Abstract
The hippocampus is essential for the formation and retrieval of memories and is a crucial neural structure sub-serving complex cognition. Adult hippocampal neurogenesis, the birth, migration and integration of new neurons, is thought to contribute to hippocampal circuit plasticity to augment function. We evaluated hippocampal volume in relation to brain volume in 375 mammal species and examined 71 mammal species for the presence of adult hippocampal neurogenesis using immunohistochemistry for doublecortin, an endogenous marker of immature neurons that can be used as a proxy marker for the presence of adult neurogenesis. We identified that the hippocampus in cetaceans (whales, dolphins and porpoises) is both absolutely and relatively small for their overall brain size, and found that the mammalian hippocampus scaled as an exponential function in relation to brain volume. In contrast, the amygdala was found to scale as a linear function of brain volume, but again, the relative size of the amygdala in cetaceans was small. The cetacean hippocampus lacks staining for doublecortin in the dentate gyrus and thus shows no clear signs of adult hippocampal neurogenesis. This lack of evidence of adult hippocampal neurogenesis, along with the small hippocampus, questions current assumptions regarding cognitive abilities associated with hippocampal function in the cetaceans. These anatomical features of the cetacean hippocampus may be related to the lack of postnatal sleep, causing a postnatal cessation of hippocampal neurogenesis.
Keywords: Adult hippocampal neurogenesis, Hippocampus, Doublecortin, Memory, Mammalia, Cognition
Introduction
The hippocampus and associated cortices are neural structures thought to be fundamentally involved in the learning and retention of facts, events and space in time (Alme et al. 2010; Buzsáki and Moser 2013). In mammals, the hippocampus is reciprocally connected, through the entorhinal cortex, to virtually all areas of the neocortex. Once neural information reaches the entorhinal cortex, it is, for the most part, processed through the hippocampal circuitry and the neural information processed by the hippocampus then flows back to the neocortex, where it can be used in cognitive processes or consolidated as memories (Andersen et al. 2007). As the hippocampus is extensively interconnected with the neocortex, an altered anatomy of the hippocampus may lead to changes in neural processing with the neocortex, and hence alter, or even impair, cognitive functions (Sweatt 2004).
Within the hippocampal circuitry, the dentate gyrus has been proposed to function as a pattern separator, a neural process that allows the distinct representation of overlapping or similar inputs within this circuitry (Treves et al. 2008; Sahay et al. 2011). In addition to this specialized function, the dentate gyrus is one of only two areas in the mammalian brain where adult neurogenesis occurs, that is, the birth, migration, maturation and integration of new neurons into the existing circuitry throughout much of the life span (Kempermann 2012). Neurogenesis in the mammalian dentate gyrus is thought to enhance cognitive adaptability, as changes in active movement, novelty and complexity within an environment appear to up- or down-regulate the rate of adult neurogenesis (Kempermann 2012). Behavioural studies in laboratory rodents have demonstrated that ablation of adult neurogenesis in the dentate gyrus leads to the impairment of the ability of an organism to undertake pattern separation (Sahay et al. 2011; Clelland et al. 2009; Tronel et al. 2010). In addition, increasing the rate of adult hippocampal neurogenesis is sufficient to improve pattern separation (Sahay et al. 2011). These studies indicate that the newly generated and integrated granule cells in the dentate gyrus are critical for the process of pattern separation and hence learning and memory formation. This concept has been expanded into the memory resolution hypothesis, which indicates that the newly born, broadly tuned, young neurons interact with the specifically tuned mature neurons to increase the fidelity of spatial and contextual discrimination (Aimone et al. 2011). Thus, the structure of the hippocampal formation, along with the presence of adult hippocampal neurogenesis in the dentate gyrus, underscores the concept that the hippocampus is one of the key regions of the brain involved in complex cognitive processing (Andersen et al. 2007) that leads to complex behavioural outcomes.
Cetaceans (whales, dolphins and porpoises) are widely believed to express behaviours reliant upon complex cognitive activity (Marino et al. 2008). Certain smaller cetaceans, of the suborder Odontoceti, are known to have brains that, relative to body mass, are the second largest to humans (Manger 2006). This observation, coupled with specific interpretations of cetacean behaviour (Manger 2013), provides the bases for the concept that cetaceans are cognitively complex (Marino et al. 2008). Thus, cetacean brains are thought to be able to generate behaviours that are beyond the cognitive capabilities present in the brains of most other mammals; however, it is clear that the cetacean brain has a morphology that is distinctly different from that of all other mammals (Glezer et al. 1988; Manger 2006; Manger et al. 2004, 2012) and thus the concept that cetaceans are cognitively complex has been questioned (Manger 2006, 2013) and vigorously defended (Marino et al. 2008). One specific aspect of the morphology of the cetacean brain that led to the questioning of the level of cognitive complexity ascribed to cetaceans was the apparently small size and loosely organized appearance of the hippocampus—a well-known feature of cetacean neuroanatomy (Filimonoff 1965; Pilleri and Gihr 1970; Jacobs et al. 1971, 1979; Morgane et al. 1980; Schwerdtfeger et al. 1984; Manger 2006). With the discovery of specific endogenous markers to visualize immature neurons and thus adult hippocampal neurogenesis (using antibodies directed against doublecortin, DCX; Kempermann 2012), we decided to look for evidence of adult hippocampal neurogenesis and evaluate the absolute and relative size of the cetacean hippocampus in comparison to a broad range of other mammalian species. Hippocampal size and the presence or absence of adult hippocampal neurogenesis in the cetaceans would provide substantive information to the debate surrounding the purported cognitive complexity of species belonging to this mammalian order (Manger 2006, 2013; Marino et al. 2008).
Materials and methods
Volumetric analysis of the hippocampus and amygdala Data for total brain (n = 375), hippocampal (n = 375) and amygdala (n = 373) volumes were taken from the literature (Pirlot and Nelson 1978; Stephan et al. 1981; Baron et al. 1996; Reep et al. 2007; Montie et al. 2008) or calculated from MRI scans of the brain of species used in the current study (Manger et al. 2010; Patzke et al. 2013a) (Table 1). Several linear and non-linear regression models were fit to the log-transformed data (of all species apart from the cetaceans, elephants, hippopotami and manatee, which were excluded from the regression calculations to specifically test whether the data from these species fit, or did not fit, the models) and then ranked using goodness of fit criteria (r2; AICC, sum of squares) with the statistical software CurveExpert Professional version 1.6.5 (Hyams 2010). Phylogenetic independent contrasts were also calculated from the data to examine scaling relationships between hippocampal volume and brain volume while controlling for the effects of phylogenetic relatedness (Felsenstein 1985). Standardized independent contrasts were calculated using the PDAP:PDTREE module (Garland and Ives 2000) of Mesquite software version 1.12 (Maddison and Maddison 2005) from data based on the mammalian super-tree (Bininda-Emonds et al. 2007, 2008). Branch lengths were transformed according to the method of Pagel (1992), which assigns all branch lengths to 1 with the constraint that tips are contemporaneous. Alternative methods of branch length transformation did not significantly alter the results and independent contrasts were uncorrelated with their standard deviations, indicating that branch lengths met statistical assumptions (Garland et al. 1992). While independent contrast analysis is commonly used when exploring cross-taxonomic relationships, this technique is known to perform poorly when the underlying relationship between characters is non-linear. In accordance with suggestions pertaining to non-linearity (Garland et al. 1992; Quader et al. 2004), we log transformed our data and performed independent contrast analysis to evaluate the scaling of hippocampal volume with brain volume if a linear model were valid.
Table 1.
Species and data used in the current study for the volumetric analysis of the hippocampus
| Order/species | M b | V b | V h | S | Order/species | M b | V b | V h | S |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Monotremata | Microchiroptera | ||||||||
| Ornithorynchus anatinus | 9.17 | 8.85 | 0.51 | 1 | Nycteris grandis | 0.71 | 0.68 | 0.04 | 6 |
| Tachyglossus aculeatus | 27.52 | 26.56 | 1.77 | 1 | Nycteris hispida | 0.28 | 0.27 | 0.02 | 6 |
| Didelphimorphia | Nycteris javanica | 0.47 | 0.45 | 0.03 | 6 | ||||
| Didelphis marsupialis | 6.89 | 6.65 | 0.53 | 1 | Nycteris nana | 0.24 | 0.23 | 0.02 | 6 |
| Soricomorphia | Nycteris thebaica | 0.32 | 0.31 | 0.02 | 6 | ||||
| Solenodon paradoxus | 4.67 | 4.51 | 0.30 | 2 | Nycteris tragata | 0.46 | 0.44 | 0.02 | 6 |
| Afrosoricida | Megaderma lyra | 0.91 | 0.88 | 0.04 | 6 | ||||
| Tenrec ecaudatus | 2.57 | 2.48 | 0.15 | 2 | Megaderma spasma | 0.64 | 0.62 | 0.03 | 6 |
| Setifer setosus | 1.51 | 1.46 | 0.10 | 2 | Macroderma gigas | 1.70 | 1.65 | 0.07 | 6 |
| Hemicentetes semispinosus | 0.83 | 0.80 | 0.06 | 2 | Cardioderma cor | 0.67 | 0.65 | 0.03 | 6 |
| Echinops telfairi | 0.62 | 0.60 | 0.05 | 2 | Lavia frons | 0.64 | 0.62 | 0.03 | 6 |
| Oryzorictes talpoides | 0.58 | 0.56 | 0.06 | 2 | Rhinolophus cornutus | 0.19 | 0.19 | 0.02 | 6 |
| Microgale cowani | 0.42 | 0.41 | 0.04 | 2 | Rhinolophus eloquens | 0.40 | 0.39 | 0.03 | 6 |
| Limnogale mergulus | 1.15 | 1.11 | 0.08 | 2 | Rhinolophus hipposerdos | 0.15 | 0.14 | 0.01 | 6 |
| Nesogale dobsoni | 0.56 | 0.54 | 0.07 | 2 | Rhinolophus landeri | 0.28 | 0.27 | 0.02 | 6 |
| Nesogale talazaci | 0.79 | 0.76 | 0.10 | 2 | Rhinolophus lepidus | 0.19 | 0.18 | 0.01 | 6 |
| Micropotamogale lamottei | 0.80 | 0.077 | 0.08 | 2 | Rhinolophus luctus | 0.62 | 0.60 | 0.03 | 6 |
| Potamogale velox | 4.16 | 4.02 | 0.30 | 2 | Rhinolophus macrotis | 0.21 | 0.20 | 0.02 | 6 |
| Chlorotalpa stuhlmanni | 0.74 | 0.71 | 0.06 | 2 | Rhinolophus malayanus | 0.20 | 0.19 | 0.01 | 6 |
| Chrysochloris asiatica | 0.70 | 0.68 | 0.07 | 2 | Rhinolophus megaphyllus | 0.26 | 0.25 | 0.02 | 6 |
| Eulipotyphla | Rhinolophus megaphyllus | 0.23 | 0.22 | 0.02 | 6 | ||||
| Aethechinus algirus | 3.20 | 3.09 | 0.23 | 2 | Rhinolophus paradoxolo. | 0.27 | 0.26 | 0.02 | 6 |
| Erinaceus europaeus | 3.35 | 3.23 | 0.24 | 2 | Rhinolophus pearsoni | 0.33 | 0.32 | 0.03 | 6 |
| Hemiechinus auritus | 1.90 | 1.83 | 0.13 | 2 | Rhinolophus pusillus | 0.16 | 0.16 | 0.01 | 6 |
| Sorex minutus | 0.11 | 0.10 | 0.01 | 2 | Rhinolophus trifoliatus | 0.35 | 0.34 | 0.02 | 6 |
| Sorex araneus | 0.20 | 0.19 | 0.02 | 2 | Rhinolophus yunanensis | 0.44 | 0.42 | 0.03 | 6 |
| Neomys fodiens | 0.32 | 0.31 | 0.03 | 2 | Hipposideros armiger | 0.80 | 0.77 | 0.05 | 6 |
| Crocidura occidentalis | 0.44 | 0.43 | 0.04 | 2 | Hipposideros calcaratus | 0.40 | 0.39 | 0.03 | 6 |
| Crocidura russula | 0.19 | 0.18 | 0.02 | 2 | Hipposideros calcaratus | 0.34 | 0.32 | 0.02 | 6 |
| Suncus murinus | 0.38 | 0.37 | 0.03 | 2 | Hipposideros cervinus | 0.21 | 0.21 | 0.01 | 6 |
| Talpa europaea | 1.02 | 0.98 | 0.09 | 2 | Hipposideros commersoni | 0.75 | 0.72 | 0.05 | 6 |
| Desmana moschata | 4.00 | 3.86 | 0.27 | 2 | Hipposideros diadema | 0.71 | 0.69 | 0.05 | 6 |
| Galemys pyrenaicus | 1.33 | 1.28 | 0.11 | 2 | Hipposideros fulvus | 0.24 | 0.23 | 0.02 | 6 |
| Macroscelidea | Hipposideros halophyllus | 0.15 | 0.15 | 0.01 | 6 | ||||
| Elephantulus fuscipes | 1.33 | 1.28 | 0.17 | 2 | Hipposideros lankadiva | 0.73 | 0.71 | 0.05 | 6 |
| Rhynchocyon stuhlmanni | 6.1 | 5.89 | 0.58 | 2 | Hipposideros larvatus | 0.41 | 0.40 | 0.03 | 6 |
| Scandentia | Hipposideros lekaguli | 0.55 | 0.53 | 0.04 | 6 | ||||
| Tupaia glis | 3.20 | 3.09 | 0.15 | 2 | Hipposideros maggietaylor. | 0.63 | 0.61 | 0.04 | 6 |
| Tupaia minor | 2.58 | 2.49 | 0.13 | 2 | Hipposideros maggietaylor. | 0.45 | 0.44 | 0.03 | 6 |
| Urogale everetti | 4.28 | 4.13 | 0.17 | 2 | Hipposideros ridleyi | 0.27 | 0.26 | 0.02 | 6 |
| Primates | Hipposideros sperois | 0.28 | 0.027 | 0.02 | 6 | ||||
| Cheirogaleus major | 6.80 | 6.56 | 0.35 | 2 | Hipposideros turpis | 0.60 | 0.58 | 0.04 | 6 |
| Cheirogaleus medius | 3.14 | 3.03 | 0.17 | 2 | Asellicus stoliczkanus | 0.15 | 0.15 | 0.01 | 6 |
| Microcebus murinus | 1.78 | 1.72 | 0.10 | 2 | Asellicus tricuspidatus | 0.13 | 0.13 | 0.01 | 6 |
| Lepilemur ruficaudatus | 7.60 | 7.34 | 0.39 | 2 | Rhinonycteris aurantius | 0.25 | 0.24 | 0.02 | 6 |
| Lemur fulvus | 23.30 | 22.49 | 0.75 | 2 | Triaenops persicus | 0.27 | 0.26 | 0.02 | 6 |
| Lemur variegatus | 31.50 | 30.41 | 1.40 | 2 | Noctilio albiventris | 0.60 | 0.58 | 0.02 | 6 |
| Avahi laniger | 10.49 | 10.13 | 0.53 | 2 | Noctilio leporinus | 1.18 | 1.14 | 0.05 | 6 |
| Avahi occidentalis | 9.67 | 9.33 | 0.48 | 2 | Pteronotus gymnonotus | 0.34 | 0.33 | 0.02 | 6 |
| Propithecus verreauxi | 26.70 | 25.77 | 1.04 | 2 | Pteronotus personatus | 0.23 | 0.22 | 0.01 | 6 |
| Indri indri | 38.30 | 36.97 | 1.52 | 2 | Pteronotus parnelli | 0.54 | 0.52 | 0.04 | 6 |
| Daubentonia madagascarie. | 45.15 | 43.58 | 1.78 | 2 | Mormoops megalophylla | 0.39 | 0.37 | 0.03 | 6 |
| Loris tardigradus | 6.60 | 6.37 | 0.19 | 2 | Micronycteris megalotis | 0.27 | 0.26 | 0.01 | 6 |
| Nycticebus coucang | 12.50 | 12.07 | 0.57 | 2 | Micronycteris minuta | 0.29 | 0.28 | 0.02 | 6 |
| Perodicticus potto | 14.00 | 13.51 | 0.61 | 2 | Micronycteris schmidtorum | 0.31 | 0.30 | 0.01 | 6 |
| Galago crassicaudatus | 10.30 | 9.94 | 0.46 | 2 | Micronycteris brachyotis | 0.41 | 0.39 | 0.02 | 6 |
| Galago demidovii | 3.38 | 3.26 | 0.15 | 2 | Macrophyllum macrophyll. | 0.32 | 0.31 | 0.02 | 6 |
| Galago senegalensis | 4.80 | 4.63 | 0.26 | 2 | Tonatia bidens | 0.79 | 0.76 | 0.04 | 6 |
| Tarsius sp. | 3.60 | 3.47 | 0.15 | 2 | Tonatia schulzi | 0.51 | 0.49 | 0.02 | 6 |
| Callithrix jacchus | 7.60 | 7.34 | 0.22 | 2 | Tonatia sylvicola | 0.76 | 0.73 | 0.03 | 6 |
| Cebuella pygmaea | 4.50 | 4.34 | 0.13 | 2 | Mimon crenulatum | 0.32 | 0.31 | 0.02 | 6 |
| Saguinus oedipus | 10.00 | 9.65 | 0.26 | 2 | Phyllostomus discolor | 1.09 | 1.05 | 0.08 | 6 |
| Saguinus tamarin | 10.30 | 9.94 | 0.28 | 2 | Phyllostomus elongates | 0.89 | 0.85 | 0.05 | 6 |
| Callimico goeldii | 11.00 | 10.61 | 0.28 | 2 | Phyllostomus hastatus | 1.52 | 1.46 | 0.08 | 6 |
| Aotus trivirgatus | 17.10 | 16.51 | 0.54 | 2 | Phylloderma stenops | 1.34 | 1.29 | 0.09 | 6 |
| Callicebus moloch | 19.00 | 18.34 | 0.59 | 2 | Trachops cirrhosus | 1.00 | 0.97 | 0.05 | 6 |
| Pithecia monachal | 35.00 | 33.78 | 0.83 | 2 | Vampyrum spectrum | 2.59 | 2.50 | 0.11 | 6 |
| Alouatta sp. | 52.00 | 50.19 | 1.32 | 2 | Glossophaga longirostris | 0.44 | 0.42 | 0.04 | 6 |
| Ateles geoffroyi | 108.00 | 104.25 | 1.37 | 2 | Glossphaga soricina | 0.39 | 0.38 | 0.03 | 6 |
| Lagothrix lagotricha | 101.00 | 97.49 | 1.59 | 2 | Monophyllus plethodon | 0.45 | 0.43 | 0.03 | 6 |
| Cebus sp. | 71.00 | 68.53 | 0.89 | 2 | Leptonycteris curasoae | 0.61 | 0.59 | 0.05 | 6 |
| Saimiri sciureus | 24.00 | 23.17 | 0.35 | 2 | Leptonycteris nivalis | 0.59 | 0.57 | 0.04 | 6 |
| Macaca mulatta | 93.00 | 89.77 | 1.35 | 2 | Lonchophylla mordax | 0.43 | 0.42 | 0.04 | 6 |
| Cercocebus albigena | 104.00 | 100.39 | 1.49 | 2 | Lonchophylla thomasi | 0.34 | 0.33 | 0.03 | 6 |
| Papio anubis | 201.00 | 194.02 | 3.40 | 2 | Lionycteris spurrelli | 0.35 | 0.34 | 0.03 | 6 |
| Cercopithecus mitis | 75.00 | 72.39 | 1.37 | 2 | Anoura caudifer | 0.41 | 0.39 | 0.03 | 6 |
| Cercopithecus ascanius | 67.00 | 64.67 | 1.19 | 2 | Anoura geoffroyi | 0.59 | 0.57 | 0.05 | 6 |
| Cercopithecus talapoin | 40.00 | 38.61 | 0.71 | 2 | Choeroniscus minor | 0.39 | 0.38 | 0.04 | 6 |
| Erythrocebus patas | 108.00 | 104.25 | 1.59 | 2 | Carollia castanea | 0.45 | 0.44 | 0.04 | 6 |
| Pygathrix nemaeus | 77.00 | 74.32 | 2.30 | 2 | Carollia perspicullata | 0.55 | 0.53 | 0.04 | 6 |
| Nasalis larvatus | 97.00 | 93.63 | 1.97 | 2 | Rhinophylla pumilio | 0.36 | 0.34 | 0.03 | 6 |
| Colobus badius | 78.00 | 75.29 | 1.67 | 2 | Sturnira lilium | 0.62 | 0.60 | 0.05 | 6 |
| Hylobates lar | 102.00 | 98.46 | 2.67 | 2 | Strunira ludovici | 0.68 | 0.65 | 0.05 | 6 |
| Pan troglodytes | 405.00 | 390.93 | 3.78 | 2 | Sturnira tildae | 0.70 | 0.68 | 0.05 | 6 |
| Gorilla gorilla | 500.00 | 482.63 | 4.78 | 2 | Uroderma bilobatum | 0.61 | 0.59 | 0.04 | 6 |
| Homo sapiens | 1,330.00 | 1,283.78 | 10.29 | 2 | Vampyrops helleri | 0.52 | 0.50 | 0.03 | 6 |
| Carnivora | Vampyrops infuscus | 1.11 | 1.07 | 0.08 | 6 | ||||
| Canis latrans | 88.30 | 85.23 | 2.16 | 3 | Vampyrops lineatus | 0.74 | 0.72 | 0.05 | 6 |
| Vulpes vulpes | 43.50 | 41.99 | 1.29 | 3 | Vampyrops vittatus | 1.18 | 1.13 | 0.07 | 6 |
| Fennicus zerda | 17.30 | 16.70 | 0.49 | 3 | Vampyrodes caraccioloi | 0.95 | 0.91 | 0.06 | 6 |
| Ursus maritimus | 458.60 | 442.66 | 5.59 | 3 | Vampyressa pusilla | 0.38 | 0.37 | 0.03 | 6 |
| Procyon cancrivorous | 61.56 | 59.42 | 1.03 | 3 | Chiroderma salvini | 0.81 | 0.79 | 0.06 | 6 |
| Nasau nasau | 37.00 | 35.71 | 0.49 | 3 | Chiroderma trinitatum | 0.54 | 0.52 | 0.03 | 6 |
| Bassaricyon gabbi | 19.30 | 18.63 | 0.58 | 3 | Chiroderma villosum | 0.71 | 0.68 | 0.04 | 6 |
| Mustela nivalis | 1.50 | 1.45 | 0.17 | 3 | Chiroderma villosum | 0.84 | 0.81 | 0.06 | 6 |
| Taxidea taxus | 49.00 | 47.29 | 1.45 | 3 | Echtophylla macconnelli | 0.34 | 0.33 | 0.03 | 6 |
| Mephitis mephitis | 10.30 | 9.94 | 0.31 | 3 | Artibeus concolor | 0.63 | 0.61 | 0.04 | 6 |
| Crocuta crocuta | 162.50 | 156.85 | 3.08 | 3 | Artibeus jamaicensis | 1.02 | 0.98 | 0.06 | 6 |
| Felis concolor | 125.50 | 121.14 | 1.80 | 3 | Artibeus lituratus | 1.23 | 1.19 | 0.08 | 6 |
| Felis pardis | 125.50 | 121.14 | 3.12 | 3 | Enchisthenes harti | 0.51 | 0.49 | 0.04 | 6 |
| Panthera leo | 258.00 | 249.03 | 4.50 | 3 | Ardops sp. | 0.57 | 0.55 | 0.04 | 6 |
| Zalophus californianus | 379.13 | 365.96 | 2.33 | 3 | Sphaeronycteris toxophyll. | 0.52 | 0.51 | 0.03 | 6 |
| Callorhinus ursinus | 328.75 | 317.33 | 1.95 | 3 | Brachyphylla cavernarum | 1.20 | 1.15 | 0.08 | 6 |
| Eumetopius jubatus | 661.25 | 638.27 | 3.53 | 3 | Desmodus rotundus | 1.00 | 0.96 | 0.04 | 6 |
| Phoca vitulina | 275.00 | 265.44 | 2.11 | 3 | Diphylla ecaudata | 0.80 | 0.77 | 0.04 | 6 |
| Artiodactyla | Natalus tumidirostris | 0.25 | 0.24 | 0.03 | 6 | ||||
| Tayassu tajacu | 80.50 | 77.70 | 2.67 | 3 | Furipterus horrens | 0.13 | 0.12 | 0.01 | 6 |
| Lama glama | 200.30 | 193.34 | 3.52 | 3 | Myotis adversus | 0.25 | 0.25 | 0.01 | 6 |
| Camelus dromedarius | 518.00 | 500.00 | 8.58 | 3 | Myotis albescens | 0.13 | 0.12 | 0.01 | 6 |
| Odocoileus virginianus | 160.00 | 154.44 | 3.26 | 3 | Myotis altarium | 0.24 | 0.23 | 0.02 | 6 |
| Bos indicus | 474.00 | 457.53 | 7.71 | 3 | Myotis annectans | 0.22 | 0.21 | 0.02 | 6 |
| Hippopotamus amphibius | 579.40 | 559.27 | 6.81 | 5 | Myotis bechsteini | 0.27 | 0.26 | 0.03 | 6 |
| Hippopotamus amphibius | 407.50 | 393.34 | 3.63 | 5 | Myotis bocagei | 0.19 | 0.18 | 0.01 | 6 |
| Cetacea | Myotis dasycneme | 0.31 | 0.30 | 0.03 | 6 | ||||
| Lagenorhynchus acutus | 1,292.20 | 1,253.90 | 1.61 | 4 | Myotis montivagus | 0.17 | 0.16 | 0.01 | 6 |
| Lagenorhynchus acutus | 1,329.70 | 1,293.60 | 1.67 | 4 | Myotis myotis | 0.49 | 0.47 | 0.04 | 6 |
| Lagenorhynchus acutus | 1,305.30 | 1,255.20 | 1.91 | 4 | Myotis nattereri | 0.22 | 0.21 | 0.02 | 6 |
| Phocoena phocoena | 503.00 | 485.52 | 0.60 | 5 | Myotis nigricans | 0.14 | 0.13 | 0.01 | 6 |
| Phocoena phocoena | 486.00 | 469.11 | 0.60 | 5 | Myotis nigricans | 0.16 | 0.15 | 0.01 | 6 |
| Tursiops truncatus | 1,530.00 | 1,476.83 | 1.05 | 5 | Myotis siligorensis | 0.10 | 0.10 | 0.01 | 6 |
| Balaenoptera acutorostrata | 2,900.00 | 2,799.23 | 1.43 | 5 | Pipistrellus babu | 0.10 | 0.09 | 0.01 | 6 |
| Balaenoptera acutorostrata | 2,800.00 | 2,702.70 | 1.42 | 5 | Pipistrellus ceylonicus | 0.18 | 0.18 | 0.01 | 6 |
| Perissodactyla | Pipistrellus circumdatus | 0.23 | 0.22 | 0.02 | 6 | ||||
| Equus burchelli | 254.99 | 246.13 | 10.34 | 3 | Pipistrellus crassulus | 0.13 | 0.13 | 0.01 | 6 |
| Diceros bicornus | 531.00 | 512.55 | 7.48 | 5 | Pipistrellus imbricatus | 0.14 | 0.13 | 0.01 | 6 |
| Xenarthra | Pipistrellus javanicus | 0.12 | 0.12 | 0.01 | 6 | ||||
| Myrmechophaga tridactyla | 58.80 | 56.76 | 2.69 | 3 | Pipistrellus mimus | 0.09 | 0.09 | 0.01 | 6 |
| Tamandua tetradactyla | 24.00 | 23.17 | 1.07 | 3 | Pipistrellus nanus | 0.10 | 0.10 | 0.01 | 6 |
| Choloepus didactylus | 25.93 | 25.03 | 1.14 | 3 | Pipistrellus papuanus | 0.11 | 0.11 | 0.01 | 6 |
| Dasypus novemcinctus | 16.25 | 15.68 | 0.93 | 3 | Pipistrellus pulveratus | 0.14 | 0.14 | 0.01 | 6 |
| Megachiroptera | Pipistrellus subflavus | 0.13 | 0.12 | 0.01 | 6 | ||||
| Eidolon helvum | 4.29 | 4.14 | 0.26 | 6 | Scotozous dormeri | 0.14 | 0.14 | 0.01 | 6 |
| Rousettus aegyptiacus | 2.28 | 2.20 | 0.15 | 6 | Nyctalus noctula | 0.36 | 0.35 | 0.02 | 6 |
| Rousettus amplexicaudatus | 1.35 | 1.31 | 0.10 | 6 | Nyctalus stenopterus | 0.24 | 0.24 | 0.01 | 6 |
| Rousettus amplexicaudatus | 1.72 | 1.66 | 0.13 | 6 | Glischropus tylopus | 0.10 | 0.10 | 0.01 | 6 |
| Myonycteris torquata | 1.16 | 1.12 | 0.09 | 6 | Eptesicus brasiliensis | 0.20 | 0.19 | 0.01 | 6 |
| Pteropus alecto | 7.04 | 6.79 | 0.36 | 6 | Eptesicus brasiliensis | 0.20 | 0.19 | 0.01 | 6 |
| Pteropus conspicillatus | 8.35 | 8.06 | 0.39 | 6 | Eptesicus flavenscens | 0.19 | 0.18 | 0.01 | 6 |
| Pteropus hypomelanus | 5.30 | 5.12 | 0.28 | 6 | Eptesicus fuscus | 0.24 | 0.23 | 0.02 | 6 |
| Pteropus lylei | 6.13 | 5.92 | 0.29 | 6 | Eptesicus pumilis | 0.12 | 0.12 | 0.01 | 6 |
| Pteropus mahaganus | 5.28 | 5.10 | 0.28 | 6 | Ia io | 0.76 | 0.74 | 0.05 | 6 |
| Pteropus neohibernicus | 9.11 | 8.80 | 0.40 | 6 | Tylonycteris pachypus | 0.08 | 0.08 | 0.01 | 6 |
| Pteropus poliocephalus | 7.23 | 7.00 | 0.36 | 6 | Tylonycteris robustula | 0.12 | 0.11 | 0.01 | 6 |
| Pteropus samoensis | 5.79 | 5.59 | 0.32 | 6 | Hesperopterus blandfordi | 0.16 | 0.15 | 0.01 | 6 |
| Pteropus scapulatus | 5.36 | 5.17 | 0.27 | 6 | Glauconycteris poensis | 0.21 | 0.20 | 0.01 | 6 |
| Pteropus temmincki | 4.89 | 4.72 | 0.28 | 6 | Chalinolobus gouldi | 0.24 | 0.23 | 0.02 | 6 |
| Pteropus tonganus | 6.08 | 5.87 | 0.31 | 6 | Chalinolobus morio | 0.18 | 0.18 | 0.02 | 6 |
| Pteropus vampyrus | 9.12 | 8.80 | 0.44 | 6 | Scotorepens sanborni | 0.16 | 0.15 | 0.01 | 6 |
| Dobsonia inermis | 2.70 | 2.61 | 0.19 | 6 | Rhogessa parvula | 0.11 | 0.11 | 0.01 | 6 |
| Dobsonia moluccensis | 5.34 | 5.16 | 0.32 | 6 | Scotomanes ornatus | 0.38 | 0.37 | 0.03 | 6 |
| Dobsonia moluccensis | 3.83 | 3.70 | 0.24 | 6 | Scotomanes sp. | 0.34 | 0.33 | 0.03 | 6 |
| Dobsonia praedatrix | 3.01 | 2.92 | 0.18 | 6 | Scotophilus dinganii | 0.44 | 0.42 | 0.02 | 6 |
| Hypsignathus monstrosus | 3.48 | 3.36 | 0.22 | 6 | Scotophilus heathi | 0.48 | 0.46 | 0.03 | 6 |
| Epomops franqueti | 2.21 | 2.13 | 0.16 | 6 | Scotophilus kuhli | 0.34 | 0.33 | 0.02 | 6 |
| Epomophorus labiatus | 1.59 | 1.53 | 0.11 | 6 | Lasiurus borealis | 0.17 | 0.16 | 0.01 | 6 |
| Micropteropus pusillus | 0.83 | 0.80 | 0.06 | 6 | Miniopterus australis | 0.20 | 0.20 | 0.02 | 6 |
| Scotonycteris zenkeri | 0.71 | 0.69 | 0.07 | 6 | Miniopterus haradai | 0.22 | 0.22 | 0.02 | 6 |
| Casinycteris argynnis | 0.84 | 0.81 | 0.07 | 6 | Miniopterus inflatus | 0.33 | 0.31 | 0.02 | 6 |
| Cynopterus brachyotis | 0.98 | 0.95 | 0.08 | 6 | Miniopterus magnater | 0.32 | 0.31 | 0.02 | 6 |
| Cynopterus horsfieldi | 1.37 | 1.33 | 0.11 | 6 | Miniopterus medius | 0.27 | 0.26 | 0.02 | 6 |
| Megaerops ecaudatus | 0.80 | 0.77 | 0.07 | 6 | Miniopterus pusillus | 0.22 | 0.21 | 0.02 | 6 |
| Chironax melanocephalus | 0.61 | 0.59 | 0.06 | 6 | Miniopterus tristis | 0.33 | 0.32 | 0.02 | 6 |
| Sphaeris blanfordi | 0.83 | 0.80 | 0.07 | 6 | Murina cyclotis | 0.25 | 0.24 | 0.02 | 6 |
| Balionycteris maculata | 0.51 | 0.49 | 0.06 | 6 | Murina cyclotis | 0.28 | 0.27 | 0.02 | 6 |
| Nyctimene albiventer | 0.83 | 0.80 | 0.07 | 6 | Murina huttoni | 0.26 | 0.25 | 0.02 | 6 |
| Nyctimene robinsoni | 1.23 | 1.19 | 0.10 | 6 | Murina turbinaris | 0.22 | 0.21 | 0.02 | 6 |
| Nyctimene vizcaccia | 0.99 | 0.95 | 0.07 | 6 | Harpiocephalus hapria | 0.44 | 0.43 | 0.04 | 6 |
| Paranyctimene raptor | 0.73 | 0.71 | 0.06 | 6 | Kerivoula papillosa | 0.31 | 0.30 | 0.04 | 6 |
| Eonycteris spelaean | 1.31 | 1.26 | 0.10 | 6 | Kerivoula pellucida | 0.20 | 0.20 | 0.01 | 6 |
| Megaloglossus woermanni | 0.68 | 0.66 | 0.06 | 6 | Kerivoula phalaena | 0.12 | 0.11 | 0.01 | 6 |
| Macroglossus minimus | 0.56 | 0.54 | 0.05 | 6 | Phoniscus atrox | 0.19 | 0.18 | 0.03 | 6 |
| Macroglossus sobrinus | 0.69 | 0.67 | 0.06 | 6 | Nyctophilus geoffroyi | 0.17 | 0.16 | 0.01 | 6 |
| Syconycteris sp. | 0.57 | 0.55 | 0.05 | 6 | Nyctophilus timoriensis | 0.25 | 0.25 | 0.02 | 6 |
| Syconycteris sp. | 0.63 | 0.61 | 0.06 | 6 | Tadarida aegyptiaca | 0.39 | 0.38 | 0.02 | 6 |
| Melonyceris melanops | 1.29 | 1.25 | 0.08 | 6 | Tadarida condylura | 0.46 | 0.44 | 0.02 | 6 |
| Nesonycteris woodfordi | 1.02 | 0.98 | 0.07 | 6 | Tadarida mops | 0.48 | 0.46 | 0.03 | 6 |
| Notopteris macdonaldi | 1.46 | 1.14 | 0.10 | 6 | Tadarida niveiventer | 0.43 | 0.42 | 0.02 | 6 |
| Microchiroptera | Tadarida beccarii | 0.23 | 0.23 | 0.01 | 6 | ||||
| Rhinopoma hardwickei | 0.28 | 0.27 | 0.02 | 6 | Tadarida jobensis | 0.36 | 0.35 | 0.02 | 6 |
| Rhinopoma microphyllum | 0.39 | 0.37 | 0.03 | 6 | Tadarida leucostigma | 0.39 | 0.38 | 0.02 | 6 |
| Emballonura monticola | 0.17 | 0.16 | 0.01 | 6 | Tadarida plicata | 0.45 | 0.43 | 0.02 | 6 |
| Emballonura raffrayana | 0.17 | 0.17 | 0.01 | 6 | Tadarida plicata | 0.33 | 0.31 | 0.01 | 6 |
| Emballonura semicaudata | 0.18 | 0.18 | 0.01 | 6 | Otomops martiensseni | 0.76 | 0.73 | 0.03 | 6 |
| Coleura afra | 0.26 | 0.25 | 0.01 | 6 | Molossops abrasus | 0.42 | 0.40 | 0.02 | 6 |
| Rhynchonycteris naso | 0.12 | 0.11 | 0.01 | 6 | Molossops greenhalli | 0.30 | 0.29 | 0.01 | 6 |
| Saccopteryx bilineata | 0.23 | 0.22 | 0.01 | 6 | Molossops planirostris | 0.19 | 0.18 | 0.01 | 6 |
| Saccopteryx canescens | 0.13 | 0.13 | 0.01 | 6 | Eumops auripendulus | 0.61 | 0.59 | 0.03 | 6 |
| Saccopteryx leptura | 0.16 | 0.16 | 0.01 | 6 | Eumops glaucinus | 0.65 | 0.63 | 0.03 | 6 |
| Cormura brevirostris | 0.22 | 0.21 | 0.01 | 6 | Molossus ater | 0.53 | 0.51 | 0.02 | 6 |
| Peropteryx macrotis | 0.16 | 0.16 | 0.01 | 6 | Molussus molossus | 0.32 | 0.31 | 0.02 | 6 |
| Peropteryx trinitatis | 0.14 | 0.13 | 0.01 | 6 | Molossus trinitatus | 0.44 | 0.42 | 0.02 | 6 |
| Taphozous australis | 0.52 | 0.50 | 0.03 | 6 | Cheiromeles torquatus | 1.36 | 1.32 | 0.06 | 6 |
| Taphozous hildegardeae | 0.55 | 0.53 | 0.03 | 6 | Proboscidea | ||||
| Taphozous mauritianus | 0.55 | 0.53 | 0.03 | 6 | Loxodonta africana (LA1) | 5,145.00 | 4,966.22 | 11.21 | 5 |
| Taphozous melanopogon | 0.54 | 0.52 | 0.02 | 6 | Loxodonta africana (LA2) | 5,250.00 | 5,067.57 | 10.74 | 5 |
| Taphozous theobaldi | 0.68 | 0.66 | 0.03 | 6 | Loxodonta africana (LA3) | 4,835.00 | 4,666.99 | 10.57 | 5 |
| Cyttarops alecto | 0.18 | 0.17 | 0.01 | 6 | Sirenia | ||||
| Craseonycteris thonglongyai | 0.09 | 0.08 | 0.01 | 6 | Trichechus manatus | 350.00 | 337.84 | 3.63 | 3 |
| Nycteris arge | 0.35 | 0.34 | 0.02 | 6 | |||||
All values are rounded to two decimal points. The volume of the brain was calculated from brain mass as described by Stephan et al. (1981) where Vb = Mb/1.036
1 Pirlot and Nelson (1978), 2 Stephan et al. (1981), 3 Reep et al. (2007), 4 Montie et al. (2008), 5 current study, 6 Baron et al. (1996), Mb mass of the brain in grams, Vb volume of the brain in millilitres, Vh volume of the hippocampus in millilitres, S source of the data
Immunohistochemistry
The brains of all animals used for immunohistochemistry were, following euthanasia, perfusion fixed with 4 % paraformaldehyde in 0.1 M phosphate buffer and then stored in an antifreeze solution until processed for immunohistochemistry (Manger et al. 2009). To investigate the presence of adult hippocampal neurogenesis, we used standard immunohistochemical procedures with an antibody directed against doublecortin (goat-anti DCX C-18 primary antibody, Santa Cruz Biotechnology) (Patzke et al. 2013a, b; Chawana et al. 2013). Using DCX immunohistochemistry, we examined the hippocampus and adjacent piriform cortex of 71 mammalian species (Table 2) from 13 mammalian orders covering a range of brain sizes (from less than 1 g through to 5 kg). This study was provided with ethical clearance by the University of the Witwatersrand Animal Ethics Committee, which uses guidelines similar to those of the NIH regarding the use of animals in scientific research. The animals used in the current study were all collected under appropriate governmental permissions.
Table 2.
Species previously reported or analysed in the current study for the presence of adult hippocampal neurogenesis (AHN) and the technique with which it was shown
| Order/species | Common name | AHN presence shown with | S |
|---|---|---|---|
|
| |||
| Dasyuromorphia | |||
| Sminthopsis crassicaudata | Fat-tailed dunnart | [3H]thymidine/PSA-NCAM | 1 |
| Sarcophilus harrisii | Tasmanian devil | DCX | 2 |
| Eulipotyphla | |||
| Erinaceus concolor | White-breasted hedgehog | DCX, Ki67 | 3 |
| Talpa europaea | European mole | DCX, Ki67 | 3 |
| Sorex araneus | Common shrew | BrdU/NeuN | 4 |
| Sorex minutus | Pygmy shrew | BrdU/NeuN | 4 |
| Afrosoricida | |||
| Echinops telfairi | Hedgehog tenrec | BrdU/DCX | 5 |
| Potomogale velox | Giant otter shrew | DCX | 2 |
| Proboscidea | |||
| Loxodonta africana | African elephant | DCX | 2 |
| Hyracoidea | |||
| Procavia capensis | Rock hyrax | DCX | 2 |
| Chrysochloridae | |||
| Amblysomus hottentotus | Hottentot golden mole | DCX | 2 |
| Macroscelididae | |||
| Petrodromus tetradactylus | Four-toed sengi | DCX | 2 |
| Elephantulus myurus | Eastern rock sengi | DCX | 2 |
| Sirenia | |||
| Trichechus manatus | West Indian manatee | DCX | 2 |
| Microchiroptera | |||
| Cardioderma cor | Heart-nosed bat | DCX | 2 |
| Chaerephon pumilus | Little free-tailed bat | DCX | 2 |
| Coleura afra | African sheath-tailed bat | DCX | 2 |
| Hipposideros commersoni | Commerson’s leaf-nosed bat | DCX | 2 |
| Miniopterus schreibersii | Schreiber’s long fingered bat | DCX | 2 |
| Triaenops persicus | Persian trident bat | DCX | 2 |
| Nycteris macrotis | Large-eared slit-faced bat | DCX | 2 |
| Hipposideros fuliginosas | Sooty round-leaf bat | DCX | 2 |
| Asellia tridens | Trident leaf-nosed bat | DCX | 2 |
| Pipistrellus kuhlii | Kuhl’s pipistrelle | DCX | 2 |
| Scandentia | |||
| Tupaia belangeri | Northern tree shrew | BrdU/NSE | 6 |
| Megachiroptera | |||
| Eidolon helvum | Straw-coloured fruit bat | DCX, Ki67 | 2 |
| Epomophorus wahlbergi | Wahlberg’s epauletted fruit bat | DCX, Ki67 | 2 |
| Casinycteris argynnis | Short-palated fruit bat | DCX, Ki67 | 2 |
| Epomops franqueti | Franquet’s epauletted fruit bat | DCX, Ki67 | 2 |
| Hypsignathus monstrosus | Hammer-headed fruit bat | DCX, Ki67 | 2 |
| Megaloglossus woermanni | Woermann’s fruit bat | DCX, Ki67 | 2 |
| Rousettus aegyptiacus | Egyptian fruit bat | DCX, Ki67 | 2 |
| Scotonycteris zenkeri | Zenker’s fruit bat | DCX, Ki67 | 2 |
| Primates | |||
| Perodicticus potto | Potto | DCX, Ki67 | 2 |
| Galagoides demidoff | Demidoff’s galago | DCX, Ki67 | 2 |
| Lemur catta | Ring-tailed lemur | DCX | 2 |
| Saimiri sciureus | Squirrel monkey | DCX | 2 |
| Callithrix jacchus | Common marmoset | BrdU/NeuN/TuJ1; BrdU/DCX/Ki67 | 7, 8 |
| Chlorocebus pygerythrus | Vervet monkey | DCX | 2 |
| Macaca mulatta | Rhesus macaque | BrdU/NeuN/TuJ1, PCNA; BrdU/NeuN, Ki67 | e.g. 9, 10 |
| Macaca fascicularis | Crab-eating macaque | BrdU/NeuN/TuJ1 | 11 |
| Papio anubis | Olive baboon | DCX | 2 |
| Homo sapiens | Human | BrdU/NeuN/CB/NSE; DCX/Ki67/PCNA/ect. | e.g. 12, 13 |
| Lagomorpha | |||
| Oryctolagus cuniculus | New Zealand albino rabbit | BrdU/RNR (M1 subunit) | 14 |
| Rodentia | |||
| Rattus norvegicus | Laboratory rat | BrdU/DCX/CB; DCX, Ki67 | e.g. 15, 16 |
| Mus musculus | Laboratory mouse | BrdU/NeuN; DCX | e.g. 17, 18 |
| Cavia porcellus | Guinea pig | BrdU/NeuN | 19 |
| Neotamias amoenus | Yellow-pine chipmunk | DCX, Ki67 | 20 |
| Sciurus carolinensis | Eastern grey squirrel | DCX, Ki67 | 20 |
| Apodemus flavicollis | Yellow-necked wood mouse | DCX, Ki67 | 21 |
| Apodemus sylvaticus | Long-tailed wood mouse | DCX, Ki67 | 21 |
| Chethrionomys glareolus | Bank vole | DCX, Ki67 | 21 |
| Microtus subterraneus | European pine vole | DCX, Ki67 | 21 |
| Tamiasciurus hudsonicus | Red squirrel | DCX, Ki67 | 22 |
| Hylomyscus stella | Stella wood mouse | DCX, Ki67 | 2 |
| Hybomys lunaris | Rwenzori striped mouse | DCX, Ki67 | 2 |
| Cricetomys emini | Emin’s pouched rat | DCX, Ki67 | 2 |
| Anomalurus beecrofti | Beecroft’s flying squirrel | DCX, Ki67 | 2 |
| Stochomys longicaudatus | Target rat | DCX, Ki67 | 2 |
| Lophuromys flavopunctatus | Yellow-spotted brush-furred rat | DCX, Ki67 | 2 |
| Mastomys natalensis | Natal multimammate rat | DCX, Ki67 | 2 |
| Jaculus jaculus | Lesser Egyptian jerboa | DCX, Ki67 | 2 |
| Acomys dimidiatus | Arabian spiny mouse | DCX, Ki67 | 2 |
| Acomys cahirinus | Cairo spiny mouse | DCX, Ki67 | 2 |
| Gerbillus dasyurus | Wagner’s gerbil | DCX, Ki67 | 2 |
| Meriones rex | King jird | DCX, Ki67 | 2 |
| Meriones libycus | Libyan jird | DCX, Ki67 | 2 |
| Eliomys melanurus | Asian garden dormouse | DCX, Ki67 | 2 |
| Xerus inauris | Cape ground squirrel | DCX, Ki67 | 2 |
| Artiodactyla | |||
| Ovis aries | Domestic sheep | BrdU/RNR (M1 subunit) | 14 |
| Tragelaphus strepsiceros | Greater kudu | DCX | 2 |
| Connochaetes taurinus | Blue wildebeest | DCX | 2 |
| Connochaetes gnou | Black wildebeest | DCX | 2 |
| Camelus dromedarius | Arabian camel | DCX | 2 |
| Syncerus caffer | African buffalo | DCX | 2 |
| Tragelaphus angasii | Nyala | DCX | 2 |
| Taurotragus oryx | Common eland | DCX | 2 |
| Giraffa camelopardalis | Giraffe | DCX | 2 |
| Damaliscus pygargus | Blesbok | DCX | 2 |
| Sus scrofa | Domestic pig | DCX | 2 |
| Antidorcas marsupialis | Springbok | DCX | 2 |
| Capra nubiana | Nubian ibex | DCX | 2 |
| Oryx dammah | Scimitar-horned oryx | DCX | 2 |
| Oryx leucoryx | Arabian oryx | DCX | 2 |
| Gazella marica | Sand gazelle | DCX | 2 |
| Hippopotamus amphibius | River hippopotamus | DCX | 2 |
| Cetacea | |||
| Balaenoptera acutorostrata | Northern minke whale | AHN absent | 2 |
| Phocoena phocoena | Harbour porpoise | AHN absent | 2 |
| Carnivora | |||
| Vulpes vulpes | Red fox | DCX, Ki67, PCNA | 23 |
| Canis lupus familiaris | Domestic dog | BrdU/DCX | 24 |
| Aonyx cinerea | Asian small-clawed otter | DCX | 2 |
| Pagophilus groenlandicus | Harp seal | DCX | 2 |
| Callorhinus ursinus | Northern fur seal | DCX | 2 |
| Mungos mungo | Banded mongoose | DCX | 2 |
| Panthera tigris altaica | Siberian tiger | DCX | 2 |
| Panthera leo | African lion | DCX | 2 |
[3H]thymidine/PSA-NCAM injection of [3H]thymidine and the polysialylated form of the neural cell adhesion molecule immunohistochemistry, BrdU 5-bromo-2-deoxyuridine injection and immunohistochemistry, CB calbindin, DCX doublecortin immunohistochemistry, Ki-67 Ki-67 immunohistochemistry, MCM2 minichromosome maintenance complex component 2, NeuN neuronal nuclei marker immunohistochemistry, NSE neuron specific enolase immunohistochemistry, PCNA proliferating-cell nuclear antigen, RNR (M1 subunit) M1 subunit of ribonucleotide reductase immunohistochemistry, S source of the data, TUJ1 beta-tubulin 3
1 Harman et al. (2003),2 current study, 3 Bartkowska et al. (2010), 4 Bartkowska et al. (2008), 5 Alpár et al. (2010), 6 Gould et al. (1997), 7 Leuner et al. (2007),8 Bunk et al. (2011), 9 Kornack and Rakic (1999), 10 Jabès et al. (2010), 11 Gould et al. (2001), 12 Eriksson et al. (1998), 13 Knoth et al. (2010), 14 Zhu et al. (2003), 15 McDonald and Wojtowicz (2005), 16 Epp et al. (2009), 17 Spampanato et al. (2012), 18 Ma et al. (2012), 19 Guidi et al. (2005), 20 Barker et al. (2005), 21 Amrein et al. (2004), 22 Johnson et al. (2010), 23 Amrein and Slomianka (2010), 24 Siwak-Tapp et al. (2007)
From each animal used in the current study, blocks of hippocampal tissue were dissected in a plane orthogonal to the ventricular surface of the hippocampus at approximately the middle portion of the hippocampus. Each tissue block was cryosectioned into 50-μm-thick sections on a freezing microtome. Consecutive sections were stained for Nissl substance and reacted immunohistochemically for DCX, with a minimum of 12 sections per stain from hippocampi from two individuals of each species. The sections used for Nissl staining were mounted on 0.5 % gelatine-coated slides, dried overnight, cleared in a 1:1 mixture of 100 % ethanol and 100 % chloroform and stained with 1 % cresyl violet.
The sections used for free-floating immunohistochemical staining were treated for 30 min in an endogenous peroxidise inhibitor (49.2 % methanol:49.2 % 0.1 M PB:1.6 % of 30 % hydrogen peroxide) followed by three 10 min rinses in 0.1 M PB. To block unspecific binding sites, the sections were then pre-incubated for 2 h, at room temperature, in blocking buffer (3 % normal rabbit serum, 2 % bovine serum albumin, BSA and 0.25 % Triton X-100 in 0.1 M PB). Thereafter, sections were incubated in the primary antibody solution, made up of the appropriate dilution of the primary antibody in blocking buffer for 48 h at 4 °C under gentle agitation. In the current study we used immunolabelling of DCX, an endogenous marker of immature neurons, to ascertain the presence or absence of adult hippocampal neurogenesis. While the presence of DCX in neurons outside of the hippocampus may not relate to adult neurogenesis in these regions, such as the piriform cortex (Klempin et al. 2011), it has been established that DCX immunolabelling of granule cells of the dentate gyrus is a good proxy for the presence of adult hippocampal neurogenesis (Rao and Shetty 2004; Couillard-Despres et al. 2005). The presence of DCX also reflects cumulative adult hippocampal neurogenesis over a period of 2 weeks to 6 months, although this period is species specific (Rao and Shetty 2004; Kohler et al. 2011). In this sense, lack of DCX staining should be a reliable indicator of the absence of adult hippocampal neurogenesis. DCX immunolabelling is therefore particularly useful when studying a wide variety of field-caught mammalian species, as no specific intervention is required to reveal adult hippocampal neurogenesis.
To visualize DCX, we used the goat-anti DCX C-18 primary antibody from Santa Cruz (catalogue number sc-8066) at a dilution of 1:300. This antibody is an affinity-purified goat polyclonal antibody raised against a peptide mapping at the C-terminus of doublecortin of human origin. The amino acid sequences of the C-terminus of the doublecortin protein are highly conserved across mammalian species based on the Protein database provided by the National Center for Biotechnology Information. The primary antibody incubation was followed by three 10 min rinses in 0.1 M PB and the sections were then incubated in a secondary antibody solution (1:1,000 dilution of biotinylated anti-goat IgG, BA 5000, Vector Labs) for 2 h at room temperature. This was followed by three 10 min rinses in 0.1 M PB, after which the sections were incubated for 1 h in an avidin–biotin solution (1:125; Vector Labs), followed by three 10 min rinses in 0.1 M PB. The sections were then placed in a solution containing 0.05 % 3,3’-diaminobenzidine (DAB) in 0.1 M PB for 5 min, followed by the addition of 3.3 μl of 30 % hydrogen peroxide per 1 ml of DAB solution. Chromatic precipitation was visually monitored under a low-power stereomicroscope. Staining continued until such time as the background stain was at a level that would allow for accurate architectonic matching to the Nissl sections without obscuring the immunoreactive structures. Development was arrested by placing sections in 0.1 M PB for 10 min, followed by two more rinses in this solution. Sections were then mounted on 0.5 % gelatine-coated glass slides, dried overnight, dehydrated in a graded series of alcohols, cleared in xylene and covers-lipped with Depex. To ensure non-specific staining of the immunohistochemical protocol, we ran tests on sections where we omitted the primary antibody, and sections where we omitted the secondary antibody. In both cases, no staining was observed. In 11 species (African elephant, four-toed sengi, Hammer-headed fruit bat, ring-tailed lemur, Beecroft’s flying squirrel, Arabian spiny mouse, greater kudu, river hippopotamus, West Indian manatee, harbour porpoise and minke whale), an absorption control in sections encompassing the dentate gyrus and piriform cortex was also run using the blocking peptide sc-8066 P (Santa Cruz) as recommended by the supplier. In all cases, no staining was evident. Digital photomicrographs were captured using a Zeiss Axioskop and Axiovision software. No pixelation adjustments or manipulation of the captured images were undertaken, except for the adjustment of contrast, brightness and levels using Adobe Photoshop 7.
Results
Hippocampal volume increases as an exponential function across mammalian species
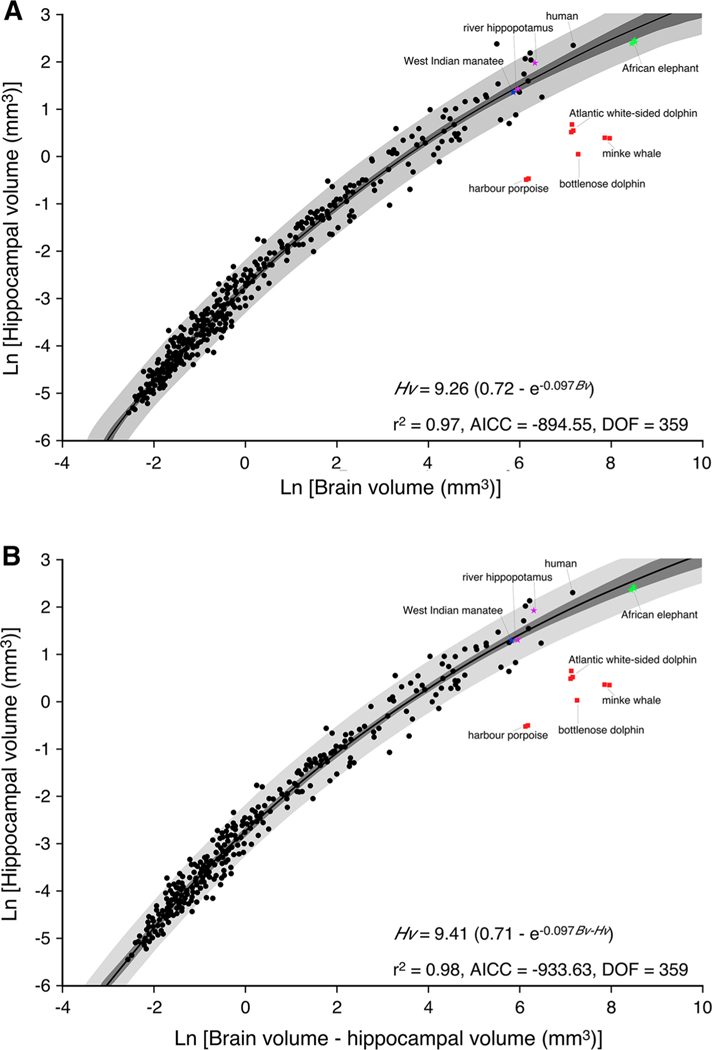
Previous studies investigating the relationship of how the hippocampus scales relative to brain size in adult mammals have used standard linear regression models (Finlay and Darlington 1995; Reep et al. 2007). In the current study, we analysed a larger database (375 species belonging to 17 orders; Table 1) and found that the relationship between brain and hippocampal volume in mature mammals was best described by an exponential function that approximated a growth curve (an exponential decay increasing form model) (Fig. 1). The exponential function depicted (Fig. 1) is based on values for chiropterans, insectivores, primates, artiodactyls, carnivores and other species for which data were available apart from the cetaceans, elephants, hippopotami and manatee (Table 1). On the basis of these tests, an exponential curve [y = a × (b - exp(−c × x)); where a = 9.26; b = 0.72 and c = 0.097] was fit across the groups as this model performed the best of all models tested(r2 = 0.97;DOF = 359; AICC =−894.55; sum of squares = 30.25; nruns = 182, P = 50.71 %). Using Akaike’s information criteria, the exponential model was shown to have a 100 % likelihood of being a better fit than the linear model (Delta = 122.50; P = 2.5 × 10−27). From this exponential function, we calculated 95 % confidence and prediction intervals, which demonstrated that the vast majority of the mammalian species fell within these statistically derived boundaries of the relationship of hippocampus to brain volume. Onto the plot, we superimposed data on hippocampal volume from African elephant, river hippopotamus, West Indian manatee and four species of cetaceans (Fig. 1; Table 1). The hippocampal volumes for the African elephant, hippopotamus and manatee all lie within the 95 % prediction intervals and close to or within the 95 % confidence intervals.
Fig. 1.
Graphical representation of the relationship between brain volume and hippocampal volume (a) and brain volume minus hippocampal volume and hippocampal volume (b) across 367 mammalian species. Note, in contrast to previous studies (Finlay and Darlington 1995; Reep et al. 2007), a function that approximates an exponential curve describes the data most efficiently and potentially reflects the presence of adult hippocampal neurogenesis in most mammalian species. Note that the hippocampal volumes of the West Indian manatee (Trichechus manatus), river hippopotamus (Hippopotamus amphibius) and African elephant (Loxodonta africana), which were not used in the determination of the descriptive function, fall within either the 95 % confidence intervals (dark grey shading) or the 95 % prediction intervals (light grey shading) determined from the data. In all cases, the cetaceans examined, harbour porpoise (Phocoena phocoena), bottlenose dolphin (Tursiops truncatus), Atlantic white-sided dolphin (Lagenorhynchus acutus) and minke whale (Balaenoptera acutorostratus), have hippocampal volumes substantially smaller than what would be predicted based on brain volume. AICC Akaike’s information criteria, Bv brain volume, Bv - Hv brain volume minus hippocampal volume, DOF degrees of freedom, Hv hippocampal volume
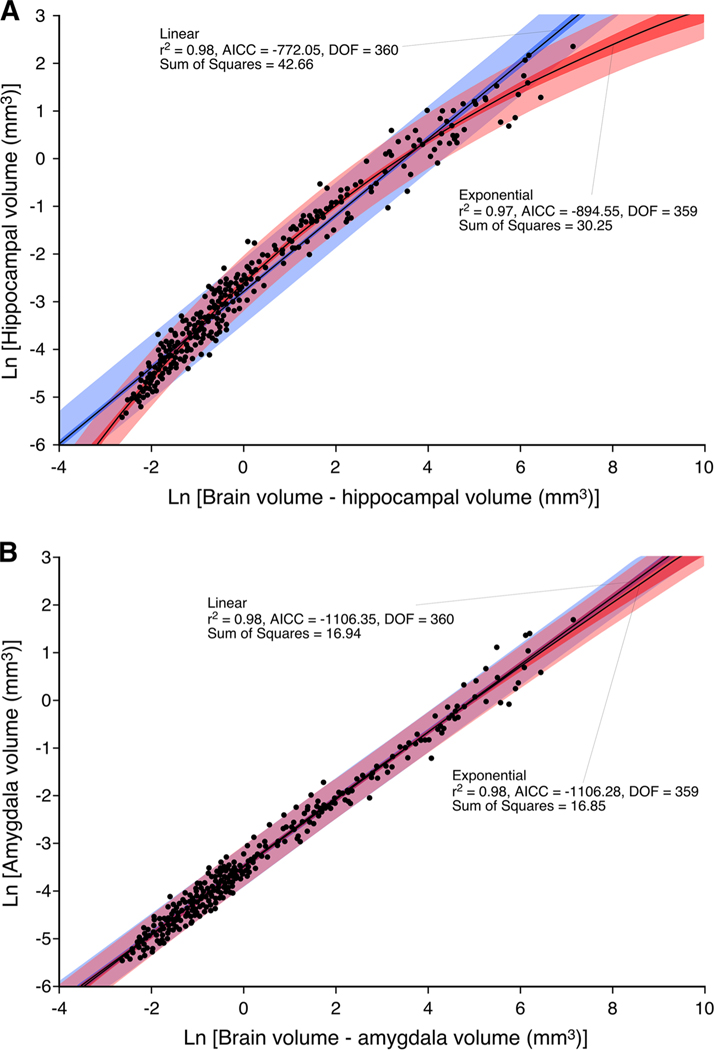
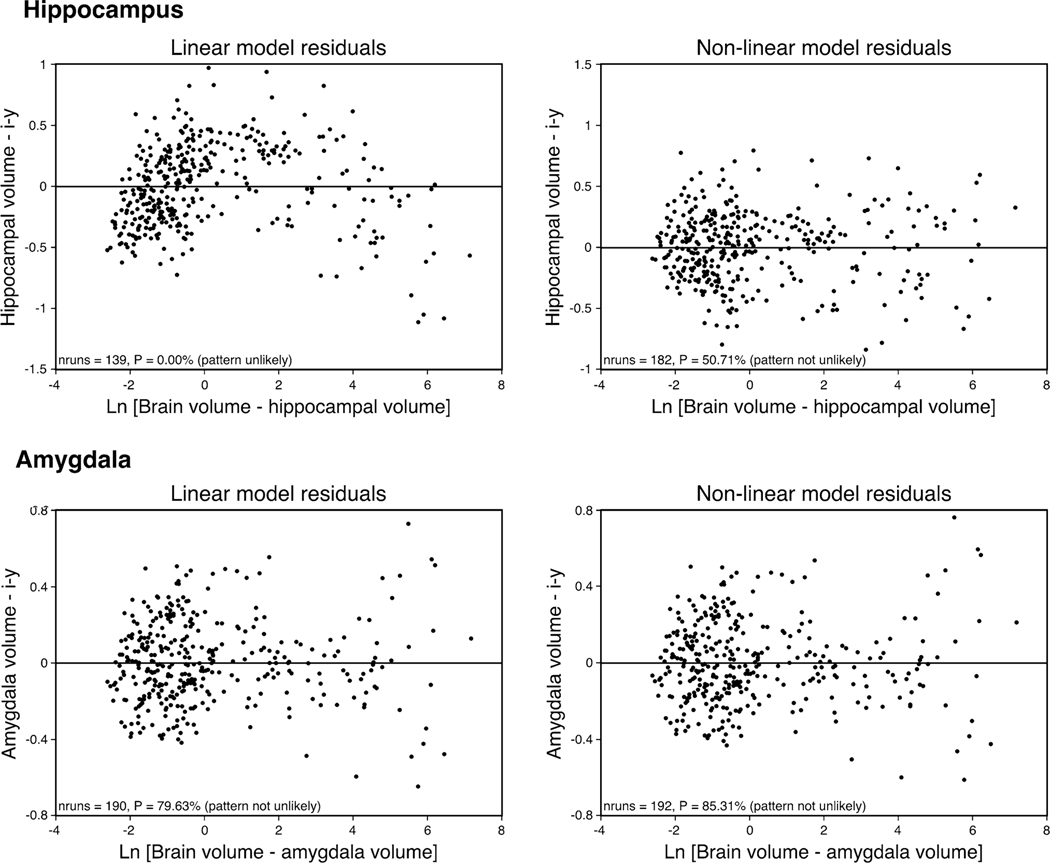
In Fig. 3a, we provide a graphical representation of the two best-performing regression models, i.e. the non-linear exponential model and the least squares linear model. The exponential model ranked best in terms of goodness of fit criteria displaying the following regression statistics (r2 = 0.97; AICC =−894.55; sum of squares = 30.25; DOF = 359) in comparison to that of the weaker-performing linear model (r2 = 0.98; AICC =−772.05; sum of squares = 42.66; DOF = 360). An F test comparing the sum of squares of the exponential model with that of the linear model indicated a 1.18 9 10−26 % (F = 147.39) probability that the exponential model was a better fit to the data than the linear model. Furthermore, both visual and statistical comparison of the accompanying residuals confirmed that a linear model was not suitable for describing these data (Fig. 4). The residuals as based on the linear model are not randomly scattered about zero as is confirmed by a runs test, while both visual and statistical comparison of the non-linear model confirms its appropriateness for this data (nruns = 182, P = 50.71 %).
Fig. 3.
Graphical representation of the relationship between brain volume minus hippocampal volume and hippocampal volume (a) and brain volume minus amygdala volume and amygdala volume (b) across mammalian species showing the contrast between the exponential function (pink shading) and the linear function (blue shading) describing these relationships. Note that the exponential function provides a more appropriate fit of the data for the hippocampus (a), while the linear function provides a more appropriate fit of the data for the amygdala (b). AICC Akaike’s information criteria, DOF degrees of freedom
Fig. 4.
Plots of the residuals obtained using both linear and non-linear regression functions to describe the relationship between brain minus hippocampal volume and hippocampal volume (upper two plots) and between brain minus amygdala volume and amygdala volume (lower two plots). The residuals as based on the linear model for the hippocampus are not randomly scattered about zero as confirmed by a runs test, while both visual and statistical comparison of the nonlinear model for the hippocampus confirms its appropriateness for this data. While both linear and exponential models describe the amygdala volume well, the less scatter observed in the linear model indicates the appropriateness of this model for the amygdala data
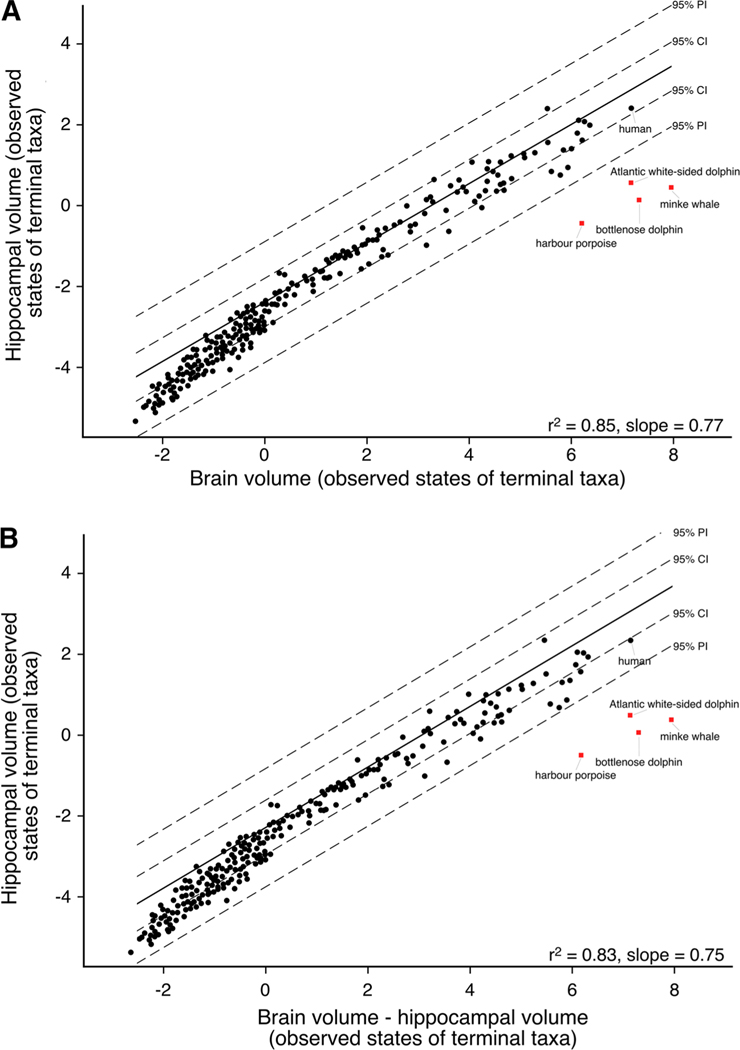
While independent contrast analysis is commonly used when exploring cross-taxonomic relationships, this technique is known to perform poorly when the underlying relationship between characters is non-linear. In accordance with suggestions pertaining to non-linearity (Garland et al. 1992; Quader et al. 2004), we log transformed our data and performed independent contrast analysis to evaluate the scaling of hippocampal volume with brain volume if a linear model were valid. In Fig. 5, we present a plot of the phylogenetic correct least square regression and associated confidence intervals and prediction intervals, mapped onto the original tip data space (Garland and Ives 2000). The resultant coefficient of determination for this model is r2 = 0.85/0.83 with a slope of 0.77/0.75. This plot indicates that even after phylogenetic correction, the cetaceans lie well below the confidence and prediction intervals of the mammalian line and are characterized by a markedly different scaling of the hippocampus relative to brain volume compared to all other mammals. In addition, the non-line-arity of the mammalian data is also evident in these plots.
Fig. 5.
Graphical representation of the phylogenetically correct least-square regression and associated confidence and prediction intervals for the brain volume compared to hippocampal volume (a) and brain volume minus hippocampal volume compared to hippocampal volume (b). The resultant coefficient of determination for these models is r2 = 0.85/0.83 with slopes of 0.77/0.75. These plots indicate that even after phylogenetic correction, the cetaceans lie well below the confidence and prediction intervals of the mammalian regression, underscoring the small size of the cetacean hippocampus. In addition, the non-linearity of the mammalian hippocampal data is also evident in these plots despite correction for phylogenetic relationships
Thus, in contrast to all other mammalian species examined to date, the data for the four species of cetaceans examined (harbour porpoise, bottlenose dolphin, Atlantic white-sided dolphin and minke whale) fall well below the 95 % prediction intervals (Figs. 1, 2, 3, 4, 5). Our data indicate that the cetaceans have hippocampal volumes that range between 8 and 20 % of the volume that would be predicted based on their brain size. Across all mammals analysed, the cetaceans were the only species that were different with regard to hippocampal size, and even their closest relative, the semi-aquatic river hippopotamus and the West Indian manatee, a species within the only other obligatorily aquatic order of mammals, did not show a trend towards a reduction of hippocampal volume.
Fig. 2.
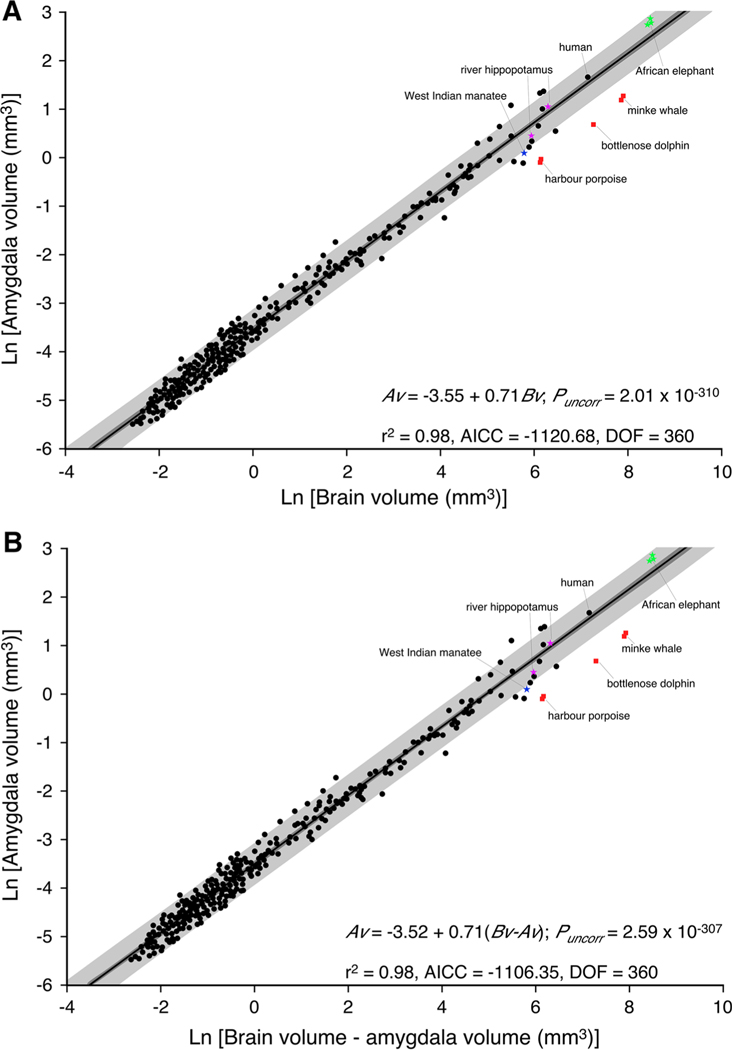
Graphical representation of the relationship between brain volume and amygdala volume (a) and brain volume minus amygdala volume and amygdala volume (b) across 364 mammalian species. Note that similar to previous studies (Finlay and Darlington 1995; Reep et al. 2007), a linear function describes the data most efficiently. Note that the amygdala volumes of the West Indian manatee (Trichechus manatus), river hippopotamus (Hippopotamus amphibius) and African elephant (Loxodonta africana), which were not used in the determination of the linear function, fall within either the 95 % confidence intervals (dark grey shading) or the 95 % prediction intervals (light grey shading) determined from the data. In all cases the cetaceans examined, harbour porpoise (Phocoena phocoena), bottlenose dolphin (Tursiops truncatus) and minke whale (Balaenoptera acutorostratus), have amygdala volumes substantially smaller than what would be predicted based on brain volume, reflecting the loss, or near loss, of the olfactory system in cetaceans. AICC Akaike’s information criteria, Av amygdala volume, Bv brain volume, Bv - Av brain volume minus amygdala volume, DOF degrees of freedom
Amygdala volume increases as a linear function across mammalian species
Previous studies investigating the relationship of how the amygdala scales relative to overall brain size in adult mammals used standard linear regression models (Finlay and Darlington 1995; Reep et al. 2007). In the current study, we analysed a larger database (373 species belonging to 17 orders) and found that the relationship between brain and amygdala volume in mature mammals was best described by a linear function (Fig. 2) as previously demonstrated (Finlay and Darlington 1995; Reep et al. 2007). On the basis of the tests undertaken, a linear function was fit across the groups as this model performed the best of all models tested (r2 = 0.98; DOF = 360; AICC = −1,120.68; nruns = 190, P = 79.63 %). From this linear function, we calculated 95 % confidence and prediction intervals, which demonstrated that the vast majority of the mammalian species fell within these statistically derived boundaries of the relationship of amygdala to brain volume. Onto the plot, we superimposed data on amygdala volume from African elephant, river hippopotamus, West Indian manatee and three species of cetaceans (Fig. 2). The amygdala volumes for the African elephant, hippopotamus and manatee all lie within the 95 % prediction intervals and close to or within the 95 % confidence intervals, but those of the three cetacean species fell below the 95 % prediction intervals.
In Fig. 3b, we provide a graphical representation of the two best-performing regression models, i.e. the least squares linear model and the non-linear exponential model. The linear model ranked best in terms of goodness of fit criteria displaying the following regression statistics (r2 = 0.98; AICC = −1,106.35; sum of squares = 16.94; DOF = 360) in comparison to that of the slightly weaker performing exponential model (r2 = 0.98; AICC = −1,106.28; sum of squares = 16.85; DOF = 359). Using Akaike’s information criteria, the linear model was shown to have a 51 % likelihood of being a better fit than the exponential model (Delta = 0.07; P = 0.49). An F test comparing the sum of squares of the exponential model with that of the linear model indicated a 16.44 % (F = 1.94) probability that the linear model was a better fit to the data. Furthermore, both visual and statistical comparison of the accompanying residuals confirmed that a linear model was more suitable for describing these data (Fig. 4).
Thus, in contrast to all other mammalian species examined, the data for the three species of cetaceans examined (harbour porpoise, bottlenose dolphin and minke whale) fall well below the 95 % prediction intervals (Figs. 2, 5). Our data indicate that the cetaceans have amygdala volumes that range between 37 and 42 % that would be predicted based on their brain size. Across all mammals analysed, the cetaceans were the only species that were different with regard to amygdala size, and even their closest relative, the semi-aquatic river hippopotamus, did not show a trend towards reduction in amygdala size; however, the West Indian manatee, a species within the only other obligatorily aquatic order of mammals, did show a trend towards a reduction of amygdala volume. For both cetaceans and the manatee, these reductions in relative amygdala volumes are likely related to the reduction/absence of the olfactory system in these species.
Adult hippocampal neurogenesis is apparent in all mammals except cetaceans
Our investigation of adult hippocampal neurogenesis across 71 species of mammals using immunohistochemistry to visualize DCX (Kempermann 2012) revealed robust staining of immature neurons across all species examined, except for the two cetacean species (Figs. 6, 7, 8). In addition, a survey of the literature (Table 2) indicates that all 93 mammalian species (from 16 different mammalian orders) studied to date, except the two cetaceans studied herein, possess robust adult hippocampal neurogenesis. Internal controls for antibody staining revealed positive staining of immature neurons in the piriform cortex of the minke whale and in the remnants of piriform cortex in the harbour porpoise (we use the term remnants as the odontocete cetaceans lack an olfactory bulb, and thus the size of the piriform/olfactory cortex is greatly reduced) (Fig. 8). The piriform cortex is known to contain neurons immunoreactive to DCX in mammals (Klempin et al. 2011). Thus, we can conclude that there are no specific problems with the cetacean tissue used or the immunohistochemical methodology. Moreover, we obtained robust staining in the only other obligatorily aquatic marine mammal investigated, the West Indian manatee, and in several species of semi-aquatic mammals, including the river hippopotamus (Fig. 7), seals from both phocid and otariid lineages (Fig. 7), Asian small-clawed otters and giant otter shrews (Patzke et al. 2013b). Given the success of DCX immunohistochemistry acting as a proxy marker for adult hippocampal neurogenesis across such a diverse array of species, we feel confident in reporting its apparent absence in the cetaceans.
Fig. 6.
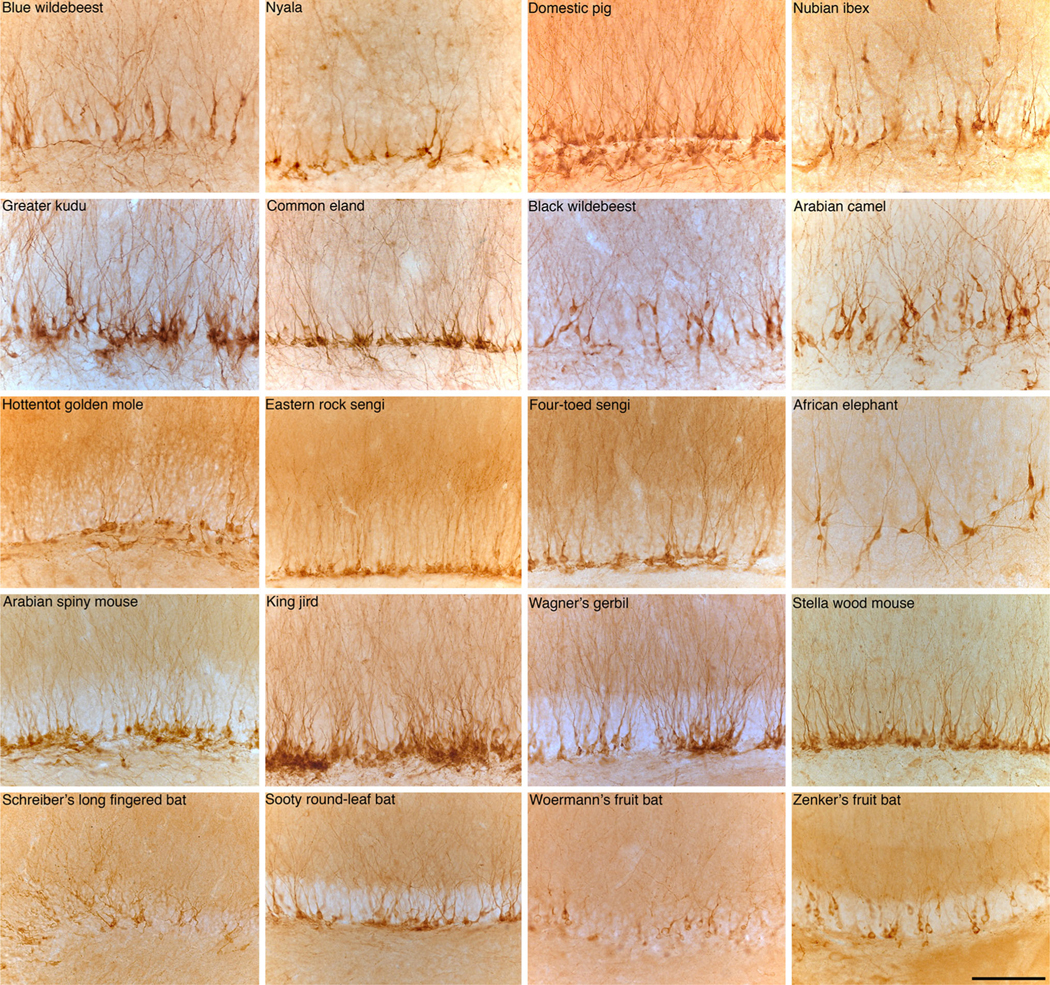
Higher-power photomicrographs of portions of the dentate gyrus immunohistochemically stained for doublecortin in a range of mammalian species. Upper two rows show artiodactyls, third row shows Afrotherians, fourth row shows rodents, and the bottom row shows Microchiropterans and Megachiropterans. Note the presence of immature neurons in all these species. Scale bar in the bottom right image 100 μm and applies to all
Fig. 7.
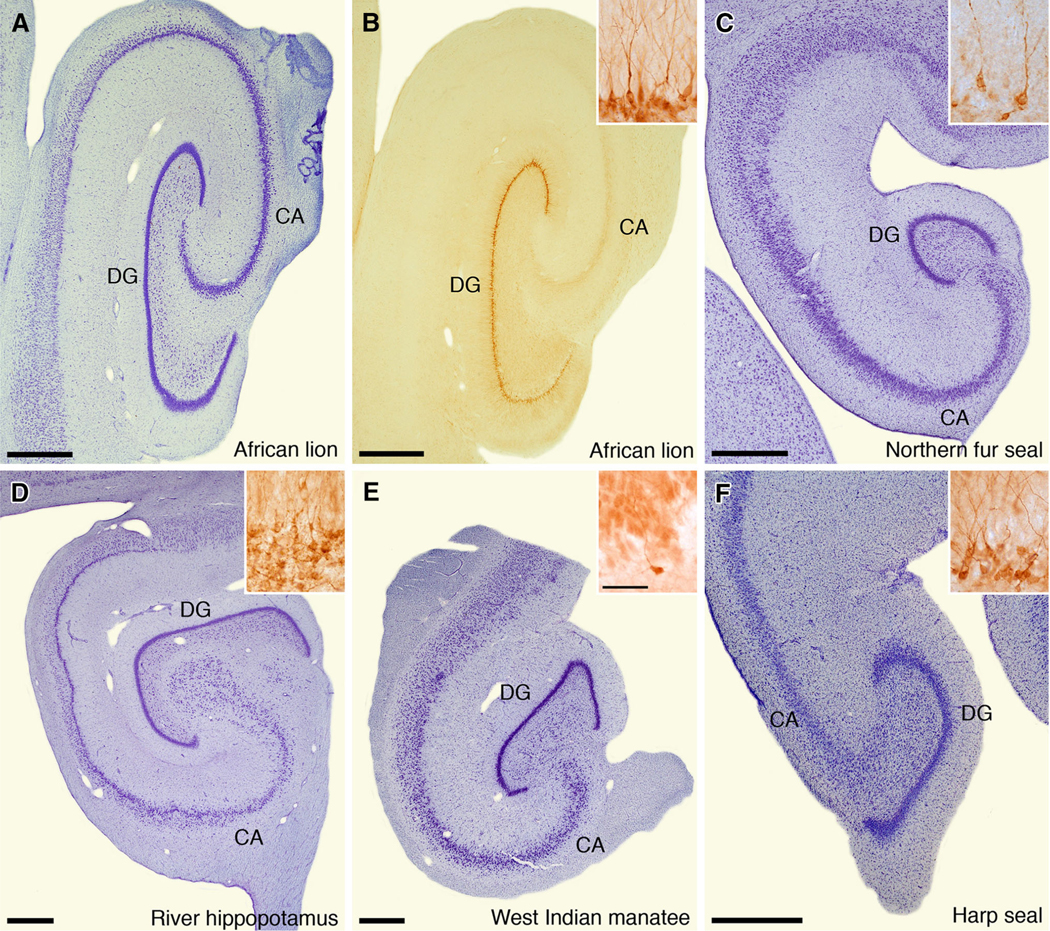
Low-power photomicrographs of the hippocampus in certain key species investigated in the current study. a African lion (Panthera leo), Nissl stain; b African lion, immunohistochemical staining for doublecortin; c Northern fur seal (Callorhinus ursinus), Nissl stain; d river hippopotamus (Hippopotamus amphibius), Nissl stain; e West Indian manatee (Trichechus manatus), Nissl stain; f harp seal (Pagophilus groenlandicus), Nissl stain. Scale bar in each low-power image 1 mm. Insets in b–e are higher-power photomicrographs of immunohistochemical staining for doublecortin in each species. Scale bar in inset e 50 μm, and applies to all insets. CA cornu ammonis, DG dentate gyrus
Fig. 8.
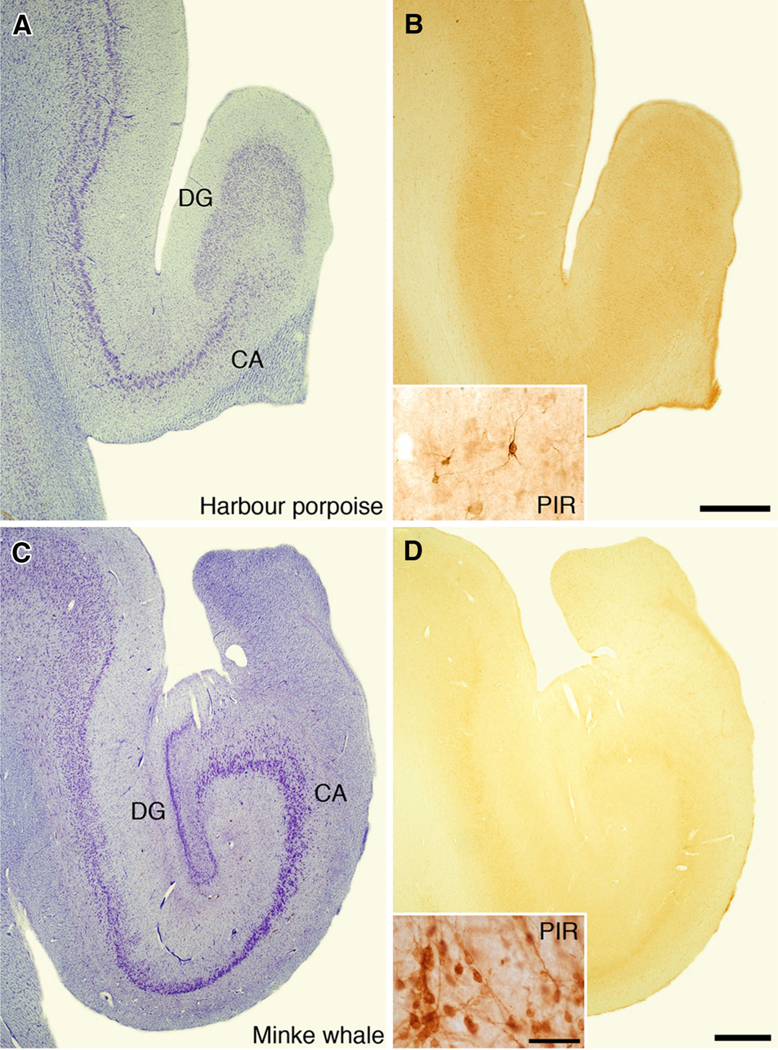
Low-power photomicrographs of the hippocampus in the harbour porpoise (Phocoena phocoena, a, b) and minke whale (Balaenoptera acutorostrata, c, d) stained for Nissl substance (a, c) or immunohistochemical staining for doublecortin (b, d). Note the loose organization of the dentate gyrus in both cetacean species (a, c) as well as the total lack of immunohistochemical staining for doublecortin in both species (b, d). Scale bar in b 1 mm and applies to a and b, scale bar in d 1 mm and applies to c and d. Insets in b and d are higher-power photomicrographs of immunohistochemical staining for doublecortin in the remnant of piriform cortex in the harbour porpoise (b) and the piriform cortex of the minke whale (d). The staining of neurons in the piriform cortex of both cetacean species acts as an internal control for the methods used and confirms the lack of adult neurogenesis in the cetacean dentate gyrus. Scale bar in inset d 50 μm, and applies to both insets. CA cornu ammonis, DG dentate gyrus, PIR piriform cortex
In addition to the apparent lack of adult hippocampal neurogenesis and the small relative and absolute size of the cetacean hippocampus, the architecture of the cetacean hippocampus contrasts with that seen in all other mammals examined. In most mammals, the granule layer of the dentate gyrus is observed to be a tightly packed layer of cells within a distinctly organized three-layered cortical region (Fig. 7); however, in the minke whale, a mysticete cetacean, while evident, the packing of the neurons in the granule cell layer of the dentate gyrus is not as dense as that seen in other mammals. In the harbour porpoise, an odontocete cetacean, the granule cell layer is so loosely organized as to be difficult to discern in normal histological preparations (Fig. 8). Thus, in contrast to all other mammals, the cetaceans have three distinct aspects of hippocampal anatomy that indicate they are neuroanatomically different to all other mammals—a small hippocampus, an apparent lack of adult hippocampal neurogenesis and a loosely organized dentate gyrus.
Discussion
The present study raises several points of interest relating to the evolution and function of the hippocampus in mammals, adult hippocampal neurogenesis, and the brains and behaviour of cetaceans. Our results demonstrate that, unlike other regions of the brain such as the amygdala, the hippocampus does not scale in a linear fashion. Rather, the scaling of the hippocampus in relation to the brain is exponential, approximating a growth curve. Is it possible that this different scaling relates to the presence of adult hippocampal neurogenesis in most mammalian species? Our survey of adult neurogenesis across many mammalian species (Table 2) indicates that adult hippocampal neurogenesis is a trait common to the vast majority of mammals, with the only species appearing to lack this neural trait being the cetaceans. Our observations of the cetacean hippocampus demonstrate that it is both absolutely and relatively small, has a loosely organized architecture, and seems to lacks adult hippocampal neurogenesis, indicating that any cognitive processes that are hippocampal/neurogenesis dependent are likely to be wanting in the cetaceans.
Hippocampal scaling and adult hippocampal neurogenesis
The current study, using a larger database than previous studies (Finlay and Darlington 1995; Reep et al. 2007), including large-brained mammals such as elephants, indicates that the manner in which the volume of the hippocampus scales with the volume of the brain is best described as an exponential function, rather than as a linear function. To date, this is the only demonstration that a component of the brain scales in a non-linear manner with overall brain volume and indeed our own calculations of the scaling of another limbic structure, the amygdala that lies in close apposition to the hippocampus, provide support for this distinction of the hippocampus. Interestingly, the only species that do not adhere to this non-linear scaling of the hippocampus are the cetaceans, which have small hippocampi and seem to lack adult hippocampal neurogenesis. Thus, it would appear that as adult hippocampal neurogenesis is a feature common to most mammals, this persistent growth phenomenon may have some bearing on the manner in which the hippocampus scales with the brain across mammalian species; however, to postulate a direct link between the two and what a potential mechanism might be is difficult at this stage. The hippocampal volume scaling relationship is likely to be affected not only by the addition of new neurons in the dentate gyrus, but also by their constituent parts (dendrites and mossy fibres), differing rates of neurogenesis across the life span, rates of apoptosis, brain size of each species and the associated neuronal density, and epigenetic and phylogenetic factors. Thus, at this stage we cannot propose any direct link between neurogenesis and hippocampal scaling, although our results indicate that this would be a potentially interesting avenue for future study.
As adult hippocampal neurogenesis appears to be a common mammalian trait (apart from cetaceans), this has important implications for the understanding of this neural phenomenon. While many factors influence the rate of proliferation and survival of newly born neurons in the adult hippocampus (e.g. Kempermann 2012), the fact that the vast majority of mammals are likely to have this trait indicates that adult hippocampal neurogenesis probably subserves an invariant function across mammalian species. Our broad survey of species examined questions concepts related to the environment and adult hippocampal neurogenesis, as the species investigated inhabit most of the environments in which mammals are found, from rainforests to deserts and terrestrial to aquatic. As mentioned earlier, newly formed neurons in the hippocampus appear to play a role in pattern separation, thus enhancing the circuits involved in learning and memory and has led to the memory resolution hypothesis for adult hippocampal neurogenesis (Sahay et al. 2011; Aimone et al. 2011). All mammalian species are likely to benefit from this circuitry enhancement, or increased memory resolution, no matter what environment they inhabit.
Why are cetaceans different from all other mammals?
Our findings raise the question of how cetaceans came to have small hippocampi that seem to lack adult neurogenesis and are loosely organized. In terms of general neuroanatomical structure (Manger 2006; Manger et al. 2004, 2012) and sleep physiology (Lyamin et al. 2008), cetaceans are different from all other mammals and the current study adds further support to this interpretation of cetacean neurobiology. Adult cetaceans lack, or have minimal, REM sleep (Lyamin et al. 2008) and appear to lack a clear sleep state for the first month of life, likely having less than 30 s of sleep during the first postnatal month (Lyamin et al. 2005), both aspects appearing to be features of their evolutionary adaptation to the thermally challenging aquatic environment. Studies of the effect of sleep deprivation on adult hippocampal neurogenesis in laboratory mammals (Meerlo et al. 2009) have shown that prolonged REM deprivation decreases cell proliferation rates and that prolonged deprivation of both NREM and REM sleep inhibits cell maturation and integration. This can occur independently of the release of adrenal stress hormones (Meerlo et al. 2009), as seen in mother cetaceans prior to and after birth (Lyamin et al. 2005). The lack of postnatal sleep and the continued lack of REM sleep throughout life may lead to a cessation of hippocampal neurogenesis immediately after birth in cetaceans, despite this not appearing to be a stress-related reduction in hippocampal neurogenesis. This cessation, sustained by a lack of REM sleep in older cetaceans, may prevent any postnatal enlargement of the hippocampus as seen in other mammals (Bayer 1980; Thompson 2012), leading to the observed small size of this structure in adult cetaceans. Moreover, if hippocampal development were arrested immediately postnatally in cetaceans as proposed, the loosely organized cetacean dentate gyrus is likely to be one result of this premature cessation of hippocampal development. It has been postulated that the risk of hypothermia in neonatal cetaceans underlies the lack of postnatal sleep (Lyamin et al. 2005, 2008), thus the current observations lend support to the thermogenesis hypothesis of cetacean brain evolution (Manger 2006).
What do these findings mean regarding cetacean cognitive capacities?
That cetaceans have small, loosely organized hippocampi that apparently lack adult hippocampal neurogenesis poses a serious problem for the hypothesis that these animals are, in comparison to most other mammals except great apes, highly cognitively complex (Marino et al. 2008). Here, we provide three examples of cognitive studies in which the hippocampus plays a central role that are instructive in understanding the results of behavioural experiments on cetaceans. In an object permanence task (invisible displacement/transposition task), cetaceans have been shown to possibly only reach Piaget stage 4 (visible displacement), whereas other mammals and birds tested readily reach stage 5 and apes achieve stage 6 (Mitchell and Hoban 2010; Jaakkola et al. 2010). Object permanence tasks are strongly hippocampus dependent, as they rely on spatial memory. Thus, the failure of cetaceans to clearly achieve higher than stage 4 on these tasks is in agreement with the lack of hippocampal development and adult neurogenesis demonstrated here. Additionally, as Piaget stage 6 of object permanence is thought to be a necessary requirement for mirror-self recognition (Mitchell and Hoban 2010), the lack of achievement of this level of object permanence by cetaceans questions the results of a previous study suggesting that dolphins have this cognitive ability (Reiss and Marino 2001). As a second example, the much lauded language comprehension studies of dolphins (Herman et al. 1984) can be appropriately contextualized. It should be noted that for a dolphin to begin to participate in the trials that probe semantic understanding requires at least 4 years of training (Herman et al. 1984). In a comparable experimental situation, sea lions were shown to reach similar levels of performance to dolphins on these tasks in 2 years or less (Schusterman and Kreiger 1984). In the current study, we have observed that pinnipeds have normal-sized hippocampi that possess adult hippocampal neurogenesis. Thus, success in these types of cognitive experiments that requires the formation and recall of hippocampus-dependent explicit memories and cognitive flexibility was clearly achieved more rapidly in sea lions than dolphins. As a third example, it has been shown that bottlenose dolphins fail to complete a spatial maze task associated with an “if and only if, then” construct on their own volition, whereas several other mammalian and vertebrate species tested readily achieved this combined maze and rule task (Nikolskaya 2005). To complete this task successfully, the animals were required to form memories of places and events, which, given the structure of the cetacean hippocampus, appears to be a cognitive task beyond their neural means.
It may be argued that the functions associated with the hippocampus with regard to complex cognition, learning, memory and spatial orientation have been subsumed into the circuitry in other parts of the cetacean brain, thus facilitating the expression of normally hippocampus dependent cognitive functions. This situation has been observed in rats, where the prefrontal cortex of rats with lesions of the dorsal hippocampus assumes hippocampal functions (Zelikowsky et al. 2013). Despite this, given the known neuroanatomy of the cetacean brain, where the prefrontal cortex appears almost absent (Manger 2006) and the entorhinal and subicular regions of the hippocampal formation appear to be proportionally smaller in the cetaceans mirroring the decrease in hippocampal size (Jacobs et al. 1971, 1979), it is difficult to speculate where this alternative circuitry might lie, how this may facilitate hippocampus-dependent functions or even if it would be as effective as the typical mammalian hippocampal circuitry in undertaking hippocampus-related tasks. Given the fact that it is far more difficult to misinterpret neuroanatomical structure than behavioural studies, the current and previous findings (Manger 2006; Manger et al. 2012) regarding cetacean brain structure appear to necessitate a reappraisal of our notions regarding the cognitive capabilities and behavioural studies of cetaceans (Manger 2013) and the evolution of relatively and absolutely large brain size in this mammalian order (Manger 2006).
Acknowledgments
This work was mainly supported by funding from the South African National Research Foundation (PRM), the Swiss-South African Joint Research Program (AOI and PRM), the King Saud University, Deanship of Scientific Research, Research Chairs Program (AA), and by a fellowship within the Postdoctoral-Program of the German Academic Exchange Service, DAAD (NP). We thank all the relevant wildlife authorities for permission to collect the material used.
Contributor Information
Nina Patzke, School of Anatomical Sciences, University of the, Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa.
Muhammad A. Spocter, School of Anatomical Sciences, University of the, Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa Department of Anatomy, Des Moines University, Des Moines, IA, USA.
Karl Æ. Karlsson, Biomedical Engineering, Reykjavik University, Menntavegur 1, 101 Reykjavik, Iceland
Mads F. Bertelsen, Centre for Zoo and Wild Animal Health, Copenhagen Zoo, Frederiksberg, Denmark
Mark Haagensen, Department of Radiology, Donald Gordon Medical Centre, University of the Witwatersrand, Parktown, Johannesburg 2193, South Africa.
Richard Chawana, School of Anatomical Sciences, University of the, Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa.
Sonja Streicher, Department of Zoology and Entomology, University of Pretoria, Pretoria 0002, South Africa.
Consolate Kaswera, Faculté des Sciences, University of Kisangani, B.P 1232, Kisangani, Democratic Republic of Congo.
Emmanuel Gilissen, Department of African Zoology, Royal Museum for Central Africa, Leuvensesteenweg 13, 3080 Tervuren, Belgium; Laboratory of Histology and Neuropathology, Université libre de Bruxelles, 1070 Brussels, Belgium.
Abdulaziz N. Alagaili, KSU Mammals Research Chair, Department of Zoology, King Saud University, Riyadh 11451, Saudi Arabia
Osama B. Mohammed, KSU Mammals Research Chair, Department of Zoology, King Saud University, Riyadh 11451, Saudi Arabia
Roger L. Reep, Department of Physiological Sciences, University of Florida, Gainsville, FL 32610, USA
Nigel C. Bennett, Department of Zoology and Entomology, University of Pretoria, Pretoria 0002, South Africa KSU Mammals Research Chair, Department of Zoology, King Saud University, Riyadh 11451, Saudi Arabia.
Jerry M. Siegel, Neurobiology Research, 151A3, Department of Psychiatry, Brain Research Institute, UCLA School of Medicine, Sepulveda VA Medical Centre, North Hills, CA 91343, USA
Amadi O. Ihunwo, School of Anatomical Sciences, University of the, Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa
Paul R. Manger, School of Anatomical Sciences, University of the, Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa
References
- Aimone JB, Deng W, Gage FH (2011) Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70:589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme CB, Buzzetti RA, Marrone DF, Leutgeb JK, Chawla MK, Schaner MJ, Bohanik JD, Khoboko T, Leutgeb S, Moser EI, Moser MB, McNaughton BL, Barnes CA (2010) Hippocampal granule cells opt for early retirement. Hippocampus 20:1109–1123 [DOI] [PubMed] [Google Scholar]
- Alpár A, Kunzle H, Gartner U, Popkova Y, Bauer U, Grosche J, Reichenbach A, Hartig W (2010) Slow age-dependent decline of doublecortin expression and BrdU labeling in the forebrain from lesser hedgehog tenrecs. Brain Res 1330:9–19 [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L (2010) A morphologically distinct granule cell type in the dentate gyrus of the red fox correlates with adult hippocampal neurogenesis. Brain Res 1328:12–24 [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP (2004) Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-livingrodents. Hippocampus 14:1000–1010 [DOI] [PubMed] [Google Scholar]
- Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J (2007) The hippocampus book. Oxford University Press, New York [Google Scholar]
- Barker JM, Wojtowicz JM, Boonstra R (2005) Where’s my dinner? Adult neurogenesis in free-living food-storing rodents. Genes Brain Behav 4:89–98 [DOI] [PubMed] [Google Scholar]
- Baron G, Stephan H, Frahm HD (1996) Comparative neurobiology in chiroptera. Birkhauser Verlag, New York [Google Scholar]
- Bartkowska K, Djavadian RL, Taylor JR, Turlejski K (2008) Generation, recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla). Eur J Neurosci 27:1710–1721 [DOI] [PubMed] [Google Scholar]
- Bartkowska K, Turlejski K, Grabiec M, Ghazaryan A, Yavruoyan E, Djavadian RL (2010) Adult neurogenesis in the hedgehog (Erinaceus concolor) and mole (Talpa europaea). Brain Behav Evol 76:128–143 [DOI] [PubMed] [Google Scholar]
- Bayer SA (1980) Development of the hippocampal regions in the rat II. Morphogenesis during embryonic and early postnatal life. J Comp Neurol 190:115–134 [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A (2007) The delayed rise of present-day mammals. Nature 446:507–512 [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A (2008) Corrigendum. The delayed rise of present-day mammals. Nature 456:274. [DOI] [PubMed] [Google Scholar]
- Bunk EC, Stelzer S, Hermann S, Schafers M, Schlatt S, Schwamborn JC (2011) Cellular organization of adult neurogenesis in the common marmoset. Aging Cell 10:28–38 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal–entorhinal system. Nat Neurosci 16:130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawana R, Patzke N, Kaswera C, Gilissen E, Ihunwo AO, Manger PR (2013) Adult neurogenesis in eight megachiropteran species. Neuroscience 244:159–172 [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14 [DOI] [PubMed] [Google Scholar]
- Epp JR, Barker JM, Galea LA (2009) Running wild: neurogenesis in the hippocampus across the lifespan in wild and laboratory-bred Norway rats. Hippocampus 19:1040–1049 [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordberg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317 [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15 [Google Scholar]
- Filimonoff IN (1965) On the so-called rhinencephalon on the dolphin. J Hirnforsch 8:1–23 [PubMed] [Google Scholar]
- Finlay BL, Darlington RB (1995) Linked regularities in the development and evolution of mammalian brains. Science 268:1578–1584 [DOI] [PubMed] [Google Scholar]
- Garland T, Ives AR (2000) Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–364 [DOI] [PubMed] [Google Scholar]
- Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32 [Google Scholar]
- Glezer II, Jacobs MS, Morgane PJ (1988) Implications of the “initial brain” concept for brain evolution in Cetacea. Behav Brain Sci 11:75–116 [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG (2001) Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA 98:10910–10917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi S, Ciani E, Severi S, Contestabile A, Bartesaghi R (2005) Postnatal neurogenesis in the dentate gyrus of the guinea pig. Hippocampus 15:285–301 [DOI] [PubMed] [Google Scholar]
- Harman A, Meyer P, Ahmat A (2003) Neurogenesis in the hippocampus of an adult marsupial. Brain Behav Evol 62:1–12 [DOI] [PubMed] [Google Scholar]
- Herman LM, Richards DG, Wolz JP (1984) Comprehension of sentences by bottlenose dolphins. Cognition 16:129–219 [DOI] [PubMed] [Google Scholar]
- Hyams DG (2010) CurveExpert software. http://www.curveexpert.net. Accessed 15 Mar 2013
- Jaakkola K, Guarino E, Rodriguez M, Erb L, Trone M (2010) What do dolphins (Tursiops truncatus) understand about hidden objects? Anim Cogn 13:103–120 [DOI] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P (2010) Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci 31:273–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MS, Morgane PJ, McFarland WL (1971) The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Rhinic lobe (Rhinencephalon) I. The paleocortex. J Comp Neurol 141:205–272 [DOI] [PubMed] [Google Scholar]
- Jacobs MS, McFarland WL, Morgane PJ (1979) The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Rhinic lobe (Rhinencephalon): the archicortex. Brain Res Bull 4(Suppl 1):1–108 [DOI] [PubMed] [Google Scholar]
- Johnson KM, Boonstra R, Wojtowicz JM (2010) Hippocampal neurogenesis in food-storing red squirrels: the impact of age and spatial behavior. Genes Brain Behav 9:583–591 [DOI] [PubMed] [Google Scholar]
- Kempermann G (2012) New neurons for ‘survival of the fittest’. Nat Rev Neurosci 13:727–736 [DOI] [PubMed] [Google Scholar]
- Klempin F, Kronenberg G, Cheung G, Kettenmann H, Kempermann G (2011) Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One 6:e25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G (2010) Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5:e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT (2011) Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci USA 108:10326–10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P (1999) Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA 96:5768–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitsky Y, Gross CG, Gould E (2007) Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci USA 104:17169–17173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Pryaslova J, Lance V, Siegel JM (2005) Continuous activity in cetaceans after birth. Nature 435:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM, Siegel JM (2008) Cetacean sleep: an unusual form of mammalian sleep. Neurosci Biobehav Rev 32:1451–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Hamadeh MJ, Christie BR, Foster JA, Tarnopolsky MA (2012) Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PLoS One 7:e36048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2005) Mesquite: a modular system for evolutionary analysis version 1.06. http://mesquiteproject.org. Accessed 28 Feb 2013
- Manger PR (2006) An examination of cetacean brain structure with a novel hypothesis correlating thermogenesis to the evolution of a big brain. Biol Rev 81:293–338 [DOI] [PubMed] [Google Scholar]
- Manger PR (2013) Questioning the interpretations of behavioural observations of cetaceans: is there really support for a special intellectual status for this mammalian order? Neuroscience 250:664–696 [DOI] [PubMed] [Google Scholar]
- Manger PR, Fuxe K, Ridgway SH, Siegel JM (2004) The distribution and morphological characteristics of catecholamine cells in the diencephalon and midbrain of the bottlenose dolphin (Tursiops truncatus). Brain Behav Evol 64:42–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger PR, Pillay P, Maseko BC, Bhagwandin A, Gravett N, Moon DJ, Jillani NE, Hemingway J (2009) Acquisition of brains from the African elephant (Loxodonta africana): perfusion-fixation and dissection. J Neurosci Methods 179:16–21 [DOI] [PubMed] [Google Scholar]
- Manger PR, Hemingway J, Haagensen M, Gilissen E (2010) Crosssectional area of the elephant corpus callosum: comparison to other eutherian mammals. Neuroscience 167:815–824 [DOI] [PubMed] [Google Scholar]
- Manger PR, Prowse M, Haagensen M, Hemingway J (2012) Quantitative analysis of neocortical gyrencephaly in African elephants (Loxodonta africana) and six species of cetaceans: comparison with other mammals. J Comp Neurol 520:2430–2439 [DOI] [PubMed] [Google Scholar]
- Marino L, Butti C, Connor RC, Fordyce RE, Herman LM, Hof PR, Lefebvre L, Lusseau D, McCowan B, Nimchinsky EA, Pack AA, Reidenberg JS, Reiss D, Rendell L, Uhen MD, van der Gutcht E, Whitehead H (2008) A claim in search of evidence: reply to Manger’s thermogenesis hypothesis of cetacean brain structure. Biol Rev 83:417–440 [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM (2005) Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett 385:70–75 [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 13:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, Hoban E (2010) Does echolocation make understanding object permanence unnecessary? Failure to find object permanence understanding in dolphins and beluga whales. In: Dolins FL, Mitchell RW (eds) Spatial cognition, spatial perception. Mapping the self and space. Cambridge University Press, Cambridge, pp 258–280 [Google Scholar]
- Montie EW, Schneider G, Ketten DR, Marino L, Touhey KE, Hahn ME (2008) Volumetric neuroimaging of the Atlantic white-sided dolphin (Lagenorhynchus acutus) brain from in situ magnetic resonance images. Anat Rec 291:263–282 [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Jacobs MS, McFarland WL (1980) The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Surface configurations of the telencephalon of the bottlenose dolphin with comparative observations in four other cetacean species. Brain Res Bull 5(Suppl. 3):1–107 [Google Scholar]
- Nikolskaya KA (2005) Evolutionary aspects of intellect in vertebrates: can intellect be a factor confining choice of the habitat? Invest Russ 8:1442–1500 [Google Scholar]
- Pagel MD (1992) A method for the analysis of comparative data. J Theor Biol 156:431–442 [Google Scholar]
- Patzke N, Olaleye O, Haagensen M, Hof PR, Ihunwo AO, Manger PR (2013a) Organization and chemical neuroanatomy of the African elephant (Loxodonta africana) hippocampus. Brain Struct Funct (in press) [DOI] [PubMed] [Google Scholar]
- Patzke N, Kaswera C, Gilissen E, Ihunwo AO, Manger PR (2013b) Adult neurogenesis in a giant otter shrew (Potamogale velox). Neuroscience 238:270–279 [DOI] [PubMed] [Google Scholar]
- Pilleri G, Gihr M (1970) The central nervous system of the Mysticete and Odontocete whales. In: Pilleri G (ed) Investigations on Cetacea, vol 2. Brain Anatomy Institute, Berne, pp 89–127 [Google Scholar]
- Pirlot P, Nelson J (1978) Volumetric analyses of monotreme brains. In: Augee ML (ed) Monotreme biology. The Royal Zoological Society of New South Wales, Syndey, pp 171–180 [Google Scholar]
- Quader S, Isvaran K, Hale RE, Miner BG, Seavy NE (2004) Nonlinear relationships and phylogenetically independent contrasts. J Evol Biol 17:709–715 [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234–246 [DOI] [PubMed] [Google Scholar]
- Reep RL, Finlay BL, Darlington RB (2007) The limbic system in mammalian brain evolution. Brain Behav Evol 70:57–70 [DOI] [PubMed] [Google Scholar]
- Reiss D, Marino L (2001) Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc Natl Acad Sci USA 98:5937–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman RJ, Kreiger K (1984) California sea lions are capable of semantic comprehension. Psychol Rec 34:3–23 [Google Scholar]
- Schwerdtfeger WK, Oelschlager HA, Stephan H (1984) Quantitative neuroanatomy of the brain of the La Plata dolphin, Pontoporia blainvillei. Anat Embryol 170:11–19 [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW (2007) Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem 88:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato J, Sullivan RK, Turpin FR, Bartlett PF, Sah P (2012) Properties of doublecortin expressing neurons in the adult mouse dentate gyrus. PLoS One 7:e41029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G (1981) New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol 35:1–29 [DOI] [PubMed] [Google Scholar]
- Sweatt JD (2004) Hippocampal function in cognition. Psychopharmacology 174:99–110 [DOI] [PubMed] [Google Scholar]
- Thompson DK (2012) Postnatal hippocampal growth in health and prematurity: modulation and implications. In: Preedy VR (ed) Handbook of growth and growth monitoring in health and disease. Springer, New York, pp 643–662 [Google Scholar]
- Treves A, Tashiro A, Witter MP, Moser EI (2008) What is the mammalian dentate gyrus good for? Neuroscience 154:1155–1172 [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN (2010) Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci USA 107:7963–7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS (2013) Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci USA 110:9938–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang ZY, Hansson HA (2003) Visualization of proliferating cells in the adult mammalian brain with the aid of ribonucleotide reductase. Brain Res 977:180–189 [DOI] [PubMed] [Google Scholar]