 Mice.
To generate floxed βarr2 mice, we inserted LoxP sites flanking exon 2 of the Arrb2 gene. The genomic region encompassing and flanking exon 2 of the Arrb2 gene was isolated from a bacterial artificial chromosome library (Coriell Institute) containing genomic DNA from the 129SvJ mouse strain. The homology arms were generated by PCR amplification of regions upstream and downstream of exon 2; inserted into the pEZ-Frt-Lox-DT vector generated in Klaus Rajewsky’s laboratory, Max Delbruck Center, Berlin; and obtained from Addgene (11736). Exon 2 of the Arrb2 gene was inserted into the pEZ-Frt-Lox-DT vector, such that it was now flanked by LoxP sites and next to the neomycin selection cassette that was flanked by flippase recognition target (FRT) sites for excision by flippase (FLP) recombinase (Fig. S4A). The targeting vector was then injected into C57BL/6J-129SvJ ES cells at the Duke University Transgenic Facility, selected for recombination by neomycin resistance, and confirmed by PCR. Two optimal ES cell clones were injected into blastocysts that were reimplanted into recipient female mice. Chimeric pups obtained were selected by coat color, crossed with C57BL/6J (Jackson Laboratories) mice to confirm germline transmission, and screened by PCR for appropriate insertion (Fig. S4B). The floxed βarr2 (+/f) mouse was then crossed with an FLPe recombinase mouse (Jackson Laboratories) to remove the neoR selection cassette. Subsequently, the floxed βarr2 (+/f) mice were either propagated to generate βarr2f/f mice for all experimental studies or crossed with a CMV-Cre mouse line (stock 006054; Jackson Laboratories) to globally delete the βarr2 allele (−/−) for confirmation of Cre-lox recombination. These mice were then propagated, and genotypes were confirmed by Western blot for deletion of the Arrb2 gene (Fig. S4F).
Prepulse Inhibition.
The βarr2f/f mice were habituated for 10 min to white noise (64 dB) in the prepulse inhibition (PPI) apparatus (San Diego Instruments). They were tested with seven pulse-alone trials (40-ms bursts of 120-dB white noise) followed by combinations of the prepulse–pulse (20-ms prepulse stimuli 4, 8, or 12 dB above the white noise background), pulse alone, and null trials (64 dB) and terminated with seven pulse-alone trials (35). PPI responses were calculated as %PPI = [1 − (prepulse trials/startle-only trials)] × 100.
Translating Ribosome Affinity Purification.
Translating ribosome affinity purification (TRAP) was performed as described previously (90, 91), with minor modifications. Briefly, WT or βarr2f/f mice harboring a Cre-inducible TRAP transgene (ribosomal L10a-GFP) were crossed with βarr2 wt/wt A2aCre+ or βarr2f/f A2aCre+ mice for cell type-specific expression of the TRAP transgene in D2R-MSNs. These mice were euthanized, striata were rapidly dissected, and TRAP was performed. The mRNAs derived from WT or βarr2−/− mice were converted into cDNA using Superscript VILO Mastermix (ThermoFisher Scientific) and then, subjected to Taqman qPCR. Two Primetime Standard qPCR Assays were designed (IDT) to specifically detect exon 2 of βarr2 (ENSMUST00000108568). Probe set 1: forward primer 5′-CCCTAACTGCAAGCTCACC-3′, reverse primer 5′-CTTTCCGGTCCTTCAAGTAGTC-3′, and probe 5′-56-FAM/AGCGCGACT/ZEN/TTGTAGATC ACCTGG/3IABkFQ/-3′ and probe set 2: forward primer 5′-GCGCACCATGGGAGAAA-3′, reverse primer 5′-AAGTACACGGTGAGCTTGC-3′, and probe 5′-/56-FAM/AGGGTCTTC/ZEN/AAGAAGTCGAGCCCTA/3IABkFQ-3′. Taqman gene expression assays (ThermoFisher) were ordered for Drd2 (MM00438545.m1), PENK (MM01212875.m1), Drd1 (MM01353211.m1), Tac1 (MM01166996.m1), and GAPDH (Mm99999915.g1). Real-time PCR was performed with TaqMan Fast Advanced Master Mix (4444557; ThermoFisher) on a StepOnePlus Real-Time PCR System (ThermoFisher). Relative mRNA abundance was determined using the ∆∆CT (threshold cycle) method (92).
cAMP Inhibition Assay.
To measure D2R Gi-mediated cAMP inhibition, HEK293T cells were cotransfected in a 1:1:2 ratio with human D2Long receptor, GRK2, or pcDNA3.1 and a split luciferase-based cAMP biosensor (GloSensor; Promega), respectively. After at least 24 h, transfected cells were plated in polylysine-coated 384-well white clear-bottom cell culture plates with DMEM and 1% dialyzed FBS at a density of 15,000–20,000 cells per 40 µL per well and incubated overnight. Next day, drug dilutions were prepared in fresh assay buffer (20 mM Hepes, 1× HBSS, 0.3% BSA, 0.03% ascorbic acid, pH 7.4) at 5× and 6× concentrations for APDs and DA, respectively. APD concentrations corresponding to dose–response curves were added in 5-µL volume to each well containing 20 µL per well assay buffer (20 mM Hepes, 1× HBSS, pH 7.4). Plates were allowed to incubate for exactly 30 min in the dark at room temperature, and immediately afterward, 5 µL DA (10 nM final concentration) was added and allowed to incubate for an additional 15 min. To stimulate endogenous cAMP production via β-adrenergic receptor-mediated Gαs activation, 10 µL 4× isoproterenol (200 nM final concentration) diluted in assay buffer supplemented with GloSensor assay reagent was added per well, and luminescence intensity was quantified 15 min later using a Wallac TriLux Microbeta (Perkin-Elmer) Luminescence Counter.
βarr BRET Assay.
To measure D2R-mediated βarr2 recruitment, HEK293T cells were cotransfected in a 1:1:15 ratio with mouse D2Long receptor fused to C-terminal renilla luciferase (RLuc8), GRK2, or pcDNA3.1 and a Venus-tagged N-terminal βarr2 (a gift from Jonathan Javitch, Columbia University, New York), respectively. After at least 24 h, transfected cells were plated in polylysine-coated 96-well white clear-bottom cell culture plates with DMEM and 1% dialyzed FBS at a density of 250,000–500,000 cells in 200 µL per well and incubated overnight. Buffers used for the BRET assay and to dilute drugs were exactly the same as for the cAMP inhibition assay. The next day, media were decanted, cells were washed twice with assay buffer, and APDs were added at concentrations corresponding to the dose–response curves and allowed to incubate for exactly 30 min. Afterward, EC80 of DA was added to each well and allowed to incubate for exactly 5 min. The RLuc substrate, coelenterazine h (5 µM final concentration; Promega), was added per well, and exactly 5 min later, both luminescence at 485 nm and fluorescent enhanced yellow fluorescent protein (eYFP) emission at 530 nm were measured for 1 s per well using a Mithras LB940 Multimode Multiplate Reader (Berthold Technologies). The ratio of eYFP/RLuc was calculated per well, and the net BRET ratio was calculated by subtracting the eYFP/RLuc per well from the eYFP/RLuc ratio without Venus-Arrestin present. The net BRET ratio was plotted as a function of drug concentration using Graphpad Prism 5 (Graphpad Software Inc.). The GRK2/3 inhibitor cpd101 (93) was purchased from Hello Bio.
Western Blot.
Western blot analyses were performed on human postmortem or drug-naïve mice brain tissue as described previously (30). Briefly, STR and frontal cortex regions were rapidly dissected from mouse brains on ice, or frozen human postmortem tissue (six patient samples from PFC area 10 and caudate) was obtained from Craig Stockmeier, Postmortem Brain Core, University of Mississippi Medical Center, Jackson, MS. Tissue samples were immediately sonicated and boiled in preheated 1% SDS solution. Loading buffer was added to the samples, which were loaded onto 10% (wt/vol) SDS gels (Nupage; Invitrogen), transferred to nitrocellulose membranes, and exposed to antibodies for GRK2/3 (1:500; 05–465; EMD Millipore), βarr1/2 (A2CT clone; gift from Robert J. Lefkowitz, Duke University, Durham, NC), and GAPDH (1:5,000; MAB374; EMD Millipore). Incubation with primary antibody was followed by exposure to IR secondary antibody (LICOR), and the blots were developed using an LICOR Odyssey Detection System and quantified using ImageJ (NIH).
Human Brain Tissue.
Human prefrontal cerebral cortex (area 10) and caudate nucleus were obtained at autopsy from psychiatrically normal individuals (Table S2), rapidly frozen, and stored at −80 °C. The procedures used for brain tissue collection, toxicology, and psychological autopsy have been published elsewhere (94, 95). Toxicology screens revealed nothing remarkable.
Table S2.
Details of origin of individual postmortem brain tissue samples
| Brain region | Duke code | Tube weight | Tube and tissue weight | Tissue, g | Tissue, mg | Age, y | Sex | PMI, h | Diagnosis |
| Area 10 | Duke 00IA | 0.9886 | 1.1003 | 0.1117 | 112 | 27 | Female | 15 | No diagnosis |
| Caudate | Duke 001B | 0.9991 | 1.1402 | 0.1411 | 142 | 27 | Female | 15 | No diagnosis |
| Area 10 | Duke 002A | 0.9841 | 1.1184 | 0.1343 | 135 | 37 | Female | 13 | No diagnosis |
| Caudate | Duke 002B | 0.9852 | 1.1046 | 0.1194 | 120 | 37 | Female | 13 | No diagnosis |
| Area 10 | Duke 003A | 0.9873 | 1.0984 | 0.1111 | 112 | 51 | Female | 22 | No diagnosis, nicotine dependence |
| Caudate | Duke 003B | 0.995 | 1.1103 | 0.1153 | 116 | 51 | Female | 22 | No diagnosis, nicotine dependence |
| Area 10 | Duke 004A | 0.9769 | 1.0937 | 0.1168 | 117 | 43 | Male | 23 | No diagnosis |
| Caudate | Duke 004B | 0.9464 | 1.0787 | 0.1323 | 133 | 43 | Male | 23 | No diagnosis |
| Area 10 | Duke 005A | 0.9806 | 1.0876 | 0.107 | 107 | 48 | Male | 9 | No diagnosis, nicotine dependence |
| Caudate | Duke 005B | 0.988 | 1.1195 | 0.1315 | 132 | 48 | Male | 9 | No diagnosis, nicotine dependence |
| Area 10 | Duke 006A | 1.0042 | 1.111 | 0.1068 | 107 | 54 | Male | 17 | No diagnosis, Hx: nicotine dependence |
| Caudate | Duke 006B | 0.9535 | 1.0544 | 0.1009 | 101 | 54 | Male | 17 | No diagnosis, Hx: nicotine dependence |
Hx, medical history; PMI, postmortem interval.
Discussion
We used neuron-specific βarr2KO mice to characterize the role of βarr2 in striatal and cortical circuits and their involvement in pharmacological models of antipsychotic-like action. The βarr2-biased D2R ligand 94A showed a paradoxical pharmacological profile in biochemical, behavioral, and electrophysiological assays; 94A reversed the hyperlocomotor responses to both AMPH and PCP through both striatal antagonism and cortical agonism. Furthermore, the agonist activity of 94A increased the firing of PFC D2R-expressing FSI in a βarr2- and GRK2-dependent manner but did not mimic quinpirole’s ability to inhibit firing of striatal D2R+ MSNs. These contrasting electrophysiological, behavioral, and biochemical effects are consistent with higher PFC expression of βarr2 and GRK2 compared with STR. These data suggest that biased agonism of D2R-βarr2 in the PFC could add a dimension of cortical benefits in addition to the striatal antagonist profile characteristic of clinically efficacious APDs.
The opposite pharmacological action of 94A in the STR and PFC in both behavioral and electrophysiological assays is consistent with the higher expression of βarr2 and GRK2 in PFC compared to STR. Although we show that both ARI and 94A lack basal agonist activity when direct D2R-βarr2 interactions are assessed by BRET in HEK293 cells, the partial agonist activity of these compounds can be revealed when using assays such as the TANGO and DiscoverX (33), which markedly amplify the signal. Similarly, when the D2R-βarr2 BRET assay is performed in the presence of overexpressed GRK2, which recapitulates PFC expression patterns, both 94A and ARI now show similar D2R-βarr2 partial agonist activity. However, somewhat unexpectedly, 94A is more efficacious than ARI not only at inhibiting PCP-induced locomotion following local PFC injection but also, in enhancing firing of PV+ FSIs of the PFC in a D2R-, GRK2-, and βarr2-dependent manner. This enhanced PFC antipsychotic-like effect and excitability of PV+ PFC FSIs by 94A can be attributed to the relative balance between Gαi/o (neuronal inhibition) and βarr2 (neuronal excitation) agonist activities; 94A has no Gαi/o agonist activity (33), whereas ARI is a Gαi/o partial agonist. In contrast to the PFC, the STR has low expression of GRK2 and βarr2, and we showed in vitro that, with low levels of GRK2 expression, 94A is an antagonist at the D2R-βarr2 pathway. Furthermore, 94A does not mimic the effect of the D2R agonist quinpirole on striatal D2R+ MSN firing and inhibits the striatal AMPH response consistent with its antagonist-like properties in vivo. Therefore, although behaviorally, 94A has the same effect (i.e., inhibition of the locomotor response), it has opposite effects pharmacologically and on neuronal firing in the PFC versus STR. Consistent with the above observations with 94A, D2R deletion also has opposite effects in PFC versus STR on the PCP response. Although the antipsychotic-like effect of 94A is selectively dependent on the D2R-βarr2 pathway, other APDs do not require βarr2 for their antipsychotic-like activity, suggesting that targeting βarr2 is sufficient but not necessary for antipsychotic-like activity (60). However, our data suggest that targeting the D2R-βarr2 pathway may generate unique antipsychotic-like actions that current APDs do not achieve.
D2R antagonist and partial agonist APDs are the primary treatment options for schizophrenia, but there are several disease domains, such as negative symptoms and cognitive deficits, that are unaffected by APDs. The prevalent DA hypothesis of schizophrenia posits that this disorder presents with reduced cortical DA tone but enhanced striatal DA release, with these spatially distinct manifestations being interdependent (20, 75–77). Brain imaging studies in schizophrenia patients have shown that AMPH-induced DA release is enhanced in the STR (78) (hyperdopaminergia), whereas a recent study has shown, for the first time, cortical hypodopaminergia in schizophrenia patients (79). Partial agonists, such as ARI, were originally developed to counteract these opposite phenomena but have largely been unsuccessful in correcting cortical dysfunction (80, 81). Our behavioral and electrophysiological data show that the ARI-derived βarr2-biased D2R ligand, 94A, can act as a D2R-βarr2 agonist in the PFC but a D2R-βarr2 antagonist on striatal D2 MSNs, highlighting the feasibility of a pharmacological approach, where a drug could potentially simultaneously counteract both the cortical hypodopaminergia with D2R-βarr2 agonism and striatal hyperdopaminergia with D2R-βarr2 antagonism in schizophrenia.
Our results indicate that 94A acts as an agonist at D2R-βarr2 signaling in GABAergic FSIs. This effect may contribute to the antipsychotic-like action that we observed in PCP-induced locomotion and most likely, will contribute to resetting excitation inhibition balance in cortical circuits. However, we cannot exclude the contribution of D2R-expressing pyramidal neurons that may also have elevated levels of βarr2 and GRK2. However, DA released by PFC-projecting mesocortical DA neurons inhibits pyramidal neurons primarily by activating interneurons (82), suggesting a temporally preceding role for FSIs. To target the exact subtype of cortical neurons for more specific behavioral analyses would require developing novel Cre driver lines involving at least triple-intersectional approaches. Appropriate selectively targeted Cre lines for various D2R neuronal subtypes in the PFC (83, 84) are currently lacking. After appropriate targeting is achieved, signaling effectors downstream of D2R-βarr2 in the PFC can be elucidated. Although the downstream cellular targets of D2R-G protein, such as PKA/DARPP32 signaling, are well-established (23, 25, 85), possible downstream D2R-βarr2 signaling effectors, such as GSK3β, AMPA, and NMDA receptors, are only now beginning to be revealed (86, 87). In conclusion, our data provide evidence suggesting that combining the concept of functional selectivity and taking into account region- and cell-specific receptor and transducer expression patterns have the potential to generate more effective therapies and at the same time, reduce side effects.
Materials and Methods
Animals and Drugs.
All mouse studies were conducted in accordance with the NIH guidelines for animal care and use and through animal protocols approved by the Duke University Animal Care and Use Committee and Pfizer’s Institutional Animal Care and Use Committee. All mice were housed in a 12-h light–dark cycle at a maximum of five per cage, provided with food and water ad libitum, and tested at 10–20 wk of age. Details of mouse lines used, generation of βarr2f/f animals, and drugs and chemicals used are in SI Materials and Methods.
Locomotor Activity.
Activity was measured in an Accuscan Activity Monitor (Accuscan Instruments) and performed as described (30, 88). Briefly, mice were allowed to habituate to the open field for 30 min, injected with various drugs, and returned to the open field. Locomotor activity was measured in 5-min intervals, and data were analyzed for the distance traveled in 5-min increments over 120 min. All APDs and tool compounds were administered i.p. and injected 10 min before AMPH or PCP injections.
IHC.
Fifty-micrometer-thick vibratome cut sections of formalin-fixed mouse brains were processed for IHC analyses as described previously (30). To assess neuron-specific deletion of βarr2, antibodies to βarr2 (generated in rabbit; at 1:300; gift from Jeff Benovic, Thomas Jefferson University, Philadelphia), Cre recombinase (mouse antibody MAB3120; at 1:500; EMD Millipore), and D2 neuron marker enkephalin (ENK; rabbit antibody; AB5026; at 1:500; EMD Millipore) were used. Because both antibodies to βarr2 and ENK were from rabbit, they were used in combination with antibodies to Cre only. Additionally, because βarr2 levels in the STR are much lower, a TSA Amplification System (Perkin-Elmer) was used to enhance detection of βarr2. Antibodies to PV (1:500; PVG-213; Swant Inc.) and GRK2/3 (1:500; 05–465; EMD Millipore) were used to label PV+ interneurons and assess the levels of GRK2, respectively, in these cells. All representative images are from IHC analyses from at least two to three mice for each group.
Western Blot.
Western blot analyses were performed on postmortem human or drug-naïve mice brain tissue as described previously (30). For human brain tissue, all procedures were carried out in compliance with an approved protocol from the University of Mississippi Medical Center Institutional Review Board. Written informed consent was obtained from legally defined next of kin for tissue collection and informant-based retrospective diagnostic interviews (Table S2). Human or mouse tissue lysates were loaded onto SDS gels followed by transfer onto nitrocellulose membranes. Membrane blots were incubated with primary antibody followed by IR secondary antibody (LICOR), and the blots were developed using an LICOR Odyssey Detection System. Details are in SI Materials and Methods.
Stereotaxic Surgeries and Virus.
Deletion of βarr2 or D2Rs in the PFC was achieved by a viral approach; βarr2f/f or D2f/f mice were stereotaxically injected bilaterally with 0.5 μL AAV serotype 2/8 (UNC Viral Vector Core). βarr2f/f (PFC βarr2KO) or A2aCre βarr2f/f (PFC + STR βarr2KO) mice were injected with mCherry-Cre AAV, whereas D2f/f mice were injected with either GFP or mCherry-Cre AAV at coordinates +2.5 mm anteroposterior (AP), ±0.3 mm mediolateral (ML), and −1.8 mm dorsoventral (DV) from bregma to target the prelimbic/infralimbic region of the PFC. Mice were allowed to recover for 3 wk to allow for viral expression of GFP or mCherry-Cre before behavioral testing.
Cannulation and Local PFC Drug Injections.
For local PFC injection of drugs, we inserted bilateral guide cannulas (Plastics One) into the PFC of WT mice. The bilateral guide cannulas were inserted at +2.0 mm AP with 1.0-mm spacing (±0.5 mm ML) and −2.0 mm DV and fixed to the skull with dental cement. Mice were allowed to recover for 2 wk, and then, drugs were injected using an automated injection system. Drugs were dissolved in VEH [10% (vol/vol) DMSO and 20% (vol/vol) hydroxypropyl cyclodextrin], and either 1 μg (Quinpirole and UNC9994A) or 0.5 μg (cpd101–GRK2/3 inhibitor; Hello Bio, Bristol, UK) per 0.5 μL were injected per side at a rate of 0.4 μL/min. After local injection, mice were placed in the activity monitors for 10 min before systemic (i.p.) injection with PCP (6 mg/kg), and then, locomotor activity was recorded.
cAMP Inhibition Assay.
To measure D2R Gαi-mediated cAMP inhibition, a split luciferase-based cAMP biosensor (GloSensor; Promega) in HEK293T cells was used. The assay was performed in a 384-well plate using a Wallac TriLux Microbeta (Perkin-Elmer) Luminescence Counter. Details are in SI Materials and Methods.
βarr BRET Assay.
To measure D2R-mediated βarr2 recruitment, a mouse D2Long receptor fused to C-terminal renilla luciferase and a Venus-tagged N-terminal βarr2 (a gift from Jonathan Javitch, Columbia University, New York) expressed in HEK293T cells were used in a BRET assay. The assay was performed in a 96-well plate using a Mithras LB940 Multimode Plate Reader (Berthold Technologies). Details are in SI Materials and Methods.
Electrophysiology.
Slice preparation.
Three hundred-micrometer-thick coronal slices were cut from 7- to 10-wk-old mice of either sex using a Leica VT1200S Microtome. Gad1-EGFP Tg (Jackson Immunoresearch Laboratories Inc.) and global βarr2−/− mice were used in our electrophysiological studies. Acute slices were secured by placing a harp along the midline between the two hemispheres.
Intracellular recording.
Whole-cell patch recordings were obtained from visually identified interneurons in layer V of infralimbic or prelimbic cortex or MSNs in the STR using differential contrast video microscopy on an upright microscope (BX51WI; Olympus). Recordings were obtained from FSI or MSNs of Gad1-eGFP adult mice (n = 1 per animal), which were further identified by their responses to hyperpolarizing and depolarizing current injections. Recordings were collected using a Multiclamp 700A (Molecular Devices). Patch electrodes (tip resistance = 4–6 MΩ) were filled with the following (in mM): 115 K-Gluconate, 10 HEPES, 20 KCl, 2 MgCl, 2 Mg-ATP, 2 Na-ATP, and 0.3 GTP. Slices were submerged in artificial cerebrospinal fluid containing the following: 125 mM NaCl, 25 mM NaHCO3, 3.5 mM KCl, NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, and 10 mM glucose. All recordings were made at 32.5 °C ± 1 °C. Series resistance was usually 15–20 MΩ, and experiments were discontinued if the series resistance exceeded 30 MΩ.
Statistical Analyses.
Data were analyzed by standard one- and two-way ANOVA or two- and three-way repeated-measures ANOVA (RMANOVA) tests for comparison between genotypes, treatments, or doses. Individual genotypes, treatments, or doses were compared using a post hoc Bonferroni’s test. Data were analyzed for normality with equal variance, and only parametric tests were used. Data points were excluded based on previously established criterion and set to ±2 SDs from the group mean. Data are presented as mean ± SEM.
Acknowledgments
We thank Xiuqin Zhang and Benjamin Phillips for maintenance of the mouse colony. Antibodies to βarr2 for Western blot analyses (A2CT) and βarr2-specific IHC antibody were generous gifts from Dr. Robert Lefkowitz (Duke University) and Dr. Jeff Benovic (Thomas Jefferson University), respectively. Human postmortem brain samples were obtained from Dr. Craig Stockmeier (Postmortem Brain Core, University of Mississippi Medical Center). We also acknowledge the assistance of Dr. James C. Overholser, Dr. George Jurjus, and Lesa Dieter in psychiatric assessments and Gouri Mahajan in tissue preparation. This work was supported, in part, by NIH Grants 5R37-MH-073853 and 5U-19-MH-082441. Support from the Sidney R. Baer Jr. Foundation (N.M.U.) and the Pall Family Foundation (M.G.C.) for parts of this work is also greatly appreciated. This study was also supported by an award from the Ruth K. Broad Biomedical Research Foundation (T.F.P.) and the National Cancer Institute (NCI) Clinical Oncology Research Career Development Program NCI 5K12-CA100639-10 (to J.C.S.). Some of the behavioral experiments were conducted with equipment and software purchased with a North Carolina Biotechnology Center grant. The Postmortem Brain core is supported by Institutional Development Award (IDeA) Centers of Biomedical Research Excellence (COBRE) Program of NIH/National Institute of General Medical Sciences Grant P30 GM103328. We acknowledge the support of the Cuyahoga County Medical Examiner’s Office.
Footnotes
Conflict of interest statement: P.O. is an employee and shareholder at Pfizer, Inc. M.G.C. has received compensation from Lundbeck as a member of their Psychopharmacology Advisory Board and is a consultant for Omeros Corp. M.G.C. also owns equity in Acadia Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614347113/-/DCSupplemental.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2011;51:117–144. doi: 10.1146/annurev-pharmtox-010510-100553. [DOI] [PubMed] [Google Scholar]
- 3.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 6.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 7.Laporte SA, et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96(7):3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attramadal H, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267(25):17882–17890. [PubMed] [Google Scholar]
- 9.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 10.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 11.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 12.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97(20):11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 14.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28(8):416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Walters RW, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119(5):1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Cyr JA, Taylor AE, Nicholson K. Behavior and the basal ganglia. Adv Neurol. 1995;65:1–28. [PubMed] [Google Scholar]
- 18.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 19.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 20.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 22.Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: An integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15(6):410–424. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- 23.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294(5544):1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 24.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 25.Svenningsson P, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302(5649):1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- 26.Bateup HS, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA. 2010;107(33):14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132(1):125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 29.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27(4):881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3β in D2R-expressing neurons reveals distinct roles for β-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA. 2012;109(50):20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 32.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 33.Allen JA, et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, et al. Structure-functional selectivity relationship studies of β-arrestin-biased dopamine D2 receptor agonists. J Med Chem. 2012;55(16):7141–7153. doi: 10.1021/jm300603y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SM, et al. Effects of β-arrestin-biased dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutamatergic mice. Neuropsychopharmacology. 2016;41(3):704–715. doi: 10.1038/npp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: Insights from brain imaging studies. Eur Psychiatry. 2005;20(1):15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: A review and meta-analysis. Schizophr Bull. 1999;25(2):201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 38.King DJ. Drug treatment of the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 1998;8(1):33–42. doi: 10.1016/s0924-977x(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 39.Klewe IV, et al. Recruitment of beta-arrestin2 to the dopamine D2 receptor: Insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology. 2008;54(8):1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masri B, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci USA. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krasel C, Bünemann M, Lorenz K, Lohse MJ. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280(10):9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- 42.Ménard L, et al. Synergistic regulation of beta2-adrenergic receptor sequestration: Intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51(5):800–808. [PubMed] [Google Scholar]
- 43.Zhang J, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95(12):7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson SM, Pack TF, Caron MG. Receptor, ligand and transducer contributions to dopamine D2 receptor functional selectivity. PLoS One. 2015;10(10):e0141637. doi: 10.1371/journal.pone.0141637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Höllt V. Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res. 2001;95(1-2):129–137. doi: 10.1016/s0006-8993(01)03046-3. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed MR, Bychkov E, Gurevich VV, Benovic JL, Gurevich EV. Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. J Neurochem. 2008;104(6):1622–1636. doi: 10.1111/j.1471-4159.2007.05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Arco A, Mora F, Mohammed AH, Fuxe K. Stimulation of D2 receptors in the prefrontal cortex reduces PCP-induced hyperactivity, acetylcholine release and dopamine metabolism in the nucleus accumbens. J Neural Transm (Vienna) 2007;114(2):185–193. doi: 10.1007/s00702-006-0533-3. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience. 2002;114(3):769–779. doi: 10.1016/s0306-4522(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 49.Fell MJ, et al. In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-bicaroxylate monohydrate (LY354740) and (-)-2-oxa-4-aminobicyclo[3.1.0] Hexane-4,6-dicarboxylic acid (LY379268) J Pharmacol Exp Ther. 2009;331(3):1126–1136. doi: 10.1124/jpet.109.160598. [DOI] [PubMed] [Google Scholar]
- 50.White IM, et al. Phencyclidine-induced increases in striatal neuron firing in behaving rats: Reversal by haloperidol and clozapine. J Neural Transm. 1995;102(2):99–112. doi: 10.1007/BF01276506. [DOI] [PubMed] [Google Scholar]
- 51.Anzalone A, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023–9034. doi: 10.1523/JNEUROSCI.0918-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: Presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9(1):53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 54.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 55.Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine D2 receptors in the cerebral cortex: Distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci USA. 1989;86(16):6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19(4):849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- 57.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13(6):2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lieberman JA, et al. Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60(3):358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powell SB, Geyer MA. Overview of animal models of schizophrenia. Curr Protoc Neurosci. 2007;9:9.24. doi: 10.1002/0471142301.ns0924s39. [DOI] [PubMed] [Google Scholar]
- 60.Schmid CL, Streicher JM, Meltzer HY, Bohn LM. Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a βarrestin2-independent activation of Akt. Neuropsychopharmacology. 2014;39(8):1902–1913. doi: 10.1038/npp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bohn LM, et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23(32):10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tseng KY, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28(48):12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24(22):5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17(5):1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 66.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43(11):970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 68.Fung SJ, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167(12):1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto T, et al. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakazawa K, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho KK, et al. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron. 2015;85(6):1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 75.Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286(5768):74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 76.Ventura R, et al. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29(1):72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- 77.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 78.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: New evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 79.Slifstein M, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: A positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72(4):316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kern RS, et al. The neurocognitive effects of aripiprazole: An open-label comparison with olanzapine. Psychopharmacology (Berl) 2006;187(3):312–320. doi: 10.1007/s00213-006-0428-x. [DOI] [PubMed] [Google Scholar]
- 81.Rajagopal L, Massey BW, Huang M, Oyamada Y, Meltzer HY. The novel object recognition test in rodents in relation to cognitive impairment in schizophrenia. Curr Pharm Des. 2014;20(31):5104–5114. doi: 10.2174/1381612819666131216114240. [DOI] [PubMed] [Google Scholar]
- 82.Kabanova A, et al. Function and developmental origin of a mesocortical inhibitory circuit. Nat Neurosci. 2015;18(6):872–882. doi: 10.1038/nn.4020. [DOI] [PubMed] [Google Scholar]
- 83.Madisen L, et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85(5):942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bateup HS, et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11(8):932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du J, et al. A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA. 2010;107(25):11573–11578. doi: 10.1073/pnas.0913138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li YC, Xi D, Roman J, Huang YQ, Gao WJ. Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29(49):15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36(3):551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP) Nat Protoc. 2014;9(6):1282–1291. doi: 10.1038/nprot.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heiman M, et al. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci USA. 2014;111(12):4578–4583. doi: 10.1073/pnas.1401819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 93.Lowe JD, et al. Role of G protein-coupled receptor kinases 2 and 3 in μ-opioid receptor desensitization and internalization. Mol Pharmacol. 2015;88(2):347–356. doi: 10.1124/mol.115.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu H, et al. Quantitative analysis of focused a-to-I RNA editing sites by ultra-high-throughput sequencing in psychiatric disorders. PLoS One. 2012;7(8):e43227. doi: 10.1371/journal.pone.0043227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cobb JA, et al. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2013;47(3):299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]