Significance
Efforts to develop an efficacious HIV vaccine have been unsuccessful to date. Efficacy trials have reported that recombinant Ad5 (rAd5)-HIV vaccines were not efficacious and unexpectedly associated with excess HIV infection in vaccine recipients. Understanding the underlying mechanisms is urgent and will further HIV vaccine design. By comparing human CD4 T cells specific to Ad5 and CMV, we report that natural exposure- or vaccine-induced Ad5-specific CD4 T cells are highly susceptible to HIV compared with CMV-specific CD4 T cells and selectively manifest a Th17-like proinflammatory phenotype. Our findings suggest a potential mechanism for rAd5-associated excess HIV infections in vaccine recipients and highlight that testing HIV susceptibility of vaccine-generated CD4 T cells may have utility before vaccine evaluation in human trials.
Keywords: AIDS, antigen-specific CD4 T cells, viral vectors
Abstract
Efficacy trials of adenovirus 5-vectored candidate HIV vaccines [recombinant Ad5 (rAd5)-HIV] were halted for futility due to lack of vaccine efficacy and unexpected excess HIV infections in the vaccine recipients. The potential immunologic basis for these observations is unclear. We comparatively evaluated the HIV susceptibility and phenotypes of human CD4 T cells specific to Ad5 and CMV, two viruses that have been used as HIV vaccine vectors. We show that Ad5-specific CD4 T cells, either induced by natural Ad5 exposure or expanded by rAd5 vaccination, are highly susceptible to HIV in vitro and are preferentially lost in HIV-infected individuals compared with CMV-specific CD4 T cells. Further investigation demonstrated that Ad5-specific CD4 T cells selectively display a proinflammatory Th17-like phenotype and express macrophage inflammatory protein 3α and α4β7 integrin, suggestive of gut mucosa homing potential of these cells. Analysis of HIV p24 and cytokine coexpression using flow cytometry revealed preferential infection of IL-17- and IL-2-producing, Ad5-specific CD4 T cells by HIV in vitro. Our data suggest a potential mechanism explaining the excess HIV infections in vaccine recipients after rAd5-HIV vaccination and highlight the importance of testing the HIV susceptibility of vaccine-generated, vector and insert-specific CD4 T cells in future HIV vaccine studies.
Adenovirus serotype 5 (Ad5) has been used as an HIV vaccine viral vector, and the candidate HIV vaccines based on Ad5 vectors [recombinant Ad5 (rAd5)-HIV] have been intensively studied (1–5). However, development of efficacious rAd5-HIV vaccines in humans has been unsuccessful to date, despite the vaccines eliciting potent cellular immune responses. Notably, the Step trial that evaluated the Merck rAd5-HIV vaccines demonstrated no vaccine efficacy and was halted due to unexpected excess HIV infections in men who have sex with men in the vaccine arm (2, 5). The Phambili study testing the same Merck rAd5 vaccines in South Africans with heterosexual risk of HIV infection was also terminated early, and a higher HIV infection rate, albeit detected more distant from vaccination, was observed in rAd5-vaccinated individuals (3). Similarly, immunization of rhesus monkeys with replication-defective rAd5 vaccine (Merck-type) was consistent with the results of the Step trial and suggested greater risk of simian immunodeficiency virus (SIV) infection in vaccinated monkeys (6). More recently, the HVTN505 study of a different rAd5 vaccine, given with a DNA prime, was also terminated early for futility; at the time of study closure there was a nonsignificant excess of infections in the vaccine arm that seemed to diminish with time (4). The potential immunologic basis for the rAd5-associated excess HIV infection in vaccine recipients is unknown.
CD4 T cells are major cellular targets by HIV for infection in vivo. Studies focusing on early immunologic events during HIV transmission revealed that virtually all initially infected cells at mucosal sites are CD4 T cells (7). Therefore, occurrence of HIV acquisition in vaccinated individuals most likely involves early exposure and infection of vulnerable CD4 T cells by HIV. Our group and others have shown that human CD4 T cells specific to different antigens demonstrate differential susceptibilities to HIV (8, 9). Because vector-specific CD4 T cells are elicited by recombinant viral vector vaccines, it should be highly informative for future HIV vaccine design to investigate whether vector antigen-specific CD4 T cells induced by a given viral vectored HIV vaccine are particularly resistant or susceptible to HIV infection. For example, in contrast to rAd5-HIV vaccines, the rhesus CMV-based SIV vaccine did not cause increase in SIV infection and was shown to stringently control SIV in vaccinated monkeys (10).
A carboxyfluorescein succinimidyl ester (CFSE)-based proliferation assay used to model T-cell antigen specificity has been adapted to determine the susceptibility of antigen-specific CD4 T cells to HIV and the associated phenotypes (8). In this study we comparatively investigated human CD4 T cells specific to Ad5 and CMV, using cell samples from Ad5/CMV naturally exposed, HIV-uninfected and -infected subjects, as well as from a phase I HIV vaccine trial that evaluated a DNA prime rAd5 boost regimen (11). We found that human Ad5-specific CD4 T cells are substantially more susceptible to HIV in vitro and are preferentially lost in HIV-infected individuals compared with CMV-specific CD4 T cells. Flow cytometric and microarray analyses revealed remarkable phenotypic differences between Ad5 and CMV-specific CD4 T cells. Our data suggest that the greater HIV susceptibility of Ad5-specific CD4 T cells may pose a potential risk in HIV vaccine trials.
Results
Ad5-Specific T-cell Responses Are Preferentially Lost or Greatly Reduced in HIV-Infected Individuals.
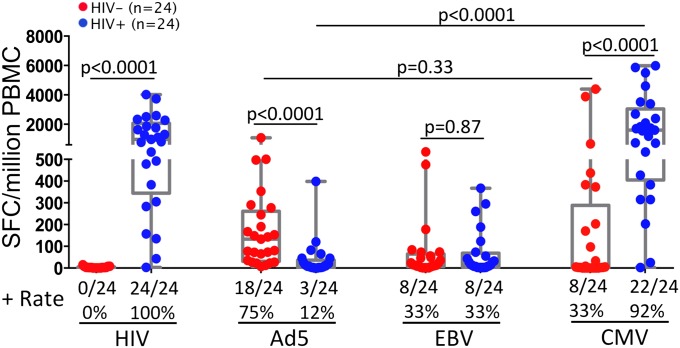
We first investigated the effect of HIV infection on Ad5- and CMV-specific T-cell immunity in vivo by measuring the magnitudes of peripheral Ad5- and CMV-specific T-cell responses in antiretroviral therapy (ART) naïve, HIV-infected individuals (n = 24) compared with HIV-uninfected volunteers (n = 24) using IFN-γ enzyme-linked immunospot (ELISPOT). Both groups of subjects were US military healthcare beneficiaries or civilians residing in the Washington, DC area (Table S1). As a control, HIV gag-specific T-cell responses were detected in 24 of 24 HIV-infected individuals (100%), and no such responses were found in HIV-uninfected volunteers (0 of 24) (Fig. 1). Of interest, Ad5 hexon-specific T-cell responses were readily detectable in the majority of HIV-uninfected volunteers (18 of 24, 75%) but were absent or greatly reduced in nearly all of the HIV-infected subjects (3 of 24, 12%) (P < 0.0001) (Fig. 1). In contrast to Ad5, the CMV-specific T-cell responses were well maintained in the HIV-infected individuals (22 of 24, 92%) at even higher magnitudes than in HIV-uninfected volunteers (P < 0.0001) (Fig. 1). As another control, the EBV-specific T-cell responses were comparable between HIV-infected (8 of 24, 33%) and HIV-uninfected (8 of 24, 33%) individuals (P = 0.87) (Fig. 1). Finally, we measured Ad5 antibody titers in sera of the HIV-infected subjects and found that greater than 71% of them were Ad5 antibody positive (Table S2), suggesting that the majority of the HIV-infected subjects had prior Ad5 exposure. Altogether, these data suggest that, compared with CMV, Ad5-specific T cells were preferentially lost in peripheral blood of untreated, HIV-infected patients.
Fig. 1.
Ad5-specific CD4 T cells are preferentially lost or greatly reduced in HIV-infected individuals. IFN-γ-ELISpot measurement of the magnitudes of HIV gag-, Ad5 hexon, EBV-LMP2, and CMV pp65-specific T-cell responses in PBMCs from HIV-uninfected (n = 24, red) and ART-naïve, HIV-infected (n = 24, blue) subjects. Results are shown as box and whisker plots showing the median and quartile spot-forming cells per 106 PBMCs. The Mann-Whitney test was used to analyze the statistical difference for the same antigen between groups, and the Wilcoxon test was used to examine the difference between antigens within the same group. Two-tailed P values were denoted.
Ad5-Specific CD4 T Cells from Ad5 Naturally Exposed Individuals Are More Susceptible to HIV than CMV-Specific CD4 T Cells.
To directly determine the susceptibilities of Ad5- and CMV-specific CD4 T cells to HIV in vitro, peripheral blood mononuclear cells (PBMCs) from the above-mentioned HIV-uninfected volunteers with positive memory CD4 responses to both Ad5 and CMV were CFSE-labeled and stimulated with Ad5 hexon or CMV pp65 peptides for 3–4 d, followed by in vitro HIV exposure for another 3 d. HIV infection of Ad5- and CMV-specific CD4 T cells in the same PBMCs was determined according to intracellular p24 expression in a CFSE-diluted (CFSE-low) CD4 T-cell population by multiparametric flow cytometry (Fig. S1A). The CFSE-low proliferating CD4 T cells were verified to be truly antigen specific on the basis of the observation that greater than 91% of these CD4 T cells expressed the same cytokine in response to the antigen restimulation (Fig. S1B). We also showed that these in vitro expanded CFSE-low, antigen-specific CD4 T cells closely resemble their in vivo phenotypes regarding memory differentiation and cytokine profile (Fig. S1C).
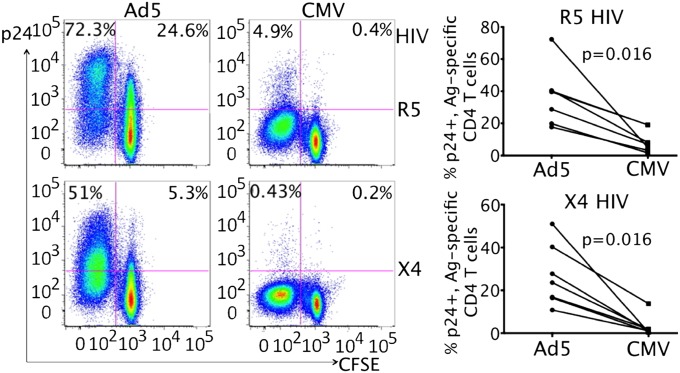
We found that Ad5-specific CD4 T cells from naturally exposed, HIV-uninfected volunteers were highly susceptible to both R5 and X4 HIV infection, whereas the matched CMV-specific CD4 T cells were resistant (Fig. 2). Representative data showed that 72.3% (R5) and 51% (X4) CFSE-low, Ad5-specific CD4 T cells expressed p24 after HIV exposure, whereas the CMV-specific CD4 T cells from the same PBMCs demonstrated much lower rates of p24 expression, with only 4.9% (R5) and 0.43% (X4) p24+ in CFSE-low CD4 T cells (Fig. 2). Cumulative results from multiple donors (n = 7) showed that the difference was statistically significant (P < 0.05 for both R5 and X4) (Fig. 2). Cell viability was monitored using an amine reactive aqua staining kit and found to be comparable between Ad5 and CMV stimulations (Fig. S2A). We examined memory phenotypes of Ad5- and CMV-specific CD4 T cells as well as the HIV infectivity within each memory subset and observed that the majority of Ad5- and CMV-specific CD4 T cells were effector memory cells (EM) (Ad5: >85%; CMV: >97%), and only a small fraction of Ad5-specific CD4 T cells demonstrate a central memory phenotype (CM) (7.7%) (Fig. S2B); no difference was found in HIV infectivity between EM and CM subsets within Ad5- or CMV-specific CD4 T cells (Fig. S2B), suggesting that the differential HIV susceptibilities seen between Ad5- and CMV-specific CD4 cells are not due to differences in CD4 T-cell memory phenotypes. The greater susceptibility of Ad5-specific CD4 T cells to HIV compared with CMV-specific CD4 T cells was also independent of CD8 T cells present in PBMCs (Fig. S3A) and was not due to higher expression of entry coreceptors (CCR5 and CXCR4) (Fig. S3B). Similar to CFSE-low, antigen-specific cells, HIV infection rates in CFSE-hi, nondividing CD4 T cells were also higher in Ad5-stimulated PBMC than in CMV-stimulated PBMC for both R5 and X4 HIV infection (R5: 24.6% vs. 0.4%; X4: 5.3% vs. 0.2%) (Fig. 2), which might be due to differential bystander activation of nonspecific CD4 T cells caused by Ad5 and CMV stimulation (Fig. S4).
Fig. 2.
Ad5-specific CD4 T cells from Ad5 naturally exposed healthy volunteers are highly susceptible to HIV in vitro compared with CMV-specific CD4 T cells. In vitro HIV infection of Ad5- and CMV-specific CD4 T cells in antigen-stimulated PBMCs of HIV- volunteers. (Left) Representative flow cytometry plots (CD3+CD8− T cells) show p24+ percentages in CFSE-low and CFSE-high CD4 T cells between Ad5 and CMV stimulation. (Right) Cumulative results (n = 7) for comparing p24+ rate in CFSE-low, CD4 T cells between Ad5 and CMV within the same individual. The Wilcoxon test was used to compare the difference. Two-tailed P values were denoted.
To understand whether such CMV-associated HIV resistance is unique to CMV or can be extended to other herpes viruses, we examined CD4 T cells specific to HSV-2, another DNA virus that is similar to CMV and has also been developed as vectors for gene delivery. The results showed that unlike CMV, the HSV-2-specific CD4 T cells are susceptible to HIV infection (Fig. S5), suggesting that closely related but different viruses may elicit qualitatively distinct T-cell responses.
Vaccine-Expanded, Ad5-Specific CD4 T Cells Are Also Highly Susceptible to HIV.
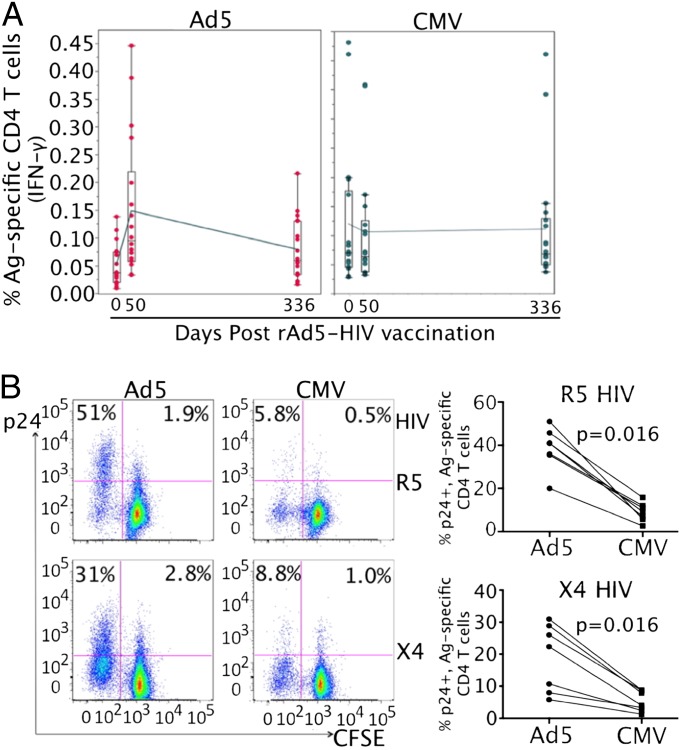
HVTN 505 was a phase II-b trial testing the efficacy of a DNA/rAd5 HIV vaccine regimen (ID: NCT00865566) (4). An earlier phase I trial evaluating a similar regimen (DNA/delayed rAd5 boost) was conducted in Uganda (ID: NCT01549509). We determined the susceptibility of vaccine-expanded, Ad5-specific CD4 T cells to HIV using PBMCs from this phase I trial. After rAd5-HIV immunization, a significant expansion of Ad5-specific CD4 T cells from baseline was measured in the majority of vaccinated individuals and remained detectable 336 d after vaccination (Fig. 3A). A stable baseline level of CMV-specific CD4 T cells was also detected in these vaccine recipients throughout the study (Fig. 3A). Accordingly, this cohort provided us with an opportunity to assess the HIV susceptibility of Ad5-specific CD4 T cells, expanded after rAd5-HIV vaccination, compared with CMV-specific CD4 T cells. Like Ad5-specific CD4 T cells from naturally exposed individuals, vaccine-expanded, rAd5-specific CD4 T cells were also substantially more susceptible to both R5 and X4 HIV, with 51% (R5) and 31% (X4) productively infected after HIV exposure compared with the matched CMV-specific CD4 T cells, where only 5.8% (R5) and 8.8% (X4) were productively infected (Fig. 3B). Evaluation of PBMCs from seven vaccine recipients showed that the difference was statistically significant (P < 0.05 for both R5 and X4) (Fig. 3B). Taken together, these results demonstrate that Ad5-specific CD4 T cells, either induced by natural exposure or expanded by vaccination, were highly susceptible to HIV infection compared with CMV-specific CD4 T cells.
Fig. 3.
HIV infection of vaccine-expanded, rAd5 vector-specific CD4 T cells compared with CMV-specific CD4 T cells from DNA/rAd5-HIV vaccinated individuals. (A) Kinetics of peripheral rAd5- (Left) and CMV-specific (Right) CD4 T-cell responses in 18 Ugandan rAd5-HIV vaccine recipients measured by intracellular IFN-γ staining. Box and whisker plots showing the median and quartile IFN-γ expression at baseline, 28 d, and 336 d after vaccination with a trend line connecting the time points at the median response are presented. (B) In vitro HIV infection of Ad5- and CMV-specific CD4 T cells in antigen-stimulated PBMCs from rAd5 vaccine recipients (n = 7). Representative flow cytometry plots (Left) and cumulative results for comparing p24+ percentages in CFSE-low CD4 T cells (n = 7) (Right) are shown. Two-tailed P values were denoted.
Ad5-Specific CD4 T Cells Selectively Manifest a Proinflammatory Th17-Like, Gut-Mucosa Homing Phenotype Compared with CMV-Specific CD4 T Cells.
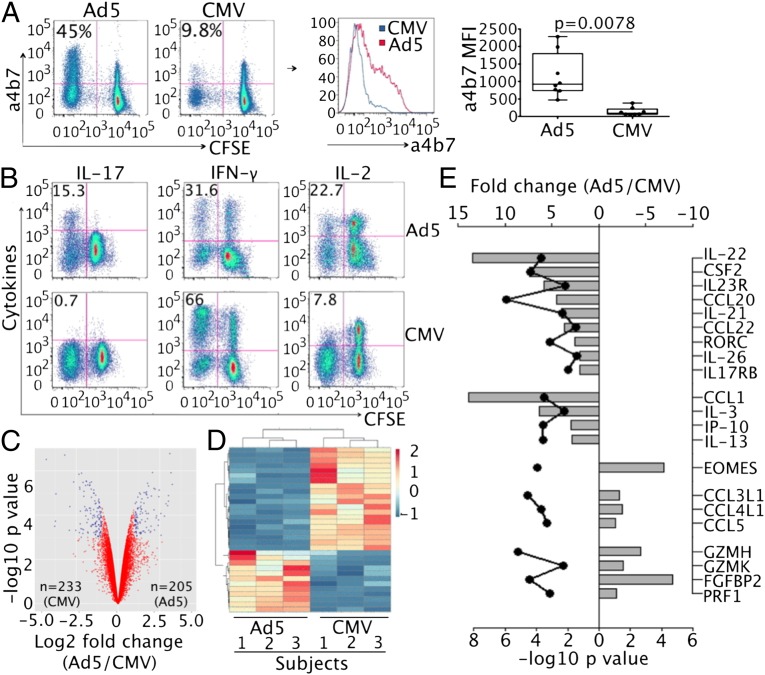
To understand the immunologic basis underlying the remarkable difference in HIV susceptibility between Ad5- and CMV-specific CD4 T cells, we next explored their phenotypes using multiparameter flow cytometry. As well as being a major site of incipient HIV infection, the gastrointestinal tract and gut-associated lymphoid tissue undergo massive CD4 T-cell depletion during acute HIV infection (12). Migration of lymphocytes to gastrointestinal tissues is dependent on expression of α4β7 integrin (13). By focusing on CFSE-low cells, we found that expression of α4β7 was significantly higher on Ad5-specific CD4 T cells (45%) than CMV-specific CD4 T cells (9.8%) in vaccine recipients [mean fluorescence intensity (MFI): 1,065 ± 194.2 vs. 179.2 ± 50.5 for Ad5 vs. CMV, respectively; n = 7, P < 0.01] (Fig. 4A). No significant difference was observed for CCR9 and CCR6 expression between Ad5- and CMV-specific CD4 T cells (Fig. S3C).
Fig. 4.
Ad5-specific CD4 T cells selectively manifest a Th17-like phenotype with gut mucosal homing potential. (A) Expression of α4β7 integrin on Ad5- and CMV-specific CD4 T cells from rAd5-vaccinated individuals. Representative flow cytometry plots comparing α4β7+ percentages (Left) or MFI (Center), and box and whisker plot (Right) comparing the median and quartile of α4β7 MFI from seven donors between Ad5 and CMV are shown. (B) Cytokine profiles of Ad5- and CMV-specific CD4 T cells. PBMCs were restimulated with PMA and ionomycin on day 6 after initial Ag-specific stimulation. Expression of IL-17A, IFN-γ, and IL-2 are shown, with number in each plot representing percentage of cytokine+ in CFSE-low CD4 T cells. (C–E) Gene expression profiling of FACS-sorted, Ad5- and CMV-specific CD4 T cells from the same PBMCs by microarray. (C) Global view of fold changes and the associated P values for genes expressed at significantly higher (n = 205) and lower (n = 233) levels, respectively, in Ad5- relative to CMV-specific CD4 T cells (P value < 0.05). (D) Heat map comparison of gene expression changes between Ad5- and CMV-specific CD4 T cells from three subjects. Fold change for higher (red) and lower (blue) expression is shown. (E) List of selected genes expressed at significantly higher or lower levels in Ad5-specific CD4 T cells compared with CMV-specific CD4 T cells. Fold change (gray bar) and P value (black dot) for each gene are shown.
We next investigated the cytokine profiles of Ad5- and CMV-specific CD4 T cells by restimulating these cells with phorbol 12-myristate 13-acetate (PMA) and ionomycin on day 6 after the initial antigen stimulation. We found that a significant fraction of CFSE-low, Ad5-specific CD4 T cells produced IL-17 (15.3%) in addition to production of IFN-γ (31.6%) and IL-2 (22.7%) (Fig. 4B). In contrast, no IL-17 was detected in the matched CMV-specific CD4 T cells from the same PBMCs, despite the ability of these cells to produce IFN-γ (66%) and low levels of IL-2 (7.8%) (Fig. 4B).
To more thoroughly understand the qualitative difference between Ad5- and CMV-specific CD4 T cells, we sorted these CFSE-low cells from the same bulk PBMCs (n = 3) and performed gene-expression profiling. PBMCs from healthy volunteers were used for microarray because much larger numbers of cells were available compared with the rAd5 vaccine recipients. Significance analysis of microarrays identified a total of 205 and 233 genes that were expressed at significantly higher and lower levels, respectively, in Ad5-specific CD4 T cells compared with CMV-specific CD4 T cells (P < 0.05) (Fig. 4C). Heat-map comparison showed distinct gene expression profiles between Ad5- and CMV-specific CD4 T cells (Fig. 4D). Specifically, in addition to production of IL-17 cytokine as shown in Fig. 4B, Ad5-specific CD4 T cells expressed significantly higher levels of mRNA for nearly all currently known Th17-associated genes, including RORC (Th17 transcription factor), IL-22, CSF2 (GM-CSF), IL-23R, CCL20 (MIP-3α), IL-21, CXCL10 (IP-10), IL-26, and IL17RB (Fig. 4E). Some non-Th17 inflammatory cytokines or chemokines also demonstrated significantly higher expression in Ad5-specific CD4 T cells, such as CCL1, IL-3, and IL-13 (Fig. 4E). Higher expression of representative Th17-associated genes (IL-17A, IL-17F, and IL-22) in activated Ad5-specific CD4 T cells was confirmed by real-time PCR (Fig. S6). In contrast, genes expressed at lower levels in Ad5-specific CD4 T cells relative to CMV-specific CD4 T cells included Th1 transcription factor EOMES, genes involved in cytolytic pathway (GZMH, GZMK, PRF1, and FGFBP2) and CCR5 ligands (CCL3L1, CCL4L1, and CCL5) (Fig. 4E). Consistent with the microarray results, flow cytometric analysis revealed that Ad5-specific CD4 T cells produced less MIP-1β (CCL4) compared with CMV-specific CD4 T cells (Fig. S7). Collectively, these data suggest that Ad5-specific CD4 T cells are qualitatively distinct from CMV-specific CD4 T cells by manifesting a proinflammatory Th17-like, gut-mucosa homing phenotype and expressing lower levels of protective β-chemokines.
HIV Preferentially Infects IL-17- and IL-2-Producing, Ad5-Specific CD4 T Cells in Vitro.
To explore the association between the above-described cellular parameters and HIV susceptibility of antigen-stimulated CD4 T cells, we established an in vitro assay in which HIV infectivity, phenotypes, and antigen specificity of CD4 T cells can be investigated altogether in a single experiment. In vitro Ad5-stimulated, HIV-exposed PBMCs were restimulated with PMA and ionomycin on day 6 after initial antigen stimulation (day 3 after HIV infection) for de novo cytokine synthesis. By overlaying p24+ cells with different cytokine-producing, CFSE-low Ad5-specific CD4 populations, we identified that HIV preferentially infects IL-17- and IL-2-producing CD4 T cells (Fig. 5): 76% of IL-17+/IFN-γ+ and 73% of IL-17+/IFN-γ− CD4 cells were p24 positive compared with 20% and 9.2% p24+ in IL-17−/IFN-γ+ and IL-17−/IFN-γ− CD4 T cells. Of IL-2 producing CD4 cells, 70% of IL-2+/IFN-γ+ and 62.5% of IL-2+/IFN-γ− were p24 positive. In contrast only 4% of IL-2−/IFN-γ+ and 4.2% of IL-2−/IFN-γ− CD4 T cells were HIV infected. The majority of p24+, IFN-γ producing CD4 T cells coexpressed either IL-17 or IL-2 (Fig. 5). This result provides in vitro evidence for preferential infection of IL-17- and IL-2-producing, Ad5 hexon-stimulated CD4 T cells by HIV and suggests a mechanism for the greater susceptibility of Ad5-specific CD4 T cells to the virus than CMV-specific CD4 T cells.
Fig. 5.
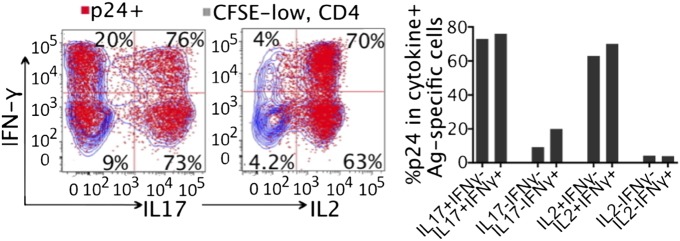
HIV preferentially infects IL-17- and IL-2-producing, Ad5-specific CD4 T cells in vitro. Antigen-stimulated, HIV-exposed PBMCs (CFSE labeled) were restimulated with PMA and ionomycin for de novo cytokine synthesis on day 6 after initial antigen stimulation. Coexpression of intracellular p24 (red) and cytokines within CFSE-low, Ad5-specific CD4 T cells (blue) was analyzed by flow cytometry. Representative plots (Left) and percentage of p24+ in each cytokine subsets (Right) are shown.
Discussion
Preferential infection of vaccine-generated CD4 T cells by HIV may undermine HIV vaccine-induced immunity and may pose a potential risk in vaccinated subjects. We here present evidence that natural exposure- or vaccine-induced Ad5-specific CD4 T cells are substantially more susceptible to HIV than CMV-specific CD4 T cells. By sorting the antigen-specific CD4 T cells from PBMCs and using cellular immunology and genomic approaches, we demonstrate that Ad5-specific CD4 T cells are qualitatively distinct from CMV-specific CD4 T cells and manifest a Th17-like proinflammatory phenotype. The findings suggest a potential mechanism for enhanced HIV infection risk in rAd5-HIV vaccine recipients in clinical trials.
The failure of rAd5-HIV vaccine efficacy trials has had significant impact on the HIV vaccine field. The mechanisms for the observed increases in HIV infection are likely complex, and a reasonable hypothesis proposes that CD4 T cells induced to the viral vector or the HIV inserts of HIV vaccine candidates may serve as susceptible targets for HIV. Despite induction of vaccine-specific CD4 T cells, excess HIV infections were not observed after gp120/alum vaccination (14) or canarypox primed, gp120 boost vaccination (15). An earlier study using Ad5 vector-pulsed dendritic cells to stimulate autologous T cells reported that in vitro Ad5 activated CD4 T cells are susceptible to HIV infection, which was defined on the basis of comparison with nonactivated resting CD4 T cells in the culture (16). This raised a concern that any CD4 T cells activated in vitro could be preferentially infected by HIV. We here directly compared HIV infection of memory CD4 T cells specific to two different vector antigens in parallel and showed that Ad5-specific CD4 T cells are highly susceptible to HIV, whereas the matched CMV-specific CD4 T cells are resistant. In addition to antigen-specific cells, we also observed higher HIV infection of nondividing CD4 T cells (CFSE-hi) by Ad5 stimulation (Fig. 2). This might be associated with the observation that Ad5 stimulation caused stronger bystander activation of CD4 T cells in PBMCs (Fig. S4), possibly as a result of proinflammatory cytokines produced after Ad5 stimulation. This may have implications for vaccine trials in which Ad5 vaccination may not only elicit Ad5-specific CD4 populations that are readily infected by HIV but may also possibly cause bystander activation of nonspecific CD4 T cells that could potentially serve HIV targets as well. Nevertheless, whether the susceptibility of different vaccine-induced CD4 T cells to HIV infection (and associated phenotypes) will be useful for screening of potential HIV vaccines or viral vectors remains unknown but can potentially be evaluated in correlates analyses using extant samples from clinical trials.
We also investigated Ad5-specific CD4 T cells from rAd5-vaccinated subjects in a phase I DNA prime, delayed rAd5-HIV vaccine trial (RV156A) and showed that vaccine-expanded, Ad5-specific CD4 T cells express substantially higher levels of mucosal homing molecules, such as α4β7 integrin and CCL-20 (MIP-3α), suggesting that Ad5-specific T cells may home to mucosal surfaces. This is consistent with the preliminary in vivo observation from HVTN 505 showing that a higher frequency of Ad5 vector-specific CD4 T cells was detected in the rectum and colon of rAd5 vaccinees compared with placebo recipients (17). However, an earlier study using experimental Ad5 infection to induce baseline Ad5 immunity before rAd5 vaccination in rhesus monkeys showed no preferential trafficking or accumulation of Ad5-specific CD4 T cells to mucosal sites in vaccinated monkeys with baseline Ad5 immunity compared with those without baseline Ad5 immunity (18). This might be related to possible differences in the biology of Ad5 infection (host range effects) and/or species-specific immune responses induced by Ad5 between human and monkeys. Further mucosal studies from vaccinated monkeys and humans are warranted to confirm whether vaccine-induced, Ad5 vector- and insert-specific CD4 T cells are preferentially localized to mucosal sites and whether they are virtually more susceptible to HIV in vivo compared with CMV-specific CD4 T cells.
Because of the high prevalence of Ad5 in humans and the effects of preexisting Ad5 immunity on reducing vaccine-specific immune responses (19), alternative adenovirus vectors such as rare human serotypes Ad26 and Ad35 have been pursued and are currently under active investigation (20). Unlike Ad5, it was shown that Ad26 vector immunization in an early-phase human trial did not induce an increase in numbers or activation status of total or vector-specific CD4 T cells in gut mucosa of vaccinated individuals (17), suggesting that the HIV susceptibility and mucosal homing phenotypes of Ad5-specific CD4 T cells may not extend to other adenovirus serotypes. Alternative Ad vectors have thus far not been evaluated in large efficacy trials. However, between 1999 and 2011 the US Army vaccinated military recruits with Ad4 and Ad7 vectors to prevent contagious respiratory diseases during training (21), and the subsequent retrospective analysis of the serologic data showed no increase in incidence of HIV infection in vaccinated individuals (17, 22). The differences between Ad5 and other Ad vectors could be explained by the major biological differences between adenovirus subgroups, such as receptor use, in vivo tropism, and distinct immune responses induced (20). Our data would argue that information with respect to the phenotypes and HIV susceptibility of vaccine- and vector-specific CD4 T cells induced by alternative adenoviruses in early phase trials is critical and should be investigated. Similarly, it would be useful to further investigate phenotypes (including in vivo localization) and SIV susceptibility of vaccine-induced CD4 T-cell populations by different vaccine vectors in monkey models where correlation analysis can be performed.
Like adenoviruses, members of the herpes virus family have also been used as HIV/SIV vaccine vectors including CMV and varicella-zoster virus (VZV) (10, 23). Of interest, unlike CMV vectors, which are associated with stringent viral control in rhesus monkeys, the VZV-vectored SIV vaccine was shown to associate with increased SIV infection and accelerated disease progression (23). These observations imply that CMV-associated HIV resistance may not be extended other herpes viruses. Indeed, examination of the HIV susceptibility of CD4 T cells specific to HSV-2, another herpes virus, showed that, unlike CMV, HSV-2-specific CD4 T cells are readily susceptible to HIV infection (Fig. S5). These results collectively indicate that immune responses elicited by closely related but different recombinant viral vectors can be qualitatively distinct and could potentially impact outcomes of vaccine efficacy.
Th17 CD4 T-cell responses are classically thought to be against extracellular bacterial and fungal infections. Preferential depletion of Th17 CD4 T cells has been found in HIV-infected individuals as well as in pathogenic SIV-infected monkeys (24). Our data showed that a significant fraction of Ad5-specific CD4 T cells, but not CMV-specific CD4 T cells, produce IL-17. This Th17-like phenotype was further confirmed by the microarray analysis revealing that nearly all known Th17-associated genes were expressed at higher levels in Ad5-specific CD4 T cells. This finding may reflect the fact that Ad5 is naturally acquired through nasopharynx or gut and can establish persistent infection in these mucosal tissues (25), which may lead to induction of CD4 responses manifesting characteristics of mucosal immunity. In a similar manner, CD4 T cells specific to HSV-2, another mucosal viral pathogen, are susceptible to HIV (Fig. S5) and interestingly manifest a phenotype typical of mucosal responses as well. These findings suggest that utilization of mucosal pathogens as vector vehicles for HIV/SIV vaccine delivery needs to be more thoroughly investigated in future studies.
β-Chemokines, including CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES), are natural CCR5 ligands that can block HIV infection of target cells at entry. We and several other groups have shown that human CD4 T cells specific to different antigens, such as HIV (26, 27), CMV (8, 28), tetanus toxoid, and Candida albicans (8), can be protected from HIV by autocrine production of β-chemokines. The microarray analysis in our study (Fig. 4 C–E) identified lower mRNA expression of β-chemokines in Ad5-specific CD4 T cells compared with CMV-specific CD4 T cells, and the data were confirmed by flow cytometric analysis of MIP-1β production (Fig. S7), suggesting that lack of sufficient production of CCR5 ligands might represent another mechanism for the greater susceptibility of Ad5-specific CD4 T cells to HIV. Mechanisms for the difference in HIV susceptibility between Ad5- and CMV-specific CD4 T cells might be multifactorial and should be further explored.
In summary, we present evidence that human CD4 T cells specific to different viral vector antigens are qualitatively distinct. More thorough investigation of HIV vaccine-generated, insert- and vector-specific CD4 T cells in terms of HIV susceptibility, phenotypes, and in vivo mucosal homing potential may be a critical parameter in the future testing of HIV/SIV vaccines.
Materials and Methods
A detailed description of materials and methods is provided in SI Materials and Methods but is briefly as follows.
Study Participants.
Three groups of human participants were included in this study: 24 ART-naïve, HIV-infected subjects, 24 HIV-uninfected healthy volunteers (Table S1), and 7 vaccine recipients from a phase I DNA/rAd5 HIV vaccine trial (ID: NCT01549509). Deidentified PBMCs and sera samples from these subjects were used.
PBMC and Sera Samples, HIV, and Antigens.
PBMCs from HIV-infected and HIV-uninfected subjects were tested for HIV-, Ad5-, EBV-, and CMV-specific T-cell responses. Sera samples from HIV-infected subjects were examined for Ad5 antibody titers. PBMCs from HIV-uninfected subjects as well as from rAd5-HIV vaccine recipients were used for in vitro HIV susceptibility assay. R5 (US1) and X4 (92/UG/029) HIV were used for infection. HIV-gag, AdV5-Hexon, EBV-LMP2, and CMV-pp65 peptide pools were used for PBMC stimulations.
IFN-γ ELISpot.
Magnitudes of HIV-, Ad5-, EBV-, and CMV-specific T-cell responses in HIV-infected and HIV-uninfected subjects were measured using a validated IFN-γ ELISpot assay, as previously reported (29).
Measurement of Serum Ad5 Antibody Titers.
Sera samples from HIV-infected subjects were evaluated to determine the relative concentration of Ad5 neutralizing antibodies at the National Institutes of Health Vaccine Research Center by previously described methods (30).
In Vitro HIV Infection and Flow Cytometric Analysis.
PBMCs (10–20 × 106) were labeled with CFSE as previously described (8). Cells were then stimulated with AdV5 Hexon or CMV pp65 peptide pools for 3–4 d, followed by exposure to R5 or X4 HIV for another 3 d. Cells were then subjected to flow cytometric analysis for intracellular HIV p24 expression. In some assays, cells were restimulated with PMA and ionomycin for de novo cytokine synthesis before flow cytometric analysis, and antibodies against cytokines (IL-2, IL-17, IFN-γ, and MIP-1β) were included as well for intracellular staining.
Cell Sorting.
CFSE-labeled and antigen-stimulated PBMCs (no HIV infection) were stained with Aqua Live/Dead Stain, followed by surface staining with antibodies (CD3, CD4, CD8, and CD14/CD19). Live, antigen-specific CD4 T cells were sorted from PBMCs according to CFSE-low, CD3+CD4+CD8−CD14−CD19− by BD FACS Aria. Cellular RNAs were extracted from the sorted cells for microarray analysis.
Microarray.
Microarray was performed using Human Gene 2.0 ST Gene-Chips (Affymetrix) according to the manufacturer’s instructions, as previously described (8). Data were analyzed using the R computing environment. Gene expression data were normalized into robust multichip average expression measures. The R package was used for gene differential expression analysis. Genes with the P values <0.05 and fold change >2 were highlighted in the Volcano plot. Genes with the P values <10−4 and fold change >2 were subjected to two-way clustering analysis.
Statistical Analysis.
Statistical analysis was performed using Prism 6.0 (GraphPad, Inc.). Mann-Whitney and Wilcoxon tests were used for statistical analyses. P values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank all the PBMC donors for their contributions to this study. The work was supported by a Robert Mapplethorpe Foundation HIV/AIDS grant (to H.H.), National Institutes of Health Grant 1R21AI110214 (to H.H.), and by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation and the US Department of Defense.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE60180).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400446111/-/DCSupplemental.
References
- 1.Shiver JW, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder SP, et al. Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray GE, et al. HVTN 503/Phambili Study Team Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11(7):507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammer SM, et al. HVTN 505 Study Team Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369(22):2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duerr A, et al. Step/HVTN 504 Study Team Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study) J Infect Dis. 2012;206(2):258–266. doi: 10.1093/infdis/jis342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi H, et al. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86(4):2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H, et al. Distinct gene-expression profiles associated with the susceptibility of pathogen-specific CD4 T cells to HIV-1 infection. Blood. 2013;121(7):1136–1144. doi: 10.1182/blood-2012-07-446278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geldmacher C, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207(13):2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eller MA, et al. Induction of HIV-specific functional immune responses by a multiclade HIV-1 DNA vaccine candidate in healthy Ugandans. Vaccine. 2007;25(45):7737–7742. doi: 10.1016/j.vaccine.2007.08.056. [DOI] [PubMed] [Google Scholar]
- 12.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 14.Flynn NM, et al. rgp120 HIV Vaccine Study Group Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191(5):654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 15.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 16.Benlahrech A, et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci USA. 2009;106(47):19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Allergy and Infectious Diseases . Mini-Summit on Adenovirus Platforms for HIV Vaccines. Bethesda, MD: National Institutes of Health; 2013. [Google Scholar]
- 18.Masek-Hammerman K, et al. Mucosal trafficking of vector-specific CD4+ T lymphocytes following vaccination of rhesus monkeys with adenovirus serotype 5. J Virol. 2010;84(19):9810–9816. doi: 10.1128/JVI.01157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frahm N, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest. 2012;122(1):359–367. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5(5):386–390. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoke CH, Jr, Snyder CE., Jr History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013;31(12):1623–1632. doi: 10.1016/j.vaccine.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP. Immunology. Immune activation with HIV vaccines. Science. 2014;344(6179):49–51. doi: 10.1126/science.1250672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staprans SI, et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci USA. 2004;101(35):13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76(21):10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur G, et al. Antigen stimulation induces HIV envelope gp120-specific CD4(+) T cells to secrete CCR5 ligands and suppress HIV infection. Virology. 2007;369(1):214–225. doi: 10.1016/j.virol.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y, Abdelwahab S, Kamin-Lewis R, DeVico AL, Lewis GK. Self-protection of individual CD4+ T cells against R5 HIV-1 infection by the synthesis of anti-viral CCR5 ligands. PLoS ONE. 2008;3(10):e3481. doi: 10.1371/journal.pone.0003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casazza JP, et al. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS Pathog. 2009;5(10):e1000646. doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Currier JR, et al. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS ONE. 2010;5(11):e13983. doi: 10.1371/journal.pone.0013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paris R, et al. Adenovirus type 4 and 7 vaccination or adenovirus type 4 respiratory infection elicits minimal cross-reactive antibody responses to nonhuman adenovirus vaccine vectors. Clin Vaccine Immunol. 2014;21(5):783–786. doi: 10.1128/CVI.00011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.