Abstract
The canonical Wnt signaling pathway (or Wnt/β-catenin pathway) plays a pivotal role in embryonic development and adult homeostasis; deregulation of the Wnt pathway contributes to the initiation and progression of human diseases including cancer. Despite its importance in human biology and disease, how regulation of the Wnt/β-catenin pathway is achieved remains largely undefined. Increasing evidence suggests that post-translational modifications (PTMs) of Wnt pathway components are essential for the activation of the Wnt/β-catenin pathway. PTMs create a highly dynamic relay system that responds to Wnt stimulation without requiring de novo protein synthesis and offer a platform for non-Wnt pathway components to be involved in the regulation of Wnt signaling, hence providing alternative opportunities for targeting the Wnt pathway. This review highlights the current status of PTM-mediated regulation of the Wnt/β-catenin pathway with a focus on factors involved in Wnt-mediated stabilization of β-catenin.
Keywords: The Wnt/β-catenin pathway, Posttranslational modification, Phosphorylaiton, Ubiquitination, Sumoylation, Acetylation, ADP-ribosylation
Introduction
Wnt proteins belong to an evolutionarily conserved family of secreted cystein-rich glycoproteins. Wnts can activate β-catenin-dependent canonical Wnt pathway and β-catenin-independent non-canonical Wnt pathways, including planar cell polarity pathway and calcium pathway [1-3]. Interdisciplinary studies in the past three decades have yielded a comprehensive understanding of Wnt molecules and their downstream effects. While signaling by Wnt proteins plays pivotal roles in a wide range of developmental and physiological processes [4-8], dysregulation of Wnt pathway is linked to many human diseases including cancers [7,9,10].
A key feature of the canonical Wnt pathway is the regulated degradation of transcription coactivator β-catenin by the β-catenin destruction complex, consisting of Glycogen Synthase Kinase 3α and 3β (GSK3α and GSK3β), Casein Kinase 1 (CK1), Adenomatous Polyposis Coli (APC), scaffold protein Axin and transcription co-factor β-catenin [11]. In the absence of Wnt, β-catenin is phosphorylated by GSK3 on serine 33 and 37 and threonine 41 (which requires priming phosphorylation by CK1) [12]. Phosphorylation triggers β-catenin recruitment of ubiquitin E3 β-TrCP (β-transducin repeats-containing proteins), causing its ubiquitination and proteasomal degradation, resulting in a low level of cytoplasmic β-catenin [13,14]. Upon Wnt stimulation, Wnt ligand forms a complex with the cell-surface receptor Frizzled (Fz) and low-density lipoprotein receptor-related protein (LRP) 5/6 [4,15], and initiates a series of molecular events ultimately causing stabilization of β-catenin by suppressing phosphorylation of β-catenin [16,17] as well as β-TrCP-mediated ubiquitination and proteasomal degradation of β-catenin [18] (summarized in Figure 1). Newly synthesized β-catenin then accumulates and enters the nucleus to interact with transcription factors TCF (T-cell factor)/LEF (lymphoid enhancing factor) to activate transcription of the Wnt target genes [18].
Figure 1.
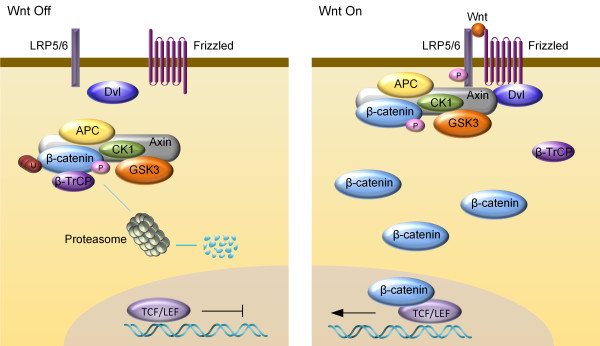
Schematic diagram of the simplified Wnt/β-catenin pathway. Left panel: in the absence of Wnt ligand, β-catenin is sequentially phosphorylated by CK1 and GSK3 in the cytoplasmic β-catenin destruction complex. Ubiquitin E3 ligase β-TrCP recognizes phosphorylated β-catenin and promotes its ubiquitination and proteasome degradation. Right panel: Wnt/β-catenin signaling is activated by the binding of Wnt ligand to Fz receptor and LRP5/6 coreceptors, resulting in the recruitment of Dvl and destruction complex to the membrane, which inactivates destruction complex, leading to stabilization of β-catenin. Accumulated β-catenin enters nucleus and activates target gene transcription.
In addition to core components of the Wnt pathway (for review, see [19,20]), non-Wnt pathway proteins also participate in the activation of Wnt signaling as regulators through modulating posttranslational modifications (PTMs) of the Wnt pathway components. By covalently adding functional groups or proteins to the target proteins, most often through enzymatic reactions, PTMs quickly change target protein’s property, relaying rapid messages in the cell, and resulting in further concerted activation of signaling cascades in response to stimuli [21]. Until now, more than 200 different types of PTM have been identified including phosphorylation, acetylation, glycosylation, methylation, ADP-ribosylation, ubiquitination and ubiquitin-like modification [22]. Besides single modifications, proteins are often modified through a combination of PTMS; different signaling pathways can be linked by PTM of shared “integrator” protein to achieve the efficient and proper cellular response. Being key mechanisms to increase proteomic diversity, PTMs are highly dynamic and largely reversible.
Most components in the Wnt/β-catenin pathway including Wnt proteins undergo one or more covalent modifications. For PTMs of Wnt proteins including glycosylation and palmitoylation, we refer the reader to two excellent reviews [23,24]. In this review, we summarize recent advances in PTM-mediated regulation of Wnt signaling with a focus on factors involved in Wnt-mediated stabilization of β-catenin and activation of β-catenin–dependent transcription (Table 1).
Table 1.
Summary of PTMs of Wnt/β-catenin pathway components
| Protein | PTM | Sites | Domains | Involved enzymes | Function | References |
|---|---|---|---|---|---|---|
| Frizzled |
Phosphorylation |
S576 (Xenopus Fz3) |
- |
- |
Reduces Fz3 activity |
[25] |
| S554/S560 (Drosophila Fz1) |
KTxxxW motif |
aPKC |
Inhibits Fz1 activity |
[26] |
||
| Ubiquitination |
- |
- |
ZNRF3/RNF43 |
Targets for degradation |
[27,28] |
|
| - |
- |
UBPY/USP8 (deubiquitinase) |
Targets for degradation |
[29] |
||
| Glycosylation |
- |
- |
- |
Important for Fz maturation |
[30] |
|
| LRP6 |
Phosphorylation |
T1479 |
Intracellular domain (ICD) |
CK1γ |
Recruits Axin and promotes Wnt/β-catenin signaling |
[31] |
| S1490 |
ICD |
GSK3/Grk5/6/MAPKs |
Recruits Axin and promotes Wnt/β-catenin signaling |
[32-35] |
||
| T1493 |
ICD |
CK1α,γ,ϵ,δ |
Recruits Axin and promotes Wnt/β-catenin signaling |
[31,32] |
||
| S1420/S1430 |
ICD |
CK1ϵ |
Suppresses LRP6-Axin interaction and β-catenin accumulation |
[36] |
||
| S1490 |
ICD |
PKA |
Essential for PT-induced β-catenin stabilization |
[37] |
||
| S1490 |
ICD |
PFTK1/Cyclin Y |
Promots Wnt/β-catenin signaling |
[38] |
||
| Palmitoylation |
C1394/C1399 |
ICD |
- |
ER exit |
[39] |
|
| Ubiquitination |
K1403 |
ICD |
- |
ER retention |
[39] |
|
| - |
- |
ZNRF3/RNF43 |
Targets for degradation |
[28] |
||
| LRP5 |
Phosphorylation |
PPPSPxS motifs |
ICD |
GSK3/CK1 |
Required for Axin binding |
[40] |
| Axin |
Phosphorylation |
S322/S326/S330/S333/T337/ S339/T341/S343 (Rat Axin) |
- |
GSK3 |
- |
[41] |
| S322/S326/S330 (Rat Axin) |
- |
GSK3 |
Increases stability |
[42] |
||
| T609/S614 (Mouse Axin) |
β-catenin binding domain |
GSK3 |
Required for Axin binding to β-catenin |
[43] |
||
| S497/S500 (Mouse Axin1 isoform 2) |
β-catenin binding domain |
GSK3/PP1cγ (phosphatase) |
Essential for Axin-β-catenin interaction |
[17] |
||
| Ubiquitination |
- (K48-linked chain) |
- |
RNF146 |
Targets for degradation |
[44,45] |
|
| K789/K821 (K29-linked chain) |
DIX domain |
Smurf1 |
Disrupts Axin interaction with LRP5/6 |
[46] |
||
| K505 (Mouse Axin1 isoform 1) |
- |
Smurf2 |
Targets for degradation |
[47] |
||
| Sumoylation |
C-terminal KVEKVD (Mouse Axin) |
DIX domain |
Likely PIAS family |
No effect on Wnt pathway |
[48] |
|
| ADP-ribosylation |
- |
- |
TNKS1/TNKS2 |
Facilitates ubiquitin E3 binding; Promotes Wnt/β-catenin signaling |
[49] |
|
| GSK3 |
Phosphorylation |
S27 (GSK3α)/S9 (GSK3β) |
- |
AKT/S6K1/RSK/PKA/PKC |
Suppresses kinase activity towards certain substrates |
[50-54] |
| Y279 (GSK3α)/Y216 (GSK3β) |
Kinase domain |
PYK2/GSK3 |
May have impact on GSK3 activity |
[55-57] |
||
| T43 (GSK3β) |
- |
ERK |
Required for phosphorylation at Ser9 which inactivates GSK3β |
[58] |
||
| T390 (GSK3β) |
- |
P38 MAPK |
Inactivates GSK3β |
[59] |
||
| Ubiquitination |
- |
- |
- |
Targets for degradation |
[60] |
|
| Sumoylation |
K292 |
Kinase domain |
- |
Critical for kinase activity, protein stability and nuclear localization |
[61] |
|
| ADP-ribosylation |
- |
- |
ARTD10 |
Inhibits activity |
[62] |
|
| APC |
Phosphorylation |
- |
- |
GSK3 |
Increases APC binding to β-catenin |
[63-65] |
| S1279/S1392 |
- |
CK1ϵ |
Essential for the regulatory activity of APC towards β-catenin |
[66] |
||
| Ubiquitination |
- |
- |
USP15 (deubiquitinase) |
Targets for degradation |
[67,68] |
|
| - (K63-linked chain) |
|
Trabid (deubiquitinase) |
- |
[69] |
||
| - (K63-linked chain) |
- |
HectD1 |
Enhances APC-Axin interaction |
[70] |
||
| Dvl |
Phosphorylation |
S139/S142 (mouse Dvl1) |
- |
CK1ϵ |
Promotes Wnt signaling |
[71] |
| - |
- |
CK1ϵ |
May enhance interaction between Dvl1 and Frat-1 |
[72] |
||
| S298/S480 (Dvl2) |
PDZ domain (S298)/DEP domain (S480) |
RIPK4 |
Essential for Wnt-induced β-catenin accumulation; promotes Dvl2 signalosome assembly |
[73] |
||
| S236 (Drosophila DSH) |
- |
CK1ϵ |
- |
[74] |
||
| - |
- |
PAR-1/CK2 |
- |
[75,76] |
||
| - |
- |
DDX3 |
May promote signalosome formation |
[77] |
||
| Ubiquitination |
K413/K444/K451/K461 (Dvl1) |
DEP domain |
USP14 (deubiquitinase) |
Suppresses Fz-Dvl interaction |
[78] |
|
| K5/K20/K34/K46/K50/K60/K69 (K63-linked chain) (Dvl1) |
DIX domain |
CYLD (deubiquitinase) |
May enhance DVL signaling activity |
[79] |
||
| - |
- |
KLHL12-Cullin-3/ITCH/NEDD4L/pVHL/Malin/NEDL1 |
Targets for degradation |
[80-85] |
||
| β-catenin |
Phosphorylation |
S45 |
- |
CK1 |
Primes phosphorylation by GSK3 |
[12] |
| S33/S37/T41 |
- |
GSK3 |
Required for β-TrCP recognition |
[12,86,87] |
||
| S675 |
- |
PKA |
Increases stability |
[88] |
||
| S552 |
Armadillo (ARM) repeats domain |
AKT |
Promotes β-catenin disassociation from cell-cell contact and accumulation in both the cytosol and nucleus |
[89] |
||
| S191/S605 |
ARM repeats domain |
JNK2 |
Critical for β-catenin nuclear localization |
[90] |
||
| T120 |
- |
PKD1 |
May suppress β-catenin transcription activity |
[91] |
||
| Ubiquitination |
K19/K49 (K48-linked chain) |
- |
β-TrCP |
Targets for degradation |
[14,92-96] |
|
| - (K11/K29-linked chain) |
- |
EDD |
Increases stability |
[97] |
||
| K394 (K63-linked chain) |
- |
Rad6B (ubiquitin conjugating enzyme) |
Increases stability |
[98,99] |
||
| - (K11/K63-linked chain) |
- |
FANCL |
May increase β-catenin expression and activity |
[100] |
||
| - |
- |
Jade-1 |
Targets for degradation |
[101] |
||
| Acetylation |
K49 |
- |
CBP |
Inhibits β-catenin ability to activate c-myc gene |
[102] |
|
| K345 |
ARM repeats domain |
P300 |
Enhances β-catenin interaction with TCF-4 |
[103] |
||
| K19/K49 |
- |
PCAF |
Increases stability |
[104] |
||
| TCF/LEF | Phosphorylation |
T155/S166 (LEF-1) |
- |
Nemo-like kinase |
Inhibits DNA binding of TCF/β-catenin complex |
[105,106] |
| T178/T189 (TCF4) | ||||||
| S154 (TCF4) |
- |
TNIK |
Required for TCF4 transcriptional activity |
[107,108] |
||
| - |
- |
GSK3/CK1ϵ |
Inhibits/enhances TCF3 interaction with β-catenin |
[109] |
||
| S42/S61 (LEF-1) |
β-catenin binding domain |
CK2 |
Enhances LEF-1 binding to β-catenin and transactivation |
[110] |
||
| S40 (murine LEF-1) |
β-catenin binding domain |
CKIδ |
Disrupts LEF-1/β-catenin complex |
[111] |
||
| S147/S149/T170/S181/ T184/S190 (Xenopus TCF3) |
- |
HIPK2 |
Promotes dissociation of TCF/LEF from promoter DNA |
[112,113] |
||
| S130/T153/S164 (mouse LEF-1) | ||||||
| Acetylation |
K25 (Drosophila TCF) |
β-catenin binding domain |
CBP |
Decreases the affinity of β-catenin to TCF |
[114] |
|
| K185/K187/K188 (POP1) |
- |
CBP/p300 |
Required for POP1 nuclear localization and biological activity |
[115] |
||
| Lys150 (TCF4E2) |
- |
CBP |
Releases inhibition by HBP1 repressor |
[116] |
||
| - |
- |
CBP/p300 |
- |
[116] |
||
| Sumoylation |
K25/K267 (Mouse LEF-1) |
β-catenin binding domain (K25) |
PIASy |
May repress LEF-1 activity by targeting LEF-1 to nuclear bodies |
[117] |
|
| K297 (TCF4) |
- |
PIASy, Axam |
Activates β-catenin-dependent transcriptional activity of TCF4 |
[118] |
||
| Ubiquitination | - | - | NARF | Targets for degradation | [119,120] |
Phosphorylation
Addition of a phosphate group to amino acid residues on serine, threonine or tyrosine residues, is one of the most important and well-studied post-translational modifications in eukaryotes. As one of the first PTMs to be described, phosphorylation plays critical roles in the regulation of many cellular processes; abnormal phosphorylation results in a variety of human diseases [121]. Many components of the Wnt/β-catenin pathway, including a G protein-coupled receptor proteins frizzled, Wnt co-receptor LRP6 (low density lipoprotein receptor-related protein-6), β-catenin destruction complex members (CK1, GSK3, Axin, APC, β-catenin) and disheveled (Dvl), are regulated by phosphorylation. Phosphorylation represents a key mechanism responsible for the tight control of β-catenin levels within normal cells and the activation of the Wnt/β-catenin pathway (Figure 2).
Figure 2.
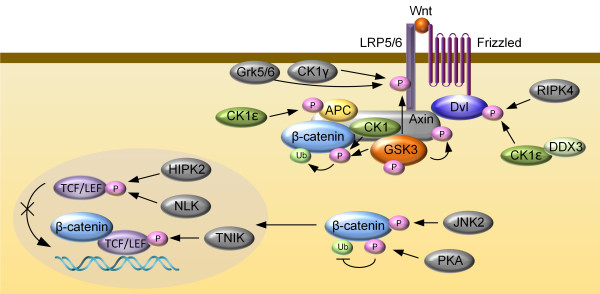
Schematic diagram of the simplified phosphorylation-mediated regulation of the core Wnt/β-catenin pathway components. Phosphorylation of LRP6 at T1479 by CK1γ and at S1490 by GSK3 and Grk5/6 promotes Wnt signaling. Dvl phosphorylation mediated by RIPK4 and CK1ϵ is essential for Wnt signaling. Phosphorylation of Axin at S497/S500 by GSK3 is suppressed by Wnt ligand, resulting in reduced association with LRP6 and β-catenin. C-terminal phosphorylation of β-catenin by PKA inhibits its ubiquitination and thus promotes β-catenin signaling activity. TNIK phosphorylates TCF4 to activate its transcriptional activity. NLK and HIPK2 phosphorylate TCF/LEF factors to inhibit their interaction with DNA.
Phosphorylation-dependent degradation of β-catenin by the β-catenin destruction complex
In the absence of Wnt, CK1α phosphorylates β-catenin at Ser45, which precedes and is required for subsequent phosphorylation of β-catenin at Ser33, Ser37 and Thr41 by GSK3 [12]. Phosphorylation of β-catenin by CK1 and GSK3 causes β-TrCP-mediated proteolysis of β-catenin, keeping the cytosolic and nuclear levels of β-catenin very low [122,123]. Upon Wnt stimulation, phosphorylation of β-catenin by GSK3 undergoes “two-phase” dynamic change: GSK3 phosphorylation of β-catenin is sharply inhibited within 30 min, phosphorylation then returns to its initial level in 2 hours [16,17] or achieve even higher level in 6 hours [17]. When normalized with respect to total β-catenin, it appears that GSK3-mediated phosphorylation of β-catenin is continuously suppressed by Wnt [12,16,17]. No significant change in CK1α-mediated phosphorylation of β-catenin is observed in 0.5-1 hour, but remarkable induction of β-catenin phosphorylation by CK1α at Ser45 is detected thereafter in different cell lines [16]. These results clearly indicate that inhibition of GSK3-medited phosphorylation of β-catenin is responsible for Wnt-induced acute stabilization of β-catenin and may contribute to Wnt-induced chronic accumulation of β-catenin. Regarding Wnt-induced long-term stabilization of β-catenin, a prior study has demonstrated that without attenuating overall GSK3-mediated β-catenin phosphorylation, Wnt abrogates β-TrCP recruitment to phosphorylated β-catenin and blocks β-catenin ubiquitination and degradation [18]. This study clearly suggests that other mechanisms are also involved in the regulation of Wnt-induced chronic stabilization of β-catenin.
Several models have been proposed to explain Wnt-mediated inhibition of β-catenin phosphorylation by GSK3: (i) Disruption of the destruction complex. Wnt induces rapid disruption of Axin/GSK3 interactions, which separates GSK3 from its substrate β-catenin, thus inhibiting β-catenin phosphorylation and causing initial stabilization of β-catenin [124]. (ii) Inhibition of GSK3 activity by LRP6. Compelling evidence indicates that Wnt-activated LRP6 can inhibit GSK3 function directly [125-128]. Results of in vitro and in vivo studies show that dually phosphorylated PPPSPxS peptides are sufficient to inhibit GSK3 kinase activity towards β-catenin and other physiological GSK3 target sites including tau and glycogen synthase [126,127]. (iii) Axin dephosphorylation. As a scaffold protein that directly interacts with other core components of the destruction complex [12,129], the scaffolding function of Axin is essential in the process of β-catenin phosphorylation by GSK3 because the interaction of GSK3β with the Axin can enhance phosphorylation of β-catenin by several orders of magnitude [130]. Axin is phosphorylated by GSK3 at Ser497/500 [17]. Upon Wnt stimulation, GSK3-mediated phosphorylation of Axin declines rapidly [17]. Dephosphorylation of Axin at Ser497/500 is carried out by PP1cγ, an isoform of PP1 catalytic subunit (PP1c) within the LRP6 signaling complex. Dephosphorylated Axin dissociates with LRP6 and β-catenin, thereby inhibiting β-catenin phosphorylation [17]. This notion is also supported by an earlier observation that phosphorylation of Axin by GSK3 increases its affinity for β-catenin [131]. Similar with this mechanism, PP1 was reported to dephosphorylates Axin at CK1-phosphorylated serine residues to reduce Axin-GSK3 interaction, contributing to β-catenin stabilization [132]. Of note, in addition to its role in β-catenin phosphorylation, phosphorylation also regulates Axin abundance: while direct phosphorylation of rat Axin on S322/S326/S330 by GSK3 stabilizes Axin [42], dephosphorylation of Axin by protein phosphatase 2C decreases the half-life of Axin [133].
GSK3β interaction with another scaffold protein APC also promotes GSK3-mediated phosphorylation of β-catenin [134]. Phosphorylation of APC by GSK3, facilitated by Axin and β-catenin and counter balanced by PP2A [63], increases APC binding affinity for β-catenin [64,65]. In addition to GSK3, APC was also reported to be phosphorylated by CK1ϵ in an Axin-dependent manner, which, in turn, confers APC’s ability to down-regulate β-catenin [66].
Propagation of Wnt signaling through LRP6 phosphorylation
The binding of Wnt ligands to the transmembrane receptors Frizzled (Fz) and co-receptor LRP5/6 initiates a signaling cascade resulting in stabilization of β-catenin and the activation of β-catenin-dependent transcription [4,135,136]. A key step in the cascade is phosphorylation of the intracellular domain (ICD) of LRP6 at five reiterated PPPSPxS motifs and adjacent Ser/Thr cluster [31-33,137]. For a detailed summary of regulation of LRP6 by phosphorylation, we refer readers to an earlier review [138]. The enzymes catalyzing LRP6 phosphorylation have been identified: PPPSPxS motifs are sequentially phosphorylated by GSK3 (e.g., at Ser1490) and CK1 (e.g., at Thr1493) [31,32], whereas the Ser/Thr cluster (e.g., at Thr1479) is phosphorylated by casein kinase 1γ (CK1γ) [31]. Wnt-induced generation of phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) at the plasma membrane is required for LRP6 phosphorylation by GSK3 and CK1γ [139]. Other key players involved in LRP6 phosphorylation have also been identified (Fz, Dvl, Axin, and PtdIns(4,5)P2) [139,140]. However, the sequence of the molecular events leading to LRP6 phosphorylation and the assembly of the LRP6 coreceptor complex remains unclear.
Different models have been proposed to depict the process: (i) Initiation and amplification of LRP6 phosphorylation [140]. In the presence of Wnt, Fz forms a complex with LRP6 and Wnt, which in turn recruits Dvl through Fz intracellular domain. Dvl directly binds to Axin [141-143], resulting in relocation of Axin and associated GSK3 to the plasma membrane to initiate LRP6 phosphorylation. The phosphorylated PPPSPxS motifs on LRP6 provide docking sites for Axin [31,137,144], leading to recruitment of additional Axin/GSK3β to form LRP6-Axin signaling complex and phosphorylate LRP6 on Ser1490 to propagate Wnt signaling [140]. (ii) LRP6 signalosome assembly [145]. Wnt induces the formation of membrane LRP6 aggregates containing Wnt pathway components, such as Fz, Dvl, Axin and GSK3 (called LRP6 signalosomes), to trigger the phosphorylation of LRP6 by GSK3 and CK1. The highly dynamic polymerization property of Dvl DIX domain, which enables Dvl self-association and co-polymerization with Axin, is important for receptor aggregation and Axin recruitment [145-147]. (iii) Wnt3a-induced PtdIns (4,5)P2 formation [139]. Upon Wnt stimulation, Fz transduces signal to Dvl, Dvl then directly interacts with and activates phosphatidylinositol-4-phosphate 5-kinase type I (PIP5KI). PIP5KI in turn induces PtdIns(4,5)P2 formation, which promotes LRP6 aggregation, LRP6 phosphorylation and Axin recruitment by unclear mechanism.
As discussed above, Dvl plays a critical role in the assembly LRP6 coreceptor complex and LRP6 phosphorylation. Dvl itself is a phosphorylation substrate: phosphorylation of Dvl can be catalyzed by RIPK4 (receptor-interacting serine/threonine-protein kinase 4), PAR-1 (Partitioning-defective 1), CK2 and CK1 [73-77]. It is known that Wnt stimulation induces Dvl phosphorylation [73,148,149], which is believed to be a critical step in Wnt signaling, however, whether Dvl phosphorylation is required for LRP6 phosphorylation or assembly of LRP6 coreceptor complex and how phosphorylation activates Dvl remain to be determined. Identification of phosphorylation sites on Dvl will help to address these questions.
The mysterious roles of GSK3 phosphorylation in Wnt signaling
Gsk3α and Gsk3β have redundant function in the Wnt/β-catenin pathway [150]. How canonical Wnt signaling regulates Gsk3 to inhibit β-catenin proteolysis remains largely elusive. The serine/threonine protein kinase GSK3 itself is a phosphoprotein, but whether and how GSK3 phosphorylation is involved in Wnt signaling remains an open question, and evidently, contradictions exist. Catalyzed by the serine/threonine protein kinase Akt or other kinases [50-54], the N-terminus of GSK3 can be phosphorylated at Ser21 on GSK3α and Ser9 on GSK3β. Structural studies indicate that the phosphorylated N-terminus competes with the priming phosphate of GSK3 substrate for the same binding sites as a “pseudosubstrate” inhibitor, resulting in GSK3 inactivation [151]. Inhibition of GSK3 activity by Ser9/Ser21phosphorylation has been well established in the insulin pathway [53,151-153]. A prior study has shown that Wnt signaling stimulates Akt, which in turn, in association with Dvl, enhances GSK3β phosphorylation at Ser9, causing increased β-catenin level [154]. Consistent with this result, overexpression of GSK3β-Ser9A (serine mutated to alanine) abolishes insulin and IGF-1 (Insulin-like growth factor-1)-induced activation of β-catenin-dependent transcription [155]. However, the observations that GSK3β-Ser9A mutant, GSK3α-Ser21A and wild type GSK3β are regulated by Wnt signaling similarly [150,156] and that the Wnt pathway is intact in GSK3α/β21A/21A/9A/9A knockin embryonic stem (ES) cells [157] appear to exclude the involvement of phosphorylation of GSK3α/β at Ser21 and Ser9 respectively in Wnt signaling.
There are several additional phosphorylation sites on GSK3 that have been reported to be associated with the regulation of β-catenin level in various biological contexts, but their role in Wnt signaling remains undetermined and elusive. Phosphorylation of GSK3β at threonine 43 by Erk (extracellular-signal-regulated kinase), primes GSK3β for phosphorylation at Ser9 by p90RSK, and mediates HBV-X protein (HBX)-induced upregulates β-catenin in human hepatocellular carcinoma cells [58]. Phosphorylation of GSK3β at threonine 390 by p38 mitogen-activated protein kinase (MAPK), which occurs primarily in the brain and thymocytes, inactivates GSK3β, leading to an accumulation of β-catenin [59]. Interestingly, Thr390 of GSK3β is not conserved in GSK3α, suggesting different regulatory mechanisms of GSK3 isoforms by phosphorylation. Consistent with the notion that p38 and phosphorylation of GSK3β at Ser9 may play a role in Wnt signaling, a prior study shows that p38 MAPK is activated upon Wnt3a stimulation and is crucial for Wnt3a-induced accumulation of β-catenin through inhibiting GSK3β a activity by inducing its phosphorylation at Ser9 [158]. Phosphorylation at tyrosine 216 in GSK3β or tyrosine 279 in GSK3α has been shown to be required for GSK3 full kinase activity using transcription factor c-Jun as an in vitro substrate [55,159]. In GSK3α/3β double knockout ES cells, expression wild type GSK3α reduces GSK3α/3β deficient-mediated elevation of β-catenin level, expression of GSK3α-Y279F (tyrosine replaced with phenylalanine) only partially reduces β-catenin level with respect to wild type level, likely due to impaired GSK3 kinase activity [156]. However, others have also shown that the C-terminal Tyr216 phosphorylation has no or minimal impact on GSK3 activity in in vitro kinase assay using myelin basic protein (MBP) or tau as substrates [160,161]. Consistent with this, it has been shown that overexpression of kinase-dead GSK3α-K148R or GSK3β–K85R remarkably enhances β-catenin-dependent transcription in the presence and absence of Wnt, whereas overexpression of GSK3α-Y279F or GSK3β–Y216F inhibits Wnt-induced activation of β-catenin-dependent transcription to a level comparable to that of WT GSK3α or GSK3β [161]. Similar to the case of kinase activity, while it has been shown that phosphorylation of GSK3β at Tyr216 impacts its binding to Axin [130,162], other evidence indicates that GSK3β with tyrosine to phenylalanine mutation at Tyr216 still retains strong binding capacity to Axin [161,163].
Activation of β-catenin-dependent gene transcription by phosphorylation of β-catenin and TCF/LEF
In contract to the N-terminus phosphorylation by CK1 and GSK3 that triggers β-catenin ubiquitination and degradation, phosphorylation of several sites on β-catenin C-terminus (e.g., Ser675 by protein kinase A, Ser552 by AKT, and Ser191/605 by JNK2) appears to stabilize β-catenin and affect its nuclear accumulation [88-90], leading to the activation of β-catenin-dependent transcription. The TCF/LEF family proteins function as transcription repressors or activators of Wnt-responsive genes by binding to different nuclear partners, Groucho and β-catenin [164-167]. Phosphorylation of TCF/LEF family by multiple kinases has been suggested to be important for the activation of the Wnt/β-catenin pathway. The Nemo-like kinase (Nlk) family of protein kinases phosphorylates human TCF4 on two threonine residues in its central domain, Thr178 and Thr189 (and the corresponding sites Thr155 and Ser166 of human LEF-1), and inhibits the DNA binding ability of the TCF/β-catenin complex [105,106]. The kinase TNIK (Traf2 and Nck-interacting kinase,) interacts directly with both TCF4 and β-catenin and phosphorylates TCF4 to activate Wnt target gene [107,108]. Phosphorylation of human LEF-1 by CK2 at Ser42 and Ser61 increases its affinity for β-catenin and enhances gene transcription [110]. Surprisingly, however, phosphorylation of murine Ser40 residue (corresponding to human Ser42) by CKIδ disrupts the β-catenin/LEF-1 complex [111]. Both GSK3 and CK1ϵ are kinases responsible for TCF3 phosphorylation [109]. Phosphorylation of TCF3 by CK1ϵ enhances, while by GSK inhibits, TCF3 binding to β-catenin [109]. Phosphorylation of multiple members of TCF family, including LEF-1, TCF3 and TCF4, is catalyzed by homeodomain-interacting protein kinase 2 (HIPK2) [112,113]. This phosphorylation causes TCF proteins dissociation from a target promoter. Notably, HIPK2-dependent phosphorylation of transcriptional repressor TCF3 is induced by Wnt8, resulting in target gene derepression and ventroposterior development [113].
Ubiquitination
Ubiquitin is an 8.5 kDa regulatory protein found in almost all tissues of eukaryotic organisms. Ubiquitination is a PTM in which an ubiquitin protein is attached to a substrate protein through an enzymatic process requiring three types of enzymes: ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s) and ubiquitin ligases (E3s) [168,169]. As an important PTM, ubiquitination is involved in the regulation of many basic cellular processes by regulating the degradation of proteins (via the proteasome and lysosome); coordinating the cellular localization of proteins; activating and inactivating proteins; and modulating protein-protein interactions [170-173]. These effects are mediated by different types of substrate ubiquitination: adding one ubiquitin molecule to one substrate lysine residue (monoubiquitination) or several lysine residues (multi-monoubiquitination); adding an ubiquitin chain on a single lysine residue on the substrate protein (polyubiquitination) [174]. Polyubiquitin chains are built by the formation of an isopeptide bond between Gly76 of one ubiquitin to the epsilon-NH2 group of one of the seven potential lysines (K6, K11, K27, K29, K33, K48 or K63) of the preceding ubiquitin [175,176]. A special polyubiquitination chain, the head-to-tail linear polyubiquitin chain, is formed by linking the N-terminal amino group of methionine on the ubiquitin conjugated with a substrate protein and the C-terminal carboxyl group of the incoming ubiquitin [177,178]. The various types of ubiquitination are linked to distinct physiological functions in cells. While lysine 48-linked chains target proteins for degradation [173]; other types of ubiquitin linkages mediates proteolytic as well as non-proteolytic functions including endocytic trafficking, lysosomal turnover and DNA repair [175,179,180]. Like phosphorylation, ubiquitin modification of Wnt pathway proteins has emerged as a key mechanism that determines the pathway activity (Figure 3).
Figure 3.
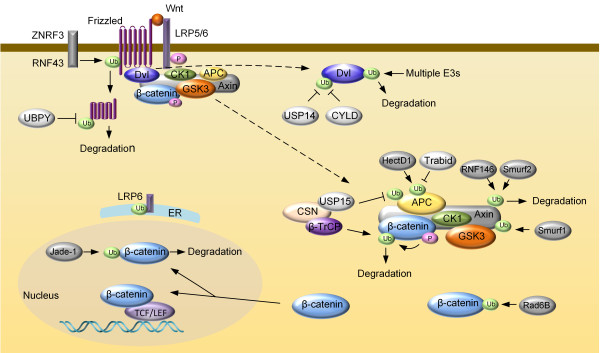
Ubiquitination-mediated regulation of the core Wnt/β-catenin pathway components. Cell-surface transmembrane ubiquitin E3 ligases ZNRF3 and RNF43 target frizzled for lysosome degradation. UBPY deubiquitinates frizzled to recycle it to the plasma membrane. Palmitolylation and monoubiquitylation regulate LRP6 exit from the endoplasmic reticulum (ER). Multiple ubiquitin E3 ligases target Dvl for degradation, thus negatively regulate Wnt signaling. CYLD and USP14 are deubiquitinases responsible for removing K63-linked polyubiquitin chain of Dvl. RNF146 and Smurf2-mediated ubuiqitination targets Axin for degradation, whereas Smurf1-mediated ubuiqitination of Axin regulates its interaction with LRP5/6. USP15 protects APC from degradative ubuiqitnation. HectD1 modifies APC with K63-linked polyubiquitin chain to promote interaction between APC and Axin. Apart from the β-TrCP-mediated degradative ubiquitination of β-catenin, ubiquitination-mediated by ubiquitin-conjugating enzyme Rad6B increases β-catenin stability. Ubiquitin ligase Jade-1, which is primarily localized in the nucleus, may regulate abundance of the nucleus pool of β-catenin.
Regulation of turnover (proteasomal and lysosomal degradation) of β-catenin, Axin, APC and Dvl by ubiquitin
Modulation of the abundance of the Wnt pathway components through ubiquitination-mediated proteasomal and lysosomal degradation plays a critical role in the regulation of Wnt signaling. A characteristic feature of the canonical Wnt pathway is tight regulation of the level of β-catenin controlled by CK1- and GSK3β-mediated phosphorylation and subsequent proteolytic degradation. In the absence of Wnt, phosphorylation of the N-terminus of β-catenin—Ser45 by CK1α, followed by GSK3-mediated phosphorylation of Ser33, Ser37 and Thr41— triggers the recruitment of the β-TrCP [12,14,86,123], the substrate-recognition subunit of a multi-protein Skp1-Cullin-F-box (SCF) RING-type E3 ligase [181]. The SCFβ-TrCP-ubiquitin ligase complex subsequently attaches K48-linked polyubiquitin chains onto lysine residues 19 and 49 at the N-terminus of β-catenin [92,93], causing its proteasomal degradation. β-TrCP recruitment to and ubiquitination of β-catenin is inhibited upon Wnt stimulation despite of phosphorylation of β-catenin by CK1 and GSK3 [18]. How this regulation is achieved remains an open question. The plant homeodomain protein (PHD) Jade-1 is also found to mediate β-catenin ubiquitination and degradation [101]. Like β-TrCP, Jade-1 directly interacts with the N-terminus of β-catenin in a phosphorylation-dependent manner. However, unlike β-TrCP which only ubiquitylates phosphorylated β-catenin, Jade-1 ubiquitylates both phosphorylated and non-phosphorylated β-catenin and therefore regulates canonical Wnt signaling in both Wnt-off and Wnt-on phases. Since Jade-1 is primarily localized in the nucleus [182,183], it may mainly regulate the nuclear pool of β-catenin, whereas β-TrCP is responsible to degrade cytoplasmic β-catenin [94]. This may explain why Jade-1 silencing cannot be completely compensated for by β-TrCP [101]. The stability of Jade-1 is dependent on the presence of a functional von Hippel-Lindau (VHL) protein [183], downregulation of Jade-1by VHL mutations is thought to be responsible for the hyperactivation of the Wnt pathway in renal cell carcinoma. In contrast to ubiquitination by β-TrCP and Jade-1, ubiquitination of β-catenin by the E2 ubiquitin conjugating enzyme Rad6B and the E3 ubiquitin ligase EDD stabilizes β-catenin [97-99]. Both Rad6B and EDD interact with β-catenin and promote its ubiquitination, leading to increased level and enhanced activity of β-catenin. EDD promotes the attachment of K29-linked and/or K11-linked polyubiquitin chains to β-catenin [97], whereas Rad6B adds K63-linked polyubiquitin chain to Lys 394 of β-catenin [98,99], suggesting that Rad6B (E2) may not couple with EDD (E3) to attach polyubiquitin chain to β-catenin. Together, it appears that different ubiquitin E2 or E3 may attach different types of ubiquitin chain to different lysine residues on β-catenin, causing context-specific functional consequences.
Axin (Axin1 and Axin2) is a scaffold protein that directly interacts with other core components of the destruction complex [129,184,185]. Being the rate-limiting factor of the destruction complex, Axin abundance is a determinant factor for the assembly of the multi-protein destruction complex [186,187], and the cellular level of Axin is tightly controlled. Wnt induces polyubiquitination-mediated proteasome degradation of Axin, an event that is believed to impair the formation of sufficient destruction complex, facilitating Wnt-induced β-catenin stabilization. A recent study has revealed that the poly-ADP-ribosylation of Axin catalyzed by poly-ADP-ribosylating enzymes tankyrase (TNKS) 1 and tankyrase 2 is a prerequisite for Axin ubiquitination [49]. Until now, two ubiquitin E3 ligases have been implicated in Axin ubiquitination and degradation: the smad ubiquitination regulatory factor 2 (Smurf2) [47] and the RNF146 RING-type ubiquitin E3 ligase [44,45]. Smurf2 directly interacts with Axin and specifically ubiquitylates lysine 505 on Axin [47]. RNF146 binds to and ubiquitinates poly-ADP-ribosylated Axin for degradation to promote Wnt signaling [44,45]. The ubiquitination-mediated degradation of Axin is counterbalanced by the ubiquitin protease USP34 [188]. Whether and how the activities of these E3 ubiquitin ligases (Smurf2 and RNF146) and the deubiquitinating enzyme USP34 are regulated upon Wnt simulation is currently unknown. Furthermore, how these ubiquitin ligases cooperate to share their responsibility for ubiquilating Axin remains to be determined. For example, do Smurf2 and RNF146 ubiquitinate Axin in different pools or different complexes? Do they couple with different E2 ubiquitin conjugating enzymes to ubiquitinate different lysine residues on Axin? Answers to these questions will help to elucidate molecular mechanism underlying Axin regulation. Nevertheless, given that small-molecule inhibitor of TNKS1 and TNKS2 exerts anti-tumor effect through downregulating Wnt signaling by function as an Axin stabilizer [49], Poly-ADP-ribosylation -dependent ubiquitination of Axin provides an alternative and promising opportunity for targeting Wnt pathway for cancer therapy.
Considered as a Wnt pathway negative regulator, the scaffold protein APC facilitates GSK3-mediated phosphorylation of β-catenin [134]. APC is also an ubiquitin substrate. Ubiquitination of APC, which targets APC for proteasome degradation, is facilitated by Axin and is suppressed by Wnt3a [67]. The responsible E3 ubiquitin ligase is currently unknown. The deubiquitinase USP15 (Ub-specific protease 15) has been implicated in the ubiquitination-mediated degradation of APC [68]. USP15 is a key component of the COP9 signalosome (CSN), which regulates the ubiquitin proteasome system (UPS) by controlling cullin-RING Ub ligases [189]. The CSN complex associates with the SCFβ-TrCP E3 complex to form a supercomplex. The CSN supercomplex regulates the balance between β-catenin and APC: while it stimulates β-catenin degradation, USP15 associated with the CSN stabilizes APC. Upon Wnt stimulation, the CSN complex dissociates from SCFβ-TrCP and the APC-Axin complexes, rendering APC susceptible for proteolysis. This model suggests that Wnt-induced degradation of APC promotes β-catenin stabilization, which is not consistent with earlier studies showing stabilization of APC upon Wnt signaling [67,190].
Dvl (three vertebrate isoforms: Dvl1, Dvl2 and Dvl3) is the decision point for downstream canonical and non-canonical Wnt signaling branches and plays a critical role in the relay of signals from the LRP6 receptor complex to downstream effectors in the Wnt/β-catenin pathway [191-194]. The level of Dvl is tightly regulated by ubiquitination-mediated degradation. Several ubiquitin ligases have been identified as negative regulator of Wnt signaling by physically interacting with Dvl to enhance its ubiquitination and subsequent degradation through proteasome or lysosome under different physiological conditions [80-84]. In a Wnt-dependent manner, the BTB-protein Kelch-like 12 (KLHL12) binds to Dvl, promoting its poly-ubiquitination and degradation and antagonizing the Wnt–β-catenin pathway in cultured cells, Xenopus and zebrafish embryos [80]. Wnt stimulation is known to hyperphosphorylates Dvl, which is required for the full activation of the Wnt pathway [71,73]. The HECT-containing Nedd4-like ubiquitin E3 ligase ITCH interacts with Dvl, the interaction requires both the PPXY motif and the DEP domain of Dvl. ITCH ubiquitinates and degrades phosphorylated Dvl and but does not appear to influence the function of nuclear Dvl in the Wnt signaling pathway [81].
NEDD4L (neural precursor cell expressed, developmentally down-regulated 4-like) is a member of the NEDD4 family ubiquitin ligases, directly binds to Dvl2 through the WW3 domain of NEDD4L and the PY motif of Dvl2, and targets Dvl2 for proteasomal degradation through K6-, K27-, and K29-linked atypical ubiquitination [82]. By promoting Dvl2 degradation, NEDD4L negatively regulates the Wnt/β-catenin pathway and antagonized Dvl2-induced axis duplication in Xenopus embryos. In a recent study autophagy was shown to negatively regulate Wnt signaling by promoting Dvl degradation through Von Hippel–Lindau protein (VHL)-mediated polyubiquitination [83]. VHL, a component of an SCF (Skp1–Cdc53–F-box)-like ubiquitin E3 ligase complex, binds to Dvl through Dvl’s DEP domain and ubiquitinates Dvl, ultimately causing Dvl degradation through the autophagy–lysosome pathway. The negative association of Dvl protein with autophagy in human colon cancer suggests a clinical relevance of this finding [83]. The RING finger domain containing ubiquitin E3 ligase Malin is also found to interact with Dvl2 and promote polyubiquitination of Dvl through K48- and K63-linked ubiquitin chains, leading to its degradation through both proteasome and autophagy [84].
The involvement of multiple ligases highlights the importance of tight control of Dvl level in cells. But how these E3 ubiquitin ligases—KLHL12, ITCH, NEDD4L, VHL and Malin—distinguish themselves from each other as an ubiquitin ligase for Dvls remains unclear. It is possible that they act on Dvl in a different format or different isoform of Dvl. For example, ITCH only ubiquitinates phosphorylated Dvl, whereas pVHL and KLHL12 ubiquitinates both phosphorylated and unphosphorylated Dvl, and KLHL12 seemed to prefer targeting Dvl3 over Dvl2 [80]. It is also possible that these ligases are regulated differently in response to stimuli including Wnt in a tissue-specific manner or within specific subcellular compartments.
Regulation of Wnt receptor LRP6 and Fz trafficking by ubiquitin
The levels of Wnt receptor LRP6 and Fz, not surprisingly, greatly impact the activation of the pathway [195,196]. The membrane level of LRP6 is largely regulated by the interplay between PTMs: palmitoylation, a covalent attachment of fatty acids to cysteine and less frequently to serine and threonine residues of proteins, and ubiquitination [39]. LRP6 is palmitoylated shortly after synthesis and remains palmitoylated, which is required for LRP6 exits from endoplasmic reticulum (ER). Without palmitoylation, LRP6 is retained in the ER due to monoubiquitination on lysine 1403. Mutation of this site leads to a full recovery of membrane targeting of palmitoylation-deficient LRP6. Notably, the responsible ubiquitin E3 ligase and deubiquitinating enzyme for LRP6 ubiquitination, and the types of ubiquitin chains have not been revealed.
Ubiquitination commonly drives cell-surface receptor internalization and lysosomal degradation [197]. The ubiquitination of Fz is mediated by cell-surface transmembrane E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3) and its homologue ring finger 43 (RNF43) [27,28]. As a negative regulator of the Wnt pathway, RNF43 interacts with FZD5, promotes its ubiquitin-mediated endocytosis, thereby negatively regulating Wnt/β-catenin signaling [27]. ZNRF3, another ubiquitin E3 ligase for Fz, forms a receptor complex with R-spondin proteins [28]. Without R-spondin, ZNRF3 ubiquitylates frizzled and promotes its degradation, therefore inhibiting Wnt signaling. When R-spondin is present, it clears out ZNRF3 from the membrane by inducing the interaction between ZNRF3 and the stem-cell marker LGR4, resulting in accumulation of frizzled and LRP6 on the plasma membrane and enhancing Wnt signaling. In contrast to ubiquitination-mediated lysosomal trafficking and degradation, de-ubiquitination controls the recycling of receptors, including frizzled receptors [29]. Fz is stabilized by UBPY/Ub-specific protease 8 (USP8)-mediated deubiquitination, which leads to led to recycling of Frizzled to the plasma membrane, thereby upregulating Wnt signaling [29].
The nonproteolytic roles of K63-linked polyubiquitination of APC and Dvl and K29-linked polyubiquitination of Axin
The most studied function of ubiquitination in the Wnt pathway relates to protein turnover. However, emerging evidence indicates that nonproteolytic function of polyubiquitination of core Wnt pathways proteins through lysine 63 plays an important role in the regulation of the pathway [69,70,79,198]. In 2008, Tran et al. reported the first direct evidence indicating a role of K63-linked ubiquitin chain during Wnt-induced transcription [69]. Trabid, a DUB enzyme, is found to preferentially binds to K63-linked ubiquitin chains with its three tandem NZF fingers (Npl4 zinc finger), and to cleaves these chains with its OTU (ovarian tumor) domain. Trabid binds to and deubiquitylates APC. Although Trabid targets APC ubiquitination and function as a positive regulator of Wnt signaling in mammalian and Drosophila cells, surprisingly, it acts below the stabilization of β-catenin. How Trabid-mediated deubiquitination of APC links to the activity of the TCF–β-catenin transcription complex remains unsolved. Two later studies shed more light on the molecular mechanisms underlying K63-linked ubiquitination of APC in Wnt signaling [70,198]. APC is modified predominantly with K63-linked ubiquitin chains when it is bound to Axin in unstimulated HEK293 cells, which requires a fully assembled APC/Axin/GSK3β/phospho-β-catenin complex [198]. Wnt3a stimulation inhibits K63-linked ubiquitination of APC in a time-dependent manner, an event coincides with the disassociation of Axin from APC and the stabilization of cytosolic β-catenin, indicating that K63-linked polyubiquitination of APC impacts the assembly and/or efficiency of the β-catenin destruction complex. This finding was further confirmed by the observation that the E3 ubiquitin ligase HectD1 modifies APC with K63-lined polyubiquitination and promotes the APC/Axin interaction to negatively regulate Wnt signaling [70]. Together, these studies have established a negative correlation between K63-linked ubiquitination of APC and activation of the Wnt pathway. Future identification of the ubiquitination site(s) in APC will enable mutational analysis and more conclusive determination of the importance of K63-lined ubiquitination of APC in Wnt signaling and other APC-regulated cellular processes.
Both positive and negative roles of K63-lined ubiquitination of Dvl in the Wnt regulation have been reported [78,79]. A prior study shows that the N-terminus of Dvl1 (K5, 20, 34, 46, 50, 60 and 69 on the DIX domain) is modified by K63-linked polyubiquitination, which requires DIX-domain- mediated polymerization of Dvl [79]. The deubiquitinating enzyme CYLD binds to and deubiquinates Dvl, inhibiting the signaling activity of Dvl and the activation of the Wnt pathway. CYLD is a familial cylindromatosis tumor suppressor gene, mutations in the CYLD gene cause human familial cylindromatosis [199]. The finding that hyperactive Wnt signaling in human cylindroma skin tumors arises from mutations in CYLD validates the clinical significance of CYLD-mediated deubiquitination of Dvl [79]. In contrast to the positive role of K63-linked ubiquitination of Dvl in Wnt signaling [79], a recent study shows that K63-linked ubiquitination of Dvl on four lysines at the C-terminus within the DEP domain (K413, 444, 451 and 461) plays a negative role in the regulation of Wnt signaling [78]. Usp14, identified as a deubiquitinase for Dvl, transiently interacts with Usp14 upon Wnt stimulation, disassembles K63-linked polyubiquitin chains attached to Dvl, an event that is required for Wnt signaling. Tissue microarray analysis of colon cancer has revealed a strong correlation between the levels of Usp14 and β-catenin, providing further support for the negative of K63-linked ubiquitination of Dvl in Wnt signaling. Together, these two studies suggest that ubiquitination of Dvl on lysines clustered in different domains (i.e., the N-terminal DIX domain vs. the C-terminal DEP domain) through K63-lined ubiquitin chains involves different deubiquitinase and plays seemingly contradictory roles (positive and negative) in Wnt signaling. Since both studies used HEK293 cells as a cellular model for most of the mechanistic experiments [78,79], the different outcome of Dvl ubiquitination appears not to result from cell-type-specific or cellular context-specific effects. The contradictory roles of K63-linked ubiquitination of Dvl add further complexity to the understanding of the molecular mechanisms underlying the still perplexing role of Dvl in Wnt signaling.
Ubiquitination of Axin by ubiquitin ligases Smurf2 and RNF146 mediates Axin degradation [44,45,47]. Smurf1 is recently identified as an additional ubiquitin E3 ligase for Axin ubiquitination [46]. Unexpectedly, smurf1-mediated Axin polyubiquitination at Lys789/821 with K29-linked polyubiquitin chain does not cause its degradation, but instead disrupts its association with LRP5/6, resulting in suppression of LRP6 phosphorylation at Ser1490. Consistent with its negative role in Wnt signaling, Axin ubiquitination with K29-linked polyubiquitin chain is significantly suppressed by Wnt3a stimulation.
Sumoylation
In addition to ubiquitin, there is a growing family of ubiquitin-like proteins (UBLs) that modify cellular targets in a pathway parallel to, but distinct from, that of ubiquitin. SUMO (Small Ubiquitin-like Modifier) proteins are a family of small proteins that are around 100 amino acids in length and 12 kDa in mass. Mammalian SUMO has four isoforms: SUMO1, SUMO2, SUMO3, and SUMO4. SUMO2 and SUMO3 share 95% sequence homology and are distinct from SUMO-1(often collectively referred to as SUMO2/3). Sumoylation refers to the process that small ubiquitin-related modifier (SUMO) is covalently attached to the target proteins through sequential enzymatic reactions involving the activity of SUMO activating enzyme (E1), SUMO conjugating enzyme (E2) and SUMO ligase (E3) [200,201]. Sumoylation is a reversible process. Removal of SUMO from its target proteins is carried out by members of SUMO-specific proteases (SENP) family [202]. SUMO was identified as a post-translational modifier almost two decades ago [203], and our knowledge of sumoylation has been greatly expanded since then. We now know that at any given time, only a small portion of a particular substrate is modified by SUMO, but the very small amount of sumoylated proteins play an important role in the regulation of diverse signaling pathways and is critical to maintain normal cell function [204,205].
The identification of Axam (Axin Associating Molecule) as a novel Axin-binding protein and a negative regulator of Wnt/β-catenin pathway provides the first evidence that sumoylation is involved in the regulation of Wnt signaling [206]. Axam belongs to the SENP family, and downregulation of β-catenin by Axam requires its DeSUMOylation activity [207]. Later, sumoylation of LEF1 is found to negatively regulate LEF1 transcriptional activity by sequestration into nuclear bodies [117]. The protein inhibitor of activated gamma (PIASy) is identified as a LEF1 SUMO E3 ligase [117]. In contrast, PIASy-dependent sumoylation of TCF4, another member of TCF/LEF family, appears to activate β-catenin-dependent transcriptional activity of TCF4, whereas Axam has opposing effect [118].
Sumoylation of the scaffold protein Axin at its C-terminal six amino acids stretch (C6 motif), a motif that is critical for Axin interaction with three SUMO-1 E3s, PIAS1, PIASxβ and PIASy, prevents its polyubiquitination, thus increasing its stability [48,208]. Given the fact that Axin is the concentration-limiting component in the β-catenin destruction complex [49,186], it is reasonable to expect that sumoylation of Axin is involved in the regulation of Wnt signaling by controlling Axin steady-state level. Surprisingly, it appears not to be the case because wt Axin and Axin sumoylation-defective mutants destabilize β-catenin and inhibit LEF1 transcriptional activity at similar level [48]. GSK3β is found to be sumoylated at lysine 292 [61]. Mutation of the lysine 292 inhibits GSK3β activity toward tau and reduces GSK3β stability. However, whether sumoylation of K292 on GSK3β plays a role in Wnt signaling remains unexplored. Transducin β-like protein 1 (TBL1) and TBL1-related protein (TBLR1) function as transcriptional coactivators in canonical Wnt pathway by recruiting β-catenin to the Wnt target gene promoters [209]. A prior study indicates that Wnt-dependent sumoylation of TBL1 and TBLR1 releases them from SMRT/N-CoR corepressor complex and enhances the formation of the TBLR1-TBL1-β-catenin complex and their recruitment to Wnt target gene promoters [210]. Sumoylation or ubiquitination fusion protein methodology is a powerful complementary strategy for mutant approach that is used to discriminate between the consequence of lacking substrate sumoylation and conformational change-related artifacts [211-215]. The observation that fusion SUMO1 to sumoylation mutants of TBL1 and TBLR1 restores the activity of TBL1 and TBLR1 thus validates the functional role of sumoylation in the regulation of TBL1 and TBLR1and Wnt target genes [210].
Acetylation
Protein acetylation is a process that an acetyl group is transferred to the ϵ-amino group of an lysine residue of target protein [216]. Acetylation is catalyzed by acetyltransferase and the reverse process known as deacetylation is catalyzed by deacetylase [217,218]. Acetylation is well known to occur on N-terminal tail of histone protein to weaken DNA-histone interaction by neutralizing its positive charge, thereby activate gene expression [219-221]. Site-specific acetylation of a growing number of non-histone proteins involved in the regulation of diverse cell functions has been shown to regulate their activity, localization, specific interactions, and stability/degradation [222,223]. Using high-resolution mass spectrometry approach, an earlier study has identified 3600 lysine acetylation sites on 1750 proteins, suggesting that this modification is one of the most abundant chemical modifications in nature [224]. Nevertheless, to date, only a few components of the Wnt/β-catenin pathway including β-catenin and TCF are found regulated by acetylation. β-catenin is acetylated by the CREB-binding protein (CBP) acetyltransferase at lysine 49, a lysine site that is frequently found mutated in cancer [102]. Mutation of K49 activates transcription of Wnt pathway target in a promoter-specific fashion, implying a negative role of acetylation of β-catenin at K49 in Wnt signaling. In contrast, a later study shows that the transcriptional coactivator p300 upregulates β-catenin-dependent gene transcription by acetylating β-catenin at lysine 345, which increases the affinity of β-catenin for Tcf4 [103]. Consistent with this, a more recent study indicates that acetylation of β-catenin at lysine 19 and 49 by p300/CBP-associated factor (PCAF) stabilizes β-catenin, induces β-catenin nuclear translocation and increases its transcriptional activity, thereby upregulating Wnt signaling [104]. The positive role of β-catenin acetylation is further supported by a study showing that the NAD-dependent deacetylase sirt1 deacetylates β-catenin, leading to inhibition of β-catenin transcriptional activity and cell proliferation [225]. Identification of sirt1 deacetylation sites on β-catenin will help to elucidate the underlying mechanism by which acetylation of particular sites on β-catenin regulates Wnt signaling. Nonetheless, the observation that sirt1expression inversely correlates with nuclear β-catenin in human colon tumor specimens suggest the importance of balanced acetylation status of β-catenin in human disease.
Members of the TCF/LEF family are also substrates of acetylation [114-116]. It has been shown that drosophila CREB-binding protein (dCBP) binds to dTCF, acetylates lysine 25 in the Armadillo (β-catenin in drosophila)-binding domain of dTCF, which in turn lowers the affinity of Armadillo binding to dTCF, thereby repressing TCF [114]. In contrast, acetylation of the Caenorhabditis elegans LEF/TCF homolog POP-1 by CBP/p300 at lysines 185, 187 and 188 is required for POP-1 nuclear localization and biological activity during C. elegans embryogenesis [115], thus validating the physiological relevance of acetylation of POP-1, though POP-1 is considered to participate in the non-canonical Wnt pathway. The positive role of TCF acetylation in Wnt signaling is further supported by a recent study showing that human TCF4E2 can be acetylated at lysine 150 by CBP, leading to relief of transcriptional repression by transcription repressor presumably by inducing conformational change of TCF::DNA complex [116].
ADP-ribosylation
ADP-ribosylation, which refers to the enzymatic transfer of one or more ADP-ribose from NAD + to the acceptor proteins [226], has been recognized as an important regulator in a wide range of biological processes, including DNA damage responses, transcriptional regulation, cell death as well as energy metabolism [227,228]. The importance of ADP-ribosylation in Wnt pathway is illustrated by the study in which TNKS1 and TNKS2 are identified to interact with Axin through its tankyrase-binding domain (a small amino-terminal region of axin 1, amino acids 19–30) and catalyze its poly-ADP-ribosylation, which in turn facilitates poly-ubiquitination and subsequent proteasome degradation of Axin [49]. Targeting TNKS by small molecule inhibitors including XAV939 [49] and WIKI4 [229] inhibits Wnt signaling and suppresses the malignant phenotypes including anchorage-independent growth in colorectal cancer cells, suggesting that TNKS is a potential target for treatment of Wnt-dependent cancers. More recently, in a screen to identify targets of ADP-ribosyltransferases ARTD10 and ARTD8, GSK3β is found to be modified by mono-ADP-ribosylation [62]. Further analysis indicates that this modification inhibits GSK3β activity in vitro. It is of great interest to understand the role of ADP-ribosylation of GSK3β in Wnt signaling in the future.
Cross-talk between posttranslational modifications
PTMs often interplay to work in concert [21]. The interconnected modifications create multiple layer of regulation to fine tune the function of target protein and to determine the functional read-out. Crosstalk between PTMs has been classified as positive type or negative type [230]. Examples of positive crosstalk include priming phosphorylation, phosphorylation-dependent ubiquitination or sumoylation, and sumoylation-dependent ubiquitination. In these events, one PTM is believed to promote the addition of the second PTM through creating a binding site or recognition motif for the protein that is essential for the second PTM. The well-known example of negative crosstalk between PTMs is the competitive modification of different modifiers on a single residue.
Although crosstalk between PTMs has emerging as an important regulatory mechanism in signal transduction [21,231-233], most studies on the regulation of Wnt pathway by PTMs have focused on signal modifications. The best studied PTM crosstalk in Wnt/β-catenin pathway is the positive and negative interplay between PTMs on β-catenin: the sequential phosphorylation and ubiquitination of β-catenin [12,14,123] and competition between acetylation and ubiquitination of overlapping lysine residues on β-catenin [104]. In the case of positive interplay, β-catenin is phosphorylated at Ser45 by CK1α, which primes β-catenin for subsequent phosphorylation at Ser33, Ser37 and Thr41 by GSK3 and then triggers the recruitment of β-TrCP to β-catenin, resulting in poly-ubiquitination and degradation of β-catenin [12,14,123]. Lysines 19 and 49 on β-catenin are β-TrCP-dependent ubiquitination sites [93]. In the negative interplay case, acetylation of β-catenin at K19 and 49 by PCAF blocks β-catenin ubiquitination thereby stabilizing β-catenin [104]. How these three modifications are coordinated to control β-catenin protein level and the activation of the Wnt pathway in biological and pathological processes remains to be determined.
Another example of PTM crosstalk in the Wnt/β-catenin pathway is the poly-ADP-ribosylation-mediated polyubiquitination of Axin [49]. A recent study combining crystallographic and biochemical analysis sheds light on the mechanism of interplay between the two PTMs of Axin [234]. RNF146, The ubiquitin E3 ligase for Axin, contains a WWE domain and a RING domain, and is the only known E3 ubiquitin ligase to date that requires poly-ADP-ribosylation of the substrate for subsequent polyubiquitination [44,45,234,235]. The WWE domain of RNF146 specifically binds to iso-ADP-ribose (iso-ADPR), the smallest internal PAR structural unit containing the characteristic ribose–ribose glycosidic bond formed during poly-ADP-ribosylation rather than mono (ADP-ribose), leading to ubiquitination of poly-ADP-ribosylated Axin [234]. Several residues in RNF146 WWE domain are identified to be critical for iso-ADPR binding; sequence alignment further indicates these residues are conserved among WWE domains. Given that many ubiquitin E3 ligases contain WWE domain, it highly possible that poly-ADP-ribosylation is a general mechanisms to target protein for ubiquitination [234].
Conclusions
Biological and clinical study over the past few years has greatly expanded our understanding of the complex Wnt signaling network, but there are still many fundamental aspects of the Wnt-related biology to be discovered and understood. Characterization, dynamic detection and quantification of chemical modifications of the pathway components are essential for us to gain deeper insight into biological control of the pathway. Although identification of PTMs involved in the Wnt/β-catenin pathway and validation of their function are far from completion—in fact a large number of PTMs are not all validated as physiological relevant, the concept that relaying Wnt signal requires dynamic changes in PTM states of the pathway components has been emerged. We now know that instead of relying solely on one particular modification, the Wnt pathway is controlled by the coordinated actions of phosphorylation, ubiquitination and other PTMs. However, little is known as to how PTM coordination is effectively achieved at the molecular and cellular level. For example, systematic analysis of PTM changes with respect to space and time remains an important future goal for PTM research in the Wnt field, and how signaling pathways or upstream enzymes that catalyze these PTMs interact combinatorially, hierarchically or reciprocally to ensure the sequence of the occurrence of the PTMs and kinetics of their durations during both “Wnt on and Wnt off” situations remains largely unexplored.
As the importance of PTMs has increasingly been appreciated, there is an increasing need for improved technologies that enable researchers to measure the state and function of PTMs in physiological situations. Characterizing the function of PTM is technically challenging due to the dynamic and reversible nature of PTM, and the complicated interplays between PTMs. A research trend in the field of PTM is the mouse knockin technology. Knockin methodology, coupled with gene knockouts and specific pharmacological inhibitors, is a powerful approach to dissect the physiological roles of individual modification at given sties on a given protein. However, it is an imperfect model because it knocks the protein permanently in one form—the protein with mimicked modification or the protein lacking modification, therefore it cannot recapitulate the dynamic and reversible nature of the PTMs.
Aberrant activation of Wnt/beta-catenin pathway contributes to development of different human cancers, especially colorectal cancers. As pointed by Nusse and Varmus [236], “among the most significant challenges in future research in the Wnt field is the identification of effective and specific Wnt pathway inhibitors for use in cancer and other diseases”. Unfortunately, Wnt signaling pathway is difficult to target [237,238]. The success of tankyrases inhibitors –XAV939 [49] and WIKI4 [229]— as Wnt pathway inhibitors by inhibiting poly-ADP-ribosylation of Axin suggests that modulating PTMs of the Wnt pathway components represents a promising alternative approach for targeting the Wnt pathway. To this end, we believe that a better understanding of the regulation of the Wnt/β-catenin pathway by PTMs could have far-reaching implications for identifying novel approaches for targeting Wnt signaling.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CG, GX, and JH wrote the review. All authors read and approved the final manuscript.
Contributor Information
Chenxi Gao, Email: gaoc@upmc.edu.
Gutian Xiao, Email: xiaog2@upmc.edu.
Jing Hu, Email: huj3@upmc.edu.
Acknowledgment
This work was supported in part by USPHS grant CA166197 awarded to Jing Hu.
References
- Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin. 2011;43(10):745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1(2):a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20(1):781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87(2):97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(1):a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391(6666):493–496. doi: 10.1038/35154. [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96(11):6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131(20):5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Hernández AR, Klein AM, Kirschner MW. Kinetic responses of β-catenin specify the sites of Wnt control. Science. 2012;338(6112):1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Abreu JG, Peng L, He X. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340(6134):867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Vivian SW, Ng Ser S, Boersema Paul J, Low Teck Y, Karthaus Wouter R, Gerlach Jan P, Mohammed S, Heck Albert JR, Maurice Madelon M, Mahmoudi T, Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14(1):59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science. 2000;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17(6):666–672. doi: 10.1038/nsmb.1842. [DOI] [PubMed] [Google Scholar]
- Jensen ON. Interpreting the protein language using proteomics. Nat Rev Mol Cell Biol. 2006;7(6):391–403. doi: 10.1038/nrm1939. [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4(9):a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J, Xu HE, Williams BO. Lipid modification in Wnt structure and function. Curr Opin Lipidol. 2013;24(2):129–133. doi: 10.1097/MOL.0b013e32835df2bf. [DOI] [PubMed] [Google Scholar]
- Yanfeng WA, Tan C, Fagan RJ, Klein PS. Phosphorylation of frizzled-3. J Biol Chem. 2006;281(17):11603–11609. doi: 10.1074/jbc.M600713200. [DOI] [PubMed] [Google Scholar]
- Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila Eye. Cell. 2005;121(4):621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJR, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488(7413):665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Mukai A, Yamamoto-Hino M, Awano W, Watanabe W, Komada M, Goto S. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J. 2010;29(13):2114–2125. doi: 10.1038/emboj.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120(2):223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438(7069):867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Yokota C, Tamai K, Zeng X, He X. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J Biol Chem. 2008;283(23):16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Philipp M, Wang J, Premont RT, Garrison TR, Caron MG, Lefkowitz RJ, Chen W. G protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J Biol Chem. 2009;284(50):35040–35048. doi: 10.1074/jbc.M109.047456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Červenka I, Wolf J, Mašek J, Krejci P, Wilcox WR, Kozubík A, Schulte G, Gutkind JS, Bryja V. Mitogen-activated protein kinases promote WNT/β-catenin signaling via phosphorylation of LRP6. Mol Cell Biol. 2011;31(1):179–189. doi: 10.1128/MCB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek W, Kang H, Garcia BA, Shabanowitz J, Coombs GS, Hunt DF, Virshup DM. Negative regulation of LRP6 function by casein kinase Iϵ phosphorylation. J Biol Chem. 2006;281(18):12233–12241. doi: 10.1074/jbc.M510580200. [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22(21):2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang YL, Su Y, Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M, Niehrs C. Cell cycle control of Wnt receptor activation. Dev Cell. 2009;17(6):788–799. doi: 10.1016/j.devcel.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105(14):5384–5389. doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Semenov MV, Huang H, He X. Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PLoS One. 2011;6(8):e23537. doi: 10.1371/journal.pone.0023537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17(5):1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem. 1999;274(16):10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Jho E, Lomvardas S, Costantini F. A GSK3β phosphorylation site in axin modulates interaction with β-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266(1):28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Mickanin C, Feng Y, Charlat O, Michaud GA, Schirle M, Shi X, Hild M, Bauer A, Myer VE, Finan PM, Porter JA, Huang SM, Cong F. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 2011;13(5):623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- Callow MG, Tran H, Phu L, Lau T, Lee J, Sandoval WN, Liu PS, Bheddah S, Tao J, Lill JR, Hongo JA, Davis D, Kirkpatrick DS, Polakis P, Costa M. Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One. 2011;6(7):e22595. doi: 10.1371/journal.pone.0022595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Li Z, Li C, Chen Y, Chen Z, He X, Mao L, Wang X, Zeng R, Li L. Smurf1-mediated Lys29-linked nonproteolytic polyubiquitination of Axin negatively regulates Wnt/β-catenin signaling. Mol Cell Biol. 2013;33(20):4095–4105. doi: 10.1128/MCB.00418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jho EH. The Protein stability of Axin, a negative regulator of Wnt signaling, is regulated by Smad ubiquitination regulatory factor 2 (Smurf2) J Biol Chem. 2010;285(47):36420–36426. doi: 10.1074/jbc.M110.137471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H-L, Fan E, Zhou H-M, Xu Z, Zhang Y, Lin S-C. SUMO-1 modification of the C-terminal KVEKVD of Axin is required for JNK activation but has no effect on Wnt signaling. J Biol Chem. 2002;277(45):42981–42986. doi: 10.1074/jbc.M208099200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97(22):11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou LM, Tian PY, Lin HY, Jiang YP, Lin RZ. Dual regulation of glycogen synthase kinase-3β by the α1A-adrenergic receptor. J Biol Chem. 2001;276(44):40910–40916. doi: 10.1074/jbc.M103480200. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24(2):185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol Biol Cell. 2006;17(4):1834–1844. doi: 10.1091/mbc.E05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004;377(Pt 1):249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol Cell. 2005;19(2):159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science. 2008;320(5876):667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failor KL, Desyatnikov Y, Finger LA, Firestone GL. Glucocorticoid-induced degradation of glycogen synthase kinase-3 protein is triggered by serum- and glucocorticoid-induced protein kinase and Akt signaling and controls β-catenin dynamics and tight junction formation in mammary epithelial tumor cells. Mol Endocrinol. 2007;21(10):2403–2415. doi: 10.1210/me.2007-0143. [DOI] [PubMed] [Google Scholar]
- Eun Jeoung L, Sung Hee H, Jaesun C, Sung Hwa S, Kwang Hum Y, Min Kyoung K, Tae Yoon P, Sang Sun K. Regulation of glycogen synthase kinase 3beta functions by modification of the small ubiquitin-like modifier. Open Biochem J. 2008;2:67–76. doi: 10.2174/1874091X00802010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijs KL, Kleine H, Braczynski A, Forst A, Herzog N, Verheugd P, Linzen U, Kremmer E, Luscher B. ARTD10 substrate identification on protein microarrays: regulation of GSK3beta by mono-ADP-ribosylation. Cell Commun Signal. 2013;11(1):5. doi: 10.1186/1478-811X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3β-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19(4):537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol Cell. 2004;15(4):511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Tice DA, Polakis P. Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1ϵ. J Biol Chem. 2001;276(42):39037–39045. doi: 10.1074/jbc.M105148200. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Costantini F, Jho E-h, Joo C-K. Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. J Biol Chem. 2004;279(47):49188–49198. doi: 10.1074/jbc.M404655200. [DOI] [PubMed] [Google Scholar]
- Huang X, Langelotz C, Hetfeld-Pěchoč BK, Schwenk W, Dubiel W. The COP9 signalosome mediates β-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol. 2009;391(4):691–702. doi: 10.1016/j.jmb.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Tran H, Hamada F, Schwarz-Romond T, Bienz M. Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 2008;22(4):528–542. doi: 10.1101/gad.463208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Bustos D, Yeh R, Rubinfeld B, Lam C, Shriver S, Zilberleyb I, Lee MW, Phu L, Sarkar AA, Zohn IE, Wertz IE, Kirkpatrick DS, Polakis P. HectD1 E3 ligase modifies adenomatous polyposis coli (APC) with polyubiquitin to promote the APC-Axin interaction. J Biol Chem. 2013;288(6):3753–3767. doi: 10.1074/jbc.M112.415240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimowski LK, Garcia BA, Shabanowitz J, Hunt DF, Virshup DM. Site-specific casein kinase 1ϵ-dependent phosphorylation of Dishevelled modulates β-catenin signaling. FEBS J. 2006;273(20):4594–4602. doi: 10.1111/j.1742-4658.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- Hino S, Michiue T, Asashima M, Kikuchi A. Casein kinase Iϵ enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of β-catenin. J Biol Chem. 2003;278(16):14066–14073. doi: 10.1074/jbc.M213265200. [DOI] [PubMed] [Google Scholar]
- Huang X, McGann JC, Liu BY, Hannoush RN, Lill JR, Pham V, Newton K, Kakunda M, Liu J, Yu C, Hymowitz SG, Hongo JA, Wynshaw-Boris A, Polakis P, Harland RM, Dixit VM. Phosphorylation of dishevelled by protein kinase RIPK4 regulates Wnt signaling. Science. 2013;339(6126):1441–1445. doi: 10.1126/science.1232253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIε/discs overgrown promotes both Wnt-Fz/β-catenin and Fz/PCP signaling in Drosophila. Curr Biol. 2006;16(13):1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng J-J, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3(7):628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates Dishevelled. EMBO J. 1997;16(11):3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat CM, Dolde C, de Groot RE, Ohkawara B, Reinhard C, Korswagen HC, Niehrs C. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt–β-catenin signaling. Science. 2013;339(6126):1436–1441. doi: 10.1126/science.1231499. [DOI] [PubMed] [Google Scholar]
- Jung H, Kim BG, Han WH, Lee JH, Cho JY, Park WS, Maurice MM, Han JK, Lee MJ, Finley D, Jho EH. Deubiquitination of Dishevelled by Usp14 is required for Wnt signaling. Oncogenesis. 2013;2:e64. doi: 10.1038/oncsis.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello DV, Haegebarth A, Kuper I, Edelmann MJ, Henraat M, Canninga-van Dijk MR, Kessler BM, Clevers H, Maurice MM. Loss of the tumor suppressor CYLD enhances Wnt/β-catenin signaling through K63-linked ubiquitination of Dvl. Mol Cell. 2010;37(5):607–619. doi: 10.1016/j.molcel.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-[beta]-catenin pathway by targeting Dishevelled for degradation. Nat Cell Biol. 2006;8(4):348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Wei W, Li M, Wang J, Nie F, Li L. The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol Cell Biol. 2012;32(19):3903–3912. doi: 10.1128/MCB.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Zhang Y, Xu C, Tao QH, Chen YG. HECT domain-containing E3 ubiquitin ligase NEDD4L negatively regulates Wnt signaling by targeting dishevelled for proteasomal degradation. J Biol Chem. 2013;288(12):8289–8298. doi: 10.1074/jbc.M112.433185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, Fu W, Zhang J, Wu W, Zhang X, Chen YG. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol. 2010;12(8):781–790. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- Sharma J, Mulherkar S, Mukherjee D, Jana NR. Malin regulates Wnt signaling pathway through degradation of dishevelled2. J Biol Chem. 2012;287(9):6830–6839. doi: 10.1074/jbc.M111.315135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Fujita T, Ozaki T, Kato C, Kurose Y, Sakamoto M, Kato S, Goto T, Itoyama Y, Aoki M, Nakagawara A. NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J Biol Chem. 2004;279(12):11327–11335. doi: 10.1074/jbc.M312389200. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10(12):1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166(2):543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase stabilizes β-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25(20):9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of β-catenin during canonical Wnt signaling. Cell. 2008;133(2):340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Zhang C, Li Z, Biswas MH, Balaji KC. β-catenin phosphorylated at threonine 120 antagonizes generation of active β-catenin by spatial localization in trans-Golgi network. PLoS One. 2012;7(4):e33830. doi: 10.1371/journal.pone.0033830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a β-TrCP1-Skp1-β-catenin complex: destruction motif binding and lysine specificity of the SCFβ-TrCP1 ubiquitin ligase. Mol Cell. 2003;11(6):1445–1456. doi: 10.1016/S1097-2765(03)00234-X. [DOI] [PubMed] [Google Scholar]
- Winer IS, Bommer GT, Gonik N, Fearon ER. Lysine residues Lys-19 and Lys-49 of β-catenin regulate its levels and function in T cell factor transcriptional activation and neoplastic transformation. J Biol Chem. 2006;281(36):26181–26187. doi: 10.1074/jbc.M604217200. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 1999;18(9):2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18(4):849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13(3):270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Koren A, Caspi M, Zilberberg A, Rosin-Arbesfeld R. The EDD E3 ubiquitin ligase ubiquitinates and up-regulates β-catenin. Mol Biol Cell. 2011;22(3):399–411. doi: 10.1091/mbc.E10-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Gerard B, Pauley RJ, Williams BO, Tait L. Rad6B is a positive regulator of β-catenin stabilization. Cancer Res. 2008;68(6):1741–1750. doi: 10.1158/0008-5472.CAN-07-2111. [DOI] [PubMed] [Google Scholar]
- Gerard B, Sanders MA, Visscher DW, Tait L, Shekhar MP. Lysine 394 is a novel Rad6B-induced ubiquitination site on β-catenin. Biochim Biophys Acta. 2012;1823(10):1686–1696. doi: 10.1016/j.bbamcr.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao KH, Rotelli MD, Petersen CL, Kaech S, Nelson WD, Yates JE, Hanlon Newell AE, Olson SB, Druker BJ, Bagby GC. FANCL ubiquitinates β-catenin and enhances its nuclear function. Blood. 2012;120(2):323–334. doi: 10.1182/blood-2011-11-388355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT. Jade-1 inhibits Wnt signalling by ubiquitylating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008;10(10):1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Rodova M, Miska EA, Calvet JP, Kouzarides T. Acetylation of β-catenin by CREB-binding protein (CBP) J Biol Chem. 2002;277(28):25562–25567. doi: 10.1074/jbc.M201196200. [DOI] [PubMed] [Google Scholar]
- Lévy L, Wei Y, Labalette C, Wu Y, Renard CA, Buendia MA, Neuveut C. Acetylation of β-catenin by p300 regulates β-catenin-Tcf4 interaction. Mol Cell Biol. 2004;24(8):3404–3414. doi: 10.1128/MCB.24.8.3404-3414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Jin Q, Zhang F, Yan T, Zhai Q. PCAF acetylates β-catenin and improves its stability. Mol Biol Cell. 2009;20(1):419–427. doi: 10.1091/mbc.E08-08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/β-catenin signaling. Mol Cell Biol. 2003;23(4):1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature. 1999;399(6738):798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Li VSW, Ng SS, Taouatas N, Vries RGJ, Mohammed S, Heck AJ, Clevers H. The kinase TNIK is an essential activator of Wnt target genes. EMBO J. 2009;28(21):3329–3340. doi: 10.1038/emboj.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitashige M, Satow R, Jigami T, Aoki K, Honda K, Shibata T, Ono M, Hirohashi S, Yamada T. Traf2- and Nck-interacting kinase is essential for Wnt signaling and colorectal cancer growth. Cancer Res. 2010;70(12):5024–5033. doi: 10.1158/0008-5472.CAN-10-0306. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of β-catenin stability by Tcf3 and CK1∈. J Cell Biol. 2001;154(5):983–994. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16(22):2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Hämmerlein A, Weiske J, Huber O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/β-catenin complex. Cell Mol Life Sci. 2005;62(5):606–618. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem. 2011;286(14):12093–12100. doi: 10.1074/jbc.M110.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19(4):521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltzer L, Bienz M. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature. 1998;395(6701):521–525. doi: 10.1038/26785. [DOI] [PubMed] [Google Scholar]
- Gay F, Calvo D, Lo MC, Ceron J, Maduro M, Lin R, Shi Y. Acetylation regulates subcellular localization of the Wnt signaling nuclear effector POP-1. Genes Dev. 2003;17(6):717–722. doi: 10.1101/gad.1042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfert S, Weise A, Bruser K, Biniossek ML, Jägle S, Senghaas N, Hecht A. Acetylation of human TCF4 (TCF7L2) proteins attenuates inhibition by the HBP1 repressor and induces a conformational change in the TCF4::DNA complex. PLoS One. 2013;8(4):e61867. doi: 10.1371/journal.pone.0061867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R. PIASy, a nuclear matrix–associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. 2001;15(23):3088–3103. doi: 10.1101/gad.944801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Ihara M, Matsuura Y, Kikuchi A. Sumoylation is involved in β-catenin-dependent activation of Tcf-4. EMBO J. 2003;22(9):2047–2059. doi: 10.1093/emboj/cdg204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Matsumoto K, Chitnis AB, Itoh M. Nrarp functions to modulate neural-crest-cell differentiation by regulating LEF1 protein stability. Nat Cell Biol. 2005;7(11):1106–1112. doi: 10.1038/ncb1311. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohnishi J, Ohkawara B, Iemura S, Satoh K, Hyodo-Miura J, Kawachi K, Natsume T, Shibuya H. NARF, an nemo-like kinase (NLK)-associated ring finger protein regulates the ubiquitylation and degradation of T cell factor/lymphoid enhancer factor (TCF/LEF) J Biol Chem. 2006;281(30):20749–20760. doi: 10.1074/jbc.M602089200. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in human health and disease. Eur J Biochem. 2001;268(19):5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, Polakis P. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–211. doi: 10.1016/S0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3β associations that regulate β-catenin stabilization are mediated by Gα proteins. Curr Biol. 2005;15(22):1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3′s phosphorylation of β-catenin. Proc Natl Acad Sci U S A. 2008;105(23):8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S, Lee SH, Kim H, Yum S, Stamos JL, Xu Y, Lee SJ, Lee J, Oh S, Han JK, Park BJ, Weis WI, Ha NC. Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signaling. PLoS One. 2008;3(12):e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Abreu JG, He X. Inhibition of GSK3 phosphorylation of β-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4(3):e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Dolan PJ, Johnson GVW. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J Biol Chem. 2006;281(8):4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A. Axin prevents Wnt-3a-induced accumulation of β-catenin. Oncogene. 1999;18(4):979–985. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH. Structural basis for recruitment of glycogen synthase kinase 3β to the axin–APC scaffold complex. EMBO J. 2003;22(3):494–501. doi: 10.1093/emboj/cdg068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of Axin releases β-catenin from the Axin complex. Genes Dev. 1999;13(14):1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the β-catenin degradation complex. EMBO J. 2007;26(6):1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strovel ET, Wu D, Sussman DJ. Protein phosphatase 2Cα dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem. 2000;275(4):2399–2403. doi: 10.1074/jbc.275.4.2399. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and Axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J Biol Chem. 2000;275(44):34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/β-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20(2):119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–156. doi: 10.1016/S1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci. 2010;67(15):2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, Abrams CS, Sokol SY, Wu D. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321(5894):1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135(2):367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 1999;18(10):2823–2835. doi: 10.1093/emboj/18.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol Cell Biol. 1999;19(6):4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc Natl Acad Sci U S A. 2011;108(5):1937–1942. doi: 10.1073/pnas.1017063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr Iii GH, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–809. doi: 10.1016/S1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Bilić J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316(5831):1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJG, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14(6):484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120(14):2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem. 1999;274(30):21464–21470. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AM. Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize β-catenin. Mol Cell Biol. 2004;24(11):4757–4768. doi: 10.1128/MCB.24.11.4757-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12(6):957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7(6):1321–1327. doi: 10.1016/S1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105(6):721–732. doi: 10.1016/S0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the β-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene. 2001;20(2):252–259. doi: 10.1038/sj.onc.1204064. [DOI] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem. 2000;275(42):32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24(8):1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikkavilli RK, Feigin ME, Malbon CC. p38 mitogen-activated protein kinase regulates canonical Wnt–β-catenin signaling by inactivation of GSK3β. J Cell Sci. 2008;121(21):3598–3607. doi: 10.1242/jcs.032854. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu CY, Li XY, Weiss SJ. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc Natl Acad Sci U S A. 2012;109(28):11312–11317. doi: 10.1073/pnas.1203015109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Tang TL, Neel BG, Sokol SY. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development. 1995;121(12):3979–3988. doi: 10.1242/dev.121.12.3979. [DOI] [PubMed] [Google Scholar]
- Buescher JL, Phiel CJ. A noncatalytic domain of glycogen synthase kinase-3 (GSK-3) is essential for activity. J Biol Chem. 2010;285(11):7957–7963. doi: 10.1074/jbc.M109.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser E, Young N, Dajani R, Franca-Koh J, Ryves J, Williams RSB, Yeo M, Webster MT, Richardson C, Smalley MJ, Pearl LH, Harwood A, Dale TC. Identification of the Axin and Frat binding region of glycogen synthase kinase-3. J Biol Chem. 2002;277(3):2176–2185. doi: 10.1074/jbc.M109462200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qiu WJ, Liu DX, Neo SY, He X, Lin SC. Differential molecular assemblies underlie the dual function of Axin in modulating the WNT and JNK pathways. J Biol Chem. 2001;276(34):32152–32159. doi: 10.1074/jbc.M104451200. [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395(6702):608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Arce L, Pate K, Waterman M. Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression. BMC Cancer. 2009;9(1):159. doi: 10.1186/1471-2407-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12(4):364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol Cell Biol. 1998;18(8):4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67(1):425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695(1–3):55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315(5809):201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278(38):35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33(3):275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Komander D. The emerging complexity of protein ubiquitination. Biochem Soc Trans. 2009;37(Pt 5):937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- Kulathu Y, Komander D. Atypical ubiquitylation — the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol. 2012;13(8):508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. EMBO Rep. 2008;9(6):536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25(20):4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F, Sakata S-i, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nat Cell Biol. 2009;11(2):123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Sorkin A. In: Intracellular traffic and neurodegenerative disorders. George-Hyslop PS, Mobley WC, Christen Y, editor. Berlin, Heidelberg: Springer; 2009. Regulation of endocytic trafficking of receptors and transporters by ubiquitination: possible role in neurodegenerative disease; pp. 141–155. [Google Scholar]
- Kravtsova-Ivantsiv Y, Ciechanover A. Non-canonical ubiquitin-based signals for proteasomal degradation. J Cell Sci. 2012;125(3):539–548. doi: 10.1242/jcs.093567. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Spiegelman VS, Suresh Kumar KG. The many faces of β-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23(11):2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- Panchenko MV, Zhou MI, Cohen HT. von Hippel-Lindau partner Jade-1 Is a transcriptional co-activator associated with histone acetyltransferase activity. J Biol Chem. 2004;279(53):56032–56041. doi: 10.1074/jbc.M410487200. [DOI] [PubMed] [Google Scholar]
- Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT. The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1. J Biol Chem. 2002;277(42):39887–39898. doi: 10.1074/jbc.M205040200. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science. 1998;280(5363):596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8(10):573–581. doi: 10.1016/S0960-9822(98)70226-X. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Krüger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1):e10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of β-catenin stability: reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5(3):523–532. doi: 10.1016/S1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Lui TT, Lacroix C, Ahmed SM, Goldenberg SJ, Leach CA, Daulat AM, Angers S. The ubiquitin-specific protease USP34 regulates axin stability and Wnt/β-catenin signaling. Mol Cell Biol. 2011;31(10):2053–2065. doi: 10.1128/MCB.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16(5):2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22(5):717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Habas R. Canonical Wnt signaling: an unexpected new player. Dev Cell. 2006;11(2):138–139. doi: 10.1016/j.devcel.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9(18):3724–3733. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sancho JM, Greer YE, Abrahams CL, Takigawa Y, Baljinnyam B, Lee KH, Lee KS, Rubin JS, Brown AM. Functional consequences of Wnt-induced dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J Biol Chem. 2013;288(13):9428–9437. doi: 10.1074/jbc.M112.448480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93(5):767–777. doi: 10.1016/S0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Liu Q, Lu W, Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2009;29(4):539–549. doi: 10.1038/onc.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125(2):265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- Tran H, Polakis P. Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the β-catenin destruction complex. J Biol Chem. 2012;287(34):28552–28563. doi: 10.1074/jbc.M112.387878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25(2):160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- Jürgen Dohmen R. SUMO protein modification. Biochim Biophys Acta. 2004;1695(1–3):113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol. 2010;11(12):861–871. doi: 10.1038/nrm3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ETH. SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem. 2009;284(13):8223–8227. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135(6):1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O. SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007;8(6):550–555. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73(1):355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kadoya T, Kishida S, Fukui A, Hinoi T, Michiue T, Asashima M, Kikuchi A. Inhibition of Wnt signaling pathway by a novel axin-binding protein. J Biol Chem. 2000;275(47):37030–37037. doi: 10.1074/jbc.M005984200. [DOI] [PubMed] [Google Scholar]
- Kadoya T, Yamamoto H, Suzuki T, Yukita A, Fukui A, Michiue T, Asahara T, Tanaka K, Asashima M, Kikuchi A. Desumoylation activity of Axam, a novel Axin-binding protein, is involved in downregulation of β-catenin. Mol Cell Biol. 2002;22(11):3803–3819. doi: 10.1128/MCB.22.11.3803-3819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chia IV, Costantini F. SUMOylation target sites at the C terminus protect Axin from ubiquitination and confer protein stability. FASEB J. 2008;22(11):3785–3794. doi: 10.1096/fj.08-113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang CY. TBL1-TBLR1 and β-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10(2):160–169. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- Choi HK, Choi KC, Yoo JY, Song M, Ko Suk J, Kim Chul H, Ahn JH, Chun KH, Yook Jong I, Yoon HG. Reversible SUMOylation of TBL1-TBLR1 regulates β-catenin-mediated Wnt signaling. Mol Cell. 2011;43(2):203–216. doi: 10.1016/j.molcel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- Versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302(5652):1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- Yurchenko V, Xue Z, Sadofsky MJ. SUMO modification of human XRCC4 regulates its localization and function in DNA double-strand break repair. Mol Cell Biol. 2006;26(5):1786–1794. doi: 10.1128/MCB.26.5.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, Mustelin T, Feng GS, Chen G, Yu J. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun. 2012;3:911. doi: 10.1038/ncomms1919. [DOI] [PubMed] [Google Scholar]
- Kubota Y, O’Grady P, Saito H, Takekawa M. Oncogenic Ras abrogates MEK SUMOylation that suppresses the ERK pathway and cell transformation. Nat Cell Biol. 2011;13(3):282–291. doi: 10.1038/ncb2169. [DOI] [PubMed] [Google Scholar]
- Carter S, Vousden KH. p53-Ubl fusions as models of ubiquitination, sumoylation and neddylation of p53. Cell Cycle. 2008;7(16):2519–2528. doi: 10.4161/cc.7.16.6422. [DOI] [PubMed] [Google Scholar]
- Lundby A, Lage K, Weinert Brian T, Bekker-Jensen Dorte B, Secher A, Skovgaard T, Kelstrup Christian D, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2(2):419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32(3):959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2011;36(2):108–116. doi: 10.1016/j.tibs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64(2):435–459. doi: 10.1128/MMBR.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12(5):599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41(1):185–198. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine Acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3(4):e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+into a nuclear signal. Genes Dev. 2005;19(17):1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70(3):789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7(7):517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- James RG, Davidson KC, Bosch KA, Biechele TL, Robin NC, Taylor RJ, Major MB, Camp ND, Fowler K, Martins TJ, Moon RT. WIKI4, a novel inhibitor of tankyrase and Wnt/β-catenin signaling. PLoS One. 2012;7(12):e50457. doi: 10.1371/journal.pone.0050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28(5):730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31(4):449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kanjanapangka J, Liu N, Liu S, Liu C, Wu Z, Wang Y, Loh T, Kowolik C, Jamsen J, Zhou M, Truong K, Chen Y, Zheng L, Shen B. Sequential posttranslational modifications program FEN1 degradation during cell-cycle progression. Mol Cell. 2012;47(3):444–456. doi: 10.1016/j.molcel.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr R. In: Advances in Genetics, Volume 70. Zdenko H, Toshikazu U, editor. Waltham, Massachusetts: Academic Press; 2010. Interplay between different epigenetic modifications and mechanisms; pp. 101–141. [DOI] [PubMed] [Google Scholar]
- Wang Z, Michaud GA, Cheng Z, Zhang Y, Hinds TR, Fan E, Cong F, Xu W. Recognition of the iso-ADP-ribose moiety in poly(ADP-ribose) by WWE domains suggests a general mechanism for poly(ADP-ribosyl)ation-dependent ubiquitination. Genes Dev. 2012;26(3):235–240. doi: 10.1101/gad.182618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Lee YI, Shin JH, Andrabi SA, Chi Z, Gagné JP, Lee Y, Ko HS, Lee BD, Poirier GG, Dawson VL, Dawson TM. Iduna is a poly(ADP-ribose) (PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. Proc Natl Acad Sci U S A. 2011;108(34):14103–14108. doi: 10.1073/pnas.1108799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31(12):2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31(12):2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]


