Abstract
Malaria accounts for an enormous burden of disease globally, with Plasmodium falciparum accounting for the majority of malaria, and P. vivax being a second important cause, especially in Asia, the Americas and the Pacific. During infection with Plasmodium spp., the merozoite form of the parasite invades red blood cells and replicates inside them. It is during the blood-stage of infection that malaria disease occurs and, therefore, understanding merozoite invasion, host immune responses to merozoite surface antigens, and targeting merozoite surface proteins and invasion ligands by novel vaccines and therapeutics have been important areas of research. Merozoite invasion involves multiple interactions and events, and substantial processing of merozoite surface proteins occurs before, during and after invasion. The merozoite surface is highly complex, presenting a multitude of antigens to the immune system. This complexity has proved challenging to our efforts to understand merozoite invasion and malaria immunity, and to developing merozoite antigens as malaria vaccines. In recent years, there has been major progress in this field, and several merozoite surface proteins show strong potential as malaria vaccines. Our current knowledge on this topic is reviewed, highlighting recent advances and research priorities.
Keywords: Plasmodium falciparum, Plasmodium vivax, merozoites, invasion, immunity, vaccines, antibodies
The authors summarize current knowledge of merozoite surface proteins of malaria parasites; their function in invasion, processing of surface proteins before, during and after invasion, their importance as targets of immunity, and the current status of malaria vaccines that target merozoite surface proteins.
INTRODUCTION
Malaria remains one of the world's leading causes of morbidity and mortality with an estimated 600 000 deaths and 200 million cases annually (World Health Organization 2014). There are several Plasmodium spp. that cause malaria in humans, with Plasmodium falciparum accounting for the majority of severe malaria and deaths, particularly in Africa. Plasmodium vivax is a second important cause of malaria with most of the burden occurring in Asia. The true burden of P. vivax infections is unclear, with estimates of between 71 and 391 million cases per year (Price et al.2007). Other causes of human malaria include P. malariae and P. ovale (recently proposed to exist as two species (Sutherland et al.2010), which account for a minor proportion of the global malaria burden. Plasmodium. knowlesi is a zoonotic infection transmitted from macaques to humans by infected mosquitoes in parts of South East Asia; direct human-to-human transmission appears rare (Singh and Daneshvar 2013).
To initiate infection in humans, sporozoite forms of Plasmodium parasites are injected into the skin by infected Anopheles mosquitoes and then migrate to the liver and infect hepatocytes. Over 7–10 days, parasites develop and divide into merozoites that are released into the bloodstream. An important feature of P. vivax is the occurrence of dormant hypnozoites in the liver that can reactivate weeks, months or years later to initiate new episodes of blood-stage infection. This does not occur with P. falciparum. During the blood-stage of infection with Plasmodium spp., the merozoite form of the parasite invades red blood cells (RBCs; reticulocytes and mature erythrocytes) and replicates inside them. Cycles of blood-stage replication take approximately 48 h for P. falciparum and P. vivax, but only 24 h for P. knowlesi. It is during the blood-stage of infection that malaria disease occurs and understanding molecular and cellular events involved in merozoite invasion and the host immune responses to merozoite antigens is crucial for the development of malaria vaccines and novel therapeutics.
Over the past 10 years, there has been substantial progress in reducing the enormous burden of malaria globally through the use of interventions such as insecticide-treated bed-nets, improved access to early diagnosis and effective treatment of malaria including the use of highly effective artemisinin combination therapy (ACT). However, emerging resistance to ACTs, increasing mosquito resistance to insecticides, and evidence of rebound increases of malaria in some regions, highlights the need for effective vaccines, new antimalarial agents and other novel control interventions. Currently, there is no malaria vaccine available. A vaccine targeting sporozoites, known as RTS,S has completed phase three trials in African children and demonstrated only modest efficacy of 29%–36% (when a booster dose was given, and varying by age group) (RTSS Clinical Trials Partnership 2015). The development of highly efficacious vaccines remains a key long-term goal. A number of merozoite antigens are promising vaccine candidates with some showing partial efficacy in clinical trials.
In this review, we will summarize recent advances in understanding proteins present on the surface of the merozoite and their role in host cell invasion and their processing before, during and after invasion. Furthermore, we will highlight the expanding knowledge on protective human immune responses to merozoite antigens and progress on developing merozoite surface antigens as vaccines. For the purposes of this review, we define merozoite surface proteins as those that are exposed on the surface of the merozoite any time prior to invasion, including proteins that relocate from organelles to the merozoite surface. Research on merozoite antigens has focussed heavily on P. falciparum as the major cause of morbidity and mortality; similar research on P. vivax has been greatly constrained by the inability to readily culture P. vivax in vitro, and available data on merozoite antigens are limited. Therefore, this review necessarily focuses on P. falciparum, including data on P. vivax and other human malaria pathogens where available.
Proteins on the merozoite surface and their role in RBC invasion
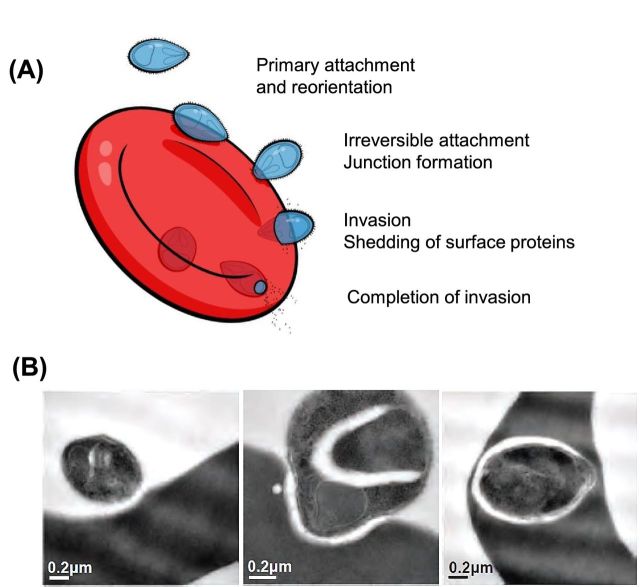
Early electron microscope images of Plasmodium merozoites revealed that they were covered in a ‘fuzzy’ fibrillar coat of surface proteins; remarkably, this coat appeared to be shed during RBC invasion (Fig. 1A) (Ladda, Aikawa and Sprinz 1969; Bannister et al.1975; Aikawa et al.1978; Langreth et al.1978). Since these initial observations, the composition and function of merozoite surface proteins (MSPs) has been of great interest because of their role in RBC invasion and potential as vaccine candidates (Richards and Beeson 2009) and, more recently, as drug targets for inhibiting blood-stage replication (Boyle, Wilson and Beeson 2013; Chandramohanadas et al.2014; Wilson et al.2015).
Figure 1.
‘Invasion of RBC by P. falciparum merozoites.’ (A) After release from schizonts, most merozoites are thought to invade RBCs within several minutes, although some may take substantially longer. Invasion commences with initial, or primary, reversible attachment of the merozoite to the RBC surface. The merozoite reorientates, if needed, so that it's apical end makes contact with the RBC surface. Secondary interactions then occur, mediating strong and irreversible attachment to the RBC, leading to the release of contents from the rhoptries and the formation of the tight junction. Merozoite invasion then proceeds via an actin-myosin motor, and processing of many merozoite surface proteins occurs. Invasion is completed by resealing of the RBC membrane, and completion of the processing and shedding of merozoite surface proteins (image modified from Richards and Beeson 2009). (B) Electron micrographs showing different stages in RBC invasion by a merozoite (from Boyle et al.2010b).
Proteins relevant to red blood cell (RBC) invasion are present on the merozoite surface, or contained within organelles known as rhoptries and micronemes at the apex of the merozoite. Merozoite surface proteins are tethered as either glycophosphatidylinositol (GPI)-anchored proteins, integral membrane proteins or as peripherally-associated proteins (held on the merozoite surface through interactions with membrane-bound proteins) (Table 1). Other merozoite proteins are maintained in the rhoptries and micronemes during schizont development, and then localize to the merozoite surface prior to, or soon after, merozoite egress from the schizont via a variety of mechanisms.
Table 1.
Merozoite surface proteins of P. falciparum.
| Function | Processing | Vaccine trials1 | Phase | |
|---|---|---|---|---|
| GPI-anchored merozoite surface proteins | ||||
| MSP1 | Thought to mediate initial attachment through binding of MSP142 to heparin-like proteoglycans or Band 3, as well as binding of the MSP1 complex to the RBC surface. MSP119 is carried into invaded RBC, and appears involved in intraerythrocytic development. MSP133 binds to S100 to supress inflammation and chemotaxis | Expressed as a high molecular mass protein (MW 180Kda) that then undergoes proteolytic process into 83-, 30-, 38-kDa and C-terminal 42-kDa fragment prior to egress, then further cleaved to MSP119 and MSP133 | (Ockenhouse et al.2006) | I |
| (Stoute et al.2007) | I | |||
| (Keitel et al.1999) | I | |||
| (Thera et al.2006) | I | |||
| (Genton et al.2003) (with MSP2 & RESA) | I/II | |||
| (Sheehy et al.2011) | Ia | |||
| (Saul et al.1999) (with MSP2 & RESA) | I | |||
| (Withers et al.2006) | Ib | |||
| (Lawrence et al.2000) (with MSP2 & RESA) | I/II | |||
| (Genton et al.2002) (with MSP2 & RESA) | I/2b | |||
| (Genton et al.2000) (with MSP2 & RESA) | I | |||
| (Hu et al.2008) (with AMA1) | I | |||
| (Ockenhouse et al.1998) (with AMA1 & RESA) | I/IIa | |||
| MSP2 | Unknown. Appears to be essential | Carried into erythrocyte then rapidly degraded | (McCarthy et al.2011) | I/IIb |
| (Saul et al.1999) (with MSP1 & RESA) | I | |||
| (Lawrence et al.2000) (with MSP1 & RESA) | I/II | |||
| (Genton et al.2002) (with MSP1 & RESA)) | I/2b | |||
| (Genton et al.2000) (with MSP1 & RESA) | I | |||
| (Genton et al.2003) (with MSP1 & RESA) | ||||
| (Sturchler et al.1995) (with CSP) | I/II | |||
| MSP4 | Unknown. Appears to be essential | Carried into erythrocyte and maintained during early ring stage | N | |
| MSP5 | Unknown. Not essential | Unknown | N | |
| MSP10 | Unknown | Processed from 80Kda to 36Kda form | N | |
| Pf12 | Unknown. Forms a heterodimer with Pf41 | Cleaved from the surface during invasion | N | |
| Pf38 | Unknown | Unknown | N | |
| Pf92 | Unknown | Unknown | N | |
| Pf113 | Unknown | Unknown | N | |
| Peripheral surface proteins | ||||
| MSP9 (ABRA) | Bind to 5ABC domain of Band 3 | Unknown | N | |
| MSP3 | Unknown. Forms a complex with MSP1 and other proteins | Cleaved from MSP362 by PfSUB1 into MSP344 and shed at tight junction during invasion | (Audran et al.2005) | I |
| (Esen et al.2009) (with GLURP) | Ia | |||
| (Sirima et al.2007) (Sirima, Cousens and Druilhe 2011) | Ib | |||
| (Mordmuller et al.2010) (with GLURP) | Ib | |||
| (Belard et al.2011) (with GLURP) | Ib | |||
| MSP6 | Unknown function. Forms protein complex with MSP1 | Cleaved from the surface during invasion | N | |
| MSP7 | Unknown function. Forms protein complex with MSP1 | Produced as a 48kDa precursor, then processed into MSP733,then processed into MSP722 by PfSUB1 and shed at tight junction during invasion | N | |
| GLURP | Unknown | Shed from the merozoite surface during invasion | (Hermsen et al.2007) | Ia |
| (Esen et al.2009) (with MSP3) | Ia | |||
| (Mordmuller et al.2010) (with MSP3) | Ib | |||
| (Belard et al.2011) (with MSP3) | Ib | |||
| SERA3 | Unknown | Unknown | N | |
| SERA4 | Unknown | Shed at tight junction during invasion | N | |
| SERA5 | Unknown. May function as a protease | Processed from 120kDa precursor into P47 and P73 fragments. P73 is further processed into P50, P6 and P18 fragments | (Horii et al.2010) | I |
| (Palacpac et al.2013) | II | |||
| SERA6 | Unknown. May function as a protease | Unknown | N | |
| Pf41 | Unknown. Complexed with Pf12 on the merozoite surface | Unknown | N | |
| Microneme proteins released onto the merozoite surface | ||||
| AMA1 | Binds to RON2 to mediate the tight junction formation and irreversible attachment | Processed from 83kDa precursor into 66 kDa species and partially cleaved during invasion | ||
| (Sheehy et al.2012b) | Ia | |||
| (Roestenberg et al.2008) | I | |||
| (Saul et al.2005) | I | |||
| (Polhemus et al.2007) | I | |||
| (Mullen et al.2008) | I | |||
| (Thera et al.2008) | I | |||
| (Dicko et al.2008) | I | |||
| (Hu et al.2008) (with MSP1) | I | |||
| (Thompson et al.2008) (with CSP) | IIa | |||
| (Genton et al.2007) (with CSP) | I | |||
| (Thera et al.2011) | II | |||
| (Sagara et al.2009a) | II | |||
| (Ockenhouse et al.1998) (with MSP1 & SERA) | I/IIa | |||
| EBA140 | Binds to glycophorin C | Shed from the surface during invasion | N | |
| EBA175 | Binds to glycophorin A, triggers release of rhoptry proteins to merozoite surface | Cleaved by ROM protease; shed from the surface during invasion | (El Sahly et al.2010) | I |
| EBA181/JESEBL | Binds to sialic acids on RBCs and band 4.1 | Shed from the surface during invasion | N | |
| EBL1 | Binds to glycophorin B | Shed from the surface during invasion | N | |
| MTRAP | Binds to aldolase, may be involved in merozoite reorientation or formation of tight junction | Unknown | N | |
| PTRAMP | Binds to aldolase and semaphorin A, may be involved in merozoite reorientation or formation of tight junction | Unknown | N | |
| GAMA | Binds to a non-sialylated receptor on the erythrocyte surface | Expressed as 85kDa precursor then processed to P37-P49 dimer and P49 further processed into P42 and residual stub. The P37-P42 dimer is shed during invasion | N | |
| CyRPA | Tethers PfRH5-PfRipr protein complex to GPI anchor | Unknown | N | |
| PfRipr | Forms complex with PfRH5 and CyRPA | Unknown | N | |
| Rhoptry proteins released onto the surface | ||||
| PfRH1 | Binds to sialic acids (unknown receptor) on the RBC surface to initiate calcium signaling leading to release of EBA175 | Unknown | N | |
| PfRH2a | Binds to unknown receptor on RBC surface | Unknown | N | |
| PfRH2b | Binds to unknown receptor on RBC surface (receptor Z) | Unknown | N | |
| PfRH4 | Binds to CR1 to mediate invasion | Unknown | N | |
| PfRH5 | Binding to basigin receptor on RBCs | Unknown | N | |
| RAMA | Binds to unknown receptor on erythrocytes | Produced as 170kDa protein during trophozoite stage then processed into a 60kDa form | N | |
| RALP1 | Binds to unknown receptor on erythrocyte surface | Expressed as 90kDa protein during late schizonts, then cleaved to 50kDa and released from the rhoptries during invasion | N | |
| Others | ||||
| SUB1 | Protease that processes merozoite surface proteins prior to invasion, including MSP1, MSP6, MSP7, and SERAs. Released from exonemes | Unknown | N | |
| SUB2 | Protease involved in shedding of merozoite surface proteins during invasion | Unknown | N | |
Only vaccine trials conducted in humans are indicated, and whether the trial was a phase I or II.
Once released from schizonts, merozoites may take several minutes before establishing contact with the surface of a RBC and commencing invasion. After primary attachment of the merozoite to the RBC surface, invasion by P. falciparum occurs within approximately 30 seconds (Gilson and Crabb 2009) and involves a sequence of extracelluar recognition events (Weiss et al.2015) (Fig. 1A and B). Primary merozoite attachment is thought to be mediated by GPI-anchored MSPs; however, specific receptor–ligand interactions remain elusive. The subsequent binding of merozoites is dependent on the erythrocyte binding antigen (EBA) and reticulocyte binding-like homologous (PfRH) protein families. Both EBA and PfRH ligands are integral-membrane proteins that allow a number of alternate pathways for merozoite invasion [reviewed in: (Tham, Healer and Cowman 2012)]. The diversification of RBC binding pathways appears to have been driven by a combination of immune selection (Persson et al.2008) and human RBC polymorphisms (Maier et al.2003). As such, while their collective role in invasion is essential, there is a level of redundancy among protein family members. After merozoites have bound to RBCs, a smaller member of the PfRH family, PfRH5, anchors the merozoite to the RBC by binding to the basigin protein receptor (Crosnier et al.2011). This anchoring appears to allow apical membrane antigen 1 (AMA1), an integral membrane bound protein, to initiate tight junction formation by binding to RON2 (part of the RON complex), which are secreted from merozoite organelles and translocated to the RBC surface prior to invasion [based on work on both P. falciaparum (Lamarque et al.2011; Srinivasan et al.2011) and Toxoplasma gondii models (Besteiro et al.2009; Tonkin et al.2011; Lamarque et al.2014). Finally, RBC invasion is driven by an actin-myosin type motor (Baum et al.2006).
The kinetics of merozoite invasion is only partially understood and largely limited to in vitro studies (Boyle, Wilson and Beeson 2013; Weiss et al.2015). From these studies, 80% of merozoites invade within 10 m of co-incubation with RBCs, but the remainder can invade after prolonged periods post-egress (Boyle et al.2010b). Similarly, the invasive capacity of merozoites appears to decline relatively quickly after schizont rupture with an estimated half-life of 8 mins at 37°C and 15 mins at room temperature, however a subpopulation of merozoites can retain their invasive capacity for extended periods (Boyle et al.2010b; Boyle, Wilson and Beeson 2013). Whether in vitro kinetics are relevant in vivo is currently unknown, nor do we know the basis for the decline in invasion capacity after egress observed in vitro (Boyle, Wilson and Beeson 2013). This knowledge of merozoite survival and invasion kinetics is relevant to understanding immune exposure, and the development of vaccines and therapeutics targeting merozoite invasion (reviewed in Boyle, Wilson and Beeson 2013).
GPI-anchored merozoite surface proteins
The fibrillar surface coat of merozoites appears to be largely composed of glycosylphosphatidy inositol (GPI)-anchored proteins, with integral membrane proteins and the peripherally-associated surface proteins representing a minor portion of the total surface protein (Gilson et al.2006). To date numerous GPI anchored merozoite surface proteins (MSPs) have been identified: these include proteins formally known as MSPs (MSP1, MSP2, MSP4, MSP5 and MSP10) and the 6-cysteine domain family proteins, Pf92, Pf38 and Pf12 (Sanders et al.2005). In addition, other GPI-anchored proteins, rhoptry associated membrane antigen (RAMA) (Topolska et al.2004a,b), microneme associated cysteine-rich protective antigen (CyRPA) (Reddy et al.2015) and GPI-anchored micronemal antigen (GAMA) (Arumugam et al.2011) migrate to the merozoite surface from organelles prior to, or during, RBC invasion. While many of these proteins contain cysteine-rich EGF domains and a variety of other globular domains predicted to mediate receptor-binding functions during the primary recognition and attachment to RBCs, very little experimental evidence exists to support this function. Exceptionally little is known about the functional interactions of GPI-anchored surface proteins; clearly this is an area ready for major advances. Most merozoite surface GPI-anchored proteins appear to play an essential role/s in merozoite invasion, as most are reported to be refractory to genetic disruption (MSP5, Pf38 and Pf12 have been successfully disrupted) (Sanders et al.2006; Arumugam et al.2011; Reddy et al.2015).
Merozoite Surface Protein 1
Merozoite Surface Protein 1 (MSP1) is the most abundant of all GPI-anchored surface proteins in terms of copy number (Gilson et al.2006). MSP1 is expressed as a high molecular mass protein (MW 180 kDa) that undergoes extensive proteolytic processing prior to egress of the merozoite from the schizont. This processing modifies the secondary struction of MSP1 so that it can bind spectrin and mediate RBC rupture (Das et al.2015). Following processing, the MSP1 protein complex consists of four polypeptide fragments of MSP1, the 83 kDa N-terminal fragment (MSP183), two internal 30 and 38 kDa fragments (MSP130 & MSP138) and the GPI- anchored C-terminal 42 kDa fragment (MSP142) along with associating proteins MSP6 and MSP7 (Stafford et al.1996; Trucco et al.2001; Pachebat et al.2007) It has been suggested that MSP1 has a role in initial contact of the merozoite with the RBC possibly by binding the cysteine rich EGF-like domains of MSP119, via some type of proteoglycan with heparin-like side chains or similar structure (Boyle et al.2010a), or Band 3 in complex with other merozoite antigens (Goel et al.2003; Kariuki et al.2005). It has also recently been reported that the N-terminal region of MSP183 interacts with glycophorin A as an essential mediator of invasion (Baldwin et al.2015). During invasion MSP1 undergoes further processing with the MSP142 fragment being cleaved to MSP119 and MSP133 (Blackman and Holder 1992; Stafford et al.1994). The MSP119 GPI anchored fragment is carried into the invaded RBC (Blackman et al.1990) where it localizes to the developing food vacuole during ring/trophozoite formation (Dluzewski et al.2008). MSP1 is broadly regarded as dimorphic, but it is highly polymorphic with substantial polymorphisms across the protein, particularly in the MSP133 (which has two allelic groups) and MSP1-block 2 regions (which as three allelic groups), whereas the C-terminal MSP119 region is relatively conserved across P. falciparum isolates (Miller et al.1993; Barry et al.2009; Holder 2009).
Merozoite surface protein 2
The second most abundant GPI anchored merozoite surface protein, by copy number, is MSP2, which is an approximately 25 kDa protein with an observed weight of 40–50 kDa (Gilson et al.2006). As is common for several other MSPs, MSP2 is dimorphic, existing in two main allelic forms (3D7-like and FC27-like), sharing N- and C-terminal regions, with strain-specific variable regions (Fenton et al.1991). The strain-specific region is made up of repeating units; 3D7-like forms contain repeating units of Gly, Ser and Ala, while FC27-like forms contain 32-, 12- and 8-mer sequence repeats. Both forms of MSP2 are largely unstructured, but full length recombinant proteins form fibrils under physiological conditions (Adda et al.2009). Fibril formation is mediated through the N-terminal region (Low et al.2007) and this region may also have membrane interaction properties (Zhang et al.2008). It is unknown whether native MSP2 forms fibril-like or other complexes; however, there is some evidence that oligomers of MSP2 are found on the surface of merozoites (Adda et al.2009), with a number of interactions between MSP2 molecules being hypothesized (Yang et al.2010). Recent studies suggest that the N-terminal region of MSP2 may interact with the lipid membrane of the merozoite (MacRaild et al.2012). MSP2 appears to be essential for invasion and is retained on the surface during invasion and degraded soon after invasion is complete (Boyle et al.2014). However, its precise role is unknown, and no receptor–ligand interactions or binding of MSP2 to RBCs have been described.
Merozoite surface proteins 4 and 10
MSP4 and MSP10 are similar to MSP1 in that they contain double EGF-like domains adjacent to their C-terminal GPI anchor attachment points, which are highly immunogenic (Black et al.1999; Black et al.2003). MSP4 is expressed as a 40 kDa protein that remains on the surface of merozoites during invasion and can be detected on the developing intraerythrocytic parasite (Boyle et al.2014). Its definitive role in merozoite invasion is unknown, but it may aid parasite development post-invasion. MSP10 is initially expressed as an 80 kDa protein and is subsequently processed down to a smaller 36 kDa form which localizes to the apical end of merozoites (Black et al.2003). No RBC receptor for either MSP4 or MSP10 has been identified.
Merozoite surface protein 5
In P. falciparum, MSP5 is closely related to MSP4 and is located between the genes encoding MSP2 and MSP4 on chromosome 2 of P. falciparum (Marshall, Tieqiao and Coppel 1998). Similar to MSP4, MSP5 encodes a protein of 272 amino acids in length and contains a GPI anchor-attachment signal and EGF-like domains at the C-terminus (Wang et al.1999). MSP5 has been localized to the surface of merozoites (Wu et al.1999), but its function is not known. MSP5 can be disrupted in P. falciparum with no apparent growth defect suggesting that it is not essential to parasite growth in vitro (Sanders et al.2006). However, MSP5 appears to be highly conserved across P. falciparum isolates (Wu et al.1999; Polson et al.2005) and PfMSP5 antibody levels have been reported to be significantly associated with reduced incidence of clinical malaria (Perraut et al.2014), making it a potential candidate for a strain-transcending blood-stage P. falciparum vaccine. Further, antibodies to PfMSP5 cross react with PvMSP5, suggesting that it is a potential cross-species vaccine target (Woodberry et al.2008).
Pf12, Pf38 and Pf92
Pf12, Pf38 and Pf92 belong to a family of 6-cysteine domain family proteins. All three GPI-anchored proteins are expressed during blood-stage replication and are found on the surface of the merozoite (Sanders et al.2006). While the role of these proteins in merozoite invasion is unknown, 6-cys domain proteins have essential roles in other stages of the parasite life-cycle and are implicated in mediating the development of transmission stages (van Dijk et al.2010; Sala et al.2015) and recognition and invasion of hepatocytes (Ishino, Chinzei and Yuda 2005). It has been suggested that 6-cys domain proteins have structural similarity to Toxoplasma SAG proteins (Gerloff et al.2005) that may interact with heparin-like surface receptors (Jacquet et al.2001; He et al.2002).
GPI-anchored rhoptry proteins released onto the merozoite surface
RAMA
RAMA is expressed as 170 kDa protein in early trophozoites where it initially localizes to the golgi membrane (Topolska et al.2004a). It is later proteolytically processed to yield a smaller p60 kDa form that is bound to the inner membrane of the rhoptry via its C-terminal GPI anchor (Topolska et al.2004a). Following egress, p60 RAMA is released from the surface, binds to an unidentified receptor on the RBC surface and becomes associated with the parasitophorous vacuole in early ring stage parasites (Topolska et al.2004a).
GPI-anchored microneme proteins released onto the merozoite surface
Cysteine-rich protective antigen
The recently described CyRPA is expressed as a 35 kDa protein in the micronemes and subsequently migrates to the apical end of the merozoite surface. CyRPA binds PfRH5 and PfRipr proteins to form a 200 kDa complex involved in binding basigin (mediated by PfRH5) on the RBC surface (Reddy et al.2015). While CyRPA does not exhibit RBC binding activity, antibodies against CyRPA appear to inhibit merozoite invasion by blocking its interaction with PfRH5 and PfRipr, which are peripheral-associated proteins that lack transmembrane domains and rely on CyRPA to tether them to the merozoite surface. Sequence analysis suggests that CyRPA is not under immune selection pressure, and is highly conserved, with only a single polymorphism detected across 18 P. falciparum strains (Dreyer et al.2012), These properties, combined with the fact that antibodies raised against recombinant CyRPA are capable of inhibiting merozoite invasion (Reddy et al.2015), make CyRPA an attractive vaccine candidate for further investigation.
GPI-anchored micronemal antigen
GAMA is an 85 kDa micronemal protein and, bioinformatic analysis suggests that GAMA subsequently migrates to the surface of merozoites where it undergoes primary and secondary processing events (Haase et al.2008; Hinds et al.2009). This generates two species of GAMA heterodimers: p37+p49 and p37+p42, which are shed from the merozoite, with only a short GPI anchored ‘stub’ left bound to the surface (Arumugam et al.2011). Studies suggest that motifs in the C-terminal third of the protein mediate binding to a non-sialylated receptor on the RBC surface (Arumugam et al.2011). Because of this, GAMA has been proposed to be involved in a sialic acid-independent invasion pathway. Antibodies to the C-terminal portion of GAMA inhibit merozoite invasion, this inhibition is significantly enhanced by the addition of antibodies to EBA 175 (Arumugam et al.2011).
Transmembrane-anchored merozoite surface proteins
Integral membrane proteins anchored to the merozoite surface by C-terminal transmembrane domains can be broadly classified into two separate classes: (i) alternative invasion ligands that are collectively essential for RBC invasion, but mediate overlapping and redundant functions, and are therefore individually dispensable; and (ii) essential invasion ligands that have non-redundant roles in RBC invasion. Defining invasion pathways largely relies on studying invasion into RBCs that have either been treated with enzymes to modify or cleave surface proteins or that have mutant or absent host receptors (Egan et al.2015). The most commonly used enzymes to study RBC receptors are neuraminidase, which removes sialic acid residues from sialoglycoproteins, and trypsin or chymotrypsin, which cleave surface proteins at specific peptide moieties. In P. falciparum, the alternative invasion ligands consists of two families, the EBAs (EBA175, EBA140, EBA181 and EBL1) and the PfRH family (PfRH1, PfRH2a, PfRH2b and PfRH4). PfRH5 is an additional member of this family, but it differs in that it appears to play an essential non-redundant role, and it is not an integral membrane protein (Baum et al.2009). All the EBA and PfRH proteins (except PfRH5) can be disrupted in P. falciparum, resulting in changes in invasion phenotype (Reed et al.2000; Duraisingh et al.2003; Maier et al.2003) and susceptibility to human antibodies (Persson et al.2008; 2013). To date, only some RBC receptors that bind these ligands have been identified.
Erythrocyte binding antigens
The EBAs are microneme-derived proteins that are released onto the merozoite surface where they localize to the apical end of merozoites. EBA175, 140 and 181 each have N-terminal Duffy binding-like domains termed F1 and F2, which share a conserved structure and are involved in binding RBC receptors (Adams et al.1992) (Adams et al.2001) and regulating the strength and specificity of the ligand-receptor interaction (Maier et al.2009). EBA175 binds to glycophorin A, a sialoglycoprotein on the RBC surface (Sim et al.1994) whereas EBA140 binds to glycophorin C (Lobo et al.2003; Maier et al.2003), EBL1 to glycophorin B (Mayer et al.2009) and EBA181 to sialic residues on the RBC surface and to band 4.1 (Gilberger et al.2003; Lanzillotti and Coetzer 2006).
PfRH proteins
PfRH1, 2 and 4 are large (>200 kDa) rhoptry-derived proteins. While the members of the PfRH protein family share a low degree of sequence homology, modelling studies based upon the solved crystal structure of the peripherally associated PfRH5 suggest that the membrane bound PfRH proteins adopt a similar ‘Kite-like’ conformation with receptor binding sites located at their tip (Wright et al.2014). PfRH1 appears to function earlier than other members, and binds to an unknown sialated receptor protein on the RBC surface (Rayner et al.2000; Rayner et al.2001) triggering a calcium flux in merozoites that leads to the release of EBA 175 from the micronemes (Gao et al.2013; Singh, More and Chitnis 2014). PfRH2a and PfRH2b are closely related proteins which share a high degree of sequence homology throughout the N-terminal and central domains of each protein, but contain highly divergent C-terminal cytoplasmic domains (Rayner et al.2000; Duraisingh et al.2003; Dvorin et al.2010). Both PfRH2a and PfRH2b appear to bind to similar receptors (Triglia et al.2011). The receptor for PfRH2b has been labelled ‘receptor Z’, which is resistant to cleavage by trypsin (Duraisingh et al.2003). PfRH4 binds to complement receptor 1 (CR1) (Tham et al.2011). Several studies have shown that P. falciparum can use alternative invasion receptor-ligand pathways by modulating the expression and utilization of EBA and PfRH proteins; for example, in-vitro loss of EBA175 has been shown to induce a compensatory up-regulation of PfRH4 (Stubbs et al.2005), and the loss of PfRH2b function was found to lead to an increased reliance on sialic acid dependent invasion ligands (Duraisingh et al.2003).
Apical membrane antigen 1
Apical membrane antigen 1 (AMA1) is a highly structured type-1 integral membrane protein (88 kDa) and is proteolytically processed into a smaller 66 kDa species within the micronemes prior to relocalization to the merozoite surface (Narum and Thomas 1994). The exposed ectodomain of AMA1 contains 16 cysteine residues which form 8 disulphide bonded pairs and mediate the formation of 3 defined domains, each with several protruding loops that are hot spots for polymorphic residues (Hodder et al.1996). The folded ectodomain also forms a distinct hydrophobic cleft which is involved in binding its parasite-derived receptor, PfRON2, after the translocation of the RON2/4/5 complex from the rhoptries into the RBC membrane (Besteiro et al.2009; Lamarque et al.2011; Srinivasan et al.2011; Tonkin et al.2011; Lamarque et al.2014). PfRON2 contains several internal transmembrane domains and spans both sides of the RBC membrane (Lamarque et al.2011). This results in a C-terminal domain of PfRON2 adopting an exposed loop conformation on the surface of RBCs that can directly interact with the hydrophobic cleft of AMA1 (Lamarque et al.2011; Srinivasan et al.2011; Tonkin et al.2011). In contrast PfRON4 and PfRON5 do not contain transmembrane domains and remain exclusively within the RBC cytosol while in complex with PfRON2 (Chen et al.2011). The interaction between AMA1 and the RON complex has been proposed to initiate the formation of the moving junction (Lamarque et al.2011). However, the process by which this occurs remains unclear. While the cytoplasmic tail of AMA1 contains indispensable amino acid sequence motifs known to interact with aldolase (Treeck et al.2009) there is no evidence that AMA1 directly engages with the actin-myosin invasion machinery of the merozoite. It is also proposed that the phosphorylation of Ser610 in the cytoplasmic domain of AMA1 by protein kinase A may also be a signalling event to coordinate merozoite invasion (Treeck et al.2009; Leykauf et al.2010). Recent AMA1 knock-down (Giovannini et al.2011) and knock out (Bargieri et al.2013) studies in Toxoplasma gondii and the rodent malaria P. berghei have questioned whether AMA1 plays an essential role in merozoite invasion. However, in P. falciparum AMA1 cannot be deleted (Triglia et al.2011), with antibodies and AMA1-binding peptides strongly inhibiting invasion (Lamarque et al.2011; Srinivasan et al.2011; Tonkin et al.2011; Lamarque et al.2014) and the knock-down of AMA1 leading to loss of invasion (Yap et al.2014), strongly suggesting that in P. falciparum AMA1 is an essential invasion ligand.
MTRAP and PTRAMP
In addition to AMA1, two non-redundant transmembrane MSPs have been identified to date: merozoite- specific thrombospondin-related anonymous protein (MTRAP) and the thrombospondin- related apical membrane protein (PTRAMP). MTRAP and PTRAMP both contain thrombospondin repeat (TSR)-like domains and are part of the TSR superfamily, which includes sporozoite expressed proteins such as circumsporozoite surface protein (CSP) (Plassmeyer et al.2009) and thrombospondin-related anonymous protein (TRAP) (Tucker 2004; Tossavainen et al.2006). On sporozoites, TRAP plays a role in mediating gliding motility by engaging the actin-myosin motor via its cytoplasmic domain and hepatocytes surface receptors through its extracellular TSR domains (Menard 2000; Buscaglia et al.2003; Morahan, Wang and Coppel 2009), and it is thought that the TSR domains of MTRAP and PTRAMP may perform similar functions. MTRAP binds to semaphorin-7A on RBCs (Bartholdson et al.2012). Neither MTRAP or PTRAMP can be disrupted (Thompson et al.2004; Baum et al.2006), and the cytoplasmic domains of both proteins bind aldolase, an actin binding protein involved in the moving junction, suggesting that they may play an essential role in merozoite reorientation or tight junction formation (Baum et al.2006), however recent imaging studies suggest that MTRAP does not play a direct role in entry of the merozoite into the RBC (Riglar et al.2015).
Peripherally-associated merozoite surface proteins
The peripherally associated merozoite surface proteins consist of a diverse array of proteins that do not have transmembrane domains or GPI anchors to directly link them to the merozoite surface; these proteins include MSP7, the MSP6/MSP3-family of proteins, SERA proteins, PfRH5/PfRipr, RALP1 and GLURP.
MSP3-MSP6 family of proteins
MSP6 is expressed as a 36 kDa protein that is released on to the merozoite surface where it then associates with the MSP138 component of the MSP1 complex prior to merozoite invasion, via a leucine zipper-like domain present at its C-terminus (Kauth et al.2003; Kauth et al.2006). MSP6 is also known as MSP3.2, and is a member of the MSP3-like multigene family that contains 8 members, all of which are arranged contiguously on chromosome 10 of P. falciparum. While the function of MSP6 is not known, MSP6 derived peptides have been reported to bind to the surface of RBCs (Lopez et al.2006). All members of the MSP3 gene family (MSP3.1-MSP3.8) contain the signature N-terminal amino acid sequence NLRNA/G, while only 6 members share a similar C-terminal sequence organization (Singh et al.2009). While it does not appear that the two MSP3 members lacking the conserved C-terminal structure (MSP3.5 and MSP3.6) are expressed during the erythrocytic stages, the remaining 6 members (MSP3/MSP3.1, MSP6/MSP3.2, MSP3.3, PfMSPDBL1/MSP3.4, MSP3.7 and MSP3.8) all appear to be simultaneously expressed as peripherally- associated merozoite surface proteins (Singh et al.2009). The function of the MSP3-family proteins is not known, however, several members of the MSP3 gene family have been genetically truncated (MSP3/MSP3.1, MSP3.3 and MSP3.6) with little or modest effects on in vitro parasite growth (Mills et al.2002), suggestion that some functional complementation may exist.
MSP3 (MSP3.1) is a 48 kDa merozoite surface protein that was initially identified by screening of a P. falciparum genome wide expression library (Oeuvray et al.1994). MSP3 has since become a strong blood-stage vaccine candidate, and its C-terminal domain is completely conserved across P. falciparum isolates (Oeuvray et al.1994). In addition to its conserved C-terminal domain structure PfMSPDBL1/MSP3.4 also possesses a Duffy-binding like domain structurally similar to those found in the EBAs (Sakamoto et al.2012). Recombinant PfMSP1DBL has been shown to bind RBCs, suggesting that it may play a role merozoite attachment. Antibodies raised against recombinant PfMSP-DBL protein inhibit parasite invasion in a dose dependant manner (Sakamoto et al.2012).
MSP7-family proteins
MSP7 is also a member of large multigene families that are found across many Plasmodium species, and is anchored to the merozoite surface non-covalently via the MSP1 complex. MSP7 expression is concurrent to that of MSP1 and both proteins become associated with each other prior to their migration to the merozoite surface (Pachebat et al.2001; Pachebat et al.2007). While MSP7 can be disrupted, the loss of MSP7 significantly reduces RBC invasion efficiently of knock out parasites, which suggests that MSP7 plays an important, but non-essential, role in merozoite invasion in vitro (Kadekoppala et al.2008). While several other members of the MSP7 gene family are transcribed in trophozoite and schizont stages (Mello et al.2002), it remains unclear whether there is a redundancy of function at a protein level that may allow parasites with disrupted MSP7 to still invade RBCs. Antibodies raised against MSP7 do not strongly inhibit RBC invasion (Kauth et al.2006). In P. falciparum, MSP7 is part of gene family consisting of 6 members, which appear to have arisen due to genetic duplication events. The N-terminal signal peptide and C-terminal domains of MSP7 family members are more highly conserved than the central domains, which in MSP7 are unstructured but highly acidic (Kadekoppala and Holder 2010). As the C-terminal domain of MSP7 mediates its interaction with MSP1 it is possible that other members of the MSP7 gene family may perform related functions. Apart from its association with MSP1, very little is known about the function of MSP7; MSP7 derived peptides show some ability to bind the RBC receptor B and 3 (Garcia et al.2007), but these experiments are not definitive.
Serine-rich antigen family
The SERA family of proteins is expressed in late stage schizonts where they localize to the parasitophorous vacuole. All SERA proteins contain a serine (SERA 1–5 and 9) or cysteine (SERA 6–8) catalytic domain, which shares modest homology to the canonical domains found in the papain-family of proteases (Hodder et al.2003). While the exact function of the SERAs is not known, anti-SERA5 antibodies or cysteine protease inhibitors inhibit schizont rupture and it has been proposed that SERA proteins may be responsible for the proteolytic cleavages required for merozoite egress (Delplace et al.1987; Knapp et al.1991). While several members of the SERA family can be genetically disrupted, as a group they appear to play an essential role in blood-stage development. Disruption of SERA4 is reported to elicit a concordant increase in SERA5 expression (McCoubrie et al.2007) and neither SERA5 nor SERA6 can be deleted (Miller et al.2002; McCoubrie et al.2007). While primarily active in schizonts, SERA5 is proteolytically processed upon schizont rupture, with the N- and C-terminal portions of SERA5 remaining associated with the merozoite surface after their release (Debrabant and Delplace 1989). However the function of these shed fragments is yet to be determined.
PfRH5 and PfRipr
Following attachment to the RBC via EBA and PfRH protein families, the next stage of invasion involves PfRH5 and the PfRH5-interacting-protein (PfRipr). While distantly related to the PfRH family (15–30% sequence similarity), PfRH5 is unique in that it is much smaller (45 kDa) and does not possess a C-terminal transmembrane domain (Baum et al.2009). The rhoptry-derived PfRH5 and microneme-associated PfRipr associate together on the surface of the merozoite by binding to the GPI-anchored CyRPA (Reddy et al.2015). Mature PfRipr is a large (123 kDa) and highly structured protein, with 10 epidermal growth factor-like domains and 87 cysteine residues distributed along its length (Chen et al.2011). It is currently thought that PfRipr acts as a scaffold for the presentation of PfRH5 to the erythroctye surface (Chen et al.2011). While all members of this complex are refractory to disruption and can be targeted by invasion-inhibitory antibodies (Baum et al.2009) (Chen et al.2011; Reddy et al.2015), only PfRH5 makes direct contact with the basigin receptor on the surface of the RBC (Crosnier et al.2011).
Rhoptry-associated lecine zipper-like protein
The rhoptry-associated, leucine zipper-like protein (RALP1) is a RBC binding protein. Expressed as a 90 kDa protein in late stage schizonts, it undergoes proteolytic cleavage to 50 kDa, which is released from the rhoptries during merozoite invasion (Haase et al.2008). While the N-terminal fragment of RALP1 is shed, the 50 kDa C-terminal fragment contains the leucine zipper-like motif predicted to mediate protein-protein interaction and binds to an unknown receptor protein on the RBC surface (Haase et al.2008; Ito et al.2013). RALP1 is refractory to gene knockout and is thought to be involved the formation of the moving junction. Anti-RALP1 antibodies raised against the C-terminal fragment inhibit merozoite invasion, but not merozoite attachment to the RBC membrane (Ito et al.2013).
Glutamate-rich protein (GLURP)
The glutamate-rich protein (GLURP) is expressed on the merozoite surface and has a predicted molecular mass of 145 kDa, with two tandem repeat regions designated R1 and R2, and an N-terminal non-repeat region which has limited diversity (R0) (Borre et al.1991; Hogh et al.1993). The function of GLURP is unknown; antibodies to it do not inhibit invasion, and it is shed from the merozoite surface during invasion (Theisen et al.1998). However, GLURP has been a strong focus of vaccine development and has progressed to phase 1 vaccine trials (Table 1).
Similarities and differences between merozoite proteins of P. vivax and P. falciparum
Much less is known about the merozoite surface proteins of P. vivax because of the inability to readily culture P. vivax in vitro and use molecular genetic approaches to study the roles of key proteins. These limitations have been a barrier to understanding P. vivax invasion biology and immunity, and the development of effective vaccines. In broad terms, many key invasion events and interactions are expected to be similar to those of P. falciparum since P. vivax has orthologous genes for many P. falciparum merozoite proteins (Moreno-Perez et al.2014). While an extensive review of P. vivax merozoite surface proteins will not be provided here, it is valuable to reflect on some key differences and similarities, as this is relevant to developing vaccines and therapeutics. Of note, obvious orthologues are absent in P. vivax for a number of essential and/or abundant P. falciparum proteins, some of which are promising P. falciparum vaccine candidates.
Unlike P. falciparum, P. vivax has a very strong preference for invasion of reticulocytes and is largely restricted to invading young reticulocytes that still bear CD71 (Malleret et al.2015). In addition, efficient invasion by P. vivax requires the Duffy antigen on reticulocytes, although invasion into Duffy-negative reticulocytes can occur (Ryan et al.2006). In contrast, P. falciparum can efficiently invade Duffy negative RBCs and mature erythrocytes.
Plasmodium vivax has orthologues of MSP1, MSP6 and MSP7, which appear to play a role in invasion (Cheng et al.2013), suggesting there is a functional MSP1-like complex as for P. falciparum. Further, there are orthologues of several other merozoite surface proteins MSP4, MSP5, MSP9, MSP10, SERA5 and the 6-cyteine-domain family of proteins. However, there is no obvious orthologue for MSP2, which is an essential and abundant protein in P. falciparum, and a promising vaccine candidate. Like P. falciparum, P. vivax as a multigene PvMSP3 family (Carlton et al.2008); however, these are not clear homologues of PfMSP3 proteins and have significant sequence and structural differences from P. falciparum (Rice et al.2014). While P. vivax lacks the same family of EBA and PfRH proteins found in P. falciparum, related proteins are present, including Duffy-binding protein (DBP) and the reticulocyte-binding proteins (RBPs). PvDBP plays an important role in invasion, binding to the Duffy blood group antigen on the surface of reticulocytes, and is related to the EBA family of proteins, each of which contain Duffy-binding-like domains (Batchelor, Zahm and Tolia 2011; Batchelor et al.2014) (Adams et al.1992) (Adams et al.2001). For some time, it was thought that PvDBP1 was essential for P. vivax invasion. However, recent studies have revealed that the PvRBP family contains 11 members, two of which (PvRBP1 and PvRBP2) can bind reticulocytes independently of the Duffy antigen, suggesting that P. vivax may also possess alternate pathways for merozoite attachment/reorientation (Li and Han 2012), as seen for P. falciparum. Although P. vivax lacks an obvious PfRH5 orthologue, it does have orthologues for PfRipr and PfCyRPA, which form an invasion complex with PfRH5. Additionally, orthologues for the PfAMA1-RON2/4/5/8 complex are present in the P. vivax genome and PvAMA1, and PfAMA1 have very similar structures (Arevalo-Pinzon et al.2011, 2013, 2015; Tonkin et al.2011; Cheng et al.2015). However, further studies are needed to confirm functional homology of AMA1 across species. Orthologues for several other P. falciparum micronemal and rhoptry proteins are present in P. vivax, including GAMA and MTRAP.
The differences between these two major species of human malaria highlight the need for caution when inferring function of P. vivax proteins from studies of P. falciparum, or other species, and emphasize the need to dramatically advance our understanding of the merozoite surface proteins of P. vivax. Although orthologues have been identified in P. vivax, the function of these proteins is yet to be established for the majority of these proteins.
Processing of merozoite proteins before, during and after invasion
Distinct processing of merozoite surface proteins before, during and after invasion, highlights the sophisticated protease machinery that has evolved for invasion and the diverse roles of different merozoite surface proteins (Fig. 2). In some cases, processing events and proteases involved are well defined. In other cases, the precise mechanisms and proteases are still unclear. Furthermore, nearly all knowledge of merozoite protein processing is based on studies with P. falciparum, and P. knowlesi, due to the difficulty in maintaining P. vivax in culture. While conservation of processes is hypothesized, these inferences are yet to be confirmed experimentally, and there may be significant differences in major events between Plasmodium species.
Figure 2.
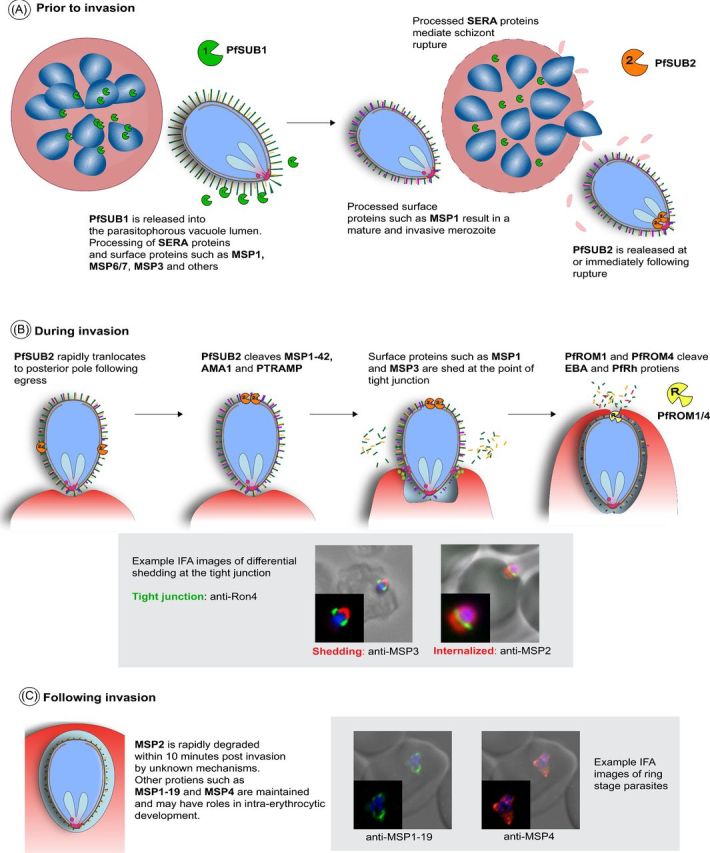
‘Processing of merozoite proteins before, during, and after invasion.’ (A) Prior to invasion, PfSUB1 is released from the merozoite into the parasitophorous vacuole lumen where it processes SERA proteins and a number of merozoite surface proteins. This activates SERA proteins so they can mediate rupture, and matures some surface proteins to functional conformations (e.g. MSP1) (B) Around the time of rupture, PfSUB2 is released and translocates to the apex of the merozoite. PfSUB2 cleaves MSP1-42, AMA1 and PTRAMP. During invasion, cleaved and peripherally-associated surface proteins are shed at the point of tight junction, while other proteins such as MSP2 and MSP4 are internalized during invasion. PfROM1 and PfROM4 are also involved and cleave EBA and PfRH proteins, as well as AMA1. (C) Following invasion, MSP2 and possibly other proteins are rapidly degraded, whereas other proteins, including MSP1-19 and MSP4, are maintained post-invasion and may have roles in intraerythrocytic parasite development.
Processing before invasion
In the final stages of merozoite maturation (Fig. 2A), multiple protein processing events occur to initiate egress of mature merozoites from the schizont and to ensure that invasion ligands are correctly processed and ready to mediate invasion.
As central player in the maturation of the merozoite prior to egress is the calcium dependent redox switch subtilisin protease, PfSUB1 (Withers-Martinez et al.2014). Around the time of egress, PfSUB1 is released from merozoite exonemes into the parasitophorous vacuole lumen (Yeoh et al.2007; Koussis et al.2009). Once released, PfSUB1 processes SERA family proteins, including processing of SERA6 to an active cysteine proteases form (Silmon de Monerri et al.2011; Ruecker et al.2012). This processing is thought to be required for the activation of SERA proteins that then mediate merozoite egress from the schizont (Ruecker et al.2012). These processes may be conserved in both P. vivax and P. knowlesi; both species express SUB1 orthologues which appear to have conserved substrate binding pockets, along with SERA cleavage sequence sites (Withers-Martinez et al.2012).
PfSUB1 is also responsible for extensive proteolytic processing of major merozoite surface proteins, including MSP1, which is processed from the high molecular mass protein (MW 180 kDa), to MSP183, MSP130, MSP138 and the C-terminal GPI anchored MSP142 fragments. Disruption of MSP1 cleavage renders the merozoite non-invasive (Child et al.2010). MSP6 and MSP7, which form a complex with MSP1 on the parasite surface, are also processed by PfSUB1, undergoing a N-terminal truncation to their mature forms (Stafford et al.1996; Pachebat et al.2001; Trucco et al.2001; Pachebat et al.2007). Prior to the translocation of the MSP1 complex to the merozoite surface, MSP7 undergoes a primary processing step, releasing an N-terminal 20 kDa fragment which is rapidly degraded and leaving a 33 kDa C-terminal fragment (MSP733) associated with MSP1. When MSP1 is processed into its four polypeptide components, MSP733 undergoes a secondary cleavage event which yield the C-terminal 22 kDa form (MSP722) (Pachebat et al. 2001, 2007). It appears likely that the PfSUB1 processing of proteins is required for the maturation of these proteins so they gain invasion function; it has been shown that only processed but not full length MSP1 can form a complex with MSP6 (Kauth et al.2006) and only the processed MSP142, but not full-length MSP1, can bind heparin-like polysaccharides (Boyle et al.2010a). Furthermore, recent studies report that MSP1 processing is required for binding to spectrin that is required for RBC rupture (Das et al.2015). Orthologues of MSP1 and MSP7 are found in P. vivax (Carlton et al.2008), and it may be likely that PvSUB1 also mediates processing of these proteins.
Bioinformatic and proteomic approaches have identified substrate preference sequence for PfSUB1 and based on this, a number of other possible target proteins have been identified including MSP3 and other MSP3-family proteins, MSP7-like family proteins (MSRP 2,3,4,5), MTRAP, RAMA, RAP1/2, SERA proteins, RhopH2/3, CLAG and falcipain-3 and plasmepsin IX and IV (Silmon de Monerri et al.2011). This suggests that PfSUB1 is a key in coordinating both egress and final maturation of the merozoite, and it has been proposed that PfSUB1, which is conserved across P. vivax and P. knowlesi, may be targetable for a pan-species antimalarial drug (for review of PfSUB1 see Withers-Martinez et al.2012)
Processing during invasion
Processing of merozoite proteins is also essential during the invasion process (Fig. 2B). Many surface proteins are cleaved and shed from the surface of the merozoite during invasion; however, not all surface proteins are cleaved or processed, with some being retained on the surface during invasion, indicating that cleavage and shedding is a selective process. Release of proteins anchored to the merozoite via GPI or other membrane anchor requires specific protein cleavage. For MSP1, cleavage of the GPI-anchored MSP1-42 fragment results in the release of MSP1-33, while the MSP1-19 fragment is carried into the RBC upon invasion (Blackman and Holder 1992). PfSUB2 also cleaves merozoite antigens AMA1 and PTRAMP (Harris et al.2005; Green et al.2006). Unlike PfSUB1, substrate specificity of PfSUB2 does not require a specific protein sequence and is instead based on proximity of substrates to the PfSUB2 protease active site (Green et al.2006; Olivieri et al.2011). SUB2 protease activity against other proteins appears to occur in a non-sequence specific manner at juxtamembrane sites via cleaving disordered regions based on relative distances from the membrane. In isolated merozoites, some shedding of MSP1 from the parasite surface occurs in the absence of invasion; however, erythrocyte invasion is required for complete shedding to occur (Blackman et al., 1990, 1993, 1994, 1996; Blackman and Holder 1992; Boyle et al.2010b). This shedding, along with other surface proteins that are not membrane-anchored (for example, MSP3, MSP7, SERA4 and SERA5) occurs at the tight junction between the invading merozoite and RBC (Blackman et al.1996; Boyle et al.2010b, 2014; Riglar et al.2011). However, following egress, PfSUB2 protease rapidly translocates in an actin and cytoplasmic domain dependent manner from the apex to the posterior of the merozoite in isolated merozoites (Harris et al.2005; Child et al.2013) and it appears that translocation occurs prior to the shedding of MSP1 (Riglar et al.2011). This suggests that cleavage by PfSUB2 and shedding at the tight junction are distinct, separate and specific mechanisms. A SUB2 orthologue is present in P. vivax, and many surface proteins have orthologues between P. falciparum and P. vivax (Carlton et al.2008), suggesting that some cleavage and shedding mechanisms may be conserved.
Along with SUB2 processing, the rhomboid protease ROM family also appears important in the shedding of merozoites surface proteins during invasion, specifically PfROM1 and PfROM4 (for a review of ROM proteases see Santos, Graindorge and Soldati-Favre 2012). ROM proteases cleave proteins in a site-specific manner within transmembrane domains and cleave merozoite proteins PfEBA175 (O'Donnell et al.2006), other PfRH and PfEBA family proteins, PfMTRAP (Baker, Wijetilaka and Urban 2006) and PfAMA1 (Howell et al.2005; Olivieri et al.2011). As with PfSUB2 mediated-processing, cleavage by PfROM is essential for invasion at least for some proteins; PfEBA175 mutants that are refractory to processing are non-viable (O'Donnell et al.2006). Little is known about the specific timing of PfROM cleavage during invasion.
Not all merozoite surface proteins are cleaved and shed during invasion (Fig. 2B and C); two essential surface proteins MSP2 and MSP4 are instead carried into the RBC during invasion, without apparent cleavage or shedding (Boyle et al.2014). Further, antibodies to MSP2, and the MSP1-19 fragment of MSP1, can be internalized into the RBC during invasion while bound to the merozoite surface (Blackman et al.1994; Dluzewski et al.2008; Moss et al.2012; Boyle et al.2014). The tight junction, while appearing as a complete ring structure in immunofluorescence microscopy using labelling of PfRON4 and AMA1 (Riglar et al.2011) and as a region of close proximity between the merozoite and RBC membranes in electron microscopy (Bannister et al.1975) is clearly not acting alone to force the shedding of proteins; otherwise, bound antibodies would not be internalized. Instead the tight junction must consist of either transient interactions, which can be broken to allow the passage of antibodies and surface proteins, or consist of gaps between receptor-ligand proteins to allow proteins to pass through. It remains possible that cleavage of surface proteins and the mechanisms of shedding require a further unknown factor to mediate the specific release of proteins from the merozoite surface.
The cleavage/shedding of surface proteins during invasion occurs in other Apicomplexa parasites including Toxoplasma, Neospora, Eimeria and Cryptosporidium, and it is hypothesized that shedding is necessary to release receptor–ligand interactions and allow for the invasion process to complete (Carruthers and Blackman 2005). Indeed, antibodies and compounds that prevent PfSUB2 function and the cleavage/shedding of MSP1, AMA1 and other surface proteins are inhibitory to the invasion process, showing that cleavage/shedding in some form is essential (Blackman et al.1994; Uthaipibull et al.2001; Dutta et al.2003; Fleck et al.2003; Woehlbier et al.2006). While the hypothesis for cleavage/shedding allowing release of receptor–ligand interactions seems plausable for merozoite antigens such as EBA and PfRH proteins that have clear ligand–receptor interactions with the RBC surface, many merozoite surface proteins are thought to mediate initial contact of the merozoite with the RBC via low affinity and reversible receptor–ligand interactions. It is possible that cleavage/shedding has other functions, such as preparing the merozoite for post-invasion functions or contributing to immune evasion (Saul 1987). Indeed, this has been suggested for the cleavage and shedding of AMA1, whereby complete shedding is not required for invasion, and may instead function to evade antibody mediated invasion inhibition (Olivieri et al.2011).
Processing after invasion
While MSP2 is carried into the RBC during invasion, it is rapidly degraded post invasion, and is absent from the ring within 10 m (Fig. 2C) (Boyle et al.2014). This result is consistent with previous reports that MSP2 protein is not detectable in ring-stage parasites nor invasion supernatants using a number of different experimental approaches (Ramasamy 1987; Clark et al.1989; Barron 1992; Pearce et al.2004). This suggests that MSP2 does have a specific role during invasion, and its processing post-invasion may be required for subsequent intraerythrocytic development. MSP2 is lacking in P. vivax (Carlton et al.2008), suggesting that the role of MSP2 in P. falciparum may be very specific. Intriguingly, antibodies bound to MSP2 can be internalized during invasion and maintained for at least 20 h of intraerythrocytic development. Like MSP2, MSP4 also remains on the merozoite surface and does not appear to be cleaved or processed during invasion (Boyle et al.2014). However, unlike MSP2, it persists in the developing intracellular parasite for several hours, but its function is currently unknown. A MSP4 orthologue is found in P. vivax, and has been confirmed as surface located (Black et al.2002). MSP119 is maintained in the developing parasite post-invasion and is thought to be involved in the formation of the food vacuole (Dluzewski et al.2008). The rapid degradation of MSP2 post invasion, but not of internalized antibodies bound to MSP2, nor of MSP4 or MSP119, points to specific, unknown, proteases that are involved in processing of merozoite proteins following invasion.
Immune targeting of merozoites
Targets of immunity
Merozoite surface proteins and invasion ligands are important targets of human immune responses that contribute to protective immunity. Antibodies to merozoite antigens are a crucial component of protective immunity and have been a major research focus. Components of cell-mediated immunity are also important including monocytes, macrophages, neutrophils and other cell types involved in antibody-mediated killing, and CD4+ T cell help for antibody generation and immune activation (Beeson, Osier and Engwerda 2008). As outlined earlier, the merozoite surface presents a complex array of antigens as potentially important antibody targets. This complexity has made it difficult to identify key targets of protective antibodies, and to quantify their relative importance. It is notable that while the merozoite surface has dozens of different proteins, this is quite different to sporozoites and parasitized RBCs where a single antigen appears to be the dominant target of antibodies (CSP and PfEMP1, respectively) (Chan et al.2012; Dups, Pepper and Cockburn 2014).
Numerous studies have demonstrated the acquisition of antibodies to P. falciparum merozoite antigens in association with malaria exposure, and some have been associated with protective immunity in longitudinal studies (reviewed in (Richards and Beeson 2009; Fowkes et al.2010)). One of the criteria used to objectively evaluate merozoite antigens as targets of protective immunity is the demonstration of protective associations between antibodies and subsequent risk of malaria in longitudinal studies (Fowkes et al.2010). Although associations with protection from malaria can vary between studies, and may be influenced by multiple factors, a systematic review demonstrated that, on the whole, antibodies to several prominent merozoite antigens (e.g. MSP119, MSP3 and AMA1) were associated with protective immunity (Fowkes et al.2010). However, at the time this study was conducted, antibodies to only a small number of antigens had been evaluated in detail in longitudinal studies.
Recent studies have begun to take a more systematic approach to evaluating antibodies to a large array of P. falciparum merozoite antigens, and these studies suggest that antibodies are acquired to most, if not all, merozoite surface proteins (Richards et al.2013; Ondigo et al.2014; Osier et al.2014b). Studies comparing the magnitude of protective associations between antibodies to different antigens have identified antigens that may be more important targets of protective antibodies, and could be prioritized for vaccine development (Richards et al.2013; Osier et al.2014b; Dent et al.2015) (Fig. 3). For example, Richards et al. (2013) evaluated over 100 purified recombinantly expressed P. falciparum merozoite proteins; after evaluating antigen quality and immunoreactivity, 46 proteins were studies in detail in a longitudinal cohort of children acquiring immunity (Fig. 3). Interestingly, protective associations were stronger for emerging vaccine candidates compared to established vaccine candidates that have already been tested in clinical trials, and were generally stronger for rhoptry and micronemal proteins. The EBA and PfRH family of proteins generally had strong protective associations; this included protective associations for the promising vaccine candidates PfRH5, its binding partner PfRipr, and EBA175, as well as a number of other recently identified proteins. Studies in other populations have also found protective associations for antibodies to EBA175 and PfRH5 (McCarra et al.2011; Dobano et al.2012; Tran et al.2014), but other members of these families have been little studied. Osier et al. (2014b) evaluated antibodies to a panel of P. falciparum merozoite antigens produced with a mammalian expression system, and found strong protective associations with several recently defined antigens, as well as established vaccine candidates MSP2, MSP3 and AMA1. A further approach to identifying important targets is the use of protein microarrays (Doolan et al.2008; Crompton et al.2010). In this platform, protein fragments are expressed with an E. coli cell-free system, and then printed onto arrays without any purification or refolding step. These arrays typically contain large numbers of proteins and only a minority of all proteins are merozoite surface proteins. Using this approach, Dent et al. (2015) found that antibodies to MSP2, MSP7 and MSP10 of P. falciparum, among other proteins, were significantly associated with protection. An important picture emerging from these studies is that a repertoire of antibodies to multiple antigens is also important in protective immunity, and antibodies to certain combinations of antigens may be particularly important in mediating protection (Gray et al.2007; Osier et al.2008, 2014b; Stanisic et al.2009; Reiling et al.2010; Richards et al.2010, 2013).
Figure 3.
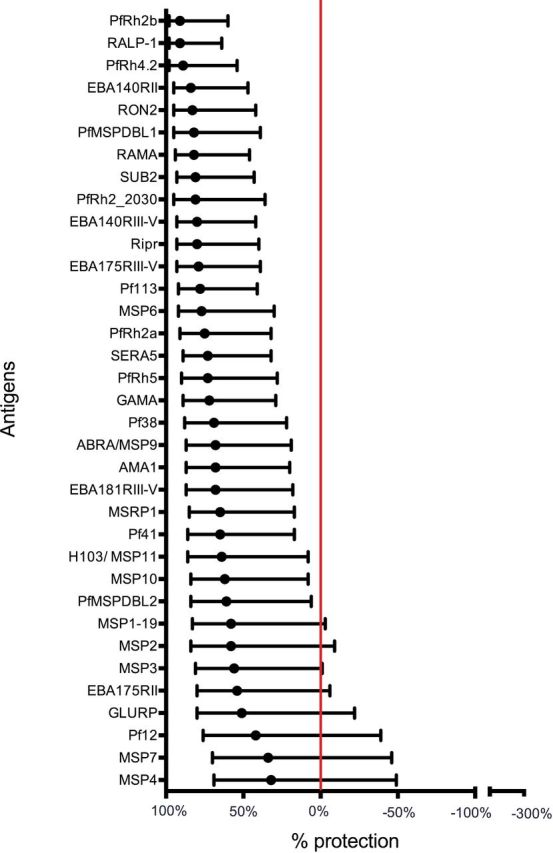
‘Association between antibodies to P. falciparum merozoite antigens and protection from malaria.’ Antibodies to a range of different merozoite proteins were evaluated in a longitudinal cohort of children living in a malaria-endemic region of Papua New Guinea. Antibody responses were prospectively related to the risk of malaria over a 6-month period of follow-up; malaria was defined as parasitemia of greater than 5000 parasites/ul of blood and fever. In the figure, antigen-specific antibodies are ranked by the strength of their association with protection (determined from hazard ratios calculated using the Cox proportional hazards model). The red line indicates no protective association. Error bars represent the 95% confidence interval. Figure was adapted from Richards et al. (2013).
Antibody responses to P. vivax antigens have also been investigated in areas endemic for P. vivax, particularly PvDBP, PvRBP and orthologues of P. falciparum merozoite surface proteins. PvDBP and PvRBP induce antibody responses in populations naturally exposed to P. vivax (Tran et al.2005; Cole-Tobian et al.2009; Souza-Silva et al.2010; Kano et al.2012). Additionally, individuals with antibodies to the essential conserved N-terminal cysteine-rich region II (PvDBPII) have been shown to be associated with protection against high-density P. vivax infections (Cole-Tobian et al.2009). It has also been reported that antibodies inhibit PvDBP binding to its receptor (King et al.2008). Antibodies to PvAMA1 are also associated with P. vivax exposure (Yildiz Zeyrek et al.2011; Fowkes et al.2012), and antibodies to PvAMA1 have been shown to inhibit merozoite invasion in vitro (Vicentin et al.2014). However, antibodies to PvAMA1 are yet to be investigated as a target of protective immunity in human cohort studies (Cutts et al.2014).
PvSERA4, which is the most dominantly expressed member of the P. vivax SERA multigene family with an expression profile similar to PfSERA5, has also been shown to stimulate antibody responses (Yildiz Zeyrek et al.2011), but no evidence for functional or protective responses is reported. Antibodies from naturally exposed individuals recognize P. vivax merozoite surface antigens representing polymorphic (PvMSP1 N-terminus, PvMSP3α Block I and II repeats, PvMSP5) and conserved regions (PvMSP119, the extreme N- and C-terminal ends of PvMSP3α and PvMSP10) (Pasay et al.1995; Soares et al.1997; Woodberry et al.2008; Yeom et al.2008; Fernandez-Becerra et al.2010; Lima-Junior et al.2011; Yildiz Zeyrek et al.2011; Kano et al.2012; Stanisic et al.2013; Versiani et al.2013). PvMSP9 contains two species-specific blocks of repeats, designated PvMSP9RI and PvMSP9RII which can induce antibody responses in naturally exposed populations (Lima-Junior et al.2008, 2012; Stanisic et al.2013).
Currently, there is very little evidence in the literature for specific associations between any one antibody response and protection from symptomatic disease, but a recent systematic review demonstrated that antibodies to PvMSP119, PvMSP3α and the N-terminals of PvMSP1 and PvMSP9 were associated with protection against P. vivax, but only in single geographical locations (Cutts et al.2014). Further research is needed to define the role of P. vivax merozoite antibody responses in protective P. vivax immunity. In the absence of methods to readily culture P. vivax to study merozoite proteins, studies on targets of human immunity may play an essential role in identifying and prioritizing vaccine candidates.
Identification of antibody targets, and how these antibodies are acquired and maintained, is also valuable for the development of serological tools for malaria surveillance. Antibodies to malaria antigens in populations can act as markers of recent or long term exposure to malaria, and population serological screening may be a valuable tool to enhance malaria control and elimination efforts (Drakeley and Cook 2009; Elliott et al.2014).
Function of antibodies
Antibodies to merozoites appear to protect against clinical disease by inhibiting blood-stage replication and preventing high-density parasitemia, rather than preventing infection per se (Stanisic et al.2009; Richards et al.2010). Antibodies to merozoite antigens mediate multiple effector mechanisms, including acting directly to inhibit replication, or through interactions with immune cells and complement (Fig. 4A). Many studies have shown that antibodies can inhibit the growth and replication of blood-stage P. falciparum (reviewed in (Beeson et al.2014)), and the mechanism for this is thought to be primarily by inhibiting RBC invasion of merozoites, although antibodies may also act by inhibiting schizont rupture and intraerythrocytic development. While little is known about the exact effector mechanism by which antibodies inhibit merozoite invasion, it is though that they may function by inhibiting receptor–ligand interactions, protein processing or conformational changes required by merozoite proteins to perform their role in invasion. Affinity-purified human antibodies to several P. falciparum merozoite antigens, including MSP119, AMA1, PfRH4, EBA175, PfRH5 and MSP-DBL1/2 have been shown to inhibit growth in vitro (Egan et al.1999; Hodder, Crewther and Anders 2001; Reiling et al.2012; Badiane et al.2013; Tran et al.2014; Chiu et al.2015). Growth-inhibition assays (GIA) have been the most widely used functional antibody assay for merozoite antigens. Growth assays using genetically-modified P. falciparum lines have been use to evaluate antigen-specific inhibitory antibodies to MSP119 and the EBAs (O'Donnell et al.2001; Persson et al.2008, 2013; Wilson et al.2011). The recent development of robust methods to purify viable invasive merozoites has enabled the development of assays that specifically measure invasion-inhibitory activity of antibodies and other inhibitors (Boyle et al.2010b, Wilson et al.2013), but these have not yet been widely applied to studies of naturally acquired or vaccine-induced immunity. Acquired antibodies have also been shown to inhibit the binding of the invasion ligands EBA175 and PvDBP1 to their respective RBC receptors, and these inhibitory antibodies have been associated with protective immunity (King et al.2008; Irani et al.2015).
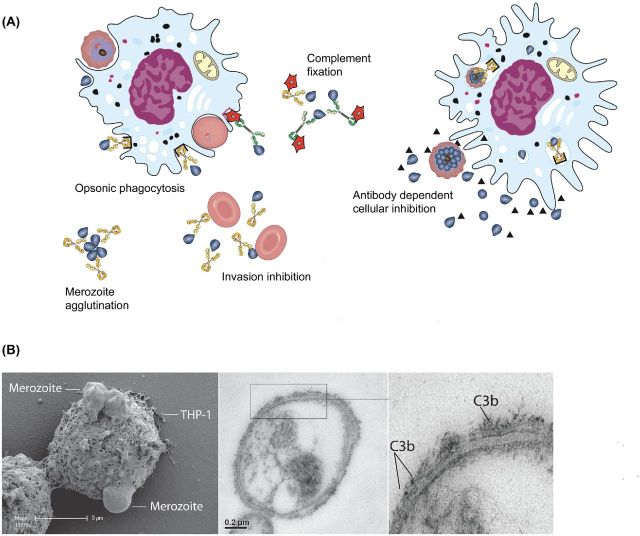
Figure 4.
‘Antibody-mediated mechanisms of immunity to merozoites.’ (A) Antibodies to merozoites may mediate immunity through a number of mechanisms. This includes the ability of antibodies to directly inhibit invasion of merozoites, interact with complement (red stars) to inhibit invasion or lyse merozoites, and agglutinate merozoites to inhibit their dispersal after egress from schizonts. Opsonization of merozoites by antibodies promotes their phagocytosis by monocytes and macrophages, and killing by neutrophils. Phagocytosis of opsonized merozoites by monocytes results in activation and the production of TNF-alpha and other cytokines, and secretion of soluble factors (represented by triangles) that inhibit parasite growth (referred to as ADCI). Different antibody types (such as different IgG subclasses) may have different functional activities, particularly for complement fixation and Fc–receptor interactions; however, these differences are not currently clearly defined. To reflect these potentially important differences, we have shown antibodies as yellow or green to represent the presence of different antibody types that can mediate functional activities. (B) Early stages of phagocytosis of antibody-opsonized merozoites by a THP1 monocyte imaged using scanning electron microscopy (adapted from (Osier et al.2014a)). (C) Antibodies to merozoites promote the activation of complement on the merozoite surface by fixing complement component C1q leading to the activation of complement through the classical cascade. A key step in the cascade is the formation of C3b, which is labelled in this figure with gold particles on the surface of merozoites by transmission electron microscopy.
Antibodies to merozoite antigens are predominantly comprised of IgG1 and IgG3 (Taylor et al.1998; Roussilhon et al.2007; Stanisic et al.2009; Reiling et al.2010; Richards et al.2010), both of which are effective at complement fixation (Boyle et al.2015) and interact with FcR receptors on phagocytic cells to mediate effector mechanisms (Stubbs et al.2011; Osier et al.2014a). A recent study demonstrated that human antibodies to P. falciparum merozoite surface antigens can fix C1q to activate complement via the classical pathway and inhibit merozoite invasion and blood-stage replication (Boyle et al.2015) (Fig. 4C). MSP1 and MSP2 were shown to be targets of these antibodies, but other merozoite antigens remain to be investigated. The mechanism of antibody-complement inhibition appears to be mediated by the binding of C1q and other complement components resulting in inhibition of merozoite invasion and through the formation of the membrane attack complex that rapidly leads to merozoite lysis. Antibodies from many malaria-exposed adults and children are either non-inhibitory or weakly inhibitory on their own, but effectively block invasion in the presence of complement. The ability of antibodies to fix C1q to the merozoite surface was very strongly associated with protection against P. falciparum malaria in children, suggesting that complement activation by antibodies to merozoite surface proteins is an important mechanism in acquired immunity. Furthermore, antibody-complement inhibitory activity was induced by immunization with a recombinant MSP2-based vaccine in a phase 1 clinical trial (Boyle et al.2015).
Opsonization of P. falciparum merozoites by antibodies has been shown to mediate important interactions with immune cells, including monocytes, macrophages and neutrophils (Fig. 4A and B). Phagocytosis of merozoites and immune complexes by monocytes can lead to the release of soluble factors that inhibit parasite growth in vitro, in a process called antibody-dependent cellular inhibition (ADCI) (Bouharoun-Tayoun et al.1995). Antibodies that mediate this activity are acquired through natural exposure to malaria, and have been induced by vaccines based on several different merozoite surface proteins, including human vaccine trials based on recombinant MSP2, MSP3 and GLURP (Druilhe et al.2005; McCarthy et al.2011; Jepsen et al.2013).
Opsonization of P. falciparum merozoites also promotes phagocytosis by monocytes (Fig. 4B) (Khusmith and Druilhe 1982; Khusmith, Druilhe and Gentilini 1982; Stubbs et al.2011; Osier et al.2014a), leading to production of TNF-alpha and monocyte activation (Bouharoun-Tayoun et al.1995; Osier et al.2014a). Opsonic phagocytosis activity of antibodies was associated with protection from malaria in longitudinal cohorts of children in Kenya (Osier et al.2014a) and Papua New Guinea (Hill et al.2013), and are effectively boosted by infections (Osier et al.2014a). This activity may contribute to immunity by direct clearance of opsonized merozoites, and indirectly through immune activation. Opsonization of P. falciparum merozoites by antibodies also appears to promote killing by neutrophils, measured in respiratory burst assays; activity in these assays was associated with protection from malaria in longitudinal cohort studies (Joos et al.2010).
The mechanisms by which antibodies to P. vivax merozoites mediate immunity have been scarcely studied, but similar mechanisms would be expected to those reported for P. falciparum. Human antibodies to PvDBP1 were shown to inhibit invasion (Grimberg et al.2007), supporting the concept that inhibition of invasion is one mechanism by which antibodies to P vivax merozoite antigens may function. As for P. falciparum, antibodies to P. vivax merozoites are predominantly IgG1 and IgG3 (Fernandez-Becerra et al.2010; Lima-Junior et al.2011), suggesting that complement and FcR mediated mechanisms will be relevant.
Polymorphims
Many key targets of protective immunity and vaccine candidates demonstrate significant polymorphisms in sequence reflective of immune selection (Barry et al.2009; Conway 2015). This sequence diversity in many merozoite antigens poses challenges in vaccine development, as strategies are required to generate broadly active antibodies that would circumvent the potential for vaccine escape. However, the significance of polymorphisms for immune escape is not well understood for most antigens. Among the most polymorphic antigens are multiple members of the MSP3-like family, and other antigens such as MSP1 and AMA1. However, for many antigens the level of antigenic diversity may be modest and could be overcome with existing vaccine approaches, such as the inclusion of multiple alleles. For example, different MSP2 sequences can be grouped into two major allelic types (Smythe et al.1991). MSP1 has significant polymorphisms through the sequence, but the relatively conserved C-terminal region of MSP1, MSP119, has only four polymorphic residues (Barry et al.2009), which has supported its potential as a vaccine candidate. The MSP142, which has been tested as a candidate vaccine in clinical trials (Ogutu et al.2009), is broadly classified as dimorphic with two protoype alleles; however, there is substantial polymorphism within these allelic types (Vijay Kumar et al.2005; Mehrizi et al.2008). For AMA1, over 200 haplotypes are present in populations; however, antigenic diversity appears much more restricted and diversity may be covered by the inclusion of just 3–4 different alleles (Drew et al.2012; Dutta et al.2013; Terheggen et al.2014) (Remarque et al.2008). Other promising candidates, such as PfRH5, have very limited polymorphism, or others such as MBA175, MSP3 and PfRH2 contain conserved regions that may be targeted (Druilhe et al.2005; Reiling et al.2010; Healer et al.2013; Drew and Beeson 2015).
Phenotypic variation and immune evasion
Plasmodium falciparum merozoites can use different pathways for RBC invasion, and these pathways are linked to variation in the use and/or expression of the EBA and PfRH invasion ligands, and this phenotypic variation appears to facilitate immune evasion (Fig. 5A). Variation in invasion phenotypes has been demonstrated by evaluating invasion of different isolates into RBCs that have been treated with enzymes to selectively cleave RBC receptors. Several studies have shown substantial variation in the ability of clinical isolates to invade RBCs treated with different enzymes (Okoyeh, Pillai and Chitnis 1999; Bowyer et al.2015) and the ability of laboratory-adapted clonal lines to switch invasion pathways has also been demonstrated (Dolan, Miller and Wellems 1990). In addition, substantial variation in the expression of EBA and PfRH invasion ligands among clinical isolates has been reported (Nery et al.2006; Reiling et al.2012; Bowyer et al.2015). Although the diversity and properties of invasion pathways have not been fully defined, invasion phenotypes can be broadly classified into two main groups: sialic acid (SA)-dependent invasion, demonstrated by poor invasion of neuraminidase-treated RBCs; and SA-independent invasion, demonstrated by efficient invasion of neuraminidase-treated erythrocytes. EBA140, EBA181, EBL-1 and PfRH1 are involved in SA-dependent invasion; neuraminidase cleaves SA on the RBC surface and inhibits the binding of these ligands to their RBC receptors. On the other hand, PfRH2 and PfRH4 are important in SA-independent invasion and interactions appear chymotrypsin-sensitive. While this classification is likely to be an oversimplification of a complex spectrum of phenotypes, it serves as valuable model to understand and study phenotypic variation and immune evasion, with relevance to vaccine development (Fig. 5A).
Figure 5.
‘Use of alternate invasion pathways and immune evasion.’ (A) In this example of alternate invasion pathways, the clonal parasite isolate gives rise to merozoites that largely use a sialic acid-dependent invasion pathway (blue merozoites); a small subpopulation of merozoites use an alternate sialic acid independent pathway (yellow merozoites). In the absence of any selective pressure, parasite replication gives rise to a population of merozoites that predominantly invade via a sialic acid dependent invasion. Selective pressure on invasion, either through immune selection (e.g. antibodies that block ligands of sialic acid dependent invasion) or phenotypic selection (invasion into RBCs with deficiency in sialic acid receptors) leads to the emergence of parasites using an alternate sialic acid independent invasion pathway. The use of an alternate pathway can also be generated artificially by genetic disruption of EBA175. The sialic acid dependent invasion pathway uses the EBA ligands and PfRH1, whereas the sialic acid independent invasion pathway has a greater reliance on PfRH4 and PfRH2. (B) Differential inhibition by antibodies from malaria-exposed individuals of P. falciparum lines using different invasion pathways. In these examples, changes in invasion pathways were generated by deletion of EBA175, EBA140 or EBA181. Results show the proportion of samples (n = 130) that differentially inhibited the invasion of the parental parasites compared to parasites with disruption of specific EBA genes. No difference in inhibition between the isolates was regarded as <25% difference in the level of inhibition (indicated in blue). The proportion of samples that inhibited the knockout line more than the 3D7wt line (by >25%) is shown in green; the proportion of samples that inhibited 3D7wt more than the knockout line (by >25%) is shown in red. Data reproduced from (Persson et al.2013).
Isolates can be switched from SA-dependent to SA-independent invasion phenotypes by extended culture in neuraminidase-treated RBCs, and the change in phenotype is associated with upregulation of PfRH4 (Stubbs et al.2005; Gaur et al.2006). Invasion phenotypes can also be modified by deletion of EBA or PfRH genes (Reed et al.2000; Duraisingh et al.2003). For example, deletion of EBA175 can be used to shift the invasion phenotype of an isolate from an SA-dependent to SA-independent invasion phenotype (Reed et al.2000). Using defined isolates generated with these approaches, studies have shown that changes in invasion phenotype can lead to changes in the susceptibility of P. falciparum to human invasion-inhibitory antibodies, suggesting that variation in invasion phenotypes facilitates immune evasion (Persson et al.2008). In a study of invasion-inhibitory antibodies in Kenyan adults and children, a phenotypic switch from SA-dependent invasion to SA-independent invasion led to a loss of antibody inhibitory activity among many samples (Persson et al.2008). Changes in the sensitivity of P. falciparum to invasion-inhibitory antibodies was also seen when EBA175, EBA140, or EBA181 were selectively deleted (Persson et al.2008, 2013) (Fig. 5B). The ability of human antibodies to inhibit isolates with EBA175, EBA140 or EBA181 gene deletions also varied, suggesting that there are immunologically significant differences between these different phenotypes and further highlighting the role of diverse invasion phenotypes in immune evasion (Persson et al.2013).
Supporting these findings, antibodies to EBA and PfRH invasion ligands are acquired with increasing age and exposure to malaria in populations (Persson et al.2008), and antibodies to the EBAs and PfRH ligands have been associated with protective immunity against malaria in prospective longitudinal studies (Reiling et al.2010; Richards et al.2010; McCarra et al.2011; Reiling et al.2012). Reduced invasion-inhibitory activity by serum antibodies of EBA knockout lines compared with parental parasites suggests that the EBAs are important targets of inhibitory antibodies (Persson et al.2013). Indeed, affinity-purified human antibodies to EBA175 have been shown to inhibit invasion (Badiane et al.2013), and human antibodies can inhibit the binding of EBA175 to glycophorin A (Ohas et al.2004; Irani et al.2015).
Further, studies are needed to understand the role of PfRH ligands in immune evasion mediated by phenotypic variation and as targets of inhibitory antibodies. Affinity-purified human antibodies to PfRH4 can inhibit invasion, and isolates lacking expression of PfRH4 can evade these inhibitory antibodies (Reiling et al.2012). Others have shown that rabbit antibodies to PfRH1 inhibit invasion in an isolate that uses SA-dependent invasion, and switching to SA-independent invasion leads to loss of inhibitory activity (Gao et al.2008). Phenotypic variation in invasion may also be important for parasite adaptation to host polymorphisms in RBC surface receptors (Dolan, Miller and Wellems 1990; Maier et al.2003; Tham et al.2010). For example, deletions in glycophorin C (receptor for EBA140) and deficiencies in CR1 (receptor for PfRH4) are common in some populations, and levels of glycophorin A (receptor for EBA175 and potentially MSP1) declines with increasing RBC age. The ability to use different receptor–ligand interactions for invasion to adapt to the availability of receptors on the RBC surface may be important for parasite survival in the host. The potential for variation in invasion phenotypes in P. vivax remains undefined because of the difficulty in maintaining in isolates in culture.
Merozoites as vaccine candidates
To date, seven P. falciparum merozoite antigens have been assessed in human vaccine trials; MSP1, MSP2, MSP3, AMA1, EBA175, GLURP and SERA5 (Table 1). Most of these have been delivered as recombinant antigens with different adjuvants, or using a viral vectored prime-boost regimen using chimpanzee adenovirus 63 (ChAd63) and modified vaccine virus Ankara (MVA). The majority of these have been phase 1 trials. A small number of vaccines have been tested in phase II field trials to assess protection against naturally acquired infections, or in phase I/II trials that assess protection against experimental infections in vaccinated malaria-naïve volunteers. In striking contrast, there has been only one human trial of a P. vivax merozoite antigen; PvDBP using the CHAd63-MVA system (unpublished, NCT01816113). Numerous merozoite antigen vaccines have shown significant efficacy in small animal models of malaria; the extensive literature on this will not be reviewed here in the interests of space. Instead the focus will be on results from clinical trials.
Single antigen vaccines
AMA1 is the most widely studied candidates and has been tested in 23 human trials on its own, and in nine studies in combination with other antigens. A vaccine combining two alleles of AMA1 (3D7 and FVO), adjuvanted with Alhydrogel, demonstrated good safety and immunogenicity in malaria-naïve adults, and Malian children and adults (Malkin et al.2005; Dicko et al.2007, 2008). Unfortunately, a phase IIb trial of this vaccine in 2–3-year-old children in Mali failed to demonstrate any reduction in frequency of high parasitaemia episodes (Sagara et al.2009a; Ouattara et al.2010). The vaccine did induce good invasion-inhibitory antibodies, but other functional activities of antibodies have not yet been reported (Miura et al.2011). Looking to enhance immunogenicity, AMA1 has also been assessed including CPG7909 oligodeoxynucleotide as an additional adjuvanting agent in the Alhydrogel formulation. This led to 3—4-fold higher antibody titers in malaria-naïve adults (Mullen et al.2008) and higher antibody levels in Malian adults (Sagara et al.2009b). However, the vaccine failed to protect malaria naïve participants in the UK when undergoing sporozoite challenge (Duncan et al.2011). The reasons for this are unclear, but it may be that antibody titres measured by ELISA and activity in standard growth inhibition assays do not adequately represent antibody specificity and functional activity required for protective immunity.
Another AMA1 vaccine included a single allele formulated with a more potent adjuvant, ASO2A, showed good immunogenicity in malaria-naïve adults, and Malian adults and children (Thera et al.2006, 2008, 2010, Polhemus et al.2007). A phase IIb trial in Malian children failed to demonstrate significant overall protection from malaria (6 month follow-up), but there was evidence of substantial strain-specific efficacy against malaria caused by vaccine-like strains (Thera et al.2011). While this vaccine is not suitable for further development in its present form, the results did establish an important proof-of-principle for AMA1 as a potential vaccine antigen.
Plasmodium falciparum MSP1-based vaccines have yet to demonstrate clear efficacy in humans. A single allele of MSP142 formulated with ASO2A induced high-level antibodies in malaria-naïve adults, and malaria-exposed children and adults, including growth-inhibitory antibodies (Ockenhouse et al.2006; Thera et al.2006; Withers et al.2006; Stoute et al.2007). However, a Phase IIb trial in Kenyan children aged 1–4 years found no protection from symptomatic malaria (Ogutu et al.2009). The reasons for the lack of efficacy are unclear, but may have been due to the inclusion of only one allele providing inadequate coverage of circulating strains. MSP142 delivered using the ChAd63 approach induced strong T-cell responses but only modest growth-inhibitory antibodies; three participants were infection challenge post vaccination and all developed blood-stage parasitemia (Sheehy et al.2012a,b).
MSP2 has mostly been assessed in combination with other antigens as part of Combination B, discussed below. A phase I study of a MSP2 vaccine that included the two allelic variants (3D7 and FC27) in malaria-naïve adults showed good immunogenicity, including the induction of ADCI activity (McCarthy et al.2011). Recent results showed that these vaccine-induced antibodies can also recruit complement components to inhibit invasion, but have little invasion-inhibitory activity in the absence of complement (Boyle et al.2015).
A P. falciparum MSP3 vaccine based on the conserved C-terminal end of the protein expressed as a long synthetic peptide inducde antibodies that had ADCI activity measured in vitro. Furthermore, passive transfer of these antibodies promoted parasite clearance in vivo in a humanized mouse model (Druilhe et al.2005). A phase I study of 1—2-year-old children in Burkina Faso reported strong cytophilic antibody responses (Sirima et al.2009). Although not designed as a phase IIb study, post-vaccine follow-up indicated significantly fewer episodes of malaria among children who received the MSP3 vaccine compared to controls (Sirima, Cousens and Druilhe 2011).
Other antigens have been assessed in phase I trials. An EBA175 vaccine based on region II of the protein, which mediates binding to glycophorin A, showed good immunogenicity, but in vitro GIA activity was modest (up to 15%) (El Sahly et al.2010). However, an EBA175-based vaccine showed efficacy in a primate model (Jones et al.2001). The N-terminal end of SERA5 (with serine repeats removed) was recently assessed in a phase Ib of children and adults in Uganda (Palacpac et al.2013). Post-vaccine follow-up found reduced incidence of high parasitemia episodes in the SERA5 vaccine group versus control. PfRH5 is emerging as a promising vaccine candidate based on numerous criteria (Drew and Beeson 2015). Following significant efficacy in a non-human primate infection challenge model (Douglas et al.2015), PfRH5-based vaccines are now progressing into clinical trials. This is supported by recent studies showing that a recombinant chimeric antibody (Ab-1) against basigin that blocks binding of PfRH5 cleared parasitemia in a humanized mouse model (Zenonos et al.2015). In summary, results from clinical trials have demonstrated that several merozoite antigens are immunogenic when used as vaccines in humans, and can induce relevant functional immune responses. However, a few have shown evidence of protective efficacy, and additional antigens and combinations need to be investigated.
Combination antigen vaccines
A phase II trial of the Combination B vaccine showed significant efficacy in reducing parasitemia in children in a malaria-endemic area of PNG (Lawrence et al.2000; Genton et al.2002, 2003). The vaccine was comprised of full-length MSP2 (3D7 allele), MSP1 (N-terminal end) and part of the ring-infected erythrocyte surface antigen (RESA). Vaccine efficacy is believed to have been mainly mediated by MSP2; infections with parasites containing the vaccine allele of MSP2 were greatly reduced, but there was no protective effect against infections having the alternate allele (FC27). These data provide important proof of concept for MSP2 as a vaccine candidate.
Numerous other antigen combinations have been tested in phase I trials, including some challenge trials. MSP1 and AMA1 have been combined as recombinant antigens (Ellis et al.2012) and using the ChAd63-MVA approach (Sheehy et al.2012a; Biswas et al.2014). Although, high levels of antibodies were induced for both antigens, subsequent experimental challenge found only one individual was protected. Chimeric MSP1-AMA1 vaccines have also been explored (Hu et al.2008; Malkin et al.2008). MSP1 has also been combined with EBA175 (F2 domain)(Chitnis et al.2015), which induced strong EBA175 responses and good invasion-inhibitory antibodies against homologous parasites, but poor MSP1 responses.
A chimeric MSP3-GLURP recombinant protein has been tested in three phase I studies in malaria-naïve adults, and adults and children in Gabon (Esen et al.2009) (Mordmuller et al.2010; Belard et al.2011). When tested in 1—5-year-old Gabonese children the vaccine induced strong IgG responses to GLURP and MSP3, including memory B-cells, but antibody levels were not maintained beyond 1 year post-immunization (Belard et al.2011). The vaccine has been shown to induce ADCI activity (Jepsen et al.2013).
The multiantigen vaccine NYVAC-Pf7 incorporated MSP1, AMA1 and SERA, as well the non-blood-stage antigens CSP, liver stage antigens 1 (LSA1), sporozoite surface protein 2 (SSP2) and the sexual stage antigen Pfs25, in an attenuated vaccinia virus (Tine et al.1996). A phase I/IIa study demonstrated a slight delay in the pre-patent period following human sporozoite challenge (Ockenhouse et al.1998). The SPf66 vaccine included a 45 amino acid chimeric peptide derived from three merozoite-derived antigens and the NANP epitope of CSP on sporozoites. It was tested in multiple phase II trials, with variable efficacy (e.g. (Valero et al.1993; Alonso et al.1994; D'Alessandro et al.1995; Nosten et al.1996)). A subsequent Cochrane review concluded that there was no justification for further trials using the same formulation (Graves and Gelband 2006).
Challenges in developing merozoite vaccines
Promising results from a small number of phase II trials in malaria-endemic populations, and data from extended follow-up from phase Ib trials, support the potential of merozoite antigens as vaccine candidates. However, results also highlight the many challenges faced. This includes identifying and prioritizing the most attractive candidates, identifying antigen combinations to further maximize protective responses, and defining immune responses required for optimal protection. There is a strong need for functional assays as correlates of protective immunity, which are currently lacking, and a greater range of functional assays need to be used in the evaluation of vaccine-induced responses. Functional assays have been largely restricted to growth-inhibition assays, and MSP3, MSP3-GLURP and MSP2 vaccines have also demonstrated activity in ADCI assays. Only the MSP2 vaccine has been show to induce antibody–complement interactions to inhibit invasion; other vaccines have not yet been tested. It also appears that more potent adjuvants or vaccine delivery platforms are needed to enhance protective efficacy and induce long-lived responses. Since merozoite immunity appears to prevent malaria by inhibiting blood-stage replication, it is unclear whether the experimental human challenge model is the right approach for evaluating these vaccines.
CONCLUSIONS
Merozoite invasion of RBCs involves multiple proteins and receptor–ligand interactions, and extensive remodelling of the merozoite surface before, during and after invasion. The merozoite surface is highly complex, presenting a multitude of antigens to the immune system. This complexity has proved challenging to our efforts to understand merozoite invasion, identify merozoite surface proteins, define antigens and effector mechanisms that are crucial to protective immunity and prioritize antigens for vaccine development. Great progress has been made in recent years towards advancing our understanding in these areas. As highlighted in this review, we now have a much more detailed and sophisticated view of the merozoite surface. However, much remains to be done and there are many major gaps in our knowledge. The major challenge remains translating this knowledge into interventions, such as vaccines and therapeutics, to address the enormous global burden of malaria. Therefore, translational research and activities that harnesses our growing knowledge of the merozoite must remain a high priority.
Acknowledgments
We thank Christine Langer for help with preparing figures and Arzum Cubuk for help with preparing the manuscript.
FUNDING
Funding to the authors was provided by the National Health and Medical Research Council of Australia (Program grant and Senior Research Fellowship to JB; Early Career Fellowships to MB and JR; Infrastructure for Research Institutes Support Scheme Grant to the Burnet Institute); Australian Research Council (Future Fellowship to F. Fowkes); Victorian State Government Operational Infrastructure Support Grant to the Burnet Institute.
Conflict of interest. None declared.
REFERENCES
- Adams JH, Sim BK, Dolan SA, et al. A family of erythrocyte binding proteins of malaria parasites. P Natl Acad Sci USA. 1992;89:7085–9. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JH, Blair PL, Kaneko O, et al. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 2001;17:297–9. doi: 10.1016/s1471-4922(01)01948-1. [DOI] [PubMed] [Google Scholar]
- Adda CG, Murphy VJ, Sunde M, et al. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol Biochem Parasit. 2009;166:159–71. doi: 10.1016/j.molbiopara.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M, Miller LH, Johnson J, et al. Erythrocyte entry by malarial parasites. a moving junction between erythrocyte and parasite. J Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso PL, Smith T, Schellenberg JR, et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania [see comments] Lancet. 1994;344:1175–81. doi: 10.1016/s0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- Arevalo-Pinzon G, Bermudez M, Curtidor H, et al. The Plasmodium vivax rhoptry neck protein 5 is expressed in the apical pole of Plasmodium vivax VCG-1 strain schizonts and binds to human reticulocytes. Malaria J. 2015;14:106. doi: 10.1186/s12936-015-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Pinzon G, Curtidor H, Patino LC, et al. PvRON2, a new Plasmodium vivax rhoptry neck antigen. Malaria J. 2011;10:60. doi: 10.1186/1475-2875-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Pinzon G, Curtidor H, Abril J, et al. Annotation and characterization of the Plasmodium vivax rhoptry neck protein 4 (PvRON4) Malar J. 2013;12:356. doi: 10.1186/1475-2875-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TU, Takeo S, Yamasaki T, et al. Discovery of GAMA, a Plasmodium falciparum merozoite micronemal protein, as a novel blood-stage vaccine candidate antigen. Infect Immun. 2011;79:4523–32. doi: 10.1128/IAI.05412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audran R, Cachat M, Lurati F, et al. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun. 2005;73:8017–26. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiane AS, Bei AK, Ahouidi AD, et al. Inhibitory humoral responses to the Plasmodium falciparum vaccine candidate EBA-175 are independent of the erythrocyte invasion pathway. Clin Vaccine Immunol. 2013;20:1238–45. doi: 10.1128/CVI.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MR, Li X, Hanada T, et al. Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells. Blood. 2015;125:2704–11. doi: 10.1182/blood-2014-11-611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister LH, Butcher GA, Dennis ED, et al. Structure and invasive behaviour of Plasmodium knowlesi merozoites in vitro. Parasitology. 1975;71:483–91. doi: 10.1017/s0031182000047247. [DOI] [PubMed] [Google Scholar]
- Bargieri DY, Andenmatten N, Lagal V, et al. Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nat Commun. 2013;4:2552. doi: 10.1038/ncomms3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron C. Transcription of the gene for the merozoite surface antigen MSA2 of the human malaria parasite Plasmodium falciparum during the asexual cycle. FEBS Lett. 1992;300:77–81. doi: 10.1016/0014-5793(92)80168-g. [DOI] [PubMed] [Google Scholar]
- Barry AE, Schultz L, Buckee CO, et al. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One. 2009;4:e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdson SJ, Bustamante LY, Crosnier C, et al. Semaphorin-7A is an erythrocyte receptor for P. falciparum merozoite-specific TRAP homolog, MTRAP. PLoS Pathog. 2012;8:e1003031. doi: 10.1371/journal.ppat.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor JD, Malpede BM, Omattage NS, et al. Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLoS Pathog. 2014;10:e1003869. doi: 10.1371/journal.ppat.1003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor JD, Zahm JA, Tolia NH. Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat Struct Mol Biol. 2011;18:908–14. doi: 10.1038/nsmb.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Richard D, Healer J, et al. A conserved molecular motor drives cell invasion and gliding motility across malaria life cycle stages and other apicomplexan parasites. J Biol Chem. 2006;281:5197–208. doi: 10.1074/jbc.M509807200. [DOI] [PubMed] [Google Scholar]
- Baum J, Chen L, Healer J, et al. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol. 2009;39:371–80. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Beeson J, Fowkes FJ, Reiling L, et al. Correlates of protection for Plasmodium falciparum malaria vaccine development: current knowledge and future research. In: Corradin G, Engers H, editors. Malaria Vaccine Development: Over 40 Years of Trials and Tribulations. London: Future Medicine; 2014. pp. 81–104. [Google Scholar]
- Beeson JG, Osier FH, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–84. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Belard S, Issifou S, Hounkpatin AB, et al. A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PLoS One. 2011;6:e22525. doi: 10.1371/journal.pone.0022525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S, Michelin A, Poncet J, et al. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog. 2009;5:e1000309. doi: 10.1371/journal.ppat.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Choudhary P, Elias SC, et al. Assessment of humoral immune responses to blood-stage malaria antigens following ChAd63-MVA immunization, controlled human malaria infection and natural exposure. PLoS One. 2014;9:e107903. doi: 10.1371/journal.pone.0107903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CG, Barnwell JW, Huber CS, et al. The Plasmodium vivax homologues of merozoite surface proteins 4 and 5 from Plasmodium falciparum are expressed at different locations in the merozoite. Mol Biochem Parasit. 2002;120:215–24. doi: 10.1016/s0166-6851(01)00458-3. [DOI] [PubMed] [Google Scholar]
- Black CG, Wang L, Hibbs AR, et al. Identification of the Plasmodium chabaudi homologue of merozoite surface proteins 4 and 5 of Plasmodium falciparum. Infect Immun. 1999;67:2075–81. doi: 10.1128/iai.67.5.2075-2081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CG, Wang L, Wu T, et al. Apical location of a novel EGF-like domain-containing protein of Plasmodium falciparum. Mol Biochem Parasit. 2003;127:59–68. doi: 10.1016/s0166-6851(02)00308-0. [DOI] [PubMed] [Google Scholar]
- Blackman MJ. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 1994;45:213–20. doi: 10.1016/s0091-679x(08)61853-1. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Chappel JA, Shai S, et al. A conserved parasite serine protease processes the Plasmodium falciparum merozoite surface protein-1. Mol Biochem Parasit. 1993;62:103–14. doi: 10.1016/0166-6851(93)90182-w. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Dennis ED, Hirst EM, et al. Plasmodium knowlesi: secondary processing of the malaria merozoite surface protein-1. Exp Parasitol. 1996;83:229–39. doi: 10.1006/expr.1996.0069. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Heidrich HG, Donachie S, et al. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–82. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman MJ, Holder AA. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasit. 1992;50:307–15. doi: 10.1016/0166-6851(92)90228-c. [DOI] [PubMed] [Google Scholar]
- Blackman MJ, Scott-Finnigan TJ, Shai S, et al. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–93. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre MB, Dziegiel M, Hogh B, et al. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasit. 1991;49:119–31. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Oeuvray C, Lunel F, et al. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–18. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer PW, Stewart LB, Aspeling-Jones H, et al. Variation in Plasmodium falciparum Erythrocyte Invasion Phenotypes and Merozoite Ligand Gene Expression across Different Populations in Areas of Malaria Endemicity. Infect Immun. 2015;83:2575–82. doi: 10.1128/IAI.03009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, Langer C, Chan JA, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun. 2014;82:924–36. doi: 10.1128/IAI.00866-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, Reiling L, Feng G, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42:580–90. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MJ, Richards JS, Gilson PR, et al. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood. 2010a;115:4559–68. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- Boyle MJ, Wilson DW, Beeson JG. New approaches to studying Plasmodium falciparum merozoite invasion and insights into invasion biology. Int J Parasitol. 2013;43:1–10. doi: 10.1016/j.ijpara.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Boyle MJ, Wilson DW, Richards JS, et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. P Natl Acad Sci USA. 2010b;107:14378–83. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaglia CA, Coppens I, Hol WG, et al. Sites of interaction between aldolase and thrombospondin-related anonymous protein in plasmodium. Mol Biol Cell. 2003;14:4947–57. doi: 10.1091/mbc.E03-06-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–63. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, Blackman MJ. A new release on life: emerging concepts in proteolysis and parasite invasion. Mol Microbiol. 2005;55:1617–30. doi: 10.1111/j.1365-2958.2005.04483.x. [DOI] [PubMed] [Google Scholar]
- Chan JA, Howell KB, Reiling L, et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest. 2012;122:3227–38. doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohanadas R, Basappa , Russell B, et al. Small molecule targeting malaria merozoite surface protein-1 (MSP-1) prevents host invasion of divergent plasmodial species. J Infect Dis. 2014;210:1616–26. doi: 10.1093/infdis/jiu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lopaticki S, Riglar DT, et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011;7:e1002199. doi: 10.1371/journal.ppat.1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Li J, Ito D, et al. Antigenicity and immunogenicity of PvRALP1, a novel Plasmodium vivax rhoptry neck protein. Malaria J. 2015;14:186. doi: 10.1186/s12936-015-0698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wang Y, Ito D, et al. The Plasmodium vivax merozoite surface protein 1 paralog is a novel erythrocyte-binding ligand of P. vivax. Infect Immun. 2013;81:1585–95. doi: 10.1128/IAI.01117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child MA, Epp C, Bujard H, et al. Regulated maturation of malaria merozoite surface protein-1 is essential for parasite growth. Mol Microbiol. 2010;78:187–202. doi: 10.1111/j.1365-2958.2010.07324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child MA, Harris PK, Collins CR, et al. Molecular determinants for subcellular trafficking of the malarial sheddase PfSUB2. Traffic. 2013;14:1053–64. doi: 10.1111/tra.12092. [DOI] [PubMed] [Google Scholar]
- Chitnis CE, Mukherjee P, Mehta S, et al. Phase I clinical trial of a recombinant blood stage vaccine candidate for Plasmodium Falciparum malaria based on MSP1 and EBA175. PLoS One. 2015;10:e0117820. doi: 10.1371/journal.pone.0117820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Hodder AN, Lin CS, et al. Antibodies to the Plasmodium falciparum proteins MSPDBL1 and MSPDBL2 opsonize merozoites, inhibit parasite growth, and predict protection from clinical malaria. J Infect Dis. 2015;212:406–15. doi: 10.1093/infdis/jiv057. [DOI] [PubMed] [Google Scholar]
- Clark JT, Donachie S, Anand R, et al. 46–53 kilodalton glycoprotein from the surface of Plasmodium falciparum merozoites. Mol Biochem Parasit. 1989;32:15–24. doi: 10.1016/0166-6851(89)90125-4. [DOI] [PubMed] [Google Scholar]
- Cole-Tobian JL, Michon P, Biasor M, et al. Strain-specific duffy binding protein antibodies correlate with protection against infection with homologous compared to heterologous Plasmodium vivax strains in Papua New Guinean children. Infect Immun. 2009;77:4009–17. doi: 10.1128/IAI.00158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DJ. Paths to a malaria vaccine illuminated by parasite genomics. Trends Genet. 2015;31:97–107. doi: 10.1016/j.tig.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton PD, Kayala MA, Traore B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. P Natl Acad Sci USA. 2010;107:6958–63. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Bustamante LY, Bartholdson SJ, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–7. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts J, Powell R, Agius P, et al. Immunological markers of P. vivax exposure and immunity: a systematic review and meta-analysis. BMC Medicine. 2014;12:150. doi: 10.1186/s12916-014-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro U, Leach A, Drakeley CJ, et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–7. doi: 10.1016/s0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- Das S, Hertrich N, Perrin AJ, et al. Processing of Plasmodium falciparum merozoite surface protein msp1 activates a spectrin-binding function enabling parasite egress from RBCs. Cell Host Microbe. 2015;18:433–44. doi: 10.1016/j.chom.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrabant A, Delplace P. Leupeptin alters the proteolytic processing of P126, the major parasitophorous vacuole antigen of Plasmodium falciparum. Mol Biochem Parasit. 1989;33:151–8. doi: 10.1016/0166-6851(89)90029-7. [DOI] [PubMed] [Google Scholar]
- Delplace P, Fortier B, Tronchin G, et al. Localization, biosynthesis, processing and isolation of a major 126 kDa antigen of the parasitophorous vacuole of Plasmodium falciparum. Mol Biochem Parasit. 1987;23:193–201. doi: 10.1016/0166-6851(87)90026-0. [DOI] [PubMed] [Google Scholar]
- Dent AE, Nakajima R, Liang L, et al. Plasmodium falciparum protein microarray antibody profiles correlate with protection from symptomatic malaria in Kenya. J Infect Dis. 2015;212:1429–38. doi: 10.1093/infdis/jiv224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A, Diemert DJ, Sagara I, et al. Impact of a Plasmodium falciparum AMA1 vaccine on antibody responses in adult Malians. PLoS One. 2007;2:e1045. doi: 10.1371/journal.pone.0001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A, Sagara I, Ellis RD, et al. Phase 1 study of a combination AMA1 blood stage malaria vaccine in Malian children. PLoS One. 2008;3:e1563. doi: 10.1371/journal.pone.0001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzewski AR, Ling IT, Hopkins JM, et al. Formation of the food vacuole in Plasmodium falciparum: a potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)) PLoS One. 2008;3:e3085. doi: 10.1371/journal.pone.0003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobano C, Quelhas D, Quinto L, et al. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin Vaccine Immunol. 2012;19:157–66. doi: 10.1128/CVI.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SA, Miller LH, Wellems TE. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990;86:618–24. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DL, Mu Y, Unal B, et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics. 2008;8:4680–94. doi: 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AD, Baldeviano GC, Lucas CM, et al. A PfRH5-based vaccine is efficacious against Heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe. 2015;17:130–9. doi: 10.1016/j.chom.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley C, Cook J. Chapter 5. Potential contribution of sero-epidemiological analysis for monitoring malaria control and elimination: historical and current perspectives. Adv Parasitol. 2009;69:299–352. doi: 10.1016/S0065-308X(09)69005-9. [DOI] [PubMed] [Google Scholar]
- Drew DR, Beeson JG. PfRH5 as a candidate vaccine for Plasmodium falciparum malaria. Trends Parasitol. 2015;31:87–8. doi: 10.1016/j.pt.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Drew DR, Hodder AN, Wilson DW, et al. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One. 2012;7:e51023. doi: 10.1371/journal.pone.0051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer AM, Matile H, Papastogiannidis P, et al. Passive immunoprotection of Plasmodium falciparum-infected mice designates the CyRPA as candidate malaria vaccine antigen. J Immunol. 2012;188:6225–37. doi: 10.4049/jimmunol.1103177. [DOI] [PubMed] [Google Scholar]
- Druilhe P, Spertini F, Soesoe D, et al. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2005;2:e344. doi: 10.1371/journal.pmed.0020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CJ, Sheehy SH, Ewer KJ, et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One. 2011;6:e22271. doi: 10.1371/journal.pone.0022271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dups JN, Pepper M, Cockburn IA. Antibody and B cell responses to Plasmodium sporozoites. Front Microbiol. 2014;5:625. doi: 10.3389/fmicb.2014.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Triglia T, Ralph SA, et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22:1047–57. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Dlugosz LS, Drew DR, et al. Overcoming antigenic diversity by enhancing the immunogenicity of conserved epitopes on the malaria vaccine candidate apical membrane antigen-1. PLoS Pathog. 2013;9:e1003840. doi: 10.1371/journal.ppat.1003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Haynes JD, Moch JK, et al. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. P Natl Acad Sci USA. 2003;100:12295–300. doi: 10.1073/pnas.2032858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorin JD, Bei AK, Coleman BI, et al. Functional diversification between two related Plasmodium falciparum merozoite invasion ligands is determined by changes in the cytoplasmic domain. Mol Microbiol. 2010;75:990–1006. doi: 10.1111/j.1365-2958.2009.07040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AF, Burghaus P, Druilhe P, et al. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 1999;21:133–9. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- Egan ES, Jiang RH, Moechtar MA, et al. Malaria. A forward genetic screen identifies erythrocyte CD55 as essential for Plasmodium falciparum invasion. Science. 2015;348:711–4. doi: 10.1126/science.aaa3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sahly HM, Patel SM, Atmar RL, et al. Safety and immunogenicity of a recombinant nonglycosylated EBA175 Region II malaria vaccine in healthy adults living in an area where malaria is not endemic. Clin Vaccine Immunol. 2010;17:1552–9. doi: 10.1128/CVI.00082-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SR, Fowkes FJ, Richards JS, et al. Research priorities for the development and implementation of serological tools for malaria surveillance. F1000 Prime Rep. 2014;6:100. doi: 10.12703/P6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RD, Wu Y, Martin LB, et al. Phase 1 study in malaria naive adults of BSAM2/Alhydrogel(R)+CPG 7909, a blood stage vaccine against P. falciparum malaria. PLoS One. 2012;7:e46094. doi: 10.1371/journal.pone.0046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen M, Kremsner PG, Schleucher R, et al. Safety and immunogenicity of GMZ2 - a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine. 2009;27:6862–8. doi: 10.1016/j.vaccine.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Fenton B, Clark JT, Khan CM, et al. Structural and antigenic polymorphism of the 35- to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol Cell Biol. 1991;11:963–71. doi: 10.1128/mcb.11.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Becerra C, Sanz S, Brucet M, et al. Naturally-acquired humoral immune responses against the N- and C-termini of the Plasmodium vivax MSP1 protein in endemic regions of Brazil and Papua New Guinea using a multiplex assay. Malaria J. 2010;9:29. doi: 10.1186/1475-2875-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck SL, Birdsall B, Babon J, et al. Suramin and suramin analogues inhibit merozoite surface protein-1 secondary processing and erythrocyte invasion by the malaria parasite Plasmodium falciparum. J Biol Chem. 2003;278:47670–7. doi: 10.1074/jbc.M306603200. [DOI] [PubMed] [Google Scholar]
- Fowkes FJ, Richards JS, Simpson JA, et al. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes FJ, McGready R, Cross NJ, et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis. 2012;206:1612–21. doi: 10.1093/infdis/jis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Gunalan K, Yap SS, et al. Triggers of key calcium signals during erythrocyte invasion by Plasmodium falciparum. Nat Commun. 2013;4:2862. doi: 10.1038/ncomms3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Yeo KP, Aw SS, et al. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog. 2008;4:e1000104. doi: 10.1371/journal.ppat.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Y, Puentes A, Curtidor H, et al. Identifying merozoite surface protein 4 and merozoite surface protein 7 Plasmodium falciparum protein family members specifically binding to human erythrocytes suggests a new malarial parasite-redundant survival mechanism. J Med Chem. 2007;50:5665–75. doi: 10.1021/jm070773z. [DOI] [PubMed] [Google Scholar]
- Gaur D, Furuya T, Mu J, et al. Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. Mol Biochem Parasit. 2006;145:205–15. doi: 10.1016/j.molbiopara.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Genton B, Al-Yaman F, Anders R, et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine in adults living in an endemic area of Papua New Guinea. Vaccine. 2000;18:2504–11. doi: 10.1016/s0264-410x(00)00036-0. [DOI] [PubMed] [Google Scholar]
- Genton B, Al-Yaman F, Betuela I, et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine (MSP1, MSP2, RESA) against Plasmodium falciparum in Papua New Guinean children. Vaccine. 2003;22:30–41. doi: 10.1016/s0264-410x(03)00536-x. [DOI] [PubMed] [Google Scholar]
- Genton B, Betuela I, Felger I, et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1–2b trial in PNG. J Infect Dis. 2002;185:820–7. doi: 10.1086/339342. [DOI] [PubMed] [Google Scholar]
- Genton B, Pluschke G, Degen L, et al. A randomized placebo-controlled phase Ia malaria vaccine trial of two virosome-formulated synthetic peptides in healthy adult volunteers. PLoS One. 2007;2:e1018. doi: 10.1371/journal.pone.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff DL, Creasey A, Maslau S, et al. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. P Natl Acad Sci USA. 2005;102:13598–603. doi: 10.1073/pnas.0502378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilberger TW, Thompson JK, Triglia T, et al. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem. 2003;278:14480–6. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- Gilson PR, Crabb BS. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int J Parasitol. 2009;39:91–6. doi: 10.1016/j.ijpara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Gilson PR, Nebl T, Vukcevic D, et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2006;5:1286–99. doi: 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- Giovannini D, Spath S, Lacroix C, et al. Independent roles of apical membrane antigen 1 and rhoptry neck proteins during host cell invasion by apicomplexa. Cell Host Microbe. 2011;10:591–602. doi: 10.1016/j.chom.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Goel VK, Li X, Chen H, et al. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. P Natl Acad Sci USA. 2003;100:5164–9. doi: 10.1073/pnas.0834959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P, Gelband H. Vaccines for preventing malaria (SPf66) Cochrane Db Syst Rev. 2006:CD005966. doi: 10.1002/14651858.CD005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, Corran PH, Mangia E, et al. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem. 2007;53:1244–53. doi: 10.1373/clinchem.2006.081695. [DOI] [PubMed] [Google Scholar]
- Green JL, Hinds L, Grainger M, et al. Plasmodium thrombospondin related apical merozoite protein (PTRAMP) is shed from the surface of merozoites by PfSUB2 upon invasion of erythrocytes. Mol Biochem Parasit. 2006;150:114–7. doi: 10.1016/j.molbiopara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Grimberg BT, Udomsangpetch R, Xainli J, et al. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med. 2007;4:e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S, Cabrera A, Langer C, et al. Characterization of a conserved rhoptry-associated leucine zipper-like protein in the malaria parasite Plasmodium falciparum. Infect Immun. 2008;76:879–87. doi: 10.1128/IAI.00144-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PK, Yeoh S, Dluzewski AR, et al. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 2005;1:241–51. doi: 10.1371/journal.ppat.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Grigg ME, Boothroyd JC, et al. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat Struct Biol. 2002;9:606–11. doi: 10.1038/nsb819. [DOI] [PubMed] [Google Scholar]
- Healer J, Thompson JK, Riglar DT, et al. Vaccination with conserved regions of erythrocyte-binding antigens induces neutralizing antibodies against multiple strains of Plasmodium falciparum. PLoS One. 2013;8:e72504. doi: 10.1371/journal.pone.0072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsen CC, Verhage DF, Telgt DS, et al. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine. 2007;25:2930–40. doi: 10.1016/j.vaccine.2006.06.081. [DOI] [PubMed] [Google Scholar]
- Hill DL, Eriksson EM, Li Wai Suen CS, et al. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One. 2013;8:e74627. doi: 10.1371/journal.pone.0074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds L, Green JL, Knuepfer E, et al. Novel putative glycosylphosphatidylinositol-anchored micronemal antigen of Plasmodium falciparum that binds to erythrocytes. Eukaryot Cell. 2009;8:1869–79. doi: 10.1128/EC.00218-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–94. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder AN, Crewther PE, Matthew ML, et al. The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem. 1996;271:29446–52. doi: 10.1074/jbc.271.46.29446. [DOI] [PubMed] [Google Scholar]
- Hodder AN, Drew DR, Epa VC, et al. Enzymic, phylogenetic, and structural characterization of the unusual papain-like protease domain of Plasmodium falciparum SERA5. J Biol Chem. 2003;278:48169–77. doi: 10.1074/jbc.M306755200. [DOI] [PubMed] [Google Scholar]
- Hogh B, Thompson R, Zakiuddin IS, et al. Glutamate rich Plasmodium falciparum antigen (GLURP) Parassitologia. 1993;35(Suppl):47–50. [PubMed] [Google Scholar]
- Holder AA. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–56. doi: 10.1017/S0031182009990515. [DOI] [PubMed] [Google Scholar]
- Horii T, Shirai H, Jie L, et al. Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol Int. 2010;59:380–6. doi: 10.1016/j.parint.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Howell SA, Hackett F, Jongco AM, et al. Distinct mechanisms govern proteolytic shedding of a key invasion protein in apicomplexan pathogens. Mol Microbiol. 2005;57:1342–56. doi: 10.1111/j.1365-2958.2005.04772.x. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen Z, Gu J, et al. Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS One. 2008;3:e1952. doi: 10.1371/journal.pone.0001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani V, Ramsland PA, Guy AJ, et al. Acquisition of functional antibodies that block the binding of erythrocyte binding antigen 175 and protection from Plasmodium falciparum malaria in children. Clin Infect Dis. 2015;61:1244–52. doi: 10.1093/cid/civ525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–75. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- Ito D, Hasegawa T, Miura K, et al. RALP1 is a rhoptry neck erythrocyte-binding protein of Plasmodium falciparum merozoites and a potential blood-stage vaccine candidate antigen. Infect Immun. 2013;81:4290–8. doi: 10.1128/IAI.00690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet A, Coulon L, De Neve J, et al. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasit. 2001;116:35–44. doi: 10.1016/s0166-6851(01)00297-3. [DOI] [PubMed] [Google Scholar]
- Jepsen MP, Jogdand PS, Singh SK, et al. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J Infect Dis. 2013;208:479–88. doi: 10.1093/infdis/jit185. [DOI] [PubMed] [Google Scholar]
- Jones TR, Narum DL, Gozalo AS, et al. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. J Infect Dis. 2001;183:303–12. doi: 10.1086/317933. [DOI] [PubMed] [Google Scholar]
- Joos C, Marrama L, Polson HE, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One. 2010;5:e9871. doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekoppala M, Holder AA. Merozoite surface proteins of the malaria parasite: the MSP1 complex and the MSP7 family. Int J Parasitol. 2010;40:1155–61. doi: 10.1016/j.ijpara.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Kadekoppala M, O'Donnell RA, Grainger M, et al. Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes. Eukaryot Cell. 2008;7:2123–32. doi: 10.1128/EC.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano FS, Sanchez BA, Sousa TN, et al. Plasmodium vivax Duffy binding protein: baseline antibody responses and parasite polymorphisms in a well-consolidated settlement of the Amazon Region. Trop Med Int Health. 2012;17:989–1000. doi: 10.1111/j.1365-3156.2012.03016.x. [DOI] [PubMed] [Google Scholar]
- Kariuki MM, Li X, Yamodo I, et al. Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Bioph Res Co. 2005;338:1690–5. doi: 10.1016/j.bbrc.2005.10.154. [DOI] [PubMed] [Google Scholar]
- Kauth CW, Epp C, Bujard H, et al. The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: interactions and arrangements of subunits. J Biol Chem. 2003;278:22257–64. doi: 10.1074/jbc.M302299200. [DOI] [PubMed] [Google Scholar]
- Kauth CW, Woehlbier U, Kern M, et al. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:31517–27. doi: 10.1074/jbc.M604641200. [DOI] [PubMed] [Google Scholar]
- Keitel WA, Kester KE, Atmar RL, et al. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (msp-1(19)) and T helper epitopes of tetanus toxoid. Vaccine. 1999;18:531–9. doi: 10.1016/s0264-410x(99)00221-2. [DOI] [PubMed] [Google Scholar]
- Khusmith S, Druilhe P. Specific arming of monocytes by cytophilic IgG promotes Plasmodium falciparum merozoite ingestion. T Roy Soc Trop Med H. 1982;76:423–4. doi: 10.1016/0035-9203(82)90208-5. [DOI] [PubMed] [Google Scholar]
- Khusmith S, Druilhe P, Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982;35:874–9. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CL, Michon P, Shakri AR, et al. Naturally acquired Duffy-binding protein-specific binding inhibitory antibodies confer protection from blood-stage Plasmodium vivax infection. P Natl Acad Sci USA. 2008;105:8363–8. doi: 10.1073/pnas.0800371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp B, Nau U, Hundt E, et al. A new blood stage antigen of Plasmodium falciparum highly homologous to the serine-stretch protein SERP. Mol Biochem Parasit. 1991;44:1–13. doi: 10.1016/0166-6851(91)90215-r. [DOI] [PubMed] [Google Scholar]
- Koussis K, Withers-Martinez C, Yeoh S, et al. A multifunctional serine protease primes the malaria parasite for red blood cell invasion. EMBO J. 2009;28:725–35. doi: 10.1038/emboj.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda R, Aikawa M, Sprinz H. Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol. 1969;55:633–44. [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin J, et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 2011;7:e1001276. doi: 10.1371/journal.ppat.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque MH, Roques M, Kong-Hap M, et al. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun. 2014;5:4098. doi: 10.1038/ncomms5098. [DOI] [PubMed] [Google Scholar]
- Langreth SG, Nguyen-Dinh P, Trager W. Plasmodium falciparum: merozoite invasion in vitro in the presence of chloroquine. Exp Parasitol. 1978;46:235–8. doi: 10.1016/0014-4894(78)90136-4. [DOI] [PubMed] [Google Scholar]
- Lanzillotti R, Coetzer TL. The 10 kDa domain of human erythrocyte protein 4.1 binds the Plasmodium falciparum EBA-181 protein. Malaria J. 2006;5:100. doi: 10.1186/1475-2875-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G, Cheng QQ, Reed C, et al. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18:1925–31. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- Leykauf K, Treeck M, Gilson PR, et al. Protein kinase a dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog. 2010;6:e1000941. doi: 10.1371/journal.ppat.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han ET. Dissection of the Plasmodium vivax reticulocyte binding-like proteins (PvRBPs) Biochem Bioph Res Co. 2012;426:1–6. doi: 10.1016/j.bbrc.2012.08.055. [DOI] [PubMed] [Google Scholar]
- Lima-Junior JC, Jiang J, Rodrigues-da-Silva RN, et al. B cell epitope mapping and characterization of naturally acquired antibodies to the Plasmodium vivax merozoite surface protein-3alpha (PvMSP-3alpha) in malaria exposed individuals from Brazilian Amazon. Vaccine. 2011;29:1801–11. doi: 10.1016/j.vaccine.2010.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior JC, Rodrigues-da-Silva RN, Banic DM, et al. Influence of HLA-DRB1 and HLA-DQB1 alleles on IgG antibody response to the P. vivax MSP-1, MSP-3alpha and MSP-9 in individuals from Brazilian endemic area. PLoS One. 2012;7:e36419. doi: 10.1371/journal.pone.0036419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Junior JC, Tran TM, Meyer EV, et al. Naturally acquired humoral and cellular immune responses to Plasmodium vivax merozoite surface protein 9 in Northwestern Amazon individuals. Vaccine. 2008;26:6645–54. doi: 10.1016/j.vaccine.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo CA, Rodriguez M, Reid M, et al. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl) Blood. 2003;101:4628–31. doi: 10.1182/blood-2002-10-3076. [DOI] [PubMed] [Google Scholar]
- Lopez R, Valbuena J, Rodriguez LE, et al. Plasmodium falciparum merozoite surface protein 6 (MSP-6) derived peptides bind erythrocytes and partially inhibit parasite invasion. Peptides. 2006;27:1685–92. doi: 10.1016/j.peptides.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Low A, Chandrashekaran IR, Adda CG, et al. Merozoite surface protein 2 of Plasmodium falciparum: expression, structure, dynamics, and fibril formation of the conserved N-terminal domain. Biopolymers. 2007;87:12–22. doi: 10.1002/bip.20764. [DOI] [PubMed] [Google Scholar]
- McCarra MB, Ayodo G, Sumba PO, et al. Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr Infect Dis J. 2011;30:1037–42. doi: 10.1097/INF.0b013e31822d1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JS, Marjason J, Elliott S, et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide(R) ISA 720. PLoS One. 2011;6:e24413. doi: 10.1371/journal.pone.0024413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoubrie JE, Miller SK, Sargeant T, et al. Evidence for a common role for the serine-type Plasmodium falciparum serine repeat antigen proteases: implications for vaccine and drug design. Infect Immun. 2007;75:5565–74. doi: 10.1128/IAI.00405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRaild CA, Pedersen MO, Anders RF, et al. Lipid interactions of the malaria antigen merozoite surface protein 2. Biochim Biophys Acta. 2012;1818:2572–8. doi: 10.1016/j.bbamem.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Maier AG, Baum J, Smith B, et al. Polymorphisms in erythrocyte binding antigens 140 and 181 affect function and binding but not receptor specificity in Plasmodium falciparum. Infect Immun. 2009;77:1689–99. doi: 10.1128/IAI.01331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier AG, Duraisingh MT, Reeder JC, et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin E, Hu J, Li Z, et al. A Phase 1 trial of PfCP2.9: An AMA1/MSP1 chimeric recombinant protein vaccine for Plasmodium falciparum malaria. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.09.081. [DOI] [PubMed] [Google Scholar]
- Malkin EM, Diemert DJ, McArthur JH, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005;73:3677–85. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Li A, Zhang R, et al. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125:1314–24. doi: 10.1182/blood-2014-08-596015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall VM, Tieqiao W, Coppel RL. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol Biochem Parasit. 1998;94:13–25. doi: 10.1016/s0166-6851(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Mayer DC, Cofie J, Jiang L, et al. Glycophorin B is the erythrocyte receptor of Plasmodium falciparum erythrocyte-binding ligand, EBL-1. P Natl Acad Sci USA. 2009;106:5348–52. doi: 10.1073/pnas.0900878106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrizi AA, Zakeri S, Salmanian AH, et al. Plasmodium falciparum: sequence analysis of the gene encoding the C-terminus region of the merozoite surface protein-1, a potential malaria vaccine antigen, in Iranian clinical isolates. Exp Parasitol. 2008;118:378–85. doi: 10.1016/j.exppara.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mello K, Daly TM, Morrisey J, et al. A multigene family that interacts with the amino terminus of plasmodium MSP-1 identified using the yeast two-hybrid system. Eukaryot Cell. 2002;1:915–25. doi: 10.1128/EC.1.6.915-925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard R. The journey of the malaria sporozoite through its hosts: two parasite proteins lead the way. Microbes Infect. 2000;2:633–42. doi: 10.1016/s1286-4579(00)00362-2. [DOI] [PubMed] [Google Scholar]
- Miller LH, Roberts T, Shahabuddin M, et al. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1) Mol Biochem Parasit. 1993;59:1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- Miller SK, Good RT, Drew DR, et al. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. J Biol Chem. 2002;277:47524–32. doi: 10.1074/jbc.M206974200. [DOI] [PubMed] [Google Scholar]
- Mills KE, Pearce JA, Crabb BS, et al. Truncation of merozoite surface protein 3 disrupts its trafficking and that of acidic-basic repeat protein to the surface of Plasmodium falciparum merozoites. Mol Microbiol. 2002;43:1401–11. doi: 10.1046/j.1365-2958.2002.02834.x. [DOI] [PubMed] [Google Scholar]
- Miura K, Zhou H, Diouf A, et al. Immunological responses against Plasmodium falciparum Apical Membrane Antigen 1 vaccines vary depending on the population immunized. Vaccine. 2011;29:2255–61. doi: 10.1016/j.vaccine.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan BJ, Wang L, Coppel RL. No TRAP, no invasion. Trends Parasitol. 2009;25:77–84. doi: 10.1016/j.pt.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Mordmuller B, Szywon K, Greutelaers B, et al. Safety and immunogenicity of the malaria vaccine candidate GMZ2 in malaria-exposed, adult individuals from Lambarene, Gabon. Vaccine. 2010;28:6698–703. doi: 10.1016/j.vaccine.2010.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Perez DA, Degano R, Ibarrola N, et al. Determining the Plasmodium vivax VCG-1 strain blood stage proteome. J Proteomics. 2014;113C:268–80. doi: 10.1016/j.jprot.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Moss DK, Remarque EJ, Faber BW, et al. Plasmodium falciparum 19-kilodalton merozoite surface protein 1 (MSP1)-specific antibodies that interfere with parasite growth in vitro can inhibit MSP1 processing, merozoite invasion, and intracellular parasite development. Infect Immun. 2012;80:1280–7. doi: 10.1128/IAI.05887-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GE, Ellis RD, Miura K, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narum DL, Thomas AW. Differential localization of full-length and processed forms of PF83/AMA-1 an apical membrane antigen of Plasmodium falciparum merozoites. Mol Biochem Parasit. 1994;67:59–68. doi: 10.1016/0166-6851(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Nery S, Deans AM, Mosobo M, et al. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. Mol Biochem Parasit. 2006;149:208–15. doi: 10.1016/j.molbiopara.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F, Luxemburger C, Kyle DE, et al. Randomised double-blind placebo-controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Shoklo SPf66 Malaria Vaccine Trial Group. Lancet. 1996;348:701–7. doi: 10.1016/s0140-6736(96)04465-0. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Angov E, Kester KE, et al. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine. 2006;24:3009–17. doi: 10.1016/j.vaccine.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Ockenhouse CF, Sun PF, Lanar DE, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum. malaria. J Infect Dis. 1998;177:1664–73. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- O'Donnell RA, de Koning-Ward TF, Burt RA, et al. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med. 2001;193:1403–12. doi: 10.1084/jem.193.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell RA, Hackett F, Howell SA, et al. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol. 2006;174:1023–33. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, et al. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–602. [PubMed] [Google Scholar]
- Ogutu BR, Apollo OJ, McKinney D, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One. 2009;4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohas EA, Adams JH, Waitumbi JN, et al. Measurement of antibody levels against region II of the erythrocyte-binding antigen 175 of Plasmodium falciparum in an area of malaria holoendemicity in western Kenya. Infect Immun. 2004;72:735–41. doi: 10.1128/IAI.72.2.735-741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoyeh JN, Pillai CR, Chitnis CE. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect Immun. 1999;67:5784–91. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A, Collins CR, Hackett F, et al. Juxtamembrane shedding of Plasmodium falciparum AMA1 is sequence independent and essential, and helps evade invasion-inhibitory antibodies. PLoS Pathog. 2011;7:e1002448. doi: 10.1371/journal.ppat.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondigo BN, Hodges JS, Ireland KF, et al. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis. 2014;210:1123–32. doi: 10.1093/infdis/jiu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier FH, Feng G, Boyle MJ, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014a;12:108. doi: 10.1186/1741-7015-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier FH, Mackinnon MJ, Crosnier C, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med. 2014b;6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara A, Mu J, Takala-Harrison S, et al. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malaria J. 2010;9:175. doi: 10.1186/1475-2875-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachebat JA, Kadekoppala M, Grainger M, et al. Extensive proteolytic processing of the malaria parasite merozoite surface protein 7 during biosynthesis and parasite release from erythrocytes. Mol Biochem Parasit. 2007;151:59–69. doi: 10.1016/j.molbiopara.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Pachebat JA, Ling IT, Grainger M, et al. The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol Biochem Parasit. 2001;117:83–9. doi: 10.1016/s0166-6851(01)00336-x. [DOI] [PubMed] [Google Scholar]
- Palacpac NM, Ntege E, Yeka A, et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS One. 2013;8:e64073. doi: 10.1371/journal.pone.0064073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasay MC, Cheng Q, Rzepczyk C, et al. Dimorphism of the C terminus of the Plasmodium vivax merozoite surface protein 1. Mol Biochem Parasit. 1995;70:217–9. doi: 10.1016/0166-6851(95)00015-s. [DOI] [PubMed] [Google Scholar]
- Pearce JA, Triglia T, Hodder AN, et al. Plasmodium falciparum merozoite surface protein 6 is a dimorphic antigen. Infect Immun. 2004;72:2321–8. doi: 10.1128/IAI.72.4.2321-2328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraut R, Joos C, Sokhna C, et al. Association of antibody responses to the conserved Plasmodium falciparum merozoite surface protein 5 with protection against clinical malaria. PLoS One. 2014;9:e101737. doi: 10.1371/journal.pone.0101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson KE, Fowkes FJ, McCallum FJ, et al. Erythrocyte-binding antigens of Plasmodium falciparum are targets of human inhibitory antibodies and function to evade naturally acquired immunity. J Immunol. 2013;191:785–94. doi: 10.4049/jimmunol.1300444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson KE, McCallum FJ, Reiling L, et al. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J Clin Invest. 2008;118:342–51. doi: 10.1172/JCI32138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmeyer ML, Reiter K, Shimp RL, Jr, et al. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J Biol Chem. 2009;284:26951–63. doi: 10.1074/jbc.M109.013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus ME, Magill AJ, Cummings JF, et al. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2007;25:4203–12. doi: 10.1016/j.vaccine.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Polson HE, Conway DJ, Fandeur T, et al. Gene polymorphism of Plasmodium falciparum merozoite surface proteins 4 and 5. Mol Biochem Parasit. 2005;142:110–5. doi: 10.1016/j.molbiopara.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Price RN, Tjitra E, Guerra CA, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R. Studies on glycoproteins in the human malaria parasite Plasmodium falciparum. Identification of a myristilated 45kDa merozoite membrane glycoprotein. Immunol Cell Biol. 1987;65(Pt 5):419–24. doi: 10.1038/icb.1987.48. [DOI] [PubMed] [Google Scholar]
- Rayner JC, Galinski MR, Ingravallo P, et al. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. P Natl Acad Sci USA. 2000;97:9648–53. doi: 10.1073/pnas.160469097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner JC, Vargas-Serrato E, Huber CS, et al. Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J Exp Med. 2001;194:1571–81. doi: 10.1084/jem.194.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KS, Amlabu E, Pandey AK, et al. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. P Natl Acad Sci USA. 2015;112:1179–84. doi: 10.1073/pnas.1415466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MB, Caruana SR, Batchelor AH, et al. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. P Natl Acad Sci USA. 2000;97:7509–14. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling L, Richards JS, Fowkes FJ, et al. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol. 2010;185:6157–67. doi: 10.4049/jimmunol.1001555. [DOI] [PubMed] [Google Scholar]
- Reiling L, Richards JS, Fowkes FJ, et al. The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS One. 2012;7:e45253. doi: 10.1371/journal.pone.0045253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarque EJ, Faber BW, Kocken CH, et al. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun. 2008;76:2660–70. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice BL, Acosta MM, Pacheco MA, et al. The origin and diversification of the merozoite surface protein 3 (msp3) multi-gene family in Plasmodium vivax and related parasites. Mol Phylogenet Evol. 2014;78:172–84. doi: 10.1016/j.ympev.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Arumugam TU, Reiling L, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–90. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- Richards JS, Stanisic DI, Fowkes FJ, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- Riglar DT, Richard D, Wilson DW, et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011;9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Riglar DT, Whitehead L, Cowman AF, et al. Localization-based imaging of malarial antigens during red cell entry reaffirms role for AMA1 but not MTRAP in invasion. J Cell Sci. 2015;129:228–42. doi: 10.1242/jcs.177741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roestenberg M, Remarque E, de Jonge E, et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS One. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussilhon C, Oeuvray C, Muller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RTSS. Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruecker A, Shea M, Hackett F, et al. Proteolytic activation of the essential parasitophorous vacuole cysteine protease SERA6 accompanies malaria parasite egress from its host erythrocyte. J Biol Chem. 2012;287:37949–63. doi: 10.1074/jbc.M112.400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JR, Stoute JA, Amon J, et al. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg. 2006;75:575–81. [PubMed] [Google Scholar]
- Sagara I, Dicko A, Ellis RD, et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine. 2009a;27:3090–8. doi: 10.1016/j.vaccine.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I, Ellis RD, Dicko A, et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel + CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine. 2009b;27:7292–8. doi: 10.1016/j.vaccine.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Takeo S, Maier AG, et al. Antibodies against a Plasmodium falciparum antigen PfMSPDBL1 inhibit merozoite invasion into human erythrocytes. Vaccine. 2012;30:1972–80. doi: 10.1016/j.vaccine.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Sala KA, Nishiura H, Upton LM, et al. The Plasmodium berghei sexual stage antigen PSOP12 induces anti-malarial transmission blocking immunity both in vivo and in vitro. Vaccine. 2015;33:437–45. doi: 10.1016/j.vaccine.2014.11.038. [DOI] [PubMed] [Google Scholar]
- Sanders PR, Gilson PR, Cantin GT, et al. Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem. 2005;280:40169–76. doi: 10.1074/jbc.M509631200. [DOI] [PubMed] [Google Scholar]
- Sanders PR, Kats LM, Drew DR, et al. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun. 2006;74:4330–8. doi: 10.1128/IAI.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J, Graindorge A, Soldati-Favre D. New insights into parasite rhomboid proteases. Mol Biochem Parasit. 2012;182:27–36. doi: 10.1016/j.molbiopara.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Saul A. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 1987;9:1–9. doi: 10.1111/j.1365-3024.1987.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Saul A, Lawrence G, Allworth A, et al. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine. 2005;23:3076–83. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Saul A, Lawrence G, Smillie A, et al. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine. 1999;17:3145–59. doi: 10.1016/s0264-410x(99)00175-9. [DOI] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther. 2011;19:2269–76. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: assessment of efficacy against mosquito bite challenge in humans. Mol Ther. 2012a;20:2355–68. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, et al. Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors. PLoS One. 2012b;7:e31208. doi: 10.1371/journal.pone.0031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silmon de Monerri NC, Flynn HR, Campos MG, et al. Global identification of multiple substrates for Plasmodium falciparum SUB1, an essential malarial processing protease. Infect Immun. 2011;79:1086–97. doi: 10.1128/IAI.00902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim BK, Chitnis CE, Wasniowska K, et al. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–4. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, More KR, Chitnis CE. Role of calcineurin and actin dynamics in regulated secretion of microneme proteins in Plasmodium falciparum merozoites during erythrocyte invasion. Cell Microbiol. 2014;16:50–63. doi: 10.1111/cmi.12177. [DOI] [PubMed] [Google Scholar]
- Singh S, Soe S, Weisman S, et al. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One. 2009;4:e5410. doi: 10.1371/journal.pone.0005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirima SB, Cousens S, Druilhe P. Protection against malaria by MSP3 candidate vaccine. New Engl J Med. 2011;365:1062–4. doi: 10.1056/NEJMc1100670. [DOI] [PubMed] [Google Scholar]
- Sirima SB, Nebie I, Ouedraogo A, et al. Safety and immunogenicity of the Plasmodium falciparum merozoite surface protein-3 long synthetic peptide (MSP3-LSP) malaria vaccine in healthy, semi-immune adult males in Burkina Faso, West Africa. Vaccine. 2007;25:2723–32. doi: 10.1016/j.vaccine.2006.05.090. [DOI] [PubMed] [Google Scholar]
- Sirima SB, Tiono AB, Ouedraogo A, et al. Safety and immunogenicity of the malaria vaccine candidate MSP3 long synthetic peptide in 12–24 months-old Burkinabe children. PLoS One. 2009;4:e7549. doi: 10.1371/journal.pone.0007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe JA, Coppel RL, Day KP, et al. Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. P Natl Acad Sci USA. 1991;88:1751–5. doi: 10.1073/pnas.88.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares IS, Levitus G, Souza JM, et al. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun. 1997;65:1606–14. doi: 10.1128/iai.65.5.1606-1614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva FA, da Silva-Nunes M, Sanchez BA, et al. Naturally acquired antibodies to Plasmodium vivax Duffy binding protein (DBP) in Brazilian Amazon. Am J Trop Med Hyg. 2010;82:185–93. doi: 10.4269/ajtmh.2010.08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Beatty WL, Diouf A, et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. P Natl Acad Sci USA. 2011;108:13275–80. doi: 10.1073/pnas.1110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford WH, Blackman MJ, Harris A, et al. N-terminal amino acid sequence of the Plasmodium falciparum merozoite surface protein-1 polypeptides. Mol Biochem Parasit. 1994;66:157–60. doi: 10.1016/0166-6851(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Stafford WH, Gunder B, Harris A, et al. A 22 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasit. 1996;80:159–69. doi: 10.1016/0166-6851(96)02696-5. [DOI] [PubMed] [Google Scholar]
- Stanisic DI, Javati S, Kiniboro B, et al. Naturally acquired immune responses to P. vivax merozoite surface protein 3alpha and merozoite surface protein 9 are associated with reduced risk of P. vivax malaria in young Papua New Guinean children. PLoS Neglect Trop D. 2013;7:e2498. doi: 10.1371/journal.pntd.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoute JA, Gombe J, Withers MR, et al. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine. 2007;25:176–84. doi: 10.1016/j.vaccine.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Stubbs J, Olugbile S, Saidou B, et al. Strain-transcending Fc-dependent killing of Plasmodium falciparum by merozoite surface protein 2 allele-specific human antibodies. Infect Immun. 2011;79:1143–52. doi: 10.1128/IAI.01034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J, Simpson KM, Triglia T, et al. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science. 2005;309:1384–7. doi: 10.1126/science.1115257. [DOI] [PubMed] [Google Scholar]
- Sturchler D, Berger R, Rudin C, et al. Safety, immunogenicity, and pilot efficacy of Plasmodium falciparum sporozoite and asexual blood-stage combination vaccine in Swiss adults. Am J Trop Med Hyg. 1995;53:423–31. doi: 10.4269/ajtmh.1995.53.423. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Tanomsing N, Nolder D, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201:1544–50. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Allen SJ, Greenwood BM, et al. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–13. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- Terheggen U, Drew DR, Hodder AN, et al. Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med. 2014;12:183. doi: 10.1186/s12916-014-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham WH, Healer J, Cowman AF. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 2012;28:23–30. doi: 10.1016/j.pt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Tham WH, Schmidt CQ, Hauhart RE, et al. Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes. Blood. 2011;118:1923–33. doi: 10.1182/blood-2011-03-341305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham WH, Wilson DW, Lopaticki S, et al. Complement receptor 1 is the host erythrocyte receptor for Plasmodium falciparum PfRh4 invasion ligand. P Natl Acad Sci USA. 2010;107:17327–32. doi: 10.1073/pnas.1008151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M, Soe S, Oeuvray C, et al. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;66:11–17. doi: 10.1128/iai.66.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera MA, Doumbo OK, Coulibaly D, et al. Safety and allele-specific immunogenicity of a malaria vaccine in malian adults: results of a phase i randomized trial. PLoS Clin Trials. 2006;1:e34. doi: 10.1371/journal.pctr.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS One. 2008;3:e1465. doi: 10.1371/journal.pone.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera MA, Doumbo OK, Coulibaly D, et al. Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial. PLoS One. 2010;5:e9041. doi: 10.1371/journal.pone.0009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thera MA, Doumbo OK, Coulibaly D, et al. A field trial to assess a blood-stage malaria vaccine. New Engl J Med. 2011;365:1004–13. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Cooke RE, Moore S, et al. PTRAMP; a conserved Plasmodium thrombospondin-related apical merozoite protein. Mol Biochem Parasit. 2004;134:225–32. doi: 10.1016/j.molbiopara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Thompson FM, Porter DW, Okitsu SL, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS One. 2008;3:e1493. doi: 10.1371/journal.pone.0001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tine JA, Lanar DE, Smith DM, et al. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996;64:3833–44. doi: 10.1128/iai.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin ML, Roques M, Lamarque MH, et al. Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science. 2011;333:463–7. doi: 10.1126/science.1204988. [DOI] [PubMed] [Google Scholar]
- Topolska AE, Lidgett A, Truman D, et al. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem. 2004a;279:4648–56. doi: 10.1074/jbc.M307859200. [DOI] [PubMed] [Google Scholar]
- Topolska AE, Richie TL, Nhan DH, et al. Associations between responses to the rhoptry-associated membrane antigen of Plasmodium falciparum and immunity to malaria infection. Infect Immun. 2004b;72:3325–30. doi: 10.1128/IAI.72.6.3325-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossavainen H, Pihlajamaa T, Huttunen TK, et al. The layered fold of the TSR domain of P. falciparum TRAP contains a heparin binding site. Protein Sci. 2006;15:1760–8. doi: 10.1110/ps.052068506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Oliveira-Ferreira J, Moreno A, et al. Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg. 2005;73:244–55. [PubMed] [Google Scholar]
- Tran TM, Ongoiba A, Coursen J, et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis. 2014;209:789–98. doi: 10.1093/infdis/jit553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck M, Zacherl S, Herrmann S, et al. Functional analysis of the leading malaria vaccine candidate AMA-1 reveals an essential role for the cytoplasmic domain in the invasion process. PLoS Pathog. 2009;5:e1000322. doi: 10.1371/journal.ppat.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T, Chen L, Lopaticki S, et al. Plasmodium falciparum merozoite invasion is inhibited by antibodies that target the PfRh2a and b binding domains. PLoS Pathog. 2011;7:e1002075. doi: 10.1371/journal.ppat.1002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco C, Fernandez-Reyes D, Howell S, et al. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasit. 2001;112:91–101. doi: 10.1016/s0166-6851(00)00350-9. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The thrombospondin type 1 repeat superfamily. Int J Biochem Cell B. 2004;36:969–74. doi: 10.1016/j.biocel.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Uthaipibull C, Aufiero B, Syed SE, et al. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J Mol Biol. 2001;307:1381–94. doi: 10.1006/jmbi.2001.4574. [DOI] [PubMed] [Google Scholar]
- Valero MV, Amador LR, Galindo C, et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia [see comments] Lancet. 1993;341:705–10. doi: 10.1016/0140-6736(93)90483-w. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, van Schaijk BC, Khan SM, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versiani FG, Almeida ME, Melo GC, et al. High levels of IgG3 anti ICB2-5 in Plasmodium vivax-infected individuals who did not develop symptoms. Malaria J. 2013;12:294. doi: 10.1186/1475-2875-12-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentin EC, Francoso KS, Rocha MV, et al. Invasion-inhibitory antibodies elicited by immunization with Plasmodium vivax apical membrane antigen-1 expressed in Pichia pastoris yeast. Infect Immun. 2014;82:1296–307. doi: 10.1128/IAI.01169-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay Kumar S, Ranjan S, Saxena V, et al. Plasmodium falciparum: genetic diversity of C-terminal region of MSP-1 in isolates from Indian sub-continent. Exp Parasitol. 2005;110:384–8. doi: 10.1016/j.exppara.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Wang L, Black CG, Marshall VM, et al. Structural and antigenic properties of merozoite surface protein 4 of Plasmodium falciparum. Infect Immun. 1999;67:2193–200. doi: 10.1128/iai.67.5.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Gilson PR, Taechalertpaisarn T, et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 2015;11:e1004670. doi: 10.1371/journal.ppat.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Fowkes FJ, Gilson PR, et al. Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. PLoS One. 2011;6:e27705. doi: 10.1371/journal.pone.0027705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Goodman CD, Sleebs BE, et al. Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 2015;13:52. doi: 10.1186/s12915-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Langer C, Goodman CD, et al. Defining the timing of action of antimalarial drugs against Plasmodium falciparum. Antimicrob Agents Ch. 2013;57:1455–67. doi: 10.1128/AAC.01881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers MR, McKinney D, Ogutu BR, et al. Safety and reactogenicity of an MSP-1 malaria vaccine candidate: a randomized phase ib dose-escalation trial in kenyan children. PLoS Clin Trials. 2006;1:e32. doi: 10.1371/journal.pctr.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers-Martinez C, Strath M, Hackett F, et al. The malaria parasite egress protease SUB1 is a calcium-dependent redox switch subtilisin. Nat Commun. 2014;5:3726. doi: 10.1038/ncomms4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers-Martinez C, Suarez C, Fulle S, et al. Plasmodium subtilisin-like protease 1 (SUB1): insights into the active-site structure, specificity and function of a pan-malaria drug target. Int J Parasitol. 2012;42:597–612. doi: 10.1016/j.ijpara.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehlbier U, Epp C, Kauth CW, et al. Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect Immun. 2006;74:1313–22. doi: 10.1128/IAI.74.2.1313-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry T, Minigo G, Piera KA, et al. Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. J Infect Dis. 2008;198:134–42. doi: 10.1086/588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World Malaria Report. Geneva: World Health Organization; 2014. [Google Scholar]
- Wright KE, Hjerrild KA, Bartlett J, et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature. 2014;515:427–30. doi: 10.1038/nature13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Black CG, Wang L, et al. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol Biochem Parasit. 1999;103:243–50. doi: 10.1016/s0166-6851(99)00134-6. [DOI] [PubMed] [Google Scholar]
- Yang X, Adda CG, MacRaild CA, et al. Identification of key residues involved in fibril formation by the conserved N-terminal region of Plasmodium falciparum merozoite surface protein 2 (MSP2) Biochimie. 2010;92:1287–95. doi: 10.1016/j.biochi.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A, Azevedo MF, Gilson PR, et al. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol. 2014;16:642–56. doi: 10.1111/cmi.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, O'Donnell RA, Koussis K, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–83. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Yeom JS, Kim ES, Lim KJ, et al. Naturally acquired IgM antibody response to the C-terminal region of the merozoite surface protein 1 of Plasmodium vivax in Korea: use for serodiagnosis of vivax malaria. J Parasitol. 2008;94:1410–4. doi: 10.1645/GE-1484.1. [DOI] [PubMed] [Google Scholar]
- Yildiz Zeyrek F, Palacpac N, Yuksel F, et al. Serologic markers in relation to parasite exposure history help to estimate transmission dynamics of Plasmodium vivax. PLoS One. 2011;6:e28126. doi: 10.1371/journal.pone.0028126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenonos ZA, Dummler SK, Muller-Sienerth N, et al. Basigin is a druggable target for host-oriented antimalarial interventions. J Exp Med. 2015;212:1145–51. doi: 10.1084/jem.20150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Perugini MA, Yao S, et al. Solution conformation, backbone dynamics and lipid interactions of the intrinsically unstructured malaria surface protein MSP2. J Mol Biol. 2008;379:105–21. doi: 10.1016/j.jmb.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]