Abstract
Bacteria live in a toxic world in which their competitors excrete hydrogen peroxide or superoxide-generating redox-cycling compounds. They protect themselves by activating regulons controlled by the OxyR, PerR, and SoxR transcription factors. OxyR and PerR sense peroxide when it oxidizes key thiolate or iron moieties, respectively; they then induce overlapping sets of proteins that defend their vulnerable metalloenzymes. An additional role for OxyR in detecting electrophilic compounds is possible. In some non-enteric bacteria SoxR appears to control the synthesis and export of redox-cycling compounds, whereas in the enteric bacteria it defends the cell against the same agents. When these compounds oxidize its iron-sulfur cluster, SoxR induces proteins that exclude, excrete, or modify them. It also induces enzymes that defend the cell against the superoxide that such compounds make. Recent work has brought new insight to the biochemistry and physiology of these responses, and comparative studies have clarified their evolutionary histories.
Keywords: hydrogen peroxide, superoxide, OxyR, PerR, SoxR, reactive oxygen species
Introduction
The most harmful effects of oxygen are mediated by its partially reduced forms—hydrogen peroxide (H2O2) and superoxide (O2−). These species continuously arise inside cells through the autoxidation of redox enzymes, and so aerobes maintain high titers of scavenging enzymes that hold these oxidants below the threshold of toxicity. But by the 1980s it was realized that a variety of natural circumstances can elevate these species above that threshold and that microbes universally maintain inducible defensive systems to counteract such events. Several such systems have now been dissected, and the goal of this review is to map out what is known and what key problems remain. The focus will be on the three tran scription factors that have been most intensively examined: OxyR and PerR, which respond to H2O2 stress, and SoxR, which defrays the toxic effects of O2−.
Detecting hydrogen peroxide: OxyR and PerR
The threat of H2O2
H2O2 arises in natural habitats through reactions between sulfur and oxygen at oxic/anoxic interfaces, the photochemical reduction of oxygen by chromophores, and the redox-cycling of pigments. More ominously, plants, animals, and certain bacteria excrete H2O2 to poison local microbes (5, 27, 77, 96). H2O2 is an ideal weapon. Because it is small and uncharged, it passively crosses membranes at rates similar to that of water, and so it cannot be excluded by the targeted cell (95, 113). Once inside it disrupts multiple aspects of iron metabolism, thereby attacking a feature of life that is virtually universal.
The ways in which H2O2 damages cells have been identified in the model bacterium Escherichia coli. Exogenous H2O2 damages DNA, so it is mutagenic. This effect arises from Fenton chemistry, in which H2O2 reacts with the intracellular pool of unincorporated iron (42):
The hydroxyl radical (HO·) reacts at diffusion-limited rates with most biomolecules. Some of the iron pool is loosely associated with DNA (92), and Fenton reactions on its surface produce the lesions that cause mutagenesis.
However, the most marked effect of H2O2 stress is to inactivate two families of iron-containing enzymes: non-redox mononuclear enzymes and [4Fe-4S]-containing dehydratases (2, 46, 101, 102). Both classes of enzymes employ a solvent-exposed reduced iron atom to bind and activate substrates, and in both cases incoming H2O2 can contact and oxidize the iron. The metal dissociates and activity is lost. Such enzymes sit within the TCA cycle, the pentose phosphate pathway, and key biosynthetic pathways, so growth stops. As little as 0.5 μM H2O2 is sufficient to poison cells in this way, so the titers of scavenging enzymes are calibrated to keep the steady-state H2O2 concentration below this level. This goal becomes much harder when bacteria enter a habitat containing extracellular H2O2. The H2O2 quickly flows across membranes and into the cytoplasm. For that circumstance virtually all bacteria maintain inducible defensive regulons governed by either the OxyR or the PerR transcription factors.
Defensive tactics: the OxyR regulon
The OxyR protein is more widely distributed. It features a sensory cysteine residue that is directly oxidized by H2O2, thereby generating a disulfide bond that alters its binding to DNA (Fig. 1A) (4, 11). In some bacteria OxyR acts as a repressor, and its oxidation inactivates this function and stimulates gene expression (39, 40, 71, 72, 103). More commonly OxyR acts as an activator protein that recruits RNA polymerase. In E. coli the oxidized protein stimulates the transcription of about two dozen genes that are well-suited to address the problems that H2O2 causes (Table 1) (118). To drive down the H2O2 level, OxyR strongly induces both an NADH peroxidase (AhpCF) and catalase (KatG)(44). A mini-ferritin, Dps, is synthesized to sequester unincorporated iron (10, 31). This has the effect of substantially suppressing the rate of DNA damage, especially in collaboration with the induction of Fur and Yaa proteins (70, 109, 117). The MntH manganese importer is induced, apparently to enable Mn—which does not react with H2O2—to displace Fe as the catalytic cofactor of mononuclear enzymes (3, 49, 101). Induction of the Suf iron-sulfur assembly system enables the repair of damaged iron-sulfur clusters. Although the cell normally uses an Isc system for this purpose, Isc does not work well when iron levels decline, a situation that ensues from the action of Dps (47, 83). Similarly, the iron-dependent enzymes in the heme biosynthetic pathway are replaced (HemF) or induced (HemH) in order to sustain this process as intracellular iron becomes scarce (75a).
Figure 1. Activation of OxyR.
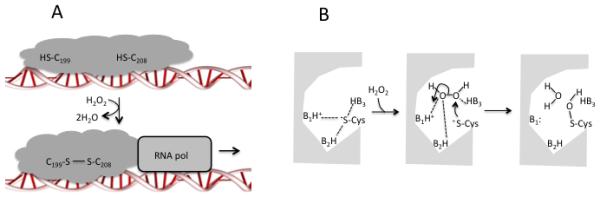
(A) Oxidation of a sensory cysteine by H2O2 perturbs global conformation. In E. coli the disulfide-bonded form of the protein stabilizes the transcription complex. (B) The acute H2O2 sensitivity of OxyR may depend upon a shift in H-bonds from the sensory cysteine thiolate to the incoming H2O2. This idea derives from models of peroxiredoxin behavior (18, 104).
Table 1.
The E. coli OxyR regulona.
| Gene | Activity | Impact |
|---|---|---|
|
katG
ahpCF |
catalase NADH peroxidase |
scavenge H2O2 |
|
dps
fur yaaA |
mini-ferritin iron-import repressor unknown biochemistry |
minimize free iron |
| mntH | manganese importer | activates mononuclear enzymes |
| sufA-E | iron-sulfur assembly | activates Fe/S enzymes |
|
hemF
hemH |
coproporphyrinogen III oxidase ferrochelatase |
sustain heme synthesis |
|
gor
trxC grxA dsbG |
glutathione reductase thioredoxin glutaredoxin protein sulfenate reductase |
thiol maintenance |
Several genes of unknown function are not included. The PerR regulon of B. subtilis includes catalase, a mini-ferritin (MrgA), Fur, and heme biosynthetic genes (7). In that bacterium Mn importers and Suf proteins are constitutively synthesized, while disulfide-reducing systems are controlled by an independent regulator, Spx, that does not respond to H2O2.
Three regulon members typically serve to reduce disulfide bonds: glutathione reductase, glutaredoxin 1, and thioredoxin 2. The glutaredoxin assists in deactivating OxyR once H2O2 has dissipated (4), but the roles of the others are less obvious. Deletion of these genes does not cause obvious sensitivity to H2O2, which inspires other ideas about their purpose in this regulon (see below.)
How does OxyR sense H2O2?
Recent studies have analyzed both the specificity and the sensitivity of OxyR. E. coli OxyR features a hyperreactive thiol (Cys199) that is quickly oxidized by H2O2 to a sulfenic acid (−SOH). The adducted residue moves from the hydrophobic cleft in which it is normally buried, and it swings into the proximity of Cys208, with which it condenses to form a disulfide bond (11). This shift locks the domain into a conformation that activates the protein as a transcription factor. The protein functions as a dimer, and it displays some cooperativity that may avoid activation when H2O2 is scant (60). The fact that OxyR uses cysteine oxidation to detect H2O2 enables it to work like a rheostat, given that disulfide formation is among the few amino acid oxidations that are reversible.
The surprising aspect of this chemistry is that cysteine per se reacts very sluggishly with H2O2 (2 M−1 s−1) (114). With such a rate constant the half-time for oxidation by 0.5 μM H2O2—an intracellular concentration that suffices to block E. coli growth (101)—is approximately one week. In sharp contrast, the sensing cysteine residue on OxyR exhibits a rate constant of 105 M−1 s−1 and detects micromolar H2O2 within seconds (4). What is the source of this kinetic improvement?
The hyper-reactive behavior of OxyR C199 has not been deconstructed in detail, but it resembles that of the catalytic cysteine residues on peroxiredoxins, the ubiquitous peroxidases (including E. coli AhpC) that degrade H2O2 through cycles of cysteine oxidation and reduction (18, 104). As with OxyR, their reaction involves a nucleophilic attack of a cysteine thiolate upon H2O2 (Fig. 1A) with cleavage of the dioxygen bond. A nearby cationic residue sets the stage by ensuring deprotonation of the cysteine (81)—but this should provide only an order-of-magnitude improvement in reactivity. A plausible explanation for the remaining stimulation is depicted in Fig. 1B. The peroxidactic cysteine residue of peroxiredoxins participates in hydrogen-bond networks that shield its charge within a largely hydrophobic cleft (89). Entry of H2O2 may shift these bonds towards the H2O2 itself, freeing the thiolate. Several aspects of this are conducive to catalysis. Release of the cysteine residue generates a nucleophile whose potency is enhanced in a low-dielectric environment. The hydrogen bonds that shift towards H2O2 likely polarize its di-oxygen bond, making it more conducive to attack, and one of these residues probably protonates the hydroxide leaving group to pull the reaction forward. The cysteine residue of OxyR is invariably flanked by arginine and histidine residues that may play the same role.
A hydrogen-bond network is configured to fit H2O2 should also enhance the rate constant by disfavoring water in the active site, given that H2O is too small a molecule to bridge a hyrogen-bond network tailored for H2O2 (91). The upshot is that thiol-based peroxidases achieve a level of reactivity (107 M−1 s−1) that is appropriate for their physiological roles, and similar features may have allowed OxyR to do so as well. A new structure of the Pseudomonas aerugionas OxyR protein suggests that H2O2 binding might engage a H-bond network that ultimately links the deprotonation of cysteine with the protonation of the product hydroxide group (47a).
In E. coli OxyR quickly responds to the sub-micromolar levels of H2O2 that threaten to disrupt metabolism. The facultative bacterium Vibrio vulnificus takes it a step further, employing two OxyR proteins that are calibrated to sense distinct levels of H2O2: The more sensitive OxyR is activated by the low levels of H2O2 that are endogenously generated upon aeration, whereas the less sensitive one responds to more-severe influxes of H2O2 from the external environment (52).
Has OxyR evolved to respond to stresses other than H2O2?
Because OxyR responds to adduction of its sensory thiol, it can also be activated by electrophiles (116). Experimenters have commonly used the synthetic chemical diamide as a proxy for such compounds. An interesting question is whether OxyR has an authentic role in sensing stressors other than H2O2. Seth et al. reported that OxyR becomes nitrosylated during anaerobic nitrate reduction, presumably by nitric oxide that escapes from the system (97). Indeed, an oxyR mutant exhibited poorer growth in this circumstance, suggesting that some elements of the regulon help the cell to tolerate this stress.
Many bacteria contain transcription factors other than OxyR that appear to be dedicated to responding to electrophiles, whose attack upon thiols can indirectly generate disulfide bonds. Both the Spx system of Bacillus subtilis and the SigR/RsrA system of Streptomyces coelicolor each induce a mixture of glutaredoxins, thioredoxins, chaperones, and proteases, which collectively avert protein aggregation from the denaturing effects of disulfide formation and thiol adduction (48, 51, 84, 94, 119). These bacteria additionally contain OxyR or PerR systems that respond to H2O2—but in these bacteria, the latter systems do not include disulfide-reducing genes that are part of the E. coli OxyR regulon. Thus it seems possible that E. coli is exceptional in multitasking OxyR with responding to both stresses: H2O2, which primarily threatens iron enzymes, and electrophiles, which react with cysteine residues much more avidly than does H2O2.
PerR of Bacillus subtilis: an alternative to OxyR
Gram-positive bacteria often employ a different sensor of H2O2: PerR. This dimeric protein belongs to the Fur family of metal-binding transcription factors, which have evolved in their various forms to sense iron (Fur proper), manganese (Mur), zinc (Zur), nickel (Nur), or H2O2 (PerR) (19, 38). Each subunit contains a structural site that irreversibly binds zinc, plus at least one regulatory site that reversibly binds the activating metal (Fig. 2A). Only the metal-bound forms of the proteins are competent to bind DNA, and they generally work as repressors. In Bacillus subtilis PerR, the regulatory metal is ferrous iron, and it is affixed in a distorted square pyramid with four co-planar ligands and a single axial ligand (45). When H2O2 levels rise, H2O2 accesses the sixth coordination site and oxidizes the iron atom. The ferryl or hydroxyl radical that is thereby formed oxidizes the histidine ligands of the iron atom to 2-oxohistidine (63). The modified residues can no longer bind the metal, and the resultant apoprotein undergoes a global conformational change that eradicates its ability to bind DNA (Fig. 2B). Because holo-PerR acts as a repressor, the effect is to derepress regulon members whenever H2O2 levels rise.
Figure 2. Structure of PerR in its (A) holoenzyme and (B) demetallated forms.
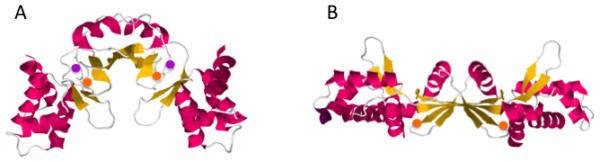
Structures were derived from the Protein Data Bank (PDB 3F8N and 2FE3) (45, 105). Orange circles represent Zn, and purple circles represent Mn.
Strikingly, B. subtilis PerR reacts with H2O2 at essentially the same rate constant as does E. coli OxyR (105 M−1 s−1) (62). Other mononuclear iron proteins, such as E. coli peptide deformylase, have similar inactivation rate constants (2), indicating that this value is largely dictated by the fundamental rate of Fenton reactions. However, B. subtilis also employs a true Fur protein whose iron cofactor seems to be resistant to oxidation by H2O2. Inspection of this discrepancy elicited evidence that in the Fur protein a coordinating glutamate residue probably acts as a bidentate ligand to the iron atom, thereby occluding its sixth coordination site (87). The analogous aspartate ligand of PerR does not. Given that H2O2 must directly contact iron in order to oxidize it (28), H2O2 oxidizes PerR but not Fur.
The PerR regulon of Bacillus subtilis includes many of the same genes that OxyR controls in other bacteria: genes encoding a catalase, a Dps homologue (MrgA), and Fur protein (7) (Table 1). Yet PerR offers several intriguing departures from the OxyR mechanism of H2O2 sensing. First, given that there is no known mechanism of oxo-histidine reduction, the reaction is presumably irreversible, perhaps making the system more of an on/off switch. This arrangement is a rarity: With few exceptions (78) bacterial regulators are reversible, and their activities are set by the dynamic equilibrium between activating and deactivating events. It is not clear whether the irreversibility of PerR deactivation provides any advantage.
Second, like other mononuclear proteins, PerR can bind manganese in place of iron, both in vitro and in vivo (9). Given that Mn is not oxidized by H2O2, the upshot is that PerR does not respond to H2O2 whenever the cytoplasm is rich in manganese. As manganese helps to defend cells against H2O2, this arrangement suggests that a primary goal of the PerR and OxyR responses may be to raise the Mn/Fe ratio (115); if that ratio is already high, no induction is needed.
Finally, PerR does not control the synthesis of disulfide-reducing redoxins, as their synthesis is governed by the Spx response regulator (119), which is not responsive to H2O2. PerR appears to be focused exclusively on the detection and prevention of Fenton chemistry.
Superoxide stress: re-thinking SoxR
The threat of redox-cycling compounds
Because O2− is a charged molecule, it cannot penetrate into bacteria from the outside (56, 73); therefore, any cytoplasmic O2− stress is due to O2− that is generated internally. Endogenous superoxide (O2−) that is formed by enzyme autoxidation is kept very scarce (~10−10 M) by the action of superoxide dismutases (SODs) (41). E. coli mutants that lack cytoplasmic SODs exhibit growth defects (8) that have been traced back to deficiencies in the same enzymes that H2O2 inactivates: [4Fe-4S] dehydratases and mononuclear iron enzymes (20, 24, 35, 59, 101). These mutants are unable to synthesize branched-chain or aromatic amino acids, and they cannot catabolize TCA-cycle substrates.
The same phenotypes arise even in wild-type E. coli if it encounters viologens (e.g., paraquat), quinones, or phenazines (37). These cyclic organic chemicals can penetrate into cells, where they directly abstract electrons from redox enzymes and transfer them to oxygen. Toxic doses of O2− are thereby produced. Indeed, quinones and phenazines are released by various bacteria and plants, often in an effort to poison microflora (43, 85, 107). In 1977 it was discovered that E. coli responds to this stress by inducing higher titers of SOD (36). This adaptation is mediated by two proteins dubbed SoxR and SoxS (superoxide) (32, 106). SoxR is a [2Fe-2S]-containing transcription factor that senses the stress; it then induces the transcription of soxS. SoxS is a second transcription factor that then activates scores of defensive genes scattered around the chromosome (6). Several of these encode proteins that help suppress the toxicity of O2−: superoxide dismutase; aconitase A and fumarase C, which are superoxide-resistant isozymes of vulnerable [4Fe-4S] enzymes (67, 108); and YggX, which facilitates cluster-repair processes (30, 34, 88). Like OxyR, the SoxRS system is not activated during normal aerobic growth and does not control the basal level of defensive systems; instead, it becomes active only when forcing conditions emerge.
The sensing mechanism of SoxR
SoxR is a homodimer that binds one [2Fe-2S]+ cluster per monomer (Fig. 3); a second domain includes a DNA-binding HTH motif (112). Electron paramagnetic resonance studies demonstrate that when cells are exposed to redox-cycling compounds, the clusters are quickly oxidized to the [2Fe-2S]2+ state (15, 26). Upon removal of the oxidant the clusters revert within minutes to their reduced forms. The E. coli soxS gene features an unusually long 20-bp spacing between its −10 and −35 promoter motifs, and the current model is that cluster oxidation contorts the SoxR dimer and thereby alters the conformation of the bound DNA, moving the promoter motifs to the same side of the helix so that RNA polymerase can productively bind. Physical studies have confirmed that changes in SoxR redox state distort bound DNA (23). Genetic studies indicate that the SoxR reduction to the deactivated form is catalyzed by Rsx/Rse proteins (55) that likely employ NADPH as a source of electrons (54). Thus these data collectively suggest that transcription of the soxS depends upon the dynamic equilibrium between the oxidation and reduction of the SoxR iron-sulfur clusters. When SoxR returns to its reduced form, the transcription of the full regulon quickly ceases owning to the rapid proteolysis of extant SoxS protein (33).
Figure 3. SoxR in association with DNA.
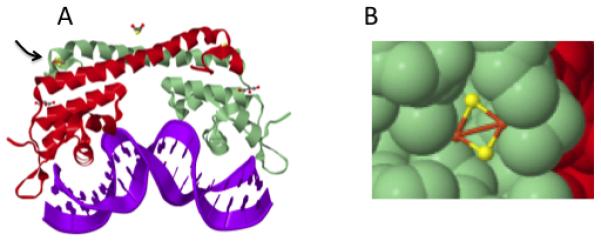
(A) Oxidation of the cluster (arrow) allows the protein to twist the DNA, improving the −10 to −35 spacing. (B) The cluster is exposed on the protein surface so that it is accessible to diverse oxidants. The structure was derived from the Protein Data Bank (PDB 2ZHG) (112).
What is the inducer that oxidizes SoxR?
The apparent parallel with H2O2 detection by OxyR suggested the obvious possibility that SoxR is activated when O2− directly oxidizes its clusters. The clusters are located near the protein surface and appear accessible to a small molecule like O2− (Fig. 3) (112). Unfortunately, two efforts to directly detect cluster oxidation by O2− in vitro led to contradictory results (21, 34), suggesting that one of these complicated experiments was compromised in some way. The in vivo data, however, are clearer and somewhat surprising. In SOD− mutants—which suffer debilitating levels of O2−—the soxS gene is induced only 2- to 4-fold (25, 34). This activation is consistent with the idea that O2− can oxidize the clusters, but it is far less than the ~40-fold induction that occurs when wild-type cells are treated with redox-cycling compounds. Similarly modest effects were observed for members of the regulon (34, 66, 68). This induction is too minor to be effectual: The SoxRS regulon provided no protection to SOD− mutants unless soxS was forcibly induced from a heterologous promoter (34). Thus high O2− concentrations are not sufficient to adequately activate SoxR.
Further, overproduction of SOD does not diminish the ability of redox agents to activate SoxR in wild-type cells (25, 29, 34, 67, 75), leading workers to conclude that these drugs oxidize SoxR independently of O2−. Indeed, exposure to redox compounds can activate SoxR even in anoxic cells, from which O2− is absolutely absent (14, 34, 57).
An alternative explanation for SoxR oxidation by redox agents is that they deplete NADPH pools and thereby disrupt the ability of Rse/Rsx to keep SoxR in its reduced form. Indeed, some genes within the E. coli SoxR regulon provide mechanisms to restore NADPH pools (58, 79). However, measurements indicate that the shift in NADPH redox status [to −.314 V, calculated from (57)] is not enough to explain the near-total oxidation of SoxR [Eo’ = −.293 V (53)], and mutations that impair cellular NADPH formation do not activate SoxR (34). Thus although NADPH depletion might augment the inducing effects of redox agents, by itself it is probably not sufficient to turn on the system.
Therefore it seems likely that the redox compounds themselves directly oxidize the clusters of SoxR, just as they do the metal and flavin moieties of typical redox enzymes. This reaction has been demonstrated in vitro, with rate constants that accommodate the rates of SoxR oxidation that are observed in vivo (34). The position of the iron atom near the SoxR protein surface ensures that it can be oxidized by diverse univalent oxidants without regard for their structures.
The constituency of the regulon supports the idea that the SoxRS response specifically arose to defend bacteria against redox-cycling compounds. A major element of the SoxRS response in E. coli is the induction of proteins that collectively block the entry and accumulation of redox-active compounds within the cell: lipopolysaccharide-modifying enzymes that diminish the ability of such compounds to penetrate the envelope, broad-specificity export systems that actively pump them out of the cytoplasm, and derivatizing enzymes that deactivate them through covalent modification (1, 61, 65, 69, 74, 93) (Fig. 4A). Interestingly, SoxR is an important defense against redox-active agents even under anoxic conditions, indicating that these agents can exert O2−-independent mechanisms of toxicity. Their primary action under these conditions is unclear, but such compounds can form adducts to proteins by acting as Michael acceptors, they can deactivate [4Fe-4S] dehydratases by directly oxidizing the clusters, and they can deplete cellular NADPH pools by oxidizing NADPH-reducible enzymes and then dumping the electrons into the anaerobic respiratory chain (34).
Figure 4. Toxic actions of a redox-cycling agent and the defensive tactics that minimize its accumulation.
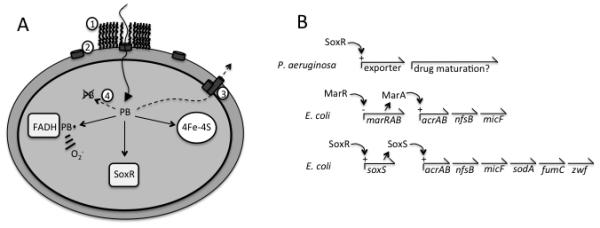
(A) Redox-cycling compounds such as plumbagin (PB) catalyze electron transfer from flavoproteins to oxygen, directly damage [4Fe-4S]-dependent dehydratases, and activate SoxR by oxidation. Components of the SoxR system exclude the agent by (1) altering the lipopolysaccharide coat (via waaYZ) (62), (2) inhibiting porin synthesis (micF) (12), (3) exporting the compound (tolC, acrAB) (74), and (4) modifying it (nfsA, ygfZ) (65, 69, 93). (B) Relationship of E. coli SoxRS to non-enteric SoxR and E. coli MarA. In non-enteric bacteria SoxR detects endogenous redox-active compounds like pyocyanin and induces exporters that excrete it. The SoxRS regulon could plausibly have been created by lateral transfer of soxR and duplication of marA, as SoxS and MarA exhibit 50% identity. The SoxRS regulon consists of MarA-controlled genes plus several genes that specifically defend against oxidizing compounds. Only representative genes are shown; for a full list, see EcoCyc (ecocyc.org).
Finally, it is worth noting that although H2O2 is an ineffective activator of SoxR (34, 118), nitric oxide (NO) can activate it by forming dinitrosyl iron complexes with the atoms of the iron-sulfur cluster (22, 82). The significance of this effect is uncertain, because dedicated NO scavenging systems are controlled by independent regulatory proteins (NsrR and NorR) that are much more responsive to NO. In one study (90) NO doses that activated the aerobic and anaerobic NO-scavenging systems by 30- and 180-fold only induced soxS 5-fold. Furthermore, this slight SoxS induction had no apparent effect upon SoxS-controlled genes, leading the authors to suggest that this degree of SoxS induction was inadequate for activation of the full regulon. That outcome dovetails with the failure of 3-fold SoxS induction to provide any protection to SOD− cells (34).
A more expansive view: SoxR beyond E. coli
Studies of SoxR and SoxS in other bacteria have prompted a substantial reappraisal of their biological role. Dietrich et al. found homologs of SoxR among α-, β-, γ-, and δ-proteobacteria and among actinobacteria, but not elsewhere (13). Strikingly, only enterics employ SoxS; in all the other bacteria SoxR directly controls the expression of all regulon members. Just as striking is the fact that in these non-enterics the SoxR regulon is quite different from that of E. coli: it typically comprises only a handful of genes of unclear function (80), and these generally do not include sodA or other genes that might confer resistance to O2− (13, 14, 50, 80, 99). The implication is that the physiological purpose of SoxR in non-enterics is quite different from its purpose in E. coli.
Evidence regarding this purpose has come from studies of Pseudomonas aeruginosa and Streptomyces coelicolor. Both bacteria synthesize and excrete redox-cycling compounds: pyocyanin for P. aeruginosa and actinorhodin for S. coelicolor. Different roles have been suggested for these compounds, including toxifying competitors, signaling, assisting electron transfer to distant respiratory substrates, and solubilizing iron (14, 110, 111). The pumps that excrete these compounds, and perhaps the enzymes that complete their synthesis, are induced in both bacteria when SoxR is oxidized by either the redox agent itself or a precursor to it (13, 99) (Fig. 4B). The SoxR protein can also respond to exogenous synthetic redox compounds such as paraquat, but this is presumably adventitious, as SoxR activation does not enhance resistance to such compounds (86). The implication is that these bacteria use SoxR to control the export of endogenous redox agents into the environment.
Whether or not it is to the advantage of the producing organism, these agents can penetrate and toxify other microbes. It is possible that the latter laterally acquired SoxR as a sensor that could perceive these substances and induce defenses. In E. coli the arriving soxR gene may have piggy-backed onto a pre-existing system that responds to other toxic chemicals (Fig. 4B). SoxS is a paralog of the MarA and Rob transcription factors, which respond to and provide protection against exogenous non-redox agents (to salicylate and bile salts, for example). The three proteins are similar enough (~ 50% identity) that they recognize common regulatory sites, and so their regulons overlap extensively (76). The simple model, then, is that E. coli acquired SoxR and, after duplication of MarA or Rob, fashioned SoxS to connect SoxR to a pre-existing regulon that serves to exclude, modify, or export hazardous compounds. SoxR provided the means to activate this system in response to redox-active compounds. Notably, the reduction potential of E. coli SoxR is lower than those of P. aeruginosa and S. coelicolor SoxR proteins, and accordingly it responds to a larger range of redox agents to which it responds (98, 100). Extant antioxidant defenses, such as sodA and fumC, may then have been added to the regulon. The latter genes are significantly more responsive to SoxS than to MarA (76).
A final word about other “antioxidant” regulons
Workers have identified distinct bacterial transcription factors that are activated by singlet oxygen [e.g., RpoE/ChrR (64)], organic hydroperoxides [OhrR (17)], disulfide stress [Spx (119) and SigR/RsrA (51)], or hypochlorous acid [HypT (16)]. Unfortunately, space restrictions preclude a discussion of these systems here, and so readers are encouraged to consult recent literature to learn more about them. Implicit in this diversity is the fact that “oxidative stress” is an overbroad term that subsumes a panoply of oxidants that (a) vary in their chemical properties, (b) attack very different targets, and (c) are sensed and defended against in completely different ways. For example, none of those systems is activated by physiological doses of H2O2 or O2−, and they provide very little cross-over resistance against them.
Prospects
Because life evolved in an anoxic world, the iron-intensive biochemistry that was inherited by contemporary organisms exhibits some incompatibility with the presence of oxygen. This vulnerability is problematic for bacteria that experience fluctuations in local oxygen tension, and it is exploited by competitors that would stymie their growth. A key goal of current work on these regulons is to identify the natural circumstances in which they are activated. As this review emphasizes, important clues can be deduced from the biochemistry of the sensor proteins themselves and from the membership of the regulons that they control. The emergence of studies in non-enteric bacteria promises to provide clarity by offering new perspectives on these familiar regulators.
Acknowledgments
Work in the author’s lab is supported by grants GM49640 and GM101012 from the National Institutes of Health.
Literature cited
- 1.Aiba H, Matsuyama S, Mizuno T, Mizushima S. Function of micF as an antisense RNA in osmoregulatory expression of the ompF gene in Escherichia coli. J Bacteriol. 1987;169:3007–12. doi: 10.1128/jb.169.7.3007-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjem A, Imlay JA. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J. Biol. Chem. 2012;287:15544–56. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–58. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 1999;96:6161–5. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard K, Lardy B, Krause K-H. NOX family NADPH oxidases: Not just in mammals. Biochemie. 2007;89:1107–12. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard JL, Wholely WY, Conlon EM, Pomposiello PJ. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and -independent transcriptional networks. PLoS ONE. 2007;2:e1186. doi: 10.1371/journal.pone.0001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–98. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life. EMBO J. 1986;5:623–30. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Keramati L, Helmann JD. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. USA. 1995;92:8190–4. doi: 10.1073/pnas.92.18.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiancone E, Ceci P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim. Biophys. Acta. 2010;1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–13. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 12.Chou JH, Greenberg JT, Demple B. Posttranscriptional repression of Escherichia coli ompF protein in response to redox stress. Positive control of the micF antisense RNA by the soxRS locus. JBact. 1993;175:1026–31. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–6. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61:1308–21. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 15.Ding H, Demple B. In vivo kinetics of a redox-regulated transcriptional switch. Proc Natl Acad Sci USA. 1997;94:8445–9. doi: 10.1073/pnas.94.16.8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drazic A, Miura H, Peschek J, Le Y, Bach NC, et al. Methionine oxidation activates a transcription factor in response to oxidative stress. Proc. Natl. Acad. Sci. USA. 2013;110:9493–8. doi: 10.1073/pnas.1300578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubbs JM, Mongkolsuk S. Peroxide-sensing transcriptional regulators in bacteria. J. Bacteriol. 2012;194:5495–503. doi: 10.1128/JB.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer-Sueta G, Manta B, Botti H, Radi R, Trujillo M, Denicola A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 2011;24:434–50. doi: 10.1021/tx100413v. [DOI] [PubMed] [Google Scholar]
- 19.Fillat MF. The FUR (ferric uptake regulator) superfamily: diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 2014;546:41–52. doi: 10.1016/j.abb.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–76. [PubMed] [Google Scholar]
- 21.Fujikawa M, Kobayashi K, Kozawa T. Direct oxidation of the [2Fe-2S] cluster in SoxR protein by superoxide: distinct differential sensitivity to superoxide-mediated signal transduction. J. Biol. Chem. 2012;287:35702–8. doi: 10.1074/jbc.M112.395079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujikawa M, Kobayashi K, Kozawa T. Mechanistic studies on formation of the dinitrosyl iron complex of the [2Fe-2S] cluster of SoxR protein. J. Biochem. 2014;156:163–72. doi: 10.1093/jb/mvu029. [DOI] [PubMed] [Google Scholar]
- 23.Fujikawa M, Kobayashi K, Kozawa T. Redox-dependent DNA distortion in a SoxR protein-promoter complex studied using fluorescent probes. J. Biochem. 2014 doi: 10.1093/jb/mvu085. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–33. [PubMed] [Google Scholar]
- 25.Gaudu P, Dubrac S, Touati D. Activation of SoxR by overproduction of desulfoferrodoxin: multiple ways to induce the soxRS regulon. J. Bacteriol. 2000;182:1761–3. doi: 10.1128/jb.182.6.1761-1763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudu P, Moon N, Weiss B. Regulation of the soxRS oxidative stress regulon. J Biol Chem. 1997;272:5082–6. doi: 10.1074/jbc.272.8.5082. [DOI] [PubMed] [Google Scholar]
- 27.Glass GA, DeLisle DM, DeTogni P, Gabig TG, Magee BH, et al. The respiratory burst oxidase of human neutrophils. Further studies of the purified enzyme. J Biol Chem. 1986;261:13247–51. [PubMed] [Google Scholar]
- 28.Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Rad. Biol. Med. 1993;15:435–45. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 29.Gort AS, Imlay JA. Balance between endogenous superoxide stress and antioxidant defenses. J Bacteriol. 1998;180:1402–10. doi: 10.1128/jb.180.6.1402-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gralnick JA, Downs DM. The YggX protein of Salmonella enterica is involved in Fe(II) trafficking and minimizes the DNA damage caused by hydroxyl radicals: residue CYS-7 is essential for YggX function. J. Biol. Chem. 2003;278:20708–15. doi: 10.1074/jbc.M301577200. [DOI] [PubMed] [Google Scholar]
- 31.Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:6181–5. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffith KL, Shah IM, Wolf RE., Jr Proteolytic degradation of Escherichia coli transcription activators Sox and MarA as the mechanism for reversing the induction of the superoxide (SoxRS) and multiple antibiotic resistance (Mar) regulons. Mol Microbiol. 2004;51:1801–16. doi: 10.1046/j.1365-2958.2003.03952.x. [DOI] [PubMed] [Google Scholar]
- 34.Gu M, Imlay JA. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 2011;79:1136–50. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol. Microbiol. 2013;89:123–34. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan HM, Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J. Biol. Chem. 1977;252:7667–72. [PubMed] [Google Scholar]
- 37.Hassan HM, Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J. Biol. Chem. 1978;253:8143–8. [PubMed] [Google Scholar]
- 38.Helmann JD. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J. Biol. Chem. 2014;289:28112–20. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo YJ, Chung IY, Cho WJ, Lee BY, Kim JH, et al. The major catalase gene (katA) of Pseudomonas aeruginosa PA14 is under both positive and negative control of the global transactivator OxyR in response to hydrogen peroxide. J Bacteriol. 2010;192:381–90. doi: 10.1128/JB.00980-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ieva R, Roncarati D, Metruccio MM, Seib KL, Scarlato V, Delany I. OxyR tightly regulates catalase expression in Neisseria meningitidis through both repression and activation mechanisms. Mol Microbiol. 2008;70:1152–65. doi: 10.1111/j.1365-2958.2008.06468.x. [DOI] [PubMed] [Google Scholar]
- 41.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11:443–54. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 43.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem Res Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson FS, Morgan RW, Christman MF, Ames BN. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. JBC. 1989;264:1488–96. [PubMed] [Google Scholar]
- 45.Jacquamet L, Traore DA, Ferrer JL, Proux O, Testemale D, et al. Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol. Microbiol. 2009;73:20–31. doi: 10.1111/j.1365-2958.2009.06753.x. [DOI] [PubMed] [Google Scholar]
- 46.Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007;282:929–37. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang S, Imlay JA. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol. 2010;78:1448–67. doi: 10.1111/j.1365-2958.2010.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Jo I, Chung I-Y, Bae H-@, Kim J-S, Song S, Cho Y-H, Ha N-C. Structural details of OxyR peroxide-sensing mechanism. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1424495112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallifidas D, Thomas D, Doughty P, Paget MSB. The sigmaR regulon of Streptomyces oelicolor A3(2) reveals a key role in protein quality control during disulphide stress. Microbiology. 2010;156:1661–72. doi: 10.1099/mic.0.037804-0. [DOI] [PubMed] [Google Scholar]
- 49.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+ J Bacteriol. 2002;184:3151–8. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Park W. Oxidative stress response in Pseudomonas putida. Appl. MIcrobiol. Biotechnol. 2014;98:6933–46. doi: 10.1007/s00253-014-5883-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim M-S, Dufour YS, Yoo JS, Cho Y-B, Park J-H, et al. Conservation of thiol-oxidative stress responses regulated by SigR orthologues in actinomycetes. Mol. Microbiol. 2012;85:326–44. doi: 10.1111/j.1365-2958.2012.08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S, Bang Y-J, Kim D, Lim JG, Oh MH, Choi SH. Distinct characteristics of OxyR2, a new OxyR-type regulator, ensuring expression of peroxiredoxin 2 detoxifying low levels of hdyrogen peroxide in Vibrio vulnificus. Mol. Microbiol. 2014;93:992–1009. doi: 10.1111/mmi.12712. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi K, Fujikawa M, Kozawa T. Binding of promoter DNA to SoxR protein decreases the reduction potential of the [2Fe-2S] cluster. Biochemistry. 2014;54:334–9. doi: 10.1021/bi500931w. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi K, Tagawa S. Isolation of reductase for SoxR that governs an oxidative response regulon from Escherichia coli. FEBS Lett. 1999;451:227–30. doi: 10.1016/s0014-5793(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 55.Koo MS, Lee JH, Ray SY, Yeo WS, Lee JW, et al. A reducing system of the superoxide sensor SoxR in Escherichia coli. Embo J. 2003;22:2614–22. doi: 10.1093/emboj/cdg252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of phagocytosed bacteria. Mol. Microbiol. 2002;43:95–106. doi: 10.1046/j.1365-2958.2002.02719.x. [DOI] [PubMed] [Google Scholar]
- 57.Krapp AR, Humbert MV, Carrillo N. The soxRS response of Escherichia coli can be induced in the absence of oxidative stress and oxygen by modulation of NADPH content. Microbiology. 2011;157:957–65. doi: 10.1099/mic.0.039461-0. [DOI] [PubMed] [Google Scholar]
- 58.Krapp AR, Rodriguez RE, Poli HU, Paladini DH, Palatnik JF, Carillo N. The flavoenzyme ferredoxin (flavodoxin)-NADP(H) reductase modulates NADP(H) homeostasis during the soxRS response of Escherichia coli. J. Bacteriol. 2002;184:1474–80. doi: 10.1128/JB.184.5.1474-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo CF, Mashino T, Fridovich I. α, β-dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J Biol Chem. 1987;262:4724–7. [PubMed] [Google Scholar]
- 60.Lee C, Lee SM, Mukhopadhyay P, Kim SJ, Lee SC, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat. Struct. Mol. Biol. 2004;11:1179–85. doi: 10.1038/nsmb856. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Lee KL, Yeo WS, Park SJ, Roe JH. SoxRS-mediated lipopolysaccharide modification enhances resistance against multiple drugs in Escherichia coli. J Bacteriol. 2009;191:4441–50. doi: 10.1128/JB.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J-W, Helmann JD. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J. Biol. Chem. 2006;281:23567–78. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- 63.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalyzed histidine oxidation. Nature. 2006;440:363–7. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 64.Lemke RSS, Peterson AC, ziegelhoffer EC, Westphall MS, Tjellstrom H, et al. Synthesis and scavenging role of furan fatty acids. Proc. Natl. Acad. Sci. USA. 2014;111:E3450–E7. doi: 10.1073/pnas.1405520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin CN, Syu WJ, Sun WS, Chen JW, Chen TH, et al. A role of ygfZ in the Escherichia coli response to plumbagin challenge. J. Biomed. Sci. 2010;17:84. doi: 10.1186/1423-0127-17-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liochev SI, Benov L, Touati D, Fridovich I. Induction of the soxRS regulon of Escherichia coli by superoxide. J. Biol. Chem. 1999;274:9479–81. doi: 10.1074/jbc.274.14.9479. [DOI] [PubMed] [Google Scholar]
- 67.Liochev SI, Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci USA. 1992;89:5892–6. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liochev SI, Fridovich I. Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1997;94:2891–6. doi: 10.1073/pnas.94.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liochev SI, Hausladen A, Fridovich I. Nitroreductase A is regulated as a member of the soxRS regulon of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:3537–9. doi: 10.1073/pnas.96.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Bauer SC, Imlay JA. The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron. J. Bacteriol. 2011;193:2186–96. doi: 10.1128/JB.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loprosert S, Fuangthong M, Whangsuk W, Atichartpongkul S, Mongkolsuk S. Molecular and physiological analysis of an OxyR-regulated ahpC promoter in Xanthomonas campestris pv. Phaseoli. Mol. Microbiol. 2000;37:1504–14. doi: 10.1046/j.1365-2958.2000.02107.x. [DOI] [PubMed] [Google Scholar]
- 72.Luo L, Qi MS, Yao SY, Cheng HP, Zhu JB, Yu GQ. Role of oxyR from Sinorhizobium meliloti in regulating the expression of catalases. Acta Biochim Biophys Sin (Shanghai) 2005;37:421–8. doi: 10.1111/j.1745-7270.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- 73.Lynch R, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J. Biol. Chem. 1978;253:4697–9. [PubMed] [Google Scholar]
- 74.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 75.Mahavihakanont A, Charoenlap N, Namchaiw P, Eiamphungporn W, Chattrakarn S, et al. Novel roles of SoxR, a transcriptional regulator from Xanthomonas campestris, in sensing redox-cycling drugs and regulating a protective gene that have overall implications for bacterial stress physiology and virulence on a host plant. J. Bacteriol. 2012;194:209–17. doi: 10.1128/JB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75a.Mancini S, Imlay JA. The induction of two biosynthetic enzymes helps Escherichia coli sustain heme synthesis and activate catalase during hydrogen peroxide stress. Mol. Microbiol. 2015 doi: 10.1111/mmi.12967. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin RG, Rosner JL. Promoter discrimination at class I MarA regulon promoters mediated by glutamic acid 89 of the MarA transcriptional activator of Escherichia coli. J. Bacteriol. 2011;193:506–15. doi: 10.1128/JB.00360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–72. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mielecki D, Grzesiuk E. Ada response--a strategy for repair of alkylated DNA in bacteria. FEMS Microbiol. Lett. 2014;355:1–11. doi: 10.1111/1574-6968.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakayama T, Yonekura S-I, Yonei S, Zhang-Akiyama Q-M. Escherichia coli pyruvate:flavodoxin oxidoreductase, YdbK--regulation of expression and biological roles in protection against oxidative stress. Genes Genet. Syst. 2013;88:175–88. doi: 10.1266/ggs.88.175. [DOI] [PubMed] [Google Scholar]
- 80.Naseer N, Shapiro JA, Chander M. RNA-Seq analysis reveals a six-gene SoxR regulon in Streptomyces coelicolor. PLoS One. 2014;9:e106181. doi: 10.1371/journal.pone.0106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson KJ, Parsonage D, Hall A, Karplus PA, Poole LB. Cysteine pK(a) values for the bacterial peroxiredoxin AhpC. Biochemistry. 2008;47:12860–8. doi: 10.1021/bi801718d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nunoshiba T, Derojaswalker T, Wishnok JS, Tannenbaum SR, Demple B. Activation by nitric oxide of an oxidative stress response that defends Escherichia coli against activated macrophages. PNAS USA. 1993;90:9993–7. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–72. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 84.Paget MS, Molle V, Cohen G, Aharonowitz Y, Buttner MJ. Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the sigmaR regulon. Mol. Microbiol. 2001;42:1007–20. doi: 10.1046/j.1365-2958.2001.02675.x. [DOI] [PubMed] [Google Scholar]
- 85.Paiva SRd, Figueiredo MR, Aragão TV, Kaplan MAC. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem Inst Oswaldo Cruz. 2003;98:959–61. doi: 10.1590/s0074-02762003000700017. [DOI] [PubMed] [Google Scholar]
- 86.Palma M, Zurita J, Ferreras JA, Worgall S, Larone DH, et al. Pseudomonas aeruginosa SoxR does not conform to the archetypal paradigm for SoxR-dependent regulation of the bacterial oxidative stress adaptive response. Infect Immun. 2005;73:2958–66. doi: 10.1128/IAI.73.5.2958-2966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parent A, Caux-Thang C, Signor L, Clemancey M, Sethu R, et al. Single glutamate to aspartate mutation makes ferric uptake regulator (Fur) as sensitive to H2O2 as peroxide resistance regulator (PerR) Angew. Chem. Int. Ed. 2013;52:10339–43. doi: 10.1002/anie.201304021. [DOI] [PubMed] [Google Scholar]
- 88.Pomposiello PJ, Koutsolioutsou A, Carrasco D, Demple B. SoxRS-regulated expression and genetic analysis of the yggX gene of Escherichia coli. J Bacteriol. 2003;185:6624–32. doi: 10.1128/JB.185.22.6624-6632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poole LB. The catalytic mechanism of peroxiredoxins. Subcell. Biochem. 2007;44:61–81. doi: 10.1007/978-1-4020-6051-9_4. [DOI] [PubMed] [Google Scholar]
- 90.Pullan ST, Gidley MD, Jones RA, Barrett J, Stevanin TM, et al. NItric oxide in chemostat-cultured Escherichia coli is sensed by Fnr and other global regulators: unaltered methionine biosynthesis indicates lack of S nitrosation. J. Bacteriol. 2007;189:1845–55. doi: 10.1128/JB.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Putnam CD, Arvai AS, Bourne Y, Tainer JA. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol. 2000;296:295–309. doi: 10.1006/jmbi.1999.3458. [DOI] [PubMed] [Google Scholar]
- 92.Rai P, Cole TD, Wemmer DE, Linn S. Localization of Fe(2+) at an RTGR sequence within a DNA duplex explains preferential cleavage by Fe(2+) and H2O2. J Mol Biol. 2001;312:1089–101. doi: 10.1006/jmbi.2001.5010. [DOI] [PubMed] [Google Scholar]
- 93.Rau J, Stolz A. Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent Azo reductases. Appl Environ Microbiol. 2003;69:3448–55. doi: 10.1128/AEM.69.6.3448-3455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Runde S, Moliere N, Heinz A, MAisonneuve E, Janczikowski A, et al. The role of thiol oxidtive stress response in heat-induced protein aggregate formation during thermotolerance in Bacillus subtilis. Mol. Microbiol. 2014;91:1036–52. doi: 10.1111/mmi.12521. [DOI] [PubMed] [Google Scholar]
- 95.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 2001;183:7182–9. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seki M, Iida K, Saito M, Nakayama H, Yoshida S. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactase oxidase and coupling with aerobic utilization of lactate. J. Bacteriol. 2004;186:2046–51. doi: 10.1128/JB.186.7.2046-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seth D, Hausladen A, Wang YJ, Stamler JS. Endogenous protein S-nitrosylation of E. coli: regulation by OxyR. Science. 2012;336:470–3. doi: 10.1126/science.1215643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheplock R, Recinos DA, Mackow N, Dietrich LE, Chander M. Species-specific residues calibrate SoxR sensitivity to redox-active molecules. Mol. Microbiol. 2012;87:368–81. doi: 10.1111/mmi.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin JH, Singh AK, Cheon DJ, Roe J-H. Activation of the SoxR regulon in Streptomyces colelicolor by the extracellular form of the pigmented antibiotic actinorhodin. J. Bacteriol. 2011;193:75–81. doi: 10.1128/JB.00965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh AK, Shin J-H, Lee K-L, Imlay JA, Roe J-H. Comparative study of SoxR activation by redox-active compounds. Mol. Microbiol. 2013;90:983–96. doi: 10.1111/mmi.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sobota JM, Gu M, Imlay JA. Intracellular hydrogen peroxide and superoxide poison 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J. Bacteriol. 2014;196:1980–91. doi: 10.1128/JB.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sobota JM, Imlay JA. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. USA. 2011;108:5402–7. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teramoto H, Inui M, Yukawa H. OxyR acts as a transcriptional repressor of hdyrgen peroxide-inducible antioxidant genes in Corynebacterium glutamicum R. FEBS J. 2013;280:3298–312. doi: 10.1111/febs.12312. [DOI] [PubMed] [Google Scholar]
- 104.Tosatto SCE, Bosello V, Fogolari F, Mauri P, Roveri A, et al. The catalytic site of glutathione peroxidases. Antiox. Redox Rep. 2008;10:1515–26. doi: 10.1089/ars.2008.2055. [DOI] [PubMed] [Google Scholar]
- 105.Traore DA, Ghaouani AE, Ilango S, Dupuy J, Jacquamet L, et al. Crystal structure of the apo-PerR-Zn protein from Bacillus subtilis. Mol. Microbiol. 2006;61:1211–9. doi: 10.1111/j.1365-2958.2006.05313.x. [DOI] [PubMed] [Google Scholar]
- 106.Tsaneva IR, Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990;172:4197–205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner JM, Messenger AJ. Occurrence, biochemistry, and physiology of phenazine pigment production. Adv Microb Physiol. 1986;27:211–75. doi: 10.1016/s0065-2911(08)60306-9. [DOI] [PubMed] [Google Scholar]
- 108.Varghese S, Tang Y, Imlay JA. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J. Bacteriol. 2003;185:221–30. doi: 10.1128/JB.185.1.221-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Varghese S, Wu A, Park S, Imlay KRC, Imlay JA. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol. 2007;64:822–30. doi: 10.1111/j.1365-2958.2007.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Kern SE, Newman DK. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J Bacteriol. 2010;192:365–9. doi: 10.1128/JB.01188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J. Bacteriol. 2011;193:3606–17. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watanabe S, Kita A, Kobayashi K, Miki K. Crystal structure of the [2Fe-2S] oxidative-stress sensor SoxR bound to DNA. Proc Natl Acad Sci USA. 2008;105:4121–6. doi: 10.1073/pnas.0709188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. Implications for microbial killing. J. Biol. Chem. 2006;281:39860–9. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 114.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Rad. Biol. Med. 1999;27:322–8. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 115.Yamamoto K, Ishihama A, Busby SJ, Grainger DC. The Escherichia coliK-12 MntR miniregulon includes dps, which encodes the major stationary-phase DNA-binding protein. J. Bacteriol. 2011;193:1477–80. doi: 10.1128/JB.01230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–21. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 117.Zheng M, Doan B, Schneider TD, Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–43. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183:4562–70. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuber P. Management of oxidative stress in Bacillus. Annu. Rev. Microbiol. 2009;63:575–97. doi: 10.1146/annurev.micro.091208.073241. [DOI] [PubMed] [Google Scholar]


