Abstract
Background & Aims
Statins decrease portal pressure in patients with cirrhosis and increase survival times of those who have bled from varices. However, statins can be hepatotoxic. It is important to determine whether long-term statin use will be beneficial or detrimental for patients with cirrhosis because physicians are reluctant to prescribe statins to patients with liver disease. We investigated effects of statins on decompensation and survival times in patients with compensated cirrhosis.
Methods
We performed a retrospective cohort using the Veteran Affairs Clinical Case Registry, which contains nationwide data from veterans infected with the hepatitis C virus (HCV). We identified patients with compensated cirrhosis from January 1996 through December 2009. Statin use was according to filled prescriptions. Cirrhosis and decompensation were determined from ICD9 codes, using a validated algorithm.
Results
Among 40,512 patients with HCV compensated cirrhosis (98% male, median age of 56 years), 2802 statin users were identified. We developed a propensity score model using variables associated with statin prescription, and new statin users were matched with up to 5 non-users; 685 statin users were matched with 2062 non-users. Discrimination of the propensity score model was 0.92. Statin users had lower risk of decompensation (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.39–0.77)] and death (HR, 0.56; 95% CI, 0.46–0.69), compared with non-users. Findings persisted after adjustment for age, FIB-4 index score, serum level of albumin, model for end-stage liver disease and Child scores (HR for decompensation, 0.55; 95% CI, 0.39–0.78) and HR for death, 0.55; 95% CI, 0.45–0.68).
Conclusions
Based on data from the Veteran Affairs Clinical Case Registry, statin use among patients with HCV and compensated cirrhosis is associated with over 40% lower risk of cirrhosis decompensation and death. Although statins cannot yet be widely recommended for these patients, their use should not be avoided.
Keywords: Simvastatin, Prognosis, Decompensation, Mortality
Cirrhosis results from any chronic liver disease and has two distinct stages: compensated and decompensated. Median survival in compensated cirrhosis is over 12 years, while it is less than 2 years once decompensation occurs, that is, when complications of cirrhosis (ascites, variceal hemorrhage, and/or encephalopathy) become clinically apparent1. Main predictors of decompensation are the presence of clinically significant portal hypertension (determined by portal pressure measurement) and a low serum albumin (an indicator of liver dysfunction)2, 3. A decrease in portal pressure of only 10% has been shown to significantly decrease the development of varices4 and reduce the incidence of first variceal hemorrhage, ascites and death in patients with compensated cirrhosis5, 6.
Most drugs currently used to decrease portal pressure do so through splanchnic vasoconstriction thereby reducing portal blood inflow. However, an important component of portal hypertension is increased intrahepatic vascular resistance partially due to sinusoidal endothelial dysfunction with decreased nitric oxide. Statins increase nitric oxide availability at the intrahepatic level7 and decrease portal pressure both in experimental animals8 and in patients with cirrhosis9. Their beneficial effect may go beyond reducing portal pressure by increasing flow into the liver, thereby potentially improving liver function9. While statins can also be hepatotoxic, a small retrospective study recently suggested that statins are safe in cirrhosis and their use was a negative predictor of death10.
The objective of this study was to assess the association between statin use, decompensation and death, in a large cohort of patients with compensated cirrhosis. We hypothesized that statins would be associated with lower risk of decompensation and death. Patients with more severe liver disease are less likely to be prescribed a statin but more likely to decompensate and die. Patients with more severe cardiovascular risk are more likely to receive a statin but may be at higher risk of death. To account for this confounding by indication we used propensity score matching. This technique provides an analysis that emulates a randomized trial.
Methods
Study design and data source
This is a retrospective cohort study, approved by the Institutional Review Board of the Veterans Affairs (VA) Connecticut Health Care System. Data was obtained from the US Departments of VA HCV Clinical Case Registry (CCR); a database of HCV infected Veterans receiving care in any VA facility nationwide. Subjects were included in the CCR if they had positive HCV antibody or an International Classification of Diseases, Ninth Revision (ICD-9) code for hepatitis C; 80% of patients had a positive HCV-RNA, a confirmatory test was not available in the remaining 20%. Data elements in the CCR include demographics, inpatient and outpatient visits (including ICD -9 diagnosis and procedure codes, and Current Procedural Terminology [CPT] codes), laboratory results and pharmacy data. Mortality was determined from the VA vital status file which is compiled from combined sources including inpatient mortality, social security data, and national death benefits data, a method shown to provide excellent mortality ascertainment.11Details on the creation and contents of CCR data have been published elsewhere12. The dataset used in the current study consisted of HCV patients in CCR in care between January 1, 1996 and December 31, 2009.
Study patients
Included patients had cirrhosis, defined by the presence of one inpatient or 2 outpatient codes (ICD 9 codes 571.2, 571.5, 571.6, as previously validated)13 and who attended, primary care/internal medicine, cardiology, endocrinology, gastroenterology, geriatrics, hepatology, infectious diseases, or women’s health clinics. These clinics were chosen because they were the source of 85% of statin use and we wanted to ensure that users and non-users came from the same source population and had an equal opportunity to receive a statin prescription. Patients with HIV (ICD-9 code 042, 044, V08) or hepatitis B infection (positive surface antigen or positive HBV DNA) were excluded.
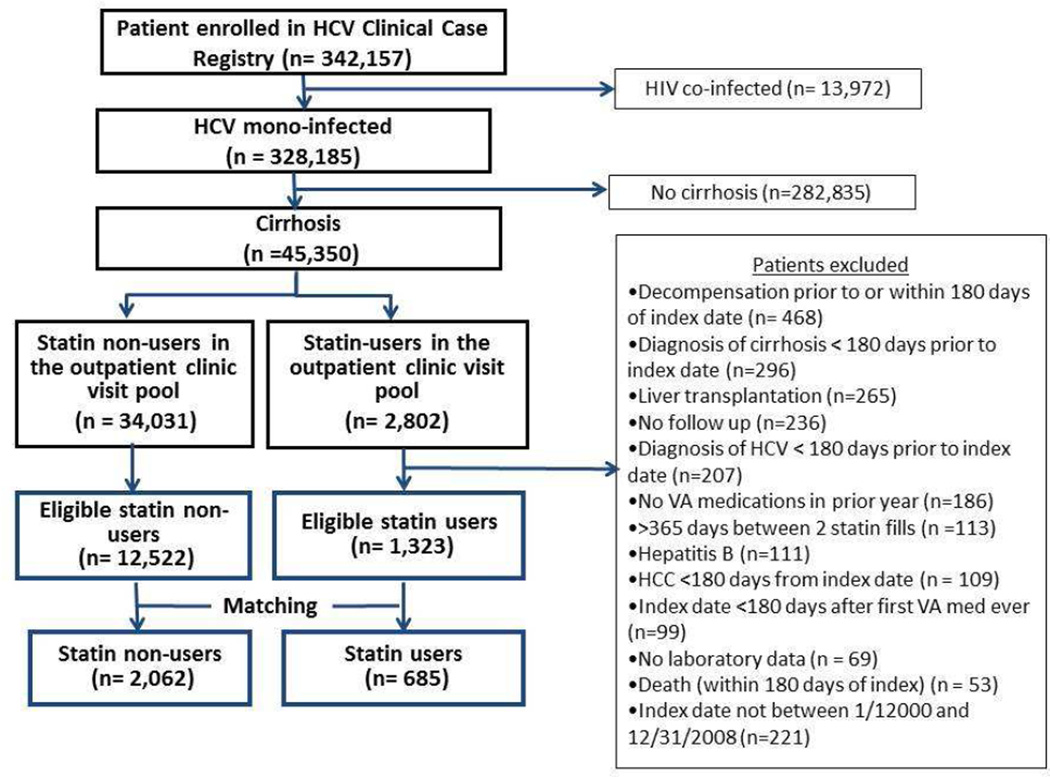
An index date was defined as date of first statin fill for statin users and a randomly chosen clinic visit date for statin non-users (Supplementary figure 1) between 2000 and 2009 (scarce statin use prior to 2000). Baseline period was defined as 365 days before the index date. Baseline labs used for the study were from this period and were the closest available to the index date. Statin users had to be newly initiating and were required to have at least 2 fills of any statin (simvastatin, lovastatin, rosuvastatin, atorvastatin, pravastatin and fluvastatin). To ensure that patients received medications from the VA (and minimize the possibility of statin prescription outside the VA) all patients had to have filled at least one VA prescription for any drug in the year before the index date (Figure 1, Supplementary Figure 1). To ensure that statin users were new initiators, the index date had to be at least 180 days after the first VA non-statin prescription of any kind. Patients were excluded if they had decompensation (as defined below) or hepatocellular carcinoma (ICD-9 code 155.0) before or within 180 days after the index date, no labs, or no follow-up. They were also excluded if they died within 180 days after the index date (Figure 1, Supplementary Figure 1). Statin users were excluded if they had only one statin prescription fill, or >365 days between first and second fill.
Figure 1.
Study Flow diagram
Study outcomes
The primary outcomes were cirrhosis decompensation and death. Decompensation was defined by the presence of one inpatient or two outpatient ICD -9 codes for (A)esophageal varices with bleeding (ICD9 code 456.0) (B) esophageal varices in diseases classified elsewhere, with bleeding (ICD9 code 456.20), (C) ascites (ICD 9 code 789.5) or (D) spontaneous bacterial peritonitis (ICD 9 code 567.23). This definition was modified from the original14 by excluding the code for esophageal varices without bleeding (ICD-9 code 456.1, 456.21) and portal hypertension (572.3) as these are not decompensating events1.
Outcomes analysis was restricted to patients with index dates prior to December 31, 2008 to allow all the opportunity for at least one year of follow-up. Follow-up began 180 days after the index date (Supplementary Figure 1) to avoid immortal time bias15. That is, as statin users were guaranteed to be alive long enough to have a second fill, a similar allowance had to be made for non-users. Most statin users had the second fill by 180 days. Follow-up for decompensation ended at the earliest of date of diagnosed decompensation liver transplant, death, or last visit recorded at the VA as of Dec 31, 2009. Follow-up for death was similar but patients were not censored at decompensation or transplant.
Data collection
Data collected included age, sex, race, body mass index (BMI), geographic site and its statin prescribing pattern, comorbid conditions and laboratory data as well as any prescription of antiviral therapy with pegylated interferon plus ribavirin during the study period. Co-morbid conditions were defined as those recorded any time prior to index date (Supplementary Figure 1). Hypertension, peripheral artery disease, chronic kidney disease, coronary artery disease, cerebrovascular disease smoking, diabetes, alcohol dependence and drug abuse were determined based on occurrence of at least one inpatient or two outpatient ICD -9 codes for the respective diagnoses. Baseline laboratory data on total cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides, albumin, total bilirubin, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), international normalized ratio (INR), platelet count and hemoglobin was collected prior to and closest to the index date and within 365 days of the same. The number of lipid and non-lipid laboratory tests performed in the year prior to the index date was also obtained. The FIB-4 index was calculated at baseline as [age in years × AST in U/L]/ [(platelet count × 109/L) × (ALT in U/L)16. Prior studies have shown that it is useful in ruling in cirrhosis in patients with chronic liver disease17 and, in compensated cirrhosis, it can identify patients with clinically significant portal hypertension with reasonable accuracy (unpublished observations). Liver transplantation was identified using ICD-9 procedure codes and diagnosis codes. Adherence to therapy was assessed by an extensively studied method, the proportion of days covered (PCD), which is calculated as the cumulative number of days during which the medication was available divided by the number of days of follow-up.18, 19
Statistical analysis
Development of propensity score
In order to minimize confounding by indication, the study employed a propensity score matched cohort design.20 Propensity scores were developed using covariates associated with statin use, both well-established predictors and also those specific to the patient population. The final multivariable logistic regression model included 33 variables and 3 interaction terms. We included age, body mass index, year of index visit, frequently occurring diagnoses in statin users (e.g. coronary artery disease, cerebrovascular disease, smoking, diabetes), laboratory values predictive of statin use (e.g. LDL, total cholesterol), healthcare utilization variables (e.g. number of laboratory tests, specialty clinic visited) and negative correlates of statin use (laboratory values indicating severity of liver disease e.g. albumin, total bilirubin, INR, platelet count and FIB-4 index. Additionally, prescribing patterns were developed by determining the proportion of patients in the visit pool who initiated statin use at each site by years (1997–2001, 2002–2003, 2004–2009). Sites below the 25th percentile were deemed as low statin-prescribing sites; those above the 75th percentile were deemed high statin-prescribing sites; all others were classified as medium. This attribute was assigned to each record and used in the propensity score model. Adding prescribing patterns by geographic site and year, and interactions with diabetes and HDL by year improved model fit and discrimination. The c-statistic for the propensity score model was 0.92, which indicates an excellent discrimination between statin users and non-users21.
Matching
Statin users were matched by propensity scores to non-users with a greedy matching algorithm22. First, all possible 5 decimal place matches were made, then 4 decimal places and so on down to 1 decimal place. Next, the 5 best matches for each user were randomly selected with each non-user only selected once. The weighted average of each set of non-users was used to represent one non-user. Statin users who could not be matched were excluded from the matched cohort analysis. Subjects were assigned their original exposure status until the end of follow-up regardless of actual statin use during follow-up to emulate an intention to treat analysis of a randomized trial.
Outcome analysis
We performed parallel analyses in 1) an unmatched sample of all eligible statin users compared to a sample of all eligible unique non-users in whom the index date was randomly selected and 2) the propensity score matched sample of users and non-users. Kaplan-Meier curves were generated to compare primary outcomes in statin users and non-users. The association between statin use and risk of mortality and decompensation was estimated using Cox proportional hazards model with adjustment for age, FIB-4 index (as a surrogate of clinically significant portal hypertension), serum albumin, Model of End Stage Liver Disease (MELD) score and Child-Turcotte-Pugh (CTP) score; parameters that have been shown to predict decompensation and/or death in compensated cirrhosis1, 2.
Six different sensitivity analyses were performed in the matched cohort: 1) in patients who did not receive HCV anti-viral therapy; 2) in patients who were HCV RNA positive; 3) excluding patients with FIB 4 <1.45 (patients who are less likely to have cirrhosis and may have been miscoded); (4) in statin users with > 50% adherence; 5) excluding statin users with >180 days before the second fill and 6) using an alternative definition of decompensated cirrhosis. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc. Cary, North Carolina).
Results
Of 342,157 HCV infected patients in the CCR, 45,350 (14%) patients had cirrhosis and no HIV infection. Among 40,512 patients with a visit to one of the included clinics, we identified 2,802 statin users. After applying exclusion criteria 1,323 statin users and 12,522 non-users were eligible for unmatched analysis (Figure 1). Most patients used simvastatin (85%), followed by lovastatin (10%), pravastatin (3%), rosuvastatin (1%) and fluvastatin (1%).
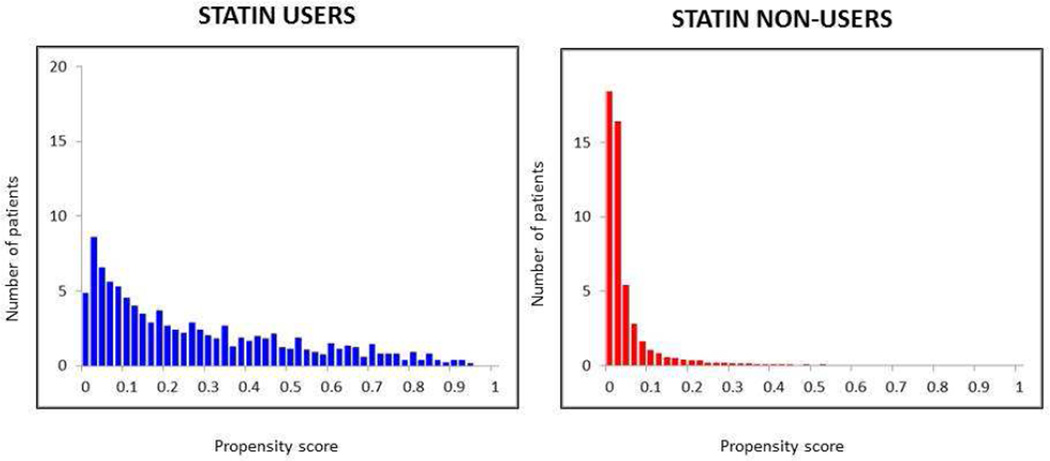
We were able to match 685 statin users with 2,062 statin non-users. As shown in Figure 2, statin users had a wider range of propensity scores than non-users and therefore we were only able to match users with propensity scores in the lower range. Only 16 % of non-users had a score >0.04, compared with 87% of users. Statin users with high propensity scores (i.e. those very likely to be prescribed statins) could not be matched and were excluded from matched analysis. Among the 2062 non-users, the propensity score of the user matched to 5 decimal places for 16%, 37% were within 0.0001, 36% to 0.001, 10% to 0.02 and 1% matched to one decimal place. Of 685 statin users, 134 were matched to 5 non-users, 238 to 4 non-users, 3 to 212 non-users 87 to 2 non-users and 14 to one non-user.
Figure 2.
Propensity score histograms for statin users and statin non-users
Statin users had a wider range of propensity scores than non-users. Stain users with propensity scores in the lower range were matched to statin non-users with similar propensity scores
Baseline characteristics of unmatched and propensity score-matched users and non-users are shown in Table 1. The weighted average of each set of non-users was used to represent one non-user. In the unmatched cohort there were many clinically and statistically significant differences between users and non-users, specifically, statin users were older, had a higher prevalence of smoking, coronary artery disease, hypertension, diabetes, chronic kidney disease, peripheral artery disease and cerebrovascular disease; as well as higher cholesterol, LDL, albumin and platelet count. As expected, in the propensity matched cohort there were no differences in these parameters between statin users and nonusers, demonstrating the validity of our model. Antiviral therapy was prescribed in 23% of the patients (20.7% of non-statin users; 25.4% of statin users; p=0.04). Median adherence, as determined by the proportion of days covered (PDC) was 0.79 (IQR 0.45, 1.00) for cirrhosis decompensation and 0.77 (IQR 0.41, 1.00) for death
Table 1.
Baseline characteristics of unmatched and matched cohort
| Unmatched | Propensity Score Matched | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-user (N = 12522) |
Statin user (N = 1323) |
p value |
Non-user (N = 685) |
Statin user (N = 685) |
p value |
||||||
| Age | <45 | 499 | (4.0) | 23 | (1.7) | <.0001 | 14 | (2.0) | 12 | (1.8) | 0.94 |
| 45–49 | 2048 | (16.4) | 114 | (8.6) | 58 | (8.4) | 65 | (9.5) | |||
| 50–55 | 4010 | (32.0) | 385 | (29.1) | 190 | (27.7) | 197 | (28.8) | |||
| 55–59 | 3488 | (27.9) | 469 | (35.4) | 248 | (36.2) | 241 | (35.2) | |||
| 60–64 | 1277 | (10.2) | 174 | (13.2) | 88 | (12.8) | 91 | (13.3) | |||
| ≥65 | 1200 | (9.6) | 158 | (11.9) | 88 | (12.8) | 79 | (11.5) | |||
| Median (IQR) | 54 | (50, 58) | 56 | (52, 60) | 56 | (52, 60) | 56 | (52, 59) | |||
| Sex | Male | 12236 | (97.7) | 1300 | (98.3) | 0.20 | 671 | (97.9) | 677 | (98.8) | 0.18 |
| Race | White | 6136 | (49.0) | 707 | (53.4) | <.0001 | 341 | (49.8) | 359 | (52.4) | 0.67 |
| Black | 1850 | (14.8) | 266 | (20.1) | 145 | (21.2) | 146 | (21.3) | |||
| Hispanic | 1064 | (8.5) | 78 | (5.9) | 45 | (6.6) | 38 | (5.5) | |||
| Other | 3472 | (27.7) | 272 | (20.6) | 154 | (22.4) | 142 | (20.7) | |||
| Year | 2000 | 1091 | (8.7) | 48 | (3.6) | <.0001 | 28 | (4.1) | 28 | (4.1) | 1.0 |
| 2001 | 1016 | (8.1) | 49 | (3.7) | 19 | (2.8) | 19 | (2.8) | |||
| 2002 | 1133 | (9.0) | 76 | (5.7) | 41 | (6.0) | 41 | (6.0) | |||
| 2003 | 1075 | (8.6) | 82 | (6.2) | 41 | (6.0) | 41 | (6.0) | |||
| 2004 | 1216 | (9.7) | 193 | (14.6) | 80 | (11.7) | 80 | (11.7) | |||
| 2005 | 1367 | (10.9) | 183 | (13.8) | 84 | (12.3) | 84 | (12.3) | |||
| 2006 | 1466 | (11.7) | 207 | (15.6) | 112 | (16.4) | 112 | (16.4) | |||
| 2007 | 1713 | (13.7) | 229 | (17.3) | 122 | (17.8) | 122 | (17.8) | |||
| 2008 | 2445 | (19.5) | 256 | (19.3) | 158 | (23.1) | 158 | (23.1) | |||
| Site prescribing pattern ** | Low | 2106 | (16.8) | 114 | (8.6) | <.0001 | 74 | (10.8) | 68 | (9.9) | 0.80 |
| Medium | 7812 | (62.4) | 747 | (56.5) | 400 | (58.3) | 396 | (57.8) | |||
| High | 2604 | (20.8) | 462 | (34.9) | 212 | (30.9) | 221 | (32.3) | |||
| Clinic | Gastroenterology, Hepatology, Infectious Disease | 3123 | (24.9) | 80 | (6.0) | <.0001 | 59 | (8.6) | 55 | (8.0) | 0.96 |
| Primary Care | 8886 | (71.0) | 1058 | (80.0) | 557 | (81.4) | 558 | (81.5) | |||
| Endocrinology, Nephrology | 274 | (2.2) | 61 | (4.6) | 31 | (4.5) | 31 | (4.5) | |||
| Cardiology | 239 | (1.9) | 124 | (9.4) | 38 | (5.5) | 41 | (6.0) | |||
| Conditions | Smoking | 7453 | (59.5) | 868 | (65.6) | <.0001 | 453 | (66.2) | 451 | (65.8) | 0.89 |
| Alcohol abuse/dependence | 7085 | (56.6) | 694 | (52.5) | 0.004 | 365 | (53.3) | 372 | (54.3) | 0.72 | |
| Drug abuse/dependence | 5401 | (43.1) | 576 | (43.5) | 0.78 | 290 | (42.3) | 322 | (47.0) | 0.08 | |
| Coronary artery disease | 1496 | (11.9) | 493 | (37.3) | <.0001 | 210 | (30.7) | 241 | (35.2) | 0.08 | |
| Hypertension | 7443 | (59.4) | 1111 | (84.0) | <.0001 | 553 | (80.7) | 568 | (82.9) | 0.29 | |
| Diabetes | 3613 | (28.9) | 725 | (54.8) | <.0001 | 357 | (52.1) | 363 | (53.0) | 0.73 | |
| Chronic kidney disease | 335 | (2.7) | 78 | (5.9) | <.0001 | 38 | (5.5) | 40 | (5.8) | 0.79 | |
| Peripheral artery disease | 466 | (3.7) | 113 | (8.5) | <.0001 | 56 | (8.2) | 41 | (6.0) | 0.11 | |
| Cerebrovascular disease | 386 | (3.1) | 75 | (5.7) | <.0001 | 39 | (5.7) | 40 | (5.8) | 0.92 | |
| Number of lipid tests | 0 | 3774 | (30.1) | 49 | (3.7) | <.0001 | 43 | (6.2) | 46 | (6.7) | 0.82 |
| 1 | 4959 | (39.6) | 461 | (34.8) | 259 | (37.9) | 267 | (39.0) | |||
| 2 or more | 3789 | (30.3) | 813 | (61.5) | 383 | (55.9) | 372 | (54.3) | |||
| Total Cholesterol | <200 | 7944 | (63.4) | 685 | (51.8) | <.0001 | 438 | (63.9) | 444 | (64.8) | 0.80 |
| ≥200 | 777 | (6.2) | 587 | (44.4) | 203 | (29.6) | 193 | (28.2) | |||
| None found | 3801 | (30.4) | 51 | (3.9) | 44 | (6.4) | 48 | (7.0) | |||
| LDL (mg/dL) | <100 | 5391 | (43.1) | 259 | (19.6) | <.0001 | 204 | (29.7) | 211 | (30.8) | 0.94 |
| 100 –129 | 1477 | (11.8) | 389 | (29.4) | 219 | (32.0) | 216 | (31.5) | |||
| 130–159 | 403 | (3.2) | 351 | (26.5) | 125 | (18.2) | 115 | (16.8) | |||
| ≥160 | 104 | (0.8) | 191 | (14.4) | 44 | (6.4) | 43 | (6.3) | |||
| None found | 5147 | (41.1) | 133 | (10.1) | 93 | (13.6) | 100 | (14.6) | |||
| HDL (mg/dL) | <40 | 3666 | (29.3) | 671 | (50.7) | <.0001 | 325 | (47.4) | 319 | (46.6) | 0.65 |
| 40–59 | 2874 | (23.0) | 451 | (34.1) | 225 | (32.9) | 212 | (30.9) | |||
| ≥60 | 5043 | (40.3) | 108 | (8.2) | 82 | (12.0) | 93 | (13.6) | |||
| None found | 939 | (7.5) | 93 | (7.0) | 53 | (7.7) | 61 | (8.9) | |||
| Triglyceride (mg/dL) | <150 | 5808 | (46.4) | 722 | (54.6) | <.0001 | 391 | (57.1) | 381 | (55.6) | 0.84 |
| ≥150 | 1873 | (15.0) | 512 | (38.7) | 221 | (32.3) | 227 | (33.1) | |||
| None found | 4841 | (38.7) | 89 | (6.7) | 73 | (10.6) | 77 | (11.2) | |||
| Number of non-lipid labs | <10 | 4344 | (34.7) | 141 | (10.7) | <.0001 | 87 | (12.7) | 90 | (13.1) | 0.75 |
| 10–12 | 3467 | (27.7) | 511 | (38.6) | 243 | (35.5) | 254 | (37.1) | |||
| ≥13 | 4711 | (37.6) | 671 | (50.7) | 355 | (51.8) | 341 | (49.8) | |||
| Albumin (g/dL) | ≥4.0 | 3066 | (24.5) | 560 | (42.3) | <.0001 | 263 | (38.4) | 269 | (39.3) | 0.99 |
| 3.6–3.9 | 2959 | (23.6) | 322 | (24.3) | 165 | (24.0) | 162 | (23.6) | |||
| 2.8–3.5 | 4289 | (34.3) | 259 | (19.6) | 166 | (24.2) | 168 | (24.5) | |||
| <2.8 | 1502 | (12.0) | 70 | (5.3) | 35 | (5.1) | 34 | (5.0) | |||
| None found | 706 | (5.6) | 112 | (8.5) | 57 | (8.3) | 52.0 | (7.6) | |||
| Median (IQR) | 3.6 | (3.1, 4.0) | 3.9 | (3.5, 4.2) | 3.8 | (3.4, 4.2) | 3.9 | (3.4, 4.2) | |||
| Total bilirubin (mg/dL) | <2 | 10012 | (80.0) | 1221 | (92.3) | <.0001 | 622 | (90.8) | 625 | (91.2) | 0.91 |
| 2–3 | 1278 | (10.2) | 28 | (2.1) | 20 | (3.0) | 22 | (3.2) | |||
| >3 | 598 | (4.8) | 6 | (0.5) | 7 | (1.1) | 5 | (0.7) | |||
| None found | 634 | (5.1) | 68 | (5.1) | 35 | (5.1) | 33 | (4.8) | |||
| Median (IQR) | 1 | (0.7, 1.6) | 0.7 | (0.5, 1.0) | 0.8 | (0.6, 1.1) | 0.7 | (0.5, 1.1) | |||
| INR | <1.7 | 8261 | (66.0) | 754 | (57.0) | <.0001 | 421 | (61.5) | 405 | (59.1) | 0.77 |
| 1.7–2.3 | 361 | (2.9) | 11 | (0.8) | 6 | (0.9) | 6 | (0.9) | |||
| >2.3 | 122 | (1.0) | 31 | (2.3) | 9 | (1.3) | 12 | (1.8) | |||
| None found | 3778 | (30.2) | 527 | (39.8) | 249 | (36.4) | 262 | (38.2) | |||
| Median (IQR) | 1.2 | (1.1, 1.3) | 1.1 | (1.0, 1.2) | 1.1 | (1.0, 1.2) | 1 | (1.0, 1.2) | |||
| Platelets (× 109 /L) | ≥150 | 3866 | (30.9) | 801 | (60.5) | <.0001 | 362 | (52.8) | 372 | (54.3) | 0.95 |
| 100–149 | 3085 | (24.6) | 273 | (20.6) | 167 | (24.4) | 160 | (23.4) | |||
| <100 | 5081 | (40.6) | 167 | (12.6) | 118 | (17.2) | 116 | (16.9) | |||
| None found | 490 | (3.9) | 82 | (6.2) | 39 | (5.6) | 37 | (5.4) | |||
| Median (IQR) | 112 | (76, 171) | 180 | (129, 233) | 155 | (107, 211) | 167 | (116, 223) | |||
| FIB4 | <1.45 | 1209 | (9.7) | 375 | (28.3) | <.0001 | 128 | (18.7) | 159 | (23.2) | 0.12 |
| 1.45–3.25 | 2792 | (22.3) | 513 | (38.8) | 262 | (38.2) | 242 | (35.3) | |||
| >3.25 | 7388 | (59.0) | 273 | (20.6) | 205 | (29.9) | 211 | (30.8) | |||
| None found | 1133 | (9.0) | 162 | (12.2) | 90 | (13.1) | 73 | (10.7) | |||
| Hemoglobin (g/dL) | ≥14 | 6298 | (50.3) | 801 | (60.5) | <.0001 | 387 | (56.4) | 414 | (60.4) | 0.25 |
| 12–13.9 | 3747 | (29.9) | 300 | (22.7) | 175 | (25.5) | 167 | (24.4) | |||
| 10–11.9 | 1438 | (11.5) | 104 | (7.9) | 62 | (9.0) | 54 | (7.9) | |||
| <10 | 340 | (2.7) | 29 | (2.2) | 21 | (3.0) | 10 | (1.5) | |||
| None found | 699 | (5.6) | 89 | (6.7) | 41 | (6.0) | 40 | (5.8) | |||
| Median (IQR) | 14.1 | (12.8, 15.3) | 14.6 | (13.3, 15.7) | 14.4 | (13.0, 15.6) | 14.6 | (13.3, 15.6) | |||
| MELD | <10 | 5640 | (45.0) | 586 | (44.3) | <0.000 1 | 318 | (46.5) | 313 | (45.7) | 0.70 |
| ≥10 | 2688 | (21.5) | 191 | (14.4) | 106 | (15.4) | 98 | (14.3) | |||
| None found | 4194 | (33.5) | 546 | (41.3) | 261 | (38.1) | 274 | (40.0) | |||
| Median (IQR) | 8.3 | (6.3, 10.9) | 7.3 | (5.6, 9.9) | 8 | (6, 10) | 7 | (6, 10) | |||
| BMI (kg/m2) | <18.5 | 165 | (1.3) | 9 | (0.7) | <.0001 | 8 | (1.2) | 8 | (1.2) | 0.51 |
| 18.5 –24.9 | 3095 | (24.7) | 249 | (18.8) | 156 | (22.7) | 129 | (18.8) | |||
| 25–29.9 | 4513 | (36.0) | 461 | (34.8) | 227 | (33.1) | 237 | (34.6) | |||
| ≥30 | 4377 | (35.0) | 583 | (44.1) | 285 | (41.6) | 299 | (43.6) | |||
| None found | 372 | (3.0) | 21 | (1.6) | 10 | (1.5) | 12 | (1.8) | |||
| Median (IQR) | 27.9 | (24.7, 31.7) | 29.3 | (25.8, 32.9) | 28.6 | (25.1, 32.5) | 29.3 | (25.7, 33.2) | |||
For the unmatched cohort, statin non-users sample were randomly selected by index date among all unique eligible non-users. For the matched cohort, the weighted average of each group of non-users was used to represent one non-user.
Prescribing patterns were developed by determining the proportion of patients in the visit pool who initiated statin use at each site by years (1997–2001, 2002–2003, 2004–2009). Sites below the25th percentile were deemed as low statin-prescribing sites; those above the 75th percentile were deemed high statin-prescribing sites; all others were classified as medium.
Cirrhosis decompensation
In the unmatched cohort, in a median follow-up of 2.5 years for statin users and 1.5 years for statin nonusers, statin use was associated with lower risk of decompensation [HR 0.22 (95% CI 0.17, 0.28)] that remained after adjusting for age, BMI, serum albumin, FIB4, MELD and CTP scores (Table 2).
Table 2.
Hazard ratios of association of statin with decompensation and death in unadjusted and adjusted cohort.
| Unmatched | Propensity Score Matched | |||||||
|---|---|---|---|---|---|---|---|---|
| Events | HR | (CI) | Events | HR | (CI) | |||
| Decompensation | Unadjusted | 2275 | 0.22 | (0.17, 0.28) | 220 | 0.55 | (0.39, 0.77) | |
| Adjusted for | Age | 0.23 | (0.18, 0.30) | 0.54 | (0.38, 0.76) | |||
| Age+ BMI | 0.23 | (0.18, 0.29) | 0.53 | (0.37, 0.75) | ||||
| Age + BMI + albumin, | 0.29 | (0.23, 0.37) | 0.53 | (0.37, 0.75) | ||||
| Age + BMI+ albumin + FIB4 | 0.40 | (0.31, 0.51) | 0.55 | (0.39, 0.78) | ||||
| Age + BMI + albumin + FIB4 + MELD | 0.37 | (0.29, 0.48) | 0.55 | (0.39, 0.78) | ||||
| Age + BMI + Child + FIB4 | 0.38 | (0.30, 0.49) | 0.55 | (0.39, 0.77) | ||||
| Death | Unadjusted | 5015 | 0.39 | (0.34, 0.44) | 667 | 0.56 | (0.46, 0.69) | |
| Adjusted for | Age | 0.36 | (0.32, 0.41) | 0.55 | (0.45, 0.66) | |||
| Age + BMI | 0.36 | (0.32, 0.42) | 0.55 | (0.45, 0.67) | ||||
| Age + BMI + albumin, | 0.45 | (0.40, 0.52) | 0.56 | (0.46, 0.69) | ||||
| Age + BMI+ albumin + FIB4 | 0.51 | (0.45, 0.59) | 0.57 | (0.47, 0.69) | ||||
| Age + BMI + albumin + FIB4 + MELD | 0.48 | (0.42, 0.55) | 0.55 | (0.45, 0.68) | ||||
| Age + BMI + Child + FIB4 | 0.49 | (0.43, 0.56) | 0.55 | (0.45, 0.67) | ||||
In the matched cohort, median follow-up for decompensation was 2.3 years for statin users and 1.7 years for statin non-users. There were 220 decompensation events (39 statin users and 181 non-users). Statin use was associated with lower risk of decompensation [HR 0.55 (95% CI 0.39, 0.77)] compared to non-use. These findings persisted after adjustment for antiviral therapy [HR 0.56 (95% CI 0.39, 0.79)].
We further analyzed the effect of statin use on the type of decompensation. As shown in Table 3, statin use was associated with a lower risk of variceal hemorrhage [HR 0.39 (95% CI 0.19, 0.78)] and ascites [HR 0.59 (95% CI 0.39, 0.91)]. Development of spontaneous bacterial peritonitis was not different between study groups [HR 0.93 (95% CI 0.29, 2.90)].
Table 3.
Rates of decompensation and specific decompensating events in statin non-users and statin users
| N | PY | Decompensation | Ascites | VH | SBP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Rate | Events | Rate | Events | Rate | Events | Rate | |||
| Non-user | 2062 | 4615 | 181 | 3.9 | 112 | 2.4 | 58 | 1.3 | 11 | 0.24 |
| User | 685 | 1842 | 39 | 2.1 | 26 | 1.4 | 9 | 0.5 | 4 | 0.22 |
| Total events | 220 | 138 | 67 | 15 | ||||||
| Hazard ratio (UCL, LCL) | 0.55 (0.39, 0.77) p=0.0007 |
0.59 (0.39, 0.91) p=0.02 |
0.39 (0.19, 0.78) p=0.01 |
0.92 (0.9, 2.9) p=0.89 |
||||||
PY= person year; VH=variceal hemorrhage; SBP= spontaneous bacterial peritonitis; UCL= 95% upper confidence limit; LCL= 95% lower confidence limit; rates are per 100 PY
Death
In the unmatched cohort, statin use was associated with a lower risk of death [HR 0.39 (95% CI 0.34, 0.44)] that remained significant after adjusting for age, BMI, serum albumin, FIB 4, MELD and CTP scores (median follow-up 2.6 years for statin users, 1.9 years for statin non-users) (Table 2).
In the matched cohort, median follow-up for death was 2.4 years for users and 1.9 years for non-users. There were 667 deaths (121 users and 546 non-users). Statin use was associated with lower risk of death [HR 0.56 (95% CI 0.46, 0.69)] compared to non-use. These findings persisted after adjustment for antiviral therapy [HR 0.57 (95% CI 0.47, 0.70)].
Statin use was also associated with a significantly lower risk of development of HCC [HR 0.42 (95% CI 0.27, 0.64)] and lower rate of liver transplantation [HR 0.37 (95% CI 0.15, 0.96)] (Table 4).
Table 4.
Risk of HCC, liver transplantation and death in statin non-users and in statin users.
| HCC | Liver transplantation | Death | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PY | Events | Rate | PY | Events | Rate | PY | Events | Rate | |
| Non-user | 4673 | 148 | 3.2 | 4771 | 34 | 0.7 | 4897 | 546 | 11.1 |
| User | 1881 | 25 | 1.3 | 1895 | 5 | 0.3 | 1913 | 121 | 6.3 |
| Total | 173 | 39 | 667 | ||||||
| Hazard ratio (UCL, LCL) | 0.42 (0.27, 0.64) P <0.001 |
0.37 (0.15, 0.96) P=0.04 |
0.56 (0.46, 0.69) p <0.001 |
||||||
HCC= hepatocellular carcinoma; PY= person year; UCL=95% upper confidence limit; CL= 95% lower confidence limit; rates are per 100 PY
Probability of decompensation and death
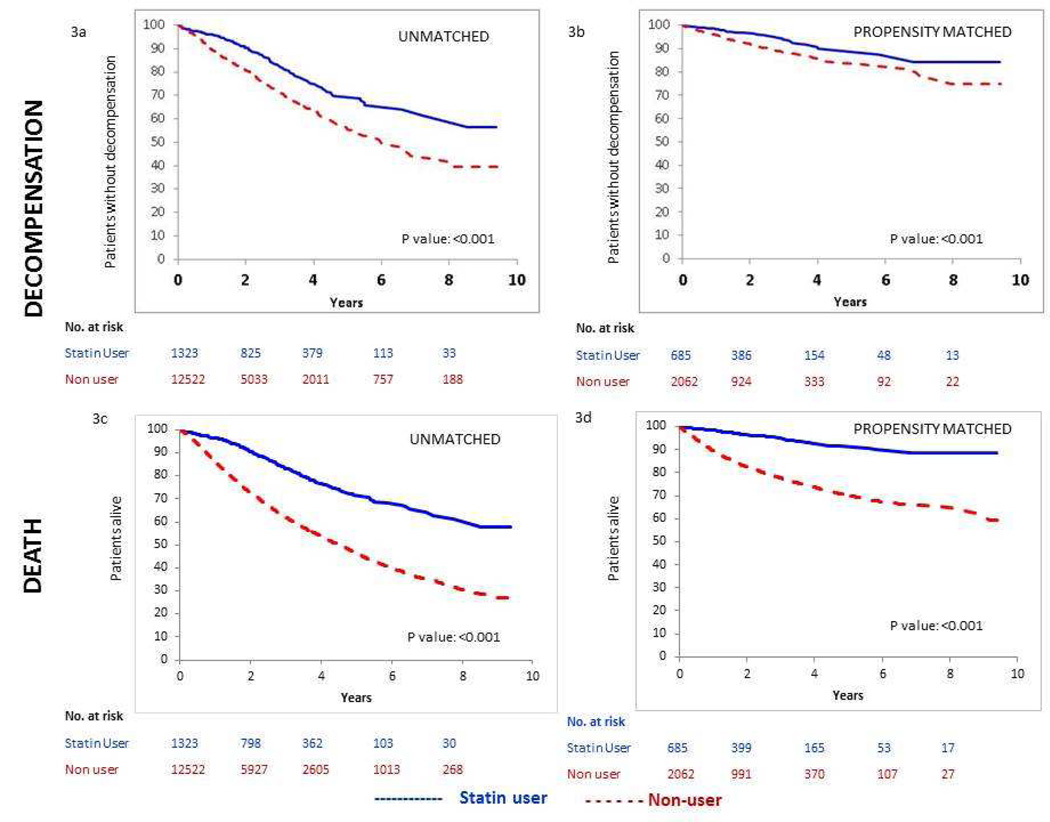
Kaplan-Meier plots for both unmatched and matched cohorts showed an advantage for statin users for both outcomes (decompensation and death) beginning about one year after the start of follow-up that persisted over the full 8 years of available follow-up time (Figure 3). Differences were attenuated in the matched cohort but remained significant, p <0.001 (log-rank test and Wilcoxon test).
Figure 3.
a, b: Kaplan Meier estimates of percentages of patients reaching decompensation for unmatched and propensity matched cohorts
c, d: Kaplan Meier estimates of percentages of patients dying for unmatched and propensity matched cohorts
Sensitivity analyses
All six sensitivity analyses yielded similar results to that of the main analysis in the matched cohort. Compared to non-users, statin users had a lower risk of decompensation and death after 1) excluding patients who received HCV antiviral therapy [HR 0.59 (95% CI 0.41, 0.85)]and [HR 0.56 (95% CI 0.45, 0.69)]; 2) excluding patients who did not have HCV RNA confirmation [HR 0.63 (95% CI 0.43, 0.93)] and [HR 0.58 (95% CI 0.46, 0.73)]; 3) excluding patients with FIB 4 < 1.45 [HR 0.61 (95% CI 0.42, 0.88)] and [HR 0.55 (95% CI 0.44, 0.70)]; 4) excluding 208 statin users with <50% adherence [HR 0.50 (95% CI 0.32, 0.77)] and [HR 0.57 (95% CI 0.45, 0.72)]; 5) excluding 55 statin users whose second fill was 181 to 365 days after the end of the first fill [HR 0.52 (95% CI 0.36, 0.75)] and [HR 0.57 (95% CI 0.46, 0.70)]; and 6) using an alternate (previously published) definition14 for decompensation [HR 0.65 (95% CI 0.47, 0.89)].
Discussion
In this propensity score matched study we demonstrate 40% lower risk of decompensation and death with statin use, in a large cohort of U.S. veterans with compensated HCV cirrhosis. As expected, given appropriate3 matching, multivariable analysis adjusting for predictors of death and decompensation did not alter the results. Unmatched analyses showed overly wide differences, illustrating the importance of properly accounting for confounding by indication.
Analysis of specific decompensating events in our study showed that statins were associated with a significant decrease in the development of ascites and variceal hemorrhage, the two main decompensating events in cirrhosis. Major determinants of decompensation in patients with compensated cirrhosis are the severity of portal hypertension (as determined by hepatic venous pressure gradient ≥10 mmHg) and slight impairment in liver synthetic function (serum albumin <4 g/dL and MELD score >10)2.
Simvastatin has been shown to reduce portal pressure both in experimental animals8, 23 and in patients with cirrhosis9. It does this by ameliorating endothelium-dependent vasorelaxation of the liver vasculature by increasing bioavailability of the vasodilator, nitric oxide7. Intrahepatic vasodilatation also improves flow to the liver and may improve liver function as demonstrated by improved clearance of indocyanine green in patients with cirrhosis9. Commonly used portal pressure- reducing agents such as non-selective beta-blockers lack this effect because they that act by decreasing portal venous inflow, without an effect on hepatic flow. In a recent placebo-controlled study performed in patients with cirrhosis who had recently bled from varices, simvastatin improved survival without an effect on recurrent variceal hemorrhage24, indicating that its beneficial effect may be mostly related to liver flow amelioration and consequent improvement in liver function. Furthermore, it has also been shown that statins may decrease liver fibrosis in experimental cirrhosis and decrease progression of fibrosis in patients with viral hepatitis25.
Statins also have important anti-inflammatory properties in hepatocytes as well as vascular cells (endothelial, smooth muscle, immune cells)26. Statins reduce IL-6 mediated C-reactive protein27, an acute phase reactant, produced mostly in the hepatocytes and marker of poor prognosis in cirrhosis28. Pre-clinical data suggest that simvastatin may attenuate liver inflammation and liver injury associated with infections or bleeding29 and data in humans show that statins are protective in ischemic hepatitis30. Statin use has also been shown to suppress hepatitis C activity in-vitro and in-vivo31, 32, with recent data suggesting that the concomitant use of statins while undergoing antiviral treatment increases the likelihood of sustained virologic response33.
Therefore, our results could be explained by various effects of statins not only in ameliorating mechanisms of portal hypertension but also potentially an anti-inflammatory and antiviral effect, although the latter effect may not be as important since a simvastatin survival benefit has been described in decompensated cirrhosis of mostly an alcoholic etiology24. Furthermore, our results could not be explained on the basis of antiviral therapy since only a minority of patients received pegylated interferon and ribavirin and sensitivity analysis excluding patients receiving antiviral therapy demonstrated the same results as in the overall group.
Ours is the first study evaluating the effect of statins in a homogeneous nationwide cohort of patients with compensated HCV cirrhosis. A previous small single-center study that combined patients with compensated and decompensated cirrhosis and that concluded that statins were not harmful, had shown that statin use was a negative predictor of death10.
In order to emulate a randomized placebo-controlled trial, propensity score matching was performed and resulted in excellent balance between statin users and non-users. This matching corrected for confounding that may have occurred because of less statin use in patients with more severe liver disease or more statin use in patients with a greater cardiovascular risk and potentially higher mortality20. As noted, only statin users with low propensity scores could be matched, thereby excluding those with a higher probability of receiving statins. These patients would have likely been ineligible to participate in a randomized trial as they would have had a strong indication for statin use and it would have been unethical to withhold a statin. Although the cause of death cannot be obtained from this database and the decrease in mortality could be due to a reduction in cardiovascular-related deaths, the fact that patients with high propensity for statin use (i.e. patients with a high risk of dying a cardiovascular-related death) were excluded reduces this possibility. Additionally, decompensation and HCC are important predictors of death in patients with cirrhosis1, 34and as both decompensation and HCC were reduced in this cohort, it seems more likely that the observed decrease in mortality was in fact due to a reduction in decompensation and HCC. Our study confirms evidence in the literature showing a decrease in HCC with statins35, although the mechanisms may be different from those by which statins would decrease decompensation.
We note other limitations as ICD 9 codes were used for diagnoses, misclassification bias is a possibility and patients without cirrhosis could have been included in the study. However, cirrhosis is more likely to be underdiagnosed and even if patients without cirrhosis had been included, one would expect them to be equally distributed between study groups. All patients in this cohort had chronic HCV infection but concomitant etiologies for cirrhosis cannot be excluded as more than 50% had an ICD 9 diagnosis of alcohol dependence and median BMI was in the overweight range. Another limitation is that the study cohort is predominantly male. This may affect the generalizability of the results but does not limit its internal validity
Although statins can be hepatotoxic, this is usually mild and self-limited and their safety has been previously demonstrated in patients with chronic liver disease.36, 37 The beneficial effect of statins in our study, as it relates to decompensation and death, further supports a lack for significant deleterious hepatotoxic effect. Statin prescription in patients with chronic liver disease remains low38 as indicated by the relatively low number of statin users in our cohort, even in those with a high cholesterol (less than half the patients with cholesterol >200 mg/dL received a statin).
This retrospective propensity-matched cohort study performed in veterans with HCV compensated cirrhosis demonstrates the association of statin use with decreased risk of cirrhosis decompensation and death. Results should lead to multicenter prospective placebo-controlled randomized trials in the general non-veteran population that should stratify patients by etiology of cirrhosis. While we cannot yet recommend statin use in all patients with compensated cirrhosis, it is important that practitioners recognize that statins should not be avoided in these patients.
Supplementary Material
Acknowledgments
Funding/support: Yale Liver Center NIH P30 DK34989
Role of the Funder/Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CCR
clinical case registry
- CPT
current procedural terminology
- CTP
Child-Turcotte-Pugh
- HBV
hepatitis B Virus
- HCV
hepatitis C virus
- HDL
high density lipoprotein
- HR
hazards ratio
- HIV
human immunodeficiency virus
- ICD
International Classification of Diseases
- INR
international normalized ratio
- LDL
low density lipoprotein
- MELD
model for end stage liver disease
- VA
Veterans Affairs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Author contributions: Dr. Garcia-Tsao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Study concept and design: Mohanty, Garcia-Tsao
Acquisition, analysis and interpretation of data: All authors
Drafting of manuscript: Mohanty
Critical revision of manuscript for intellectual content: All authors
Statistical analysis: Tate
Study supervision: Garcia-Tsao
Approval of final version of manuscript: All authors
References
- 1.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Ripoll C, Bari K, Garcia-Tsao G. Serum Albumin Can Identify Patients With Compensated Cirrhosis With a Good Prognosis. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Gea V, Aracil C, Colomo A, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol. 2012;107:418–427. doi: 10.1038/ajg.2011.456. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Aracil C, Colomo A, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119–128. doi: 10.1053/j.gastro.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Zafra C, Abraldes JG, Turnes J, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Abraldes JG, Albillos A, Bañares R, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 11.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 12.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 14.Lo Re V, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20:689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 17.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 18.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. discussion 575–7. [DOI] [PubMed] [Google Scholar]
- 19.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 20.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465–476. doi: 10.1002/pds.1062. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer D, Lemeshow S. Applied Logistic Regression. New York: John Wiley and Sons; 2000. [Google Scholar]
- 22.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrone G, Maeso-Díaz R, García-Cardena G, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015 Sep;64(9):1434–1443. doi: 10.1136/gutjnl-2014-308338. [DOI] [PubMed] [Google Scholar]
- 24.Abraldes JG, Villanueva C, Aracil C, et al. Addition of simvastatin to standard treatment improves survival after variceal bleeding in patients with cirrhosis. A double-blind randomized trial ( NCT01095185). J Hepatol. 2014;60:S525. [Google Scholar]
- 25.Simon TG, King LY, Zheng H, et al. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–1236. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen C, Andersen O, Krag A, et al. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:619–626. doi: 10.1097/MEG.0b013e328351db6e. [DOI] [PubMed] [Google Scholar]
- 29.La Mura V, Pasarín M, Meireles CZ, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology. 2013;57:1172–1181. doi: 10.1002/hep.26127. [DOI] [PubMed] [Google Scholar]
- 30.Drolz A, Horvatits T, Michl B, et al. Statin therapy is associated with reduced incidence of hypoxic hepatitis in critically ill patients. J Hepatol. 2014;60:1187–1193. doi: 10.1016/j.jhep.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Bader T, Fazili J, Madhoun M, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 32.Delang L, Paeshuyse J, Vliegen I, et al. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology. 2009;50:6–16. doi: 10.1002/hep.22916. [DOI] [PubMed] [Google Scholar]
- 33.Butt AA, Yan P, Bonilla H, et al. Effect of addition of statins to antiviral therapy in HCV infected persons: Results from ERCHIVES. Hepatology. 2015 doi: 10.1002/hep.27835. [DOI] [PubMed] [Google Scholar]
- 34.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Singh PP, Singh AG, et al. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 37.Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4:902–907. doi: 10.1016/j.cgh.2006.03.014. quiz 806. [DOI] [PubMed] [Google Scholar]
- 38.Rzouq FS, Volk ML, Hatoum HH, et al. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340:89–93. doi: 10.1097/MAJ.0b013e3181e15da8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.