Abstract
The mechanism controlling tissue-specific expression of estrogen receptor 1 (ESR1) is unclear. In other genes, DNA methylation of a region called the tissue-dependent and differentially methylated region (T-DMR) has been associated with tissue-specific gene expression. This study investigated whether human ESR1 has a T-DMR and whether DNA methylation of the T-DMR regulates its expression. ESR1 expression was tissue-specific, being high in the endometrium and mammary gland and low/nil in the placenta and skin. Therefore, DNA methylation profiles of the promoter of ESR1 were analyzed in these tissues and in breast cancer tissues. In all of the normal tissues, the proximal promoter regions were unmethylated. On the other hand, the distal regions (T-DMR) were unmethylated in the endometrium and mammary gland, but were moderately methylated and hypermethylated in the placenta and skin, respectively. T-DMR-methylated reporter assay was performed to examine whether DNA methylation at the T-DMR suppresses ESR1 transcription. T-DMR, but not the promoter region, had transcriptional activities and DNA methylation of the T-DMR suppressed ESR1 transcription. Early growth response protein 1 was shown to be a possible transcription factor to bind the T-DMR and up-regulate ESR1 expression. ESR1 has several upstream exons, and each upstream exon, Exon-A/Exon-B/Exon-C, had its own T-DMR. In some breast cancer cases and breast cancer cell lines, ESR1 expression was not regulated by DNA methylation at T-DMR as it is in normal tissues. In conclusion, ESR1 has a T-DMR. DNA methylation status at the T-DMR is involved in tissue-specific ESR1 expression in normal tissues but not always in breast cancer.
The estrogen receptor (ER) is a transcription factor that mediates estrogen hormone action in many physiological and pathological processes. Expression of human estrogen receptor 1 (ESR1), which codes ER-α, is tissue specific (1, 2). For example, ESR1 expression is high in the endometrium and mammary gland and low in the placenta and skin. This assures that estrogen molecules have effects only in specific tissues. However, the mechanism controlling tissue-specific expression of ESR1 is unclear.
DNA methylation is one of the most characterized epigenetic marks, and occurs at CpG sites. CpG islands, which are CpG site-rich regions, are located in the gene promoter near the transcription start site (TSS) and are hypomethylated in normal tissues. DNA methylation of the gene promoter interrupts the recognition and binding of transcription factors (3–6), recruits methyl CpG binding proteins that interact with transcription repressors (7), and induces chromatin condensation via recruitment of histone deacetylases (8). DNA methylation is also associated with trimethylation of the site of lysine 27 on histone H3 (H3K27me3), which is a repressive histone modification (9, 10). Thus, it is thought that DNA methylation of the gene promoter plays a central role in gene silencing.
In addition, the cell specificity of normal cells or even abnormal cells can be defined and distinguished by their DNA methylation profile (11–17). DNA methylation of a specific region of the gene has an important role in determining tissue- and cell-specific gene expression. The region regulating cell-specific gene expression is called the tissue-dependent and differentially methylated region (T-DMR) (16). Recent genome-wide analyses have identified many T-DMRs in mammalian genomes (16, 18, 19).
We previously found a possible link between the mRNA expression of ESR1 and the DNA methylation status of a region distant from the TSS of ESR1 (−1188 to −790 bp) (20). In human uterine leiomyomas, ESR1 expression was elevated and the region from −1188 to −790 bp was less methylated in comparison with normal myometrium (20). These findings, together with the finding that the DNA methylation status of the promoter region including the CpG island around TSS (−566 to +229 bp) was hypomethylated in both leiomyoma tissues and normal myometrium (20), suggest that the region from −1188 to −790 bp distant from TSS is a T-DMR regulating ESR1 expression via DNA methylation.
ESR1 has several TSSs corresponding to upstream Exon-A to upstream Exon-E1 (21). The transcription of ESR1 starts from any of these upstream exons, and the upstream exons are used in a tissue-dependent manner (22). For example, upstream Exon-A is mainly used in MCF-7 cells, whereas upstream Exon-E is used in liver (21). In tissues with high ESR1 expression, upstream Exon-A, Exon-B, and Exon-C are often used (21). Glucocorticoid receptor and progesterone receptor also have several TSSs, and DNA methylation of the region close to each TSS contributes to the regulation of transcription from each TSS (23–26). These findings led us to investigate whether a T-DMR is present in each upstream exon of ESR1, and if present, whether its DNA methylation status is involved in regulating transcription of the exon.
Regarding the ESR1 expression in breast cancer, down-regulation of ESR1 expression has been associated with a poor prognosis (27) and DNA methylation of the ESR1 promoter down-regulates ESR1 transcription (28–32). However, it is unclear why some cases of breast cancer show various levels of ESR1 expression despite DNA hypomethylation in the promoter region (30, 32). Only 25% of ER-α-negative breast cancer tissues show DNA methylation in the promoter region (30). In addition, upstream exons used for ESR1 expression are different among individuals and different upstream exons are associated with clinicopathological variations (33). These findings raise the question whether DNA methylation of T-DMR contributes to the regulation of ESR1 expression in transcription levels in breast cancer.
In the present study, we first examined the DNA methylation status in the promoter region of ESR1 in normal tissues with or without ESR1 expression, and further investigated whether ESR1 has a T-DMR for ESR1 expression and DNA methylation of this T-DMR actually contributes to the tissue-specific ESR1 expression. Next, in order to clarify the different ESR1 expressions in breast cancer, we examined the DNA methylation status of upstream Exon-A, -B, and -C and their transcription levels in breast cancer tissues and ER-α-positive and ER-α-negative breast cancer cell lines.
Materials and Methods
This study was reviewed and approved by the Institutional Review Board of Yamaguchi University Graduate School of Medicine. Written informed consent was obtained from the participants before the collection of any samples, and the specimens were irreversibly deidentified. All experiments handling human tissues were performed in accordance with Tenets of the Declaration of Helsinki.
Tissue samples
Specimens of endometrium, mammary gland, placenta, and skin were obtained from 35 Japanese women, and breast cancer tissue was obtained from 17 Japanese women. Endometrium was obtained from patients who underwent surgery for uterine leiomyoma. Mammary gland tissue and breast cancer tissue were obtained from patients who underwent mastectomy for breast cancer. Placenta tissue was obtained from patients with normal delivery. Skin tissue was obtained from operation scar with a small piece of normal skin tissue at repeated cesarean section. Specimens were dissected immediately after the removal, immersed in liquid nitrogen and stored at −80°C until DNA/RNA extraction as previously reported (13, 14).
Cell culture
ER-α-positive breast cancer cell line (MCF7) was obtained from the Department of Yamaguchi University Center for Gene Research and was cultured in DMEM (Sigma) supplemented with 10% fetal calf serum (FCS) (Biological Industries Ltd), 0.1mM nonessential amino acids (GIBCO BRL), 1mM sodium pyruvate (GIBCO), 100-U/mL penicillin, and 100-mg/mL streptomycin at 37°C under 5% CO2 in a humidified atmosphere. ER-α-negative breast cancer cell line (MDA-MB-231; ATCC) was cultured in L-15 medium (ATCC) supplemented with 10% FCS, 100-U/mL penicillin, and 100-mg/mL streptomycin under the same conditions. Endometrial stromal cells (ESCs) were isolated from the human proliferative phase endometrium as previously reported (34) and cultured in DMEM (Sigma) supplemented with 10% FCS, 4mM glutamine, 50-μg/mL streptomycin, and 50-U/mL penicillin.
Sodium bisulfite sequencing
Bisulfite reactions were performed using a EpiTect Bisulfite kit (QIAGEN) according to the conditions as follows: 95°C for 5 minutes, 65°C for 85 minutes, 95°C for 5 minutes, and 65°C for 175 minutes as previously reported (15, 35). The bisulfite-converted DNA was amplified by PCR using 6 primer pairs for region I to VI shown in Supplemental Table 1 under the thermocycling conditions (95°C for 10 min, and 40 cycles of 94°C for 30 se, 60°C for 30 s, and 72°C for 1 min followed by 10 min of final extension at 72°C). The resulting products were cloned into pGEM-T easy vector (Promega). After sequencing reaction using a BigDye Terminator V3.1 kit (Applied Biosystems), sequencing was performed with a 3130xl Genetic Analyzer (Applied Biosystems) as previously reported (11, 13, 14). QUMA (http://quma.cdb.riken.jp/) was used to analyze the bisulfite sequencing data (36). The percentage of the methylated CpG sites in a total of the examined CpG sites was calculated and defined as follows: 5%>: unmethylation, 5%–30%: hypomethylation, 31%–70%: moderate methylation, and 71%<: hypermethylation.
RT-PCR and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated from tissues using Isogen (Wako Pure Chemical Industries Ltd), and 1-μg total RNA was reverse-transcribed using a Quantitect Reverse Transcription kit (QIAGEN) according to the manufacturer's protocol as previously reported (13). To distinguish the transcribed products derived from each upstream exon (see Figure 3 below), we synthesized 3 primer pairs as shown in Figure 3 below and Supplemental Table 2 for ESR1. We also made the primer pair inside of Exon1 to amplify any types of transcription variants (Supplemental Table 2). A primer pair for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control (Supplemental Table 2). PCR was performed under the thermocycling conditions (initial cycle of 94°C for 10 min, then 22–35 cycles of 94°C for 30 s, 55°C–60°C for 30 s and 72°C for 1 min followed by final extension for 10 min at 72°C). The resulting products were subjected to agarose gel electrophoresis. Real-time qRT-PCR was performed using SYBR Premix Ex Taq (TAKARA) and a LightCycler (Roche Applied Science) in ESR1 variants and DNA methyltransferases (DNMTs), including DNMT1, DNMT3A, and DNMT3B, using the primer pairs shown in Supplemental Table 2. All samples were run in duplicate. Melting curves of the products were obtained after cycling by a stepwise increase of temperature form 55°C to 95°C.
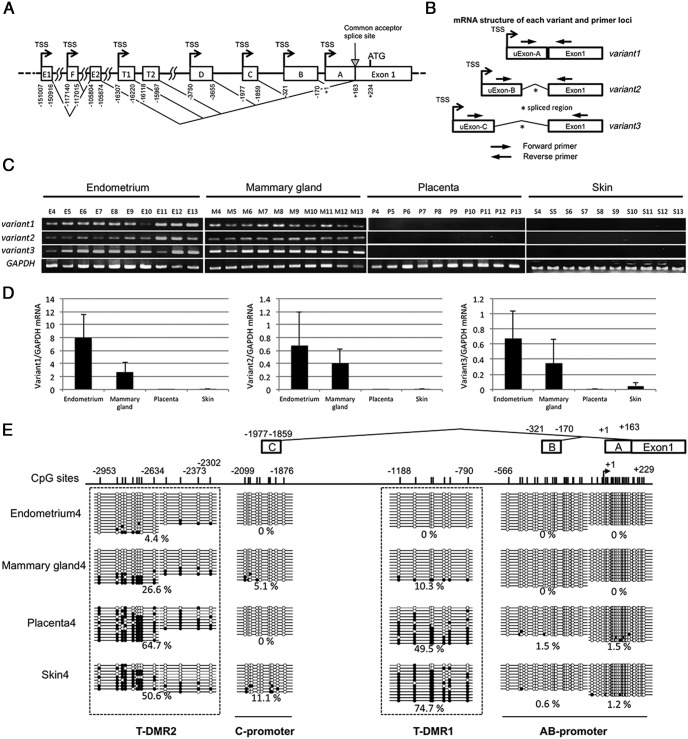
Figure 3. DNA methylation statuses around upstream exons and mRNA expression of ESR1 variants in normal tissues.
A, Genomic organization of upstream exons and corresponding TSSs of ESR1. The upstream exons are shown by boxes, and the corresponding TSSs are indicated by arrows. The numbers below the upstream exon boxes indicate 5′ start sites, splice donor site and acceptor sites, which are involved in generating mature ESR1 mRNA with the distance from the originally described TSS at +1. All 5′ upstream exons are spliced at the common acceptor splice site (+163 bp). B, The primer design to investigate the transcribed mRNAs of variant1, variant2, and variant3, separately. C and D, ESR1 mRNA expression of variant1, variant2, and variant3 was analyzed by RT-PCR and qRT-PCR using primers shown in Figure 3B and Supplemental Table 2. GAPDH was used as an internal control. The value of mRNA of each variant was normalized to that of the internal control (GADPH). Data were expressed as a ratio of mRNA of each variant to GADPH. Each bar represents the mean ± SEM of the number of tissue samples; endometrium (n = 10), mammary gland (n = 10), placenta (n = 12), and skin (n = 3). E, DNA methylation statuses of AB-promoter (−556 to +229 bp), T-DMR1 (−1188 to −790 bp), C-promoter (−2099 to −1876 bp), and T-DMR2 (−2953 to −2302 bp) were analyzed by sodium bisulfite genomic sequencing in the tissue samples of endometrium, mammary gland, placenta, and skin. Open and filled circles indicate unmethylated and methylated CpG status, respectively. The percentage of the methylated CpG sites in a total of the examined CpG sites was shown. The location of each upstream exon and CpG sites are shown with the distance from TSS of upstream Exon-A. A–C are upstream Exon-A, Exon-B, and Exon-C, respectively.
Reporter assay for the promoter activity and T-DMR-methylated reporter assay
First, we investigated whether the promoter region has transcriptional activities in the absence of the T-DMR1. To make a construct which only has the promoter region of ESR1, the promoter region was inserted into pGL3 basic vector (Promega), and the construct was designated as pGL3-Promoter. The construct with both the promoter region and the T-DMR was also made and designated as pGL3-Promoter-TDMR. Uterine smooth muscle cells (Lonza) were cultured on a 24-well plate (8 × 104 cells/well) for 24 hours and then transfected with 450 ng of the reporter constructs linearized by Aor5HI (Takara) and 30 ng of pRL-TK vector (Promega) as a normalization control using Lipofectamine2000 (Invitrogen). Twenty-four hours after the transfection, the luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer instruction. The assays were performed in duplicate and repeated 3 times.
Secondly, we examined the effect of DNA methylation at the T-DMR1 on the luciferase activity in the presence of the promoter region (corresponding to AB-promoter). For this purpose, T-DMR-methylated reporter assays were performed as previously reported (see Figure 2 below and Supplemental Figure 1) (15). Briefly, a 5′-flanking region (−651 to +37) of the ESR1 gene and the T-DMR1 region from −1244 to −638 were amplified and designated as ESR1-promoter and ESR1-Tdmr, respectively. Both ESR1-promoter and ESR1-Tdmr fragments were inserted together into pGL3 basic vector (Promega). The construct was amplified using the dam− and dcm− Escherichia coli strain SCS110 (Stratagene). The Tdmr fragment (−1244 to −638), excised from the construct, was mock treated or treated with SssI methylase (New England Biolabs). The resultant unmethylated and methylated Tdmr fragments were religated with the linealized vector fragment to create 2 types of reporter constructs: pGL3-TDMR-M (methylated) and pGL3-TDMR_U (unmethylated). The transfection with the constructs was done in uterine smooth muscle cells and the luciferase activities were measured as described above.
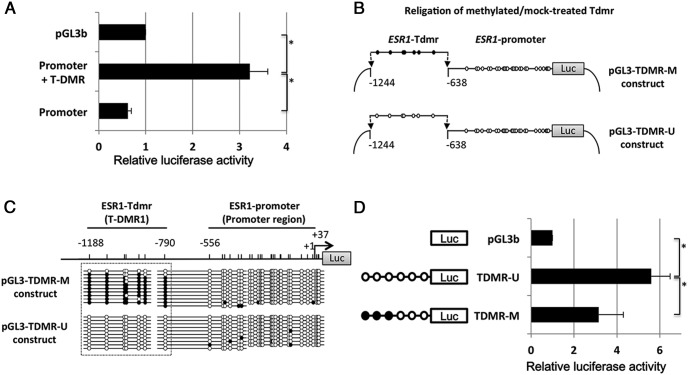
Figure 2. T-DMR-methylated reporter assay.
A, Reporter assay to compare the promoter activity between the promoter+T-DMR and the promoter of ESR1. Horizontal bars show the relative luciferase activities to pGL-3 basic vector. The values are mean ± SEM of 3 independent assays. *, P < .05. B, Production of T-DMR-methylated reporter constructs (see Supplemental Figure 1). A 5′-flanking promoter region (−651 to +37 bp) and the T-DMR1 (−1244 to −638 bp) of ESR1 were amplified and designated as ESR1-promoter and ESR1-Tdmr, respectively. Both ESR1-promoter and ESR1-Tdmr fragments were inserted together into pGL3 basic vector. After the amplification of this vector using E.coli strain SCS110 (Stratagene), the ESR1-Tdmr fragment was excised from the vector and mock treated or treated with SssI methylase (New England Biolabs). The resultant unmethylated and methylated ESR1-Tdmr fragments were religated with the linearized vector fragment to create 2 types of reporter constructs: pGL-TDMR-U (unmethylated) and pGL3-TDMR-M (methylated). C, DNA methylation status of T-DMR1 (−1188 to −790 bp) and proximal promoter region (−556 to +37 bp) of pGL-TDMR-M and pGL3-TDMR-U constructs were analyzed by sodium bisulfite genomic sequencing. Open and filled circles indicate unmethylated and methylated CpG status, respectively. D, T-DMR-methylated reporter assay to assess the effect of DNA methylation at T-DMR on the transcriptional activity of the ESR1 promoter. Horizontal bars show the relative luciferase activities to pGL-3 basic vector. The values are mean ± SEM of 3 independent assays. *, P < .05.
Western blotting
Western blotting was performed as previously reported (37). Briefly, whole-cell lysates were prepared using loading buffer reagents (Santa Cruz Biotechnology, Inc) without trypsin treatment. Equal amounts of total protein were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel. After SDS-PAGE was completed, proteins were transferred to the polyvinylidene difluoride membrane (ATTO) with semidry-type blotting system. The transferred membranes were stained by immunochemical technique consisting of the next procedure: after blocking the membrane with blocking solution (5% skimmed milk with 0.1% Tween-20 dissolved in Tris-buffered saline; pH 7.5), the blotted membranes were incubated with antibodies for DNMT1, DNMT3A, Early growth response protein 1 (EGR1) (Cell Signaling Technology), and β-tubulin (Sigma), which were diluted in blocking solution. Then, these membranes were incubated with the peroxidase-conjugated second antibody diluted in blocking solution. Finally, an enhanced chemiluminescence-Western blotting detection system (Amersham) was applied according to the manufacturer's protocol, after which the membranes were exposed to hyperfilm-enhanced chemiluminescence (Amersham). To reuse the blot, the membranes were stripped in Restore Western stripping buffer (Pierce Biotechnology).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the SimpleChIP Enzymatic Chromatin IP kit (Cell Signaling Technology) as previously reported (11). Cells were cross-linked by addition of formaldehyde into the medium, and cross-linking was terminated by addition of glycine (0.125M, final concentration). After the nuclear extraction, Micrococcal Nulease was added to digest DNA. Two percent of the supernatant was kept as input controls. The chromatin was incubated with the next antibodies at 4°C overnight: a transcription factor, EGR1 (Cell Signaling Technology) and H3K27me3 (generous gift from Dr Kimura, Tokyo Institute of Technology) (38). Normal rabbit IgG (Cell Signaling Technology and Invitrogen, respectively) was used as a negative control. Immune complexes were collected, and cross-linkings of the immunoprecipitated chromatin complexes (IP) and input controls (INPUT; 2% of the total soluble chromatin) were reversed by heating the samples at 65°C overnight. The DNA fragments were purified and subjected as a template for PCR amplification. The specific primers used for ChIP-PCR analysis to amplify the regions in the T-DMR1 and T-DMR2 (Supplemental Table 3). The relative levels of each target sequence were analyzed by real-time PCR. The cycle threshold (Ct) of IP sample was used for quantification and the value were normalized for their corresponding INPUT value using the next formula (IP/INPUT = 2 CtINPUTDNA − CtIPDNA) as we reported previously (34).
Lipid-mediated transfection of small interfering RNA (siRNA) duplexes
Knockdowns of EGR1 were performed using siRNA method as reported previously (34). EGR1 ON-TARGET plus SMART pool and ON-TARGET plus Non-Targeting pool siRNA were purchased from Dharmacon. ESCs were plated in the medium lacking antibiotics at approximately 3 × 105 cells in 25-cm2 tissue culture flasks and incubated for 2 days. At 30%–50% confluence, siRNA duplexes (20nM) and RNAi MAX (2.5 μL/well; Invitrogen) diluted in Optimem (Invitrogen) were transfected to ESCs at 60% confluence. After the medium was changed 4 hours later, cells were incubated for 72 hours.
Statistical analyses
A paired t test was performed for the comparison between the 2 groups in Figures 3 and 5 below using SPSS for Windows version 11 (SPSS, Inc). Differences were considered significant at P < .05.
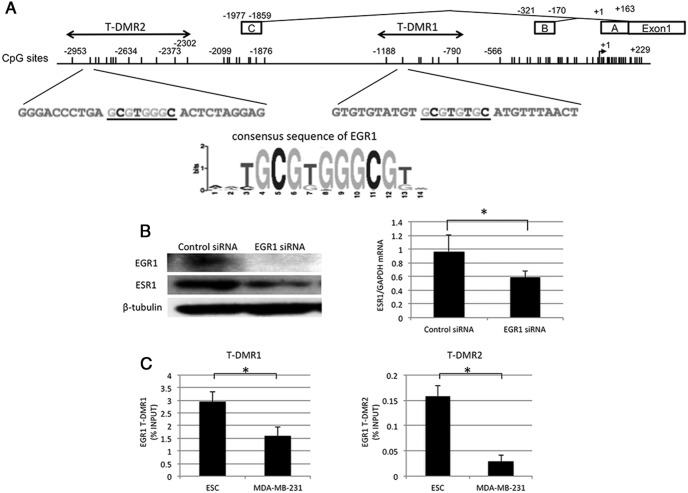
Figure 5. Regulation of ESR1 expression by EGR1 and binding statuses of EGR1 at T-DMRs.
A, Motif analysis was performed in T-DMR1 and T-DMR2 using Multiple Em for Motif Eliciation (MEME), which is a motif-based sequence analysis tool. The underlined sequences are the possible binding sites of EGR1, whose consensus sequence is shown below. B, Effects of EGR1 knockdown on ESR1 expression. EGR1 siRNA was transfected into human ESCs, which express ESR1. Protein expressions of EGR1 and ESR1 were analyzed by Western blotting in ESC treated with EGR1 siRNA or control siRNA. β-Tubulin was used as an internal control. The immunoblot is a representative of 3 independent incubations. ESR1 mRNA expression was analyzed by quantitative RT-PCR. The values are mean ± SEM of 3 independent incubations. *, P < .05. C, The binding statuses of EGR1 to T-DMR1 and T-DMR2 in ESC and MDA-MB-231. The binding statuses were examined by a ChIP assay. *, P < .05.
Results
ESR1 mRNA expression and DNA methylation statuses of ESR1 promoter
ESR1 mRNA expression was examined in the endometrium, mammary gland, placenta, and skin. ESR1 mRNA expression was high in the endometrium and mammary gland and low or negligible in the placenta and skin (Figure 1A), indicating that ESR1 expression is tissue-specific. We then tested the DNA methylation status of the promoter region (−566 to +229 bp) and the distal region (−1188 to −790 bp) from TSS by sodium bisulfite sequencing in these tissues (Figure 1B). In all of the tissues, the promoter region from −566 to +229 bp was unmethylated, whereas in the distal region from −1188 to −790 bp, the DNA methylation status was variable among the tissues (Figure 1B). Endometrium and mammary gland showed unmethylation and hypomethylation statuses, whereas placenta and skin showed moderate methylation and hypermethylation statuses, respectively. An analysis of additional samples confirmed that the DNA methylation status in the distal region varied among these tissues (Figure 1C). The DNA methylation status in the distal region was strongly associated with ESR1 expression, which suggests that the distal region is a T-DMR. We thus designated it as T-DMR1.
Figure 1. ESR1 mRNA expression and DNA methylation statuses of the ESR1 promoter.
A, mRNA expression levels of ESR1 in endometrium, mammary gland, placenta, and skin were determined by RT-PCR and quantified by densitometry. The data were normalized to GAPDH (an internal control). Three tissue samples were examined in each tissue type. B, The diagram shows a detailed map of approximately 1.5-kb region around the TSS (arrow) at the promoter region of ESR1. The position of the TSS is designated as +1. The vertical lines indicate positions of CpG sites. The thick horizontal lines indicate the distal region (T-DMR1, −1188 to −790 bp) and promoter region (−556 to +229 bp). DNA methylation status of these CpG sites is shown. Methylation status of all the CpG sites between −1188 and +229 bp (56CpG sites) was analyzed by sodium bisulfite genomic sequencing in endometrium, mammary gland, placenta, and skin. Open and filled circles indicate unmethylated and methylated CpG status, respectively. The percentage of the methylated CpG sites in a total of the examined CpG sites was shown. C, DNA methylation status of CpG sites in the distal region (T-DMR1, −1188 to −790 bp) was analyzed in additional 2 tissue samples of endometrium, mammary gland, placenta, and skin.
T-DMR-methylated reporter assay
As shown in Figure 2A, the pGL3-Promoter+T-DMR construct had significantly higher reporter activities (P < .05) compared with the empty vector, whereas the promoter alone did not induce the reporter activity, indicating T-DMR1 is essential for the enough reporter activity.
Because the results of Figures 1 and 2A indicated that T-DMR1 is essential for the transcriptional activity of ESR1 and the DNA methylation status of T-DMR1 is associated with ESR1 expression, we examined whether DNA methylation of T-DMR1 actually has an inhibitory effect on ESR1 expression. For this purpose, T-DMR-methylated reporter assay was performed to demonstrate that the DNA methylation of T-DMR1 alone, whereas keeping the promoter region hypomethylated has a suppressive effect on reporter activities. A DNA fragment corresponding to the T-DMR1 was methylated in vitro and inserted into a reporter construct with an unmethylated promoter region (pGL3-TDMR-M construct) (Figure 2B). Unmethylated T-DMR1 was used as a control (pGL3-TDMR-U construct). The unmethylated and methylated statuses of T-DMR1 in the pGL3-TDMR-U and pGL3-TDMR-M constructs were confirmed by sodium bisulfite sequencing (Figure 2C). The pGL3-TDMR-U construct (control) had 5-fold higher reporter activities compared with the empty vector, whereas the pGL3-TDMR-M construct reporter activity was significantly reduced by 47.5% when compared to the pGL3-TDMR-U construct (P < .05) (Figure 2D). These results indicate that DNA methylation of T-DMR1 suppresses ESR1 expression. Therefore, the distal region (−1188 to −790 bp) was determined as the T-DMR that regulates ESR1 gene expression.
DNA methylation statuses around upstream exons and mRNA expression of ESR1 variants in normal tissues
ESR1 has several upstream exons (upstream Exon-A to upstream Exon-E1), in which transcription starts from the corresponding TSSs (Figure 3A) (21). Three upstream exons, Exon-A, Exon-B, and Exon-C, are often used in the tissues with high ESR1 expression (21). The transcription products from upstream Exon-A, Exon-B, and Exon-C are spliced to generate mature ESR1 mRNAs, called variant1, variant2, and variant3, respectively (Figure 3B). ESR1 mRNA expression of variant1, variant2, and variant3 was analyzed by RT-PCR and qRT-PCR. All 3 variants were highly expressed in the endometrium and mammary gland, whereas none of the variants were expressed in the placenta and skin (Figure 3, C and D), indicating that the transcription from each upstream exon is tissue-specific.
Next, we analyzed DNA methylation status around upstream Exon-A, Exon-B, and Exon-C (−2953 to +229 bp). CpG sites located from −566 to +229 bp were unmethylated in all the tissues, which was similar to the result shown in Figure 1 (Figure 3E). We designated this region as AB-promoter. CpG sites of T-DMR1 (−1188 to −790 bp) were unmethylated in the endometrium, hypermethylated in the mammary gland, moderately methylated in the placenta and hypermethylated in the skin, which was also similar to the result shown in Figure 1 (Figure 3E). CpG sites in the region from −2099 to −1876 bp, corresponding to upstream Exon-C, were unmethylated or hypomethylated in all the tissues (Figure 3E). We designated this region as C-promoter. The region from −2953 to −2302 bp was unmethylated in the endometrium, hypomethylated in the mammary gland, and moderately methylated in the placenta and skin (Figure 3E), indicating that the region from −2953 to −2302 bp is another T-DMR that regulates variant3 expression. We designated this T-DMR as T-DMR2 (Figure 3E). From these findings, it is suggested that each upstream exon has its own T-DMR.
DNA methylation statuses around upstream exons and mRNA expression of ESR1 variants in breast cancer
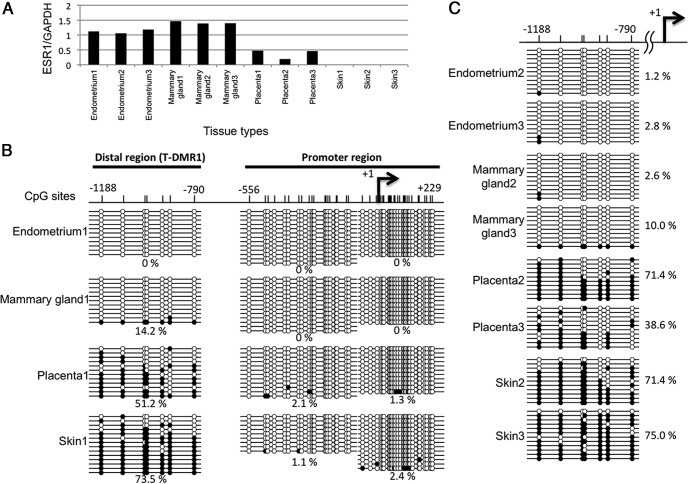
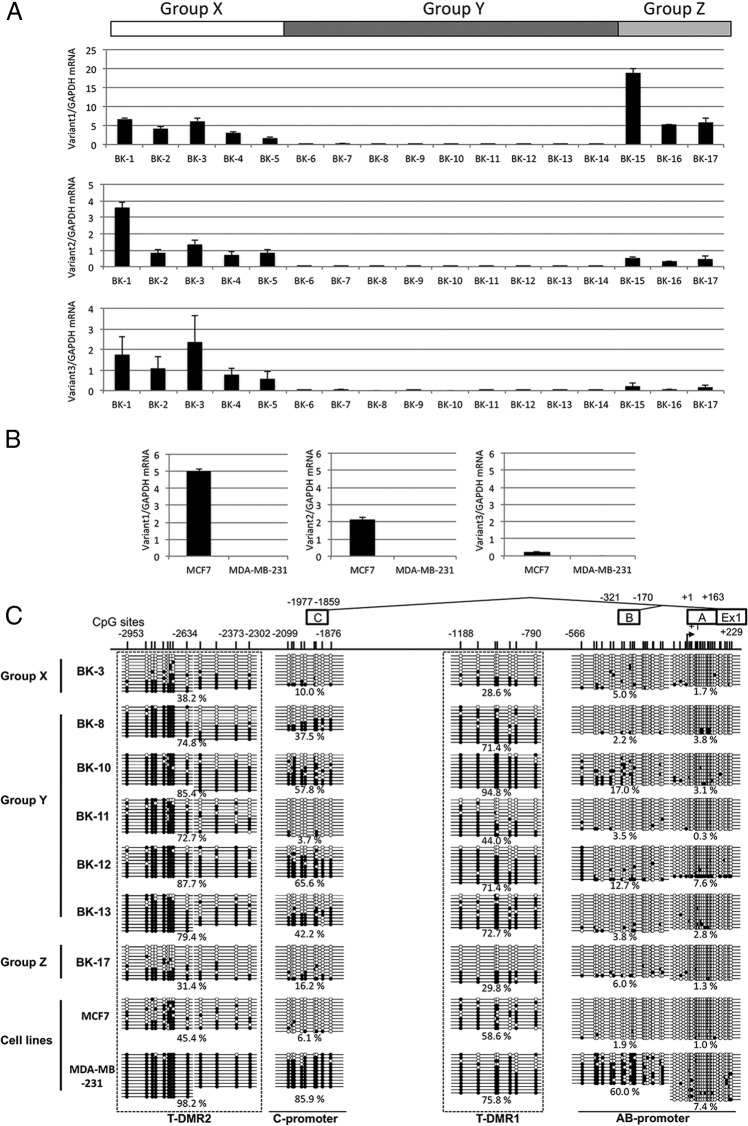
To investigate whether DNA methylation of the T-DMRs is involved in the regulation of transcription of the ESR1 mRNA variants in breast cancer, we examined the mRNA expression levels of variant1, variant2, and variant3 in 17 breast cancer tissues by real-time qRT-PCR and DNA methylation statuses around upstream Exon-A, Exon-B, and Exon-C by sodium bisulfite sequencing. Seventeen tissue samples were classified into 3 groups (Figure 4A); all 3 variants were expressed (group X), none of the variants were expressed (group Y), and variant1 and variant2 were expressed (group Z). In this study, mRNA expression was defined as positive when the ratio of mRNA of each variant to GADPH is more than 0.3 according to the expression level of the mammary gland shown in Figure 3D. We also examined the mRNA expression of the 3 variants in MCF7 and MDA-MB-231 cells, which are known as an ER-α-positive and an ER-α-negative breast cancer cell line, respectively. As shown in Figure 4B, in MCF7, variant1 and variant2, but not variant3, were expressed whereas any of the variants were not expressed in MDA-MB-231. Then, we examined DNA methylation statuses of AB-promoter, C-promoter, T-DMR1, and T-DMR2 in each group and both cell lines (Figure 4C). In case BK-3 of group X expressing all 3 variants, the AB-promoter and C-promoter were unmethylated and hypomethylated, and T-DMR1 and T-DMR2 were hypomethylated and moderately methylated, respectively, similar to what was observed in the DNA methylation profiles of the endometrium and mammary gland. In group Y expressing none of the variants, T-DMR1 and T-DMR2 were hypermethylated as was observed in the placenta and skin, but interestingly, the methylation statuses of the C-promoter varied among the cases such as moderate methylation in cases BK-8, BK-10, BK-12, and BK-13, and unmethylation in case BK-11. In group Z, the DNA methylation pattern of case BK-17 was similar to that of case BK-3 of group X, but interestingly, case BK-17 expressed only variant1 and variant2. In addition, in MCF7, although both T-DMR1 and T-DMR2 were moderately methylated, MCF7 expressed variant1 and variant2 but not variant3. These findings indicate that transcriptional regulation of ESR1 expression is abnormal in some breast cancers. In other words, some cases of breast cancer may have other regulation mechanisms in ESR1 expression than DNA methylation at T-DMRs. Furthermore, in MDA-MB-231 expressing none of the variants, even B-promoter and C-promoter in addition to T-DMR1 and T-DMR2 were moderately methylated and hypermethylated, respectively. It is also suggested that DNA methylation aberrantly occurs in breast cancer.
Figure 4. DNA methylation statuses around upstream exons and mRNA expression of ESR1 variants in breast cancer.
A, ESR1 mRNA expressions of variant1, variant2, and variant3 were determined by real-time qRT-PCR using primers shown in Figure 3B in 17 breast cancer tissues. According to the mRNA expression pattern of the variants, 17 tissue samples were classified into 3 groups. Group X is the group which showed transcription of all 3 variants. Group Y is the group which showed no transcription of any variants. Group Z is the group which showed transcription of variant1 and variant2. mRNA expression was defined as positive when the ratio of mRNA of each variant to GAPDH is more than 0.3 according to the expression level of the mammary gland shown in Figure 3D. B, ESR1 mRNA expression levels in MCF7 and MDA-MB-231. Expression levels were examined by qRT-PCR. C, DNA methylation statuses of AB-promoter (−556 to +229 bp), T-DMR1 (−1188 to −790 bp), C-promoter (−2099 to −1876 bp), and T-DMR2 (−2953 to −2302 bp) were analyzed by sodium bisulfite genomic sequencing in the tissue/cellular samples from group X (BK-3), group Y (BK-8, BK-10, BK-11, BK-12, and BK-13), group Z (BK-17), MCF7, and MDA-MB-231. Open and filled circles indicate unmethylated and methylated CpG status, respectively. The percentage of the methylated CpG sites in a total of the examined CpG sites was shown. The location of each upstream Exon and CpG sites are shown with the distance from TSS of upstream Exon-A. A–C are upstream Exon-A, Exon-B, and Exon-C, respectively.
DNMTs expression
ESR1 expression is reported to be activated in response to 5-aza-2′-deoxycytidine, an inhibitor of DNMTs, in ER-negative breast cancer cells (28), suggesting that DNMTs are involved in the regulation of DNA methylation of the ESR1 promoter region. Single nucleotide polymorphisms of DNMT1 are associated with the risk of triple-negative (ER-negative, progesterone receptor-negative, and Her2/neu-negative) breast cancers (39). These reports suggest that DNMTs are associated with the DNA methylation in ESR1 gene. Therefore, to investigate whether the expression levels of DNMTs are involved in the regulation of DNA methylation levels of the T-DMRs, we examined the mRNA and protein levels of DNMTs in the normal tissues and breast cancer tissues. Endometrium, mammary gland, placenta, skin, and breast cancer tissues expressed the mRNA of DNMT1 and DNMT3A, but the expression level of DNMT3B was low compared with DNMT1 and DNMT3A (Supplemental Figure 2A). There was no association between the mRNA or protein levels of DNMTs and the DNA methylation levels at the T-DMRs in normal tissues and breast cancer tissues (Supplemental Figure 2).
Potential transcription factors and histone modifications at T-DMRs
We searched the transcription factor that binds to the T-DMRs analyzing a transcription factor motif using The Multiple Em for Motif Eliciation (MEME) suite (http://meme-suite.org) (40), and EGR1 was extracted as a potential transcription factor. EGR1 has the consensus DNA sequence (GCGTGGGCG) in both T-DMR1 and T-DMR2 with extremely low P value and q value (0.00000561399 and 0.00343731, respectively) (Figure 5A). EGR1 belongs to the EGR family of C2H2-type zinc-finger proteins (41). EGR1 is a nuclear protein and functions as a transcriptional regulator (41). Therefore, to show that EGR1 actually regulates ESR1 expression, the effect of EGR1 knockdown on ESR1 expression was examined by transfecting EGR1 siRNA into human ESC, which express ESR1. As shown in Figure 5B, EGR1 was clearly suppressed by EGR1 siRNA transfection, and mRNA and protein expressions of ESR1 were significantly suppressed by EGR1 knockdown.
Because DNA hypomethylation within the promoter region of the gene facilitates the EGR1 binding to its consensus motif (42), we next investigated using a ChIP assay whether the binding of EGR1 to the T-DMRs is affected by DNA methylation at the T-DMRs. For this purpose, ESC isolated from the human endometrium expressing all 3 ESR1 variants and MDA-MB-231 expressing none of the ESR1 variants were used. As shown in Figure 5C, the EGR1 bindings to the T-DMR1 and T-DMR2 were significantly higher in ESC than in MDA-MB-231, suggesting that EGR1 binds to T-DMR1 and T-DMR2 in the human endometrium in which T-DMR1 and T-DMR2 are unmethylated, but the EGR1 bindings are inhibited in MDA-MB-231 in which T-DMR1 and T-DMR2 are hypermethylated.
DNA methylation is associated with a repressive histone modification, H3K27me3, which is involved in gene silencing (9, 10, 43). Therefore, we investigated the H3K27me3 status in T-DMR1 and T-DMR2 in ESC, in which T-DMRs are hypomethylated, and MDA-MB-231, in which T-DMRs are hypermethylated. The H3K27me3 levels in both T-DMR1 and T-DMR2 were significantly lower in ESC than in MDA-MB-231 (Supplemental Figure 3).
Discussion
The present study shows that ESR1 expression is tissue-specific (eg, high expression in the endometrium and mammary gland and low or no expression in the placenta and skin) and that it is regulated by DNA methylation at T-DMR1, which is distant from TSS, rather than by DNA methylation at the promoter region. DNA methylation of T-DMR1 was strongly associated with down-regulation of ESR1 expression in normal tissues, and suppressed ESR1 transcription in vitro. In addition, DNA methylation of T-DMR2 appears to regulate transcription of the upstream Exon-C, because variant3 mRNA was not expressed when the C-promoter was hypomethylated and T-DMR2 was moderately methylated and hypermethylated in the placenta and skin, although we did not directly show that DNA methylation at T-DMR2 down-regulates transcription of the upstream Exon-C.
Recent genome-wide analyses have shown that T-DMR is distributed throughout mammalian genomes (16, 18, 19). The T-DMRs identified in this study have 3 well-known characteristics of T-DMR: most of the T-DMR is outside of the promoter region including CpG islands (44), T-DMR exists close to the CpG-rich sequences, and most of the tissue-specific DNA methylation occurs within 2 kb distant from the CpG islands (45). Our results are also consistent with the tissue-specific expression of Gsg2, in which the T-DMR at the distal region, although keeping the CpG-rich promoter regions hypomethylated, acts as a transcription modulator that regulates gene expression levels (46).
ESR1 has several upstream exons and their corresponding TSSs. Although tissue-specific usage of these upstream exons is observed in humans and in other species (21, 22, 47), how a particular TSS is selected is still unclear. In this study, to investigate the mechanism of tissue-specific usage of these upstream exons for ESR1 expression, we focused on the association of DNA methylation statuses in the regions around upstream Exon-A, Exon-B, and Exon-C with mRNA expression of their corresponding variants, because they are often used in the tissues with high ESR1 expression (21). Transcription started from upstream Exon-A, Exon-B, and Exon-C in the endometrium and mammary gland, whereas it did not occur from any of the 3 upstream exons in the placenta and skin (Figure 3C). T-DMR1 and T-DMR2 were unmethylated and hypomethylated in the endometrium and mammary gland and were moderately methylated and hypermethylated in the placenta and skin while keeping the promoter regions hypomethylated. These findings suggest that each T-DMR regulates transcription from the corresponding upstream exons via DNA methylation for ESR1 expression in normal tissues.
Like ESR1, glucocorticoid/progesterone receptors, which are nuclear hormone receptors, have several TSSs (24, 26). However, the usage of these TSSs is regulated by DNA methylation in the promoter regions of each TSS, but not by DNA methylation at the T-DMR (23, 25, 26), indicating that the mechanism of tissue-specific usage of the upstream exons in ESR1 is different from that in glucocorticoid/progesterone receptors.
The reporter assay in this study revealed that T-DMR1, but not the proximal promoter, was essential for the enough reporter activity. In addition, DNA methylation at the T-DMR1 inhibited the transcription activity. These findings indicate that T-DMR1 regulates transcription. Recently, not only the regions near TSS but also the distal promoter regions that are far from TSS are considered as important regions for transcription (48). The recruitment of a complex consisting of basal transcription factors and RNA polymerase II to the proximal promoter region triggers transcription (49, 50). Long-range chromatin interactions, such as distal enhancer-proximal promoter interactions, are involved in the recruitment of the transcription factor complex, and recognized as an important mechanism to regulate gene expression levels (51, 52). From these findings, we speculate that the T-DMR1 interacts with the proximal promoter region and regulates the transcription of ESR1.
It is unclear how DNA methylation at the TDMRs down-regulates the ESR1 expression in normal tissues. DNA methylation of the gene promoter interrupts the recognition and binding of transcription factors (3–6). EGR1 is a potential transcription factor that binds to T-DMR1 and T-DMR2 for ESR1 expression. In fact, EGR1 was shown to up-regulate ESR1 expression in this study. The binding of EGR1 to the T-DMRs may be interrupted by DNA methylation, because EGR1 bound to T-DMR1 and T-DMR2 in ESC isolated from the human endometrium in which T-DMR1 and T-DMR2 are unmethylated, but the bindings of EGR1 were inhibited in MDA-MB-231 cells in which T-DMR1 and T-DMR2 are hypermethylated. However, because DNA methylation of T-DMR1 did not completely suppress the luciferase activity in the reporter assay, we cannot neglect the other regulatory mechanism in ESR1 mRNA expression than DNA methylation at the T-DMRs. Our result also showed that DNA methylation at the T-DMRs is associated with a repressive histone modification, H3K27me3, suggesting an interaction between DNA methylation and H3K27me3 to induce chromatin condensation. H3K27me3 has been reported to be associated with DNA methylation and involved in gene silencing by inducing chromatin condensation (9, 10). Further studies are needed about the interaction of DNA methylation and H3K27me3 at the T-DMRs and their significances in ESR1 expression.
Because there was no association between the mRNA/protein levels of DNMTs and the DNA methylation levels at the T-DMRs, it is unlikely that the expression level of DNMTs is involved in the regulation of DNA methylation level at the T-DMRs. This result is not surprising because the DNMTs levels are the total amount in the tissues, and do not reflect the local levels at the T-DMRs. Although it is important to know how tissue-specific DNA methylation is regulated or how DNMTs are preferentially recruited to the T-DMRs, these questions are not solved at present.
Regarding ESR1 expression in breast cancer, it has been reported that DNA methylation of the promoter is involved in repression of ESR1 expression (28–32). However, a long-standing question is why breast cancer cases with hypomethylated ESR1 promoters show various levels of ESR1 expression (30, 32). Our results show that breast cancer has a variety of DNA methylation profiles in the T-DMRs and promoter regions. In some cases of breast cancer, T-DMRs regulate ESR1 transcription via DNA methylation in a manner similar to normal tissues. Group X showed the DNA methylation status similar to what was observed in the endometrium and mammary gland, and group Y showed the DNA methylation status as was observed in placenta and skin (Figure 4C). However, in other cases, the regulation was different from that in normal tissues. In group Z (Figure 4C), although the DNA methylation level of T-DMR2 was similar to group X, the variant3 expression was repressed. In addition, in MCF7, both T-DMR1 and T-DMR2 were moderately methylated, but variant1 and variant2 were expressed and variant3 was not expressed. These results indicate that there are some cases of breast cancer in which transcriptional regulation for ESR1 expression deviates from the regulation seen in normal tissues. Some cases of breast cancer may have other regulation mechanisms in ESR1 expression than DNA methylation at the T-DMRs. In fact, about 70% of the ER-α-negative breast cancer tissues showed ESR1 mRNA expression (53–55). Src promotes estrogen-dependent ER-α proteolysis, leading to the absence of ESR1 expression in breast cancer (56). These reports suggest a posttranscriptional or posttranslational control in ER-α levels in breast cancer. The amount of ER-α expression is also important from the point of view of the response to hormone therapy. Further studies are needed to clarify how the expression levels of mRNA and protein of ER-α are regulated in breast cancer.
It is interesting to note that none of the breast cancer cases had both DNA hypomethylation in T-DMRs and DNA hypermethylation in the promoter regions. In addition, the DNA methylation statuses of the C-promoter varied among the breast cancer cases (group Y in Figure 4C), in which T-DMR1 and T-DMR2 were hypermethylated. These findings suggest that aberrant DNA methylation occurs at the T-DMR first and then extends to the promoter region in breast cancer. In MDA-MB-231, an ER-negative breast cancer cell line, B-promoter and C-promoter in addition to T-DMR1 and T-DMR2 were moderately methylated and hypermethylated, respectively. This is not surprising, because aberrant DNA methylation often occurs in a variety of cancers including breast cancer (57, 58). Previous reports have shown that some breast cancer cases have DNA hypermethylation in the promoter region and low or no ESR1 expression (30–32). However, the present study did not include the breast cancer cases with DNA hypermethylation in AB-promoter. Further studies with more samples may explain the association between the DNA methylation pattern in T-DMRs and promoter regions and ESR1 expression in breast cancer.
In conclusion, this is the first report to demonstrate that ESR1 has T-DMRs, and that the T-DMRs regulate tissue-specific ESR1 expression via DNA methylation in normal tissues. We also found that each upstream exon has a corresponding T-DMR, which regulates transcription from the upstream exon. Furthermore, our results show some breast cancer cases deviate from the normal regulatory mechanism of the transcription regulation of ESR1.
Acknowledgments
We thank Dr Hiroshi Kimura, Tokyo Institute of Technology, for the gift of anti-H3K27me3 antibody.
This work was supported in part by JSPS KAKENHI Grants 24592471, 24791704, 24791705, 25293343, 25462559, 25462560, 25861495, 26670726, 26861328, 26861329, 26861330, and 26462492 for Scientific Research from the Ministry of Education, Science, and Culture, Japan, New Yobimizu project of Yamaguchi University, and Takeda Science Foundation.
Disclosure Summary: The authors have nothing to disclose.
Funding Statement
This work was supported in part by JSPS KAKENHI Grants 24592471, 24791704, 24791705, 25293343, 25462559, 25462560, 25861495, 26670726, 26861328, 26861329, 26861330, and 26462492 for Scientific Research from the Ministry of Education, Science, and Culture, Japan, New Yobimizu project of Yamaguchi University, and Takeda Science Foundation.
Footnotes
- ChIP
- chromatin immunoprecipitation
- Ct
- cycle threshold
- DNMT
- DNA methyltransferase
- EGR1
- early growth response protein 1
- ER
- estrogen receptor
- ESC
- endometrial stromal cell
- ESR1
- estrogen receptor 1
- FCS
- fetal calf serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- H3K27me3
- trimethylation of the site of lysine 27 on histone H3
- qRT-PCR
- quantitative real-time RT-PCR
- siRNA
- small interfering RNA
- T-DMR
- tissue-dependent and differentially methylated region
- TSS
- transcription start site.
References
- 1. Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev. 1995;16:35–62. [DOI] [PubMed] [Google Scholar]
- 2. Grandien K, Berkenstam A, Gustafsson JA. The estrogen receptor gene: promoter organization and expression. Int J Biochem Cell Biol. 1997;29:1343–1369. [DOI] [PubMed] [Google Scholar]
- 3. Takizawa T, Nakashima K, Namihira M, et al. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. [DOI] [PubMed] [Google Scholar]
- 4. Maier H, Colbert J, Fitzsimmons D, Clark DR, Hagman J. Activation of the early B-cell-specific mb-1 (Ig-α) gene by Pax-5 is dependent on an unmethylated Ets binding site. Mol Cell Biol. 2003;23:1946–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. [DOI] [PubMed] [Google Scholar]
- 6. Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. [DOI] [PubMed] [Google Scholar]
- 7. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. [DOI] [PubMed] [Google Scholar]
- 8. Wade PA. Methyl CpG-binding proteins and transcriptional repression. Bioessays. 2001;23:1131–1137. [DOI] [PubMed] [Google Scholar]
- 9. Ning X, Shi Z, Liu X, et al. DNMT1 and EZH2 mediated methylation silences the microRNA-200b/a/429 gene and promotes tumor progression. Cancer Lett. 2015;359:198–205. [DOI] [PubMed] [Google Scholar]
- 10. Wong CM, Wong CC, Ng YL, Au SL, Ko FC, Ng IO. Transcriptional repressive H3K9 and H3K27 methylations contribute to DNMT1-mediated DNA methylation recovery. PLoS One. 2011;6:e16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee L, Asada H, Kizuka F, et al. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology. 2013;154:458–470. [DOI] [PubMed] [Google Scholar]
- 12. Lieb JD, Beck S, Bulyk ML, et al. Applying whole-genome studies of epigenetic regulation to study human disease. Cytogenet Genome Res. 2006;114:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maekawa R, Sato S, Yamagata Y, et al. Genome-wide DNA methylation analysis reveals a potential mechanism for the pathogenesis and development of uterine leiomyomas. PLoS One. 2013;8:e66632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maekawa R, Yagi S, Ohgane J, et al. Disease-dependent differently methylated regions (D-DMRs) of DNA are enriched on the X chromosome in uterine leiomyoma. J Reprod Dev. 2011;57:604–612. [DOI] [PubMed] [Google Scholar]
- 15. Sato S, Maekawa R, Yamagata Y, et al. Potential mechanisms of aberrant DNA hypomethylation on the x chromosome in uterine leiomyomas. J Reprod Dev. 2014;60:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiota K, Kogo Y, Ohgane J, et al. Epigenetic marks by DNA methylation specific to stem, germ and somatic cells in mice. Genes Cells. 2002;7:961–969. [DOI] [PubMed] [Google Scholar]
- 17. Shiota K, Yanagimachi R. Epigenetics by DNA methylation for development of normal and cloned animals. Differentiation. 2002;69:162–166. [DOI] [PubMed] [Google Scholar]
- 18. Sakamoto H, Suzuki M, Abe T, et al. Cell type-specific methylation profiles occurring disproportionately in CpG-less regions that delineate developmental similarity. Genes Cells. 2007;12:1123–1132. [DOI] [PubMed] [Google Scholar]
- 19. Yagi S, Hirabayashi K, Sato S, et al. DNA methylation profile of tissue-dependent and differentially methylated regions (T-DMRs) in mouse promoter regions demonstrating tissue-specific gene expression. Genome Res. 2008;18:1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asada H, Yamagata Y, Taketani T, et al. Potential link between estrogen receptor-α gene hypomethylation and uterine fibroid formation. Mol Hum Reprod. 2008;14:539–545. [DOI] [PubMed] [Google Scholar]
- 21. Reid G, Denger S, Kos M, Gannon F. Human estrogen receptor-α: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERα gene promoter region. Mol Endocrinol. 2001;15:2057–2063. [DOI] [PubMed] [Google Scholar]
- 23. Hansberg-Pastor V, González-Arenas A, Peña-Ortiz MA, García-Gómez E, Rodríguez-Dorantes M, Camacho-Arroyo I. The role of DNA methylation and histone acetylation in the regulation of progesterone receptor isoforms expression in human astrocytoma cell lines. Steroids. 2013;78:500–507. [DOI] [PubMed] [Google Scholar]
- 24. Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendoza-Garcés L, Rodríguez-Dorantes M, Alvarez-Delgado C, Vázquez-Martínez ER, Garcia-Tobilla P, Cerbón MA. Differential DNA methylation pattern in the A and B promoters of the progesterone receptor is associated with differential mRNA expression in the female rat hypothalamus during proestrus. Brain Res. 2013;1535:71–77. [DOI] [PubMed] [Google Scholar]
- 26. Turner JD, Alt SR, Cao L, et al. Transcriptional control of the glucocorticoid receptor: CpG islands, epigenetics and more. Biochem Pharmacol. 2010;80:1860–1868. [DOI] [PubMed] [Google Scholar]
- 27. McGuire WL. Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol. 1978;5:428–433. [PubMed] [Google Scholar]
- 28. Ferguson AT, Vertino PM, Spitzner JR, Baylin SB, Muller MT, Davidson NE. Role of estrogen receptor gene demethylation and DNA methyltransferase.DNA adduct formation in 5-aza-2′deoxycytidine-induced cytotoxicity in human breast cancer cells. J Biol Chem. 1997;272:32260–32266. [DOI] [PubMed] [Google Scholar]
- 29. Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor α expression in breast cancer. Oncologist. 2006;11:1–8. [DOI] [PubMed] [Google Scholar]
- 30. Lapidus RG, Ferguson AT, Ottaviano YL, et al. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res. 1996;2:805–810. [PubMed] [Google Scholar]
- 31. Yan L, Yang X, Davidson NE. Role of DNA methylation and histone acetylation in steroid receptor expression in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:183–192. [DOI] [PubMed] [Google Scholar]
- 32. Yoshida T, Eguchi H, Nakachi K, et al. Distinct mechanisms of loss of estrogen receptor α gene expression in human breast cancer: methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–2201. [DOI] [PubMed] [Google Scholar]
- 33. Higuchi T, Gohno T, Nagatomo T, et al. Variation in use of estrogen receptor-α gene promoters in breast cancer compared by quantification of promoter-specific messenger RNA. Clin Breast Cancer. 2014;14:249–257. [DOI] [PubMed] [Google Scholar]
- 34. Tamura I, Taketani T, Lee L, et al. Differential effects of progesterone on COX-2 and Mn-SOD expressions are associated with histone acetylation status of the promoter region in human endometrial stromal cells. J Clin Endocrinol Metab. 2011;96:E1073–E1082. [DOI] [PubMed] [Google Scholar]
- 35. Yamagata Y, Nishino K, Takaki E, et al. Genome-wide DNA methylation profiling in cultured eutopic and ectopic endometrial stromal cells. PLoS One. 2014;9:e83612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumaki Y, Oda M, Okano M. QUMA: quantification tool for methylation analysis. Nucleic Acids Res. 2008;36:W170–W175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura I, Asada H, Maekawa R, et al. Induction of IGFBP-1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology. 2012;153:5612–5621. [DOI] [PubMed] [Google Scholar]
- 38. Kimura H, Hayashi-Takanaka Y, Goto Y, Takizawa N, Nozaki N. The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct Funct. 2008;33:61–73. [DOI] [PubMed] [Google Scholar]
- 39. Tao R, Chen Z, Wu P, et al. The possible role of EZH2 and DNMT1 polymorphisms in sporadic triple-negative breast carcinoma in southern Chinese females. Tumour Biol. 2015;36:9849–9855. [DOI] [PubMed] [Google Scholar]
- 40. Bailey TL, Boden M, Buske FA, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poirier R, Cheval H, Mailhes C, et al. Distinct functions of egr gene family members in cognitive processes. Front Neurosci. 2008;2:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogishima T, Shiina H, Breault JE, et al. Promoter CpG hypomethylation and transcription factor EGR1 hyperactivate heparanase expression in bladder cancer. Oncogene. 2005;24:6765–6772. [DOI] [PubMed] [Google Scholar]
- 43. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song F, Smith JF, Kimura MT, et al. Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression. Proc Natl Acad Sci USA. 2005;102:3336–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irizarry RA, Ladd-Acosta C, Wen B, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato S, Maeda C, Hattori N, Yagi S, Tanaka S, Shiota K. DNA methylation-dependent modulator of Gsg2/Haspin gene expression. J Reprod Dev. 2011;57:526–533. [DOI] [PubMed] [Google Scholar]
- 47. Wilson ME, Westberry JM, Prewitt AK. Dynamic regulation of estrogen receptor-α gene expression in the brain: a role for promoter methylation? Front Neuroendocrinol. 2008;29:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tamura I, Ohkawa Y, Sato T, et al. Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol Endocrinol. 2014;28:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Y, Jorgensen M, Kolde R, et al. Prediction of RNA Polymerase II recruitment, elongation and stalling from histone modification data. BMC Genomics. 2011;12:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Auerbach RK, Euskirchen G, Rozowsky J, et al. Mapping accessible chromatin regions using Sono-Seq. Proc Natl Acad Sci USA. 2009;106:14926–14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Wong CH, Birnbaum RY, et al. Chromatin connectivity maps reveal dynamic promoter-enhancer long-range associations. Nature. 2013;504:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li G, Ruan X, Auerbach RK, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carmeci C, deConinck EC, Lawton T, Bloch DA, Weigel RJ. Analysis of estrogen receptor messenger RNA in breast carcinomas from archival specimens is predictive of tumor biology. Am J Pathol. 1997;150:1563–1570. [PMC free article] [PubMed] [Google Scholar]
- 54. Garcia T, Lehrer S, Bloomer WD, Schachter B. A variant estrogen receptor messenger ribonucleic acid is associated with reduced levels of estrogen binding in human mammary tumors. Mol Endocrinol. 1988;2:785–791. [DOI] [PubMed] [Google Scholar]
- 55. Henry JA, Nicholson S, Farndon JR, Westley BR, May FE. Measurement of oestrogen receptor mRNA levels in human breast tumours. Br J Cancer. 1988;58:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chu I, Arnaout A, Loiseau S, et al. Src promotes estrogen-dependent estrogen receptor α proteolysis in human breast cancer. J Clin Invest. 2007;117:2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochem Biophys Res Commun. 2014;455:70–83. [DOI] [PubMed] [Google Scholar]
- 58. Hattori N, Ushijima T. Compendium of aberrant DNA methylation and histone modifications in cancer. Biochem Biophys Res Commun. 2014;455:3–9. [DOI] [PubMed] [Google Scholar]