Abstract
The skin is a complex organ that, in addition to providing a strong barrier against external insults, serves as an arena for a wide variety of inflammatory processes, including immunity against infections, tumor immunity, autoimmunity, and allergy. A variety of cells collaborate to mount functional immune responses, which are initiated by resident populations and evolve through the recruitment of additional cell populations to the skin. Inflammatory responses are quite diverse, resulting in a wide range of signs and symptoms that depend on the initiating signals, characteristics of the infiltrating cell populations, and cytokines that are produced (cytokines are secreted protein that allows for cell–cell communication; usually refers to communication between immune–immune cells or stromal–immune cells). In this work, we will review the skin architecture and resident and recruited cell populations and discuss how these populations contribute to inflammation using human diseases and treatments when possible to illustrate their importance within a clinical context.
The skin provides a strong physical barrier against external insults. But it also serves as an arena for diverse inflammatory processes, involving both resident and recruited cells that combat a multitude of pathogens.
Tissues at the interface of the host and the environment, including the skin, gut, and other mucosal surfaces, present the first line of defense against pathogens. The barrier function of the skin is of critical importance, which is evident when this barrier is disrupted following injury, or in atopic dermatitis, ichthyosis, or irritant contact dermatitis. Once the barrier is disrupted, the rapid but nonspecific innate immune response is recruited in defense, a process that relies on detection of both self and foreign “danger signals” as the initial alarm. Next, the slower, but specific adaptive immune response may be required for definitive clearance of a pathogen.
In addition to providing protection against invading pathogens, the skin is also an arena where sterile inflammation, including tumor immunity, allergy, and autoimmune responses, may participate in disease. Tumor immunity is defective in organ transplant patients who are immunosuppressed, but is co-opted during imiquimod treatment of various skin cancers and warts. Allergic responses, which likely evolved to protect against parasitic invasion, may cause disease when directed against innocuous foreign materials, as in allergic contact dermatitis. Autoimmunity, possibly caused by antipathogen or antitumor immunity misdirected against self, causes a wide range of pathology in the skin, including vitiligo, lupus, psoriasis, and other diseases.
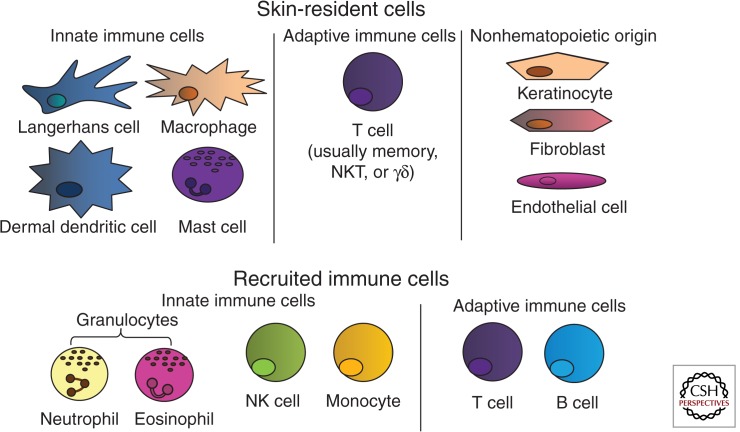
Appropriate functioning of the skin in these roles requires close communication and collaboration among a number of various cell types, including stromal cells (keratinocytes, fibroblasts, endothelial cells, and adipocytes) as well as those derived from the bone marrow (dendritic cells, macrophages, natural killer cells, mast cells, T cells, and others). Of the bone marrow–derived cells that can be found in the skin, some are resident cell populations that migrate to the skin where they terminally differentiate and primarily reside there, some recirculate continuously and perform a surveillance role, some are recruited to fight infection for the short term, and others are recruited and maintained as memory cells to protect against future reinvasion. Bone marrow–derived cells can be further subdivided into innate and adaptive immune populations (see Fig. 1). Innate cells form rapid, but nonspecific, responses to infection. They generally recognize non-self-molecular patterns on pathogens or pathogen-associated molecular patterns (PAMPs), through receptors called pattern-recognition receptors (PRRs are intracellular or cell surface receptors activated by DAMPs to induce inflammation). Adaptive immune populations form slower, but pathogen-specific, responses to infection through specialized and unique antigen-specific receptors formed via genetic rearrangement (an antigen is any molecule capable of inducing an antibody response or T-cell response; it can be protein, lipid, or carbohydrate). These receptors permit immune cells to mount a more targeted attack on invaders, and these cells can then become long-lived and capable of a rapid, specific response against reinvasion of a pathogen, known as a memory response.
Figure 1.
Immune populations in the skin. (Top) Langerhans cells, dermal dendritic cells, macrophages, other innate cells (mast cells, NK cells, NKT cells, γδ-T cells), and memory T cells comprise the skin-resident immune system under the steady state. Langerhans cells, dermal dendritic cells, macrophages, and γδ-T cells first sense infection or injury and initiate a rapid, innate response that includes the recruitment of effector cells. Innate effectors (NK populations) provide a rapid, antigen-nonspecific response, whereas memory T cells provide a rapid, antigen-specific memory response to previously encountered pathogens. Stromal cell populations, such as keratinocytes, fibroblasts, and endothelial cells, also participate in immune responses by sensing tissue damage and producing inflammatory cytokines. (Bottom) On activation of the skin-resident immune system, additional immune cells are recruited to help contain and fight infection and/or to remove cellular debris to aid in the healing process. These include additional innate cells like neutrophils and eosinophils, as well as adaptive populations like naïve or central memory T cells and B cells.
Initiation of an adaptive immune response is performed by antigen-presenting cells (APCs), which efficiently take up (phagocytose) proteins, process them into recognizable peptides (antigens), and present them to T cells on surface human leukocyte antigen (HLA) I and HLA II molecules (human leukocyte antigen class I and II; typically, class I presents intracellular antigens and class II presents extracellular antigens). All nucleated cells in the body express HLA I, providing an important mechanism for detection of viral infections as well as malignant transformation through direct communication with immune cells. HLA I presents antigens that are produced within the cell and permits their recognition by antigen-specific T cells through their T-cell receptor, making the cells susceptible to cytotoxic T-cell-mediated killing. Typically, if those antigens are from normal self-proteins, they will be spared, a process called immunological tolerance. However, if those antigens are derived from intracellular pathogens (like viruses), or if the antigen is an abnormal self-protein generated following malignant transformation of the cell, then that cell may be killed to prevent further injury to the host. Unlike HLA I, HLA II is typically only expressed on APCs, and is capable of presenting antigens derived from extracellular proteins acquired through phagocytosis (cell eating; the process by which antigen-presenting cells and other phagocytic cells take up pathogens or other debris). Dendritic cells (DCs) are recognized as professional APCs because their main function is to take up antigens, thereby linking innate and adaptive immune responses. If DCs phagocytose a pathogen in the presence of a PAMP that alerts them to danger, they then produce proinflammatory mediators to recruit innate immune cells to initiate the defense, and then typically migrate out of the skin through lymphatic channels into draining lymph nodes. There they position themselves to directly encounter T cells, and each T cell will briefly probe the DC to determine whether it is presenting an antigen that the T cell recognizes. If so, the T cell will become activated and initiate a search for the location of the infection.
In this article, we will describe how all of the cell populations within the skin contribute to immune responses, and the general principles and tools required for effective immunity. We will use specific examples of common skin diseases and treatments when possible to illustrate how the immune system works within a clinical context.
NORMAL SKIN IMMUNE SYSTEM
Tissue Architecture and Composition
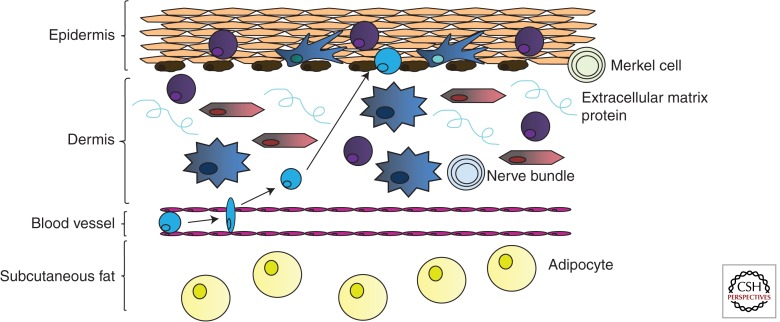
Normal skin architecture includes the epidermis, dermis, and subcutaneous fat (see Fig. 2). The resident cell populations that make up these strata can be broadly divided into immune and nonimmune cells. Nonimmune cell populations are important for the structure and function of the skin, but also contribute to skin immunity, as they first provide a general barrier to invasion by foreign materials. They also directly participate in inflammation, shaping the immune response as it develops within the tissue.
Figure 2.
Location of immune cells within normal skin architecture. Distinct populations of immune cells inhabit local niches within the skin. The epidermis contains Langerhans cells to provide immune surveillance. Memory T cells are also retained in the epidermis, presumably for early detection and control of re-encountered pathogens. Keratinocytes may sense pathogens or other damage-associated signals and communicate this to the immune system through cytokines. The dermis contains dermal dendritic cells, T cells, and fibroblasts. In this example, we depict a T cell being recruited out of the blood and into the dermis and potentially the epidermis, depending on the site of infection/injury. Recruitment of cells to peripheral tissues requires production of chemoattractants. Endothelial cells, which form the walls of the vasculature, may present chemokines and/or adhesion molecules to immune cells to direct their transmigration into the skin.
Epidermis
The epidermis is comprised primarily of keratinocytes, which are tightly connected to one another and act similarly to a brick wall to limit access to the internal environment. As a consequence, these tight connections limit movement within this layer and therefore other cell types that reside there, which include both stromal and bone marrow–derived cells, are primarily fixed in position. However, when damage occurs and immune cells sense danger, they release proinflammatory mediators to recruit innate effector cells, and then exit to draining lymph nodes where they encounter T cells and other members of the adaptive immune system.
Cellular Components of the Epidermis
Keratinocytes maintain tight junctions and form the stratum corneum, which is critical for the barrier function of the epidermis (see Box 1). They may also contribute to inflammation, as they can express HLA II and secrete cytokines.
Melanocytes are pigment-producing cells in the skin. The melanin pigment they produce and distribute to keratinocytes helps to shield DNA from ultraviolet radiation, which can induce DNA damage. Melanocytes are also capable of expressing MHC II, and are the targets of autoimmunity in vitiligo.
Merkel cells are specialized cells that communicate with cutaneous neurons in skin sensation. A clear contribution of Merkel cells to cutaneous immunity has not yet been described.
BOX 1. SKIN BARRIER DYSFUNCTION.
Filaggrin is a protein that multimerizes, binds to keratin fibers, and strengthens connective tissues, helping to form a tight barrier against exposure to environmental agents. It is essential to barrier function within the epidermis. In atopic dermatitis and ichthyosis, filaggrin mutations cause barrier dysfunction, resulting in chronic inflammation and opportunistic infection.
Bone Marrow–Derived Cells of the Epidermis
Langerhans cells (LHCs) are dendritic cells (DCs) that spend the majority of their time in the epidermis. LHCs are tightly connected to keratinocytes through dendritic processes that radiate in all directions, allowing them to probe throughout the entire epidermis. The exact role of LHCs is not entirely clear, although they may promote tolerance to environmental antigens, including commensal bacteria and fungi, and help to polarize T cells into a particular inflammatory response as described below. Although keratinocytes and melanocytes may also be capable of acting as APCs, the implication of this function is not yet clear.
Memory T cells may reside within the epidermis for very long periods of time. Whereas neutrophils and T cells can infiltrate the epidermis under some circumstances (atopic and contact dermatitis, cutaneous T-cell lymphoma, psoriasis, and vitiligo), they are typically excluded.
Dermis
The dermis is primarily comprised of extracellular matrix proteins that give the skin structure and elasticity. Unlike the epidermis, it permits the free migration of cell populations. The dermis and epidermis are separated by the basement membrane, a thin, tight sheet of extracellular matrix proteins that regulates movement of cells and proteins in between these two layers.
Cellular Components of the Dermis
Fibroblasts produce structural proteins, which in addition to providing a supporting scaffold, serve as highway systems for migratory immune cells. As in the lymph node, these highways ensure that immune cells frequently contact each other, which is important in communication during immune responses. Like keratinocytes, fibroblasts are capable of producing cytokines.
Endothelial cells form the innermost layer of the blood vessels in the skin, and regulate the passage of immune cells into the skin through the production of adhesion molecules, cytokines, and chemokines (a cytokine that acts as a chemoattractant to induce cell migration) (see also Box 2).
Neurons form nerve bundles in the skin, which allow for sensation. Recently, it has been shown that memory T cells can interact with neurons to regulate innate immunity (Rosas-Ballina et al. 2011). Neuroimmunology is a relatively new field, and the relationship between skin neurons and immune cells is not yet fully appreciated.
BOX 2. LFA-1 AND LEUKOCYTE ADHESION.
LFA-1 is expressed by endothelial cells and promotes adhesion of leukocytes, permitting their migration into peripheral tissues. A defect in the adhesion molecule CD18, a component of LFA-1, results in leukocyte adhesion deficiency (LAD). LAD is characterized by chronic bacterial skin infections owing to impaired neutrophil chemotaxis. In addition, the CD11a component of LFA-1 was the target of the drug efalizumab for psoriasis, which presumably functioned by inhibiting immune cell migration into the skin. Predictable side effects were infections of multiple organs, including a potentially lethal JC virus infection within the brain, which led to its withdrawal from the market in 2009.
Bone Marrow–Derived Cells of the Dermis
Innate populations of the dermis:
Dermal DCs (dDCs) and plasmacytoid DCs (pDCs), two distinct populations of dendritic cells, are located in the dermis. They have fewer dendrites but increased motility compared with LHCs, and are capable of migrating on collagen paths to monitor the dermis. Each DC population has been characterized by the production of different cytokines, and may initiate distinct inflammatory responses following activation. For example, pDCs have been reported to initiate antifungal immunity (Ramirez-Ortiz et al. 2011), whereas dDCs initiate antiviral immunity (Kaplan 2010). These roles are not likely to be exclusive, such that different DC populations may contribute to different immune responses in multiple ways.
Macrophages are skin-resident immune cells with high phagocytic capacity and motility. Although they are less likely than DCs to present antigen to T cells, due to relative cell numbers, they are capable of activating immune responses through PRRs and cytokine secretion. They also “clean up” debris from dead or dying cells, invading pathogens, or environmental insults, including tattoo ink. For example, “melanophages” represent macrophages that have phagocytosed melanocyte fragments and melanin released into the dermis following epidermal inflammation.
Monocytes are a type of immature macrophages that are usually found in the circulation. They can be recruited to the skin to maintain homeostasis or in response to infection/injury, where they receive cues to differentiate into macrophages or myeloid DCs, a DC population that is not well understood.
Granulocytes include neutrophils (also called polymorphonuclear cells or PMNs), eosinophils, basophils, and mast cells. These innate immune cell populations can be recruited to the skin following activation of a tissue-resident cell and subsequent release of chemokines and activation of the endothelium. Granulocytes are named for their cytoplasmic granules that are filled with proteases, vasoactive peptides, and antimicrobial peptides, which are released during degranulation. Neutrophils are typically the first cell type recruited to the skin following activation of dendritic cells and/or macrophages in response to PAMPs encountered during infection, and are capable of efficiently phagocytosing and killing pathogens (see Box 3). Neutrophils have also recently been shown to make “sticky” extracellular traps (NETs) by expelling the DNA from their nucleus, effectively trapping pathogens like flies caught in a spider web. Eosinophils and basophils contribute to antiparasitic and allergic responses, although we are just beginning to understand their unique roles in immunity. Eosinophils degranulate when their IgE receptors become cross-linked, and they release proteases, chemokines, and vasoactive proteins. Basophils, which also degranulate in this manner, can act as potential APCs. Mast cells are typically skin-resident granulocytes that mediate immune responses against parasites following binding and cross-linking of IgE antibodies bound to parasitic invaders, followed by degranulation of histamines and other proinflammatory proteins. They have also been implicated in the pathogenesis of allergic responses when IgE antibodies are produced against innocuous environmental antigens, like dust mite proteins and animal dander.
Natural killer (NK) cells are classified as innate cells because of their pattern-recognition functions, but may also confer memory, like adaptive populations. NK cells detect the level of self-HLA I expressed on the surface of cells, and are activated when expression levels are too low, which occurs when either malignant or virally infected cells impair the expression of HLA I to escape immune detection by T cells. NK cells can also perform antibody-dependent cellular cytotoxicity (ADCC) by binding IgG-coated pathogens and cells, releasing granules containing perforin and granzyme.
BOX 3. CHRONIC GRANULOMATOUS DISEASE.
Neutrophils kill pathogens through the use of a “respiratory burst,” which depends on NADPH oxidase to generate oxygen free radicals. This gene is defective in chronic granulomatous disease (CGD), and patients with CGD develop chronic skin bacterial and fungal infections.
Adaptive populations of the dermis:
Most of the skin-resident adaptive cells are T lymphocytes (T cells) of different subsets. There are approximately 20 billion T cells present in the skin, nearly twice the number of T cells in the blood (Clark 2010) (a lymphocyte is a type of adaptive immune cell including T and B cells, capable of genetically rearranging antigen-specific receptors). CD8+ cytotoxic T cells (CTLs) are effector cells that recognize a specific antigen presented on a cell surface by HLA I, and subsequently kill that cell. CD4+ helper T cells (TH cells) “help” effector cell populations like CTLs and B cells through the production of cytokines and promaturation signals to DCs, which both license the effector cells to carry on an attack, and steer the response in a particular direction, either antiviral, antitumor, antibacterial, or antiparasitic, as described in more detail below. This TH-cell help provides an important check to the development of an immune response, essentially licensing other antigen-specific T and B cells before allowing the response to develop. T-regulatory cells (Tregs) are typically CD4+ and express FoxP3, a critical transcription factor for their development (see Box 4). Their role is to suppress immune responses as a major contributor to peripheral tolerance, helping to prevent autoimmunity and to resolve inflammation once a threat has been controlled. As mentioned above, any of these cell populations may be temporary, migrating through the skin for surveillance, or may remain long term as skin-resident memory T cells (TRM) to prevent reinfection.
γδ-T cells, which have a limited repertoire of T-cell receptors (TCRs), and natural killer T cells (NKTs), which are classified by their expression of both NK cell receptors and the universal T-cell marker CD3, are other T-cell populations of the dermis. Both populations respond to lipid antigens.
Once activated, B lymphocytes (B cells) mature into antibody-producing plasma cells that usually reside in the lymph nodes and bone marrow, producing antibodies that become passively delivered to the skin through the circulation. These antibodies may mediate either infectious or autoimmune responses, depending on their specificity. Whereas B cells and plasma cells can occasionally be found in the skin (syphilitic infection, lupus, and other diseases), the reason for this localization to the skin is unknown.
BOX 4. IPEX SYNDROME.
Immunodysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) is a genetic disruption of the FoxP3 transcription factor, which is important for the generation of Tregs. Tregs are critical for the maintenance of peripheral tolerance to self-tissues and thus in the prevention of autoimmunity. Without this, patients develop multiple autoimmune diseases, including enteropathy; insulin-dependent diabetes mellitus, thyroid disease, and other endocrinopathies; and skin disease including eczema, psoriasiform dermatitis, urticaria, and alopecia universalis, among others.
Subcutaneous Fat
The subcutaneous fat layer is mainly comprised of adipocytes, but also contains nerves, blood, and lymphatic vessels. Adipocytes are fat cells that sequester potentially inflammatory fatty acids in the form of lipids. They are also capable of producing proinflammatory cytokines.
Soluble Proteins of the Skin Immune System
There are several families of proteins that are important for skin immunity. Complement is a family of soluble plasma proteins that can bind to pathogens, self-catalyze to form soluble chemoattractants and membrane-bound opsonins that promote phagocytosis, and membrane attack complexes that can puncture bacteria (see Box 5). Complement fixation to the bacterium can occur by three different pathways: classical, alternative, or lectin. Classical activation of complement is catalyzed by antibodies bound to the pathogen, alternative activation occurs through direct binding of complement to the pathogen, and lectin activation is catalyzed by mannose binding lectin (MBL) bound to the pathogen. Phagocytes express complement receptors that recognize complement bound to an invading organism, which facilitates phagocytosis of the invader regardless of how complement was first fixed to the surface (a form of opsonization, which is the process of coating with proteins that facilitate phagocytosis).
Antimicrobial peptides are another family of proteins that contribute to skin immunity. Examples include β-defensins, psoriasin, and lysozyme, which have varying but direct antimicrobial activity against bacteria and fungi. Keratinocytes are one of the main sources of antimicrobial peptides, which are induced by TLR ligation and/or cytokine production.
Interferons (IFN) belong to a family of cytokines that are proinflammatory and also directly “interfere” with viral replication through reducing protein translation and increasing p53 levels, which promotes apoptosis.
Antibodies bind with high specificity and affinity to both foreign proteins during an infectious immune response, or self-proteins in autoimmunity. They can injure cells by promoting phagocytosis through opsonization, complement-mediated lysis, activation of signaling cascades, and through neutralization of surface proteins.
BOX 5. COMPLEMENT ABNORMALITIES.
Genetic defects in complement can result in too much or too little complement activity. Loss-of-function mutations ultimately result in recurrent infections because of decreased opsonization and/or decreased lytic activity. Common infections resulting from complement deficiency include recurrent meningococcal infection (Neisseria subspecies), Streptococcus pneumoniae infection, or other encapsulated bacterial infections. Hereditary or autoimmune angioedema results from too much complement activity caused by a loss of the C1 esterase inhibitor. As a result, complement activation occurs spontaneously, which induces production of bradykinin. Bradykinin is a vasoactive peptide that results in blood vessel dilation, thereby causing the edema and other symptoms of the disease.
INITIATION AND EVOLUTION OF AN IMMUNE RESPONSE IN THE SKIN
DCs regularly take up proteins within the skin, but must distinguish whether they are presented in a dangerous context like infection or malignancy, or a safe context like normal cell turnover during tissue homeostasis. A number of molecular patterns alert immune cells to danger. These patterns are typically present only when normal cellular processes have been disrupted, as seen in infection, malignancy, or necrotic cell death. These patterns are broadly referred to as danger-associated molecular patterns (DAMPs; can be pathogen-derived or altered-self signals), microbe-associated molecular patterns (MAMPs), viral-associated molecular patterns (VAMPs), or pathogen-associated molecular patterns (PAMPs), depending on from where they are derived. Examples include lipopolysaccharide (LPS), flagellin, and peptidoglycan from bacteria, double-stranded RNA from viruses, or mislocalized (extranuclear) double-stranded DNA during necrotic cell death. The receptors that recognize these patterns include Toll-like receptors (TLRs) and NOD-like receptors (NLRs), and are expressed by DCs, other immune cells, and sometimes stromal cells. There are both surface and intracellular PRRs to allow for both extra- and intracellular recognition of pathogens. Activation of these receptors by DAMPs promotes antigen processing and presentation, up-regulation of costimulatory receptors for T-cell activation, and secretion of proinflammatory cytokines including IL-6, IL-1β, TNF-α, and others. Therefore, it is these “danger signals” that initiate a proinflammatory response.
On activation, tissue-resident cells secrete proinflammatory cytokines that promote the recruitment of innate effector cells, including neutrophils, monocytes, and NK cells. These first responders can begin the attack using the mechanisms described above. However, an effective immune response usually requires the subsequent involvement of the adaptive immune system, and so DCs must interact with a multitude of T cells to identify the correct cells with the capacity to recognize the specific invading pathogen. Because very few T cells are capable of responding to any particular challenge, DCs must migrate to a draining lymph node, the equivalent of Grand Central Station in New York, to most efficiently survey the millions of potential responders and find those that can participate in any particular response.
Following the successful activation of an antigen-specific T cell, that cell will proliferate, exit the lymph node into the blood stream, and search for the location in the body that contains its target. The DC will help to direct the T cell to the skin through a mechanism that is not fully understood, called “imprinting”; however, it appears that vitamins play a role. Vitamin D is produced primarily within the skin following exposure to UV light, and therefore skin DCs contain large amounts of this vitamin. When T cells are activated by DCs containing vitamin D, the T cells express skin-homing receptors, including CCR4, CCR10, and CLA. A similar T-cell educational program occurs via intestinal DCs that contain vitamin A, which is acquired through the diet, inducing α6β4 protein, a gut-homing receptor. Once the T cells are in the bloodstream and express skin-homing receptors, the initial cytokines and adhesion molecules on endothelial cells at the site of inflammation help to guide those cells to the correct site. Although activated B cells, which produce antibodies, may also be recruited to the skin during inflammation, they more often remain within the lymph nodes or bone marrow, secreting the antibody into the blood where it is passively carried to the skin and contributes to the immune response.
As described above, T-cell subsets have specialized functions, including those that are directly cytotoxic, others that suppress responses, and still others that oversee and help to shape the response through the secretion of cytokines. TH subsets are usually designated with a number to delineate which types of cytokines they produce. For example, TH1 cells produce interferon-γ (IFN-γ) and tumor necrosis factor (TNF); TH17 cells produce IL-17, IL-21, and IL-22; and TH2 cells produce IL-4, IL-5, and IL-13. These different cytokine patterns are usually associated with recruitment of slightly different types of immune effector populations. For example, TH2 responses recruit basophils, eosinophils, and mast cells to coordinate an antiparasitic response, whereas TH1 responses result in recruitment of CTLs for an antiviral or antitumor response, and TH17 promotes an antibacterial or antifungal response through the recruitment of neutrophils and production of cytokines and antimicrobial peptides. TH1 and TH17 responses may also promote autoimmunity, whereas TH2 responses may mediate allergy (see also the section on Diseased Skin). For efficiency and efficacy, immune responses often polarize toward a single specific pathway; however, mixed responses may also occur, creating a significant level of complexity that has yet to be fully understood for all such responses in vivo.
The subpopulation of DC that initiates the immune response and the signals present during the initiation can influence the nature of that response. For example, LHC may suppress immune responses, acting as tolerogenic mediators because they regularly sample foreign proteins and organisms present on the intact skin surface, which are primarily nonthreatening. When LHC-deficient mice are exposed to a contact allergen, the response is exacerbated, supporting this suppressive role of LHCs in the skin. In contrast, dDC-deficient mice elicit a dampened response, suggesting this dDC population is proinflammatory in this context. This role is intuitive, because exposure of dermal DCs to a foreign antigen would require disruption of the epidermis, which is a potentially dangerous event. Recent studies also support a nuanced proinflammatory role for these two populations. Whereas activated LHCs seem to promote an antibacterial/antifungal immune response through the production of TH17-specific cytokines, dDC promote antiviral responses through the production of IFNs and other TH1-specific cytokines. This may be because of the fact that viruses often enter the skin systemically or through epidermal disruption and therefore primarily enter the dermis, whereas bacteria most often enter through the epidermis. Little is known about the mechanism by which DCs distinguish these stimuli, although it is likely mediated through PRRs.
Turning off Inflammation and Initiating Wound Healing
An important part of normal immune responses is turning off inflammation once the infection is cleared or injury is healed. Skin-resident Treg populations have been shown to be activated by epidermal LHC (Seneschal et al. 2012), and play an important role in dampening inflammation. There are several different Treg populations that are usually subdivided into central and peripheral subtypes. Whereas central Tregs develop in the thymus, peripheral Tregs are generated during immune responses in lymphoid organs and/or tissues, where asymmetric division of effector cells allows for generation of a small population of Tregs. Examples of these include TH3 and TR1 cells, which produce the anti-inflammatory cytokine IL-10. Both central and peripheral Tregs are capable of down-modulating inflammation via cytokine production, uptake, and removal of the T-cell growth factor IL-2, and through DC interactions. Interleukin-10 (IL-10) is an anti-inflammatory cytokine that will down-regulate the expression of other cytokines, MHC II, and costimulatory molecules. Tregs express the high-affinity IL-2 receptor, CD25, and will therefore preferentially bind and “sop up” IL-2 in the milieu, thereby removing the T-cell growth and survival signal. Tregs are also capable of directly interacting with DCs and inducing down-regulation of costimulatory molecules through cell–cell interactions. Tregs also produce transforming growth factor-β (TGF-β), which can both inhibit immune cell proliferation and stimulate fibroblast production of extracellular matrix proteins (see Box 6). In both the skin and the gut, Tregs have been shown to play an important role in maintaining tolerance to normal bacterial flora. It has recently been postulated that Treg sensing of flora is also important for normal wound healing (Chen et al. 2013).
BOX 6. IMIQUIMOD TREATMENT OF WARTS.
Imiquimod is a synthetic TLR-7/8 agonist that can be used as adjuvant therapy. In the case of HPV, imiquimod can induce production of type I IFN, IL-6, and TNF to overcome the lack of inflammatory mediator production and actively suppress Treg function. An additional approach to treating warts is intradermal injection of Candida antigens, which are natural TLR ligands.
DISEASED SKIN
Proper Immune Responses in Disease—Infectious Immunity
Infectious Immunity: Part I. Examples of Bacterial Immunity
Staphylococcus aureus is a prevalent human skin commensal and pathogen that can cause superficial skin infections (impetigo and exacerbation of atopic dermatitis), infection of the hair follicle (folliculitis and furunculosis), as well as deep infections (ecthyma and abscesses). Like most other bacterial infections of the skin, these conditions are characterized clinically by tender, red, inflamed pustules and abscesses that form as a result of cytokine expression, neutrophil recruitment, and an epidermal response, including keratinocyte proliferation and production of antimicrobial peptides. Twenty percent of the population is colonized with S. aureus, and infections like those described above are quite common.
Methicillin-resistant S. aureus (MRSA) is a particularly recalcitrant infection due both to its antibiotic resistance as well as multiple virulence factors (a component of a pathogen that permits infection and survival; can be protein, lipid, carbohydrate, or nucleic acid) (reviewed by Foster 2005). S. aureus is a Gram-positive coccus, which has an outer cell wall. Innate immune receptors and PRRs important in the detection of Gram-positive bacteria include Toll-like receptor 2 (TLR-2), and mannose-binding lectin (MBL), which are capable of detecting the sugars and lipids that comprise bacterial cell walls. As described in detail above, ligation of PRRs associated with skin-resident immune cells induces activation and maturation of DCs, keratinocyte proliferation and antimicrobial peptide production, and the production of cytokines and chemokines to promote the recruitment of neutrophils and macrophages to the site of infection. Once in the skin, neutrophils and macrophages phagocytose and destroy the bacteria. As mentioned above, phagocytosis is aided by opsonization with antibodies and the binding of complement. Once bacteria are taken into the phagosome, it fuses with the lysosome resulting in acidification, activation of proteases, and production of reactive oxygen species, all of which are capable of breaking down the pathogens. This process also results in the generation of antigens, which can be presented on HLA II to activate the adaptive immune response. During this process, some macrophages and DCs will migrate to the draining lymph node to activate T and B cells via the HLA II–antigen complexes for initiation of the adaptive immune response. Activation of T cells and TH17 skewing seems to be particularly important for antibacterial immunity. This is attributable to the functions of TH17-associated cytokines: IL-17, 22, and 23 can promote thickening of the epidermis, production of antimicrobial peptides, and recruitment of neutrophils, which further enhances bacterial clearance.
S. aureus has developed several mechanisms to avoid the host immune response. It can avoid phagocytosis through the virulence factors Protein A (interferes with IgG binding to its FcγR), ClfA (promotes coating with fibrinogen, which outcompetes the binding of opsonins including complement), and capsule formation (promotes biofilm formation via polysaccharide intercellular adhesion), which inhibits complement and antibody binding. It can also prevent phagolysosomal fusion via the virulence factor SarA, thereby evading death and surviving within the immune cells themselves. Inside the host phagocytes, S. aureus can also scavenge free radicals via superoxide dismutases. The unique structure of its wall is resistant to degradation by lysozyme. These factors may in part explain why it is so difficult to induce good adaptive immune responses to S. aureus, as there could be less efficient antigen generation and presentation.
S. aureus produces the chemotaxis inhibitory protein of staphylococci (CHIPS), which can bind to and inhibit chemoattractant receptors on the surface of neutrophils. It also produces Eap, a protein that disrupts LFA-1 and ICAM-1 interactions that are required for neutrophils to adhere to endothelial cells and transmigrate into the skin (see also Box 2). The bacteria are also capable of preventing complement fixation through production of a Staphylococcus complement inhibitor (SCIN), Efb, or through Staphylokinase, an enzyme that promotes degradation of complement, antibodies, and clots. As mentioned above, complement is important not only for phagocytosis of bacteria, but also for neutrophil chemotaxis. By inhibiting complement fixation, therefore, the bacteria are not only avoiding complement-mediated lysis and opsonization, but also potentially reducing the number of neutrophils that are recruited to the skin by the complement proteins C3a and C5a. S. aureus is capable of expressing nucleases, which can cut neutrophilic NETs comprised of DNA discussed above, in addition to lipases and proteases, which supports their dissemination.
Another way that S. aureus can avoid the host immune response is by invading epithelial and endothelial cells and living inside them in a semi-dormant state. Intracellular pathogens usually require cell-mediated immunity to clear infection. However, S. aureus has also developed mechanisms to evade T cell and other leukocyte responses, namely through production of toxins and superantigens. The α-toxin is capable of inserting into host membranes and multimerizing to form a pore, much like the way complement can form a pore in bacterial cell walls. Other toxins include the Panton–Valentine leukocidin (PVL), which can lyse leukocytes, and the leukocidin D/E, leukocidin M/F, and the γ-hemolysin (Hlg), which can lyse erythrocytes and leukocytes. Recurrent furunculosis and other conditions have been associated with PVL expression by S. aureus. S. aureus also produces one of the best-characterized superantigens, which nonspecifically activates T cells via strong binding of both the MHC II and the T-cell receptor. Toxic shock syndrome toxin-1 (TSST-1, which can initiate tampon-associated toxic shock syndrome) prevents the generation of normal T-cell responses against the bacterium and also affects the ability to generate antibody responses by B cells, which often need T cell help for appropriate class switching and activation.
In light of all of the ways in which S. aureus can evade immune responses, it is easy to see why drug-resistant strains pose a threat. Immunocompromised patients are especially vulnerable (see Box 3), making it of utmost importance to understand skin immunity to bacteria.
Infectious Immunity: Part II. Examples of Viral Immunity
Antiviral immunity largely depends on NK and CTL responses. Both NKs and CTLs kill their targets via perforin/granzyme-induced apoptosis. The inflammatory response to most viral infections of the skin consists of minimal inflammation and symptoms caused by the targeted destruction of virally infected cells and therefore usually lack redness, swelling, or pus that are characteristic of bacterial or fungal infections. TH1-type cytokines are important for antiviral immunity because they drive CTL responses and antiviral mechanisms through production of IFN-γ. We will discuss human papilloma virus (HPV) as an example of antiviral immunity in the skin. HPV is the most common STD; it is estimated that 50% of sexually active men and women get infected. The majority of people who contract HPV clear the infection within 2 yr, and not everyone that has HPV will develop cancer, as this is strain dependent. In this section, we will highlight concepts of antiviral immunity and ways in which HPV can subvert the host immune response.
More than 70 different strains of HPV exist. HPV belongs to the papillomaviridae family of nonenveloped DNA viruses. HPV productively infects keratinocytes, and takes over the cellular machinery to manufacture progeny viruses. The HPV replication cycle is tied to keratinocyte differentiation, in which HPV early proteins (“E” 1–7) are produced in undifferentiated keratinocytes and late proteins (“L” 1 and 2), which are involved in capsid formation, are produced in more superficial cells to promote sloughing of virus. Some HPV strains can be transmitted sexually and cause genital warts, whereas some can cause warts on other parts of the skin. Different HPV strains are adapted to infect specific anatomic locations. For example, strain 1 usually infects the soles of the feet, strain 2 the palms of the hands, strains 6 and 11 are associated with genital warts, and strains 16 and 18 can cause cervical cancer. Recently, two vaccines were developed against HPV: Gardasil (types 6, 11, 16, and 18) and Cervarix (types 16 and 18) in hopes of preventing most cervical cancers (90% contain HPV DNA).
HPV produces proteins E6 and E7 that inhibit host p53 and Rb, respectively, which normally are involved in sensing DNA damage and repair mechanisms to halt cell-cycle progression. This is accomplished via ubiquitin-mediated proteasomal degradation. P53 and Rb are known as the “guardians of the genome” because the DNA damage-sensing mechanism is important for cells to maintain the health of the genome and to avoid improper growth. Because viruses need to use the host cell machinery to replicate, overriding these proteins allows them to replicate more efficiently. A byproduct of this is the hyperproliferation of keratinocytes that characterizes a wart. HPV can also avoid IFN-mediated antiviral responses through E7, which can bind to and inhibit the promoters of type-I IFN-related genes. Treatment with IFN-α can be used for genital warts, although patients with higher E7 levels tend to respond less well than patients with low E7. E6 can down-regulate IL-18 expression, which is important for the generation of TH1 and CTL responses.
Productive antiviral immunity depends on NK cell responses, CTL responses, and antibody production. NK cells look for the presence or absence of self HLA I on the surface of cells, whereas CTLs respond to specific HLA I–peptide complexes. To avoid detection by CTLs, many viruses have developed the ability to down-regulate HLA I. For example, the E5 protein of HPV is capable of down-regulating HLA I expression via inhibition of tapasin and HLA I promoter binding by transcription factors. Yet even though the chance of activating a CTL is decreased by E5, an NK cell could still kill an HPV-infected target cell. NK cells receive activating signals through killer activating receptors (KARs), whereas inhibitory signals are transmitted through killer inhibitory receptors (KIRs), which detect presence of HLA I on the cell’s surface. Therefore, it is the balance of positive and negative signals that the NK cell receives that will determine whether or not it will kill the target cell. However, not many NK cells are recruited to sites of HPV infection, as the virus has devised other mechanisms to subvert inflammatory responses. Part of this is because of the sequestration of the virus inside primarily differentiated keratinocytes, which are located high in the epidermis. HPV induces the recruitment of CD4+CD25+FoxP3+ Tregs via CCL17 and CCL22 production by LHC and macrophages within the wart. Consistent with their function discussed above, these Tregs can dampen inflammation and promote viral survival. In addition, Tregs specific for HPV antigens have been found in cervical cancer patients (see Box 6).
Antibody responses to viruses like HPV help prevent initial infection or spread of infection via neutralization of binding of the virus to the host cells. Yet the availability of HPV antigens during infection is often low owing to the fact that, unlike other viruses, HPV does not induce lysis of its target cells to allow for release of virions thereby producing free antigens. Additionally, the L proteins are most immunogenic but are primarily produced in superficial keratinocytes, thereby allowing the virus to avoid detection by most LHC, which reside closer to the basement membrane. Therefore, antibody responses to HPV are often delayed and inadequate. Some LHC and other APCs are able to take up HPV antigens either through phagocytosis of cellular debris or possibly through exosomes, which are nanometer-sized structures secreted by the cell. However, HPV infection is not a highly inflammatory process, and therefore lacks stimulation of APCs to mature, secrete proinflammatory cytokines or promote effector T-cell activation. Several HPV proteins mimic host proteins and therefore can be tolerogenic. (Subversion of host immunity by HPV is nicely summarized by Tindle [2002].)
Infectious immunity: Part III. Examples of fungal and yeast immunity
Antifungal immunity relies heavily on the innate immune system, and protective immunity depends on both TH1 and TH17 responses as well as antibody production. Like S. aureus, Candida albicans colonizes much of the population, but is primarily pathogenic in immunocompromised patients, where disease incidence is 24 cases per 100,000. Treatments for Candida are antifungal drugs, such as miconazole and fluconazole, which inhibit formation of fungal cell membranes. Several antifungal vaccines have made it to phase I clinical trials, however achieving both efficacy and safety has proven difficult.
PRRs that are important for recognition of Candida include TLRs 2, 4, and 6, as well as lectin-like receptors including dectins, galectins, DC-SIGN and the mannose receptor. All of these PRRs recognize sugar and lipid components of the fungal cell wall, such as β-glucan, zymosan, and chitin. Complement receptor 3 (CR3) is important for recognizing opsonized Candida and other pathogens. TLR-9, an intracellular PRR, is important for recognition of fungal DNA, which is hypomethylated compared with mammalian DNA (recognition of Candida is summarized by Netea et al. 2008). Skin-resident macrophages and DCs use these PRRs to detect Candida, and initiate the recruitment of neutrophils and monocytes via cytokine and chemokine production. Recruited populations of neutrophils and monocytes phagocytose Candida and kill it via generation of reactive oxygen species. Extracellular killing is also induced, although the mechanism for this is not yet known.
As described above, skin-resident LHCs have been reported to initiate TH17 cell responses. Interestingly, ligation of different PRRs generates different TH profiles: ligation of the mannose receptor usually results in IFN-γ production and TH1 responses, chitin recognition and fungal DNA recognition results in IL-4 and IL-13 production and TH2 responses, and hyphae recognition by dectin 1 results in IL-17 production and TH17 responses. Treg responses can also be generated through tolerogenic DC populations and TRIF-dependent signaling downstream of some PRRs. Most of these associations have been determined using PRR knockout mouse models of Candidiasis. However, little is known about how these mixed T-cell responses ultimately influence the host’s ability to clear the pathogen, although it seems that early generation of Treg responses allows Candida to subvert host immunity. It is possible that confusing the immune system by inducing these mixed T-cell responses also dampens host immunity owing to a lack of positive-feedback loops. One possible mechanism for this differential induction of immune responses would be the morphological changes that occur when Candida transitions from yeast to hyphae, which results in differences in bioavailability of PAMPs from the cell wall. Understanding this modulation of DCs by different fungal epitopes is important for the design of effective antifungal vaccines.
Infectious Immunity: Part IV. Examples of Parasite Immunity
Immunity against parasites is mediated by a mixture of innate and adaptive immune responses that rely on IgE, granulocytes, and TH2 cells. Parasites present challenges to the immune system because of several factors, including their size and complexity, migration to different tissues within the body, and ability to subvert the host immune response. These immune evasion mechanisms have made it notoriously difficult to develop vaccines against parasites. We will discuss skin immune responses to the hookworm as an example of antiendoparasite immunity (see Box 7 for a note on ectoparasites). Hookworms are nematodes that belong to the family Ancylostomatidae, and infect approximately 20% of the world population. The larvae burrow into the skin, often through the feet that come into contact with contaminated soil or water. As they mature, they travel through the blood to the lungs and eventually the intestines, where they feed off of blood and reproduce. Hookworm eggs are secreted in feces, and embryos develop and hatch in the soil or water where they consume bacteria until they develop into the infective worm stage, L3, thus completing the life cycle (reviewed by Loukas and Prociv 2001).
BOX 7. ARTHROPOD BITES AND IMMUNITY TO VECTOR-BORNE DISEASES.
Many insects have specialized salivary proteins, which allow them to consume a blood meal without inducing coagulation, and some have developed proteins that inhibit immune responses. Research is being conducted to examine the effects of an insect bite on cytokine and chemokine production in the skin.
On entering the skin, L3 hookworm larvae shed their outer cuticle and begin expressing enzymes that permit their movement through tissues. Cuticle or sheath antigens can be taken up by APCs and presented to T and B cells. Initially following skin invasion, zoonotic species elicit inflammatory responses that cause a creeping eruption or ground itch, whereas anthropophilic species can do so silently. Antibody responses have been detected to the cuticle as well as the exsheathing fluid, and it has been suggested that this allows the worm to misdirect the immune response, similar to countermeasure decoys to distract a heat-seeking missile. Antibody responses to fluid and cuticle proteins may be used clinically as a diagnostic tool for detecting infection. Although T-cell responses seem to be weak and their exact specificities are unknown, TH2-cell responses against hookworms result in production of IL-4 and IL-13, which induce B-cell class switching to IgE. Interleukin-5 (IL-5), a major eosinophil survival and activation factor, is also produced by TH2 cells. Eosinophils are recruited to sites of infection and are major players in antiparasite immunity. They bind IgE-opsonized larvae via the FcεR, causing them to degranulate. Enzymes, and the reactive oxygen species that they release, degrade larvae. Once the larvae matures into an adult worm in the intestine, immune responses seem to become elevated most likely in response to the greater availability of worm antigens. Coinciding with this, peripheral eosinophilia is often seen in patients with hookworm infections. Tissue-resident mast cells also degranulate on encountering a larvae or worm, which may result in the recruitment of more eosinophils. Their mechanism of activation is slightly different from that of eosinophils in that they may have IgE preloaded in their FcεRs. IL-9 seems to be important for mast-cell activation and production of proteases.
In addition to misdirecting antibody responses to their cuticle, hookworms have developed several other mechanisms to subvert the host immune response. They produce a neutrophil inhibitory factor protein (NIF), which interferes with neutrophil recognition of opsonized parasites; C-type lectins that mimic those of the host, which interfere with immune recognition and coagulation; metalloproteinases and peptides that interfere with coagulation and permit feeding; cysteine proteases that cleave Igs and the low-affinity IgE receptor; and aspartic proteases, which help them digest hemoglobin but also may be involved in cleaving Igs and complement. They also produce protease inhibitors and antioxidants that help them withstand the effects of degranulation by eosinophils and mast cells. Hookworms also produce acetylcholinesterases, which are thought to both inhibit gut peristalsis and interfere with immune function.
Improper Immune Responses in Disease
Improper Immune Responses: Part I. Allergy
Allergy is an improper immune response to an otherwise innocuous antigen. The incidence of allergic disease is rising in developed countries, and treatments cost $400 million annually in the United States alone (see Box 8). Allergic contact dermatitis occurs following chemical or environmental exposure, resulting in generation of neoantigens via haptenization of self-molecules (reviewed by Kaplan et al. 2012). Haptens themselves can cause oxidative stress in keratinocytes, resulting in release of reactive oxygen species and danger signals, such as ATP. Neoantigens produced in response to hapten exposure, such as byproducts of hyaluronic acid degradation, can activate TLRs, resulting in production of proinflammatory cytokines by skin-resident immune cells. Studies in mice have indicated that recognition of neoantigens is mediated by TLR-2 and/or TLR-4 in IL-12-dependent and -independent mechanisms. It is also thought that NLRs and the inflammasome are capable of recognizing haptens and neoantigens, although many ligand-receptor partners have yet to be determined. Repeated exposure induces sensitization and subsequent type IV hypersensitivity (see Box 9). This response is characterized by an influx of T cells to the skin, resulting in tissue damage and an inflammatory eruption.
BOX 8. HYGIENE HYPOTHESIS.
The hygiene hypothesis, which was first proposed by Strachan in 1989, states that exposure to pathogens in childhood helps to develop normal, balanced immune responses. Growing up in ultraclean environments, therefore, results in the failure to appropriately modulate Th2 responses in childhood, thereby creating a predisposition to allergic immune responses.
BOX 9. HYPERSENSITIVITY REACTIONS.
Type I hypersensitivity—immediate hypersensitivity (15–30 min, but can be delayed up to 10–12 h); IgE-mediated, involves mast cells, basophils, and eosinophils (e.g., asthma).
Type II hypersensitivity—cytotoxic hypersensitivity (min-h); IgG and IgM antibody plus complement-mediated (e.g., blood group incompatibility).
Type III hypersensitivity—immune-complex hypersensitivity (3–10 h); circulating IgG complexed with antigen deposits on basement membranes (e.g., arthus reaction/serum sickness).
Type IV hypersensitivity—delayed type hypersensitivity (peaks at 48 h); memory T-cell mediated (e.g., contact dermatitis).
Tissue penetration of haptens is crucial for induction of allergic contact dermatitis. Close to 3000 compounds have been discovered that are capable of inducing contact dermatitis. Small molecular weight compounds can diffuse through the epidermis, although disruption of the barrier function of keratinocytes is thought to speed up the sensitization process (see Box 1). Examples of compounds that can elicit allergic contact dermatitis include nickel, which is the most common contact allergen and can bind directly to TLR-4 (see Box 10 and Schmidt et al. 2010), and the dust mite allergens Der p 2 and Der f 2, which are homologs of the endogenous TLR-4-binding protein MD2. Dust mites, or Dermatophagoides subspecies, consume sloughed keratinocytes, and are often found in clothing, bedding, and on the skin. Dust mites often cause allergic responses attributable to the type of immune responses they elicit: IgG and IgE responses confer immunity but also hypersensitivity reactions. Tissue-resident mast cells also play a role in skin allergy, caused by their ability to degranulate after their surface-loaded IgE is cross-linked on an allergen encounter. Whereas systemic and topical steroids are sometimes used to manage acute symptoms, the preferred treatment for contact dermatitis is avoidance of the allergen.
BOX 10. NICKEL ALLERGY.
One of the most common triggers of contact hypersensitivity is nickel, which is present in jewelry, orthopedic materials, and coins. Schmidt et al. (2010) showed that nickel is an inorganic ligand for the TLR-4–MD2 complex. On binding to histidine residues in human TLR-4, nickel can induce crosslinking of the receptor that induces a proinflammatory signal via the MyD88 adapter protein. This leads to subsequent cytokine production and elicitation of contact hypersensitivity.
Improper Immune Responses: Part II. Autoimmunity
Autoimmunity results when the immune system targets self-tissues, resulting in destruction, and, potentially, organ failure. There are checkpoints to prevent the immune system from targeting self-tissues; and, therefore, autoimmune disease is believed to require multiple hits that deactivate those checkpoints (similar to the multihit hypothesis of cancer development). These mechanisms include central tolerance through deletion of autoreactive T and B cells in the thymus (see Box 11), peripheral tolerance through the action of CD25+ Tregs (see Box 4), production of anti-inflammatory cytokines, such as IL-10 and TGF-β, and down-modulation of proinflammatory cytokine production by ligation of certain PRRs on innate and tissue-resident immune cells, such as the phosphatidyl serine receptor on macrophages, which aids in uptake of apoptotic cells in a physiologic context. Because autoimmunity is a destructive process and does not appear to be beneficial for survival, these responses likely evolved as overzealous anti-infectious or antitumor responses. For example, Japanese have a very low incidence of psoriasis compared with U.S. or European populations, whereas their risk for tuberculosis is much higher, suggesting that their genetic makeup puts them at low risk for psoriasis but high risk for tuberculosis. IFIT1 is a component of interferon signaling, and is a risk allele for type I diabetes, but the high-risk allele for diabetes is protective against coxsackie viral infection, suggesting that this allele evolved to protect from infection. Native Americans were devastated by tuberculosis infection on arrival of Europeans—the descendants of those who survived now have a very high risk for rheumatoid arthritis, suggesting that tuberculosis survivors possessed aggressive immune responses that now promote autoimmunity. These examples support the hypothesis that autoimmunity developed as a consequence of evolving potent anti-infectious responses. Therefore, we will categorize autoimmune responses accordingly. In addition to improving our understanding of pathogenesis, an advantage to thinking about autoimmunity in this way is that treatments used to interfere with autoimmunity are likely to increase the risk for infections controlled by that response.
BOX 11. APECED SYNDROME.
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome results from a genetic disruption of the transcription factor autoimmune regulator (AIRE). AIRE is expressed by specialized cells in the thymus and induces the expression of tissue-specific self-antigens from peripheral tissues to allow for negative selection of autoreactive T cells as they develop. Without AIRE, patients are unable to delete autoreactive T cells, resulting in development of multiple autoimmune diseases including autoimmune skin diseases, such as alopecia, vitiligo, nail dystrophy, and enamel hypoplasia.
In this section, we will discuss both T-cell-driven autoimmune disease and B-cell-driven autoantibody-mediated disease in the context of improper antiviral/tumor, or antibacterial/fungal immune responses.
Antibacterial-Like Autoimmunity
Psoriasis, which afflicts 2%–3% of the world population, is a highly inflammatory disease of the skin that presents with pruritus, pain, erythema, occasional pustules, and thickened scales. Histologically, it is characterized by epidermal acanthosis, vascular proliferation, and a significant inflammatory infiltrate consisting of neutrophils, T cells, DCs, and other populations. Keratinocytes produce significant levels of antimicrobial peptides, and the cytokines IL-23, IL-17, IL-6, and IL-22 appear to play a prominent role in pathogenesis. These characteristics mirror those seen in an antibacterial response and, in fact, lesions of psoriasis rarely become superinfected, unlike in atopic dermatitis. This resistance to infection is probably caused by this overzealous antibacterial-like response.
Treatment for psoriasis includes local, general immunosuppressive medications like topical steroids and calcineurin inhibitors, systemic general immunosuppressants like cyclosporine A and methotrexate, as well as newer, more targeted systemic biologic medications like TNF-α inhibitors, p40 (a component of IL-23) inhibitors, and IL-17 inhibitors. Although these treatments are likely to have improved safety profiles over more general immunosuppressants, they still have rare, but predictable, side effects based on their ability to interfere with the antibacterial response, including an increased incidence of bacterial and fungal infections. A more detailed discussion concerning psoriasis and its treatments is addressed in the literature.
Antiviral-Like Autoimmunity
Vitiligo is an autoimmune disease of the skin that results in the loss of melanocytes from the epidermis, which can be quite psychologically devastating for patients. It affects ∼0.5% of the population worldwide, without preference for race or gender, and targets all areas of the skin, with a preference for the face and genitals greater than hands and feet greater than trunk and proximal extremities. The patchy depigmentation is typically symmetrical except in the case of segmental vitiligo, in which unilateral depigmentation typically remains localized and limited by the midline. Segmental vitiligo affects ∼5% of all vitiligo patients but is responsible for a larger component of childhood vitiligo, affecting ∼20%. Patients with vitiligo are typically asymptomatic, with only ∼20% complaining of mild itching in lesional skin.
On physical exam, there is typically no erythema or scale, signs seen only in the rare inflammatory subtype. Histological examination may reveal a subtle infiltrate made up of CD4+ and CD8+ T cells in the absence of neutrophils or epidermal proliferation. Often, lesional skin contains few T cells. Because keratinocytes retain the pigment that they acquire from melanocytes as they differentiate through the layers of the epidermis until they are sloughed off, melanocyte destruction does not result in visible depigmentation until complete turnover of the overlying keratinocytes, a process that requires 14–48 d. During this time, destructive T cells likely migrate laterally through the skin to perpetuate disease, and therefore are not present once the depigmented epidermis becomes clinically visible. Thus, biopsy recommendations to “catch” an immune infiltrate include selecting perilesional skin up to 1 cm beyond the visible border, a slightly hyperpigmented region beyond the border, or better, an elliptical biopsy radiating outward from the lesion, including the border itself and up to 1 cm beyond.
Vitiligo is mediated by CTLs and IFN-γ production, reflecting a TH1 immune response within the skin. Unlike some autoimmune diseases where it has been difficult to determine which antigens the autoreactive T cells are responding to, the TCR specificities in vitiligo have been determined for a number of peptides, including MART-1/Melan-A (melanoma antigen recognized by T cells), tyrosinase (an enzyme required for melanin production), and gp100 (also known as premelanosome protein, a transmembrane protein expressed on the surface of pigment-producing cells in the skin and eye). This clinical, histological, and mechanistic picture is similar to what is seen in response to a viral infection or antitumor response, in contrast to psoriasis. Consequently, attempts to treat vitiligo using anti-TNF-α inhibitors have been disappointing, and no other systemic therapies for vitiligo are widely available. Typical treatments for vitiligo include topical steroids and calcineurin inhibitors, as well as narrow-band UVB light therapy. New approaches to treatment will likely benefit from targeting IFN-γ and other components of the TH1 inflammatory pathway.
Antibody-Mediated Autoimmunity
The skin is affected by a number of autoimmune diseases that are clearly mediated by antibodies. These diseases can be subdivided according to whether they are tissue specific, or tissue non-specific. Tissue-specific diseases are relatively straightforward, as the antibodies interfere with protein–protein binding, and the symptoms develop from a lack of protein function. Tissue-specific antibody-mediated autoimmune diseases of the skin include pemphigus vulgaris, pemphigus foliaceus, bullous pemphigoid, epidermolysis bullosa acquisita, linear IgA, and others. Pemphigus vulgaris is mediated by antibody production against desmoglein 3, which is predominantly expressed in the basal epidermis and acts as a glue to hold keratinocytes together. The result is separation of the epidermis just superior to the basal keratinocyte layer, which then accumulates fluid and forms a bulla on the surface of the skin. In contrast, pemphigus foliaceus is mediated by desmoglein 1 antibodies, forming bullae in the superficial layers of the epidermis and mucosae, because of predominant expression of desmoglein 1 at that location. Because these diseases are primarily antibody mediated, they require little contribution from immune cells during the effector phase apart from the plasma cells that produce the antibodies. As mentioned above, despite being a B-cell-driven autoimmune disease of the skin, B cells are not typically found within the skin, but reside within the lymph nodes and bone marrow, secreting antibody into the blood stream, which is then delivered passively to the skin and sterically disrupts protein–protein binding. Consequently, there is little inflammation present within lesional skin, and topical immunosuppression has limited efficacy. Treatment is aimed at systemic general immunosuppression, including prednisone, mycophenylate mofetil, azathioprine, cyclophosphamide, and/or cyclosporine, which ultimately results in decreased antibody production. Recent studies suggest that rituximab, an antibody that targets B cells but not plasma cells, may be an effective treatment, so it is not yet clear exactly how it modulates this plasma cell-driven disease.
Tissue nonspecific antibody-mediated autoimmune diseases are best characterized by the connective tissue diseases. Lupus erythematosus is probably the best understood of these diseases, yet mechanisms of pathogenesis are far from clear. Autoantibodies in lupus appear to target self-proteins that are not limited to a specific tissue, unlike the tissue-specific diseases. Common targets include DNA, RNA, and their associated proteins. It is likely that necrotic cell death initiates the inflammatory response following release of nuclear contents, a process that is prevented during programmed cell death, or apoptosis. Sun exposure may contribute to pathogenesis through damage of keratinocytes and subsequent release of nuclear contents. Antibody–protein complexes form within the vasculature and precipitate onto small vessel endothelium in specific organs, including the skin, kidney, joints, and others. Therefore, inflammation may appear in any of these organs, resulting in a wide variety in clinical presentation and symptoms, even within the same individual. Recent data suggest that nuclear components act as self-DAMPs (see above) and initiate immune responses through TLRs and NLRs within the tissues. More research will be required to understand the detailed pathogenesis of these complex systemic diseases.
Malignancy
Proper immune responses to combat malignancy are similar to antiviral responses, often involving NK cells and CTLs, which are capable of killing altered-self cells. Often, tumors down-regulate HLA I expression and avoid CTL-mediate killing, yet they then become susceptible to NK cells. They are also often stressed because of hyperproliferation, and can present altered self-peptides on HLA I that can be recognized by CTLs. For in-depth discussions of malignancies of the skin, please refer to the chapters covering melanoma and cutaneous T-cell lymphoma.
CONCLUDING REMARKS
Skin structural, stromal, and hematopoietic cells are crucial for protective immunity to a multitude of pathogens. Genetic susceptibility or subversion of the host immune response can lead to chronic, continued infections. Similarly, genetic susceptibility in addition to environmental triggers can lead to development of conditions like allergy and autoimmunity. Understanding the relationships between the cells and proteins that confer protective immunity in the skin will ultimately shed light on new potential treatments for a myriad of diseases.
ACKNOWLEDGMENTS
J.E.H. is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR061437 and research grants from the Charles H. Hood Foundation, Vitiligo Research Foundation, and Dermatology Foundation. Conflicts of interest: J.E.H. has received grant support from Combe Inc., Abbvie, and Sanofi-Genzyme.
Footnotes
Editors: Anthony E. Oro and Fiona M. Watt
Additional Perspectives on The Skin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Chen L, Guo S, Ranzer MJ, Dipietro LA 2013. Toll-like receptor 4 has an essential role in early skin wound healing. J Invest Dermatol 133: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA 2010. Skin-resident T cells: The ups and downs of on site immunity. J Invest Dermatol 130: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ 2005. Immune evasion by staphylococci. Nat Rev Microbiol 3: 948–958. [DOI] [PubMed] [Google Scholar]
- Kaplan DH 2010. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol 31: 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Igyarto BZ, Gaspari AA 2012. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Prociv P 2001. Immune responses in hookworm infections. Clin Microbiol Rev 14: 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6: 67–78. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM 2011. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 9: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, et al. 2011. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, et al. 2010. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol 11: 814–819. [DOI] [PubMed] [Google Scholar]
- Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS 2012. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin-resident regulatory T cells. Immunity 36: 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindle RW 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2: 59–65. [DOI] [PubMed] [Google Scholar]