Abstract
Background and aims
Neutrophil to lymphocyte ratio (NLR) is an inflammatory-based marker. A systematic review and meta-analysis was performed to explore the prognostic role of NLR in patients with hepatocellular carcinoma (HCC).
Results
Overall, 598 papers were identified, of which 90 papers including 20,475 HCC patients were finally included. Low baseline NLR was significantly associated with better overall survival (HR = 1.80, 95% CI: 1.59–2.04, p < 0.00001) and recurrence-free or disease-free survival (HR = 2.23, 95% CI: 1.80–2.76, p < 0.00001). Low post- treatment NLR was significantly associated with better overall survival (HR = 1.90, 95% CI: 1.22–2.94, p = 0.004). Decreased NLR was significantly associated with overall survival (HR = 2.23, 95%CI: 1.83–2.72, p < 0.00001) and recurrence-free or disease-free survival (HR = 2.23, 95% CI: 1.83–2.72, p < 0.00001). The findings from most of subgroup meta-analyses were consistent with those from the overall meta-analyses.
Materials and Methods
All relevant literatures were identified via PubMed, EMBASE, and Cochrane library databases. Hazard ratio (HR) with 95% confidence interval (95%CI) was calculated. Subgroup meta-analyses were performed according to the treatment options, NLR cut-off value ranges, and regions.
Conclusions
NLR should be a major prognostic factor for HCC patients. NLR might be further incorporated into the prognostic model of HCC.
Keywords: hepatocellular carcinoma, inflammatory, neutrophil, lymphocyte, prognosis
INTRODUCTION
Prognostic assessment of hepatocellular carcinoma (HCC) is very important for clinicians and patients. The relevant knowledge is being rapidly accumulated. Traditional prognostic variables mainly include portal vein thrombosis, tumor size, and alpha-fetoprotein, etc. [1]. As for the prognostic staging of HCC, the Barcelona Clinic Liver Cancer (BCLC) system is the most frequently used tool with 5 major parameters, such as tumor size, tumor number, Child-Pugh class, physical status, and tumor metastasis [2]. Several alternative staging systems include the Cancer of the Liver Italian Program (CLIP) system [3], the Hong Kong Liver Cancer (HKLC) system [4], and the Japan Integrated Scoring (JIS) system [5]. As for the liver function assessment of HCC, Child-Pugh class is the most frequently used tool with 5 variables, such as bilirubin, albumin, international normalized ratio, ascites, and hepatic encephalopathy [6]. Albumin-bilirubin score is a recently developed and more convenient tool [7]. More recently, the associations of inflammation-based markers with the prognosis of HCC have been actively explored. Neutrophil to lymphocyte ratio (NLR), which refers to the ratio of neutrophil to lymphocyte count, is a readily available marker for assessing the systemic inflammatory changes. NLR reflects the potential balance between neutrophil-associated pro-tumor inflammation and lymphocyte-dependent anti-tumor immune function [8–11]. An elevated NLR may represent a trend towards increased pro-tumor inflammation and decreased anti-tumor immune function. Herein, we have conducted a systematic review and meta-analysis to analyze the prognostic role of NLR in HCC patients treated with different treatment options. This work was registered at PROSPERO database (registration number: CRD CRD42016033409).
RESULTS
Study selection and characteristics
A total of 598 papers were identified. Among them, 90 papers with 20,475 HCC patients were included in the systematic review (Figure 1) [12–101]. Study characteristics were summarized in Table 1. According to the publication type, 21 and 69 papers were published in abstract and full-text forms, respectively. According to the study design, 60 and 5 papers were retrospective and prospective, respectively; 2 papers were both retrospective and prospective; and the study design was not available in 23 papers. According to the regions, 63, 14, and 13 studies were conducted by Asian, European, and American researchers, respectively.
Figure 1. Flowchart of study inclusion.
Table 1. Study characteristics.
| First author | Journal (Year) | Type of publication | Study design | Regions | Enrollment period | Study population | No. Pts |
|---|---|---|---|---|---|---|---|
| Abdelmessih RM | Hepatology (2011) | Abstract | Retrospective | NY, US | 1999.3– 2010.4 | HCC patients who were downstaged with TACE prior to LT | 200 |
| Afshar M | Journal of Hepatology (2015) | Abstract | Retrospective | Birmingham, UK | 2009.4– 2014.3 | HCC patients treated with sorafenib | 217 |
| Agopian VG | Journal of the American College of Surgeons (2015) | Full-text | Retrospective | CA, US | 1984– 2013 | HCC patients treated with LT | 865 |
| Aino H | Molecular and Clinical Oncology (2014) | Full-text | NA | Fukuoka, Japan | 1998.4– 2012.4 | Advanced HCC patients with extrahepatic metastasis | 419 |
| Bertuzzo VR | Transplantation (2011) | Full-text | Retrospective | Bologna, Italy | 1997– 2009 | HCC patients treated with LT | 219 |
| Bodzin A | American Journal of Transplantation (2015) | Abstract | Retrospective | CA, US | 1984– 2014 | Recurrent HCC after LT | 106 |
| Bronson N | HPB (2012) | Abstract | Retrospective | PA, US | 2002.6– 2011.7 | HCC patients treated with resection | 68 |
| Bruixola G | Journal of Clinical Oncology (2015) | Abstract | Retrospective | Valencia, Spain | 2008– 2014 | HCC patients treated with sorafenib | 145 |
| Chan AW | Annals of Surgical Oncology (2011) | Full-text | Retrospective | Hong Kong, China | 2001.1– 2011.12 | BCLC stage 0/A primary HCC patients treated with surgical resection | 597 |
| Chang JX | Annals of Oncology (2014) | Abstract | Retrospective | Beijing, China | 2008– 2009 | Advanced HCC patients treated with cryoablation | 150 |
| Chen TM | Journal of Gastroenterology and Hepatology (2012) | Full-text | Retrospective | Taiwan, China | 2003.7– 2010.12 | Early HCC patients treated with RFA | 158 |
| Chen X | British Journal of Surgery (2012) | Full-text | Prospective | Hong Kong, China | 2009.4– 2011.5 | HCC patients with Child-Pugh grade A who underwent partial hepatectomy | 190 |
| Chen Z | Supportive Care in Cancer (2014) | Abstract | NA | Guangzhou, China | 2008.9– 2010.6 | Advanced HCC patients without fever or signs of infection | 219 |
| da Fonseca LG | Medical Oncology (2014) | Full-text | Retrospective | Sao Paulo, Brazil | 2009.7– 2013.11 | HCC patients who received sorafenib as initial systemic treatment | 120 |
| Dan J | PLoS ONE (2013) | Full-text | Retrospective | Guangzhou, China | 2005.5– 2008.8 | Small HCC patients treated with RFA | 178 |
| Facciorusso A | Journal of Gastroenterology and Hepatology (2014) | Full-text | NA | Foggia, Italy | 2005.4– 2010.2 | HCC patients treated with RFA | 103 |
| Fan W | PLoS ONE (2015) | Full-text | Retrospective | Guangzhou, China | 2003.1– 2012.12 | Recurrent HCC patients treated with TACE | 132 |
| Fu SJ | Medical Oncology (2013) | Full-text | NA | Guangzhou, China | 2006.1– 2009.4 | HBV-associated HCC patients treated with radical hepatectomy | 282 |
| Fu YP | Liver Cancer (2015) | Abstract | NA | Guangzhou, China | NA | HCC patients treated with curative resection | 772 |
| Gao F | Medicine (2015) | Full-text | Retrospective | Beijing, China | 2008.10– 2012.5 | Newly diagnosed with HCC | 825 |
| Gomez D; Farid S | World Journal of Surgery (2008); HPB (2010, Abstract) | Full-text | NA | Leeds, UK | 1994.1– 2007.4 | HCC patients treated with curative resection | 96 |
| Guo ZX | Chinese Journal of Cancer (2009) | Full-text | Retrospective | Guangzhou, China | 2000– 2005 | HCC patients treated with curative resection (age <35 years old) | 91 |
| Halazun KJ | Annals of Surgery (2009) | Full-text | Retrospective | NY, US | 2001– 2007 | HCC patients treated with LT | 150 |
| Harimoto N | Transplantation (2013) | Full-text | Retrospective | Fukuoka, Japan | 1996.10– 2012.8 | HCC patients treated with LDLT | 167 |
| Higashi T | Annals of Surgical Oncology (2015) | Full-text | Prospective | Kumamoto, Japan | 2008– 2012 | HCC patients treated with resection | 215 |
| Hu B | Clinical Cancer Research (2014) | Full-text | Retrospective/Prospective | Shanghai, China | 2005– 2006/2010– 2011 | HCC patients treated with curative resection | 133/123 |
| Huang GQ | Oncotarget (2015) | Full-text | Retrospective | Wenzhou, China | 2007.1– 2014.1 | HCC patients treated with curative resection | 508 |
| Huang J | Medical Oncology (2014) | Full-text | Prospective | Guangzhou, China | 2008– 2009 | HCC patients treated with hepatectomy as initial treatment | 349 |
| Huang ZL | Journal of Vascular and Interventional Radiology (2011) | Full-text | Retrospective | Guangzhou, China | 2001– 2004 | HCC patients treated with TACE | 145 |
| Kanno Y | Clinical Nutrition (2014) | Abstract | NA | Mibu, Japan | 2000– 2012 | HCC patients treated with curative surgery | 418 |
| Kim DG | Hepatology (2013) | Abstract | NA | Seoul, South Korea | 2000.10– 2011.11 | HCC patients treated with LDLT | 224 |
| Kinoshita A | Annals of Surgical Oncology (2015) | Full-text | Prospective; Retrospective | Tokyo, Japan | 2005.1– 2012.8 | Newly diagnosed HCC | 186 |
| Lai Q | Transplantation International (2014) | Full-text | NA | Brussels, Belgium | 1994.1– 2012.3 | Patients with pre-LT proven diagnosis of HCC who entered the waiting list for LT | 181 |
| Li C | Journal of Surgical Research (2015) | Full-text | NA | Chengdu, China | 2007– 2014 | HBV-associated HCC patients treated with resection | 236 |
| Li JP | Chinese Journal of Cancer Prevention and Treatment (2013) | Full-text | Retrospective | Jinan, China | 2006.2– 2009.2 | Unresectable HCC patients treated with TACE | 154 |
| Li X | Tumor Biology (2014) | Full-text | Retrospective | Guangzhou, China | 2008.11– 2010.4 | Advanced HCC patients (BCLC stages C and D) who did not receive sorafenib | 205 |
| Li X | PLoS ONE (2014) | Full-text | Retrospective | Beijing, China | 2006.4– 2014.4 | Recurrent HCC patients treated with curative thermal ablation | 506 |
| Liao R | World Journal of Surgical Oncology (2015) | Full-text | Retrospective | Chongqing, China | 2007.1– 2010.12 | Single-nodule small HCC patients treated with curative resection | 222 |
| Liao W | Translational Oncology (2014) | Full-text | Retrospective | Guilin, China | 1999.9– 2007.6 | HCC patients treated with curative resection | 256 |
| Liese J | Transplantation (2014) | Abstract | Retrospective | Frankfurt, Germany | 2007.1– 2012.12 | HCC patients treated with LT | 92 |
| Limaye AR | Hepatology Research (2013) | Full-text | Retrospective | FL, US | 2000– 2008 | HCC patients treated with LT | 160 |
| Long J | Hepatology International (2016) | Full-text | Prospective | Beijing, China | 2010.8– 2014.7 | HCC with PVTT patients treated with microwave ablation after TACE | 60 |
| Lu D | Transplantation (2015) | Abstract | NA | Hangzhou, China | 2002– 2012 | Small HCC patients treated with LT | 140 |
| Luè A | Journal of Hepatology (2014) | Abstract | NA | 4 different hospitals, Spain | 2005.8– 2013.10 | HCC patients treated with sorafenib | 186 |
| Mano Y | Annals of Surgery (2013) | Full-text | Retrospective | 3 different hospitals, Japan | 1996.1– 20009.12 | HCC patients treated with curative resection | 958 |
| McNally ME | Annals of Surgical Oncology (2013) | Full-text | Retrospective | OH, US | A 10–year period | HCC patients treated with TACE | 104 |
| Mizukoshi E | Hepatology (2015) | Abstract | NA | Kanazawa, Japan | NA | HCC patients treated with hepatic arterial infusion chemotherapy | 36 |
| Motomura T | Journal of Hepatology (2013) | Abstract | NA | Fukuoka, Japan | 1999.7– 2011.3 | HCC patients treated with LT | 158 |
| Na GH | World Journal of Gastroenterology (2014) | Full-text | Retrospective | Seoul, South Korea | 2000.10– 2011.11 | HCC patients treated with LDLT | 224 |
| Nagai S | Transplantation (2015) | Abstract | NA | IN, US | 2001– 2012 | HCC patients treated with LT | 268 |
| Ni XC | Medicine (2015) | Full-text | Retrospective | Shanghai, China | 2010.12– 2012.1 | HCC patients treated with resection (test cohort) | 367 |
| Oh BS | BMC Cancer (2013) | Full-text | Retrospective | Seoul, South Korea | 2007.1– 2010.12 | Newly diagnosed HCC | 318 |
| Okamura Y | World Journal of Surgery (2015) | Full-text | Retrospective | Shizuoka, Japan | 2002.9– 2012.11 | HCC patients treated with resection | 256 |
| Parisi I | Liver Transplantation (2014) | Full-text | NA | London, UK | 1996– 2010 | HCC patients treated with LT | 150 |
| Peng W | Journal of Surgical Research (2014) | Full-text | Retrospective | Chengdu, China | 2007.2– 2012.3 | Small HCC patients treated with curative resection | 189 |
| Pinato DJ | Translational Research (2012) | Full-text | Retrospective | London, UK | NA | HCC patients treated with TACE | 54 |
| Pinato DJ | Journal of Hepatology (2012) | Full-text | Retrospective | London, UK | 1993– 2011 | HCC patients (training set) | 112 |
| Ruan DY | World Journal of Gastroenterology (2015) | Full-text | Retrospective | Guangzhou, China | 2003.9– 2011.6 | HCC patients treated with curative resection | 200 |
| Shindoh J | Transplant International (2014) | Full-text | Retrospective | Tokyo, Japan | 1996.1– 2012.12 | HCC patients treated with LDLT | 124 |
| Sirin G | Hepatology International (2015) | Abstract | Retrospective | Kocaeli, Japan | 2007– mid–2012 | HCC patients treated with segmental resection and/or RFA | 49 |
| Sukato DC | Journal of Vascular and Interventional Radiology (2015) | Full-text | Retrospective | PA, US | 2000.8– 2012.11 | Intermediate- or advanced-stage HCC patients treated with radioembolization | 176 |
| Sullivan KM | Journal of Surgical Oncology (2014) | Full-text | NA | WI, US | 2011.7– 2012.4 | HCC patients | 75 |
| Sun Q | Biomedical Research (2014) | Full-text | Retrospective | Beijing, China | 2003– 2008 | HCC patients treated with resection | 80 |
| Tajiri K | Journal of Gastroenterology and Hepatology (2016) | Full-text | Retrospective | Toyama, Japan | 2003– 2014 | HCC patients treated with RFA | 163 |
| Tajiri K | Hepatology Research (2015) | Full-text | Retrospective | Toyama, Japan | 2010– 2013 | Advanced HCC patients treated with hepatic arterial infusion chemotherapy | 26 |
| Terashima T | Hepatology Research (2015) | Full-text | Retrospective | Ishikawa, Japan | 2003.3– 2012.12 | Advanced HCC patients treated with hepatic arterial infusion chemotherapy | 266 |
| Uchida K | American Journal of Transplantation (2012) | Abstract | NA | FL, US | 2002.3– 2010.12 | HCC patients treated with DDLT | 275 |
| Wang GY | PLoS ONE (2011) | Full-text | Retrospective | Guangzhou, China | 2003.10– 2009.6 | HBV-associated HCC patients treated with LT | 101 |
| Wang K | Liver Transplantation (2013) | Abstract | Retrospective | Hangzhou, China | NA | HCC patients treated with LT | 235 |
| Wang Q | Annals of Surgical Oncology (2015) | Full-text | NA | NY, US | 1983– 2013 | HBV-associated HCC patients treated with resection | 234 |
| Wang W | Hepatology Research (2015) | Full-text | Retrospective | Hangzhou, China | 2002.1– 2012.12 | Male HCC patients treated with LT | 248 |
| Wei K | Medical Oncology (2014) | Full-text | Retrospective | Tianjin, China | 2010.1.1– 2013.5.31 | Intermediate-advanced HCC patients treated with concurrent TAE in combination with sorafenib | 40 |
| Weinmann AJ | Hepatology (2015) | Abstract | Retrospective | Mainz, Germany | 2007– 2013 | HCC patients treated with sorafenib | 148 |
| Xiao GQ | Hepatobiliary and Pancreatic Diseases International (2015); | Full-text | Retrospective | Chengdu, China | 1999.2– 2012.9 | HCC patients treated with LT | 305 |
| Xu X | Chinese Medical Journal (2014) | Full-text | Retrospective | Xi'an, China | 2003.7– 2012.9 | HCC patients treated with TACE | 178 |
| Xue TC | Tumor Biology (2015) | Full-text | Retrospective | Shanghai, China | 2008.1– 2011.3 | Huge HCC patients treated with TACE | 165 |
| Yamamura K | Journal of Hepato-Biliary-Pancreatic Sciences (2014) | Full-text | Prospective | Aichi, Japan | 2003.1– 2012.12 | HCC patients treated with resection | 113 |
| Yang X | Chinese Journal of Radiology (2015) | Full-text | Retrospective | Chengdu, China | 2000– 2010 | HBV-associated HCC patients treated with TACE | 546 |
| Yang Z | Oncotarget (2015) | Full-text | Retrospective | Shanghai, China | 2009.9– 2015.5 | HBV-associated HCC patients treated with TACE | 189 |
| Yip V | HPB (2011) | Abstract | NA | Liverpool, UK | 1997– 2008 | HCC patients treated with resection | 47 |
| Yoshizumi T | Anticancer Research (2016) | Full-text | NA | Fukuoka, Japan | 1999.4– 2015.3 | HCC patients within Milan criteria treated with LDLT | 129 |
| Yoshizumi T | Transplantation Proceedings (2013) | Full-text | NA | Fukuoka, Japan | 1999.4– 2011.12 | HCC patients within Kyushu University criteria treated with LDLT | 152 |
| Yoshizumi T | Hepatology Research (2013) | Full-text | NA | Fukuoka, Japan | 1999.4– 2012.8 | Recurrent HCC adult patients treated with LDLT | 104 |
| Young AL | Journal of American College of Surgeons (2012) | Full-text | Retrospective | Leeds, UK | 1994.1.1– 2008.12.31 | HCC patients treated with resecction | 142 |
| Zhang J | Oncology Letters (2014) | Full-text | Retrospective | Wuhan, China | 2002.3– 2012.8 | Non-viral HCC patients treated with TACE | 138 |
| Zhang W | Medical Oncology (2015) | Full-text | Retrospective | Tianjin, China | 2009.8.1– 2012.3.28 | HCC patients who received sorafenib after resection | 38 |
| Zheng YB | Asian Pacific Journal of Cancer Prevention (2013) | Full-text | Retrospective | Guangzhou, China | 2011.1– 2012.12 | HCC patients treated with sorafenib monotherapy | 65 |
| Zheng YB | Chinese Journal of Interventional Imaging and Therapy (2013) | Full-text | Retrospective | Guangzhou, China | 2008.1– 2012.12 | HCC patients treated with TACE | 77 |
| Zhou D | Scientific Reports (2015) | Full-text | Retrospective | Guangzhou, China | 2007– 2009 | HCC patients treated with surgical resection, ablative therapy, and TACE | 1061 |
| Zhou DS | World Journal of Gastroenterology (2015) | Full-text | Retrospective | Guangzhou, China | 2009.9– 2011.11 | HBV–related HCC patients treated with TACE | 224 |
Abbreviations: DDLT, deceased donor liver transplantation; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; LT, liver transplantation; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Notes:
Some data from Kinoshita A, Annals of Surgical Oncology (2015) is also published by the same authors in British Journal of Cancer (2012).
Some data from Wang GY, PLoS ONE (2011) is also published by the same authors in National Medical Journal of China (2011).
Some data from Xiao GQ, Hepatobiliary and Pancreatic Diseases International (2015) is also published by the same authors in World Journal of Gastroenterology (2013) and Hepato-gastroenterology (2014).
Study quality
Quality of included studies was summarized in Supplementary Table 1. Three, 18, 12, 30, and 27 studies had 7, 6, 5, 4, and ≤ 3 points, respectively.
Meta analyses
Association of baseline NLR with overall survival
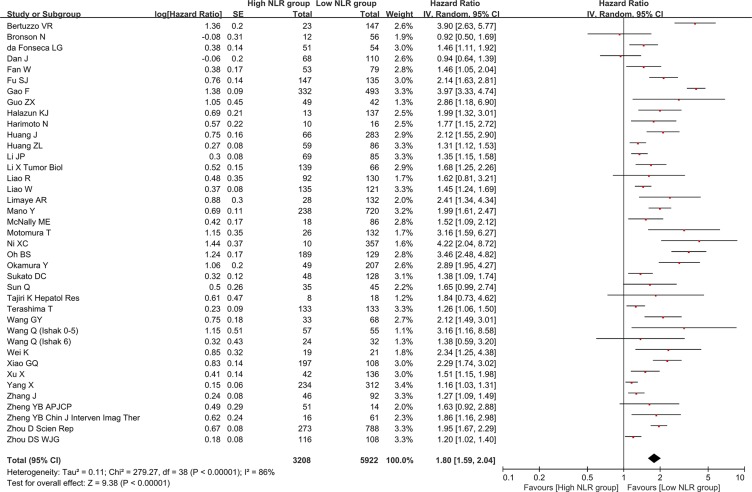
There were 39 groups of individual data regarding the association of baseline NLR with overall survival. They were extracted from 38 papers. HR was 1.80 (95% CI: 1.59–2.04, p < 0.00001), suggesting that low baseline NLR group had a significantly better overall survival than high baseline NLR group (Figure 2). Heterogeneity among studies was statistically significant (I2 = 86%, p < 0.00001). Funnel plot suggested a potential publication bias (Supplementary Figure 1).
Figure 2. Forest plot evaluating the association between baseline NLR and overall survival in HCC patients.
Association of post-treatment NLR with overall survival
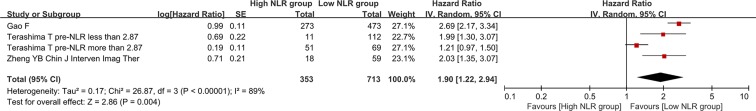
There were 4 groups of individual data regarding the association of post-treatment NLR with overall survival. They were extracted from 3 papers. HR was 1.90 (95% CI: 1.22–2.94, p = 0.004), suggesting that low post-treatment NLR group had a significantly better overall survival than high post-treatment NLR group (Figure 3). Heterogeneity among studies was statistically significant (I2 = 89%, p < 0.00001).
Figure 3. Forest plot evaluating the association between post-treatment NLR and overall survival in HCC patients.
Association of NLR change with overall survival
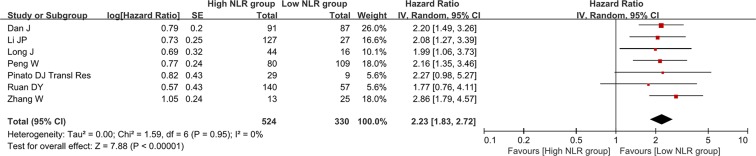
There were 7 groups of individual data regarding the association of NLR change with overall survival. They were extracted from 7 papers. HR was 2.23 (95% CI: 1.83–2.72, p < 0.00001), suggesting that decreased NLR group had a significantly better overall survival than increased NLR group (Figure 4). Heterogeneity among studies was not statistically significant (I2 = 0%, p = 0.95).
Figure 4. Forest plot evaluating the association between NLR change and overall survival in HCC patients.
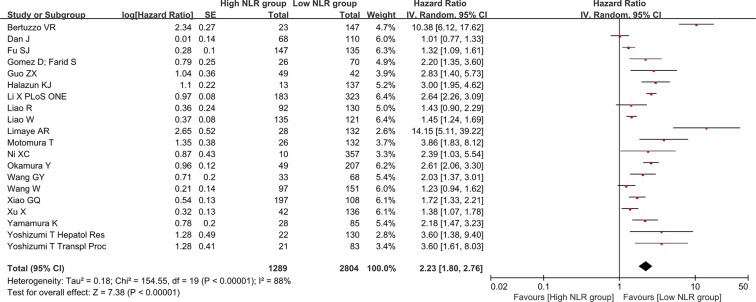
Association of baseline NLR with recurrence-free or disease-free survival
There were 20 groups of individual data regarding the association of baseline NLR with recurrence-free or disease-free survival. They were extracted from 20 papers. HR was 2.23 (95% CI: 1.80–2.76, p < 0.00001), suggesting that low baseline NLR group had a significantly better recurrence-free or disease-free survival than high baseline NLR group (Figure 5). Heterogeneity among studies was statistically significant (I2 = 88%, p < 0.00001). Funnel plot suggested a potential publication bias (Supplementary Figure 2).
Figure 5. Forest plot evaluating the association between baseline NLR and recurrence-free or disease-free survival in HCC patients.
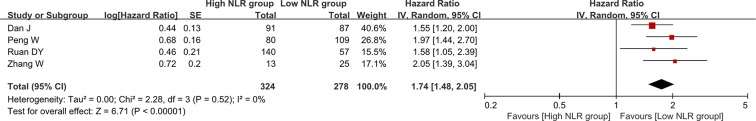
Association of NLR change with recurrence-free or disease-free survival
There were 4 groups of individual data regarding the association of NLR change with recurrence-free or disease-free survival. They were extracted from 4 papers. HR was 2.23 (95% CI: 1.83–2.72, p < 0.00001), suggesting that decreased NLR group had a significantly better overall survival than increased NLR group (Figure 6). Heterogeneity among studies was not statistically significant (I2 = 0%, p = 0.52).
Figure 6. Forest plot evaluating the association between NLR change and recurrence-free or disease-free survival in HCC patients.
Subgroup meta-analyses
Results of subgroup meta-analyses were summarized in Table 2.
Table 2. Results of subgroup meta-analyses.
| Items | No. groups of data | No. Pts in High NLR group | No. Pts in Low NLR group | Hazard ratio (95% CI) | P value | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 | P value | ||||||
| Overall survival & baseline NLR | |||||||
| Subgroup analysis according to treatment option | |||||||
| Liver transplantation | 7 | 330 | 740 | 2.38 (1.95–2.91) | < 0.00001 | 38% | 0.14 |
| Surgical resection | 12 | 914 | 2183 | 1.95 (1.61–2.37) | < 0.00001 | 62% | 0.002 |
| Radiofrequency ablation | 1 | 68 | 110 | 0.94 (0.64–1.39) | 0.76 | NA | NA |
| Transarterial chemoembolization | 9 | 653 | 1045 | 1.29 (1.20–1.38) | < 0.00001 | 14% | 0.32 |
| Radioembolization | 1 | 48 | 128 | 1.38 (1.09–1.74) | 0.008 | NA | NA |
| Hepatic arterial infusion chemotherapy | 2 | 141 | 151 | 1.28 (1.07–1.52) | 0.006 | 0% | 0.43 |
| Transarterial embolization + sorafenib | 1 | 19 | 21 | 2.34 (1.25–4.38) | 0.008 | NA | NA |
| Sorafenib | 2 | 102 | 68 | 1.49 (1.17–1.91) | 0.001 | 0% | 0.73 |
| Mixed | 4 | 933 | 1476 | 2.59 (1.68–4.00) | < 0.0001 | 94% | < 0.00001 |
| Subgroup analysis according to NLR cut-off value range | |||||||
| NLR cut-off value ≥ 1, < 2 | 2 | 110 | 246 | 1.22 (0.77–1.93) | 0.4 | 73% | 0.05 |
| NLR cut-off value ≥ 2, < 3 | 16 | 2077 | 3289 | 1.93 (1.56–2.39) | < 0.00001 | 91% | < 0.00001 |
| NLR cut-off value ≥ 3, < 4 | 7 | 515 | 903 | 1.55 (1.28–1.88) | < 0.00001 | 75% | 0.0005 |
| NLR cut-off value = 4 | 6 | 308 | 349 | 2.07 (1.73–2.49) | < 0.00001 | 0% | 0.63 |
| NLR cut-off value = 5 | 8 | 198 | 1135 | 1.86 (1.37–2.52) | < 0.0001 | 83% | < 0.00001 |
| Subgroup analysis according to regions | |||||||
| America | 8 | 251 | 680 | 1.55 (1.30–1.84) | < 0.00001 | 26% | 0.22 |
| Asia | 30 | 2934 | 5095 | 1.81 (1.57–2.08) | < 0.00001 | 88% | < 0.00001 |
| Europe | 1 | 23 | 147 | 3.9 (2.63–5.77) | < 0.00001 | NA | NA |
| Overall survival & post-treatment NLR | |||||||
| Subgroup analysis according to treatment option | |||||||
| Transarterial chemoembolization | 1 | 18 | 59 | 2.03 (1.35–3.07) | 0.0007 | NA | NA |
| Hepatic arterial infusion chemotherapy | 2 | 62 | 181 | 1.5 (0.92–2.43) | 0.1 | 76% | 0.04 |
| Mixed | 1 | 273 | 473 | 2.69 (2.17–3.34) | < 0.00001 | NA | NA |
| Subgroup analysis according to NLR cut-off value range | |||||||
| NLR cut-off value ≥ 2, < 3 | 3 | 335 | 654 | 1.86 (1.06–3.26) | 0.03 | 92% | < 0.00001 |
| NLR cut-off value = 4 | 1 | 18 | 59 | 2.03 (1.35–3.07) | 0.0007 | NA | NA |
| Subgroup analysis according to regions | |||||||
| Asia | 4 | 353 | 713 | 1.9 (1.22–2.94) | 0.004 | 89% | < 0.00001 |
| Overall survival & NLR change | |||||||
| Subgroup analysis according to treatment option | |||||||
| Surgical resection | 2 | 220 | 166 | 2.06 (1.37–3.11) | 0.0006 | 0% | 0.68 |
| Radiofrequency ablation | 1 | 91 | 87 | 2.2 (1.49–3.26) | < 0.00001 | NA | NA |
| Microwave ablation | 1 | 44 | 16 | 1.99 (1.06–3.73) | 0.03 | NA | NA |
| Transarterial chemoembolization | 2 | 156 | 36 | 2.12 (1.39–3.24) | 0.0005 | 0% | 0.86 |
| Sorafenib | 1 | 13 | 25 | 2.86 (1.79–4.57) | < 0.00001 | NA | NA |
| Subgroup analysis according to NLR cut-off value change | |||||||
| Increase or decrease | 6 | 384 | 273 | 2.26 (1.84–2.78) | < 0.00001 | 0% | 0.94 |
| Delta | 1 | 140 | 57 | 1.77 (0.76–4.11) | 0.18 | NA | NA |
| Subgroup analysis according to regions | |||||||
| Asia | 6 | 495 | 321 | 2.23 (1.81–2.74) | < 0.00001 | 0% | 0.9 |
| Europe | 1 | 29 | 9 | 2.27 (0.98–5.27) | 0.06 | NA | NA |
| RFS/DFS & baseline NLR | |||||||
| Subgroup analysis according to treatment option | |||||||
| Liver transplantation | 9 | 460 | 1088 | 3.31 (2.05–5.32) | < 0.00001 | 89% | < 0.00001 |
| Surgical resection | 8 | 536 | 1147 | 1.87 (1.47–2.37) | < 0.00001 | 76% | 0.0002 |
| Radiofrequency ablation | 1 | 68 | 110 | 1.01 (0.77–1.33) | 0.94 | NA | NA |
| Thermal ablation | 1 | 183 | 323 | 2.64 (2.26–3.09) | < 0.00001 | NA | NA |
| Transarterial chemoembolization | 1 | 42 | 136 | 1.38 (1.07–1.78) | 0.01 | NA | NA |
| Subgroup analysis according to NLR cut-off value range | |||||||
| NLR cut-off value ≥ 1, < 2 | 2 | 110 | 246 | 1.18 (0.87–1.6) | 0.27 | 62% | 0.1 |
| NLR cut-off value ≥ 2, < 3 | 6 | 655 | 958 | 1.9 (1.4–2.59) | < 0.0001 | 90% | < 0.00001 |
| NLR cut-off value ≥ 3, < 4 | 3 | 158 | 304 | 1.72 (1.17–2.54) | 0.006 | 73% | 0.03 |
| NLR cut-off value = 4 | 4 | 266 | 453 | 2.75 (1.63–4.63) | 0.001 | 62% | 0.05 |
| NLR cut-off value = 5 | 5 | 100 | 843 | 4.51 (2.24–9.12) | < 0.0001 | 85% | < 0.0001 |
| Subgroup analysis according to regions | |||||||
| America | 2 | 41 | 269 | 6.07 (1.34–27.55) | 0.02 | 87% | 0.006 |
| Asia | 16 | 1199 | 2318 | 1.85 (1.53–2.24) | < 0.00001 | 83% | < 0.00001 |
| Europe | 2 | 49 | 217 | 4.77 (1.04–21.77) | 0.04 | 94% | < 0.0001 |
| RFS/DFS & NLR change | |||||||
| Subgroup analysis according to treatment option | |||||||
| Surgical resection | 2 | 220 | 166 | 1.82 (1.42–2.34) | < 0.00001 | 0% | 0.4 |
| Radiofrequency ablation | 1 | 91 | 87 | 1.55 (1.20–2.00) | 0.007 | NA | NA |
| Sorafenib | 1 | 13 | 25 | 2.05 (1.39–3.04) | 0.0003 | NA | NA |
| Subgroup analysis according to NLR cut-off value change | |||||||
| Increase or decrease | 3 | 184 | 221 | 1.77 (1.48–2.12) | < 0.00001 | 2% | 0.36 |
| Delta | 1 | 140 | 57 | 1.58 (1.05–2.39) | 0.03 | NA | NA |
| Subgroup analysis according to regions | |||||||
| Asia | 4 | 324 | 278 | 1.74 (1.48–2.05) | < 0.00001 | 0% | 0.52 |
DISCUSSION
The present study systematically reviewed the role of NLR in the assessment of prognosis of HCC patients. To our knowledge, two previous meta-analyses also explored the association of NLR with prognosis of HCC [102–103]. Both of them were published in 2014. In the first meta-analysis, Xiao et al. searched the relevant literatures in August 2013 and identified 15 studies with 3,094 patients [102]. In the second meta-analysis, Xue et al. searched the relevant literatures in October 2013 and identified 26 studies with 4,461 patients [103]. Several advantages and features of our work should be acknowledged: 1) the relevant literatures were identified more recently (January 2016), and a larger number of relevant studies were included (90 papers with 20,475 patients); 2) according to the different time points when NLR values were obtained, we divided into baseline NLR, post-treatment NLR, and NLR change; 3) overall survival and recurrence-free or disease-free survival were selected as the primary outcomes; and 4) according to the treatment options, NLR cut-off values, and regions, we performed subgroup meta-analyses.
The major finding of our study was that low baseline NLR was significantly associated with better overall survival and recurrence-free or disease-free survival of HCC patients. This was based on a relatively large number of relevant data (38 papers for overall survival and 20 papers for recurrence-free or disease-free survival). Therefore, in our opinion, the relationship of baseline NLR with survival of HCC patients should be stable. This consideration was also confirmed by the subgroup meta-analyses: 1) except for one subgroup meta-analysis in patients undergoing radiofrequency ablation, other subgroup meta-analyses in patients undergoing different treatment modalities supported such an inverse association between them; 2) except for one subgroup meta-analysis with a NLR cut-off value of ≥ 1 and < 2, other subgroup meta-analyses with other NLR cut-off value ranges supported such an inverse association between them; and 3) regardless of regions, subgroup meta-analyses supported such an inverse association between them. Certainly, two following issues should be acknowledged. First, only one study focused on the patients undergoing radiofrequency ablation. Thus, more data might be necessary for the validation of our findings. Second, only two studies employed a NLR cut-off value of ≥ 1 and < 2. Given such a small NLR cut-off value, the survival difference between high and low NLR groups might be hardly achieved.
Another finding was that low post-treatment NLR was significantly associated with better overall survival of HCC patients. However, due to a small number of included studies, the subgroup meta-analyses were performed in patients undergoing transarterial chemoembolization and hepatic arterial infusion chemotherapy, studies with a NLR cut-off value of ≥ 2 and < 3 and NLR cut-off value of 4, and Asian studies. Except for one subgroup meta-analysis in HCC patients undergoing hepatic arterial infusion chemotherapy, other subgroup meta-analyses supported statistically significant associations. Similarly, we also found that decreased NLR after treatment was significantly associated with better recurrence-free or disease-free survival of HCC patients. Notably, such an inverse association was maintained regardless of treatment modalities.
Several limitations should be clarified. First, HR value for the association of NLR with overall survival was relatively small. Thus, their relationship might be weak. Whether the prognostic assessment of HCC can be guided by baseline NLR value should be further explored. Second, all included studies were observational, and most of them were retrospective. The quality of included studies was relatively low according to the NEWCASTLE-OTTAWA quality assessment scale. A major concern was a low comparability of patient characteristics between low and high NLR groups. This was primarily because all included studies were observational and NLR was only one of many variables included in univariate or multivariate analyses in a majority of original studies. Third, the heterogeneity was statistically significant in several meta-analyses. Random-effect model was employed to produce more conservative results. Fourth, because the researchers paid close attention on the prognostic role of NLR, some relevant paper has been published after this paper was finished [104].
In conclusion, the importance of NLR for assessing the overall survival and recurrence-free or disease-free survival should be acknowledged. Thus, we would like to suggest that NLR may be incorporated into the algorithm regarding the prognostic assessment of HCC. Further studies should confirm the prognostic ability of NLR in different specific settings according to the stage of HCC and treatment options and explore the superiority of NLR over other traditional prognostic scores or models. Additionally, considering that NLR change was associated with prognosis of HCC patients, future studies should explore how to prolong the survival of HCC patients by improving the inflammatory conditions.
MATERIALS AND METHODS
We searched 3 major databases, including PubMed, EMBASE, and Cochrane library databases from the inception of databases. Search items were as follows: ((hepatocellular carcinoma) OR (liver cancer)) AND ((NLR) OR ((neutrophil) AND lymphocyte)). The last search was performed on January 20, 2016. All relevant literatures regarding the prognostic role of NLR in HCC patients were identified. Exclusion criteria were as follows: 1) duplicates; 2) comments; 3) erratum; 4) reviews; 5) case reports; 6) experimental studies; and 7) original studies did not evaluate the prognostic role of NLR in HCC patients. Publication language was not restricted.
We extracted the following data from the included studies: first author, journal, publication year, publication type, study design, regions, enrollment period, study population, number of patients, NLR cut-off values, and overall survival and recurrence-free or disease-free survival data according to the NLR value. In cases of uncertainty, we communicated with the authors and/or journal editors to validate the accuracy of data.
Given the nature of included studies, the study quality was assessed according to the NEWCASTLE-OTTAWA quality assessment scale for cohort studies [105]. This scale consisted of 8 questions with a maximum of 9 points. A study with more points would be of higher quality.
Data analysis was described as previously [106–108]. Briefly, only random-effects models were employed. Hazard ratios (HRs) were calculated because the overall survival and recurrence-free or disease-free survival were time-dependent data. I2 statistic and the Chi-square test were used to evaluate the heterogeneity among studies. Funnel plots were performed to evaluate the publication bias, if there were ≥ 10 groups of individual data included in the meta-analysis.
Notably, the meta-analyses were performed according to the times when NLR values were obtained (i.e. baseline NLR, post-treatment NLR, and NLR change). As for the baseline and post-treatment NLR, the patients were divided into two groups (i.e., low and high NLR group) according to the definitions of original studies. If the patients were divided into ≥ 3 groups in the original studies, the relevant data would not be included in the meta-analyses. Additionally, subgroup meta-analyses were performed according to the treatment options (i.e., liver transplantation, surgical resection, radiofrequency ablation, transarterial chemoembolization, radioembolization, hepatic arterial infusion chemotherapy, transarterial embolization plus sorafenib, sorafenib, and mixed treatments), NLR cut-off value ranges, and regions (i.e., America, Asia, and Europe).
SUPPLEMENTARY MATERIALS FIGURES AND TABLE
Footnotes
CONFLICTS OF INTERESTS
None.
Authors’ Contributions
XQ: designed the study, performed the literature search and selection, data extraction, quality assessment, and statistical analysis, and drafted the manuscript; JL and HD: performed the literature selection, data extraction, and quality assessment; CS: performed the literature search; HL and XG: gave critical comments and revised the manuscript. All authors have made an intellectual contribution to the manuscript and approved the submission.
REFERENCES
- 1.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 3.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 4.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691–1700. doi: 10.1053/j.gastro.2014.02.032. e1693. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 6.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 7.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol. 2011;225:438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- 10.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nind AP, Nairn RC, Rolland JM, Guli EP, Hughes ES. Lymphocyte anergy in patients with carcinoma. Br J Cancer. 1973;28:108–117. doi: 10.1038/bjc.1973.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelmessih RM, Verna EC, Brubaker WD, Halazun KJ, Siegel A, Brown RS. Hepatocellular carcinoma tumor staging at the time of liver transplant but not at diagnosis are predictive of tumor recurrence in patients who are downstaged with chemoembolization. Hepatology. 2011;54:1380A–1381A. [Google Scholar]
- 13.Afshar M, Clarke H, Jackson-Wilding A, Ahmed A, Ma YT, Punia P. Neutrophil lymphocyte ratio (NLR) at diagnosis is a predictor for survival in patients receiving sorafenib for advanced hepatocellular carcinoma (HCC): A large UK cohort. J Hepatol. 2015;62:S442. [Google Scholar]
- 14.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Aino H, Sumie S, Niizeki T, Kuromatsu R, Tajiri N, Nakano M, Satani M, Yamada S, Okamura S, Shimose S, Sumie H, Torimura T, Sata M. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol Clin Oncol. 2014;2:393–398. doi: 10.3892/mco.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, D'Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 17.Bodzin A, Reino D, Lunsford K, Harlander-Locke M, Markovic D, Zarrinpar A, Kaldas F, Farmer D, Yersiz H, Busuttil R, Agopian V. Predictors of time to recurrence and mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2015;15:89. [Google Scholar]
- 18.Bronson N, Enestvedt K, Thomas E, Vermette D, Orloff S. Preoperative neutrophil to lymphocyte ratio does not predict recurrence or prognosis after resection for hepatocellular carcinoma. HPB. 2012;14:456. [Google Scholar]
- 19.Bruixola G, Niño OM, Diaz-Beveridge R, Reche E, Salvador C, Escoin C, Akhoundova D, Segura A, Gimenez A, Aparicio J. Baseline neutrophil-to-lymphocyte ratio (NLR) and early toxicity as prognostic factors in advanced hepatocellular carcinoma patients treated with sorafenib. J Clin Oncol. 2015:33. [Google Scholar]
- 20.Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, Chan HL, To KF. Prognostic Nutritional Index (PNI) Predicts Tumor Recurrence of Very Early/Early Stage Hepatocellular Carcinoma After Surgical Resection. Ann Surg Oncol. 2015;22:4138–4148. doi: 10.1245/s10434-015-4516-1. [DOI] [PubMed] [Google Scholar]
- 21.Chang JX, Zeng Z, Lu YY, Qu HJ, Xu LG, Gao X, Wang H, Lou M, Wang C, Yang Y. Nlr of pro-cryoablation can predict the prognosis of patients with advanced hepatocellular carcinoma. Ann Oncol. 2014;25:v45. [Google Scholar]
- 22.Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553–561. doi: 10.1111/j.1440-1746.2011.06910.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Zhai J, Cai X, Zhang Y, Wei L, Shi L, Wu D, Shen F, Lau WY, Wu M. Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with Child-Pugh grade A cirrhosis. Br J Surg. 2012;99:1701–1710. doi: 10.1002/bjs.8951. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Xy LI, Min DONG, Qu L, Xiaokun M, Xiangyuan WU. Identification of prognostic value of neutrophil-to-lymphocyte ratio (NLR) in patients with advanced hepatocellular carcinoma (HCC) Support Care Cancer. 2014;22:S43. [Google Scholar]
- 25.da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, Hoff PM, Sabbaga J. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31:264. doi: 10.1007/s12032-014-0264-5. [DOI] [PubMed] [Google Scholar]
- 26.Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M. Postoperative Neutrophil-to-Lymphocyte Ratio Change Predicts Survival of Patients with Small Hepatocellular Carcinoma Undergoing Radiofrequency Ablation. PLoS ONE. 2013:8. doi: 10.1371/journal.pone.0058184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, Di Leo A, Carr BI, Barone M. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol. 2014;29:1905–1910. doi: 10.1111/jgh.12618. [DOI] [PubMed] [Google Scholar]
- 28.Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS ONE. 2015:10. doi: 10.1371/journal.pone.0119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu SJ, Shen SL, Li SQ, Hua YP, Hu WJ, Liang LJ, Peng BG. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol. 2013:30. doi: 10.1007/s12032-013-0721-6. [DOI] [PubMed] [Google Scholar]
- 30.Fu YP, Ni XC, Yi Y, Cai XY, He HW, Wang JX, Lu ZF, Zhou J, Fan J, Qiu SJ. A novel and validated inflammation based score (IBS) derived from neutrophil to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma following curative surgical resection. Liver Cancer. 2015;4:224. doi: 10.1097/MD.0000000000002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao F, Li X, Geng M, Ye X, Liu H, Liu Y, Wan G, Wang X. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Medicine. 2015;94:e639. doi: 10.1097/MD.0000000000000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 33.Guo ZX, Wei W, Zhong C, Shi M, Chen MS, Guo RP. Correlation of preoperative neutrophil-to-lymphocyte ratio to prognosis of young patients with hepatocellular carcinoma. Chin J Cancer. 2009;28:75–80. doi: 10.5732/cjc.009.10073. [DOI] [PubMed] [Google Scholar]
- 34.Halazun KJ, Hardy MA, Rana AA, Woodland DCt, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Jr, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 35.Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013;96:1008–1012. doi: 10.1097/TP.0b013e3182a53f2b. [DOI] [PubMed] [Google Scholar]
- 36.Higashi T, Hayashi H, Kaida T, Arima K, Takeyama H, Taki K, Izumi D, Tokunaga R, Kosumi K, Nakagawa S, Okabe H, Imai K, Nitta H, et al. Prognostic Impact of Visceral Fat Amount and Branched-Chain Amino Acids (BCAA) in Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22:1041–1047. doi: 10.1245/s10434-015-4796-5. [DOI] [PubMed] [Google Scholar]
- 37.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 38.Huang GQ, Zhu GQ, Liu YL, Wang LR, Braddock M, Zheng MH, Zhou MT. Stratified neutrophil-to-lymphocyte ratio accurately predict mortality risk in hepatocellular carcinoma patients following curative liver resection. Oncotarget. 2016;7:5429–39. doi: 10.18632/oncotarget.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J, Xu L, Luo Y, He F, Zhang Y, Chen M. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol. 2014:31. doi: 10.1007/s12032-014-0883-x. [DOI] [PubMed] [Google Scholar]
- 40.Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702–709. doi: 10.1016/j.jvir.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 41.Kanno Y, Ishizuka M, Kubota K. Neutrophil to lymphocyte ratio is associated with postoperative survival of primary hepatocellular carcinoma patients undergoing curative surgery. Clin Nutr. 2014;33:S242. [Google Scholar]
- 42.Kim DG. Neutrophil-lymphocyte ratio and serum C-reactive protein predict overall and recurrence free survival after liver transplantation in patients with hepatocellular carcinoma. Hepatology. 2013;58:782A–783A. [Google Scholar]
- 43.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 44.Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32–41. doi: 10.1111/tri.12191. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res. 2015;198:73–79. doi: 10.1016/j.jss.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Li JP, Hu SL, Chen H, Bu WZ, Song JL. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Chinese Journal of Cancer Prevention and Treatment. 2013;20:522–525. [Google Scholar]
- 47.Li X, Chen ZH, Ma XK, Chen J, Wu DH, Lin Q, Dong M, Wei L, Wang TT, Ruan DY, Lin ZX, Xing YF, Deng Y, et al. Neutrophil-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2014;35:11057–11063. doi: 10.1007/s13277-014-2360-8. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Han Z, Cheng Z, Yu J, Liu S, Yu X, Liang P. Preoperative neutrophil-to-lymphocyte ratio is a predictor of recurrence following thermal ablation for recurrent hepatocellular carcinoma: A retrospective analysis. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0110546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao R, Tang ZW, Li DW, Luo SQ, Huang P, Du CY. Preoperative neutrophil-to-lymphocyte ratio predicts recurrence of patients with single-nodule small hepatocellular carcinoma following curative resection: A retrospective report. World J Surg Oncol. 2015:13. doi: 10.1186/s12957-015-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, Shi W, Yuan S, Tahir SA, Jin J, He S. Preoperative neutrophil-to-lymphocyte ratio as a new prognostic marker in hepatocellular carcinoma after curative resection. Transl Oncol. 2014;7:248–255. doi: 10.1016/j.tranon.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liese J, Peveling-Oberhag J, Moench C, Schnitzbauer A, Welker M, Zeuzem S, Bechstein W, Ulrich F. A possible role of miRNAs as predicitve markers for the recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2014;98:696. doi: 10.1111/tri.12733. [DOI] [PubMed] [Google Scholar]
- 52.Limaye AR, Clark V, Soldevila-Pico C, Morelli G, Suman A, Firpi R, Nelson DR, Cabrera R. Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatol Res. 2013;43:757–764. doi: 10.1111/hepr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long J, Zheng JS, Sun B, Lu N. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int. 2016:1–10. doi: 10.1007/s12072-015-9673-6. [DOI] [PubMed] [Google Scholar]
- 54.Lu D, Xu X, Zheng S. The prognostic capacity of micro-vascular invasion in liver transplantation for small hepatocellular carcinoma. Transplantation. 2015;99:161–162. [Google Scholar]
- 55.Luè A, Bustamante FJ, Iñarrairaegui M, Arenas JI, Serrano MT, Testillano M, Lorente S, Gil C, De La Torre M, Gómez A, Sangro B. Neutrophil-to-lymphocyte ratio is a predictor of one-year survival in patients with hepatocellular carcinoma receiving sorafenib. J Hepatol. 2014;60:S401. doi: 10.18632/oncotarget.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T, Maehara Y. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–305. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 57.McNally ME, Martinez A, Khabiri H, Guy G, Michaels AJ, Hanje J, Kirkpatrick R, Bloomston M, Schmidt CR. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013;20:923–928. doi: 10.1245/s10434-012-2639-1. [DOI] [PubMed] [Google Scholar]
- 58.Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Hepatology. 2015;62:408A. doi: 10.1007/s00262-016-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, Soejima Y, Maehara Y. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Na GH, Kim DG, Han JH, Kim EY, Lee SH, Hong TH, You YK. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol. 2014;20:6594–6601. doi: 10.3748/wjg.v20.i21.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai S, Mangus RS, Frost E, Kubal CA, Ekser B, Fridell JA, Kwo P, Maluccio MA, Tector AJ. Predictors for outcome of recurrence of hepatocellular carcinoma after liver transplantation: Successful treatment and long survival. Transplantation. 2015;99:225. doi: 10.1111/ctr.12644. [DOI] [PubMed] [Google Scholar]
- 62.Ni XC, Yi Y, Fu YP, He HW, Cai XY, Wang JX, Zhou J, Cheng YF, Jin JJ, Fan J, Qiu SJ. Prognostic value of the modified glasgow prognostic score in patients undergoing radical surgery for hepatocellular carcinoma. Medicine. 2015:94. doi: 10.1097/MD.0000000000001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013:13. doi: 10.1186/1471-2407-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39:1501–1509. doi: 10.1007/s00268-015-2982-z. [DOI] [PubMed] [Google Scholar]
- 65.Parisi I, Tsochatzis E, Wijewantha H, Rodriguez-Peralvarez M, De Luca L, Manousou P, Fatourou E, Pieri G, Papastergiou V, Davies N, Yu D, Luong T, Dhillon AP, et al. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within Milan criteria. Liver Transpl. 2014;20:1327–1335. doi: 10.1002/lt.23969. [DOI] [PubMed] [Google Scholar]
- 66.Peng W, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res. 2014;192:402–408. doi: 10.1016/j.jss.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 67.Pinato DJ, Sharma R. An inflammation-based prognostic index predicts survival advantage after transarterial chemoembolization in hepatocellular carcinoma. Transl Res. 2012;160:146–152. doi: 10.1016/j.trsl.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, Kubota K, Sharma R. A novel and validated prognostic index in hepatocellular carcinoma: The inflammation based index (IBI) J Hepatol. 2012;57:1013–1020. doi: 10.1016/j.jhep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 69.Ruan DY, Lin ZX, Li Y, Jiang N, Li X, Wu DH, Wang TT, Chen J, Lin Q, Wu XY. Poor oncologic outcomes of hepatocellular carcinoma patients with intra-abdominal infection after hepatectomy. World J Gastroenterol. 2015;21:5598–5606. doi: 10.3748/wjg.v21.i18.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shindoh J, Sugawara Y, Nagata R, Kaneko J, Tamura S, Aoki T, Sakamoto Y, Hasegawa K, Tanaka T, Kokudo N. Evaluation methods for pretransplant oncologic markers and their prognostic impacts in patient undergoing living donor liver transplantation for hepatocellular carcinoma. Transpl Int. 2014;27:391–398. doi: 10.1111/tri.12274. [DOI] [PubMed] [Google Scholar]
- 71.Sirin G, Senturk O, Yilmaz H, Celebi A, Hulagu S. Are inflammatory markers more useful than noninvasive fibrosis panels for prediction of hepatocellular carcinoma recurrence. Hepatol Int. 2015;9:S290. [Google Scholar]
- 72.Sukato DC, Tohme S, Chalhoub D, Han K, Zajko A, Amesur N, Orons P, Marsh JW, Geller DA, Tsung A. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol. 2015;26:816–824. doi: 10.1016/j.jvir.2015.01.038. e811. [DOI] [PubMed] [Google Scholar]
- 73.Sullivan KM, Groeschl RT, Turaga KK, Tsai S, Christians KK, White SB, Rilling WS, Pilgrim CH, Gamblin TC. Neutrophil-to-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma: a Western perspective. J Surg Oncol. 2014;109:95–97. doi: 10.1002/jso.23448. [DOI] [PubMed] [Google Scholar]
- 74.Sun Q, Jiao SC, Wu JY, Long YY, Chen L. Pretreatment haematological laboratory values: The new prognostic factors in patients undergoing hepatectomy for hepatocellular carcinoma. Biomedical Research (India) 2014;25:580–587. [Google Scholar]
- 75.Tajiri K, Baba H, Kawai K, Minemura M, Yasumura S, Takahara T, Sugiyama T. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol. 2016 doi: 10.1111/jgh.13287. [DOI] [PubMed] [Google Scholar]
- 76.Tajiri K, Kawai K, Minemura M, Yasumura S, Hosokawa A, Kawabe H, Tomizawa G, Sugiyama T. Neutrophil/lymphocyte ratio as a prognostic indicator of hepatic arterial infusion chemotherapy with arterial cisplatin plus continuous 5-fluorouracil. Hepatol Res. 2015;45:755–763. doi: 10.1111/hepr.12417. [DOI] [PubMed] [Google Scholar]
- 77.Terashima T, Yamashita T, Iida N, Nakagawa H, Arai K, Kitamura K, Kagaya T, Sakai Y, Mizukoshi E, Honda M, Kaneko S. Blood neutrophil to lymphocyte ratio as a predictor in patients with advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy. Hepatol Res. 2015;45:949–959. doi: 10.1111/hepr.12436. [DOI] [PubMed] [Google Scholar]
- 78.Uchida K, Levi D, Nishida S, Selvaggi G, Tekin A, Taizo H, Garcia M, Feun L, Tzakis AG. Recurrence pattern and prognosis after liver transplant for hepatocellular carcinoma in the MELD ERA. Am J Transplant. 2012;12:199–200. [Google Scholar]
- 79.Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q, Chen GH. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0025295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Wang K, Ling Q, Xu X, Wei Q, Chen J, Wu L, Zheng S. Does neutrophil to lymphocyte ratio improve hangzhou criteria for predicting tumor recurrence after liver transplantation? Liver Transplant. 2013;19:S280–S281. [Google Scholar]
- 81.Wang Q, Blank S, Fiel MI, Kadri H, Luan W, Warren L, Zhu A, Deaderick PA, Sarpel U, Labow DM, Hiotis SP. The Severity of Liver Fibrosis Influences the Prognostic Value of Inflammation-Based Scores in Hepatitis B-Associated Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22:1125–1132. doi: 10.1245/s10434-015-4598-9. [DOI] [PubMed] [Google Scholar]
- 82.Wang W, Ye Y, Wang T, Zhang F, Geng L, Yu J, Zhou L, Yan S, Zheng S. Prognostic prediction of male recipients selected for liver transplantation: With special attention to neutrophil-lymphocyte ratio. Hepatol Res. 2015 doi: 10.1111/hepr.12633. [DOI] [PubMed] [Google Scholar]
- 83.Wei K, Wang M, Zhang W, Mu H, Song TQ. Neutrophil-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma undergoing TAE combined with Sorafenib. Med Oncol. 2014:31. doi: 10.1007/s12032-014-0969-5. [DOI] [PubMed] [Google Scholar]
- 84.Weinmann AJ, Koch S, Sprinzl MF, Galle PR, Wörns M. Prognostic significance of inflammation based scores for patients with hepatocellular carcinoma treated with sorafenib in a western collective. Hepatology. 2015;62:400A–401A. [Google Scholar]
- 85.Xiao GQ, Yang JY, Yan LN. Combined Hangzhou criteria with neutrophil-lymphocyte ratio is superior to other criteria in selecting liver transplantation candidates with HBV-related hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:588–595. doi: 10.1016/s1499-3872(15)60416-7. [DOI] [PubMed] [Google Scholar]
- 86.Xu X, Chen W, Zhang L, Miao R, Zhou Y, Wan Y, Dong Y, Liu C. Prognostic significance of neutrophil to lymphocyte ratio in patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. Chin Med J (Engl) 2014;127:4204–4209. [PubMed] [Google Scholar]
- 87.Xue TC, Jia QA, Ge NL, Chen Y, Zhang BH, Ye SL. Imbalance in systemic inflammation and immune response following transarterial chemoembolization potentially increases metastatic risk in huge hepatocellular carcinoma. Tumour Biol. 2015;36:8797–8803. doi: 10.1007/s13277-015-3632-7. [DOI] [PubMed] [Google Scholar]
- 88.Yamamura K, Sugimoto H, Kanda M, Yamada S, Nomoto S, Nakayama G, Fujii T, Koike M, Fujiwara M, Kodera Y. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 2014;21:682–688. doi: 10.1002/jhbp.114. [DOI] [PubMed] [Google Scholar]
- 89.Yang X, Zhou S, Wen H, Li W, Li Z, Wu G, Sun Y, Wu H, Xu G. Preoperative neutrophil-lymphocyte ratio as a prognostic predictor after transarterial chemoembolization for HBV-associated hepatocellular carcinoma. Chin J Radiol. 2015;49:769–773. [Google Scholar]
- 90.Yang Z, Zhang J, Lu Y, Xu Q, Tang B, Wang Q, Zhang W, Chen S, Lu L, Chen X. Aspartate aminotransferase-lymphocyte ratio index and systemic immune-inflammation index predict overall survival in HBV-related hepatocellular carcinoma patients after transcatheter arterial chemoembolizations. Oncotarget. 2015;6:43090–43098. doi: 10.18632/oncotarget.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yip V, Fenwick S, Malik H, Ghaneh P, Sarno G, Wu A, Terlizzo M, Poston G. Resection of hepatocellular carcinoma arising in noncirrhotic/non-fibrotic liver. HPB. 2011;13:100–101. [Google Scholar]
- 92.Yoshizumi T, Harimoto N, Itoh S, Okabe H, Kimura K, Uchiyama H, Ikegami T, Ikeda T, Maehara Y. Living Donor Liver Transplantation for Hepatocellular Carcinoma within Milan Criteria in the Present Era. Anticancer Res. 2016;36:439–445. [PubMed] [Google Scholar]
- 93.Yoshizumi T, Ikegami T, Toshima T, Harimoto N, Uchiyama H, Soejima Y, Yamashita Y, Shirabe K, Maehara Y. Two-step selection criteria for living donor liver transplantation in patients with hepatocellular carcinoma. Transplant Proc. 2013;45:3310–3313. doi: 10.1016/j.transproceed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Yoshizumi T, Ikegami T, Yoshiya S, Motomura T, Mano Y, Muto J, Ikeda T, Soejima Y, Shirabe K, Maehara Y. Impact of tumor size, number of tumors and neutrophil-to-lymphocyte ratio in liver transplantation for recurrent hepatocellular carcinoma. Hepatol Res. 2013;43:709–716. doi: 10.1111/hepr.12016. [DOI] [PubMed] [Google Scholar]
- 95.Young AL, Adair R, Prasad KR, Toogood GJ, Lodge JP. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg. 2012;214:174–183. doi: 10.1016/j.jamcollsurg.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, Gong F, Li L, Zhao M, Song J. Diabetes mellitus and the neutrophil to lymphocyte ratio predict overall survival in non-viral hepatocellular carcinoma treated with transarterial chemoembolization. Oncol Lett. 2014;7:1704–1710. doi: 10.3892/ol.2014.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang W, Zhao G, Wei K, Zhang Q, Ma W, Wu Q, Zhang T, Kong D, Li Q, Song T. Adjuvant sorafenib therapy in patients with resected hepatocellular carcinoma: evaluation of predictive factors. Med Oncol. 2015:32. doi: 10.1007/s12032-015-0549-3. [DOI] [PubMed] [Google Scholar]
- 98.Zheng YB, Zhao W, Liu B, Li Y, Hu BS, Lu LG. Prognostic significance of blood neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing TACE. Chinese Journal of Interventional Imaging and Therapy. 2013;10:523–526. [Google Scholar]
- 99.Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, Li Y, Hu BS. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013;14:5527–5531. doi: 10.7314/apjcp.2013.14.9.5527. [DOI] [PubMed] [Google Scholar]
- 100.Zhou D, Zhang Y, Xu L, Zhou Z, Huang J, Chen M. A monocyte/granulocyte to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Sci Rep. 2015;5:15263. doi: 10.1038/srep15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou DS, Xu L, Luo YL, He FY, Huang JT, Zhang YJ, Chen MS. Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization. World J Gastroenterol. 2015;21:5582–5590. doi: 10.3748/wjg.v21.i18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer. 2014:14. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: A meta-analysis. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu SD, Wang YY, Peng NF, Peng YC, Zhong JH, Qin HG, Xiang BD, You XM, Ma L, Li LQ. Preoperative Ratio of Neutrophils to Lymphocytes Predicts Postresection Survival in Selected Patients With Early or Intermediate Stage Hepatocellular Carcinoma. Medicine. 2016;95:e2722. doi: 10.1097/MD.0000000000002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordasp
- 106.Qi X, Liu L, Wang D, Li H, Su C, Guo X. Hepatic resection alone versus in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:36838–36859. doi: 10.18632/oncotarget.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qi X, Wang D, Su C, Li H, Guo X. Hepatic resection versus transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:18715–18733. doi: 10.18632/oncotarget.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qi X, Tang Y, An D, Bai M, Shi X, Wang J, Han G, Fan D. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014;48:450–457. doi: 10.1097/MCG.0000000000000008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.