Abstract
Abnormal involuntary movements or dyskinesias are a serious complication of long-term l-DOPA treatment of Parkinson’s disease, for which there are few treatment options. Accumulating preclinical data show that nicotine decreases l-DOPA–induced dyskinesias (LIDs), suggesting that it may be a useful antidyskinetic therapy for Parkinson’s disease. Here, we investigated whether nicotinic acetylcholine receptor (nAChR) agonists reduced LIDs in nonhuman primates. We first tested the nonselective nAChR agonist 1, 6,7,8,9-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine (varenicline), which offers the advantage that it is approved by the U.S. Food and Drug Administration for use in humans. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–lesioned monkeys (n = 23) were first administered l-DOPA/carbidopa (10/2.5 mg/kg) twice daily 5 days/week until stably dyskinetic. Oral varenicline (0.03–0.10 mg/kg) decreased LIDs ∼50% compared with vehicle-treated monkeys, whereas nicotine treatment (300 µg/ml in drinking water) reduced LIDs by 70% in a parallel group of animals. We next tested the selective α4β2*/α6β2* nAChR agonist TC-8831 [3-cyclopropylcarbonyl-3,6-diazabicyclo[3.1.1]heptane] on LIDs in the same set of monkeys after a 10-week washout. We also tested TC-8831 in another set of MPTP-lesioned monkeys (n = 16) that were nAChR drug–naïve. Oral TC-8831 (0.03–0.3 mg/kg) reduced LIDs in both sets by 30–50%. After a washout period, repeat TC-8831 dosing led to a greater decline in LIDs (60%) in both sets of monkeys that was similar to the effect of nicotine. Tolerance to any nAChR drug did not develop over the course of the study (3–4 months). NAChR drug treatment did not worsen parkinsonism or cognitive ability. These data suggest that nAChR agonists may be useful for the management of dyskinesias in l-DOPA–treated Parkinson’s disease patients.
Introduction
Although l-DOPA is one of the most effective therapies for Parkinson’s disease, its continued use leads to abnormal involuntary movements or dyskinesias that may severely affect the quality of life of Parkinson’s disease patients (Cenci and Konradi, 2010; Brotchie and Jenner, 2011; Carta and Bezard, 2011; Fisone and Bezard, 2011; Iravani and Jenner, 2011; Prashanth et al., 2011; Rascol et al., 2011; Poewe et al., 2012; Huot et al., 2013). As there are few treatment options to manage l-DOPA–induced dyskinesias (LIDs), novel therapeutic approaches are critical. The observation that the nicotinic cholinergic system shares a close anatomical relationship with the striatal dopaminergic system and that nicotinic acetylcholine receptor (nAChR) activation modulates dopaminergic function prompted work to investigate the effect of nicotine on LIDs.
Nicotine treatment decreased LIDs in parkinsonian monkey, rat, and mouse models, suggesting that it may represent a useful treatment approach (Quik et al., 2007; Bordia et al., 2008, 2010; Huang et al., 2011a). Moreover, various modes of nicotine administration successfully attenuated LIDs, including injection, oral treatment, and minipump infusion, some of which could readily be used in the clinic. Tolerance to the effect of nicotine did not develop in any species with study periods up to 6 months (Quik et al., 2007; Bordia et al., 2008, 2010; Huang et al., 2011a).
The primary nAChRs in the basal ganglia are the α6β2* and α4β2* subtypes, with a smaller subset of α7 nAChRs (Millar and Gotti, 2009; Gotti et al., 2010; Quik and Wonnacott, 2011). Studies in parkinsonian null mutant mice suggest that nicotine may reduce LIDs by acting at one or several of these nAChR populations. The use of β2 nAChR subunit knockout mice, which lack both the α4β2* and α6β2* nAChR subtypes, showed that LIDs develop to a lesser extent in such mice, and that nicotine no longer had an antidyskinetic effect (Huang et al., 2011a). Similar results were obtained with α6 nAChR subunit knockout mice (Quik et al., 2012), as well as α4 nAChR subunit knockout mice (Quik et al., 2013b). These combined data indicate that the α4, α6, and β2 nAChR subunits (that is, α4β2* and α6β2* nAChR populations) play a role in LIDs. The use of α7 nAChR knockout mice showed that this subtype also modulated the development of LIDs, although in a manner distinct from that mediated by α4β2* and α6β2* nAChRs (Quik et al., 2013b). α7 nAChR subunit deletion increased baseline LIDs, suggesting that this subtype exerts an inhibitory effect. The development of LIDs may thus involve a concerted action of acetylcholine at α4β2*, α6β2*, and α7 nAChRs.
Pharmacological studies with nAChR agonists and antagonists also point to an involvement of α4β2* and α6β2* nAChRs in LIDs. nAChR agonists, including 1, 6,7,8,9-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine (varenicline), A85380, and compounds, such as TC-8831 (Targacept, Inc., Winston-Salem, NC), that act at both α4β2* and α6β2* subtypes decreased the occurrence of LIDs in 6-OHDA–lesioned rats (Huang et al., 2011b; Quik et al., 2013a). In addition, a recent study showed that TC-8831 reduced LIDs in parkinsonian monkeys (Johnston et al., 2013).
The objective of the current study was to determine whether the general nAChR agonist varenicline, which is approved by the U.S. Food and Drug Administration for use in humans for smoking cessation, reduces LIDs in a nonhuman primate model of Parkinson’s disease. This drug offers the advantage that it could readily be investigated in the clinic for the treatment of LIDs (Rollema et al., 2007; Cahill et al., 2011). The second objective was to extend studies with the selective central nervous system (CNS) nAChR agonist TC-8831, as previous work demonstrated a small decline in LIDs which did not persist with continued dosing (Johnston et al., 2013). TC-8831 offers the advantage that there is little interaction at peripheral nervous system receptors—that is, the cardiovascular, gastrointestinal, and other autonomic systems. We also tested the effect of the agonists on a memory task, as nAChR agonists have been shown to enhance various aspects of memory and/or attention in clinical trials (Rhodes et al., 2012; Shim et al., 2012). Results show that both varenicline and TC-8831 reduced LIDs by 30–70% with no development of tolerance over several months. Furthermore, TC-8831 reduced LIDs as effectively as nicotine with repeated use. These data suggest that nAChR agonists such as TC-8831 may be useful in the treatment of LIDs that arise in Parkinson’s disease.
Materials and Methods
Animals.
Squirrel monkeys (Saimiri sciureus), purchased from Worldwide Primates (Miami, FL), were quarantined after arrival for a 1-month period, as required by California state regulations. The monkeys were of both sexes and weighed between 0.6 and 1.2 kg. The animals were housed under a 12-hour light/dark cycle, in a temperature- and humidity-controlled room. They were provided with water ad libitum and fed monkey chow, fruits, and vegetables. All studies were performed according to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
MPTP Administration and Parkinsonian Ratings.
After quarantine, the monkeys were individually housed and trained in a variety of motor tasks to allow for future assessment of parkinsonism (Quik et al., 2007). Parkinsonism was scored on a scale from 0 (normal) to 4 (severe) for each category, and included spatial hypokinesia (use of available cage space), body bradykinesia (slowness in body movement), balance (ability to maintain posture), freezing, tremor, and left and right manual dexterity. After training, 2.0 mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Sigma-Aldrich, St. Louis, MO) dissolved in saline was injected subcutaneously (Quik et al., 2007). MPTP injection was repeated once (1.9 mg/kg s.c.) 3–4 weeks later, and parkinsonism was rated once weekly (Monday) before and 1–2 hours after l-DOPA administration throughout the course of the study.
l-DOPA Treatment and Dyskinesia Ratings.
All monkeys were gavaged two times per day at 3.5-hour intervals with l-DOPA (10 mg/kg) and carbidopa (2.5 mg/kg) for 5 days per week for at least 4 weeks, such that they were stably dyskinetic. During the course of l-DOPA treatment, monkeys were given only fruits and vegetables in the morning to allow optimal absorption of l-DOPA. Monkey chow, fruits, and vegetables were given in the afternoon 4 hours after the second l-DOPA dose. The monkeys were video recorded for the entire day (8:00 AM to 4:00 PM), with baseline recording performed Tuesday, Wednesday, and Thursday from 8:00 to 8:30 AM before the first dose of l-DOPA. l-DOPA was then gavaged at 8:30 AM and again at noon. The dyskinesia scores provided in the figures and tables were all obtained during the afternoon l-DOPA dosing period (noon to 4:00 PM). Dyskinesias were scored by a blinded rater from the afternoon recordings for a 1-minute period at 30-minute intervals. The total LID scores provided in the figures represent the average ratings performed on Wednesday and Thursday afternoon of each week for 1–4 weeks, as depicted in the timelines. Dyskinesias were rated on a scale of 0 (no dyskinesias) to 4 (severe dyskinesias) as follows: 1 = subtle dyskinesias that were not sustained (<3 trunk movements in a row), 2 = sustained dyskinesias (≥3 trunk movements in a row), 3 = moderate dyskinesias that impaired ability to remain stationary, and 4 = severe dyskinesias that were generalized and incapacitating (Tan et al., 2002; Quik et al., 2007, 2013c).
nAChR Drug Treatments.
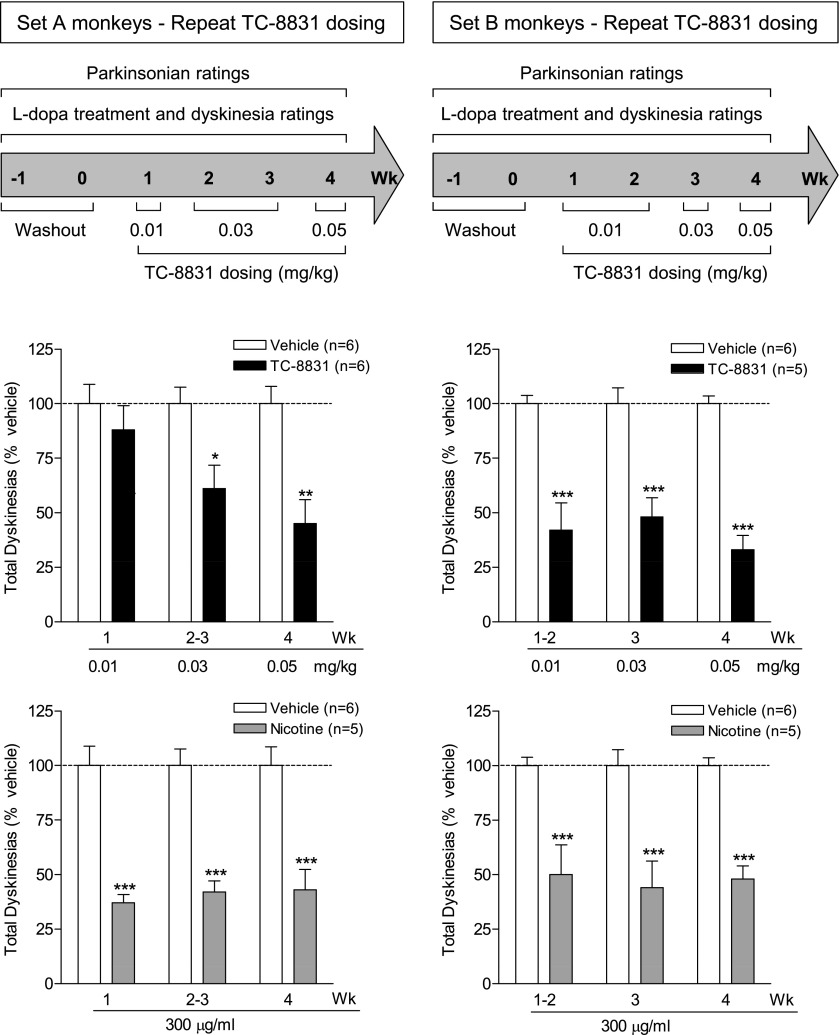
The nAChR drug treatments involved two sets of MPTP-lesioned monkeys designated set A (n = 23) and set B (n = 16). Set A monkeys had previously been in studies involving administration of nicotine and/or nAChR drugs, followed by a 1-month washout period (nAChR drug primed). Set B monkeys were nAChR drug–naïve at the start of the current study. We used nAChR drug–primed and nAChR drug–naïve monkeys to assess whether previous nAChR drug treatment modulated the ability of subsequent agonists to reduce LIDs. For both set A (nAChR drug primed) and set B (nAChR drug naïve), there were three experimental groups of monkeys. These included a vehicle-treated group (n = 6), a nicotine-treated group (n = 5), and an nAChR drug–treated group (n = 5–12).
All monkeys in set A and set B were given 50% diluted orange Gatorade in the drinking water for 1–2 weeks as a control for the nicotine-treated group, since nicotine was administered in Gatorade. Gatorade was included in the drinking water to mask the bitter taste of nicotine [(−)-nicotine, free base; Sigma-Aldrich). The control and nAChR agonist–treated monkeys were continued on Gatorade for the remainder of the study, whereas the nicotine-treated group received Gatorade plus nicotine. Nicotine was initially given in the Gatorade starting at 50 μg/ml for 2–3 days and was then increased to 150 µg/ml for an additional 3–4 days. The monkeys were maintained at a final concentration of 300 μg/ml nicotine in the drinking water. This dose of oral nicotine was used as our previous studies have shown that it yields monkey plasma nicotine levels of 10–15 ng/ml, which are similar to those in moderate smokers (Quik et al., 2006). Twenty-five milliliters of Gatorade plus nicotine or Gatorade only was also added to the monkey chow.
For the nAChR drug–treated groups in set A and/or set B, the lower doses of varenicline (0.003, 0.01, and 0.03 mg/kg) and TC-8831 (0.01 and 0.03 mg/kg) were given orally in a cracker, twice daily, 30 minutes before the monkeys were gavaged with l-DOPA. Varenicline was obtained from F.I.C. and TC-8831 from Targacept (Winston-Salem, NC; structure published in Quik et al., 2013a). The drugs were applied to the cracker in a 30–40 µl aliquot of water. The monkeys refused to take the crackers containing the higher doses of varenicline (0.06 and 0.1 mg/kg) and TC-8831 (0.05, 0.1, and 0.3 mg/kg). The drugs were therefore given orally at the same time as the l-DOPA gavage.
Cognitive Testing.
The test used to evaluate learning/memory involved the ability to inhibit goal-directed behavior and is thought to reflect a component of spatially delayed working memory in monkeys, which may involve precortical areas (Lyons et al., 2000, 2004). The apparatus used for evaluating cognitive performance was similar to that previously described for use with squirrel monkeys (Lyons et al., 2000, 2004). It consists of a clear Plexiglas box (8 × 8 × 8 cm) with one open side, containing a small piece of marshmallow. The box is locked into place on a metallic platform hooked onto the front of the monkey cage. The test was conducted prior to the morning feeding. Control and MPTP-lesioned monkeys were trained for ∼1 month with the box open at various orientations, and were scored for the average time and number of trials required to retrieve the marshmallow piece within a 30-second period (Lyons et al., 2000; Lyons and Schatzberg, 2003). When the monkey performance on these tasks had plateaued to a steady-state level, the effect of nAChR drug administration was tested once weekly (Friday).
Data Analyses.
l-DOPA was given on a 5-days-on/2-days-off schedule with parkinsonian ratings performed weekly on Monday and cognitive testing on Friday. Dyskinesia ratings were determined by averaging the scores on Wednesday and Thursday afternoon of each week. Total dyskinesias were determined by calculating area under the curve, and the data were analyzed using two-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test. Differences in rating scores between groups were analyzed using nonparametric tests (Mann–Whitney test). The data are the median or the mean ± S.E.M. of the number of animals, as indicated in the legends. P ≤ 0.05 was used for statistical significance.
Results
Varenicline Treatment Reduces LIDs.
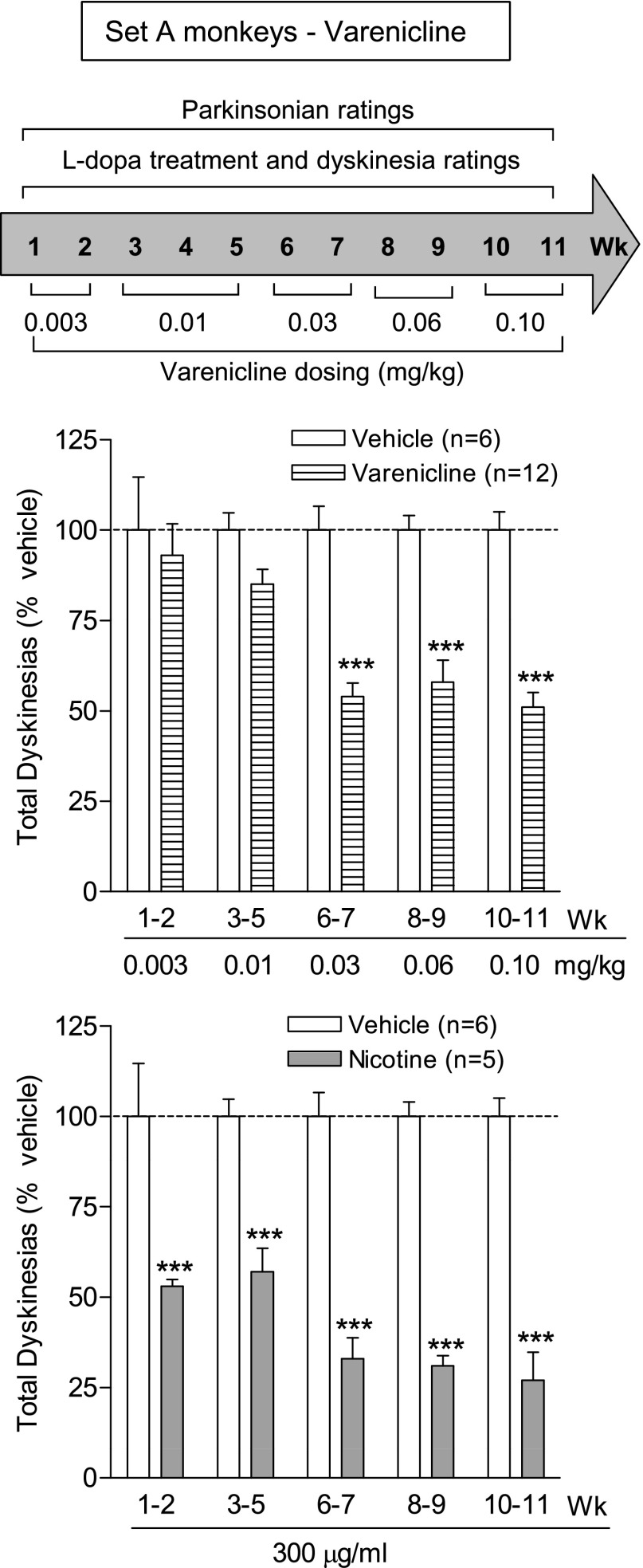
Varenicline administration was initiated at a very low dose (0.003 mg/kg), as preliminary experiments indicated that vomiting developed with a higher starting dose. Each dose of varenicline (0.003–0.10 mg/kg) was given twice daily, 5 days per week for the number of weeks indicated in the timelines above the data (Fig. 1), with the data representing the mean total LID score (expressed as % vehicle) for the specific dose over the 2–3-week period. Varenicline treatment decreased LIDs at 0.03 mg/kg by ∼45%, with a similar decline at the two higher doses (0.06 and 0.10 mg/kg). There was a significant main effect of treatment (F1,80 = 52.08, P < 0.001), dose (F4,80 = 3.86, P < 0.01), and a significant interaction (F4,80 = 3.86, P < 0.01) using two-way ANOVA. Concurrent nicotine treatment in another group of monkeys led to a somewhat greater decline in LIDs, with a significant main effect of treatment (F1,45 = 172.7, P < 0.001), but not dose (F4,45 = 1.83, P > 0.05), and no significant interaction (F 4,45 = 1.83, P > 0.05) using two-way ANOVA (Fig. 1).
Fig. 1.
Varenicline treatment reduces LIDs. MPTP-lesioned monkeys were gavaged with l-DOPA twice daily, 5 days per week. Varenicline was given orally twice daily at a 3.5-hour interval at the same time as l-DOPA. The timeline is depicted in the upper panel. The middle panel shows the total dyskinesia scores (expressed as % vehicle) averaged over 2–3 days during the 4-hour period following the afternoon dose of l-DOPA (middle panel). For comparison, the lower panel illustrates the effect of nicotine (300 µg/ml in the drinking water) on LIDs for the same time period as the varenicline data. Values are the mean ± S.E.M. of 5–12 monkeys. Significance of difference of drug treatment from vehicle: ***P < 0.001 using two-way ANOVA followed by a Bonferroni post-hoc test.
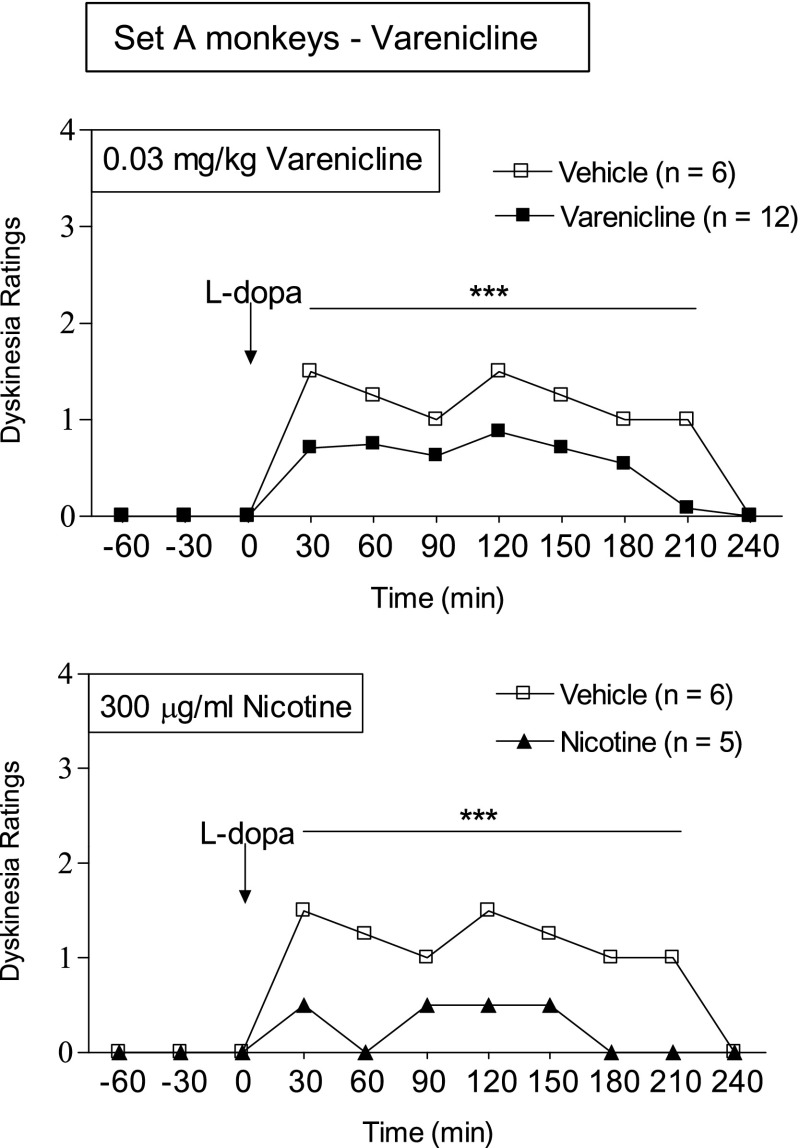
The afternoon hourly time course of the development of LIDs after l-DOPA gavage is shown in Fig. 2. l-DOPA treatment maximally increased LIDs in vehicle-treated monkeys 30 minutes after gavage, with the response lasting 3.5 hours. Varenicline dosing reduced LIDs over the entire 3.5-hour period. The data shown are for the 0.03 mg/kg dose, with the same pattern for the 0.06 and 0.10 mg/kg doses of varenicline. The effect of nicotine is shown for comparison. It yielded a greater decline in LIDs (Fig. 2), consistent with the total LIDs scores provided in Fig. 1.
Fig. 2.
Varenicline treatment decreases the hourly time course of LIDs. Monkeys were treated as described in the legend of Fig. 1. The data shown are for the afternoon 0.03 mg/kg varenicline dose averaged over several days. The effect of nicotine on LIDs is shown in the lower panel for comparison. The symbols depict the median of 5–12 monkeys. Significance of difference from vehicle using a Mann–Whitney test: ***P < 0.001.
Experiments were conducted next to determine whether the varenicline-induced reduction in LIDs persisted with drug removal. Five weeks of varenicline washout were required before LIDs returned to the levels seen in vehicle-treated monkeys, with a similar time period required for LIDs to return to vehicle levels with nicotine removal (Table 1).
TABLE 1.
Loss of the nAChR-mediated improvement in LIDs with drug removal
MPTP-lesioned monkeys were gavaged with l-DOPA and given vehicle, nicotine, varenicline, or TC-8831 according to the treatment regimens depicted in Fig. 1 for varenicline and Fig. 3 for TC-8831. Drug removal led to a return of LIDs to vehicle-treated values by 1–6 weeks after washout. Values are the mean ± S.E.M. of the number of monkeys indicated in parentheses.
| nAChR Drug and Time Point | Total LID Scores (% Vehicle) |
|
|---|---|---|
| Vehicle | nAChR Drug | |
| Varenicline | ||
| Last week treatment | 100 ± 8.8 (6) | 50 ± 4.9 (12)** |
| Week 1 washout | 100 ± 4.2 (6) | 66 ± 8.4 (12)* |
| Week 3 washout | 100 ± 7.5 (6) | 70 ± 8.6 (12) |
| Week 5 washout | 100 ± 14.9 (6) | 90 ± 4.5 (12) |
| Nicotine | ||
| Last week treatment | 100 ± 8.8 (6) | 22 ± 2.8 (5)*** |
| Week 1 washout | 100 ± 4.2 (6) | 48 ± 8.0 (5)*** |
| Week 3 washout | 100 ± 7.5 (6) | 61 ± 7.3 (5)** |
| Week 6 washout | 100 ± 14.9 (6) | 92 ± 9.6 (5) |
| TC-8831 | ||
| Last week treatment | 100 ± 8.5 (6) | 45 ± 11 (6)** |
| Week 1 washout | 100 ± 6.2 (6) | 102 ± 4.1 (6) |
P < 0.05; **P < 0.01; ***P < 0.001 using a t test (significance of difference from vehicle).
Varenicline had no significant effect on parkinsonian ratings as compared with the vehicle-treated group (Table 2). These data are consistent with those previously observed with nicotine treatment, which also does not alter parkinsonism (Quik et al., 2007, 2013c). There were no adverse effects of varenicline on fluid intake, body weight, or general behaviors, except for the vomiting mentionedearlier.
TABLE 2.
nAChR agonist treatment does not affect parkinsonism
Monkeys were rated for parkinsonism once weekly throughout the study, starting 3–4 weeks after the last MPTP injection. The parkinsonian ratings shown were obtained in the same week as the data provided in Fig. 2 for varenicline and Fig. 4 for TC-8831, with similar results throughout the study. Values are the mean ± S.E.M. of the number of monkeys indicated in parentheses.
| Study | Parkinsonian Scores |
||
|---|---|---|---|
| Vehicle | Nicotine | nAChR Agonist | |
| Set A: varenicline | 4.7 ± 0.4 (6) | 3.6 ± 0.2 (5) | 4.0 ± 0.3 (12) |
| Set A: TC-8831 | 6.0 ± 1.3 (6) | 5.2 ± 0.4 (5) | 6.6 ± 0.8 (5) |
| Set B: TC-8831 | 5.3 ± 1.1 (6) | 5.0 ± 0.4 (5) | 5.8 ± 1.0 (5) |
TC-8831 Treatment Reduces LIDs.
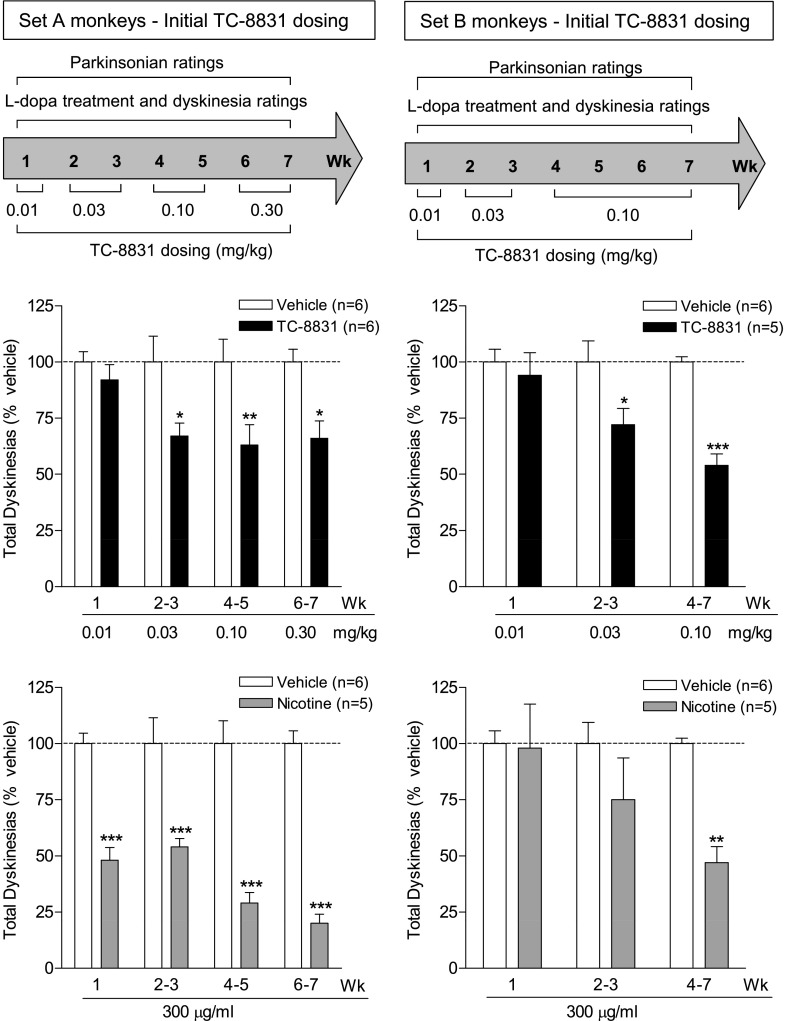
Two sets of MPTP-lesioned monkeys were used to investigate the effect of TC-8831 on LIDs. Set A monkeys (nAChR drug primed, n = 17) had previously been treated with nicotine and varenicline, followed by a 10-week washout period. Set B monkeys (n = 16) were nAChR drug naïve. Each set of monkeys was subdivided into three experimental groups as follows: vehicle-treated (n = 6), nicotine-treated (n = 5), and TC-8831–treated (n = 5–6). All TC-8831 doses were given twice daily, 5 days per week for the number of weeks indicated in the timelines above the data, with the data representing the mean total LID score (expressed as % vehicle) for the specific dose over the designated number of weeks. TC-8831 administration was started at 0.01 mg/kg; this dose had no effect on total LID scores averaged during the week (Fig. 3). The TC-8831 dose was then increased to 0.03 mg/kg for 2 weeks (Fig. 3). Significant declines (∼30%) were observed in LIDs in both sets of monkeys (Fig. 3). In set A, a maximal decline in LIDs was obtained with the 0.10 mg/kg dose, with similar results with 0.30 mg/kg (each dose given for 2 weeks). There was a significant main effect of treatment (F1,40 = 24.41, P < 0.001), but not dose (F3,40 = 1.41, P > 0.05), and no significant interaction (F3,40 = 1.41, P > 0.05) using two-way ANOVA. A decline was also observed in the set B monkeys administered 0.10 mg/kg TC-8831 for 4 weeks, with a significant main effect of treatment (F1,27 = 21.26, P < 0.001), dose (F2,27 = 3.99, P < 0.01), and a significant interaction (F2,27 = 3.99, P < 0.01) using two-way ANOVA. However, the 0.30 mg/kg dose induced vomiting in the set B monkeys; the data were therefore not included, as the emesis could interfere with uptake of both l-DOPA and TC-8831 and, thus, LIDs.
Fig. 3.
TC-8831 treatment decreases LIDs. The effect of TC-8831 was tested in a set of MPTP-lesioned monkeys previously exposed to nicotine and nAChR drugs (set A), as well as in a set of nAChR drug–naïve MPTP-lesioned monkeys (set B). The monkeys were gavaged with l-DOPA twice daily, 5 days per week. TC-8831 (0.01–0.30 mg/kg) was given orally twice daily at a 3.5-hour interval at the same time as l-DOPA. The timeline is depicted in the upper panel. The middle panel shows the total dyskinesia scores (expressed as % vehicle) averaged over 2–3 days during the 4-hour period following the afternoon dose of l-DOPA. For comparison, the lower panel illustrates the effect of nicotine (300 µg/ml in the drinking water) on LIDs for the same time period as the TC-8831 data. Values are the mean ± S.E.M. of 5–6 monkeys. Significance of difference of drug treatment from vehicle: *P < 0.05; **P < 0.01; ***P < 0.001 using two-way ANOVA followed by a Bonferroni post-hoc test.
Nicotine treatment led to 50%–80% declines in LIDs in set A (Fig. 3), with a significant main effect of treatment (F1,36 = 146.7, P < 0.001), but not dose (F3,36 = 2.4, P > 0.05), and no significant interaction (F3,36 = 2.4, P > 0.05) using two-way ANOVA. These data are consistent with previous results with long-term nicotine dosing (Quik et al., 2013c,d). These earlier studies also showed that up to 8 weeks of nicotine treatment is required for a maximal decline in LIDs (Quik et al., 2013c,d). The data in the right-hand panels in Fig. 3 are consistent with these previous studies, with a smaller nicotine-mediated decline in LIDs in the set B monkeys that had not previously received nicotine (Quik et al., 2013c,d). In set B, there was a significant main effect of treatment (F1,27 = 8.172, P < 0.01), but not dose (F2,27 = 2.49, P > 0.05), and no significant interaction (F2,27 = 2.49, P > 0.05) using two-way ANOVA.
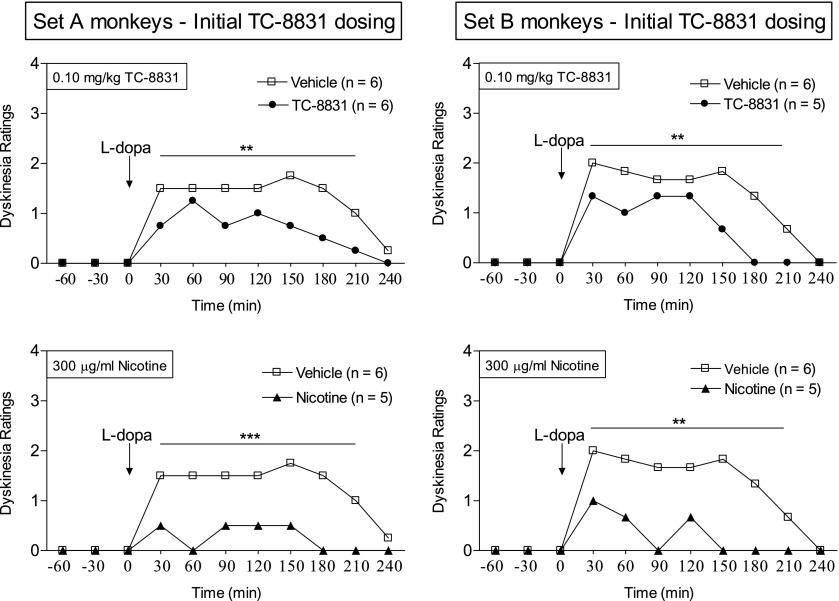
The afternoon hourly time courses demonstrate that TC-8831 reduced LIDs throughout the course of l-DOPA administration in both sets of monkeys (Fig. 4). The effect of TC-8831 was thus similar to that of nicotine, shown for comparison (Fig. 4). The data provided are for the 0.10 mg/kg dose (depicted in Fig. 3), with results similar to the other doses of TC-8831 tested.
Fig. 4.
TC-8831 treatment decreases the hourly time course of LIDs. TC-8831 was tested in a set of MPTP-lesioned monkeys previously exposed to nicotine and nAChR drugs (set A), as well as in a set of nAChR drug–naïve MPTP-lesioned monkeys (set B). Drug treatments were as described in the legend of Fig. 3, with the data shown for the afternoon 0.10 mg/kg TC-8831 dose averaged over several days. The effect of nicotine on LIDs is shown in the lower panels for comparison. The symbols depict the median of 5–6 monkeys. Significance of difference from vehicle using a Mann–Whitney test: **P < 0.01; ***P < 0.001.
After the initial dose-response studies depicted in Figs. 3 and 4, we repeated the 0.10 and/or 0.30 mg/kg doses for several weeks each. However, vomiting was observed and the data were therefore not used. The animals were then placed on a drug washout, with LIDs returning to the levels seen in vehicle-treated monkeys after 1 week (Table 1).
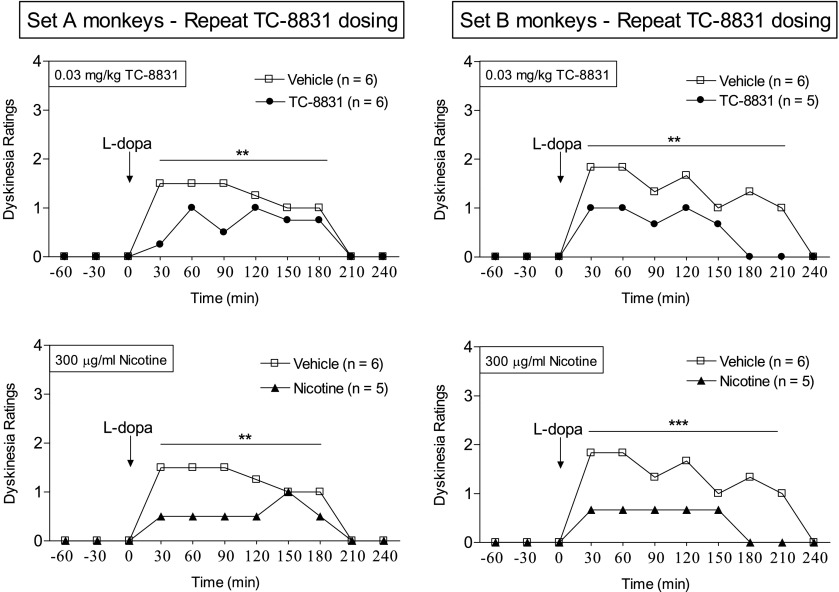
TC-8831 was subsequently retested for its effect on LIDs at the lowest doses initially used (Fig. 5). In the repeat TC-8831 dosing regimen, the maximal decline in LIDs was somewhat greater (50–70%) than in the initial study shown in Fig. 3. In set A, there was a significant main effect of treatment (F1,30 = 19.92, P < 0.001), but not dose (F2,30 = 2.51, P > 0.05), and no significant interaction (F2,30 = 2.51, P > 0.05) using two-way ANOVA. In set B, there was a significant main effect of treatment (F1,26 = 104.9, P < 0.001), but not dose (F2,26 = 0.57, P > 0.05), and no significant interaction (F2,26 = 0.57, P > 0.05) using two-way ANOVA. Interestingly, the decline in LIDs was similar to that of nicotine. In set A, there was a significant main effect of treatment (F1,27 = 87.70, P < 0.001), but not dose (F2,27 = 0.086, P > 0.05), and no significant interaction (F2,27 = 0.086, P > 0.05) using two-way ANOVA. In set B, there was a significant main effect of treatment (F1,27 = 62.07, P < 0.001), but not dose (F2,27 = 0.069, P > 0.05), and no significant interaction (F2,27 = 0.069, P > 0.05) using two-way ANOVA. There was no vomiting with the lower doses (0.01 and 0.03 mg/kg) of TC-8831. However, intermittent vomiting developed with the 0.05 mg/kg dose; higher TC-8831 doses were therefore not tested. The afternoon hourly time course of the development of LIDs shows that both TC-8831 and nicotine reduced LIDs with a similar time course (Fig. 6).
Fig. 5.
No tolerance to the TC-8831–mediated decline in LIDs with repeated dosing. TC-8831 administration was retested after a 2–3-week washout period in set A (nAChR drug primed) and set B (nAChR drug naïve). The monkeys were gavaged with l-DOPA twice daily, 5 days per week. TC-8831 (0.01–0.05 mg/kg) was given orally twice daily at a 3.5-hour interval at the same time as l-DOPA. The timeline is depicted in the upper panel. The middle panel shows the total dyskinesia scores (expressed as % vehicle) averaged over 2–3 days during the 4-hour period following the afternoon dose of l-DOPA. For comparison, the lower panel illustrates the effect of nicotine (300 µg/ml in the drinking water) on LIDs for the same time period as the TC-8831 data. Values are the mean ± S.E.M. of 5–6 monkeys. Significance of difference of drug treatment from vehicle: *P < 0.05; **P < 0.01; ***P < 0.001 using two-way ANOVA followed by a Bonferroni post-hoc test.
Fig. 6.
Hourly time course showing no tolerance to the TC-8831–mediated decline in LIDs with continued dosing. TC-8831 was retested in a set of MPTP-lesioned monkeys previously exposed to nicotine and nAChR drugs (set A), as well as in a set of nAChR drug–naïve MPTP-lesioned monkeys (set B). Drug treatments were as described in the legend of Fig. 5, with the data shown for the afternoon 0.03 mg/kg TC-8831 dose averaged over several days. The effect of nicotine on LIDs is shown in the lower panels for comparison. The symbol depicts the median of 5–6 monkeys. Significance of difference from vehicle using a Mann–Whitney test: **P < 0.01; ***P < 0.001.
TC-8831 readministration did not affect parkinsonism, similar to the results obtained with the initial dosing regimen (Table 2). TC-8831 also had no appreciable effect on a cognitive task designed for squirrel monkeys (Lyons et al., 2000; Lyons and Schatzberg, 2003). The data in Table 3 show no change in the average time and number of trials required for the monkeys to retrieve a marshmallow piece. Since parkinsonism was only mild to moderate, performance in the cognitive task was not affected by motor impairment. There were no adverse effects of TC-8831 on fluid intake, body weight, or general behavior, except for the vomiting at higher drug doses, as notedpreviously.
TABLE 3.
TC-8831 and nicotine treatment did not modulate cognitive performance
MPTP-lesioned monkeys were gavaged with l-DOPA and given vehicle, nicotine, or TC-8831 according to the treatment regimens depicted in Fig. 3 for TC-8831. The data shown were obtained in the same week as those provided in Fig. 4, with similar results throughout the study. Values are the mean ± S.E.M. of the number of monkeys indicated in parentheses.
| Set | Time to Retrieve Treat |
Number of Trials to Retrieve Treat |
||||
|---|---|---|---|---|---|---|
| Vehicle | Nicotine | TC-8831 | Vehicle | Nicotine | TC-8831 | |
| sec | sec | sec | ||||
| A | 8.3 ± 2.2 (6) | 8.0 ± 1.6 (5) | 8.0 ± 2.0 (6) | 6.7 ± 1.4 (6) | 6.6 ± 1.6 (5) | 7.7 ± 2.2 (6) |
| B | 13.2 ± 3.4 (6) | 13.8 ± 4.1 (5) | 12.0 ± 3.0 (5) | 14.6 ± 3.2 (6) | 13.7 ± 2.6 (5) | 13.6 ± 2.7 (5) |
Discussion
The present results show that two nAChR agonists with distinct pharmacological profiles reduced LIDs (30–60%) in parkinsonian monkeys. We selected the nAChR agonist varenicline because it is approved by the U.S. Food and Drug Administration and thus offered the benefit that it could be extended to the clinic with minimal delay. TC-8831 has the advantage that it is a CNS-selective nAChR agonist at α4β2* and α6β2* nAChRs, and thus may be associated with fewer peripheral side effects. Notably, there was no tolerance to the effect of either drug to reduce LIDs for the entire length of the study (3–4 months). Moreover, the drugs did not worsen parkinsonism or cognition. These data suggest that varenicline and TC-8831 may be useful for the treatment of LIDs in Parkinson’s disease patients.
The ability of nAChR agonists to reduce LIDs supports the idea that nicotine exerts its antidyskinetic effect via an interaction at nAChRs. As mentioned earlier, there are multiple nAChRs throughout the body, with the predominant populations in the striatum being the α4β2* and α6β2* nAChR subtypes (Gotti et al., 2009; Millar and Gotti, 2009; Quik and Wonnacott, 2011). Although initial studies suggested that varenicline was selective for α4β2* nAChRs, subsequent work showed that it also interacted with α6β2*, α3β4*, and α7 nAChRs (Coe et al., 2005; Mihalak et al., 2006; Grady et al., 2010; Ween et al., 2010; Chatterjee et al., 2011; Bordia et al., 2012). Varenicline’s biological effects may thus be more similar to those of nicotine, which also stimulates multiple nAChR populations. With respect to the clinical relevance of the current dosing regimen, the dose of varenicline prescribed for smoking cessation is 1 mg twice daily. The maximal effective dose of varenicline in our studies was 0.03 mg/kg twice daily, which is similar to the dose used for smoking cessation on a per-weight basis. In humans, varenicline is associated with nausea and vomiting in ∼25% of users (Cahill et al., 2011; Swan et al., 2012). However, there were no issues with vomiting in our studies when varenicline treatment was initiated at a low dose and incrementally increased in half-log intervals, suggesting it may be useful for the treatment of LIDs. Notably, a recent clinical trial showed that varenicline also improved gait and stance in patients with spinocerebellar ataxia and was fairly well tolerated (Zesiewicz et al., 2012).
Our data with TC-8831 show that a drug that interacts specifically with CNS α4β2* and α6β2* nAChRs, those most densely expressed in the striatum, also reduced LIDs. These data are consistent with previous work showing that TC-8831 attenuated LIDs in MPTP-lesioned macaques (Johnston et al., 2013). Our initial dose-response curves are similar to those of Johnston and coworkers, indicating that the effect of the nAChR agonist TC-8831 is comparable in different monkey species. However, there appeared to be a U-shaped dose-response curve in the macaque, suggesting tolerance with time and/or increasing dose (Johnston et al., 2013). The present repeat-dosing regimen clearly shows that no tolerance developed with increased TC-8831 administration. In fact, a somewhat greater decline in LIDs was observed with a repeat TC-8831 regimen, such that the reduction in LIDs approached that obtained with nicotine. These results suggest that stimulation of α4β2* and/or α6β2* nAChRs may be sufficient to reduce LIDs.
With respect to the issue of tolerance, the antidyskinetic effect of both varenicline and TC-8831 persisted over the entire 3–4 months of the study. These data are similar to those previously obtained with nicotine, which also consistently reduced LIDs over ∼6 months with no indication of a diminished response (Quik et al., 2013d).
Our data further show that previous nAChR drug treatment does not impact the effect of subsequent dosing with other nAChR agonists on LIDs—that is, there is no sensitization or desensitization among the agonists tested, at least after a washout period (10 weeks). By contrast, prolonged use appeared to enhance drug effectiveness in some cases, as nicotine best reduced LIDs after 2 or 3 months of treatment.In addition, TC-8831 treatment led to a greater decline in LIDs with repeated dosing. However, with the repeat TC-8831 dosing regimen, the monkeys were also more sensitive to vomiting, which now occurred at lower doses.
With respect to mechanism of action, nAChR agonist may improve LIDs in parkinsonian animals by continuously desensitizing or inactivating nAChR-mediated function. Our recent studies show that long-term nicotine treatment decreased dopamine release in parkinsonian rats (Bordia et al., 2013). Thus, nicotine treatment may dampen the aberrant dopamine release which is thought to underlie the development of LIDs (Cenci, 2007; Lindgren et al., 2010; Carta and Bezard, 2011; Fisone and Bezard, 2011). Support for a role for desensitization stems from studies showing that both nAChR agonists and antagonists reduced LIDs to a similar extent in parkinsonian rats (Bordia et al., 2010). In addition, intermittent nicotine exposure via injection or continuous treatment via a slow-release minipump that promotes receptor desensitization both reduce LIDs to a similar extent (Bordia et al., 2008, 2010). The idea that nicotine affects LIDs via desensitization is consistent with its effects in depression, addiction, and pain (Picciotto et al., 2008; Buccafusco et al., 2009; Dopico and Lovinger, 2009) (Mineur and Picciotto, 2010; Zhang et al., 2012).
An extensive literature indicates that nicotine and nAChR drugs may enhance cognition in experimental animal models (Terry and Decker, 2011; Gao et al., 2012). The current data show that varenicline and TC-8831 did not influence performance on a working memory task in MPTP-lesioned monkeys. This finding provides indirect support for the contention that nAChR agonists do not cause sedation.
Parkinsonism was not worsened with either varenicline or TC-8831, in agreement with our previous results showing no effect of nicotine on motor function in parkinsonian animal models (Quik et al., 2007, 2013a,c). This finding is consistent with the results of clinical reports and trials in Parkinson’s disease patients, which also report no consistent improvement in motor tasks. Although the open-label studies generally demonstrated a positive effect of nicotine on motor symptoms (Marshall and Schnieden, 1966; Ishikawa and Miyatake, 1993; Kelton et al., 2000; Mitsuoka et al., 2002; Lemay et al., 2004; Hanagasi et al., 2007; Villafane et al., 2007), there was very little clinical efficacy of nicotine in the double-blinded trials, which also included significantly larger groups of patients (Fagerstrom et al., 1994; Clemens et al., 1995; Ebersbach et al., 1999; Vieregge et al., 2001; Shoulson, 2006). Although the variability of outcomes in the clinical studies may be due to differences in nicotine dosing, duration of treatment, outcome measure evaluated, small cohorts, and/or clinical stage, the nature of the type of trial, that is, open-label versus double-blinded, may be the important variable. The finding that nicotine and nAChR agonists improve LIDs with no apparent effect on parkinsonism is consistent with preclinical studies, suggesting that these two behaviors may be differentially regulated by the direct and indirect pathways. Activation of the direct pathway may be more closely associated with LIDs, whereas parkinsonism may be linked to activation of the indirect pathway (Santini et al., 2008; Kravitz et al., 2010; Huot et al., 2013).
In summary, the present results show that nAChR agonists reduce LIDs in a nonhuman primate parkinsonian model. These data provide proof of concept for the idea that drugs that interact with CNS nAChRs may be of value as antidyskinetic agents in Parkinson’s disease. Moreover, both drugs were effective following oral administration, a mode of administration that readily extends to human use.
Acknowledgments
The authors thank Tanuja Bordia and Xiomara Perez for helpful comments concerning the manuscript, and Jason Ly for excellent technical assistance.
Abbreviations
- A85380
3-[(2S)-2-azetidinylmethoxy-5-iodo-pyridine dihydrochloride
- ANOVA
analysis of variance
- CNS
central nervous system
- LIDs
l-DOPA–induced dyskinesias
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- nAChR
nicotinic acetylcholine receptor
- OHDA
6-hydroxydopamine
- TC-8831
3-cyclopropylcarbonyl-3,6-diazabicyclo[3.1.1]heptane
- *
the possible presence of other nicotinic subunits in the receptor complex
Authorship Contributions
Participated in research design: Quik, Zhang, Mallela, Letchworth.
Conducted experiments: Zhang, Mallela, Sohn, Quik.
Contributed new agents or analytic tools: Carroll, Bencherif, Letchworth.
Performed data analysis: Zhang, Mallela, Sohn, Quik.
Wrote or contributed to the writing of the manuscript: Quik, Zhang, Mallela.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS59910, NS65851].
References
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 327:239–247 [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. (2010) Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther 333:929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Hrachova M, Chin M, McIntosh JM, Quik M. (2012) Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther 342:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, McIntosh JM, and Quik M (2013) The nicotine-mediated decline in L-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem DOI: 10.1111/jnc.12179 [published ahead of print]. [DOI] [PMC free article] [PubMed]
- Brotchie J, Jenner P. (2011) New approaches to therapy. Int Rev Neurobiol 98:123–150 [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV., Jr (2009) Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther 328:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. (2011) Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev (2):CD006103. [DOI] [PubMed] [Google Scholar]
- Carta M, Bezard E. (2011) Contribution of pre-synaptic mechanisms to L-DOPA-induced dyskinesia. Neuroscience 198:245–251 [DOI] [PubMed] [Google Scholar]
- Cenci MA. (2007) Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci 30:236–243 [DOI] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. (2010) Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 183:209–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE. (2011) Partial agonists of the α3β4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology 36:603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens P, Baron JA, Coffey D, Reeves A. (1995) The short-term effect of nicotine chewing gum in patients with Parkinson’s disease. Psychopharmacology (Berl) 117:253–256 [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, et al. (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477 [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. (2009) Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev 61:98–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G, Stöck M, Müller J, Wenning G, Wissel J, Poewe W. (1999) Worsening of motor performance in patients with Parkinson’s disease following transdermal nicotine administration. Mov Disord 14:1011–1013 [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Pomerleau O, Giordani B, Stelson F. (1994) Nicotine may relieve symptoms of Parkinson’s disease. Psychopharmacology (Berl) 116:117–119 [DOI] [PubMed] [Google Scholar]
- Fisone G, Bezard E. (2011) Molecular mechanisms of l-DOPA-induced dyskinesia. Int Rev Neurobiol 98:95–122 [DOI] [PubMed] [Google Scholar]
- Gao X, Simon KC, Schwarzschild MA, Ascherio A. (2012) Prospective study of statin use and risk of Parkinson disease. Arch Neurol 69:380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78:703–711 [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, et al. (2010) Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30:5311–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, et al. (2010) Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology 58:1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanagasi HA, Lees A, Johnson JO, Singleton A, Emre M. (2007) Smoking-responsive juvenile-onset Parkinsonism. Mov Disord 22:115–119 [DOI] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. (2011b) Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology 60:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Grady SR, Quik M. (2011a) Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors. J Pharmacol Exp Ther 338:932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. (2013) The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65:171–222 [DOI] [PubMed] [Google Scholar]
- Iravani MM, Jenner P. (2011) Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm 118:1661–1690 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Miyatake T. (1993) Effects of smoking in patients with early-onset Parkinson’s disease. J Neurol Sci 117:28–32 [DOI] [PubMed] [Google Scholar]
- Johnston TH, Huot P, Fox SH, Koprich JB, Szeliga KT, James JW, Graef JD, Letchworth SR, Jordan KG, Hill MP, et al. (2013) TC-8831, a nicotinic acetylcholine receptor agonist, reduces l-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology 73C:337–347 [DOI] [PubMed] [Google Scholar]
- Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. (2000) The effects of nicotine on Parkinson’s disease. Brain Cogn 43:274–282 [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay S, Chouinard S, Blanchet P, Masson H, Soland V, Beuter A, Bédard MA. (2004) Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 28:31–39 [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. (2010) L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1465–1476 [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF. (2000) Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J Neurosci 20:7816–7821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Schatzberg AF. (2003) Early maternal availability and prefrontal correlates of reward-related memory. Neurobiol Learn Mem 80:97–104 [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Eliez S, Reiss AL, Schatzberg AF. (2004) Cognitive correlates of white matter growth and stress hormones in female squirrel monkey adults. J Neurosci 24:3655–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Schnieden H. (1966) Effect of adrenaline, noradrenaline, atropine, and nicotine on some types of human tremor. J Neurol Neurosurg Psychiatry 29:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. (2010) Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka T, Kaseda Y, Yamashita H, Kohriyama T, Kawakami H, Nakamura S, Yamamura Y. (2002) Effects of nicotine chewing gum on UPDRS score and P300 in early-onset parkinsonism. Hiroshima J Med Sci 51:33–39 [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84:329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Mahlknecht P, Jankovic J. (2012) Emerging therapies for Parkinson’s disease. Curr Opin Neurol 25:448–459 [DOI] [PubMed] [Google Scholar]
- Prashanth LK, Fox S, Meissner WG. (2011) l-Dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol 98:31–54 [DOI] [PubMed] [Google Scholar]
- Quik M, Campos C, Bordia T, Strachan JP, Zhang J, McIntosh JM, Letchworth S, Jordan K. (2013a) Α4β2 nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology 71:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Campos C, Grady SR. (2013b) Multiple CNS nicotinic receptors mediate l-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol DOI: 10.1016/j.bcp.2013.06.027 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D. (2007) Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol 62:588–596 [DOI] [PubMed] [Google Scholar]
- Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. (2013c) Nicotine-mediated improvement in L-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis 50:30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Mallela A, Ly J, Zhang D. (2013d) Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord DOI: 10.1002/mds.25594 (published ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA. (2006) Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem 98:1866–1875 [DOI] [PubMed] [Google Scholar]
- Quik M, Park KM, Hrachova M, Mallela A, Huang LZ, McIntosh JM, Grady SR. (2012) Role for α6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology 63:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. (2011) α6β2* and α4β2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev 63:938–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Lozano A, Stern M, Poewe W. (2011) Milestones in Parkinson’s disease therapeutics. Mov Disord 26:1072–1082 [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW, Jr, Ashare RL, Schlienz NJ, Mahoney MC. (2012) The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 223:131–138 [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. (2007) Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci 28:316–325 [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Fisone G. (2008) Parkinson’s disease: levodopa-induced dyskinesia and signal transduction. FEBS J 275:1392–1399 [DOI] [PubMed] [Google Scholar]
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, Lee SW, Kong BG, Kang JW, Oh MK, et al. (2012) Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology 37:660–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulson I, Parkinson Study Group (2006) Randomized placebo-controlled study of the nicotinic agonist SIB-1508Y in Parkinson disease. Neurology 66:408–410 [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Wessel J, Michel M, Hinds DA, Stokowksi RP, McClure JB, Catz SL, Richards J, et al. (2012) Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics J 12:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LC, Protell PH, Langston JW, Togasaki DM. (2002) The hyperkinetic abnormal movements scale: a tool for measuring levodopa-induced abnormal movements in squirrel monkeys. Mov Disord 17:902–909 [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Decker MW. (2011) Neurobiology of nAChRs and cognition: a mini review of Dr. Jerry J. Buccafusco’s contributions over a 25 year career. Biochem Pharmacol 82:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P. (2001) Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology 57:1032–1035 [DOI] [PubMed] [Google Scholar]
- Villafane G, Cesaro P, Rialland A, Baloul S, Azimi S, Bourdet C, Le Houezec J, Macquin-Mavier I, Maison P. (2007) Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur J Neurol 14:1313–1316 [DOI] [PubMed] [Google Scholar]
- Ween H, Thorin-Hagene K, Andersen E, Grønlien JH, Lee CH, Gopalakrishnan M, Malysz J. (2010) Alpha3* and alpha 7 nAChR-mediated Ca2+ transient generation in IMR-32 neuroblastoma cells. Neurochem Int 57:269–277 [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, Chen R, Perlman SL. (2012) A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology 78:545–550 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xiao YD, Jordan KG, Hammond PS, Van Dyke KM, Mazurov AA, Speake JD, Lippiello PM, James JW, Letchworth SR, et al. (2012) Analgesic effects mediated by neuronal nicotinic acetylcholine receptor agonists: correlation with desensitization of α4β2* receptors. Eur J Pharm Sci 47:813–823 [DOI] [PubMed] [Google Scholar]