Abstract
Individuals differ consistently in the magnitude of their inflammatory responses to acute stressors, with females often showing larger responses than males. While the clinical significance of these individual differences remains unclear, it may be that greater inflammatory responses relate to increased systemic inflammation and thereby risk for chronic inflammatory disease. Here, we examined whether acute stressor-evoked interleukin (IL)-6 responses associate with resting levels of C-reactive protein (CRP), a marker of systemic inflammation, and whether this association differs by sex. Subjects were 57 healthy midlife adults (30–51 years; 33% female; 68% white). Blood was drawn before and 30-min after two mental stress tasks: a multisource interference task and a Stroop color word task. Hierarchical regressions controlling for age, sex, race, and BMI tested whether stressor-evoked IL-6 responses were associated with resting CRP and whether this association differed by sex. Results indicated that sex and stressor-evoked IL-6 responses interacted to predict CRP (ΔR2 = .08, B = −1.33, β = −.39, p = .02). In males, larger stressor-evoked IL-6 responses associated with higher CRP, whereas in females, stressor-evoked IL-6 responses showed a non-significant negative association with CRP. These findings indicate that inflammatory responses to acute stressors associate with resting levels of CRP; however, this association differs by sex. Previous literature suggests that there are sex differences in stressor-evoked IL-6 responses, but this is the first study to show sex differences in the relationship between acute inflammatory responses and systemic inflammation. The contribution of these sex differences to inflammatory disease risk warrants further investigation.
Keywords: inflammation, sex differences, acute stress, reactivity, interleukin-6, individual differences
1.0 Introduction
Cardiovascular disease (CVD) is the leading cause of death in the US and other developed nations (Mozaffarian et al., 2015). Inflammation plays a key role in CVD pathophysiology. Long before the onset of clinical symptoms, chronic inflammatory processes occur in arterial walls, resulting in the development of atherosclerosis (Libby et al., 2002; Ross, 1999). These processes include the recruitment and activation of immune cells that release proinflammatory cytokines, such as interleukin (IL)-6, which coordinate both local and systemic inflammatory responses. Systemically, proinflammatory cytokines enter the bloodstream and stimulate the production of acute phase proteins, such as C-reactive protein (CRP) (Steptoe and Brydon, 2005). Levels of these proteins can be reliably detected in peripheral circulation and are thought to reflect systemic levels of inflammation. Higher basal levels of inflammatory markers in peripheral circulation, such as IL-6 and CRP, predict increased risk for CVD (Danesh et al., 2008, 2000; Kaptoge et al., 2010). Despite this relationship, the physiological mechanisms underlying systemic elevations in circulating inflammatory markers are not entirely clear.
Although numerous factors contribute to systemic levels of inflammation, recent attention has focused on the possibility that psychological stress plays a role. In this regard, levels of circulating inflammatory markers increase after exposure to acute psychological stress (Rohleder, 2014; Steptoe et al., 2007). Laboratory studies show that individuals differ consistently in the magnitude of their immune responses to acute stress, with some individuals showing large responses across occasions of testing and others little or no response (Black, 2003; Cohen et al., 2000; Marsland et al., 2002, 1995). Stressor-evoked inflammatory responses are related to a number of psychosocial and biological factors, including state negative affect (Carroll et al., 2011), socioeconomic status (Brydon et al., 2004; Derry et al., 2013) and adiposity (Brydon et al., 2008; McInnis et al., 2014). However, little is known about how stressor-evoked inflammatory responses are related to disease risk. Stable individual differences in magnitude of stressor-evoked inflammatory responses may have implications for susceptibility to disease (Miller et al., 2011), with individuals prone to larger acute stressor-evoked inflammatory responses at presumably increased risk for inflammatory diseases like CVD. Thus, to the extent that larger acute stressor-evoked inflammatory responses are stable biological phenotypes of individuals, they may relate to heightened basal levels of systemic inflammation and greater CVD risk.
Critically, however, there are major gaps in our knowledge about the extent to which stressor-evoked changes in circulating inflammatory markers relate across individuals to known predictors of CVD risk. To date, only two studies have tested whether stressor-evoked inflammatory responses are associated with CVD risk. The first of these studies reported that acute stressor-evoked IL-6 and fibrinogen responses predicted ambulatory blood pressure at a 3-year follow-up (Brydon and Steptoe, 2005). The second study found that tumor necrosis factor alpha responses, but not IL-6 responses, predicted carotid artery stiffness at a 3-year follow-up (Ellins et al., 2008). Although more work is needed in this area, these findings are consistent with the possibility that stressor-evoked inflammatory responses predict vulnerability to CVD.
Additionally, there may be sex differences in the association between stressor-evoked IL-6 responses and basal inflammation. Although there are not consistent sex differences in basal levels of IL-6 (Chapman et al., 2009; Gruenewald et al., 2006; Sadeghi et al., 2005), previous reports indicate that there are sex differences in stressor-evoked IL-6 responses. Specifically, females tend to show larger stressor-evoked IL-6 responses compared with males (Hackett et al., 2012; Steptoe et al., 2002) and may show more prolonged elevations in IL-6 compared with males (Edwards et al., 2006). This finding is not consistent across all studies (Brydon and Steptoe, 2005; Carroll et al., 2011), but it does suggest that sex differences in stressor-evoked IL-6 responses should be considered. In addition, there are sex differences in CVD incidence (Lerner and Kannel, 1986; Roger et al., 2012), suggesting that there are sex-related biological factors that impact CVD risk; sex differences in the association between stressor-evoked IL-6 responses and basal inflammation may be one such factor.
Importantly, IL-6 triggers downstream production of CRP (Heinrich et al., 1990; Kerr et al., 2001), which is an established marker of CVD risk (Parrinello et al., 2015). Here, we focus on this downstream biomarker of the inflammatory process that is more proximal to the development of CVD. Accordingly, the current study examined whether individuals who show larger increases in IL-6 following acute psychological stress have higher circulating levels of CRP. Specifically, we hypothesized that larger acute stressor-evoked IL-6 responses would be associated with higher basal levels of CRP. Based on evidence for sex differences in both stressor-evoked IL-6 responses and patterns of CVD incidence, we also tested if the magnitude of IL-6 response differs by sex and whether sex moderates the association between IL-6 response and CRP. These questions were examined in healthy midlife adults who participated in a laboratory study on the physiological correlates of stress and CVD risk.
2.0 Methods
2.1 Participants
Participants were drawn from the Pittsburgh Imaging Project, a study of 331 healthy adult volunteers residing in Allegheny County, Pennsylvania. The phase of data collection for the work reported here occurred between September 2011–October 2014. A subsample of 91 participants completed a separate protocol at the end of the recruitment period (September 2013–October 2014) to assess psychophysiological and inflammatory responses to laboratory stressors (see Supplementary Figure 1 for participant flow from recruitment through analysis). Participants were between the ages of 30–51 and recruited through mass mail solicitations. The analytic sample was composed of participants who had CRP data and both pre- and post-task measures of IL-6. A total of 32 participants were not included in the analytic sample due to missing IL-6 data (N = 27) or missing CRP data (N = 5). The 27 participants were missing IL-6 data due to refusal to undergo intravenous catheterization (N=9) or blood sampling problems (N=18) at the time of the laboratory stress session. There were no statistically significant differences in age, sex, or BMI between participants who completed the blood draw at the laboratory visit and those who did not. In addition, one participant was excluded from the analytic sample due to relatively high basal IL-6 (11.33pg/mL) and another was excluded due to a high IL-6 raw change score (4.95pg/mL); these values deviated substantially (SD > 6) from the means for basal IL-6 and IL-6 raw change scores.
Descriptive statistics were assessed for the 57 remaining participants (Table 1). The sample included 38 males and 19 females. The majority of participants identified as White (68%) and 25% identified as Black. For analytic purposes, the four participants who did not identify as White or Black were grouped with Black participants; the new group was thus comprised of non-White individuals. Average annual family income was $61,119, though there was a sizable range (SD = $44,245). A large proportion of participants had completed college (52.3%) or graduate education (33.3%). The sample was slightly overweight, with a mean BMI of 26.17. The majority of participants had never smoked (74%). On average, participants had low levels of IL-6 (M = 1.24pg/mL, median = 1.08, interquartile range = 0.74) and CRP (M = 0.17 mg/dL, median =0.09, interquartile range = 0.15).
Table 1.
Descriptive statistics
| Overall | Males | Females | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean or n | SD or % | Mean or n | SD or % | Mean or n | SD or % | p | |
| Age (years) | 40.39 | 6.58 | 38.71 | 6.12 | 43.74 | 6.31 | .005 |
| Sex (female) | 19 | 33.30% | 0 | 0% | 19 | 100% | |
| Race | .08a | ||||||
| White | 39 | 68.40% | 23 | 60.50% | 16 | 84.20% | |
| Black | 14 | 24.60% | 11 | 28.90% | 3 | 15.80% | |
| Asian | 3 | 5.30% | 3 | 7.90% | 0 | 0% | |
| Multiracial | 1 | 1.80% | 1 | 2.60% | 0 | 0% | |
| BMI (kg/m2) | 26.17 | 4.4 | 26.65 | 4.58 | 25.21 | 3.96 | .25 |
| Smoking Status | .52a | ||||||
| Never | 42 | 73.70% | 27 | 71.10% | 15 | 78.90% | |
| Former | 8 | 14.00% | 5 | 13.20% | 3 | 15.80% | |
| Current | 7 | 12.30% | 6 | 15.80% | 1 | 5.30% | |
| Education | .21 | ||||||
| High School | 7 | 12.30% | 6 | 15.80% | 1 | 5.30% | |
| College | 28 | 49.10% | 20 | 52.60% | 8 | 42.20% | |
| Graduate | 22 | 38.60% | 12 | 31.60% | 10 | 52.60% | |
| Annual family income | $50,750 | $15,800 | $45,499 | $17,500 | $61,099 | $13,399 | .23 |
| Baseline IL-6 (pg/mL) | 1.24 | 0.95 | 1.25 | 1.04 | 1.24 | 0.77 | .72 |
| CRP (mg/dL) | 0.17 | 0.2 | 0.19 | 0.24 | 0.12 | 0.09 | .10 |
Note: p-values denote significance of t-tests for differences between males and females on continuous variables. BMI = body mass index; IL-6 = interleukin-6; CRP = C-reactive protein.
Due to small cell numbers, significance testing for race and smoking status was conducted by grouping participants into two groups (White vs. non-White; Never vs. Former or Current) and using a Chi-square test.
Exclusion criteria for the study included: history of any cardiovascular disease (including hypertension); prior cardiovascular or cerebrovascular surgery; chronic kidney or liver conditions; Type I or II diabetes; and any pulmonary or respiratory diseases. Participants in the analytic sample were also free of inflammatory disorders. Individuals were excluded if they reported a history of treatment for mental health problems or a problem with alcohol or substance abuse during an initial phone screen. Exclusion criteria also included regular use of corticosteroid inhalers or use of any cardiovascular, psychotropic, or lipid lowering medications. None of the participants in the analytic sample reported using prescription medications known to affect inflammatory markers within the past six months. This study had a magnetic resonance imaging component completed on a separate day from the stress reactivity protocol reported here and thus excluded participants with: history of neurosurgery or a neurological condition; head trauma leading to loss of consciousness; pregnancy; and claustrophobia or metallic implants. Participants received monetary compensation for participating in the study and informed consent procedures were carried out following guidelines of the University of Pittsburgh Institutional Review Board.
2.2 Procedures
2.2.1 Protocol Overview
Data collection took place during three study visits over an approximately 2-month long period. At study visits 1 and 2, all study participants completed questionnaire measures, assessments of anthropometric measures and biomarkers of cardiovascular risk (e.g., fasting blood sample for lipid levels and CRP), and functional magnetic resonance imaging. In the last year of data collection, a 91-person subsample returned for a third visit to complete a laboratory stress session. Previous participants also returned for a third visit, but completed a different, unrelated protocol.
2.2.2 Laboratory Testing
Acute stressor-evoked inflammatory response data were collected during a laboratory session that began between 12:00 and 1:00 PM. Participants were instructed to abstain from caffeine (12 hours), strenuous physical activity (24 hours), non-prescription medications (24 hours), and alcohol (48 hours) before the session. On arrival, participants completed an acute illness-screening questionnaire; to avoid heightened inflammation due to acute illness, those with cold or flu symptoms within 48 hours were rescheduled. Next, an intravenous catheter was inserted into the antecubital vein of the left arm for the collection of blood samples. Participants then rested quietly for a 30-min baseline period, after which the first blood sample was drawn. After the baseline, participants performed two stressor tasks, separated by a 4-min rest period. Participants then rested quietly for a 30-min recovery period. The post-stressor blood sample was collected 30-min after completion of the second stressor task, based on evidence that stressor-evoked increases in IL-6 show a delayed response (Steptoe et al., 2007; von Kanel et al., 2006). After each protocol period (i.e., baseline, task, and recovery), participants provided affective ratings.
2.2.3 Stress Tasks
Participants completed two standardized mental stress tasks validated for laboratory studies of stress reactivity: a modified Stroop color-word task (Sheu, Jennings, & Gianaros, 2012) and a modified multi-source interference task (MSIT) (Bush & Shin, 2006; Sheu et al., 2012). Both tasks have elements of conflict, time pressure, error feedback, and uncontrollability. Each task included alternating difficult (incongruent) and easy (congruent) conditions. In the Stroop task, participants saw one target word and four identifier words and were instructed to identify the color of the target word by selecting the correct identifying word. In the MSIT task, participants saw with three numbers and were instructed to select the number that differed from the other two numbers. To increase task difficulty during incongruent trials, a loud pre-recorded voice stated a random answer choice; the voice stated the correct answer during congruent trials. During each task, incorrect or delayed responses elicited automated negative feedback. Each task lasted approximately 9 minutes with adaptive inter-trial intervals so that accuracy was titrated to <60% for all participants (for further detail, see Sheu et al, 2012). These tasks have been shown to reliably elicit cardiovascular responses in a comparable sample, with intra-class correlation coefficients of 0.75–0.85 (Sheu et al., 2012). Task order was randomized across participants.
2.3 Measures
2.3.1 Participant Characteristics
Participants self-reported their age, sex, race, education, and family income on a standard demographics questionnaire.
2.3.2 IL-6
Baseline and 30-min post-task blood samples were used to assess circulating IL-6. Immediately after each blood draw, whole blood was centrifuged at room temperature at 2500 rpm for 10 minutes and plasma was removed and stored at −80 degrees Celsius. Plasma IL-6 levels were determined using high sensitivity ELISA kits (R&D Systems, Minneapolis). Samples were run in duplicate and average intra-assay coefficients of variation for Baseline and 30-min post-task were 4% and 5%, respectively. The two samples from each participant were run on the same plate.
2.3.3 CRP
Resting levels of high-sensitivity CRP (mg/dL) were measured from a fasting blood sample taken during the second study visit (M = 44.1, SD = 41.6 days prior; range = 5–208 days). Because CRP levels are stable across relatively long periods (1–5 years) (Ridker, 2007), we do not expect that CRP levels would have changed significantly between sampling (visit 2) and the laboratory session (visit 3). CRP was assayed by a CRPH reagent on a SYNCHRON LX System (Beckman Coulter, Inc.) in the Clinical Services Laboratory of the Western Psychiatric Institute and Clinic. As no participants had CRP levels indicating acute illness ( >10 mg/L), all participants with CRP data were included in analyses.
2.3.4 Affective Ratings
Participants provided affective ratings after the baseline, task, and 30-minute final recovery periods using a 9-point self-assessment manikin scale (Bradley and Lang, 1994). Participants rated their level of arousal (1-very calm; 9-very aroused), perceived control (1-very little control, 9-very much control), and emotional valence (1-very unhappy; 9-very happy).
2.3.5 Additional Covariates
Smoking status and BMI were measured and included as covariates, given their associations with systemic inflammation (O’Connor et al., 2009). Smoking status was measured during the first study visit via self-report of smoking habits and history. Participants were categorized as “never”, “former”, or “current” smokers; “former” smokers had quit at least two years prior to blood sampling and smoked less than one pack per day when they were active smokers. None of the current smokers reported smoking more than one pack per day. BMI was determined at the second study visit using participants’ height and weight measurements and calculated as weight (kg)/height (m2).
2.4 Analytic Plan
Analyses were carried out using SPSS (Version 22.0, IBM Corp). Resting CRP, baseline IL-6 and 30-min post-task IL-6 values were non-normally distributed and log transformed prior to analyses. A log-10 transformation was used for CRP and a natural log transformation was used for IL-6. Stressor task effects for IL-6 were determined with a paired samples t-test, which tested for a significant difference in IL-6 levels from baseline to 30-min post task. Task-evoked affective changes were tested using a repeated measures ANOVA, with planned contrasts assessing significant differences in arousal, control, and valence from baseline to immediately and 30-min post-task. Sex differences in the magnitude of stressor-evoked IL-6 responses and affective changes were tested using repeated-measures ANOVAs, with sex as a between-subjects factor.
Hierarchical linear regressions tested primary study hypotheses. Residualized change scores were created for IL-6 by regressing 30-min post-task IL-6 levels on baseline IL-6 levels; these residualized change scores were normally distributed. Age, sex, race, smoking status, and BMI were used as covariates. Dummy coding was used for sex (0 = male, 1 = female), race (0 = white, 1 = nonwhite), and smoking status (0 = never, 1 = former, 2 = current).
To test whether acute stressor-evoked IL-6 changes associate with resting levels of CRP, we conducted two regression models predicting CRP with residualized IL-6 change scores. The first model was minimally adjusted, with age sex and race on Step 1 and residualized IL-6 change scores on Step 2. The second, fully adjusted model included age sex, and race on Step 1, BMI and smoking status on Step 2, and residualized IL-6 changes scores on Step 3. Additional regression models tested whether the relationship between stressor-evoked IL-6 response and CRP varied by sex. For these analyses, the minimal model included sex and residualized IL-6 change scores on Step 1 and a “sex x IL-6 change” interaction term on Step 2. The fully adjusted model included sex and residualized IL-6 change scores on Step 1, covariates (age, BMI, race, and smoking status) on Step 2, and the sex x IL-6 change interaction term on Step 3. All continuous variables were mean centered prior to interaction regression analyses.
3.0 Results
3.1 Bivariate Correlations
Correlations between primary variables are shown in Table 2. Higher BMI was significantly correlated with higher baseline IL-6 (r = .36, p= .005) and higher CRP (r = .37, p = .004). The association between baseline IL-6 and resting CRP did not reach significance (r = .23, p = .08). Stressor-evoked IL-6 change scores were not significantly correlated with any primary study variables.
Table 2.
Bivariate Correlations
| Age | BMI | IL-6 | Δ IL-6 | CRP | |
|---|---|---|---|---|---|
| 1. Age (years) | - | ||||
| 2. BMI (kg/m2) | .14 | - | |||
| 3. IL-6 Baseline | .01 | .36** | - | ||
| 4. IL-6 Change | .02 | −.09 | −.02 | - | |
| 5. CRP (mg/dL) | −.11 | .37** | .23 | .14 | - |
Note: BMI = body mass index; IL-6 = interleukin-6; CRP = C-reactive protein
p < .01
p < .05
3.2 Stressor Task Effects
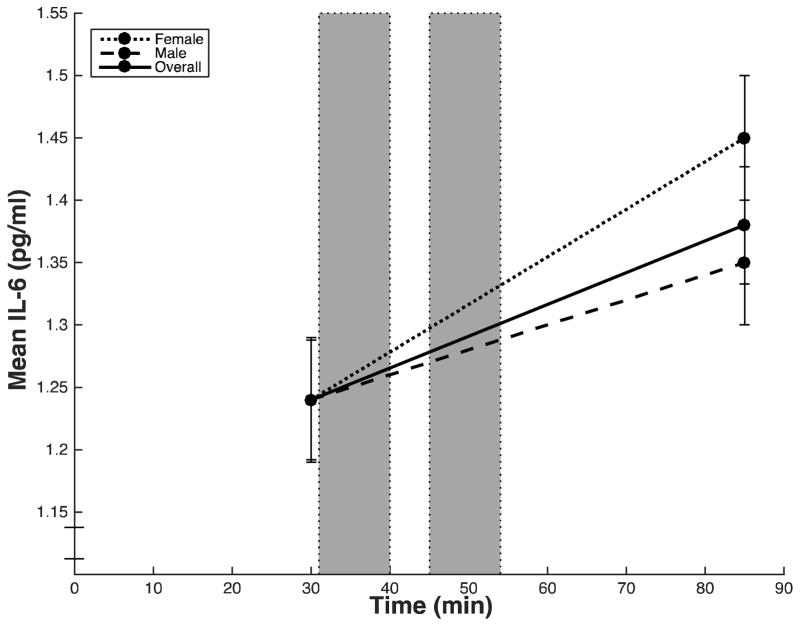
Across subjects, mean IL-6 values increased significantly from baseline to 30-min post-task (t (56) = −4.96, p < .001) (Table 3; Figure 1). There were significant sex differences in the magnitude of stressor-evoked IL-6 responses (F(1, 55) = 4.17, p = .041, ηp2 = .07) (Table 3). Specifically, females showed a greater increase in IL-6 from baseline to 30-min post-task compared with males (Figure 1). Because females in this sample were older than males, we ran an additional model with age as a covariate and found that the result remained significant (F(1, 54) = 4.53, p = .038, ηp2 = .08).
Table 3.
Mean IL-6
| Baseline | 30-min post task | Raw Change | Residualized Change | N | |
|---|---|---|---|---|---|
| Overall | 1.24 (0.95) | 1.38 (0.98) | 0.14 (0.19) | −0.07 (0.19) | 57 |
| Males | 1.24 (1.04) | 1.35 (1.10) | 0.11 (0.18) | −0.11 (0.18) | 38 |
| Females | 1.24 (0.77) | 1.45 (0.72) | 0.21 (0.19) | −0.01 (0.19) | 19 |
Note: IL-6 = interleukin-6; values in parentheses are standard deviations.
Figure 1.
Mean circulating IL-6 (pg/mL) at each time point for overall sample (solid line), males (dashed line), and females (dotted line). Blood samples were taken after a baseline period (30 min) and after the final 30-min post-task rest period (85 min). Error bars indicate standard error of the mean of IL-6.
In the full sample, self-reported affect also changed significantly over the laboratory protocol. Mean arousal changed significantly (F(2, 112) = 88.35, p < .001, ηp2 = .61), with planned contrasts showing that arousal increased from baseline to immediately post-task (F(1, 56) = 139.98, p <.001, ηp2 = .71) and returned to baseline levels at 30-min post-task (F(1, 56) = 1.75, p = .191, ηp2 = .03). Mean perceived control also changed significantly (F(2, 112) = 29.27, p < .001, ηp2 = .34); planned contrasts showed that self-reported control decreased significantly from baseline to immediately post-task (F(1, 56) = 53.41, p <.001, ηp2 = .49) and returned to baseline levels at 30-min post-task (F(1, 56) = 3.27, p = .076, ηp2 = .06). Finally, mean participant reports of emotional valence also changed significantly (F(2, 112) = 17.55, p < .001, ηp2 = .24); specifically, participants reported feeling significantly more unhappy immediately after the tasks compared to baseline (F(1, 56) = 21.73, p <.001, ηp2 = .28), with valence returning to baseline levels at 30-min post-task (F(1, 56) = 0.35, p =.554, ηp2 = .01). There were no significant sex differences in affective responses to the stressor tasks (all p’s > .50).
3.3 Stressor-Evoked IL-6 Response and CRP
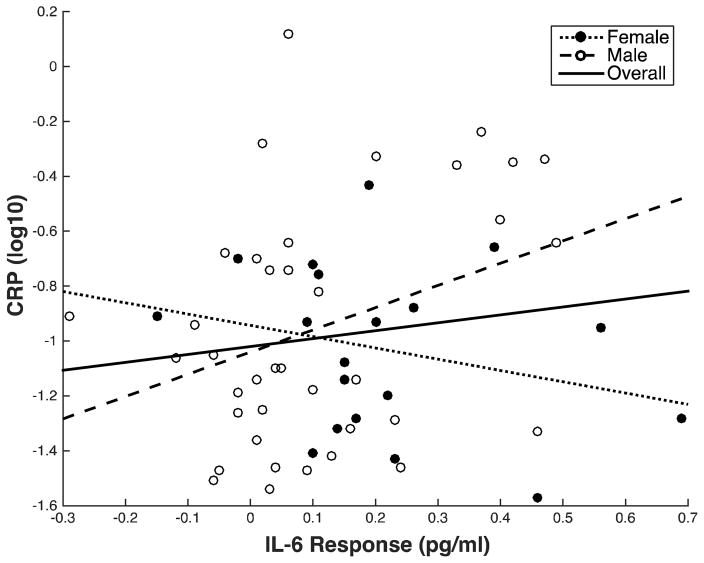
Across all subjects, the hypothesis that larger stressor-evoked IL-6 responses are associated with higher levels of CRP was not supported at a conventional level of statistical significance (p < .05). Hence, stressor-evoked IL-6 change scores were not significantly associated with CRP in the minimally adjusted model (β= .19, p = .171) or the fully adjusted model (Table 4). In the fully adjusted model, demographic variables (Step 1) did not predict significant variance in CRP. BMI and smoking status (Step 2) accounted for 17% of the variance in CRP (ΔR2 = .17, p = .007), largely driven by the positive association between BMI and CRP (β = .37, p = .01). Stressor-evoked IL-6 responses (Step 3) were not significantly with CRP (β = .22, p = .09) (Figure 2).
Table 4.
Regressions testing the association between stressor-evoked IL-6 responses and CRP
| N = 57
| ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | R2 | ΔR2 | ΔF | Sig. ΔF | |
| Step 1 | .04 | .04 | .82 | .49 | ||||
| Age | −.01 | .01 | −.07 | .63 | ||||
| Sex | .02 | .12 | −.02 | .87 | ||||
| Race | .15 | .12 | .18 | .21 | ||||
| Step 2 | .21 | .17 | 5.41 | .007 | ||||
| BMI | .03 | .01 | .37 | .01 | ||||
| Smoking Status | .11 | .08 | .19 | .15 | ||||
| Step 3 | .26 | .04 | 2.95 | .09 | ||||
| IL-6 Change | .45 | .27 | .22 | .09 | ||||
Note: BMI = body mass index; IL-6 = interleukin-6; CRP = C-reactive protein
Figure 2.
Association of stressor-evoked IL-6 response with resting CRP for overall sample (solid line), males (dashed line), and females (dotted line). IL-6 response is shown as raw change for illustrative purposes. CRP values are log10 transformed.
The relationship between stressor-evoked IL-6 response and resting CRP, however, varied by sex. Specifically, there was a significant interaction between sex and stressor-evoked IL-6 responses in predicting CRP in the minimal model (β = −.36, p = .04) and the fully adjusted model (Table 5). In the fully adjusted model, covariates (Step 2) accounted for 21% of the variance in CRP (ΔR2 = .21, p = .01), with higher BMI significantly associated with higher CRP (β = .37, p = .006). The sex x IL-6 change interaction term (Step 3), accounted for 8% of the overall variance in CRP (ΔR2 = .08, B = −1.33, β = −.39, p = .02). This indicates that the slope of the relationship between IL-6 change and CRP differed by sex (Figure 2). In males, the slope of the association between stressor-evoked IL-6 change and CRP was positive and significant (β = 0.39, p = .01); for every unit increase in IL-6 change, there was a 0.94 increase in resting CRP. In females, the slope was negative and non-significant (β = −0.21, p = .40).1 Thus, the slope coefficient in males indicates the relationship we hypothesized (i.e., greater stressor-evoked IL-6 responses are associated with higher levels of CRP). The slope coefficient in females indicates that there is no significant relationship between stressor-evoked IL-6 responses and CRP. These results remained significant after excluding current smokers from the analyses.
Table 5.
Regressions testing the interaction of sex x stressor-evoked IL-6 response in predicting CRP.
| N = 57
| ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | R2 | ΔR2 | ΔF | Sig. ΔF | |
| Minimal Model
| ||||||||
| Step 1 | .04 | .04 | 1.16 | .32 | ||||
| Sex | −0.12 | .12 | −.14 | .31 | ||||
| IL-6 Change | 0.39 | .29 | .19 | .18 | ||||
| Step 2 | .12 | .08 | 4.47 | .04 | ||||
| Sex x IL-6 Change | −1.23 | .58 | −.36 | .04 | ||||
|
| ||||||||
| Fully Adjusted Model
| ||||||||
| Step 1 | .04 | .04 | 1.16 | .32 | ||||
| Sex | −0.12 | .12 | −.14 | .31 | ||||
| IL-6 Change | 0.39 | .29 | .19 | .18 | ||||
| Step 2 | .26 | .21 | 3.60 | .01 | ||||
| Age | −0.01 | .01 | −.15 | .26 | ||||
| Race | 0.05 | .11 | .06 | .64 | ||||
| BMI | 0.03 | .01 | .37 | .006 | ||||
| Smoking Status | 0.12 | .07 | .21 | .11 | ||||
| Step 3 | .34 | .08 | 6.11 | .02 | ||||
| Sex x IL-6 Change | −1.33 | .54 | −.39 | .02 | ||||
Note: BMI = body mass index; IL-6 = interleukin-6; CRP = C-reactive protein
4.0 Discussion
This study examined whether the magnitude of acute stressor-evoked IL-6 responses was associated with resting levels of CRP, a marker of systemic inflammation. Stressor-evoked IL-6 responses were not significantly associated with resting CRP when males and females were analyzed together. However, our analyses revealed a significant interaction between sex and stressor-evoked IL-6 responses, such that males showed a positive association between magnitude of IL-6 responses and resting CRP while females showed a non-significant negative association. In addition, females showed greater stressor-evoked IL-6 responses compared with males, replicating previously reported sex differences in stressor-evoked IL-6 responses (Hackett et al., 2012; Steptoe et al., 2002).
Although basal IL-6 does not differ consistently by sex (Chapman et al., 2009; Gruenewald et al., 2006; Thorand et al., 2006; Sadeghi et al., 2005), females often show larger stressor-evoked IL-6 responses (Edwards et al., 2006; Hackett et al., 2012; Steptoe et al., 2002). In the present study, females showed significantly larger stressor-evoked IL-6 responses than males. Two other laboratory stress studies found similar differences (Hackett et al., 2012; Steptoe et al., 2002) and third study found that males had initially higher levels of IL-6 at 30-min post-stress but females showed higher levels than males at 60-min post-stress (Edwards et al., 2006). Our results diverge somewhat from the work of Edwards et al., (2006), as females in our sample had higher IL-6 at 30-min post-stress. In sum, although timing varies across studies, our findings support other reports that females show greater stressor-evoked IL-6 responses.
4.1 Mechanisms and Relevance to CVD Risk
Sex differences in stressor-evoked IL-6 responses are not well understood. These sex differences may be due to differences in affective responses to the stressor task, as prior work has shown that sex differences in task-evoked anger are associated with magnitude of IL-6 response to a social evaluative stressor (Carroll et al., 2011). In the present sample, we did not find sex differences in arousal, control, and emotional valence responses to the task. Because we did not include a measure of task-evoked anger in this study, we cannot determine whether sex differences in IL-6 responses reported here are driven by sex differences in task-evoked anger. However, the mental stress tasks used in the present study may have evoked different affective responses than the social evaluative stressor used by Carroll et al. (2011).
A number of possible biological mechanisms may account for sex differences in magnitude of IL-6 response to stress. First, sex differences in hypothalamic-pituitary-adrenal (HPA) axis responses to stress may play a role. A review of the literature on stressor-evoked cortisol responses indicates that males show greater HPA-axis responses compared to females (Kudielka and Kirschbaum, 2005). Thus, larger cortisol responses in males may result in smaller stressor-evoked IL-6 responses. In addition to sex differences in cortisol responses, males and females may differ in cellular sensitivity to the anti-inflammatory effects of glucocorticoids following stress (Dickerson et al., 2009; Miller et al., 2002). For example, prior work shows that males exhibit acute stressor-evoked increases in the sensitivity of immune cells to the anti-inflammatory effects of glucocorticoids while females do not show this increase (Rohleder et al., 2001). Thus, stressor-evoked differences in HPA axis responses to stress may be one mechanism contributing to larger stressor-evoked IL-6 responses in females.
Sex differences in IL-6 responses may also relate to availability of reproductive hormones, such as estrogens. Estrogens are thought to have an anti-inflammatory effect (Gubbels Bupp, 2015; Straub, 2007), inhibiting IL-6 production and gene expression (Liu et al., 2005). Estrogen levels differ both by sex and by menopausal status (Gubbels Bupp, 2015), suggesting that higher levels of estrogens in pre-menopausal females provide greater inhibition of inflammation. In the present study, participants provided self-reports of menopausal status, but only one female reported being menopausal. Thus, we were not powered to conduct analyses based on menopausal status. Future work should explore the possibility that estrogen levels contribute to sex differences in stressor-evoked IL-6 responses.
These same mechanisms may also be relevant to sex differences in the association between IL-6 responses and CRP. Our analyses revealed that the relationship between stressor-evoked IL-6 responses and resting CRP differs by sex. It is surprising that, even though females showed larger stressor-evoked IL-6 responses, only males showed the hypothesized positive association between IL-6 responses and CRP. In males, our results support the hypothesis that larger acute stressor-evoked inflammatory responses are linked with higher resting levels of systemic inflammation. These results are consistent with previous work showing that larger stressor-evoked IL-6 responses are associated with other CVD risk factors (Brydon & Steptoe, 2005). Importantly, these results are cross-sectional and prevent us from making any claims about the direction of the relationship between stressor-evoked IL-6 responses and CRP. As IL-6 is a precursor of CRP (Heinrich et al., 1990; Kerr et al., 2001), larger stressor-evoked IL-6 responses over time may cause heightened systemic levels of CRP. In vitro models show that IL-6 can stimulate phasic production of CRP from human hepatocytes within hours (Castell et al., 1990), but it is unclear how long it would take for transient increases in IL-6 to cause increases in systemic levels of CRP in humans in vivo. Conversely, heightened levels of systemic CRP may prime immune cells to produce larger amounts of IL-6 in response to acute stress; however, there is less theoretical and biological basis for this hypothesis. Future longitudinal work is warranted to elucidate the temporal relationship between stressor-evoked IL-6 responses, CRP, and CVD risk. Such work should consider that longitudinal associations may also differ by sex.
In contrast to males, females showed a non-significant negative association between stressor-evoked IL-6 responses and CRP. These sex differences were not driven by demographic or biobehavioral characteristics (e.g., age, race, BMI, and smoking status). The observed pattern may be related to sex differences in CVD. Incidence of CVD among females shows an approximate 10-year lag behind incidence in males (Gubbels Bupp, 2015; Lerner and Kannel, 1986; Roger et al., 2012). This lag is often attributed to hormonal changes that occur during and after menopause. The present sample included predominantly premenopausal females who may be protected against inflammatory atherosclerotic processes by higher estrogen levels, as higher concentrations of estrogens tend to have anti-inflammatory effects (Gubbels Bupp, 2015). Thus, although females show greater acute stressor-evoked IL-6 responses, this may not result in increased resting levels of systemic inflammation (i.e., CRP) due to the anti-inflammatory effect of estrogens.
4.2 Limitations, Strengths, and Future Directions
There are a number of considerations to address in future work. This sample included a relatively smaller group of females (N=19) who may not be representative of the broader population because of our exclusionary criteria. Although we did not find any systematic differences between these females and the rest of our analytic sample, the small sample size is an important interpretative consideration for our novel findings. The current results are considered preliminary and warrant replication in a larger, representative sample. Data collection for this study was cross-sectional, preventing any causal inferences about the relationships between study variables. In addition, this study did not include a non-stress control group. Although a control group could strengthen the interpretations of these data, previous work has shown that IL-6 levels do not increase significantly over time in non-stress control participants (Steptoe et al., 2001). This study also did not assess stressor-evoked IL-6 responses on multiple occasions. Previous research indicates that stressor-evoked IL-6 responses do not habituate over repeated testing sessions (von Kanel et al., 2006) but may sensitize on exposure to repeated stressors (McInnis et al., 2014). Repeated measurements would more accurately characterize stable individual differences in stressor-evoked inflammatory responses, possibly identifying individuals at risk for inflammatory diseases.
This study also has a number of notable strengths. First, this is a healthy sample where confounding influences of medication use and disease are absent. We were also were able to control for a number of factors that may have influenced the effects. In addition, we used a protocol known to elicit reliable and stable individual differences in stress reactivity (Sheu et al., 2012). As prior work has shown that stressor-evoked immune responses are relatively stable (Marsland et al., 2002, 1995), it is likely that we have observed stable individual differences that plausibly relate to disease pathophysiology.
To summarize, the clinical significance of acute stress-induced changes in inflammatory mediators is unclear. Individuals vary markedly in the magnitude of these stressor-evoked responses, with growing evidence suggesting that this variability represents a trait of the individual. It is conceivable that there is a meaningful distribution of differences in stressor-evoked inflammatory responses that contribute to risk for inflammatory disease. The current findings suggest that sex relates to the magnitude of stressor-evoked inflammatory responses and contributes to their associations with circulating levels of CRP, a known marker of CVD risk. Although females show larger increases in IL-6 in response to stress, midlife males with larger stressor-evoked inflammatory responses may be at increased CVD risk when compared to their female counterparts. Prospective studies using stressor-evoked inflammatory responses to predict disease outcomes will enable us to conclude whether magnitude of stressor-evoked inflammatory response is a vulnerability factor for CVD risk.
Supplementary Material
Sex differences in the association between IL-6 reactivity and basal CRP were tested
Females showed larger stressor-evoked IL-6 responses compared with males
In the full sample, stressor-evoked IL-6 responses were not associated with CRP
Among males, larger stressor-evoked IL-6 responses were associated with higher CRP
Among females, there was no significant association between IL-6 responses and CRP
Acknowledgments
This work was supported by grant NIH R01 HL089850 (PJG), HL07560, and the National Science Foundation Graduate Research Fellowship Program (DGE-1247842). We would also like to Sara Snyder and Julie Johnson for their contributions to data collection.
Footnotes
Note that both stressor-evoked IL-6 and resting CRP values used in regression analyses do not reflect the raw values of these variables and interpretation of slopes does not reflect raw units of these variables; IL-6 change scores are residualized scores accounting for baseline levels of IL-6, while the CRP values are log-10 transformed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Brydon L, Steptoe A. Stress-induced increases in interluekin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow up. J Hypertens. 2005;23:1001–1007. doi: 10.1016/j.hjh.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Brydon L, Wright CE, O’Donnell K, Zachary I, Wardle J, Steptoe a. Stress-induced cytokine responses and central adiposity in young women. Int J Obes (Lond) 2008;32:443–450. doi: 10.1038/sj.ijo.0803767. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM. The multi-source interference task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive attention network. Nat Protoc. 2006;1:208–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell JV, Gómez-lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: Regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, Moynihan J. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain Behav Immun. 2009;23:636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin B, Manuck SB. The stability of an intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med. 2000;22:171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup P, Lowe G, Gudnason V. Long-term interleukin-6 levels and subsequent risk of cornary heart disease: Two new prospective studies and a systematic review. PLoS Med. 2008;5:600–610. doi: 10.1371/journal.pmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Bmj. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–2685. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychol Sci. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Ring C, Carroll D. Sex differences in the interleukin-6 response to acute psychological stress. Biol Psychol. 2006;71:236–239. doi: 10.1016/j.biopsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ellins E, Halcox J, Donald A, Field B, Brydon L, Deanfield J, Steptoe A. Arterial stiffness and inflammatory response to psychophysiological stress. Brain Behav Immun. 2008;22:941–948. doi: 10.1016/j.bbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman T, Ryff CD, Karlamangla A, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294:102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Hamer M, Endrighi R, Brydon L, Steptoe A. Loneliness and stress-related inflammatory and neuroendocrine responses in older men and women. Psychoneuroendocrinology. 2012;37:1801–1809. doi: 10.1016/j.psyneuen.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. Emerging risk factors collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor jB transactivation. Cytokine. 2005;31:251–257. doi: 10.1016/j.cyto.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav. 2002;77:711–716. doi: 10.1016/s0031-9384(02)00923-x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosom Med. 1995;57:295–298. doi: 10.1097/00006842-199505000-00012. [DOI] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, Rohleder N. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun. 2014;42:33–40. doi: 10.1016/j.bbi.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Heal Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, De Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2015 update : A report from the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170:380–9. doi: 10.1016/j.ahj.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of Systemic Low-Grade Inflammation by Psychosocial Stress. Psychosom Med. 2014 doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Daniel V, Naujokat C, Weimer R, Opelz G. Strikingly higher interleukin (IL)-1α, IL-1β and soluble interleukin-1 receptor antagonist (sIL-1RA) but similar IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-α, transforming growth factor (TGF)-β2 and interferon IFN-γ urine. Clin Exp Immunol. 2005;142:312–317. doi: 10.1111/j.1365-2249.2005.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu LK, Jennings JR, Gianaros PJ. Test-retest reliability of an fMRI paradigm for studies of cardiovascular reactivity. Psychophysiology. 2012;49:873–884. doi: 10.1111/j.1469-8986.2012.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe, Brydon L. Psychoneuroimmunology and coronary heart disease. In: Vedhara K, Irwin MR, editors. Human Psychoneuroimmunology. Oxford University Press; Oxford: 2005. [Google Scholar]
- Steptoe, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemson G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci. 2001;101 [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, Giani G, Koenig W. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184:216–224. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.