Abstract
The azole antifungals block ergosterol biosynthesis by inhibiting lanosterol demethylase (Erg11p). The resulting depletion of cellular ergosterol and the accumulation of “toxic” sterol intermediates are both thought to compromise plasma membrane function. However, the effects of ergosterol depletion upon the function of intracellular membranes and organelles are not well described. The purpose of this study was to characterize the effects of azole treatment upon the integrity of the Candida albicans vacuole and to determine whether, in turn, vacuolar trafficking influences azole susceptibility. Profound fragmentation of the C. albicans vacuole can be observed as an early consequence of azole treatment, and it precedes significant growth inhibition. In addition, a C. albicans vps21Δ/Δ mutant, blocked in membrane trafficking through the late endosomal prevacuolar compartment (PVC), is able to grow significantly more than the wild type in the presence of several azole antifungals under standard susceptibility testing conditions. Furthermore, the vps21Δ/Δ mutant is able to grow despite the depletion of cellular ergosterol. This phenotype resembles an exaggerated form of “trailing growth” that has been described for some clinical isolates. In contrast, the vps21Δ/Δ mutant is hypersensitive to drugs that block alternate steps in ergosterol biosynthesis. On the basis of these results, we propose that endosomal trafficking defects may lead to the cellular “redistribution” of the sterol intermediates that accumulate following inhibition of ergosterol biosynthesis. Furthermore, the destination of these intermediates, or the precise cellular compartments in which they accumulate, may be an important determinant of their toxicity and thus ultimately antifungal efficacy.
INTRODUCTION
The development of resistance to the most important class of antifungal drugs, the azoles, is a problem of increasing medical significance (1). Various mechanisms of resistance have been reported for clinical isolates of the prevalent human fungal pathogen Candida albicans. These mechanisms include increased expression of the target enzyme lanosterol 14α-demethylase and point mutations that alter the target enzyme's affinity for azoles (2, 3). Increased expression of drug efflux pumps, such as Cdr1p (4), Cdr2p (5), and Mdr1p (6), is also known to contribute to azole resistance. However, a combination of these mechanisms is usually necessary to confer a significant reduction in azole susceptibility (3), and the resistance of many fungal isolates is not fully accounted for by these established mechanisms (7).
Azole-mediated inhibition of lanosterol 14α-demethylase (Erg11p) blocks ergosterol biosynthesis (2). The resulting depletion of cellular ergosterol and the accumulation of “toxic” intermediate sterol species are thought to compromise the function of the plasma membrane (8). While the plasma membrane typically contains the highest concentration of ergosterol, a smaller fraction can be found in intracellular membranes, including the fungal vacuole (9, 10). However, the impact of ergosterol depletion following azole treatment upon the function and integrity of intracellular organelles is not well characterized. We recently used a high-throughput screen to identify chemical agents that interfere with the accumulation of an endogenously produced pigment within the vacuole of a C. albicans ade2 mutant strain (11). Several antifungal imidazoles were identified including miconazole and ketoconazole (unpublished results). Previous studies have also indicated that ergosterol is important for endocytic trafficking from the plasma membrane to the fungal vacuole (12) and to support homotypic vacuole-vacuole fusion in an in vitro biochemical assay (13). Furthermore, several Saccharomyces cerevisiae ergosterol biosynthetic mutants are known to have an aberrant vacuole morphology (13, 14). Finally, it has recently been reported that the activity of the vacuolar proton pump (V-ATPase) responsible for vacuolar acidification is dependent upon ergosterol and is reduced following azole treatment (15). Collectively, these observations suggest that ergosterol depletion has a major impact upon vacuolar function. This is significant as defects in vacuolar function and acidification are known to substantially diminish the capacity of C. albicans to endure physiological stress, produce hyphae that can invade tissue, and cause lethal infections in mice (16–19).
The purpose of this study was to characterize the impact of azole treatment upon the integrity of the C. albicans vacuole and determine whether, in turn, vacuolar trafficking influences azole susceptibility. Our results reveal that the vacuole is rapidly and profoundly compromised following exposure to the azole antifungals. Furthermore, trafficking through the late endosomal prevacuolar compartment (PVC) is an important determinant of C. albicans' ability to tolerate antifungal agents that target ergosterol biosynthesis.
MATERIALS AND METHODS
Growth conditions.
C. albicans was routinely grown on yeast extract-peptone-dextrose (YPD) at 30°C, supplemented with 50 μg/ml uridine when necessary. Transformant selection was carried out on minimal YNB medium (6.75 g/liter yeast nitrogen base without amino acids, 2% dextrose, 2% Bacto agar), supplemented with the appropriate auxotrophic requirements as described for S. cerevisiae (20) or 50 μg/ml uridine.
For growth curves, overnight cultures in YPD at 30°C were subcultured into 15 ml fresh YNB medium at an initial cell concentration of 1 × 106 cells/ml in the presence of 1 μg/ml fluconazole or 0.5% dimethyl sulfoxide (DMSO) (drug-free control), and incubated at 30°C with shaking. Samples were then taken at 30-min intervals, optical density at 600 nm (OD600) was determined spectroscopically, and cell viability was measured by counting the number of CFU on YPD agar plates. A third sample was taken simultaneously to observe vacuolar morphology (see below).
Plasmid construction.
Plasmid pLUX (21) was kindly provided by William Fonzi (Georgetown University). Plasmids pLUXVPS21 (16) and pKE1 (22) have been previously described. All oligonucleotides used in this study are listed in Table S1 in the supplemental material. The construction of additional plasmids is described in the supplemental material.
C. albicans strains.
The vps21Δ/Δ, ypt72Δ/Δ, atg9Δ/Δ, aps3Δ/Δ, ypt52Δ/Δ, ypt53Δ/Δ, vps21Δ/Δ ypt52Δ/Δ, vps21Δ/Δ ypt53Δ/Δ, ypt52Δ/Δ ypt53Δ/Δ and vps21Δ/Δ ypt52Δ/Δ ypt53Δ/YPT53T27N (the T-to-N change at position 27 encoded by YPT53 [YPT53T27N]) mutants were constructed in previous studies (16, 22–24). The C. albicans pep12Δ/Δ mutant and isogenic control strains (25) were kindly provided by Samuel Lee (University of New Mexico). The azole-susceptible clinical isolate TW1 (isolate 1 in reference 4) and the matched azole-resistant isolate TW17 (isolate 17 in reference 4) were kindly provided by Theodore C. White (University of Missouri—Kansas City). Control strain YJB6284 (26) was kindly provided by Judith Berman (University of Minnesota), and strain SC5314 has been described previously (27). C. albicans was transformed with DNA constructs using the lithium acetate procedure (28). Gene deletion strains were constructed by the PCR-based approach of Wilson et al. (29), using the ura3Δ/Δ his1Δ/Δ arg4Δ/Δ strain BWP17 (kindly provided by Aaron Mitchell, Carnegie Mellon University). The doxycycline-repressible tetO-ERG11 strains were made using the system described by Nakayama and colleagues (30). For a detailed description of the construction of each C. albicans gene deletion and doxycycline-repressible strain, please refer to the supplemental material.
Antifungal susceptibility testing.
Antifungal susceptibility testing of all the strains included in this study was performed using the broth microdilution method described in CLSI (Clinical and Laboratory Standards Institute) document M27-A3 (31) in a 96-well plate format. All drugs for susceptibility testing used in this study were diluted in DMSO in 2-fold dilutions at 200 times the final concentration. The following antifungal drugs and concentration ranges were tested in this study: fluconazole (Sigma-Aldrich) from 64 μg/ml to 0.0313 μg/ml, miconazole (Sigma-Aldrich) from 50 μg/ml to 0.024 μg/ml, ketoconazole (Sigma-Aldrich) from 32 μg/ml to 0.015 μg/ml, itraconazole (Sigma-Aldrich) from 16 μg/ml to 0.007 μg/ml, and amphotericin B (LKT Laboratories, Inc.) from 16 μg/ml to 0.001 μg/ml. RPMI 1640 medium (Sigma-Aldrich) was prepared according to the CLSI document; the medium was buffered with morpholinepropanesulfonic acid (MOPS), and the pH was adjusted using KOH and HCl. The cell inoculum was ∼1 × 103 cells per well. The plates were incubated without shaking at 35°C for 24 or 48 h unless otherwise stated. The content of each well was carefully resuspended by pipetting up and down before OD600 was measured using a Biotek Synergy Mx plate reader.
Quantification of cellular ergosterol.
Fifty milliliters of YNB broth plus either 4 μg/ml fluconazole or 0.5% DMSO was inoculated with 2 × 106 C. albicans cells/ml and grown at 35°C for 24 h with shaking at 200 rpm. Total cellular sterols were then extracted using n-heptane by the method of Arthington-Skaggs et al. (32). n-Heptane from the organic phase was evaporated under reduced pressure (0.03 mm Hg) at −20°C. The white powdery residue was dissolved in 0.5 ml CDCl3 (99.8% D contains 1% [vol/vol] tetramethylsilane [TMS]), and it was subjected to proton nuclear magnetic resonance (NMR) analysis. The NMR was recorded on a Varian 500-MHz Inova instrument. The amounts of ergosterol in these samples were estimated by comparing the NMR integrals of the methyl C-18 group at 0.562 ppm to that of the internal TMS standard. The amount of ergosterol in these samples was calculated by standardizing these integrals with the C-18 methyl integral of the standard ergosterol sample (1 mg/ml; Sigma-Aldrich) that was prepared, and the NMR was recorded by the same procedure.
Fluorescence microscopy to determine vacuole morphology.
Vacuole morphology was determined using C. albicans strains expressing either green fluorescent protein (GFP)-Ypt72p (16) or Cpy1p-GFP (Cpy1p prepropeptide [CPP]-GFP; see supplemental material) fusion protein. In some experiments, cells were prelabeled with the dye FM4-64 to label the vacuolar membrane as previously described (33). Cells were observed with an Olympus BX51 fluorescence microscope with a 100× objective and a fluorescein isothiocyanate (FITC) filter set to detect GFP fluorescence or tetramethyl rhodamine isothiocyanate (TRIT-C) to observe FM4-64. For each field, matching phase-contrast and fluorescence images were acquired using a QImaging MicroPublisher 3 real-time viewing (RTV) camera and the CellSens digital imaging software (Olympus). Adjustments in the brightness and/or contrast of images were made using the CellSens digital imaging software. All images presented for a given time point/condition in each experiment were scaled identically to permit direct comparison.
RESULTS
Inhibition of lanosterol demethylase causes significant vacuolar defects in C. albicans.
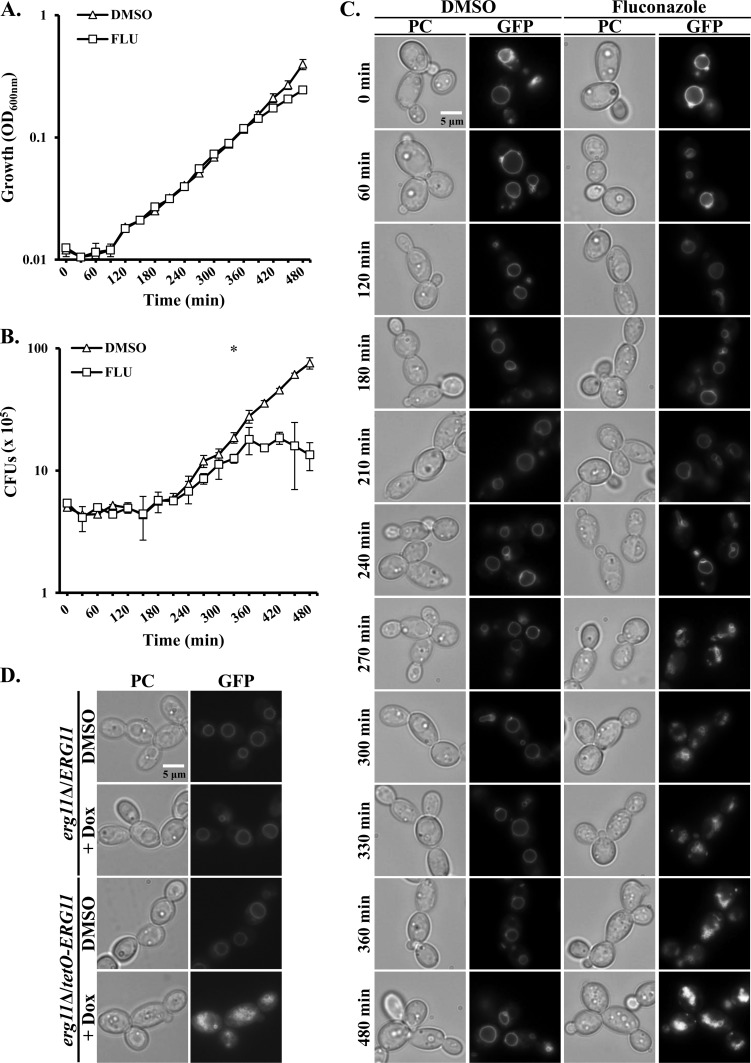
Using a high-throughput screening assay, we recently identified a variety of imidazole antifungals, including miconazole and ketoconazole as disrupting the integrity of the C. albicans vacuole (unpublished results). Some imidazoles have been proposed to possess a secondary antifungal mechanism that is independent of their inhibition of ergosterol biosynthesis and that accounts for their fungicidal activity at high concentrations (34–37). We therefore tested whether the fungistatic triazole fluconazole caused similar perturbation of the C. albicans vacuole. This was investigated using a strain expressing a GFP-tagged GTPase, Ypt72p, that localizes to the surface of the vacuole (16). Significant vacuolar disruption was observed at fluconazole concentrations of ≥0.0625 μg/ml. In order to examine the relationship between azole-mediated vacuolar disruption and growth inhibition, a time course experiment was performed. The GFP-Ypt72p-tagged strain was subcultured into YNB medium with and without 1 μg/ml fluconazole, and the growth, cell viability, and vacuolar morphology were compared at 30-min intervals. When growth was measured as OD600, statistically significant growth inhibition was initially observed in the presence of fluconazole after 420 min (Fig. 1A). However, when cell viability was compared in the same cultures as CFU, fluconazole was found to significantly reduce CFU versus the drug-free control at the 330-min time point (Fig. 1B). This apparent inconsistency may be explained by the tendency of fluconazole-treated cells to form clumps, indicating a defect in cell separation (38). These changes presumably occur before the rate of biomass production (OD600) is affected. In the presence of fluconazole, vacuolar fragmentation was initially observed after 240 min, and severe disruption occurred in all cells by the 270-min time point (Fig. 1C). This precedes the reduction in CFU and OD600, suggesting that vacuolar degeneration is an early event following azole treatment and not merely a secondary consequence of prolonged growth arrest. Similar effects on vacuolar integrity were observed using a C. albicans strain expressing a GFP-tagged version of carboxypeptidase Y (see Fig. S1 in the supplemental material), which labels the vacuolar lumen, or when vacuoles were prelabeled with FM4-64 (Fig. S2), a dye that is taken up by endocytosis and accumulates to label the vacuolar membrane.
FIG 1.
Inhibition of Erg11p causes vacuolar fragmentation in C. albicans. (A to C) A C. albicans strain expressing the GFP-Ypt72p fusion protein was subcultured into YNB broth supplemented with either 1 μg/ml fluconazole (FLU) or 0.5% DMSO (no-drug control) at ∼1 × 106 cells/ml and incubated at 30°C with shaking. Samples were taken at 30-min intervals, and growth was measured as OD600 (A) and cell viability was determined by the number of CFU. Vacuolar integrity was also observed by fluorescence microscopy using a FITC filter set (C), and matching phase-contrast (PC) images were also obtained. The values in panels A and B are the means ± standard deviations (error bars) from two independent experiments, and the images in panel C are representative of each time point in the same two experiments. Growth and CFU were compared in the presence and absence of fluconazole for each time point using a two-tailed t test. Values that are significantly different are indicated as follows: *, P < 0.05; §, P < 0.001. (D) The C. albicans ERG11 gene was placed under the transcriptional control of a doxycycline-repressible promoter, and the GFP-Ypt72p expression construct was introduced into the tetO-ERG11 strain. The tetO-ERG11 strain was then subcultured into YNB medium in the presence of 5 μg/ml of doxycycline (+ Dox) or in the absence of doxycycline at 1 × 105 cells/ml and incubated at 30°C with shaking. After 8 h, vacuole morphology was observed as described above.
In order to confirm that the observed vacuolar defects were a direct consequence of Erg11p inhibition rather than an “off-target” effect of the azoles, we constructed a strain in which the transcription of the ERG11 gene could be shut down using a doxycycline-repressible promoter. In the presence of doxycycline, the tetO-ERG11 strain exhibits vacuolar defects similar to those observed following fluconazole treatment (Fig. 1D). Thus, Erg11p inhibition is sufficient to cause loss of vacuolar integrity, presumably as a consequence of ergosterol depletion.
Vps21p-mediated trafficking through the prevacuolar compartment affects fungal growth in the presence of azoles.
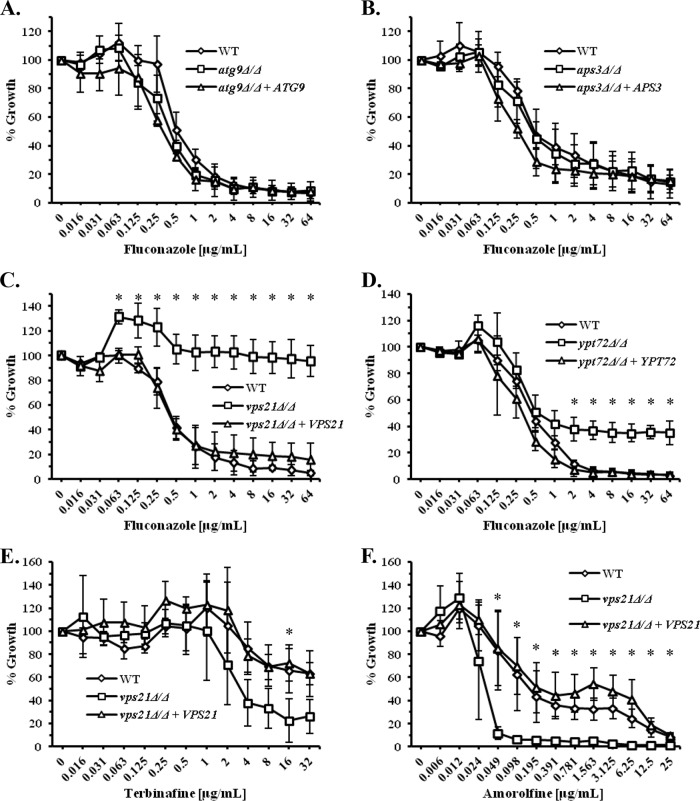
To gain further insight into the interaction between the antifungal activity of the azoles and vacuolar integrity, we tested the susceptibility of C. albicans mutants blocked in four distinct vacuolar trafficking steps (see Fig. S3 in the supplemental material), using the standard CLSI (Clinical and Laboratory Standards Institute) protocol for antifungal susceptibility testing (31). Mutants blocked in either autophagy (atg9Δ/Δ) (23) or in a direct Golgi-to-vacuole trafficking pathway (aps3Δ/Δ) (24) have susceptibilities to fluconazole (Fig. 2A and B) and miconazole (see Fig. S4A and S4B in the supplemental material) that are similar to those of the isogenic controls. However, a vps21Δ/Δ mutant lacking a Rab GTPase that regulates membrane fusion and trafficking through the prevacuolar compartment (PVC) is significantly less sensitive to fluconazole (Fig. 2C), miconazole, itraconazole, and ketoconazole (see Fig. S4C, S5A, and S5B in the supplemental material). Interestingly, a ypt72Δ/Δ mutant lacking a Rab GTPase that localizes to the vacuole also shows slightly elevated growth in the presence of fluconazole (Fig. 2D), but the difference is less dramatic than for the vps21Δ/Δ mutant. This suggests that trafficking through the PVC is more important with respect to determining azole sensitivity than trafficking to the vacuole per se.
FIG 2.
Endosomal trafficking affects C. albicans growth in the presence of ergosterol biosynthesis inhibitors. (A to D) The susceptibility of several C. albicans vacuolar trafficking mutants to fluconazole was compared to “wild-type” (WT) (YJB6284) and “reconstituted” control strains using the standard CLSI broth microdilution protocol. After 48-h incubation, growth was measured as OD600 and expressed as a percentage of the growth in the no-drug (DMSO alone) control wells. (A) An atg9Δ/Δ mutant blocked in autophagy. (B) An aps3Δ/Δ mutant blocked in a direct Golgi-to-vacuole trafficking pathway. (C) A vps21Δ/Δ mutant blocked in membrane fusion and trafficking through the PVC. (D) An ypt72Δ/Δ mutant blocked in membrane fusion at the vacuolar membrane. (E and F) The susceptibility of the C. albicans vps21Δ/Δ mutant to the allylamine terbinafine (E) and the morpholine amorolfine (F) was also determined as described above, except that growth was measured after 24-h incubation. The means ± standard deviations (error bars) from three independent experiments are presented in each panel. The growth of each mutant was compared to that of the “wild-type” control at each drug concentration using a two-tailed t test. Values that are significantly different (P < 0.05) are indicated by an asterisk.
The susceptibility of the vps21Δ/Δ mutant to amphotericin B, which acts by directly binding to ergosterol, is not significantly different from those of the controls (see Fig. S5C in the supplemental material). Furthermore, the vps21Δ/Δ mutant has a slight increase in susceptibility to the allylamine antifungal terbinafine (Fig. 2E), which inhibits an earlier step of the ergosterol biosynthetic pathway, and to amorolfine (Fig. 2F), which acts downstream of Erg11p. Thus, the phenotype of the vps21Δ/Δ mutant seems to be specific to the azoles, rather than a general “drug resistance” phenotype.
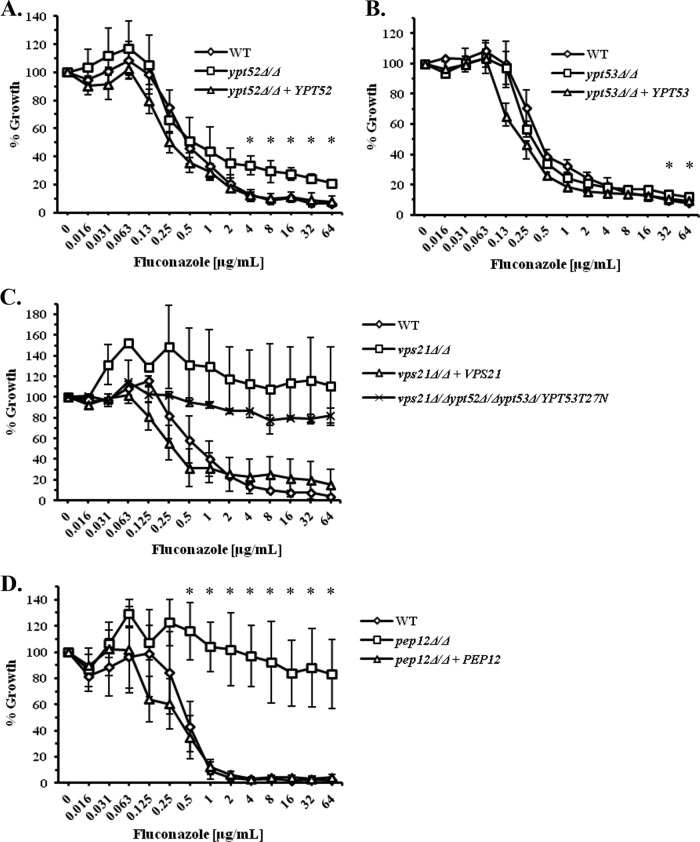
The VPS21 paralogs YPT52 and YPT53 do not significantly affect azole susceptibility.
We recently described two additional Rab GTPases that localize to the PVC, are closely related to, and share significant functional overlap with Vps21p (22). However, the ypt52Δ/Δ and ypt53Δ/Δ mutants lacking these GTPases are unaffected in their susceptibility to miconazole (see Fig. S6A and S6B in the supplemental material), as is a ypt52Δ/Δ ypt53Δ/Δ double mutant (Fig. S6C). While the ypt52Δ/Δ single mutant shows marginally increased growth in the presence of fluconazole compared to the isogenic control strain (Fig. 3A), the ypt53Δ/Δ mutant is unaffected (Fig. 3B). Furthermore, the vps21Δ/Δ ypt52Δ/Δ mutant (Fig. S6E), vps21Δ/Δ ypt53Δ/Δ mutant (Fig. S6F), and a triple GTPase mutant (Fig. 3C) are all affected by fluconazole and miconazole (data not shown) to an extent similar to that of the vps21Δ/Δ single mutant. Thus, despite significant functional overlap between these three Rab GTPases, it seems that the Vps21p GTPase has the greatest effect upon the ability of C. albicans to grow in the presence of the azoles under standard testing conditions.
FIG 3.
The Vps21p paralogs Ypt52p and Ypt53p have minimal effect upon C. albicans growth in the presence of fluconazole. (A to D) C. albicans ypt52Δ/Δ (A), ypt53Δ/Δ (B), vps21Δ/Δ ypt52Δ/Δ ypt53Δ/YPT53-YPT53T27N (triple GTPase mutant) (C), and pep12Δ/Δ (D) mutants and their isogenic control strains were tested for their susceptibility to fluconazole as described in the legend to Fig. 2. The values in each panel are the means ± standard deviations from three independent experiments. The growth of each mutant was compared to that of the “wild-type” control at each drug concentration using a two-tailed t test. *, P < 0.05.
To further determine whether this phenotype represents a specific function of the Vps21p GTPase or is a feature of the trafficking step controlled by Vps21p, we tested the azole susceptibility of a C. albicans pep12Δ/Δ mutant (25). Pep12p is a t-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein that is a downstream effector of Vps21p and that also facilitates membrane fusion at the PVC (39). The pep12Δ/Δ mutant shows a similar dose response to fluconazole as that described above for the vps21Δ/Δ mutant (Fig. 3D), suggesting that this phenotype is the result of a block in PVC transport rather than a specific function of the Vps21p GTPase.
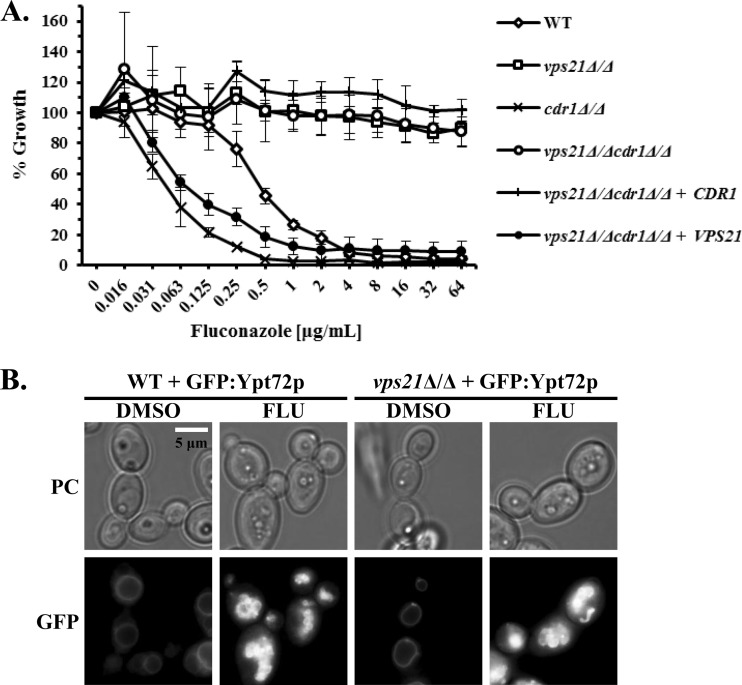
Elevated growth of the vps21Δ/Δ mutant in the presence of the azoles is independent of the Cdr1p and Mdr1p efflux pumps.
We previously reported that the C. albicans vps21Δ/Δ mutant has reduced rates of endocytosis (16). Many plasma membrane proteins are targeted for degradation within the fungal vacuole following endocytic trafficking via the PVC. Thus, we considered that reduced rates of uptake and degradation may extend the “half-lives” of the well-described Cdr1p and Mdr1p drug efflux pumps at the cell surface, and this may contribute to the reduced sensitivity of the vps21Δ/Δ mutant to fluconazole. To test this, we examined the susceptibility of vps21Δ/Δ cdr1Δ/Δ and vps21Δ/Δ mdr1Δ/Δ double mutants to fluconazole. However, loss of either CDR1 (Fig. 4A) or MDR1 (data not shown) does not significantly affect the growth of the vps21Δ/Δ mutant in the presence of fluconazole. Thus, the elevated growth of the vps21Δ/Δ mutant in the presence of the azoles does not depend upon the activity of either the Mdr1p or Cdr1p efflux pump.
FIG 4.
Growth of the C. albicans vps21Δ/Δ mutant in the presence of fluconazole does not depend upon the Cdr1p efflux pump. (A) Both alleles of the CDR1 gene were deleted from either “wild-type” (VPS21/VPS21) or the vps21Δ/Δ mutant, and the susceptibility of the resulting strains to fluconazole was tested as described in the legend to Fig. 2. The values are the means ± standard deviations (error bars) from three independent experiments. No statistically significant differences were found between the growth of the vps21Δ/Δ and vps21Δ/Δ cdr1Δ/Δ mutants. (B) C. albicans strains expressing GFP-Ypt72p were subcultured into YNB medium with and without 1 μg/ml fluconazole at ∼1 × 106 cells/ml and incubated at 30°C with shaking for 6 h. Vacuole morphology was then observed by fluorescence microscopy as described in the legend to Fig. 1.
To ensure that the vps21Δ/Δ mutant had not acquired any point mutations within the gene encoding the target enzyme, Erg11p, that could impact azole susceptibility, we amplified and sequenced the ERG11 open reading frame (ORF) from two independently constructed vps21Δ/Δ mutant strains and their isogenic controls. No polymorphisms were detected, and the sequence matched the sequence in the C. albicans genome sequence database. Interestingly, vacuolar disruption was observed in the vps21Δ/Δ mutant upon fluconazole treatment (Fig. 4B), indicating that the mechanisms that enable the vps21Δ/Δ mutant to grow in the presence of the azoles do not depend upon maintaining an intact vacuole.
The C. albicans vps21Δ/Δ mutant exhibits an exaggerated “trailing growth” phenotype.
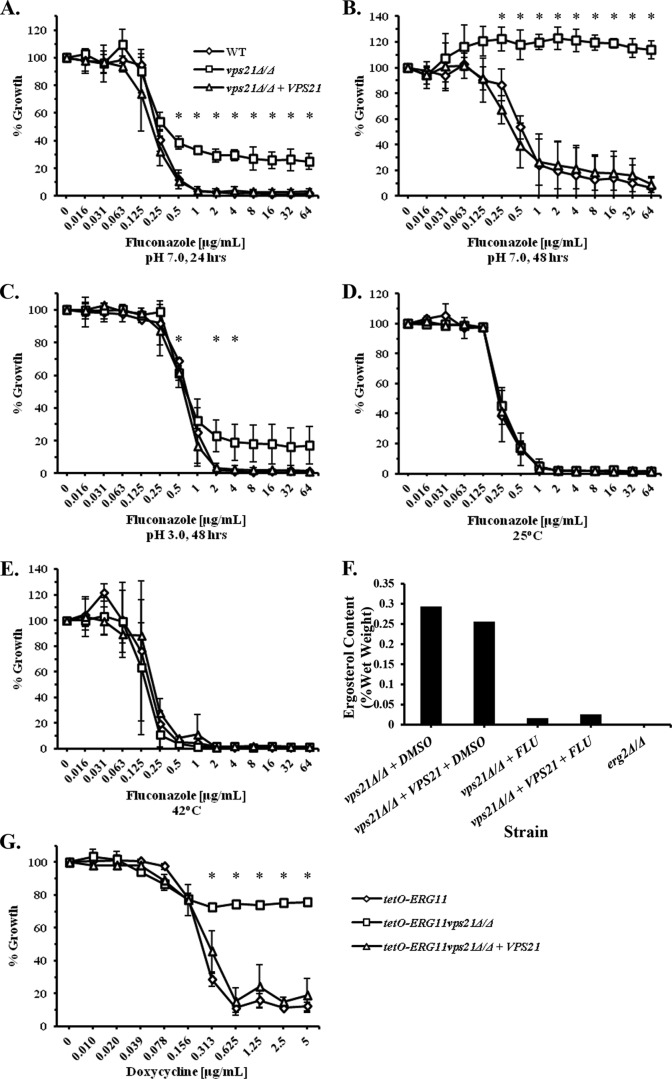
The dose-response profile of the vps21Δ/Δ mutant to fluconazole is distinct from that of a well-characterized azole-resistant isolate (4) (see Fig. S7A and S7B in the supplemental material). When growth was compared at the earlier time point of 24 h, it could be discerned that the growth of the vps21Δ/Δ mutant was inhibited at fluconazole concentrations similar to those of the control strains, but the degree of growth inhibition was significantly less (Fig. 5A). In addition, above concentrations of 0.5 μg/ml, no further “dose-dependent” decrease in growth was observed. The differential dose-response profiles at 24 versus 48 h (Fig. 5B) are characteristic of the “trailing growth” phenomenon (40). Clinical isolates exhibiting “trailing growth” typically exhibit MICs of ≤1 μg/ml at the 24-h time point, but they show MICs of ≥64 μg/ml at 48 h. Using a strict cutoff of 80% growth inhibition (MIC80), the vps21Δ/Δ mutant would be considered azole resistant at both 24- and 48-h time points; however, if we adopt a less-stringent criteria of 50% growth inhibition (MIC50), the differential response of the vps21Δ/Δ mutant at 24 h versus 48 h conforms to that of “trailing growth.” While trailing isolates are generally thought to be susceptible to azole treatment in vivo (41, 42), the phenomenon can complicate in vitro antifungal susceptibility testing. Furthermore, the mechanisms underlying trailing growth are not well understood. We therefore examined whether the increased growth of the vps21Δ/Δ mutant in the presence of the azoles was true resistance or an exaggerated “trailing growth” phenotype. It has been previously reported that adjusting the pH of the RPMI 1640 growth medium from 7 to a more acidic pH is sufficient to eliminate trailing growth, but not true azole resistance (40). At pH 3, the vps21Δ/Δ mutant still grows slightly more than the control strains in the presence of both fluconazole (Fig. 5C) and miconazole (not shown), but the differential was substantially diminished. It has also been reported that adjusting the incubation temperature from 35°C to either 25 or 42°C eliminates trailing growth (43). The vps21Δ/Δ mutant was completely resensitized to fluconazole at either 25 or 42°C (Fig. 5D and E), further supporting the idea that the elevated growth of the vps21Δ/Δ mutant in the presence of the azoles is an exaggerated form of the trailing growth phenomenon, rather than true azole resistance.
FIG 5.
The C. albicans vps21Δ/Δ mutant shows features consistent with an exaggerated trailing growth-like phenotype. (A and B) The susceptibility of the vps21Δ/Δ mutant and control strains to fluconazole was tested using the standard CLSI protocol, and growth was compared at either 24 h (A) or 48 h (B) as described in the legend to Fig. 2. (C to E) The same experiment was also conducted with RPMI 1640 medium that was adjusted to a pH of 3 (C) or pH 7 with incubation at either 25°C (D) or 42°C (E), and growth was compared after 48 h of incubation. Values in panels A to E are the means ± standard deviations from three independent experiments. The growth of the vps21Δ/Δ mutant was compared to the WT control at each concentration of fluconazole using a two-tailed t test; *, P < 0.05. (F) The vps21Δ/Δ mutant and isogenic control strain were cultured in YNB broth with and without 4 μg/ml of fluconazole at 35°C for 24 h, before total cellular sterols were extracted and ergosterol content was quantified by NMR analysis. Ergosterol content is expressed as a percentage of the sample weight (wet weight). A C. albicans erg2Δ/Δ mutant (no fluconazole) was used as a negative control for ergosterol. (G) Both VPS21 alleles were deleted from the tetO-ERG11 strain. The growth of the resulting vps21Δ/Δ, “reconstituted,” and “wild-type” (YJB6284) control strains was then compared using a broth microdilution assay in YNB medium supplemented with a range of doxycycline concentrations. Growth was measured as OD600 and expressed as a percentage of growth occurring in the no-doxycycline control wells. The means ± standard deviations of three independent experiments are indicated, and statistical analysis was performed as described above.
The vps21Δ/Δ mutant is more tolerant of Erg11p inhibition and ergosterol depletion than VPS21+ strains.
The dose-response data for the 24-h time point (Fig. 5A) suggest that the potency of the azoles with respect to Erg11p inhibition is unchanged in the vps21Δ/Δ mutant but that the in vitro antifungal efficacy (i.e., impact on growth) is reduced under standard testing conditions. We therefore considered the possibility that the vps21Δ/Δ mutant has a decreased dependence upon ergosterol for growth or that it is more tolerant than the wild type of the toxic sterol intermediates that accumulate upon azole treatment. To test this, we compared cellular ergosterol levels in the vps21Δ/Δ mutant and isogenic control strain in the presence and absence of fluconazole. This confirmed that both the vps21Δ/Δ mutant and control strains are similarly depleted of ergosterol following treatment with fluconazole (Fig. 5F). Furthermore, NMR analysis of cellular n-heptane extracts from mutant and control cultures indicates that there are no significant differences in the sterol intermediates that accumulate upon fluconazole treatment (see Fig. S8A and S8B in the supplemental material). Thus, the elevated growth of the vps21Δ/Δ mutant in the presence of the azoles is unlikely to be accounted for by altered drug uptake, efflux, or target inhibition.
The above results suggest that despite fluconazole inhibiting Erg11p (and ergosterol biosynthesis), the vps21Δ/Δ mutant is able to grow more than its isogenic control under standard MIC testing conditions. To provide further support for this argument, we deleted both VPS21 alleles from the tetO-ERG11 strain described above. This enabled us to repress Erg11p activity at a transcriptional level and independently of the azole drugs. Doxycycline suppressed the growth of the VPS21+ control strains, but growth was suppressed to a much lesser extent in the vps21Δ/Δ background (Fig. 5G). Together, these data suggest that the elevated growth of the vps21Δ/Δ mutant in the presence of the azoles relates to a tolerance of Erg11p inhibition and ergosterol depletion, rather than a reduction in Erg11p inhibition.
DISCUSSION
The inhibition of fungal growth by azoles is often assumed to be a consequence of plasma membrane dysfunction following depletion of cellular ergosterol and the accumulation of “toxic” sterol intermediates (44). In this study, we were able to establish that inhibition of lanosterol demethylase (Erg11p) by azoles or through transcriptional repression led to significant perturbation of vacuolar integrity in the fungal pathogen C. albicans. Moreover, vacuolar degeneration is an early consequence of azole treatment. We have previously demonstrated that C. albicans mutants with severe defects in vacuolar biogenesis have impaired growth, stress tolerance, polarized hyphal growth and pathogenicity (16, 18, 19, 24). Thus, it is possible that vacuolar dysfunction may make a significant contribution to the overall antifungal activity of the azoles. Previous studies have noted that several nonessential Saccharomyces cerevisiae ergosterol biosynthetic mutants have a fragmented vacuole morphology, and ergosterol is required for homotypic vacuole-vacuole fusion (13, 14). Recent studies by Zhang et al. (15) have also suggested that ergosterol is required for the activity of the V-ATPase responsible for vacuolar acidification, which in turn is required for normal vacuolar membrane fusion (45, 46). However, it is also likely that ergosterol depletion itself affects the structural integrity of the vacuole, and potentially the activity of a multitude of other vacuolar membrane proteins. Thus, the precise interdependence between ergosterol availability, V-ATPase activity, vacuolar integrity, and the antifungal efficacy of the azoles remains to be elucidated.
A second important finding of this study is that vacuolar trafficking pathways through the PVC are an important determinant of antifungal tolerance. Interestingly, blocking the PVC trafficking steps controlled by the Vps21p Rab GTPase and t-SNARE Pep12p does not seem to affect the capacity of azoles to inhibit ergosterol biosynthesis (i.e., potency), but it significantly reduces the resulting impact upon fungal growth under standard susceptibility testing conditions (35°C, pH 7). The differential dose-response curves at 24 versus 48 h, as well as the pH and temperature dependence of the vps21Δ/Δ mutants' decreased susceptibility to the azole antifungals are consistent with it being an exaggerated form of the “trailing growth”' phenotype that has been described for about 18% of C. albicans isolates (47). The mechanism(s) that underlie trailing growth is not well understood, and whether altered endosomal trafficking is a contributing factor to the trailing growth observed in these clinical isolates remains to be established. However, the results presented herein suggest that the trailing growth of the vps21Δ/Δ mutant is not the result of altered azole efflux, target inhibition, or sterol metabolism. Tests on a limited number of clinical isolates have suggested that the trailing growth phenotype is usually associated with in vivo susceptibility to azole therapy (41, 42). Thus, we would expect the vps21Δ/Δ and pep12Δ/Δ mutants to be susceptible in vivo, although this will need to be confirmed using mouse models of disseminated and/or mucosal candidiasis. Similarly, it will be important to determine whether altered endosomal trafficking contributes to the differences in in vitro and in vivo antifungal efficacy that are observed between clinical isolates.
Others have reported that a C. albicans vps15Δ/Δ mutant defective in retrograde trafficking from the PVC back to the Golgi compartment is highly sensitive to fluconazole (48). Furthermore, two mutants lacking components of the endosomal sorting complex required for transport (ESCRT) that facilitates the formation of intralumenal multivesicular bodies from the PVC's surface membrane are also sensitive to fluconazole (49). A further finding of potential clinical relevance is that the vps21Δ/Δ mutant is more susceptible to terbinafine and amorolfine, antifungal medications that target alternate steps within the ergosterol biosynthetic pathway. This suggests that membrane trafficking through the PVC compartment is an important determinant of susceptibility to antifungal agents that target ergosterol biosynthesis. Furthermore, chemical agents that interfere with specific endosomal trafficking steps may sensitize pathogenic fungi and enhance the efficacy of therapies that target the ergosterol biosynthetic pathway.
Interestingly, at sub-growth inhibitory concentrations of fluconazole, the vps21Δ/Δ mutant grew to a slightly (but reproducibly) higher cell density (as determined by OD600) than the no-drug control wells (Fig. 2C). One potential explanation for this curious effect is that slight reductions in ergosterol or a slight increase in the cellular lanosterol content may somehow ameliorate some of the phenotypic consequences of endosomal dysfunction in these mutants. This is again consistent with some kind of functional interaction between endosomal trafficking and/or function and cellular sterol composition.
Several major steps in sterol biosynthesis occur within the endoplasmic reticulum (ER) (10). While the mechanisms responsible for sterol redistribution from the ER to other cellular membranes are poorly understood (50), they are believed to involve both vesicular membrane transport and nonvesicular carrier protein-mediated processes (50–52). It is therefore possible that membrane trafficking defects at the PVC may lead to the subcellular redistribution of cellular sterol species. The methylated sterols that accumulate upon azole treatment are thought to contribute to cellular membrane dysfunction and growth inhibition (44). Thus, redistribution of these potentially toxic sterols to distinct compartments within the fungal cell could determine the specific organelles affected, and thus the cellular consequences of antifungal treatment. For example, blocking Vps21p-mediated trafficking through the PVC may divert the “toxic” sterol intermediates produced upon azole treatment away from an organelle that is especially sensitive and redirect them to a compartment that can better tolerate them. Conversely, the same mechanism may render the vps21Δ/Δ mutant more sensitive to the sterol species that accumulate following treatment with terbinafine or amorolfine. Viewed in this light, the impact of antifungal treatment upon the fungal cell is determined not only by ergosterol depletion but by a combination of the chemical and physical properties of the specific sterol intermediate that accumulates (i.e., which steps of the ergosterol biosynthesis pathway that are blocked) and the cellular destination to which it is transported. Future studies will investigate whether the cellular distribution of the potentially toxic sterol intermediates that accumulate upon treatment with the azoles, allylamines, and morpholines are affected in the above-described endosomal trafficking mutants.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under awards R21AI097664 and R01AI099080.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank H. Nakayama and M. Arisawa (Nippon Roche) for providing the materials required for the C. albicans tetracycline-regulatable (TR) system including C. albicans strain THE1 and p97CAU. We also thank Theodore White (University of Missouri—Kansas City), Samuel Lee (University of New Mexico Health Sciences Center), Judith Berman (University of Minnesota), Aaron Mitchell (Carnegie Mellon University), and William Fonzi (Georgetown University) for providing strains and plasmids that were used in this study.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04239-14.
REFERENCES
- 1.Vandeputte P, Ferrari S, Coste AT. 2012. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol 2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marichal P, Koymans L, Willemsens S, Bellens D, Verhasselt P, Luyten WH, Borgers M, Ramaekers F, Odds FC, Bossche HV. 1999. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145:2701–2713. [DOI] [PubMed] [Google Scholar]
- 3.Perea S, Lopez-Ribot JL, Kirkpatrick WR, McAtee RK, Santillan RA, Martinez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White TC. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41:1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanglard D, Ischer F, Monod M, Bille J. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 6.Goldway M, Teff D, Schmidt R, Oppenheim AB, Koltin Y. 1995. Multidrug resistance in Candida albicans: disruption of the BENr gene. Antimicrob Agents Chemother 39:422–426. doi: 10.1128/AAC.39.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14α-methylergosta-8,24(28)-dien-3β,6α-diol. Biochem Biophys Res Commun 207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- 9.Zinser E, Sperka-Gottlieb CD, Fash EV, Kohlwein SD, Paltauf F, Daum G. 1991. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173:2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinser E, Paltauf E, Daum G. 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J Bacteriol 175:2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisman LS, Bacallao R, Wickner W. 1987. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol 105:1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heese-Peck A, Pichler H, Zanolari B, Watanabe R, Daum G, Riezman H. 2002. Multiple functions of sterols in yeast endocytosis. Mol Biol Cell 13:2664–2680. doi: 10.1091/mbc.E02-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato M, Wickner WT. 2001. Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. EMBO J 20:4035–4040. doi: 10.1093/emboj/20.15.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G. 2002. Genomic analysis of homotypic vacuole fusion. Mol Biol Cell 13:782–794. doi: 10.1091/mbc.01-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YQ, Gamarra S, Garcia-Effron G, Park S, Perlin DS, Rao R. 2010. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog 6:e1000939. doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston DA, Eberle KE, Sturtevant JE, Palmer GE. 2009. Role for endosomal and vacuolar GTPases in Candida albicans pathogenesis. Infect Immun 77:2343–2355. doi: 10.1128/IAI.01458-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer GE. 2011. Vacuolar trafficking and Candida albicans pathogenesis. Commun Integr Biol 4:240–242. doi: 10.4161/cib.4.2.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer GE, Cashmore A, Sturtevant J. 2003. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot Cell 2:411–421. doi: 10.1128/EC.2.3.411-421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer GE, Kelly MN, Sturtevant JE. 2005. The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot Cell 4:1677–1686. doi: 10.1128/EC.4.10.1677-1686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke D, Dawson D, Stearns T. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 21.Ramon AM, Fonzi WA. 2003. Diverged binding specificity of Rim101p, the Candida albicans ortholog of PacC. Eukaryot Cell 2:718–728. doi: 10.1128/EC.2.4.718-728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston DA, Luna-Tapia A, Eberle KE, Palmer GE. 2013. Three prevacuolar compartment Rab GTPases impact Candida albicans hyphal growth. Eukaryot Cell 12:1039–1050. doi: 10.1128/EC.00359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer GE, Kelly MN, Sturtevant JE. 2007. Autophagy in the pathogen Candida albicans. Microbiology 153:51–58. doi: 10.1099/mic.0.2006/001610-0. [DOI] [PubMed] [Google Scholar]
- 24.Palmer GE. 2010. Endosomal and AP-3-dependent vacuolar trafficking routes make additive contributions to Candida albicans hyphal growth and pathogenesis. Eukaryot Cell 9:1755–1765. doi: 10.1128/EC.00029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palanisamy SK, Ramirez MA, Lorenz M, Lee SA. 2010. Candida albicans PEP12 is required for biofilm integrity and in vivo virulence. Eukaryot Cell 9:266–277. doi: 10.1128/EC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bensen ES, Filler SG, Berman J. 2002. A Forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot Cell 1:787–798. doi: 10.1128/EC.1.5.787-798.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 28.Gietz D, St Jean A, Woods RA, Schiestl RH. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181:1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama H, Mio T, Nagahashi S, Kokado M, Arisawa M, Aoki Y. 2000. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun 68:6712–6719. doi: 10.1128/IAI.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ. 1999. Quantitation of ergosterol content novel method for determination of fuconazole susceptibility of Candida albicans. J Clin Microbiol 37:3332–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vida TA, Emr SD. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cope JE. 1980. Mode of action of miconazole on Candida albicans: effect on growth, viability and K+ release. J Gen Microbiol 119:245–251. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi D, Kondo K, Uehara N, Otokozawa S, Tsuji N, Yagihashi A, Watanabe N. 2002. Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob Agents Chemother 46:3113–3117. doi: 10.1128/AAC.46.10.3113-3117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portillo F, Gancedo C. 1984. Mode of action of miconazole on yeasts: inhibition of the mitochondrial ATPase. Eur J Biochem 143:273–276. doi: 10.1111/j.1432-1033.1984.tb08369.x. [DOI] [PubMed] [Google Scholar]
- 37.Thevissen K, Ayscough KR, Aerts AM, Du W, De Brucker K, Meert EM, Ausma J, Borgers M, Cammue BP, Francois IE. 2007. Miconazole induces changes in actin cytoskeleton prior to reactive oxygen species induction in yeast. J Biol Chem 282:21592–21597. doi: 10.1074/jbc.M608505200. [DOI] [PubMed] [Google Scholar]
- 38.Sanglard D, White TC. 2006. Molecular principles of antifungal drug resistance, p 197–212. In Heitman J, Filler SG, Edwards JE, Mitchell AP (ed), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC. [Google Scholar]
- 39.Becherer KA, Rieder SE, Emr SD, Jones EW. 1996. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell 7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marr KA, Rustad TR, Rex JH, White TC. 1999. The trailing end point phenotype in antifungal suceptibility testing is pH dependent. Antimicrob Agents Chemother 43:1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex JH, Nelson PW, Paetznick VL, Lozano-Chiu M, Espinel-Ingroff A, Anaissie EJ. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother 42:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthington-Skaggs BA, Warnock DW, Morrison CJ. 2000. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome. Antimicrob Agents Chemother 44:2081–2085. doi: 10.1128/AAC.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal D, Patterson TF, Rinaldi MG, Revankar SG. 2007. Trailing end-point phenotype of Candida spp. in antifungal susceptibility testing to fluconazole is eliminated by altering incubation temperature. J Med Microbiol 56:1003–1004. doi: 10.1099/jmm.0.47168-0. [DOI] [PubMed] [Google Scholar]
- 44.François IEJA, Cammue BPA, Borgers M, Ausma J, Dispersyn GD, Thevissen K. 2006. Azoles: mode of antifungal action and resistance development. Effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect Agents Med Chem 5:3–13. doi: 10.2174/187152106774755554. [DOI] [Google Scholar]
- 45.Bayer MJ, Reese C, Buhler S, Peters C, Mayer A. 2003. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol 162:211–222. doi: 10.1083/jcb.200212004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baars TL, Petri S, Peters C, Mayer A. 2007. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol Biol Cell 18:3873–3882. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arthington-Skaggs BA, Lee-Yang W, Ciblak MA, Frade JP, Brandt ME, Hajjeh RA, Harrison LH, Sofair AN, Warnock DW, Candidemia Active Surveillance Group . 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother 46:2477–2481. doi: 10.1128/AAC.46.8.2477-2481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Solis NV, Heilmann CJ, Phan QT, Mitchell AP, Klis FM, Filler SG. 2014. Role of retrograde trafficking in stress response, host cell interactions, and virulence of Candida albicans. Eukaryot Cell 13:279–287. doi: 10.1128/EC.00295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornet M, Gaillardin C, Richard ML. 2006. Deletions of the endocytic components VPS28 and VPS32 in Candida albicans lead to echinocandin and azole hypersensitivity. Antimicrob Agents Chemother 50:3492–3495. doi: 10.1128/AAC.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prinz WA. 2007. Non-vesicular sterol transport in cells. Prog Lipid Res 46:297–314. doi: 10.1016/j.plipres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumann NA, Sullivan DJ, Ohvo-Rekila H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK. 2005. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry 44:5816–5826. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 52.Schulz TA, Prinz WA. 2007. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta 1771:769–780. doi: 10.1016/j.bbalip.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.