Significance
The entry of inorganic carbon (Ci; CO2 and HCO3–) into cells involves many biological processes in both animals and plants, and aquaporins as well as bicarbonate transporters play roles in Ci transport. Although transporting external HCO3– into the stroma through the chloroplast envelope is one of the rate-limiting factors for aquatic photosynthetic organisms, specific molecular components in this process have not yet been identified experimentally. Molecular identification of proteins essential for Ci uptake located in the chloroplast envelope and in the plasma membrane documented in this study helps in understanding how aquatic photosynthetic organisms developed machinery to acclimate to CO2-limiting environment and to maintain adequate levels of photosynthesis for survival or growth.
Keywords: bicarbonate uptake, Chlamydomonas, chloroplast envelope, CO2-concentrating mechanism, photosynthesis
Abstract
The supply of inorganic carbon (Ci; CO2 and HCO3–) is an environmental rate-limiting factor in aquatic photosynthetic organisms. To overcome the difficulty in acquiring Ci in limiting-CO2 conditions, an active Ci uptake system called the CO2-concentrating mechanism (CCM) is induced to increase CO2 concentrations in the chloroplast stroma. An ATP-binding cassette transporter, HLA3, and a formate/nitrite transporter homolog, LCIA, are reported to be associated with HCO3– uptake [Wang and Spalding (2014) Plant Physiol 166(4):2040–2050]. However, direct evidence of the route of HCO3– uptake from the outside of cells to the chloroplast stroma remains elusive owing to a lack of information on HLA3 localization and comparative analyses of the contribution of HLA3 and LCIA to the CCM. In this study, we revealed that HLA3 and LCIA are localized to the plasma membrane and chloroplast envelope, respectively. Insertion mutants of HLA3 and/or LCIA showed decreased Ci affinities/accumulation, especially in alkaline conditions where HCO3– is the predominant form of Ci. HLA3 and LCIA formed protein complexes independently, and the absence of LCIA decreased HLA3 mRNA accumulation, suggesting the presence of unidentified retrograde signals from the chloroplast to the nucleus to maintain HLA3 mRNA expression. Furthermore, although single overexpression of HLA3 or LCIA in high CO2 conditions did not affect Ci affinity, simultaneous overexpression of HLA3 with LCIA significantly increased Ci affinity/accumulation. These results highlight the HLA3/LCIA-driven cooperative uptake of HCO3– and a key role of LCIA in the maintenance of HLA3 stability as well as Ci affinity/accumulation in the CCM.
Inorganic carbon (Ci; CO2 and HCO3–) transport is essential for a wide range of biological processes such as CO2 metabolism, cellular pH homeostasis, and photosynthesis. Because HCO3– is not freely permeable to biological membranes, it must be transported across membranes by HCO3– transporters or channels. HCO3– transporters have been studied extensively in mammals and been found to cluster into solute carrier (SLC) 4 and SLC 26 families (1). In cyanobacteria, five types of Ci transporters have been identified (2), including three HCO3– transporters and two NAD(P)H dehydrogenase-dependent CO2 uptake systems. In land plants, aquaporin-mediated CO2 permeation has been suggested to play physiological roles in photosynthesis (3), and in a marine diatom, SLC4 family protein localized to the plasma membrane (PM) facilitates HCO3– uptake (4). However, no studies have validated the entire route of HCO3– transport from the outside of cells to the chloroplast stroma through the PM and chloroplast envelope (CE) in photosynthetic organisms.
Aquatic conditions are not well suited for efficient photosynthesis because the CO2 diffusion rate is ∼10,000-fold lower compared with that in atmospheric conditions (5). Therefore, aquatic photosynthetic organisms, including microalgae, are frequently exposed to limiting CO2 stress. To acclimate to this stress, most microalgae possess a CO2-concentrating mechanism (CCM) to accumulate CO2 around the CO2 fixation enzyme ribulose 1, 5-bisphosphate carboxylase/oxygenase (Rubisco) and to maintain adequate photosynthetic efficiency (6, 7).
The green alga Chlamydomonas reinhardtii has been used as a model organism for molecular and physiological studies of the CCM since it was first identified (8). A model of the CCM has been proposed based on the subcellular structure of C. reinhardtii (9, 10). Environmental Ci is transported to the chloroplast stroma by Ci transporters localized to the PM and CE. Carbonic anhydrase (CA) localized to the chloroplast stroma is predicted to contribute to the maintenance of the Ci pool, in the form of HCO3–, by rapid conversion of CO2 to HCO3–, thereby preventing the loss of CO2 by diffusion (11). It is known that tubule-like thylakoid membranes penetrate into the pyrenoid (12), a Rubisco-enriched structure in the chloroplast. HCO3– in the stroma is transported into the acidic thylakoid lumen by a putative channel or transporter localized to the thylakoid membrane, and HCO3– is rapidly converted to CO2 by a constitutively expressed CA (13, 14). Then, CO2 diffuses from the thylakoid lumen into the pyrenoid matrix and is fixed by Rubisco. It was also reported that C. reinhardtii acclimates to two distinct limiting CO2 conditions, termed low CO2 (LC; ∼0.03–0.5% CO2 or 7–70 µM CO2) and very low CO2 (VLC; <0.02% CO2 or <7 µM CO2) (15, 16), and different types of Ci uptake systems could function in the CCM in these separate conditions (16).
To identify CCM-associated components, several transcriptome analyses have been performed (17–22), and several genes encoding membrane proteins were focused on as candidate Ci transporter genes, including LCI1 (low CO2 inducible gene 1) (23), LCIA (low CO2 inducible gene A) (19), and HLA3 (high light activated 3) (24).
LCI1 is localized to the PM (25), and its expression is regulated by the MYB-transcription factor LCR1 (low CO2 stress response 1) (26). When LCI1 was artificially expressed in HC conditions, the cells showed increases in the internal Ci pool, suggesting that LCI1 is directly or indirectly associated with Ci uptake (25). LCIA (also known as NAR1.2) is a homolog of the nitrate transporter NAR1 and belongs to the formate/nitrite transporter family (27). Although the expression of other NAR1 family genes of C. reinhardtii is mainly regulated by nitrogen source, LCIA is specifically induced in LC conditions and is not under the control of nitrogen source (19). LCIA was predicted to localize to the CE (19), and this prediction was supported by indirect immunofluorescence assay evidence (16). Functional expression analysis using Xenopus oocytes showed transport activity of LCIA for both HCO3– and NO2– (27), and LCIA appears to be associated with HCO3– uptake in VLC conditions from analysis of an insertion mutant (16). HLA3 is an ATP-binding cassette (ABC) transporter of the multidrug resistance-related protein subfamily, and its transcription is induced by high light and LC conditions (19, 24). Although HLA3 is predicted to localize to the PM (10), no experimental data are available at present. Knockdown (KD) of HLA3 mRNA expression resulted in modest decreases in photosynthesis affinity, but simultaneous KD of LCIA and HLA3 mRNAs caused a dramatic decrease in growth rate, Ci uptake activity, and photosynthetic Ci affinity, especially in alkaline conditions, where HCO3– is the predominant form of Ci (28).
In this study, by use of indirect immunofluorescence assays and membrane fractionation, the subcellular localization of HLA3 was elucidated. In addition, by analyses of the photosynthetic characteristics of HLA3 and LCIA single insertion mutants, an HLA3/LCIA double insertion mutant, and overexpressing strains of HLA3 and/or LCIA, we concluded that HLA3 and LCIA are cooperatively associated with HCO3– uptake across the PM and CE, respectively.
Results
Accumulation of HLA3 and LCIA in Very Low CO2 Conditions.
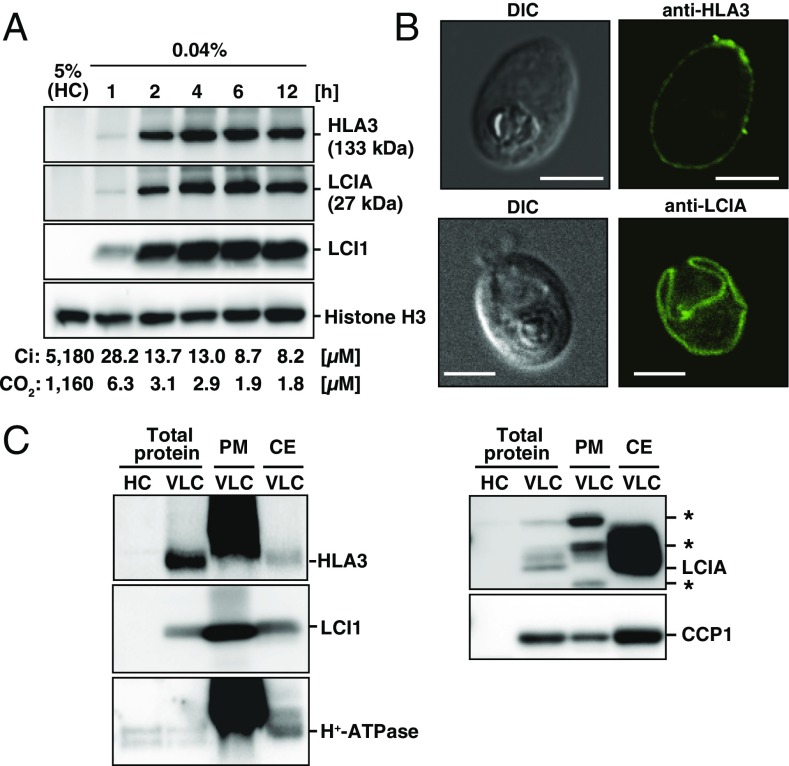
First, to define the acclimated states of limiting CO2 conditions (LC or VLC) of cells grown in liquid culture, total Ci concentration in the culture medium at pH 7.0 was measured, and consequent CO2 concentrations were calculated (Fig. 1A). CO2 concentrations supplied with 0.04% CO2 for 1, 2, 4, 6, and 12 h were estimated as 6.3, 3.1, 2.9, 1.9, and 1.8 µM, respectively, which correspond to the range for VLC (<7 µM CO2) (16). Thus, we defined the limiting CO2 conditions of liquid culture as VLC throughout this study. Next, the time course accumulation of HLA3 and LCIA after VLC induction was examined (Fig. 1A). The accumulation of these proteins started within 1 h and reached their maximum levels within 4 h, as was the case for LCI1 used as a control of VLC induction. The molecular masses of HLA3 and LCIA were detected at sizes of ∼133 and 27 kDa, respectively (Fig. S1A and SI Results and Discussion).
Fig. 1.
Accumulation and subcellular localization of HLA3 and LCIA. (A) Time-course of accumulation of HLA3, LCIA, and LCI1 proteins in WT cells. For induction of limiting-CO2 conditions, cells supplied with 5% CO2 (high CO2; HC) were centrifuged, suspended in new fresh medium, and cultured with 0.04% CO2 for 1, 2, 4, 6, and 12 h. Histone H3 was used as a loading control. The total Ci concentrations and calculated CO2 concentrations after each induction time are also indicated below the figures. Using an HCO3–/CO2 ratio of 4.47 at pH 7.0, CO2 concentrations were calculated using the equation (pH = pKa + log10 [HCO3–]/[CO2]), where pKa was an acid dissociation constant of 6.35. (B) Subcellular localization of HLA3 and LCIA by an indirect immunofluorescence assay. WT cells were grown in very low CO2 (VLC) for 12 h. DIC, differential interference contrast. (Scale bars, 5 µm.) (C) Immunoblot analysis in isolated plasma membrane (PM) and chloroplast envelope (CE) fractions with antibodies against HLA3, LCI1, H+-ATPase, LCIA, and CCP1. Asterisks indicate nonspecific bands.
Subcellular Localization of HLA3 and LCIA.
To analyze the subcellular localization of HLA3, an indirect immunofluorescence assay was performed (Fig. 1B). Fluorescence signals from an anti-HLA3 antibody were detected peripherally, suggesting the localization of HLA3 to the PM. Fluorescence signals from an anti-LCIA antibody were detected as a single cup-shaped structure (Fig. 1B), as in the previous study (16). To further clarify the localization of HLA3 and LCIA biochemically, protein samples from total cell, PM, and CE fractions were probed with antibodies against HLA3, LCI1, H+-ATPase, LCIA, and CCP1 (Fig. 1C and SI Results and Discussion). LCI1 and H+-ATPase were enriched in the PM fraction, consistent with the PM localization of these proteins (25, 29). Similarly, a notable enrichment of HLA3 was observed in the PM fraction. LCIA was highly enriched in the CE fraction, where CE protein CCP1 (30) was also enriched. From these results, we concluded that HLA3 and LCIA were localized to the PM and CE, respectively.
Isolation of an HLA3 Insertion Mutant and Photosynthetic Characteristics.
To evaluate the degree of contribution of HLA3 to the CCM, we isolated an HLA3 insertion mutant from our paromomycin resistance gene-tagged mutant library by PCR-based screening, as described previously (31), and designated the strain Hin-1 (Fig. S2 A–C and SI Results and Discussion).
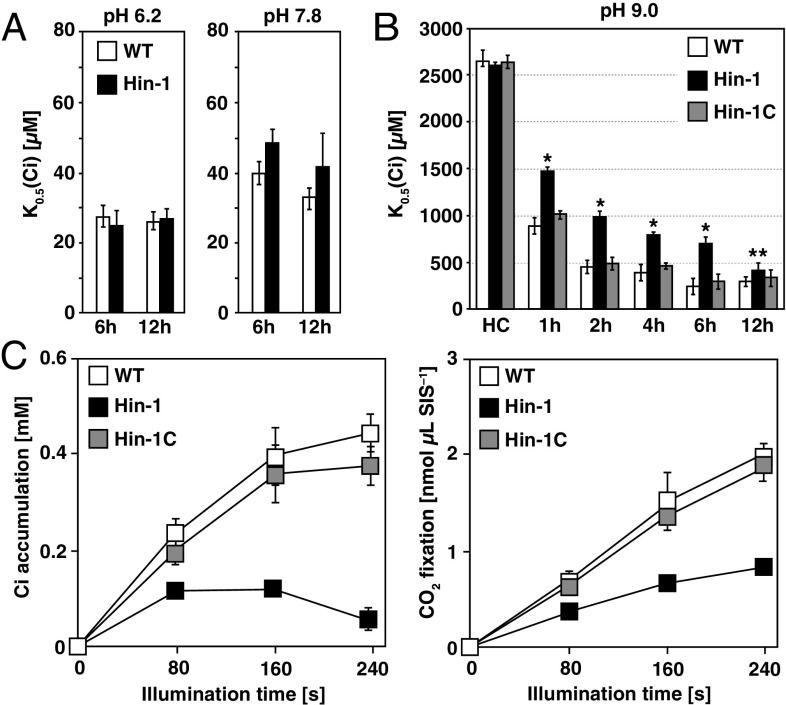
Next, the photosynthetic characteristics were evaluated by measuring the rates of Ci-dependent O2 evolution of WT, Hin-1, and the complemented strain Hin-1C grown in VLC at different pH. K0.5 (Ci) values, the Ci concentration required for half maximal O2-evolving activity, of WT and Hin-1 were similar at pH 6.2 (ratio of HCO3–:CO2 = 0.7:1) and pH 7.8 (HCO3–:CO2 = 28:1), indicating that the difference in photosynthetic Ci affinity between WT and Hin-1 was not significant (Fig. 2A). Because HLA3 KD strains showed retarded growth rates at pH 9.0 (28) where the ratio of HCO3–:CO2 = 446:1 and HCO3– was the predominant form of Ci, we evaluated the changes in Ci affinity during acclimation to VLC at pH 9.0 in a time course analysis (Fig. 2B). Both WT and Hin-1 showed a gradual decrease in K0.5 (Ci) during acclimation to VLC. However, although WT in VLC at 6 h showed almost the same Ci affinity compared with that at 12 h (241 ± 87 µM at 6 h and 290 ± 50 µM at 12 h), Hin-1 still showed much lower Ci affinity especially at 6 h (691 ± 143 µM at 6 h and 405 ± 57 µM at 12 h), and the decreased Ci affinity was restored in Hin-1C (296 ± 78 µM at 6 h and 333 ± 89 µM at 12 h). These results suggested that other Ci uptake systems could compensate for the absence of HLA3 and contribute to the increase in Ci affinity at 12 h and that measuring photosynthetic characteristics at 6 h was appropriate for evaluating the contribution of HLA3 to the CCM.
Fig. 2.
Characterization of an HLA3 insertion mutant. (A) Inorganic carbon (Ci) affinity of WT and HLA3 insertion mutant (Hin-1) grown in very low CO2 (VLC) for 6 or 12 h. Photosynthetic O2-evolving activity was measured with different external Ci concentrations at pH 6.2 or 7.8, and the respective K0.5 (Ci) values, the Ci concentration required for half maximum O2-evolving activity, were calculated. (B) Ci affinity of WT, Hin-1, and complemented Hin-1 (Hin-1C) grown in high CO2 (HC) or VLC for 1, 2, 4, 6, and 12 h. O2-evolving activity was measured at pH 9.0. *P < 0.01 and **P < 0.05 by Student t test. (C) Accumulation and fixation of Ci in WT, Hin-1, and Hin-1C. Cells were grown in VLC for 6 h, and intracellular Ci accumulation (Left) and CO2 fixation (Right) at pH 9.0 were measured using a silicone oil layer method. SIS, sorbitol impermeable space.
To evaluate the contribution of HLA3 to actual Ci uptake activity, the accumulation and fixation of [14C]-labeled Ci in WT, Hin-1, and Hin-1C grown in VLC for 6 h were measured (Fig. 2C). Hin-1 showed significantly lower levels of Ci accumulation of 0.12 mM (0.57-fold of Hin-1C), 0.12 mM (0.32-fold), and 0.06 mM (0.17-fold) after 80, 160, and 240 s of illumination, respectively, and CO2 fixation of 0.37 nmol⋅μL SIS–1 (0.59-fold), 0.67 nmol⋅μL SIS–1 (0.49-fold), and 0.83 nmol⋅μL SIS–1 (0.44-fold), respectively, compared with that of Hin-1C. These results indicated that HLA3 has a meaningful role in HCO3– uptake in VLC conditions.
Isolation of LCIA Insertion Mutants and Photosynthetic Characteristics.
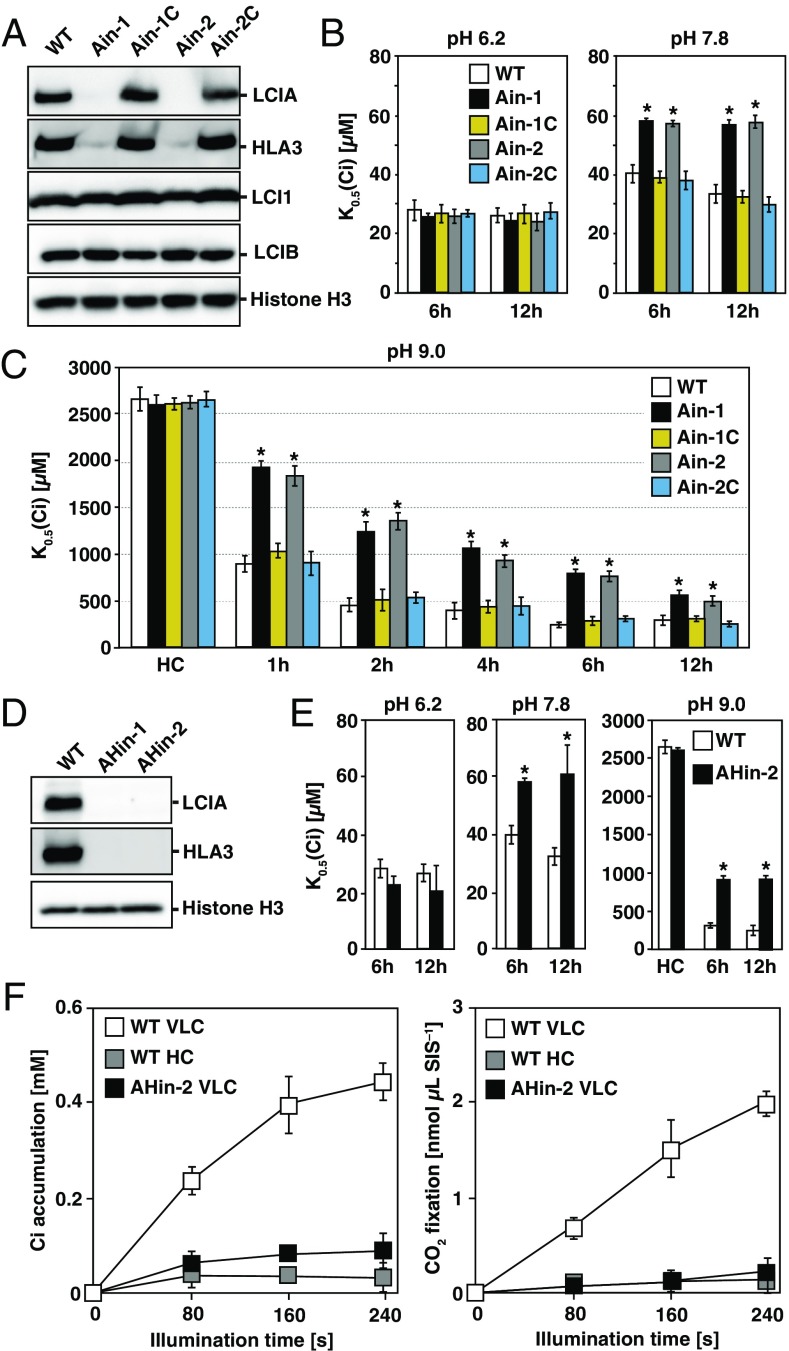
A disruption mutant of LCIA has been characterized, and the contribution of LCIA to the CCM has been reported (16). To compare the degrees of the contributions of HLA3 and LCIA to the CCM, we also isolated two LCIA insertion mutants (Fig. S2 D–G and SI Results and Discussion), designated as Ain (Ain-1 and Ain-2), and compared the photosynthetic characteristics with Hin-1. Interestingly, accumulation of HLA3 was much lower in Ain compared with that in WT, and this decreased accumulation of HLA3 was restored in the complemented strains Ain-1C and Ain-2C (Fig. 3A). This result was in sharp contrast to that of LCI1 and LCIB (32), which were not affected by the impairment of the LCIA (Fig. 3A).
Fig. 3.
Characterization of LCIA insertion mutants and an LCIA/HLA3 double-insertion mutant. (A) Accumulation of LCIA, HLA3, LCI1, and LCIB in WT, LCIA insertion mutants (Ain-1 and Ain-2), and their complemented strains (Ain-1C and Ain-2C). Cells were grown in very low CO2 (VLC) for 12 h. (B) Inorganic carbon (Ci) affinity of WT, Ain-1, Ain-2, Ain-1C, and Ain-2C grown in VLC for 6 or 12 h. Photosynthetic O2-evolving activity was measured with different external Ci concentrations at pH 6.2 or 7.8, and the respective K0.5 (Ci) values, the Ci concentration required for half maximum O2-evolving activity, were calculated. *P < 0.01. (C) Ci affinity of WT, Ain-1, Ain-2, Ain-1C, and Ain-2C grown in high CO2 (HC) or VLC for 1, 2, 4, 6, and 12 h. O2-evolving activity was measured at pH 9.0. *P < 0.01. (D) Accumulation of HLA3 and LCIA in WT and LCIA/HLA3 double-insertion mutants (AHin-1 and AHin-2) grown in VLC for 12 h. (E) Ci affinity of WT and AHin-2 grown in HC or VLC for 6 or 12 h. O2-evolving activity was measured at pH 6.2, 7.8, or 9.0. *P < 0.01. (F) Accumulation and fixation of Ci in WT and AHin-2. Cells were grown in HC or VLC for 6 h, and intracellular Ci accumulation (Left) and CO2 fixation (Right) were measured at pH 9.0. SIS, sorbitol impermeable space.
Next, the photosynthetic characteristics of Ain-1, Ain-2, Ain-1C, and Ain-2C were evaluated. As in the case of Hin-1, the K0.5 (Ci) of Ain-1 and Ain-2 was similar to WT at pH 6.2 (Fig. 3B). However, in contrast to Hin-1, the K0.5 (Ci) of Ain-1 (57 ± 2 µM at 6 h and 56 ± 3 µM at 12 h) and Ain-2 (57 ± 1 µM at 6 h and 57 ± 2 µM at 12 h) was significantly higher than that of WT (40 ± 3 µM at 6 h and 33 ± 3 µM at 12 h), Ain-1C (38 ± 2 µM at 6 h and 32 ± 2 µM at 12 h), and Ain-2C (37 ± 3 µM at 6 h and 29 ± 2 µM at 12 h), even at pH 7.8 (Fig. 3B). At pH 9.0, although Ain also showed gradual decreases in K0.5 (Ci) during acclimation to VLC, these cells always showed lower Ci affinity than Hin-1 (Fig. 2B), as well as WT and complemented strains (Fig. 3C). These results suggested a significant contribution of LCIA to increases in Ci affinity and to maintaining HLA3 stability in the CCM.
Isolation of LCIA/HLA3 Double-Insertion Mutants and Photosynthetic Characteristics.
Because Ci affinity in VLC at 12 h was higher than that at 6 h in both HLA3 and LCIA single mutants, either protein could partially complement each other to increase Ci affinity. Thus, we expected that LCIA/HLA3 double-insertion mutants would show an additive decrease in Ci affinity compared with the single-insertion mutants. Thus, we isolated double-insertion mutants by crossing one of the Ain-2 progeny with Hin-1 and designated these as AHin (AHin-1 and AHin-2; Fig. 3D, Fig. S2 H–K, and SI Results and Discussion).
Next, the photosynthetic characteristics of AHin-2 were evaluated (Fig. 3E). As in the case of Hin-1 and Ain, the K0.5 (Ci) of AHin-2 was similar to WT at pH 6.2. At pH 7.8, the K0.5 (Ci) of AHin-2 (58 ± 2 µM at 6 h and 61 ± 10 µM at 12 h) was significantly higher than that of WT, but it was similar to Ain. At pH 9.0, AHin-2 showed lower Ci affinity than both Hin-1 and Ain, and Ci affinity was not increased even at 12 h (898 ± 78 µM at 6 h and 901 ± 94 µM at 12 h). Ci accumulation and fixation in AHin-2 grown in VLC at 6 h was also measured (Fig. 3F). After 80, 160, and 240 s of illumination, AHin-2 showed substantially decreased Ci accumulation of 0.05 mM (0.21-fold of WT and 0.41-fold of Hin-1), 0.06 mM (0.16-fold and 0.53-fold), and 0.06 mM (0.15-fold and 1.0-fold), respectively, and CO2 fixation of 0.07 nmol⋅μL SIS–1 (0.1-fold and 0.2-fold), 0.12 nmol⋅μL SIS–1 (0.08-fold and 0.17-fold), and 0.14 nmol⋅μL SIS–1 (0.07-fold and 0.17-fold), respectively, compared with that of WT and Hin-1.
Finally, the effect of absence of LCIA and/or HLA3 on cell growth was examined. Growth rates were measured in VLC at pH 8.4 (Fig. S2L) because there were no significant differences at pH 7.8, and none of the cell lines could grow at pH 9.0. The doubling time of WT was 7.2 h and that of Hin-1, Ain-1, Ain-2, and AHin-2 increased significantly to 7.6, 9.5, 9.3, and 12.7 h, respectively, reflecting the degree of decreased Ci affinity of each cell line. These results highlighted an additive decrease in Ci affinity/accumulation/growth rates of the double-insertion mutant compared with the HLA3 or LCIA single-insertion mutants.
Isolation of LCIA and/or HLA3 Overexpressing Strains and Photosynthetic Characteristics.
To demonstrate the physiological function of LCIA and HLA3 more directly, the photosynthetic characteristics of cells overexpressing LCIA and/or HLA3 were examined in HC conditions where other VLC-inducible proteins were not induced. For overexpression, two chimeric plasmids, pTY2b-LCIA and pTY2b-HLA3, were constructed (Fig. S3A). These plasmids allowed the induction of LCIA and HLA3 transcripts by switching the nitrogen source from NH4+ to NO3– irrespective of the CO2 conditions. In this study, we cultured the cells with four combinations of nitrogen sources in the medium and CO2 concentrations, designated as HC-NH4+, HC-NO3–, VLC-NH4+, and VLC-NO3–.
First, we transformed WT cells with pTY2b-LCIA or pTY2b-HLA3 separately. The transformants showed accumulation of LCIA or HLA3 when grown in HC-NO3– conditions and were designated as Aox (Aox-1 and Aox-2) and Hox (Hox-1 and Hox-2), respectively (Fig. S3 B and C and SI Results and Discussion). Next, by introducing pTY2b-HLA3 into Aox-1, we generated two independent transformants expressing LCIA and HLA3 simultaneously and designated these as AHox (AHox-1 and AHox-2; Fig. S3D). Accumulation of HLA3 in AHox-1 and AHox-2 was the same as that of VLC-grown WT. To isolate a strain overexpressing both LCIA and HLA3 with greater abundance, the progeny of Aox-1 was crossed with Hox-1 and a strain designated as AHox-3 was obtained (Fig. S3E).
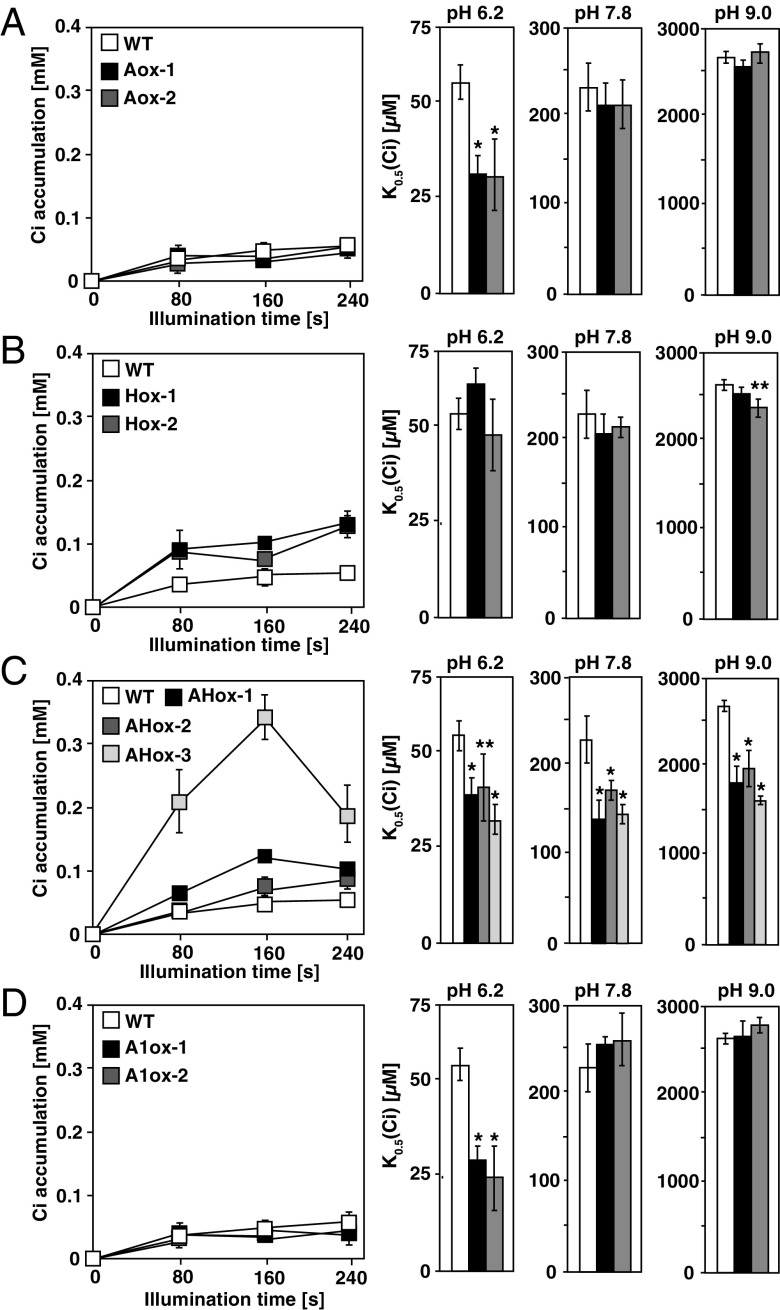
Next, to evaluate the effect of LCIA and/or HLA3 overexpression on the photosynthetic characteristics, rates of O2 evoluton at pH 6.2, 7.8, and 9.0 and Ci accumulation at pH 9.0 of these strains were measured. In Aox, there were no differences in Ci affinity at pH 7.8 and pH 9.0, as well as Ci accumulation compared with WT (Fig. 4A and Table S1–S3). In contrast, HC-NO3–-grown Hox showed a small but significant increase of Ci accumulation of 0.08 mM (2.5-fold of WT at 80 s), 0.07 mM (1.5-fold at 160 s), and 0.13 mM (2.4-fold at 240 s) in Hox-2, compared with that of HC-NO3–-grown WT, but the phenotype led to a slight increase in Ci affinity only at pH 9.0 in Hox-2 (Fig. 4B), suggesting that Ci in the cytosol transported by HLA3 could not efficiently enter the chloroplast stroma in the absence of LCIA. On the other hand, Ci affinity at pH 6.2 was increased in LCIA-overexpressing Aox (Fig. 4A) and AHox (Fig. 4C), but not in Hox (Fig. 4B).
Fig. 4.
Characterization of LCIA- and HLA3-overexpressing strains. Accumulation of inorganic carbon (Ci) (Left) and Ci affinity (Right) in WT and in strains overexpressing LCIA (A), HLA3 (B), LCIA/HLA3 (C), and LCIA/LCI1 (D). Cells were grown in high CO2-NO3– for 12 h, and Ci accumulation was measured at pH 9.0. For Ci affinity, O2-evolving activity was measured with different external Ci concentrations at pH 6.2, 7.8, or 9.0 and the respective K0.5 (Ci) values, the Ci concentration required for half maximum O2-evolving activity, were calculated. *P < 0.01 and **P < 0.05.
In contrast to Aox and Hox, AHox showed a significant increase in Ci affinity and Ci accumulation compared with WT at alkaline conditions (Fig. 4C and Tables S2 and S3). In particular, HC-NO3–-grown AHox-3 showed substantially increased Ci accumulation of 0.21 mM (6.3-fold of WT at 80 s), 0.34 mM (6.8-fold at 160 s), and 0.19 mM (3.6-fold at 240 s) compared with that of HC-NO3–-grown WT. Consequently, the respective K0.5 (Ci) of AHox-1, AHox-2, and AHox-3 decreased to 141 ± 20 (0.61-fold of WT), 174 ± 20 (0.76-fold), and 147 ± 19 µM (0.64-fold) at pH 7.8 and to 1,821 ± 201 (0.68-fold of WT), 1,980 ± 198 (0.75-fold), and 1,626 ± 49 µM (0.61-fold) at pH 9.0. In HC-NH4+ conditions at pH 7.8 where LCIA and HLA3 were not induced, the respective K0.5 (Ci) of 257 ± 28, 250 ± 30, and 262 ± 29 µM in AHox-1, AHox-2, and AHox-3 was not significantly different from that of 273 ± 31 µM in WT (Table S2). These results indicated that NO3–-induced overexpression of LCIA and HLA3 could enhance HCO3– accumulation in the chloroplast stroma and increase Ci affinity.
Although PM-localized LCI1 could be associated with Ci uptake (25), the preferred Ci species of LCI1 remained elusive. To evaluate the degree of LCIA/HLA3-driven HCO3– uptake activity, we also isolated six transformants expressing LCIA with LCI1 by introducing pTY2b-LCI1 (Fig. S3A) into Aox-1 and designated two representatives as A1ox (A1ox-1 and A1ox-2; Fig. S3F). There were no differences in Ci accumulation and affinity in alkaline conditions compared with WT (Fig. 4D and Table S2 and S3), suggesting that LCI1 was not related to direct HCO3– uptake along with LCIA.
A Defect in LCIA Led to a Decrease in HLA3 Accumulation Caused by Suppression of HLA3 mRNA Accumulation.
As described above, accumulation of HLA3 was much lower in Ain compared with that in WT (Fig. 3A). This result suggested two possibilities. First, HLA3 and LCIA undergo a physical interaction where the PM is associated with the CE and the absence of LCIA causes instability of HLA3. Second, the absence of LCIA causes the repression of HLA3 mRNA accumulation.
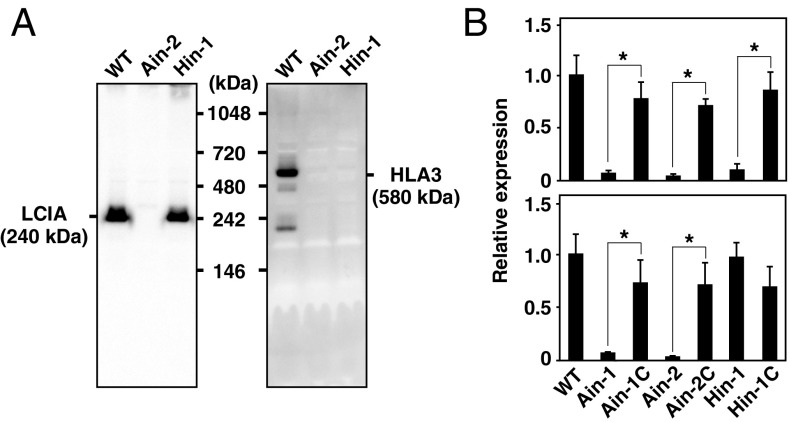
To examine the former possibility, the molecular masses of LCIA and HLA3 in vivo were estimated by Blue Native-PAGE. We expected that LCIA and HLA3 should be detected with the same molecular mass in nondenaturing conditions if these two proteins interact and form a complex. However, using 1.0% n-dodecyl β-d-maltoside (DDM) as a detergent, LCIA and HLA3 were detected with different sizes of ∼240 and 580 kDa, respectively (Fig. 5A). We also estimated the molecular masses using different DDM concentrations (0.25%, 0.5%, 1.0%, or 2.0%) or using formaldehyde cross-linker, and LCIA and HLA3 were still detected at 240 and 580 kDa, respectively (Fig. S4 A and B). Furthermore, LCIA and HLA3 could form respective complexes with the same molecular masses even in Aox, Hox, and AHox cells grown in HC-NO3– conditions (Fig. S4C). These results strongly suggested that LCIA and HLA3 did not interact physically in vivo and at least VLC-inducible proteins other than LCIA and HLA3 were not associated with the formation of the respective protein complexes.
Fig. 5.
Molecular masses of LCIA and HLA3 in nondenaturing conditions and effect of the absence of LCIA on HLA3 mRNA accumulation. (A) Molecular masses of LCIA and HLA3 in nondenaturing conditions. Total proteins were solubilized using 1.0% n-dodecyl β-d-maltoside and separated by blue-native PAGE. (B) Quantitative real-time PCR analyses of HLA3 (Upper) and LCIA (Lower) in WT, Ain-1, Ain-2, Ain-1C, Ain-2C, Hin-1, and Hin-1C. These cells were grown in very low CO2 conditions for 4 h. Expression of each gene was normalized to CBLP. Data in all experiments indicate mean value ± SD from three biological replicates. *P < 0.01.
For the latter possibility, HLA3 mRNA accumulation was evaluated by quantitative real-time PCR (Fig. 5B). The sequences of primers used are listed in Table S4. HLA3 mRNA levels were significantly reduced in Ain-1 and Ain-2 grown in VLC, but mRNA accumulation was restored in the complemented strains. In contrast, the mRNA levels of LCIA were not affected in Hin-1 (Fig. 5B), and those of other VLC-inducible genes LCIB and LCI1 were also largely unchanged in Ain-1 and Ain-2, as well as Hin-1 (Fig. S4D). These results suggested that LCIA localized to the CE could affect the mRNA expression level of HLA3 and subsequently caused a decrease in HLA3 protein accumulation.
Discussion
In this study, by characterizing the photosynthetic phenotype of LCIA and HLA3 insertion/overexpressing strains, it was revealed that HLA3 and LCIA are parts of the mechanism of HCO3– uptake through the PM and CE. These results elucidated a route of HCO3– uptake from the outside of cells to the chloroplast stroma by the cooperative function of HLA3 and LCIA.
Although LCIA could be associated with HCO3– uptake, the molecular mechanism remains elusive. LCIA is a homolog of formate transporter FocA and contains five amino acid residues (Fig. S2F) corresponding to those shown to form the pore of FocA (19, 33). FocA forms a symmetric pentamer that closely resembles the structure of aquaporin (33) and facilitates formate transport as a channel. Considering that LCIA was detected at 240 kDa in nondenaturing conditions (Fig. 5A), LCIA forms a protein complex as in the case of FocA. Furthermore, considering that the capacity for formate passage by FocA is increased by mutations of the aforementioned amino acids to smaller residues (33), examining the effect of similar mutations in LCIA could be helpful in elucidating the function of LCIA as a potential HCO3– channel. Relating to this hypothesis, a significant increase in Ci affinity at pH 6.2 was observed in LCIA-overexpressing strains (Fig. 4 A, C, and D). Considering that external CO2 at pH 6.2 should enter the cytoplasm continuously by passive influx, LCIA could function as a channel and cause an increase in the apparent Ci conductance with a minimal concentration gradient without waiting for a notable increase in Ci accumulation in the cytoplasm. In contrast, endogenous levels of HLA3 in HC conditions were not sufficient for Ci permeation toward the chloroplast stroma even with increased cytosolic Ci accumulation (Fig. 4B). These results suggested the functional importance of LCIA as a bottle neck step for increases in photosynthetic conductance across the CE.
By measuring the Ci accumulation and affinity of LCIA/LCI1-overexpressing strains and comparing the results with those of LCIA/HLA3-overexpressing strains, the degree of LCIA/HLA3-dirven HCO3– uptake activity was evaluated (Fig. 4D). However, there were no differences in Ci accumulation and affinity at pH 9.0 compared with WT, suggesting that LCI1 was not related to the direct HCO3– uptake along with LCIA. Furthermore, although it was reported that Ci affinity was increased by the single overexpression of LCI1 at pH 7.8 (25), A1ox did not show a significant increase in Ci affinity in the same pH conditions. This discrepancy could be caused by the difference in K0.5 (Ci) values of the strains examined. For overexpressing LCI1 in the previous report, strain lcr1 deficient in mRNA expression for at least three genes, LCI1, CAH1, and LCI6 (26), was used, and its K0.5 (Ci) was 445 ± 38 µM in HC conditions at pH 7.8 (25). In contrast, the K0.5 (Ci) of strain C9 used as WT in this study was 230 ± 27 µM in the same conditions, which was almost the same as 245 ± 38 µM when LCI1 was overexpressed in lcr1 (25). Thus, the effect of overexpressing LCI1 could be masked in A1ox cells.
By means of LCIA insertion mutant analyses, it was shown that LCIA localized to the CE affected HLA3 mRNA expression in the nucleus (Fig. 5B), which could throw new light on understanding the regulation of LCIA and HLA3. Considering that LCIA expression was not affected by the absence of HLA3 (Fig. 5B), there may be unidentified retrograde signals from the chloroplast to the nucleus for maintaining HLA3 mRNA expression. This possibility is supported by the recent study showing that transcript levels of LCIA and HLA3 were simultaneously impaired in an HC-requiring mutant containing a disrupted CAS gene encoding a putative chloroplast calcium sensor protein and that other LC-inducible genes, such as CAH1, LCI1, LCIB, and LCIC, were unaffected in the CAS mutant (34). Furthermore, this suggested that LCIA and HLA3 could function cooperatively as part of the CCM and that LCIA has a key role in guaranteeing the maintenance of the HCO3– uptake system. Because LCIA and HLA3 are conserved among aquatic algae, and owing to the structural relationship of LCIA homologs with aquaporin (33), the LCIA and HLA3 genes may have potential for genetic improvement of photosynthesis in land plants and algae.
Materials and Methods
C. reinhardtii strain C9 (photosynthetically WT strain available from the National Institute for Environmental Studies, Japan, as strain NIES-2235) was cultured in Tris-acetate-phosphate (TAP) medium for maintenance. For physiological experiments, cells were grown in liquid TAP medium for precultivation and diluted with modified high-salt medium [HSM (NH4+)] containing 9.35 mM NH4Cl supplemented with 20 mM Mops (pH 7.0) to an OD730 of ∼0.05 for photoautotrophic growth. To induce the expression of exogenous genes, cells grown in HSM (NH4+) medium for ∼24 h to an OD730 of ∼0.3 were collected by centrifugation and resuspended in fresh HSM (NO3–) containing 9.35 mM KNO3 aerated with air enriched with 5% CO2 (HC) or ordinary air containing 0.04% CO2 (VLC). The culture conditions with combinations of medium and CO2 concentrations are described as HC-NH4+, HC-NO3–, VLC-NH4+, and VLC-NO3–. For all culture conditions, cells were cultured at 25 °C with illumination at 80 μmol photons⋅m–2⋅s–1.
Additional experimental procedures and methods are listed in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank James V. Moroney for providing the anti-LCI1 antibody and Haruaki Yanagisawa for pGenD-aphVIII. We also thank Ryohei Kitada, Ryota Sakai, and Koki Kise for technical assistance. This work was supported by the Japan Society for the Promotion of Science KAKENHI Grants 25120714 (to H.F.) and 25840109 (to T.Y.) and the Japan Science and Technology Agency Advanced Low Carbon Technology Research and Development Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501659112/-/DCSupplemental.
References
- 1.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417(2):423–439. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- 2.Price GD, Badger MR, Woodger FJ, Long BM. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot. 2008;59(7):1441–1461. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- 3.Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425(6959):734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima K, Tanaka A, Matsuda Y. SLC4 family transporters in a marine diatom directly pump bicarbonate from seawater. Proc Natl Acad Sci USA. 2013;110(5):1767–1772. doi: 10.1073/pnas.1216234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones HG. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology. 2nd Ed Cambridge Univ Press, Cambridge, UK; 1992. [Google Scholar]
- 6.Badger MR, Price GD. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J Exp Bot. 2003;54(383):609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 7.Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- 8.Badger MR, Kaplan A, Berry JA. Internal inorganic carbon pool of Chlamydomonas reinhardtii: Evidence for a carbon-dioxide concentrating mechanism. Plant Physiol. 1980;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moroney JV, Ynalvez RA. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell. 2007;6(8):1251–1259. doi: 10.1128/EC.00064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spalding MH. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot. 2008;59(7):1463–1473. doi: 10.1093/jxb/erm128. [DOI] [PubMed] [Google Scholar]
- 11.Moroney JV, et al. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth Res. 2011;109(1-3):133–149. doi: 10.1007/s11120-011-9635-3. [DOI] [PubMed] [Google Scholar]
- 12.Ohad I, Siekevitz P, Palade GE. Biogenesis of chloroplast membranes. I. Plastid dedifferentiation in a dark-grown algal mutant (Chlamydomonas reinhardi) J Cell Biol. 1967;35(3):521–552. doi: 10.1083/jcb.35.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson J, et al. A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998;17(5):1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raven JA. Putting the C in phycology. Eur J Phycol. 1997;32(4):319–333. [Google Scholar]
- 15.Vance P, Spalding MH. Growth, photosynthesis, and gene expression in Chlamydomonas over a range of CO2 concentrations and CO2/O2 ratios: CO2 regulates multiple acclimation states. Can J Bot. 2005;83(7):796–809. [Google Scholar]
- 16.Wang Y, Spalding MH. Acclimation to very low CO2: Contribution of limiting CO2 inducible proteins, LCIB and LCIA, to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol. 2014;166(4):2040–2050. doi: 10.1104/pp.114.248294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuzawa H, et al. Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA. 2001;98(9):5347–5352. doi: 10.1073/pnas.081593498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang Y, Zhang J, Weeks DP. The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2001;98(9):5341–5346. doi: 10.1073/pnas.101534498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, et al. Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2004;135(3):1595–1607. doi: 10.1104/pp.104.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamano T, Miura K, Fukuzawa H. Expression analysis of genes associated with the induction of the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2008;147(1):340–354. doi: 10.1104/pp.107.114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brueggeman AJ, et al. Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. Plant Cell. 2012;24(5):1860–1875. doi: 10.1105/tpc.111.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang W, et al. Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell. 2012;24(5):1876–1893. doi: 10.1105/tpc.112.097949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burow MD, Chen ZY, Mouton TM, Moroney JV. Isolation of cDNA clones of genes induced upon transfer of Chlamydomonas reinhardtii cells to low CO2. Plant Mol Biol. 1996;31(2):443–448. doi: 10.1007/BF00021807. [DOI] [PubMed] [Google Scholar]
- 24.Im CS, Grossman AR. Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J. 2002;30(3):301–313. doi: 10.1046/j.1365-313x.2001.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi N, et al. Expression of a low CO₂-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell. 2010;22(9):3105–3117. doi: 10.1105/tpc.109.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshioka S, et al. The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Cell. 2004;16(6):1466–1477. doi: 10.1105/tpc.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariscal V, et al. Differential regulation of the Chlamydomonas Nar1 gene family by carbon and nitrogen. Protist. 2006;157(4):421–433. doi: 10.1016/j.protis.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3- transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2009;106(14):5990–5995. doi: 10.1073/pnas.0812885106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norling B, Nurani G, Franzen LG. Characterization of the H+-ATPase in plasma membranes isolated from the green alga Chlamydomonas reinhardtii. Physiol Plant. 1996;97(3):445–453. [Google Scholar]
- 30.Ramazanov Z, Mason CB, Geraghty AM, Spalding MH, Moroney JV. The low CO2-inducible 36-kilodalton protein is localized to the chloroplast envelope of Chlamydomonas reinhardtii. Plant Physiol. 1993;101(4):1195–1199. doi: 10.1104/pp.101.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Ballester D, et al. Reverse genetics in Chlamydomonas: A platform for isolating insertional mutants. Plant Methods. 2011;7:24. doi: 10.1186/1746-4811-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamano T, et al. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 2010;51(9):1453–1468. doi: 10.1093/pcp/pcq105. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, et al. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature. 2009;462(7272):467–472. doi: 10.1038/nature08610. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Yamano T, Kajikawa M, Hirono M, Fukuzawa H. Isolation and characterization of novel high-CO2-requiring mutants of Chlamydomonas reinhardtii. Photosynth Res. 2014;121(2-3):175–184. doi: 10.1007/s11120-014-9983-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.