Abstract
Background and Aims Iron is an essential micronutrient for all organisms and its uptake, translocation, distribution and utilization are regulated in a complex manner in plants. FER, isolated from tomato (Solanum lycopersicum), was the first transcription factor involved in the iron homeostasis of higher plants to be identified. A FER defect in the T3238fer mutant drastically downregulates the expression of iron uptake genes, such as ferric-chelate reductase 1 (LeFRO1) and iron-regulated transporter 1 (LeIRT1); however, the molecular mechanism by which FER regulates genes downstream remains unknown. The aim of this work was therefore to identify the gene that interacts with FER to regulate the iron-deficiency response in tomato.
Methods The homologue of the Arabidopsis Ib subgroup of the basic helix–loop–helix (bHLH) proteins, SlbHLH068, was identified by using the program BLASTP against the AtbHLH39 amino acid sequence in the tomato genome. The interaction between SlbHLH068 and FER was detected using yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays. In addition, virus-induced gene silencing (VIGS) was used to generate tomato plants in which SlbHLH068 expression was downregulated. The expression of genes was analysed using northern blot hybridization and multiple RT-PCR analysis. Seedlings of wild-type and mutant plants were grown under conditions of different nutrient deficiency.
Key Results SlbHLH068 is highly upregulated in roots, leaves and stems in response to iron deficiency. An interaction between SlbHLH068 and FER was demonstrated using yeast two-hybrid and BiFC assays. The heterodimer formed by FER with SlbHLH068 directly bound to the promoter of LeFRO1 and activated the expression of its reporter gene in the yeast assay. The downregulation of SlbHLH068 expression by VIGS resulted in a reduction of LeFRO1 and LeIRT1 expression and iron accumulation in leaves and roots.
Conclusions The results indicate that SlbHLH068, as a putative transcription factor, is involved in iron homeostasis in tomato via an interaction with FER.
Keywords: Tomato, Solanum lycopersicum, FER, SlbHLH068, iron homeostasis, transcription factor, iron deficiency, ferric-chelate reductase 1, iron-regulated transporter 1
INTRODUCTION
Iron is an essential micronutrient for plant growth and development. Its deficiency is one of the most common nutrient deficits in the world due to its low availability in alkaline soils, which comprise about one-third of the world’s soils (Mori, 1999). Under iron-limiting stress, plants show typical iron-deficient symptoms, such as leaf chlorosis and subsequent inhibition of plant growth and development. The uptake, translocation, internal distribution and utilization of iron are genetically controlled, and different plant species and genotypes possess various efficiencies (Kong et al., 2013). Under iron limitation, all plants except grasses use the strategy I mechanism (including the extrusion of protons to increase the solubility of ferric iron in the soil, reduction of Fe3+ to Fe2+ by ferric-chelate reductase on the root surface, and activation of high-affinity transporter IRT1 expression to enhance Fe2+ transport into roots) to mobilize and take up iron from the soil to meet their growth and development demands (Marschner et al., 1986; Römheld, 1987; Schmidt, 2003).
In the model plant Arabidopsis thaliana, ferric-chelate reductase 2 (AtFRO2) and iron transporter 1 (AtIRT1) are two key proteins involved in iron reduction and uptake (Eide et al., 1996; Robinson et al., 1999). The knockout mutant of either gene shows increased sensitivity to iron limitation (Robinson et al., 1999; Connolly and Guerinot, 2002; Henriques et al., 2002; Varotto et al., 2002; Vert et al., 2002). Several studies have determined that the expression of AtFRO2 and AtIRT1 is controlled by a set of basic helix–loop–helix (bHLH) transcriptional factors (Colangelo and Guerinot, 2004; Yuan et al., 2005, 2008; Wang et al., 2013). Among these bHLH regulators, FIT is a homologue of tomato FER in Arabidopsis and plays a key role in this regulatory cascade (Ling et al., 2002; Yuan et al., 2005). Plants lacking a functional FIT protein failed to induce the expression of AtFRO2, dramatically downregulated the accumulation of AtIRT1 mRNA and its protein, and exhibited seedling lethality under iron-limiting stress (Colangelo and Guerinot, 2004; Jakoby et al., 2004; Yuan et al., 2005). Furthermore, FIT interacts with the Ib subgroup of the bHLH protein family (AtbHLH38, AtbHLH39, AtbHLH100 and AtbHLH101), modulating the expression of AtFRO2 and AtIRT1 (Yuan et al., 2008; Wang et al., 2013).
Tomato (Solanum lycopersicum) is both an economically important crop and a model plant for studying iron homeostasis in strategy I plants. A few of the genes involved in iron uptake in tomato have been isolated and characterized. LeFRO1 is the homologue of AtFRO2 and is required for iron homeostasis in roots, shoots and reproductive organs (Li et al., 2004). LeIRT1 and LeIRT2 share high sequence similarity and are expressed in a root-specific manner, but only LeIRT1 is strongly induced by iron starvation (Eckhardt et al., 2001). LeNRAMP1 and LeNRAMP3, two other iron transporters, belong to the natural resistance-associated macrophage protein (NRAMP) family. LeNRAMP1 is significantly upregulated under iron limitation in roots, while LeNRAMP3 transcripts were detected in the root, leaf and cotyledon, and its expression was slightly elevated by iron starvation (Bereczky et al., 2003).
The identification and characterization of chloronerva (CHLN) and FER in tomato advanced our understanding of the regulation and signal transduction of iron metabolism. The CHLN gene encodes a nicotianamine (NA) synthase, which is required to make non-protein amino acid NA in the plant. NA is able to form a complex with Fe2+ (NA–Fe) for the distribution of iron in plants. In the chln mutant, although the iron-deficiency response and uptake were constitutively stimulated, seedlings exhibited typical symptoms of iron deficiency with severe intervein chlorosis (Ling et al., 1996, 1999; Bereczky et al., 2003). The NA–Fe complex was speculated to be a repressor of the iron-deficient response and uptake (Becker et al., 1992). FER was the first identified transcriptional factor involved in iron homeostasis in higher plants (Ling et al., 2002). The expression of FER was stimulated by iron starvation at both the transcriptional and the post-transcriptional levels (Ling et al., 2002; Brumbarova and Bauer, 2005). Under low iron conditions, the spontaneous mutant T3238fer is unable to activate iron-deficiency responses, such as developing transfer cells, secreting protons, and enhancing the expression of LeFRO1, LeIRT1 and LeNRAMP1, and the reduction activity of ferric-chelate reductase (Ling et al., 2002; Bereczky et al., 2003; Li et al., 2004). However, the molecular mechanism underlying FER regulation of its downstream genes remains to be revealed. In this study, we report the isolation and characterization of SlbHLH068, another member of the regulatory pathway in the strategy I mechanism. We show that SlbHLH068 interacts with FER and functions in the regulation of iron-uptake gene expression and iron homeostasis in tomato.
MATERIALS AND METHODS
Plant materials and culture conditions
Wild-type tomato (Solanum lycopersicum ‘Moneymaker’) was used for gene cloning and expression profiling. The cultivar ‘Micro-Tom’ was used in the virus-induced gene silencing (VIGS) experiment. The expression pattern of SlbHLH068 was also analysed in iron-inefficient mutants, chln, fer, chln/fer, together with the corresponding wild-types Solanum lycopersicum ‘Bonner Beste’ (BB) and ‘T3238FER’ (BF), respectively. The double mutant chln/fer was described previously by Ling et al. (1996).
For treatment with different nutrient supplies, surface-sterilized seeds were incubated on moist filter paper at 25 °C until germination. After a further 3 d, the young seedlings were transferred to pots filled with quartz sand and grown in a growth chamber with a light/dark cycle of 16/8 h and a relative humidity of 75 %. They were irrigated with half-strength Hoagland’s solution. When the fourth true leaves emerged, the seedlings were gently removed from the pots, and the roots were completely washed with deionized water. Individual seedlings were transferred to 1·2-litre plastic pots containing Hoagland’s solution. The complete Hoagland’s solution contained 5·0 mm KNO3, 5·0 mm Ca(NO3)2, 1·0 mm MgSO4.7H2O, 5·0 mm KH2PO4, 0·1 mm Fe(III)–EDTA, 46 µm H3BO3, 0·3 µm CuSO4, 4·5 µm MnCl2, 0·1 µm (NH4)6Mo7O24.4H2O and 3·8 µm ZnSO4.7H2O. To investigate the response of the SlbHLH068 gene to the different levels of elemental nutrients, the plants were treated with nitrogen deficiency (0 µm), phosphorus deficiency (0 µm), potassium deficiency (0 µm), iron deficiency (0·1 µm), copper deficiency (0 µm), high copper (32 µm), zinc deficiency (0 µm), high zinc (100 µm), manganese deficiency (0 µm) or high manganese (450 µm) in the medium for 6 d. The nutrient solution was renewed every 3 d. Normally, iron-deficient symptoms, such as interveinal chlorosis in young leaves, were clearly observed after the low-iron treatment for 4 d.
The seedlings used for Agrobacterium infiltration were grown in pots for 3 weeks. In total, three and 20 plants were infiltrated with Agrobacterium containing TRV1/TRV2 and TRV1/TRV2-bHLH068, respectively. After injection, they were grown under normal conditions for 2 weeks until the appearance of the typical silencing phenotype was observed in the control plants infiltrated with Agrobacterium containing TRV1/TRV2-CH42. They were then transferred into the hydroponic system for an iron-deficient treatment (0·1 µm Fe(III)–EDTA) for 6 d. Reverse transcriptase PCR (RT-PCR) was then performed in the plants infiltrated with Agrobacterium containing TRV1/TRV2-bHLH068. Thirteen out of 20 plants showed suppressed expression of SlbHLH068, and were selected for determination of iron content.
Total RNA extraction and cDNA synthesis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA samples were treated with RNase-free DNase Ι (New England Biolabs, Ipswich, MA, USA) to remove genomic DNA. Then, the RNA concentration was measured using a spectrophotometer (NanoDrop 2000C; Thermo Scientific, Wilmington, DE, USA). First-strand cDNA was synthesized using the SuperScript II First-Strand RT-PCR Kit (Invitrogen) from 500 ng of DNase I-treated total RNA.
Northern blot analysis
About 10 µg of RNA was separated on a 1·1 % formaldehyde agarose gel and transferred onto Hybond-N+ membrane (Amersham, Little Chalfont, UK) by capillary blotting. In total, 25 ng of a gel-purified probe was labelled with [32P]dCTP following the manual of the Prime-a-Gene labelling system (Promega, Madison, WI, USA). The membrane was hybridized overnight with an isotope-labelled probe at 65 °C. Subsequently, the membrane was washed until the radioactivity was lower than 10 c.p.m. and wrapped with plastic wrap for autoradiography.
Multiplex RT-PCR analysis
The quantitative analysis of gene expression in VIGS plants was performed using Multiplex RT-PCR (GenomeLab GeXP Analysis System; Beckman Coulter, Brea, CA, USA; Chen et al., 2007; Yuan et al., 2008). Primer design and multiplex optimization were carried out using the GeXP Expression Profiler (http://www.beckman.com. September, 2010). The housekeeping gene Leef-1 a was used as an internal control to normalize expression levels of the other genes. All primers used in this experiment are listed in Supplementary Data Table S1.
Interaction analysis of SlbHLH068 and FER using a yeast two-hybrid assay
Specific primers (Supplementary Data Table S1) containing the restriction sites EcoRΙ and SalΙ were used to amplify the full-length coding sequence of FER and SlbHLH068 from a cDNA template generated from iron-deficiency-treated tomato roots using the proofreading DNA polymerase PrimeSTAR HS (TaKaRa, Dalian, China). PCR fragments were recovered and inserted into the pGEM T-Easy Vector (Promega) for sequence confirmation. Subsequently, the coding sequence fragments were introduced between the EcoRΙ and SalΙ restriction sites in the pAD-GAL4-2.1 and pBD-GAL4 Cam plasmids of the HybriZAP-2.1 system (Stratagene, La Jolla, CA, USA) to generate the pAD-FER, pBD-bHLH068, pAD-bHLH068 and pBD-FER constructs. To determine whether FER and bHLH068 can form a heterodimer or homodimer, the plasmid combinations pAD-FER/pBD-bHLH068, pAD-FER/pBD-FER and pAD-bHLH068/pBD-bHLH068 were co-transformed into competent cells of yeast (Saccharomyces cerevisiae) strain YRG-2 (Matα ura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3112 gal4-542 gal80-538 LYS2::UASGAL1-TATAGAL1-HIS3 URA3:: UASGAL4 17mers(x3)-TATACYC1-lacZ). Expression assays of reporter genes HIS3 and LacZ were carried out as follows. Cells transformed with the desired plasmid pair were grown in liquid medium to an OD600 of 0·1 and diluted in a 10× dilution series. For each dilution, 5 µL of cell suspension was spotted on an synthetic dextrose (SD) agar plate without tryptophan (Trp), leucine (Leu) or histidine (His) for interaction screening. Cells inoculated on SD plates without Trp and Leu were subjected to transformation screening. The transformants were then transferred to a nitrocellulose membrane. lacZ expression was determined by adding Z buffer containing X-gal. Yeast cells transformed with the plasmid pairs pAD-FER/pBD, pAD/pBD-bHLH068, pAD/pBD-FER and pAD-bHLH068/pBD were used as negative controls.
Bimolecular fluorescence complementation (BiFC) assay in Arabidopsis protoplasts
The complete coding sequences of FER and SlbHLH068 were amplified using specific primers (Supplementary Data Table S1) fused with the appropriate adapter for the Gateway system (Invitrogen). When the fragment was used for N-terminal fusion with a fluorescent protein, the stop codon was removed. Each amplified product was integrated in pDONR201 by a BP reaction (PCR product with attB sites in the ends is integrated with donor vector containing attP sites) according to the standard protocol of the Gateway system. Through an LR reaction (the entry clone with attL sites is recombined with destination vector harbouring attR sites), these fragments were recombined with destination vectors to generate the pFER-cCFP, pbHLH068-nYFP, pcCFP-FER, pnYFP-bHLH068, pFER-nYFP and pbHLH068-cCFP constructs. Protoplasts were prepared from leaves of 3-week-old Arabidopsis thaliana (ectotype Columbia) and transiently transformed by the polyethylene glycol (PEG)-mediated method according to Sheen (2001). The plasmid combinations pFER-cCFP/pbHLH068-nYFP, pcCFP-FER/pnYFP-bHLH068, pFER-cCFP/pFER-nYFP and pbHLH068-cCFP/pbHLH068-nYFP were separately introduced into protoplasts. For the negative control, the empty vectors pX-nYFP and pX-cCFP were co-transformed into protoplasts. Protoplasts transformed with the pA7-YFP plasmid containing the complete fluorescent protein were used as the positive control, which was assumed to show fluorescent signal in entire cells (Gampala et al., 2007; Yuan et al., 2008; Wang et al., 2013). After incubation for 16–20 h, the protoplast fluorescence was verified using a confocal microscope (FluoView 1000; Olympus, Tokyo, Japan).
Activation assay of SlbHLH068 on the LeFRO1 promoter
Based on the genomic DNA sequence of LeFRO1, specific primers (Supplementary Data Table S1) were designed to amplify the 995-bp upstream sequence of the open reading frame (ORF). The sequence was then cloned into the pGEM T-Easy Vector (Promega) for sequence verification and referred as pTPLeFRO1. After SacΙ/HindIII digestion of pTPLeFRO1, the promoter fragment of LeFRO1 was recovered and used to replace the 2 × 35S promoter in the pJIT166 vector (pGreen, http://www.pgreen.ac.uk. September, 2009) to generate the pPLeFRO1::GUS construct, which contains the complete expression cassette of the reporter gene GUS driven by the LeFRO1 promoter. Following cleavage by SacΙ/XhoΙ and blunting of the ends, the GUS expression cassette was inserted into the PmaCI restriction site of the pBD and pBD-FER vectors to generate the pBD-PLeFRO1::GUS and pBD-FER-PLeFRO1::GUS constructs. AtbHLH40, which is unable to interact with FIT (Yuan et al., 2008), was used as a negative control. The pAD vector and its derivatives pAD-SlbHLH068 and pAD-AtbHLH40 were co-transformed with pBD-PLeFRO1::GUS or pBD-FER-PLeFRO1::GUS into yeast cells. GUS activity in yeast cells was visualized using the filter lift assay as described in the yeast two-hybrid experiment, except for the use of GUS staining buffer instead of Z buffer (Jefferson et al., 1987). Quantitative measurement of GUS activity was performed according to Yuan et al. (2008).
TRV-based vector construction and Agrobacterium infiltration
The viral vector used in this study was derived from the Tobacco Rattle Virus (TRV), which can develop a PDS silencing phenotype with 50 % efficiency 10 d following Agrobacterium infiltration in tomato (Liu et al., 2002). TRV-based VIGS is most widely used in tobacco (Ratcliff et al., 2001; Liu et al., 2004), tomato (Fu et al., 2005), Arabidopsis (Turnage et al., 2002), pepper (Chung et al., 2004) and petunia (Chen et al., 2004). Previous studies determined that the TRV-based viral system is capable of silencing genes expressed in the leaf (Ratcliff et al., 2001), flower (Ratcliff et al., 2001), meristem (Peele et al., 2001), fruit (Jia et al., 2011) and potato tuber (Faivre-Rampant et al., 2004). A gene-specific primer pair (Supplementary Data Table S1) spanning 68 bp of the 5′-untranslated region (UTR) and 132 bp of the first exon sequence was used to amplify a 200-bp fragment of SlbHLH068. This fragment was subcloned into the pGEM T-Easy Vector (Promega) for sequence verification. The fragment was then digested by XbaΙ/SacΙ and recombined with linearized pTRV2 vector to generate pTRV2-bHLH068. All the constructs used for VIGS are shown in Supplementary Data Fig. S1. The pTRV1 and pTRV2 plasmids and derivatives were introduced into Agrobacterium strain GV3101 by heat shock. The infiltration procedure is as follows. A 5-mL initial culture was obtained by shaking overnight at 28 °C in Luria broth (LB) medium containing 100 µg mL−1 rifampicin and 50 µg mL−1 kanamycin. Then the culture was inoculated into 50-mL LB medium containing antibiotics, 10 mm 2-(N-morpholino)ethanesulfonic acid (MES) and 20 mm acetosyringone. After overnight growth, the Agrobacterium cells were harvested by centrifugation at 4000 g for 5 min and resuspended in buffer containing 10 mm MgCl2, 10 mm MES and 200 mm acetosyringone. After growth to an OD600 of 2.0, the cell suspension was left in the dark at room temperature for 3 h. Then the TRV1 and TRV2 suspensions were mixed in equal proportion and infiltrated into tomato leaves using a 1-mL syringe. The infiltrated plants were covered overnight.
Measurement of mineral concentration
Mineral concentration was analysed in tomato seedlings infected with Agrobacterium containing the TRV vectors. An individual plant was used as a single sample. Three and 13 plants infected with Agrobacterium containing pTRV1/pTRV2 and pTRV1/pTRV2-bHLH068, respectively, were included in the analysis. Following treatment with a limited iron supply for 6 d, the roots and leaves were harvested separately followed by complete washing with deionized water and drying overnight in a 65 °C oven. The procedure for the mineral concentration analysis was as previously described (Du et al., 2013). Each sample was measured twice.
RESULTS
Identification and isolation of the SlbHLH068 gene
FIT, the homologue of FER in Arabidopsis, interacts with the Ib subgroup of bHLH proteins (AtbHLH38, AtbHLH39, AtbHLH100 and AtbHLH101) to modulate the expression of downstream genes such as FRO2, IRT1, HMA3, MTP3, IRT2, NAS1 and NAS2 in A. thaliana (Yuan et al., 2008; Wu et al., 2012; Wang et al., 2013). We speculated that the homologue(s) of the Ιb subgroup of bHLH proteins in tomato might also interact with FER and play an important role in iron homeostasis. To identify such genes, the genome sequence of tomato was searched against the deduced amino acid sequence of AtbHLH39 using the program BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Three putative proteins, SlbHLH066 (XP_004249748), SlbHLH067 (XP_004249749) and SlbHLH068 (XP_004249750), were identified. In a previous study, these three proteins were grouped closely with the Ib subgroup of bHLH proteins in Arabidopsis by phylogenetic analysis, indicating that they may share conserved biological function with AtbHLH38/AtbHLH39/AtbHLH100/AtbHLH101 (Sun et al., 2015). Among the three tomato bHLH proteins, SlbHLH066 and SlbHLH067 were grouped together and showed high homology with AtbHLH38/AtbHLH39/AtbHLH100, while SlbHLH068 was separate from another two tomato homologues and tightly grouped with AtbHLH101. Considering the high sequence similarity and possible functional redundancy between SlbHLH066 and SlbHLH067, we chose SlbHLH068 for further study.
To determine the full-length transcript of SlbHLH068, PCR-based screening was performed in the cDNA library of iron-deficiency-induced tomato roots (Ling et al., 2002) using specific primers spanning the SlbHLH068 sequence. Additionally, the Bacterial Artificial Chromosome (BAC) clones containing SlbHLH068 were identified and sequenced. The full-length mRNA of SlbHLH068 was 1129 bp, including an ORF of 810 bp, 5′-UTR of 129 bp and 3′-UTR of 190 bp. This gene encodes a deduced polypeptide of 269 amino acids with a calculated molecular mass of 31 kDa.
Multiple sequence alignment of the deduced amino acid sequence of SlbHLH068 with the Ib subgroup of the bHLH proteins in Arabidopsis revealed that SlbHLH068 shares 40·4 % similarity with AtbHLH101, 39·8 % with AtbHLH100, 38·8 % with AtbHLH38 and 38.3 % with AtbHLH39. Conserved domain analysis (http://www.ncbi.nlm.nih.gov/Structure/cdd/docs/cdd_search.html) showed that SlbHLH068 contains a highly conserved bHLH motif (Fig. 1A). Comparison of the coding sequence and genomic DNA sequences indicated that SlbHLH068, like AtbHLH101, contained three exons and two introns (Fig. 1B).
Fig. 1.
Gene structure and sequence comparison of AtbHLH38, AtbHLH39, AtbHLH100, AtbHLH101 and SlbHLH068 (XP_004249750). (A) Alignment of the amino acid sequences of SlbHLH068 and four Arabidopsis homologues. Multiple sequence alignment was performed using the ClustalW program in the Lasergene software. Identical residues are shown in black. Underlined amino acid sequences are conserved bHLH domains predicted by the CDD program (http://www.ncbi.nlm.nih.gov/Structure/cdd/ March, 2015). (B) Gene structure comparison of SlbHLH068 and its four homologues in Arabidopsis. Gene structure was predicted by aligning coding sequence and genome DNA sequence of each gene. Grey boxes represent upstream/downstream untranslated region, black boxes denote exons and thin lines indicate introns.
Expression profile of SlbHLH068
To investigate the expression pattern and response of SlbHLH068 to iron status, northern blotting was performed in various tissues, including roots, leaves, stems, flowers and fruits, under both iron-deficient and iron-sufficient conditions. As shown in Fig. 2A, abundant SlbHLH068 mRNA was detected in roots, leaves and stems under iron deficiency, while no hybridization signal was observed in any tissue investigated under iron-sufficient conditions. These results indicate that the expression of SlbHLH068 is highly induced under iron deficiency. A time-course experiment of iron deficiency [treatment with 0·1 µm Fe(III)–EDTA from 6 h up to 8 d] was carried out with young tomato seedlings to investigate the expression response of SlbHLH068 to iron availability. The results showed that the expression of SlbHLH068 was obviously induced by the day 4 and continuously increased until the day 8. After resupplying Fe [100 µm Fe(III)–EDTA] to the culture solution, the expression of SlbHLH068 decreased dramatically and dropped to undetectable levels by the day 2 (Fig. 2B). Additionally, we analysed the response of SlbHLH068 to other nutrient conditions. As shown in Fig. 2C, SlbHLH068 expression was specifically upregulated under iron deficiency, but was not affected by various levels of other nutrients. These results suggest that SlbHLH068 may function specifically in iron homeostasis in tomato.
Fig. 2.
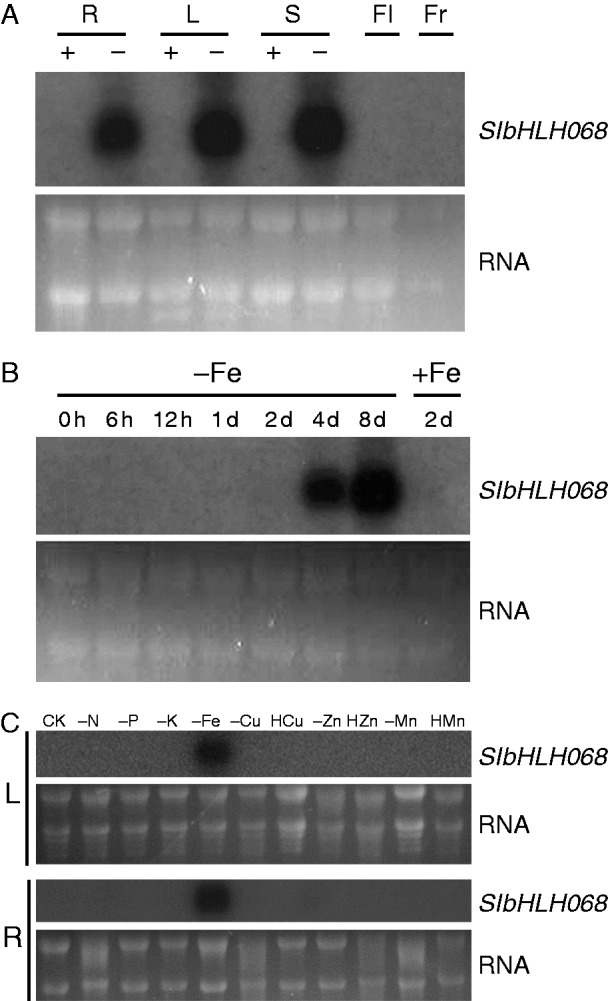
Expression profiling of SlbHLH068 in wild-type tomato (Lycopersicon esculentum ‘Moneymaker’). (A) Northern blot analysis of SlbHLH068 expression in roots (R), leaves (L), stems (S), flowers (Fl) and fruits (Fr) under differing iron supplies. Roots, leaves and flowers were collected from seedlings treated hydroponically for 6 d with sufficient (+) or deficient (−) iron. Flowers and young fruits were harvested from seedlings grown in a field with a sufficient iron supply. (B) Time-course experiment of SlbHLH068 expression in roots. The + and – signs indicate plants grown under conditions with 100 µm and 0·1 µm Fe(III)-EDTA, respectively. (C) Response of SlbHLH068 in leaves (L) and roots (R) to different elemental supply. – and H indicate deficient and high elemental levels, respectively. CK (complete nutrient condition), N (nitrogen), P (phosphorus), K (potassium), Fe (iron), Cu (copper), Zn (zinc), Mn (manganese). RNA stained with ethidium bromide was used as a control for equal loading.
SlbHLH068 interacts with FER and forms a heterodimer in yeast
To determine whether SlbHLH068 interacts with FER, a yeast two-hybrid experiment was performed using the HybriZAP-2.1 system (Stratagene). The full-length coding sequences of SlbHLH068 and FER were separately amplified and fused to the C terminals of both the GAL4 activation domain (AD) and binding domain (BD). Yeast cells transformed with different plasmid combinations were used for an auxotrophic assay and expression assay of β-galactosidase (lacZ). Three transformed yeast strains harbouring the plasmids pAD-FER/pBD-bHLH068, pAD/pBD-FER and pAD-FER/pBD-FER were able to grow on the SD agar plates lacking Trp, Leu and His, whereas the strains containing other plasmid combinations did not grow on these plates (Fig. 3A). The filter lift assay showed that the three strains could also activate lacZ expression and produce the blue colour in the membrane soaked in assay solution (Fig. 3B). These results confirmed that SlbHLH68 interacts with FER in yeast cells. As the negative control transformed with pAD/pBD-FER was able to initiate expression of the reporter gene, the yeast cells containing pAD-FER/pBD-FER grew on the selective plate and showed β-galactosidase activity, as expected. The yeast two-hybrid system appears to be unsuitable for determining whether FER can interact with itself, but SlbHLH068 obviously could not interact with itself to form a homodimer in yeast cells because yeast cells containing pAD-bHLH068/pBD-bHLH068 did not activate the transcription of reporter genes (Fig. 3A).
Fig. 3.
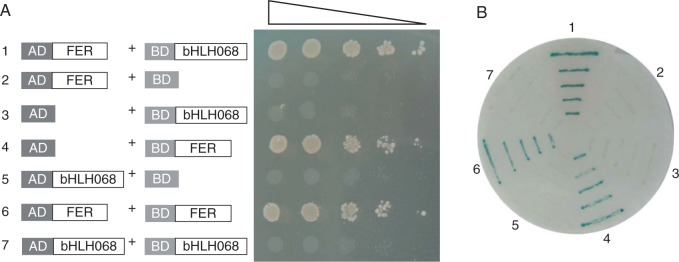
Interaction analysis of SlbHLH068 and FER by a yeast two-hybrid assay. (A) Yeast cells grown on SD plates without Trp, Leu and His. (B) Filter lift assay of lacZ expression for yeast cells grown on SD plates without Trp and Leu. The numbers 1–7 indicate yeast cells transformed with the following plasmid combinations: 1, pAD-FER/pBD-bHLH068; 2, pAD-FER/pBD; 3, pAD/pBD-bHLH068; 4, pAD/pBD-FER; 5, pAD-bHLH068/pBD; 6, pAD-FER/pBD-FER; 7, pAD-bHLH068/pBD-bHLH068.
The FER/SlbHLH068 complex can bind the LeFRO1 promoter and activate gene expression in yeast
SlbHLH068 interacted with FER and formed a heterodimer in yeast cells. To examine whether the heterodimer controls the expression of iron uptake genes, such as LeFRO1 in tomato, a transcription activation assay was performed using the yeast system described by Yuan et al. (2008). The plasmid pairs pAD/pBD-PLeFRO1::GUS, pAD/pBD-FER-PLeFRO1::GUS, pAD-SlbHLH068/pBD-PLeFRO1::GUS and pAD-SlbHLH068/pBD-FER-PLeFRO1::GUS were introduced into yeast cells. As shown by Yuan et al. (2008), AtbHLH40 was not able to interact with FIT to initiate activity of the AtFRO2 promoter. Assuming no interaction between AtbHLH40 and FER, the pAD-AtbHLH40 plasmid combined with either pBD-PLeFRO1::GUS or pBD-FER-PLeFRO1::GUS was also co-transformed into yeast cells as negative controls. Filter lift assays for GUS staining showed that a strong blue colour appeared in yeast cells containing the plasmid pair pAD-SlbHLH068/pBD-FER-PLeFRO1::GUS, whereas no blue colour was observed in the negative controls and cells transformed with other plasmid combinations (Fig. 4A). Consistent with the result of filter lift assays, GUS activity in the yeast cells containing the pAD-SlbHLH068/pBD-FER-PLeFRO1::GUS plasmids was significantly higher than that of yeast cells with other plasmid combinations, which exhibited little GUS activity, as in the negative controls (Fig. 4B). These results indicate that the interaction of FER and SlbHLH068 activated GUS expression driven by the FRO1 promoter in yeast cells.
Fig. 4.
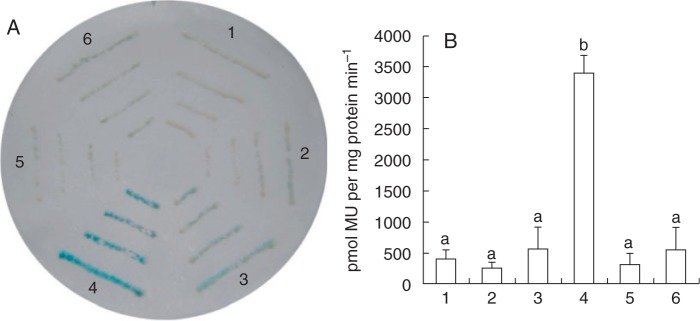
Transcriptional activation assay of the GUS gene driven by the LeFRO1 promoter in yeast. (A) Filter lift assay of GUS activity in yeast cells grown on SD plates without Trp and Leu. (B) Quantitative analysis of GUS activity in yeast cells cultured in liquid SD medium without Trp and Leu. Values are the mean of three replicates ±s.d. Different lowercase letters indicate significance as determined using ANOVA and Tukey’s post-hoc multiple comparison test at P < 0.05. The numbers 1–6 indicate plasmid combinations as follows: 1, pAD/pBD-PLeFRO1::GUS; 2, pAD/pBD-FER-PLeFRO1::GUS; 3, pAD-SlbHLH068/pBD-PLeFRO1::GUS; 4, pAD-SlbHLH068/pBD-FER-PLeFRO1::GUS; 5, pAD-AtbHLH40/pBD-PLeFRO1::GUS; 6, pAD-AtbHLH40/pBD-FER-PLeFRO1::GUS.
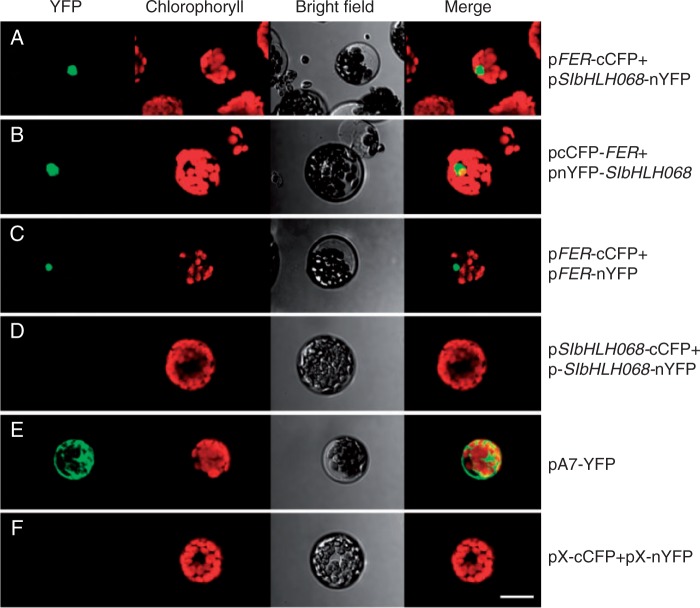
SlbHLH068 interacts with FER in the nucleus of Arabidopsis protoplasts
To further confirm the interaction between the FER and SlbHLH068 proteins in plant cells, a BiFC assay was performed in Arabidopsis protoplasts. The full-length coding sequences of FER and SlbHLH068 were cloned into vectors pX-nYFP and pX-cCFP to generate constructs pFER-cCFP, pFER-nYFP, pSlbHLH068-cCFP and pSlbHLH068-nYFP. These constructs were then transformed in pairs into Arabidopsis protoplasts. Meanwhile, the plasmid pA7-YFP and the empty plasmid pair pX-cCFP/pX-nYFP were applied as positive and negative control, respectively. As shown in Fig. 5, a strong fluorescence signal was detected in the nucleus of protoplasts transformed with the pFER-cCFP/pSlbHLH068-nYFP (Fig. 5A), pcCFP-FER/pnYFP-SlbHLH068 (Fig. 5B) and pFER-cCFP/pFER-nYFP (Fig. 5C) plasmids, whereas no signal was observed in the protoplasts transformed with the pSlbHLH068-cCFP/pSlbHLH068-nYFP (Fig. 5D) constructs or the empty vectors pX-nYFP/pX-cCFP (Fig. 5F). These results indicate that FER interacts with SlbHLH068 in plant cells. In addition, FER showed interaction with itself, but self-interaction did not occur with SlbHLH068. Consistent with the prediction for FER and SlbHLH068 as transcription factors, the fluorescent signal stimulated by a protein–protein interaction was specifically localized in the nucleus (Fig. 5A–C), but the signal representing an intact yellow fluorescent protein (YFP) was distributed throughout the whole cell (Fig. 5E).
Fig. 5.
BiFC assay of the interaction between FER and SlbHLH068 in Arabidopsis protoplast cells. Arabidopsis mesophyll protoplasts were transformed with different plasmid or plasmid pairs as follows. pFER-cCFP/pbHLH068-nYFP: co-transformation of FER and SlbHLH068 integrated in the N terminus of fluorescent protein; pcCFP-FER/pnYFP-bHLH068: co-transformation of FER and SlbHLH068 integrated in the C terminus of fluorescent protein; pFER-cCFP/pFER-nYFP: co-transformation of cCFP- and nYFP-fused FER proteins; pbHLH068-cCFP/pbHLH068-nYFP: co-transformation of cCFP- and nYFP-fused SlbHLH068 proteins; pA7-YFP: a positive control containing full-length fluorescent protein; pX-cCFP/pX-nYFP: empty plasmid pair as a negative control. Images were captured 16–20 h after transient expression using an Olympus confocal microscope. The protoplasts transformed with pFER-cCFP/pbHLH068-nYFP, pcCFP-FER/pnYFP-bHLH068 and pFER-cCFP/pFER-nYFP displayed fluorescent signals in the nucleus, whereas no fluorescent signal was observed in the nucleus of protoplasts transformed with other plasmid combinations. Scale bar = 20.0 µm.
Reduced expression of SlbHLH068 results in decreased iron accumulation
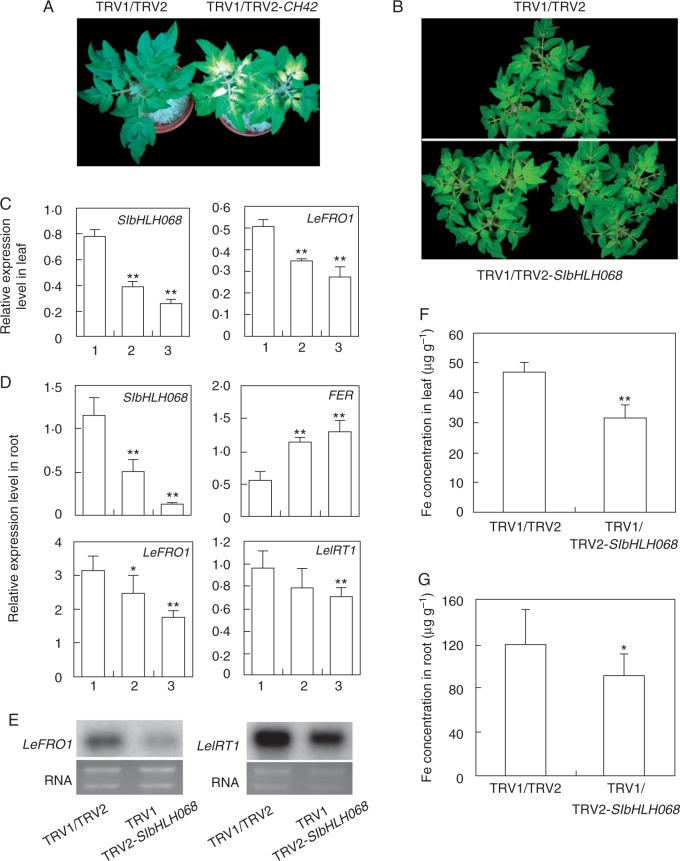
To address the physiological role of SlbHLH068 in iron homeostasis, we generated tomato plants in which SlbHLH068 expression was downregulated by VIGS. A mixture of Agrobacterium cultures containing the viral constructs pTRV1/pTRV2-bHLH068, pTRV1/pTRV2-CH42 (as a positive control) or pTRV1/pTRV2 (as a negative control) was infiltrated into the leaves of 3-week-old tomato seedlings. Two weeks later, the positive control plants developed a photo-bleached phenotype in the upper un-infiltrated leaves, which is consistent with the expected phenotype for the loss function of the CH42 gene (Fitzmaurice et al., 1999; Huang and Li, 2009; Kjemtrup et al., 1998). Meanwhile, no bleached phenotype appeared in the negative control plants. This indicated that gene silencing was initiated in tomato seedlings via the systemic spreading of TRV and that the VIGS system acted in the specific downregulation of the target gene (Fig. 6A).
Fig. 6.
Iron-deficiency response in SlbHLH068-silenced tomato plants. (A) CH42-silenced tomato with pale green leaves. The plant on the left is infiltrated with the empty vector. The plant on the right is infiltrated with pTRV1/pTRV2-CH42. (B) Tomato infiltrated with an empty vector (upper) or pTRV1/pTRV2-bHLH068 (lower) after 6 d of iron-deficiency treatment. (C) Multiplex RT-PCR analysis of SlbHLH068 and LeFRO1 expression in leaves of control and SlbHLH068-silenced plants. 1, plant infiltrated with empty vector; 2 and 3, plants infiltrated with TRV1/TRV2-bHLH068. Values are the mean of five replicates ±s.d. (D) Multiplex RT-PCR analysis of SlbHLH068, FER, LeFRO1 and LeIRT1 expression in the roots of plants infiltrated with an empty vector or TRV1/TRV2-bHLH068. (E) Northern blot analysis of LeFRO1 and LeIRT1 expression in the roots of plants infiltrated with an empty vector or TRV1/TRV2-bHLH068. RNA stained with ethidium bromide was used as a control for equal loading. (F,G) Iron content in leaves and roots of control and silenced plants. Values are the mean ± s.d. in three control plants, and 13 out of 20 plants treated with pTRV1/pTRV2-bHLH068 showed reduced expression of bHLH068. Asterisks indicate significant difference at **P < 0.01 and *P < 0.05, respectively.
For the iron-deficiency treatment, plants infected by Agrobacterium with either pTRV1/pTRV2-bHLH068 or pTRV1/pTRV2 were transferred into Hoagland’s solution with 0·1 µm Fe(III)–EDTA. Six days later, the control plants infected with empty vectors showed a typical iron-deficiency phenotype with chlorotic young leaves. Compared with the control plants, more pronounced chlorosis was observed in plants infected with pTRV1/pTRV2-bHLH068 (Fig. 6B). To further investigate the physiological and molecular changes in the infected plants, roots and upper un-infiltrated leaves were harvested to examine gene expression and determine the mineral concentration. As shown in Fig. 6C and D, SlbHLH068 transcript accumulation was significantly diminished in the plants infected with pTRV1/pTRV2-bHLH068 under iron deficiency. Meanwhile, we also observed decreased LeFRO1 transcript levels in leaves and roots, and decreased LeIRT1 transcripts in roots. FER expression was markedly increased compared with control plants (Fig. 6D). The gene expression change was further confirmed by northern blot analysis. The mRNA abundance of LeFRO1 and LeIRT1, consistent with the results of multiplex RT-PCR, was much lower in the roots of plants infiltrated with pTRV1/pTRV2-bHLH068 (Fig. 6E). Additionally, we measured the iron concentration in the 13 pTRV1/pTRV2-bHLH068-infiltrated plants, which showed reduced bHLH068 expression (determined by RT-PCR, data not shown). The iron concentration of SlbHLH068-VIGS plants (infiltrated with TRV1/TRV2-SlbHLH068) was 33 % lower in leaves and 23·5 % lower in roots than in the controls (infiltrated with TRV1/TRV2) under iron deficiency. These results imply that the suppression of SlbHLH068 expression resulted in the reduction of LeFRO1 and LeIRT1 expression and iron accumulation in leaves and roots under iron deficiency.
Expression analysis of SlbHLH068 in iron-inefficient tomato mutants
In tomato, two iron-inefficient mutants, T3238fer (fer) and chloronerva (chln), were reported, and their corresponding genes have been isolated (Ling et al., 1996, 1999). In the fer mutant, the iron-deficiency response is not switched on under iron limitation (Ling et al., 1996, 2002), while the chln mutant exhibits constitutive activation of iron-deficiency responses and accumulates excess iron in the mutant plants (Ling et al., 1999). To reveal the relationship between SlbHLH068 and FER/CHLN, we investigated the expression of SlbHLH068 in fer, chln, the double mutant fer/chln and their corresponding wild-type plants. As shown in Fig. 7, the expression of SlbHLH068, as in the wild-type, was consistently induced in roots and leaves under low-iron growth conditions in fer, chln and fer/chln. The transcription level of SlbHLH068 in the roots of fer and chln mutants was higher than in the wild-type under iron deficiency. The enhanced expression was even more pronounced in the leaves of double mutant fer/chln than in the single mutants. Based on these results, we speculate that the iron-deficiency-induced expression of SlbHLH068 is not regulated by FER or CHLN, and that the enhanced expression of SlbHLH068 in the single and double mutants under iron deficiency may be due to the increased iron-deficiency signal in the mutant plants.
Fig. 7.
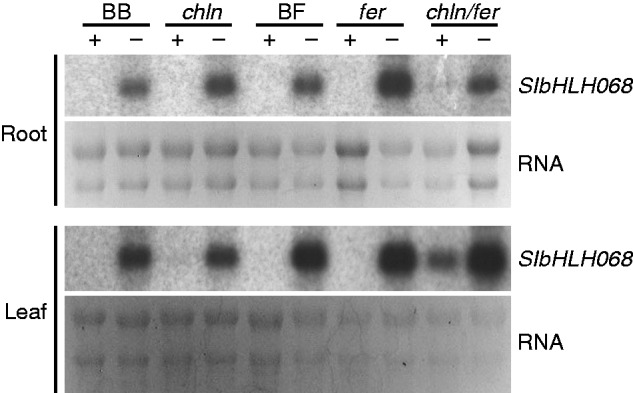
Northern blot analysis of SlbHLH068 in roots and leaves of the tomato mutants. chln, fer, chln/fer and the corresponding wild-types BB (Bonner Beste) and BF (T3238FER). Young wild-type and mutant seedlings were grown on half-strength Hoagland’s solution until emergence of the fourth true leaf. The roots were completely washed with deionized water, and plants were transferred into culture pots containing Hoagland’s solution with 100 µm (+) or 0·1 µm (−) Fe(III)–EDTA for 6 d. Total RNA was extracted from roots and leaves, and the expression intensity of SlbHLH068 was determined by northern blotting. RNA stained with ethidium bromide was used as a control for equal loading.
DISCUSSION
We isolated a bHLH protein-encoding gene, SlbHLH068, which is highly and specifically induced at the transcriptional level by a low iron supply (Fig. 2). SlbHLH068 regulates iron-deficiency response genes through interactions with FER in tomato (Figs 3–5). Knockdown of SlbHLH068 expression significantly reduced the expression of the major iron uptake genes LeFRO1 and LeIRT1, and the iron concentration in shoots and roots under iron deficiency. The SlbHLH068-silenced plants exhibited increased sensitivity to iron limitation stress compared with the wild-type (Fig. 6).
Although reduced expression of SlbHLH068 led to the downregulation of the iron-deficiency response in tomato, the effect in SlbHLH068-silenced plants was obviously not as severe as in the T3238fer mutant (Fig. 6B). Without a sufficient iron supply, T3238fer plants die at the seedling stage (Ling et al., 2002). Although the VIGS plants grew poorly, they were still alive. One possible reason is that T3238fer is a null mutant, while the SlbHLH068-silenced plants generated by VIGS still partially express SlbHLH068 (Fig. 6C, D). Another possibility is that because SlbHLH068-silenced plants have grown 5 weeks before iron deficiency treatment, the 5-week-old plants had already accumulated some iron and possessed the ability to tolerate limited iron stress. Additionally, we demonstrated the important role of SlbHLH068 in the manipulation of iron uptake, although we cannot completely rule out the possibility of the functional redundancy of SlbHLH068 in this study. A bHLH subgroup comprising functionally redundant bHLH proteins may be present in tomato, as observed in Arabidopsis (Yuan et al., 2008; Wu et al., 2012; Wang et al., 2013). Two genes (SlbHLH066 and SlbHLH067) identified by BLAST against the AtbHLH39 sequence support this hypothesis. The presence of these homologous genes may partially complement the function of SlbHLH068 and recover the defect in the regulatory pathway.
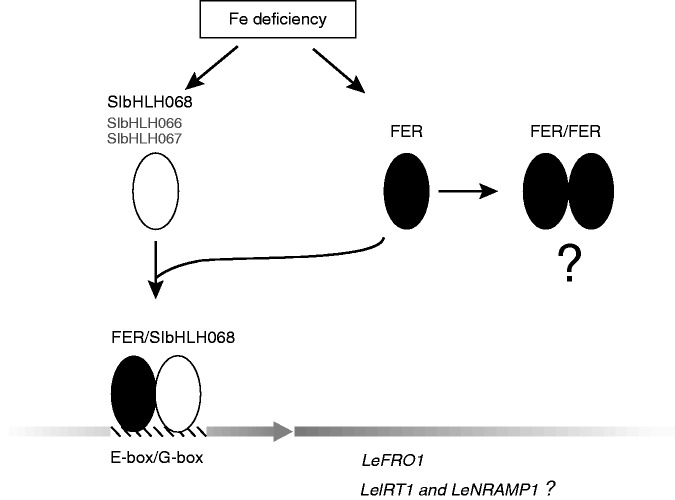
The predicted regulatory mechanism of the bHLH protein is the formation of a dimer that binds with a consensus DNA sequence (Toledo-Ortiz et al., 2003). In our study, yeast two-hybrid and BiFC experiments demonstrated that FER can form both a heterodimer with SlbHLH068 and a homodimer with itself in the nucleus. We speculate that FER may play various roles in tomato nutrition homeostasis via the formation of dimers with different partners. As described in previous studies, the core consensus sequence recognized by the bHLH protein is the E-box (CANNTG), of which the G-box (CACGTG) is the most common type (Ellenberger et al., 1994; Ferre-D’Amare et al., 1994; Shimizu et al., 1997; Toledo-Ortiz et al., 2003; Sun et al., 2015). Motif analysis indicated that one E-box and one G-box were present in the LeFRO1 promoter (Supplementary Data Fig. S2), which implies direct binding of the bHLH protein to the promoter region. Furthermore, the binding assay of the promoter of LeFRO1 revealed that the FER/SlbHLH068 heterodimer, but not the FER/FER homodimer, can directly bind and activate LeFRO1 (Fig. 4). The function of the FER/FER homodimer remains to be investigated. It is possible that the homodimer functions as a competitor of the heterodimer to achieve the fine tuning of iron homeostasis in tomato roots. As both LeFRO1 and LeIRT1 are deregulated by iron deficiency in the T3238fer mutant, FER/SlbHLH068 may also control LeIRT1 in a similar manner to LeFRO1, but a binding assay would be necessary to confirm this idea. Another two proteins, SlbHLH066 and SlbHLH067, were identified by BLAST search against the protein sequence of the Arabidopsis Ib subgroup of bHLH protein in this study. They were also closely grouped with SlbHLH068 and Arabidopsis homologues by phylogenetic analysis in the study of Sun et al. (2015). The transcriptional products of these genes, like SlbHLH068, are induced under iron-deficiency conditions. We propose the subfamily of bHLH protein in tomato, consisting of SlbHLH066, SlbHLH067 and SlbHLH068, may play a similar role to AtbHLH38/AtbHLH39/AtbHLH100/AtbHLH101 in Arabidopsis. Further study on these two genes will help us understand the functional conservation and divergence of bHLH proteins in iron homeostasis. The hypothesized regulatory network in which FER and SlbHLH068 are involved is summarized in Fig. 8.
Fig. 8.
Regulatory model of transcription factors FER and SlbHLH068 in tomato. The iron-deficient signal initiates expression of the transcription activator genes FER and SlbHLH068 in root cells. The heterodimers formed by FER and SlbHLH068 directly bind to the LeFRO1 promoter and activate its transcription. LeIRT1 and LeNRAMP1 may be regulated in the same way. FER is able to form homodimers, but their biological function remains to be investigated. SlbHLH066 and SlbHLH067, closely grouped with Ib subgroup of bHLH proteins in Arabidopsis by phylogenetic analysis, were determined as iron-deficiency responsive proteins (Sun et al., 2015). They may play redundant roles with SlbHLH068 in tomato, as AtbHLH38/AtbHLH39/AtbHLH100/AtbHLH101 in Arabidopsis. Further experimental evidence is needed to prove this hypothesis.
From previous studies, we know that both the FER mRNA and protein are expressed in a root-specific manner in response to a low iron supply (Ling et al., 2002; Li et al., 2004; Brumbarova and Bauer, 2005). SlbHLH068, as an interaction partner of FER, shares some similarity with the expression pattern of FER. The SlbHLH068 mRNA expression pattern in the root was similar to FER, which is significantly induced by iron starvation; however, the expression pattern of these two genes in other tissues is very different. In the leaf and stem, SlbHLH068 transcripts accumulated in response to a low iron supply, while the expression of FER was undetectable in the leaf regardless of the iron supply (Ling et al., 2002). As no antibody against SlbHLH068 currently exists, we have no data on the regulation of SlbHLH068 at the post-transcriptional level. Although whether the SlbHLH068 protein is stable with the absence of its FER interactor in leaf and stem is not clear, some bHLH proteins other than FER may interact with SlbHLH068 and regulate iron homeostasis genes in the above-ground parts of the tomato.
Ferric chelate reductase is required for iron metabolism in plant roots and shoots (Li et al., 2004; Wu et al., 2005). In the T3238fer mutant, the LeFRO1 mRNA level in leaves was similar to that in wild-type plants, while the level in the root was much lower than in wild-type plants (Li et al., 2004). It was suggested that the expression of LeFRO1 in roots and shoots was separately regulated and the regulation of ferric-chelate reductase in shoots was independent of FER. In SlbHLH068 VIGS plants, the expression of LeFRO1 and iron accumulation in leaves and roots were significantly diminished compared with control plants, indicating a regulatory role for SlbHLH068 with regard to LeFRO1 in both roots and shoots. As homodimers are not formed by SlbHLH068, we speculate that SlbHLH068 is involved in the modulation of iron homeostasis in the shoot via an interaction with an unknown bHLH protein(s) instead of FER.
SUPPLEMENTARY DATA
Supplementary data are available online at http://www.aob.oxfordjournals.org and consist of the following. Table S1: primers used in this study. Figure S1: diagrams of the plasmid constructs for VIGS. Figure S2: the sequence of the LeFRO1 promoter
ACKNOWLEDGMENTS
We thank Prof. Yule Liu (School of Life Sciences, Tsinghua University, Beijing, China) for kindly providing the empty pTRV1 and pTRV2 vectors and for technical assistance with the VIGS experiment. We thank Prof. Daowen Wang and Dr Weiqiang Qian (State Key Laboratory of Plant Cell and Chromosome Engineering, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China) for generating the positive control plasmid, pTRV2-CH42, used in the VIGS. This work was supported by the National Nature Science Foundation of China (grant no. 31270294) and the Ministry of Science and Technology of China (grant no. 2011CB100304).
LITERATURE CITED
- Becker R, Grün M, Scholz G. 1992. Nicotianamine and the distribution of iron into the apoplasm and symplasm of tomato (Lycopersicon esculentum Mill.): I. Determination of the apoplasmic and symplasmic iron pools in roots and leaves of the cultivar Bonner Beste and its nicotianamine-less mutant chloronerva. Planta 187: 48–52. [DOI] [PubMed] [Google Scholar]
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P. 2003. Differential regulation of nramp and irt metal transporter genes in wild-type and iron uptake mutants of tomato. The Journal of Biological Chemistry 278: 24697–24704. [DOI] [PubMed] [Google Scholar]
- Brumbarova T, Bauer P. 2005. Iron-mediated control of the basic helix–loop–helix protein FER, a regulator of iron uptake in tomato. Plant Physiology 137: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS. 2004. Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Molecular Biology 55: 521–530. [DOI] [PubMed] [Google Scholar]
- Chen QR, Vansant G, Oades K, et al. 2007. Diagnosis of the small round blue cell tumors using multiplex polymerase chain reaction. Journal of Molecular Diagnostics 9: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Seong E, Kim YC, et al. 2004. A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang). Molecular Cells 17: 377–380. [PubMed] [Google Scholar]
- Colangelo EP, Guerinot ML. 2004. The essential basic helix–loop–helix protein FIT1 is required for the iron deficiency response. The Plant Cell 16: 3400–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Guerinot M. 2002. Iron stress in plants. Genome Biology 3: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zeng D, Wang B, Qian Q, Zheng S, Ling HQ. 2013. Environmental effects on mineral accumulation in rice grains and identification of ecological specific QTLs. Environmental Geochemistry and Health 35: 161–170. [DOI] [PubMed] [Google Scholar]
- Eckhardt U, Mas Marques A, Buckhout TJ. 2001. Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Molecular Biology 45: 437–448. [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. 1996. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences USA 93: 5624–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T, Fass D, Arnaud M, Harrison SC. 1994. Crystal structure of transcription factor E47: E-box recognition by a basic region helix–loop–helix dimer. Genes and Development 8: 970–980. [DOI] [PubMed] [Google Scholar]
- Faivre-Rampant O, Gilroy EM, Hrubikova K, et al. 2004. Potato virus X-induced gene silencing in leaves and tubers of potato. Plant Physiology 134: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre-D’Amare AR, Pognonec P, Roeder R G, Burley SK. 1994. Structure and function of the b/HLH/Z domain of USF. EMBO Journal 13: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice WP, Nguyen LV, Wernsman EA, Thompson WF, Conkling MA. 1999. Transposon tagging of the sulfur gene of tobacco using engineered maize Ac/Ds elements. Genetics 153: 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. 2005. Virus-induced gene silencing in tomato fruit. The Plant Journal 43: 299–308. [DOI] [PubMed] [Google Scholar]
- Gampala SS, Kim TW, He JX, et al. 2007. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Developmental Cell 13: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques R, Jasik J, Klein M, et al. 2002. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Molecular Biology 50: 587–597. [DOI] [PubMed] [Google Scholar]
- Huang YS, Li HM. 2009. Arabidopsis CHLI2 can substitute for CHLI1. Plant Physiology 150: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P. 2004. FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Letters 577: 528–534. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HF, Chai YM, Li CL, et al. 2011. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiology 157: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjemtrup S, Sampson KS, Peele CG, et al. 1998. Gene silencing from plant DNA carried by a Geminivirus. The Plant Journal 14: 91–100. [DOI] [PubMed] [Google Scholar]
- Kong D, Chen C, Wu H, Li Y, Li J, Ling HQ. 2013. Sequence diversity and enzyme activity of ferric-chelate reductase LeFRO1 in tomato. Journal of Genetics and Genomics 40: 565–573. [DOI] [PubMed] [Google Scholar]
- Li L, Cheng X, Ling HQ. 2004. Isolation and characterization of Fe(III)-chelate reductase gene LeFRO1 in tomato. Plant Molecular Biology 54: 125–136. [DOI] [PubMed] [Google Scholar]
- Ling HQ, Pich A, Scholz G, Ganal MW. 1996. Genetic analysis of two tomato mutants affected in the regulation of iron metabolism. Molecular and General Genetics 252: 87–92. [DOI] [PubMed] [Google Scholar]
- Ling HQ, Koch G, Baumlein H, Ganal MW. 1999. Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proceedings of the National Academy of Sciences USA 96: 7098–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HQ, Bauer P, Bereczky Z, Keller B, Ganal MW. 2002. The tomato fer gene encoding a bHLH protein controls iron-uptake responses in roots. Proceedings of the National Academy of Sciences USA 99: 13938–13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31: 777–786. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nakayama N, Schiff M, Litt A, Irish VF, Dinesh-Kumar SP. 2004. Virus induced gene silencing of a DEFICIENS ortholog in Nicotiana benthamiana. Plant Molecular Biology 54: 701–711. [DOI] [PubMed] [Google Scholar]
- Marschner H, Römhelda V, Kissela M. 1986. Different strategies in higher plants in mobilization and uptake of iron. Journal of Plant Nutrition 9: 695–713. [Google Scholar]
- Mori S. 1999. Iron acquisition by plants. Current Opinion of Plant Biology 2: 250–253. [DOI] [PubMed] [Google Scholar]
- Peele C, Jordan CV, Muangsan N, et al. 2001. Silencing of a meristematic gene using geminivirus-derived vectors. The Plant Journal 27: 357–366. [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC. 2001. Tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal 25: 237–245. [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. 1999. A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697. [DOI] [PubMed] [Google Scholar]
- Römheld V. 1987. Different strategies for iron acquisition in higher plants. Plant Physiology 70: 231–234. [Google Scholar]
- Schmidt W. 2003. Iron solutions: acquisition strategies and signaling pathways in plants. Trends in Plant Science 8: 188–193. [DOI] [PubMed] [Google Scholar]
- Sheen J. 2001. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiology 127: 1466–1475. [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Toumoto A, Ihara K, et al. 1997. Crystal structure of PHO4 bHLH domain-DNA complex: flanking base recognition. EMBO Journal 16: 4689–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Fan HJ, Ling HQ. 2015. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genomics 16(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH. 2003. The Arabidopsis basic/helix–loop–helix transcription factor family. The Plant Cell 15: 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnage MA, Muangsan N, Peele CG, Robertson D. 2002. Geminivirus-based vectors for gene silencing in Arabidopsis. The Plant Journal 30: 107–114. [DOI] [PubMed] [Google Scholar]
- Varotto C, Maiwald D, Pesaresi P, Jahns P, Salamini F, Leister D. 2002. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. The Plant Journal 31: 589–599. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, et al. 2002. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. The Plant Cell 14: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Cui Y, Liu Y, et al. 2013. Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Molecular Plant 6: 503–513. [DOI] [PubMed] [Google Scholar]
- Wu HL, Li LH, Du J, Yuan YX, Cheng XD, Ling HQ. 2005. Molecular and biochemical characterization of the Fe(III) chelate reductase gene family in Arabidopsis thaliana. Plant Cell Physiology 46: 1505–1514. [DOI] [PubMed] [Google Scholar]
- Wu HL, Chen CL, Du J, et al. 2012. Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis-enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiology 158: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YX, Zhang J, Wang DW, Ling HQ. 2005. AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Research 15: 613–621. [DOI] [PubMed] [Google Scholar]
- Yuan YX, Wu HL, Wang N, et al. 2008. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Research 18: 385–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.