Abstract
Cell expansion techniques commonly utilize exogenous factors to increase cell proliferation and create a larger cell population for use in cell-based therapies. One strategy for cartilage regenerative therapies is autologous stem cell expansion and fibroblast growth factor (FGF) supplementation during cell expansion, particularly FGF-2. However, it is unknown whether FGF-10, another FGF implicated in limb and skeletal development, can elicit the same rejuvenation responses in terms of proliferation and differentiation of human synovium-derived stem cells (SDSCs). In this study, we expanded SDSCs in either FGF-2 or FGF-10 for 7 days; a control group had no treatment. FGF-2 and FGF-10 supplementation was also exclusively tested during the differentiation phase. Expanded SDSCs were evaluated for their ability to successfully engage in chondrogenic and osteogenic differentiation. We found that FGF-2 supplementation during proliferation, but not differentiation, was able to increase glycosaminoglycan deposition, pellet size, and chondrogenic gene expression following chondrogenic induction, as well as increased calcium deposition, alkaline phosphatase activity, and expression of vital osteogenic differentiation genes following osteogenic induction. FGF-10 did not elicit a similar preconditioning effect. We also observed changes of both Wnt signals and mitogen-activated protein kinase expression during SDSC chondrogenesis, which occurred in a manner dependent upon the supplementation phase of FGF-2 administration. These results indicated that FGF-2, but not FGF-10, may be supplemented during stem cell expansion to prime cells for successful chondrogenesis and osteogenesis.
Introduction
Adult mesenchymal stem cells (MSCs) are promising alternative cell sources for cartilage tissue engineering due to the shortage of autologous chondrocytes for cell-based regenerative therapies.1 However, adult stem cells obtained from tissues have either less chondrogenic potential despite large amounts such as adipose stem cells or higher endochondral ossification and limited sample size such as bone marrow stromal cells (BMSCs).2 One promising MSC population found in the joint, known as synovium-derived stem cells (SDSCs), has recently been characterized as tissue-specific stem cells for chondrogenesis.3 Strategies which allow for MSC proliferation may be necessary to achieve a substantial and usable cell number for therapies; however, the initial MSC numbers are low and their differentiation potential can be compromised following excessive ex vivo expansion, with notable increases in cell senescence marker expression and decreased proliferative capacity.4 This situation can be further complicated when combined with other preexisting and potentially detrimental factors such as donor age and disease pathology.5
It is known that the fibroblast growth factor (FGF) family is involved in limb and joint development, as well as various stages of skeletal and cartilage formation and maturation.6 For instance, FGF-2 is involved in early cartilage development and can cause dramatic increases in cell proliferation in chondrocytes and osteoblasts.7 In addition, another member of the FGF family, FGF-10, is known to be vital to limb bud initiation and development,8,9 but less is known about the ability of FGF-10 to modulate chondrogenic activity, although a recent report indicated that FGF-10 promoted Meckel's cartilage regeneration in rats.10 Interestingly, FGF signaling has been shown to maintain MSCs in an undifferentiated state during proliferation while preserving their multipotentiality,11 which further establishes the FGF family's potential benefits for preconditioning strategies.
Our recent findings suggest that decellularized extracellular matrix (dECM) deposited by stem cells could also provide a preconditioning strategy on which stem cells could be greatly expanded with enhanced chondrogenic potential12 or endochondral ossification.13 Interestingly, microarray data from these dECM studies have shown that, among all FGFs, FGF-2 and FGF-10 are the most significantly regulated factors in human SDSCs following their expansion on dECM (Fig. 1). It is unknown whether a similar rejuvenating effect can be achieved on human SDSC chondrogenesis and osteogenesis through FGF-2 and FGF-10 supplementation in the cell expansion phase, as well as supplementation in the differentiation phase. Due to the importance and potential impacts in stem cell-mediated chondrogenesis,14,15 the content of both Wnt and mitogen-activated protein kinase (MAPK) activation, which have been demonstrated in our earlier dECM preconditioning study,16 will also be assessed in this study following FGF ligand rejuvenation.
FIG. 1.
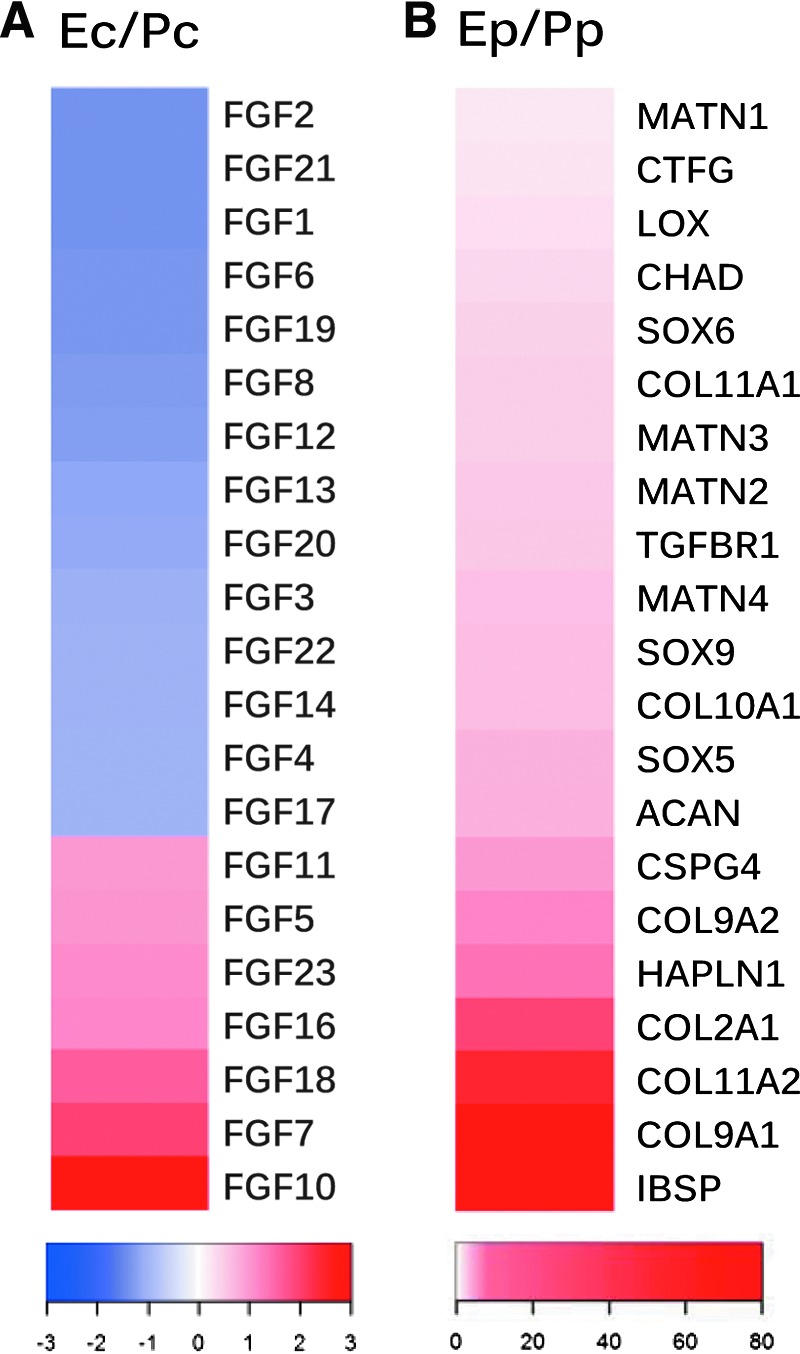
Gene expression of FGF ligands in cell expansion phase and chondrogenic markers in chondrogenic induction phase following the dECM pretreatment. The study design was detailed previously.18 Briefly, human SDSCs were expanded on either dECM or Plastic for one passage followed by a 2-week chondrogenic induction. Microarray analysis was used to evaluate FGF ligand genes in expanded cells (A) and chondrogenic marker genes in differentiated cells (B). The raw data were uploaded into Partek (St. Louis, MO) software for initial analysis. After raw intensity was background-subtracted, robust multiarray analysis was normalized, log transformed, and fold changes were determined. Heatmap.2 in R was used to show the effects on FGF ligand and chondrogenic genes, annotated in Ingenuity Pathway Analysis (IPA, Redwood City, CA) as affecting cell proliferation and chondrogenic differentiation, respectively. dECM expanded cells were referred to as Ec, while Plastic expanded cells were referred to as Pc (A). The pellets from dECM expanded cells were referred to as Ep, while those from Plastic expanded cells were referred to as Pp (B). FGF, fibroblast growth factor; dECM, decellularized extracellular matrix; SDSC, synovium-derived stem cell. Color images available online at www.liebertpub.com/tea
Materials and Methods
Evaluation of cell proliferation, surface phenotypes, and differentiation genes
Cell culture and proliferation
Human adult SDSCs obtained from Asterand (North America Laboratories, Detroit, MI) were cultured in growth medium containing alpha-minimum essential medium, 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone as described previously.16–18 Passage 4 SDSCs were treated with 10 ng/mL of FGF-2 or FGF-10 (PeproTech, Inc., Rocky Hill, NJ) during cell expansion/proliferation (F2P and F10P, respectively) or differentiation (F2D and F10D, respectively) (Fig. 2A). Cells cultured with no FGF treatment acted as a control (CNTL). Cell number was counted in 175 cm2 flasks (n = 6) using a hemocytometer. To determine proliferation index, before cell expansion, SDSCs were labeled with CellVue® Claret at 2 × 10−6 M for 5 min according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO). After 6 days of proliferation, expanded cells were collected and measured using a BD FACSCalibur™ flow cytometer (dual laser) (BD Biosciences, San Jose, CA). Twenty thousand events of each sample were collected using CellQuest Pro software (BD Biosciences), and cell proliferation index was analyzed by ModFit LT™ version 3.1 (Verity Software House, Topsham, ME).
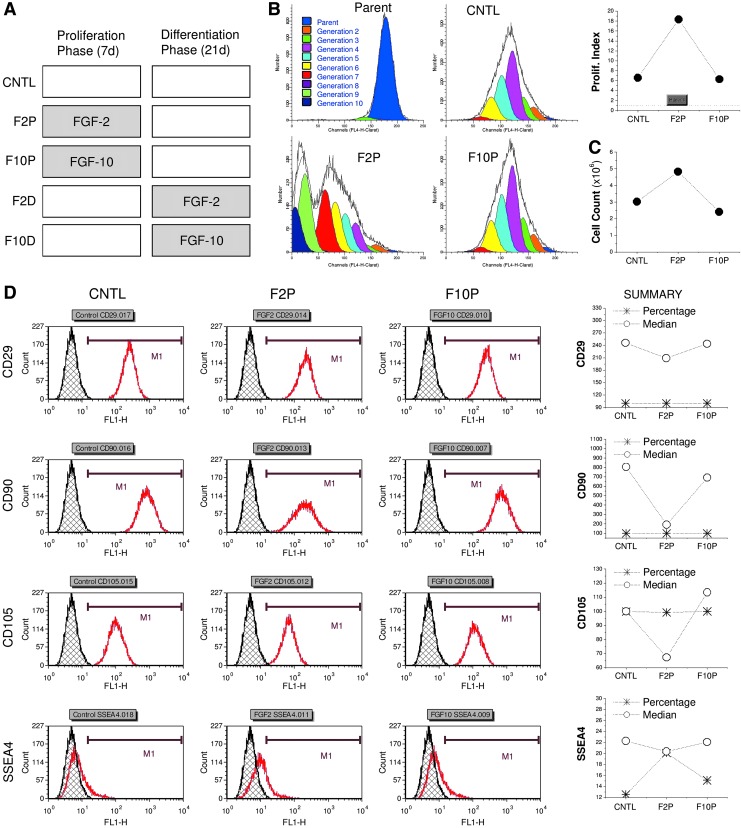
FIG. 2.
FGF ligand mediated human SDSC proliferation. (A) Experimental design. Five groups in total: FGF-2 and FGF-10 were only included in a 7-day cell expansion phase (F2P and F10P, respectively) or in a 21-day differentiation phase (F2D and F10D, respectively) with non-FGF treatment as a control (CNTL). (B) Flow cytometry was used to measure proliferation (prolif.) index of expanded SDSCs. (C) Cell number was counted ( × 106) after a 7-day cell expansion on 175 cm2 flasks (n = 6). (D). Flow cytometry was used to measure both percentage and median fluorescence intensity of mesenchymal stem cell surface markers (CD29, CD90, CD105, and SSEA4) of expanded SDSCs. SSEA4, stage-specific embryonic antigen 4. Color images available online at www.liebertpub.com/tea
Surface phenotypes of expanded cells
The following primary antibodies were used in flow cytometry analysis to detect expanded SDSC surface immunophenotype profiles: CD29 (Abcam, Cambridge, MA), CD90 (BD Pharmingen, San Jose, CA), CD105 (BioLegend, San Diego, CA), the stage-specific embryonic antigen 4 (SSEA4; BioLegend), and isotype-matched immunoglobulin Gs (IgGs; Beckman Coulter, Fullerton, CA). The secondary antibody was goat anti-mouse IgG (H + L) R-phycoerythrin conjugated (Life Technologies, Carlsbad, CA). Samples (n = 3) of each 2 × 105 expanded cells were incubated on ice in cold phosphate-buffered saline containing 0.1% ChromPure Human IgG whole molecule (Jackson ImmunoResearch Laboratories, West Grove, PA) and 1% NaN3 (Sigma-Aldrich) for 30 min. The cells were then sequentially incubated in the dark in the primary and secondary antibodies for 30 min. Fluorescence was analyzed by a FACSCalibur (BD Biosciences) using FCS Express 4 software package (De Novo Software, Los Angeles, CA).
Senescence and differentiation gene expression
Expanded cells were evaluated using real-time polymerase chain reaction (PCR) for senescence and differentiation-related gene changes. Total RNA was extracted from representative samples (n = 4) using an RNase-free pestle in TRIzol® (Life Technologies). Two micrograms of mRNA were used for reverse transcriptase with a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) at 37°C for 120 min. Senescence-related genes (cyclin-dependent kinase inhibitor 2A [P16; Assay ID Hs00923894_m1], cyclin-dependent kinase inhibitor 1A [P21; Assay ID Hs00355782_m1], and tumor protein p53 [TP53; Assay ID Hs01034249_m1]), chondrogenic marker genes (SRY [sex determining region Y]-box 9 [SOX9; Assay ID Hs00165814_m1], aggrecan [ACAN; Assay ID AIQJAP5], and type II collagen [COL2A1; Assay ID Hs00156568_m1]), and osteogenic genes (Runt-related transcription factor 2 [RUNX2; Assay ID Hs00231692_m1]; Osterix [SP7; Assay ID Hs01866874_s1]; Secreted Phosphoprotein 1 [SPP1; Assay ID hs00959010_m1]; Bone sialoprotein [IBSP; Assay ID hs00173720_m1]; and Bone Gamma-Carboxyglutamate [Gla] Protein [BGLAP; Assay ID Hs01587814_g1]) were customized by Applied Biosystems as part of their Custom TaqMan® Gene Expression Assays. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH; Assay ID Hs02758991_g1) was carried out as the endogenous control gene. Real-time PCR was performed with the iCycler iQ™ Multicolor RT-PCR Detection and calculated by computer software (PerkinElmer, Wellesley, MA). Relative transcript levels were calculated as χ = 2−ΔΔCt, in which ΔΔCt = ΔE − ΔC, ΔE = Ctexp − CtGAPDH, and ΔC = Ctct1 − CtGAPDH.
Chondrogenic induction and evaluation
Chondrogenic induction
Expanded cells (3.0 × 105) were centrifuged at 500 g for 5 min in a 15-mL polypropylene tube to form a pellet. After overnight incubation (day 0), the pellets were cultured in a serum-free chondrogenic medium consisting of high-glucose Dulbecco's modified Eagle's medium, 40 μg/mL proline, 100 nM dexamethasone, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.1 mM ascorbic acid-2-phosphate, and 1 × ITS™ Premix (BD Biosciences) with the supplementation of 10 ng/mL transforming growth factor beta3 (TGF-β3, PeproTech, Inc.) in a 5% O2 incubator as long as 21 days. F2D and F10D groups were also supplemented with 10 ng/mL FGF-2 or FGF-10, respectively. The pellets were evaluated using real-time PCR for chondrogenic marker genes (ACAN and COL2A1), type I collagen (COL1A1; Assay ID Hs00164004_m1), hypertrophic genes (type X collagen [COLXA1; Assay ID Hs00166657_m1] and alkaline phosphatase [ALP; Assay ID Hs01029144_m1]), histology and immunohistochemistry (IHC) for staining, and biochemical analysis for both DNA and glycosaminoglycan (GAG) amounts.
Histology and immunostaining
Representative pellets (n = 2) were fixed in 4% paraformaldehyde at 4°C overnight, followed by dehydrating in a gradient ethanol series, clearing with xylene, and embedding in paraffin blocks. Five-micrometer thick sections were stained with Alcian blue (counterstained with fast red) for sulfated GAGs. For IHC, the sections were immunolabeled with primary antibody against type II collagen (Col2; II-II6B3, Developmental Studies Hybridoma Bank, Iowa City, IA], followed by the secondary antibody of biotinylated horse anti-mouse IgG (Vector, Burlingame, CA). Immunoactivity was detected using VECTASTAIN ABC reagent (Vector) with 3, 3′-diaminobenzidine as a substrate.
Biochemical analysis for DNA and GAG contents
Representative pellets (n = 4) were digested at 60°C for 4 h with 125 μg/mL papain in PBE buffer (100 mM phosphate, 10 mM ethylenediaminetetraacetic acid, pH 6.5) containing 10 mM cysteine. To quantify cell density, the amount of DNA in the papain digestion was measured using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Life Technologies) with a CytoFluor® Series 4000 (Applied Biosystems). GAG was measured using dimethylmethylene blue dye and a Spectronic™ BioMate™ 3 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA) with bovine chondroitin sulfate (Sigma-Aldrich) as a standard.
Expression of both Wnt and MAPK signals following FGF-mediated chondrogenesis
Expanded cells and subsequent pellets were dissolved in the lysis buffer (Cell Signaling, Danvers, MA) with protease inhibitors. Total proteins were quantified using BCA™ Protein Assay Kit (Thermo Fisher Scientific). Thirty micrograms of protein from each sample were denatured and separated using NuPAGE® Novex® Bis-Tris Mini Gels in the XCell SureLock™ Mini-Cell (Life Technologies) at 120 V at 4°C for 3 h. Bands were transferred onto a nitrocellulose membrane using an XCell II™ Blot module (Life Technologies) at 15 V at 4°C overnight. The membrane was incubated with primary monoclonal antibodies in 5% bovine serum albumin in TBST buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% TWEEN 20) for 1 h (β-actin served as an internal control), followed by the secondary antibody of horseradish peroxidase–conjugated goat anti-mouse (Thermo Fisher Scientific) for 1 h. SuperSignal West Femto Maximum Sensitivity Substrate and CL-XPosure Film (Thermo Fisher Scientific) were used for exposure. The primary antibodies used in immunoblotting included the MAPK Family Antibody Sampler Kit (extracellular signal-regulated protein kinases 1 and 2 [Erk1/2], Jun N-terminal kinase [Jnk], and p38), phosphorylated (p-) MAPK Family Antibody Sampler Kit, and Wnt Signaling Antibody Sampler Kit (Cell Signaling). Wnt11 polyclonal antibody was obtained from Thermo Fisher Scientific. Wnt signals were also evaluated using real-time PCR (WNT3A; Assay ID Hs00263977_m1, WNT5A; Assay ID Hs00998537_m1, and WNT11; Assay ID Hs00182986_m1) following cell expansion and chondrogenic differentiation.
Osteogenic induction and evaluation
Expanded cells (n = 3) cultured for 21 days in osteogenic medium (growth medium supplemented with 0.1 μM dexamethasone, 10 mM β-glycerol phosphate, 50 μM ascorbate-2-phosphate, and 0.01 μM 1,25-dihydroxyvitamin D3) were collected for ALP activity assay with a reagent kit (Sigma-Aldrich) by measuring the formation of p-Nitrophenol from p-nitrophenyl phosphate following the manufacturer's instructions. For evaluation of calcium deposition, induced cells (n = 3) were fixed with 70% ice-cold ethanol for 1 h and then incubated in 40 mM Alizarin Red S (ARS) at pH 4.2 for 20 min with agitation. After rinsing, matrix mineral-bound staining was photographed. Quantification of staining was performed by staining density using ImageJ software.
Statistical analyses
Analysis of variance with pairwise comparison and t-test was used to compare measurements between different groups. All statistical analyses were performed with SPSS 13.0 statistical software (SPSS, Inc., Chicago, IL). p-Values <0.05 were considered statistically significant.
Results
Addition of FGF-2 but not FGF-10 during the expansion phase promoted SDSC proliferation
After 7 days of monolayer expansion, SDSCs without FGF treatment exhibited enlarged cell morphology which, in the presence of FGF-2, became notably smaller and fibroblast-like shaped cells with a glistening outline. Both proliferation index (Fig. 2B) and cell number counting data (Fig. 2C) suggested that FGF-2 significantly enhanced SDSC proliferation, while FGF-10 supplementation did not induce greater cell proliferation compared to the control group. This result is also supported by SSEA4 expression levels evaluated by flow cytometry (Fig. 2D). Surprisingly, other stem cell markers (Fig. 2D), including CD29, CD90, and CD105, exhibited decreases in median fluorescence intensity when SDSCs were expanded in the presence of FGF-2. Similar to the cell proliferation data, FGF-10 treatment did not elicit any meaningful differences in the expression of stem cell markers.
Addition of FGF-2 led to changes in expression of senescence and differentiation-related genes
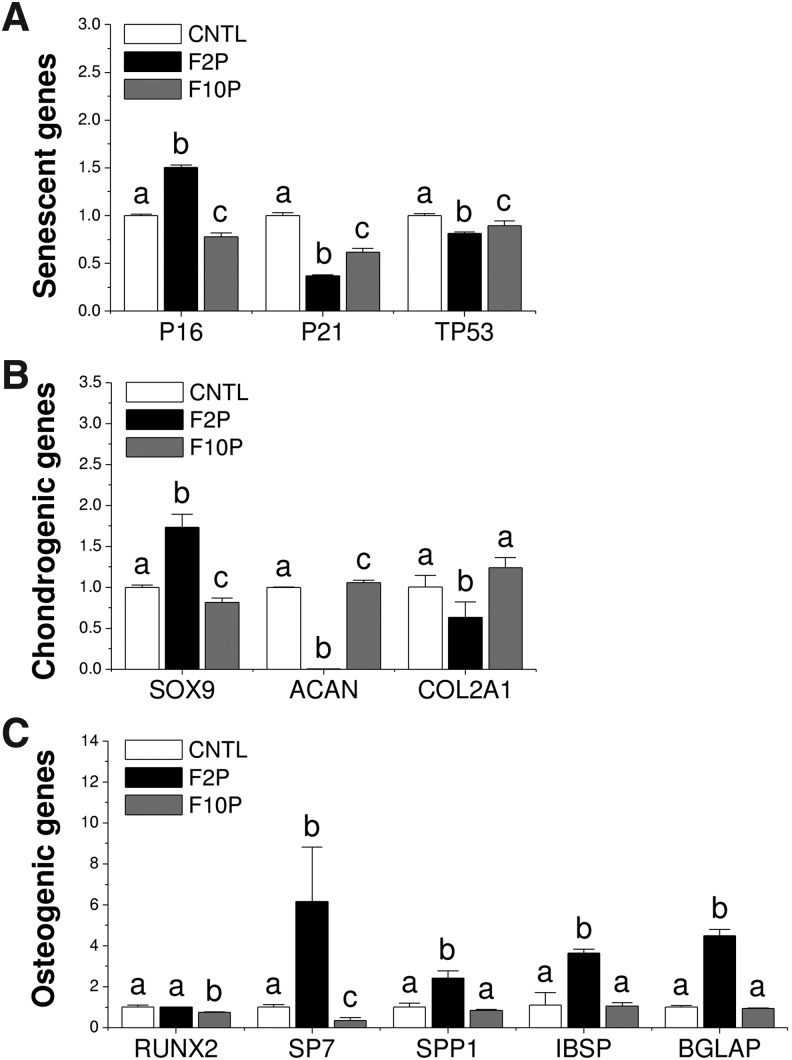
TaqMan® real-time PCR data showed that FGF-2 treatment upregulated P16 expression level in expanded cells compared with the control group but downregulated senescent genes P21 and TP53 expression levels (Fig. 3A). We also found that FGF-2 treatment significantly increased transcriptional factor SOX9, and FGF-10 treatment decreased SOX9 expression. This result was also accompanied by early and dramatic decreases in chondrogenic marker gene expression (ACAN and COL2A1) (Fig. 3B). For osteogenic genes, FGF-10 preconditioning significantly decreased RUNX2 expression, while FGF-2 produced similar RUNX2 expression levels as the control. Interestingly, FGF-2 supplementation, but not FGF-10, led to significant increases in SP7, SPP1, IBSP, and BGLAP gene expression versus other groups (Fig. 3C).
FIG. 3.
FGF ligand mediated senescence and differentiation-related gene expression in expanded SDSCs. TaqMan® real-time PCR was used to quantify mRNA levels of senescent genes (A): P16, P21, and TP53, chondrogenic genes (B): SOX9, ACAN, and COL2A1, and osteogenic genes (C): RUNX2, SP7, SPP1, IBSP, and BGLAP. Data are shown as average ± SD for n = 4. Groups not connected by the same letter are significantly different (p < 0.05). PCR, polymerase chain reaction; SD, standard deviation; SOX9, SRY (sex determining region Y)-box 9; ACAN, aggrecan; COL2A1, type II collagen; RUNX2, Runt-related transcription factor 2; SP7, Osterix; SPP1, secreted phosphoprotein 1; IBSP, bone sialoprotein; BGLAP, bone gamma-carboxyglutamate (Gla) protein.
Addition of FGF-2 but not FGF-10 during the expansion phase promoted SDSC chondrogenic potential
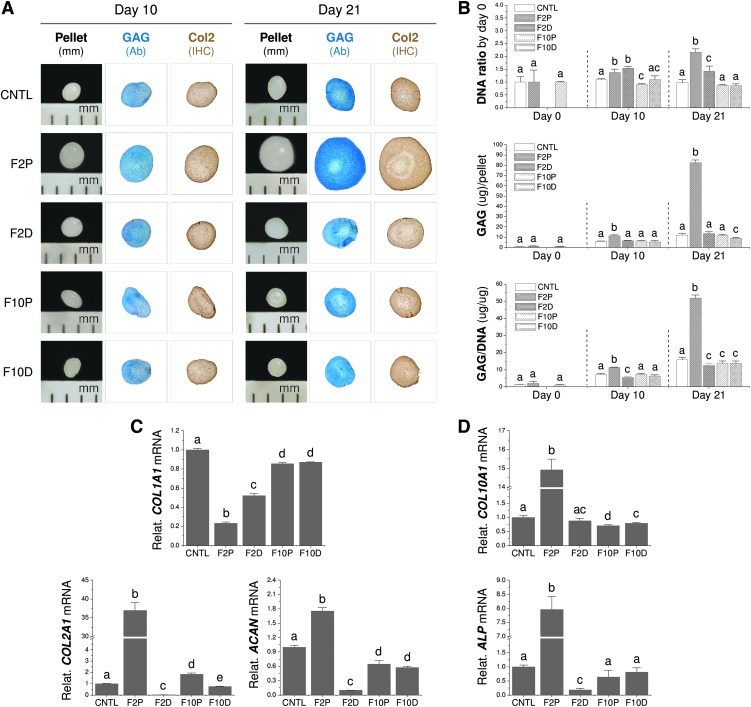
After 21-day chondrogenic induction, FGF-2 pretreated SDSCs yielded pellets with the largest size and most intense staining for sulfated GAGs by Alcian blue and Col2 by IHC compared to the other four groups, which exhibited similar pellet sizes and staining intensities (Fig. 4A). These findings were confirmed by biochemical analysis data in which the FGF-2 pretreatment group yielded the highest DNA ratio by day 0, GAG amount per pellet, and ratio of GAG to DNA, known as the chondrogenic index (Fig. 4B). TaqMan real-time PCR data showed that, after 21-day chondrogenic induction, FGF-2 pretreated cells yielded the lowest expression of COL1A1, but the highest levels of COL2A1 and ACAN, while FGF-2 treated cell pellets yielded the second lowest level of COL1A1 and the lowest levels of COL2A1 and ACAN. Both FGF-10 treatment groups, regardless of supplementation phase, yielded similar expression of COL1A1 and ACAN, which was lower than the control group (Fig. 4C), but still significantly greater than either FGF-2 treatment group. We also found that FGF-2 pretreatment yielded cells with the highest level of COL10A1 and ALP, while FGF-2 treatment yielded cells with the lowest level of ALP mRNA (Fig. 4D).
FIG. 4.
FGF ligand mediated SDSC chondrogenic differentiation. After a 21-day chondrogenic induction, SDSC pellets were evaluated using Alcian blue staining for sulfated GAGs and immunohistochemical staining for Col2 (A), biochemical analysis for both DNA and GAG amounts in a pellet (B), and TaqMan real-time PCR for mRNA levels of chondrogenic genes (C): COL1A1, COL2A1, and ACAN and hypertrophic genes (D): COL10A1 and ALP. Data are shown as average ± SD for n = 4. Groups not connected by the same letter are significantly different (p < 0.05). GAG, glycosaminoglycan; Col2, type II collagen; ALP, alkaline phosphatase. Color images available online at www.liebertpub.com/tea
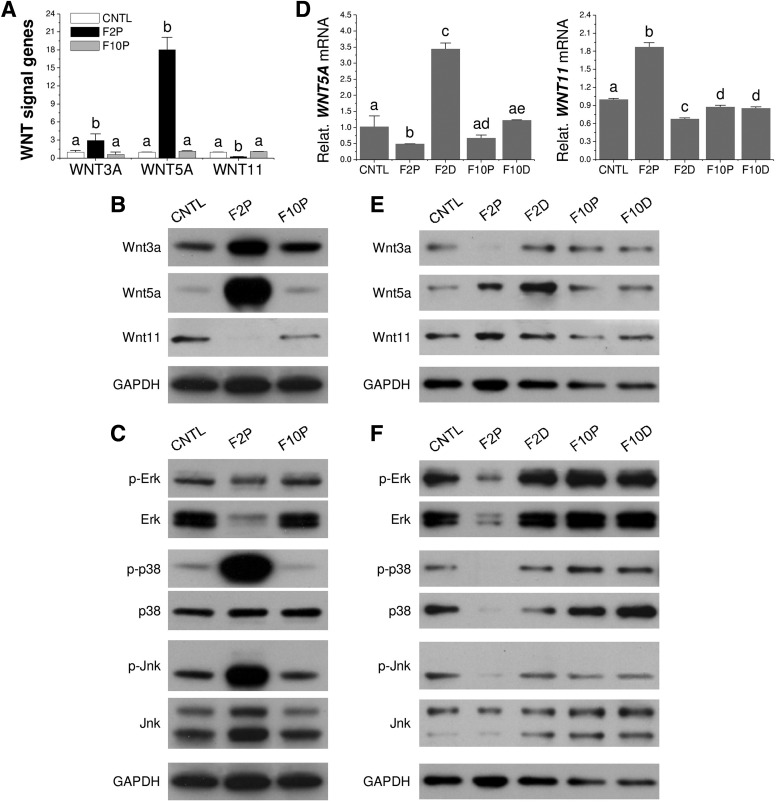
Wnt and MAPK signals following FGF-2 preconditioning and chondrogenic differentiation
TaqMan real-time PCR data showed that FGF-2 pretreated SDSCs displayed a significant upregulation of WNT5A and WNT3A and downregulation of WNT11 (Fig. 5A), which was confirmed by Western blot data (Fig. 5B); compared to significant upregulation of both p-p38 and p-Jnk signals in FGF-2 pretreated SDSCs, total Erk1/2 was markedly reduced with a modest suppression in p-Erk (Fig. 5C). Following chondrogenic differentiation, FGF-2 pretreated SDSCs showed decreased levels of Wnt5a, but increased levels of Wnt11; however, FGF-2 treatment in chondrogenic induction exhibited an opposite trend (Fig. 5D, E). Interestingly, FGF-2 pretreated SDSCs also showed decreased levels of p38, Jnk, and Erk1/2 compared to other groups (Fig. 5F).
FIG. 5.
FGF ligand mediated Wnt and MAPK signal changes in SDSC chondrogenesis. During cell expansion (A–C) and chondrogenic induction (D–F), Wnt signals were evaluated using both TaqMan real-time PCR (A, D) and Western blot (B, E), while MAPK signals were evaluated using Western blot (C, F). GAPDH was used as an internal control. Data are shown as average ± SD for n = 4. Groups not connected by the same letter are significantly different (p < 0.05). MAPK, mitogen-activated protein kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of FGF-2 and FGF-10 on SDSC osteogenic differentiation
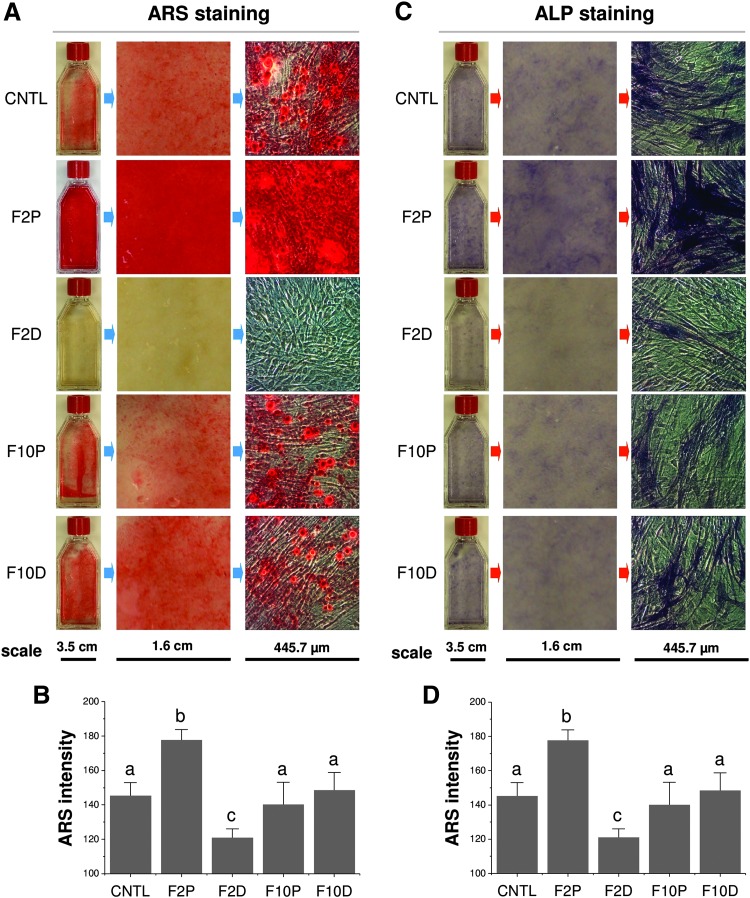
After 21-day osteogenic induction, FGF-2 pretreated SDSCs exhibited the highest density of both ARS (Fig. 6A) and ALP staining (Fig. 6B), indicating stronger calcium deposition and ALP expression, respectively. Interestingly, FGF-2 treatment in osteogenic induction significantly decreased ARS staining compared to other groups, while FGF-10 treatment either during cell expansion or osteogenic induction had no evident effect on osteogenic differentiation of expanded SDSCs (Fig. 6C, D).
FIG. 6.
FGF ligand mediated SDSC osteogenic differentiation. After a 21-day osteogenic induction, both ARS (A) and ALP staining (C) were used to evaluate osteogenic differentiation. ImageJ software was used to semiquantify the density of staining (B, D, respectively). Data are shown as average ± SD for n = 4. Groups not connected by the same letter are significantly different (p < 0.05). ARS, Alizarin Red S. Color images available online at www.liebertpub.com/tea
Discussion
In this study, we sought to determine whether FGF-2 or FGF-10 supplementation during the proliferation phase or the differentiation phase could improve human SDSC chondrogenesis and osteogenesis. In addition, we wanted to determine if both Wnt and MAPK signals were involved in FGF ligand-mediated SDSC proliferation and lineage differentiation. The present study suggested that FGF-2 supplementation during the proliferation phase can precondition SDSCs to undergo more successful chondrogenesis and osteogenesis following differentiation induction, which was superior to FGF-10 supplementation, no treatment, and the addition of either FGF during differentiation. The early upregulation of Wnt3a and Wnt5a and early downregulation of Wnt11 are likely to be influential mechanisms involved in FGF preconditioning and successful differentiation along the chondrogenic and osteogenic lineages. Chondrogenic differentiation was complimented by an upregulation of SOX9, a known transcription factor for chondrogenic activity, with FGF-2 supplementation during proliferation. Interestingly, FGF-2 preconditioning during the proliferation phase led to a RUNX2-independent mRNA upregulation of several vital osteogenic genes and more successful osteogenesis in vitro than all other groups. These findings suggested that FGF-2 supplementation during the early proliferation phase can prime human SDSCs for successful chondrogenic and osteogenic differentiation, which may be, in part, modulated through Wnt and MAPK signaling, but not when supplemented with FGF-2 during differentiation or with FGF-10 at any stage.
Unlike human germ cells, most human somatic cells do not express telomerase and, therefore, lose telomeric DNA during each round of DNA replication.19 Stress conditions could cause cell senescence20 resulting from DNA damage triggered by telomere shortening through the p53-mediated signaling pathway; on the contrary, p53 inactivation prolongs the lifespan of human fibroblasts.21 The expression of p21, a p53 target gene, increases in senescent cells.22 Since targeted deletion of the p21 gene is sufficient to escape senescence in human fibroblasts,23 p21 may have a major role in the induction of cellular senescence in human fibroblasts by inhibiting the activity of cyclin-dependent kinases. In this study, preconditioning using FGF-2 dramatically decreased P21 and TP53, indicating a rescue of telomere-dependent intrinsic senescence. Interestingly, both FGF-2 and FGF-10 pretreatment dramatically increased P16 expression in expanded cells, which is usually raised through extrinsic senescence such as reactive oxygen,19 suggesting that the rescue dominated by FGF-2 pretreatment was independent of the p16/pRb pathway. Future studies will be necessary to fully validate these trends, determine whether these changes are consistent over several cell passages, and discover a possible mechanism in which FGF-2 may be regulating cell senescence.
In this study, we found that FGF-2 rather than FGF-10 promoted SDSC proliferation. The potential underlying mechanisms could be selection of a particular subset of cells by telomere length in FGF-2-expanded MSCs.24,25 Despite the role in regulating the proliferation and maintenance of dental epithelial stem cells,26 in this study, FGF-10 did not act as a proliferation promoter like FGF-2. Interestingly, FGF-2 pretreatment decreased CD29, CD90, and CD105 expression, but increased SSEA4. This finding is consistent with a report by Hagmann et al. in which they found that CD90, CD105, and CD146 were significantly decreased in human BMSCs after treatment with FGF-2.27 One potential explanation is that FGF-2 could decrease the expression of TGF-β, which is a promoter of cell senescence.28 Sacchetti et al. found that FGF-2 pretreatment decreased the expression of CD105 (endoglin), a TGF-β coreceptor.29 Furthermore, Ito et al. found that downregulation of TGF-β signaling was responsible for the rejuvenating effect of FGF-2 pretreatment in terms of osteogenic and chondrogenic potential in human MSCs.30 A similar trend in surface phenotypes was observed in human SDSCs after expansion on dECM deposited by stem cells.17,18 Like pretreatment with FGF-2, this dECM expansion approach also plays a role in the preconditioning of expanded stem cells in both proliferation and differentiation capacity.31 Since CD29, CD90, and CD105 are considered traditional surface markers for stem cells,32 the decrease in these markers during stem cell rejuvenation raises questions regarding their defined roles as they pertain to stem cell “stemness.”
Despite the fact that both chondrogenic and osteogenic potentials were significantly increased after FGF-2 pretreatment, we did find a discrepancy in the expression of transcriptional and differentiation genes for a specific lineage in expanded cells. In terms of osteogenesis, FGF-2 pretreated SDSCs exhibited an upregulation of both transcriptional gene (SP7) and matrix genes (SPP1, IBSP, and BGLAP), despite no significant change in another transcriptional gene (RUNX2). This finding might be explained by negative feedback modulation through addition of exogenous FGF-2, which can lower endogenous FGF-2 expression,33 resulting in a decrease of the osteogenesis inhibitory effect exerted by FGF-2 during subsequent osteogenic induction.34,35 In terms of chondrogenesis, however, FGF-2 pretreated cells exhibited an upregulation of a transcriptional gene (SOX9), but downregulation of matrix genes (ACAN and COL2A1). This finding could be explained by a recent report in which pretreatment with FGF-2 could significantly enhance reprogramming efficiency by downregulating matrix genes such as collagen.36
Due to the importance of both Wnt and MAPK signaling in cartilage regeneration,15 the signals associated with these two pathways were characterized during FGF-2 pretreatment and subsequent chondrogenic induction. Following FGF-2 supplementation during the proliferative phase, we found that WNT3A and WNT5A mRNAs were markedly increased, while WNT11 mRNA was decreased. Interestingly, a contrasting trend, with WNT5A mRNA expression significantly decreased and WNT11 mRNA significantly increased versus other groups, occurred following 21-day pellet differentiation culture. This result appears to support the notion that Wnt signals are stage-specifically expressed during chondrogenesis.37 For instance, Wnt5a has been previously implicated as an early promoter38 and Wnt11, which has been shown to stimulate Col2 deposition,39 is a late promoter of chondrogenesis.40 Another study reported that Wnt3a-canonical and noncanonical Wnt pathways counteracted one another in MSC chondrogenesis.41 This notion seems well supported by other studies, which suggest that Wnt3a can prevent or inhibit chondrogenesis, while Wnt5a supports cartilage formation, but can impede Col2 expression and induce dedifferentiation.39,42,43 These findings support the Wnt expression trends observed in our data, where both Wnt3a and Wnt5a were expressed in earlier stages following proliferation and FGF-2 supplementation but, following chondrogenic differentiation, Wnt3a and Wnt5a mRNAs and proteins were either significantly decreased or undetected. This expression is likely important in maintaining the “stemness” of the SDSCs, which may promote successful responses to chondrogenic media, as well as later expression of Col2, aggrecan, and greater GAG deposition. In addition, it is worth noting that preconditioning methods, which directly or indirectly regulate Wnt signaling, could hold great potential; however, a study by Hoang44 raises a valid point that therapies targeting Wnt may have significant side effects due to their defined roles in tissue regeneration and stem cell self-renewal, as well as osteosarcoma. Although aberrant Wnt signaling does not seem to be a concern in this study, specifically with FGF-2 treatment of SDSCs, as evidenced by the reversed trend in the Wnt real-time PCR results from the expansion phase to the differentiation phase, it is a caveat which should be noted when considering therapies which modify Wnt signaling.
Interestingly, RUNX2 was not significantly increased in either of the FGF supplemented groups versus the control in expanded cells before osteogenic induction. Furthermore, Western blotting revealed that expanded cells treated with FGF-2 during proliferation had robust levels of p-p38 and p-Jnk, but little p-Erk1/2 or Erk1/2. It is known that RUNX2, a transcriptional regulator of osteogenesis, is heavily regulated through the Erk signaling pathway45,46; however, it may not be required for osteogenic differentiation to occur. A study using rat BMSCs reported results demonstrating that inhibition of p38 activity alone did not interfere with osteogenesis and suggested that mechanical strain-induced osteogenic differentiation was a result of Erk1/2 and RUNX2 activation.47 Additional studies have reported that the inhibition of Erk1/2 and Jnk, but not p38, has resulted in inhibited osteogenic differentiation48; also, decreased RUNX2 expression was observed in BMSCs with the inhibition of Erk1/2 phosphorylation.46 Furthermore, it has been confirmed that RUNX2 activation occurs through the Erk pathway.45 Although p38 is undeniably implicated in osteogenesis, studies targeting the Erk and Jnk pathways through inhibition seem to suggest that the Erk pathway may be more closely related to RUNX2 activation and regulation. Given the growing number of studies reporting RUNX2 activation without p38 signaling, the lack of RUNX2 expression could very likely be directly related to Erk1/2 signaling, which was significantly decreased in our current study compared to robust levels of p-p38 and p-Jnk in the FGF-2 pretreatment group following cell expansion, as evidenced by Western blotting. It is very possible that increases in ALP and ARS staining following successful osteogenic induction in the FGF-2 pretreatment group are the result of increased gene expression of other key osteogenic genes such as SP7, SPP1, IBSP, and BGLAP, which were all significantly increased in the FGF-2 pretreatment group versus control and FGF-10 pretreatment groups, but not through mechanisms related to RUNX2 or Erk1/2 activation. Overall, it seems that the strong ALP activity, calcium deposition, and upregulation of several key osteogenic genes in the FGF-2 pretreatment group were primarily driven through RUNX2-independent and Erk1/2-independent signaling and likely occurred through p38, Jnk, and potentially other signaling mechanisms. More studies need to be performed to fully assess the necessity of Erk1/2 and RUNX2 roles in SDSC osteogenesis.
In conclusion, FGF-2 preconditioning led to superior cell proliferation, chondrogenesis, osteogenesis, and overall rejuvenation versus no treatment or FGF-10 preconditioning. Cells preconditioned with FGF-2 during the proliferative phase led to robust chondrogenic pellet formation, accompanied by significant GAG deposition and upregulation of vital chondrogenic gene expression. Likewise, FGF-2 preconditioning produced unmatched osteogenesis, leading to cells with increased gene expression following proliferation, and led to significant increases in ARS and ALP staining for the differentiated cell cultures versus other groups. FGF-2, but not FGF-10, preconditioning was able to elicit significant changes in Wnt signal expression, as well as pronounced p38 signaling. FGF-2 seems to be an optimal choice as a preconditioning stimulus for human SDSC chondrogenesis and osteogenesis.
Acknowledgments
The authors thank Suzanne Danley for editing the article. This project was partially supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (AR062763-01A1 and P20GM103434).
Disclosure Statement
No competing financial interests exist.
References
- 1.Karnes J., Zhang Y., and Pei M. Cell therapy for the creation of cartilage and related clinical trials. In: Templeton N.S., ed. Gene and Cell Therapy: Therapeutic Mechanisms and Strategies. 4th edition. Boca Raton, FL: Taylor & Francis/CRC Press, 2014, pp. 1123–1135 [Google Scholar]
- 2.Pizzute T., Lynch K., and Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev 11, 119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones B.A., and Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B 18, 301, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Li J.T., and Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B 18, 270, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Lynch K., and Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis 10, 289, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ornitz D.M., and Marie P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev 29, 1463, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ornitz D.M. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev 16, 205, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohuchi H., Nakagawa T., Yamamoto A., Araga A., Ohata T., Ishimaru Y., et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 124, 2235, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Sekine K., Ohuchi H., Fujiwara M., Yamasaki M., Yoshizawa T., Sato T., et al. FGF-10 is essential for limb and lung formation. Nat Genet 21, 138, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Terao F., Takahashi I., Mitani H., Haruyama N., Sasano Y., Suzuki O., et al. Fibroblast growth factor 10 regulates Meckel's cartilage formation during early mandibular morphogenesis in rats. Dev Biol 350, 337, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Coutu D.L., and Galipeau J. Roles of FGF signaling in stem cell self-renewal, senescence and aging. Aging (Albany NY) 3, 920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He F., Chen X., and Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 15, 3809, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Pei M., He F., and Kish V.L. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17, 3067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh C.D., Chang S.H., Yoon Y.M., Lee S.J., Lee Y.S., Kang S.S., et al. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem 275, 5613, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Pizzute T., and Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res 358, 633, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Hansen K.C., Zhang Y., Dong C., Dinu C.Z., Dzieciatkowska M., et al. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 35, 642, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Pizzute T., Li J.T., He F., and Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment—a feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials 64, 88, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Li J.T., Davis M.E., and Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater 20, 39, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itahana K., Campisi J., and Dimri G.P. Mechanisms of cellular senescence in human and mouse cells. Biogerontology 5, 1, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Atadja P., Wong H., Garkavtsev I., Veillette C., and Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci U S A 92, 8348, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itahana K., Dimri G., and Campisi J. Regulation of cellular senescence by p53. Eur J Biochem 268, 2784, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Noda A., Ning Y., Venable S.F., Pereira-Smith O.M., and Smith J.R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 211, 90, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Brown J.P., Wei W., and Sedivy J.M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277, 831, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Bianchi G., Banfi A., Mastrogiacomo M., Notaro R., Luzzatto L., Cancedda R., et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res 287, 98, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Yanada S., Ochi M., Kojima K., Sharman P., Yasunaga Y., and Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif 39, 575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H., Li S., Han D., Kaartinen V., and Chai Y. Alk5-mediated transforming growth factor β signaling acts upstream of fibroblast growth factor 10 to regulate the proliferation and maintenance of dental epithelial stem cells. Mol Cell Biol 31, 2079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagmann S., Moradi B., Frank S., Dreher T., Kämmerer P.W., Richter W., et al. FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif 46, 396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito T., Sawada R., Fujiwara Y., Seyama Y., and Tsuchiya T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun 359, 108, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324, 2007. Erratum in: Cell 133, 928, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Ito T., Sawada R., Fujiwara Y., and Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology 56, 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei M., Li J.T., Shoukry M., and Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater 22, 333, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Campbell D.D., and Pei M. Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells 1, 1107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim S., Cho H., Lee E., Won Y., Kim C., Ahn W., et al. Osteogenic stimulation of human adipose-derived stem cells by pre-treatment with fibroblast growth factor 2. Cell Tissue Res 364, 137, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Quarto N., and Longaker M.T. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng 12, 1405, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Quarto N., Wan D.C., and Longaker M.T. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs). Bone 42, 1040, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Jiao J., Dang Y., Yang Y., Gao R., Zhang Y., Kou Z., et al. Promoting reprogramming by FGF2 reveals that the extracellular matrix is a barrier for reprogramming fibroblasts to pluripotency. Stem Cells 31, 729, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Hosseini-Farahabadi S., Geetha-Loganathan P., Fu K., Nimmagadda S., Yang H.J., and Richman J.M. Dual functions for WNT5A during cartilage development and in disease. Matrix Biol 32, 252, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Church V., Nohno T., Linker C., Marcelle C., and Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci 115, 4809, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ryu J.H., and Chun J.S. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem 281, 22039, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sekiya I., Vuoristo J.T., Larson B.L., and Prockop D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A 99, 4397, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu F., Wang J., Xu N., Liu C., Li S., Wang N., et al. WNT3A modulates chondrogenesis via canonical and non-canonical Wnt pathways in MSCs. Front Biosci (Landmark Ed) 18, 493, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Kawakami Y., Wada N., Nishimatsu S.I., Ishikawa T., Noji S., and Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ 41, 29, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Surmann-Schmitt C., Widmann N., Dietz U., Saeger B., Eitzinger N., Nakamura Y., et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci 122, 3627, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Hoang B.H. Wnt, osteosarcoma, and future therapy. J Am Acad Orthop Surg 20, 58, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Qian X., Zhang C., Chen G., Tang Z., Liu Q., Chen J., et al. Effects of BMP-2 and FGF-2 on the osteogenesis of bone marrow-derived mesenchymal stem cells in hindlimb-unloaded rats. Cell Biochem Biophys 70, 1127, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Ye N., and Jiang D. Ghrelin accelerates the growth and osteogenic differentiation of rabbit mesenchymal stem cells through the ERK1/2 pathway. BMC Biotechnol 15, 51, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P., Dai Q., Ouyang N., Yang X., Wang J., Zhou S., et al. Mechanical strain promotes osteogenesis of BMSCs from ovariectomized rats via the ERK1/2 but not p38 or JNK-MAPK signaling pathways. Curr Mol Med 15, 780, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Kim B.S., Kang H.J., Park J.Y., and Lee J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med 47, e128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]