SUMMARY
Cutaneous mechanosensory neurons detect mechanical stimuli that generate touch and pain sensation. Although opioids are generally associated only with the control of pain, here we report that the opioid system in fact broadly regulates cutaneous mechanosensation, including touch. This function is predominantly subserved by the delta opioid receptor (DOR), which is expressed by myelinated mechanoreceptors that form Meissner corpuscles, Merkel cell-neurite complexes, and circumferential hair follicle endings. These afferents also include a small population of CGRP-expressing myelinated nociceptors that we now identify as the somatosensory neurons that coexpress mu and delta opioid receptors. We further demonstrate that DOR activation at the central terminals of myelinated mechanoreceptors depresses synaptic input to the spinal dorsal horn, via the inhibition of voltage-gated calcium channels. Collectively our results uncover a molecular mechanism by which opioids modulate cutaneous mechanosensation and provide a rationale for targeting DOR to alleviate injury-induced mechanical hypersensitivity.
INTRODUCTION
The cutaneous mechanosensory system is critical for the detection and discrimination of innocuous and noxious mechanical stimuli that elicit sensations of touch and pain, respectively (Basbaum et al., 2009; Delmas et al., 2011; Lewin and Moshourab, 2004). However, innocuous stimuli in the setting of injury can also evoke pain. Indeed, skin hypersensitivity to light mechanical stimuli (also called mechanical allodynia or touch-evoked pain) is one of the most common and distressing symptoms of nerve injury-induced neuropathic pain (Costigan et al., 2009).
Of particular importance is the identification of the primary sensory neurons of the dorsal root ganglion (DRG) that mediate mechanical allodynia. We and others have shown that ablation or silencing of several populations of unmyelinated nociceptors (C fibers) does not alter nerve injury-induced mechanical hypersensitivity in rodents (Abrahamsen et al., 2008; Cavanaugh et al., 2009; Scherrer et al., 2010). By contrast, selective compression block of myelinated axons (A fibers), which eliminates the normal sense of touch while preserving C fiber function, abolishes touch-evoked neuropathic pain in humans (Campbell et al., 1988). Electrophysiological studies demonstrate that pharmacological disinhibition of spinal cord circuits or peripheral injuries that cause mechanical hypersensitivity strengthen Aβ and Aδ fiber input to nociceptive lamina I spinal neurons, uncovering a mechanism by which activation of low-threshold A fibers by normally innocuous mechanical stimuli can cause pain (Torsney, 2011; Torsney and MacDermott, 2006). Together these results indicate that cutaneous mechanosensitive A fibers contribute to touch-evoked pain, and that drugs that dampen the function of these neurons might be an effective treatment.
Delta, kappa and mu opioid receptors (DOR, KOR and MOR, respectively) are G protein-coupled receptors that regulate neurotransmission, including at the level of primary afferent DRG neurons (Williams et al., 2001). Opioids that preferentially activate MORs (e.g. morphine, oxycodone, fentanyl) are widely used to treat severe pain, but their efficacy in chronic neuropathic pain is subject to considerable uncertainty (McNicol et al., 2013). A better understanding of the neural circuits and molecular mechanisms underlying opioid analgesia is necessary for a more rational use of opioids in the clinic.
The expression pattern of DOR in DRG remains a subject of substantial controversy. We recently showed that DOR is predominantly expressed by DRG neurons with myelinated axons (Scherrer et al., 2009). Furthermore, DOR-selective agonists display anti-allodynic properties in murine models of touch-evoked neuropathic and inflammatory pain, and DOR null mice exhibit increased mechanical hypersensitivity after peripheral injury (reviewed in (Gaveriaux-Ruff and Kieffer, 2011; Ossipov et al., 2004)). These findings suggested that DOR may be expressed by cutaneous mechanosensory A fibers and that DOR-mediated regulation of these afferents could counteract nerve injury-associated mechanical hypersensitivity.
With the recent discovery that functionally distinct classes of A fibers depend on distinct neurotrophins for their development and survival, it is now possible to test this hypothesis. Thus cutaneous Aβ low-threshold mechanoreceptors (LTMRs) express the neurotrophin receptors TrkC and/or Ret (Bourane et al., 2009; Funfschilling et al., 2004; Li et al., 2011; Luo et al., 2009; Senzaki et al., 2010), while Aδ D-hair LTMRs express TrkB (Li et al., 2011; Stucky et al., 1998). This molecular characterization distinguishes touch-encoding cutaneous A fibers from myelinated nociceptors, which most often express TrkA (Fang et al., 2005) and the neuropeptide CGRP (Lawson et al., 2008) and from proprioceptors that coexpress parvalbumin, TrkC and the transcription factor Runx3 (de Nooij et al., 2013).
Building on the ability to classify subpopulations of myelinated afferents, here we conducted a neuroanatomical and electrophysiological analysis to resolve the functional organization of opioid receptors in DRG.
RESULTS
DORGFP knockin mouse faithfully reports DOR distribution in DRG and CNS
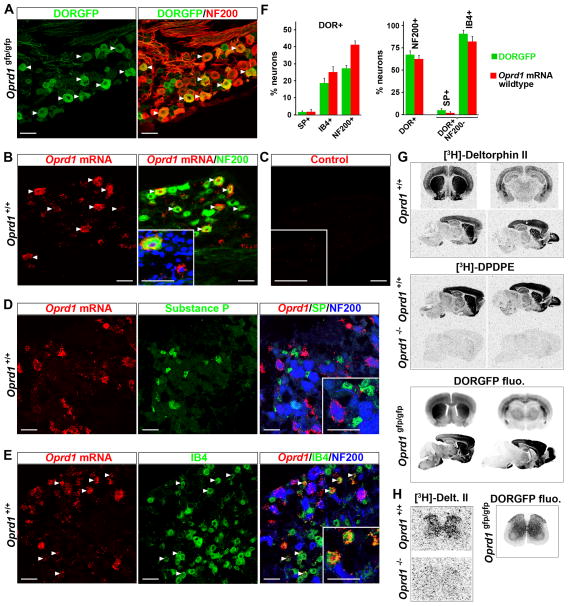
We previously used a DORGFP knockin mouse (Scherrer et al., 2006) to establish the expression pattern of DOR in DRG (Scherrer et al., 2009). Most DORGFP+ DRG neurons have large-diameter cell bodies and express the marker of myelinated afferents neurofilament 200 (NF200) (67% of DORGFP+ cells are NF200+) (Figures 1A, 1F and Table S1). DORGFP is expressed by 27% of all NF200+ DRG neurons. The majority of DORGFP+ NF200- cells bind the isolectin B4 (IB4) (91% IB4+), and 19% of IB4+ cells express DORGFP (Figures 1F, S1A, and Table S1), indicating that DOR+ small-diameter neurons comprise a subset of nonpeptidergic C nociceptors. Accordingly, very few DORGFP+ NF200− cells express substance P (SP) (~5%), and only about 2% of SP+ neurons express DORGFP (Figures 1F, S1B and Table S1).
Figure 1. DORGFP mouse faithfully reports DOR expression in CNS and DRG, where DOR is predominantly expressed by large-diameter, myelinated (NF200+) neurons.
(A) Co-staining with anti-GFP and -NF200 antibodies shows that 67% of DORGFP+ DRG neurons express NF200.
(B) Oprd1 mRNA is also predominantly present in NF200+ neurons in DRG from wildtype mice.
(C) Control showing that in the absence of the specific probe against Oprd1 sequence there is no in situ hybridization signal in DRG neurons.
(D) Substance P+ (SP+) cells almost never contain Oprd1 mRNA in DRG neurons from wildtype mice.
(E) The great majority of NF200− cells that contain Oprd1 mRNA bind the isolectin B4 (IB4).
(F) Quantification of A–E and of A–B from Figure S1. The left panel indicates neurons that coexpress DOR among those that express SP, or bind IB4, or express NF200. The right panel indicates DOR+ neurons that coexpress NF200, and DOR+ NF200− neurons that coexpress SP or bind IB4.
(G) The distribution of fluorescence in brain slices from DORGFP mice is identical to the binding pattern of DOR radioligands [3H]-Deltorphin II and [3H]-DPDPE in wildtype mice. Radiolabeling is lost in Oprd1 null mice, demonstrating specificity.
(H) In wildtype mouse spinal cord [3H]-Deltorphin II binds to DORs that are present throughout the grey matter, consistent with DORGFP fluorescence pattern.
Data are represented as means ± SEM. Scale bars equal 50 μm. See also Figure S1.
This DORGFP expression differs drastically from the immunoreactivity (ir) pattern generated in wildtype mice by an antibody that purportedly recognizes the N-terminal domain of the receptor (anti-DOR3-17). DOR-ir was observed in a larger cell population (about half of DRG neurons), mainly of small diameter (Bao et al., 2003; He et al., 2011; Wang et al., 2010). Furthermore, DOR-ir was always found to colocalize with SP in peptidergic C nociceptors, an essential requirement for a SP-DOR direct interaction that was proposed to sort DOR in large dense-core vesicles and regulate its surface expression (Guan et al., 2005). In situ hybridization studies performed by the same group suggested that Oprd1 mRNA, which encodes DOR, might be present in 71% of small-diameter DRG neurons.
To address this controversy we took advantage of the recent development of an ultra-sensitive and specific method to detect single mRNA molecules in tissues (QuantiGene ViewRNA, Panomics). We found that in DRG from wildtype mice Oprd1 mRNA is concentrated in large-diameter neurons that express NF200 (Figures 1B, 1F, S1C and S1D). These DRG cells often contain hundreds of labeled dots in their cytoplasm. Thus 62% of DRG neurons with Oprd1 mRNA are NF200+. We then asked whether the small-diameter, NF200− DRG neurons that express Oprd1 mRNA belong to the peptidergic or nonpeptidergic class of C nociceptors. We found that among 219 SP+ profiles examined, 204 did not contain a single labeled dot. Only 3 out of 219 cells displayed 5 or more labeled dots in their cytoplasm and were considered positive (~2%, Figures 1F and S1E), while 12 had only 1 or 2 dots, which was comparable to the background level. Only about 2% of NF200− DRG neurons with Oprd1 mRNA express SP, while the great majority (~82%) of these cells bound IB4 (Figures 1D–F).
We next compared the DORGFP fluorescence pattern in the CNS to the binding pattern of selective DOR radioligands, [3H]-Deltorphin II and [3H]-DPDPE, in wildtype mice. We observed that DORGFP distribution is strikingly similar to the binding pattern of both DOR radioligands throughout the CNS. Thus DORGFP fluorescence and radioligand binding are particularly intense in basolateral amygdala, striatum, cortex, pontine nucleus, olfactory bulb, and olfactory tubercle (Figure 1G). In the spinal cord, both DORGFP fluorescence and radioligand binding are seen throughout the grey matter (Figure 1H). This distribution pattern differs profoundly from that generated by the anti-DOR3-17 antibody, which labels exclusively the terminals of peptidergic afferents in superficial dorsal horn (Guan et al., 2005). Finally, note that binding of radioligands is lost in tissues from Oprd1 knockout mice, demonstrating the specificity of DOR labeling and localization (Figure 1G).
We conclude that the remarkably high correspondence between the DORGFP distribution pattern and that of Oprd1 mRNA and DOR protein in wildtype mouse, which is consistent with previous studies (Cahill et al., 2001; Goody et al., 2002; Mansour et al., 1994; Mansour et al., 1987; Mennicken et al., 2003; Minami et al., 1995) argues very strongly that DORGFP expression faithfully recapitulates that of native DOR.
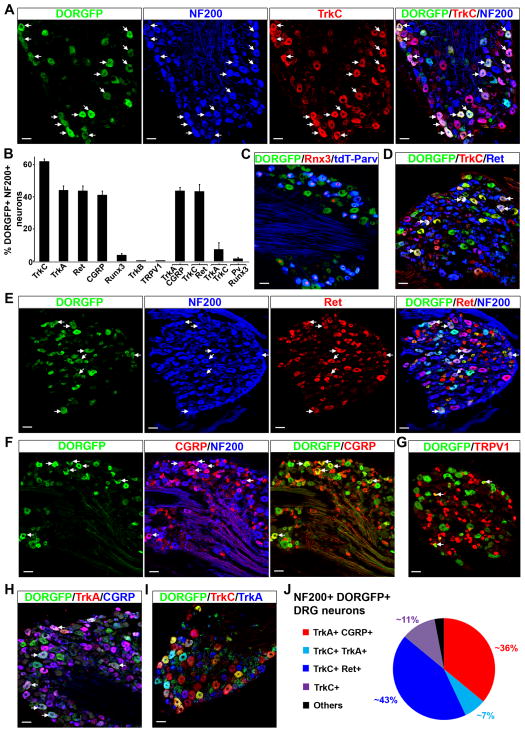
TrkA, TrkC and Ret coexpression reveals that DOR+ NF200+ neurons are heterogeneous and include nociceptors and LTMRs
We next resolved the molecular identity of DORGFP+ NF200+ neurons using a host of markers that define DRG neuron subpopulations. Opioid receptor-expressing DRG neurons are generally considered to be nociceptors, but given the anti-allodynic properties of DOR agonists, we hypothesized that DOR+ DRG neurons could include LTMRs. Consistently, we found that most of the DORGFP+ NF200+ neurons (~62%) in fact express TrkC (Figures 2A, 2B and Table S1). To characterize these neurons further and identify proprioceptors we crossed DORGFP animals with reporter mice in which the tdTomato fluorescent protein is expressed by parvalbumin+ DRG neurons. Figure 2C shows that DORGFP+ neurons do not include proprioceptors, marked by the coexpression of parvalbumin and the transcription factor Runx3 (de Nooij et al., 2013) (Figures 2B, 2C and Table S1). Rather, we found that many DORGFP+ TrkC+ DRG neurons coexpress Ret, indicating that they are likely cutaneous mechanoreceptors, including Aβ LTMRs (Figures 2B, 2D–E, and Table S1) (Bourane et al., 2009; Funfschilling et al., 2004; Luo et al., 2009; Senzaki et al., 2010). We also determined the identity of the remaining ~36% of DORGFP+ NF200+ neurons. This population coexpresses TrkA and the neuropeptide CGRP, but not TRPV1 (Figures 2B, 2F–H and Table S1), indicating that they correspond to a particular class of capsaicin-insensitive A nociceptors. Finally, we did not observe any DORGFP+ NF200+ neurons that express the D-hair marker TrkB (Figures 2B, S2B and Table S1).
Figure 2. DOR-expressing myelinated DRG neurons express the markers of Aβ low-threshold mechanoreceptors TrkC and Ret.
(A) TrkC is expressed by about 62% of NF200+ DORGFP+ DRG neurons. Arrows indicate cells where costaining occurs.
(B) Quantification of (A) and (C)–(I).
(C) Proprioceptors, identified by the coexpression of parvalbumin and Runx3, do not express DORGFP. Note that some DORGFP+ neurons individually express either parvalbumin or Runx3.
(D) Approximately 70% of DORGFP+ TrkC+ neurons coexpress Ret (arrows) indicating that these neurons are likely myelinated cutaneous mechanoreceptors, including Aβ LTMRs.
(E) About 40% of large-diameter myelinated (NF200+) DORGFP+ neurons (arrows) and almost all small-diameter DORGFP+ neurons express Ret.
(F) Approximately 36% of NF200+ DORGFP+ neurons express CGRP (arrows).
(G) Large-diameter DORGFP+ DRG neurons do not express the noxious heat sensor TRPV1. A very small number of small-diameter neurons coexpress DOR and TRPV1 (arrows).
(H) Large-diameter DORGFP+ neurons that express CGRP also immunostain for the nociceptor marker TrkA (arrows).
(I) Almost all large-diameter DORGFP+ neurons express either TrkC or TrkA.
(J) Molecular identity of myelinated (NF200+) DORGFP+ neuron subpopulations.
Data are represented as means ± SEM. Scale bars equal 50 μm.
Based on this anatomical analysis we conclude that DORGFP+ myelinated DRG neurons are remarkably more heterogeneous than previously thought, and segregate into two functionally distinct classes: a TrkA+ CGRP+ nociceptive population (~36%) and a TrkC+ and/or Ret+ population of cutaneous mechanoreceptors, which likely includes Aβ LTMRs (~62%) (Figure 2J).
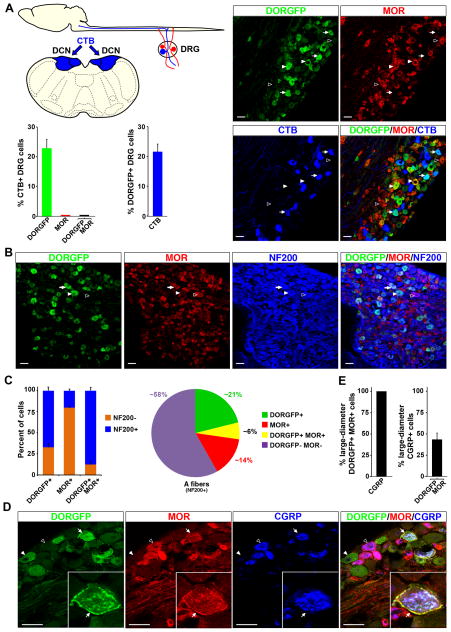
MOR is expressed by myelinated nociceptors, and colocalizes with DOR in CGRP+ NF200+ DRG neurons
Although MOR agonists regulate neurotransmitter release from both C and A nociceptors (Heinke et al., 2011), the precise molecular identity of the myelinated afferents that express MOR has so far remained elusive. Here we used an anti-MOR antibody with demonstrated specificity in mice null for MOR expression (Scherrer et al., 2009) to characterize these cells. We found that almost all NF200+ MOR+ neurons express CGRP (~99%), but very rarely TrkC (~3%), Ret (~5%) or TrkB (0%) (Figures 3A–G and Table S2). About 8% of NF200+ MOR+ neurons also express TRPV1. Because of antibody incompatibility we could not test directly whether NF200+ MOR+ neurons coexpress TrkA. Nevertheless, since ~95% of CGRP+ DRG neurons belong to the TrkA+ population of nociceptors in rodents (Figure S2C), we conclude that all NF200+ MOR+ DRG neurons are peptidergic A nociceptors, which distinguishes the MOR+ population of NF200+ DRG neurons from those that express DOR.
Figure 3. Myelinated DRG neurons that express MOR coexpress CGRP, but not markers of Aβ low-threshold mechanoreceptors.
(A) Virtually all MOR+ myelinated neurons (NF200+) express CGRP (arrows indicate NF200+ MOR+ neurons), indicating that these neurons are TrkA+ nociceptors (see Figure S2C).
(B) NF200+ MOR+ neurons (arrows) rarely express TrkC.
(C) The great majority of NF200+ MOR+ neurons express neither Ret nor TrkC (see (B)), indicating that they are neither proprioceptors nor Aβ LTMRs.
(D) A small subpopulation of NF200+ MOR+ neurons also expresses TRPV1 (arrows).
(E) MOR+ neurons do not express the D-hair receptor marker TrkB.
(F) Quantification of (A)–(E).
(G) Molecular identity of myelinated (NF200+) MOR+ neuron subpopulations.
Data are represented as means ± SEM. Scale bars equal 50 μm.
To test this hypothesis further, we took advantage of the characteristic anatomy of Aβ LTMRs. Some Aβ LTMRs, unlike any other DRG neurons, project not only to the spinal cord dorsal horn, but also to the dorsal column nuclei (DCN) (Giuffrida and Rustioni, 1992), brainstem regions critical for integration of touch information. We stereotaxically microinjected a retrograde tracer, the B fragment of cholera toxin (CTB), into the DCN of mice. This protocol labeled Aβ LTMR cell bodies in lumbar DRGs, which we then coimmunostained for DORGFP and MOR (Figure 4A). We found that among 294 CTB+ DRG neurons, 53 expressed DORGFP (~21%). By contrast, we never observed CTB+ MOR+ cells, which is consistent with our contention that only DOR, but not MOR, is expressed by Aβ LTMRs.
Figure 4. DOR and MOR are colocalized at the plasma membrane of CGRP+ A nociceptors.
(A) Stereotaxic injection of the B fragment of cholera toxin (CTB) into the dorsal column nuclei (DCN) labels Aβ LTMR cell bodies in lumbar DRGs. Only DORGFP+ DRG neurons display CTB immunoreactivity (22.9% ± 2.9%), while MOR+ (0.0% ± 0.0%) and DORGFP+ MOR+ cells do not (n=294 CTB+ cells).
(B) DORGFP and MOR are expressed by largely non-overlapping populations of DRG neurons. Some NF200+ neurons express only DOR (white arrowhead) or MOR (black arrowhead); a third population coexpresses both receptors (arrow).
(C) Quantification of (B) shows that 87.8% ± 3.9% of the DRG neurons that coexpress DOR and MOR are NF200+ (bar graph) and that 6.2% ± 1.1% of all NF200+ DRG neurons coexpress both receptors (pie-chart) (n=1091). DOR predominates in NF200+ neurons (67.3% ± 4.3%), MOR in NF200− neurons (79.8% ± 1.9%) (bar graphs).
(D) DORGFP and MOR are coexpressed at the plasma membrane of large-diameter CGRP+ DRG neurons, which are most often mechanonociceptors (arrow) (Lawson et al., 2008). White arrowhead points to a large-diameter DORGFP+ CGRP− MOR− DRG neuron that likely belongs to the TrkC+ population described in Figure 1. Black arrowhead illustrates that all large-diameter MOR+ DRG neurons, and the vast majority of MOR+ small-diameter cells, coexpress CGRP.
(E) All DORGFP+ MOR+ large-diameter DRG neurons coexpress CGRP (100.0% ± 0.0%) and this population corresponds to 43.3% ± 7.7% of all CGRP+ large-diameter DRG neurons (n=229).
Data are represented as means ± SEM. Scale bars equal 50 μm.
We next investigated the identity of the DRG cells that coexpress DOR and MOR. Previous studies provided biochemical and behavioral evidence that DOR-MOR functional interactions occur in neurons of the pain pathway (Costantino et al., 2012), but to date failed to identify the sensory neurons in which these interactions might take place (although see (He et al., 2011; Wang et al., 2010)). As we previously reported (Scherrer et al., 2009), the incidence of coexpression of these two opioid receptors in all DRG neurons is, in fact, low (less than 5%). However, our analysis now establishes that most DORGFP+ MOR+ DRG neurons express NF200 (~88%), corresponding to ~6% of all myelinated DRG neurons (Figures 4A–C). About one third of NF200+ DORGFP+ or NF200+ MOR+ express the other opioid receptor, while coexpression in NF200− neurons is very rare (Tables S1 and S2). Moreover, all the NF200+ DORGFP+ MOR+ neurons belong to the CGRP+ class of A nociceptors, and the two receptors are colocalized at the plasma membrane in these cells (Figure 4D–E).
To complete our analysis, we next examined small-diameter unmyelinated (NF200−) DRG neurons and tested whether DOR or MOR are expressed by a population of C LTMRs that express tyrosine hydroxylase (TH) (Li et al., 2011). We found that DOR+ and MOR+ unmyelinated DRG neurons are predominantly IB4-binding MrgprD+ mechanonociceptors and peptidergic TRPV1+ thermonociceptors, respectively, but not TH+ C LTMRs (Figure S3).
DOR+ myelinated DRG neurons innervate Merkel cells, hair follicles, and Meissner corpuscles, mechanosensory organs in the skin
To clarify the identity of NF200+ DRG neurons that express DOR, we next analyzed the morphology of the peripheral terminals of DORGFP-expressing DRG neurons using a whole-mount cleared skin preparation.
We focused on three distinct skin structures that are major contributors to cutaneous mechanosensation: Merkel cells, Meissner corpuscles, and hair follicles. We first immunostained hairy skin of the back of DORGFP mice with an antibody against cytokeratin 8 (CK8) and observed the characteristic distribution of Merkel cells in touch domes associated with guard hairs. Furthermore, as illustrated in Figures 5A, 5C and S4A, we found that the majority of Merkel cells are innervated by DORGFP+ NF200+ axons that terminate as flattened disks surrounding the base of Merkel cells. This anatomical organization is characteristic of the Merkel cell-neurite complex and establishes that DOR is expressed by slowly adapting I (SAI) Aβ LTMRs that detect light skin indentation.
Figure 5. The terminals of DORGFP-expressing DRG neurons form touch-detecting mechanosensory organs in the skin.
(A) Peripheral axons of DORGFP+ DRG neurons (green, arrowheads) innervate cytokeratin 8 (CK8)-immunoreactive Merkel cells grouped in touch domes associated with guard hairs (autofluorescent hair shaft, white arrow).
(B) DORGFP+ terminals form circumferential, but not longitudinal, endings (arrowheads) around follicles of most pelage hairs, including guard hairs (arrow, center of the image).
(C) DORGFP+ axons end as NF200+ flattened disks (arrowheads) surrounding the base of Merkel cells (arrows).
(D) DORGFP+ axons that form circumferential endings around follicles are myelinated (NF200+) and are distinct from CGRP+ terminals. Innervation of a guard hair is shown.
(E) DORGFP+ NF200+ axons form Meissner corpuscles in the dermal papillae of the mouse footpad. The Meissner corpuscles are localized by immunoreactivity of the glial protein S100 (arrows). DORGFP+ NF200− axons penetrate further into the epidermis (white arrowheads), consistent with their nonpeptidergic MrgprD+ identity. Black arrowhead indicates the boundary between stratum corneum (autofluorescent dead cells) and stratum granulosum.
(F) Myelinated DORGFP+ afferents with epidermal free nerve endings often coexpress CGRP (arrowheads). Circumferential endings around the follicle of the small caliber hairs (arrow) express DORGFP and NF200, but not CGRP.
Autofluorescence generates the fluorescence of the hair shafts (yellow in (A), green in (B) and (D)).
We next examined cutaneous mechanosensory organs innervated by rapidly adapting (RA) Aβ LTMRs, which include Meissner corpuscle afferents, and hair follicle longitudinal lanceolate endings. Recent studies demonstrated that Ret expression in large diameter DRG neurons early during development marks these RA Aβ LTMRs (Bourane et al., 2009; Luo et al., 2009). Consistent with our demonstration of Ret and DORGFP coexpression in large-diameter DRG neurons (Figure 2), we found that some DORGFP+ NF200+ axons innervate Meissner corpuscles, visualized with S100 glial protein immunostaining in dermal papillae of the footpad (Figure 5E).
We then analyzed the innervation of hair follicles by DORGFP+ afferents. Strikingly, DORGFP+ NF200+ axons formed circumferential endings around follicles of the great majority of guard and smaller pelage hairs, but very rarely longitudinal lanceolate endings (Figures 5B, 5D, and S4A–C). The physiological correlate of circumferential endings is still unclear; however their association with hair follicles suggests a function in mechanosensation. Because DORGFP+ terminals that innervate hair follicles are distinct from CGRP+ circumferential endings (Figures 5D, 5F and S4D), we propose that the DORGFP+ TrkC+ DRG neurons, which often coexpress Ret, form circumferential endings, and that DOR represents a marker of this poorly characterized population of DRG neurons.
Finally, we observed that numerous DORGFP+ axons terminate as free nerve endings in the skin, a morphology that is characteristic of nociceptors (Figure 5F and S4E). Based on the typical morphology of the DORGFP+ NF200− axons that extend to the stratum granulosum (Zylka et al., 2005), we suggest that these correspond to the peripheral terminals of DORGFP+ MrgprD+ C mechanociceptors (Figure 5E and S4D–E). We presume that the NF200+ DORGFP+ CGRP+ free nerve endings arise from the population of TrkA+ DORGFP+ myelinated nociceptors that coexpress MOR (Figure 5F and S4E).
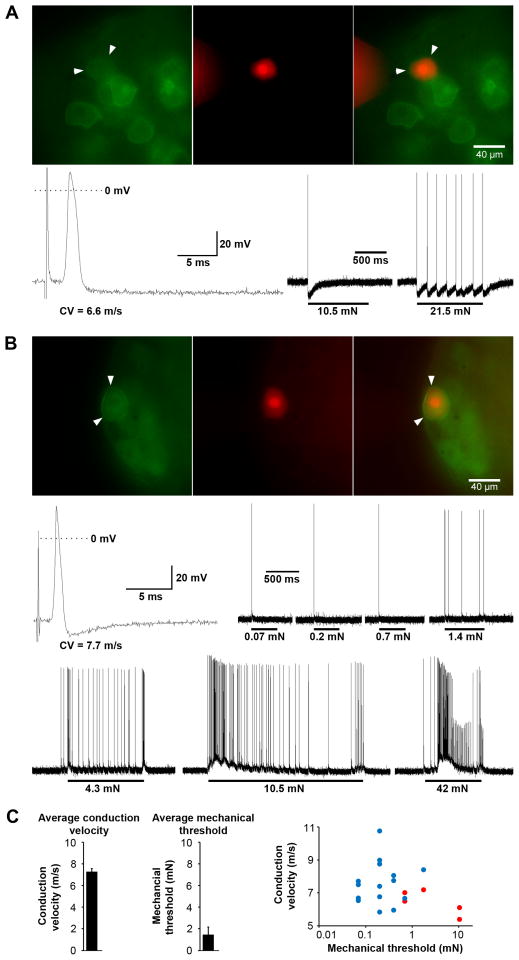
DORGFP+ myelinated afferents respond to innocuous and noxious mechanical stimuli
Our neuroanatomical analysis demonstrating that Meissner corpuscle and Merkel cell afferents express DORGFP suggested that these DORGFP+ DRG neurons are Aβ LTMRs. However, neither the physiological properties nor the mechanosensitivity of other NF200+ DORGFP+ DRG neurons forming circumferential or free nerve endings are established. To address this question we used a largely intact somatosensory system preparation, and characterized 20 randomly selected large-diameter DORGFP+ DRG neurons. These neurons had conduction velocities (CVs) between 5.4 and 10.7 m/s and relatively broad somal action potentials, with inflections on the falling phase of the spike, a property of Aδ nociceptors (Koerber and Woodbury, 2002; Lawson, 2002) (Figure 6). Importantly, all 20 of these DORGFP+ neurons responded to mechanical stimulation. We further tested nine of these 20 cells for heat sensitivity and found that, in contrast, only one cell responded to noxious heat and did so weakly. DORGFP+ Aδ fibers are thus AM mechanonociceptors, and probably do not contribute to thermosensation. Furthermore, the DORGFP+ Aδ nociceptors were not uniform, but rather consisted of at least two distinct populations. One population included cells with broad somal spikes, high mechanical threshold (including cells responding only to noxious stimuli >10mN), single spot-like small receptive fields (RFs), and pronounced long-lasting activity-induced hyperpolarization (Figures 6A and 6C). These features are characteristic of myelinated high-threshold mechanonociceptors that project to lamina I-IIo and V of the dorsal horn (Woodbury and Koerber, 2003).
Figure 6. DORGFP-expressing DRG neurons are mechanosensitive and include high- and low-threshold Aδ mechanonociceptors.
(A) The conduction velocity (CV, 6.6 m/s), broad action potential (bottom left), high mechanical threshold (10.5 mN, bottom right), and activity-induced hyperpolarization of the DORGFP+ neuron (green; red: neurobiotin) in (A) are characteristics of high-threshold Aδ mechanonociceptors.
(B) In comparison to (A) the DORGFP+ Aδ fiber recorded in (B) exhibits a relatively narrow action potential and low mechanical threshold (0.07 mN, similar to LTMRs), indicating that this myelinated nociceptor might also participate in touch sensation.
(C) Conduction velocity (7.3 m/s ± 0.3 m/s) and mechanical threshold (1.5 mN ± 0.7 mN) of 20 DORGFP+ DRG neurons show that most neurons that project to the trunk skin are low-threshold Aδ mechanonociceptors (see example in (B)). These cells (blue symbols in right panel) generally have large receptive fields composed of multiple isolated spots, while the other, comparatively high-threshold, population of DORGFP+ mechanonociceptors (red; see example in (A)) tends to have smaller receptive fields composed of a single spot.
Data are represented as means ± SEM.
The second, larger population of DORGFP+ Aδ nociceptors comprised cells with relatively low mechanical thresholds, from 0.07 to 0.7 mN, narrower action potentials, and large RFs composed of multiple isolated spots (Figures 6B and 6C). These afferents likely correspond to a population of myelinated mechanoreceptors that project widely from spinal laminae I to V (Woodbury and Koerber, 2003). Thus, while the latter DORGFP+ cells encode stimulus intensity and fire vigorously in response to noxious mechanical stimuli, which defines them as nociceptors, these afferents also respond to light touch and innocuous mechanical force (<5 mN), project to the deep dorsal horn, and thus might participate in both touch and pain sensation.
Note that a few DORGFP+ neurons had Aβ CVs and very narrow, uninflected spikes indicating that they were putative low-threshold mechanoreceptors. We could not identify and characterize the physiological properties of these DORGFP+ Aβ fibers because their axons did not travel out the intact dorsal cutaneous nerves and instead were cut during preparation (see Supplemental Experimental Procedures), but we presume that they may correspond to DORGFP+ SAI or hair follicle afferents (Figure 5).
Presynaptic DORs depress glutamate release from myelinated mechanoreceptors onto laminae III–V neurons of the spinal dorsal horn
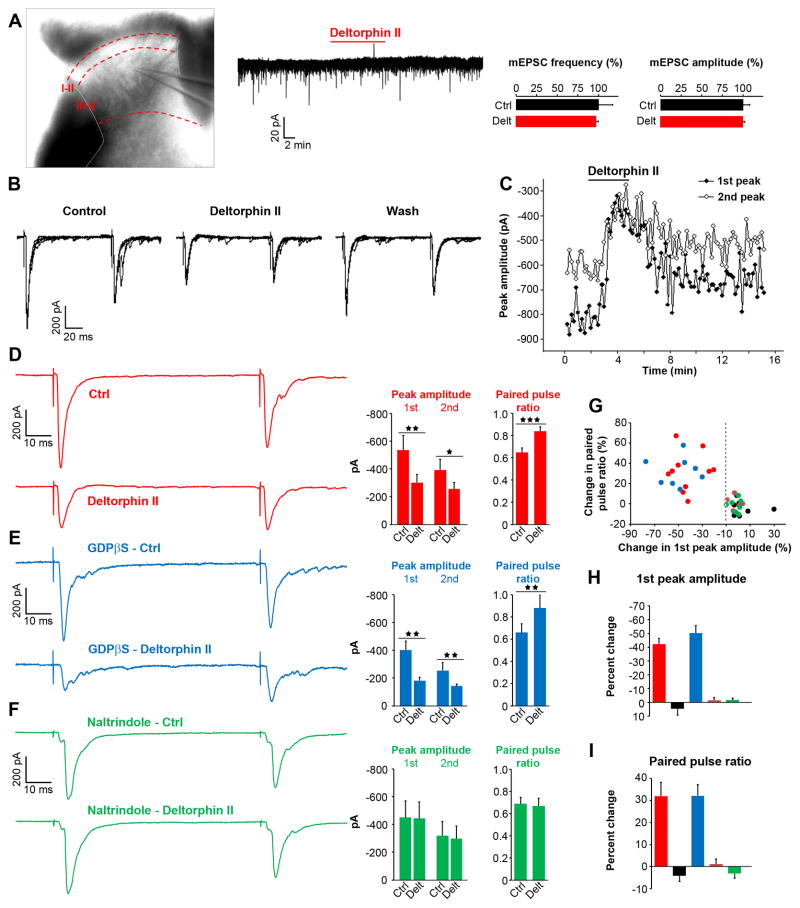
The dominant action of opioid receptors in the nervous system is to depress neurotransmission by Gi/o protein βγ subunit-mediated modulation of ion channel conductances (Williams et al., 2001). In sensory neurons, MOR agonists inhibit voltage-gated calcium channels (VGCCs) to reduce C fiber-derived glutamatergic neurotransmission to laminae I–II spinal neurons (Heinke et al., 2011; Taddese et al., 1995), consistent with MOR expression in peptidergic C nociceptors that project to superficial laminae of the dorsal horn. We reasoned that DOR function in myelinated mechanoreceptors might parallel that characteristic action of MOR on C nociceptors. To test this hypothesis, we examined the effect of the DOR-selective agonist deltorphin II on glutamatergic neurotransmission between A fibers and spinal neurons in laminae III–V. Indeed the myelinated afferents that express DORGFP, including SAI Aβ afferents, RA Meissner corpuscles Aβ afferents (Luo et al., 2009), and myelinated mechanonociceptors (Woodbury and Koerber, 2003), project to this area of the spinal cord dorsal horn.
We used spinal cord slices from wildtype mice to test the presence of DOR at the terminals of myelinated mechanoreceptors. We first recorded miniature excitatory postsynaptic currents (mEPSCs), reflecting spontaneous release of glutamate from sensory neurons and excitatory interneurons, and found that deltorphin II (500 nM) altered neither the amplitude nor the frequency of mEPSCs (Figure 7A). We therefore took a different approach and recorded EPSCs evoked by stimulation of the dorsal root at an intensity that triggers glutamate release from Aβ fibers. We used a paired pulse protocol to examine deltorphin II’s presynaptic effects on monosynaptic eEPSCs. We observed that deltorphin II significantly reduced the peak amplitude of the first eEPSC by ~56% in 10 of the 17 recorded cells (i.e. 10 responsive neurons in which deltorphin II reduced peak amplitude by at least 10%, Figures 7B–D and 7G–H). This effect was reversible with wash (Figures 7B–C). The ratio of the peak amplitude of the second eEPSC over the first is generally related to the probability of neurotransmitter release. We found that deltorphin II significantly increased the paired pulse ratio in responsive cells (~32%, Figure 7D, 7G and 7I), consistent with deltorphin II reducing glutamate release from Aβ fibers. To test for a possible contribution of DOR expressed by the postsynaptic neuron under study, we interrupted G protein activation in the recorded cells by adding GDP-βS to the pipette solution. We again observed a deltorphin II-induced decrease in first eEPSC peak amplitude and an increase in paired pulse ratio in a similar proportion of spinal neurons (8 of the 14 recorded cells), indicating that deltorphin II’s effect does not require postsynaptic activation of G proteins (Figures 7E and 7G–I). Importantly, application of the selective DOR antagonist naltrindole blocked the deltorphin II effect. Thus, in the presence of naltrindole, deltorphin II altered neither the first peak amplitude nor the paired pulse ratio in any of the 9 recorded cells (Figures 7F–I), while naltrindole alone had no effect (Figure S5). Taken together, our findings support the conclusion that a presynaptic activation of DORs on the central terminals of Aβ fibers underlies deltorphin II’s action, and that DOR functions as a regulator of innocuous mechanical input to the spinal cord.
Figure 7. Presynaptic DORs depress Aβ fiber input to laminae III–V spinal neurons.
(A) Voltage-clamp recordings of laminae III–V neurons in spinal cord slices from wildtype mice show that the DOR agonist deltorphin II (500 nM) did not significantly change miniature EPSC (mEPSC) frequency or amplitude (n=15).
(B) Example of EPSCs evoked in a lamina III spinal neuron by stimulation of monosynaptically connected Aβ fibers using a paired pulse protocol. Deltorphin II (500 nM) depressed EPSC peak amplitude and altered the ratio between the amplitudes of the 2nd and 1st peaks.
(C) Time course of the deltorphin II inhibitory effect on EPSC peak amplitudes.
(D) Deltorphin II (500 nM) significantly reduced the first peak amplitude and increased the paired pulse ratio in 10 of the 17 recorded cells (responsive neurons).
(E) GDPβS (1 mM in internal solution) did not block deltorphin II’s effect (8/14 recorded neurons with GDPβS responded to deltorphin II, a proportion similar to (D)), which confirms a presynaptic mechanism of action.
(F) The DOR antagonist naltrindole (1 μM) prevented deltorphin II’s effect in all recorded neurons (n=9).
(G) Summary showing all recorded cells. Red, deltorphin II responsive neurons; black, non-responsive neurons; blue, deltorphin II responsive neurons with GDPβS; brown, non-responsive neurons with GDPβS; green, cells recorded in the presence of naltrindole. The dotted line represents the threshold value (−10%) of percent change of 1st peak amplitude at which cells were considered responsive.
(H) Average deltorphin II effect on 1st peak amplitude. Color code as in (G).
(I) Average deltorphin II effect on paired pulse ratio. Color code as in (G).
Data are represented as means ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001.
DOR activation inhibits VGCCs in myelinated mechanoreceptors
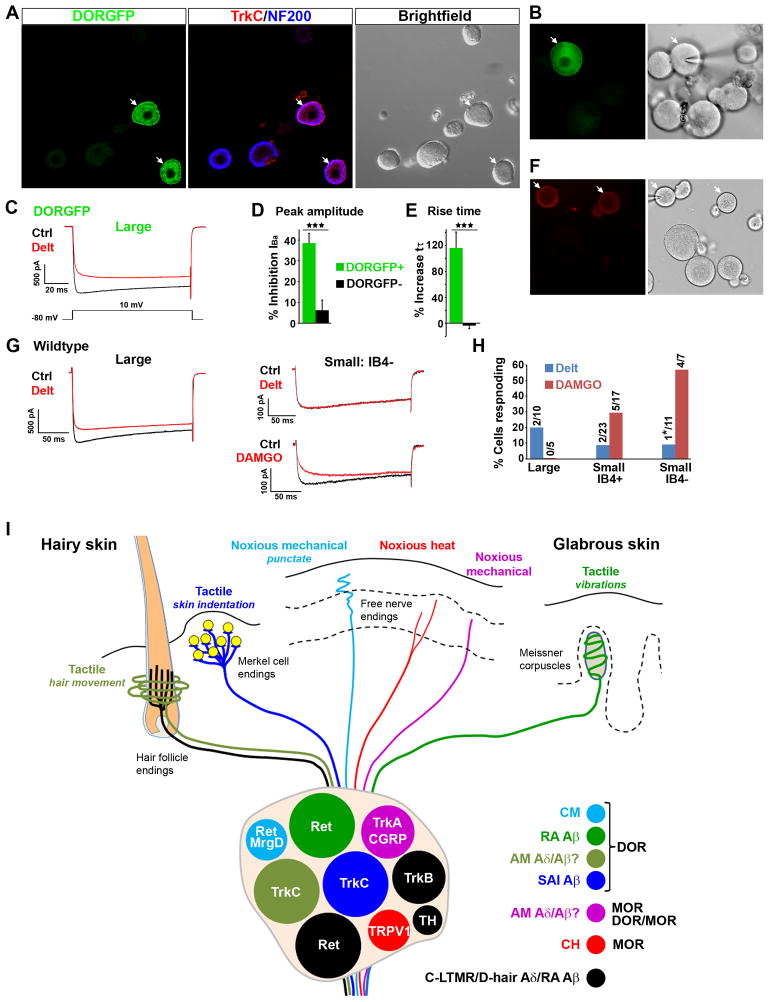
We next investigated the molecular mechanism by which DOR modulates glutamate release from myelinated mechanoreceptors using DRG primary culture from DORGFP mice. Figures 8A and S6 show that the neurochemistry of DORGFP+ cultured cells corresponds to what we observed in DRG sections: most large-diameter DORGFP+ neurons coexpressed NF200 and TrkC (Figure 8A), and the great majority of small-diameter NF200− DORGFP+ neurons bound IB4 (Figure S6).
Figure 8. DOR-mediated inhibition of voltage-gated calcium channels in cultured large-diameter DRG neurons and functional organization of DOR and MOR in cutaneous somatosensory neurons.
(A) Large-diameter NF200+ DORGFP+ cells express TrkC in primary DRG culture (arrows).
(B) DOR-expressing large-diameter DRG neurons (brightfield image at right) are identified by direct visualization of DORGFP fluorescence (arrow, left image).
(C) Deltorphin II (500 nM) reduces amplitude and increases rise time of voltage-activated calcium currents in DORGFP+ DRG neurons, but not in DORGFP- DRG neurons.
(D) Average effect of deltorphin II on current amplitude in DORGFP+ (n=11) and DORGFP− large-diameter neurons (n=7).
(E) Average effect of deltorphin II on current rise time in DORGFP+ (n=11) and DORGFP− large-diameter neurons (n=7). Results are expressed as mean ± SEM, *** p < 0.001.
(F) Labeling of IB4+ neurons in DRG culture from a wildtype mouse.
(G) In the DRG culture shown in (F) deltorphin II (500 nM) predominantly inhibited VGCCs in large-diameter neurons (> 40 pF), while DAMGO (500 nM) predominantly inhibited VGCCs in small-diameter (< 40 pF) IB4− neurons.
(H) Occurrence of deltorphin II- and DAMGO-mediated inhibition of VGCCs in 44 randomly selected neurons in DRG cultures from wildtype mice. Only one cell out of 29 tested for deltorphin II- and DAMGO-responsiveness (*) showed VGCC inhibition by both drugs.
(I) Schematic of the functional organization of DOR and MOR in cutaneous somatosensory neurons. DOR is expressed by populations of TrkC+ and/or Ret+ myelinated mechanoreceptors, which include SAI and RA Aβ LTMRs that form the Merkel cell-neurite complex (yellow) and Meissner corpuscles, respectively. DOR is also expressed by myelinated mechanonociceptors (AM), some of which are Aδ fibers, have low threshold, and respond to innocuous mechanical stimuli. The axons derived from DOR+ TrkC+ AM Aδ mechanonociceptors, which often coexpress Ret, may form circumferential endings around hair follicle, although this remains speculative. Additionally, DOR is expressed by Ret+ MrgprD+ C mechanonociceptors (CM) that innervate epidermal stratum granulosum. By contrast MOR is predominantly expressed by capsaicin- and heat-sensitive TRPV1+ C nociceptors (CH) (many of which are visceral, not cutaneous, afferents) and regulates heat pain perception. Colocalization of DOR and MOR occurs in myelinated TrkA- and CGRP-expressing neurons, which are presumptive high-threshold Aδ or Aβ mechanonociceptors. Most TH+ C LTMRs, TrkB+ D-hair mechanoreceptors, and Ret+ Aβ LTMRs that form longitudinal endings around hair follicles do not appear to express DOR or MOR.
Data are represented as means ± SEM.
To determine whether DOR activation by deltorphin II affects VGCC function in myelinated mechanoreceptors, we recorded voltage-activated calcium currents in large-diameter DORGFP+ neurons (Figure 8B). Figures 8C–E illustrate that deltorphin II (500 nM) strongly inhibited VGCCs in all DORGFP+ cells recorded, with peak amplitude decreased by ~38% compared to control. Furthermore, current rise time was significantly increased by deltorphin II (~116% of control) (Figures 8C–E). In DORGFP-negative large-diameter neurons, deltorphin II had no effect on current amplitude or rise time. We next used a DRG primary culture from wildtype mice to test whether deltorphin II also inhibits VGCCs in large-diameter neurons in this preparation. We labeled cultured neurons with IB4-biotin and streptavidin-Alexa Fluor 555 and recorded deltorphin II effects in a total of 43 wildtype DRG neurons (Figure 8F). Deltorphin II (500 nM) inhibited VGCCs in 2 of 10 large-diameter DRG neurons, in 2 of 23 IB4+ small-diameter DRG neurons, and in 1 of 11 IB4− small-diameter DRG neurons (Figures 8G and 8H). When a high-resistance seal could be maintained, we applied the MOR agonist DAMGO (500 nM) after deltorphin II and wash. DAMGO inhibited VGCCs in 4 of 7 IB4− small-diameter DRG neurons, in 5 of 17 IB4+ small-diameter DRG neurons, and in none of the 5 large-diameter DRG neurons tested (Figures 8G–H). Thus small-diameter neurons frequently responded to DAMGO (9/24, 38%), but rarely to deltorphin II (3/34, 9%). This distinction was even more pronounced when considering specifically the IB4− subpopulation that comprise peptidergic C nociceptors. Deltorphin II-responding neurons represent a discrete population of DRG neurons, most of which have large-diameter cell bodies, consistent with the idea that DOR regulates neurotransmitter release from myelinated mechanoreceptors. Only 1 cell out of 29, an IB4− small-diameter DRG neuron, responded to both drugs.
Taken together, our histological and electrophysiological results in DORGFP and wildtype mice support a model of the functional organization of the opioid system in primary afferent sensory neurons in which DOR and MOR engage common molecular mechanisms to regulate neurotransmitter release from functionally distinct primary afferent DRG neurons. The product of this segregated opioid receptor organization is a modality-specific regulation of somatosensation.
DISCUSSION
DOR expression pattern in DRG and CNS
Central to the understanding of how opioids influence somatosensation is an analysis of the distribution profile of opioid receptors in DRG neurons. Here we localized DOR in wildtype mouse tissues using in situ hybridization, radioligand binding, calcium current inhibition in DRG neurons, and inhibition of neurotransmitter release by Aβ fibers in spinal cord slices. The results clearly indicate that, as we proposed based on the analysis of DORGFP knockin mice (Scherrer et al., 2009), DOR is predominantly expressed by large-diameter myelinated DRG neurons, as well as by a subpopulation of IB4-binding nonpeptidergic unmyelinated nociceptors, but very rarely by SP+ unmyelinated nociceptors. Importantly, our conclusions are consistent with previous studies performed in wildtype mice and rats that reported that “intense DOR mRNA hybridization was primarily observed over large ganglion cells immunopositive for neurofilament 200” (Mennicken et al., 2003), and that “among the neurons which highly expressed mu-, delta- or kappa-OPR mRNA, PPTA mRNA was not expressed in about 58%, 95% or 24% of those neurons, respectively” (Minami et al., 1995).
On the other hand conclusions derived from use of the anti-DOR3-17 antibody disagree with these earlier findings, and with ours. In those studies, strong DOR3-17-ir is found in small-diameter DRG neurons, and colocalized with SP (Bao et al., 2003; Guan et al., 2005). The mismatch is even more striking in the spinal cord, where DOR3-17-ir was found to be restricted to the terminals of peptidergic afferents in laminae I–II. By contrast DOR ligand binding in wildtype mice, as well as DORGFP fluorescence, are observed throughout the spinal grey matter, consistent with in situ hybridization and electrophysiological recordings in spinal cord from rats that established the existence of DOR-expressing spinal neurons (Cahill et al., 2001; Eckert et al., 2001; Mennicken et al., 2003). Because DOR3-17-ir is intact in two strains of Oprd1 knockout mice, we proposed that anti-DOR3-17 antibody recognizes a different molecule and misrepresents DOR distribution (Scherrer et al., 2009). This statement applies to several other commercial antibodies that we have evaluated (Scherrer et al., 2009), including an anti-DOR2-18 antibody (Wang et al., 2010) that recognizes an unidentified surface molecule expressed mostly in large-diameter, Runx3+ DORGFP− DRG neurons (Figure S7). To explain the discrepancy between the DOR expression pattern obtained with DORGFP mice and with the anti-DOR3-17 antibody, Wang et al. proposed that the GFP tag altered subcellular localization and trafficking of the receptor in DORGFP mouse. Note that here DORGFP knockin mice were used to identify DOR-expressing DRG neurons, not to study receptor distribution inside the cell. Because a variety of experimental conditions differ between DORGFP expression in the reporter mouse and the heterologous overexpression of distinct DOR-GFP fusion constructs in in vitro systems, it is unclear that differences in trafficking reported by Wang et al. are relevant to the DORGFP receptor in vivo. In fact, the agreement between the DORGFP fluorescence pattern and the DOR radioligand binding pattern in wildtype mice, both of which notably arise from labeling of receptors trafficked to cellular compartments such as dendrites and axon terminals, together with the intact antinociceptive and prolocomotive properties of DOR agonists in DORGFP mice (Pradhan et al., 2009; Scherrer et al., 2006) strongly argues that the DORGFP traffics and functions as does the native DOR.
Surprisingly, in situ hybridization and electrophysiological experiments performed by Wang et al. also suggested that DOR was expressed by the majority of small-diameter neurons, including peptidergic C nociceptors (Wang et al., 2010). It is possible that inadequate in situ hybridization probes and/or excessive probe concentration or reaction time, led to non-specific labeling of DOR-negative DRG neurons. As for the electrophysiological experiments, seemingly excessive concentrations of DOR agonists (10 μM of deltorphin II or SNC80) were used for ligands with KD in the nanomolar range. At these high concentrations, off-target effects cannot be excluded, in particular cross-activation of MOR by these ligands, a finding already documented using Oprd1 and Oprm1 knockout mice (Gendron et al., 2007; Scherrer et al., 2004). Note also that Wang et al. omitted to consider the possibility that convergent inputs from distinct DOR+ and MOR+ fibers can modulate neurotransmitter release onto a given spinal neuron, without requiring coexpression of the two receptors in the same primary afferent neurons. Similarly, data on the inhibition of spinal SP release by intrathecal DOR agonists (for example (Beaudry et al., 2011; Kouchek et al., 2013)) should be interpreted carefully, because both SP and DOR are expressed, and possibly coexpressed, by numerous spinal neurons, thus preventing definitive conclusions to be drawn regarding DOR and SP coexpression in DRG neurons. By contrast, CGRP is restricted to primary afferent terminals in the dorsal horn, and our data showing DOR and CGRP coexpression in large-diameter NF200+ DRG neurons, and DOR-mediated inhibition of VGCCs in these cells, are consistent with the idea that DOR agonists can reduce CGRP release (Overland et al., 2009) and further suggest this effect results mostly from an action on myelinated nociceptors.
Functional organization of opioid receptors in primary afferent somatosensory neurons
We showed here that most DORGFP+ NF200+ DRG neurons coexpress TrkC and/or Ret, which mark several categories of myelinated mechanoreceptors (Bourane et al., 2009; Funfschilling et al., 2004; Luo et al., 2009; Senzaki et al., 2010) (Figure 8I). In retrograde labeling experiments, we found that many myelinated DORGFP+ neurons project to the dorsal column nuclei. Analysis of sensory neruon terminals in the skin demonstrated that DORGFP+ DRG neurons innervate hair follicles, Merkel cells and Meissner corpuscles, forming mechanosensory organs essential for touch. Recordings from DORGFP+ DRG neurons further showed that DOR is expressed by myelinated mechanonociceptors, including some with mechanical thresholds in the innocuous range. Electrophysiological experiments in spinal cord slices also established that DOR is present on the central terminals of Aβ fibers. Altogether these data indicate that DOR+ DRG neurons are mechanoreceptors, and include populations of low-threshold afferents that generate touch sensation (Figure 8I).
In sharp contrast with DOR+ DRG neurons, we found that the majority of MOR+ DRG neurons display molecular and anatomical features characteristic of NGF-dependent peptidergic nociceptors (lack of expression of Ret, TrkC, and TrkB; no projection to the DCN; coexpression of CGRP and TRPV1). Our analysis in DRG agrees with the fact that MOR agonists reduce synaptic transmission between C and Aδ nociceptors and superficial dorsal horn neurons (Heinke et al., 2011). Furthermore, morphine alters neither responses of gracile nucleus neurons evoked by Aβ fiber stimulation or brushing of the receptive field (Suzuki and Dickenson, 2002), touch-evoked Fos expression in the dorsal horn (Catheline et al., 2001), nor dynamic allodynia induced by strychnine-mediated disinhibition of dorsal horn circuits (Miraucourt et al., 2009). We propose, therefore, that MOR regulates neurotransmitter release from cutaneous nociceptive peptidergic TRPV1+ afferents that are heat sensitive and mechanically insensitive (Lawson et al., 2008) and, perhaps of particular clinical relevance, from TRPV1+ visceral afferents that signal injuries to deep tissues (e.g. postsurgical pain) (Figure 8I). Note, however, that MOR+ NF200+ DRG neurons might include some Aβ nociceptive fibers (Lawson et al., 2002) or Aβ LTMRs that do not project to the DCN. In our electrophysiological experiments in cultured DRG neurons from wildtype mouse, we found that DAMGO had no effect on VGCCs in the 5 large-diameter DRG neurons tested (Figure 8). This result is consistent with the idea that MOR is expressed by a relatively small population of NF200+ neurons (about 20%) (Figures 3 and 4), and suggests that blind recordings from many more DRG neurons would be necessary to determine precisely the proportion of myelinated neurons that express MOR. Note also that the DRG culture might not be fully representative of the neuronal diversity and proportions found in vivo because some neurons might be more sensitive than others to the dissociation procedure. Such factors might explain the subtle differences between electrophysiological results in DRG culture and those from our histological analysis.
Since DOR inhibits VGCCs in large-diameter NF200+ neurons, as does MOR in C fibers, our results suggest that in DRG the specific function of DOR and MOR in the control of somatosensation originates mostly from expression in distinct neuronal populations. Of course this arrangement does not exclude the existence of additional cell- or receptor-specific signaling mechanisms, such as those that regulate DOR surface expression and functional competence (Hack et al., 2005; Patwardhan et al., 2005; van Rijn et al., 2012). Note that it is possible to isolate the main action of intrathecal DOR and MOR agonists against mechanical and heat pain, respectively, when using low doses of SNC80 and DAMGO (Scherrer et al., 2009). On the other hand, it is well established that DOR and MOR agonists can reduce both mechanical and heat pain (reviewed in Gaveriaux-Ruff and Kieffer, 2011; Ossipov et al., 2004), especially when higher doses are used. Future studies will clarify whether this antinociceptive action across pain modalities results from the co-recruitment of rarer populations of afferents (i.e. heat-sensitive DOR+ and mechano-sensitive MOR+), polymodal spinal and descending control systems, or the combined activation of multiple opioid receptor types, possibly including multimers.
It is significant that we have identified discrete populations of neurons that do coexpress both receptors. First, as we reported previously (Scherrer et al., 2009), about 10% of DOR+ NF200− small-diameter DRG neurons are TRPV1+ peptidergic C nociceptors, all of which express MOR. Second we now establish that the vast majority of DOR/MOR coexpressing DRG neurons are in fact DRG neurons with myelinated axons (NF200+) that express CGRP and TrkA. Analogous to the MrgprA3-expressing DRG cells that only represent about 4% of DRG neurons but are entirely responsible for chloroquine-induced pruritus (Liu et al., 2009), the DOR/MOR-coexpressing cells likely have a significant and selective function in somatosensation despite their small number (<5% of all DRG neurons). Because CGRP+ cutaneous A fibers are mostly high-threshold mechanonociceptors (AM class (Koerber and Woodbury, 2002; Lawson et al., 2008)), our results suggest that studies examining the functional relevance of interactions between DOR and MOR should include behavioral assays of Aδ or Aβ fiber-mediated mechanical pain. Our model (Figure 8I) thus proposes that opportunities for DOR/MOR interaction in DRG, including DOR/MOR heterodimerization, is likely restricted to discrete cell populations, with the corollary that agents modulating these interactions may be useful to treat pain disorders resulting from dysfunction of these particular neurons, with limited side effects.
DOR-mediated control of cutaneous mechanosensation
Numerous studies have demonstrated that DOR-selective agonists reduce mechanical hypersensitivity induced by nerve or tissue injury in rodents (Gaveriaux-Ruff and Kieffer, 2011). Because selective activation of DOR has limited utility against acute heat pain these results were considered to reflect the prime function of DOR in the control of chronic, but not acute, pain. In light of accumulating evidence supporting the existence of peripheral labeled lines for distinct pain modalities (Basbaum et al., 2009), we rather propose that it is the preferential association of DOR with myelinated mechanonociceptors and LTMRs that underlies the particular analgesic profile of DOR agonists against acute mechanical pain and chronic mechanical hypersensitivity. A previous study using mice with a conditional deletion of Oprd1 in DRG neurons expressing the sodium channel Nav1.8 suggested that DOR+ unmyelinated nociceptors, rather than NF200+ neurons, were essential for the anti-allodynic effect of DOR agonists (Gaveriaux-Ruff et al., 2011). However, a fate map analysis has since demonstrated that Cre expression in DRG from Nav1.8-Cre mice is, in fact, not restricted to nociceptors, but rather is also observed in several categories of C and A LTMRs (Shields et al., 2012). As a result, the precise population of DRG neurons that underlies DOR-mediated anti-allodynia remains to be identified.
Our demonstration that DOR+ NF200− DRG neurons belong to the MrgprD+ subset of nonpeptidergic, IB4-binding, Ret+ C nociceptors (Figures S3E and 8I) agrees with a recent qPCR study that showed that Ret is essential for the normal expression of Oprd1 in the DRG (Franck et al., 2011) and the observation that a DOR agonist inhibits GDNF-induced hyperalgesia (Joseph and Levine, 2010). MrgprD+ DRG neurons respond to punctate noxious mechanical stimulation and are essential for acute mechanical pain, but not persistent injury-induced mechanical hypersensitivity (Cavanaugh et al., 2009). Conceivably, these DOR+ C nociceptors underlie the effect of DOR agonists on basal mechanical thresholds, but are unlikely to mediate their anti-allodynic effects in models of inflammatory or neuropathic pain.
By contrast, DOR+ myelinated mechanoreceptors emerge as strong candidate neurons to signal mechanical allodynia. First, sensory testing in neuropathic pain patients indicates that large-diameter myelinated fibers, not C fibers, are essential for mechanical allodynia (Campbell et al., 1988). Second, the sensory modalities encoded by DOR+ myelinated mechanoreceptors precisely correspond to the innocuous stimuli that evoke pain in allodynic patients. Thus movement of objects across the skin or light pressure that typically generate pain (dynamic or static allodynia, respectively), such as is caused by clothing, are expected to engage DOR+ hair follicle, Meissner corpuscle and/or SAI afferents (Figure 8I). These DOR+ afferents could fire more vigorously in response to light touch after injury, causing pain. Alternatively, their normal response to innocuous mechanical stimulation could cause pain after injury if central disinhibition mechanisms are in effect. These two scenarios are not mutually exclusive, and future studies will investigate the mechanisms by which DORs present on the peripheral terminals of mechanoreceptors may regulate threshold and firing pattern to contribute to opioid analgesia (Stein and Machelska, 2011).
In summary our results provide a new conceptual framework for the functional organization of opioid receptors in primary afferent neurons and the inhibitory mechanisms by which opioids control mechanosensation. This model provides a cellular basis for a more rational use of MOR agonists in the clinic and will foster the design of alternative analgesic strategies, including DOR targeting, based on the identity of the primary afferents engaged and the nature of the stimulus causing pain, particularly for touch-evoked neuropathic pain and movement/pressure-evoked pain, two major clinical problems.
EXPERIMENTAL PROCEDURES
See Supplemental Experimental Methods for further details.
Animals
All procedures were approved by the Stanford, Columbia, or UCSF Institutional Animal Care and Use Committees. See Supplemental Experimental Methods for a description of the mice used.
Spinal cord slice preparation and electrophysiology
The lumbar spinal cord of P15–22 C57BL/6 mice was isolated, and transverse slices with dorsal roots attached were made using a vibrating microtome. Patch-clamp recording in whole-cell configuration and voltage-clamp mode at a holding potential of −70 mV was performed at RT on laminae III–V neurons. EPSCs were evoked (eEPSCs) by stimulating the dorsal root with a suction electrode (25 to 50 μA intensity, 0.1 ms duration). eEPSCs with constant latency and no failures during a train of 20 stimuli at 50 Hz were considered monosynaptic responses mediated by Aβ fibers. Paired-pulse recordings were obtained by stimulating the dorsal root continuously at 0.1 Hz.
Primary DRG culture and electrophysiology
Lumbar and thoracic DRGs from P21–42 DORGFP or C57BL/6 wildtype mice were collected, digested, centrifuged, resuspended in MEM complete medium, and plated onto Poly-D,L,ornithine hydrobromide coated coverslips. Patch-clamp recording in whole-cell configuration was performed 2 to 6 hours after plating. Cells were classified as small or large based on capacitance (small < 40 pF < large). Recordings were performed in voltage-clamp mode with −80 mV holding potential and a 100 or 200 ms depolarization to 10 mV was used to activate VGCCs before and after perfusion of deltorphin II (500 nM) or DAMGO (500 nM). Current amplitude and rise time (20%–80%) were determined using Clampfit software.
Histology
Immunostaining was performed as described previously (Li et al., 2011; Scherrer et al., 2009). Briefly, mice were anesthetized and perfused transcardially with phosphate-buffered saline followed by 10% formalin solution. Lumbar DRGs were dissected and cryoprotected in sucrose solution. Skin was dissected, postfixed in 10% formalin, cryoprotected and cut at 20 μm or processed for whole mount staining. Tissue sections were incubated in blocking solution for one hour at RT, primary antibody overnight at 4°C, and secondary antibody for 2 hours at RT. Whole mount staining was performed as described by Li et al. (Li et al., 2011). Images were acquired with a confocal microscope. Methods for in situ hybridization are described in detail in Supplemental Experimental Methods. Radioligand binding experiments have been performed as previously described (Scherrer et al., 2009).
Largely intact somatosensory system preparation and electrophysiology
This preparation has been described previously (Koerber and Woodbury, 2003). Briefly, mice were anesthetized and perfused transcardially with oxygenated artificial CSF. The spinal cord, thoracic DRGs, dorsal cutaneous nerve, and trunk skin were dissected in continuity and pinned out in a recording chamber. DORGFP+ somata were impaled with micropipettes, and electrical search stimuli were delivered to the intact dorsal cutaneous nerve through an en passant suction electrode to identify cells with intact peripheral axons. Peripheral receptive fields (RFs) were located with a fine sable paint brush or blunt glass stylus, and mechanical thresholds were determined with calibrated von Frey filaments. Thermal sensitivity was tested by applying heated (52 °C) aCSF to the RF. Conduction velocity was calculated from spike latency and the distance between stimulating and recording electrodes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DA031777 (G.S.), DA029204 (A.I.B), NS029797 (A.B.M), NS044094 (C.J.W.), MIUR (PRIN 2008) and Fondazione Cassa di Risparmio di Modena (R.B. and C.B), Stanford University Department of Anesthesiology, Perioperative and Pain Medicine and Stanford Institute for Neuro-Innovation and Translational Neurosciences start-up funds (G.S), by an IASP International Trainee Fellowship funded by the Scan|Design Foundation BY INGER & JENS BRUUN (G.S.), and by a FAER Research Fellowship Grant (V.L.T.). We thank David Anderson (Caltech) for providing MrgprD-DTR mice, Thomas Jessell (Columbia University) for providing anti-TrkC antibody, anti-Runx3 antibody and Parvalbumin-Cre; (Gt)Rosa26Sor floxed-stop tdTomato reporter mice, David Julius (UCSF), Chris Evans (UCLA), and Louis Reichardt (UCSF) for providing various antibodies (see Supplemental Experimental Procedures for details). We thank Ellen Lumpkin (Columbia University) for stimulating discussions about skin biology, Jay Bikoff (Columbia University) for help with imaging brain sections from DORGFP mice, and Shannon Shields (Yale University) for helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011;31:13068–13077. doi: 10.1523/JNEUROSCI.1817-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourane S, Garces A, Venteo S, Pattyn A, Hubert T, Fichard A, Puech S, Boukhaddaoui H, Baudet C, Takahashi S, et al. Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron. 2009;64:857–870. doi: 10.1016/j.neuron.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Catheline G, Le Guen S, Besson JM. Intravenous morphine does not modify dorsal horn touch-evoked allodynia in the mononeuropathic rat: a Fos study. Pain. 2001;92:389–398. doi: 10.1016/S0304-3959(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert reviews in molecular medicine. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Doobar S, Jessell TM. Etv1 Inactivation Reveals Proprioceptor Subclasses that Reflect the Level of NT3 Expression in Muscle Targets. Neuron. 2013;77:1055–1068. doi: 10.1016/j.neuron.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- Eckert WA, 3rd, Willcockson HH, Light AR. Interference of biocytin with opioid-evoked hyperpolarization and membrane properties of rat spinal substantia gelatinosa neurons. Neuroscience letters. 2001;297:117–120. doi: 10.1016/s0304-3940(00)01684-0. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25:4868–4878. doi: 10.1523/JNEUROSCI.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck MC, Stenqvist A, Li L, Hao J, Usoskin D, Xu X, Wiesenfeld-Hallin Z, Ernfors P. Essential role of Ret for defining non-peptidergic nociceptor phenotypes and functions in the adult mouse. Eur J Neurosci. 2011;33:1385–1400. doi: 10.1111/j.1460-9568.2011.07634.x. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Ng YG, Zang K, Miyazaki J, Reichardt LF, Rice FL. TrkC kinase expression in distinct subsets of cutaneous trigeminal innervation and nonneuronal cells. J Comp Neurol. 2004;480:392–414. doi: 10.1002/cne.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, Reiss D, Filliol D, Nassar MA, Wood JN, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida R, Rustioni A. Dorsal root ganglion neurons projecting to the dorsal column nuclei of rats. J Comp Neurol. 1992;316:206–220. doi: 10.1002/cne.903160206. [DOI] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H, Luo J, Li ZY, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkuhler J. Multiple targets of mu-opioid receptor-mediated presynaptic inhibition at primary afferent Adelta- and C-fibers. J Neurosci. 2011;31:1313–1322. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience. 2010;171:344–350. doi: 10.1016/j.neuroscience.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav. 2002;77:589–594. doi: 10.1016/s0031-9384(02)00904-6. [DOI] [PubMed] [Google Scholar]
- Kouchek M, Takasusuki T, Terashima T, Yaksh TL, Xu Q. Effects of Intrathecal SNC80, a Delta Receptor Ligand, on Nociceptive Threshold and Dorsal Horn Substance P Release. The Journal of pharmacology and experimental therapeutics. 2013;347:258–264. doi: 10.1124/jpet.113.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61:30–44. doi: 10.1002/neu.20078. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. The Cochrane database of systematic reviews. 2013;8:CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of mu-, delta- and kappa-opioid receptor mRNAs with preprotachykinin A mRNA in the rat dorsal root ganglia. Brain Res Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor- independent mechanical allodynia. J Neurosci. 2009;29:2519–2527. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Vanderah TW, Porreca F. The delta opioid receptor subtypes and pain modulation. In: Chang K, Porreca F, Woods J, editors. The delta receptor. New York: Marcel Dekker; 2004. pp. 297–329. [Google Scholar]
- Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord. J Neurosci. 2009;29:13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopian AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4(5):e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Low SA, Wang X, Zhang J, Yamanaka H, Urban R, Solorzano C, Harper B, Hnasko TS, Edwards RH, Basbaum AI. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc Natl Acad Sci USA. 2010;107:22296–22301. doi: 10.1073/pnas.1013413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzaki K, Ozaki S, Yoshikawa M, Ito Y, Shiga T. Runx3 is required for the specification of TrkC-expressing mechanoreceptive trigeminal ganglion neurons. Mol Cell Neurosci. 2010;43:296–307. doi: 10.1016/j.mcn.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Stein C, Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacological reviews. 2011;63:860–881. doi: 10.1124/pr.110.003145. [DOI] [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci. 1998;18:7040–7046. doi: 10.1523/JNEUROSCI.18-17-07040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Dickenson AH. Nerve injury-induced changes in opioid modulation of wide dynamic range dorsal column nuclei neurones. Neuroscience. 2002;111:215–228. doi: 10.1016/s0306-4522(01)00617-0. [DOI] [PubMed] [Google Scholar]
- Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995;270:1366–1369. doi: 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- Torsney C. Inflammatory pain unmasks heterosynaptic facilitation in lamina I neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2011;31:5158–5168. doi: 10.1523/JNEUROSCI.6241-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Emergence of functional spinal delta opioid receptors after chronic ethanol exposure. Biological psychiatry. 2012;71:232–238. doi: 10.1016/j.biopsych.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci. 2003;23:601–610. doi: 10.1523/JNEUROSCI.23-02-00601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.