Abstract
Stimulation of Type II taste receptor cells (TRCs) with T1R taste receptors causes sweet or umami taste, whereas T2Rs elicit bitter taste. Type II TRCs contain the calcium channel, calcium homeostasis modulator protein 1 (CALHM1), which releases adenosine triphosphate (ATP) transmitter to taste fibers. We have previously demonstrated with chorda tympani nerve recordings and two-bottle preference (TBP) tests that mice with genetically deleted Calhm1 (knockout [KO]) have severely impaired perception of sweet, bitter, and umami compounds, whereas their sour and salty tasting ability is unaltered. Here, we present data from KO mice of effects on glossopharyngeal (NG) nerve responses, TBP, food intake, body weight, and life span. KO mice have no NG response to sweet and a suppressed response to bitter compared with control (wild-type [WT]) mice. KO mice showed some NG response to umami, suggesting that umami taste involves both CALHM1- and non-CALHM1-modulated signals. NG responses to sour and salty were not significantly different between KO and WT mice. Behavioral data conformed in general with the NG data. Adult KO mice consumed less food, weighed significantly less, and lived almost a year longer than WT mice. Taken together, these data demonstrate that sweet taste majorly influences food intake, body weight, and life span.
Key words: bitter, CALHM1, food intake, glossopharyngeal nerve, life span, mouse, obesity, overweight, Q fibers, S fibers, sweet, taste, umami
Introduction
Since antiquity, taste has been divided into the taste qualities: salty, sweet, sour, and bitter. When electrophysiological techniques became available to record from taste nerves, the responsiveness of single taste fibers to stimuli representing taste qualities could be tested (Adrian and Zotterman 1926). It was evident early on that few taste fibers of cats, dogs, rabbits, and rats responded to only one taste quality (Pfaffmann 1941; Andersson et al. 1950; Cohen et al. 1955). Thus, Pfaffmann wrote: “every element (fiber) responded to more than one of the four basic taste stimuli,” (Pfaffmann 1955). These observations gave rise to the across-fiber pattern coding theory (Erickson 1963, 1967).
It was not until taste fiber recordings in macaques were accomplished that a considerably closer relationship between the human taste qualities and taste fiber responsiveness was observed (Gordon et al. 1959; Sato et al. 1975). This relationship was further strengthened with single taste fiber recordings in other catarrhina species, including chimpanzees, using a large number of stimuli representing human taste qualities and taste modifiers (Brouwer et al. 1983; Ninomiya and Hellekant 1991; Hellekant and Ninomiya 1994; Hellekant et al. 1997a, 1998; Danilova et al. 1998a, 1998b, 1999). Together, these studies suggested that, in higher primates, taste fibers cluster according to human taste qualities indicating that each taste quality is conveyed by a separate group of taste fibers, known as labeled-line coding.
More recently, molecular data have linked different taste receptor cells (TRCs) in taste buds to human taste qualities (Adler et al. 2000; Chandrashekar et al. 2000). For bitter and sweet taste qualities, 2 families of G protein receptors have been identified in Type II TRCs; one family of some 30 T2R G protein receptors gives rise to bitter taste (cf. Meyerhof 2005) and are linked to impulses in one group of taste fibers, called Q fibers, whereas stimulation of a combination of 2 out of 3 T1R receptors on the TRCs elicits a sweet/umami taste linked to impulses in S/M fibers. Thus, molecular and single fiber data have demonstrated a strong match between TRC types and taste fiber clusters (grouping).
Type II TRCs contain high concentrations of the calcium channel CALHM1 (Dreses-Werringloer et al. 2008; Moyer et al. 2009). When Type II TRCs are stimulated, they release the transmitter ATP to activate taste fibers (Finger et al. 2005). We demonstrated recently that mice with deleted CALHM1 channels do not release ATP. This causes absence of behavioral and chorda tympani (CT) nerve responses to sweet and diminished responses to bitter compounds (Taruno et al. 2013).
In this study, we report further impacts of CALHM1 knockout (KO) using two-bottle preference (TBP) tests and glossopharyngeal (NG) nerve recordings. These show a lack of responses to sweet and a greatly depressed response to bitter and umami in the KO mice, which corroborate the TBP data. We also present our findings on the effects of CALHM1 KO on food intake, body weight, and life span. Our results show that KO mice eat and weigh significantly less than normal (wild-type [WT]) mice. Our data also indicate that KO mice live longer than the WT mice. We suggest that without functioning T1R receptors and S fibers intake is determined by energy needs. This averts excessive consumption and abrogates weight gain in KO mice compared with WT mice. Mice with decreased or no sweet tasting ability present an opportunity to elucidate the influence of S fibers on food intake, body weight, and ultimately health and life. The importance of this study is in its relevance to the human condition, as will be discussed in the following.
Materials and methods
Animals
Calhm1 +/− founder mice were generated at GenOway. Calhm1 exon 1 deletion was performed by homologous recombination in 129Sv cells. The resultant embryonic stem cells were injected into blastocysts derived from C57BL/6J mice to obtain chimeric mice that possess germ line transmission of the targeted Calhm1 locus. WT (+/+) and Calhm1 KO (−/−) mice on the mixed 129Sv×C57BL/6J genetic background of F1 obtained from littermate mice were used in this study. Loss of Calhm1 expression in Calhm1 −/− taste buds was verified by real-time polymerase chain reaction and immunohistochemistry (cf. Taruno et al. 2013). The WT and Calhm1 null mice (KO) males and females were housed individually in the same room and given the same feed (Purina 5001). These studies were conducted in accordance to institutional and national guidelines for the care and use of animals and were approved by the University of Minnesota IACUC.
Stimuli and stimulation
Our choice of compounds and concentrations was influenced by our and other studies in mice and we strived to include a variety of compounds to represent all taste qualities. Hence, we used NaCl and NH4Cl for salty taste; ascorbic and citric acid as sour tastants; quinine hydrochloride, denatonium benzoate (DB), and sparteine as bitter compounds; SC45647, acesulfame-K, saccharin, 2 concentrations of sucrose, fructose, glucose as sweet tastants; and monosodium glutamate (MSG), monocalcium glutamate, and disodium-5′-inosinate (IMP) as umami tasting compounds (see Table 1).
Table 1.
Solutions used in experiments
| Compound | Concentration (mM) |
|---|---|
| NH4Cl | 100 |
| NaCl | 300 |
| Sc45647 | 8 |
| IMP | 30 |
| Ace-K | 25 |
| Citric acid | 20 |
| Saccharin Na salt | 30 |
| QHCl | 10 |
| Sucrose | 100 |
| DB | 5 |
| Sucrose | 300 |
| Ascorbic acid | 20 |
| MSG | 100 |
| Fructose | 300 |
| MSG | 300 |
| Glucose D-(+) dextrose | 100 |
| Monocalcium di-L-glutamate 4H2O | 100 |
| Sparteine (−)- | 50 |
The same TBP method and equipment were used as in our previous studies in mice (e.g., Finger et al. 2005). Hence, one bottle contained the tastant, the other water. The side of the cage for which the bottles were presented was switched every 24 h. Intake was measured every 24 h for at least two readings.
During taste nerve recordings, the stimuli were delivered to the tongue with an open flow system, controlled by a computer under conditions of constant flow and 33°C (cf. Hellekant and Roberts 1995; Danilova et al. 2002). A 10-s stimulus time was used and a 50-s rinse between stimuli. Generally, the order of Table 1 was followed to minimize cross-adaptation (Hellekant 1968).
Nerve recordings
The mice were anesthetized; a tracheotube was inserted and ligated caudally to the larynx. Isoflurane or halothane anesthesia was maintained at surgical levels to prevent muscle artifacts. Body temperature and temperature of the surgical table, blood oxygen and CO2, anesthesia concentration, and heart rate (electrocardiogram) were continuously monitored.
The lingual branch of the NG nerve was exposed after mobilizing and freeing M. digastricus’ caudal belly and dissection along the hypoglossal nerve until the NG nerve was seen dorsally as it dips under hyoglossal muscle. The central end was then cut and the nerve mobilized.
Nerve impulses were recorded between a silver wire electrode and an indifferent electrode touching the walls of the wound, fed into a custom-made amplifier, monitored over a loudspeaker and an oscilloscope. The nerve impulses were processed by a smoothed absolute value circuit (Hellekant and Roberts 1995), which generates a direct current potential, the product of nerve impulse frequency and amplitude, here called the summated signal. This signal and a binary code for the tastant used were fed to an oscilloscope and computer. Stimulus code, individual nerve impulses, and summated signal were preserved on a recorder (Gould ES 1000). The response to a stimulus was calculated by subtracting the level of nerve activity preceding stimulation from the peak response during stimulation.
In each mouse, the responses to all compounds were expressed relative to the response to 0.1M NH4Cl. The average in each animal and group was calculated, variance determined, and significance between the NG responses of KO and WT mice determined. Student’s t-test of log-transformed electrophysiological data was performed with significance level set at P < 0.05.
Measurements of food intake and body weight
Food intake and body weight in adult KO and WT mice were measured once a week for several weeks. We repeated these measurements in 3 different groups of WT and KO mice and found the differences between KO and WT mice consistent.
Results
TBP tests
We used TBP tests to elucidate differences in ability to taste between KO and WT mice. These data complement also taste nerve data by providing insight to the hedonic value of a taste created by a compound. Figure 1 presents a summary of the results of the two-bottle tests using these compounds. The mean preference ratio was calculated for each mouse and then averaged for the WT and KO mice groups. The left staple in each pair displays averaged data from the KO mice, whereas the right staple shows the average of the WT group. Asterisks indicate statistically significant differences between the responses of the 2 groups (*P < 0.05). Error bars show standard error (SE). A value between 40 and 60 indicates no preference or rejection of the compound.
Figure 1.
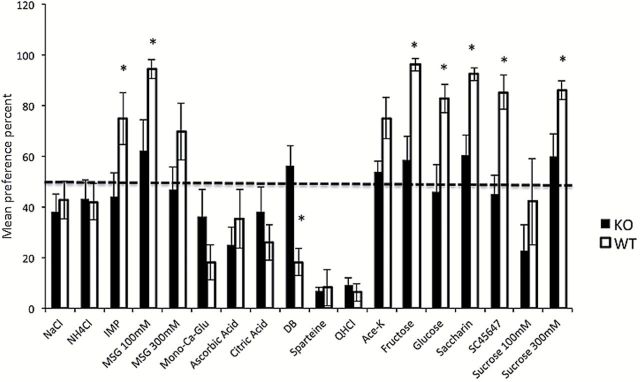
Behavioral results of ≥48-h TBP tests in CALHM1 KO and WT mice. The y-axis displays the mean preference ratio in percentage of total intake of each tastant listed in Table 1. Asterisks indicate statistically significant differences between the mean responses of the 2 mouse groups (*P < 0.05). Error bars represent SE. The plot shows that significant differences between umami and sweet compounds were present in KO mice compared with WT. With regard to bitter tastants, there was a significant difference in preference for DB between KO and WT mice.
Nerve responses to sweet, sour, bitter, umami, and salty compounds
Figure 2 presents a continuous sequence of summated responses from the NG nerve to NaCl, SC47647, IMP, acesulfame-K, citric acid, QHCl, sucrose, saccharin, and MSG. The upper trace was obtained from a WT mouse and the lower trace was obtained from a KO mouse. Approximately 20 s of recordings during rinses were not included in the figure. The recordings demonstrate complete loss of NG response to all sweeteners in the KO mouse, but no significant difference in responses of the WT and KO mouse to stimulation of salts and acids. The umami compounds, IMP and MSG, elicited a smaller response and the bitter compound, QHCl, almost no response in the KO mouse.
Figure 2.

Summated responses of NG nerve of a WT mouse (top trace) and a KO mouse (bottom trace) during 10-s stimulation with salt (NaCl), sweet (SC45647, acesulfame-K, sucrose, and saccharin), sour (citric acid), bitter (QHCl), and umami (IMP and MSG) compounds. The recordings are consecutive with 20 s of the trace during rinsing between stimulations removed to save space. The binary code of the bottom line shows when and how long each stimulus was applied.
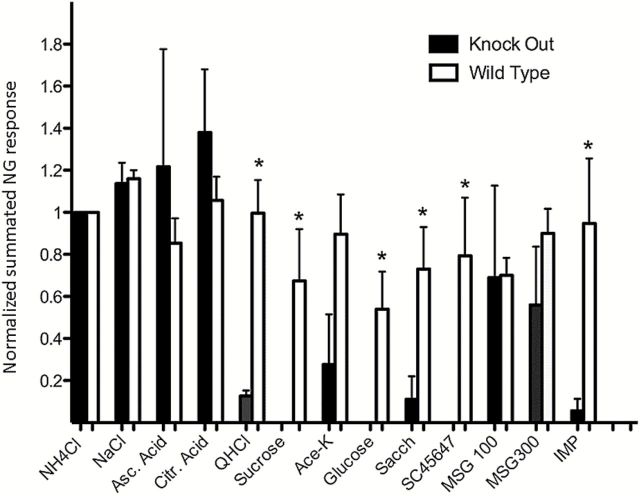
Figure 3 presents averages of 9 consecutive recordings of NG in KO mice (dark columns) and 9 NG recordings in WT mice (light columns). All responses were normalized to the response of NH4Cl. The columns depict peak responses to each compound with the baseline activity deducted. The columns show a significant difference between WT and KO mice for sweet and bitter stimuli, indicated with asterisks, but none for salts and acids. Two out of the 3 umami compounds gave a diminished response in KO mice. The exception is that MSG gave a significant response in the NG recordings but not in our previous recordings of the CT (Taruno et al. 2013). This will be discussed later.
Figure 3.
Each pair of staples represents averages of 9 summated NG recordings in WT and KO mice to the compounds denoted below. The responses of each mouse were normalized to the NH4Cl response. Asterisks denote a significant difference. The data show that although there was a significant difference between the responses of WT and KO mice to sweet and bitter (P < 0.05), no significant difference could be recorded in the responses to the salty and sour stimuli between WT and KO mice.
Comparison between non-normalized data from KO and WT mice does not change these conclusions, features already evident in the 2 recordings of previous Figure 2.
Body weight, food intake, and survival times
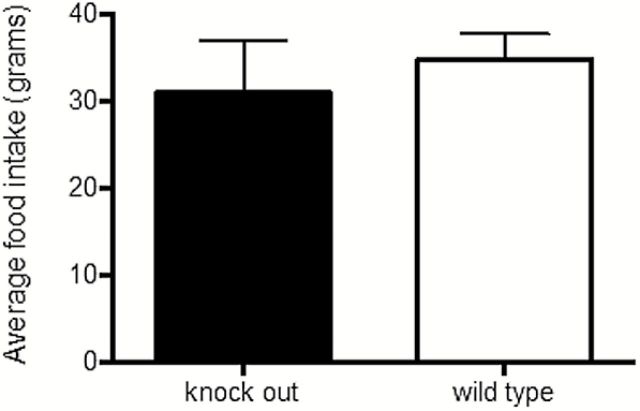
We discovered that CALHM1 KO mice had leaner bodies than WT mice. We therefore began weighing the mice. We also reasoned that differences in food intake caused the difference in body weight between WT and KO mice. In Figure 4, we present measurements of food intake over 12 weeks of 8 adult WT (black columns) and 15 adult KO mice (white columns). The columns show that WT mice consumed more food.
Figure 4.
The staples illustrate weekly food intake of WT and KO mice measured over 12 weeks. The data indicate that KO mice consumed less food than WT mice (P = 0.0822).
A difference in food intake between KO and WT mice should over time increase the difference in body weight between the 2 groups, so we measured and then calculated the mean body weights of 11 adult WT and 12 adult KO mice <1-year old and 14 WT and 7 KO >1-year old. Figure 5 shows that the KO mice weighed less than the WT mice and the weight difference increased with time. In the mice >1-year old, the difference between KO and WT mice was statistically significant.
Figure 5.
The staples summarize mean body weight of adult KO mice and WT littermates <1 year and >1 year of age. Data show that the difference between KO and WT mice increased with age and became significant after 1 year (P < 0.05).
Given the association between life span and excessive body weight, we compared life span of 9 WT and 6 KO mice, which coexisted in the same room and type of cages. The KO mice lived on the average 981 days, SE 41, whereas the average life span of the WT mice was 571 days, SE 91.
In summary, the study focuses on effects of CALHM1 on the posterior tongue. We evaluated NG nerve responses in CALHM1 KO and WT mice to an array of taste solutions. The NG in KO mice showed no response to sweet, a slight response to umami, a much smaller response to bitter, but no significant difference in responses to salty and sour compounds. These data are similar to our results of CALHM1 KO in CT nerve recordings except in regard to the umami response (Taruno et al. 2013).
Discussion
Sweet, bitter, and umami tastants bind to specific taste receptors on the tongue stimulating taste nerve fibers. Type II TRCs have receptors that when stimulated give rise to sweet/umami and bitter taste. Transmission of this information to the central nervous system (CNS) from the anterior tongue is mediated by fibers of the CT nerve, whereas information from the posterior tongue is mediated by nerve fibers of the NG nerve. The study has consequences for our understanding of taste transduction and some of them will be discussed in the following. The possibility that the taste effects were caused by CALHM1 CNS deficiency is discussed in the section: CALHM1 and CNS. The relationship between Type II TRCs and taste fiber classes will be discussed in the section: CALHM1 and sweet taste coding. The calcium channel CALHM1 is necessary for taste signal transduction in Type II TRCs and our previous investigation in CALHM1 KO mice involved the analysis of defective receptor signals that are mediated by the CT nerve (Taruno et al. 2013). The analysis suggested that CALHM1 had a critical role in the release of ATP transmitter to S, M (umami), and Q fibers. This is discussed in sections: CALHM1 and bitter taste and CALHM1 and umami taste. Finally, we present findings suggesting that loss or decreased functions of Type II TRCs and loss of S fiber input to CNS strongly influences food intake and possibly life span. This will be discussed in sections: CALHM1, food intake and body weight, and CALHM1 and life span.
CALHM1 and CNS
Given the expression of CALHM1 in the brain of humans, and particularly in hippocampus, with the latter’s central role in short term memory, it can be surmised that the behavioral data of the KO mice were caused by memory deficits (Khachaturian 1994; Dreses-Werringloer et al. 2008; Lambert et al. 2008; Yu et al. 2009). This is however an unlikely explanation because our taste nerve recordings here and in (Taruno et al. 2013) clearly show that the primary defects of CALHM1 deficiency are at the periphery, at least for sweet, bitter, and umami taste sensing. Consequently, regardless of the CNS effects of deletion of CALHM1, no taste nerve impulses were conveyed to the CNS of KO mice, which therefore had no ability to react to sweet.
CALHM1 and sweet taste coding
Our previous study (Taruno et al. 2013) and the present one show that blocking transmitter release of Type II TRCs in KO mice abolishes the taste nerve response to compounds that, lacking for better description, are sweet to WT mice. The absence of impulses of taste fibers that respond to compounds described as sweet by humans brings up 2 points. One is, can these compounds be labeled as sweet to a mouse? Mice are phylogenetically distant to humans, as are their food choices. However, a sweet taste quality seems to exist in mice because Ninomiya and Imoto (1995) using the sweet taste blocker gurmarin demonstrated that sweet compounds stimulate a dedicated group of taste fibers. Further, calcium imaging of geniculate ganglion neurons demonstrates a relationship between tastants and subsets of tastes fibers, of which one subset responded to sweet (Barretto et al. 2015). It is likely that these were S fibers synapsing with Type II TRCs. There is no definite answer to the question if taste qualities exist in mice, but the sweeteners used here trigger intake in both mouse and human. Together, these data justify the conclusion of a taste quality also in mice, which, for lack of better description and in analogy with human perception, can be called sweet.
The second point is the long-lived question of how taste information is coded in nerves. Defining the relationship between TRCs and their sensory fibers is critical for understanding how compounds on the tongue are perceived in the CNS. The importance of elucidating taste coding may be prioritized if impulses in sweet taste fibers are found to determine the borderline between necessary consumption and excessive consumption. This knowledge can lead to a better understanding of factors that lead to obesity.
A brief recapitulation of the 2 theories of taste fiber coding is provided in the following. This has been an open-ended question long before the discovery of receptors for sweet/umami and bitter taste qualities. As mentioned, earlier investigators found, using laboratory species that taste fibers generally responded to stimuli representing more than one human taste quality. Thus, there was no group of fibers that responded only to a single taste quality, for example, sweet. This gave rise to the across-fiber pattern theory in which taste qualities are thought to be the result of responses across a large number of fibers (Pfaffmann 1941, 1955; Erickson 1963).
The first single fiber recordings of rhesus monkeys showed a better relationship between taste fibers and human taste qualities (Gordon et al. 1959) than earlier studies. Similar conclusions could be drawn from recordings of Japanese macaques (Sato et al. 1975; Sato et al. 1977). But the limitations of these early studies were that a compound might elicit more than one taste quality. For example, saccharin elicits, in addition to sweet taste, a bitter taste. Other sweeteners, such as acesulfame-K, stevioside, sucralose, or even carbohydrates suffered of similar limitations. The answer to this problem was the identification of a compound that elicits sweet taste without side tastes. Miraculin fulfills this requirement because it adds a sweet taste quality to sour taste (Brouwer et al. 1968; Kurihara and Beidler 1968). This effect was first identified in humans, but because we cannot record single taste fibers in humans, we searched for other species. We found that miraculin has similar effects in simian primates (Diamant et al. 1972; Hellekant et al. 1981). We therefore used rhesus monkeys to elucidate taste coding by combining results of behavioral tests with single taste fiber recordings before and after miraculin (Brouwer et al. 1983); after miraculin exposure, the same cluster of fibers responding to sweet compounds also responded to acids. Thus, the existence of taste fibers, which specifically conveyed the sweet taste quality, was demonstrated.
Later investigations involving single taste fiber studies especially in primates, using a larger number of stimuli representing human taste qualities alone (Hellekant et al. 1974, 1976; Danilova et al. 1998b, 1998c), or in combination with gymnemic acid (GA; Hellekant et al. 1996, 1997b, 1998), miraculin (Danilova et al. 1998a; Hellekant et al. 1998; Danilova and Hellekant 2006), or lactisole (Wang et al. 2009), demonstrated the existence of taste fiber clusters with high specificity to sweet, umami, sour, bitter, and salty compounds. Together, these studies indicate that a one-to-one relationship exists between taste fibers responding to sweet and T1R receptors or bitter and T2R receptors in the Type II TRCs. These data demonstrated that taste coding in animals is similar to human; in all species the labeled-line theory is supported, although the compounds inside a taste quality may differ from one species to another (Kare 1960, 1961, 1966; Danilova et al. 1999; Hellekant and Danilova 1999; Hellekant et al. 2010).
CALHM1 and bitter taste
The NG recordings showed that KO mice have an impaired but not completely absent sense of bitter taste. This seems to contradict TBP data in Figure 1, which showed essentially the same rejection in both KO and WT mice. However, it should be kept in mind that a TBP test is a method to determine the hedonic value of a compound and is less a means to determine how strong the taste is. The subject can choose between paying attention to, or neglecting, taste differences of the compounds. As a result, preference curves for bitter compounds usually suddenly drop from no discrimination to complete rejection (cf. Taruno et al. 2013). This complicates determination of thresholds for aversive compounds more than for attractive compounds. Here, this explains the near identical rejection of QHCl by both WT and KO mice in TBP tests of Figure 1, although the NG responses to QHCl in Figures 2 and 3 differed significantly between KO and WT.
CALHM1 and umami taste
The response to umami was greatly suppressed in CT recordings (Taruno et al. 2013), whereas less suppressed in NG recordings presented here. Figure 2 showed a residual response to umami in the NG recordings of KO mice. This result concurs with data from a number of studies indicating that TRCs with mGluR1 and mGluR4 receptors respond to umami compounds and exist on the posterior tongue of mice (Ninomiya et al. 2000; Shindo et al. 2008; Yasuo et al. 2008; Chaudhari et al. 2009; Kinnamon and Vandenbeuch 2009). These TRCs are not depending on the presence of CALHM1 for their function and gave therefore responses to umami in the NG recordings of KO mice.
CALHM1, food intake, and body weight
The connection between sweet taste and liking exists from birth and the powerful influence of S fibers on intake has been demonstrated in many species, including mice (e.g., Danilova et al. 2006; Taruno et al. 2013), hamsters (Frank et al. 1988; Faurion and Vayssettes-Courchay 1990; Rehnberg et al. 1990; Frank et al. 2005), monkeys (Brouwer et al. 1983; Hellekant and Danilova 1996; Danilova et al. 1998a; Danilova and Hellekant 2004, 2006), and humans (Steiner et al. 2001). The effects of miraculin and GA on S fiber response and intake have been summarized already. Similarly, bitter taste, which is the effect of increased impulses in Q fibers, causes rejection in all species, including human infants (Steiner et al. 2001). Hence, intake is affected by the ratio between S and Q fiber activity.
One example is presented in a study of the common marmoset, where the ratios of S and Q fiber responses in both CT and NG to 32 tastants were recorded and calculated. The results showed that the ratio of S/Q fiber activity was linearly related to consumption of the compounds (Danilova and Hellekant 2006).
Here, the loss of S fiber impulses lowered food palatability and consequently intake. This effect dominated over the effect of diminished Q activity, which should have stimulated food intake, because any aversive bitter taste of the food would be diminished. But loss of pleasant taste, due to the absence of S fiber input to appetite stimulating areas of CNS, appears to be the principal factor responsible for diminished intake in KO mice. In WT mice, with functioning Type II TRCs with T1R1 receptors, the food stimulated S fibers, which gave rise to hedonically positive sensations, triggering further intake in WT mice, even after the energy needs had been satisfied.
A recent study (Damak et al. 2012) in TRPM5 KO mice reports similar effects on food intake as we describe for CALHM1 here. TRPM5 is a calcium-activated cation channel necessary in taste transduction that colocalizes with a high frequency with CALHM1 in Type II TRCs. The authors reported that TRPM5-KO mice weigh less and are leaner than WT mice (Damak et al. 2012). Together, these data indicate a role of Type II TRCs and S fiber activity in food intake and body weight.
CALHM1 and life span
A number of studies in rodents (McCay et al. 1935) and primates indicate that limitation of food intake decreases morbidity and increases life span (Colman et al. 1998; Colman et al. 2009). Here, the WT mice were significantly heavier than the KO and ate more. We suggest that this led to shorter life span of WT than in KO mice. However, final conclusions must wait for the results in larger cohorts of mice.
In summary, our results add corroborative behavioral and NG data to our previous study of CALHM1 deletion on taste. In addition, it demonstrates the close relationship between sweet taste and food intake, body weight, and ultimately mortality. The connection between sweet taste and liking exists from birth, but linking sweet taste and excessive consumption has not generally yielded conclusive results. Our data indicate that the loss of S fiber impulses lowered food palatability and consequently intake. Our results suggest an important link between sweet taste, Type II TRCs with T1R receptors, S fiber activity, and excessive consumption that merits further study.
Funding
This work was supported by the National Institutes of Health [R01AG042508 to P.M.].
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. 2000. A novel family of mammalian taste receptors. Cell. 100:693–702. [DOI] [PubMed] [Google Scholar]
- Adrian ED, Zotterman Y. 1926. The impulses produced by sensory nerve-endings. J Physiol. 61:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B, Landgren S, Olsson L, Zotterman Y. 1950. The sweet taste fibres of the dog. Acta Physiology Scand 21:105–119. [DOI] [PubMed] [Google Scholar]
- Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. 2015. The neural representation of taste quality at the periphery. Nature. 517:373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JN, Glaser D, Hard Af Segerstad C, Hellekant G, Ninomiya Y, Van der Wel H. 1983. The sweetness-inducing effect of miraculin; behavioural and neurophysiological experiments in the rhesus monkey Macaca mulatta . J Physiol. 337:221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer JN, van der Wel H, Francke A, Henning GJ. 1968. Mieraculin, the sweetness-inducing protein from miracle fruit. Nature. 220:373–374. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. 2000. T2Rs function as bitter taste receptors. Cell. 100:703–711. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Pereira E, Roper SD. 2009. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr. 90:738S–742S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ, Hagiwara S, Zotterman Y. 1955. The response spectrum of taste fibres in the cat: a single fibre analysis. Acta Physiol Scand. 33:316–332. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. 1998. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging (Milano). 10:83–92. [DOI] [PubMed] [Google Scholar]
- Damak S, le Coutre J, Bezencon C. 2012. Deletion or inhibition of Trpm5 decreases the gain in body weight and fat mass in mice. AChems 2012; Los Angeles. [Google Scholar]
- Danilova V, Damak S, Margolskee RF, Hellekant G. 2006. Taste responses to sweet stimuli in alpha-gustducin knockout and wild-type mice. Chem Senses. 31:573–580. [DOI] [PubMed] [Google Scholar]
- Danilova V, Danilov Y, Roberts T, Elmer D, Hellekant G. 2002. Electrophysiological recordings of mammalian taste nerves. In: Simon SA, Nicolelis MAL, editors. Methods and Frontiers in Neuroscience. Boca Raton (FL): CRC Press; p. 239–264. [Google Scholar]
- Danilova V, Hellekant G. 2004. Sense of taste in a New World monkey, the common marmoset. II. Link between behavior and nerve activity. J Neurophysiol. 92:1067–1076. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. 2006. Elucidating coding of taste qualities with the taste modifier miraculin in the common marmoset. Brain Res Bull. 68:315–321. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Jin Z. 1998a. Effect of miraculin on behavioral and single taste fibers responses in common marmoset, Callithrix jacchus jacchus . Achems Sarasota FL. 550 Available from: http://www.achems.org/i4a/pages/index.cfm?pageid=3608. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Roberts R, Tinti JM, Nofre C. 1998b. Behavioral and single chorda tympani taste fiber responses in the common marmoset, Callithrix jacchus jacchus . Ann N Y Acad Sci. 855:160–164. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Tinti JM, Nofre C. 1998c. Gustatory responses of the hamster Mesocricetus auratus to various compounds considered sweet by humans. J Neurophysiol. 80:2102–2112. [DOI] [PubMed] [Google Scholar]
- Danilova V, Roberts T, Hellekant G. 1999. Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chem Senses. 24:301–316. [DOI] [PubMed] [Google Scholar]
- Diamant H, Hellekant G, Zotterman Y. 1972. The effect of miraculin on the taste buds of man, monkey and rat. In: Schneider D, editor. Olfaction and Taste IV. Stuttgart (Germany): Wissenschaftlige Verlagsgesellschaft MB4 Stuttgart; p. 241–244. [Google Scholar]
- Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, et al. 2008. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 133:1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP. 1963. Sensory neural patterns and gustation. In: Zotterman Y, editor. Olfaction and Taste. Oxford: Pergamon Press; p. 205–213. [Google Scholar]
- Erickson RP. 1967. Neural coding of taste quality. In: Kare MR, Maller O, editors. The Chemical Senses and Nutrition. Baltimore (MD): John Hopkins Press; p. 313–327. [Google Scholar]
- Faurion A, Vayssettes-Courchay C. 1990. Taste as a highly discriminative system: a hamster intrapapillar single unit study with 18 compounds. Brain Res. 512:317–332. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. 2005. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310:1495–1499. [DOI] [PubMed] [Google Scholar]
- Frank ME, Bieber SL, Smith DV. 1988. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J Gen Physiol. 91:861–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. 2005. Peripheral gustatory processing of sweet stimuli by golden hamsters. Brain Res Bull. 66:70–84. [DOI] [PubMed] [Google Scholar]
- Gordon G, Kitchell R, Strom L, Zotterman Y. 1959. The response pattern of taste fibres in the chorda tympani of the monkey. Acta Physiol Scand. 46:119–132. [DOI] [PubMed] [Google Scholar]
- Hellekant G. 1968. Postexcitatory depression of gustatory receptors. Acta Physiol Scand. 74:1–9. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Danilova V. 1996. Species differences toward sweeteners. Food Chem. 56:323–328. [Google Scholar]
- Hellekant G, Danilova V. 1999. Taste in domestic pig, Sus scrofa . Anim Physiol Anim Nutr. 63:139–144. [Google Scholar]
- Hellekant G, Danilova V, Ninomiya Y. 1997. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta . J Neurophysiol. 77:978–993. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Glaser D, Brouwer JN, van der Wel H. 1976. Gustatory effects of miraculin, monellin and thaumatin in the Saguinus midas tamarin monkey studied with electrophysiological and behavioural techniques. Acta Physiol Scand. 97:241–250. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Glaser D, Brouwer J, van der Wel H. 1981. Gustatory responses in three prosimian and two simian primate species (Tupaia glis, Nycticebus coucang, Galago senegalensis, Callithrix jacchus jacchus and Saginus midas niger) to six sweeteners and miraculin and their phylogenetic implications. Chem Senses. 6:165–173. [Google Scholar]
- Hellekant G, Hagstrom EC, Kasahara Y, Zotterman Y. 1974. On the gustatory effects of miraculin and gymnemic acid in monkey. Chem Senses Flavor. 1:137–145. [Google Scholar]
- Hellekant G, Ninomiya Y. 1994. Umami taste in chimpanzee (Pan troglodytes) and rhesus monkey (M. mulatta). In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and Taste XI. Tokyo (Japan): Springer Verlag; p. 365–368. [Google Scholar]
- Hellekant G, Ninomiya Y, Danilova V. 1997. Taste in chimpanzees II: single chorda tympani fibers. Physiol Behav. 61:829–841. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y, Danilova V. 1998. Taste in chimpanzees. III: labeled-line coding in sweet taste. Physiol Behav. 65:191–200. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Ninomiya Y, DuBois GE, Danilova V, Roberts TW. 1996. Taste in chimpanzee: I. The summated response to sweeteners and the effect of gymnemic acid. Physiol Behav. 60:469–479. [DOI] [PubMed] [Google Scholar]
- Hellekant G, Roberts TW. 1995. Whole nerve and single fiber recordings in non-human primates. In: Spielman AI, Brand JG, editors. Experimental Cell Biology of Taste and Olfaction: Current Techniques and Protocols. Boca Raton (FL): CRC Press; p. 277–290. [Google Scholar]
- Hellekant G, Roberts TW, Elmer DG, Cragin T, Danilova V. 2010. Responses of single chorda tympani taste fibers of the calf (Bos taurus). Chem Senses. 35:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kare MR. 1960. Senses of animals differ from man’s. Farm Res. 26:8–9. [Google Scholar]
- Kare MR. 1961. Comparative aspects of the sense of taste. In: Kare MR, Halpern BP, editors. Physiological and Behavioral Aspects of Taste. Chicago: University of Ghicago Press; p. 6–15. [Google Scholar]
- Kare MR. 1966. Taste perception in animals. Agr Sci Rev. 4:10–15. [Google Scholar]
- Khachaturian ZS. 1994. Calcium hypothesis of Alzheimer’s disease and brain aging. Ann N Y Acad Sci. 747:1–11. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC, Vandenbeuch A. 2009. Receptors and transduction of umami taste stimuli. Ann N Y Acad Sci. 1170:55–59. [DOI] [PubMed] [Google Scholar]
- Kurihara K, Beidler LM. 1968. Taste-modfying protein from miracle fruit. Science. 161:1241–1243. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Campagne F, Marambaud P. 2008. [CALHM1, a novel gene to blame in Alzheimer disease]. Med Sci (Paris). 24:923–924. [DOI] [PubMed] [Google Scholar]
- McCay C, Crowell M, Maynard L. 1935. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1:63–79. [PubMed] [Google Scholar]
- Meyerhof W. 2005. Elucidation of mammalian bitter taste. Rev Physiol Biochem Pharmacol. 154:37–72. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Hevezi P, Gao N, Lu M, Kalabat D, Soto H, Echeverri F, Laita B, Yeh SA, Zoller M, et al. 2009. Expression of genes encoding multi-transmembrane proteins in specific primate taste cell populations. PLoS One. 4:e7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Hellekant G. 1991. Specific taste sensitivity of single chorda tympani fibers in chimpanzee. Proc Jpn Symp Taste Smell. 25:313–316. [Google Scholar]
- Ninomiya Y, Imoto T. 1995. Gurmarin inhibition of sweet taste responses in mice. Am J Physiol. 268:R1019–R1025. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Nakashima K, Fukuda A, Nishino H, Sugimura T, Hino A, Danilova V, Hellekant G. 2000. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr. 130:950S–953S. [DOI] [PubMed] [Google Scholar]
- Pfaffmann C. 1941. Gustatory afferent impulses. J Cell Comp Physiol. 17:243–258. [Google Scholar]
- Pfaffmann C. 1955. Gustatory nerve impulses in rat, cat and rabbit. J Neurophysiol. 18:429–440. [DOI] [PubMed] [Google Scholar]
- Rehnberg BG, Hettinger TP, Frank ME. 1990. The role of sucrose-sensitive neurons in ingestion of sweet stimuli by hamsters. Physiol Behav. 48:459–466. [DOI] [PubMed] [Google Scholar]
- Sato M, Hiji Y, Ito H, Imoto T. 1977. Sweet taste sensitivity in Japanese macaques. In: Kare MR, Maller O, editors. The Chemical Senses and Nutrition. New York: Academic Press; p. 327–342. [Google Scholar]
- Sato M, Ogawa H, Yamashita S. 1975. Response properties of macaque monkey chorda tympani fibers. J Gen Physiol. 66:781–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y, Miura H, Carninci P, Kawai J, Hayashizaki Y, Ninomiya Y, Hino A, Kanda T, Kusakabe Y. 2008. G alpha14 is a candidate mediator of sweet/umami signal transduction in the posterior region of the mouse tongue. Biochem Biophys Res Commun. 376:504–508. [DOI] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. 2001. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 25:53–74. [DOI] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Danilova V, Cragin T, Roberts TW, Koposov A, Hellekant G. 2009. The sweet taste quality is linked to a cluster of taste fibers in primates: lactisole diminishes preference and responses to sweet in S fibers (sweet best) chorda tympani fibers of M. fascicularis monkey. BMC Physiol. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. 2008. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull. 31:1833–1837. [DOI] [PubMed] [Google Scholar]
- Yu JT, Chang RC, Tan L. 2009. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol. 89:240–255. [DOI] [PubMed] [Google Scholar]