The pan-HER kinase inhibitor dacomitinib produced durable partial regressions in patients with HER2-mutant lung cancers, but only in individuals with certain specific mutations. More of these precisely defined mutation types are being identified by next-generation sequencing platforms which are rapidly becoming the standard method for genotyping lung cancers at diagnosis.
Keywords: dacomitinib, HER2 mutations, HER2 amplification, lung cancers, tyrosine kinase inhibitors
Abstract
Background
HER2 mutations and amplifications have been identified as oncogenic drivers in lung cancers. Dacomitinib, an irreversible inhibitor of HER2, EGFR (HER1), and HER4 tyrosine kinases, has demonstrated activity in cell-line models with HER2 exon 20 insertions or amplifications. Here, we studied dacomitinib in patients with HER2-mutant or amplified lung cancers.
Patients and methods
As a prespecified cohort of a phase II study, we included patients with stage IIIB/IV lung cancers with HER2 mutations or amplification. We gave oral dacomitinib at 30–45 mg daily in 28-day cycles. End points included partial response rate, overall survival, and toxicity.
Results
We enrolled 30 patients with HER2-mutant (n = 26, all in exon 20 including 25 insertions and 1 missense mutation) or HER2-amplified lung cancers (n = 4). Three of 26 patients with tumors harboring HER2 exon 20 mutations [12%; 95% confidence interval (CI) 2% to 30%] had partial responses lasting 3+, 11, and 14 months. No partial responses occurred in four patients with tumors with HER2 amplifications. The median overall survival was 9 months from the start of dacomitinib (95% CI 7–21 months) for patients with HER2 mutations and ranged from 5 to 22 months with amplifications. Treatment-related toxicities included diarrhea (90%; grade 3/4: 20%/3%), dermatitis (73%; grade 3/4: 3%/0%), and fatigue (57%; grade 3/4: 3%/0%). One patient died on study likely due to an interaction of dacomitinib with mirtazapine.
Conclusions
Dacomitinib produced objective responses in patients with lung cancers with specific HER2 exon 20 insertions. This observation validates HER2 exon 20 insertions as actionable targets and justifies further study of HER2-targeted agents in specific HER2-driven lung cancers.
ClinicalTrials.gov
introduction
Aberrations in human epidermal growth factor receptor 2 (HER2, ERBB2) have emerged as oncogenic drivers and therapeutic targets in lung cancers, with HER2 mutations occurring in ∼1%–4% [1–5], and HER2 amplifications occurring in 2%–5% [6–8] of lung adenocarcinomas. Preclinical studies have described the antitumor activity of HER2-targeted therapies, inhibiting both HER2-mutant and HER2-amplified lung cancer cell lines [9, 10], including mutations in the HER2 extracellular domain [11]. The growing use of multiplexed genomic assays that can detect both mutations and amplifications has led to the identification of an increasing number of individuals with HER2-driven lung cancers [12]. The availability of HER2-targeted agents makes HER2 an ‘actionable’ target, further spurring the implementation of assays to detect it. Several reports have demonstrated the activity of HER2-directed therapies in patients with HER2-mutant [13–16] and HER2-amplified [15] lung cancers.
Dacomitinib (PF-00299804; Pfizer, New York, NY) is a pan-HER inhibitor that irreversibly binds to HER2, HER1 (EGFR), and HER4 tyrosine kinases [17, 18]. We have reported the results of the phase II trial of dacomitinib in patients with lung adenocarcinomas clinically and/or molecularly selected for the presence of epidermal growth factor receptor (EGFR) mutations [19]. As part of this larger effort, we studied a separate molecularly selected cohort of individuals with tumors harboring HER2 mutations or amplification.
methods
patients and treatments
We conducted a multicenter, phase II study of adults with pathologically confirmed stage IIIB or IV lung adenocarcinomas, Eastern Cooperative Oncology Group performance status 0–2, and measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST version 1.0) [20]. Results in the group with EGFR-driven tumors have been reported [19]. Inclusion and exclusion criteria for this HER2 cohort were identical to the EGFR cohort with the exception of the target and including patients with any prior systemic therapy [19]. Molecular selection was carried out by fluorescence in situ hybridization (FISH) or sequencing by multiplexed testing of tumor samples in CLIA laboratories at individual study sites. HER2 amplification was defined as a HER2/centromere of chromosome 17 signal ratio ≥2 [6, 21]. HER2 mutations included exon 20 insertions, deletions, and point mutations in intra- or extracellular domains. The study was approved by an Institutional Review Board at each site. All patients provided written, informed consent. This trial was linked to the Lung Cancer Mutation Consortium (LCMC) project (National Cancer Institute, USA, 1RC2CA148394-010, NCT0114286) [4].
We gave dacomitinib 45 mg orally, once-a-day, in 28-day cycles. In five individuals who had received no prior systemic therapy, an initial dose of 30 mg was given. Treatment was continued until disease progression or unacceptable toxicity. Dacomitinib was not continued beyond disease progression.
Tumor assessments were done at baseline, 4 weeks, 8 weeks, and then every 8 weeks. Investigators determined response per RECIST version 1.0 [20]. Toxicities were assessed continuously, classified using the Medical Dictionary for Regulatory Activities (MeDRA; version 15.0) and graded by the National Cancer Institute (USA) Common Terminology Criteria for Adverse Events, version 3.0.1 (NCI CTCAE v3.0.1).
outcomes and assessments
Study end points reported included the proportions of patients with HER2 mutations and amplifications achieving partial responses, duration of responses, progression-free survival at data analysis cutoff, overall survival at data analysis cutoff, and the frequencies of treatment-related toxicities. The duration of responses was measured from the time of the first documentation of response to the date of disease progression. Progression-free survival was defined as the interval from the date of the first dose of dacomitinib to the date of disease progression or death. Overall survival was defined as the interval from the date of the first dose of dacomitinib to the date of death. The information presented reflects information in the database as of 30 May 2014.
statistical methods
The study proposed to enroll about 25 patients with HER2-mutant or amplified lung cancers to explore the activity of dacomitinib in this molecularly defined group. No statistical hypothesis was tested. Time-to-event end points were analyzed using the Kaplan–Meier method. All patients who received any study drug were included in both the response and toxicity analyses. We used SAS version 9.1.3 for all statistical analyses.
results
We enrolled and treated 30 individuals with pathologically confirmed recurrent or de-novo lung cancers between January 2011 and February 2013. Patient characteristics are detailed in supplementary Table S1, available at Annals of Oncology online. Half were women, 60% were never smokers, and all but two had stage IV disease. Dacomitinib was the first systemic therapy for 17% and 83% had received at least one prior i.v. cytotoxic chemotherapy. Two patients (7%) were previously treated with trastuzumab. Twenty-eight patients were enrolled from Lung Cancer Mutation Consortium sites.
The specific aberrations detected in the 26 patients with HER2 mutations are detailed in Table 1. Thirteen tumors harbored identical 12-bp exon 20 insertions [A775_G776insYVMA, alternative nomenclature p.Y772_A775dup(c.2313_2324dup)]. There were four 9-bp insertions and three 3-bp insertions. Two patients had HER2 exon 20 indels and one a missense mutation. Mutation details were not specified in three cases with exon 20 insertions. Data on the four HER2-amplified cases is detailed in supplementary Table S2, available at Annals of Oncology online. Only one case had high-level amplification. Of the 10 patients with HER2 mutations that were also tested for HER2 amplification, none were amplified.
Table 1.
Description of HER2 mutations and clinical outcomes of patients treated with dacomitinib
|
HER2 mutation type Partial responses noted |
HER2 amplification status by FISH (HER2/CEP17 ratio) | Mutation Amino acid change (and nucleotide change) |
Alternate nomenclature (based on HGVS guidelines) |
Survival from start of dacomitinib (months) |
|---|---|---|---|---|
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 23 |
| Exon 20 insertion (12-bp duplication) | <2.0 | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 10 |
| Exon 20 insertion (12-bp duplication) | <2.0 | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 16 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 3 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 2 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 9 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 9 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 29 |
| Exon 20 insertion (12-bp duplication) | <2.0 | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 5 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 2+ |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 7 |
| Exon 20 insertion (12-bp duplication) | Not tested | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 14 |
| Exon 20 insertion (12-bp duplication) | <2.0 | p. A775_G776insYVMA (c. 2324_2325ins12) | p.Y772_A775dup (c.2313_2324dup) | 26+ |
| Exon 20 insertion (9-bp duplication) Partial response Duration 14 months |
<2.0 | p. P780_Y781insGSP (c. 2339_2340ins GGCTCCCCA) | p.G778_P780dup (c.2331_2339dup) |
27 |
| Exon 20 insertion (9-bp duplication) Partial response Duration 11 months |
<2.0 | p. P780_Y781insGSP (c. 2339_2340ins GGCTCCCCA) |
p.G778_P780dup (c.2331_2339dup) |
25+ |
| Exon 20 insertion (9 bp) | Not tested | Not specified | Nor specified | 4 |
| Exon 20 insertion (9 bp) | <2.0 | Not specified | Not specified | 8 |
| Exon 20 insertion (3 bp) | <2.0 | Not specified | Not specified | 3 |
| Exon 20 insertion (3 bp) | Not tested | p. G776 > VC (c.2326_2327insTGT) | p.G776delinsVC (c.2326_2327insTGT) |
21 |
| Exon 20 insertion (3 bp) | Not tested | p. G776 > VC (c.2326_2327insTGT) | p.G776delinsVC (c.2326_2327insTGT) |
2 |
| Exon 20 insertion | Not tested | Not specified | Not specified | 7 |
| Exon 20 insertion | <2.0 | Not specified | Not specified | 18+ |
| Exon 20 insertion | Not tested | Not specified | Not specified | 21+ |
| Exon 20 indel Partial response Duration 3+ months |
Not tested | p. M774delinsWLV (c.2320A > TGGCTGG) |
p. M774delinsWLV (c.2320delins TGGCTGG) |
23+ |
| Exon 20 indel | <2.0 | p. G776 > LC (c. 2326G > TTGT) |
p.G776delinsLC (c.2326delinsTTGT) |
9 |
| Exon 20 missense mutation | Not tested | p. V777L (c.2329G > T) | p. V777L (c.2329G > T) | 3 |
HGVS, Human Genome Variation Society.
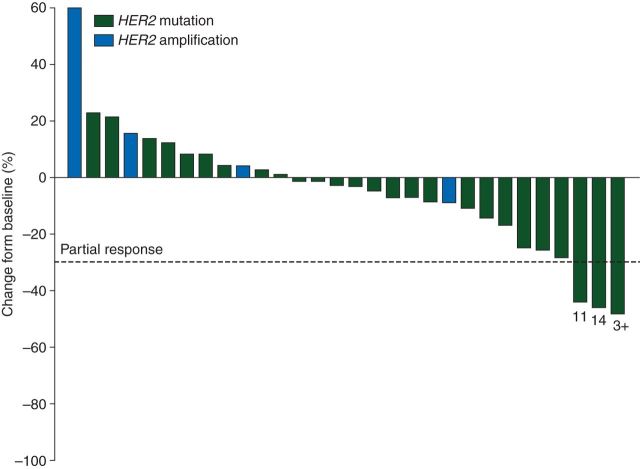
Confirmed partial responses were seen in three patients, two with tumors harboring a 9-bp exon 20 insertion, lasting 11 and 14 months and one with a tumor harboring an exon 20 indel lasting 3+ months. The two patients with the exon 20 insertions that had the longest responses had identical amino acid changes (p. P780_Y781insGSP, Table 1). The overall response for the patients with HER2-mutant disease was 12% (95% CI 2% to 30%) and 0% (95% CI 0% to 60%) for the four patients with HER2-amplified tumors. Figure 1 is a waterfall plot depicting the greatest change in target lesion size for each patient.
Figure 1.
Best change from baseline for each patient (n = 30): waterfall. The duration of responses are noted below the bar for each of the three patients with partial responses (RECIST 1.0). Numbers below the bars note the duration of the partial responses in months.
For the HER2-mutant cohort, the median progression-free survival was 3 months (95% CI 2–4 months). The four individuals with HER2-amplified tumors remained progression free for 1, 1, 5, and 5 months. The median overall survival was 9 months from the start of dacomitinib (95% CI 7–21 months) for patients with HER2-mutant cancers and their 1-year survival was 44% (95% CI 25–62). Overall survivals were 5, 7, 15, and 22 months for the individuals with HER2-amplified tumors. The patient with the highest degree of amplification (HER2/CEP17 ratio 17) had the longest survival. Kaplan–Meier survival curves from the start of dacomitinib for the HER-mutant and HER2-amplified groups are shown in supplementary Figure S1, available at Annals of Oncology online.
Treatment-related toxicities are listed in supplementary Table S3, available at Annals of Oncology online, reporting the maximum toxicity seen at any time while on study for all 30 patients. Some diarrhea was seen in 90% and skin rash in 73%. There was one case of grade 4 diarrhea and no grade 4 rash. Rash and diarrhea resolved with either supportive measures or dacomitinib cessation. Due to treatment-related toxicity, the dose of dacomitinib was reduced in 17% and in 13%, dacomitinib was stopped. There was one drug-related death. This individual developed hepatic failure likely due to an interaction of mirtazapine and dacomitinib. Mirtazapine is metabolized by CYP2D6 and dacomitinib is a strong inhibitor of the same cytochrome. Mirtazapine was started 6 days after the initiation of dacomitinib. Pharmacokinetic evaluations after development of liver failure revealed plasma mirtazapine levels increased 26-fold over expected. The patient died 13 days after starting mirtazapine.
discussion
This is the first phase II trial of a HER2-targeted agent in patients with lung cancers molecularly selected for study based on the presence of HER2 mutations or amplification in their tumors. Durable partial responses were documented in 12% of individuals with HER2 mutations using dacomitinib, an inhibitor of the HER2 tyrosine kinase.
Stephens et al. [1] and Shigematsu et al. [2] independently identified mutations in HER2 in lung cancers that were mutually exclusive with EGFR and KRAS. Reports exploring the association between HER2 amplification and HER2 mutation in lung cancers have yielded divergent results [3, 22]. Kinase domain mutations, mainly exon 20 insertions and point mutations [3] lead to constitutive HER2 kinase activation which is felt to underlie the sensitivity of cancers driven by these mutations to kinase inhibitors [9]. Subsequently, there have been several reports that HER2 kinase inhibitors, including afatinib [13, 14], lapatinib [23], neratinib [24], and neratinib plus temsirolimus [16, 24], can lead to regressions in patients with tumors harboring these mutations. Cappuzzo et al. [15] also reported a partial response in a patient with lung cancer harboring both a HER2 exon 20 (G776 > LC) mutation and HER2 amplification, following treatment with the combination of trastuzumab and paclitaxel. The clinical series by Mazieres et al. [13] reported 10 of 16 patients with tumors with YMVA HER2 exon 20 insertions experienced partial responses to HER2-targeted therapies, 9 of them with concurrent chemotherapy and 8 with trastuzumab. One partial response with trastuzumab alone was reported. Tomizawa reported a response with trastuzumab plus vinorelbine in a similar patient [25]. While a randomized phase II trial by Gatzemeier et al. [21] concluded that the addition of trastuzumab to chemotherapy produced no added activity in HER2 protein overexpressed lung cancers, the six patients with either 3+ HER2 overexpression or HER2 amplification had a response rate of 83%. A trial of ado-trastuzumab emtansine (TDM-1) in patients with lung cancers with HER2 protein overexpression is underway (ClinicalTrials.gov, NCT02289833). Reviews of this topic have recently been published [26, 27]. But despite these reviews and series, no prospective trials have explored single-agent HER2-targeted agents in patients molecularly selected by the presence of HER2 mutations or amplifications. The majority of the responses reported were using a HER2-targeted agent with chemotherapy.
The activity of dacomitinib in patients with HER2 mutations in this trial confirms the earlier reports of benefit with HER2 kinase inhibitors in similar patients. While the responses all provided clinical benefit, they were few in number and were seen only in two of four patients with 9-bp HER2 exon 20 insertions and one of two patients with indels in HER2 exon 20. Preclinical studies demonstrate that a number of HER2 mutations are oncogenic both in vitro and in vivo, similar to EGFR mutations [9, 28]. The sensitivity to dacomitinib among the HER2 mutations may vary, in part explaining the clinical findings. The most common HER2 mutation is located in the analogous position as EGFR exon 20 insertion mutations and some but not all of these are also dacomitinib sensitive. No partial responses were documented here in patients with the most common HER2 mutation, a 12-bp insertion in exon 20 (p. A775_G776insYVMA (c. 2324_2325ins12). In contrast, the responses reported with afatinib were described in patients with tumors with the YVMA exon 20 insertion found in 13/26 of the patients in this trial [13, 14]. These observations suggest that the benefits of an individual agent may be confined to specific aberrations and the term ‘HER2-positive lung cancers’ does not adequately define these illnesses. The specific type of HER2 mutation, presence and degree of HER2 amplification, and HER2 protein expression should be precisely defined for each patient in future studies of HER2-targeted agents.
Advances in technology have made it possible to routinely identify oncogenic drivers in patients with lung cancers using multiplex panels [29] which are today recommended for use in all patients with adenocarcinomas in treatment guidelines [30]. The Lung Cancer Mutation Consortium in the United States and the French Cooperative Thoracic Intergroup have detected mutually exclusive HER2 mutations in 3% (95% CI 2% to 4%) and 1% (95% CI 1% to 2%) of tumors from patients with lung cancers respectively [4, 5]. Next-generation sequencing (NGS) is quickly replacing current multiplex panels. With regard to HER2, in addition to identifying mutations in exon 20, NGS panels can assess extracellular domain mutations and the presence of amplifications [12]. These expanding capabilities are increasing the number of patients with HER2 aberrations that can treated by both approved and investigational agents.
The data reported here have limitations. This was an exploratory study with no predefined primary end point, study outcome, or sample size specific to this HER2 cohort. The end points chosen for this report were the same as for the EGFR cohort previously reported [19]. Because we were uncertain about the frequency of HER2 mutations and amplifications, no sample sizes were prespecified for either group. Central confirmation of molecular abnormalities was not mandated since HER2 amplification testing was a standard test for breast cancer specimens and multiplex testing for oncologic drivers carried out within CLIA standards were in place at all study sites. Tissue was not collected to determine HER2 protein expression.
These results with dacomitinib emphasize both the promise and pitfalls of testing drugs in patients with tumors harboring HER2 aberrations. While durable responses are documented, their frequency is a fraction of what we have observed with kinase inhibitors targeting EGFR and ALK in mutation-positive cases where response rates in excess of 50% are seen, including a 76% partial response rate and 18 months progression-free survival with dacomitinib in patients with sensitizing EGFR mutations [19]. Beyond the diversity of the specific molecular aberrations in HER2 in lung cancers, the variable effectiveness of HER2 kinase inhibitors may also reflect the inherent activity of the drugs themselves. Although we demonstrated partial responses here with dacomitinib in HER2-mutant lung cancers, no responses were reported with the HER2 kinase inhibitor neratinib in similar patients [24]. Care must be taken before concluding that the results for any one agent define the overall effectiveness of ‘HER2-targeted therapies’ in lung cancers. Future trials must assess adequate numbers of cases with precise molecular alterations for each agent under study. The relationships among HER2 amplification, HER2 protein expression, and HER2 mutation are largely unknown. The impact of these abnormalities on therapeutic efficacy of HER2-targeted agents either alone or in the context of HER2 mutations is equally unclear. Activation of other pathways, particularly PI3KCA/mTOR, may also play a role as suggested by the observation that the combination of the mTOR inhibitor temsirolimus plus neratinib (a HER2 kinase inhibitor) produced a higher response rate in patients with HER2-mutant lung cancers than with neratinib alone [24]. Further complicating the picture is the fact that in other cancers, HER2-targeted antibodies show greater clinical benefit than tyrosine kinase inhibitors in HER2-amplified cancers despite being far weaker inhibitors of oncogenic HER2 signaling [31]. These knowledge gaps for an important target in a rapidly growing patient group make further research and comprehensive assessment of HER2 mutation, HER2 copy number, and HER2 protein expression in all cases enrolled on trials of HER2-targeted agents.
This study also emphasizes the complexity of determining the existence of ‘actionable’ mutations in lung cancers. Precise molecular targets for ‘actionability’ require that they are of sufficient frequency to make their study in prospective clinical trials feasible. If the activating mutations of EGFR had represented only 15% of all EGFR mutations, rather than the 80+ percent that have been found, could clinical trials detect a ‘signal’ of sufficient clarity to justify drug development? The challenges and resources to identify a drug and prove that it has meaningful clinical activity are substantial. Tracking an activity ‘signal’ in a ‘segment of a segment’ of a molecularly defined patient population could prove to be challenging in today's drug development and testing environment. A commitment to sharing precise molecular profiles of tumors using a standardized nomenclature and a searchable database that bridges institutional and pharmaceutical company efforts could go a long way in unraveling these complexities.
This phase II trial of dacomitinib demonstrated that trials of agents targeting HER2 in patients with lung cancers molecularly selected for tumors with HER2 aberrations are possible and can lead to meaningful responses, but only for selected genotypes. We advocate that single-agent activity for HER2-targeted therapies be documented before proceeding to combination trials and that study of trastuzumab, pertuzumab, and TDM-1 be pursued in patients with HER2-amplified tumors. The results presented here can help guide future studies and further raise the hope that the significance of HER2 as a target in lung cancers will continue to grow, both in the numbers of individuals treated and the magnitude of the benefits achieved.
funding
This work was supported by Pfizer, Inc. No grant applied.
disclosure
MGK has received consultancy fees from AstraZeneca, Clovis Oncology, Daiichi Sankyo, and Genentech/Roche; BTL has received consultancy fees from Roche and Biosceptre International. JO, IT, HZ, and ZG are employees and stockholders of Pfizer. MEA has received consultancy fees from AstraZeneca. PAJ has received consultancy fees from Pfizer, AstraZeneca, Boehringer Ingelheim, Clovis Oncology, Chugai, and Genentech; DRC, GG, and TH declare no competing interests.
Supplementary Material
references
- 1.Stephens P, Hunter C, Bignell G et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004; 431: 525–526. [DOI] [PubMed] [Google Scholar]
- 2.Shigematsu H, Takahashi T, Nomura M et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005; 65: 1642–1646. [DOI] [PubMed] [Google Scholar]
- 3.Arcila ME, Chaft JE, Nafa K et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012; 18: 4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311: 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowak F, Calvo F, Soria J. Europe does it better: molecular testing across a national health care system-the French example. Am Soc Clin Oncol Educ Book 2013: 332–337. [DOI] [PubMed] [Google Scholar]
- 6.Heinmoller P, Gross C, Beyser K et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 2003; 9: 5238–5243. [PubMed] [Google Scholar]
- 7.Cappuzzo F, Cho YG, Sacconi A et al. p95HER2 truncated form in resected non-small cell lung cancer. J Thorac Oncol 2012; 7: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SE, Narasanna A, Perez-Torres M et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006; 10: 25–38. [DOI] [PubMed] [Google Scholar]
- 10.Bunn PA Jr, Helfrich B, Soriano AF et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res 2001; 7: 3239–3250. [PubMed] [Google Scholar]
- 11.Greulich H, Kaplan B, Mertins P et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci USA 2012; 109: 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013; 31: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazieres J, Peters S, Lepage B et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013; 31: 1997–2003. [DOI] [PubMed] [Google Scholar]
- 14.De Greve J, Teugels E, Geers C et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012; 76: 123–127. [DOI] [PubMed] [Google Scholar]
- 15.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med 2006; 354: 2619–2621. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi L, Bahleda R, Tolaney SM et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol 2014; 32: 68–75. [DOI] [PubMed] [Google Scholar]
- 17.Engelman JA, Zejnullahu K, Gale CM et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007; 67: 11924–11932. [DOI] [PubMed] [Google Scholar]
- 18.Janne PA, Boss DS, Camidge DR et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 2011; 17: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janne PA, Ou SI, Kim D et al. Phase 2 trial of dacomitinib as initial treatment in patients with clinically and/or molecularly selected advanced non-small cell lung cancer. Lancet Oncol 2014; 15: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216. [DOI] [PubMed] [Google Scholar]
- 21.Gatzemeier U, Groth G, Butts C et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004; 15: 19–27. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Sun Y, Fang R et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012; 7: 85–89. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RJ, Carter CA, Giaccone G. HER2 mutations in non-small-cell lung cancer can be continually targeted. J Clin Oncol 2012; 30: 3318–3319. [DOI] [PubMed] [Google Scholar]
- 24.Besse B, Soria J-C, Yao B et al. Neratinib (N) with or without Temsirolimus (TEM) in patiens (PTS) with non-small cell lung cancer (NSCLC) carrying HER2 somatic mutations: an international randomized phase II study. Ann Oncol 2014; 25(suppl 4): v1–v41. [Google Scholar]
- 25.Tomizawa K, Suda K, Onozato R et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer 2011; 74: 139–144. [DOI] [PubMed] [Google Scholar]
- 26.Landi L, Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther 2013; 13: 1219–1228. [DOI] [PubMed] [Google Scholar]
- 27.Ricciardi GR, Russo A, Franchina T et al. NSCLC and HER2: between lights and shadows. J Thorac Oncol 2014; 9: 1750–1762. [DOI] [PubMed] [Google Scholar]
- 28.Perera SA, Li D, Shimamura T et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci USA 2009; 106: 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pao W, Kris MG, Iafrate AJ et al. Integration of molecular profiling into the lung cancer clinic. Clin Cancer Res 2009; 15: 5317–5322. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. http://wwwnccnorg/professionals/physician_gls/pdf/nsclpdf 2014; Version 2.2015 (13 February 2015, date last accessed).
- 31.Moasser MM. Two dimensions in targeting HER2. J Clin Oncol 2014; 32: 2074–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.