Highlights
-
•
Delayed brain tumor (DBT) mouse cell lines stably expressing the murine angiotensin converting enzyme 2 (mACE2) have been generated.
-
•
The cell lines are highly susceptible to mouse-adapted SARS-CoV infection.
-
•
SARS-CoV-MA15 efficiently induced the expression of proinflammatory cytokines and IFN-β in DBT-mACE2 cells.
-
•
DBT-mACE2 cells provide a good experimental system that is species-homologous to the in vivo systems for evaluating SARS-CoV-host interaction studies.
Keywords: SARS, Coronavirus, Mouse adapted, Stably transformed murine cells, SARS-CoV receptor ACE2, Proinflammatory cytokines
Abstract
Infection of conventional mice with a mouse adapted (MA15) severe acute respiratory syndrome (SARS) coronavirus (CoV) reproduces many aspects of human SARS such as pathological changes in lung, viremia, neutrophilia, and lethality. However, established mouse cell lines highly susceptible to mouse-adapted SARS-CoV infection are not available. In this work, efficiently transfectable mouse cell lines stably expressing the murine SARS-CoV receptor angiotensin converting enzyme 2 (ACE2) have been generated. These cells yielded high SARS-CoV-MA15 titers and also served as excellent tools for plaque assays. In addition, in these cell lines, SARS-CoV-MA15 induced the expression of proinflammatory cytokines and IFN-β, mimicking what has been observed in experimental animal models infected with SARS-CoV and SARS patients. These cell lines are valuable tools to perform in vitro studies in a mouse cell system that reflects the species used for in vivo studies of SARS-CoV-MA15 pathogenesis.
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV) is an enveloped plus-strand RNA virus of the Coronaviridae family, genus β, within the Nidovirales order (de Groot et al., 2012, Enjuanes et al., 2008). SARS-CoV was first detected in late 2002 in Guangdong province, China and spread to more than 30 countries in a few months, causing 8000 infections and 800 deaths (Drosten et al., 2003, Fouchier et al., 2003, Ksiazek et al., 2003, Kuiken et al., 2003, Marra et al., 2003, Peiris et al., 2003, Rota et al., 2003). The spread of the virus was ultimately controlled by the isolation of infected individuals, and the WHO declared the end of the SARS epidemic in July 2003. However, SARS-like coronaviruses remain and are circulating in bats all over the world, making virus reemergence a realistic possibility (Lau et al., 2005, Li et al., 2005, Woo et al., 2006).
Coronaviruses encode two overlapping open reading frames (ORFs 1a and 1b) that are translated into two polyproteins which are processed by two viral proteases to yield 16 non-structural replicase proteins (Ziebuhr et al., 2000). These proteins are involved in genome replication and transcription of subgenomic mRNAs encoding the structural proteins nucleocapsid (N), envelope (E), membrane (M) and spike (S), as well as a set of CoV species-specific proteins. The spike protein is localized at the surface of the virion, and is responsible for the attachment to the cellular receptor, and for virus-cell membrane fusion, to facilitate virus entry (Gallagher and Buchmeier, 2001). The cellular receptor for SARS-CoV is the angiontesin convertin enzyme 2 (ACE2) (Li et al., 2003, Wong et al., 2004), although the glycoprotein CD209L (L-SIGN) may also be used as a weaker alternative receptor (Jeffers et al., 2004).
SARS-CoV infects many experimental animals such as mice, ferrets, cats, hamsters and non-human primates (cynomolgus and rhesus macaques, African green monkeys and marmosets) (Roberts et al., 2008, Subbarao and Roberts, 2006). However, none of the infection models completely reproduce human clinical disease and pathological findings. To overcome these limitations, SARS-CoV was adapted to grow in mice by passing the virus in lung for 10, 15, or 25 times (Day et al., 2009, Nagata et al., 2008, Roberts et al., 2007). Infection of Balb/c mice with the resulting mouse adapted (MA) viruses reproduced many aspects of human SARS, including pathological changes in the lung, viremia, neutrophilia, and lethality (Day et al., 2009, Nagata et al., 2008, Roberts et al., 2007). This inbred mouse model of human SARS disease has many advantages compared to the other animal models, such as small animal size, low cost, availability of the animals, the possibility to genetically manipulate the host animals (i.e. to develop gene knock-outs and knock-ins), and the availability of immunological and molecular biology reagents specific to the host animals.
Coronaviruses generally do not induce a high interferon response (Frieman et al., 2008). At least two mechanisms have been proposed to explain the low levels of type I interferon (IFN-α and -β) during coronavirus infections: the sequestering of viral RNA in double membrane vesicles (Gosert et al., 2002, Knoops et al., 2008), which prevents or reduces recognition by pattern recognition receptors (PRRs); and the expression of viral proteins that antagonize the innate response. In fact, SARS-CoV proteins nsp1, nsp3, 3b, 6, M and N act as interferon antagonists (Devaraj et al., 2007, Frieman et al., 2007, Kopecky-Bromberg et al., 2007, Narayanan et al., 2008, Siu et al., 2009, Sun et al., 2012, Wathelet et al., 2007). However, even with these viral strategies of defensive evasion and offensive antagonism of interferons, there are well-described host proinflammatory responses to in vivo SARS-CoV infections. Inflammatory mediators such as interleukin (IL)-1, -6, and -8, CXCL10/interferon-inducible protein (IP)-10, CCL2/monocyte chemoattractant protein (MCP)-1, CCL5/protein regulated and normal T expressed and secreted (RANTES), and CXCL9/monokine induced by interferon gamma (MIG) have been recognized in lungs of patients affected by SARS (Cameron et al., 2007, Huang et al., 2005, Jiang et al., 2005, Reghunathan et al., 2005, Tang et al., 2005, Wong et al., 2003, Zhang et al., 2004). Upregulation of genes mediating inflammation has also been described after infection with SARS-CoV in different animal models such as cynomolgus macaques and African green monkeys (de Lang et al., 2007, Smits et al., 2010, Smits et al., 2011) and mice (Baas et al., 2008). Accordingly, the expression of several proinflammatory genes is considered to be a strong correlate of SARS-CoV induced pathology.
Several established cell lines from different species, including monkey cells Vero E6, MA104 and FRhK-4, human cells Caco-2, CL-14, LoVo and Huh-7, pig cells PK-15, POEK and PS, and mink cells Mv 1 Lu (Chan et al., 2004, Cinatl et al., 2004, Hattermann et al., 2005, Mossel et al., 2005, Ng et al., 2003) are susceptible to SARS-CoV infection. However, not all of these cell lines possess the full complement of genes encoding innate immune signaling and response factors, and as such, some of the cell lines are not suitable for evaluating the innate host response to SARS-CoV. Mouse DBT cells have been transfected with human or civet SARS-CoV receptor ACE2 (Becker et al., 2008, Sheahan et al., 2008a, Sheahan et al., 2008b). However, as far as we know, the susceptibility of these cell lines to a mouse adapted SARS-CoV has not been determined. In addition, mouse cell lines have never been stably transfected with the mouse ACE2, which is the natural receptor used by the mouse adapted SARS-CoV.
In this work, we established mouse cell lines stably expressing the murine SARS-CoV receptor ACE2 (Li et al., 2003, Wong et al., 2004). These cell lines were highly susceptible to mouse adapted SARS-CoV infection and were also transfectable with high efficiency. Using these cell lines, we demonstrated that a mouse adapted SARS-CoV (SARS-CoV-MA15) induced the expression of genes leading to inflammation, as shown in SARS patients and experimental animal models infected with SARS-CoV. In addition, SARS-CoV-MA15 induced a weak IFN-β response, as generally shown in coronavirus infections, preventing the robust production of type I IFNs. These mouse cell lines are valuable tools to perform in vitro studies that could be further developed in the species-homologous SARS mouse models.
2. Materials and methods
2.1. Cells
Delayed brain tumor (DBT) cells were originally obtained from an intracerebral tumor induced in an adult BALB/c mouse by intracerebral injection of Rous sarcoma virus (Kumanishi, 1967). The African green monkey kidney-derived Vero E6 cells were kindly provided by Snijder (University of Leiden, The Netherlands). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO) supplemented with 25 mM HEPES, 10% fetal bovine serum (FBS, Biowhittaker), and 1% non-essential amino acids (SIGMA) and incubated in a 5% CO2 atmosphere, at 37 °C.
2.2. Plasmids
The plasmid pcDNA3.1 encoding the murine ACE2 gene (GeneBank sequence NM_001130513.1) fused to 5′ myc tag codons (pcDNA3.1-myc-mACE2), and a gene providing geneticin (G418) resistance in prokaryotic and eukaryotic cells, was kindly provided by Farzan (Harvard Medical School, USA) (Li et al., 2004). SARS-CoV full-length cDNAs encoding the Urbani strain or the mouse adapted strain (MA15) were assembled in bacterial artificial chromosomes (BACs) under the control of the cytomegalovirus (CMV) immediate-early promoter to allow the expression of the viral RNA in the nucleus by cellular RNA polymerase II. At the 3′ end, these cDNAs were flanked by a 25-bp poly(A) tail, followed by the hepatitis delta virus ribozyme and the bovine growth hormone and termination and polyadenylation sequences, to generate a correct 3′ end (Almazan et al., 2006, Almazan et al., 2000).
2.3. Viruses
SARS-CoV-Urbani and SARS-CoV-MA15 viruses (Roberts et al., 2007) were rescued from infectious cDNA clones generated (Almazan et al., 2006, Fett et al., 2013). To recover infectious viruses, BHK cells were grown to 95% confluence in a 25-cm2 flask and transfected with the cDNA clones by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's specifications. At 6 h posttransfection (hpt), cells were trypsinized, plated over a confluent monolayer of Vero E6 cells grown in a 25-cm2 flask, and incubated at 37 °C for 72 h. After one passage in Vero E6 cells, the recovered viruses were cloned by three rounds of plaque purification. All work with infectious viruses was performed in biosafety level (BSL) 3 plus facilities. All personnel were equipped with positive-pressure air purifying respirators (3 M HEPA AirMate, St. Paul, MN).
2.4. Generation of DBT cells stably expressing mACE2
To transfect DBT-mACE2 with high efficiency, 3 × 105 DBT-mACE2 cells were transfected using the program EN-158 of Amaxa 4D-Nucleofector and 1 μg of the linearized plasmid pcDNA3.1-myc-mACE2 in buffer SG (Lonza). Nucleofected cells were seeded on 24-well plates. After nucleofection, cells were incubated for 10 min at room temperature (RT), and then the cells were added to DMEM (GIBCO) supplemented with 25 mM HEPES, 10% fetal bovine serum (FBS, Biowhittaker) and 1% non-essential amino acids (SIGMA) and incubated at 37 °C. 24 h post-nucleofection, G418 was added to the culture medium, to a final concentration of 800 μg/ml, to select for geneticin resistance. The selective medium was changed every 3–4 days for 2 weeks, and the cells were cloned three times by limiting dilution (1 cell/well) in the presence of G418 (800 μg/ml). Subsequently, ten clones of DBT-mACE2 were amplified in the presence of 800 μg/ml of G418.
2.5. Transfection of DBT-mACE2 clones
To analyze the efficiency of transfection, DBT-mACE2 cells were nucleofected as described above, using a GFP-encoding plasmid. 24 h post nucleofection GFP expression was analyzed by fluorescence activated cell sorting (FACS). DBT-mACE2 cells were transfected with an efficiency higher than 90%.
2.6. Indirect immunofluorescence microscopy
To detect viral N proteins and the myc tag fused to mACE2, DBT-mACE2 cells were grown to 80% confluence on glass coverslips and infected with rSARS-CoV-MA15 at a moi of 0.1. At 24 hpi, media were removed and cells were washed twice with PBS and fixed and permeabilized with ice-cold 100% methanol for 20 min at −20 °C or with 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min at room temperature, in the case of non-permeabilized cells. Primary antibody incubations were performed in PBS containing 10% FBS for 90 min at room temperature. The mouse mAb SA46-4 specific for N protein, kindly provided by Fang (South Dakota State University, Brookings, USA) (dilution 1:500), and a mouse anti-myc (dilution 1:500, Millipore, Ref. 05-724), were used. Coverslips were washed two times with PBS between primary and secondary antibody incubations. Secondary antibodies (Invitrogen) were Alexa 488 (for detection of N protein) or Alexa 594 (for detection of myc tag) conjugates and were incubated for 45 min at room temperature at 1:500 dilutions in PBS containing 10% FBS. Nuclei were stained using DAPI (dilution 1:200, Sigma). Coverslips were mounted in Prolong Gold anti-fade reagent (Invitrogen) and examined on a Leica SP5 confocal microscope (Leica Microsystems). The percentage of mACE2 and viral N-positive cells were calculated by analyzing 10 random fields, each one containing at least 30 cells.
2.7. Virus production in different DBT-mACE2 clones
DBT-mACE2 clones grown to densities of 3.0 × 105 cells/cm2 were infected at a moi of 0.1 with SARS-CoV-MA15 or SARS-CoV-Urbani. Culture supernatants were collected at 72 hpi, and virus titers were determined in Vero E6 cells as previously described (DeDiego et al., 2007).
To analyze the effect of the moi on virus production, DBT-mACE2 clone 6 cells, which supported the highest viral titers, were infected at mois ranging from 0.0001 to 1 pfu per cell with the rSARS-CoV-MA15 virus. Culture supernatants were collected at the indicated times post-infection and titrated on Vero E6 cells.
To determine the effect of cell density on virus production, DBT-mACE2 clone 6 cells at different cell densities were infected at a moi of 0.1. Culture supernatants were collected at 72 hpi and titrated in Vero E6 cells.
2.8. Virus titration in DBT-mACE2 cells
DBT-mACE2 clone 6 cells at a cell density of 2.5 × 105 cells/cm2 were infected with SARS-CoV-MA15. Cell cultures were incubated at 37 °C for 60 min for virus adsorption and then overlaid with DMEM containing 0.6% low melting agarose and 4% FBS. 48 hpi, cells were fixed with 10% formaldehyde in PBS and stained with a solution containing 0.1% (w/v) crystal violet and 20% methanol.
2.9. Expression of inflammation-related cytokines and IFN-β and -γ in rSARS-CoV-MA15 infected cells
DBT-mACE2 clone 6 cells were infected with rSARS-CoV at a moi of 0.1. Total RNAs from DBT-mACE2 infected cells were extracted at 48 hpi using the Qiagen RNeasy kit according to the manufacturer's instructions. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) reactions were performed at 37 °C for 2 h with a High Capacity cDNA transcription kit (Applied Biosystems) using 100 ng of total RNA and random hexamer oligonucleotides. Cellular gene expressions were analyzed using TaqMan gene expression assays (Applied Biosystems) specific for Mus musculus genes (Table 1 ). Data were acquired with an ABI PRISM 7000 sequence detection system (Applied Biosystems) and analyzed with ABI PRISM 7000 SDS version 1.0 software. Gene expression in mock-infected cells and rSARS-CoV-MA15-infected cells was compared. Quantification was achieved using the 2 − ΔΔCt method, which analyzes relative changes in gene expression in qPCR experiments (Livak and Schmittgen, 2001). The results of three independent experiments were analyzed. DBT-mACE2 clone 6 cells were infected at a moi of 0.1 and cell extracts in lysis buffer (1% NP-40, 50 mM Tris–HCl, pH 7.6, 2 mM NaCl, 2 mM EDTA and protease inhibitors) were prepared at 48 hpi. Cell extracts were diluted 1:5 in assay buffer (Millipore). The expression of mouse CXCL10/IP-10 and CXCL2/macrophage inflammatory protein 2 (MIP-2) was evaluated with the Luminex technology and a mouse cytokine antibody bead kit (Milliplex map kit; Millipore), following the manufacturer's instructions. Data were collected from three independent infections.
Table 1.
Taqman assays used to analyze the expression of cellular genes by quantitative RT-PCR.
| Gene name | Taqman assaya | Description |
|---|---|---|
| TNF | Mm00443258-m1 | Tumor necrosis factor |
| IL-1α | Mm00439620-m1 | Interleukin 1α |
| IL-1β | Mm01336189-m1 | Interleukin 1β |
| IL-6 | Mm00446190-m1 | Interleukin 6 |
| CCL2/MCP-1 | Mm00441242-m1 | Monocyte chemotactic protein 1 |
| CCL5/RANTES | Mm01302428-m1 | Regulated upon activation, normal T-cell expressed, and secreted |
| CXCL1/NAP-3 | Mm04207460-m1 | Neutrophil activating protein 3 |
| CXCL2/MIP-2 | Mm00436450-m1 | Macrophage inflammatory protein 2 |
| CXCL10/IP-10 | Mm00445235-m1 | Interferon inducible protein 10 |
| IFN-β | Mm00439552-s1 | Interferon β |
| IFN-γ | Mm01168134-m1 | Interferon γ |
| 18S | Mm03928990-g1 | Ribosomic RNA 18S |
Mm, means Mus musculus.
3. Results
3.1. Generation of mouse DBT cells expressing the SARS-CoV receptor mACE2
To generate a mouse cell line susceptible to SARS-CoV infection, DBT cells were nucleofected with a plasmid encoding the SARS-CoV receptor mACE2 fused N-terminally to a myc tag (myc-mACE2) along with an antibiotic resistance marker. Transfected cells were selected by using geneticin (G418). Ten independently-derived cell clones were generated. mACE2 expression in the different clones was analyzed by immunofluorescence using an antibody specific for the myc tag. The ten selected clones showed high mACE2 expression in more than 70% of the cells (Fig. 1A and B). The myc-mACE2 expression was stable for at least 20 passages in tissue culture (data not shown). Myc-mACE2 was detected in the plasma membrane even in non-permeabilized cells, confirming that mACE2 is present at the cell surface. As expected, myc-mACE2 was undetectable in untransfected DBT cells (Fig. 1A and B). To determine whether the DBT-mACE2 clones were easily transfected with plasmid DNAs (a useful method for future studies), the cells were nucleofected with a plasmid encoding green fluorescent protein (GFP). Interestingly, the efficiency of DBT-mACE2 nucleofection was greater than 90% (data not shown).
Fig. 1.
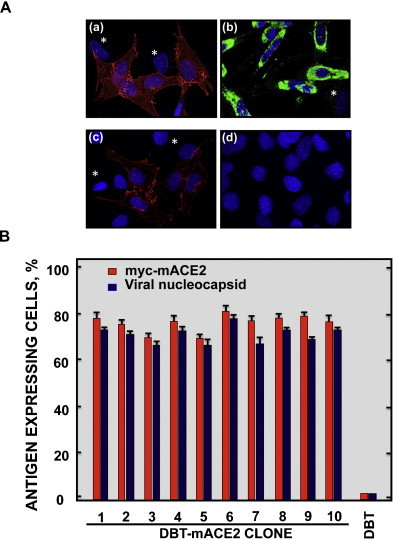
Expression of myc-mACE2 and viral nucleoprotein in DBT-mACE2-infected clones. (A) Untransformed DBT and DBT-mACE2 cells were infected at a moi of 0.1 with rSARS-CoV-MA15. Myc-mACE2 and viral nucleoprotein expression was analyzed by indirect immunofluorescence using specific myc and nucleoprotein antibodies followed by A549 and A488-conjugated secondary mouse antibodies. Asterisks indicate cells that do not express myc-mACE2 or viral nucleoprotein, as controls. (a) myc-mACE2 in permeabilized cells, (b) viral nucleoprotein in permeabilized cells, (c) myc-mACE2 in non-permeabilized cells and (d) myc-mACE2 and nucleoprotein in untransformed DBT cells. (B) The percentage of myc-mACE2 positive and viral N protein-positive cells was calculated by analyzing 10 random fields, each one containing 30 cells.
3.2. Susceptibility of DBT-mACE2 to SARS-CoV infection
To determine whether the DBT-mACE2 cells were susceptible to SARS-CoV, the 10 selected clones, untransfected DBT cells, and positive-control Vero E6 cells were infected with the mouse adapted SARS-CoV-MA15 strain at a moi of 0.1. No infectious virus was recovered from untransfected DBT cells at 72 hpi (Fig. 2A). Interestingly, in transfected cells, all DBT-mACE2 clones showed high viral titers at 72 hpi, ranging from 5.0 × 106 to 1.2 × 107 pfu/ml, respectively. DBT-mACE2 clones 1 and 6 produced the highest virus outputs (9.2 × 106 and 1.2 × 107 pfu/ml), which were as high as those obtained in Vero E6 cells (Fig. 2A).
Fig. 2.
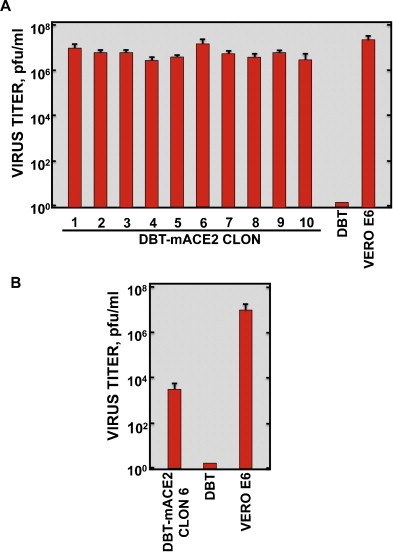
SARS-CoV production in DBT-mACE2 cell clones. Cells were infected at a moi of 0.1 with rSARS-CoV-MA15 (A) or rSARS-CoV-Urbani. (B) Viral titers in cell supernatants at 72 hpi were measured using a plaque assay on Vero E6 cells. Error bars represent standard deviations of the mean from three experiments.
To analyze whether DBT-mACE2 cells were also susceptible to a non-mouse adapted SARS-CoV, the clone 6 of DBT-mACE2 cells, producing the highest SARS-CoV-MA15 titers, untransfected DBT cells and Vero E6 cells were infected with SARS-CoV-Urbani. Infectious viruses were also recovered in DBT-mACE2 cells, although with titers more than 103-fold lower than those for SARS-CoV-MA15 (Fig. 2B). In contrast, no infectious virus was recovered from untransfected DBT cells. SARS-CoV-Urbani and MA15 virus titers in Vero E6 cells were similar for both viruses (Fig. 2A and B).
To identify conditions for optimal virus production, moi and cell density parameters were varied. Firstly, DBT-mACE2 clone 6 cells were infected with SARS-CoV-MA15 at mois ranging from 0.0001 to 1. Virus titers were determined at 0, 4, 24, 48 and 72 hpi (Fig. 3 ). The highest viral titer (>107 pfu/ml) was obtained at moi of 0.1. Virus titers in cells infected with a moi of 1 rapidly decreased after 24 hpi, probably due to extensive cell death. Indeed, considerable cytopathic effect (CPE) was observed by microscopy. DBT-mACE2 clone 6 cells were also infected at cells densities ranging from 0.5 × 105 and 4 × 105 cells/cm2, at the optimal moi of 0.1, and virus titers were determined at 72 hpi. The highest viral titers (>107 pfu/ml) were observed at a cell density of 3 × 105 cells/cm2 (Fig. 4 ).
Fig. 3.
Effect of moi on the growth kinetics of SARS-CoV-MA15 in DBT-mACE2 clone 6 cells. DBT-mACE2 clone 6 cells were infected at the indicated input mois. Viral titers in cell supernatants at the indicated times post-infection were measured by plaque assay on Vero E6 cells. Error bars represent standard deviations of the mean from three experiments.
Fig. 4.
Effect of cell density on the growth of SARS-CoV-MA15 in DBT-mACE2 clone 6 cells. DBT-mACE2 clone 6 cells were seeded at different cell densities and infected at a moi of 0.1. Viral titers in cell supernatants at 72 hpi were measured by plaque assay on Vero E6 cells. Error bars represent standard deviations of the mean from three experiments.
To determine the percentage of infected cells, untransfected DBT cells, and DBT-mACE2 clones were infected with SARS-CoV-MA15, and analyzed by immunofluorescence with an antibody specific for the viral N protein (Fig. 1A and B). The percentage of infected cells, detected by the presence of N protein, was higher than 70% in all the DBT-mACE2 transfected clones.
SARS-CoV infection caused CPE in DBT-mACE2 cells. At input mois of 0.1, cell rounding and detachment was evident at 48 hpi, being almost total at 72 hpi. Control uninfected cells showed no morphological changes (Fig. 5A). In fact, SARS-CoV-MA15 formed clear, easily identifiable plaques in DBT-mACE2 cell monolayers. Lysis plaques on DBT-mACE2 cells and Vero E6 cells were compared. Smaller plaques were observed using Vero E6 cells in comparison to DBT-mACE2 cells (Fig. 5B). Virus titers obtained by plaque assays on Vero E6 and DBT-mACE2 indicator cells were very similar (4.3 × 107 and 4.0 × 107, respectively).
Fig. 5.
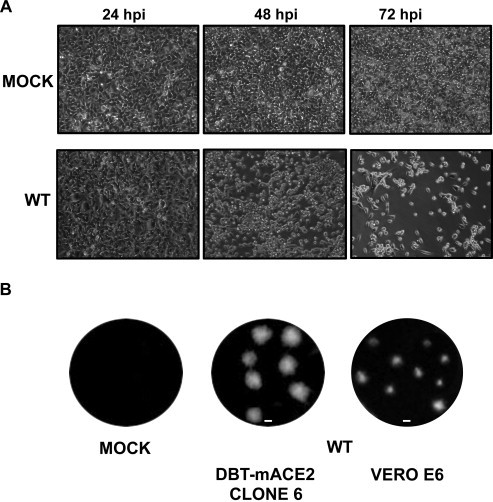
Cytopathic effect and lysis plaques produced by SARS-CoV-MA15 on DBT-mACE2 clone 6 cells. (A) DBT-mACE2 clone 6 cells were mock-infected or infected with SARS-CoV-MA15 at a moi of 0.1. CPE was visualized at 24, 48, and 72 hpi. (B) Lysis plaques produced after 2 days by SARS-CoV-MA15 infection in DBT-mACE2 and Vero E6 cells.
3.3. Expression of inflammation related cytokines
Cytokines and IFNs are important mediators in the regulation of the immune response. To determine whether DBT-mACE2 cells are good models to evaluate the inflammatory host response to SARS-CoV infection in vitro, the mRNA expression levels of several inflammation-related cytokines and that of IFN-β and IFN-γ were analyzed by quantitative RT-PCR. Specifically, CCL2/MCP-1, tumor necrosis factor (TNF), CXCL10/IP-10, CXCL1/neutrophil activating protein 3 (NAP-3), IFN-β, CXCL2/MIP-2, CCL5/RANTES, IL6, IL1B, IL1A, and IFN-γ transcripts, and 18S rRNA, as control, were compared in both uninfected and SARS-CoV-MA15-infected cells at 24, 48 and 72 hpi. The levels of cytokine mRNAs at 24 hpi, were lower compared to 48 hpi, showing fold-increases of 1.2 to 3-fold over mock. Similar levels were observed for 48 and 72 hpi (data not shown). CCL2/MCP-1, TNF, CXCL10/IP-10 and CXCL1/NAP-3 were the most upregulated cytokines 48 h after SARS-CoV infection (between 22- and 50-fold increase; Fig. 6A). Less striking upregulations were observed for IFN-β, CXCL2/MIP-2, CCL5/RANTES and IL6, whereas more limited changes were obtained for IL 1B, IL 1A, and IFN-γ (Fig. 6A). The expression of control 18S rRNA did not change in SARS-CoV or mock-infected cells as expected (Fig. 6A).
Fig. 6.
Expression of proinflammatory cytokines in SARS-CoV-MA15-infected cells. DBT-mACE2 clone 6 cells were infected at a moi of 0.1 with rSARS-CoV-MA15. (A) Cellular RNAs were extracted at 48 hpi. The expression of the indicated cytokines and interferons, and that of 18S rRNA as a control, was determined by qRT-PCR. In each case, the corresponding mRNA expression levels in SARS-CoV-MA15-infected cells were plotted as fold-change relative to expression levels in uninfected cells. (B) Cell extracts were prepared at 48 hpi. Expression of the cytokines CXCL10 and CXCL2 at the protein level was evaluated in mock and DBT-mACE2-infected cells.
To evaluate whether the induction of cytokine mRNAs in SARS-CoV-MA15-infected cells correlate with an induction at the protein level, the levels of CXCL10 and CXCL2 proteins were determined in mock and DBT-mACE2-infected cells. In agreement with the results obtained with the mRNAs, CXCL10 and CXCL2 were also upregulated at the protein level, showing fold-increases of 3 and 1.8-fold over mock, respectively (Fig. 6B). These data indicated that DBT-mACE2 cells were useful to study proinflammatory cytokine expression in vitro.
4. Discussion
The development of established mouse cell lines highly susceptible to mouse-adapted SARS-CoV infection is described in this study. The best current small animal model for SARS-CoV is the infection of Balb/c mice with a mouse adapted SARS-CoV (Day et al., 2009, Nagata et al., 2008, Roberts et al., 2007). The establishment of mouse cell lines susceptible to SARS-CoV was therefore of high interest, so that in vitro and in vivo evaluations with the mouse adapted SARS-CoV could take place in the same species environment and using the same cellular receptor.
The expression of mACE2 was sufficient to convert DBT cells, which were not susceptible to SARS-CoV infection, into cells producing high SARS-CoV-MA15 titers. Similarly, the transient expression of mACE2 in mouse 3T3 cells generated susceptibility to SARS-CoV infection, although these cells were not stably transformed (Li et al., 2004). In addition, the expression of civet and human ACE2 in DBT cells (Becker et al., 2008, Sheahan et al., 2008a, Sheahan et al., 2008b), and the expression of human ACE2 in mice also led to cells susceptible to SARS-CoV and to an animal model more susceptible to the infection by a human SARS-CoV (McCray et al., 2007, Yang et al., 2007). All these data indicated that ACE2 is clearly a required SARS-CoV receptor.
SARS-CoV-Urbani titers in DBT-mACE2 cells were more than 103-fold lower than titers obtained for SARS-CoV-MA15. Critical mutations differentiating these two virus strains map in the gene encoding the spike protein (Roberts et al., 2007) that binds to the cellular ACE2 and facilitates virus entry (Li et al., 2003). The affinity of SARS-CoV-Urbani spike protein binding to mACE2 is lower that the affinity of SARS-CoV-MA15 spike protein binding to mACE2, making the Urbani virus entrance less efficient (Li et al., 2004).
SARS-CoV infection in DBT-mACE2 cells induced high levels of CPE, probably due to apoptosis, as it has been observed in other SARS-CoV-infected cell lines, such as Vero (DeDiego et al., 2011, Ren et al., 2005, Yan et al., 2004). This virus-induced cytopathology provides an additional experimental advantage of DBT-mACE2 cell lines, as the infection outcomes are overt and are similar to what happens to the epithelial respiratory cells in SARS patients (Lang et al., 2003, Zhang et al., 2003). Accordingly, plaque assays of SARS-CoV-MA15 were efficient in DBT-ACE2 cells.
Gene expressions leading to acute inflammation are hallmarks of SARS-CoV infection and have been associated with SARS-CoV-induced pathology (Rockx et al., 2009, Smits et al., 2010). Using genomic analysis, it has been shown that gene expressions encoding proinflammatory cytokines are increased in DBT-mACE2 cells, compared to mock-infected cells. These results correlate with the data obtained in SARS patients (Cameron et al., 2007, Huang et al., 2005, Jiang et al., 2005, Reghunathan et al., 2005, Tang et al., 2005, Wong et al., 2003, Zhang et al., 2004) and in SARS-CoV-infected monkeys and mice (Baas et al., 2008, de Lang et al., 2007, Smits et al., 2010, Smits et al., 2011). In addition, it has been shown that SARS-CoV-MA15 infection induced a limited IFN-β production in DBT-mACE2 cells. This weak induction could be due to the viral RNA sequestering in double membrane vesicles described in coronaviruses (Gosert et al., 2002, Knoops et al., 2008), which prevents or reduces recognition by PRRs, and to the expression of SARS-CoV proteins that antagonize the innate response (Devaraj et al., 2007, Frieman et al., 2007, Kopecky-Bromberg et al., 2007, Narayanan et al., 2008, Siu et al., 2009, Sun et al., 2012, Wathelet et al., 2007).
The DBT-mACE2 cells generated in this work, could be used in the context of virus adaptation to a homologous receptor of another host species, such as for example hamsters or rats and in the context of mouse adaptation to the murine receptor. In addition, DBT-mACE2 cells may be useful to grow mouse adapted SARS-CoV, with reduced likelihood of undesired changes in the SARS-CoV genome that may occur during replication in non-murine cells.
Acknowledgments
This work was supported by grants from the Ministry of Science and Innovation of Spain (BIO2010-16705), the European Community's Seventh Framework Programme (FP7/2007–2013) under the project “EMPERIE” EC Grant Agreement number 223498, and U.S. National Institutes of Health (NIH) (2P01AI060699-06A1, W000306844). JAR received a fellowship from the Fundacion La Caixa. We thank Marga Gonzalez for her technical assistance.
References
- Almazan F., DeDiego M.L., Galan C., Escors D., Alvarez E., Ortego J., Sola I., Zuñiga S., Alonso S., Moreno J.L., Nogales A., Capiscol C., Enjuanes L. Construction of a SARS-CoV infectious cDNA clone and a replicon to study coronavirus RNA synthesis. Journal of Virology. 2006;80:10900–10906. doi: 10.1128/JVI.00385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazan F., Gonzalez J.M., Penzes Z., Izeta A., Calvo E., Plana-Duran J., Enjuanes L. Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5516–5521. doi: 10.1073/pnas.97.10.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas T., Roberts A., Teal T.H., Vogel L., Chen J., Tumpey T.M., Katze M.G., Subbarao K. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. Journal of Virology. 2008;82:9465–9476. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M.M., Graham R.L., Donaldson E.F., Rockx B., Sims A.C., Sheahan T., Pickles R.J., Corti D., Johnston R.E., Baric R.S., Denison M.R. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19944–19949. doi: 10.1073/pnas.0808116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Kelvin D.J. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. Journal of Virology. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.K., To K.F., Lo A.W., Cheung J.L., Chu I., Au F.W., Tong J.H., Tam J.S., Sung J.J., Ng H.K. Persistent infection of SARS coronavirus in colonic cells in vitro. Journal of Medical Virology. 2004;74:1–7. doi: 10.1002/jmv.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J., Jr., Hoever G., Morgenstern B., Preiser W., Vogel J.U., Hofmann W.K., Bauer G., Michaelis M., Rabenau H.F., Doerr H.W. Infection of cultured intestinal epithelial cells with severe acute respiratory syndrome coronavirus. Cellular and Molecular Life Sciences. 2004;61:2100–2112. doi: 10.1007/s00018-004-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.W., Baric R., Cai S.X., Frieman M., Kumaki Y., Morrey J.D., Smee D.F., Barnard D.L. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R.J., Baker S.C., Baric R., Enjuanes L., Gorbalenya A.E., Holmes K.V., Perlman S., Poon L., Rottier P.J.M., Talbot P.J., Woo P.C.Y., Ziebuhr J. Coronaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; San Diego: 2012. pp. 774–796. [Google Scholar]
- de Lang A., Baas T., Teal T., Leijten L.M., Rain B., Osterhaus A.D., Haagmans B.L., Katze M.G. Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathogens. 2007;3:e112. doi: 10.1371/journal.ppat.0030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M.L., Alvarez E., Almazan F., Rejas M.T., Lamirande E., Roberts A., Shieh W.J., Zaki S.R., Subbarao K., Enjuanes L. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. Journal of Virology. 2007;81:1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Alvarez E., Oliveros J.C., Zhao J., Fett C., Perlman S., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathogens. 2011;7:e1002315. doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S.G., Wang N., Chen Z., Chen Z., Tseng M., Barretto N., Lin R., Peters C.J., Tseng C.T., Baker S.C., Li K. Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. Journal of Biological Chemistry. 2007;282:32208–32221. doi: 10.1074/jbc.M704870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Enjuanes L., Gorbalenya A.E., de Groot R.J., Cowley J.A., Ziebuhr J., Snijder E.J. The nidovirales. In: Mahy B.W.J., Van Regenmortel M., Walker P., Majumder-Russell D., editors. Encyclopedia of Virology. 3rd ed. Elsevier Ltd.; Oxford: 2008. pp. 419–430. [Google Scholar]
- Fett C., DeDiego M.L., Regla-Nava J.A., Enjuanes L., Perlman S. Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein. Journal of Virology. 2013;87:6551–6559. doi: 10.1128/JVI.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stohr K., Osterhaus A.D. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Heise M., Baric R. SARS coronavirus and innate immunity. Virus Research. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M., Yount B., Heise M., Kopecky-Bromberg S.A., Palese P., Baric R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. Journal of Virology. 2007;81:9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. Journal of Virology. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann K., Muller M.A., Nitsche A., Wendt S., Donoso Mantke O., Niedrig M. Susceptibility of different eukaryotic cell lines to SARS-coronavirus. Archives of Virology. 2005;150:1023–1031. doi: 10.1007/s00705-004-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-gamma-related cytokine storm in SARS patients. Journal of Medical Virology. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. American Journal of Respiratory and Critical Care Medicine. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biology. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. Journal of Virology. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C., Zaki S., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S., Ling A.-E., Humphrey C., Shieh W.-J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A.M., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K.S., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.-C., Stohr K., Peiris J.S.M. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanishi T. Brain tumors induced with Rous sarcoma virus, Schmidt-Ruppin strain. I. Induction of brain tumors in adult mice with Rous chicken sarcoma cells. Japanese Journal of Experimental Medicine. 1967;37:461–474. [PubMed] [Google Scholar]
- Lang Z.W., Zhang L.J., Zhang S.J., Meng X., Li J.Q., Song C.Z., Sun L., Zhou Y.S., Dwyer D.E. A clinicopathological study of three cases of severe acute respiratory syndrome (SARS) Pathology. 2003;35:526–531. doi: 10.1080/00313020310001619118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li W., Greenough T.C., Moore M.J., Vasilieva N., Somasundaran M., Sullivan J.L., Farzan M., Choe H. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. Journal of Virology. 2004;78:11429–11433. doi: 10.1128/JVI.78.20.11429-11433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for de SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J.M., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., Meyerholz D.K., Kirby P., Look D.C., Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. Journal of Virology. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. Journal of Virology. 2005;79:3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Iwata N., Hasegawa H., Fukushi S., Harashima A., Sato Y., Saijo M., Taguchi F., Morikawa S., Sata T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. American Journal of Pathology. 2008;172:1625–1637. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K., Huang C., Lokugamage K., Kamitani W., Ikegami T., Tseng C.T., Makino S. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. Journal of Virology. 2008;82:4471–4479. doi: 10.1128/JVI.02472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. Journal of General Virology. 2003;84:3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reghunathan R., Jayapal M., Hsu L.Y., Chng H.H., Tai D., Leung B.P., Melendez A.J. Expression profile of immune response genes in patients with Severe Acute Respiratory Syndrome. BMC Immunology. 2005;6:2. doi: 10.1186/1471-2172-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Yang R., Guo L., Qu J., Wang J., Hung T. Apoptosis induced by the SARS-associated coronavirus in Vero cells is replication-dependent and involves caspase. DNA and Cell Biology. 2005;24:496–502. doi: 10.1089/dna.2005.24.496. [DOI] [PubMed] [Google Scholar]
- Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., Zaki S.R., Baric R., Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathogens. 2007;3:23–37. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Lamirande E.W., Vogel L., Jackson J.P., Paddock C.D., Guarner J., Zaki S.R., Sheahan T., Baric R., Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Research. 2008;133:20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., van den Brand J., Baric R., Katze M.G. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. Journal of Virology. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campganoli R., Icenogle J.P., Peñaranda S., Bankamp B., Maher K., Chen M.-H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.d., Peret T.C.T., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rassmussen M., Fouchier R., Gunther S., Osterhaus A.D.M.E., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- Sheahan T., Rockx B., Donaldson E., Corti D., Baric R. Pathways of cross-species transmission of synthetically reconstructed zoonotic severe acute respiratory syndrome coronavirus. Journal of Virology. 2008;82:8721–8732. doi: 10.1128/JVI.00818-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T., Rockx B., Donaldson E., Sims A., Pickles R., Corti D., Baric R. Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. Journal of Virology. 2008;82:2274–2285. doi: 10.1128/JVI.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu K.L., Kok K.H., Ng M.H., Poon V.K., Yuen K.Y., Zheng B.J., Jin D.Y. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. Journal of Biological Chemistry. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van I.W.F., Eijkemans M.J., van Amerongen G., Kuiken T., Andeweg A.C., Osterhaus A.D., Haagmans B.L. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathogens. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., van den Brand J.M., de Lang A., Leijten L.M., van Ijcken W.F., van Amerongen G., Osterhaus A.D., Andeweg A.C., Haagmans B.L. Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species. Journal of Virology. 2011;85:4234–4245. doi: 10.1128/JVI.02395-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K., Roberts A. Is there an ideal animal model for SARS? Trends in Microbiology. 2006;14:299–303. doi: 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PloS ONE. 2012;7:e30802. doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N.L., Chan P.K., Wong C.K., To K.F., Wu A.K., Sung Y.M., Hui D.S., Sung J.J., Lam C.W. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clinical Chemistry. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. Journal of Virology. 2007;81:11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W., Wong C.K., Chan P.K., Ng M.H., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. Journal of Biological Chemistry. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Li K.S., Poon R.W., Wong B.H., Tsoi H.W., Yip B.C., Huang Y., Chan K.H., Yuen K.Y. Molecular diversity of coronaviruses in bats. Virology. 2006;351:180–187. doi: 10.1016/j.virol.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Xiao G., Zhang J., Hu Y., Yuan F., Cole D.K., Zheng C., Gao G.F. SARS coronavirus induces apoptosis in Vero E6 cells. Journal of Medical Virology. 2004;73:323–331. doi: 10.1002/jmv.20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., Xu Y.F., Ding M.X., Deng H.K., Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comparative Medicine. 2007;57:450–459. [PubMed] [Google Scholar]
- Zhang Q.L., Ding Y.Q., He L., Wang W., Zhang J.H., Wang H.J., Cai J.J., Geng J., Lu Y.D., Luo Y.L. Detection of cell apoptosis in the pathological tissues of patients with SARS and its significance. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:770–773. [PubMed] [Google Scholar]
- Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., Ye L., Xu S., Sun R., Wang Y., Lou J. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infection and Immunity. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. Journal of General Virology. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]


