ABSTRACT
The continual threat to global health posed by influenza has led to increased efforts to improve the effectiveness of influenza vaccines for use in epidemics and pandemics. We show in this study that formulation of a low dose of inactivated detergent-split influenza vaccine with a Toll-like receptor 2 (TLR2) agonist-based lipopeptide adjuvant (R4Pam2Cys) provides (i) immediate, antigen-independent immunity mediated by the innate immune system and (ii) significant enhancement of antigen-dependent immunity which exhibits an increased breadth of effector function. Intranasal administration of mice with vaccine formulated with R4Pam2Cys but not vaccine alone provides protection against both homologous and serologically distinct (heterologous) viral strains within a day of administration. Vaccination in the presence of R4Pam2Cys subsequently also induces high levels of systemic IgM, IgG1, and IgG2b antibodies and pulmonary IgA antibodies that inhibit hemagglutination (HA) and neuraminidase (NA) activities of homologous but not heterologous virus. Improved primary virus nucleoprotein (NP)-specific CD8+ T cell responses are also induced by the use of R4Pam2Cys and are associated with robust recall responses to provide heterologous protection. These protective effects are demonstrated in wild-type and antibody-deficient animals but not in those depleted of CD8+ T cells. Using a contact-dependent virus transmission model, we also found that heterologous virus transmission from vaccinated mice to naive mice is significantly reduced. These results demonstrate the potential of adding a TLR2 agonist to an existing seasonal influenza vaccine to improve its utility by inducing immediate short-term nonspecific antiviral protection and also antigen-specific responses to provide homologous and heterologous immunity.
IMPORTANCE
The innate and adaptive immune systems differ in mechanisms, specificities, and times at which they take effect. The innate immune system responds within hours of exposure to infectious agents, while adaptive immunity takes several days to become effective. Here we show, by using a simple lipopeptide-based TLR2 agonist, that an influenza detergent-split vaccine can be made to simultaneously stimulate and amplify both systems to provide immediate antiviral protection while giving the adaptive immune system time to implement long-term immunity. Both types of immunity induced by this approach protect against vaccine-matched as well as unrelated virus strains and potentially even against strains yet to be encountered. Conferring dual functionality to influenza vaccines is beneficial for improving community protection, particularly during periods between the onset of an outbreak and the time when a vaccine becomes available or in scenarios in which mass vaccination with a strain to which the population is immunologically naive is imperative.
INTRODUCTION
The most effective way to curb the seasonal impact of influenza, which causes an average of 250,000 to 500,000 deaths annually (1), is vaccination. The “split” influenza virus vaccine, which is prepared from purified virions that have been inactivated and then disrupted (split) with detergent, induces neutralizing antibodies directed against viral hemagglutinin (HA) and neuraminidase (NA). Because circulating viruses mutate readily and new antigenically distinct strains are continuously selected in the presence of neutralizing antibody elicited by previous strains, the effectiveness of a seasonal vaccine diminishes with time. These vaccines must therefore be reformulated annually to include the HA and NA of the viral strains that are predicted to circulate in the forthcoming influenza season.
The recent emergence of swine-origin H1N1 virus as well as ongoing zoonotic spillover events involving H7N9 and highly pathogenic H5N1 viruses have highlighted the real and potential burden that pandemics pose to global health and national economies in the absence of an effective vaccine. Although interventions, such as those employing the neuraminidase inhibitors, against novel strains can be effective at alleviating symptoms and at reducing disease severity when used preemptively (2, 3) or when initiated promptly after illness onset (4, 5), their efficacy is reliant on a daily dosing regimen (6), and their benefit does not extend beyond the course of treatment. Furthermore, widespread or prolonged use is associated with the emergence of drug-resistant strains (7, 8). The need for continual development of effective short-term intervention strategies as well as vaccination approaches that can induce broad cross-reactive immunity, particularly against heterologous virus subtypes, therefore remains a priority.
The induction of regulated mucosal cellular innate responses, particularly those mediated by macrophages (9), neutrophils (10, 11), and NK cells (12) as well as cytokines (13, 14), during the early stages of infection plays a crucial role in limiting viral replication and disease severity. This has led to the idea that an influenza vaccine that can be designed to induce short-acting innate protection before longer-lasting adaptive responses come into effect could be even more effective in limiting infection (15). Moreover, vaccines that prime or boost memory CD8+ T cell responses to epitopes conserved across heterologous strains, i.e., those not subject to antibody-mediated selection pressure, could provide an additional level of cross-protective immunity to serologically diverse influenza virus strains, including novel subtypes with pandemic potential (16, 17). Cross-reactive CD8+ cytotoxic T lymphocyte (CTL) responses induced in humans as a result of infection (18, 19) are closely associated with better prognosis after reinfections (20, 21), but these responses can wane over time (22) and can differ among different age groups (23, 24). Although CTL immunity does not prevent viral infection, it can significantly reduce viral load and moderate disease severity (25).
A number of studies have investigated ways of improving the level of protection that can be elicited by inactivated split virus vaccines in both mice and ferrets (26–31). While these approaches are effective at inducing primarily antibody-mediated heterosubtypic or cross-clade protection, the modification of a split virus vaccine formulation to induce innate immunity to provide immediate protection as well as subsequent adaptive immunity, including heterologous CD8+ T cell-mediated responses, has yet to be realized. Such an intervention would extend the spectrum of immunity that can be induced by a seasonal influenza vaccine and would be beneficial in reducing disease burden during the period between the onset of a pandemic and the time at which a vaccine becomes available.
We have previously shown that inoculation of soluble protein antigens formulated with anionic or cationic lipopeptide-based adjuvants containing the Toll-like receptor 2 (TLR2) agonist S-[2,3-bis(palmitoyloxy)propyl]cysteine (Pam2Cys) induces not only robust antibody but also strong CD8+ T cell responses (32–34). Electrostatic association between the cationic lipopeptide R4Pam2Cys and the model antigen ovalbumin (OVA) induced endogenous OVA257–264-specific CD8+ T cell responses that mediated rapid clearance of a chimeric influenza virus containing the KbOVA257–264 epitope following viral challenge (34). In addition, our studies in animals have since demonstrated that intranasal (i.n.) inoculation of Pam2Cys alone can rapidly induce pulmonary innate responses that protect against challenge with different influenza A virus (IAV) strains (35). These effects are induced within a day of inoculation and can reduce disease severity, lung viral titers, and the transmission of virus for up to a week. Importantly, the generation of adaptive immune responses is not compromised by intranasal treatment with Pam2Cys (35).
In this study, we evaluated the advantages of formulating a split influenza virus vaccine with a modification of Pam2Cys and examined its ability to confer immediate short-term protection against challenge with the homologous virus and with a serologically distinct virus as well as its ability to confer long-term protection by the induction of both virus-specific antibody and CD8+ T cell responses. We also investigated the role of induced adaptive responses in mediating vaccine efficacy against these viral strains and report on the effects of vaccination on transmissibility of virus from infected animals.
RESULTS
Inoculation with split IAV vaccine formulated with R4Pam2Cys confers rapid antiviral protection.
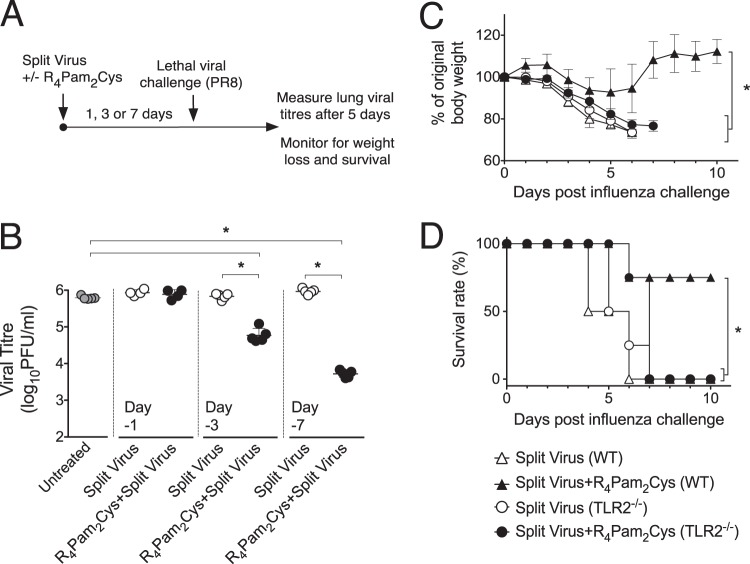
Our previous work (35) demonstrated that treatment of animals with Pam2Cys in the absence of antigen at up to 7 days prior to influenza virus challenge results in a reduction in viral loads postchallenge. To assess whether these protective effects extended to the use of an influenza vaccine formulation containing R4Pam2Cys, we used a split influenza virus vaccine derived from PR8 influenza virus. This vaccine preparation contained proteins corresponding to the molecular weights of the major viral surface glycoproteins, as well as the internal nucleoprotein (NP) and the M1 matrix protein (see Fig. S1 in the supplemental material). By determining lung viral titers in mice challenged within 7 days of vaccination, i.e., before any adaptive immune response would have a significant effect, we were able to determine any protective effects mediated by the innate immune system (Fig. 1A).
FIG 1 .
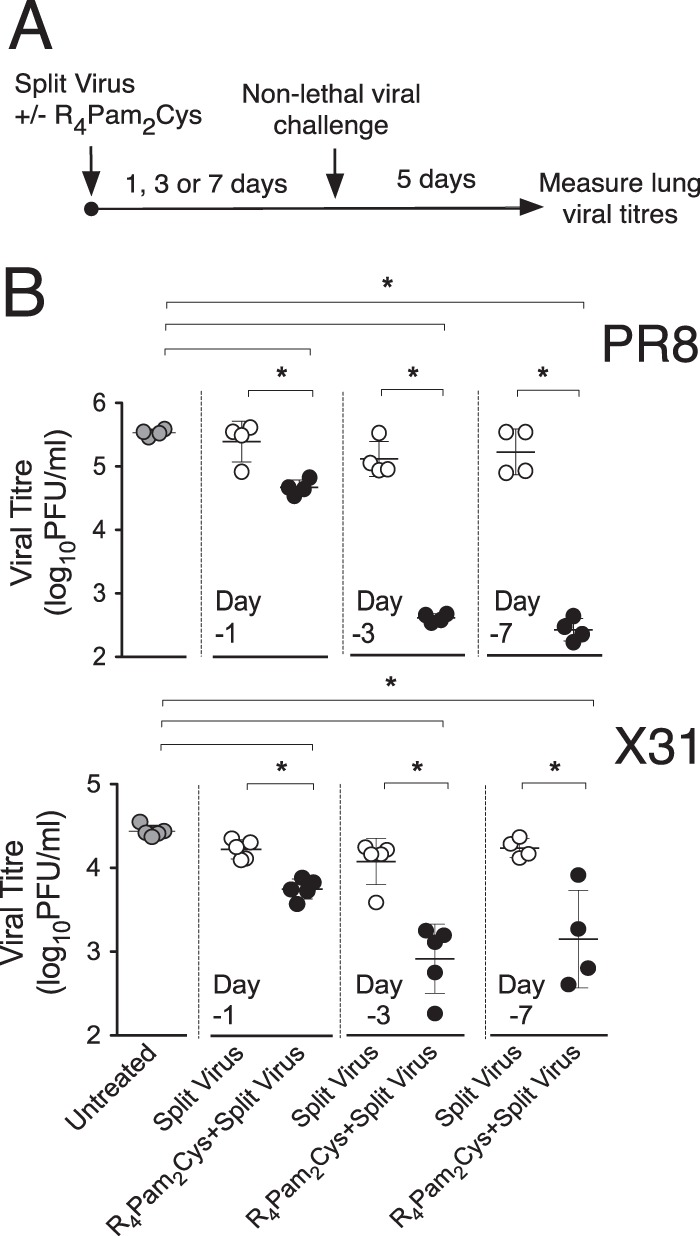
Rapid protective antiviral effects induced by inoculation with split virus vaccine formulated with R4Pam2Cys. (A) BALB/c mice (n = 4 to 5 per group) were inoculated via the intranasal route with PR8-derived split virus vaccine alone or combined with 5 nmol of R4Pam2Cys at 1 day (day −1), 3 days (day −3), or 7 days (day −7) prior to challenge with either 50 PFU of PR8 or 104.5 PFU of X31. (B) Lungs were harvested 5 days later and viral titers determined. Symbols represent the titers obtained from individual mice, and horizontal lines indicate the mean virus titers (± standard deviations [SD]) of the group. Groups are defined at the bottom of panel B. Asterisks (*) indicate P values of <0.05.
The results (Fig. 1B) show that mice inoculated with a single dose of split virus vaccine formulated with R4Pam2Cys and challenged after 24 h with PR8 or X31 virus had significantly reduced lung viral titers 5 days postinfection compared to naive mice or those inoculated with split virus in the absence of R4Pam2Cys. A greater reduction in viral titers in mice inoculated with split virus plus R4Pam2Cys was detected when vaccinations were carried out 3 days prior to challenge. These protective effects were still evident 7 days following vaccination.
To evaluate protection against a lethal viral infection, vaccinated animals were challenged with a higher dose (500 PFU) of PR8 (Fig. 2A). While lung viral titers were not reduced in mice vaccinated in the presence of R4Pam2Cys and challenged a day after vaccination (Fig. 2B), significant reductions in viral titers in these mice compared to the control animals were detected when vaccinations were carried out 3 to 7 days prior to challenge.
FIG 2 .
Inoculation with split virus vaccine formulated with R4Pam2Cys also provides rapid protection against lethal viral challenge in a TLR2-dependent manner. (A) BALB/c mice (n = 5 per group) were inoculated via the intranasal route with PR8-derived split virus vaccine alone or combined with 5 nmol of R4Pam2Cys 3 days prior to challenge with 500 PFU of PR8. (B) Lungs were harvested 5 days later, and viral titers were determined. Symbols represent the titers obtained from individual mice, and horizontal lines indicate the mean virus titers (± SD) for the group. C57BL/6 or TLR2−/− mice (n = 4 per group) were similarly inoculated 3 days prior to viral challenge and monitored daily for signs of illness and weight loss. Mice were killed when a humane endpoint was reached as characterized by >20% weight loss accompanied by signs of severe disease. (C and D) The mean body weight of mice is represented as a percentage of the original weight at the time of challenge as depicted in panel C, and survival over a 10-day period is shown in panel D. Asterisks (*) indicate P values of <0.05.
Mice vaccinated with split virus plus R4Pam2Cys 3 days before lethal viral challenge were also protected from the substantial weight loss (Fig. 2C) and development of severe disease symptoms associated with infection compared to mice that received split virus alone, which by day 7 all had to be culled, having reached the defined humane endpoint (Fig. 2D). No protective effects were observed in similarly vaccinated TLR2−/− mice, indicating that protection mediated by R4Pam2Cys was dependent on recognition and signaling operating through TLR2. These observations demonstrate that an influenza vaccine delivered with Pam2Cys can induce rapid protection to reduce the impact of infection irrespective of the viral subtype. These protective effects are also achieved with a lesser dose of Pam2Cys than was previously used to confer antiviral protection (35).
Antibody responses induced by vaccination of minimal doses of split IAV vaccine formulated with R4Pam2Cys.
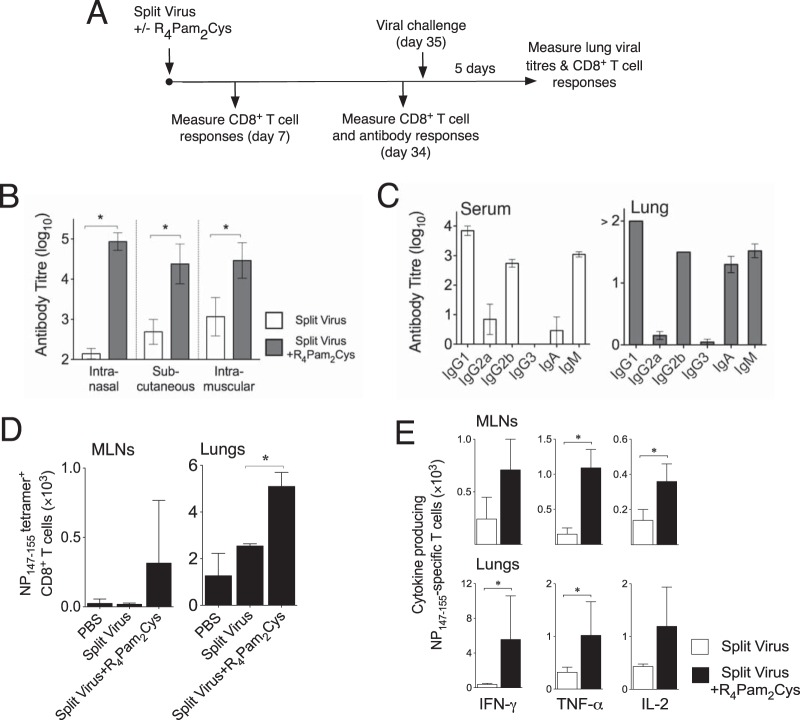
The induction of subsequent adaptive (antigen-specific) immunity was studied by measuring CD8+ T cell and antibody responses at several time points after vaccination. Animals were also challenged 35 days after vaccination, and recall CD8+ T cell responses and viral loads were analyzed 5 days postinfection (Fig. 3A). To evaluate antibody responses, mice were inoculated via the intranasal, subcutaneous, or intramuscular route. A low dose of split vaccine alone was unable to induce titers of antibody as high as those seen with adjuvanted split vaccine irrespective of the inoculation route used (Fig. 3B). In contrast, administration of the vaccine with R4Pam2Cys resulted in significant titers of antibody, with an ~600-fold improvement in titers following inoculation via the intranasal route and an ~20-fold to 60-fold improvement following inoculation by the subcutaneous or intramuscular route.
FIG 3 .
Induction of antibody and CD8+ T cell responses by vaccination. (A) Adaptive immunity induced by vaccination was studied by measuring antigen-specific immune responses at various time points after inoculation and viral loads following challenge 35 days postvaccination. (B) To measure antibody titers, BALB/c mice (n = 5 per group) were inoculated via the intranasal, subcutaneous, or intramuscular route with split virus vaccine alone or with split virus combined with R4Pam2Cys. Sera were obtained from blood taken 34 days later, and vaccine-specific antibody levels were determined by ELISA using split virus vaccine as a coating antigen. (C) Isotypes of vaccine-specific antibodies in sera and lung homogenate supernatants from intranasally vaccinated mice were also assayed using isotype-specific detection antibodies. Results are presented as the mean antibody titer (± SD) from all serum samples in each group. (D) To analyze CD8+ T cell responses, BALB/c mice (n = 3 per group) were inoculated via the intranasal route and lymphocytes in the lungs and MLNs were stained with fluorochrome-conjugated anti-CD8 antibody and H2Kd tetramer to detect NP147–155-specific cells. (E) Lymphocytes were also restimulated in vitro in the presence of NP147–155 for 7 days before analysis for IFN-γ-, TNF-α-, and IL-2-producing CD8+ T cells by ICS. Bar graphs depict the means numbers of NP147–155 tetramer+-producing (D) and antigen-specific cytokine-producing (E) CD8+ T cells per group, and asterisks (*) indicate P values of <0.05.
Serum antibody isotype profiles induced by intranasal inoculation of R4Pam2Cys-formulated vaccine were dominated by IgM, IgG1, and IgG2b (Fig. 3C). Similar isotype profiles were observed in the lung with the additional presence of IgA, indicating a significant mucosal antibody response. Taken together, these results indicate that formulation of an otherwise ineffective low dose of split virus vaccine combined with R4Pam2Cys elicits strong antibody responses characterized by the presence of a number of antibody isotypes, including significant levels of IgA, especially when administered intranasally.
Hemagglutination inhibition (HI) and neuraminidase inhibition (NI) titers in sera obtained from mice inoculated with split virus plus R4Pam2Cys were found to be comparable to or higher than the antibody titers induced by PR8 infection (Table 1). In contrast, titers were not detected in sera of animals vaccinated with split virus alone. Not surprisingly, no detectable HI or NI reactivity was observed using sera from mice inoculated with vaccine alone or with vaccine formulated with R4Pam2Cys when tested against the serologically distinct H3N2 X31 virus. These results demonstrate that antibodies induced by inoculation of split virus can prevent viral HA and NA activity of homologous but not heterologous strains.
TABLE 1 .
Hemagglutination and neuraminidase inhibition (HI and NI) titers of sera from animals vaccinated against or infected with PR8 or X31a
| Mouse vaccination or infection category | Titer (log10) of virus strainb: |
|||
|---|---|---|---|---|
| PR8 |
X31 |
|||
| HI | NI | HI | NI | |
| PBS | <1.0 | <1.5 | <1.0 | <1.5 |
| Split virus | <1.0 | <1.5 | <1.0 | <1.5 |
| Split virus + R4Pam2Cys | 2.92 ± 0.3 | 2.58 ± 0.3 | <1.0 | <1.5 |
| PR8 infected | 2.21 ± 0.0 | 2.71 ± 0.0 | ND | ND |
| X31 infected | ND | ND | 2.58 ± 0.4 | 2.98 ± 0.0 |
Hemagglutination (HI) and neuraminidase inhibition (NI) tests were performed with PR8 or X31 virus on sera (n = 5) obtained 35 days after vaccination or infection.
HI titers (log10) are expressed as the reciprocal of the highest dilution of sample that inhibited 4 hemagglutinating units of virus. NI titers (log10) are expressed as the reciprocal of the highest dilution of sample that caused a 50% inhibition of NA activity.
To investigate the induction of T cell responses, lymphocytes from the mediastinal lymph nodes (MLNs) and lungs were analyzed for the presence of NP147–155-specific CD8+ T cells by tetramer straining 7 days after vaccination. Compared to vaccination of split virus alone, vaccination of split virus in the presence of R4Pam2Cys resulted in the detection of higher numbers of NP147–155-specific CD8+ T cells at both these sites, especially in the lungs (Fig. 3D). Furthermore, higher numbers of gamma interferon (IFN-γ)-, tumor necrosis factor alpha (TNF-α)-, and interleukin-2 (IL-2)-producing NP147–155-specific CD8+ T cells were also detected in lymphocyte populations derived from animals vaccinated with split virus plus R4Pam2Cys following in vitro restimulation with NP147–155 peptide for a further 7 days (Fig. 3E).
Protection against homologous and heterologous viral challenge.
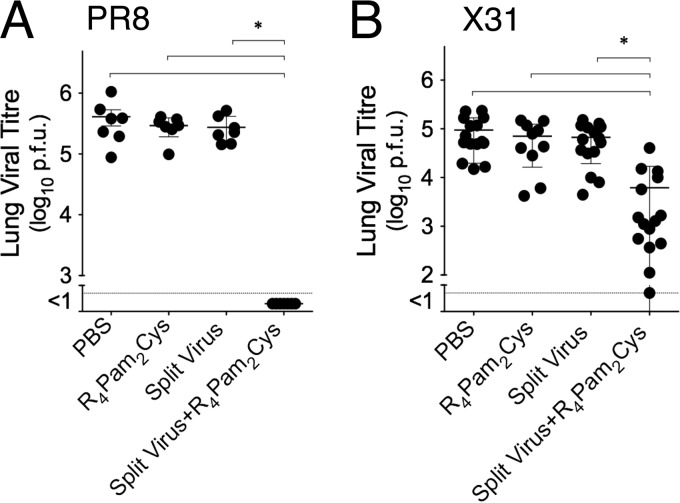
To evaluate the in vivo protective efficacy of split virus formulations, mice were challenged 35 days following inoculation with split virus vaccine alone or formulated with R4Pam2Cys and lung viral titers were determined 5 days later. Mice challenged with the homologous PR8 virus (Fig. 4A) were not protected if vaccinated with split virus or with R4Pam2Cys alone as these groups of mice had lung viral titers not significantly different from those of phosphate-buffered saline (PBS)-vaccinated control mice. In contrast, animals that received low-dose split virus formulated with R4Pam2Cys showed complete elimination of virus from the lungs.
FIG 4 .
Protection of vaccinated animals against homologous and heterologous virus. BALB/c mice (n = 7 to 15 per group) were intranasally inoculated with PR8-derived split virus vaccine alone or formulated with R4Pam2Cys. A separate group of mice also received a similar dose of R4Pam2Cys alone. Animals were challenged intranasally 35 days later with 50 PFU of PR8 (A) or 104.5 PFU of X31 (B). Titers of virus in lung homogenates collected 5 days after viral challenge were determined by plaque formation. Viral titers of individual animals are presented, with the mean value of the group represented by the horizontal bar. Error bars represent SD, and asterisks (*) indicate P values of <0.05.
A similar approach was used to determine if vaccination could clear infection with X31 (Fig. 4B). As with PR8 challenge, inoculation of mice with split virus alone or R4Pam2Cys alone provided no significant reduction of viral loads in lungs. The titers of virus in the lungs of mice that had been inoculated with split vaccine formulated with R4Pam2Cys, however, were significantly reduced. These results demonstrate that the presence of R4Pam2Cys in a split virus formulation not only enhances virus-clearing adaptive immune responses to the homologous virus strain but also enables the vaccine to provide cross-reactive virus-clearing immunity to heterologous virus.
Ability of antibodies induced by vaccination to mediate heterosubtypic protection.
To investigate the possible presence of X31-neutralizing antibodies that were not detected in standard HI and NI assays, sera from vaccinated mice were tested for their ability to inhibit infection of MDCK cell monolayers in an in vitro virus neutralization assay. The results from these experiments (Table 2) demonstrated that sera obtained from naive mice or from mice inoculated with split virus alone were ineffective at specifically preventing PR8 and X31 infection of cells. In contrast, sera obtained from mice inoculated with split virus administered with R4Pam2Cys or from mice previously primed with PR8 virus effectively neutralized PR8 infection. They did not, however, inhibit infection by X31, indicating the absence of cross-neutralizing antibodies. Neutralization of X31 was achieved only using sera from mice previously infected with the homologous (X31) virus strain. These results are consistent with the presence of HI and NI antibodies in these sera (Table 1). We conclude that, although coformulation of the split vaccine with R4Pam2Cys results in a strong homologous neutralizing antibody response, detectable levels of neutralizing antibodies against a heterologous strain could not be demonstrated in vitro.
TABLE 2 .
Ability of sera from vaccinated or infected mice to neutralize X31 and PR8 infection of MDCK cellsa
| Mouse vaccination or infection category | Titer (log10) of virus strainb: |
|
|---|---|---|
| PR8 | X31 | |
| PBS | <1 | <1 |
| Split virus | <1 | <1 |
| Split virus + R4Pam2Cys | 3.95 ± 0.4 | <1 |
| PR8 infected | 4.50 ± 0.0 | <1 |
| X31 infected | <1 | 3.52 ± 0.2 |
Viral neutralization tests were performed using sera (n = 5) taken 35 days after vaccination or infection.
Titers (log10) are expressed as the highest average dilution (± standard deviation) that reduced plaque numbers to 50% of the titers obtained in the presence of serum from unvaccinated mice.
Protection against heterosubtypic viral challenge in µMT antibody-deficient mice.
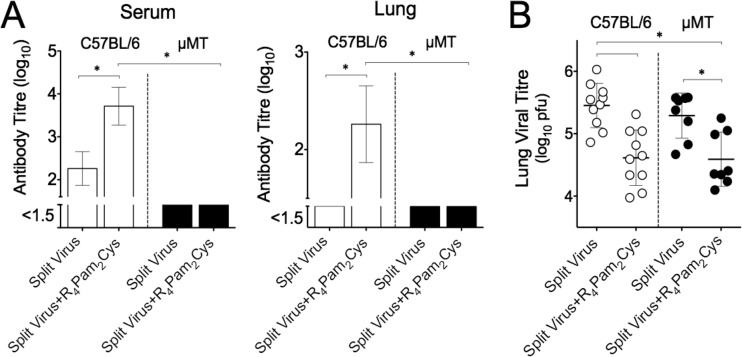
To provide evidence that the immunity to heterologous virus challenge occurred independently of any antibody-mediated effects, µMT B cell-deficient mice were vaccinated and we evaluated their ability to clear virus following challenge with X31. Prior to challenge, vaccine-specific antibody titers in these mice were compared to those obtained in congenic C57BL/6 mice. In contrast to the significant levels of antibody detected in the serum and lung homogenates of C57BL/6 mice inoculated with split virus formulated with R4Pam2Cys, antibody levels in µMT mice that had been similarly immunized were below the limits of detection of the assay (Fig. 5A).
FIG 5 .
Cross-reactive immunity and antibody responses in vaccinated µMT mice. (A) C57BL/6 mice (white bars) or congenic µMT mice (black bars) were inoculated via the intranasal route with split virus vaccine alone or combined with R4Pam2Cys (n = 8 to 10 mice per group). Vaccine-specific antibody levels in serum and lung homogenates were measured 34 days later by ELISA. The means and SDs of the results determined for each group are shown. (B) Animals were challenged 35 days after vaccination with 104.5 PFU of X31. Titers of virus in individual lung homogenates collected from C57BL/6 mice (white circles) and µMT mice (black circles) 5 days after viral challenge were determined by plaque formation. The mean values are represented by a horizontal bar. Error bars represent SD, and asterisks (*) indicate P values of <0.05.
When pulmonary viral titers were measured in vaccinated µMT mice following challenge with X31, significantly lower viral levels were present in mice vaccinated with split virus in the presence of R4Pam2Cys than in its absence (Fig. 5B). It therefore appears that, despite a lack of antibody, µMT mice can mount a virus-clearing response to a heterologous virus, indicating that the cross-protective immunity generated in the presence of R4 Pam2Cys is antibody independent.
Pulmonary CD8+ T cell responses in vaccinated animals following viral challenge.
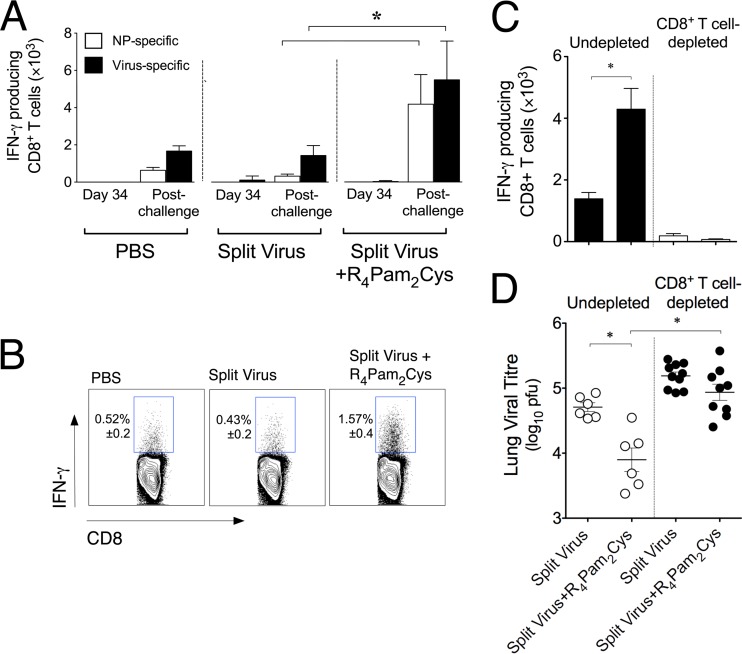
We next investigated whether protection against X31 was associated with CD8+ T cell responses. Following vaccination, lymphocytes in the lungs were analyzed for virus-specific IFN-γ-producing CD8+ T cells following ex vivo stimulation with syngeneic antigen-presenting cells (APCs) infected with X31 or pulsed with the nucleoprotein-derived H-2Kd-restricted immunodominant epitope NP147–155. Our analyses found very few virus- or NP-specific IFN-γ-producing CD8+ T cells in the lungs of mice 34 days after vaccination with split virus in the absence or presence of R4Pam2Cys (Fig. 6A). Following challenge with X31, however, the number of virus-specific IFN-γ-producing CD8+ T cells that were detected in mice inoculated with split virus formulated with R4Pam2Cys was approximately 3-fold higher than the number detected in mice that received split virus alone or PBS (Fig. 6A and B). A large proportion of this recall response also appeared to be specific for NP147–155 (Fig. 6A).
FIG 6 .
Induction of CD8+ T cell responses in vaccinated mice following X31 challenge and its role in mediating cross-reactivity immunity. (A) BALB/c mice (n = 5 per group) were inoculated with split virus vaccine alone or premixed with R4Pam2Cys. Lymphocytes in lungs of vaccinated mice were analyzed for virus- or NP147–155-specific IFN-γ-producing T cells in an ICS assay on day 34 postvaccination. Animals were then challenged on day 35 with 104.5 PFU of X31, and specific IFN-γ-producing T cells in the lungs were enumerated 5 days later. Each bar represents the mean and SD of the results determined for each group. (B) Concatenated dot plots of samples from virus-challenged animals are also shown, depicting the percentages (± SD) of responsive IFN-γ+ CD8+ T cells in the lung following stimulation with virus-infected APCs. (C) Vaccinated mice (n = 5 to 10) were also depleted of CD8+ T cells prior to X31 challenge. The total numbers of NP147–155-specific IFN-γ+ CD8+ T cells in BAL fluid samples from CD8+ T cell-depleted or undepleted vaccinated mice were enumerated in an ICS assay 5 days later. (D) Titers of virus in lung homogenates were also determined by plaque formation. Titers in individual animals are presented, with the mean values for the groups represented by the horizontal bars. All error bars represent SD, and asterisks (*) indicate P values of <0.05.
To determine the importance of these responses in mediating heterologous protection, vaccinated animals were depleted of CD8+ T cells prior to X31 challenge. Non-CD8+ T cell-depleted mice vaccinated with split virus plus R4Pam2Cys exhibited significantly higher recall responses to NP147–155 than mice vaccinated with split virus alone (Fig. 6C) at 5 days postinfection. In comparison, responses in similarly vaccinated but CD8+ T cell-depleted animals were absent and correlated with an absence of a reduction in viral titers (Fig. 6D). Taken together, these results support the view that the ability of mice inoculated with split virus formulated with R4Pam2Cys to clear virus from the lung is at least in part due to the presence of a strong NP-specific CD8+ T cell-mediated recall response.
Reduction in pulmonary viral loads of contacts cohoused with R4Pam2Cys-vaccinated index mice.
To determine if the cross-protective responses observed in mice inoculated with split virus plus R4Pam2Cys (Fig. 4B) also inhibited transmission of virus to an unvaccinated population, we used a mouse contact-dependent transmission model (36). Mice vaccinated with split virus alone or combined with R4Pam2Cys were infected with a transmissible strain (X31) 35 days after vaccination (vaccinated index mice) and were cohoused for 54 h with naive animals (unvaccinated contact mice). Irrespective of whether mice were vaccinated with split virus in the presence or absence of R4Pam2Cys, we found similar viral titers (>6 log10 PFU/ml) in the lungs of all vaccinated index mice (Fig. 7) after the cohousing period. This was expected because the recall and virus-clearing effects of pulmonary cytotoxic T cells take at least that long to manifest. Nevertheless, unvaccinated contact mice cohoused with index animals vaccinated with split virus plus R4Pam2Cys had undetectable or very low levels of virus in the lung. This is in contrast to the 1 to 5.4 log10 PFU/ml of virus found in more than 60% of contacts cohoused with index mice vaccinated with split virus alone or combined with PBS. It should be noted that transmissibility in this model is known to be dependent on the level of virus present in the saliva of index mice. These results therefore suggest that, despite equivalent pulmonary viral loads early after challenge, reduced levels of virus may be present in the saliva of mice vaccinated with split virus plus R4Pam2Cys compared to mice vaccinated with split virus alone (36), resulting in less viral shedding.
FIG 7 .

Pulmonary infection of unvaccinated contact mice cohoused with vaccinated index mice. BALB/c mice (n = 4 per group) were inoculated with PBS or with split virus vaccine alone or premixed with R4Pam2Cys. Mice were then challenged 35 days later with 104.5 PFU of X31. After 6 h, 2 vaccinated index mice were cohoused with 3 naive unvaccinated contact mice for 48 h in a box that allowed direct contact. Vaccinated index mice were culled at 54 h postchallenge, while contact mice were culled at 96 h after the cohousing. Lungs were collected to assess viral loads as determined by plaque assay. Averaged geometric mean viral titers (± SD) in the lungs of mice determined in two separate experiments that included 8 index mice and 12 contact mice are shown.
DISCUSSION
R4Pam2Cys associates electrostatically with oppositely charged regions on vaccine antigens, providing a means of forming complexes of the antigen and the TLR2 agonist without resorting to the complexities of covalent chemistry. Directing antigens to TLR2, particularly on dendritic cells (DCs), using R4Pam2Cys results in improved antigen uptake and maturation of the DC and promotes proinflammatory cytokine secretion, resulting in the induction of both humoral and cellular immunity (32–34). By formulating inactivated detergent-split influenza virus vaccine with R4Pam2Cys, significant enhancement of vaccine immunogenicity through the induction of stronger neutralizing antibody responses and CD8+ T cell-mediated cross-protective immunity results. Such a vaccination regimen also mitigates contact-dependent transmission.
These findings build on our previous work, which showed that the breadth and effectiveness of responses induced with a suboptimal dose of this same vaccine are improved when the vaccine is coadministered with a Pam2Cys-peptide immunogen containing a cross-protective T cell epitope (37). The difference in this study was that, when R4Pam2Cys was used, the addition of extra antigen was not required to induce the immunogenic and protective effects observed. An additional benefit of a vaccine formulation that contains Pam2Cys is its ability to stimulate innate cellular and cytokine-mediated responses which provide short-lived resistance to different influenza virus strains in an antigen-independent manner (35). This protective effect has been shown to last for up to 7 days and is characterized by neutrophils and macrophages infiltrating the lung as well as by elevated levels of IL-2, IL-6, IFN-γ, and TNF-α, among other inflammatory mediators (35). Unlike the use of antivirals such as oseltamivir, which has been reported to affect the induction of adaptive immune responses (38, 39), the use of Pam2Cys, besides conferring immediate protection, also permits the generation of subsequent and robust adaptive immune responses. Furthermore, and because the immediate protective effect of Pam2Cys is mediated by host responses rather than by targeting a structural feature of the virus, resistance to the use of Pam2Cys is less likely to occur. One could therefore envisage the use of a split virus formulation containing R4Pam2Cys which would be administered intranasally as a spray to provide immediate short-term antiviral protection in the event of an outbreak while the antigenic component of the vaccine would extend protection well beyond this time frame via the establishment of adaptive immunity.
In our efforts to define the immune mechanisms mediating cross-protection, we investigated the role of antibodies that were induced by vaccination using R4Pam2Cys. Although antibodies that were capable of inhibiting the HA and NA activity showed no cross-reactivity with heterologous virus, it was possible that antibodies against other conserved regions of the virus, e.g., the highly conserved stalk domain of HA (40–42) and the fusion active subunit of HA2 (43, 44), may have been involved. Antibodies against NP (45) and the ectodomain of the virus M2 protein (46), which exert their activity by binding to infected host cell surface-associated viral antigens and engage antibody-dependent cellular cytotoxicity (47), have also been implicated in facilitating cross-protective immunity. Nonetheless, results from our in vitro neutralization assay and the fact that cross-protection was demonstrated in antibody-deficient animals but not in CD8+ T cell-depleted animals indicated that the cross-protective immunity afforded by the presence of R4Pam2Cys is CD8+ T cell mediated.
The induction of NP-specific primary CD8+ T cell responses following vaccination using split virus in the presence of R4Pam2Cys is associated with a strong recall response elicited following challenge, suggesting that T cells are recalled from a pool of memory cells established following vaccination and recruited from extrapulmonary sites such as the mediastinal lymph nodes to help facilitate viral clearance. Moreover, it is also possible that CD4+ T cell responses to HA and NA are primed by vaccination to help support both antibody- and cell-mediated responses. Support for this hypothesis is based on our unpublished observations which showed that vaccination of OVA formulated with R4Pam2Cys also results in the proliferation of CD4+ T cells and promotes their differentiation into T follicular helper cells to help drive germinal B cell formation. Moreover, both vaccine-induced OVA-specific antibody responses and CD8+ T cell responses are absent in CD4+ T cell-deficient animals (GK1.5 mice).
The use of virus-infected APCs in the intracellular cytokine staining (ICS) assays to examine CD8+ T cell recall responses allows presentation of an array of virus-derived CTL cell epitopes potentially induced by the multiple antigens contained in the vaccine formulation. Although it appears that the CTL responses detected are directed largely against an epitope derived from NP that has been shown to be immunodominant in the response to viral infection, it remains to be seen what other T cell specificities are involved in this response; the presence of multiple and different viral proteins in the split virus vaccine suggests that an array of other subdominant responses (48) could also be elicited. The potential to boost cross-reactive CD8+ T cell responses in humans by vaccination was exemplified in recent phase I and 2a clinical trials demonstrating the use of an NP and M1 proteins delivered by a vaccinia virus vector to increase antigen-specific T cell responses (49) and reduce disease symptoms and viral shedding following exposure to an influenza virus strain to which subjects had not previously been exposed (50). Virus vector-based vaccines that target conserved antigens have also been shown to reduce transmission in animals (51). The use of Pam2Cys described here therefore not only provides similar benefits but also has advantages over such delivery systems due to its nonbiological nature and ease of manufacture and use.
The adaptive responses induced using R4Pam2Cys were achieved using a considerably smaller dose of split virus vaccine than is usually required to induce biologically active antibody responses in mice. This dose-sparing effect of R4Pam2Cys is an important point to be considered in the economy of vaccine manufacture, distribution, and administration in the event of influenza pandemics and epidemics. Alterations to current seasonal vaccine formulations to include R4Pam2Cys might also be attractive to manufacturers because such alterations would not require development of a new vaccine per se and could have the potential to significantly reduce the time periods that are usually associated with new vaccine development, expediting introduction.
These findings highlight the benefits of using a TLR agonist to improve the utility and also to extend the spectrum of immunity induced by influenza split virus vaccines against homologous and heterologous subtypes. A cost-effective method for dose sparing and at the same time extending efficacy could be of great benefit in scenarios in which there is an imperative for mass vaccination with a virus strain to which the population is immunologically naive. The innate- and adaptive-immune-system-mediated protective effects achieved through use of R4Pam2Cys could be especially beneficial for improving community protection, particularly during the period between the time of an outbreak and the time when a vaccine becomes available.
MATERIALS AND METHODS
Synthesis of the cationic lipopeptide R4Pam2Cys.
The synthesis of the cationic lipopeptide R4Pam2Cys was carried out manually using conventional solid-phase methodologies as described previously (34). Synthetic lipopeptide preparations contained no detectable lipopolysaccharide (LPS) (<0.05 endotoxin units [EU]/ml) as determined using the Limulus amebocyte lysate assay (Lonza, Walkersville, MD).
Vaccination and infection of mice.
All animal experimentation was performed with approval from the University of Melbourne’s Animal Ethics Committee. C57BL/6, BALB/c, TLR2−/−, and B cell-deficient (µMT) mice (52) were bred and maintained under specific-pathogen-free conditions and used when the mice were 6 to 10 weeks of age. µMT mice carry a disrupted gene encoding the μ-chain constant region of IgM which arrests B cell development and the ability to mount antibody responses (52). Mice were anesthetized with isoflurane and inoculated via the intranasal (i.n.) route with split virus vaccine that was provided by bioCSL Limited, Australia. The vaccine contained 1 µg of HA and was derived from A/Puerto Rico/8/34 (PR8; H1N1) virus. The vaccine was administered either in saline solution or formulated with 5 nmol of R4Pam2Cys (9.91 µg) in a total volume of 40 µl. For some experiments, mice were inoculated via the subcutaneous route at the base of the tail (50 µl at each side) or via the intramuscular route (40 µl into each quadriceps muscle).
After 1, 3, 7, or 35 days, mice were challenged intranasally with 104.5 PFU of A/HK×31 (X31) (H3N2) influenza virus or with 50 or 500 PFU of PR8 influenza virus. X31 is a recombinant virus containing the HA and NA segments from a 1968 Hong Kong influenza virus but sharing the internal viral proteins of the PR8 virus. Lungs were harvested 5 days later, homogenized in 3 ml RPMI 1640 (Invitrogen, Australia), and centrifuged at 300 × g for 30 s, and supernatants were stored at −80°C. Titers of virus in the supernatants of lung homogenates were then determined using a Madin-Darby canine kidney (MDCK) plaque assay as previously described (53).
ELISAs.
Levels of antibody present in serial dilutions of sera or the supernatants of lung homogenates obtained from mice 34 days following vaccination were determined by an enzyme-linked immunosorbent assay (ELISA) as previously described (34). A panel of horseradish peroxidase (HRP)-conjugated rat anti-mouse IgG1-, IgG2a-, IgG2b-, IgG3-, IgA-, and IgM-specific antibodies (Southern Biotech, USA) were used in a separate assay to determine the isotypes of antibodies. Titers of antibody are expressed as the reciprocal of the highest dilution of serum required to achieve an optical density of 0.2.
Hemagglutination inhibition (HI), neuraminidase inhibition (NI), and virus neutralization assays.
The presence of hemagglutination inhibition (HI) antibodies and of neuraminidase inhibition (NI) antibodies was determined as described elsewhere (54, 55). Virus neutralization assays were carried out as described previously (55).
In vivo depletion of CD8+ T cells.
Mice were inoculated via the intraperitoneal route with 100 µg (200 µl) of anti-CD8α antibody (clone 2.43; National Cell Culture Center, USA) 29 days after vaccination. Antibody was administered daily for 3 consecutive days and then once every 3 days thereafter for the duration of the experiment.
Intracellular cytokine staining (ICS) assay and tetramer staining.
Lungs from mice were finely minced and treated with 2 mg collagenase A (Roche Diagnostics, Germany)–2 ml RPMI 1640 per lung for 30 min at 37°C. Cell suspensions were passed through a wire sieve before centrifugal sedimentation of cells and treatment with 0.15 M NH4Cl–17 mM Tris-HCl at pH 7.2 for 5 min at 37°C. In some experiments, cells were obtained from bronchoalveolar lavage (BAL) fluids or mediastinal lymph nodes (MLNs). Lymphocyte cell suspensions (1 × 106) were cultured in the presence of the H2Kd-restricted influenza virus nucleoprotein (NP)-derived immunodominant epitope NP147–155 (TYQRTRALV; 1 mg/ml) at 37°C in a mixture with 200 µl of supplemented RPMI 1640 containing 55 mM 2-mercaptoethanol (2-ME), BD GolgiPlug (1 mg/ml) from a Cytofix/Cytoperm Plus kit (Becton, Dickinson), and recombinant IL-2 (Roche, Mannheim, Germany) (10 U/ml). For some experiments, lymphocyte cell suspensions were cultured in the presence of 2 × 105 virus-infected syngeneic P815 target cells. These cells (4 × 106/ml) had been infected previously with X31 (at a multiplicity of infection of 20) for 1 h at 37°C in Opti-MEM (Life Technologies, Australia) supplemented with gentamicin, glutamine, penicillin, and streptomycin at the concentrations described above and had been washed extensively in fetal calf serum (FCS) before use. Infection of cells was confirmed by the phenotypic expression of surface HA as verified by flow cytometry.
After 6 h, lymphocytes were washed with fluorescence-activated cell sorter (FACS) wash buffer (1% FCS–5 mM EDTA–PBS) and stained with a peridinin chlorophyll protein (PerCP) Cy5.5-conjugated rat anti-mouse CD8 antibody (clone 53-6.7; Becton, Dickinson) for 30 min at 4°C. Fixation and permeabilization were then performed for 20 min at 4°C using Cytofix/Cytoperm solution (Becton, Dickinson) according to the manufacturer’s instructions. Cells were washed once and stained for intracellular IFN-γ, IL-2, or TNF-α with fluorochrome-conjugated antibodies for 30 min at 4°C before flow cytometric analysis (FACSCanto II or LSR II; BD Biosciences). Data analysis was performed using FlowJo software (Treestar, USA).
For in vitro restimulation of lymphocytes over 7 days, cells (2 × 106) were cultured in 200 µl of supplemented RPMI 1640 media containing NP147–155 peptide (5 µg/ml) and IL-2 (10 U/ml) with medium changes performed every 2 days. ICS assays were then performed as indicated above.
Tetramer staining of lymphocytes from the MLNs and lungs was performed at room temperature using a 1:200 dilution in a mixture with 50 µl of 10% FCS–PBS for 30 min followed by the addition of fluorochrome-conjugated anti-CD8 antibody for 30 min. Cells were extensively washed before analysis.
Contact-dependent virus transmission.
The transmissibility of virus was evaluated in a mouse model developed for the study of contact-dependent viral transmission (36). Vaccinated mice were challenged after 35 days by the i.n. route with 104.5 PFU of X31 virus. After 6 h, these index mice were introduced into a clean cage and cohoused with naive contact mice (2 index mice for 3 contact mice) for 2 days. Index mice were euthanized directly after the cohousing period, and contact mice were euthanized 4 days after their initial exposure to the index mice. Viral titers in lung homogenates of all animals were then determined in a standard plaque assay.
Statistical analyses.
Analysis of variance (ANOVA) and P values were obtained using nonparametric one-way ANOVA; Tukey’s post hoc range tests, two-way ANOVA, and Bonferroni posttests were performed using the Prism 5 software package (GraphPad Software, La Jolla, CA, USA).
SUPPLEMENTAL MATERIAL
Components in PR8 split virus vaccine. (A) SDS-PAGE analysis of viral components (PB1/2, polymerase basic protein subunits 1 and 2; PA, acid polymerase subunit; NP, nucleoprotein; NA, neuraminidase; HA, hemagglutinin; M, matrix protein) present in PR8 split virus vaccine (1 µg). (B) Western blot analysis of the vaccine (1 µg) probed with a murine anti-NP-specific (lane 1) or anti-HA-specific (lane 2) antibody followed by an HRP-conjugated murine Ig-specific detection antibody and development using a chemiluminescent substrate. Download
ACKNOWLEDGMENTS
This work was supported by funding from program grant 567122 from the National Health and Medical Research Council of Australia and a University of Melbourne Early Career Researcher grant and a University of Melbourne Researcher Grant Support Scheme awarded to B.Y.C. The Melbourne WHO Collaborating Centre for Reference Research on Influenza is supported by the Australian government Department of Health.
We thank Claerwen Jones for providing the antibody used for the CD8+ T cell depletion experiments and Damien Zanker for help with the tetramer staining assay.
Footnotes
Citation Chua BY, Wong C. Y, Mifsud EJ, Edenborough KM, Sekiya T, Tan ACL, Mercuri F, Rockman S, Chen W, Turner SJ, Doherty PC, Kelso A, Brown LE, Jackson DC. 2015. Inactivated influenza vaccine that provides rapid, innate-immune-system-mediated protection and subsequent long-term adaptive immunity. mBio 6(6):e01024-15. doi:10.1128/mBio.01024-15.
REFERENCES
- 1.WHO 2009, posting date Influenza (seasonal) fact sheet no. 211. World Health Organization, Geneva, Switzerland: www.who.int/mediacentre/factsheets/fs211/en/index.html. [Google Scholar]
- 2.Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. 2003. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 326:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson RJ, Cooper KL, Tappenden P, Rees A, Simpson EL, Read RC, Nicholson KG. 2011. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect 62:14–25. doi: 10.1016/j.jinf.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, Foxlee R. 2010. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev 4:001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng S, Cowling B, Fang V, Chan K, Ip D, Cheng C, Uyeki T, Houck P, Malik Peiris J, Leung G. 2010. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis 50:707–714. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schünemann HJ, Hill SR, Kakad M, Vist GE, Bellamy R, Stockman L, Wisløff TF, Del Mar C, Hayden F, Uyeki TM, Farrar J, Yazdanpanah Y, Zucker H, Beigel J, Chotpitayasunondh T, Hien TT, Ozbay B, Sugaya N, Oxman AD. 2007. Transparent development of the WHO rapid advice guidelines. PLoS Med 4:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore C, Galiano M, Lackenby A, Abdelrahman T, Barnes R, Evans MR, Fegan C, Froude S, Hastings M, Knapper S, Litt E, Price N, Salmon R, Temple M, Davies E. 2011. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 203:18–24. doi: 10.1093/infdis/jiq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Vries E, Stelma FF, Boucher CAB. 2010. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med 363:1381–1382. doi: 10.1056/NEJMc1003749. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. 1987. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol 68:425–432. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa H. 2001. Inhibitory role of neutrophils on influenza virus multiplication in the lungs of mice. Microbiol Immunol 45:679–688. doi: 10.1111/j.1348-0421.2001.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 11.Tate MD, Deng Y-, Jones JE, Anderson GP, Brooks AG, Reading PC. 2009. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 12.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol 7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 13.Tsurita M, Kurokawa M, Imakita M, Fukuda Y, Watanabe Y, Shiraki K. 2001. Early augmentation of interleukin (IL)-12 level in the airway of mice administered orally with clarithromycin or intranasally with IL-12 results in alleviation of influenza infection. J Pharmacol Exp Ther 298:362–368. [PubMed] [Google Scholar]
- 14.Weiss ID, Wald O, Wald H, Beider K, Abraham M, Galun E, Nagler A, Peled A. 2010. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res 30:439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Tarbet EB, Feng T, Shi Z, Van Kampen KR, Tang DC. 2011. Adenovirus-vectored drug-vaccine duo as a rapid-response tool for conferring seamless protection against influenza. PLoS One 6:e22605. doi: 10.1371/journal.pone.0022605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kees U, Krammer PH. 1984. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med 159:365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yewdell JW, Bennink JR, Smith GL, Moss B. 1985. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A 82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon ACM, de Mutsert G, van Baarle D, Smith DJ, Lapedes AS, Fouchier RAM, Sintnicolaas K, Osterhaus ADME, Rimmelzwaan GF. 2004. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J Immunol 172:2453–2460. doi: 10.4049/jimmunol.172.4.2453. [DOI] [PubMed] [Google Scholar]
- 19.Jameson J, Cruz J, Terajima M, Ennis FA. 1999. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol 162:7578–7583. [PubMed] [Google Scholar]
- 20.Epstein S. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis 193:49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 21.McMichael AJ, Gotch FM, Noble GR, Beare PAS. 1983. Cytotoxic T-cell immunity to influenza. N Engl J Med 309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 22.McMichael AJ, Gotch FM, Dongworth DW, Clark A, Potter CW. 1983. Declining T-cell immunity to influenza, 1977–82. Lancet ii:762–764. doi: 10.1016/S0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- 23.Boon ACM, Fringuelli E, Graus YMF, Fouchier RAM, Sintnicolaas K, Iorio AM, Rimmelzwaan GF, Osterhaus ADME. 2002. Influenza A virus specific T cell immunity in humans during aging. Virology 299:100–108. doi: 10.1006/viro.2002.1491. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y, Jing Y, Campbell AE, Gravenstein S. 2004. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol 172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 25.Yap KL, Ada GL, McKenzie IFC. 1978. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 26.De Haan A, Haijema BJ, Voorn P, Meijerhof T, van Roosmalen ML, Leenhouts K. 2012. Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine 30:4884–4891. doi: 10.1016/j.vaccine.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Dong L, Liu F, Fairman J, Hong DK, Lewis DB, Monath T, Warner JF, Belser JA, Patel J, Hancock K, Katz JM, Lu X. 2012. Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice. Vaccine 30:254–264. doi: 10.1016/j.vaccine.2011.10.103. [DOI] [PubMed] [Google Scholar]
- 28.Nagai H, Ikematsu H, Tenjinbaru K, Maeda A, Dramé M, Roman FP. 2010. A phase II, open-label, multicentre study to evaluate the immunogenicity and safety of an adjuvanted prepandemic (H5N1) influenza vaccine in healthy Japanese adults. BMC Infect Dis 10:338. doi: 10.1186/1471-2334-10-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto S, Matsuoka S, Takenaka N, Haredy AM, Tanimoto T, Gomi Y, Ishikawa T, Akagi T, Akashi M, Okuno Y, Mori Y, Yamanishi K. 2012. Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split virion vaccine with mucosal adjuvants show similar levels of cross-protection. Clin Vaccine Immunol 19:979–990. doi: 10.1128/CVI.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockman S, Brown LE, Barr IG, Gilbertson B, Lowther S, Kachurin A, Kachurina O, Klippel J, Bodle J, Pearse M, Middleton D. 2013. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J Virol 87:3053–3061. doi: 10.1128/JVI.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Duan Y, Zhang P, Li Z, Wang C, Dong M, Tang C, Xing L, Gu H, Zhao Z, Liu X, Zhang S, Wang X. 2012. Multiple-clade H5N1 influenza split vaccine elicits broad cross protection against lethal influenza virus challenge in mice by intranasal vaccination. PLoS One 7:e30252. doi: 10.1371/journal.pone.0030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua BY, Johnson D, Tan A, Earnest-Silveira L, Sekiya T, Chin R, Torresi J, Jackson DC. 2012. Hepatitis C VLPs delivered to dendritic cells by a TLR2 targeting lipopeptide results in enhanced antibody and cell-mediated responses. PLoS One 7:e47492. doi: 10.1371/journal.pone.0047492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua BY, Olson MR, Bedoui S, Sekiya T, Wong CY, Turner SJ, Jackson DC. 2014. The use of a TLR2 agonist-based adjuvant for enhancing effector and memory CD8 T-cell responses. Immunol Cell Biol 92:377–383. doi: 10.1038/icb.2013.102. [DOI] [PubMed] [Google Scholar]
- 34.Chua BY, Pejoski D, Turner SJ, Zeng W, Jackson DC. 2011. Soluble proteins induce strong CD8+ T cell and antibody responses through electrostatic association with simple cationic or anionic lipopeptides that target TLR2. J Immunol 187:1692–1701. doi: 10.4049/jimmunol.1100486. [DOI] [PubMed] [Google Scholar]
- 35.Tan ACL, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, Jackson DC. 2012. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm 9:2710–2718. doi: 10.1021/mp300257x. [DOI] [PubMed] [Google Scholar]
- 36.Edenborough KM, Gilbertson BP, Brown LE. 2012. A mouse model for the study of contact-dependent transmission of influenza A virus and the factors that govern transmissibility. J Virol 86:12544–12551. doi: 10.1128/JVI.00859-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobbin JCA, Zeng W, Jackson DC, Brown LE. 2014. Different arms of the adaptive immune system induced by a combination vaccine work in concert to provide enhanced clearance of influenza. PLoS One 9:e115356. doi: 10.1371/journal.pone.0115356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung I, To K, Lee C, Lin C, Chan J, Tse H, Cheng V, Chen H, Ho P, Tse C, Ng T, Que T, Chan K, Yuen K. 2010. Effect of clinical and virological parameters on the level of neutralizing antibody against pandemic influenza A virus H1N1 2009. Clin Infect Dis 51:274–279. doi: 10.1086/653940. [DOI] [PubMed] [Google Scholar]
- 39.Ijichi S, Ijichi N. 2003. Too early cure of influenza: recurrence in oseltamivir-treated children. J. Paediatr Child Health 39:480–481. doi: 10.1046/j.1440-1754.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 40.Ekiert DC, Bhabha G, Elsliger M-, Friesen RHE, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabhu N, Prabakaran M, Ho H-, Velumani S, Qiang J, Goutama M, Kwang J. 2009. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J Virol 83:2553–2562. doi: 10.1128/JVI.02165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stropkovská A, Mucha V, Fislová T, Gocník M, Kostolanský F, Varečková E. 2009. Broadly cross-reactive monoclonal antibodies against HA2 glycopeptide of influenza A virus hemagglutinin of H3 subtype reduce replication of influenza A viruses of human and avian origin. Acta Virol 53:15–20. doi: 10.4149/av_2009_01_15. [DOI] [PubMed] [Google Scholar]
- 45.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol 181:4168–4176. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Song A, Levin J, Dennis D, Zhang N, Yoshida H, Koriazova L, Madura L, Shapiro L, Matsumoto A, Yoshida H, Mikayama T, Kubo RT, Sarawar S, Cheroutre H, Kato S. 2008. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res 80:168–177. doi: 10.1016/j.antiviral.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, Kent SJ. 2013. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 190:1837–1848. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 48.La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. 2010. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest 120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ, Milicic A, Poyntz HC, Lambe T, Fletcher HA, Hill AVS, Gilbert SC. 2011. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis 52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, Peng Y, Blais M-, Duncan CJA, Sheehy SH, Havelock T, Faust SN, Williams RL, Gilbert A, Oxford J, Dong T, Hill AVS, Gilbert SC. 2012. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price GE, Lo C, Misplon JA, Epstein SL. 2014. Mucosal immunization with a candidate universal influenza vaccine reduces virus transmission in a mouse model. J Virol 88:6019–6030. doi: 10.1128/JVI.03101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitamura D, Roes J, Kühn R, Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 53.Tannock GA, Paul JA, Barry RD. 1984. Relative immunogenicity of the cold-adapted influenza virus A/Ann Arbor/6/60 (A/AA/6/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect Immun 43:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambré CR, Chauvaux S, Pilatte Y. 1989. Fluorometric assay for the measurement of viral neuraminidase in influenza vaccines. Vaccine 7:104–105. doi: 10.1016/0264-410X(89)90045-5. [DOI] [PubMed] [Google Scholar]
- 55.Ng WC, Wong V, Muller B, Rawlin G, Brown LE. 2010. Prevention and treatment of influenza with hyperimmune bovine colostrum antibody. PLoS One 5:e13622. doi: 10.1371/journal.pone.0013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Components in PR8 split virus vaccine. (A) SDS-PAGE analysis of viral components (PB1/2, polymerase basic protein subunits 1 and 2; PA, acid polymerase subunit; NP, nucleoprotein; NA, neuraminidase; HA, hemagglutinin; M, matrix protein) present in PR8 split virus vaccine (1 µg). (B) Western blot analysis of the vaccine (1 µg) probed with a murine anti-NP-specific (lane 1) or anti-HA-specific (lane 2) antibody followed by an HRP-conjugated murine Ig-specific detection antibody and development using a chemiluminescent substrate. Download