Abstract
The identification of immunosuppressive factors within human tumor microenvironments, and the ability to block these factors, would be expected to enhance patients’ anti-tumor immune responses. We previously established that an unidentified factor, or factors, present in ovarian tumor ascites fluids reversibly inhibited the activation of T cells by arresting the T cell signaling cascade. Ultracentrifugation of the tumor ascites fluid has now revealed a pellet that contains small extracellular vesicles (EV) with an average diameter of 80nm. The T cell arrest was determined to be causally linked to phosphatidylserine (PS) that is present on the outer leaflet of the vesicle bilayer, as a depletion of PS expressing EV or a blockade of PS with anti-PS antibody significantly inhibits the vesicle induced signaling arrest. The inhibitory EV were also isolated from solid tumor tissues. The presence of immune suppressive vesicles in the microenvironments of ovarian tumors and our ability to block their inhibition of T cell function represent a potential therapeutic target for patients with ovarian cancer.
Keywords: Immune suppressive extracellular vesicles, T cells, Human ovarian cancer, diacylglycerol kinase, phosphatidylserine
Introduction
Recent successes with clinical trials designed to enhance immune killing of tumors have led to an increased optimism and focus upon immunotherapy for cancer (1). For example, positive therapeutic effects in some cancer patients have been reported with the use of antibodies that block the inhibitory effects of PD-1/PDL-1 (2, 3) and CTLA-4 (4). Also, dramatic and durable tumor reductions have been seen in some patients with hematological malignancies with the adoptive transfer of tumor-specific chimeric antigen receptor (CAR) T cells (5, 6). While these results are highly encouraging, many patients fail to exhibit significant reductions in tumor or tumor reductions are only short-lived. Ultimately the success of these and other immunological approaches to the treatment of cancer will depend on a more comprehensive understanding of multiple complex cellular and molecular mechanisms that contribute to the inhibition of anti-tumor immune responses (7).
T cells present within tumor microenvironments have been found to be hyporesponsive to activation via the T cell receptor (8–12). And it has been reported that human T cells derived from peripheral blood (13) and tumor-specific CAR T cells (14) become functionally arrested following their entry into human tumor xenografts. Several immunological checkpoints have been identified that may contribute to the loss of function of tumor-associated T cells (15). Investigations into such checkpoints have focused largely upon those that are mediated by T regulatory cells, myeloid derived suppressor cells, immunosuppressive dendritic cells, immunoinhibitory receptors, and inhibitory factors including TGFβ, prostaglandins, and adenosine (16). The mechanisms by which these different checkpoints suppress T cell function in human cancer have not yet been completely determined. It is possible that some checkpoints are mediated by acellular factors that act directly upon T cells to induce a functional arrest.
We previously reported that activation of T cells derived from ovarian solid tumor tissues or patient tumor ascites fluids is rapidly and reversibly arrested by cell-free ovarian tumor ascites fluids (17). Tumor ascites fluids also re-establish anergy in ovarian tumor-associated T cells in which the T cell receptor (TCR) signaling arrest had been reversed (17). It was determined that the tumor ascites fluids blocked the activation of both NFκB and NFAT in T cells receiving a TCR activation signal, and that the signaling arrest occurred at or just proximal to PLC-γ. Based upon these earlier results we concluded that a factor or factors present in tumor ascites fluids may represent yet another T cell checkpoint. However, the factors present in the tumor ascites fluids responsible for the induction of the TCR signaling arrest were not identified (17).
We have now determined and report here that the T cell inhibitory activity of tumor ascites fluids is mediated by very small, 50–120nm, extracellular vesicles (EV) derived from ovarian tumor ascites fluids and from solid ovarian tumors, and that the TCR signaling arrest is dependent, in part, on a phospholipid, phosphatidylserine, that is expressed on the surface of the lipid bilayer that surrounds the EV. We propose and present evidence that is consistent with a mechanism for the EV-induced TCR signaling arrest and we suggest that EV present within ovarian tumor microenvironments represent a novel T cell checkpoint and a potentially viable therapeutic target.
Materials and Methods
Specimens
Ovarian tumor ascites fluids were received from the Roswell Park Cancer Institute (RPCI) Tissue Procurement Facility. Experiments were done using ascites fluids that had been stored at −80°C before being thawed. Fresh ascites and EV isolated from the unfrozen fluids have also been tested and shown to have no difference in their inhibitory activity. All ascites fluids were depleted of cells by centrifugation and passed through a 0.22µm Millipore filter. Normal donor peripheral blood was provided by the Flow and Image Cytometry Facility at RPCI. Normal donor peripheral blood lymphocytes (NDPBL) were obtained by monocyte depletion and Ficoll-Hypaque density separation. Ascites fluids were frozen and stored at −80°C and cells were frozen and stored in liquid nitrogen until use, as previously reported (8, 17). All specimens were obtained under sterile conditions and using IRB approved protocols.
Delipidation of tumor ascites fluid
The tumor ascites fluid was treated with 800µg/mL pronase (EMD Millipore, Billerica, MA; USA) overnight at room temperature. The reaction was stopped by putting into a boiling water bath for 10min. The ascites fluid was then added to 2 volumes of methanol:diethyl ether (20:80 v/v) in an extraction funnel. The funnel was rotated end over end at 30rpm for 2hr, and then centrifuged at 1500rpm for 2min to separate the aqueous and organic phases. The aqueous phase was isolated by careful suction with a needle and syringe and the residual methanol was washed out with 2 volumes of Diethyl ether. The aqueous phase was then placed in a rotavap at 37°C for 5–10min to fully remove the residual organics.
Isolation and quantification of extracellular vesicles (EV)
Undiluted cell supernatant fluids from patient derived solid tumors or 50% diluted ascites fluids (with RPMI 1640 + 2% HSA) were ultracentrifuged at 200,000×g for 90min. The pellets were resuspended in RPMI 1640 + 1% HSA. Protein quantification of EV suspended in HBSS was determined by BCA assay (18).
Immunofluorescence staining of human T cells for CD4+ and CD8+ for isolation by flow cytometry
Human NDPBL were thawed in complete medium (RPMI 1640 + heat inactivated 10% fetal bovine serum). The cells (8 × 107 cells) were brought up in 1× PBS and added to a sterile 12 × 75 polystyrene capped tube. The cells were pelleted and then resuspended in the residual fluid. The cells were blocked with NMIgG (50µL of 3mg/mL stock) for 10 minutes and then stained with 100µL anti-human CD4-APC and 100µL anti-human CD8-PE (BD Biosciences, San Jose, CA; USA) for 20 minutes. The cells were washed with 1× PBS and brought up to 2 × 107 cells/mL in 1× PBS for isolation by flow cytometry using a FACS Aria IIU cell sorter (BD Biosciences, San Jose, CA). After the sort, the cells were allowed to recover overnight at 37°C, 5% CO2 in complete medium.
T cell activation
Human NDPBL or sorted T cells were activated for 2 hrs at 37°C with immobilized anti-huCD3/CD28 with or without liposomes, anti-PS antibody, 25µM DGK Inhibitors I [R59022] and II [R59949] (Santa Cruz Biotechnology Inc., Dallas, TX; USA), 50% tumor ascites fluid, 50% delipidated ovarian ascites fluid, or exosomes derived from tumor ascites fluids or solid ovarian tumors. When the effect of anti-PS antibody on EV inhibition was determined, the EV were incubated with 10 µg/ml of clone 1H6 anti-PS antibody (Upstate - Cell Signaling Solutions, Lake Placid, NY; USA) for 1 hr at 37°C and diluted to 1× for the activation step. This antibody has been shown to be specific for PS and did not show cross-reactivity with other phospholipids (19)PS on the EV was also blocked using 10ug/ml of Annexin V (BD Biosciences San Jose, CA). The percentage of activated T cells was determined by monitoring the translocation of NFκB from the cytosol into the nucleus using fluorescence confocal microscopy as previously reported (17).
Immunofluorescence staining of human T cells for confocal imaging
After activation, cells were attached to alcian blue coverslips in a humid chamber (10min) and fixed in 2% Formaldehyde in 1× PBS (40min), the cells were permeablized and blocked with 30µg NMIgG in 5% normal mouse serum in 1× PBS + 0.4% Triton X-100. The cells were then stained for intracellular CD3 (mouse anti-human CD3-Alexa Fluor 647, BD Biosciences) for 20 minutes. After washing once with NGS block (5% normal goat serum in 1× PBS), the cells were blocked with 30µg NGIgG in NGS + 0.4% Triton X-100 (NGS block/perm) for 10 minutes. After blotting off the NGIgG, the cells were incubated with 2µg/mL purified rabbit anti-human NFκB p65 (Santa Cruz Biotechnology, Inc.) in NGS block/perm for 1 hour. After washing twice with NGS block, the cells were incubated with 2µg/mL goat anti-rabbit IgG-Alexa Fluor 488 (Life Technologies, Grand Island, NY; USA) in 100µL NGS block/perm for 30 minutes. The cells were washed twice with NGS block and twice with 1× PBS before mounting the coverslips on glass slides with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA; USA). Cells were then observed on a Zeiss LSM 510 Confocal Microscope with at least 100 CD3+ cells counted per condition.
Immunophenotyping of extracellular vesicles
An 18-parameter LSR Fortessa (BD Biosciences) was employed to measure phenotypic characteristics of ascites-derived EV and engineered liposomes. The cytometer was mechanically and electronically adapted in order to optimize the detection of submicron particles (See Supplemental Methods).
For the immunophenotyping of EV, PE-Annexin V labeled or unlabeled EV were separately incubated with saturating amounts of BV421-conjugated isotype control, BV421-conjugated anti-CD90, BV421-conjugated anti-CD326, or BV510-conjugated anti-CD45, BV421-conjugated anti-CD41, BV510-conjugated anti-CD71, FITC-conjugated anti-CD235a (all from BD Biosciences), or PE-conjugated MHC Class I (Caltag) for 30 minutes at room temperature in the dark.
For the analysis of flow cytometric data, WinList software (version 7.1.1; Verity Software House) was employed, with no gating strategy applied to the raw data files. Background fluorescence intensity was measured from a sample that contained microparticles which were not labeled with fluorescently-conjugated reagents. Events that were brighter than this background level of fluorescence intensity were considered to be positive for the marker of interest.
Depletion of PS expressing EV
50 µg of anti-PS antibody (Upstate - Cell Signaling Solutions) or isotype control (mouse IgG, Caltag) was conjugated to 5 mg Dynabeads M-280 Tosylactivated (Life Technologies) according to manufacturer’s instructions. The conjugated beads were incubated with 0.34mg of ovarian tumor ascites fluid derived EV with tilting and rotation for 1 hour at 4°C to capture PS expressing EV. The unbound EV (not expressing PS) were separated from the EV-bead complex using a magnet (BD Biosciences).
Preparation of PS liposomes
The PS containing liposomes were prepared using thin film method as described previously (20). Briefly, chloroform solutions of phosphatidylcholine (PC) and brain phosphatidylserine (PS) were mixed in a molar ratio of 70:30 in a glass tube and the solvent was evaporated using a roto evaporator. The thin film thus obtained was further flushed with nitrogen to ensure that there was no residual solvent. The dried film was then re-suspended with 1× PBS and incubated at 37 °C for 5 minutes to form multi lamellar vesicles (MLVs). The vesicles were sonicated for 5 minutes and were then extruded using 80 nm poly carbonate filter multiple times to form small unilamellar vesicles (ULVs).
Statistics
All statistics were calculated using SigmaPlot 12 (Systat Software, San Jose, CA; USA). Differences between groups were considered significant if p<0.05.
Results
Inhibitory factor present in ovarian tumor ascites fluids is a lipid
We previously established that T cells present in ovarian tumor ascites fluids were hyporesponsive to activation and that this was due to an unidentified factor or factors present in the tumor ascites fluid (17). The ascites fluids also induced a rapid and reversible arrest in the T cell signaling cascade that occurred downstream of diacylglycerol (17). It was determined that the inhibitory activity in the tumor ascites fluid was heat stable (30 minutes in a boiling water bath) and resistant to proteolytic digestion with pronase. Based on these findings, we considered that lipids may contribute to the TCR signaling arrest.
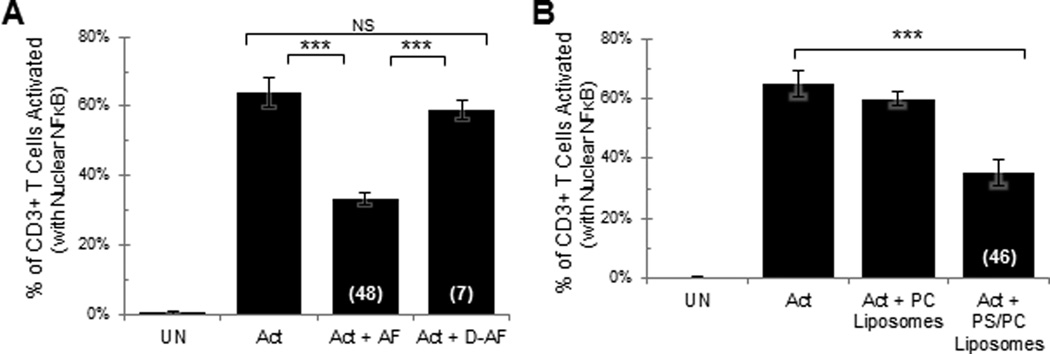
Ovarian tumor ascites fluids were pronase-treated, boiled, and delipidated by extracting with diethyl ether. As expected, the vast majority of lipid material was retained in the organic fraction as visualized by multiple bands (Supplemental Fig. 1). The aqueous fraction retained only a small quantity of a single band, which was tentatively identified as a water-soluble disialoganglioside. The aqueous (delipidated) phase was assayed for its ability to inhibit the induction of the T cell signaling arrest. The activation of T cells in a mixed population of peripheral blood leukocytes following their incubation with immobilized anti-CD3 and CD28 was monitored by immunofluorescence confocal microscopy for the translocation of NFκB into the nucleus. T cell activation (translocation of NFκB) was significantly inhibited by the tumor ascites fluid prior to delipidation, but this inhibition was largely eliminated in the delipidated tumor ascites fluid (Fig. 1A).
Fig 1. Lipids present in tumor ascites fluid and phosphatidylserine (PS) containing liposomes induce T cell signaling arrest.
Human normal donor peripheral blood lymphocytes (NDPBL) were incubated in medium only (UN) or with immobilized anti-CD3/CD28 in medium only (Act), A. in medium with 50% ovarian tumor ascites fluid (Act + AF), or in medium with the aqueous phase of delipidated 50% ovarian tumor ascites fluid (Act + D-AF) (n=4, mean ± SEM) B. in medium with PC liposomes or with PS/PC liposomes (n=7, mean ± SEM). Activation was monitored by confocal microscopy to quantify the percentage of CD3+ T cells with nuclear NFκB. The numbers in parentheses indicate percent inhibition. NS = Not Significant; * = p<0.05; ** = p<0.01; *** = p<0.005 – Same for all figures.
To identify possible lipids that could be responsible for the T cell signaling arrest, tumor ascites fluids were extracted with hexane and isopropyl alcohol and the lipids present in the organic phase analyzed and identified by thin layer chromatography (TLC). We identified both non-polar lipids (cholesterol esters, triglycerides, and free fatty acids) and polar lipids. A two dimensional TLC analysis of the polar lipids revealed the presence of the following phospholipids: lysophophatidylcholine (LPC), sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylinositol (PI), cardiolipin (CL) and phosphatidylserine (PS).
Phosphatidylserine (PS) induces a signaling arrest in T cells
PS is known to play important roles in many biological processes resulting in anti-inflammatory effects. For example, exposure of PS on the outer leaflet of apoptotic lymphocytes induces their phagocytosis by macrophages leading to an active suppression of their inflammatory mediators (21). We have shown that a normally immunogenic protein is converted into a tolerogen when used together with PS (20). Others have reported that PS significantly inhibits adaptive immune responses by a variety of different (as of yet poorly defined) mechanisms (22). In view of these findings, PS was tested for its ability to inhibit T cell activation.
Because of the relative insolubility of PS in water, we began by formulating PS containing liposomes. As the liposomes formulated with 100% PS aggregated in the cation-containing buffers required for our activation experiments, we prepared liposomes formulated with 30% PS and 70% PC that we found remained in suspension in the calcium containing activation buffer. Control liposomes were formulated with 100% PC and did not aggregate in the activation buffer. Peripheral blood T cells were activated in the presence of either the PS/PC or PC only liposomes. As shown in Figure 1B, the PS/PC liposomes significantly inhibited T cell activation while PC only liposomes failed to inhibit the activation. Also, it was established that the PS/PC liposomes inhibited T cell activation in a dose dependent fashion (Supplemental Fig. 2). We conclude that PS has the capacity to induce the same signature TCR signaling arrest that we have observed previously with tumor ascites fluids (17).
Tumor ascites fluid acts directly upon CD4+ and CD8+ T cells to induce the TCR signaling arrest
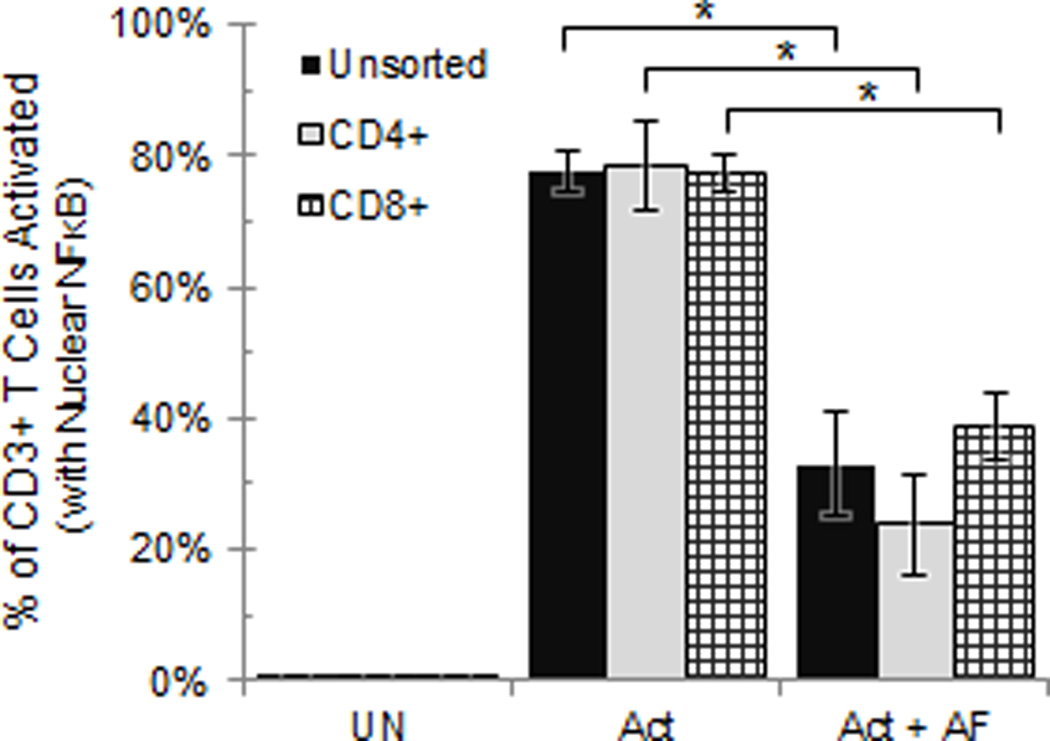
Because our evaluations of T cell activation were made on mixed populations of leukocytes, it was possible that the T cell signaling arrest was mediated by other cell types. To address this question, CD4+ and CD8+ cells were isolated from normal donor peripheral blood lymphocytes (NDPBL) and sorted by flow cytometry (Supplemental Fig. 3). It was determined that the tumor ascites fluid inhibited the isolated CD4+ and CD8+ T cells to the same degree as that which was observed with the unsorted PBL (Fig. 2). Because no other cells were present in the sorted population, we conclude that the tumor ascites fluids directly inhibit TCR signaling of both CD4+ and CD8+ T cells.
Fig 2. Tumor ascites fluid directly inhibits CD4+ and CD8+ T cell activation.
NDPBL sorted for CD4+ or CD8+ were incubated in media only (UN), with immobilized anti-CD3/CD28 in medium only (Act), or in medium with 50% ascites fluid (Act + AF) (n=3, mean ± SEM). * = p<0.05. See also Figure S2.
Small 50–120nm extracellular vesicles are present in ovarian tumor ascites fluids
The presence of PS in the tumor ascites fluids, and the ability of PS containing liposomes to induce the signature TCR signaling arrest, led us to explore the possibility that PS was one of the inhibitory factors present in the tumor ascites and that it was present in lipoprotein complexes, micelles, large extracellular vesicles (apoptotic bodies), uni- or multi-lamellar liposome like bodies, or very small extracellular microvesicles (possibly exosomes). If it were present within extracellular vesicles (large or small) one would expect to be able to recover them from the pellet following the ultracentrifugation of the tumor ascites fluids that had been subjected to low-speed centrifugation and 0.2µm filtration.
Following ultracentrifugation of these tumor ascites fluids, an examination of the resultant pellet by quasi-elastic light scatter analysis revealed the presence of homogenous (by size and lamellarity) extracellular vesicles (EV) with an average diameter of 80nm (Supplemental Fig. 4A). A broad phase transition from solid to fluid phase, centered around 37°C, was observed (Supplemental Fig. 4B) indicating that the EV were surrounded by a lipid bilayer. The size and lipid bilayer composition of the isolated vesicles were confirmed by transmission electron microscopy (Supplemental Fig. 4A - insert).
Extracellular vesicles isolated from ovarian tumor ascites fluids induce the signature TCR signaling arrest in peripheral blood T cells
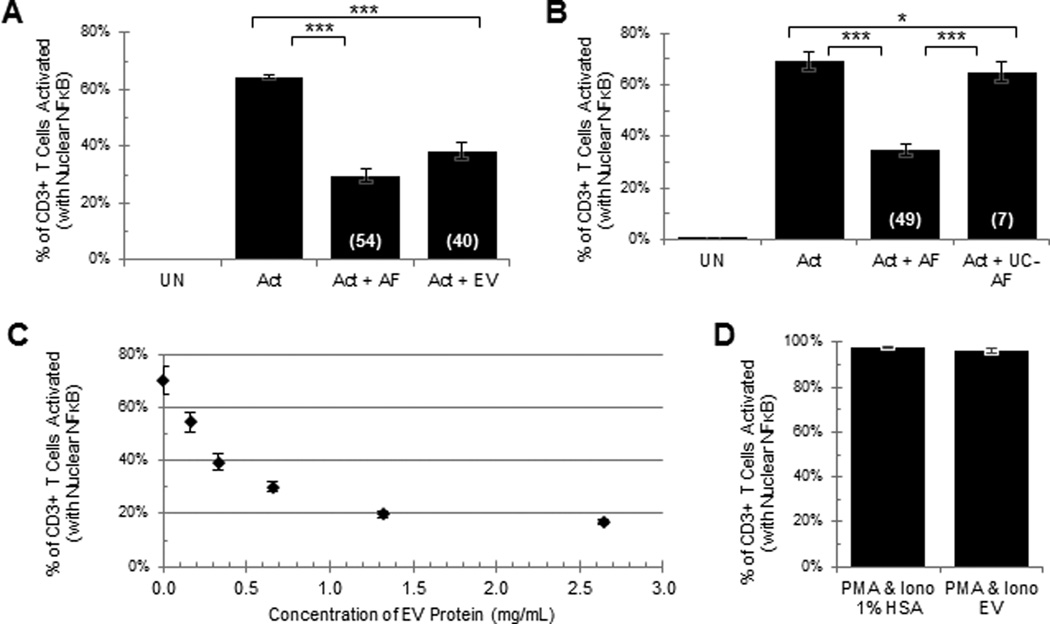
As shown in Fig. 3A, a two-fold dilution of the EV in the resuspended pellet (equivalent to 50% of the tumor ascites fluid) inhibited the activation of the T cells, slightly less than that is observed in 50% tumor ascites fluid. The EV-induced inhibition has been repeated 8 times with EV derived from different tumor ascites fluids and found to induce significant inhibition of T cell activation. The readout for T cell activation reported in Figs. 1–6 is the translocation of NFκB from the cytosol into the nucleus. We have seen very similar ascites and EV induced inhibition of T cell activation using other readout systems including the up-regulation of CD69 (supplemental Fig. 5), up-regulation of CD25 (data not shown) and a translocation of CD107a from the cytosol to the plasma membrane (data not shown). We have consistently observed, with the ultracentrifugation of several different tumor ascites fluids, that the inhibitory activity in the supernatant fluids was significantly reduced or completely eliminated (Fig. 3B). As shown in Fig. 3C, the EV inhibited the T cell signaling in a dose dependent fashion. We further establish that the EV, like the tumor ascites fluids, are acting directly on CD4+ and CD8+ T cells to arrest the T cell signaling (Supplemental Fig 6) and that EV do not inhibit the activation of the T cells with PMA and Ionomycin (Fig. 3D). This is consistent with the possibility that the EV are acting at or just upstream of diacylglycerol. As we have previously established with the tumor ascites fluids (17), the inhibition of the T cell signaling is reversible because T cells exposed to EV fully recover their activation potential following an overnight incubation without EV (data not shown).
Fig 3. Extracellular vesicles derived from ovarian tumor ascites fluid by ultracentrifugation inhibit the activation of T cells in a dose dependent manner.
NDPBL were incubated in medium only (UN) or with immobilized anti-CD3/CD28 in medium only (Act), in medium with 50% ovarian tumor ascites fluid (Act + AF), A. in medium with extracellular vesicles derived from 50% ovarian tumor ascites fluid (Act + EV) (n=8, mean ± SEM), B. in medium with the supernatant of 50% ovarian tumor ascites fluid after ultracentrifugation (Act + UC-AF) (n=11, mean ± SEM). C. NDPBL were incubated with increasing concentrations of extracellular vesicles based on EV protein weight (n=3, mean ± SEM). D. NDPBL were incubated with PMA and Ionomycin in the presence or absence of extracellular vesicles (n=8, mean ± SEM).
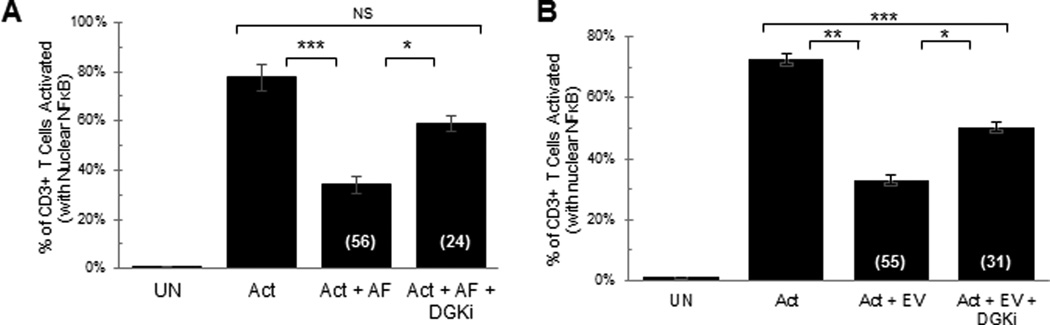
Fig 6. Inhibition of diacylglycerol kinase reverses the tumor ascites fluid- and extracellular vesicle-induced T cell signaling arrest.
NDPBL were incubated in medium only (UN) or with immobilized anti-CD3/CD28 in media only (Act), A. in media containing 25% ovarian ascites fluid with (Act + AF + DGKi) or without (Act + AF) 100µM DGK inhibitors (n=3, mean ± SEM), B. in ascites fluid-derived extracellular vesicles with or without each of the DGK inhibitors. (n=3, mean ± SEM). [DGK inhibitors used are a combination of R59949 and R59022].
PS is expressed on the surface of the tumor-associated extracellular vesicles
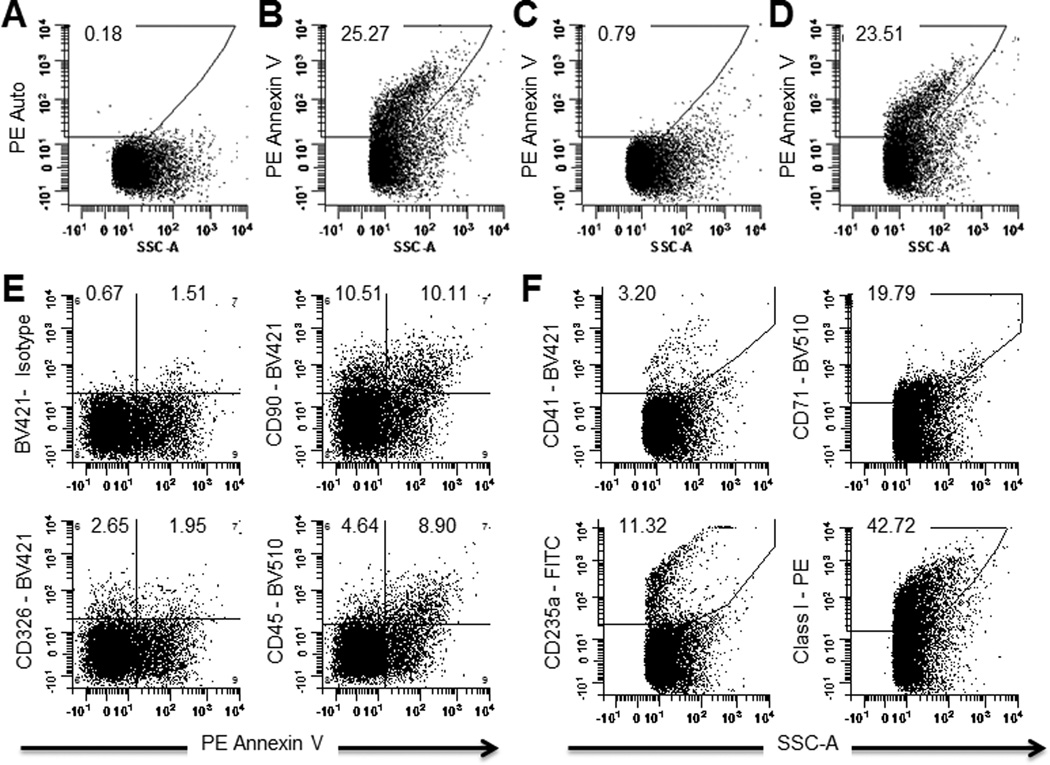
EV isolated from ovarian tumor ascites fluid were stained with fluorescently labeled Annexin V and assayed using an LSR Fortessa flow cytometer that was configured to resolve and quantify the very small EV for evidence of Annexin V binding. A portion (23–26%) of the EV bound the fluorescently labeled Annexin V (Fig. 4B). The binding was shown to be specific, as it was completely inhibited by unlabeled Annexin V, but was not inhibited by bovine serum albumin (Fig. 4C and D).
Fig 4. Flow cytometric analysis demonstrates that extracellular vesicles derived from ascites fluids bind to fluorescently labeled Annexin V.
A. Unlabeled EV. B. EV labeled with R-phycoerythrin (PE) conjugated Annexin V (PE-Annexin V). C. EV labeled with PE-Annexin V, competed with 100× unlabeled Annexin V. D. EV labeled with PE-Annexin V, competed with 100× unlabeled bovine serum albumin. E. EV stained with PE-Annexin V and labeled CD90 (Endothelial cell marker), CD326 (EpCAM), or CD45 (Leukocyte marker) antibodies. F. EV stained with labeled CD41 (platelet marker), CD71 (transferin), CD235 (erythrocyte marker), or MHC Class I antibodies.
Extracellular vesicles are derived from tumor cells and tumor-associated fibroblasts, leukocytes, and erythrocytes
Phenotypic analysis with fluorescently labeled antibodies identified EV derived from ovarian tumor ascites fluid that expressed a tumor marker (CD326; EpCAM), a fibroblast marker (CD90), a leukocyte marker (CD45), and a portion of each of these microvesicles also expressed PS on the surface (by binding Annexin V) (Fig. 4E). Other cell markers found on the EV surfaces include an erythrocyte marker (CD235a; glycophorin), a marker of most nucleated cells (Class I MHC), a platelet marker (CD41), and a marker for proliferating cells that could include both tumor cells and tumor-associated cells (CD71; transferrin receptor) (Fig. 4F).
Inhibitory activity of extracellular vesicles derived from ovarian tumor ascites fluids and from solid ovarian tumor tissues is blocked by anti-PS antibody and by a depletion of the PS expressing subset of EV
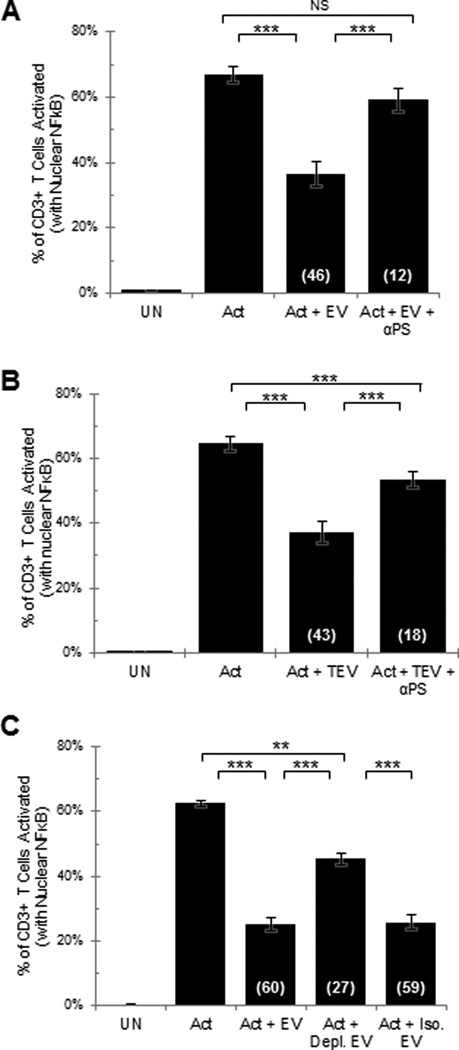
As PS containing liposomes were found to inhibit T cell activation (Fig. 1B) and because a portion of the EV express PS on their surface (Fig. 4A–D), we predicted that PS plays a significant role in the EV-induced signaling arrest of T cells. Consistent with this prediction, it was determined that the inhibitory activity that is observed with EV derived from ovarian tumor ascites fluids was blocked by anti-PS antibody (Fig. 5A). Another approach to blocking PS on the EV was to incubate EV with Annexin V. It was established in three separate experiments that Annexin V blocked the EV induced T cell activation inhibition by 52 +/− 8.8%.
Fig 5. Antibodies to phosphatidylserine block the extracellular vesicle induced T cell signaling arrest.
NDPBL were incubated in medium only (UN) or with immobilized anti-CD3/CD28 in media only (Act), A. in media containing extracellular vesicles derived from 50% ovarian tumor ascites fluid with (Act + EV + αPS) or without (Act + EV) phosphatidylserine antibody (n=3, mean ± SEM), B. in extracellular vesicles derived from solid ovarian tumor with (Act + TEV + αPS) or without phosphatidylserine antibody (Act + TEV) (n=3, mean ± SEM). C. Human NDPBL were incubated in medium only (UN) or with immobilized antibodies to CD3 and CD28 in media only (Act) or in media containing extracellular vesicles derived from 50% ovarian tumor ascites fluid without (Act+ EV) or with depletion using anti-phosphatidylserine antibody coupled magnetic beads (Act + Depl. EV) or in a negative control depletion using isotype labeled magnetic beads (Act + Iso. EV) (n=3, mean ± SEM).
Because T cells derived from solid tumor tissues, like those isolated from the tumor ascites fluids, have been shown to have a reversible TCR signaling arrest (8, 11), we further predicted that inhibitory EV would be present within solid tumor microenvironments. To address this possibility, tissues derived from solid ovarian tissues were mechanically disrupted into viable cell suspensions and the tissue suspensions subjected to the same isolation protocol that was used to isolate EV from ovarian tumor ascites fluid. We determined that EV were present in the ultracentrifuged pellet, and that these EV derived from the solid tumors similarly inhibited the activation of T cells (Fig. 5B). And as we found with the ascites fluid-derived EV, the inhibitory activity induced by the solid tumor-derived EV was also blocked by the addition of anti-PS antibody (Fig. 5B).
These data establish that tumor-associated immunoinhibitory EV exist in both fluid and solid ovarian tumor microenvironments. The blockade of the inhibitory activity with anti-PS antibody suggests that PS is causally linked to the microvesicle-induced T cell signaling arrest. Consistent with this possibility, we determined that the depletion of the PS expressing subset of EV using anti-PS antibody coated magnetic beads resulted in the loss of the EV-induced inhibition of T cell function (Fig. 5C).
Inhibition of diacylglycerol kinase (DGK) blocks the T cell inhibitory effect of tumor ascites fluids and tumor-associated extracellular vesicles
Our discovery that the tumor ascites fluid-induced TCR signaling arrest (17) and the inhibition with EV (Fig. 3D) are overcome by the addition of a diacylglycerol (DAG) analog (PMA) (17) and the findings of others that the inactivation of DAG is observed in anergic T cells (23) suggest that EV interacting with T cells may lead to an inactivation of DAG. The inactivation of DAG has been reported to occur in anergic T cells resulting from a phosphorylation of DAG into the inactive phosphatidic acid, a reaction that is mediated by diacylglycerol kinases (DGK) (24). We hypothesized that the inhibitory activity of tumor ascites fluids and EV on fully functional T cells derived from normal donor PBL was mediated by the same or similar mechanism that is observed in anergic T cells. Consistent with this hypothesis, we found that two diacylglycerol kinase inhibitors (DGKi) [R59949 and R59022] reverse the tumor ascites-induced inhibition of the TCR signaling arrest (Fig. 6A) and significantly blocked the EV-induced inhibitory activity (Fig. 6B).
We conclude that the induction of the TCR signaling arrest by PS present in tumor ascites fluids and PS expressing EV derived from the tumor ascites fluids results from an inactivation of DAG that is mediated by DGK.
Discussion
The release of very small extracellular vesicles by viable tumor cells was initially reported over three decades ago (25). We subsequently reported that extracellular vesicles are released from normal as well as neoplastic cells and characterized these vesicles ultrastructurally and biochemically (26). We report here that EV present in the ascites fluids and solid tumors of ovarian cancer patients induce a rapid and reversible arrest in the T cell receptor signaling cascade of CD4+ and CD8+ T cells that is dependent in part on PS. And our data support a likely mechanism by which this signaling arrest is achieved.
The lamellarity, nanometer size, and presence of PS on the outer leaflet of the EV that we report here are similar to that which have been reported for extracellular vesicles called exosomes isolated from bodily fluids (27). However, further characterization of our EV will be needed to determine if they conform to the current definition of exosomes as defined by the International Society for Extracellular Vesicles (27). Others have reported finding PS+ exosomes in ovarian cancer patients’ tumor ascites fluids and blood and have suggested that the exosomes may play a role in tumor progression (28).
Previous reports have suggested that extracellular vesicles isolated from tumor microenvironments suppress antitumor responses indirectly by augmenting the function or preventing the apoptosis of T regulatory cells, generating myeloid derived suppressor cells, and blocking the maturation of dendritic cells and macrophages (29–33). However, our findings establish that the EV derived from ovarian tumor ascites fluids and solid tumors are acting directly upon T cells to arrest their function. It has been postulated by others that tumor extracellular vesicles/exosomes may modulate lymphocyte function directly by mimicking activation-induced cell death (34, 35) by the induction of apoptosis resulting from suppression of CD3-ζ chain, or through the expression of apoptosis inducing ligands such as FasL, PDL and TRAIL on the vesicles’/exosomes’ surface (36). However, all of these suggested mechanisms would result in a rather slow and non-reversible inhibition of T cell function. We present evidence that is consistent with a mechanism that is compatible with the initial rapid and reversible T cell inhibition that we observe with the EV. While the T cell inhibition that we have observed here in vitro with EV is reversible, it is possible that T cells that may be chronically exposed to EV in vivo could ultimately become irreversibly inhibited. It has been suggested that the T cell exhaustion that arises in chronic infections and cancer progresses from a reversible to a non-reversible T cell arrest that coincides with the accumulation of the number and different types of checkpoint molecules on the cell surface (37).
The findings that the inhibition of diacylglycerol kinase (DGK) completely blocks the tumor ascites fluid-induced TCR signaling arrest and partially blocks the EV-induced T cell inhibition are consistent with the notion that the arrest in the activation of T cells is mediated by a DGK phosphorylation of diacylglycerol (DAG) converting it into the inactive phosphatidic acid. This mechanism accounts for both the rapid and reversible inhibition seen with the ascites fluid and the EV derived from ascites fluids, and for our finding that a diacylglycerol analog (PMA) bypasses the inhibition induced by tumor ascites fluid (17) and by the EV derived from ascites fluids (Fig. 3D). The regulation of DAG by DGK has been shown by others to be critical in determining whether activation or anergy ensues after T cell receptor stimulation (23, 24, 38, 39). The finding that an antibody to PS significantly blocks the inhibitory activity of both tumor ascites fluid and the tumor-associated EV establishes a role for this phospholipid in the inhibitory process. This causal link of the inhibitory activity of tumor-associated EV to PS was further documented by our finding that the depletion of the PS positive EV significantly reduced the inhibitory activity of the EV. Others have reported that PS enhances the metabolic activity of DGK (40). Thus PS on the tumor-associated EV may work in a similar fashion to enhance DGK activity resulting in the T cell receptor signaling arrest.
The ability of PS liposomes to induce the TCR signaling arrest suggests that PS, itself, has the capacity to modulate the T cell function. This is significant as others have suggested that PS may participate only as a way for extracellular vesicles (exosomes) to bind to and deliver their immune regulatory molecules to PS binding target cells (28, 41). The presence of PS on the surface of the EV suggests that the inhibitory process may begin with the binding of PS expressing EV to a PS receptor on T cells. The binding of PS by T cells to a PS receptor has been previously reported by others and shown to result in the inhibition of immune responses in vivo (22). An immunomodulatory capability of PS to convert a known immunogen into a tolerogen has been reported (20).
The presence of the immunosuppressive EV in both tumor ascites fluid and solid ovarian tumor tissues suggest that they may contribute to the hyporesponsiveness of tumor-associated T cells that has been previously reported (8, 10–12, 17, 42, 43). The anergy and hyporesponsiveness of T cells present in ovarian tumor microenvironments is known to be reversed when these cells are removed from the tumor microenvironment (17). The ability to block or reverse the EV-induced T cell arrest with anti-PS antibodies or with diacylglycerol kinase inhibitors (DGKi) represents two potential approaches that could be exploited therapeutically to enhance patients’ T cell responses to their tumor.
A report showing that regulatory T cells secrete microvesicles/exosomes that are capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma (44), represents an elegant and intriguing report on the growing inventory of examples of tumor-associated EV that regulate anti-tumor immune responses. We recognize that lipids other than PS have been shown to modulate T cell function and immunity. Polyunsaturated fatty acids (PUFA) were shown to directly modify T cell signaling proteins (45) and to inhibit T cell signaling by displacing Lck, Fyn, and LAT from detergent resistant to detergent sensitive fractions (46, 47). Elevated levels of gangliosides from renal cell carcinoma patients have been linked to tumor-associated T cell dysfunction possibly by inducing apoptosis (48, 49) or by promoting immune deviation in favor of Th2 T cell responses (50). In view of our inability to completely block the EV-induced inhibition of T cells signaling with antibodies to PS and with the depletion of PS-expressing EV, it is possible that one or more other lipids may act in concert or synergistically with the PS exosomes to suppress T cell functions.
We conclude that by targeting and eliminating the immunosuppressive tumor-associated PS-containing EV, it will be possible to enhance patients’ anti-tumor immunity by reversing the TCR signaling arrest of T cells in the tumor and by preventing the arrest of T cells that enter the tumor microenvironment.
Supplementary Material
Acknowledgements
We thank Anthony Miliotto and the Tissue Procurement Facility of Roswell Park Cancer Institute (RPCI) for their assistance in providing tumor tissues. Flow cytometry and confocal microscopy services were provided by the Confocal Microscopy and Flow Cytometry Core Facility at the University at Buffalo. Additional cytometry services were provided by the Flow and Image Cytometry Core facility at the Roswell Park Cancer Institute.
Financial Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers R01CA108970 and R01CA131407 (RBB); the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL70227 (SB-I); and the National Institutes of Health under award numbers P50CA159981 and R01CA158318 (KO). The Flow and Image Cytometry Core facility at the Roswell Park Cancer Institute is supported in part by the NCI Cancer Center Support Grant 5P30 CA016056.
Footnotes
Conflict of Interest: No conflicts to disclose.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305(5681):197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 8.Broderick L, Brooks SP, Takita H, Baer AN, Bernstein JM, Bankert RB. IL-12 reverses anergy to T cell receptor triggering in human lung tumor-associated memory T cells. Clin Immunol. 2006;118(2–3):159–169. doi: 10.1016/j.clim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Koneru M, Schaer D, Monu N, Ayala A, Frey AB. Defective proximal TCR signaling inhibits CD8+ tumor-infiltrating lymphocyte lytic function. J Immunol. 2005;174(4):1830–1840. doi: 10.4049/jimmunol.174.4.1830. [DOI] [PubMed] [Google Scholar]
- 10.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67(23):11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson-Abelson M, Bankert RB. Targeting the TCR signaling checkpoint: a therapeutic strategy to reactivate memory T cells in the tumor microenvironment. Expert Opin Ther Targets. 2008;12(4):477–490. doi: 10.1517/14728222.12.4.477. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185(12):7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota SJ, Facciponte JG, Kelleher RJ, Jr, Shultz LD, Loyall JL, Parsons RR, et al. Changes in ovarian tumor cell number, tumor vasculature, and T cell function monitored in vivo using a novel xenograft model. Cancer Immun. 2013;13(2):11.11–11.11. [PMC free article] [PubMed] [Google Scholar]
- 14.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20(16):4262–4273. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 16.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–118. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson-Abelson MR, Loyall JL, Lehman HK, Barnas JL, Minderman H, O'Loughlin KL, et al. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14.11–14.10. [PMC free article] [PubMed] [Google Scholar]
- 18.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. 30:33.22:33.22.31–33.22.29. [DOI] [PubMed] [Google Scholar]
- 19.Mourdjeva M, Kyurkchiev D, Mandinova A, Altankova I, Kehayov I, Kyurkchiev S. Dynamics of membrane translocation of phosphatidylserine during apoptosis detected by a monoclonal antibody. Apoptosis : an international journal on programmed cell death. 2005;10(1):209–217. doi: 10.1007/s10495-005-6076-5. [DOI] [PubMed] [Google Scholar]
- 20.Gaitonde P, Ramakrishnan R, Chin J, Kelleher RJ, Jr, Bankert RB, Balu-Iyer SV. Exposure to factor VIII protein in the presence of phosphatidylserine induces hypo-responsiveness toward factor VIII challenge in hemophilia A mice. J Biol Chem. 2013;288(24):17051–17056. doi: 10.1074/jbc.C112.396325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174(3):1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 23.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4(9):882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 24.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7(11):1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 25.Taylor DD, Doellgast GJ. Quantitation of peroxidase-antibody binding to membrane fragments using column chromatography. Anal Biochem. 1979;98(1):53–59. doi: 10.1016/0003-2697(79)90704-8. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro PK, Repasky EA, Black J, Kubo RT, Bankert RB. Biochemical and ultrastructural characterization of a novel cell structure associated with immunoglobulin secretion in B-lymphocytes. J Mol Cell Immunol. 1987;3(5):293–306. [PubMed] [Google Scholar]
- 27.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller S, Konig A-K, Marme F, Runz S, Wolterink S, Koensgen D, et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer letters. 2009;278(1):73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer. 2005;92(2):305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33(5):441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 32.Tickner JA, Urquhart AJ, Stephenson SA, Richard DJ, O'Byrne KJ. Functions and Therapeutic Roles of Exosomes in Cancer. Front Oncol. 2014;4:127.121–127.128. doi: 10.3389/fonc.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41(1):245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168(7):3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 35.Perone MJ, Larregina AT, Shufesky WJ, Papworth GD, Sullivan ML, Zahorchak AF, et al. Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses in naive and activated T cells. J Immunol. 2006;176(12):7207–7220. doi: 10.4049/jimmunol.176.12.7207. [DOI] [PubMed] [Google Scholar]
- 36.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11(3):1010–1020. [PubMed] [Google Scholar]
- 37.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 38.Mueller DL. Linking diacylglycerol kinase to T cell anergy. Nat Immunol. 2006;7(11):1132–1134. doi: 10.1038/ni1106-1132. [DOI] [PubMed] [Google Scholar]
- 39.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006;7(11):1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 40.Abe T, Lu X, Jiang Y, Boccone CE, Qian S, Vattem KM, et al. Site-directed mutagenesis of the active site of diacylglycerol kinase alpha: calcium and phosphatidylserine stimulate enzyme activity via distinct mechanisms. Biochem J. 2003;375(Pt 3):673–680. doi: 10.1042/BJ20031052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205(6):1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med. 2000;6(6):465–479. [PMC free article] [PubMed] [Google Scholar]
- 43.Broderick L, Bankert RB. Memory T cells in human tumor and chronic inflammatory microenvironments: sleeping beauties re-awakened by a cytokine kiss. Immunol Invest. 2006;35(3–4):419–436. doi: 10.1080/08820130600755066. [DOI] [PubMed] [Google Scholar]
- 44.Xie Y, Zhang X, Zhao T, Li W, Xiang J. Natural CD8(+)25(+) regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem Biophys Res Commun. 2013;438(1):152–155. doi: 10.1016/j.bbrc.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 45.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275(1):261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 46.Zeyda M, Staffler G, Horejsi V, Waldhausl W, Stulnig TM. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem. 2002;277(32):28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- 47.Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am J Clin Nutr. 2006;84(6):1277–1289. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- 48.Biswas S, Biswas K, Richmond A, Ko J, Ghosh S, Simmons M, et al. Elevated levels of select gangliosides in T cells from renal cell carcinoma patients is associated with T cell dysfunction. J Immunol. 2009;183(8):5050–5058. doi: 10.4049/jimmunol.0900259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornton MV, Kudo D, Rayman P, Horton C, Molto L, Cathcart MK, et al. Degradation of NF-kappa B in T cells by gangliosides expressed on renal cell carcinomas. J Immunol. 2004;172(6):3480–3490. doi: 10.4049/jimmunol.172.6.3480. [DOI] [PubMed] [Google Scholar]
- 50.Crespo FA, Sun X, Cripps JG, Fernandez-Botran R. The immunoregulatory effects of gangliosides involve immune deviation favoring type-2 T cell responses. J Leukoc Biol. 2006;79(3):586–595. doi: 10.1189/jlb.0705395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.