ABSTRACT
Although several signaling pathways in oriented cell division have been well characterized such as delta/notch inductions or wnt/frizzled-based anterior-posterior polarity, there is strong evidence for additional signal pathways controlling early anterior-posterior polarity decisions. The homolog of the adhesion G protein-coupled receptor latrophilin, LAT-1 has been identified as a receptor essential for oriented cell division in an anterior-posterior direction of specific blastomeres in the early C. elegans embryo. We recently conducted a study aiming at clarifying the signals involved in LAT-1 function. We identified a Gs protein/adenylyl cyclase/cAMP pathway in vitro and demonstrated its physiological relevance in oriented cell division. By interaction with a Gs protein LAT-1 elevates cAMP levels. These data indicate that G-protein signaling in oriented cell division is not solely GPCR-independent. This commentary will discuss our findings in the context of the current knowledge of mechanisms controlling oriented cell division and anterior-posterior polarity. Further, we identify open questions which need to be addressed in the future.
KEYWORDS: early C. elegans embryo, G protein-coupled receptor, latrophilin, signaling pathway, spindle orientation
Introduction
Multicellular organisms are highly dependent on their cells to be spatially organized to form different tissues and organs and to develop and maintain specialized functions. For this organization cells require a sense of orientation and a form of polarity, they need to know their position and how to get to the correct place in a group of cells. From the fertilized oocyte onwards a tightly regulated set of mechanisms controls the processes coordinating the arrangement of cells during development involving cell fate specification, differentiation, orientation of mitotic spindles and cell migration. While the molecular mechanisms regulating polarity and mitotic spindle orientation in asymmetric cell divisions have been intensely studied (reviewed in refs.1,2), the cues involved in spindle orientation of symmetric divisions and in propagating signals in groups of cells to align cell polarity and division plane orientation are less well understood. In the early Caenorhabditis elegans embryo spindle orientations during asymmetric and symmetric cell divisions are controlled by a network of only partially characterized signaling pathways including Wnt proteins1 and PAR proteins.3-5 The mechanistic details of the signals involved in cell polarity of the first 3 rounds of embryonic cell divisions establishing the characteristic geometry of blastomeres are considerably well understood.1,3 After the third round of cell cleavage creating an 8-cell stage embryo Wnt/Frizzled (Fz), constitutes an instructive signal specifying polarity. A posterior polarizing center is located in the descendants of the founder blastomere P14 and can orient the division planes of immediately adjacent cells.5 However, the propagation of this signal in subsequent rounds of cell divisions is barely characterized. The latrophilin/ADGRL homolog LAT-1 has been recently identified as a novel candidate molecule being involved in this process as it is required for the coordination of spindle orientation at this stage of embryonic development.6 Being originally described as receptor for the black widow spider's (Latrodectus mactans) toxin7,8 LAT-1 belongs to the adhesion G protein-coupled receptors (adhesion GPCRs). This class of receptors is considered to play dual roles in cell-cell or cell-matrix adhesion and transmembrane signaling,9-11 making them interesting molecules in the establishment and maintenance of polarity. An adhesion GPCR with a characterized role in planar cell polarity is the cadherin-like flamingo/starry night (FMI) and its vertebrate homologs ADGRC/CELSR (reviewed in refs.12,13).
Our studies focus on elucidating the function of LAT-1 and especially its signaling mechanisms required to exert its role in oriented cell division and polarity in the early embryo.
LAT-1 in tissue polarity of anterior blastomeres and oriented cell division
Previous studies have provided clear evidence that LAT-1 exerts several different functions in embryogenesis as well as in post-embryonic processes.6,14 However, its role in oriented cell division and anterior-posterior polarity is the most thoroughly studied one. Nematodes homozygous for the null mutant allele lat-1(ok1465) (referred to lat-1 hereafter) show severe defects in embryonic and larval development leading to only a small number of individuals passing developmental stages and reaching adulthood.6 Previous studies revealed that the spindle of ABal, one of the AB4 blastomeres in the 8-cell stage embryo, is turned almost perpendicular to the anterior-posterior axis in which this cell normally divides, suggesting that anterior-posterior polarity is disturbed (Fig. 1). This skewed orientation causes both daughter cells, ABala and ABalp, to contact the neighboring blastomere MS after division instead of only ABalp.6 A result of these incorrect cell-cell contacts is embryonic lethality. The arrangement of cells at the 12-cell stage does not solely depend on the cleavage direction of ABal but also on that of ABar. Under specific circumstances ABar may push ABala away from MS, whose position also varies in embryos. However, the effect of LAT-1 on division of other AB descendants such as ABar has not been fully analyzed yet and remains elusive.
Figure 1.
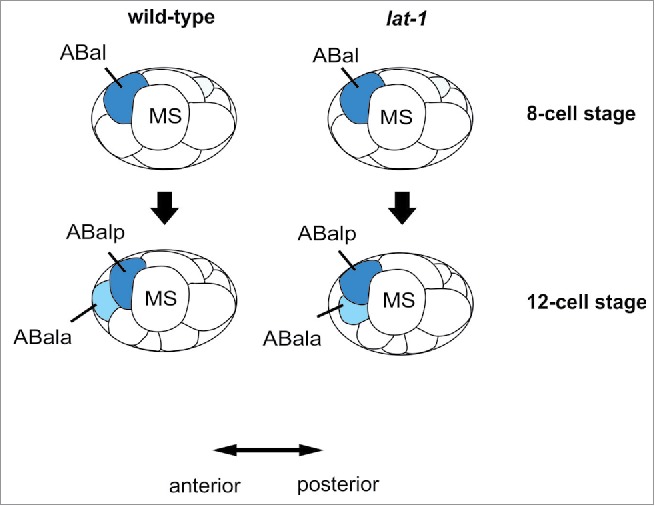
Schematic depiction of cleavage plane orientations in early wild-type and lat-1 mutant C. elegans embryos. In the 8-cell stage of wild-type embryos, ABal divides in a 128° angle to the anterior-posterior axis. This slightly skewed angled leads to the arising daughter cell ABala being displaced to the most anterior location of the embryo not contacting the MS blastomere whereas the ABalp daughter touches its posterior neighbor MS. In lat-1 mutant individuals the ABal cleavage plane is displaced to approximately 90° resulting in ABalp and ABala blastomeres which both contact MS.
These data indicate that LAT-1 is involved in orienting cleavage planes in an anterior-posterior direction in descendants of the ABal blastomere. For this function, maternal as well as zygotic contribution of LAT-1 is essential.6 One of the major pathways involved in polarity decisions at this stage in the early embryo is Wnt/Fz which is engaged in spindle rotation of some of the dividing AB descendants ensuring correct contacts of daughter cells to neighboring blastomeres.15-17 The relation of a LAT-1-mediated signal and a Wnt/Fz cascade remains unclear. It can be speculated that LAT-1 signaling acts in parallel of cues mediated by Wnt/Fz and aids its propagation.
LAT-1 mediates a Gs protein/adenylyl cyclase/cAMP signal upon intrinsic activation
For an in-depth understanding of polarity and oriented cell division knowledge on the molecular mechanisms underlying these processes is indispensable. Like for many other adhesion GPCRs the modes of LAT-1 signaling are poorly understood. In our recent study, we sought to assess the signals mediated by LAT-1 and their physiological relevance in oriented cell division.18 Our previous analyses revealed that the receptor mediates its function in spindle orientation via a signaling mode depending on its 7 transmembrane domain suggesting a classical GPCR signal involving heterotrimeric G proteins.6,14 To address this question we employed an in vitro assay system based on heterologous expression of lat-1 and observed functional G-protein coupling of the receptor to the 3 major G proteins Gs, Gq and Gi by measuring second messengers. Due to the high conservation of these G proteins among metazoan species19 heterologous coupling of these to the nematode receptor is possible. As no agonist of LAT-1 has been described yet, basal levels of receptor activity were measured. We identified a Gs signal leading to an increase in intracellular cyclic AMP (cAMP) levels, whereas no coupling to Gq or Gi proteins was observed. However, it cannot be excluded that LAT-1 activates several other G proteins besides the 3 tested in this study, especially as in total 21 Gα subunits exist in C. elegans, with most of them having no clear mammalian homolog. Signaling of LAT-1 was further characterized by analyses of the activation mechanism of LAT-1. We demonstrated that the receptor is activated by a 13 amino acid short intrinsic sequence located in the extracellular N terminus. Based on several lines of experimental evidence14 we speculate that this intrinsic activation occurs by structural changes within the receptor upon binding to a yet unknown extracellular agonist and interaction of this sequence with the 7 transmembrane region (Fig. 2). However, the details of activation still need to be investigated. Studies on the signaling mechanisms of other adhesion GPCRs which also contain an intrinsic agonistic sequence suggest the involvement of mechanical forces prior to activation.20,21 As the role of mechanosensation in polarity has been firmly shown (reviewed in ref.22), a possible activation of LAT-1 by this mechanism needs to be elucidated.
Figure 2.
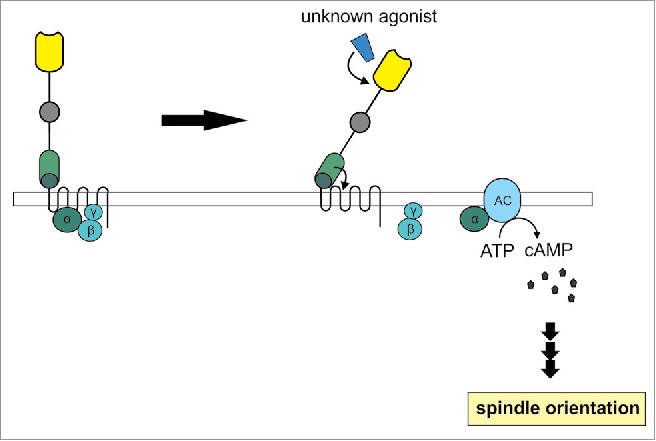
Model of LAT-1 signaling. In the absence of an extracellular agonist LAT-1 resides in an inactive state. As a consequence of interaction of LAT-1 with a so far unknown agonist, LAT-1 is activated by an intramolecular sequence in the extracellular domain adjacent to the transmembrane domain. This sequence possibly interacts with the 7 transmembrane domain. Due to further conformational changes a Gαs protein dissociates from its βγ subunit and is able to activate adenylyl cyclases (AC) which subsequently produce cAMP. This second messenger is essential for the control of correct cell division plane orientation in the ABal bastomere.
These experiments add a new layer of mechanistic understanding of LAT-1 as a novel receptor involved in oriented cell division in an anterior-posterior direction in ABal descendants and as a GPCR signaling via G proteins. So far, several studies have very elegantly demonstrated the involvement of G proteins, but in a GPCR-independent context, for instance via GEF proteins23 and G-protein regulators.24 Although a GPCR-dependent and G protein-mediated signaling pathway in these processes has not been unambiguously defined there are some clear indications for their existence.25,26 LAT-1 might fill this gap and contribute an additional signal via a Gs protein/adenylyl cyclase introducing a new level of regulation. From a molecular standpoint, controlling vital processes, which need to be tightly regulated, such as polarity by several pathways which are very different in conveying molecular information is highly advantageous as this concept avoids intersection of the multitude of signaling pathways.
cAMP-mediated LAT-1 signaling is essential for anterior-posterior cell division in AB descendants
The connection between the signaling characteristics of LAT-1 observed in vitro and their physiological relevance was first shown by the identification of GSA-1 as a candidate G protein LAT-1 possibly activates. GSA-1, which is also expressed in embryos27, is the closest homolog of Gαs in C. elegans displaying 66% identity to mammalian Gαs proteins.28 RNAi analyses of gsa-1 in lat-1 mutant embryos indicated a potential interaction of the receptor and the G protein which is consistent with the putative role of LAT-1 as a regulator of cAMP.
The impact of the identified Gs/adenylyl cyclase/cAMP-mediated mechanism of LAT-1 on ABal spindle orientation was evaluated by specifically modulating the proposed cascade in the absence of the receptor. Interestingly, a lack of receptor yields lower cAMP levels in embryos compared to wild-type controls. Elevation of cAMP by the adenylyl cyclase activator forskolin corrects the tilted ABal division plane in lat-1 mutant embryos, restores the correct contacts between the daughter cells and the MS blastomere and, subsequently, rescues lethality. While in lat-1 embryos the ABal division plane is tilted toward a position almost perpendicular to the anterior-posterior axis (Fig. 1) compared to the more oblique position (approximately 128°) in wild-type nematodes, forskolin treatment yields an almost wild-type ABal spindle orientation (approximately 114°). Other compounds elevating intracellular cAMP levels such as 8-bromoadenosine or 3-isobutyl-1-methylxanthine (IBMX) show similar positive effects on the survival rate lat-1 nematodes indicating that ABal division plane orientation is also corrected.
These data suggest that an increase in cAMP levels is a key signal for the anterior-posterior orientation of cleavage planes in the ABal cell in the C. elegans embryo. Despite this finding it cannot be fully excluded that the G protein subunits themselves are involved in parallel mechanisms. Interestingly, this metabotropic signal is not polarized although leading to a polarized outcome on the cellular level. This is in contrast to many other polarity-mediating molecules which are for instance asymmetrically distributed within cells29 and might indicate that LAT-1 is not directly or not solely mediating polarity in ABal division. However, it is well possible that the signal transduced by LAT-1 to mediate an effect on polarity is polarized further downstream through effectors of cAMP such as protein kinases A or A kinase anchoring proteins.30,31 It is also conceivable that polarity information in achieved by temporally restricting cues activating LAT-1.
Concluding remarks
The latrophilin homolog LAT-1 has been implicated in defining a mechanism required for the alignment of cell division planes in the early C. elegans embryo. The data we have provided on the signaling mechanism give first insights in the molecular functions of LAT-1. Reviewing the existing data, we believe that the receptor regulates intracellular cAMP levels via a G protein-mediated cascade. Further studies will be required to define the up- and especially downstream components of this novel receptor to gain information on the polarizing events within the cells. Another line of experiments need to address possible interactions with other polarity defining mechanisms such as the Wnt/Fz pathway.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (FOR2149, Pr1534/1-1, Sfb610), the European Social Funds and by the Medical Faculty, Leipzig University (Formel 1 junior research grant).
References
- [1].Gonczy P, Rose LS. Asymmetric cell division and axis formation in the embryo. WormBook 2005:1-20; http://dx.doi.org/ 10.1895/wormbook.1.30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol 2009; 11:365-74; PMID:19337318; http://dx.doi.org/ 10.1038/ncb0409-365 [DOI] [PubMed] [Google Scholar]
- [3].Walston TD, Hardin J. Wnt-dependent spindle polarization in the early C. elegans embryo. Semin Cell Dev Biol 2006; 17:204-13; PMID:16765610; http://dx.doi.org/ 10.1016/j.semcdb.2006.04.005 [DOI] [PubMed] [Google Scholar]
- [4].Hutter H, Schnabel R. glp-1 and inductions establishing embryonic axes in C. elegans. Development 1994; 120:2051-64; PMID:7925009 [DOI] [PubMed] [Google Scholar]
- [5].Goldstein B. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J Cell Biol 1995; 129:1071-80; PMID:7744956; http://dx.doi.org/ 10.1083/jcb.129.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Langenhan T, Prömel S, Mestek L, Esmaeili B, Waller-Evans H, Hennig C, Kohara Y, Avery L, Vakonakis I, Schnabel R, et al.. Latrophilin signaling links anterior-posterior tissue polarity and oriented cell divisions in the C. elegans embryo. Dev Cell 2009; 17:494-504; PMID:19853563; http://dx.doi.org/ 10.1016/j.devcel.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krasnoperov VG, Beavis R, Chepurny OG, Little AR, Plotnikov AN, Petrenko AG. The calcium-independent receptor of α-latrotoxin is not a neurexin. Biochem Biophys Res Commun 1996; 227:868-75; PMID:8886023; http://dx.doi.org/ 10.1006/bbrc.1996.1598 [DOI] [PubMed] [Google Scholar]
- [8].Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, et al.. α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 1997; 18:925-37; PMID:9208860; http://dx.doi.org/ 10.1016/S0896-6273(00)80332-3 [DOI] [PubMed] [Google Scholar]
- [9].Hamann J, Vogel B, van Schijndel GM, van Lier RA. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 1996; 184:1185-9; PMID:9064337; http://dx.doi.org/ 10.1084/jem.184.3.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stacey M, Chang GW, Davies JQ, Kwakkenbos MJ, Sanderson RD, Hamann J, Gordon S, Lin HH. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 2003; 102:2916-24; PMID:12829604; http://dx.doi.org/ 10.1182/blood-2002-11-3540 [DOI] [PubMed] [Google Scholar]
- [11].Hamann J, Aust G, Arac D, Engel FB, Formstone C, Fredriksson R, Hall RA, Harty BL, Kirchhoff C, Knapp B, et al.. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev 2015; 67:338-67; PMID:25713288; http://dx.doi.org/ 10.1124/pr.114.009647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet 2007; 8:555-63.; PMID:17563758; http://dx.doi.org/ 10.1038/nrg2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Strutt D. The planar polarity pathway. Curr Biol 2008; 18:R898-902; PMID:18957230; http://dx.doi.org/ 10.1016/j.cub.2008.07.055 [DOI] [PubMed] [Google Scholar]
- [14].Prömel S, Frickenhaus M, Hughes S, Mestek L, Staunton D, Woollard A, Vakonakis I, Schöneberg T, Schnabel R, Russ AP, et al.. The GPS motif is a molecular switch for bimodal activities of adhesion class G protein-coupled receptors. Cell Rep 2012; 2:321-31; PMID:22938866; http://dx.doi.org/ 10.1016/j.celrep.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 1997; 90:707-16; PMID:9288750; http://dx.doi.org/ 10.1016/S0092-8674(00)80531-0 [DOI] [PubMed] [Google Scholar]
- [16].Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell 1997; 90:695-705; PMID:9288749; http://dx.doi.org/ 10.1016/S0092-8674(00)80530-9 [DOI] [PubMed] [Google Scholar]
- [17].Walston T, Tuskey C, Edgar L, Hawkins N, Ellis G, Bowerman B, Wood W, Hardin J. Multiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryos. Dev Cell 2004; 7:831-41; PMID:15572126; http://dx.doi.org/ 10.1016/j.devcel.2004.10.008 [DOI] [PubMed] [Google Scholar]
- [18].Müller A, Winkler J, Fiedler F, Sastradihardja T, Binder C, Schnabel R, Kungel J, Rothemund S, Hennig C, Schöneberg T, et al.. Oriented Cell Division in the C. elegans Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1. PLoS Genet 2015; 11:e1005624; PMID:26505631; http://dx.doi.org/ 10.1371/journal.pgen.1005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].de Mendoza A, Sebe-Pedros A, Ruiz-Trillo I. The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity. Genome Biol Evol 2014; 6:606-19; PMID:24567306; http://dx.doi.org/ 10.1093/gbe/evu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schoneberg T, Piao X, et al.. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 2015; 85:755-69; PMID:25695270; http://dx.doi.org/ 10.1016/j.neuron.2014.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scholz N, Gehring J, Guan C, Ljaschenko D, Fischer R, Lakshmanan V, Kittel RJ, Langenhan T. The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep 2015; 11:866-74; PMID:25937282; http://dx.doi.org/ 10.1016/j.celrep.2015.04.008 [DOI] [PubMed] [Google Scholar]
- [22].Asnacios A, Hamant O. The mechanics behind cell polarity. Trends Cell Biol 2012; 22:584-91; PMID:22980034; http://dx.doi.org/ 10.1016/j.tcb.2012.08.005 [DOI] [PubMed] [Google Scholar]
- [23].Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gonczy P. RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 2004; 119:219-30; PMID:15479639; http://dx.doi.org/ 10.1016/j.cell.2004.09.026 [DOI] [PubMed] [Google Scholar]
- [24].Tsou MF, Hayashi A, Rose LS. LET-99 opposes Galpha/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling. Development 2003; 130:5717-30; PMID:14534135; http://dx.doi.org/ 10.1242/dev.00790 [DOI] [PubMed] [Google Scholar]
- [25].Cabello J, Neukomm LJ, Gunesdogan U, Burkart K, Charette SJ, Lochnit G, Hengartner MO, Schnabel R. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol 2010; 8:e1000297; PMID:20126385; http://dx.doi.org/ 10.1371/journal.pbio.1000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fievet BT, Rodriguez J, Naganathan S, Lee C, Zeiser E, Ishidate T, Shirayama M, Grill S, Ahringer J. Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat Cell Biol 2013; 15:103-12; PMID:23242217; http://dx.doi.org/ 10.1038/ncb2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Korswagen HC, Park JH, Ohshima Y, Plasterk RH. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes Dev 1997; 11:1493-503; PMID:9203577; http://dx.doi.org/ 10.1101/gad.11.12.1493 [DOI] [PubMed] [Google Scholar]
- [28].Park JH, Ohshima S, Tani T, Ohshima Y. Structure and expression of the gsa-1 gene encoding a G protein α(s) subunit in C. elegans. Gene 1997; 194:183-90; PMID:9272860; http://dx.doi.org/ 10.1016/S0378-1119(97)00122-4 [DOI] [PubMed] [Google Scholar]
- [29].Segalen M, Bellaiche Y. Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 2009; 20:972-7; PMID:19447051; http://dx.doi.org/ 10.1016/j.semcdb.2009.03.018 [DOI] [PubMed] [Google Scholar]
- [30].Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol 2008; 18:44-52; PMID:18514505; http://dx.doi.org/ 10.1016/j.conb.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zallen JA. Planar polarity and tissue morphogenesis. Cell 2007; 129:1051-63; PMID:17574020; http://dx.doi.org/ 10.1016/j.cell.2007.05.050 [DOI] [PubMed] [Google Scholar]


