ABSTRACT
Epstein-Barr virus (EBV) is a human gammaherpesvirus associated with a variety of tumor types. EBV can establish latency or undergo lytic replication in host cells. In general, EBV remains latent in tumors and expresses a limited repertoire of latent proteins to avoid host immune surveillance. When the lytic cycle is triggered by some as-yet-unknown form of stimulation, lytic gene expression and progeny virus production commence. Thus far, the exact mechanism of EBV latency maintenance and the in vivo triggering signal for lytic induction have yet to be elucidated. Previously, we have shown that the EBV microRNA miR-BART20-5p directly targets the immediate early genes BRLF1 and BZLF1 as well as Bcl-2-associated death promoter (BAD) in EBV-associated gastric carcinoma. In this study, we found that both mRNA and protein levels of BRLF1 and BZLF1 were suppressed in cells following BAD knockdown and increased after BAD overexpression. Progeny virus production was also downregulated by specific knockdown of BAD. Our results demonstrated that caspase-3-dependent apoptosis is a prerequisite for BAD-mediated EBV lytic cycle induction. Therefore, our data suggest that miR-BART20-5p plays an important role in latency maintenance and tumor persistence of EBV-associated gastric carcinoma by inhibiting BAD-mediated caspase-3-dependent apoptosis, which would trigger immediate early gene expression.
IMPORTANCE EBV has an ability to remain latent in host cells, including EBV-associated tumor cells hiding from immune surveillance. However, the exact molecular mechanisms of EBV latency maintenance remain poorly understood. Here, we demonstrated that miR-BART20-5p inhibited the expression of EBV immediate early genes indirectly, by suppressing BAD-induced caspase-3-dependent apoptosis, in addition to directly, as we previously reported. Our study suggests that EBV-associated tumor cells might endure apoptotic stress to some extent and remain latent with the aid of miR-BART20-5p. Blocking the expression or function of BART20-5p may expedite EBV-associated tumor cell death via immune attack and apoptosis.
INTRODUCTION
While Epstein-Barr virus (EBV) can either establish latent infection or undergo a lytic cycle in host cells (1), EBV usually remains latent by expressing a limited number of viral genes, including noncoding RNAs (2). The conversion from latency to a lytic cycle is heralded by the expression of two immediate early genes, BZLF1 and BRLF1, which encode the transcription factors Zta and Rta, respectively (3–5). Zta and Rta can trans-activate each other as well as themselves (6, 7). They also sequentially activate early and late lytic genes, leading to the production of the progeny virus (8). In vitro, lytic replication can be induced by various stimuli, such as hypoxia (9), transforming growth factor beta (10–12), cross-linking of surface immunoglobulin (13), and 12-O-tetradecanoylphorbol-13-acetate (TPA) (14). In vivo, latency can be converted to a lytic cycle by triggering factors that remain unknown.
In cases where the host cell is forced to undergo apoptosis, lytic replication may be induced to successfully produce progeny virus. Human herpesviruses such as herpes simplex virus 1 (HSV-1) and Kaposi's sarcoma-associated herpesvirus (KSHV) were reported to have an apoptosis-initiated alternative replication program (15, 16). Furthermore, we and others reported that chemotherapeutic agents such as 5-fluorouracil (5-FU) (17) and docetaxel (18) as well as apoptosis-inducing reagents such as 2[[3-(2,3-dichlorophenoxy)propyl]amino]ethanol (DCPE) (15) and sodium arsenite (19) could induce EBV reactivation.
EBV expresses >40 mature microRNAs (miRNAs), including the BamHI fragment A rightward transcript (BART) and BamHI fragment H rightward open reading frame 1 (20, 21). BART miRNAs are expressed at higher levels in epithelial malignancies than in lymphomas (21–24). Several BART miRNAs have been shown to regulate cellular pathways and EBV latency. More specifically, miR-BART18-5p was shown to maintain latency by reducing the levels of mitogen-activated protein (MAP) kinase kinase kinase 2 (MAP3K2), which is involved in lytic cycle induction in latently infected memory B cells (25). Additionally, suppression of miR-BART6-5p by an antagomir has been shown to activate the expression of the Zta, Rta, EBV nuclear antigen 2 (EBNA2), and latent membrane protein 1 (LMP1) genes (26).
We previously reported that miR-BART20-5p suppressed lytic replication and progeny virus production by directly targeting BRLF1 and BZLF1 (27). We also found that miR-BART20-5p targets the 3′ untranslated region (UTR) of BAD (28), a BH3-only protein belonging to the proapoptotic subgroup of the B-cell lymphoma 2 (Bcl-2) family (29).
Recently, some miRNA targets have been proposed to act as competitive endogenous RNAs (ceRNAs) to modulate the repression of other targets of the same miRNA (30). Noncoding RNAs and the 3′ UTR of the target mRNA generate a large-scale ceRNA regulatory network by competing for endogenous miRNAs (31, 32) and affect the functions of miRNAs (33, 34). For instance, PTEN and its pseudogene act as ceRNAs (35). In addition, the 3′ UTRs of Versican and Fibronectin, two targets of miR-199a*, induced cell, tissue, and organ adhesion by arresting miR-199a* functions (36). It would be interesting to test if EBV immediate early genes and BAD function as ceRNAs, as they all bind with miR-BART20-5p sequence specifically.
BAD blocks the ability of antiapoptotic Bcl-2 family proteins and Bcl-2-associated X (BAX) to form mitochondrial permeability pores, leading to the activation of caspase-3 (37). The BAD-BAX cascade has been implicated as being necessary for strong and prolonged activation of caspase-3 in order to induce apoptosis (38). Indeed, inhibition of BAD by using a miR-BART20-5p mimic promoted cell proliferation and inhibited basal as well as 5-FU-induced apoptosis in our previous study (28). As replication of herpesviruses, including EBV, can be activated by apoptosis (15, 17–19, 39), it is intriguing that miR-BART20-5p targets both BAD and EBV immediate early genes. In this study, we analyzed the relationship between BAD and BRLF1/BZLF1 in the context of miR-BART20-5p function.
MATERIALS AND METHODS
Cell culture and reagents.
AGS-EBV is a gastric carcinoma cell line derived from AGS cells infected with a recombinant Akata virus (40–42). Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 400 μg/ml of G418 (Gibco, Carlsbad, CA, USA). BAD expression plasmid-transfected cells were cultured in RPMI 1640 medium supplemented with 200 μg/ml hygromycin B (Invitrogen, San Diego, CA, USA) in order to select transfectants for 2 weeks before analysis. All cells were maintained at 37°C in a 5% CO2 incubator.
Plasmid construction.
The BAD coding sequences (BAD-CDSs) with or without the 3′ UTR (NCBI GenBank accession number NM_004322) were amplified by using cDNA prepared from AGS-EBV cells to obtain BAD-CDS+3′-UTR and BAD-CDS, respectively. The amplicons were cloned into the HindIII/BamHI sites of the pCEP4 vector (Invitrogen) by using an EZ-Fusion cloning kit (Enzynomics, Daejeon, South Korea). The constructed BAD expression vectors (pCEP4-BAD-CDS and pCEP4-BAD-CDS+3′-UTR) contained a hygromycin selection marker for enrichment of transfected cells. The sequences of the primers used for each plasmid construct were as follows: 5′-CCAGCTGCTAGCAAGCTTATGTTCCAGATCCCAGAGTT-3′ and 5′-CTTATCATGTCTGGATCCTCACTGGGAGGGGGCGGAGC-3′ for pCEP4-BAD-CDS and 5′-CCAGCTGCTAGCAAGCTTATGTTCCAGATCCCAGAGTT-3′ and 5′-CTTATCATGTCTGGATCCCGGCGGCACAGACGCGGGCTT-3′ for pCEP4-BAD-CDS+3′-UTR.
Transfection and TPA treatment.
The locked nucleic acid (LNA)–miR-BART20-5p inhibitor [LNA-miR-BART20-5p(i)] (5′-GAATGAAGACATGCCTGCT-3′) (catalog number 426096-00) and the control LNA-miRNA inhibitor (control LNA) (5′-GTGTAACACGTCTATACGCCCA-3′) (catalog number 199004-00) were purchased from Exiqon (Vedbaek, Denmark). The scrambled control (5′-ACGUGACACGUUCGGAGAAUU-3′) was purchased from Genolution Pharmaceuticals (Seoul, South Korea). The scrambled control and control LNAs were used as negative controls for miRNA mimics and LNA inhibitors, respectively. For experiments, 1 × 106 cells were seeded into 100-mm-diameter dishes containing 10 ml culture medium 24 h prior to transfection. All transfection experiments were performed by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocols. After 24 h, cells were treated with 5 nM TPA for 48 or 72 h to induce the EBV lytic cycle.
Quantitative reverse transcription-PCR (RT-PCR).
AGS-EBV cells were harvested and total RNA was extracted by using RNAzol B reagent (Tel-Test, Friendswood, TX, USA) according to the manufacturer's instructions. cDNA was synthesized by using 1 μg total RNA, oligo(dT) primers (Ahram Biosystems, Seoul, South Korea), and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Real-time PCR was carried out by using a SYBR green quantitative PCR (qPCR) kit (TaKaRa, Tokyo, Japan) with an Mx3000p real-time PCR system (Stratagene, La Jolla, CA, USA). The sequences of the primers used for each gene were as follows: 5′-GCTCCGGCAAGCATCATC-3′ and 5′-GGTAGGAGCTGTGGCGACT-3′ for BAD, 5′-GGCTAACCAAGGACAACAGC-3′ and 5′-GAAGCCACCCGATTCTTGTA-3′ for BZLF1, 5′-GTGTTCCACAGCCTGCAC-3′ and 5′-GAAGCCACCCGATTCTTGTA-3′ for BRLF1, 5′-TCGGTCTGGTACAGATGTCG-3′ and 5′-GGCTCAGAAGCACACAAACA-3′for caspase-3, and 5′-ATGGGGAAGGTGAAGGTCG-3′ and 5′-GGGGTCATTGATGGCAACAATA-3′ for the glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH). PCR conditions were 95°C for 5 min followed by 40 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. To confirm the purity of the PCR products, dissociation curves were checked routinely. To accomplish this, reaction mixtures were incubated at 95°C for 60 s and ramped from 60°C to 95°C at a rate of 0.1°C/s with continuous measurement of fluorescence. Relative gene expression was calculated by the comparative threshold cycle (CT) method using GAPDH as an internal loading control.
Cell proliferation assay.
Cell proliferation was analyzed by using Cell Counting kit 8 (CCK-8; Dojindo Molecular Technologies, Tokyo, Japan). BAD-overexpressing cells (1 × 103 cells/well) were seeded into a 96-well plate. After the indicated periods, 10 μl of CCK-8 solution was added to each well. The absorbance at a wavelength of 450 nm was measured after 2 h by using a SoftMax apparatus (Molecular Devices, Sunnyvale, CA, USA).
Small interfering RNA sequences.
Small interfering RNAs (siRNAs) and a negative-control siRNA lacking any known target gene product were synthesized by Genolution Pharmaceuticals. The siBAD siRNA was specific for BAD, while siBRLF1/BZLF1 had dual specificity for BRLF1 and BZLF1. The siRNA specific for caspase-3 (siCASP3) was purchased from Bioneer Corporation (Daejeon, South Korea). The sequence of the negative-control siRNA was 5′-ACGUGACACGUUCGGAGAAUU-3′. The sequences of the siRNAs were as follows: 5′-CGACAUAACCCAGAAUCAACA-3′ for siBRLF1/BZLF1, 5′-GUACUUCCCUCAGGCCUAU-3′ for siBAD, and 5′-AGUAUGCCGACAAGCUUGA-3′ for siCASP3.
Western blot analysis.
Cells were lysed in radioimmunoprecipitation assay buffer containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, and 10 μg/ml aprotinin). The cell lysate (50 μg protein) was mixed with 5× loading buffer (Fermentas, Waltham, MA, USA) and heated at 95°C for 5 min. Samples were separated by electrophoresis on 12.5% sodium dodecyl sulfate-polyacrylamide gels, and the separated proteins were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). To detect the expression of EBV lytic proteins and apoptosis-related genes, anti-BZLF1 (1:500; Dako, Glostrup, Denmark), anti-BRLF1 (1:500; Argene, Verniolle, France), anti-BAD (1:1,000; Cell Signaling Technology, Beverly, MA, USA), anti-poly(ADP-ribose) polymerase (PARP) (1:500; Becton Dickinson, San Diego, CA, USA), and anti-cleaved caspase-3 (1:500; Cell Signaling Technology) antibodies were used. After washing, the blots were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Amersham Biosciences, Piscataway, NJ, USA) at a dilution of 1:5,000 for 1 h at room temperature. Anti-α-tubulin antibody (Cell Signaling Technology) was used to confirm comparable loading between gel lanes. Protein bands were visualized by using an enhanced chemiluminescence detection system (Amersham Bioscience), and the membrane was exposed to X-ray film (Agfa, Mortsel, Belgium). The density of each protein band was quantified by using Fujifilm Multi Gauge software (version 3.0).
Quantitative PCR to assess EBV genome copy numbers.
AGS-EBV cells (1 × 106) were seeded into a 100-mm-diameter dish. After 24 h, the cells were transfected with siRNAs (30 nM) or the LNA-miR-BART20-5p inhibitor (50 nM). After 24 h, the cultures were refreshed with new RPMI 1640 medium containing 5 nM TPA to induce EBV lytic replication. The cells were incubated at 37°C in a 5% CO2 incubator for 3 days, allowing the production of progeny viruses. Cells were removed by centrifugation for 5 min at 800 × g at room temperature, and the supernatant was then passed through a 0.45-μm-pore-size filter (Nalgene, Rochester, NY, USA). The filtrate was ultracentrifuged by using an SW41 rotor (Beckman Instruments, Fullerton, CA, USA) at 75,000 × g for 2 h at 4°C. The pellet was then resuspended in 200 μl 0.2× phosphate-buffered saline and heated to 95°C for 15 min. Next, 20 μl of 20 mg/ml proteinase K was added to the suspension, and the mixture was incubated at 56°C for 1 h, followed by heat inactivation at 95°C for 30 min. Real-time PCR amplification of EBNA1 was carried out by using a SYBR green qPCR kit (TaKaRa, Tokyo, Japan) with an Mx3000P real-time PCR system (Stratagene). The sequences of the EBNA-1 primers were 5′-AGTCGTCTCCCCTTTGGAAT-3′ (sense) and 5′-TCCTCACCCTCATCTCCATC-3′ (antisense). The relative viral copy number was calculated as described previously (27).
Statistical analyses.
Data were analyzed by using the Student t test. Cell proliferation assay results were analyzed by two-way repeated-measure analysis of variance. Curve fitting and analyses were performed by using GraphPad Prism (GraphPad Software, San Diego, CA, USA). P values of <0.05 were considered to reflect statistically significant differences. All results were expressed as means ± standard deviations (SD).
RESULTS
siBAD abrogates the enhancing effect of the miR-BART20-5p inhibitor on expression of EBV immediate early genes.
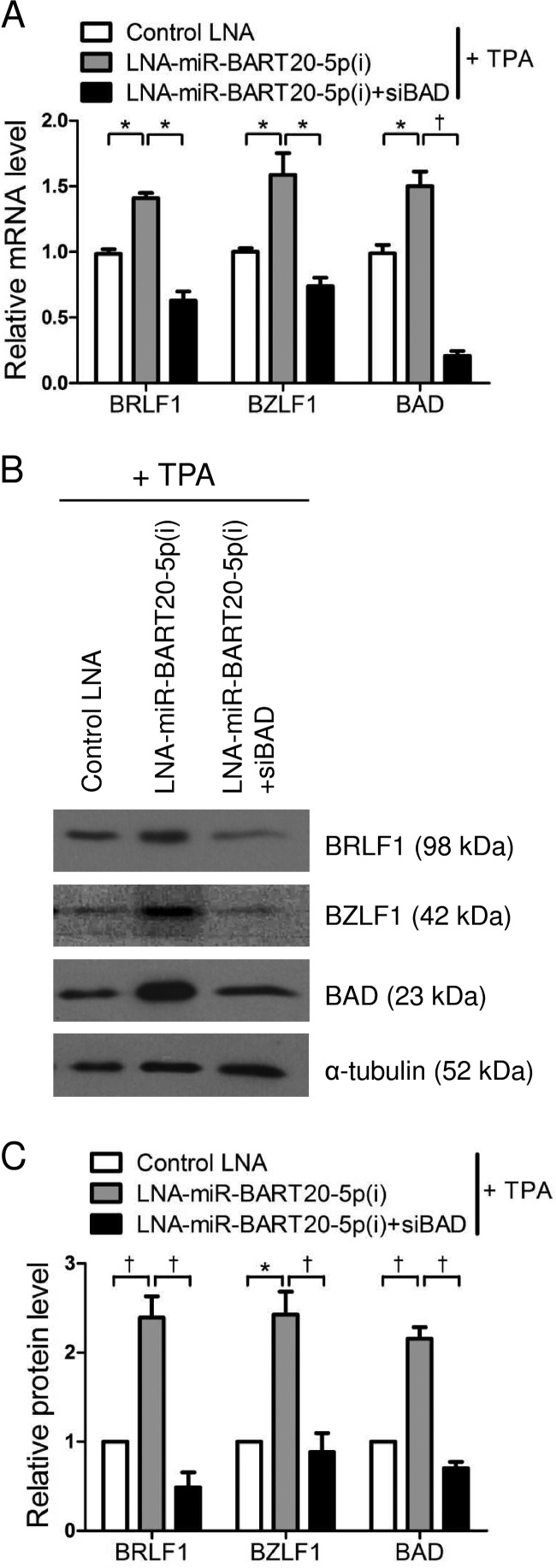
To confirm that miR-BART20-5p targeted the EBV immediate early genes and BAD, AGS-EBV cells were transfected with LNA-miR-BART20-5p(i). After induction of the EBV lytic cycle by TPA treatment, expression levels of the EBV immediate early genes BRLF1 and BZLF1, as well as the BH3-only protein gene BAD, were analyzed by real-time RT-PCR and Western blotting. Expressions of the BRLF1 and BZLF1 proteins in AGS-EBV cells were readily detected by Western blotting after 48 h when tested at different time points after treatment with 5 nM TPA. In addition, when cells were treated with TPA for 48 h, the most clear effect of miR-BART20-5p was observed (data not shown). Both mRNA and protein levels of BRLF1, BZLF1, and BAD were increased following transfection with LNA-miR-BART20-5p(i) compared with the control LNA (Fig. 1A to C).
FIG 1.
Effect of LNA-miR-BART20-5p(i) and siBAD on BRLF1, BZLF1, and BAD expression. AGS-EBV cells were transfected with 50 nM control LNA, LNA-miR-BART20-5p(i), or LNA-miR-BART20-5p(i) plus siBAD. After 24 h, the cells were treated with 5 nM TPA for 48 h and then harvested for RNA and protein preparation. (A) Real-time RT-PCR analysis of BRLF1, BZLF1, and BAD was carried out by using a SYBR green qPCR kit. The RT-PCR results from three independent experiments were normalized to GAPDH levels and are expressed as ratios relative to the values obtained from control LNA-transfected cells. (B) BRLF1, BZLF1, and BAD protein levels were analyzed by Western blot analysis. (C) Western blot results similar to those shown in panel B were obtained by using two additional sets of independently transfected AGS-EBV cells. Western blot results were normalized to α- tubulin values and are expressed as ratios relative to the values obtained from control LNA-transfected cells. Mean values from all three experiments are plotted. Error bars indicate SD (n = 3). *, P < 0.05; †, P < 0.01.
When AGS-EBV cells were cotransfected with siBAD and LNA-miR-BART20-5p(i), siBAD abrogated the enhancing effect of LNA-miR-BART20-5p(i) on the mRNA expression of BRLF1, BZLF1, and BAD (Fig. 1A). Western blot results also showed that the effect of LNA-miR-BART20-5p(i) on the expression of these three genes was completely abolished by siBAD (Fig. 1B and C).
BAD affects the expression of BRLF1 and BZLF1.
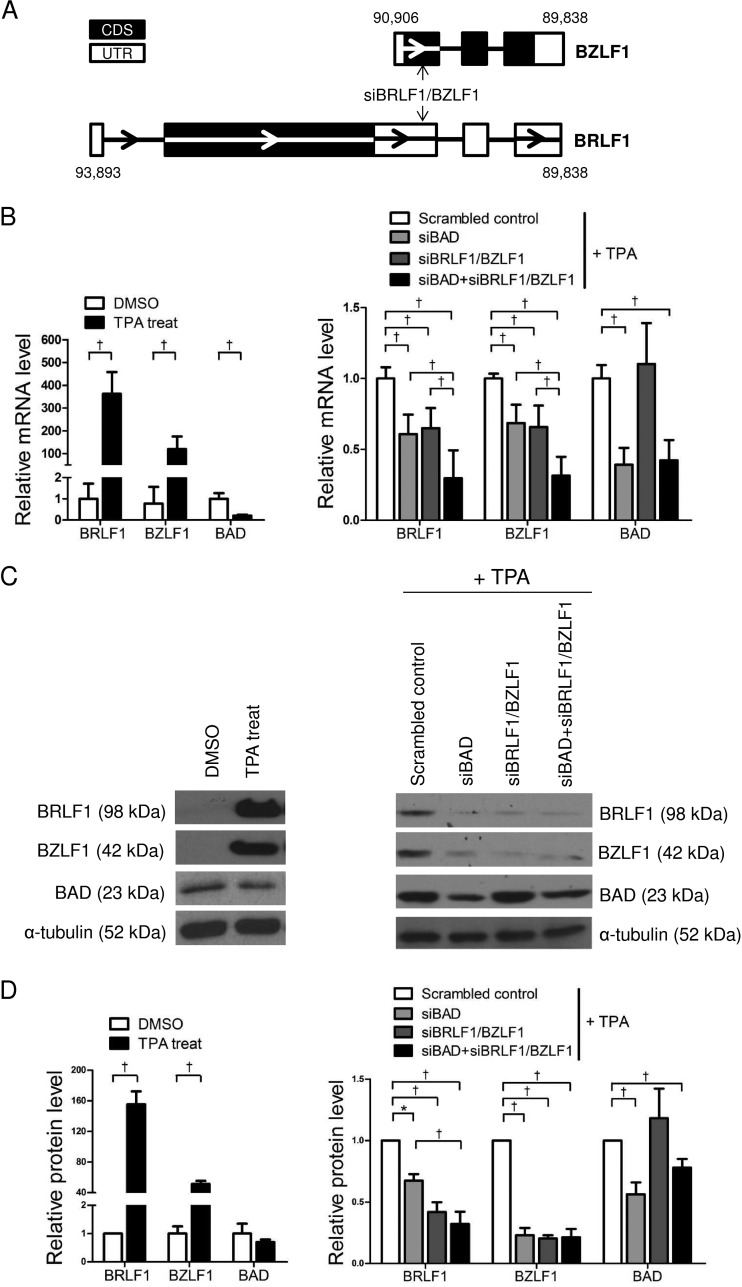
To investigate whether BAD affected the expression of BRLF1 and BZLF1 in AGS-EBV cells, siBAD and siBRLF1/BZLF1 were used. It should be noted that siBRLF1/BZLF1 is capable of targeting the two genes simultaneously, as BZLF1 is encoded within the 3′ UTR of BRLF1 (Fig. 2A). Effective induction of the EBV lytic cycle in AGS-EBV cells was confirmed by the expression of BRLF1 and BZLF1 following TPA treatment (Fig. 2B to D, left). TPA treatment showed a tendency to reduce BAD mRNA and protein levels slightly. However, no statistical significance was found on the protein level.
FIG 2.
Effect of dually specific siBRLF1/BZLF1 and siBAD on BRLF1, BZLF1, and BAD expression. (A) Schematic drawing showing the binding sites of siBRLF1/BZLF1 on BRLF1 and BZLF1. (B to D, left) AGS-EBV cells were treated with the vehicle (dimethyl sulfoxide [DMSO]) or 5 nM TPA for 48 h and harvested for RNA (B) and protein (C and D) preparation. AGS-EBV cells were transfected with 30 nM siBAD, siBRLF1/BZLF1, siBAD plus siBRLF1/BZLF1, or the scrambled control. (Right) After 24 h, transfected cells were stimulated with TPA for 48 h. (B) BRLF1, BZLF1, and BAD mRNA levels were detected by real-time RT-PCR. The RT-PCR results from three independent experiments were normalized to GAPDH values and are expressed as ratios relative to the values obtained from scrambled control-transfected cells. (C) BRLF1, BZLF1, and BAD protein levels were analyzed by Western blot analysis. (D) Western blot results similar to those shown in panel C were obtained by using two additional sets of independently transfected AGS-EBV cells. Mean values from all three experiments are plotted. Error bars indicate SD (n = 3). *, P < 0.05; †, P < 0.01.
The expression levels of BAD, BRLF1, and BZLF1 were determined by real-time RT-PCR and Western blotting of TPA-treated AGS-EBV cells following transfection with the scrambled control, siBAD, siBRLF1/BZLF1, or siBAD plus siBRLF1/BZLF1. The expression levels of BRLF1 and BZLF1 were reduced to similar levels by siBAD and siBRLF1/BZLF1 (Fig. 2B to D, right). An additive effect on the expression of the BRLF1 and BZLF1 mRNAs was observed when AGS-EBV cells were cotransfected with siBAD and siBRLF1/BZLF1 compared to cells transfected with either of these siRNAs (Fig. 2B, right). In contrast, siBRLF1/BZLF1 did not affect the expression of BAD (Fig. 2B, right). Similar results were observed in Western blot experiments (Fig. 2C and D, right). However, the additive effect of siBAD and siBRLF1/BZLF1 shown for the mRNA levels of the two EBV immediate early genes was not obvious when the protein levels of BRLF1 and BZLF1 were assessed by Western blotting (Fig. 2C and D, right).
Overexpressed BAD upregulates the expression of BRLF1 and BZLF1.
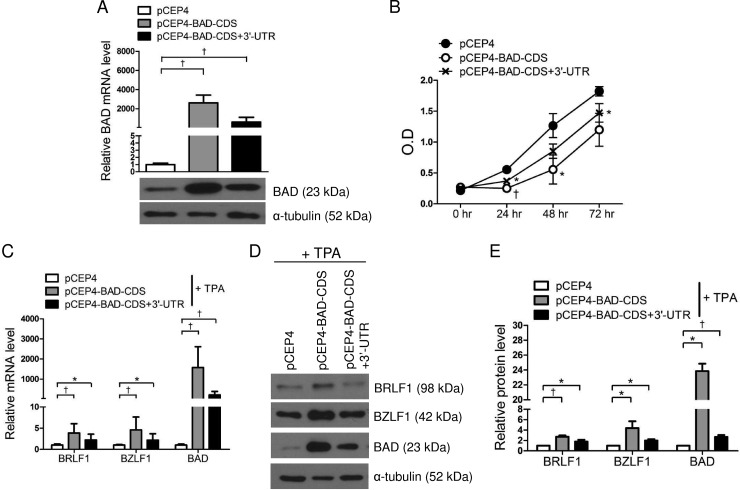
The BAD expression vectors pCEP4-BAD-CDS and pCEP4-BAD-CDS+3′-UTR were used to examine the effect of BAD overexpression on the expression of BRLF1 and BZLF1. AGS-EBV cells were transfected with each of the BAD expression vectors or an empty vector (pCEP4) as a control. After selection of transfected cells following 2 weeks of treatment with 200 μg/ml hygromycin, the cells were harvested to assess BAD expression levels. Real-time RT-PCR and Western blotting revealed that transfection with pCEP4-BAD-CDS and pCEP4-BAD-CDS+3′UTR significantly upregulated BAD expression in AGS-EBV cells (Fig. 3A). The BAD expression level was slightly higher following transfection with pCEP4-BAD-CDS than with pCEP4-BAD-CDS+3′-UTR (Fig. 3A). We then investigated the effect of BAD overexpression on cell proliferation. A CCK-8 assay showed that cell proliferation decreased following BAD overexpression and that cell proliferation seemed to inversely correlate with the level of BAD expression (Fig. 3B).
FIG 3.
Overexpression of BAD upregulates BRLF1 and BZLF1 expression. AGS-EBV cells were transfected with pCEP4-BAD-CDS, pCEP4-BAD-CDS+3′-UTR, or the empty vector (pCEP4). (A) Real-time RT-PCR and Western blot results confirm that BAD is overexpressed in AGS-EBV cells transfected with pCEP4-BAD-CDS or pCEP4-BAD-CDS+3′-UTR. RT-PCR results have been normalized to GAPDH values and are expressed as ratios relative to the values obtained from cells transfected with pCEP4. (B) At the indicated times after transfection, 10 μl of CCK-8 solution was added to each well to assess cell proliferation. O.D, optical density. (C and D) Appropriately transfected cells were treated with 5 nM TPA for 48 h and harvested for real-time RT-PCR (C) or Western blotting (D). (E) Western blot results similar to those shown in panel D were obtained by using two additional sets of independently transfected AGS-EBV cells. Mean values from all three experiments are plotted. Error bars indicate SD (n = 3). *, P < 0.05; †, P < 0.01.
Next, we performed real-time RT-PCR and Western blot analysis to assess the expression levels of BRLF1 and BZLF1 in 5 nM TPA-treated BAD-overexpressing cells. BAD expression levels in BAD-overexpressing cells were reduced by TPA treatment (Fig. 3A versus C). This is similar to the results shown in Fig. 2B (left). Real-time RT-PCR and Western blotting revealed that the expression levels of BRLF1, BZLF1, and BAD in BAD-overexpressing cells were significantly higher than those in control cells. The expression levels of BRLF1, BZLF1, and BAD were noticeably higher in cells transfected with pCEP4-BAD-CDS than in those transfected with pCEP4-BAD-CDS+3′-UTR (Fig. 3C to E).
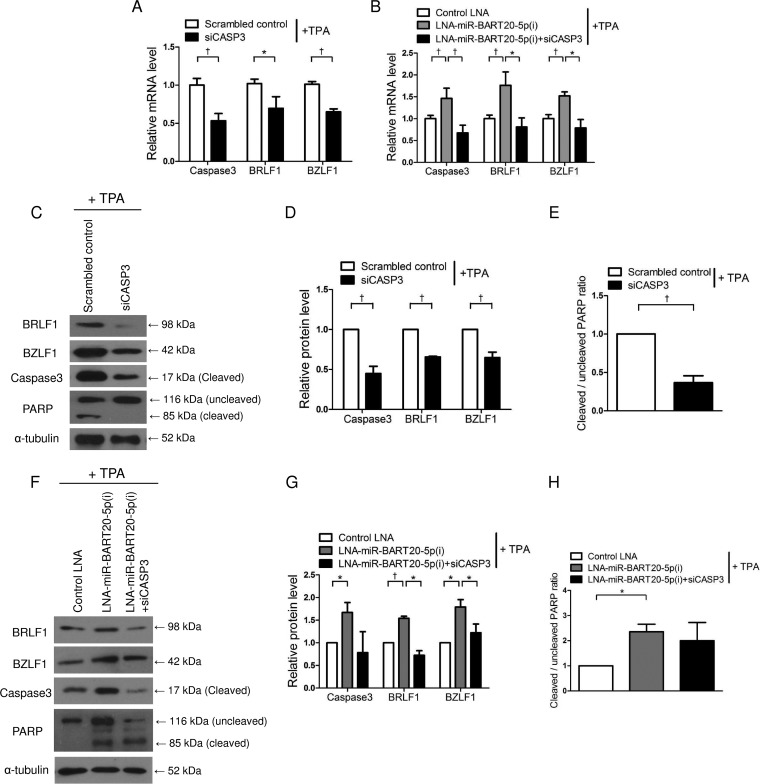
Induction of BRLF1 and BZLF1 by BAD is mediated by caspase-3.
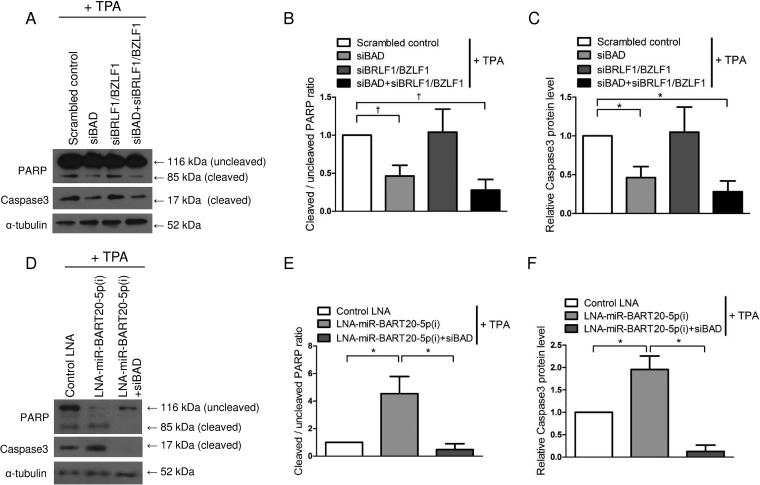
To investigate the effect of EBV immediate early genes and BAD on apoptosis, the expression levels of PARP and caspase-3 were analyzed by Western blotting following TPA treatment in cells transfected with the siRNAs for BAD and/or BRLF1/BZLF1. When AGS-EBV cells were transfected with siBAD, the cleaved forms of PARP and caspase-3 were reduced by 60%. In contrast, siBRLF1/BZLF1 did not affect the ratios of cleaved PARP and caspase-3 (Fig. 4A to C). In addition, expression levels of both cleaved PARP and caspase-3 were increased following transfection with LNA-miR-BART20-5p(i), and this LNA inhibitor effect was completely counteracted by siBAD cotransfection (Fig. 4D to F).
FIG 4.
Effect of BAD, BRLF1, and BZLF1 on apoptosis. (A to C) AGS-EBV cells were transfected with 30 nM siBAD, siBRLF1/BZLF1, siBAD plus siBRLF1/BZLF1, or the scrambled control. (D to F) AGS-EBV cells were transfected with 50 nM control LNA, LNA-miR-BART20-5p(i), or LNA-miR-BART20-5p(i) plus siBAD. Twenty-four hours after transfection, transfected cells were treated with 5 nM TPA for 48 h. (A and D) Expression levels of the cleaved forms of the PARP and caspase-3 proteins were detected by Western blot analysis. (B and E) The ratio of cleaved PARP to the uncleaved form observed in the three independent Western blot experiments shown in panels A and D. (C and F) The expression levels of cleaved caspase-3 in transfected cells were normalized to α-tubulin levels and are expressed as ratios relative to the values obtained from the control. Error bars indicate SD (n = 3). *, P < 0.05; †, P < 0.01.
We also conducted experiments to test whether the expression of the EBV immediate early genes was dependent on caspase-3. To accomplish this, AGS-EBV cells were transfected with a siRNA specific for caspase-3 (siCASP3) or a scrambled control and then treated with TPA before the expression levels of caspase-3, BRLF1, and BZLF1 were compared. Real-time RT-PCR results showed that the expression levels of BRLF1, BZLF1, and caspase-3 were reduced by siCASP3 (Fig. 5A). Similarly, AGS-EBV cells treated with TPA showed reduced BRLF1 and BZLF1 protein levels when transfected with siCASP3 compared with those transfected with the scrambled control (Fig. 5C and D). Similar reductions were also observed for cleaved PARP and caspase-3 following siCASP3 transfection (Fig. 5C). The ratio of cleaved PARP to the uncleaved form was decreased by up to 60% by siCASP3 (Fig. 5E). In contrast, transfection with LNA-miR-BART20-5p(i) increased the expression levels of cleaved caspase-3 and PARP as well as BRLF1 and BZLF1; however, these effects were eliminated by cotransfection with siCASP3 (Fig. 5B and F to H).
FIG 5.
Regulation of BRLF1 and BZLF1 by caspase-3-dependent apoptosis. (A and C to E) AGS-EBV cells were transfected with 30 nM siCASP3 or the scrambled control. (B and F to H) AGS-EBV cells were transfected with 50 nM control LNA, LNA-miR-BART20-5p(i), or LNA-miR-BART20-5p(i) plus siCASP3. After 24 h, transfected cells were treated with 5 nM TPA for 48 h. (A and B) BRLF1, BZLF1, and caspase-3 mRNA levels were quantified by real-time RT-PCR. (C and F) Protein levels of BRLF1 and BZLF1, as well as cleaved forms of PARP and caspase-3, were measured by Western blotting. (D and G) Expression levels of cleaved caspase-3, BRLF1, and BZLF1 in transfected cells were normalized to α-tubulin levels and are expressed as ratios relative to the values obtained from the control. (E and H) Ratios of cleaved PARP to the uncleaved form observed in three independent Western blot experiments shown in panels C and F. Error bars indicate SD (n = 3). *, P < 0.05; †, P < 0.01.
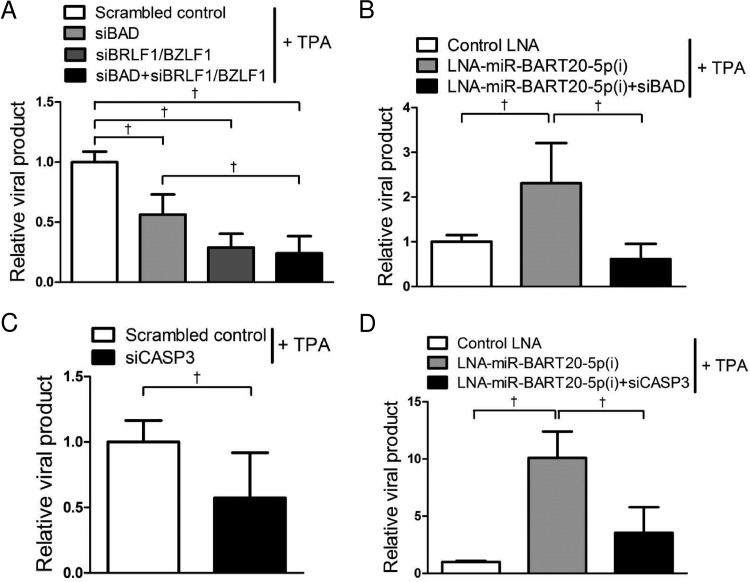
miR-BART20-5p inhibits virus production by targeting viral and cellular genes.
In order to determine whether apoptosis induced not only the expression of EBV lytic genes but also viral particle production, EBV genome copy numbers were analyzed. The level of viral particle production was significantly decreased following TPA treatment when cells were transfected with siBAD and/or siBRLF1/BZLF1 (Fig. 6A). Conversely, EBV genome copy numbers were increased >2.5-fold by TPA treatment in cells transfected with LNA-miR-BART20-5p(i) compared with cells transfected with the control LNA (Fig. 6B). When AGS-EBV cells were cotransfected with LNA-miR-BART20-5p(i) and siBAD, the level of viral particle production was significantly decreased compared with that in cells transfected with LNA-miR-BART20-5p(i) only (Fig. 6B). Results similar to those shown in Fig. 6A and B were also obtained when digital droplet PCR was used to measure viral DNA titers (data not shown).
FIG 6.
Suppression of viral production by siRNAs for BAD and caspase-3. (A and C) AGS-EBV cells were transfected with 30 nM siRNAs or the scrambled control. (B and D) AGS-EBV cells were transfected with 50 nM control LNA, LNA-miR-BART20-5p(i), or LNA-miR-BART20-5p(i) plus siRNA. Twenty-four hours after transfection, cells were treated with 5 nM TPA for 72 h. Cell supernatants were assayed in order to detect the EBV genome by real-time PCR. The ratios of the levels of viral production to the amount obtained from the control are shown. Error bars indicate SD (n = 3). †, P < 0.01.
The effect of siCASP3 on progeny virus production was also assessed following TPA treatment. The level of viral particle production was reduced by 50% when cells were transfected with siCASP3 compared to that in cells transfected with the scrambled control (Fig. 6C). In addition, cotransfected siCASP3 completely abolished the enhancing effect of LNA-miR-BART20-5p(i) on viral production (Fig. 6D).
DISCUSSION
We previously reported that miR-BART20-5p directly targeted BAD (28) and two EBV immediate early genes, BRLF1 and BZLF1 (27). In this study, the relationship between BAD and BRLF1/BZLF1 in the context of miR-BART20-5p function was investigated. We found that miR-BART20-5p inhibited the expression of EBV immediate early genes indirectly by suppressing BAD-induced caspase-3-dependent apoptosis. Thus, EBV-associated gastric carcinoma cells may be able to survive under mild apoptotic stress and remain latent with the aid of miR-BART20-5p.
Apoptosis is not a prerequisite for EBV lytic activation. For example, TPA does not induce apoptosis but successfully induces the EBV lytic cycle (39). However, when the host cell is forced to undergo apoptosis, the virus may undergo lytic replication and produce progeny viruses rather than perishing with the host. Accordingly, apoptosis-induced viral replication was observed in cells infected with human herpesviruses, including HSV-1, KSHV, and EBV (15, 16). In addition, caspase-3 activity was shown to play a key role in the induction of lytic activation under apoptotic conditions (39). Our data also showed that BAD-mediated caspase-3-dependent apoptosis played a key role in the activation of lytic replication. Further studies are warranted to clarify whether BAD, caspase-3, or apoptosis activates the BRLF1 and BZLF1 promoters directly or indirectly by some other mechanisms.
The expression levels of BRLF1, BZLF1, and BAD were significantly increased in BAD-overexpressing cells. This effect was greater in cells transfected with pCEP4-BAD-CDS than in those transfected with pCEP4-BAD-CDS+3′-UTR. These differences may be attributable to the fact that pCEP4-BAD-CDS+3′-UTR, unlike pCEP4-BAD-CDS, which does not contain the 3′-UTR sequence, can be downregulated by miR-BART20-5p or other human miRNAs.
The 3′ UTR of BAD may have functioned as a ceRNA for miR-BART20-5p, resulting in the upregulation of the EBV immediate early genes BRLF1 and BZLF1. However, this is less likely, as the BAD expression vector without the 3′ UTR increased the expression levels of BRLF1 and BZLF1 more than the BAD expression vector with the 3′ UTR. Meanwhile, BRLF1 and BZLF1 did not affect the expression of BAD, suggesting that the 3′ UTRs of BRLF1 and BZLF1 do not compete with BAD for miR-BART20-5p. Thus, BRLF1/BZLF1, and BAD do not appear to exert a ceRNA effect on each other. ceRNA effects are argued to be induced only in miRNA families with low total miRNA-to-target ratios (43). Furthermore, it was recently suggested that modulation of miRNA target abundance is unlikely to cause significant effects on gene expression and metabolism through a ceRNA effect, based on stoichiometric analysis of miR-122 and its well-known targets (44).
siBAD and siBRLF1/BZLF1 produced similar reductions in the expression levels of BRLF1 and BZLF1 mRNAs. Furthermore, siBAD and siBRLF1/BZLF1 showed an additive effect on the reduction of BRLF1 and BZLF1 mRNA levels, suggesting that the direct and indirect regulatory mechanisms of BRLF1 and BZLF1 for EBV latency maintenance do not have overlapping effects. This additive effect of these two siRNAs was not observed when protein levels of the EBV immediate early genes were measured or when progeny virus production was assessed. For some genes, large differences in mRNA expression levels are not mirrored by protein expression levels (45).
We observed that the expression level of BAD was decreased when the lytic cycle was induced by TPA treatment. This was expected considering that the level of miR-BART20-5p has been shown to be significantly increased following lytic induction (21, 27) and that miR-BART20-5p directly targets the 3′ UTR of BAD (28).
Several reported targets of miR-BART20-5p seem to facilitate the survival of EBV-infected cells. miR-BART20-5p targets the T-box transcription factor TBX21 (46) and interferon (47), leading to the progression of EBV-associated tumors. In addition, miR-BART20-5p may inhibit the EBV immediate early genes BRLF1 and BZLF1 to maintain latency and avoid apoptosis as well as immune attack of EBV-infected cells. Because miR-BART20-5p seems to play an important role in EBV-infected tumor cell growth and EBV latency maintenance, specific inhibitors for miR-BART20-5p would be potential therapeutic candidates that can affect a variety of EBV-associated tumors.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP [Ministry of Science, ICT and Future Planning]) (2015M2B2A9032172) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2014R1A2A1A11051682).
REFERENCES
- 1.Kenney SC, Mertz JE. 2014. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol 26:60–68. doi: 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI. 2000. Epstein-Barr virus infection. N Engl J Med 343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 3.Rooney CM, Rowe DT, Ragot T, Farrell PJ. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol 63:3109–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooney C, Taylor N, Countryman J, Jenson H, Kolman J, Miller G. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc Natl Acad Sci U S A 85:9801–9805. doi: 10.1073/pnas.85.24.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse HJ. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J 19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holley-Guthrie EA, Quinlivan EB, Mar EC, Kenney S. 1990. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol 64:3753–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Speck S. 2003. Synergistic autoactivation of the Epstein-Barr virus immediate-early BRLF1 promoter by Rta and Zta. Virology 310:199–206. doi: 10.1016/S0042-6822(03)00145-4. [DOI] [PubMed] [Google Scholar]
- 8.Pattle S, Farrell P. 2006. The role of Epstein-Barr virus in cancer. Expert Opin Biol Ther 6:1193–1205. doi: 10.1517/14712598.6.11.1193. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J-H, Wang N, Li A, Liao W-T, Pan Z-G, Mai S-J, Li D-J, Zeng M-S, Wen J-M, Zeng Y-X. 2006. Hypoxia can contribute to the induction of the Epstein-Barr virus (EBV) lytic cycle. J Clin Virol 37:98–103. doi: 10.1016/j.jcv.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.di Renzo L, Altiok A, Klein G, Klein E. 1994. Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma cell lines. Int J Cancer 57:914–919. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 11.Fahmi H, Cochet C, Hmama Z, Opolon P, Joab I. 2000. Transforming growth factor beta 1 stimulates expression of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J Virol 74:5810–5818. doi: 10.1128/JVI.74.13.5810-5818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iempridee T, Das S, Xu I, Mertz J. 2011. Transforming growth factor beta-induced reactivation of Epstein-Barr virus involves multiple Smad-binding elements cooperatively activating expression of the latent-lytic switch BZLF1 gene. J Virol 85:7836–7848. doi: 10.1128/JVI.01197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takada K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer 33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Wu F, Chen H, Shamay M, Zheng Q, Hayward G. 2004. Early activation of the Kaposi's sarcoma-associated herpesvirus RTA, RAP, and MTA promoters by the tetradecanoyl phorbol acetate-induced AP1 pathway. J Virol 78:4248–4267. doi: 10.1128/JVI.78.8.4248-4267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du T, Zhou G, Roizman B. 2012. Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures. Proc Natl Acad Sci U S A 109:14616–14621. doi: 10.1073/pnas.1212661109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad A, Lu M, Lukac D, Zeichner S. 2012. An alternative Kaposi's sarcoma-associated herpesvirus replication program triggered by host cell apoptosis. J Virol 86:4404–4419. doi: 10.1128/JVI.06617-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo J, Kim T-G, Hong Y, Chen J-Y, Lee S. 2011. Contribution of Epstein-Barr virus infection to chemoresistance of gastric carcinoma cells to 5-fluorouracil. Arch Pharm Res 34:635–643. doi: 10.1007/s12272-011-0414-7. [DOI] [PubMed] [Google Scholar]
- 18.Shin H, Kim D, Lee S. 2011. Association between Epstein-Barr virus infection and chemoresistance to docetaxel in gastric carcinoma. Mol Cells 32:173–179. doi: 10.1007/s10059-011-0066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zebboudj A, Maroui MA, Dutrieux J, Touil-Boukoffa C, Bourouba M, Chelbi-Alix MK, Nisole S. 2014. Sodium arsenite induces apoptosis and Epstein-Barr virus reactivation in lymphoblastoid cells. Biochimie 107 (Part B):247–256. doi: 10.1016/j.biochi.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer S, Zavolan M, Grässer F, Chien M, Russo J, Ju J, John B, Enright A, Marks D, Sander C, Tuschl T. 2004. Identification of virus-encoded microRNAs. Science 304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Schäfer A, Lu S, Bilello J, Desrosiers R, Edwards R, Raab-Traub N, Cullen B. 2006. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog 2:e23–e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S-J, Chen G-H, Chen Y-H, Liu C-Y, Chang K-P, Chang Y-S, Chen H-C. 2010. Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One 5:e12745. doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Chae H-S, Oh S, Kang J-H, Park C, Park W, Takada K, Lee J, Lee W-K, Lee S. 2007. Expression of viral microRNAs in Epstein-Barr virus-associated gastric carcinoma. J Virol 81:1033–1036. doi: 10.1128/JVI.02271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Seo M, Choi H, Kim S, Shin H, Yoon AR, Tao Q, Rha S, Lee S. 2013. Characterization of naturally Epstein-Barr virus-infected gastric carcinoma cell line YCCEL1. J Gen Virol 94:497–506. doi: 10.1099/vir.0.045237-0. [DOI] [PubMed] [Google Scholar]
- 25.Qiu J, Thorley-Lawson DA. 2014. EBV microRNA BART 18-5p targets MAP3K2 to facilitate persistence in vivo by inhibiting viral replication in B cells. Proc Natl Acad Sci U S A 111:11157–11162. doi: 10.1073/pnas.1406136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iizasa H, Wulff B-E, Alla N, Maragkakis M, Megraw M, Hatzigeorgiou A, Iwakiri D, Takada K, Wiedmer A, Showe L, Lieberman P, Nishikura K. 2010. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem 285:33358–33370. doi: 10.1074/jbc.M110.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung YJ, Choi H, Kim H, Lee SK. 2014. MicroRNA miR-BART20-5p stabilizes Epstein-Barr virus latency by directly targeting BZLF1 and BRLF1. J Virol 88:9027–9037. doi: 10.1128/JVI.00721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Choi H, Lee S. 2015. Epstein-Barr virus miR-BART20-5p regulates cell proliferation and apoptosis by targeting BAD. Cancer Lett 356:733–742. doi: 10.1016/j.canlet.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Lomonosova E, Chinnadurai G. 2008. BH3-only proteins in apoptosis and beyond: an overview. Oncogene 27(Suppl 1):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. 2011. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L, Du W, Yang X, Chen K, Ghanekar A, Levy G, Yang W, Yee A, Lu W-Y, Xuan J, Gao Z, Xie F, He C, Deng Z, Yang B. 2013. Versican 3′-untranslated region (3′-UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. FASEB J 27:907–919. doi: 10.1096/fj.12-220905. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Yu H, Xi M, Ma D, Lu X. 2015. The SNAI1 3′UTR functions as a sponge for multiple migration-/invasion-related microRNAs. Tumour Biol 36:1067–1072. doi: 10.1007/s13277-014-2733-z. [DOI] [PubMed] [Google Scholar]
- 33.Poliseno L, Salmena L, Zhang J, Carver B, Haveman W, Pandolfi P. 2010. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swami M. 2010. Small RNAs: pseudogenes act as microRNA decoys. Nat Rev Cancer 10:535. doi: 10.1038/nrc2898. [DOI] [PubMed] [Google Scholar]
- 35.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP. 2011. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D, Shatseva T, Jeyapalan Z, Du W, Deng Z, Yang B. 2009. A 3′-untranslated region (3′UTR) induces organ adhesion by regulating miR-199a* functions. PLoS One 4:e4527. doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elkholi R, Floros K, Chipuk J. 2011. The role of BH3-only proteins in tumor cell development, signaling, and treatment. Genes Cancer 2:523–537. doi: 10.1177/1947601911417177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cregan SP, MacLaurin JG, Craig CG, Robertson GS, Nicholson DW, Park DS, Slack RS. 1999. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J Neurosci 19:7860–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad A, Remick J, Zeichner S. 2013. Activation of human herpesvirus replication by apoptosis. J Virol 87:10641–10650. doi: 10.1128/JVI.01178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borza CM, Hutt-Fletcher LM. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med 8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Liao G, Chen H, Wu FY, Hutt-Fletcher L, Hayward GS, Hayward SD. 2006. Contribution of C/EBP proteins to Epstein-Barr virus lytic gene expression and replication in epithelial cells. J Virol 80:1098–1109. doi: 10.1128/JVI.80.3.1098-1109.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshiyama H, Imai S, Shimizu N, Takada K. 1997. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol 71:5688–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosson AD, Zamudio JR, Sharp PA. 2014. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol Cell 56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M. 2014. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell 54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maier T, Güell M, Serrano L. 2009. Correlation of mRNA and protein in complex biological samples. FEBS Lett 583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 46.Lin T-C, Liu T-Y, Hsu S-M, Lin C-W. 2013. Epstein-Barr virus-encoded miR-BART20-5p inhibits T-bet translation with secondary suppression of p53 in invasive nasal NK/T-cell lymphoma. Am J Pathol 182:1865–1875. doi: 10.1016/j.ajpath.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Huang W-T, Lin C-W. 2014. EBV-encoded miR-BART20-5p and miR-BART8 inhibit the IFN-γ-STAT1 pathway associated with disease progression in nasal NK-cell lymphoma. Am J Pathol 184:1185–1197. doi: 10.1016/j.ajpath.2013.12.024. [DOI] [PubMed] [Google Scholar]